Role of monocytes in the pathogenesis of antiphospholipid syndrome and potential therapeutic targets (Review)
- Authors:
- Published online on: September 2, 2025 https://doi.org/10.3892/mmr.2025.13670
- Article Number: 305
-
Copyright: © Huo et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Antiphospholipid syndrome (APS) is an autoimmune disorder characterized by arterial and/or venous thrombosis, obstetric complications and persistently positive antiphospholipid antibodies (aPLs) (1). The aPLs include lupus anticoagulants, anticardiolipin antibodies and anti-β-2-glycoprotein-I (β2GPI) antibodies (2). Thrombosis can be present in any tissue or organ, such as the skin, eyes, heart, lungs, kidneys and the micro-vessels of other organs, with the deep veins of the lower extremities and the cerebral arteries being the most commonly affected. Notably, APS can cause damage and endanger life, thus imposing a considerable health burden (3,4). Furthermore, pregnancy-related complications associated with APS, such as fetal death and eclampsia, have a notable impact on health and quality of life (5).
The mechanisms underlying thrombosis and obstetric complications in APS have not yet been fully elucidated. At present, various pathogenic mechanisms underlying this disorder have been proposed, including activation of vascular endothelial cells (6), activation of complement and complement-mediated injury (7,8), activation of platelets (9), activation of the coagulation coupling system, inhibition of the fibrinolysis system (10,11) and activation of neutrophils (12), T cells (13) and extracellular vesicles have pro-coagulant effects (14,15).
In addition to the aforementioned pathogenic immune cells, current studies have shown that monocytes are involved in thrombosis and obstetric complications (16,17), but evidence regarding their role in the development of APS is limited (17). Monocyte activation can be induced in patients with APS through different pathways, including the direct binding of aPLs to monocytes and activation through the complement pathway. Monocytes can differentiate into other forms of immune cells by themselves, and secrete pro-inflammatory and pro-coagulant factors, thus disrupting the balance of the immune system, which is closely related to thrombosis and obstetric complications in APS (18,19). However, further related studies are required. Therefore, the aim of the present study was to review the role of monocytes in APS, thereby providing further understanding of the pathogenesis of APS and to potentially identify therapeutic targets for the treatment of APS. Fig. 1 shows a schematic diagram summarizing how monocytes can act as potential therapeutic targets for APS.
Epidemiology of APS
At present, based on recently summarized data, the estimated annual incidence rate of APS is 1–2 cases/100,000 individuals within the global population, and the prevalence rate is 40–50 cases/100,000 individuals (20). Notably, there are differences in these rates among countries and regions (21–23). According to different regional studies (including the United States, South Korea, Argentina, Northwest Italy and Spain), the peak age of onset of APS in men is 55–79 years and it is 30–39 years in women (20,21), and the ratio of female to male patients with primary APS is 2–4:1 (20,21). Secondary APS is mainly related to systemic lupus erythematosus, with a ratio of 1:7. Catastrophic APS (CAPS) accounts for <1% of all patients with APS (20,24). The 10-year survival rate of APS ranges between 80 and 90.7%, and the standardized mortality rate is 1.61–1.8/100,000 individuals (23,25).
Diagnosis of APS
In 1999, an expert panel formulated the classification criteria for APS (1), and the classification criteria were revised in 2006 in Sydney, Australia (1). Regarding the clinical manifestations of APS, thrombosis and obstetric complications are predominant, and the main serological manifestation is positivity for aPLs (Table I) (26). In addition to the aforementioned typical clinical manifestations, some patients are persistently positive for aPLs and present only with ‘non-standard’ manifestations. Non-standard manifestations occur in other organs; for example, when the kidneys are involved, APS can present as hypertension and proteinuria (27). When the skin and corresponding blood vessels are involved, APS may present as reticular blue spots (1). When the cardiovascular system is involved, APS manifests as myocarditis, valvular heart disease or diastolic dysfunction (28). Neurological diseases can manifest as dementia, epilepsy, migraine, myelitis and chorea (28). In addition to the standard aPLs listed in Table I, non-standard aPLs, such as antiphosphatidylserine/prothrombin, anti-dome-specific anti-β2GPI, anti-annexin A5 and immunoglobulin A antibodies (29), serve an important role in supplementing the diagnosis of APS; however, more studies are required for further confirmation. In the latest APS classification criteria (1), which were jointly developed in 2023 by the American College of Rheumatology and the European League Against Rheumatism, clinical manifestations such as heart valve and hematological (thrombocytopenia) pathologies were included in the classification criteria. Compared with the Sapporo classification criteria revised in 2006 (30), the specificity was 99 vs. 86%, and the sensitivity was 84 vs. 99% (1). CAPS is rare; however, due to microvascular thrombosis, it can lead to multiple organ failure and is life-threatening. The diagnosis of CAPS should meet the following four classification criteria: Clinical manifestations of damage to at least three organs and systems; clinical manifestations developed simultaneously and rapidly within 1 week; laboratory-confirmed presence of aPLs; and histopathological evidence of small-diameter vascular obstruction (31).
Monocytes
Monocytes are an important immune cell, accounting for ~10% of total nucleated cells in circulating blood (32,33), and they are the main components in autoimmune responses. When entering tissues, monocytes trigger endogenous inflammatory processes (34), and in response to damage or infection, monocytes rapidly participate in regulating the inflammatory response and promoting the repair of damaged tissues (35). Monocytes are not only heterogeneous but they can also self-renew. Their membranes express various molecular markers, such as lipopolysaccharide (LPS)-related receptors [Toll-like receptors (TLRs)], complement receptors and cytokine receptors, and perform multiple functions, including participating in thrombosis and destroying pathogens (36). Monocytes can be classified into the following three subgroups based on their expression of CD14 (LPS-related receptor) and CD16 (FcγIII receptor) (37,38): i) Classical monocytes, which express the surface markers CD14++CD16− and C-X-C chemokine receptor 2+CX3CR1−, and account for 80–95% of monocytes; this subgroup mainly performs phagocytosis and tissue repair functions, and secretes cytokines, such as IL-1, IL-10, IL-12 and TNF-α (39,40). ii) Intermediate monocytes, which express the surface markers CD14++CD16+ and C-C chemokine receptor (CCR)2−CX3CR1+, and account 2–11% of monocytes; this subgroup has a highly pro-inflammatory cellular effect, generates a high level of reactive oxygen species (ROS) and inflammatory mediators, and can secrete cytokines, such as IL-1β, IL-6 and TNF-α (40,41). iii) Non-classical monocytes, which express the surface markers CD14++CD16+ and CCR2−CX3CR1+, and account for 2–8% of monocytes; this subgroup mainly serves roles in patrolling, clearing debris, eliminating apoptotic cells and in the antiviral response, and can secrete cytokines, such as IL-1β, IL-6 and TNF-α (40,42). Among patients with APS, the classic and intermediate types of monocytes are dominant, and at the same time, their surface adhesion is enhanced (43), participating in activation of complement and endothelial cell injury, and thus they are related to the pathogenesis of thrombosis and adverse pregnancy outcomes in APS.
Role of monocytes in normal coagulation
As aforementioned, monocytes have receptors that enable sensing of vascular damage, inflammation or infection, leading to activation. They can then migrate into tissues and differentiate into dendritic cells and macrophage subtypes. In addition to serving a role in innate immune responses and tissue repair, monocytes are actively involved in hemostasis (44). When blood vessels are damaged, platelets are recruited and they interact with monocytes, thus activating each other. Simultaneously, cytokines (such as TNF-α, IL-8 and IL-1β) are released, and the two form heterogeneous cell complexes (45). After platelets are activated, they can express P-selectin (CD62P) on their surface, which binds to the related receptors expressed by monocytes. Meanwhile, platelets can also adhere to monocytes through bridging molecules, such as fibrinogen and thrombomodulin, thereby contributing to the formation of more complexes (46,47). Under the mediation of CCR1, this complex increases the binding of platelet factor 4 to the surface of monocytes and markedly enhances the arrest of monocytes on endothelial cells induced by CCL5, thereby recruiting more platelets to aggregate at the site of injured endothelial cells (48–50), which is conducive to hemostasis. Furthermore, under normal circumstances, the activity of tissue factor (TF) expressed by monocytes is low and can be transformed into fully pro-coagulant TF through specific inflammatory agonist pathways. For example, when CD162P binds to its receptor, it can rapidly increase TF exposure and TF-dependent coagulation activity in monocytes, even in the absence of protein synthesis. This may be related to the conformation or orientation changes of the extracellular domain of the protein when exposed to anionic phospholipids on the surface of monocytes (51). In this scenario, monocytes roll on the surface of blood vessels and adhere in large quantities to the luminal surface of the affected blood vessels. Simultaneously, they deposit together with other white blood cells under the mediation of CD62P to initiate thrombosis (52). The TF formed by monocytes also initiates in vivo coagulation through factor VII, promotes the release of thrombin, and converts fibrinogen into fibrin. Activation of coagulation factor XIII is beneficial for maintaining the stability of fibrin clots, and the coagulation process is further amplified by the activation of factors V, VIII and XI (53), thereby exerting hemostasis. Notably, a previous study has shown that in monocytes of healthy individuals, when platelets and CD62P are co-cultured, there is a marked upregulation of the expression of TF. Meanwhile, monocytes can further enhance the activation of platelets, thereby enabling the secretion of more platelet-derived growth factors and soluble CD62P (54). When platelets and CD62P combine with polystyrene beads, they effectively increase the exposure of TF in monocytes, thereby unlocking potential TF activity (54) and participating in hemostasis.
Role of monocytes in normal pregnancy
During pregnancy, the innate immune response usually changes. Compared with monocytes in non-pregnant individuals, the monocytes in pregnant individuals exhibit increased expression of CD14 and CD64, increased number and activation, and functional changes, indicating phenotypic activation of monocytes during pregnancy (55). During a healthy pregnancy, intermediate monocytes increase in number, while classical monocytes decrease in number and release inflammatory cytokines such as TNF-α and interferon (IFN)-γ. In a normal pregnancy, an appropriate amount of TNF-α can not only protect the placental unit, but also change the adhesion of trophoblast cells to laminin and inhibit their mobility, thereby regulating the invasion of trophoblast cells (56). IFN-γ not only downregulates protease activity and increases apoptosis in extravillous trophoblasts (EVTs), thereby preventing excessive invasion of EVT, but also contribute to vascular remodeling during implantation, improving nutrient delivery to the implanted cells (57,58). Therefore, IFN-γ serves a key role in early placental development and trophoblast invasion. After the implantation period ends, the T helper (Th)1-dominant immune advantage in the decidua gradually shifts toward a Th2-dominant profile (59). This advantage is related to IL-4 released by monocytes, which can bias T-cell subsets towards Th2, and Th2 cells can induce a local Th2 advantage by releasing Th2 cytokines after infiltrating into the decidua basalis (60). For example, the release of IL-4, IL-10 and IL-13 inhibits the development of Th1 and Th17 immunity, and promotes allograft tolerance, thus facilitating pregnancy (61).
In addition, trophoblasts express and secrete chemokines, and recruit monocytes into the decidua, where monocytes differentiate into dendritic cells and can be functionally reprogrammed by decidua stromal cells under the action of IL-1β and granulocyte colony-stimulating factors, thus altering the cytokine profile secreted by monocyte-derived dendritic cells. The secretion of IL-1β, IL-6 and IL-10 is increased, and the secretion of IL-8 and TNF-α is decreased, thus differentiating lymphocytes to the Th2 spectrum (62,63). IL-1β activity is positively associated with the enzyme activities of matrix metalloproteinase (MMP)2 and MMP9, thereby enhancing the phagocytic ability of macrophages and degrading the extracellular matrix to promote trophoblast invasion (64). In addition, monocyte-derived decidua macrophages secret vascular endothelial growth factor (VEGF) and placental growth factor, which are considered to serve an important role in the early stage of spiral artery remodeling (65). Therefore, monocytes have an important role in normal pregnancy.
Relationship between monocytes and thrombosis in APS
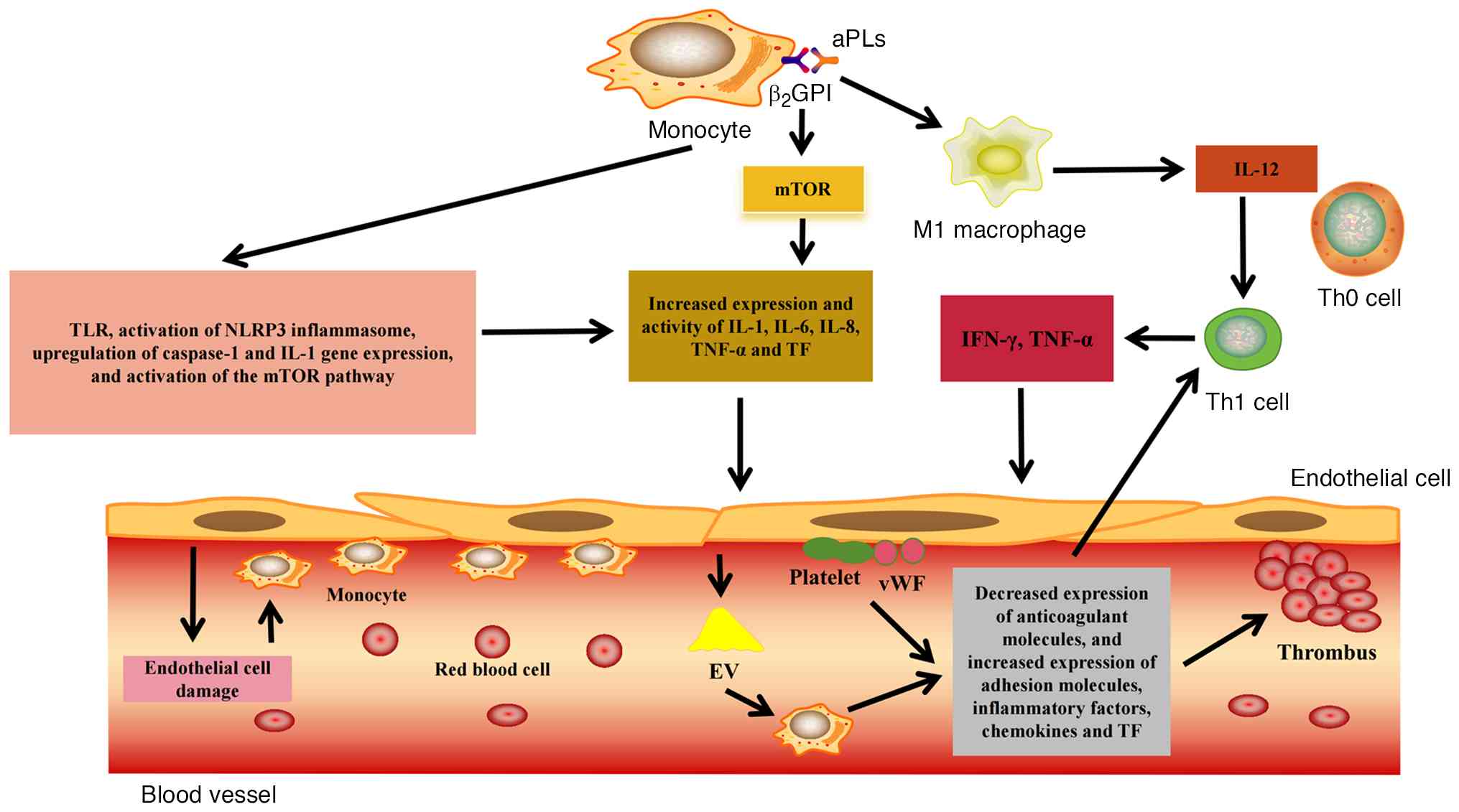
In vitro studies of APS have revealed that, compared with controls (anti-β2GPI peptide-treated monocytes that were isolated from the venous blood of 30 healthy, age and sex-matched subjects), monoclonal aPLs isolated from patients with APS (that were then co-cultured with mononuclear cells from healthy donors), result in elevated TNF-α levels (66,67). When aPLs bind to the β2GPI protein on monocytes, TLR-4 activates the myeloid differentiation primary reactive protein signaling pathway, thus leading to the release of various pro-inflammatory cytokines, including IL-1, IL-6, IL-8 and TNF-α (68,69). Studies using monoclonal aPLs co-cultured with healthy mouse monocytes have revealed that aPLs activate the NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome in monocytes, inducing expression of caspase-1 and increasing its enzymatic activity. This simultaneously upregulates IL-1 gene expression and IL-1 protein production, thereby increasing TF expression and activity in monocytes (62,70,71). Through monocytes, APS induces inflammation, particularly when monocytes adhere to endothelial cells, causing chronic endothelial inflammation and damage. This process recruits more monocytes, which roll along, firmly adhering to the injured vessel wall, migrating into tissues and differentiating (72). After differentiation into M1 macrophages, they can phagocytize excessive oxidized low-density lipoproteins and other immune complexes, leading to apoptosis, necrosis, lipid deposition, and the release of numerous inflammatory cytokines and TFs that further recruit circulating monocytes (72). Inflammatory cytokines produced by M1 macrophages, such as IL-12, promote differentiation of T cells into Th1 cells. Locally produced IFN-γ and TNF-α by activated Th1 cells increase the expression of class II major histocompatibility complex molecules on endothelial cells, enabling these cells to present β2GPI to T cells. This process further amplifies differentiation into Th1 effector cells (73), exacerbating endothelial cell damage. Moreover, anti-β2GPI antibodies can activate the mammalian target of rapamycin (mTOR) pathway in monocytes, thereby upregulating the expression of TF and IL-8, which is closely related to the formation of pro-inflammatory and pro-coagulant phenotypes in monocytes (74).
Normally, when the vascular endothelium is damaged, collagen is exposed to the circulation under high shear flow, and platelet-rich thrombosis is formed under the action of monocytes (64). The activation of monocytes, platelets and endothelial cells under the interaction of various receptors and ligands can lead to increased expression of adhesion molecules, such as E-selectin, increased inflammatory response, and a pro-coagulant effect by reducing the expression of anti-coagulant molecules, such as thrombomodulin (64). In addition, endothelial cells can transfer related substances, such as microRNA (miR)-125a-5p and miR-222, to monocytes via extracellular vesicles, while releasing cytokines, chemokines and TF (75). Since anti-β2GPI antibodies hinder clearance of extracellular vesicles, they can further trigger the immune complex to induce monocyte activation, resulting in the secretion of inflammatory cytokines and triggering a coagulation cascade (64). In patients with APS (67), and in an in vitro study simulating CAPS (43), it has been reported that monocytes express heterodimers of CD49d that can bind to ligand vascular cell adhesion molecule 1, causing monocytes to adhere to damaged endothelial cells (76), which is associated with thrombosis. Fig. 2 summarizes the potential role of monocytes in thrombosis in APS, as aforementioned.
Relationship between monocytes and obstetric complications in APS
In APS, monocytes evolve into dendritic cells and secrete cytokines, such as IL-4, to induce activation of autoreactive B cells, thereby producing aPLs (35,77). After binding with an autoantigen, these antibodies form immune complexes, and their deposition can induce the activation of complement. Meanwhile, activation of monocytes can reduce complement regulatory proteins, resulting in the production of the allergic toxin C5a (78,79). After C5a binds to the C5a receptor on monocytes, it not only leads to the release of inflammatory mediators, such as chemokines, cytokines and C3, but it also attracts a large number of monocytes to infiltrate the decidua. This results in a substantial inflammatory response, which enhances the activation and deposition of C3, leading to activated monocytes entering the placenta and causing placental inflammation (75,80); this eventually leads to fetal damage. In the decidua, monocytes not only participate in the expression of TF, but also produce a large number of ROS, and the induction of oxidation can cause fetal membrane injury and fetal death (64). Activated monocytes can also secrete monocyte chemoattractant protein 1 (MCP-1), which binds to CCR2 when there is an immune imbalance, and mediates the transformation of T cells to Th1 and the production of Th1 cytokines, such as TNF-α and INF-γ (81). Activated monocytes in the placenta secrete cytokines, such as IL-1, causing T cells to differentiate into Th17 cells. This further activates natural killer cells and secretes IFN-γ, TNF-α, perforin and granzyme, mediating their cytotoxic activity against target cells (82); this may damage placental vascular endothelial cells and lead to micro-thrombotic events in the placenta. This subsequently leads to obstetric complications. Excess Th17 cells and their secreted cytokines may also lead to uncontrolled infiltration of neutrophils at the maternal-fetal interface (83), which can lead to a further elevated local TNF-α level, decreased essential angiogenic factors and VEGF, and ultimately abnormal placenta formation and fetal death.
TNF-α increases the plasminogen activator inhibitor-1 level from trophoblastic cells, reduces the invasive capability of trophoblastic cells, activates endothelial cells, induces the expression of MMP-9 in the decidual membrane and destroys the decidual extracellular matrix, thus interfering with normal EVT invasion (81). TNF-α can also increase the expression of MCP-1. With an increase in MCP-1 levels, more M1-type macrophages are recruited and stimulated to secrete more pro-inflammatory factors, thus further promoting the expression of MCP-1, forming a positive feedback loop and a vicious cycle (17,84), which can result in miscarriage. When MCP-1 levels increase, the number of CD14+CD11c+CD163− monocytes is markedly increased, which enhances Fas-mediated EVT cell apoptosis and interferes with placental implantation (85,86). In addition, MCP-1 and IL-8 attract circulating monocytes to the wall of the placental blood vessels and damage the vascular endothelium in a manner similar that seen in atherosclerotic lesions, resulting in hypoxia caused by insufficient blood supply to the placenta, thereby reducing the expression of atypical chemokine receptor 2, and promoting MCP-1 upregulation and trophoblast apoptosis through negative feedback. This is associated with pre-eclampsia (87,88). Soluble fms-like tyrosine kinase 1 (sFlt-1) is an anti-angiogenic factor, which has an association with the development of pre-eclampsia. In mouse models with an inducible form of human sFlt-1, the expression of this protein in the early stage of pregnancy triggers fetal growth restriction, a finding accompanied by serious defects in placental formation (89). Furthermore, increased levels of anti-angiogenic factors (such as sFlt-1) cause maternal endothelial dysfunction (89). Excessive release of sFlt-1 selectively binds to VEGF, thereby inhibiting the binding of VEGF to its membrane receptors, while inhibiting TGF-β1 signaling, which can lead to reduced production of nitric oxide, endothelial cell dysfunction and vasodilatory disorders. In this scenario, a hypoxic environment causes placental cells to activate hypoxia-inducible factor-1 (90). In patients with APS, the aforementioned pathways may lead to fetal resorption by continuously amplifying the inflammatory response, inhibiting uterine spiral artery remodeling, damaging the placenta and reducing the number of fetal capillaries. Fig. 3 summarizes the potential role of monocytes in obstetric complications of APS, as discussed in the present study.
Potential therapeutic targets for monocyte-induced immune disorders in APS
The pathogenesis of APS is complex, and the specific, targeted treatment remains unclear. Currently, heparin, aspirin and hydroxychloroquine (HCQ) are considered key therapeutic agents in managing APS (91). The mechanism by which these drugs act in APS has been explored in previous studies. For example, aspirin may exert its effect by modulating lipoproteins and blocking the impact of aPL on human trophoblast migration and endothelial cell interactions (92). Heparin has been shown to prevent APL-induced fetal loss by inhibiting the activation of complement and regulating the balance between T-cell subsets (93). HCQ can not only reduce the production of inflammatory cytokines, such as TNF-α, IFN-γ and IL-6, by peripheral blood monocytes, but also reduce TF expression, and the plasma levels of soluble cell adhesion molecules, such as E-selectin, and vascular cell adhesion molecules, while improving the formation of endothelial nitric oxide synthase (94–97). As aforementioned, monocyte-induced immune dysregulation serves a notable role in the development and progression of APS, contributing to both obstetric complications and thrombotic events. Therefore, pharmacological strategies aimed at correcting monocyte-related immune abnormalities, particularly their pro-inflammatory and pro-coagulant phenotypes, are essential for improving patient outcomes. Identifying new therapeutic targets to address monocyte-driven immune dysfunction is urgently needed and may provide valuable insights for advancing APS treatment (Table II) (74,98–106).
TNF-α blockers can completely inhibit β2GPI-induced TF expression in monocytes, while improving endothelial dysfunction and preventing fetal resorption. A previous study evaluated their efficacy in 18 women with persistent aPL positivity and recurrent adverse pregnancy outcomes despite treatment with low molecular weight heparin (LMWH) combined with low-dose aspirin (LDA) and HCQ. Of these patients, 16 initiated adalimumab and 2 initiated certolizumab in addition to the original regimen; 12 women achieved successful pregnancy outcomes, including 9 term deliveries and 3 preterm births. This suggests that the combination of LMWH, LDA and TNF-α blockers may be a promising therapeutic approach for refractory obstetric manifestations associated with aPL, with good tolerability and no reported adverse reactions (107). However, the limited sample size in this previous study necessitates larger-scale trials to further evaluate the efficacy and safety of this regimen.
Activation of the mTOR pathway is associated with vascular injury. In the renal context, the vascular endothelium of proliferative intrarenal vessels in patients with APS nephropathy has demonstrated evidence of mTOR complex pathway activation (100). The use of the mTOR inhibitor sirolimus has been associated with preserved renal function in aPL-positive renal transplant recipients (100). In addition, a previous study revealed that patients with APS nephropathy treated with sirolimus exhibited no recurrence of vasculopathy, with biopsy findings showing reduced vascular proliferation. Among 10 patients receiving sirolimus therapy, 7 (70%) maintained normal renal allograft function at 144 months post-transplantation, compared with only 3 of the 27 untreated patients (11%) (100). These findings hold notable implications for aPL-induced mTOR activation in monocytes. In vitro studies have also demonstrated that rapamycin completely blocks anti-β2GPI antibody-induced monocyte activation, resulting in notable reductions in IL-8 expression, TF mRNA levels and TF activity (74,98). Notably, rapamycin fails to inhibit anticardiolipin antibody-mediated signaling, suggesting potential needs for aPL subtype stratification when utilizing these agents (98). However, further large-scale investigations are required to validate these observations.
The NLRP3 inflammasome in monocytes represents another potential therapeutic target. MCC950, a highly selective NLRP3 inhibitor, significantly reduces TNF-α and IL-6 levels in endotoxemic mice while markedly decreasing extracellular vesicle TF activity and thrombin-antithrombin complexes, demonstrating dual anti-inflammatory and anticoagulant properties (108). A previous study also identified the anti-inflammatory effects of endogenous Krebs cycle metabolites, such as itaconate, which suppresses inflammasome activation through NLRP3 modification (102). Colchicine additionally blocks NLRP3 and improves thromboinflammation (98). These agents represent promising therapeutic candidates for APS; however, these interventions remain unexplored in the context of APS and require systematic investigation.
ROS may represent another potential therapeutic target in APS, being closely associated with inflammation-driven thrombosis. Adequate ubiquinol levels serve crucial roles in protecting cells against protein oxidation and lipid peroxidation (109). In vitro experiments have demonstrated that ubiquinol supplementation to peripheral blood cells from patients with APS can not only reduce oxidative stress, but also improve mitochondrial dysfunction, revealing notable anti-inflammatory properties (105). A randomized double-blind controlled trial involving 36 patients with APS showed that ubiquinol (200 mg/day) for 1 month enhanced vascular endothelial function, decreased monocyte expression of prothrombotic and proinflammatory mediators (IL-1β, TNF-α, IL-6 and IL-8), inhibited phosphorylation of thrombosis-associated protein kinases, and reduced peroxides along with the proportion of depolarized mitochondria in monocytes (106). These findings suggest the potential therapeutic benefits of ubiquinol in APS, possibly serving as adjuvant therapy to standard APS treatment.
Conclusion
In recent years, the role of monocytes in the pathogenesis of APS has been continuously studied; however, their role in the underlying pathogenic mechanisms of thrombosis and obstetric complications is still unresolved, because the signaling pathways and molecular-level specific mechanisms of action have not been clearly elucidated. In addition, interactions between monocytes and other immune cells, such as endothelial cells, platelets and T cells, can further complicate the situation. aPLs induce pro-inflammatory and pro-coagulant states in monocytes. Monocytes are activated by different pathways and are the most important source of TF in APS, and exposure to TF on monocytes is associated with pathways that promote coagulation and inflammatory responses, indicating that these pathways may be promising therapeutic targets. Therefore, the role of monocytes in APS may be an important area of study, and further studies are required to investigate the specific molecular mechanisms of the association between the activation of monocytes, and thrombosis and obstetric complications, as a reference for the development of new targeted interventions for APS.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
RXH, CCW and YTY wrote the manuscript. YY, XCH, BQW, DLM, RJH and YJH acquired and interpreted the data. RXH conceptualized and designed the study. JYL and XXH edited the manuscript for intellectual content. Data authentication is not applicable. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Barbhaiya M, Zuily S, Naden R, Hendry A, Manneville F, Amigo MC, Amoura Z, Andrade D, Andreoli L, Artim-Esen B, et al: 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Ann Rheum Dis. 82:1258–1270. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Radic M and Pattanaik D: Cellular and molecular mechanisms of anti-phospholipid syndrome. Front Immunol. 9:9692018. View Article : Google Scholar : PubMed/NCBI | |
|
Knight JS and Kanthi Y: Mechanisms of immunothrombosis and vasculopathy in antiphospholipid syndrome. Semin Immunopathol. 44:347–362. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Hernández-Molina G, González-Pérez I, Pacheco-Molina C and Cabral AR: Quality of life in patients with antiphospholipid syndrome is related to disease burden and anticoagulant therapy. Int J Rheum Dis. 20:755–759. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Chighizola CB, Crisafulli F, Hoxha A, Carubbi F, Bellan M, Monti S, Costa L, Baldi C, Radin M, Praino E, et al: Psychosocial burden in young patients with primary anti-phospholipid syndrome: An Italian nationwide survey (The AQUEOUS study). Clin Exp Rheumatol. 39:938–946. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Velásquez M, Peláez LF, Rojas M, Narváez-Sánchez R, Velásquez JA, Escudero C, San Martín S and Cadavid ÁP: Differences in endothelial activation and dysfunction induced by antiphospholipid antibodies among groups of patients with thrombotic, refractory, and non-refractory antiphospholipid syndrome. Front Physiol. 12:7647022021. View Article : Google Scholar : PubMed/NCBI | |
|
Redecha P, Tilley R, Tencati M, Salmon JE, Kirchhofer D, Mackman N and Girardi G: Tissue factor: A link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood. 110:2423–2431. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Salmon JE and Girardi G: Antiphospholipid antibodies and pregnancy loss: A disorder of inflammation. J Reprod Immunol. 77:51–56. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Proulle V, Furie RA, Merrill-Skoloff G, Furie BC and Furie B: Platelets are required for enhanced activation of the endothelium and fibrinogen in a mouse thrombosis model of APS. Blood. 124:611–622. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Wahl D, Membre A, Perret-Guillaume C, Regnault V and Lecompte T: Mechanisms of antiphospholipid-induced thrombosis: Effects on the protein C system. Curr Rheumatol Rep. 11:77–81. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Bu C, Gao L, Xie W, Zhang J, He Y, Cai G and McCrae KR: beta2-glycoprotein i is a cofactor for tissue plasminogen activator-mediated plasminogen activation. Arthritis Rheum. 60:559–568. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Yalavarthi S, Gould TJ, Rao AN, Mazza LF, Morris AE, Núñez-Álvarez C, Hernández-Ramírez D, Bockenstedt PL, Liaw PC, Cabral AR and Knight JS: Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: A newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol. 67:2990–3003. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Kuwana M: Beta2-glycoprotein I: Antiphospholipid syndrome and T-cell reactivity. Thromb Res. 114:347–355. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Chaturvedi S, Alluri R and McCrae KR: Extracellular vesicles in the antiphospholipid syndrome. Semin Thromb Hemost. 44:493–504. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Rand JH, Wu XX, Guller S, Gil J, Guha A, Scher J and Lockwood CJ: Reduction of annexin-V (placental anticoagulant protein-I) on placental villi of women with antiphospholipid antibodies and recurrent spontaneous abortion. Am J Obstet Gynecol. 171:1566–1572. 1994. View Article : Google Scholar : PubMed/NCBI | |
|
Doğan Z, Bektaşoğlu G, Dümür Ş, Uzun H, Erden İ and Yurtdaş M: Evaluation of the relationship between monocyte to high-density lipoprotein cholesterol ratio and thrombus burden in patients with deep vein thrombosis. Rev Assoc Med Bras (1992). 69:e202212112023. View Article : Google Scholar : PubMed/NCBI | |
|
Chen P, Zhou L, Chen J, Lu Y, Cao C, Lv S, Wei Z, Wang L, Chen J, Hu X, et al: The immune atlas of human deciduas with unexplained recurrent pregnancy loss. Front Immunol. 12:6890192021. View Article : Google Scholar : PubMed/NCBI | |
|
Oku K, Amengual O and Atsumi T: Pathophysiology of thrombosis and pregnancy morbidity in the antiphospholipid syndrome. Eur J Clin Invest. 42:1126–1135. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Xourgia E and Tektonidou MG: An update on antiphospholipid syndrome. Curr Rheumatol Rep. 23:842022. View Article : Google Scholar : PubMed/NCBI | |
|
Dabit JY, Valenzuela-Almada MO, Vallejo-Ramos S and Duarte-García A: Epidemiology of antiphospholipid syndrome in the general population. Curr Rheumatol Rep. 23:852022. View Article : Google Scholar : PubMed/NCBI | |
|
Hwang JJ, Shin SH, Kim YJ, Oh YM, Lee SD, Kim YH, Choi CW and Lee JS: Epidemiology of antiphospholipid syndrome in Korea: A nationwide population-based study. J Korean Med Sci. 35:e352020. View Article : Google Scholar : PubMed/NCBI | |
|
Radin M, Sciascia S, Bazzan M, Bertero T, Carignola R, Montabone E, Montaruli B, Vaccarino A, Cecchi I, Rubini E, et al: Antiphospholipid syndrome is still a rare disease-estimated prevalence in the piedmont and aosta valley regions of northwest Italy: Comment on the article by Duarte-García et al. Arthritis Rheumatol. 72:1774–1776. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Duarte-García A, Pham MM, Crowson CS, Amin S, Moder KG, Pruthi RK, Warrington KJ and Matteson EL: The epidemiology of antiphospholipid syndrome: A population-based study. Arthritis Rheumatol. 71:1545–1552. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Petri M: Antiphospholipid syndrome. Transl Res. 225:70–81. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Cervera R, Serrano R, Pons-Estel GJ, Ceberio-Hualde L, Shoenfeld Y, de Ramón E, Buonaiuto V, Jacobsen S, Zeher MM, Tarr T, et al: Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: A multicentre prospective study of 1000 patients. Ann Rheum Dis. 74:1011–1018. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
D'Ippolito S, Barbaro G, Paciullo C, Tersigni C, Scambia G and Di Simone N: Antiphospholipid syndrome in pregnancy: New and old pathogenetic mechanisms. Int J Mol Sci. 24:31952023. View Article : Google Scholar : PubMed/NCBI | |
|
Turrent-Carriles A, Herrera-Félix JP and Amigo MC: Renal involvement in antiphospholipid syndrome. Front Immunol. 9:10082018. View Article : Google Scholar : PubMed/NCBI | |
|
Abreu MM, Danowski A, Wahl DG, Amigo MC, Tektonidou M, Pacheco MS, Fleming N, Domingues V, Sciascia S, Lyra JO, et al: The relevance of ‘non-criteria’ clinical manifestations of antiphospholipid syndrome: 14th international congress on antiphospholipid antibodies technical task force report on antiphospholipid syndrome clinical features. Autoimmun Rev. 14:401–414. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Pignatelli P, Ettorre E, Menichelli D, Pani A, Violi F and Pastori D: Seronegative antiphospholipid syndrome: Refining the value of ‘non-criteria’ antibodies for diagnosis and clinical management. Haematologica. 105:562–572. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, DE Groot PG, Koike T, Meroni PL, et al: International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 4:295–306. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Asherson RA, Cervera R, de Groot PG, Erkan D, Boffa MC, Piette JC, Khamashta MA and Shoenfeld Y; Catastrophic Antiphospholipid Syndrome Registry Project Group, : Catastrophic antiphospholipid syndrome: International consensus statement on classification criteria and treatment guidelines. Lupus. 12:530–534. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Auffray C, Sieweke MH and Geissmann F: Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 27:669–692. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Ziegler-Heitbrock L: Blood monocytes and their subsets: Established features and open questions. Front Immunol. 6:4232015. View Article : Google Scholar : PubMed/NCBI | |
|
Kzhyshkowska J, Gudima A, Moganti K, Gratchev A and Orekhov A: Perspectives for monocyte/macrophage-based diagnostics of chronic inflammation. Transfus Med Hemother. 43:66–77. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M and Ley K: Development of monocytes, macrophages, and dendritic cells. Science. 327:656–561. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Dash SP, Gupta S and Sarangi PP: Monocytes and macrophages: Origin, homing, differentiation, and functionality during inflammation. Heliyon. 10:e296862024. View Article : Google Scholar : PubMed/NCBI | |
|
Cormican S and Griffin MD: Human monocyte subset distinctions and function: Insights from gene expression analysis. Front Immunol. 11:10702020. View Article : Google Scholar : PubMed/NCBI | |
|
Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, et al: Nomenclature of monocytes and dendritic cells in blood. Blood. 116:e74–e80. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM and Wong SC: The three human monocyte subsets: Implications for health and disease. Immunol Res. 53:41–57. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Medrano-Bosch M, Simón-Codina B, Jiménez W, Edelman ER and Melgar-Lesmes P: Monocyte-endothelial cell interactions in vascular and tissue remodeling. Front Immunol. 14:11960332023. View Article : Google Scholar : PubMed/NCBI | |
|
Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P and Wong SC: Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 118:e16–e31. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, et al: Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 33:375–386. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Štok U, Štucin N, Blokar E, Ambrožič A, Sodin-Šemrl S, Čučnik S and Žigon P: Antiphospholipid antibody syndrome-associated increased surface expression of VLA4 integrin on human monocytes. Biomedicines. 10:23412022. View Article : Google Scholar : PubMed/NCBI | |
|
Mandel J, Casari M, Stepanyan M, Martyanov A and Deppermann C: Beyond hemostasis: Platelet innate immune interactions and thromboinflammation. Int J Mol Sci. 23:38682022. View Article : Google Scholar : PubMed/NCBI | |
|
Hui H, Fuller KA, Erber WN and Linden MD: Imaging flow cytometry in the assessment of leukocyte-platelet aggregates. Methods. 112:46–54. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Gawaz MP, Loftus JC, Bajt ML, Frojmovic MM, Plow EF and Ginsberg MH: Ligand bridging mediates integrin alpha IIb beta 3 (platelet GPIIB-IIIA) dependent homotypic and heterotypic cell-cell interactions. J Clin Invest. 88:1128–1134. 1991. View Article : Google Scholar : PubMed/NCBI | |
|
Silverstein RL, Asch AS and Nachman RL: Glycoprotein IV mediates thrombospondin-dependent platelet-monocyte and platelet-U937 cell adhesion. J Clin Invest. 84:546–552. 1989. View Article : Google Scholar : PubMed/NCBI | |
|
Han Z, Liu Q, Li H, Zhang M, You L, Lin Y, Wang K, Gou Q, Wang Z, Zhou S, et al: The role of monocytes in thrombotic diseases: A review. Front Cardiovasc Med. 10:11138272023. View Article : Google Scholar : PubMed/NCBI | |
|
von Hundelshausen P, Weber KS, Huo Y, Proudfoot AE, Nelson PJ, Ley K and Weber C: RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation. 103:1772–1777. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Moore KL, Stults NL, Diaz S, Smith DF, Cummings RD, Varki A and McEver RP: Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol. 118:445–456. 1992. View Article : Google Scholar : PubMed/NCBI | |
|
Ivanov II, Apta BHR, Bonna AM and Harper MT: Platelet P-selectin triggers rapid surface exposure of tissue factor in monocytes. Sci Rep. 9:133972019. View Article : Google Scholar : PubMed/NCBI | |
|
Purdy M, Obi A, Myers D and Wakefield T: P- and E-selectin in venous thrombosis and non-venous pathologies. J Thromb Haemost. 20:1056–1066. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Maugeri N, Brambilla M, Camera M, Carbone A, Tremoli E, Donati MB, de Gaetano G and Cerletti C: Human polymorphonuclear leukocytes produce and express functional tissue factor upon stimulation. J Thromb Haemost. 4:1323–1330. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Hottz ED, Martins-Gonçalves R, Palhinha L, Azevedo-Quintanilha IG, de Campos MM, Sacramento CQ, Temerozo JR, Soares VC, Dias SSG, Teixeira L, et al: Platelet-monocyte interaction amplifies thromboinflammation through tissue factor signaling in COVID-19. Blood Adv. 6:5085–5099. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Melgert BN, Spaans F, Borghuis T, Klok PA, Groen B, Bolt A, de Vos P, van Pampus MG, Wong TY, van Goor H, et al: Pregnancy and preeclampsia affect monocyte subsets in humans and rats. PLoS One. 7:e452292012. View Article : Google Scholar : PubMed/NCBI | |
|
Torchinsky A, Shepshelovich J, Orenstein H, Zaslavsky Z, Savion S, Carp H, Fain A and Toder V: TNF-alpha protects embryos exposed to developmental toxicants. Am J Reprod Immunol. 49:159–168. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Piccinni MP, Raghupathy R, Saito S and Szekeres-Bartho J: Cytokines, hormones and cellular regulatory mechanisms favoring successful reproduction. Front Immunol. 12:7178082021. View Article : Google Scholar : PubMed/NCBI | |
|
Casazza RL, Lazear HM and Miner JJ: Protective and pathogenic effects of interferon signaling during pregnancy. Viral Immunol. 33:3–11. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Yang X, Tian Y, Zheng L, Luu T and Kwak-Kim J: The update immune-regulatory role of pro- and anti-inflammatory cytokines in recurrent pregnancy losses. Int J Mol Sci. 24:1322022. View Article : Google Scholar : PubMed/NCBI | |
|
Michimata T, Tsuda H, Sakai M, Fujimura M, Nagata K, Nakamura M and Saito S: Accumulation of CRTH2-positive T-helper 2 and T-cytotoxic 2 cells at implantation sites of human decidua in a prostaglandin D(2)-mediated manner. Mol Hum Reprod. 8:181–187. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Mitchell RE, Hassan M, Burton BR, Britton G, Hill EV, Verhagen J and Wraith DC: IL-4 enhances IL-10 production in Th1 cells: implications for Th1 and Th2 regulation. Sci Rep. 7:113152017. View Article : Google Scholar : PubMed/NCBI | |
|
Shi JW, Yang HL, Fan DX, Yang SL, Qiu XM, Wang Y, Lai ZZ, Ha SY, Ruan LY, Shen HH, et al: The role of CXC chemokine ligand 16 in physiological and pathological pregnancies. Am J Reprod Immunol. 83:e132232020. View Article : Google Scholar : PubMed/NCBI | |
|
Shao Q, Liu X, Huang Y, Chen X and Wang H: Human decidual stromal cells in early pregnancy induce functional re-programming of monocyte-derived dendritic cells via crosstalk between G-CSF and IL-1β. Front Immunol. 11:5742702020. View Article : Google Scholar : PubMed/NCBI | |
|
Álvarez D, Morales-Prieto DM and Cadavid ÁP: Interaction between endothelial cell-derived extracellular vesicles and monocytes: A potential link between vascular thrombosis and pregnancy-related morbidity in antiphospholipid syndrome. Autoimmun Rev. 22:1032742023. View Article : Google Scholar : PubMed/NCBI | |
|
Erez O, Romero R, Jung E, Chaemsaithong P, Bosco M, Suksai M, Gallo DM and Gotsch F: Preeclampsia and eclampsia: The conceptual evolution of a syndrome. Am J Obstet Gynecol. 226((2S)): S786–S803. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Xie H, Zhou H, Wang H, Chen D, Xia L, Wang T and Yan J: Anti-β(2)GPI/β(2)GPI induced TF and TNF-α expression in monocytes involving both TLR4/MyD88 and TLR4/TRIF signaling pathways. Mol Immunol. 53:246–254. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Colasanti T, Alessandri C, Capozzi A, Sorice M, Delunardo F, Longo A, Pierdominici M, Conti F, Truglia S, Siracusano A, et al: Autoantibodies specific to a peptide of β2-glycoprotein I cross-react with TLR4, inducing a proinflammatory phenotype in endothelial cells and monocytes. Blood. 120:3360–3370. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Raschi E, Chighizola CB, Grossi C, Ronda N, Gatti R, Meroni PL and Borghi MO: β2-glycoprotein I, lipopolysaccharide and endothelial TLR4: Three players in the two hit theory for anti-phospholipid-mediated thrombosis. J Autoimmun. 55:42–50. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Brandt KJ, Fickentscher C, Boehlen F, Kruithof EKO and de Moerloose P: NF-κB is activated from endosomal compartments in antiphospholipid antibodies-treated human monocytes. J Thromb Haemost. 12:779–791. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Müller-Calleja N, Köhler A, Siebald B, Canisius A, Orning C, Radsak M, Stein P, Mönnikes R and Lackner KJ: Cofactor-independent antiphospholipid antibodies activate the NLRP3-inflammasome via endosomal NADPH-oxidase: Implications for the antiphospholipid syndrome. Thromb Haemost. 113:1071–1083. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Hurst J, Prinz N, Lorenz M, Bauer S, Chapman J, Lackner KJ and von Landenberg P: TLR7 and TLR8 ligands and antiphospholipid antibodies show synergistic effects on the induction of IL-1beta and caspase-1 in monocytes and dendritic cells. Immunobiology. 214:683–691. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Tektonidou MG: Cardiovascular disease risk in antiphospholipid syndrome: Thrombo-inflammation and atherothrombosis. J Autoimmun. 128:1028132022. View Article : Google Scholar : PubMed/NCBI | |
|
Benagiano M, Borghi MO, Romagnoli J, Mahler M, Bella CD, Grassi A, Capitani N, Emmi G, Troilo A, Silvestri E, et al: Interleukin-17/Interleukin-21 and Interferon-γ producing T cells specific for β2 Glycoprotein I in atherosclerosis inflammation of systemic lupus erythematosus patients with antiphospholipid syndrome. Haematologica. 104:2519–2527. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Xia L, Zhou H, Wang T, Xie Y, Wang T, Wang X and Yan J: Activation of mTOR is involved in anti-β2GPI/β2GPI-induced expression of tissue factor and IL-8 in monocytes. Thromb Res. 157:103–110. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Niyonzima N, Rahman J, Kunz N, West EE, Freiwald T, Desai JV, Merle NS, Gidon A, Sporsheim B, Lionakis MS, et al: Mitochondrial C5aR1 activity in macrophages controls IL-1β production underlying sterile inflammation. Sci Immunol. 6:eabf24892021. View Article : Google Scholar : PubMed/NCBI | |
|
Khoy K, Mariotte D, Defer G, Petit G, Toutirais O and Le Mauff B: Natalizumab in multiple sclerosis treatment: from biological effects to immune monitoring. Front Immunol. 11:5498422020. View Article : Google Scholar : PubMed/NCBI | |
|
Cheng S, Wang H and Zhou H: The role of TLR4 on B cell activation and anti-β2GPI antibody production in the antiphospholipid syndrome. J Immunol Res. 2016:17197202016. View Article : Google Scholar : PubMed/NCBI | |
|
Chaturvedi S, Brodsky RA and McCrae KR: Complement in the pathophysiology of the antiphospholipid syndrome. Front Immunol. 10:4492019. View Article : Google Scholar : PubMed/NCBI | |
|
Kiss MG, Papac-Miličević N, Porsch F, Tsiantoulas D, Hendrikx T, Takaoka M, Dinh HQ, Narzt MS, Göderle L, Ozsvár-Kozma M, et al: Cell-autonomous regulation of complement C3 by factor H limits macrophage efferocytosis and exacerbates atherosclerosis. Immunity. 56:1809–1824.e10. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Girardi G, Yarilin D, Thurman JM, Holers VM and Salmon JE: Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 203:2165–2175. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Lin Z, Shi JL, Chen M, Zheng ZM, Li MQ and Shao J: CCL2: An important cytokine in normal and pathological pregnancies: A review. Front Immunol. 13:10534572023. View Article : Google Scholar : PubMed/NCBI | |
|
Shields CA, McCalmon M, Ibrahim T, White DL, Williams JM, LaMarca B and Cornelius DC: Placental ischemia-stimulated T-helper 17 cells induce preeclampsia-associated cytolytic natural killer cells during pregnancy. Am J Physiol Regul Integr Comp Physiol. 315:R336–R343. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Wang W, Sung N, Gilman-Sachs A and Kwak-Kim J: T helper (Th) cell profiles in pregnancy and recurrent pregnancy losses: Th1/Th2/Th9/Th17/Th22/Tfh cells. Front Immunol. 11:20252020. View Article : Google Scholar : PubMed/NCBI | |
|
Wu Z, Wang M, Liang G, Jin P, Wang P, Xu Y, Qian Y, Jiang X, Qian J and Dong M: Pro-inflammatory signature in decidua of recurrent pregnancy loss regardless of embryonic chromosomal abnormalities. Front Immunol. 12:7727292021. View Article : Google Scholar : PubMed/NCBI | |
|
Vishnyakova P, Elchaninov A, Fatkhudinov T and Sukhikh G: Role of the monocyte-macrophage system in normal pregnancy and preeclampsia. Int J Mol Sci. 20:36952019. View Article : Google Scholar : PubMed/NCBI | |
|
Huang SJ, Schatz F, Masch R, Rahman M, Buchwalder L, Niven-Fairchild T, Tang C, Abrahams VM, Krikun G and Lockwood CJ: Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. J Reprod Immunol. 72:60–73. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Gowhari Shabgah A, Jadidi-Niaragh F, Mohammadi H, Ebrahimzadeh F, Oveisee M, Jahanara A and Gholizadeh Navashenaq J: The role of atypical chemokine receptor D6 (ACKR2) in physiological and pathological conditions; friend, foe, or both? Front Immunol. 13:8619312022. View Article : Google Scholar : PubMed/NCBI | |
|
Yan S, Cui S, Zhang L, Yang B, Yuan Y, Lv X, Fu H, Li Y, Huang C and Wang P: Expression of ACKR2 in placentas from different types of preeclampsia. Placenta. 90:121–127. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Rybak-Krzyszkowska M, Staniczek J, Kondracka A, Bogusławska J, Kwiatkowski S, Góra T, Strus M and Górczewski W: From biomarkers to the molecular mechanism of preeclampsia-A comprehensive literature review. Int J Mol Sci. 24:132522023. View Article : Google Scholar : PubMed/NCBI | |
|
Jena MK, Sharma NR, Petitt M, Maulik D and Nayak NR: Pathogenesis of preeclampsia and therapeutic approaches targeting the placenta. Biomolecules. 10:9532020. View Article : Google Scholar : PubMed/NCBI | |
|
Knight JS, Branch DW and Ortel TL: Antiphospholipid syndrome: Advances in diagnosis, pathogenesis, and management. BMJ. 380:e0697172023. View Article : Google Scholar : PubMed/NCBI | |
|
Alvarez AM, Mulla MJ, Chamley LW, Cadavid AP and Abrahams VM: Aspirin-triggered lipoxin prevents antiphospholipid antibody effects on human trophoblast migration and endothelial cell interactions. Arthritis Rheumatol. 67:488–497. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Bruno V, Svensson-Arvelund J, Rubér M, Berg G, Piccione E, Jenmalm MC and Ernerudh J: Effects of low molecular weight heparin on the polarization and cytokine profile of macrophages and T helper cells in vitro. Sci Rep. 8:41662018. View Article : Google Scholar : PubMed/NCBI | |
|
Saraiva-Mangolin S, Vaz CDO, Ruiz T, Mazetto BM and Orsi FA: Use of hydroxychloroquine to control immune response and hypercoagulability in patients with primary antiphospholipid syndrome. Eur J Intern Med. 90:114–115. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Tishler M, Yaron I, Shirazi I and Yaron M: Hydroxychloroquine treatment for primary Sjögren's syndrome: Its effect on salivary and serum inflammatory markers. Ann Rheum Dis. 58:253–256. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Miranda S, Billoir P, Damian L, Thiebaut PA, Schapman D, Le Besnerais M, Jouen F, Galas L, Levesque H, Le Cam-Duchez V, et al: Hydroxychloroquine reverses the prothrombotic state in a mouse model of antiphospholipid syndrome: Role of reduced inflammation and endothelial dysfunction. PLoS One. 14:e02126142019. View Article : Google Scholar : PubMed/NCBI | |
|
Arachchillage DJ, Laffan M and Pericleous C: Hydroxy-chloroquine as an immunomodulatory and antithrombotic treatment in antiphospholipid syndrome. Int J Mol Sci. 24:13312023. View Article : Google Scholar : PubMed/NCBI | |
|
Müller-Calleja N, Hollerbach A, Häuser F, Canisius A, Orning C and Lackner KJ: Antiphospholipid antibody-induced cellular responses depend on epitope specificity: Implications for treatment of antiphospholipid syndrome. J Thromb Haemost. 15:2367–2376. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Hollerbach A, Müller-Calleja N, Canisius A, Orning C and Lackner KJ: Induction of tissue factor expression by anti-β2-glycoprotein I is mediated by tumor necrosis factor α. J Thromb Thrombolysis. 49:228–234. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Canaud G, Bienaimé F, Tabarin F, Bataillon G, Seilhean D, Noël LH, Dragon-Durey MA, Snanoudj R, Friedlander G, Halbwachs-Mecarelli L, et al: Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med. 371:303–312. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Cornelius DC, Travis OK, Tramel RW, Borges-Rodriguez M, Baik CH, Greer M, Giachelli CA, Tardo GA and Williams JM: NLRP3 inflammasome inhibition attenuates sepsis-induced platelet activation and prevents multi-organ injury in cecal-ligation puncture. PLoS One. 15:e02340392020. View Article : Google Scholar : PubMed/NCBI | |
|
Hooftman A, Angiari S, Hester S, Corcoran SE, Runtsch MC, Ling C, Ruzek MC, Slivka PF, McGettrick AF, Banahan K, et al: The immunomodulatory metabolite itaconate modifies NLRP3 and inhibits inflammasome activation. Cell Metab. 32:468–478.e7. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Chen X, Zhang H, Xiao J, Yang C, Chen W, Wei Z, Chen X and Liu J: 4-Octyl itaconate alleviates lipopolysaccharide-induced acute lung injury in mice by inhibiting oxidative stress and inflammation. Drug Des Devel Ther. 14:5547–5558. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, et al: Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 381:2497–2505. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Perez-Sanchez C, Ruiz-Limon P, Aguirre MA, Bertolaccini ML, Khamashta MA, Rodriguez-Ariza A, Segui P, Collantes-Estevez E, Barbarroja N, Khraiwesh H, et al: Mitochondrial dysfunction in antiphospholipid syndrome: Implications in the pathogenesis of the disease and effects of coenzyme Q(10) treatment. Blood. 119:5859–5870. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Pérez-Sánchez C, Aguirre MÁ, Ruiz-Limón P, Ábalos-Aguilera MC, Jiménez-Gómez Y, Arias-de la Rosa I, Rodriguez-Ariza A, Fernández-Del Río L, González-Reyes JA, Segui P, et al: Ubiquinol effects on antiphospholipid syndrome prothrombotic profile: A randomized, placebo-controlled trial. Arterioscler Thromb Vasc Biol. 37:1923–1932. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Alijotas-Reig J, Esteve-Valverde E, Llurba E and Gris JM: Treatment of refractory poor aPL-related obstetric outcomes with TNF-alpha blockers: Maternal-fetal outcomes in a series of 18 cases. Semin Arthritis Rheum. 49:314–318. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Sachetto ATA, Archibald SJ, Perkins M, Zhang G, Zhang Y, Ye D, Grover SP, Wu C, Li Z and Mackman N: Pathways regulating the levels of tissue factor-positive extracellular vesicles and activation of coagulation in endotoxemic mice. J Thromb Haemost. 23:2422–2435. 2025. View Article : Google Scholar : PubMed/NCBI | |
|
Mantle D and Hargreaves IP: Coenzyme Q10 and autoimmune disorders: An overview. Int J Mol Sci. 25:45762024. View Article : Google Scholar : PubMed/NCBI |