Efficacy of tacrolimus and other immunosuppressants in the treatment of systemic lupus erythematosus: A systematic review and meta‑analysis
- Authors:
- Published online on: June 23, 2025 https://doi.org/10.3892/br.2025.2023
- Article Number: 145
-
Copyright: © Li et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Systemic lupus erythematosus (SLE) is recognized globally as one of the three major intractable diseases. It can affect multiple organs, including the heart, lungs and kidneys, and severe cases it can also damage the central nervous system (1). Global epidemiological data (2) show that the annual incidence is 5.14 per 100,000 individuals, with 8.82 and 1.53 cases for women and men, respectively. Significant regional variations have been observed, with higher incidence in Poland, United States and Barbados but higher prevalence in the UAE, Barbados and Brazil. Although the exact cause of SLE remains unknown, it is considered to be influenced by a combination of genetic factors, environmental exposures (such as ultraviolet radiation, toxins and radiation), viral infections, hormone levels and gut microbiota (3-5).
Patients with SLE often experience B-cells overactivation and impaired T-cell function, leading to immune dysregulation and systemic inflammatory responses that disrupt multiple organ functions (6). Currently, the treatment of SLE primarily relies on immunosuppressive agents and corticosteroids. Tacrolimus (TAC), as a calcineurin inhibitor, has high lipophilicity and its mechanism of action is similar to that of cyclosporine. Once inside the cell, TAC binds to the FK506-binding protein in T lymphocytes, forming a complex that disrupts the calcineurin signaling pathway and inhibits the influx of calcium ions (7). This mechanism helps prevent the release of various inflammatory mediators, regulates the function of T lymphocyte subsets, reduces disease activity, and controls SLE progression (8).
TAC has gained increasing attention in recent years. A systematic evaluation of its efficacy compared with other immunosuppressants in the treatment of SLE could provide a more scientific basis for clinical decision-making. In the present study, the efficacy of TAC in treating SLE was systematically assessed by analyzing the existing literature and comparing it with other immunosuppressants.
Materials and methods
Literature search
A literature search was conducted in the databases of PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (https://www.embase.com), Cochrane Library (https://www.cochranelibrary.com/) and Web of Science (https://clarivate.com) for articles regarding the treatment of SLE with TAC published from database inception up to December 2024. In addition, the reference lists were manually searched to ensure the validity of the included studies. The main search key words included TAC, SLE, lupus nephritis and immunosuppressants, among others. The search strategy involved a combination of subject headings and free terms. The search strategy in PubMed is shown in Table I.
Inclusion and exclusion criteria
Inclusion criteria were as follows: i) The study designs included randomized controlled trials (RCT), cohort studies, single-arm studies; ii) the study participants were patients with clear diagnosis of SLE (9), with no restrictions on sex, age, or region; iii) The treatment group used TAC, and the control group used immunosuppressive agents, such as cyclophosphamide (CYC), mycophenolate mofetil (MMF) and azathioprine (AZA); and iv) the studies analyzed at least one of the following outcomes: complete remission (CR) rate, partial response (PR) rate, SLE disease activity index (SLEDAI) and adverse reactions.
Exclusion criteria were as follows: i) Published similar or duplicate literature; ii) literature from which valid data cannot be extracted or full text cannot be obtained; iii) systematic reviews, reviews, or animal experiment literature; and iv) literature that does not separate the control and treatment groups (Table II).
Literature screening and data extraction
Two researchers independently reviewed all reports and excluded articles that were obviously irrelevant based on the title, abstract and full text. If consensus was not achieved, a third researcher was consulted. Data extraction encompassed the following content: i) basic information; ii) baseline characteristics; iii) interventions; iv) elements of bias risk assessment and v) outcome measures.
Quality evaluation standard
Two researchers independently assessed the risk of bias in the included studies and cross-checked their findings with each other. In the case of disagreements, a third researcher was consulted to resolve the issues. Corresponding quality assessment tools were applied based on the study design. For RCTs, the Cochrane Risk of Bias Tool was used, which is specifically designed for RCTs and allows for a comprehensive assessment of bias risks related to random sequence generation, allocation concealment, blinding, data integrity and selective reporting. For non-RCTs, the Newcastle-Ottawa Scale (NOS) was used, as this tool is specifically designed for non-random studies (such as cohort or case-control studies) and systematically assesses bias risks related to selection, comparability and outcome measurement.
For RCTs, the Cochrane Collaboration's recommended risk-of-bias assessment tool was utilized (10). The assessment encompassed six domains and seven items, namely, random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), completeness of outcome data (attrition bias), selective reporting of results (reporting bias) and other sources of bias (other biases). Each domain was rated as having a low, high, or unclear risk of bias.
For non-RCT, the NOS was used to assess the risk of bias (11). The evaluation content included the risk of bias in patient selection, between-group comparability, and outcome exposure or results. The NOS consists of eight aspects and nine entries, with each entry scoring 1 or 0 points. Based on the scores, the studies were categorized into having low quality (<5 points), medium (5-8 points), and high (8-9 points) quality.
Statistical methods
The meta-analysis was conducted using StataMP 17 (StataCorp LLC). For dichotomous variables, odds ratios (OR) and 95% confidence intervals (CI) were used as effect sizes. The random-effects model was used for the meta-analysis, regardless of heterogeneity. Publication bias was assessed by constructing a funnel plot. The significance level was set at α=0.05.
Results
Included literature
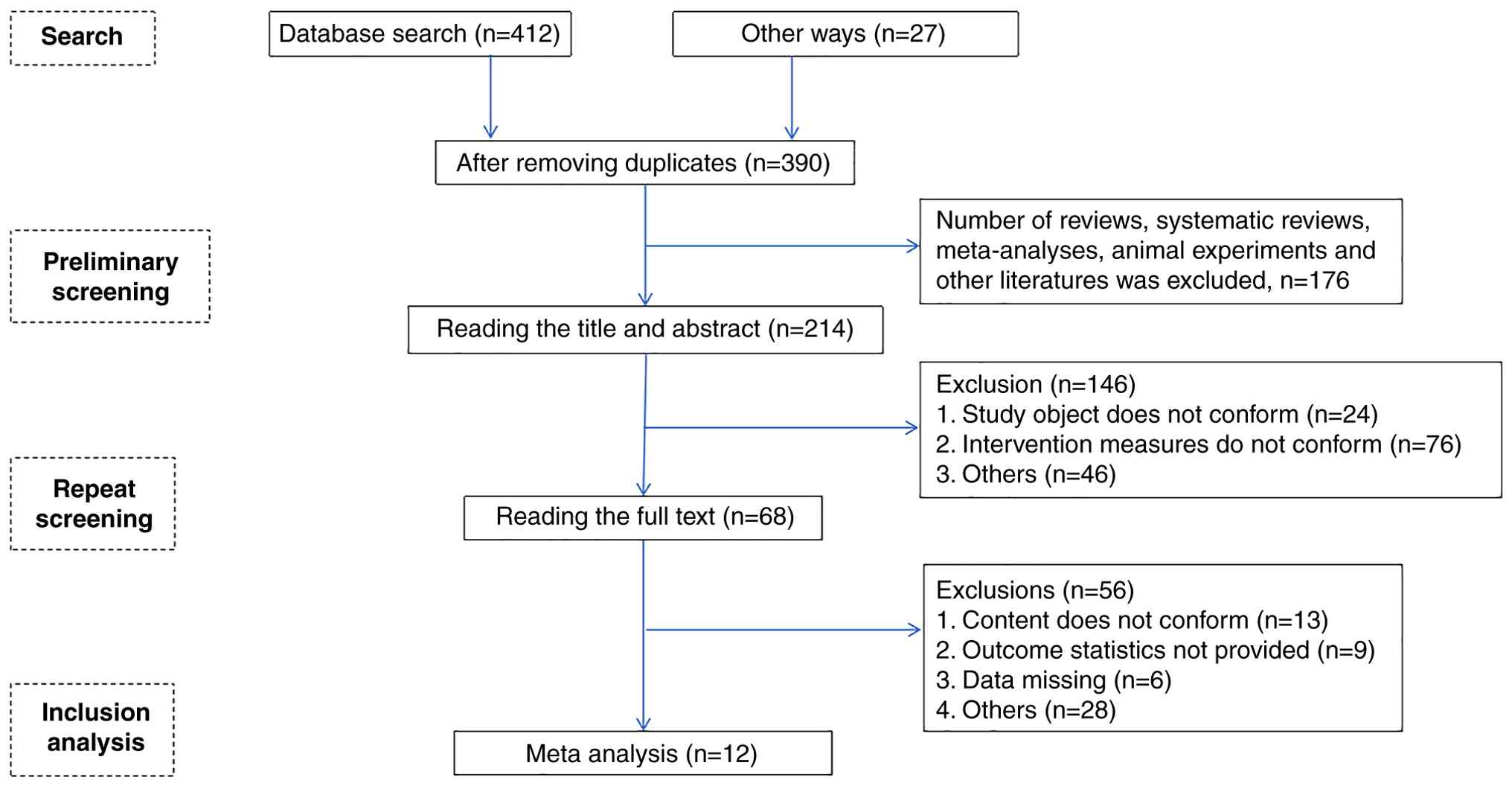
Of the total of 439 studies searched, 12 (12-23) were finally included after layer-by-layer screening, including 10 RCTs (12-14,16-22) and 2 cohort studies (15,23). The literature screening process and results are shown in Fig. 1.
General characteristics
A total of 12 studies (12-23) enrolled 1,217 patients, which included 582 in the treatment group and 635 in the control group (Table III).
Literature quality evaluation
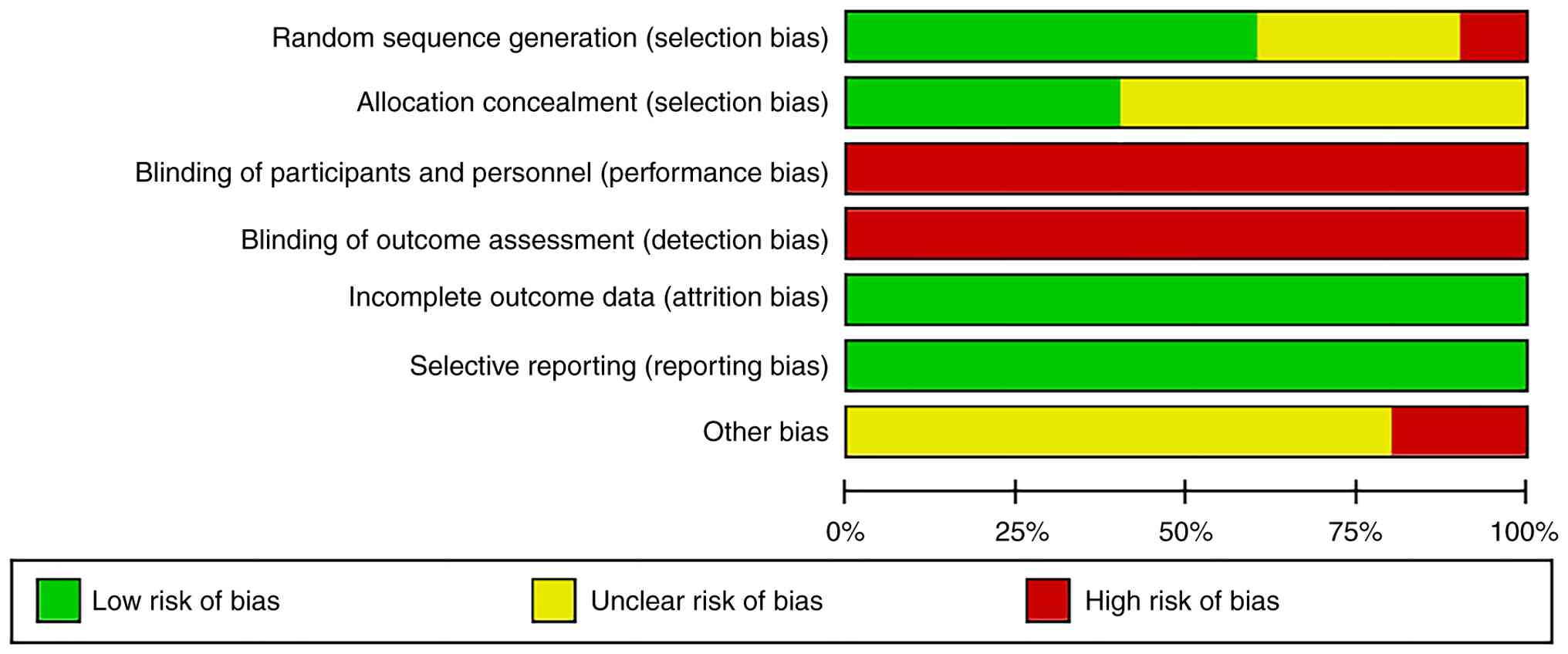
One RCT (16) did not describe the randomization method; thus, it was rated as ‘high risk’. A total of three RCTs (13,14,19) mentioned the methods used, such as sealed envelopes to achieve allocation concealment, therefore they were rated as ‘low risk’. For studies that did not employ blinding or did not clearly describe the blinding method (12-23), considering the potential performance bias and detection bias, these studies were assessed as having a ‘high risk’ of bias. Specifically, in unblinded studies, participants and researchers may be influenced by subjective factors, which could affect the validity and reliability of the study results. All studies (12-23) have reported all the expected collected data and predefined primary and secondary outcome measures, therefore they were rated as ‘low risk.’ However, whether there were other sources of bias was unclear, thus the risk of other bias was rated as unclear (Fig. 2).
One cohort study (23) had an NOS score of 9, whereas the other cohort study (15) did not report matching or adjusting for the exposed and unexposed groups in the analysis, resulting in an NOS score of 8 (Table IV).
Results of meta-analysis. CR
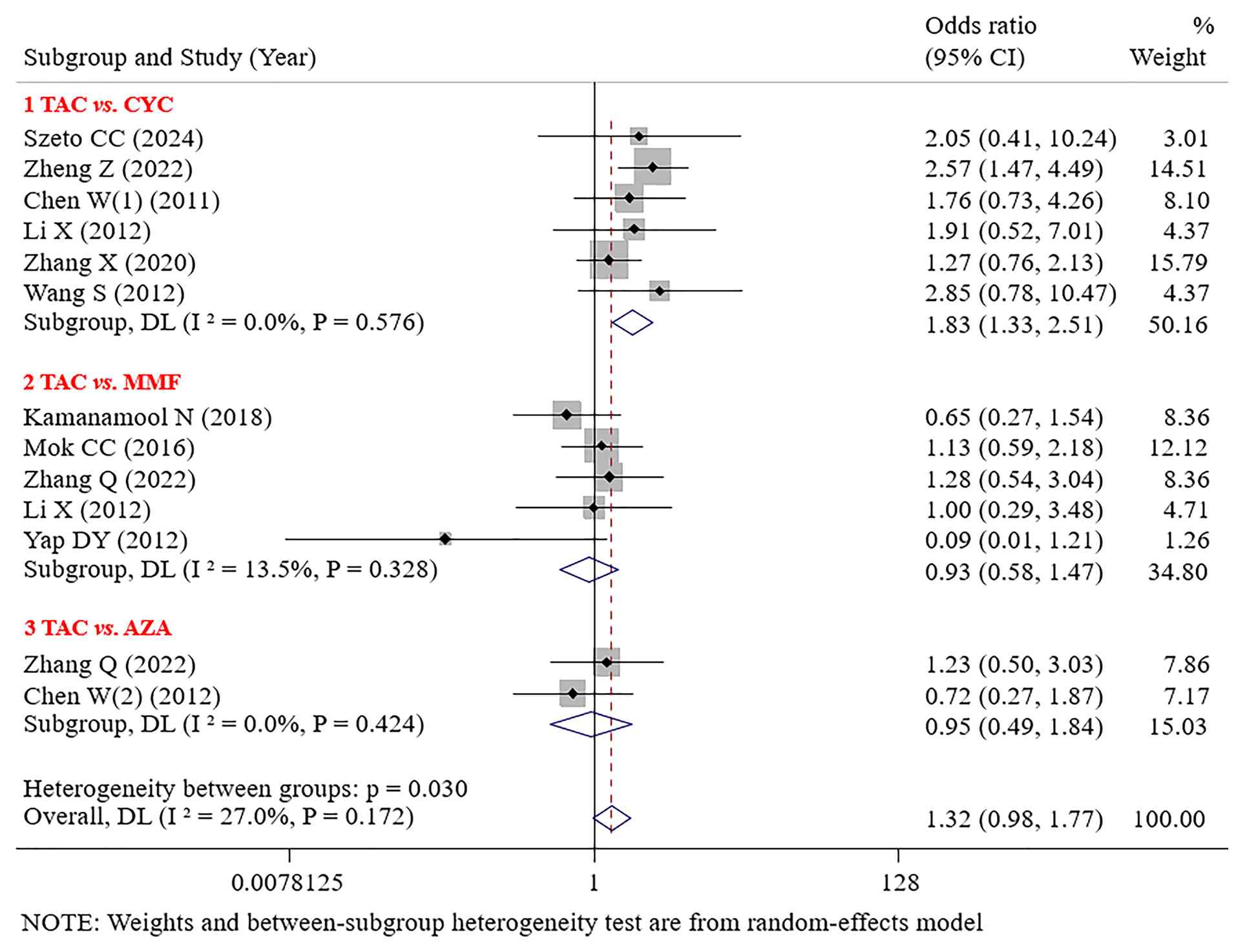
TAC demonstrated significantly superior CR to CYC (OR=1.83; 95% CI: 1.33-2.51; P<0.001). No significant difference in CR was found between TAC and MMF or AZA [(OR=0.93; 95% CI: 0.58-1.47; P=0.753) OR=0.95; 95% CI: 0.49-1.84; P=0.884; Fig. 3].
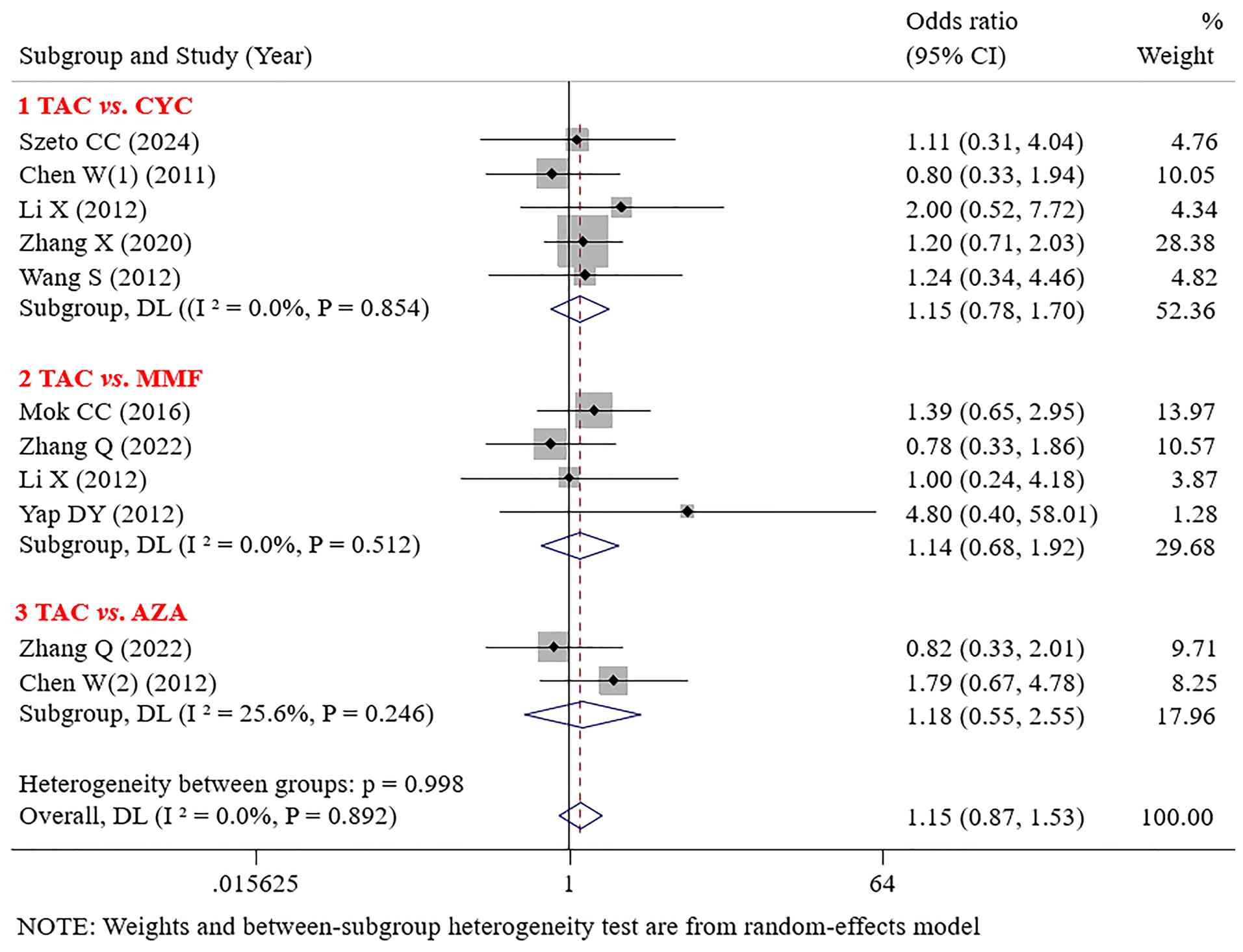
PR. No significant differences in PR were found between TAC and CYC (OR=1.15; 95% CI: 0.78-1.70; P=0.476), MMF (OR=1.14; 95% CI: 0.68-1.92; P=0.610), or AZA (OR=1.18; 95% CI: 0.55-2.55; P=0.672) (Fig. 4).
SLEDAI score. The changes in the SLEDAI scores from eight studies (12,14,15,17-19,21,23) are summarized in Table V. TAC demonstrated therapeutic advantages in reducing the SLEDAI score; however, the results varied across different studies. Szeto et al (12) employed a general linear model for repeated measurements, with results indicating no significant difference in SLEDAI scores between the CYC and TAC groups throughout the follow-up period. Wang et al (15) revealed that the TAC group showed an improved effect on improving the SLEDAI score compared with the CYC group. Zheng et al (21) indicated that at week 24 of treatment, the least squares mean change in the SLEDAI score for the TAC group was -8.6, whereas for the CYC group, it was -6.4, with the TAC group demonstrating significantly improved efficacy. Mok et al (18) did not find a significant difference in the effect of TAC and MMF on improving the SLEDAI score after 6 months of treatment. Kamanamool et al (19) observed that TAC showed a more pronounced short-term improvement in the SLEDAI score; however, its long-term effect was slightly inferior to that of MMF. Suzuki et al (23) demonstrated that both TAC and MMF significantly improved the SLEDAI score, with no significant difference between the two groups, suggesting the comparable efficacy of TAC and MMF in improving the SLEDAI score. Chen et al (14) found that TAC and AZA exhibited significant efficacy in improving the SLEDAI score; however, no significant difference was found between them. Li et al (17) reported that CYC, TAC and MMF were all effective in improving the SLEDAI score; however, no significant difference was observed in disease activity control among the three treatment regimens.
Serum albumin. The results of five studies (13,14,16-18) regarding the TAC-induced changes in serum albumin are summarized in Table VI. The results of Chen et al (14) are consistent with the conclusions of Mok et al (18), showing no significant difference in serum albumin changes between TAC and AZA. Chen et al (13) found that TAC was superior to CYC in reducing proteinuria in the short term (1 month) but slightly increased serum albumin levels. However, in the long-term follow-up (6 months), the two drugs did not show significant differences in proteinuria and serum albumin levels. Li et al (17) observed that with short-term treatment (4-8 weeks), TAC is more effective in improving urine protein and albumin levels, whereas MMF is more beneficial in maintaining higher serum albumin levels with long-term treatment (24 weeks). However, Yap et al (16) did not find a significant difference in the serum albumin levels between TAC and MMF in their 24-month study (39.7±5.3 vs. 43.4±2.2; P=0.189).
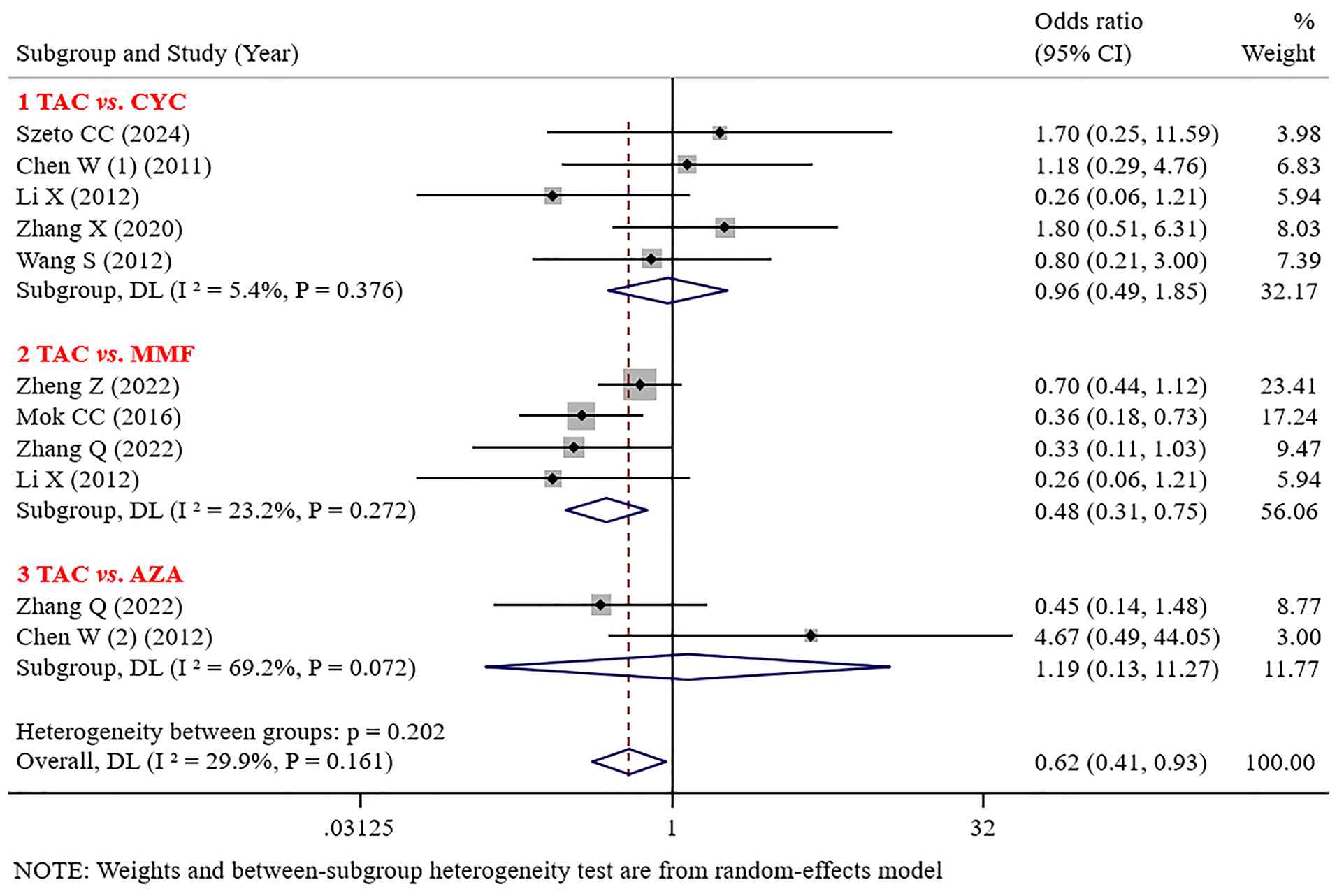
Infection. No significant differences were found in infections (such as herpes zoster, urinary tract infections, lung infections, and upper respiratory infections) between TAC and CYC or AZA [(OR=0.96, 95% CI: 0.49-1.85; P=0.894) (OR=1.19; 95% CI: 0.13-11.27; P=0.881)]. The incidence of infections with TAC was lower than that with MMF (OR=0.48; 95% CI: 0.31-0.75; P=0.001; Fig. 5).
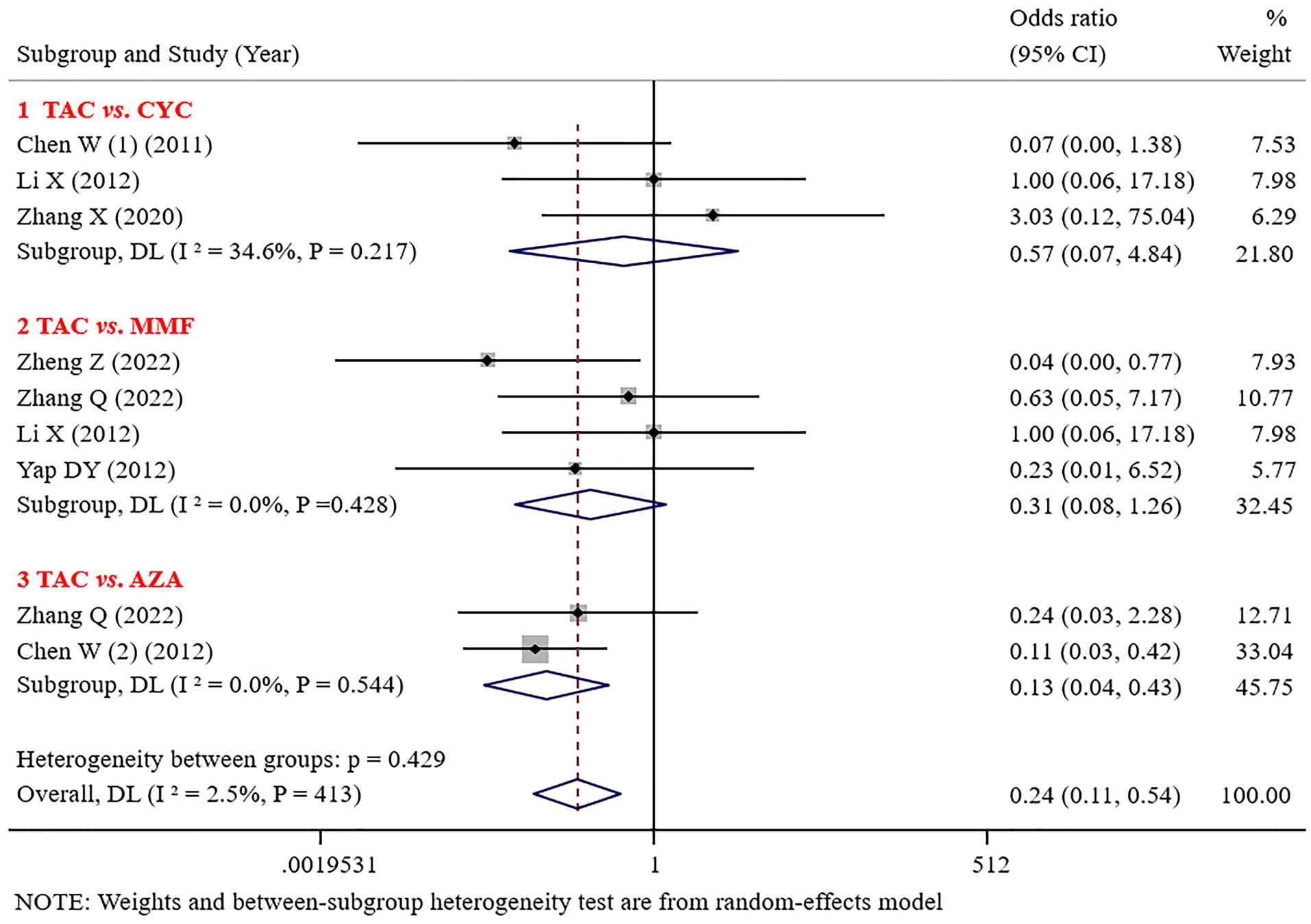
Leukopenia. No significant difference was noted in the incidence of leukopenia between TAC and CYC or MMF [(OR=0.57; 95% CI: 0.07-4.84; P=0.609); (OR=0.31; 95% CI: 0.08-1.26; P=0.102)]. The incidence of leukopenia caused by TAC was significantly lower than that caused by MMF and AZA (OR=0.13; 95% CI: 0.04-0.43; P=0.001; Fig. 6).
Publication bias assessment
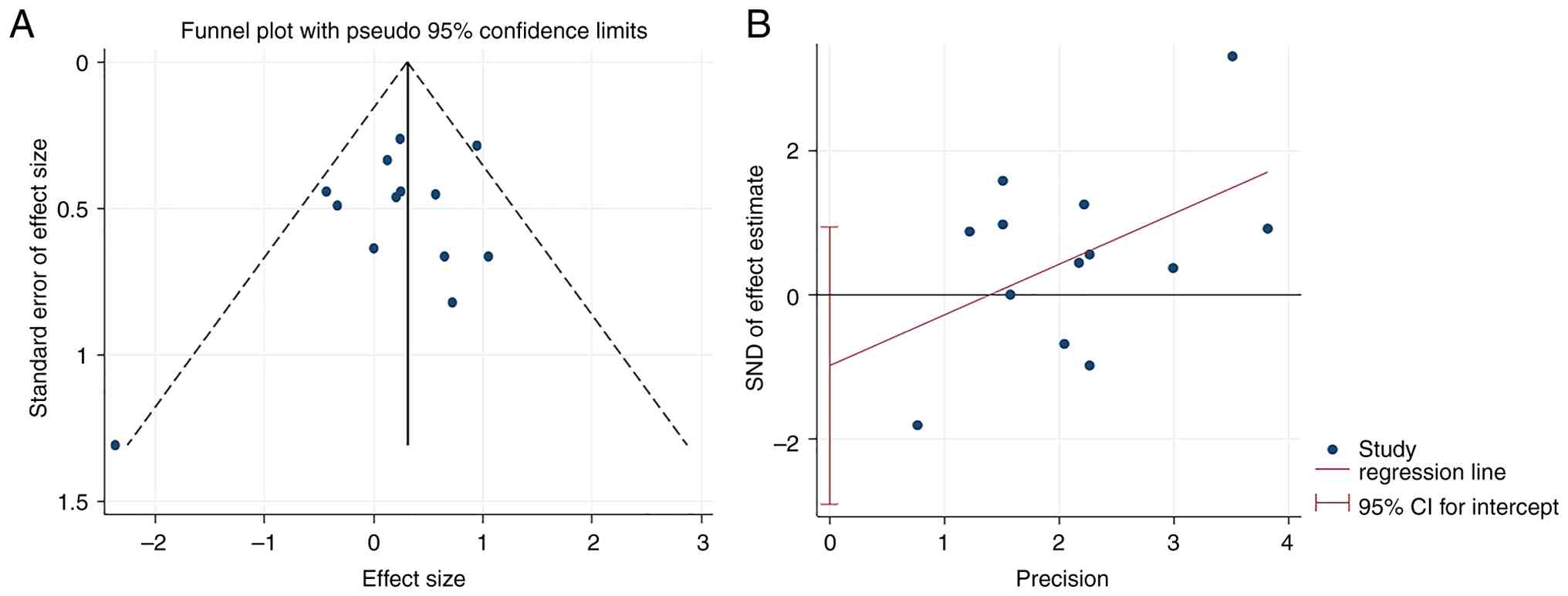
A funnel plot was constructed to assess the publication bias for the CR. The funnel plot was approximately symmetrical, indicating no publication bias. The results of the Begg (P=0.807) and Egger (P=0.284) tests also suggest the absence of publication bias (Fig. 7).
Discussion
SLE predominantly affects reproductive women, with renal involvement as a frequent and serious complication. Lupus nephritis is one of the most severe manifestations of SLE, with progresses to end-stage renal disease (ESRD) in ~10% of cases, significantly compromising patient outcomes and quality of life (24). Although no definitive cure exists for lupus nephritis, early therapeutic intervention can markedly slow disease progression and improve renal function prognosis, potentially preventing the development of renal failure. MMF and CYC are commonly used immunosuppressive drugs. However, CYC's alkylating agents have adverse reactions such as bone marrow suppression, infection, gonadal suppression, or increased risk of tumors. Meanwhile, certain studies have suggested that MMF may increase the risk of progression to ESRD in certain patients (25), whereas other studies have indicated that MMF could offer improved renal outcomes compared with CYC (26).
Multiple studies have demonstrated the significant efficacy of TAC in treating SLE, as evidenced by improvements in SLEDAI scores, proteinuria reduction, and alleviation of other clinical symptoms (for example, arthritis and skin rash) (8,27-28). Long-term, low-dose TAC therapy has shown favorable outcomes in certain patient populations with relatively fewer adverse effects (29,30). However, low-dose induction therapy may still carry an increased risk of acute flares. Mechanistic studies have revealed that TAC enhances therapeutic efficacy by inhibiting P-glycoprotein (P-gp) activity, thereby overcoming P-gp-mediated drug resistance (31). Regarding clinical outcomes, 67% of patients achieved a renal response (complete or partial) within 3 months of TAC treatment, with the response rate increasing to 92% by 6 months. Moreover, urine protein levels significantly decreased, and the expression and function of P-gp were also improved (31). Notably, TAC has demonstrated safety for both the mother and fetus during pregnancy (32), and pediatric use has shown comparable efficacy and safety profiles (33). Emerging evidence on TAC-based maintenance therapy for pediatric-onset lupus nephritis indicates that long-term TAC-based immunosuppression demonstrates favorable efficacy and lower cytotoxicity in with pediatric-onset lupus nephritis, suggesting significant potential as maintenance therapy in clinical practice (33). Overall, TAC has shown significant potential in the treating SLE. Owing to the involvement of multiple factors in the treatment of SLE, such as disease activity, organ involvement and patient tolerance, commonly used clinical immunosuppressants include MMF, CYC and AZA. However, these drugs have different efficacy and safety profiles. To clarify the role of TAC in SLE, its effectiveness and safety were systematically analyzed and compared with those of MMF, CYC and AZA to provide clinicians with a more comprehensive treatment option and optimization strategy.
The present systematic review revealed that TAC demonstrated significantly superior CR rates to CYC, consistent with previous findings (34). Specifically, the advantage of TAC in CR may be related to its lower immunosuppressive toxicity and improved clinical efficacy. Therefore, for patients who do not respond well to CYC, TAC may offer greater potential as an alternative, particularly in avoiding CYC-associated infectious complications. In addition, TAC did not show significant differences in PR from MMF or AZA, suggesting that it may exert similar effects to these drugs during the maintenance phase of treatment. The results further support findings from previous meta-analyses (35,36), indicating that TAC and MMF have comparable efficacy in CR and PR, particularly with long-term treatment, where both drugs may contribute similarly to the control of the immune response. Although TAC demonstrated improved short-term efficacy, the differences in its effect compared with those of MMF and AZA during the maintenance phase were not significant. Notably, the MMF group showed a lower relapse rate, suggesting the potential advantages of MMF in long-term immunosuppressive therapy (35). Therefore, for patients who have successfully controlled immune response, MMF or AZA may be considered for maintenance therapy to reduce the relapse risk. Overall, TAC is superior to CYC in terms of CR and has potential advantages in personalized treatment strategies, particularly for patients who have an inadequate response to CYC. Moreover, the combination of TAC with MMF, AZA, or other immunosuppressive drugs may become an ideal treatment option in long-term therapy (37,38). Therefore, to explore the best treatment combinations and individualized treatment strategies for patients, future studies could focus on the combined use of TAC with other immunosuppressive agents.
The key to therapeutic intervention lies in the complete or partial improvement of the clinical manifestations of kidney injury. The present study indicated that although results across different studies vary, TAC demonstrates certain short-term efficacy advantages in improving the SLEDAI score and serum albumin levels in patients with SLE. In terms of SLEDAI score improvement, the present findings align with those of Wang et al (15), indicating that the TAC group performance was improved compared with the CYC group. However, Szeto et al (12) did not observe a significant difference. This may be attributed to differences in the baseline characteristics of patients, disease severity and treatment regimens across studies. In addition, while the SLEDAI score is sensitive in assessing disease activity, its ability to predict treatment response may be influenced by various factors. Regarding serum albumin levels, TAC exhibited some improvement in the short term compared with AZA and MMF; however, long-term follow-up results revealed no significant differences in efficacy. This finding is consistent with the results of previous meta-analyses (36), which concluded the lack of significant differences between TAC and MMF in terms of proteinuria, serum albumin, serum creatinine, creatinine clearance and SLEDAI score. The early efficacy advantage of TAC may stem from its rapid immune modulation, whereas the convergence of long-term efficacy suggests that various immunosuppressive agents can ultimately achieve disease stabilization. This finding indicates that TAC is more suitable as a short-term induction therapy, whereas maintenance therapy may require a combination with other immunosuppressive agents. This conclusion aligns with the results of previous research and supports the necessity of personalized treatment strategies.
For continuous outcomes such as SLEDAI scores and serum albumin levels, a narrative review was preferred over a meta-analysis, primarily due to the significant heterogeneity in the available research data. Specifically, considerable differences were observed in the assessment criteria, time points and data collection methods for SLEDAI scores and serum albumin levels across studies, making data integration challenging. In addition, some studies had small sample sizes and missing data, particularly in the measurement of serum albumin levels, which could compromise the accuracy and robustness of the meta-analysis results. The use of different statistical analysis methods across studies further complicated the direct application of a meta-analysis. Therefore, to ensure the reliability of the findings and avoid bias arising from data heterogeneity, a narrative review was employed. This approach allows for a clearer presentation of the differences between studies and ensures a more accurate interpretation of the research outcomes.
Regarding safety, Li et al (34) considered TAC as the safest treatment option, with the lowest likelihood of infection events. The results of the present study demonstrate a lower incidence of infection events (such as herpes zoster and urinary tract infections) in the TAC group than in the MMF group, with no significant difference when compared with those in the CYC and AZA groups. An observational study found that MMF was significantly associated with the overall infection risk in SLE (adjusted OR=1.90), as well as with specific types of infections, such as bacterial (adjusted OR=2.07), viral (adjusted OR=1.92) and opportunistic (adjusted OR=2.13) infections (39). Moreover, the longer the duration and the higher the MMF dosage, the higher the infection risk (39). As a calcineurin inhibitor, TAC reduces excessive immune responses by inhibiting T-cell activation and proliferation. By suppressing the production of interleukin-2, TAC helps control inflammatory reactions and alleviates immune-mediated damage, thereby limiting T-cell proliferation (7,8,40). Although MMF and CYC also alleviate inflammatory reactions through immunosuppression, their mechanisms and modes of action differ. MMF primarily inhibits immune cell proliferation by suppressing deoxyadenosine triphosphate synthesis (41), whereas CYC directly targets immune cells by inhibiting DNA synthesis (42). By contrast, the immunosuppressive effect of TAC is more specific, avoiding the broad suppression of the immune system, which may reduce the risk of infection. The meta-analysis showed that leukopenia was significantly less reported in the TAC groups. The results of the present study align with the analysis finding of Kraaij et al (43), who also found a lower incidence of leukopenia in the TAC treatment group than in the AZA groups. This may be related to the mechanism of TAC, which selectively inhibits T-cell function, thus avoiding widespread effects on bone marrow production, thereby reducing the incidence of leukopenia. By contrast, AZA, by inhibiting cell proliferation, affects a broader range of immune cells, including B cells, T cells and bone marrow hematopoietic cells, making them more likely to induce leukopenia. However, owing to the limited number of the included studies, this conclusion still needs further validation.
In the present meta-analysis, a total of 12 studies were included. It is important to note that none of the included studies employed blinding. This design choice was determined by the respective research teams and may have been influenced by the initial objectives of the studies, resource constraints, or ethical considerations at the time of their design. The absence of blinding could potentially introduce bias, either overestimating or underestimating the treatment effects. Specifically, without blinding, there may be a risk of performance or detection bias, which could lead to an overestimation of the therapeutic benefits due to subjective assessment or reporting. However, the results of these studies were carefully assessed and found to be consistent across multiple trials, providing some confidence in the validity of the findings despite the absence of blinding.
The present study also has some limitations that need to be addressed in future research. First, the long-term efficacy of TAC remains uncertain. Among the studies included, only one analyzed the relapse rates at 3 and 5 years. Although TAC demonstrates favorable efficacy in short-term treatment, its long-term effects, particularly in maintaining remission and preventing disease relapse, have not been fully validated. Thus, further validating these findings and establishing robust evidence for clinical practice require large-scale RCT with long-term follow-up. These studies should focus on standardized dosing protocols and employ a prospective cohort design to evaluate relapse rates, safety profiles and long-term renal outcomes. Second, regarding the TAC dosage, existing studies have not provided a detailed discussion on its optimal dosage. In immunosuppressive therapy, choosing the correct drug dosage is crucial for both efficacy and safety; however, no standardized dosage has been established for TAC in clinical practice. Third, given the limited reporting in the current studies, a comprehensive analysis of other adverse events has not been conducted. Fourth, although TAC demonstrates similar long-term efficacy to other immunosuppressive agents such as MMF, CYC and AZA, selecting the most appropriate immunosuppressive drug based on the characteristics of patient groups remains an important issue for further exploration. Finally, the studies included lacked blinding, which may introduce bias into the results and affect the validity and reliability of the outcomes. Non-blinded studies may be influenced by observer or participant bias, leading to the overestimation or underestimation of treatment effects. Nevertheless, therapeutic effect data in these studies are consistent across multiple studies, and most of them have strictly controlled other variables. Although the lack of a blinded design may affect the accuracy of some data, the overall therapeutic effect still demonstrates a higher credibility. Of course, future research should strive to adopt double-blind or at least single-blind designs to reduce the effect of observer and participant bias. Strictly controlling these bias factors may yield more accurate and reliable estimates of treatment effects.
In conclusion, TAC has demonstrated significant short-term efficacy in the treatment of SLE, particularly in improving the CR, SLEDAI scores, and serum albumin levels. Its long-term efficacy is comparable with those MMF, CYC and AZA. However, it exhibits a lower risk of infections and leukopenia when considering safety. Nevertheless, potential limitations should be acknowledged in clinical applications, and continuous monitoring remains essential.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
XL contributed to the conception and design of the study and drafted the manuscript. JZ and YL performed the data collection and statistical analysis. All authors read and approved the final version of the manuscript. XL and JZ confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Balogh L, Oláh K, Sánta S, Majerhoffer N and Németh T: Novel and potential future therapeutic options in systemic autoimmune diseases. Front Immunol. 15(1249500)2024.PubMed/NCBI View Article : Google Scholar | |
|
Tian J, Zhang D, Yao X, Huang Y and Lu Q: Global epidemiology of systemic lupus erythematosus: A comprehensive systematic analysis and modelling study. Ann Rheum Dis. 82:351–356. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Wang YF, Zhang Y, Lin Z, Zhang H, Wang TY, Cao Y, Morris DL, Sheng Y, Yin X, Zhong SL, et al: Identification of 38 novel loci for systemic lupus erythematosus and genetic heterogeneity between ancestral groups. Nat Commun. 12(772)2021.PubMed/NCBI View Article : Google Scholar | |
|
Xiang K, Wang P, Xu Z, Hu YQ, He YS, Chen Y, Feng YT, Yin KJ, Huang JX, Wang J, et al: Causal effects of gut microbiome on systemic lupus erythematosus: A two-sample mendelian randomization study. Front Immunol. 12(667097)2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhang Y, Zhao C, Lei Y, Li Q, Jin H and Lu Q: Development of a predictive model for systemic lupus erythematosus incidence risk based on environmental exposure factors. Lupus Sci Med. 11(e001311)2024.PubMed/NCBI View Article : Google Scholar | |
|
Su X, Yu H, Lei Q, Chen X, Tong Y, Zhang Z, Yang W, Guo Y and Lin L: Systemic lupus erythematosus: Pathogenesis and targeted therapy. Mol Biomed. 5(54)2024.PubMed/NCBI View Article : Google Scholar | |
|
Wang M, Zhou J, Niu Q and Wang H: Mechanism of tacrolimus in the treatment of lupus nephritis. Front Pharmacol. 15(1331800)2024.PubMed/NCBI View Article : Google Scholar | |
|
Bai W, Li M, Zhou S, Peng L, Zhao J, Tian X, Wang Q, Leng X, Zhang S, Wang Y, et al: Clinical efficacy of tacrolimus in systemic lupus erythematosus with various manifestations: A real-world study. Chin Med J (Engl). 135:2245–2247. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Li M, Zhao Y, Zhang Z, Huang C, Liu Y, Gu J, Zhang X, Xu H, Li X, Wu L, et al: 2020 Chinese guidelines for the diagnosis and treatment of systemic lupus erythematosus. Rheumatol Immunol Res. 1:5–23. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et al: The cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar | |
|
Stang A: Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 25:603–605. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Szeto CC, Kwan BC, Lai FM, Tam LS, Li EK, Chow KM, Gang W and Li PK: Tacrolimus for the treatment of systemic lupus erythematosus with pure class V nephritis. Rheumatology (Oxford). 47:1678–1681. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Chen W, Tang X, Liu Q, Chen W, Fu P, Liu F, Liao Y, Yang Z, Zhang J, Chen J, et al: Short-term outcomes of induction therapy with tacrolimus versus cyclophosphamide for active lupus nephritis: A multicenter randomized clinical trial. Am J Kidney Dis. 57:235–244. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Chen W, Liu Q, Chen W, Tang X, Fu P, Liu F, Liao Y, Yang Z, Zhang J, Chen J, et al: Outcomes of maintenance therapy with tacrolimus versus azathioprine for active lupus nephritis: A multicenter randomized clinical trial. Lupus. 21:944–952. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Wang S, Li X, Qu L, Wang R, Chen Y, Li Q, He X, Zhang X, Wang H, Wu J, et al: Tacrolimus versus cyclophosphamide as treatment for diffuse proliferative or membranous lupus nephritis: A non-randomized prospective cohort study. Lupus. 21:1025–1035. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Yap DY, Yu X, Chen XM, Lu F, Chen N, Li XW, Tang CS and Chan TM: Pilot 24 month study to compare mycophenolate mofetil and tacrolimus in the treatment of membranous lupus nephritis with nephrotic syndrome. Nephrology (Carlton). 17:352–357. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Li X, Ren H, Zhang Q, Zhang W, Wu X, Xu Y, Shen P and Chen N: Mycophenolate mofetil or tacrolimus compared with intravenous cyclophosphamide in the induction treatment for active lupus nephritis. Nephrol Dial Transplant. 27:1467–1472. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Mok CC, Ying KY, Yim CW, Siu YP, Tong KH, To CH and Ng WL: Tacrolimus versus mycophenolate mofetil for induction therapy of lupus nephritis: A randomised controlled trial and long-term follow-up. Ann Rheum Dis. 75:30–36. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Kamanamool N, Ingsathit A, Rattanasiri S, Ngamjanyaporn P, Kasitanont N, Chawanasuntorapoj R, Pichaiwong W, Anutrakulchai S, Sangthawan P, Ophascharoensuk V, et al: Comparison of disease activity between tacrolimus and mycophenolate mofetil in lupus nephritis: A randomized controlled trial. Lupus. 27:647–656. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Zhang X, Liu P and Zhang Z: Analysis of the clinical effects of the combination of mycophenolate mofetil with either tacrolimus or cyclophosphamide. Clinics (Sao Paulo). 75(e1820)2020.PubMed/NCBI View Article : Google Scholar | |
|
Zheng Z, Zhang H, Peng X, Zhang C, Xing C, Xu G, Fu P, Ni Z, Chen J, Xu Z, et al: Effect of tacrolimus vs intravenous cyclophosphamide on complete or partial response in patients with lupus nephritis: A randomized clinical trial. JAMA Netw Open. 5(e224492)2022.PubMed/NCBI View Article : Google Scholar | |
|
Zhang Q, Xing P, Ren H, Chen X, Xie J, Zhang W, Shen P, Li X and Chen N: Mycophenolate mofetil or tacrolimus compared with azathioprine in long-term maintenance treatment for active lupus nephritis. Front Med. 16:799–807. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Suzuki S, Otani T, Ikeda K, Tamura N and Morimoto S: Additional benefits of belimumab in chronic phase of systemic lupus erythematosus and efficacy of tacrolimus combination therapy. Immunol Med. 27:1–7. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Almaani S, Meara A and Rovin BH: Update on lupus nephritis. Clin J Am Soc Nephrol. 12:825–835. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Deng J and Xie H, Zhu L, Luo L and Xie H: Maintenance therapy for lupus nephritis with mycophenolate mofetil or azathioprine. A meta-analysis. Clin Nephrol. 91:172–179. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Prasad N, Kurian J, Agarwal V, Bhadauria D, Behera M, Yacha M, Kushwaha R, Agrawal V, Jain M and Gupta A: Long-term outcomes of lupus nephritis treated with regimens based on cyclophosphamide and mycophenolate mofetil. Lupus. 29:845–853. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Suzuki K, Kameda H, Amano K, Nagasawa H, Takei H, Nishi E, Okuyama A, Tsuzaka K and Takeuchi T: Single center prospective study of tacrolimus efficacy and safety in the treatment of various manifestations in systemic lupus erythematosus. Rheumatol Int. 31:757–763. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Takahashi S, Hiromura K, Sakurai N, Matsumoto T, Ikeuchi H, Maeshima A, Kaneko Y, Kuroiwa T and Nojima Y: Efficacy and safety of tacrolimus for induction therapy in patients with active lupus nephritis. Mod Rheumatol. 21:282–289. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Yap DY, Ma MK, Mok MM, Kwan LP, Chan GC and Chan TM: Long-term data on tacrolimus treatment in lupus nephritis. Rheumatology (Oxford). 53:2232–2237. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Tanaka H, Watanabe S, Aizawa-Yashiro T, Oki E, Kumagai N, Tsuruga K and Ito E: Long-term tacrolimus-based immunosuppressive treatment for young patients with lupus nephritis: A prospective study in daily clinical practice. Nephron Clin Pract. 121:C165–C173. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Edavalath S, Rai MK, Gupta V, Mishra R, Misra DP, Gupta L and Agarwal V: Tacrolimus induces remission in refractory and relapsing lupus nephritis by decreasing P-glycoprotein expression and function on peripheral blood lymphocytes. Rheumatol Int. 42:1347–1354. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Hiramatsu Y, Yoshida S, Kotani T, Nakamura E, Kimura Y, Fujita D, Nagayasu Y, Shabana K, Makino S, Takeuchi T and Arawaka S: Changes in the blood level, efficacy, and safety of tacrolimus in pregnancy and the lactation period in patients with systemic lupus erythematosus. Lupus. 27:2245–2252. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Tanaka H, Aizawa T and Endo M: Long-term outcome of tacrolimus-based immunosuppressive treatment for patients with paediatric-onset lupus nephritis. Nephrology (Carlton). 29:901–908. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Li K, Yu Y, Gao Y, Zhao F, Liang Z and Gao J: Comparative effectiveness of rituximab and common induction therapies for lupus nephritis: A systematic review and network meta-analysis. Front Immunol. 13(859380)2022.PubMed/NCBI View Article : Google Scholar | |
|
Shin JI, Li H, Park S, Yang JW, Lee KH, Jo Y, Park S, Oh J, Kim H, An HJ, et al: Induction and maintenance treatment of lupus nephritis: A comprehensive review of meta-analyses. J Clin Med. 11(343)2022.PubMed/NCBI View Article : Google Scholar | |
|
Lee YH and Song GG: Efficacy and safety of tacrolimus versus mycophenolate mofetil as induction treatment and low-dose tacrolimus as treatment for lupus nephritis: A meta-analysis. Z Rheumatol. 82:754–762. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Dong Y, Shi J, Wang S, Liu Y, Yu S and Zhao L: The efficacy of immunosuppressive drugs induction therapy for lupus nephritis: A systematic review and network meta-analysis. Ren Fail. 45(2290365)2023.PubMed/NCBI View Article : Google Scholar | |
|
Jiang N, Jin S, Yu C, Zhao J, Wang Q, Tian X, Li M and Zeng X: Efficacy and safety of immunosuppressive agents for adults with lupus nephritis: A systematic review and network meta-analysis. Front Immunol. 14(1232244)2023.PubMed/NCBI View Article : Google Scholar | |
|
Guo Q, Zhang X, Sun S, Tang X, Shen W, Liang J, Yao G, Geng L, Ding S, Chen H, et al: Association between mycophenolate mofetil use and subsequent infections among hospitalized patients with systemic lupus erythematosus: A nested case-control study. Rheumatol Ther. 10:1535–1554. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Yoon KH: Efficacy and cytokine modulating effects of tacrolimus in systemic lupus erythematosus: A review. J Biomed Biotechnol. 2010(686480)2010.PubMed/NCBI View Article : Google Scholar | |
|
Shigesaka M, Ito T, Inaba M, Imai K, Yamanaka H, Azuma Y, Tanaka A, Amuro H, Nishizawa T, Son Y, et al: Mycophenolic acid, the active form of mycophenolate mofetil, interferes with IRF7 nuclear translocation and type I IFN production by plasmacytoid dendritic cells. Arthritis Res Ther. 22(264)2020.PubMed/NCBI View Article : Google Scholar | |
|
Hussenbocus YAAM, Jin Z, Pan W, Liu L, Wu M, Hu H, Ding X, Wei H, Zou Y, Qian X, et al: Low dosage use of cyclophosphamide improves the survival of patients with systemic lupus erythematosus. Clin Rheumatol. 41:2043–2052. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Kraaij T, Bredewold OW, Trompet S, Huizinga TW, Rabelink TJ, de Craen AJ and Teng YK: TAC-TIC use of tacrolimus-based regimens in lupus nephritis. Lupus Sci Med. 3(e000169)2016.PubMed/NCBI View Article : Google Scholar |