Smoke signals in the genome: Epigenetic consequences of parental tobacco exposure (Review)
- Authors:
- Published online on: June 23, 2025 https://doi.org/10.3892/br.2025.2024
- Article Number: 146
-
Copyright: © Vlachou et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
1. Introduction
Epigenetics is a rapidly evolving field within genetic science, which investigates heritable changes in gene expression that occur independently of alterations in the underlying DNA sequence (1). These changes are instead driven by environmental influences, such as air pollution, nutritional status, medication use, alcohol consumption and tobacco smoking. Such epigenetic alterations, primarily DNA methylation, histone modification and the regulation of gene expression by microRNAs (miRNAs/miRs), serve a crucial role in fetal development, particularly through their impact on the placenta, an organ fundamental to nutrient and oxygen exchange during pregnancy (2,3). Proper epigenetic regulation of the placenta is essential for embryonic growth and survival. Disruptions in this finely tuned regulatory system can result in long-term adverse outcomes for the offspring, establishing a developmental trajectory that may predispose individuals to disease later in life (4).
Research has underscored the placenta as both a target and a mediator of environmental exposures. Maternal tobacco smoking during pregnancy has been shown to significantly modulate placental miRNA expression, such as miR-16, miR-21 and miR-146a, potentially altering cellular differentiation, immune signaling and apoptotic pathways essential to fetal development (5). Nicotine, the principal addictive component of cigarette smoke, induces marked epigenetic changes, including gene-specific DNA methylation shifts and oxidative damage. These modifications have been implicated in a range of pathological conditions, such as intrauterine growth restriction, impaired pulmonary development, childhood asthma and cognitive dysfunction (6,7).
Moreover, epigenetic vulnerability is not limited to the intrauterine environment. Mounting evidence supports the notion that environmental exposures even prior to conception can affect the germline. Tobacco exposure has been associated with nuclear and mitochondrial DNA alterations in gametes, leading to downstream consequences in the embryo (8). These findings challenge the traditional view that only maternal exposures during gestation influence fetal outcomes, suggesting instead that both maternal and paternal environmental histories are critical determinants of offspring health. Notably, paternal smoking has been linked to epigenetic modifications in sperm, particularly altered DNA methylation patterns and non-coding RNA expression, which may be transmitted to the zygote and influence the trajectory of embryonic development (9,10).
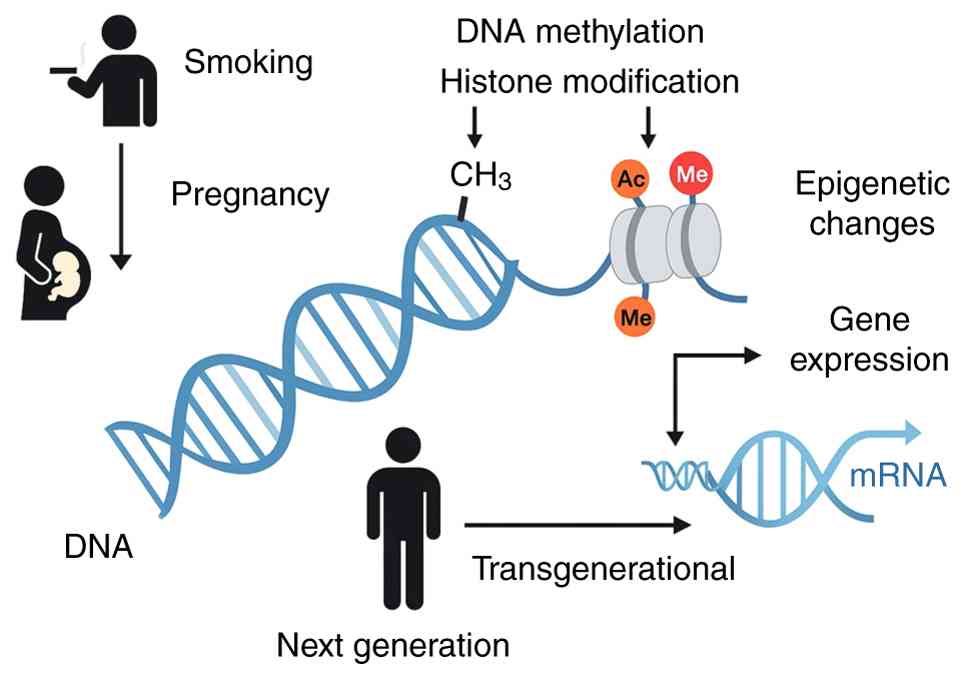
An epidemiological study has also raised the possibility of transgenerational epigenetic inheritance. This phenomenon implies that exposure to harmful environmental agents such as tobacco smoke may affect not only the directly exposed individual but also subsequent generations. For example, maternal smoking during pregnancy has been associated with an increased risk of asthma and impaired pulmonary function in grandchildren, even when the intermediate generation did not smoke (11). These observations emphasize the long-term consequences of environmental insults, potentially mediated by stable epigenetic marks that persist across generations.
In light of the increasing public health concern regarding prenatal and preconception exposures, understanding the epigenetic mechanisms through which tobacco use exerts its effects on offspring is of paramount importance. The present review aims to summarize the existing evidence on the epigenetic effects of maternal, paternal and ancestral smoking. It focuses on identifying the key molecular pathways involved, characterizing the specific genomic regions affected by these exposures, and examining the consequences of such changes on offspring health, from birth through adulthood. Through an integrative examination of molecular and population-level data, this work highlights the critical role of epigenetic programming in mediating the intergenerational and transgenerational effects of tobacco smoke exposure.
Although epigenetic regulation encompasses mechanisms such as histone modifications and non-coding RNAs, the present review focuses specifically on DNA methylation. This emphasis reflects the greater volume of evidence linking DNA methylation to tobacco-related epigenetic changes and its clearer mechanistic relevance to heritable disease risk. Histone modifications and non-coding RNAs are acknowledged as important but fall outside the scope of the present review.
2. Fetal programming theory (Barker hypothesis)
The theory of fetal programming, commonly referred to as the Barker hypothesis, was first proposed by Barker and Clark in 1997, and highlights the critical interaction between genetic predisposition and environmental factors during intrauterine development (12). It posits that the intrauterine environment serves a formative role in determining the molecular characteristics of fetal growth and that adverse exposures during gestation may predispose the individual to disease in later life (12). The initial formulation of the hypothesis suggested that poor maternal nutrition during pregnancy could lead to permanent physiological and metabolic adaptations in the fetus. These adaptations, although aimed at ensuring survival under conditions of limited nutrient availability, may subsequently increase susceptibility to chronic diseases such as cardiovascular disease, diabetes and hypertension in adulthood (12). The terms ‘fetal programming’ and ‘developmental reprogramming’ are used to describe lasting effects of early-life environmental exposures on long-term health. Fetal programming refers specifically to in utero processes that shape disease susceptibility, whereas developmental reprogramming encompasses broader mechanisms, including epigenetic modifications such as DNA methylation, which may occur throughout early development. In the present review, the term ‘fetal programming’ is primarily used to maintain consistency, except where epigenetic reprogramming more precisely describes molecular changes.
The implications of this theory have since been broadened to include a range of environmental stressors beyond nutrition, including maternal stress, exposure to environmental toxins, and notably, tobacco smoking during pregnancy. These exposures may induce adaptive epigenetic responses in the fetus, such as changes in gene expression, DNA methylation patterns and chromatin structure, which enable survival under suboptimal conditions but may lead to long-term alterations in organ development, metabolic regulation and neuroendocrine function. Such modifications are often irreversible and can persist across the lifespan, thereby establishing a developmental origin for adult-onset disease (13,14). In this context, the Barker hypothesis provides a compelling framework for understanding how early-life exposures, particularly to maternal behaviors such as smoking, can program biological systems in ways that affect long-term health outcomes.
3. Components of cigarette smoke
Cigarette smoke contains a wide array of harmful compounds, several of which are known to exert significant toxic and epigenetic effects on the developing fetus. Among the primary toxicants are nicotine, carbon monoxide (CO), polycyclic aromatic hydrocarbons (PAHs), formaldehyde and various heavy metals, including cadmium (Cd) and lead (15).
Nicotine is a potent vasoconstrictor that induces the narrowing of maternal blood vessels, which in turn reduces uteroplacental blood flow. This diminished circulation compromises the oxygen and nutrient supply to the fetus, resulting in chronic intrauterine hypoxia (16,17). Nicotine exposure has been shown to alter gene expression and induce DNA methylation changes in pathways associated with neuronal development. For example, maternal nicotine exposure disrupts the hippocampal GM13530/miR-7119-3p/myocyte enhancer factor 2C competing endogenous RNA axis, leading to impaired synaptic plasticity (18). Furthermore, nicotine enhances the formation of the N-methyl-D-aspartate receptor/neuronal nitric oxide synthase/postsynaptic density protein-95 complex, promoting calcium influx, nitric oxide production and downstream p38 MAPK phosphorylation, mechanisms that contribute to excitotoxic neuronal damage, and deficits in learning and social behavior (18). In parallel, prenatal nicotine exposure results in epigenetic modifications in the testis and pituitary gland, including altered DNA methylation of genes involved in peripheral nervous system signaling such as those encoding transcription factors nuclear respiratory factor 1 and E26 transformation-specific variant 4, as well as γ-aminobutyric acid-related signaling components (19). Nicotine exposure also affects respiratory function through epigenetic mechanisms, as demonstrated by Onuzulu et al (20); this previous study identified widespread DNA methylation changes in the lungs of mice chronically exposed to cigarette smoke. These included hypermethylation of genes such as forkhead box J1, a key regulator of motile ciliogenesis, potentially impairing mucociliary clearance. Additional methylation alterations in IL6 receptor and genes involved in Wnt/β-catenin signaling suggest disrupted immune regulation and epithelial development, contributing to persistent lung function decline even after smoking cessation (20). CO poses an additional and distinct threat; it binds to hemoglobin with an affinity >200-fold greater than that of oxygen, forming carboxyhemoglobin and effectively displacing oxygen from its transport pathway (21). The developing fetus is particularly vulnerable to the effects of CO due to the high oxygen affinity of fetal hemoglobin, which further enhances the uptake of CO and exacerbates tissue hypoxia. Chronic fetal hypoxia has been linked to a spectrum of developmental impairments, including growth restriction and neurodevelopmental deficits (22,23).
Heavy metals such as Cd also have a notable role in the toxicological burden of tobacco smoke. Exposure to Cd has been associated with sex-specific alterations in DNA methylation patterns, suggesting a complex interaction between metal toxicity, sex hormones and epigenetic regulation. In a study by Virani et al (24), urinary Cd concentrations were found to be 7-fold higher in exposed populations compared with controls. The researchers reported hypermethylation of Cd-associated DNA markers in men, whereas women exhibited elevated Cd levels alongside hypomethylation of the same markers. These findings underscore the differential biological response to Cd and suggest that epigenetic susceptibility may vary based on sex and smoking status (24).
PAHs, such as benzo[a]pyrene, are another class of toxicants found in cigarette smoke. These compounds are well-established carcinogens and genotoxins capable of forming DNA adducts and causing mutations (25). Intrauterine exposure to PAHs has been implicated in both somatic and germline genomic damage, potentially laying the groundwork for carcinogenesis later in life (26,27).
Formaldehyde, a volatile organic compound present in cigarette smoke, is associated with a broad spectrum of teratogenic and neurodevelopmental effects. Prenatal exposure has been linked to congenital cardiovascular and musculoskeletal anomalies, memory deficits, learning disabilities and an increased risk of neurological disorders (28). Additionally, lead, another heavy metal commonly found in tobacco smoke, has neurotoxic effects that are particularly detrimental during early brain development. Lead interferes with neurotransmitter signaling and has been associated with cognitive impairment and behavioral disorders (29).
4. Smoking during pregnancy
Tobacco use during pregnancy is a well-established risk factor for a broad range of adverse outcomes in offspring. It compromises fetal development and has been linked to both short-term complications and long-term disease susceptibility, often through complex interactions involving toxicological and epigenetic mechanisms. Prenatal exposure to tobacco smoke introduces a cascade of molecular disruptions that begin during gestation and can manifest throughout the lifespan of individuals (30,31).
Maternal smoking impairs fetal growth by compromising placental function and oxygen delivery, and it exerts toxic effects on the central nervous system. Nicotine and other smoke-derived compounds interfere with neurodevelopmental pathways, disrupting the formation and function of key brain structures. These insults are mediated in part by epigenetic mechanisms that modulate gene expression without altering the DNA sequence. As a result, maternal smoking during pregnancy has been associated with elevated risks of several neuropsychiatric disorders in offspring (32,33). According to Knopik et al (32), prenatal smoking can increase the odds of attention-deficit/hyperactivity disorder (ADHD) by 2-3-fold, with odds ratios (ORs) ranging from 1.8 to 2.7 depending on study design and control for familial confounding. The risk for conduct problems and externalizing behaviors was similarly elevated (ORs, 1.5-2.6), while associations with depression, anxiety and schizophrenia were also observed, though with more variability and potential confounding influences (32). These outcomes are considered to stem from altered neurogenesis and dysregulated neurotransmitter receptor formation, with effects that persist into adolescence and potentially adulthood (33).
The detrimental influence of tobacco exposure during gestation extends beyond direct maternal inhalation. An experimental study in animal models has shown that secondhand smoke exposure during pregnancy can also induce notable biological effects in the fetus. In a study using rats, gestational exposure to sidestream cigarette smoke was shown to be associated with an increased incidence of allergic asthma and bronchopulmonary dysplasia in both first- and second-generation offspring, suggesting the possibility of transgenerational transmission of harm (34). Notably, these effects were accompanied by measurable changes in the expression of critical miRNAs, specifically reduced levels of miR-130a, and elevated levels of miR-16 and miR-221. These miRNAs are key regulators of apoptosis, angiogenesis and immune signaling in pulmonary tissues, indicating that epigenetic dysregulation serves a central role in mediating the respiratory consequences of prenatal tobacco exposure (34).
5. DNA methylation
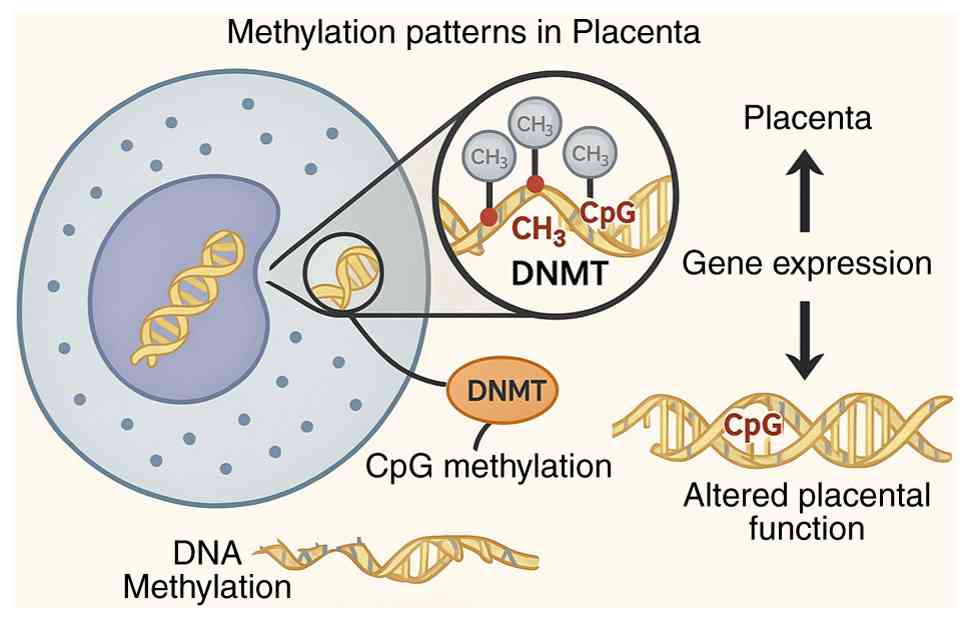
DNA methylation is one of the most well-studied epigenetic mechanisms by which environmental exposures, including maternal smoking, influence gene expression. This modification involves the addition of methyl groups (-CH3) to cytosine residues, typically at CpG dinucleotide sites, and is catalyzed by a family of enzymes known as DNA methyltransferases (DNMTs) (35,36). Components of cigarette smoke can induce oxidative stress and inflammatory responses, both of which have been shown to alter DNMT activity. These changes can result in either hypermethylation, leading to the suppression of gene expression, such as that of tumor suppressor genes, or hypomethylation, which may result in uncontrolled expression of genes, including oncogenes (37).
One of the most concerning aspects of these epigenetic changes is their impact on fetal brain development. In a comparative study of fetuses exposed to cigarette smoke in utero compared with those who were not, DNA methylation patterns in brain tissue were found to differ significantly. The smoke-exposed group showed evidence of impaired neuronal development and reduced density of brain neurons, suggesting that tobacco-related epigenetic alterations may underlie later neurocognitive dysfunction (38).
Beyond neurological effects, altered DNA methylation resulting from prenatal tobacco exposure has been linked to a variety of pathologies in childhood. These include pediatric cancer, orofacial clefts and asthma, all of which may arise from disrupted gene expression patterns linked to maternal smoking during pregnancy (39). Additionally, methylation changes have been associated with an increased risk of several chronic diseases in adulthood, such as hypertension, atherosclerosis, chronic obstructive pulmonary disease, type 2 diabetes, insulin resistance, and obesity; conditions that together constitute the metabolic syndrome (40,41).
DNA methylation in umbilical cord blood has emerged as a critical biomarker reflecting the impact of environmental conditions on intrauterine development. For example, a recent study has demonstrated that maternal smoking during pregnancy is associated with lower birth weight and smaller body size, effects that correlate with distinct methylation patterns in fetal tissues. Notably, hypomethylation at cg05575921 in the AHRR gene, a locus consistently linked to tobacco smoke exposure, has been strongly associated with reduced birth weight. Additional alterations include hypermethylation of MYO1G (cg12803068) and hypomethylation of GFI1 (cg09935388), both of which have been implicated in developmental regulation and hematopoiesis. These epigenetic modifications are believed to reflect disrupted fetal growth trajectories in response to intrauterine tobacco exposure, and they may serve as early indicators of future health risks, including cardiometabolic and respiratory disorders (42). Notably, these methylation changes are not transient; they can persist into early childhood and beyond. Research by Bauer et al (43) revealed that DNA methylation changes associated with maternal smoking remain detectable in offspring years after birth and are linked to chromatin remodeling and diminished lung function.
Several cohort studies have provided compelling evidence of this phenomenon. Ivorra et al (44) examined full-term neonates born to mothers who smoked throughout pregnancy, with exposure verified by cotinine levels in maternal and umbilical cord blood. Their epigenome-wide association study revealed 31 differentially methylated CpG sites across 25 genes in exposed vs. non-exposed newborns, raising concerns about potential long-term health consequences. Notably, cg05727225 within the ADM (Adrenomedullin) gene was the most significantly hypermethylated site, a gene involved in vascular tone and metabolic regulation. Additional examples include hypermethylation of cg00387170 in FOXP4 (involved in lung development), cg04402350 in ZNF827 (a zinc finger transcription factor), and cg12331332 in CASP7 (a gene related to apoptosis). Some sites, such as cg03529555 in NAV2, were found to be hypomethylated. Xu et al (45) conducted a large-scale study involving 954 mother-infant pairs, revealing that infants exposed to maternal smoking had an average birth weight 300 g lower than their unexposed counterparts. Moreover, the researchers identified 38 differentially methylated CpG sites associated with maternal smoking, several of which were also associated with birth weight, highlighting DNA methylation as a key mechanistic link between tobacco exposure and impaired fetal growth.
Similarly, Antoun et al (46) assessed DNA methylation in cord blood from 1,040 newborns and found that alterations in specific genes associated with birth weight could be traced to maternal smoking, pregnancy-induced hypertension and folate status during gestation. These findings reinforce the notion that the prenatal epigenetic environment is highly responsive to maternal health and behavior, with smoking acting as a particularly potent disruptor.
Interventions aimed at mitigating these effects have shown some promise. Nutritional strategies, including maternal vitamin C supplementation during pregnancy, have been associated with improvements in placental DNA methylation patterns adversely affected by tobacco smoke. This, in turn, supports more appropriate expression of genes involved in placental and fetal respiratory development (47). Additionally, adequate maternal folate levels may partially buffer against the methylation-altering effects of tobacco smoke exposure, suggesting that nutritional optimization may reduce epigenetic harm in exposed pregnancies (48). Fig. 1 illustrates how DNA methylation in the placenta modulates gene expression, highlighting pathways affected by maternal smoking that may influence fetal development and disease risk.
6. Influence of paternal smoking
Emerging evidence has highlighted the significant role of paternal environmental exposures, particularly tobacco use prior to conception, in shaping the epigenetic profile of offspring. Research has indicated that the father's smoking history, including the timing of smoking initiation, may have lasting biological consequences for future generations through epigenetic modifications in sperm (49,50).
Specifically, paternal smoking that begins during adolescence, particularly before the age of 15 years, has been associated with altered DNA methylation patterns in the offspring. Kitaba et al (49) identified 14 hypermethylated CpG sites in the offspring of fathers who smoked during early adolescence. These epigenetic changes were found in genes associated with the development of asthma, wheezing, impaired pulmonary function and obesity in childhood, suggesting a direct link between early paternal smoking and respiratory and metabolic dysregulation in the next generation.
Beyond specific CpG alterations, paternal smoking has been shown to influence the sperm epigenome, a collective term describing the set of epigenetic modifications, including DNA methylation, histone modifications and the activity of non-coding RNAs such as miRNAs, that regulate gene expression without altering the DNA sequence (9,10). These modifications can be profoundly affected by exposure to tobacco-related toxins, which interfere with the programming of sperm cells and may transmit altered gene regulation patterns to the zygote at conception.
The consequences of these paternal epigenetic changes have been increasingly recognized in human and animal studies. Epidemiological findings have linked preconception paternal smoking to an increased risk of childhood asthma (51). An experimental study in animal models has corroborated these findings, showing that paternal nicotine exposure results in altered DNA methylation in the lungs of offspring, impairing pulmonary development and enhancing susceptibility to airway inflammation (37). In a large population-based cohort, the children of fathers who smoked prior to conception demonstrated a higher incidence of ADHD, implicating paternal epigenetic contributions in the etiology of neurodevelopmental disorders (52).
Furthermore, prenatal exposure to tobacco toxins has been linked to an increased risk of autism spectrum disorder. A previous study identified sperm epigenetic alterations in smoking fathers that affect genes involved in synaptic plasticity and brain development, offering a potential mechanistic explanation for these neurodevelopmental effects (53). Paternal smoking has also been associated with insulin resistance and an elevated risk of childhood obesity in offspring (54), likely mediated by disruptions in the epigenetic regulation of metabolic pathways (9).
These findings challenge the conventional maternal-centric perspective of prenatal risk and emphasize the need to consider paternal health and exposures when evaluating intergenerational disease risk. The sperm epigenome, far from being a passive carrier of genetic material, emerges as a dynamic and environmentally sensitive system capable of transmitting acquired risk across generations.
7. Influence of smoking in previous generations
Research has attracted attention to the concept of transgenerational epigenetic inheritance, where environmental exposures such as tobacco use by previous generations can have biological effects on descendants through heritable epigenetic modifications. These findings emphasize the deeply rooted and multigenerational consequences of smoking, particularly regarding respiratory outcomes such as asthma (55-59).
One of the earliest observations supporting this notion came from epidemiological studies showing that grandmaternal smoking during pregnancy is associated with an increased risk of asthma in grandchildren, independent of the mother's own smoking behavior. For example, Li et al (55) reported that the smoking status of the maternal grandmother was linked to a higher prevalence of early childhood asthma in grandchildren, regardless of whether the daughter (the mother of the child) smoked. Similarly, Magnus et al (56) revealed that grandmaternal tobacco use during pregnancy conferred a significantly increased asthma risk to the grandchild, reinforcing the hypothesis that intrauterine exposure can induce heritable changes that persist beyond the directly exposed generation.
Further supporting this theory, Golding et al (57) identified that both paternal grandparents who smoked, specifically grandfathers during adolescence and grandmothers during pregnancy, were linked to elevated asthma risk in their grandchildren. This suggests that the timing of exposure, including during gametogenesis and gestation, is critical for the establishment of epigenetic marks capable of transgenerational transmission.
Evidence from a molecular study has corroborated these epidemiological findings. Luo et al (58) demonstrated that smoking during pregnancy by grandmothers was associated with altered DNA methylation patterns in their grandchildren, highlighting a potential biological mechanism for this intergenerational risk. DNA methylation is known to be relatively stable and heritable during cell division, making it a plausible mediator of long-term, cross-generational effects.
A large-scale cohort analysis by Lodge et al (59), which followed >44,000 grandmothers, 46,000 mothers and 66,000 grandchildren, concluded that the grandchildren of women who smoked during pregnancy had a higher risk of developing asthma during the first 6 years of life, regardless of whether their mothers smoked. The aforementioned study provided robust population-level evidence for transgenerational respiratory consequences of prenatal smoke exposure.
One of the most detailed investigations into this topic was the three-generation Dutch Lifelines cohort study (11), which included 25,747 adults and 11,544 children. Researchers analyzed the relationship between grandmaternal smoking and the respiratory health of grandchildren, stratifying the data by sex and generation. The results showed that maternal grandmother smoking was associated with an increased risk of asthma and reduced lung function in male grandchildren of the more recent generations, particularly when their mothers also smoked. Conversely, in older generations, the same exposure appeared to have a reverse or neutral effect, potentially due to survivor bias or differences in environmental factors across generations. Notably, female grandchildren exhibited a higher risk for early-onset asthma if their grandmothers smoked, but this risk diminished with age. In some cases, smoking by the grandmother appeared to have a protective effect in more recent generations when the mother was a non-smoker, indicating complex, potentially compensatory epigenetic dynamics. Fig. 2 demonstrates how smoking-related epigenetic changes in germ cells or during fetal development can propagate across generations, influencing gene expression and disease risk in children.
8. Epigenetic interventions and public health implications
Understanding the epigenetic consequences of tobacco exposure has not only deepened insight into disease etiology but also opened new avenues for prevention and intervention. As epigenetic modifications such as DNA methylation and histone modifications are potentially reversible, there is increasing interest in identifying strategies that can mitigate or even reverse the heritable effects of smoking.
Although some epigenetic changes induced by tobacco smoke appear to be stable and persist into childhood or even adulthood (39,43), it has been suggested that cessation of smoking, especially before or during early pregnancy, can reduce the extent of fetal exposure and limit epigenetic disruptions. For example, methylation patterns in cord blood differ significantly between infants of current smokers and those of mothers who quit smoking before the second trimester, indicating partial reversibility with early cessation (39).
Certain nutrients, particularly those involved in one-carbon metabolism, have been proposed as modifiers of the epigenetic landscape. Folate, vitamin B12, choline and betaine contribute to DNA methylation by donating methyl groups. Maternal supplementation with folic acid has been shown to counteract some of the adverse methylation changes induced by smoking (48). Similarly, vitamin C supplementation during pregnancy has been associated with improved DNA methylation profiles in the placenta and with improved pulmonary outcomes in the infants of smokers (47).
Antioxidants are also under investigation for their potential to counteract oxidative stress-related epigenetic changes. Tobacco smoke induces reactive oxygen species, which in turn can modulate the activity of DNMTs and histone-modifying enzymes (7). Therefore, antioxidant supplementation may attenuate these effects and protect the integrity of the fetal epigenome.
Public health campaigns have traditionally emphasized the immediate risks of smoking during pregnancy, such as low birth weight or preterm birth; however, the emerging evidence of intergenerational and transgenerational epigenetic effects adds urgency and depth to these messages. Educating both prospective mothers and fathers about the epigenetic risks posed by tobacco use, even prior to conception, could improve participation in cessation programs and promote healthier preconception behaviors (9,10).
Policy actions may also be informed by epigenetic evidence. For example, stricter regulations on smoking in multi-unit housing or in vehicles with minors could be justified not only by secondhand smoke risks but also by potential transgenerational impacts. Moreover, integrating epigenetic screening (for example, methylation biomarkers in cord blood) into maternal-fetal medicine might eventually allow risk stratification and tailored early-life interventions (42).
The potential heritability of epigenetic damage raises ethical questions regarding reproductive choices, intergenerational responsibility and health equity. Populations with higher smoking rates due to socioeconomic disadvantage may face compounded intergenerational health risks, thus requiring targeted interventions. At the same time, the concept of ‘epigenetic guilt’ or stigmatization of parental behavior should be avoided in favor of empowering and supportive strategies that emphasize modifiable risk factors (14).
Future research should focus on longitudinal monitoring of epigenetic changes post-cessation, controlled trials evaluating the efficacy of dietary and pharmacological interventions, and broader application of epigenomic biomarkers in public health surveillance. Such efforts will be essential in developing epigenetically informed prevention strategies that transcend traditional generational boundaries. A summary of the main epigenetic mechanisms and their potential fetal consequences is presented in Table I.
Table ISummary of epigenetic mechanisms affected by parental tobacco exposure and potential consequences to the fetus. |
9. Conclusions
The evidence reviewed in the present review highlights the profound and enduring effects of tobacco exposure on the epigenome across generations. Maternal smoking during pregnancy has been shown to alter DNA methylation patterns in the placenta and fetal tissues, with long-term consequences for growth, neurodevelopment and disease risk. Notably, these epigenetic modifications are not confined to maternal behavior; paternal smoking, particularly when initiated in adolescence, can disrupt the sperm epigenome, influencing offspring gene expression and health outcomes. Furthermore, transgenerational inheritance of smoking-induced epigenetic changes, observed in the grandchildren of smokers, emphasizes the extended reach of environmental exposures. These findings challenge the traditional view of genetic inheritance by introducing epigenetic memory as a crucial mediator of disease risk. They also reinforce the importance of early and even preconception interventions to protect future generations.
Despite the increasing body of evidence, several limitations persist in current research. Numerous findings are derived from observational studies, which may be subject to residual confounding and limited in establishing causality. Additionally, there is a need for more mechanistic studies in humans and standardized epigenetic biomarkers to improve understanding of the persistence and functional consequences of tobacco-induced epigenetic changes. While the reversibility of certain epigenetic marks offers hope, the persistence of others calls for urgent public health action. Cessation programs, nutritional supplementation, and policy reforms grounded in epigenetic science should be prioritized to prevent the propagation of tobacco-related harm through the germline. In summary, addressing tobacco exposure from an epigenetic perspective reframes it not only as a personal health issue but as a transgenerational public health imperative.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
MV and AD conceptualized the study. MV, AK, VV, AD, GK, VEG and DAS made a substantial contribution to data interpretation and analysis, and wrote and prepared the draft of the manuscript. AD and MV analyzed the data and provided critical revisions. All authors contributed to manuscript revision, and read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were used to improve the readability and language of the manuscript, and subsequently, the authors revised and edited the content produced by the AI tool as necessary, taking full responsibility for the ultimate content of the present manuscript.
References
|
Aboud NM, Tupper C and Jialal I: Genetics, Epigenetic Mechanism. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL, 2025. https://www.ncbi.nlm.nih.gov/books/NBK532999/. | |
|
Ashraf UM, Hall DL, Rawls AZ and Alexander BT: Epigenetic processes during preeclampsia and effects on fetal development and chronic health. Clin Sci (Lond). 135:2307–2327. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Koukoura O, Sifakis S and Spandidos DA: DNA methylation in the human placenta and fetal growth (review). Mol Med Rep. 5:883–889. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Maccani MA and Marsit CJ: Epigenetics in the placenta. Am J Reprod Immunol. 62:78–89. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Maccani MA, Avissar-Whiting M, Banister CE, McGonnigal B, Padbury JF and Marsit CJ: Maternal cigarette smoking during pregnancy is associated with downregulation of miR-16, miR-21, and miR-146a in the placenta. Epigenetics. 5:583–589. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Ochoa-Avilés C, Ochoa-Avilés A, Rivas-Párraga R, Escandón S, Santos-Jesus TD, Silva MJ, Leão V, Salinas M, Vicuña Y, Baldeón L, et al: Mother's smoking habits affects IL10 methylation but not asthma in Ecuadorian children. Mol Genet Genomic Med. 12(e2438)2024.PubMed/NCBI View Article : Google Scholar | |
|
Upadhyaya P, Milillo C, Bruno A, Anaclerio F, Buccolini C, Dell'Elice A, Angilletta I, Gatta M, Ballerini P and Antonucci I: Nicotine-induced genetic and epigenetic modifications in primary human amniotic fluid stem cells. Curr Pharm Des. 30:1995–2006. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Pirini F, Guida E, Lawson F, Mancinelli A and Guerrero-Preston R: Nuclear and mitochondrial DNA alterations in newborns with prenatal exposure to cigarette smoke. Int J Environ Res Public Health. 12:1135–1155. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Soubry A, Hoyo C, Jirtle RL and Murphy SK: A paternal environmental legacy: Evidence for epigenetic inheritance through the male germ line. Bioessays. 36:359–371. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Sharma U: Paternal contributions to offspring health: Role of sperm small RNAs in intergenerational transmission of epigenetic information. Front Cell Dev Biol. 7(215)2019.PubMed/NCBI View Article : Google Scholar | |
|
Mahon GM, Koppelman GH and Vonk JM: Grandmaternal smoking, asthma and lung function in the offspring: The Lifelines cohort study. Thorax. 76:441–447. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Barker DJ and Clark PM: Fetal undernutrition and disease in later life. Rev Reprod. 2:105–112. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Chen M and Zhang L: Epigenetic mechanisms in developmental programming of adult disease. Drug Discov Today. 16:1007–1018. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Banik A, Kandilya D, Ramya S, Stünkel W, Chong YS and Dheen ST: Maternal factors that induce epigenetic changes contribute to neurological disorders in offspring. Genes (Basel). 8(150)2017.PubMed/NCBI View Article : Google Scholar | |
|
Breton CV, Byun HM, Wenten M, Pan F, Yang A and Gilliland FD: Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 180:462–467. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Birnbaum SC, Kien N, Martucci RW, Gelzleichter TR, Witschi H, Hendrickx AG and Last JA: Nicotine- or epinephrine-induced uteroplacental vasoconstriction and fetal growth in the rat. Toxicology. 94:69–80. 1994.PubMed/NCBI View Article : Google Scholar | |
|
Pintican D, Poienar AA, Strilciuc S and Mihu D: Effects of maternal smoking on human placental vascularization: A systematic review. Taiwan J Obstet Gynecol. 58:454–459. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Zhao Y, Li B, Cao H, Wang F, Mu M, Jin H, Liu J, Fan Z and Tao X: Maternal nicotine exposure promotes hippocampal CeRNA-mediated excitotoxicity and social barriers in adolescent offspring mice. Ecotoxicol Environ Saf. 273(116079)2024.PubMed/NCBI View Article : Google Scholar | |
|
Dali O, Muriel-Muriel JA, Vargas-Baco A, Tevosian S, Zubcevic J, Smagulova F and Hayward LF: Prenatal nicotine exposure leads to epigenetic alterations in peripheral nervous system signaling genes in the testis of the rat. Epigenetics Chromatin. 17(14)2024.PubMed/NCBI View Article : Google Scholar | |
|
Onuzulu CD, Lee S, Basu S, Comte J, Hai Y, Hizon N, Chadha S, Fauni MS, Halayko AJ, Pascoe CD and Jones MJ: Novel DNA methylation changes in mouse lungs associated with chronic smoking. Epigenetics. 19(2322386)2024.PubMed/NCBI View Article : Google Scholar | |
|
Kosaki Y, Maeyama H, Nojima T, Obara T, Nakao A and Naito H: Carbon monoxide poisoning during pregnancy treated with hyperbaric oxygen. Clin Case Rep. 9(e04138)2021.PubMed/NCBI View Article : Google Scholar | |
|
Piešová M and Mach M: Impact of perinatal hypoxia on the developing brain. Physiol Res. 69:199–213. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Ramirez Zegarra R, Dall'Asta A and Ghi T: Mechanisms of fetal adaptation to chronic hypoxia following placental insufficiency: A review. Fetal Diagn Ther. 49:279–292. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Virani S, Rentschler KM, Nishijo M, Ruangyuttikarn W, Swaddiwudhipong W, Basu N and Rozek LS: DNA methylation is differentially associated with environmental cadmium exposure based on sex and smoking status. Chemosphere. 145:284–290. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Bukowska B, Mokra K and Michałowicz J: Benzo[a]pyrene-Environmental occurrence, human exposure, and mechanisms of toxicity. Int J Mol Sci. 23(6348)2022.PubMed/NCBI View Article : Google Scholar | |
|
Cao C, Jia Z, Shao M, Li R, Sun Q and Liu D: Prenatal exposure to polycyclic aromatic hydrocarbons could increase the risk of low birth weight by affecting the DNA methylation states in a Chinese cohort. Reprod Biol. 21(100574)2021.PubMed/NCBI View Article : Google Scholar | |
|
Yang L, Shang L, Wang S, Yang W, Huang L, Qi C, Gurcan A, Yang Z and Chung MC: The association between prenatal exposure to polycyclic aromatic hydrocarbons and birth weight: A meta-analysis. PLoS One. 15(e0236708)2020.PubMed/NCBI View Article : Google Scholar | |
|
Duong A, Steinmaus C, McHale CM, Vaughan CP and Zhang L: Reproductive and developmental toxicity of formaldehyde: A systematic review. Mutat Res. 728:118–138. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Gundacker C, Forsthuber M, Szigeti T, Kakucs R, Mustieles V, Fernandez MF, Bengtsen E, Vogel U, Hougaard KS and Saber AT: Lead (Pb) and neurodevelopment: A review on exposure and biomarkers of effect (BDNF, HDL) and susceptibility. Int J Hyg Environ Health. 238(113855)2021.PubMed/NCBI View Article : Google Scholar | |
|
Banderali G, Martelli A, Landi M, Moretti F, Betti F, Radaelli G, Lassandro C and Verduci E: Short and long term health effects of parental tobacco smoking during pregnancy and lactation: A descriptive review. J Transl Med. 13(327)2015.PubMed/NCBI View Article : Google Scholar | |
|
Scott-Goodwin AC, Puerto M and Moreno I: Toxic effects of prenatal exposure to alcohol, tobacco and other drugs. Reprod Toxicol. 61:120–130. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Knopik VS, Maccani MA, Francazio S and McGeary JE: The epigenetics of maternal cigarette smoking during pregnancy and effects on child development. Dev Psychopathol. 24:1377–1390. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Toledo-Rodriguez M, Lotfipour S, Leonard G, Perron M, Richer L, Veillette S, Pausova Z and Paus T: Maternal smoking during pregnancy is associated with epigenetic modifications of the brain-derived neurotrophic factor-6 exon in adolescent offspring. Am J Med Genet B Neuropsychiatr Genet. 153B:1350–1354. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Singh SP, Chand HS, Langley RJ, Mishra N, Barrett T, Rudolph K, Tellez C, Filipczak PT, Belinsky S, Saeed AI, et al: Gestational exposure to sidestream (Secondhand) cigarette smoke promotes transgenerational epigenetic transmission of exacerbated allergic asthma and bronchopulmonary dysplasia. J Immunol. 198:3815–3822. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Spandidos DA: A unified theory for the development of cancer. Biosci Rep. 6:691–708. 1986.PubMed/NCBI View Article : Google Scholar | |
|
Koukoura O, Spandidos DA, Daponte A and Sifakis S: DNA methylation profiles in ovarian cancer: Implication in diagnosis and therapy (Review). Mol Med Rep. 10:3–9. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Rehan VK, Liu J, Naeem E, Tian J, Sakurai R, Kwong K, Akbari O and Torday JS: Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med. 10(129)2012.PubMed/NCBI View Article : Google Scholar | |
|
Chatterton Z, Hartley BJ, Seok MH, Mendelev N, Chen S, Milekic M, Rosoklija G, Stankov A, Trencevsja-Ivanovska I, Brennand K, et al: In utero exposure to maternal smoking is associated with DNA methylation alterations and reduced neuronal content in the developing fetal brain. Epigenetics Chromatin. 10(4)2017.PubMed/NCBI View Article : Google Scholar | |
|
Joubert BR, Felix JF, Yousefi P, Bakulski KM, Just AC, Breton C, Reese SE, Markunas CA, Richmond RC, Xu CJ, et al: DNA methylation in newborns and maternal smoking in pregnancy: Genome-wide consortium meta-analysis. Am J Hum Genet. 98:680–696. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Barcelona V, Huang Y, Brown K, Liu J, Zhao W, Yu M, Kardia SLR, Smith JA, Taylor JY and Sun YV: Novel DNA methylation sites associated with cigarette smoking among African Americans. Epigenetics. 14:383–391. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Rogers JM: Smoking and pregnancy: Epigenetics and developmental origins of the metabolic syndrome. Birth Defects Res. 111:1259–1269. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Deng WQ, Cawte N, Campbell N, Azab SM, de Souza RJ, Lamri A, Morrison KM, Atkinson SA, Subbarao P, Turvey SE, et al: Maternal smoking DNA methylation risk score associated with health outcomes in offspring of European and South Asian ancestry. Elife. 13(RP93260)2024.PubMed/NCBI View Article : Google Scholar | |
|
Bauer T, Trump S, Ishaque N, Thürmann L, Gu L, Bauer M, Bieg M, Gu Z, Weichenhan D, Mallm JP, et al: Environment-induced epigenetic reprogramming in genomic regulatory elements in smoking mothers and their children. Mol Syst Biol. 12(861)2016.PubMed/NCBI View Article : Google Scholar | |
|
Ivorra C, Fraga MF, Bayón GF, Fernández AF, Garcia-Vicent C, Chaves FJ, Redon J and Lurbe E: DNA methylation patterns in newborns exposed to tobacco in utero. J Transl Med. 13(25)2015.PubMed/NCBI View Article : Google Scholar | |
|
Xu R, Hong X, Zhang B, Huang W, Hou W, Wang G, Wang X, Igusa T, Liang L and Ji H: DNA methylation mediates the effect of maternal smoking on offspring birthweight: A birth cohort study of multi-ethnic US mother-newborn pairs. Clin Epigenetics. 13(47)2021.PubMed/NCBI View Article : Google Scholar | |
|
Antoun E, Titcombe P, Dalrymple K, Kitaba NT, Barton SJ, Flynn A, Murray R, Garratt ES, Seed PT, White SL, et al: DNA methylation signatures in cord blood associated with birthweight are enriched for dmCpGs previously associated with maternal hypertension or pre-eclampsia, smoking and folic acid intake. Epigenetics. 17:405–421. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Shorey-Kendrick LE, McEvoy CT, O'Sullivan SM, Milner K, Vuylsteke B, Tepper RS, Haas DM, Park B, Gao L, Vu A, et al: Impact of vitamin C supplementation on placental DNA methylation changes related to maternal smoking: Association with gene expression and respiratory outcomes. Clin Epigenetics. 13(177)2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhang B, Hong X, Ji H, Tang WY, Kimmel M, Ji Y, Pearson C, Zuckerman B, Surkan PJ and Wang X: Maternal smoking during pregnancy and cord blood DNA methylation: New insight on sex differences and effect modification by maternal folate levels. Epigenetics. 13:505–518. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Kitaba NT, Knudsen GTM, Johannessen A, Rezwan FI, Malinovschi A, Oudin A, Benediktsdottir B, Martino D, González FJC, Gómez LP, et al: Fathers' preconception smoking and offspring DNA methylation. Clin Epigenetics. 15(131)2023.PubMed/NCBI View Article : Google Scholar | |
|
Bhadsavle SS and Golding MC: Paternal epigenetic influences on placental health and their impacts on offspring development and disease. Front Genet. 13(1068408)2022.PubMed/NCBI View Article : Google Scholar | |
|
Svanes C, Koplin J, Skulstad SM, Johannessen A, Bertelsen RJ, Benediktsdottir B, Bråbäck L, Elie Carsin A, Dharmage S, Dratva J, et al: Father's environment before conception and asthma risk in his children: A multi-generation analysis of the Respiratory Health In Northern Europe study. Int J Epidemiol. 46:235–245. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Langley K, Heron J, Smith GD and Thapar A: Maternal and paternal smoking during pregnancy and risk of ADHD symptoms in offspring: testing for intrauterine effects. Am J Epidemiol. 176:261–268. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Kim B, Ha M, Kim YS, Koh YJ, Dong S, Kwon HJ, Kim YS, Lim MH, Paik KC, Yoo SJ, et al: Prenatal exposure to paternal smoking and likelihood for autism spectrum disorder. Autism. 25:1946–1959. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Chang CH and Chuang LM: Fetal exposure to parental smoking and the risk of type 2 diabetes: Are lifestyle-related factors more important? J Diabetes Investig. 7:472–475. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Li YF, Langholz B, Salam MT and Gilliland FD: Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest. 127:1232–1241. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Magnus MC, Håberg SE, Karlstad Ø, Nafstad P, London SJ and Nystad W: Grandmother's smoking when pregnant with the mother and asthma in the grandchild: The Norwegian Mother and Child Cohort Study. Thorax. 70:237–243. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Golding J, Tunstall H, Gregory S, Granell R, Dodd JW, Iles-Caven Y, Watkins S and Suderman M: A history of asthma may be associated with grandparents' exposures to stress and cigarette smoking. Front Toxicol. 5(1253442)2023.PubMed/NCBI View Article : Google Scholar | |
|
Luo R, Zhang H, Mukherjee N, Karmaus W, Patil V, Arshad H and Mzayek F: Association of grandmaternal smoking during pregnancy with DNA methylation of grandchildren: the Isle of Wight study. Epigenomics. 13:1473–1483. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Lodge CJ, Bråbäck L, Lowe AJ, Dharmage SC, Olsson D and Forsberg B: Grandmaternal smoking increases asthma risk in grandchildren: A nationwide Swedish cohort. Clin Exp Allergy. 48167–174. 2018.PubMed/NCBI View Article : Google Scholar |