Imiquimod promotes Th1 and Th17 responses via NF‑κB‑driven IL‑12 and IL‑6 production in an in vitro co‑culture model
- Authors:
- Published online on: July 21, 2025 https://doi.org/10.3892/etm.2025.12925
- Article Number: 175
Abstract
Introduction
Psoriasis is a common, chronic autoimmune skin disease affecting approximately 2-3% of the global population. Beyond its cutaneous manifestations, psoriasis imposes a considerable burden on patients' quality of life and increases the risk of systemic comorbidities, making it a pressing public health concern (1-6). Immunologically, the disease is driven by a dysregulated interplay between innate and adaptive immune responses (7). Among immune cell populations, dendritic cells (DCs) are central players in the initiation and amplification of inflammation by activating effector T cells and producing pro-inflammatory mediators (8). This process creates a pathogenic feedback loop that leads to persistent skin inflammation and keratinocyte hyperproliferation (5-8).
Imiquimod (IMQ) is widely used to induce psoriasis-like skin inflammation in both murine models and human studies. IMQ is a well-known agonist of Toll-like receptor 7 (TLR7), and its immunostimulatory effects are predominantly mediated through TLR7 signaling (9-12). Topical application of IMQ results in DC activation and subsequent polarization of CD4+ T cells into various Th subsets, aggravating the inflammatory response (12,13). While the IMQ-induced model has provided key insights into psoriasis pathogenesis and therapeutic responses, the interpretation of in vivo findings is often complicated by systemic immune interactions. Most studies using IMQ have been conducted in vivo, where the involvement of diverse immune cell populations and systemic factors may make it difficult to dissect the direct effects of IMQ on dendritic cells (12,13). Thus, the direct immunomodulatory effects of IMQ on DCs under controlled in vitro conditions remain poorly defined. Psoriasis remains clinically challenging to manage due to its heterogeneous immune landscape and chronic inflammatory nature (14). In this context, DCs and CD4+ T cells represent critical immunological hubs driving disease progression (4). A more precise understanding of how IMQ directly influences DC activation and the downstream differentiation of naïve CD4+ T cells could inform the development of targeted immunotherapies.
DCs serve as professional antigen-presenting cells (APCs) and are essential for initiating adaptive immune responses. Upon antigen encounter, they undergo phenotypic maturation, characterized by the upregulation of co-stimulatory molecules (CD40, CD80, and CD86), major histocompatibility complex (MHC) molecules, and the production of pro-inflammatory cytokines (8,15). These mature DCs migrate to secondary lymphoid organs where they present antigens to naïve CD4+ T cells, initiating lineage-specific T cell polarization (16,17). Aberrant activation or dysregulation of this process is implicated in several autoimmune diseases, including psoriasis (18), rheumatoid arthritis (19), and multiple sclerosis (20). Among the molecular pathways involved, the nuclear factor kappa B (NF-κB) pathway plays a pivotal role in orchestrating DC activation and cytokine production (21). IL-12 secreted by activated DCs promotes Th1 differentiation (22,23), whereas IL-6 and IL-23 are essential for Th17 development via activation of STAT3 and RORγt (24,25). Nevertheless, whether IMQ induces DC activation through NF-κB signaling and how these activated DCs modulate T cell fate in vitro remains unclear. In addition to the well-characterized roles of Th1 and Th17 cells, recent evidence suggests that Th9 cells may also contribute to psoriatic inflammation (26), highlighting the need for broader evaluation of pathogenic Th subsets in IMQ-induced responses.
In this study, we aimed to elucidate the direct effects of IMQ on DC maturation and its downstream influence on CD4+ T cell polarization using a reductionist in vitro co-culture model. Unlike previous studies that focused on complex in vivo outcomes, this approach allowed us to isolate cell-intrinsic effects of IMQ on DCs and define a clear mechanistic link to Th1 and Th17 differentiation via the NF-κB pathway. Our findings address a key gap in understanding the DC-T cell axis in psoriasis and provide a foundation for targeted immunotherapy development.
Materials and methods
Mice
Female C57BL/6N mice (7-10 weeks old) were procured from Orient Bio, and ovalbumin (OVA)-specific OT-II transgenic mice on a C57BL/6 background were obtained from Jackson Laboratory. All mice were maintained under specific pathogen-free conditions. Mice were euthanized by gradually introducing CO2 at a flow rate that displaced 30-50% of the chamber vol/min, following the AVMA Guidelines for the Euthanasia of Animals (2020 Edition). Following the cessation of respiration, CO2 exposure was continued for at least 1 min, and the absence of both breathing and cardiac activity was used to confirm death. Femurs, tibias, and lymph nodes were then collected for further analysis. All animal experiments were approved by the Institutional Animal Care and Use Committee of Korea University (KUIACUC-2021-0094) and were performed in accordance with institutional guidelines.
Generation of bone marrow-derived dendritic cells
Bone marrow-derived dendritic cells (DCs) were obtained from C57BL/6N mice and cultured according to the protocol outlined by Inaba et al (27). Briefly, bone marrow cells were isolated from the femurs and tibias of mice and cultured in RPMI-1640 medium (Corning, 10-043-CVR) supplemented with 10% fetal bovine serum (Gibco, 16000-044), 20 ng/ml granulocyte/macrophage colony-stimulating factor (GM-CSF), HEPES (Corning, 25-060-CI), penicillin/streptomycin solution (Corning, 30-002-CI), and 50 µM 2-mercaptoethanol (Sigma, M6250-10M). The culture medium was refreshed by replacing half of it every alternate day. On day 7, the semi-adherent cells were gently pipetted and harvested for use as immature GM-CSF-derived dendritic cells. To generate Flt3L-derived dendritic cells, bone marrow (BM) cells were resuspended at 6x106 cells/ml in RPMI-1640 medium containing 200 ng/ml of human recombinant FMS-like tyrosine kinase 3 ligand (Flt3L, BioLegend, 550602). Subsequently, the cell suspensions were seeded at 5 ml per well in 6 well plates and incubated for 9 days without adding additional media.
CD4+ T cell isolation and in vitro polarization of CD4+ T cells with DCs
For in vitro syngeneic co-culture of CD4+ T cells and DCs, the DCs (5x105 cells/well) cultured for 7 days were pulsed with 20 ng/ml OVA323-339 peptide for 2 h. Following this, the DCs were treated for 6 h with imiquimod (IMQ, 1 µg/ml), lipopolysaccharide (LPS, 100 ng/ml) or not. Subsequently, CD4+ T cells (1x106 cells/well) were co-cultured with OVA323-339 peptide-pulsed DCs or OVA323-339 peptide-pulsed IMQ- or LPS-stimulated DCs at a 1:10 ratio. After 3 days, the cells were harvested to analyze the T cell population, and the supernatant was collected to detect levels of IFN-γ, IL-17A, IL-9, and IL-10. CD4+ T cells were isolated from the lymph nodes of OT-II mice. The whole lymph nodes were minced using a slide glass and then filtered using a 40 µm cell strainer (Falcon, 352340). Subsequently, CD4+ T cells were purified using a CD4 (L3T4) microbead purification kit (Miltenyi Biotec, 130-117-043), ensuring a purity level of over 95%. Purified CD4+ T cells were then stimulated with anti-CD3 Ab (1 µg/ml) and anti-CD28 Ab (1 µg/ml) for 3 days.
Flow cytometric analysis
The dendritic cells (1x106 cells/well) were washed with PBS and harvested using FACS buffer (0.5% FBS and filtered 0.05% NaN3 in PBS). The purity of BMDCs was assessed by flow cytometry using CD11c staining, with >90% of cells gated as CD11c+. Subsequently, DCs were blocked with mouse IgG (Sigma, I5381) for 20 min at 4˚C to minimize nonspecific binding. Following this, the cells were stained with APC-conjugated anti-mouse CD11c antibody (eBioscience, 17-0114-82), PE-conjugated anti-mouse CD40 (eBioscience, 12-0401-81) and CD80 (BD PharmingenTM, 553769), FITC-conjugated anti-mouse CD86 (eBioscience, 11-0862-82) and MHCII (eBioscience, 11-5322-82) for 20 min at 4˚C while protected from light. For intracellular staining, CD4+ T cells (1x106 cells/well) were re-stimulated with PMA (50 ng/ml), ionomycin (1 µg/ml), and GolgiPlug containing Brefeldin A (BD sciences, 555029) for 5 h. The cells were harvested using FACS buffer and incubated with FITC-conjugated anti-mouse CD4 RM4-5 (eBioscience, 11-0042-82) for 20 min at 4˚C. The T cells were fixed and permeabilized using Cytofix/Cytoperm solution (BD sciences, 51-2090KZ). Intracellular cytokines were stained with PE-conjugated anti-IFN-γ XMG1.2 (eBioscience, 12-7311-82) and anti-IL-17A eBio17B7 (eBioscience, 12-7177-81), APC-conjugated anti-mouse IL-9 RM9A4 (BioLegend, 514105) and anti-mouse Foxp3 FJK-16s (eBioscience, 17-5773-82) for 1 h at 4˚C. The labeled cells were analyzed using a FACSymphony™ A1 flow cytometer (BD Sciences), and the FCS data were analyzed using FlowJo software (BD Sciences).
Reverse transcription polymerase chain reaction
Total mRNA was extracted from DCs (1x106 cells/well) using RiboEX reagent (GeneAll, 301-001), following the manufacturer's protocol. RNA purity was assessed using a NanoDrop™ 2000/2000c spectrophotometer (Thermo Fisher Scientific, Inc.), with A260/A280 ratios above 1.8 considered pure. The isolated total RNA was then reverse transcribed into cDNA using RocketScript Reverse Transcriptase (Bioneer). The cDNA was subsequently amplified by PCR. Following PCR amplification, the products were resolved on 1.5% agarose gels and visualized with ethidium bromide staining. The sequences of the RT-PCR primers used in this study were as follows: mouse TNF-α forward, 5'-GGCAGGTCTACTTTGGAGTC-3' and reverse, 5'-ACATTCGAGGCTCCAGTGAA-3'; mouse IL-12p40 forward, 5'-TGTGGAATGGCGTCTCTGTC-3' and reverse, 5'-CCTTTGCATTGGACTTCGGTAG-3'; mouse IL-12p35 forward, 5'-TCAGCGTTCCAACAGCCTC-3' and reverse, 5'-CCAAGGCACAGGGTCATCATCA-3'; mouse IL-1β forward, 5'-CTGAAGCAGCTATGGCAACT-3' and reverse, 5'-ACAGGACAGGTATAGATTC-3'; mouse IL-6 forward, 5'-TGAACAACGATGATGCACTT-3' and reverse, 5'-CGTAGAGAACAACATAAGTC-3'; mouse TGF-β forward, 5'-TATAGCAACAATTCCTGGCGT-3' and reverse, 5'-TCCTAAAGTCAATGTACAGCT-3'; mouse GAPDH forward, 5'-ACATCAAGAAGGTGGTGAAG-3' and reverse, 5'-ATTCAAGAGAGTAGGGAGGG-3'.
Enzyme-linked immunosorbent assay
The levels of IL-12p40, IL-12p70, IL-6, TNF-α, IL-1β, IL-17A, IL-9, and IL-10 in the culture supernatants were detected using ELISA kits (eBioscience). The concentration of IFN-γ was measured using a specific mouse anti-IFN-γ R4-6A2 (BD sciences, 554430) capture antibody and biotinylated anti-IFN-γ XMG1.2 (BD sciences, 554410) detection antibody. A standard curve was established using recombinant mouse IFN-γ (BD sciences, 554587). The wells were washed with PBST (0.05% Tween-20 in PBS), and then o-phenylenediamine (OPD) containing citrate and H2O2 was added to each well and incubated for 15 min at room temperature. To stop the reaction, 2 N H2SO4 was added to each well. Detection limits for all cytokines were as specified by the manufacturers' protocols.
Immunofluorescent staining assay
The DCs (1x106 cells/well) were gently washed with serum-free medium. Then, the DCs were fixed with 4% paraformaldehyde (PFA) and permeabilized using 0.1% Triton X-100. After permeabilization, the cells were washed with 0.1% BSA in PBS and then blocked with 0.5% BSA in PBS for 30 min at room temperature. The cells were incubated with rabbit anti-mouse NF-κB p65 (1:100 dilution) at 4˚C overnight, and subsequently stained with Alexa Fluor 488-conjugated anti-rabbit IgG Ab (1:300 dilution) for 1 h at room temperature. Nuclei were counterstained with DAPI (Molecular Probes, 3 µM) for 3 min and imaged using a confocal laser scanning microscope (LSM 800, Carl Zeiss).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 8.4.3 (GraphPad Software, Inc.). One-way ANOVA followed by the Bonferroni post hoc test was used to compare multiple groups. Results with P-values <0.05 were considered statistically significant. Data are presented as mean ± SD from three independent experiments.
Results
IMQ enhances the activation and maturation of bone marrow-derived DCs
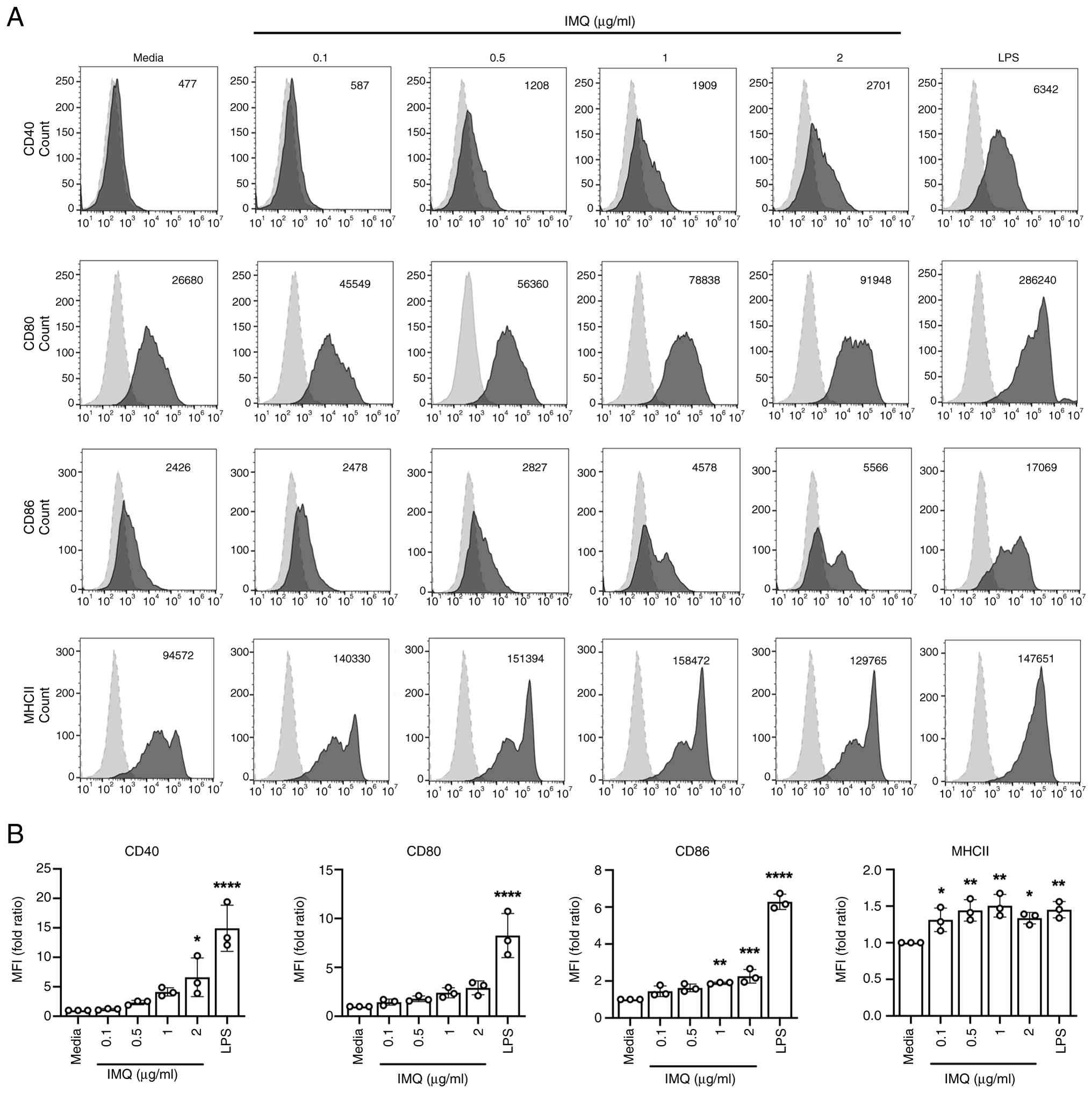
When immature dendritic cells (iDCs) undergo maturation, they acquire the capacity to activate and direct the differentiation of CD4+ T cells through various mechanisms, including co-stimulatory molecules, MHC class molecules, and the secretion of cytokines (28,29). To investigate whether imiquimod (IMQ) affects the activation and maturation of bone marrow-derived dendritic cells in a dose-dependent manner, iDCs were cultured for 20 h in the presence or absence of IMQ. The expression levels of cell surface molecules were subsequently analyzed using flow cytometry to confirm the impact of IMQ on the modulation of crucial DCs maturation markers such as CD40, CD80, CD86, and MHC class II. As illustrated in Fig. 1, IMQ markedly increased the expression of co-stimulatory molecules CD40, CD80, and CD86 on CD11c+ BMDCs in a dose-dependent manner. Additionally, MHC class II expression also increased upon treatment with IMQ (Fig. 1). The high expression of these key surface markers suggests that IMQ significantly enhances the activation and maturation processes of DCs. CD40, CD80, CD86, and MHC class II are critical surface molecules involved in the interaction between DCs and CD4+ T cells. Overall, these results indicate that IMQ significantly enhanced the activation and maturation of GM-CSF-DCs.
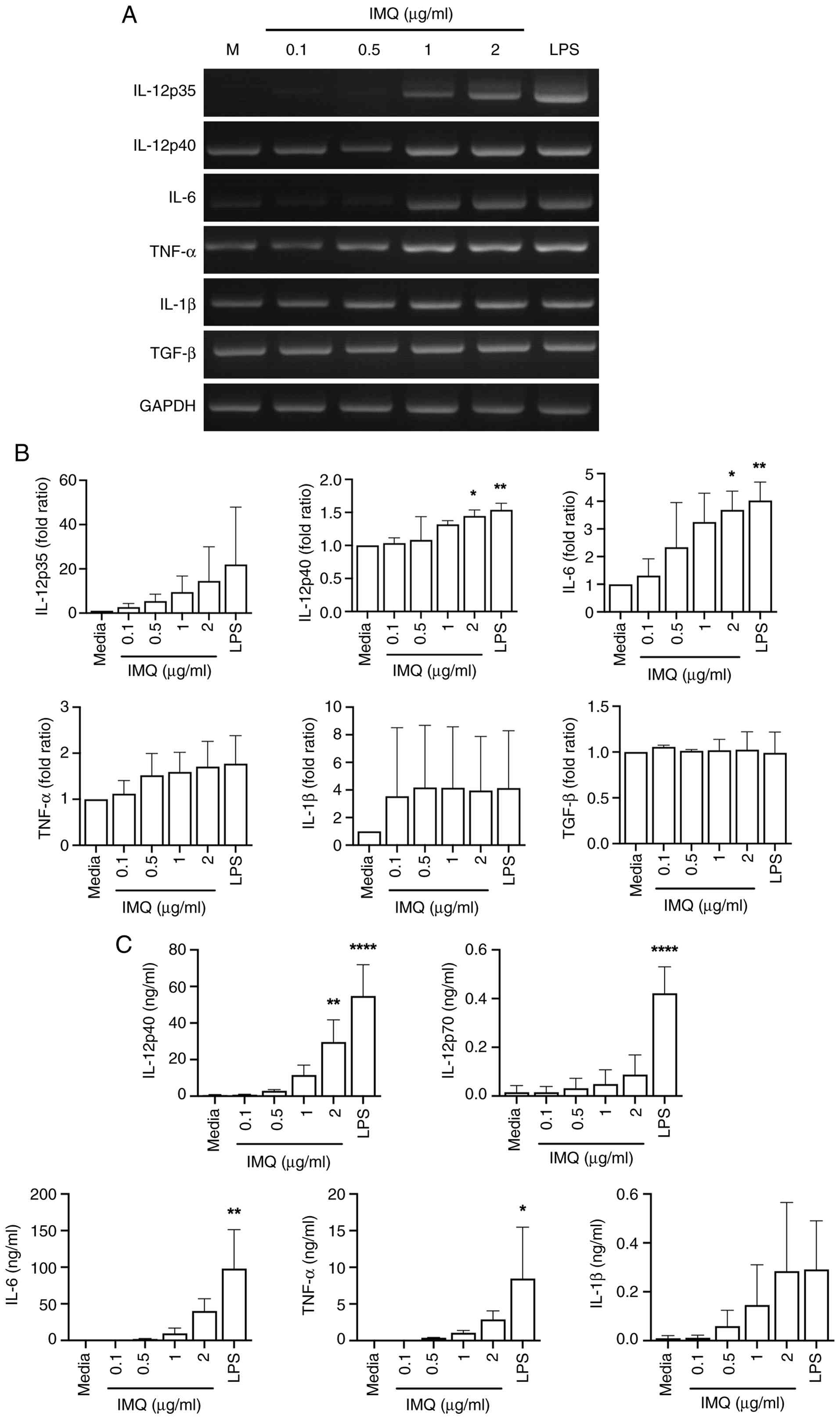
The activation and maturation of DCs is intricately linked to the modulation of pro-inflammatory cytokines, presenting a crucial aspect of immune response orchestration. Upon activation, DCs undergo a transformation that involves heightened expression of co-stimulatory molecules and the secretion of various cytokines, including pro-inflammatory mediators (29). Therefore, to evaluate whether IMQ induces an elevation in the cytokine gene expression levels of DCs, DCs were treated with IMQ, and the cytokine expression levels were assessed at both mRNA and protein levels. The mRNA levels of pro-inflammatory cytokines, such as IL-12p35, IL-12p40, IL-6, TNF-α, and IL-1β were upregulated in IMQ-treated DCs (Fig. 2A and B). IMQ treatment generally increased the secretion of IL-12p40, IL-12p70, IL-6, TNF-α, and IL-1β, with varying degrees of responsiveness among cytokines (Fig. 2C). Collectively, these results suggest that IMQ increases the mRNA levels and secretion levels of pro-inflammatory cytokines by activating and maturing DCs, similar to the increase observed in surface marker data.
Recent studies have highlighted the distinct strategies for generating DCs using GM-CSF or Flt3L, resulting in different DC subsets. GM-CSF-derived DCs, often referred to as inflammatory DCs, lack the cDC1 subset and are characterized by their robust inflammatory responses. In contrast, Flt3L-derived DCs represent a steady-state DC population that includes both pDCs and cDCs, playing critical roles in maintaining immune homeostasis (30-33). In our study, we examined the effects of IMQ on both GM-CSF-derived DCs and Flt3L-derived DCs to comprehensively understand its impact on various DC subsets. Consistent with our findings in GM-CSF-derived DCs, IMQ treatment of Flt3L-derived DCs also resulted in a significant dose-dependent increase in the expression of co-stimulatory molecules (CD40, CD80, and CD86) and MHC class II, as well as the secretion levels of pro-inflammatory cytokines, except for IL-12p40 (Fig. S1).
Nuclear factor-kappa B (NF-κB) signaling pathway is involved in the imiquimod-induced activation and maturation of DCs
Nuclear factor-kappa B (NF-κB) signaling plays a central role in dendritic cell (DC) activation by regulating the expression of cytokines, co-stimulatory molecules, and immune response mediators. This pathway is closely associated with the induction of adaptive immunity via T cell activation and polarization (34-36). Although IMQ is known to activate the NF-κB pathway and promote DC maturation, the precise molecular components involved in this process remain poorly characterized. In particular, no prior study has evaluated the expression of DC surface molecules using flow cytometry in vitro following NF-κB inhibition and subsequent IMQ stimulation.
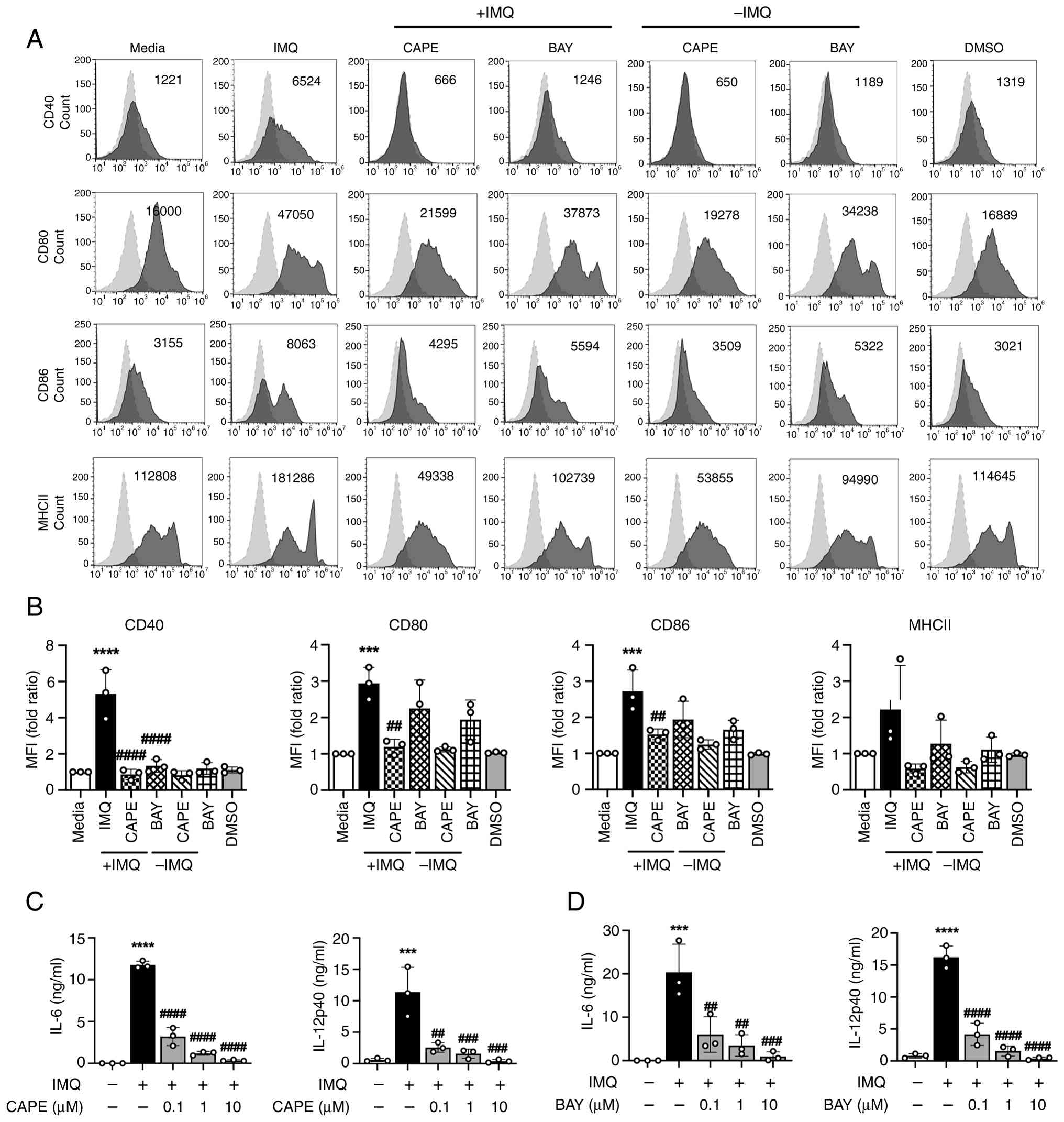
To investigate this gap in knowledge, we utilized two NF-κB pathway inhibitors, Caffeic Acid Phenethyl Ester (CAPE) and Bay 11-7082 (BAY), each targeting distinct molecular steps within the NF-κB cascade. CAPE inhibits NF-κB activation by preventing the phosphorylation and degradation of IκB, thereby blocking the nuclear translocation of NF-κB (37). In contrast, BAY acts upstream by inhibiting the IκB kinase (IKK) complex, which is responsible for IκB phosphorylation and subsequent degradation, effectively preventing NF-κB signaling (38). To assess the role of this pathway in IMQ-induced DC maturation, DCs were pre-treated with CAPE or BAY and then stimulated with IMQ. The expression levels of DC surface markers and pro-inflammatory cytokines were subsequently analyzed using flow cytometry and ELISA. The surface molecules associated with DC activation and maturation, including co-stimulatory molecules CD40, CD80 and CD86, were significantly suppressed by CAPE. Conversely, BAY significantly reduced CD40 expression on DCs compared with the IMQ group, while showing a non-significant trend toward decreased expression of CD80 and CD86. Neither treatment significantly affected MHC class II expression on DCs (Fig. 3A and B). Additionally, both inhibitors significantly decreased the secretion levels of IL-12p40 and IL-6 in a dose-dependent manner, which are two key cytokines involved in the polarization of CD4+ T cells into Th1 and Th17 subsets (Fig. 3C and D). In conclusion, these data demonstrate that IMQ induces the activation and maturation of DCs via the NF-κB-dependent signaling pathway.
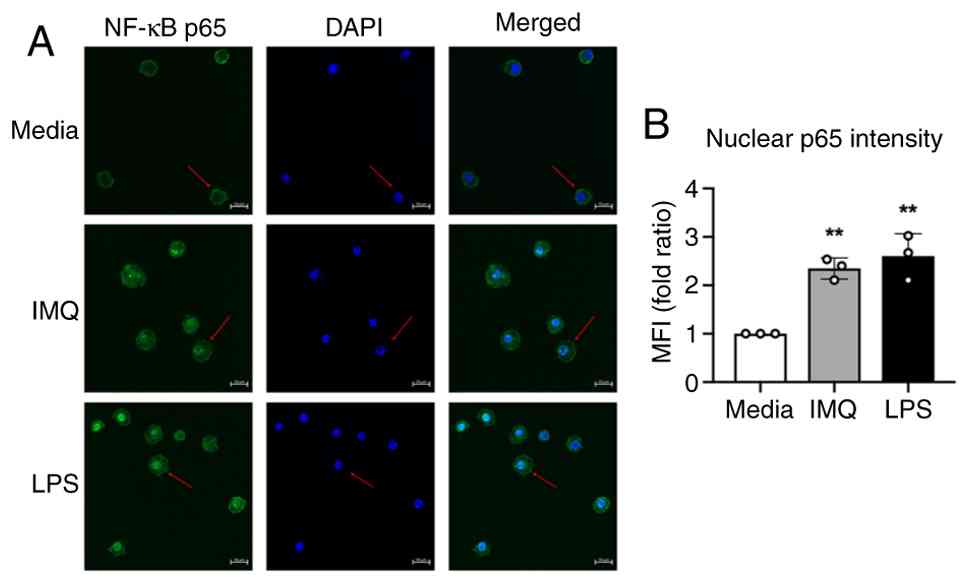
NF-κB p65 nuclear translocation is a key step in pathway activation and serves as a hallmark of immune stimulus-induced transcriptional activity. As shown in Fig. 3, the expression of DC surface molecules and secretion of IL-12p40 and IL-6 were substantially diminished when NF-κB signaling was inhibited during IMQ treatment, supporting a central role for NF-κB p65. To directly confirm whether IMQ-induced DC activation is accompanied by NF-κB p65 nuclear translocation, we performed an immunofluorescence (IF) assay. Following IMQ exposure, NF-κB p65 was detected in the nucleus of DCs, confirming translocation (Fig. 4). Taken together, our findings provide a deeper understanding of the molecular mechanisms underlying IMQ-induced DC activation, clarifying the roles of NF-κB pathway components targeted by CAPE and BAY, and revealing the direct activation of DCs by IMQ in vitro.
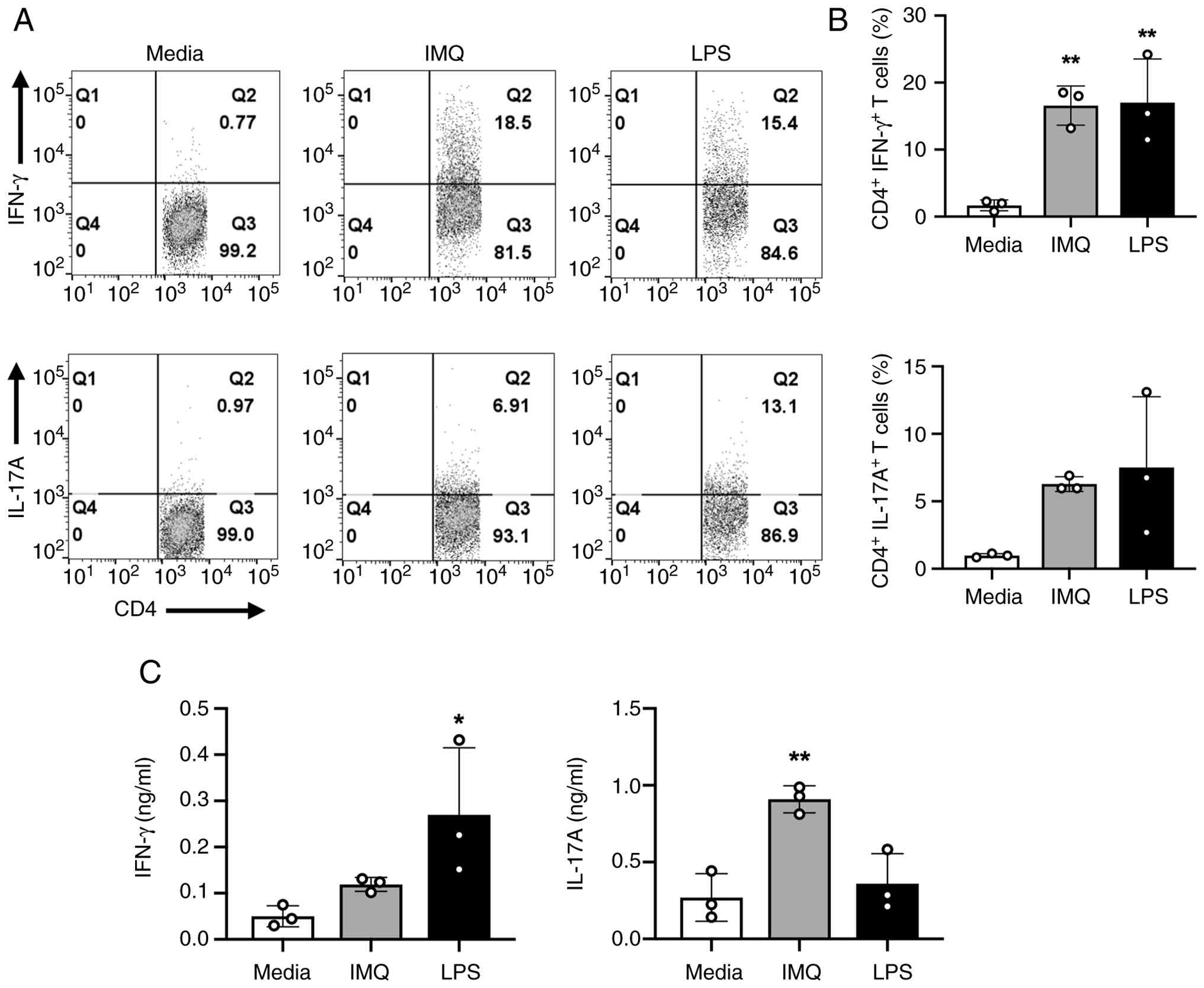
IMQ-treated DCs can induce the polarization of naïve CD4+ T cells into pathogenic CD4+ T cells
DCs play a crucial role in the immune system by capturing and presenting antigens to T cells, thereby initiating adaptive immune responses. Upon activation and maturation, DCs upregulate surface and co-stimulatory molecules, enhancing their antigen-presenting capabilities and enabling more effective interactions with T cells. As a result, the interaction between mature DCs and naïve CD4+ T cells leads to increased T cell activation and differentiation into various Th cell subsets, including Th1 and Th17 (22-25,39-43). This enhanced antigen-presenting cell (APC) function of DCs is essential for mounting a robust and targeted immune response. As previously shown in Fig. 2C, IMQ-treated DCs increased the secretion of IL-12p40 and IL-6, which are critical for Th1 and Th17 polarization. To investigate whether IMQ-stimulated DCs polarize naïve CD4+ T cells into various effector Th subsets, including Th1, Th17, Th9, and Treg, DCs were incubated with ovalbumin (OVA) (10 ng/ml) for 2 h. Subsequently, they were treated with IMQ (1 µg/ml) for 6 h and then co-cultured with naïve CD4+ T cells isolated from OT-II mice for 3 days. The population of Th subsets was assessed by flow cytometry. As depicted in Fig. 5A and B, IMQ-treated DCs notably increased the percentage of CD4+ IFN-γ+ T cells (Th1) and CD4+ IL-17A+ T cells (Th17).
Recent studies have highlighted the involvement of other Th subsets, such as Th9 cells, in autoimmune diseases like psoriasis and rheumatoid arthritis. Th9 cells, characterized by IL-9 production, have been implicated in the pathogenesis of several autoimmune diseases (44-46). Building on these studies, we observed a significant increase in the population of CD4+ IL-9+ T cells (Th9) when IMQ-treated DCs were co-cultured with naïve OT-II CD4+ T cells. IL-9 secretion tended to increase but was not statistically significant. Additionally, IMQ-treated DCs induced an increase in the population of CD4+ Foxp3+ Tregs and IL-10 secretion; however, these changes were not statistically significant (Fig. S2).
IFN-γ and IL-17A are well-recognized signature cytokines predominantly secreted by Th1 and Th17 cells, respectively. To confirm whether IMQ-stimulated DCs enhance the production of IFN-γ and IL-17A in co-cultured CD4+ T cells, cytokine production levels in the supernatants of the co-cultures were determined using ELISA. IMQ-stimulated DCs increased IFN-γ and IL-17A production in co-cultured OT-II CD4+ T cells (Fig. 5C). Additionally, IMQ-treated DCs notably tended to increase IL-9 secretion in co-cultured OT-II CD4+ T cells, with IL-10 exhibiting a similar trend (Fig. S2C). These results are the first to demonstrate that IMQ-treated DCs can polarize naïve CD4+ T cells into specific effector T helper cell subsets, such as Th1, Th17, and Th9. The substantial increase in IL-9 secretion, along with elevated Th9 population levels, suggests a key role for IL-9 in Th9 differentiation. This novel finding highlights the unique capability of IMQ-activated DCs to influence T cell differentiation, providing a new perspective on the immunomodulatory effects of IMQ.
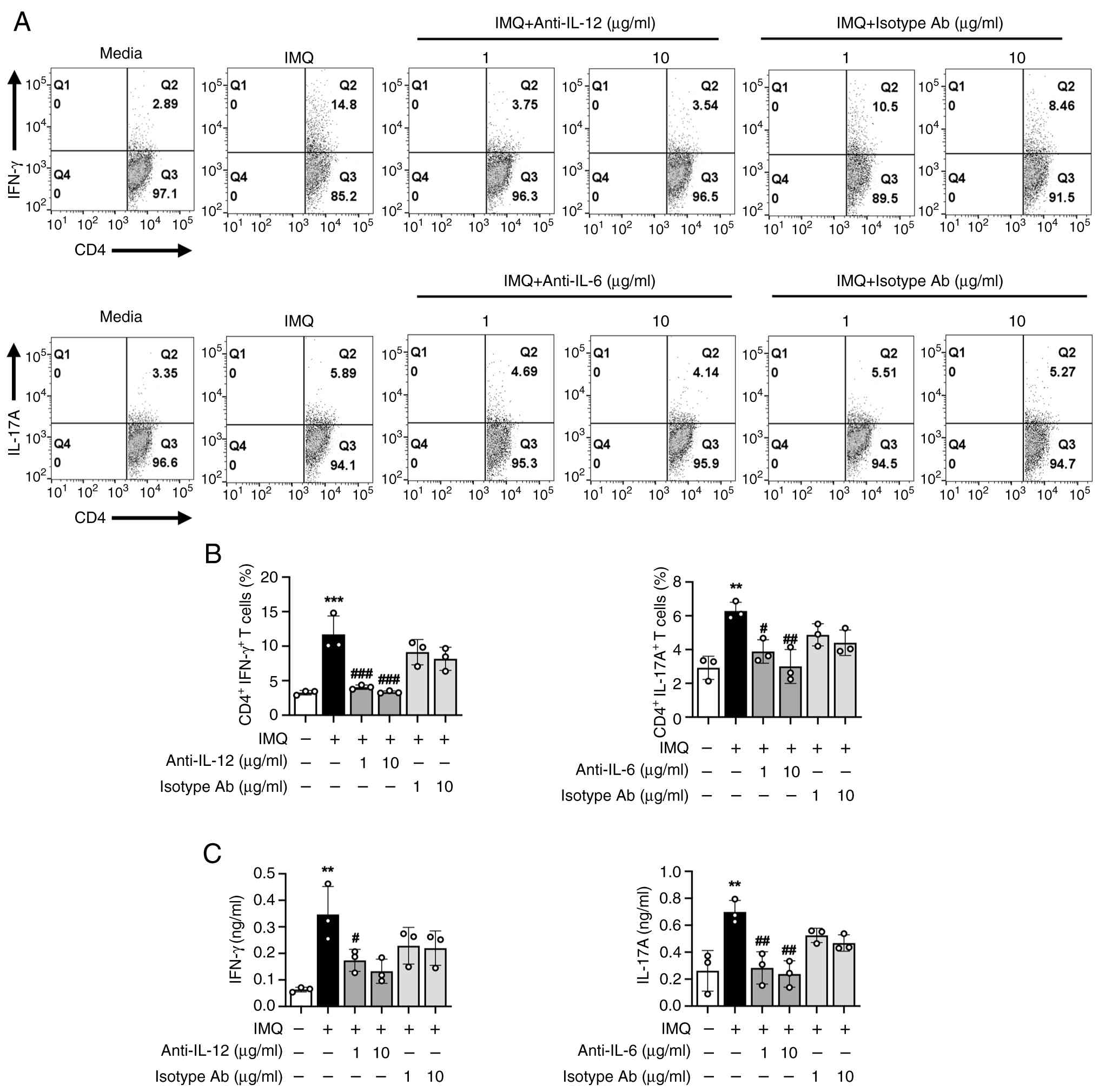
IMQ promotes Th1 and Th17 responses via DC-derived IL-12 and IL-6
DCs secrete cytokines essential for T cell polarization. Specifically, IL-12 and IL-6 are key drivers of Th1 and Th17 cell differentiation, respectively. These cytokines direct the differentiation of naïve CD4+ T cells and shape adaptive immune responses. To determine whether IL-12 and IL-6 secreted from IMQ-stimulated DCs contribute to the enhancement of Th1 and Th17 responses, neutralizing antibodies against IL-12 and IL-6 were added to co-cultures of IMQ-treated DCs and naïve CD4+ T cells isolated from OT-II transgenic mice. The frequencies of Th1 and Th17 cells, along with IFN-γ and IL-17A production, were subsequently analyzed. As illustrated in Fig. 6A and B, blockade of IL-12 or IL-6 led to a significant reduction in the frequencies of CD4+ IFN-γ+ (Th1) and CD4+ IL-17A+ (Th17) cells. Consistent with these findings, IFN-γ and IL-17A levels in culture supernatants were also decreased (Fig. 6C). In contrast, IgG isotype control antibodies had no effect on these responses. These data demonstrate that IMQ-activated DCs promote Th1 and Th17 polarization via IL-12 and IL-6 secretion, underscoring the critical role of these cytokines in mediating the immunomodulatory effects of IMQ. This novel finding highlights the therapeutic potential of targeting DC-derived IL-12 and IL-6 pathways to modulate pathogenic T cell responses.
Discussion
Psoriasis is a common chronic autoimmune disorder characterized by significant dysregulation of both innate and adaptive immune responses (7). A critical factor in psoriasis pathogenesis is the activation and maturation of DCs, which play a pivotal role in initiating and sustaining inflammatory responses (8). IMQ, an immunomodulatory agent, is widely used to induce psoriasis-like skin inflammation in mouse models. Topical IMQ application in in vivo activates DCs and drives CD4+ T cell differentiation into Th subsets, exacerbating skin inflammation (12,13). However, whether these effects result from direct DC activation by IMQ or complex immune interactions in in vivo environment remains unclear.
Our study demonstrates that IMQ markedly enhances the expression of maturation markers such as CD40, CD80, CD86, and MHC class II on GM-CSF- and Flt3L-derived DCs in a dose-dependent manner. This upregulation is crucial for both the phenotypic and functional maturation of DCs, enabling effective antigen presentation and Th cell activation. Activated DCs secrete cytokines that direct the differentiation of naïve CD4+ T cells into various effector subsets, including Th1 and Th17 cells. Th1 cells, characterized by their production of IFN-γ (22,23), and Th17 cells, which produce IL-17 (24,25), are both considered pathogenic T cell subsets in autoimmune diseases (39-42). Both subsets play crucial roles in maintaining the chronic inflammatory environment in psoriasis and other autoimmune conditions (43).
The direct effects of IMQ on DC maturation and IL-12/IL-6 production were clearly demonstrated in in vitro experiments. Pharmacological inhibition of the NF-κB signaling pathway significantly reduced IMQ-mediated DC maturation and the production of IL-12 and IL-6, highlighting the central role of NF-κB in these processes. Moreover, recent studies showed that topical thioredoxin alleviates IMQ-induced skin inflammation by inhibiting NF-κB-mediated IL-6 production (47), supporting the therapeutic potential of targeting this pathway in psoriasis. Furthermore, immunofluorescence analysis confirmed that IMQ treatment induced the translocation of the NF-κB p65 subunit to the nucleus, directly activating the NF-κB pathway in DCs. These findings demonstrate that IMQ directly influences DC behavior, bypassing the confounding factors present in in vivo systems. Furthermore, our findings suggest that IMQ-induced DC maturation and cytokine production are regulated by distinct upstream components of the NF-κB pathway. While CAPE and BAY effectively inhibited these processes, further studies targeting other NF-κB pathway elements, such as alternative IKK subunits or non-canonical signaling, could provide deeper insights into the mechanisms underlying IMQ's effects on DCs.
In this study, IMQ treatment resulted in a significant increase in both mRNA expression and secretion levels of cytokines, including IL-12 and IL-6, in DCs. As described in the introduction, these cytokines are key drivers of Th1 and Th17 differentiation, both central to psoriasis pathology. This suggests that IMQ-treated DCs are not only phenotypically mature but also primed to drive pathogenic T cell responses, contributing to the chronic inflammation in psoriasis. Our findings reveal that IMQ directly activates DCs to produce IL-12 and IL-6, leading to robust Th1 and Th17 responses. Our in vitro findings are consistent with previous in vivo IMQ-induced psoriasis models, which also report elevated Th1/Th17 responses and increased levels of IFN-γ and IL-17A (48). These results underscore the pivotal role of the DC-cytokine axis in psoriasis pathogenesis and highlight its potential as a therapeutic target for developing precision immunotherapies. While IMQ is primarily used to model psoriasis-like skin inflammation, the immune pathways it activates-particularly the DC-Th1/Th17 axis mediated via NF-κB signaling-are also implicated in other autoimmune diseases such as multiple sclerosis (MS) (49). Although IMQ itself does not induce MS, the cytokine profiles and pathogenic T cell responses observed in IMQ-treated models offer valuable insights into broader autoimmune mechanisms. These findings suggest that therapeutic strategies targeting DC activation and NF-κB signaling may be applicable not only to psoriasis but also to other Th1/Th17-driven autoimmune disorders.
In addition to Th1 and Th17 cells, the growing importance of Th9 cells in autoimmune conditions like psoriasis has been increasingly recognized in recent studies (50). While our study primarily focused on Th1/Th17 polarization, when IMQ-treated DCs were co-cultured with naïve CD4+ T cells, the Th9 cell population significantly increased, accompanied by a tendency toward elevated IL-9 secretion. This provides the first evidence that IMQ-treated DCs can induce Th9 polarization, further implicating Th9 cells in psoriasis pathogenesis. These findings highlight a previously underappreciated role of Th9 cells in psoriasis and suggest that DC-Th9 interactions may serve as a novel therapeutic target. These insights deepen our understanding of IMQ's mechanisms and offer valuable implications for treating autoimmune diseases. Previous studies have shown that Th9 differentiation typically requires IL-4 and TGF-β, which cooperatively induce IL-9 expression by activating Th9-associated transcription factors (51). Therefore, we consider it unlikely that IL-6 plays a major role in Th9 differentiation. Further investigation is necessary to identify the exact pathways responsible for the observed increase in Th9 cells, particularly through experiments employing IL-4 and TGF-β neutralization. Additionally, we observed an increase in the percentage of CD4+ Foxp3+ T cells following IMQ treatment; however, this expansion did not correspond with elevated IL-10 production. Foxp3+ regulatory T cells (Tregs) are a heterogeneous population, and not all subsets produce IL-10(52). Consequently, an increase in Foxp3+ Tregs does not necessarily correlate with elevated IL-10 levels. Furthermore, the functional status of Tregs should be considered. Although IMQ treatment increased the population of CD4+ Foxp3+ Tregs, these cells may not exhibit full suppressive function, including IL-10 production (53). Thus, the expansion of Foxp3+ Tregs does not always reflect their activation state or immunosuppressive capacity, which may be limited under the experimental conditions. Although murine GM-CSF-derived DCs are widely used as a model system, they may not fully recapitulate the phenotype and function of human dermal DCs. Therefore, caution is warranted when extrapolating our findings to human psoriasis.
Given that IMQ is commonly used in preclinical psoriasis models, its effects on DC maturation and T cell differentiation should also be compared with other psoriasis mouse models, such as IL-23-induced or mannan-induced models (54,55). These models may induce distinct immune responses, emphasizing the activation of different T helper subsets, which could reveal additional mechanisms contributing to psoriasis and help identify more specific therapeutic targets. Such comparisons are essential for developing targeted therapies, ensuring that specific immune pathways can be modulated to improve clinical outcomes in psoriasis and other autoimmune diseases. Together, our data provide a comprehensive understanding of IMQ-mediated DC activation and its downstream implications for Th subset-driven inflammation in psoriasis.
Supplementary Material
IMQ increases the expression of surface molecules in Flt3L-DCs. Bone marrow-derived DCs were isolated from C57BL/6 mice and cultured for 9 days in the presence of recombinant human Flt3L at 200 ng/ml, without adding additional media. Subsequently, the Flt3L-DCs (6x106 cells/well) were harvested and treated with IMQ (0.1, 0.5, 1 and 2 μg/ml) or LPS (100 ng/ml) for 20 h. (A) CD40, CD80, CD86 and MHCII expression on CD11c+ cells was analyzed by flow cytometry. (B) Bar graphs showing fold changes in the MFI relative to the medium group. (C) IL-12p40 and IL-6 levels in supernatants were quantified using ELISAs. Data are presented as the mean ± SD (n=3; biological replicates). *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001 vs. medium group; one-way ANOVA with the Bonferroni post hoc test. DC, dendritic cell; Flt3L, FMS-like tyrosine kinase 3 ligand; Flt3L-DCs, Flt3L-derived DCs; IMQ, imiquimod; LPS, lipopolysaccharide; MFI, mean fluorescence intensity; MHC, major histocompatibility complex.
IMQ-treated DCs promote Th9 polarization of naïve CD4+ T cells. Immature DCs were treated with OVA (20 ng/ml) for 2 h and OVA-DCs were then stimulated with IMQ (1 μg/ml) or LPS (100 ng/ml) for 6 h. Following this, the DCs were co-cultured with OT-II naïve CD4+ T cells at a 1:10 ratio for 3 days. (A) IL-9+ and Foxp3+ CD4+ T cells were measured by flow cytometry. (B) Proportions of IL-9+ and Foxp3+ CD4+ T cells are presented as the mean ± SD (n=3; biological replicates). (C) IL-9 and IL-10 levels in supernatants were quantified by ELISAs. **P<0.01 and ****P<0.0001 compared with the medium group; as determined using one-way ANOVA with the Bonferroni post hoc test. DC, dendritic cell; IMQ, imiquimod; LPS, lipopolysaccharide; OVA, ovalbumin; Th, T helper.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Research Foundation of Korea (NRF; grant no. NRF-2017R1A2B 2009442) and Korea University grants.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
YC designed and performed all the experiments. YC and JK contributed to acquiring the experimental results. YC and TSK conducted the analysis and interpretation of the experimental data. YC and TSK collaborated on manuscript writing. TSK supervised the study and corrected the manuscript. YC and TSK confirm the authenticity of all the raw data. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
All animal experiments were approved by the Institutional Animal Care and Use Committee of Korea University (approval no. KUIACUC-2021-0094; Seoul, South Korea) and were performed in accordance with institutional guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Damiani G, Bragazzi NL, Karimkhani Aksut C, Wu D, Alicandro G, McGonagle D, Guo C, Dellavalle R, Grada A, Wong P, et al: The global, regional, and national burden of psoriasis: Results and insights from the global burden of disease 2019 study. Front Med (Lausanne). 8(743180)2021.PubMed/NCBI View Article : Google Scholar | |
|
Parisi R, Iskandar IY, Kontopantelis E, Augustin M, Griffiths CE and Ashcroft DM: Global Psoriasis Atlas. National, regional, and worldwide epidemiology of psoriasis: Systematic analysis and modelling study. BMJ. 369(m1590)2020.PubMed/NCBI View Article : Google Scholar | |
|
Armstrong AW and Read C: Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA. 323:1945–1960. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Griffiths CE, Armstrong AW, Gudjonsson JE and Barker JNWN: Psoriasis. Lancet. 397:1301–1315. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhou X, Chen Y, Cui L, Shi Y and Guo C: Advances in the pathogenesis of psoriasis: From keratinocyte perspective. Cell Death Dis. 13(81)2022.PubMed/NCBI View Article : Google Scholar | |
|
Man AM, Orăsan MS, Hoteiuc OA, Olănescu-Vaida-Voevod MC and Mocan T: Inflammation and psoriasis: A comprehensive review. Int J Mol Sci. 24(16095)2023.PubMed/NCBI View Article : Google Scholar | |
|
Schön MP: Adaptive and innate immunity in psoriasis and other inflammatory disorders. Front Immunol. 10(1764)2019.PubMed/NCBI View Article : Google Scholar | |
|
Kamata M and Tada Y: Dendritic cells and macrophages in the pathogenesis of psoriasis. Front Immunol. 13(941071)2022.PubMed/NCBI View Article : Google Scholar | |
|
Patel U, Mark NM, Machler BC and Levine VJ: Imiquimod 5% cream induced psoriasis: A case report, summary of the literature and mechanism. Br J Dermatol. 164:670–672. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Wu JK, Siller G and Strutton G: Psoriasis induced by topical imiquimod. Australas J Dermatol. 45:47–50. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Parab S and Doshi G: The experimental animal models in psoriasis research: A comprehensive review. Int Immunopharmacol. 117(109897)2023.PubMed/NCBI View Article : Google Scholar | |
|
Van Der Fits L, Mourits S, Voerman JSA, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP and Lubberts E: Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 182:5836–5845. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Wohn C, Ober-Blöbaum JL, Haak S, Pantelyushin S, Cheong C, Zahner SP, Onderwater S, Kant M, Weighardt H, Holzmann B, et al: Langerinneg conventional dendritic cells produce IL-23 to drive psoriatic plaque formation in mice. Proc Natl Acad Sci USA. 110:10723–10728. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Rendon A and Schäkel K: Psoriasis pathogenesis and treatment. Int J Mol Sci. 20(1475)2019.PubMed/NCBI View Article : Google Scholar | |
|
Yin X, Chen S and Eisenbarth SC: Dendritic cell regulation of T helper cells. Annu Rev Immunol. 39:759–790. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Gutcher I and Becher B: APC-derived cytokines and T cell polarization in autoimmune inflammation. J Clin Invest. 117:1119–1127. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Tai Y, Wang Q, Korner H, Zhang L and Wei W: Molecular mechanisms of T cells activation by dendritic cells in autoimmune diseases. Front Pharmacol. 9(642)2018.PubMed/NCBI View Article : Google Scholar | |
|
Qu Y, Li D, Xiong H and Shi D: Transcriptional regulation on effector T cells in the pathogenesis of psoriasis. Eur J Med Res. 28(182)2023.PubMed/NCBI View Article : Google Scholar | |
|
Jang S, Kwon EJ and Lee JJ: Rheumatoid arthritis: Pathogenic roles of diverse immune cells. Int J Mol Sci. 23(905)2022.PubMed/NCBI View Article : Google Scholar | |
|
Wagner CA, Roqué PJ and Goverman JM: Pathogenic T cell cytokines in multiple sclerosis. J Exp Med. 217(e20190460)2020.PubMed/NCBI View Article : Google Scholar | |
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB signaling in inflammation. Signal Transduct Target Ther. 2(17023)2017.PubMed/NCBI View Article : Google Scholar | |
|
Athie-Morales V, Smits HH, Cantrell DA and Hilkens CMU: Sustained IL-12 signaling is required for Th1 development. J Immunol. 172:61–69. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Pidala J, Beato F, Kim J, Betts B, Jim H, Sagatys E, Levine JE, Ferrara JLM, Ozbek U, Ayala E, et al: In vivo IL-12/IL-23p40 neutralization blocks Th1/Th17 response after allogeneic hematopoietic cell transplantation. Haematologica. 103:531–539. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Zhou L, Lopes JE, Chong MMW, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al: TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 453:236–240. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Cao L, Deng J, Chen W, He M, Zhao N, Huang H, Ling L, Li Q, Zhu X and Wang L: CTRP4/interleukin-6 receptor signaling ameliorates autoimmune encephalomyelitis by suppressing Th17 cell differentiation. J Clin Invest. 134(e168384)2023.PubMed/NCBI View Article : Google Scholar | |
|
Deng Y, Wang Z, Chang C, Lu L, Lau CS and Lu Q: Th9 cells and IL-9 in autoimmune disorders: Pathogenesis and therapeutic potentials. Hum Immunol. 78:120–128. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S and Steinman RM: Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 176:1693–1702. 1992.PubMed/NCBI View Article : Google Scholar | |
|
Hilligan KL and Ronchese F: Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses. Cell Mol Immunol. 17:587–599. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Bol KF, Schreibelt G, Rabold K, Wculek SK, Schwarze JK, Dzionek A, Teijeira A, Kandalaft LE, Romero P, Coukos G, et al: The clinical application of cancer immunotherapy based on naturally circulating dendritic cells. J Immunother Cancer. 7(109)2019.PubMed/NCBI View Article : Google Scholar | |
|
Brasel K, De Smedt T, Smith JL and Maliszewski CR: Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood. 96:3029–3039. 2000.PubMed/NCBI | |
|
Shortman K and Naik SH: Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 7:19–30. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Xu Y, Zhan Y, Lew AM, Naik SH and Kershaw MH: Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J Immunol. 179:7577–7584. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Lellahi SM, Azeem W, Hua Y, Gabriel B, Rye KP, Reikvam H and Kalland KH: GM-CSF, Flt3-L and IL-4 affect viability and function of conventional dendritic cell types 1 and 2. Front Immunol. 13(1058963)2023.PubMed/NCBI View Article : Google Scholar | |
|
Ouaaz F, Arron J, Zheng Y, Choi Y and Beg AA: Dendritic cell development and survival require distinct NF-kappaB subunits. Immunity. 16:257–270. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Andres-Ejarque R, Ale HB, Grys K, Tosi I, Solanky S, Ainali C, Catak Z, Sreeneebus H, Saklatvala J, Dand N, et al: Enhanced NF-κB signaling in type-2 dendritic cells at baseline predicts non-response to adalimumab in psoriasis. Nat Commun. 12(4741)2021.PubMed/NCBI View Article : Google Scholar | |
|
Alam MM, Yang D, Trivett A, Meyer TJ and Oppenheim JJ: HMGN1 and R848 synergistically activate dendritic cells using multiple signaling pathways. Front Immunol. 9(2982)2018.PubMed/NCBI View Article : Google Scholar | |
|
Natarajan K, Singh S, Burke TR Jr, Grunberger D and Aggarwal BB: Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci USA. 93:9090–9095. 1996.PubMed/NCBI View Article : Google Scholar | |
|
Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T and Gerritsen ME: Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 272:21096–21103. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM and Weaver CT: Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 6:1123–1132. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Schön MP and Erpenbeck L: The interleukin-23/interleukin-17 axis links adaptive and innate immunity in psoriasis. Front Immunol. 9(1323)2018.PubMed/NCBI View Article : Google Scholar | |
|
Wu R, Zeng J, Yuan J, Deng X, Huang Y, Chen L, Zhang P, Feng H, Liu Z, Wang Z, et al: MicroRNA-210 overexpression promotes psoriasis-like inflammation by inducing Th1 and Th17 cell differentiation. J Clin Invest. 128:2551–2568. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Zeng J, Lei L, Zeng Q, Yao Y, Wu Y, Li Q, Gao L, Du H, Xie Y, Huang J, et al: Ozone therapy attenuates NF-κB-mediated local inflammatory response and activation of Th17 cells in treatment for psoriasis. Int J Biol Sci. 16:1833–1845. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Leung S, Liu X, Fang L, Chen X, Guo T and Zhang J: The cytokine milieu in the interplay of pathogenic Th1/Th17 cells and regulatory T cells in autoimmune disease. Cell Mol Immunol. 7:182–189. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Murugaiyan G, Beynon V, Pires Da Cunha A, Joller N and Weiner HL: IFN-γ limits Th9-mediated autoimmune inflammation through dendritic cell modulation of IL-27. J Immunol. 189:5277–5283. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Kaplan MH: Th9 cells: Differentiation and disease. Immunol Rev. 252:104–115. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Goswami R and Kaplan MH: A brief history of IL-9. J Immunol. 186:3283–3288. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Mostafa A, Sakurai K, Murata T, Dainichi T, Tian H, Yodoi J and Kabashima K: Recombinant human thioredoxin ameliorates imiquimod-induced psoriasis-like dermatitis in mice. J Dermatol Sci. 116:55–58. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Kim N, Lee S, Kang J, Choi YA, Lee B, Kwon TK, Jang YH and Kim SH: Hispidulin alleviates imiquimod-induced psoriasis-like skin inflammation by inhibiting splenic Th1/Th17 cell population and keratinocyte activation. Int Immunopharmacol. 87(106767)2020.PubMed/NCBI View Article : Google Scholar | |
|
Liu R, Du S, Zhao L, Jain S, Sahay K, Rizvanov A, Lezhnyova V, Khaibullin T, Martynova E, Khaiboullina S and Baranwal M: Autoreactive lymphocytes in multiple sclerosis: Pathogenesis and treatment target. Front Immunol. 13(996469)2022.PubMed/NCBI View Article : Google Scholar | |
|
Clark RA and Schlapbach C: TH9 cells in skin disorders. Semin Immunopathol. 39:47–54. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Jabeen R and Kaplan MH: The symphony of the ninth: The development and function of Th9 cells. Curr Opin Immunol. 24:303–307. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Xu L, Kitani A, Fuss I and Strober W: Cutting edge: Regulatory T cells induce CD4+CD25-Foxp3-T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 178:6725–6729. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Zhou X, Bailey-Bucktrout S, Jeker LT and Bluestone JA, Martínez-Llordella M, Ashby M, Nakayama M, Rosenthal W and Bluestone JA: Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 10:1000–1007. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Khmaladze I, Kelkka T, Guerard S, Wing K, Pizzolla A, Saxena A, Lundqvist K, Holmdahl M, Nandakumar KS and Holmdahl R: Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice. Proc Natl Acad Sci USA. 111:E3669–E3678. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Singh TP, Zhang HH, Hwang ST and Farber JM: IL-23- and imiquimod-induced models of experimental psoriasis in mice. Curr Protoc Immunol. 125(e71)2019.PubMed/NCBI View Article : Google Scholar |