Enhanced SLC2A4 expression mitigates insulin resistance in gestational diabetes mellitus via the FoxO signaling pathway in vitro
- Authors:
- Published online on: August 22, 2025 https://doi.org/10.3892/etm.2025.12956
- Article Number: 206
-
Copyright: © Jin et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Gestational diabetes mellitus (GDM) is a form of diabetes occurring in pregnant women, typically in the second or third trimester (1). Although GDM often resolves after childbirth (2,3), it poses risks to both maternal and fetal health (4,5). This condition elevates both immediate obstetric risks, including pregnancy/delivery complications (6), and long-term metabolic sequelae for mothers and offspring, such as type 2 diabetes predisposition (7) and childhood obesity (8,9), respectively. Exogenous insulin (INS) therapy remains the first-line pharmacological intervention for GDM management (10), despite gestational hormonal shifts inducing progressive INS resistance (11), which complicates treatment efficacy. Emerging evidence has highlighted placental INS signaling as a critical pathophysiological contribution to GDM progression, with impaired INS receptor (INSR) substrate phosphorylation disrupting maternal-fetal glucose homeostasis (12). Therefore, deciphering the molecular crosstalk between placental INS signaling and gestational metabolic adaptation is imperative for developing novel therapeutic approaches and precision management protocols for GDM.
Solute carrier family 2 member 4 (SLC2A4), encoding the glucose transporter 4 (GLUT4) protein, serves as the principal mediator of INS-dependent glucose uptake in peripheral tissues such as skeletal muscle and adipose tissue (13,14). Compelling evidence from both molecular studies and clinical observations has demonstrated a downregulation of SLC2A4 expression in patients with GDM compared with that in normoglycemic controls, indicating that SLC2A4 is particularly relevant to gestational glucose homeostasis as its expression in INS-sensitive tissues indicates the regulation of adaptive pregnancy, which refers to the process during pregnancy where the mother dynamically adjusts the expression and function of SLC2A4/GLUT4 to optimize the insulin sensitivity of peripheral tissues (such as skeletal muscle and adipose tissue) (15,16). However, critical mechanistic gaps persist regarding three key aspects: i) Tissue-specific transcriptional regulation of SLC2A4 during gestational INS resistance; ii) post-translational modifications affecting SLC2A4 membrane trafficking in GDM; and iii) epigenetic modulation of SLC2A4 expression via hyperglycemic memory effects. A systematic investigation of these mechanisms through multi-omics integration is essential to elucidate the pathophysiological cascade linking SLC2A4 dysfunction to GDM progression. Although SLC2A4 is associated with GDM, the underlying mechanisms are not fully understood.
The Forkhead box O (FoxO) signaling pathway serves as a pivotal regulator of cellular metabolism and is predominantly governed by INS-mediated phosphorylation cascades (17). A systematic bioinformatics analysis in arterial and venous human feto-placental endothelial cells demonstrated that GDM-associated differentially expressed genes were enriched in the FoxO pathway (18). Complementing this finding, a longitudinal clinical study revealed that placenta-derived microRNAs (miRNAs) with differential expression in patients with GDM compared with control subjects preferentially targeted FoxO signaling pathway (19). Emerging evidence further underscores the interplay between FoxO signaling and SLC2A4-mediated glucose transport mechanisms, with FoxO1 nuclear translocation modulating the expression of INS-responsive gene clusters (such as INS, INSR) during metabolic adaptation (20,21). However, whether SLC2A4 plays a role in placental cells in GDM through the FoxO signaling pathway remains unknown. Understanding the dysregulation of the FoxO signaling pathway and SLC2A4 in GDM may provide insights into the mechanisms underlying INS resistance and impaired glucose metabolism during pregnancy. Therefore, the present study aimed to investigate the role of SLC2A4 in INS resistance in an in vitro model of GDM and explore the involvement of the FoxO signaling pathway.
Materials and methods
Database analysis
The list of genes associated with GDM was retrieved from GeneCards (https://www.genecards.org/), Comparative Toxicogenomics Database (CTD) (https://ctdbase.org/) and DisGeNet (https://disgenet.com/) database. By performing jvenn analysis (https://www.home-for-researchers.com/#/), five genes were identified in all three databases: INSR, PPARG, SLC2A4, TNF and LEPR. Subsequently, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of these five genes was performed using the STRING database (https://string-db.org/). Briefly, these five genes were input into the STRING interface with the organism specified as Homo sapiens. A protein-protein interaction (PPI) network was generated using default parameters (medium confidence score ≥0.4). Subsequently, KEGG pathway enrichment analysis was conducted via the ‘Analysis’ tab, with statistical significance set at a false discovery rate (FDR) <0.05 and no minimum gene count restriction. Pathways were ranked by FDR-adjusted P-values, and those directly relevant to metabolic regulation were prioritized for discussion.
Cell culture and treatment
The trophoblast cell line, HTR-8/SVneo, obtained from Honorgene (Changsha Abiwei Biotechnology Co., Ltd.), was cultured in RPMI-1640 medium (Beijing Solarbio Science & Technology Co., Ltd.) supplemented with 5% fetal bovine serum (Beijing Solarbio Science & Technology Co., Ltd.). Cells were maintained under standard culture conditions at 37˚C with 5% CO2. In the control group, the cells were cultured in medium containing 5 mM glucose, whereas in the high glucose (HG) group, cells were cultured in medium with an elevated glucose concentration of 25 mM (22). After a 24-h incubation period at 37˚C, the cells were harvested for further analysis.
For transfection experiments, overexpression of the target genes was achieved using pc-SLC2A4 or pc-FoxO1, which are plasmid constructs based on the pcDNA3.1 (Anhui General Bio Co., Ltd.) vector backbone [pc-negative control (NC)]. These sequences are included in the original data, available from the corresponding author. These plasmids (5 µg/ml) were transfected into HTR-8/SVneo cells using Lipofectamine® 2000 (Gibco; Thermo Fisher Scientific, Inc.). For transfection, the plasmid working concentration was adjusted to 5 µg/ml and mixed with Lipofectamine® 2000 at a ratio of 1:2.5. Transfection was performed for 24 h under standard culture conditions (37˚C, 5% CO2). This procedure facilitated the introduction and expression of the target genes into the HTR-8/SVneo cell line. The transfection efficiency is shown in Fig. S1. The in vitro experiments were divided into five groups: Control, HG, HG + pc-NC, HG + pc-SLC2A4 and HG + pc-SLC2A4 + pc-FoxO1. Subsequently, 48 h later, reverse transcription-quantitative PCR (RT-qPCR) was performed to confirm the successful transfection, and cell functional analysis was performed, and RNA was extracted for RT-qPCR and western blot analysis, respectively.
Lactate dehydrogenase (LDH) detection
Cells were seeded at a density of 200,000 cells/well in 24-well plates and allowed to adhere overnight. Following the application of the treatments, cells were lysed using 100 µl LDH assay buffer (Beyotime Institute of Biotechnology). The LDH assay buffer facilitated the release of LDH from the cytoplasm into the assay medium, where it catalyzed the conversion of lactate to pyruvate and reduced NAD+ to NADH. LDH activity was measured using an LDH test kit (cat. no. C0016; Beyotime Institute of Biotechnology), according to the manufacturer's instructions. Spectrophotometric analysis at 340 nm was performed to quantify NADH production, which directly reflects the LDH enzymatic activity in the samples.
Glucose uptake assay
Glucose uptake was assessed using the Glucose Uptake Fluorometric Assay Kit (cat. no. E-BC-F041; Elabscience®; Elabscience Bionovation Inc.) according to the manufacturer's instructions. Cells were seeded in 96-well cell culture plates with ~2,000 cells per well and starved overnight in serum-free culture medium. The medium was aspirated and discarded, and the cells were washed twice with 200 µl Krebs-Ringer-Phosphate-HEPES Buffer (KRPH) solution from the kit (pH 7.4; containing 2% BSA; Elabscience®; Elabscience Bionovation Inc.). Subsequently, 100 µl KRPH solution (containing 2% BSA) were added to the control and the assay wells, while 10 µl 2-Deoxy-D-glucose (10 mmol/l) were added to the assay wells and 10 µl KRPH solution were added to the control well, followed by incubation at 37˚C for 30 min. Cells were washed three times with 100 µl KRPH solution and other reagents were added according to the kit instructions. A total of 30 µl was taken from the corresponding wells and added to a microplate, and 170 µl working solution was added to each well and incubated at 37˚C for 30 min. The fluorescence value of each well was detected using a fluorescent microplate reader with an excitation wavelength of 530 nm and an emission wavelength of 590 nm.
Cell viability
Cells were seeded at a density of 1x104 cells/well in a 96-well plate and cultured in RPMI-1640 medium at 37˚C with 5% CO2. After transfection, cell viability was evaluated using a Cell Counting Kit-8 (CCK-8) assay kit (Beyotime Institute of Biotechnology), according to the manufacturer's instructions. The optical density was measured at 450 nm using a microplate reader. Each experiment was independently repeated three times.
RT-qPCR
RNA was extracted from cells using Trizol reagent (Beyotime Institute of Biotechnology), followed by RT into cDNA using a BeyoRT™II First Strand cDNA Synthesis Kit (cat. no. D7168S; Beyotime Institute of Biotechnology). The RT kit was used according to the manufacturer's protocol. Subsequently, qPCR was performed using an ABI 7900 RT-qPCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The primer sequences used for amplification were as follows: SLC2A4 (human) forward, 5'-GCCATGAGCTACGTCTCCATT-3' and reverse, 5'-GGCCACGATGAACCAAGGAA-3'; INSR (human) forward, 5'-GAACAAAGATGACAACGAGGAG-3' and reverse, 5'-CTTACAGATGGTCGGGCAAA-3'' INS (human) forward, 5'-GGGAACGAGGCTTCTTCTACA-3' and reverse, 5'-AGAGGGAGCAGATGCTGGTAC-3'; FoxO1 (human) forward, 5'-GGATGTGCATTCTATGGTGTACC-3' and reverse, 5'-TTTCGGGATTGCTTATCTCAGAC-3'; and GAPDH (human) forward, 5'-CATCATCCCTGCCTCTACTGG-3' and reverse, 5'-GTGGGTGTCGCTGTTGAAGTC-3'. The primers were designed using Primer Premier 5 (PREMIER Biosoft) and verified using Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). Conditions of one cycle: 94˚C for 20 sec, 55˚C for 20 sec, 72˚C for 20 sec, and 40 cycles were performed. For relative quantification of gene expression, the 2-ΔΔCq method was employed, with GAPDH serving as the reference gene for normalization of mRNA expression levels.
Western blotting
Cells were lysed using RIPA buffer (Beyotime Institute of Biotechnology) and the total protein concentration was determined using a BCA kit (Beyotime Institute of Biotechnology). Proteins (60 µg/sample) were separated using an 11% SDS-PAGE gel and subsequently transferred onto a PVDF membrane (cat. no. ISEQ00010; Sigma-Aldrich; Merck KGaA). Non-specific binding sites were blocked with Pierce protein-free blocking buffer (cat. no. 37573; Thermo Fisher Scientific, Inc.). The membranes were then incubated overnight at 4˚C with primary antibodies against SLC2A4 (cat. no. 2213S; Cell Signaling Technology, Inc.), INSR (cat. no. 3025S; Cell Signaling Technology, Inc.), INS (cat. no. ab181547; Abcam), phosphorylated (p)-FoxO1 (cat. no. 9461S; Cell Signaling Technology, Inc.), FoxO1 (cat. no. 2880S; Cell Signaling Technology, Inc.), p-FoxO3a (cat. no. 13129S; Cell Signaling Technology, Inc.), FoxO3a (cat. no. 12829S; Cell Signaling Technology, Inc.) and GAPDH (cat. no. 97166; Cell Signaling Technology, Inc.) (all 1:1,000 dilution). After three washes with TBST, the membranes were incubated with HRP-conjugated goat anti-rabbit or goat anti-mouse secondary antibodies (1:1,000 dilution; cat. nos. A0208 and A0216, respectively; Beyotime Institute of Biotechnology) for 1 h at room temperature. Protein bands were visualized using a SignalFire Plus ECL reagent (cat. no. 12630S; Cell Signaling Technology, Inc.), with GAPDH serving as an internal reference. Protein expression levels were semi-quantified through densitometry analysis using ImageJ V1.8.0 software (National Institutes of Health).
Statistical analysis
Statistical analyses were conducted using SPSS software (v26.0; IBM Corp.). Experimental data were derived from three independent biological replicates, with continuous variables presented as the mean ± SD. Intergroup comparisons were performed using unpaired Student's t-test for comparisons between two groups and one-way ANOVA followed by Tukey's post hoc test for multi-group comparisons (n≥3). P<0.05 was considered to indicate a statistically significant difference.
Results
Expression pattern of SLC2A4 in HG-induced HTR-8/SVneo cells
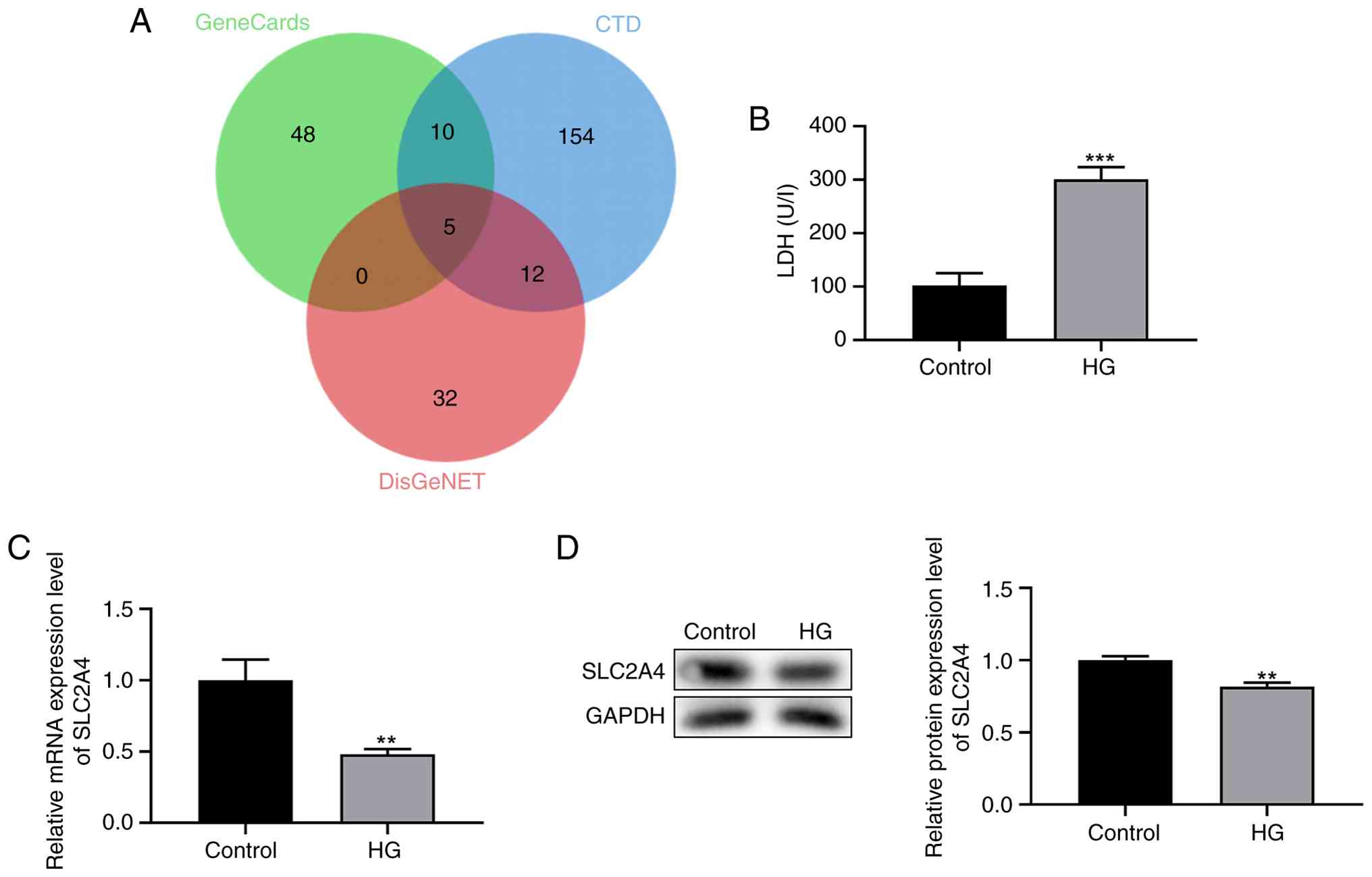
The genes implicated in GDM were investigated using the CTD, DisGeNet and Genecards databases. This comprehensive analysis led to consistent identification of a subset of genes, including INSR, PPARG, SLC2A4, TNF and LEPR across all three databases (Fig. 1A). LDH levels were measured using an LDH assay to assess cellular stress in response to HG conditions. The results indicated a significant increase in LDH levels in the HG group compared with those in the control group (P<0.001; Fig. 1B).
To delineate the specific role of SLC2A4 in GDM, RT-qPCR and western blot analyses were conducted to examine SLC2A4 expression in HTR-8/SVneo cells under HG conditions. The data revealed a significant downregulation of SLC2A4 expression at both the mRNA (P<0.01; Fig. 1C) and protein levels in HG-induced cells (P<0.01; Fig. 1D). These findings suggest a potentially important role for SLC2A4 in the cellular response to HG environments, particularly in the context of GDM.
Effects of overexpression of SLC2A4 on INS and INSR levels, glucose uptake and cell viability in HG-induced HTR-8/SVneo cells
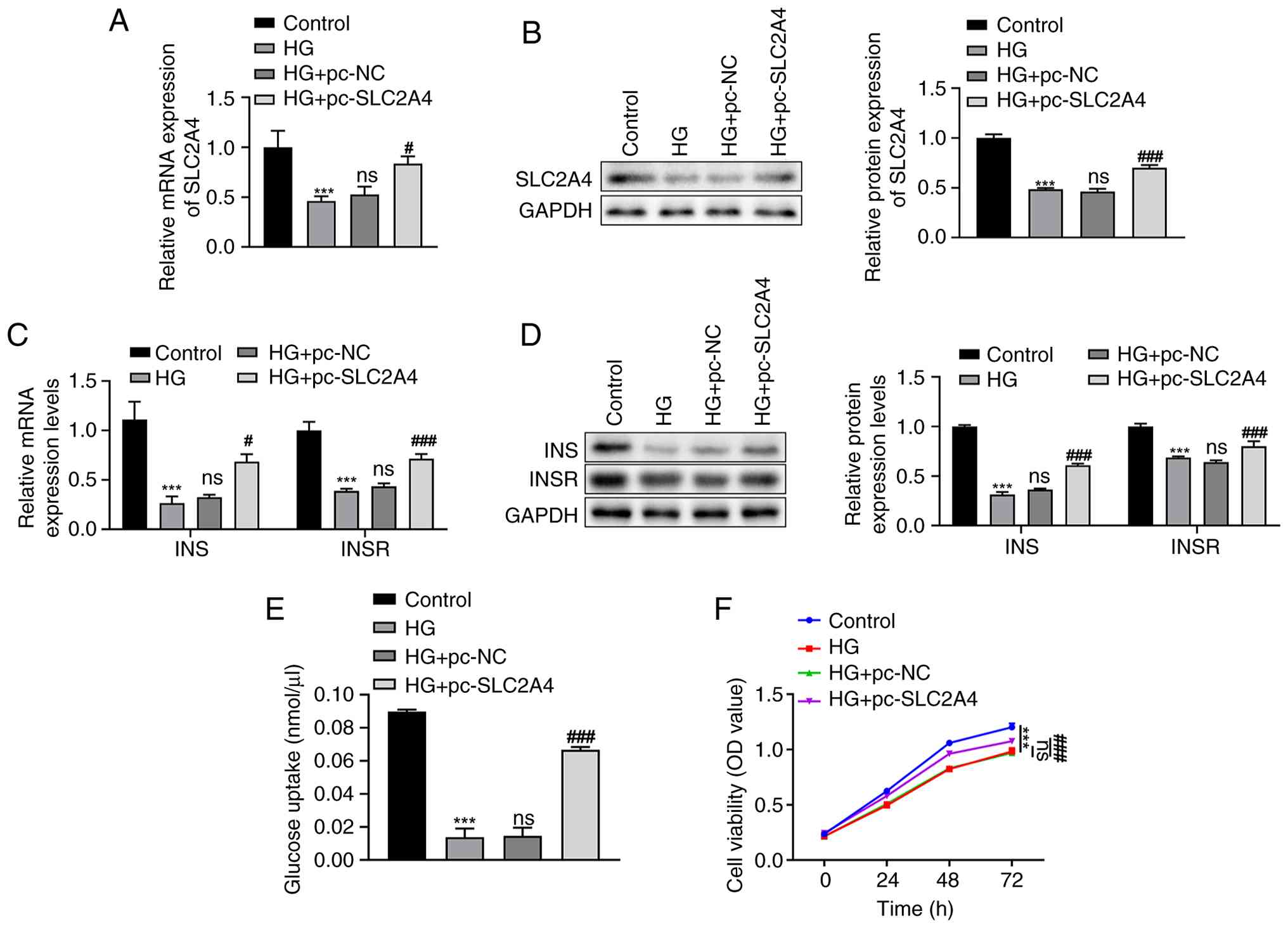
Post-transfection of either pc-NC or pc-SLC2A4 in HG-induced HTR-8/SVneo cells, SLC2A4 expression levels were measured using RT-qPCR (Fig. 2A) and western blotting (Fig. 2B). The results demonstrated a significant reduction in SLC2A4 mRNA and protein expression in the HG group compared with that in the control group (P<0.001). However, there were no significant differences in the mRNA and protein levels of SLC2A4 between the HG and HG + pc-NC groups. Transfection with pc-SLC2A4 resulted in a significant increase in SLC2A4 levels compared with the pc-NC group (P<0.05; Fig. 2A and B).
Subsequently, the expression levels of INS and INSR were assessed using the aforementioned techniques. Both INS and INSR levels were significantly decreased in the HG group compared with those in the control group (P<0.001; Fig. 2C and D). No significant differences were observed between the HG and HG + pc-NC groups in terms of mRNA (Fig. 2C) or protein (Fig. 2D) expression levels. Notably, the mRNA and protein levels of INS and INSR were significantly upregulated following transfection with pc-SLC2A4 compared with the pc-NC group (P<0.05; Fig. 2C and D).
Additionally, a glucose uptake assay was conducted to quantify glucose absorption. A significant decrease in glucose uptake was observed in the HG group compared with that in the control group (P<0.001); however, this reduction was effectively reversed by transfection with pc-SLC2A4 (P<0.001) (Fig. 2E). Cell viability, as assessed using the CCK-8 assay, decreased in HG-induced HTR-8/SVneo cells compared with that in the control group (P<0.001); however, this decrease was ameliorated following treatment with pc-SLC2A4 (P<0.001) (Fig. 2F).
These findings highlight the role of SLC2A4 in modulating INS signaling and cellular responses to HG environments in trophoblasts, as evidenced by the changes in INS and INSR expression, glucose uptake and cell viability.
Effects of overexpression of SLC2A4 on the FoxO signaling pathway in HG-induced HTR-8/SVneo cells
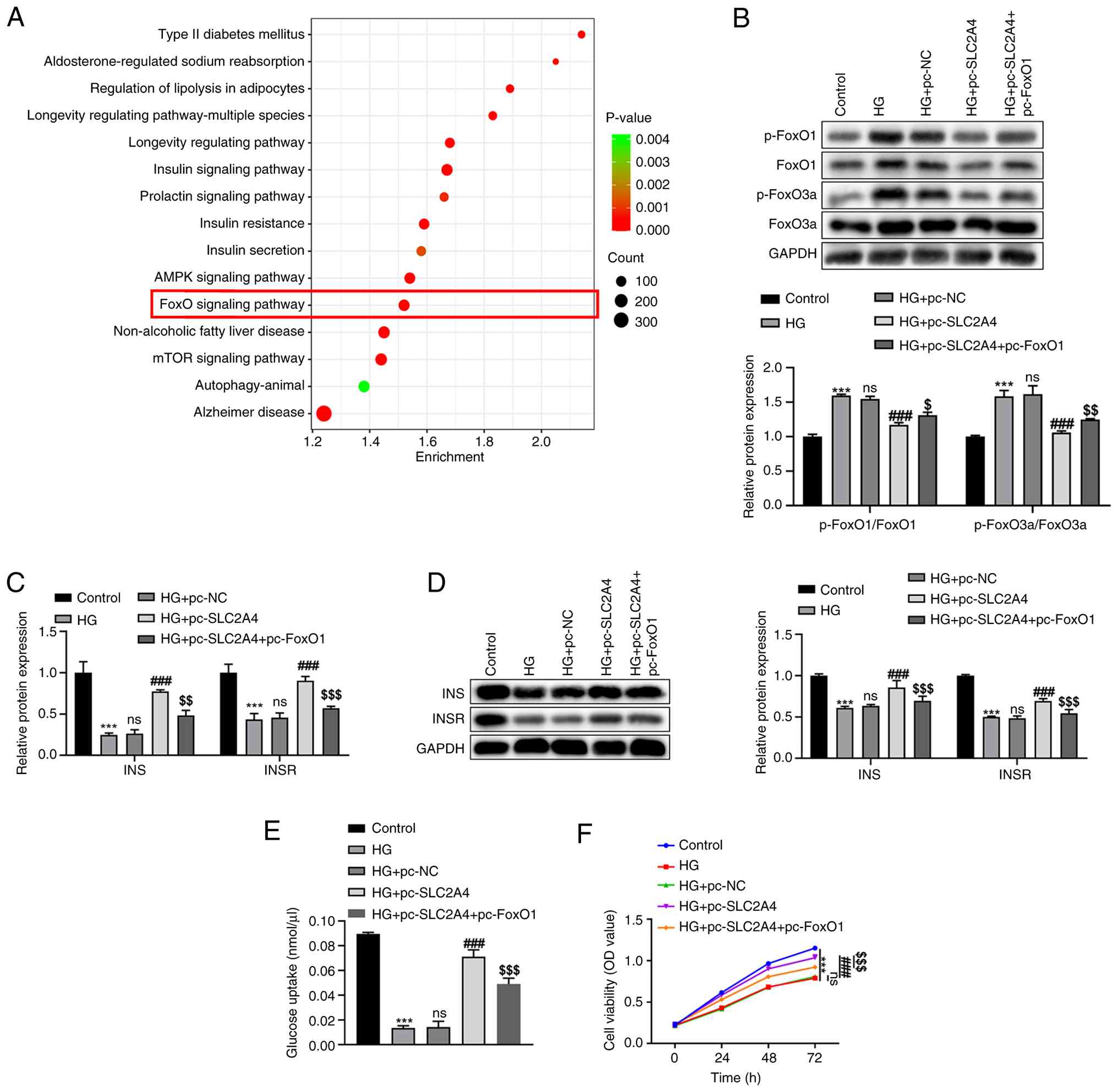
Following KEGG pathway analysis, numerous pathways associated only with SLC2A4 and GDM were identified, among which the ‘FoxO signaling pathway’ was highlighted as a key pathway of interest (Fig. 3A). To investigate the regulatory relationship between SLC2A4 and the FoxO signaling pathway, the levels of p-FoxO1, FoxO1, p-FoxO3a and FoxO3a were semi-quantified using western blot analysis (Fig. 3B). In the HG group, significantly elevated levels of p-FoxO1 (relative to FoxO1) and p-FoxO3a (relative to FoxO3a) were observed compared with those in the control group (P<0.001). However, the overexpression of SLC2A4 in HG-induced HTR-8/SVneo cells led to a reduction in the levels of these proteins (P<0.001). This downregulation suggested a suppressive effect of SLC2A4 overexpression on the FoxO signaling pathway. However, the restoration of FoxO1 protein levels by pc-FoxO1 introduction partially restored p-FoxO1 levels, and the levels of FoxO3a and p-FoxO3a were also restored (HG + pc-SLC2A4 + pc-FoxO1 vs. HG + pc-SLC2A4, P<0.05; Fig. 3B), indicating that pc-FoxO1 both increased expression of FoxO1 protein and increased phosphorylation of FoxO1/activation of the pathway.
Role of the FoxO signaling pathway in the pc-SLC2A4-induced expression changes of INS and INSR and the alteration of glucose uptake and cell viability in HG-induced HTR-8/SVneo cells
To elucidate the role of the FoxO signaling pathway in the SLC2A4-induced enhancement of INS and INSR expression, pc-FoxO1 was introduced into HG-treated HTR-8/SVneo cells. The expression levels of INS and INSR were determined through RT-qPCR (Fig. 3C) and western blotting (Fig. 3D). In the HG group, a significant reduction in INS and INSR mRNA and protein levels was observed compared with those in the control group (P<0.001). However, this decrease was counteracted in the HG + pc-SLC2A4 group, where INS and INSR expression levels were significantly elevated compared with those in both the HG + pc-NC and HG groups (P<0.001). Introduction of pc-FoxO1 prevented this increase in INS and INSR mRNA and protein expression levels (P<0.01; Fig. 3C and D).
To investigate the involvement of the FoxO signaling pathway in the increased glucose uptake and cell viability induced by SLC2A4 overexpression, pc-FoxO1 was introduced into HG-induced cells. Glucose uptake and cell viability were assessed using glucose uptake and CCK-8 assays, respectively. In the HG group, glucose uptake (Fig. 3E) and cell viability (Fig. 3F) were significantly reduced compared with those in the control group (P<0.001). However, in the HG + pc-SLC2A4 group, glucose uptake and cell viability were significantly increased compared with those in the HG + pc-NC and HG groups (P<0.001). This restoration of glucose uptake and cell viability by SLC2A4 overexpression was partially prevented when pc-FoxO1 was introduced (P<0.001; Fig. 3E and F).
Discussion
GDM, a metabolic disorder associated with increased risk of maternal-fetal complications, including perinatal morbidity (23) and placental dysplasia (24), has drawn significant research attention onto trophoblast biology. Trophoblasts are specialized epithelial cells, critical for placental morphogenesis and fetal development (25,26), that have emerged as key cellular targets in GDM pathogenesis (27-29). The experimental model of HTR-8/SVneo trophoblasts under hyperglycemic conditions used in the present study revealed significant alterations in the INS signaling cascade. The expression levels of SLC2A4, INSR and INS were significantly decreased in HTR-8/SVneo cells under high glucose condition, while overexpression of SLC2A4 could increase the expression levels of INS and INSR and improve glucose uptake in high glucose environment. Overall, this supported the hypothesis that glucose-mediated INS resistance in trophoblasts may exacerbate maternal hyperglycemia through a pathogenic feedback loop, thereby amplifying adverse pregnancy outcomes.
The association between GDM and placental oxidative stress as well as mitochondrial dysfunction is particularly pronounced when the fetus is male (30,31). Emerging evidence highlights sex-specific variations in mitochondrial bioenergetics (30) and endoplasmic reticulum stress activation (31) within placental trophoblasts during GDM progression. Building upon these findings, future research can be further developed in the following aspects: i) Mitochondrial respiratory chain dysfunction; ii) unfolded protein response dynamics; and iii) fetal sex-dependent molecular adaptations in trophoblasts under INS-resistant conditions. This will help to elucidate the pathophysiological continuum linking metabolic dysregulation to cellular stress responses in GDM-affected placentas, thereby advancing the understanding of trophoblast pathophysiology and identifying potential therapeutic targets for clinical intervention.
The SLC2/GLUT family, as a member of the major facilitator superfamily of membrane transport proteins (32), plays a pivotal role in INS resistance mechanisms (33) and is an important research focus in GDM. Xiao et al (34) has recently demonstrated that GDM regulates GLUT3 translocation via AMP kinase signaling, thereby modulating glucose uptake in trophoblasts. Additionally, Stanirowski et al (35) reported that, in well-controlled GDM/pre-GDM (PGDM) pregnancies reaching the end of gestation, the expression levels of GLUT3, GLUT8 and GLUT12 in placental tissue remain unaffected, whereas GLUT1 is significantly upregulated in type 1 PGDM. Moreover, the altered expression of GLUT1, GLUT4 and GLUT9 in placental tissue has been associated with accelerated fetal growth in GDM/PGDM (36). In the present study, reduced SLC2A4 expression in HG-induced HTR-8/SVneo cells was observed. In addition, overexpression of SLC2A4 improved INS resistance, suggesting the need for further investigation into the association between the SLC2 family and GDM pathophysiology. Furthermore, comparative analyses of other GLUT family members along with SLC2A4 in future studies may provide deeper insights into their roles in GDM-associated INS resistance and lead to the identification of novel clinical biomarkers.
INS resistance in trophoblasts is primarily attributed to the dysregulation or dysfunction of the INS signaling pathway (37,38), which involves key components, such as INS and INSR (39). Previous studies have demonstrated that INS and INSR contribute to INS resistance in MIN6(40) and HTR-8/SVneo cells (22) induced by HG. INS and INSR contribute to insulin resistance through negative feedback caused by hyperinsulinemia (reducing the expression of INSR), inflammation, lipotoxicity and other interfering signal transductions (37,38). INS is increased in the early stage and decreased in the later stage, while INSR is continuously downregulated due to negative feedback and inflammation (39). The findings in the present study revealed that SLC2A4 overexpression significantly increased INS and INSR levels, enhanced glucose uptake and improved HTR-8/SVneo cell viability. These results suggest that SLC2A4 may modulate GDM pathogenesis and INS resistance via the INS signaling pathway, although further validation through mice experiments is required to confirm this conclusion. The relevant literature indicates that SLC2A4 is a downstream effector of INS in diabetes (40-42). The present study suggested that SLC2A4 overexpression may activate the INS signaling pathway by inhibiting the FoxO signaling pathway. A recent study has demonstrated that FoxO and FoxK proteins are related to the switching of insulin signals during fasting and feeding states (43). Therefore, SLC2A4 may not directly regulate the expression or activity of INS or INSR but rather achieve this through an indirect feedback loop via the FoxO signaling pathway. The novelty of the present study lies to the fact that SLC2A4, as a downstream effector of INS, may activate the INS signaling pathway through the FoxO signaling pathway, thereby forming a regulatory loop.
Moreover, RNA-sequencing analysis has indicated that, in addition to genes involved in INSR signaling and glucose metabolism pathways, the FoxO signaling pathway plays a pivotal role in INS resistance (44). The FoxO signaling pathway has also been implicated in GDM pathophysiology via adipogenic pathways (45), yet its specific role in GDM-related INS resistance remains unexplored. In the present study, the overexpression of SLC2A4 inhibited the FoxO signaling pathway by suppressing the phosphorylation of FoxO1 and FoxO3a. Conversely, overexpression of FoxO1 prevented the upregulation of INS and INSR and decreased glucose uptake and cell viability induced by SLC2A4 overexpression. These findings indicate that SLC2A4 may regulate GDM pathogenesis through modulation of the FoxO signaling pathway. It could be hypothesized that SLC2A4 may directly affect FoxO signaling through metabolic reprogramming, INS signaling feedback, regulation of the membrane signaling microenvironment or crosstalk between epigenetics and miRNAs. Further research should focus on the tissue-specific localization, phosphorylation and target gene expression changes of FoxO proteins in SLC2A4 knockout or overexpression models, as well as the verification of the direct interaction between metabolic intermediates (such as acetyl-coA, lactic acid and pyruvate) and FoxO.
Clinically, it has been reported that patients with GDM and normal pre-pregnancy BMI who develop macrosomia may exhibit HG-induced trophoblast proliferation mediated by the ERK1/2 signaling pathway (46). Abnormal activation of the Wnt/β-catenin pathway in patients with GDM has also been associated with trophoblast dysfunction (47). Furthermore, recent studies have highlighted the involvement of the NLR family pyrin domain containing 3/apoptosis-associated speck-like protein containing a CARD/caspase-1 signaling pathway in trophoblast pyroptosis (48) and the INSR substrate 1/PI3K/Akt signaling pathway in trophoblast autophagy (49) in patients with GDM. Additionally, the JNK signaling pathway has been shown to promote INS resistance in trophoblasts in GDM (38). Collectively, these findings underscore the potential regulatory roles of multiple signaling pathways in GDM pathogenesis and suggest that broader investigations into the interplay between SLC2A4 and these pathways are warranted.
Moreover, there are several limitations to the present study relative to the HTR-8/SVneo cellular model in GDM research: i) Hormonal complexity: GDM pathophysiology involves dynamic hormonal interplay (including INS, estrogen and progesterone) that the HTR-8/SVneo cell line cannot fully recapitulate due to its static culture system nature; ii) functional specificity: HTR-8/SVneo cells are primarily used to investigate trophoblast migration and invasion, while their limited applicability in INS signaling pathway studies may compromise the accuracy of GDM-associated INS resistance modelling; iii) phenotypic drift: Prolonged in vitro propagation induces cellular adaptations divergent from primary trophoblast phenotypes, potentially diminishing the physiological relevance of the experimental outcomes; and iv) microenvironmental simplification: This monoculture system neglects critical multi-cellular interactions within placental niches, particularly immunomodulatory networks and angiogenic crosstalk at the maternal-fetal interface. It is expected that these limitations will be addressed in the future. For instance, a multifactorial stimulation model based on high sugar, lipid and hormone levels can be used to simulate hyperglycemia, high free fatty acid and pregnancy hormone levels (such as estradiol and progesterone) in patients with GDM. Alternatively, primary placental trophoblasts or organoids can be used to retain tissue-specific responses. Conversely, intermittent high sugar exposure (simulating post-meal blood glucose fluctuations) or progressive concentration increments can be adopted to simulate the metabolic adaptation process during pregnancy. Moreover, at the multi-omics integration level, the transcriptome and metabolome data of the placenta from in vitro models and patients with GDM can be compared to screen for consistent pathways (such as INS signaling and lipid metabolism).
In conclusion, the present study indicated that overexpression of SLC2A4 can effectively improve the impaired INS and INSR levels, glucose uptake and cell viability of HTR-8/SVneo cells under HG conditions and inhibit the activation of the FoxO signaling pathway. SLC2A4 may activate the INS signaling pathway by inhibiting the FoxO signaling pathway, thus alleviating INS resistance in patients with GDM. The present study examined the role of SLC2A4 and the FoxO signaling pathway in the INS resistance of trophoblasts, providing a research basis for clarifying the mechanism of GDM pathogenesis and suggesting SLC2A4 as a potential intervention target for GDM. In the future, it may be possible to explore SLC2A4 activators or FoxO inhibitors as potential treatment strategies for GDM to improve blood glucose control and fetal health in pregnant women. The present study further supports the use of SLC2A4 as a genetic marker for GDM, which may be helpful for the early screening of high-risk pregnant women. Combined with the detection of FoxO pathway activity, this may help to assess the degree of INS resistance in patients with GDM and guide precise treatment strategies, such as lifestyle intervention or drug selection. However, further studies on placenta tissues from patients with GDM should be performed to support this.
Supplementary Material
Plasmid transfection efficiency verified by reverse transcription-quantitative PCR. Transfection efficiency of (A) pc-SLC2A4 and (B) pc-FoxO1 in HTR-8/SVneo cells. **P<0.01 vs. pc-NC. NC, negative control; SLC2A4, solute carrier family 2 member 4; FoxO, Forkhead box O.
Acknowledgements
Not applicable.
Funding
Funding: The present work was supported by the Nantong Basic Science Research and Social Livelihood Science and Technology Plan Project (grant no. MSZ2022084) and Nantong Medical Key Disciplines (grant no. NTXK202105).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
YJ and CC were involved in the conception and design of the study. XC, JW, YZ and HX were involved in the analysis and interpretation of the data. YJ drafted the manuscript and CC revised it critically. YJ, XC, JW, YZ, HX and CC confirmed the authenticity of all the raw data, and read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Lende M and Rijhsinghani A: Gestational diabetes: Overview with emphasis on medical management. Int J Environ Res Public Health. 17(9573)2020.PubMed/NCBI View Article : Google Scholar | |
|
Pinto Y, Frishman S, Turjeman S, Eshel A, Nuriel-Ohayon M, Shrossel O, Ziv O, Walters W, Parsonnet J, Ley C, et al: Gestational diabetes is driven by microbiota-induced inflammation months before diagnosis. Gut. 72:918–928. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Shi P, Tang J and Yin X: Association between second- and third-trimester maternal lipid profiles and adverse perinatal outcomes among women with GDM and non-GDM: A retrospective cohort study. BMC Pregnancy Childbirth. 23(318)2023.PubMed/NCBI View Article : Google Scholar | |
|
Weir TL, Majumder M and Glastras SJ: A systematic review of the effects of maternal obesity on neonatal outcomes in women with gestational diabetes. Obes Rev. 25(e13747)2024.PubMed/NCBI View Article : Google Scholar | |
|
Moon JH and Jang HC: Gestational diabetes mellitus: Diagnostic approaches and maternal-offspring complications. Diabetes Metab J. 46:3–14. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Ye W, Luo C, Huang J, Li C, Liu Z and Liu F: Gestational diabetes mellitus and adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ. 377(e067946)2022.PubMed/NCBI View Article : Google Scholar | |
|
Tsokkou S, Konstantinidis I, Georgaki MN, Kavvadas D, Papadopoulou K, Keramas A, Sioga A, Papamitsou T and Karachrysafi S: Gestational diabetes mellitus and its correlation in the development of pancreatic cancer: A 10-year systematic review. Cancers (Basel). 16(1840)2024.PubMed/NCBI View Article : Google Scholar | |
|
Yovera L, Zaharia M, Jachymski T, Velicu-Scraba O, Coronel C, de Paco Matallana C, Georgiopoulos G, Nicolaides KH and Charakida M: Impact of gestational diabetes mellitus on fetal cardiac morphology and function: Cohort comparison of second- and third-trimester fetuses. Ultrasound Obstet Gynecol. 57:607–613. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Szmuilowicz ED, Josefson JL and Metzger BE: Gestational diabetes mellitus. Endocrinol Metab Clin North Am. 48:479–493. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Castorino K, Osumili B, Lakiang T, Banerjee KK, Goldyn A and Piras de Oliveira C: Insulin use during gestational and pre-existing diabetes in pregnancy: A systematic review of study design. Diabetes Ther. 15:929–1045. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Mitra T, Gulati R, Ramachandran K, Rajiv R, Enninga EAL, Pierret CK, Kumari RS and Janardhanan R: Endocrine disrupting chemicals: Gestational diabetes and beyond. Diabetol Metab Syndr. 16(95)2024.PubMed/NCBI View Article : Google Scholar | |
|
Calvo MJ, Parra H, Santeliz R, Bautista J, Luzardo E, Villasmil N, Martínez MS, Chacín M, Cano C, Checa-Ros A, et al: The placental role in gestational diabetes mellitus: A molecular perspective. touchREV Endocrinol. 20:10–18. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Klip A, McGraw TE and James DE: Thirty sweet years of GLUT4. J Biol Chem. 294:11369–11381. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Wang T, Wang J, Hu X, Huang XJ and Chen GX: Current understanding of glucose transporter 4 expression and functional mechanisms. World J Biol Chem. 11:76–98. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Hu S, Ma S, Li X, Tian Z, Liang H, Yan J, Chen M and Tan H: Relationships of SLC2A4, RBP4, PCK1, and PI3K gene polymorphisms with gestational diabetes mellitus in a chinese population. Biomed Res Int. 2019(7398063)2019.PubMed/NCBI View Article : Google Scholar | |
|
Li W, Yuan X, He X, Yang L, Wu Y, Deng X, Zeng Y, Hu K and Tang B: The downregulation of miR-22 and miR-372 may contribute to gestational diabetes mellitus through regulating glucose metabolism via the PI3K/AKT/GLUT4 pathway. J Clin Lab Anal. 36(e24557)2022.PubMed/NCBI View Article : Google Scholar | |
|
Lee S and Dong HH: FoxO integration of insulin signaling with glucose and lipid metabolism. J Endocrinol. 233:R67–R79. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Liu Y, Wang Y, Wang Y, Lv Y, Zhang Y and Wang H: Gene expression changes in arterial and venous endothelial cells exposed to gestational diabetes mellitus. Gynecol Endocrinol. 36:791–795. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Thamotharan S, Ghosh S, James-Allan L, Lei MYY, Janzen C and Devaskar SU: Circulating extracellular vesicles exhibit a differential miRNA profile in gestational diabetes mellitus pregnancies. PLoS One. 17(e0267564)2022.PubMed/NCBI View Article : Google Scholar | |
|
De Meyts P: The insulin receptor and its signal transduction network. In: Endotext [Internet]. Feingold KR, Ahmed SF, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K et al (eds). MDText.com, Inc., South Dartmouth, MA, 2000. | |
|
Lundell LS, Massart J, Altintas A, Krook A and Zierath JR: Regulation of glucose uptake and inflammation markers by FOXO1 and FOXO3 in skeletal muscle. Mol Metab. 20:79–88. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Lin L, Wang X, Zhao W and Chen Y: Upregulation of klotho aggravates insulin resistance in gestational diabetes mellitus trophoblast cells. Genet Res (Camb). 2022(1500768)2022.PubMed/NCBI View Article : Google Scholar | |
|
Malaza N, Masete M, Adam S, Dias S, Nyawo T and Pheiffer C: A systematic review to compare adverse pregnancy outcomes in women with pregestational diabetes and gestational diabetes. Int J Environ Res Public Health. 19(10846)2022.PubMed/NCBI View Article : Google Scholar | |
|
Shen D, Lu Y, Li G, Hu M, Li S, Ju H, Zhang M and Wang X: Mechanism of neutrophil extracellular traps generation and their role in trophoblasts apoptosis in gestational diabetes mellitus. Cell Signal. 88(110168)2021.PubMed/NCBI View Article : Google Scholar | |
|
Xiao Z, Yan L, Liang X and Wang H: Progress in deciphering trophoblast cell differentiation during human placentation. Curr Opin Cell Biol. 67:86–91. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Knöfler M, Haider S, Saleh L, Pollheimer J, Gamage TKJB and James J: Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell Mol Life Sci. 76:3479–3496. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Sa R, Ma J, Yang J, Li DF, Du J, Jia JC, Li ZY, Huang N, A L, Sha R, et al: High TXNIP expression accelerates the migration and invasion of the GDM placenta trophoblast. BMC Pregnancy Childbirth. 23(235)2023.PubMed/NCBI View Article : Google Scholar | |
|
Musa E, Salazar-Petres E, Vatish M, Levitt N, Sferruzzi-Perri AN and Matjila MJ: Kisspeptin signalling and its correlation with placental ultrastructure and clinical outcomes in pregnant South African women with obesity and gestational diabetes. Placenta. 154:49–59. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Liu Q, Gui J and Wu L: Study on the regulation of trophoblast activity by abnormally expressed hsa_circ_0024838/miR-543/HIF1A in patients with gestational diabetes mellitus. Placenta. 151:27–36. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Hebert JF and Myatt L: Placental mitochondrial dysfunction with metabolic diseases: Therapeutic approaches. Biochim Biophys Acta Mol Basis Dis. 1867(165967)2021.PubMed/NCBI View Article : Google Scholar | |
|
Kuo CH, Wang SH, Juan HC, Chen SC, Kuo CH, Kuo HC, Lin SY and Li HY: Angiopoietin-like protein 4 induces growth hormone variant secretion and aggravates insulin resistance during pregnancy, linking obesity to gestational diabetes mellitus. Biofactors. 50:1176–1191. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Holman GD: Structure, function and regulation of mammalian glucose transporters of the SLC2 family. Pflugers Arch. 472:1155–1175. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Yaribeygi H, Farrokhi FR, Butler AE and Sahebkar A: Insulin resistance: Review of the underlying molecular mechanisms. J Cell Physiol. 234:8152–8161. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Xiao Z, Liu X, Luan X, Duan R, Peng W, Tong C, Qiao J and Qi H: Glucose uptake in trophoblasts of GDM mice is regulated by the AMPK-CLUT3 signaling pathway. Sci Rep. 14(12051)2024.PubMed/NCBI View Article : Google Scholar | |
|
Stanirowski PJ, Szukiewicz D, Majewska A, Wątroba M, Pyzlak M, Bomba-Opoń D and Wielgoś M: Placental expression of glucose transporters GLUT-1, GLUT-3, GLUT-8 and GLUT-12 in pregnancies complicated by gestational and type 1 diabetes mellitus. J Diabetes Investig. 13:560–570. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Stanirowski PJ, Szukiewicz D, Pyzlak M, Abdalla N, Sawicki W and Cendrowski K: Analysis of correlations between the placental expression of glucose transporters GLUT-1, GLUT-4 and GLUT-9 and selected maternal and fetal parameters in pregnancies complicated by diabetes mellitus. J Matern Fetal Neonatal Med. 32:650–659. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Le TKC, Dao XD, Nguyen DV, Luu DH, Bui TMH, Le TH, Nguyen HT, Le TN, Hosaka T and Nguyen TTT: Insulin signaling and its application. Front Endocrinol (Lausanne). 14(1226655)2023.PubMed/NCBI View Article : Google Scholar | |
|
Ju Y, Shen T, Guo Z, Kong Y, Huang Y and Hu J: Vitronectin promotes insulin resistance in trophoblast cells by activating JNK in gestational diabetes mellitus. Cell Biol Int: April 23, 2024 (Epub ahead of print). | |
|
Chakraborty C, Roy SS, Hsu MJ and Agoramoorthy G: Landscape mapping of functional proteins in insulin signal transduction and insulin resistance: A network-based protein-protein interaction analysis. PLoS One. 6(e16388)2011.PubMed/NCBI View Article : Google Scholar | |
|
Bao S, Wang X, Cho SB, Wu YL, Wei C, Han S, Bao L, Wu Q, Ao W and Nan JX: Agriophyllum oligosaccharides ameliorate diabetic insulin resistance through INS-R/IRS/Glut4-mediated insulin pathway in db/db mice and MIN6 cells. Front Pharmacol. 12(656220)2021.PubMed/NCBI View Article : Google Scholar | |
|
Zolfaghari N, Soheili ZS, Samiei S, Latifi-Navid H, Hafezi-Moghadam A, Ahmadieh H and Rezaei-Kanavi M: microRNA-96 targets the INS/AKT/GLUT4 signaling axis: Association with and effect on diabetic retinopathy. Heliyon. 9(e15539)2023.PubMed/NCBI View Article : Google Scholar | |
|
Bao S, Wu YL, Wang X, Han S, Cho S, Ao W and Nan JX: Agriophyllum oligosaccharides ameliorate hepatic injury in type 2 diabetic db/db mice targeting INS-R/IRS-2/PI3K/AKT/PPAR-γ/Glut4 signal pathway. J Ethnopharmacol. 257(112863)2020.PubMed/NCBI View Article : Google Scholar | |
|
Sakaguchi M: The role of insulin signaling with FOXO and FOXK transcription factors. Endocr J. 71:939–944. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Cen HH, Hussein B, Botezelli JD, Wang S, Zhang JA, Noursadeghi N, Jessen N, Rodrigues B, Timmons JA and Johnson JD: Human and mouse muscle transcriptomic analyses identify insulin receptor mRNA downregulation in hyperinsulinemia-associated insulin resistance. FASEB J. 36(e22088)2022.PubMed/NCBI View Article : Google Scholar | |
|
Kunte P, Barberio M, Tiwari P, Sukla K, Harmon B, Epstein S, Bhat D, Authelet K, Goldberg M, Rao S, et al: Neonatal adiposity is associated with microRNAs in adipocyte-derived extracellular vesicles in maternal and cord blood, a discovery analysis. Int J Obes (Lond). 48:403–413. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Zheng Y, Huang M, Lu X, Xu J and Han Y, Ji J and Han Y: Association of hyperglycaemia with the placenta of GDM-induced macrosomia with normal pre-pregnancy BMI and the proliferation of trophoblast cells. J Obstet Gynaecol. 42:1759–1768. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Cui SS, Zhang P, Sun L, Yuan YL, Wang J, Zhang FX and Li R: Mucin1 induced trophoblast dysfunction in gestational diabetes mellitus via Wnt/β-catenin pathway. Biol Res. 56(48)2023.PubMed/NCBI View Article : Google Scholar | |
|
Guo J, Zhou M, Zhao M, Li S, Fang Z, Li A and Zhang M: TIGAR deficiency induces caspase-1-dependent trophoblasts pyroptosis through NLRP3-ASC inflammasome. Front Immunol. 14(1114620)2023.PubMed/NCBI View Article : Google Scholar | |
|
Qu X, Li XY, Feng Y, Wang X, Li L, Wang YP and Chu YL: sh-Ambra1 inhibits IRS-1/PI3K/Akt signalling pathway to reduce autophagy in gestational diabetes. Endokrynol Pol. 75:61–70. 2024.PubMed/NCBI View Article : Google Scholar |