Emerging threat of environmental microplastics: A comprehensive analysis of hepatic metabolic dysregulation and hepatocellular damage (Review)
- Authors:
- Published online on: July 14, 2025 https://doi.org/10.3892/ijmm.2025.5584
- Article Number: 144
-
Copyright: © Yue et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
The lightweight, stable and durable properties of plastic mean that it is widely used in industrial production and daily life (1). Plastic use began in the 19th century and has since underwent substantial growth and development over the previous two centuries. Specifically, 390 million tonnes of plastic were produced globally in 2022 and the cumulative global plastic production from 1950-2017 was ~9.2 billion tonnes. The global production of plastics has led to the release of a substantial volume of plastic particles into the environment. These particles come from industrial production, as well as small particles released from plastic products such as plastic containers and personal care items. Microplastic (MP) particles are also produced by the natural peeling and breaking down of plastic waste, and this pollution is of growing concern (2). A study in 2004 first introduced the concept of MPs when investigating plastic debris in the ocean (3). At present, it is generally accepted that MPs are plastic particles, fibres or fragments directly derived from the plastics industry or the degradation of plastic waste, with sizes ranging from 5 mm to 100 nm. Among them, MPs with particle sizes <1,000 nm are termed nanoplastics (NPs) (4). In Europe, ~4-5 million tonnes of sewage and sludge containing MPs are applied to farmland annually (5,6), and research estimates that 60-64% of land-based MPs enter the ocean from coastal regions (7). Another study also found that MPs can be transported long distances in the atmosphere, further indicating that MP pollution has become a global problem (8).
MPs are widely used throughout the world and have caused pollution on a global scale. Therefore, numerous organisms in the ecological environment consume these MPs, with a number of studies demonstrating that fish, crustaceans, plankton and invertebrates in aquatic environments ingest MPs, and that residual plastic fragments have been found in seabirds (9-11). Furthermore, previous studies have confirmed that fish indirectly exposed to MPs through nutrient transfer exhibit the same toxic reactions as those directly exposed (12,13). This suggests that MPs may be transferred through the food chain, thereby affecting organisms at higher trophic levels. As humans are at a high nutrient level in natural nutrient transfer, MPs may enter the human body through nutrient transfer, as well as through inhalation and drinking water (14). One study found an exposure concentration of 60/m3 MPs in air samples collected near the city of Bremen in Germany. Based on normal adult ventilation rates of 5-8 l/min, the estimated daily intake of MPs is 432-691 (15). Drinking water is also a notable route for MPs to enter the human body. Kosuth et al (16) conducted a study on 159 tap water samples from different regions worldwide and found that 81% of the samples contained plastic particles, with an average of 5.45 particles/l.
MPs accumulate in different organs after being consumed by animals. Research has found that MPs ingested by fish and mice appear in the gills, brain, gastrointestinal tract, liver and spleen, with the liver and gastrointestinal tract being the main sites of MPs accumulation (17-19). In addition, MPs are also found to accumulate in human organs (20,21). For instance, Horvatits et al (22) identified six different MPs (4-30 µm in size) in human liver tissue and quantitative analysis showed that the number of MPs was significantly increased in the livers of patients with cirrhosis. This indicates that the consumption of MPs may have a negative impact on liver function and may increase the risk of liver disease. The fact that MPs accumulate in animal and human livers and cause adverse effects has led to more research into the hepatic effects of MPs. These effects include induction of oxidative stress in the liver, disruption of the balance of liver lipid metabolism and exacerbation of the inflammatory response in the liver. It has been shown that MP exposure triggers redox imbalance in hepatocytes through two pathways: i) The induction of reactive oxygen species (ROS) overproduction while inhibiting the activity of antioxidant defense enzymes (23-25); and ii) the promotion of peroxisome proliferator-activated receptor (PPAR) γ-mediated dysregulation of lipid homeostasis (26,27). Concurrently, MPs are capable of enhancing hepatic TNF-α/ IL-6-driven inflammatory responses through activation of hepatocyte NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasomes (28-30). Hepatic oxidative stress, disorders of lipid metabolism and inflammatory responses are central mechanisms driving the development of liver diseases such as nonalcoholic fatty liver disease, alcoholic liver disease and cirrhosis (31). Since the 21st century, the global spread of MPs has been accompanied by a rising global incidence of liver disease. The World Health Organization estimates that by 2030 there will be 9.5 million new viral hepatitis infections, 2.1 million new cases of liver cancer and 2.8 million new deaths worldwide (32). According to the latest statistics for 2024, the global adult prevalence of metabolic dysfunction-associated fatty liver disease is approaching 30%, with a 5% annual increase in chronic liver disease in Latin America and Southeast Asia (33,34). The widespread global distribution of MPs and the rising global incidence of liver disease exhibit overlapping temporal and spatial trajectories (35). This suggests that exposure to MPs may be an important environmental factor contributing to the global epidemic of liver disease by inducing oxidative stress, disrupting lipid metabolism and triggering inflammatory cascade responses. Although researchers have made progress in understanding the liver impact of MPs, the exploration of their mechanisms of action is still insufficient. Furthermore, there are still limitations related to research methods such as the overreliance on a single experimental animal strain, insufficient exploration of long-term exposure effects and a lack of differentiation research on multiple material MPs. The aim of the present review was to provide an overview of the current published research and to present the effects of MPs on animal and human livers in order to encourage further research and exploration of the hepatic effects of MPs.
Entry of environmental MPs into the liver and hepatocytes
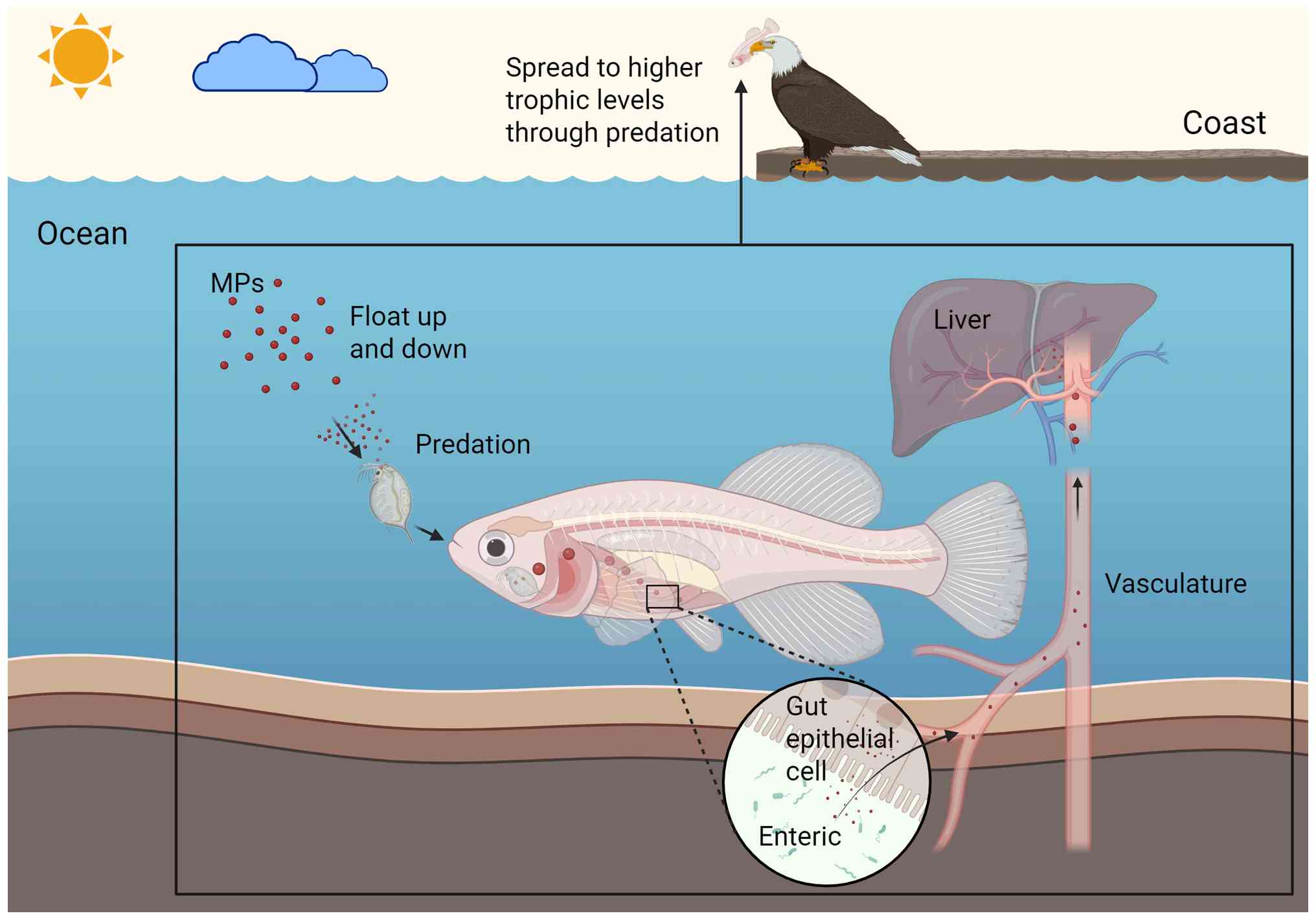
The maintenance of aquatic and terrestrial ecosystems is key for protecting animal habitats (36). In aquatic ecosystems, MPs are transported through biological activity. For instance, zooplankton and benthos acquire MPs suspended in the water column through predation and are considered to be primary consumers of MPs (37,38). MPs can then be passed along the food chain, from zooplankton and benthos to higher trophic levels. However, the majority of MPs are excreted by these organisms and return to the water column (39), where they are secondarily ingested by other aquatic organisms in the water column through gills and skin (40-43). The mechanisms by which MPs enter organisms in terrestrial ecosystems remain to be fully elucidated. However, the presence of MPs has been detected in the digestive tracts of invertebrates, insects and birds, suggesting that predation may be a key pathway for MPs to enter terrestrial animals (Fig. 1) (44-46).
After ingestion by animals, MPs are transported through the digestive tract to the intestine. The intestine serves as an innate protective barrier, with its selective permeability preventing the invasion of pathogens and toxins into the bloodstream (47-49). Existing studies present conflicting evidence on whether MPs can penetrate the intestinal tract and enter the circulation. A study by Vagner et al (50) discovered that NPs can penetrate the sea bass intestine and enter the bloodstream. However, Gao et al (51) observed minimal accumulation of NPs in the livers, spleens and kidneys of mice after intragastric administration. By contrast, the study noted that NPs were present in significant quantities in the livers and spleens of mice following intravenous injection. Consequently, it was deduced that MPs are unable to penetrate the mouse intestine (51). Despite the absence of definitive evidence regarding the mechanism of intestinal penetration, the accumulation of MPs has been widely documented in the livers of mammals, fish and birds (52-55). The accumulation of MPs in hepatic tissue is associated with the concentration and duration of exposure and oral exposure has been found to cause significantly higher levels of MP accumulation than other routes of exposure (56). Of note, the liver appears to take up MPs at a constant rate/unit time after oral exposure, following pseudo-primary kinetics (57).
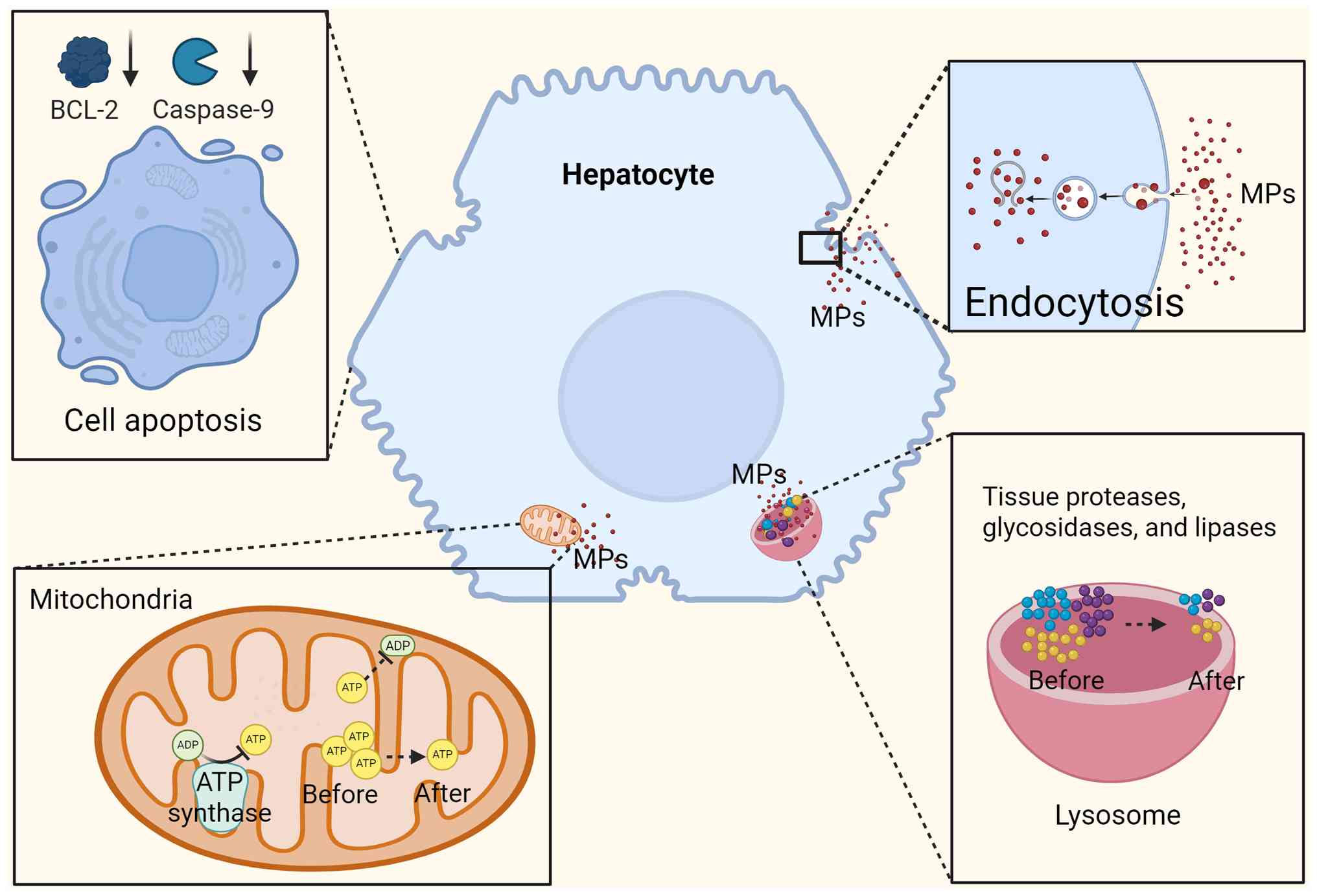
MPs enter hepatocytes following an enrichment process in the liver and the internalization mechanism of MPs shows particle size dependence. MPs with larger diameters are internalized by hepatocytes mainly through phagocytosis (58). By contrast, MPs with smaller diameters are internalized by hepatocytes through endocytosis of lattice proteins, which is mediated by motor proteins (59). Upon entering hepatocytes, MPs are initially degraded by lysosomes, which are responsible for degrading foreign substances. Thereafter, MPs are released into the cytoplasm, where they trigger mitochondrial damage. Mitochondria are the central regulatory units of fatty acid metabolism and oxidative homeostasis. The damaging effects of MPs on mitochondria directly disrupt the metabolic homeostasis of hepatocytes (60). Notably, Prietl et al (61) observed a substantial decrease in peripheral blood macrophage activity following the ingestion of MPs. Given the critical role of macrophages in hepatic immune defense, it is hypothesized that MP-induced inhibition of these cells could amplify hepatic immune injury. Furthermore, microorganisms in the environment have been shown to modify the surface chemical structure of MPs. This modification leads to a significant enhancement in the endocytosis efficiency of NPs and augments their cytotoxic effects (62,63).
In conclusion, MPs pass along the food chain in aquatic and terrestrial ecosystems and concentrate in the liver of organisms. These particles enter hepatocytes via endocytosis, subsequently inducing mitochondrial damage and metabolic disorders within the hepatocytes. The cytotoxicity of MPs can be potentiated through microbial-mediated chemical modification. These findings have prompted researchers to initiate studies on the influences of MPs on the liver.
Effects of MPs on the liver
The liver is a vital digestive gland, responsible for key functions such as metabolism and detoxification. Therefore, studying the hepatic effects of MPs is of great importance in order to obtain a full understanding of their effects on organisms. Several studies have shown that the accumulation of MPs in the livers of animals can not only lead to morphological changes in hepatocytes, but also induce oxidative stress, as well as inflammatory and immune responses (Fig. 2) (64-66). However, given the inherent interspecies variations in metabolic pathways and physiological barriers, the observed phenomena in animal models may not always accurately reflect those observed in human cells. Furthermore, data from in vitro cellular experiments and human in vivo experiments are insufficient to support existing conclusions, as they are highly dependent on animal models. Therefore, more research on the consequences of human exposure is necessary to support relevant public health decisions.
The duration of exposure is a key influencing factor of the hepatic effects of MPs, warranting exploration. Exposure of the liver to MPs for a period of time between 24 h and 7 days is termed acute exposure, while exposure lasting between 7 and 28 days is termed subacute exposure and exposure for >28 days is termed chronic exposure (67).
Acute effects in in vivo and ex vivo animal modeling Morphologic changes
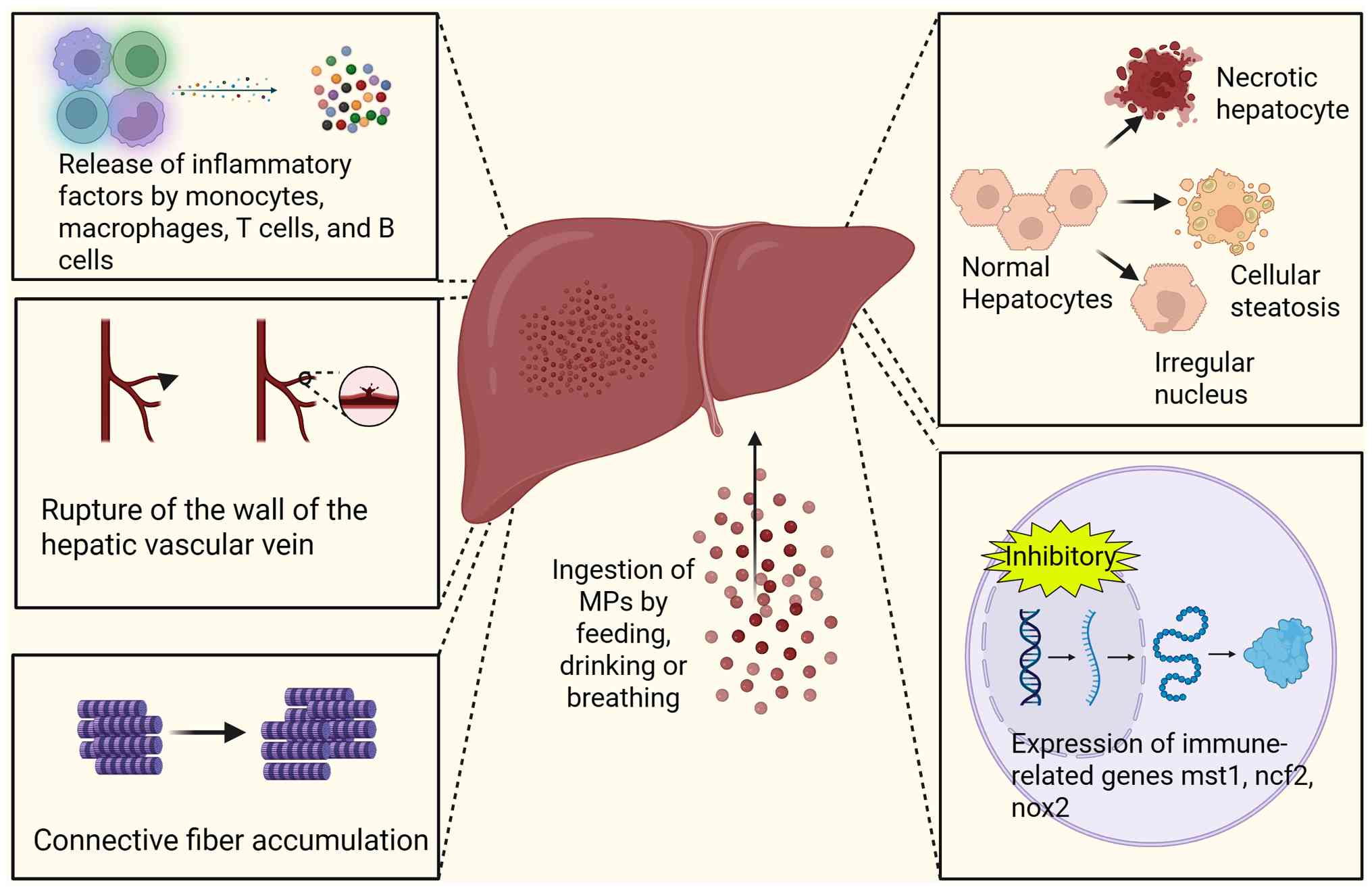
Liver morphological changes are a common pathological feature of acute and chronic liver injury, as well as numerous liver diseases. For instance, a typical pathological characteristic of acute hepatitis is hepatic congestion. This refers to a disruption in liver circulation and the development of local blood stasis (68,69). Toxicity and immune-induced liver injury can increase the water content of hepatocytes, resulting in a loose cytoplasm and cellular oedema (70). Hepatic steatosis is the pathological phenomenon of lipid droplet accumulation in hepatocytes during hepatic hypoxia, ischemia or infection (71). Research has found that, similar to the consequences of numerous liver injuries, exposure to MPs can also lead to pathological liver changes, including congestion, oedema, lipid droplet formation, steatosis, necrosis and fibrosis (72-75). Furthermore, it can also lead to the expansion and tearing of the wall of the central hepatic vein and accumulation of connective tissue fibers in the hepatic sinusoids (76).
In addition to altering liver tissue morphology, MPs also cause structural changes to hepatocytes. For instance, exposure of fish liver to MPs can cause hepatocyte swelling, irregular nuclear shape and cytoplasmic vacuolization (77). These liver lesions can significantly impair the normal function of hepatocytes. Mammalian liver exposure to MPs causes hepatocyte structural disorganization and necrosis (29,36). In summary, MP exposure compromises hepatic system homeostasis through dual impairment of functional dynamics and cytoarchitectural organization. The manifestation of morphological changes in liver tissue and cells suggests that MPs may have additional effects on the liver.
Oxidative stress
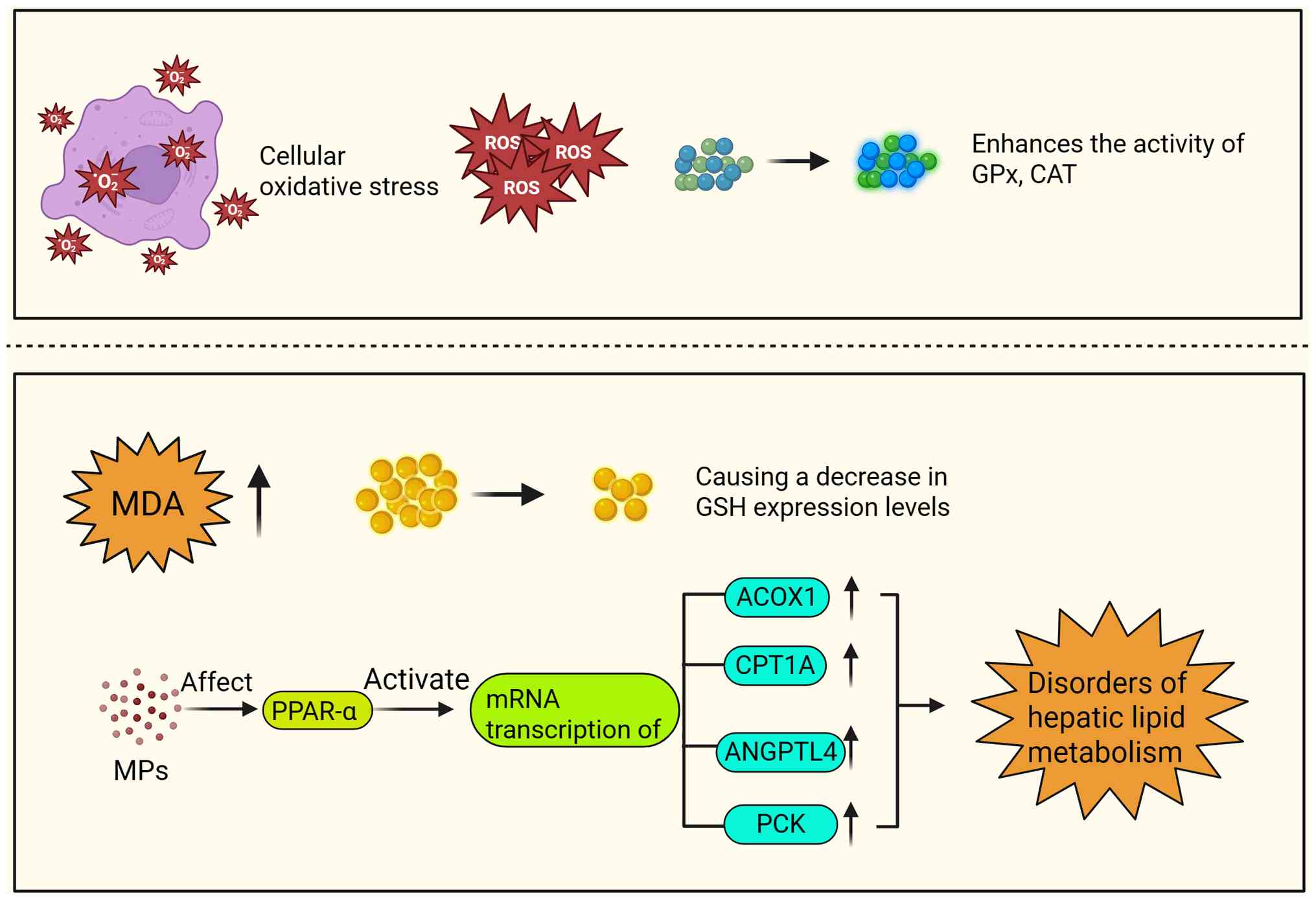
Oxidative stress is the generation of highly reactive molecules due to external stimuli, disrupting the balance between the oxidative and antioxidant systems (78,79), and constitutes a significant physiological and pathological basis in various liver diseases. In addition, it is the primary manifestation of acute liver injury (80). The key hepatic effect of MPs is triggering the oxidative stress response, and this has been demonstrated consistently in recent studies. Research has shown that short-term exposure to MPs can induce oxidative stress reactions in the liver of freshwater fish, as shown by an increase in the activities of glutathione peroxidase (GPx), catalase (CAT) and malondialdehyde (MDA) levels (81,82). GSH and CAT are important components of the hepatic antioxidant defense matrix and MDA is a key biomarker reflecting the completion of the lipid peroxidation cascade in the mammalian hepatic system (83,84). The aforementioned hepatic effects of MPs indicate that they induce oxidative stress. Of note, several studies have investigated the regulatory effect of MPs on the levels and activity of CAT. Samal et al (85) conducted an experiment with cows and discovered that MPs can bind to bovine liver CAT and alter its conformation, leading to a reduction in its activity. Banaei et al (82) found that the expression of metallothionein 1 (Mt1), carboxylesterase 2 (Ces2) and cytochrome P450 (P450) genes increased in carp hepatocytes when exposed to polyethylene (PE) MPs, while the expression of the Mt2 gene showed a significant decrease. Alterations in the expression of oxidative stress-related detoxification genes in hepatocytes of fish provide additional evidence that MPs can induce oxidative stress responses in animal livers (Fig. 3).
Inflammatory response
The inflammatory response is a pathological process in which the body defends itself against various external injuries and stimuli (86). This process is characterized by pathological changes, including local tissue proliferation, exudation and deterioration (87). Upon inflammatory stimulation of the liver, the monocyte/macrophage system is activated, resulting in the release of a large number of inflammatory molecules and cytokines (including IFN-γ, TNF-α, IL-6 and IL-1β) (88,89). Thus, increased expression of these cytokines in the liver can be used as an indicator of hepatic inflammation. The pro-inflammatory properties of MPs in the liver have been widely investigated. A growing number of studies have shown elevated expression levels of inflammatory molecules and pro-inflammatory cytokines, including TNF-α, inducible nitric oxide synthase, cyclooxygenase 2, IL-1β and IL-6, in MP-exposed livers, indicating the presence of inflammation in the liver (26,64,65).
Immune function
The liver is the most robust reticuloendothelial cell phagocytic system observed in mammals and has a powerful immune function. Macrophages are a crucial element of the hepatic phagocytic system, as well as an essential conduit for the innate immunological capabilities of the liver (90-92). MPs have been shown to damage macrophages, which in turn affect liver immune function (90). The mechanisms underlying the effect of MPs on hepatic immune cells have not yet been elucidated. However, researchers have conducted in-depth research on this issue.
Research has found that MPs have an impact on hepatic immune function, particularly innate immune function. For instance, rainbow trout exhibited impaired innate immune function following exposure to PE-MPs, evidenced by an increase in hepatic mucus cells and a decrease in white blood cells (66). It has been established that intravenous injection of polystyrene (PS)-NPs mediates transcriptional repression of key innate immunity effectors in minnow liver, including macrophage stimulation 1, neutrophil cytoplasmic factor 2, NADPH oxidase 2 and complement component 3 (93,94). Liu et al (64) demonstrated that 10 and 20% polyvinyl chloride-MPs (PVC-MPs; a common material of MPs) induced downregulation of IFNγ and TNFα genes, which regulate the function of immune cells, and upregulation of IL-1β and IL-6 genes, which promote innate immunity. In addition, they observed an increase in the activity of the non-specific immune factor, lysozyme in the liver of the common carp, indicating that PVC-MPs also affected hepatic immune regulation (64). Furthermore, PS-NPs have been demonstrated to specifically induce the biosynthesis pathway of steroid hormones, resulting in the production of related metabolites. Steroid hormones are potent endocrine regulatory hormones, impacting the activity of immune cells. Therefore, MPs may affect liver immune cell activity by promoting the synthesis of steroid hormones (95). In conclusion, research has identified that MPs of various materials can influence the immune function of the liver, with a particular impact on innate immune function. However, at present, the majority of studies regarding the effects of MPs on liver immune function have concentrated on freshwater fish and there are few studies on the effects of MPs on mammalian liver immune function, highlighting the need for further research in this area.
Lipid metabolism
By regulating gene expression and signal transduction, lipids can affect various life processes in organisms, including immune processes, as well as provide energy for survival (96). The liver plays a pivotal role in maintaining lipid homeostasis, is the primary site for cholesterol synthesis and transport, and is the most important organ for lipid metabolism (96). Research has found that exposure to NPs increases the mRNA transcription of several genes involved in the breakdown and oxidation of fatty acids in the liver of eels. These include acyl CoA oxidase 1, carnitine palmitoyltransferase 1a (CPT1A), angiopoietin-like 4 and phosphoenolpyruvate carboxyl kinase (PCK). This indicates that lipid metabolism disorders may be occurring in the liver (97,98).
One study suggested that the adverse effects of MPs disrupting gut microbiota can potentially be transmitted to the liver via the gut-liver axis, which refers to the material flow and functional effects between the two organs; this could be a contributing factor in liver lipid metabolism disorder associated with MPs (99). Further research has revealed that modulating PPAR represents a pivotal mechanism by which MPs induce hepatic lipid metabolic disorders. PPARs are ligand-activated receptors of the nuclear hormone receptor family, which have three subtypes, including α, β and γ. Among them, PPARα is abundantly present in the liver and plays an important regulatory role in liver lipid metabolism processes (100). To date, multiple studies have demonstrated that liver exposure to MPs (particularly PS particles and PVC particles) can significantly activate PPARα and its multiple downstream target genes, thus affecting the expression of various proteins related to hepatocyte lipid metabolism (101,102). By acting on PPAR and altering the expression of hepatocyte lipid metabolism-related proteins, MPs can ultimately trigger the disruption of hepatocyte lipid metabolism (Fig. 3).
In conclusion, MPs are able to disrupt lipid metabolism in the liver through specific mechanisms. Of note, mice administered with MPs showed a dose-dependent increase in the levels of total free fatty acids (FFAs), which are mainly derived from the hydrolysis of neutral fats (a type of lipid prevalent in mammals) and are a key metabolic currency of the lipid cycle in hepatocytes. Of note, the composition of FFAs remained stable, with the mono- and polyunsaturated fatty acid subpopulations balanced across exposure groups (103). By contrast, there is a notable increase in saturated fatty acids (SFAs). It has been postulated that high levels of SFA may be a contributing factor in the disruption of endoplasmic reticulum homeostasis in hepatocytes (30). It can be observed that the disturbance of hepatic lipid metabolism resulting from exposure to MPs may facilitate the generation of additional liver-related effects by MPs.
Acute response in a human in vitro model
It has been demonstrated that PS-MPs can induce an acute toxic response in human hepatocytes in vitro through multiple pathways. For instance, acute and subacute exposure of MPs in vitro activates the mitochondria-dependent apoptotic pathway in HepG2 cells, resulting in the elevation of caspase-9 activity and a significant increase in the ratio of the pro-apoptotic protein Bax to the anti-apoptotic protein Bcl-2, suggesting that apoptotic processes are induced (104). Of note, PS-NP exposure was found to significantly inhibit the expression of tumor-related genes such as polo-like kinase 1 (PLK1) and mitotic checkpoint serine/threonine kinase (BUB) 1 (105). The PLK1 and BUB1 enzymes are involved in the regulation of human hepatocyte apoptosis as well as liver tumorigenesis and progression. These findings suggest that exposure to MPs may affect liver tumor formation and development, while inducing apoptosis in human hepatocytes (Fig. 4).
Acute and subacute exposure to MPs in vitro can also damage hepatocyte organelles. For instance, JC-1 fluorescent probe assay results showed that the intracellular mitochondrial membrane potential (MMP) was decreased when hepatocytes were exposed to 25 µg/ml PS-MPs (105). Reduced MMP impairs the ability of mitochondria to produce ATP, leading to impaired mitochondrial energy production (106). It was also found that mitochondrial ATP levels in hepatocellular carcinoma cells were further reduced with increasing concentrations of PS-NP exposure (101). In conclusion, MPs can affect the energy supply of hepatocyte mitochondria and antioxidants can attenuate this effect. MPs also affect lysosomes, another important organelle within hepatocytes, that degrade cellular metabolites and waste products. Hepatocyte stability and function depend on the efficient operation of lysosomes (107). It has been shown that MPs can interact with hydrolases and other enzymes in lysosomes, and act to reduce lysosomal viability and further inhibit lysosomal phagocytosis (108). A noteworthy study found a correlation between exposure to MPs and enhanced lysosomal perinuclear localization, which was consistent with the effect of inhibiting mammalian target of rapamycin 1 (mTORC1) enzyme activity (109). mTORC1 responds to metabolic regulatory signals both inside and outside of the cell and is primarily recruited to the surface of the lysosome where it functions (110). Given the role of the mTORC1 pathway in regulating hepatic inflammation and interfering with hepatic lipid metabolism within hepatocytes, the effects of MPs on lysosomal function may result in increased inflammatory responses and disruption of lipid metabolism, reminiscent of inhibition of the mTORC1 pathway.
Although animal model studies have systematically revealed the hepatotoxicity of MPs in vivo and in vitro, human toxicological data are still highly dependent on in vitro cellular models. Although in vitro experiments can elucidate the molecular mechanisms, they cannot mimic the complex metabolic clearance, tissue barrier and systemic compensatory mechanisms that occur in the human body. Variability in key regulatory pathways (such as nuclear receptor signaling) between species may influence the severity of the toxic response (101,109). These research gaps make it challenging to translate current findings for public health decision-making. On the one hand, quantitative associations between in vitro markers of toxicity (such as mitochondrial damage and elevated inflammatory factors) and actual health damage in humans are still not established. On the other hand, population exposure heterogeneity (e.g., differences in age and metabolic status) may significantly alter toxicity risk thresholds.
Chronic exposure
Unlike acute/subacute exposures, chronic exposure to MPs can cause multidimensional toxic effects on the liver, which dynamically evolve with differences in exposure duration. At exposure durations of 40-60 days, the effects of MPs on the liver were more consistent with acute/subacute exposures. At this exposure duration, MPs were able to significantly increase hepatic cholesterol and triglyceride levels and promote hepatic steatosis by upregulating the expression of fatty acid uptake genes [such as CD36 and fatty acid-binding protein 1 (FABP1)] and synthesis genes [such as acetyl-coenzyme A carboxylase α and peroxisome proliferator-activated receptor γ (PPARγ)] (111). In addition, MP exposure at this duration induced cellular release of pro-inflammatory factors (such as TNF and IL-6), as well as hepatic stellate cell activation, exacerbating liver inflammation and fibrosis (112). When the duration of exposure was 60-90 days, the primary effect of MPs on the liver included the induction of fibrosis and metabolic disorders. At this exposure, MPs promoted hepatic stellate cell activation and collagen deposition through mitochondrial DNA leakage, activating the cyclic GMP-AMP synthase/stimulator of interferon genes (cGAS/ STING) pathway and driving NF-κB nuclear translocation, ultimately exacerbating liver fibrosis (113). After 90 days of exposure, hepatocyte AMP-activated protein kinase (AMPK) phosphorylation was inhibited and gluconeogenic gene (such as glucose-6-phosphatase catalytic subunit and phosphoenolpyruvate carboxykinase) expression was enhanced. Meanwhile, the expression of the hepatocyte lipid synthesis gene FABP4 was upregulated, demonstrating that 90 days of MP exposure caused a dual disorder of hepatic glucose-lipid metabolism (114).
The hepatic mechanism of action of MPs becomes more complex under ultra-long-term exposure (120-180 days). At an exposure duration of 180 days, MPs activated cellular autophagosome formation via ERK/mTOR signaling, while inhibiting lysosomal function, leading to the disruption of cellular autophagy and lipid droplet accumulation (115). Xia et al (78) conducted a study in which they exposed carp larvae to PVC for 30 and 60 days on a chronic diet. They observed that after 30 days, with an increase in the PVC concentration, the activity of GPx changed from an increasing trend to a decreasing trend. After 30 days of exposure to high concentrations of MPs, the transcription of antioxidant-related genes cytochrome P450 1A and glutathione s-transferase (GST) α in carp liver also changed from increasing to decreasing (78). Therefore, providing animals with a purification period after long-term exposure to MPs may alleviate hepatic oxidative stress. Furthermore, Solomando et al (86) demonstrated that following 90 days of exposure to low-density PE-MPs, a 30-day purification period in herring was sufficient to facilitate the gradual return of elevated oxidative stress biomarkers (including MDA and GSH) to normal levels. This suggests that chronic exposure to MPs can reverse the stress state of fish livers during acute/subacute MP exposure. Furthermore, it indicates that a recovery period after prolonged exposure can have a restorative effect on the oxidative stress caused by MPs.
Interactions between MP exposure and liver disease
The deleterious effects of acute and chronic exposure to MPs have been demonstrated to be a contributing factor to the onset and progression of liver diseases, particularly those associated with liver metabolic dysfunction. Due to the widespread contamination of ecosystems with MPs, the interaction between MPs and the onset and progression of liver disease may be one of the key drivers of the rising global incidence of liver disease.
Metabolic dysfunction-associated fatty liver disease (MAFLD)
MPs have the capacity to trigger the onset and progression of MAFLD by exacerbating lipid accumulation, promoting inflammatory responses and inducing oxidative stress. One study revealed that MPs induce activation of the PPARγ pathway, leading to the upregulation of the expression of key lipid synthesizing genes, including sterol regulatory element-binding protein 1c and fatty acid synthase (FASN). In addition, MPs inhibit the activity of enzymes, such as carnitine palmitoyltransferase 1A, involved in fatty acid β-oxidation (116). These observations suggest that the accumulation of lipids within hepatocytes is augmented by the actions of MPs. MPs also indirectly exacerbate hepatic lipid deposition by inducing intestinal flora dysbiosis (117), and stimulate the release of pro-inflammatory factors such as TNF-α and IL-6 from hepatocytes through the engagement of the NF-κB signaling pathway, thus promoting hepatic inflammatory responses (118). Furthermore, acute exposure to MPs induced a significant increase in hepatocyte ROS levels, which markedly promoted hepatic lipid peroxidation and aggravated hepatic steatosis (119).
Liver fibrosis
The interaction between MPs and liver fibrosis is a noteworthy recent finding. MPs were shown to promote hepatic fibrosis in both direct and indirect ways. Firstly, MPs activate hepatic stellate cells through the TGF-β/Smad and Wnt/β-catenin pathways and upregulate fibrosis markers such as α-smooth muscle actin and collagen 1a1 (120). In addition, it was found that MPs induce mitochondrial DNA leakage, and the leaked mitochondrial DNA activated the cGAS/STING pathway, which increased fibronectin deposition in hepatocytes and indirectly contributed to the process of liver fibrosis (121).
The interaction between MPs and liver disease further illustrates the evolution of MP pollution from an environmental concern to a global health threat. Therefore, it is imperative that further research is conducted to establish whether MPs have a potential hepatotoxic effect in order to address the pressing threats to liver health in this era of plastic pollution.
Biomarkers for human exposure to MPs
A significant body of evidence indicates that exposure to MPs may be hazardous to human health. Current methodological limitations in monitoring the bioaccumulation of human MPs, coupled with the lack of translatable in vivo exposure models, fundamentally restrict mechanistic characterization of the pathogenic cascade and prevent quantitative characterization of the dose-response relationship, which is essential for evidence-based risk assessment. The establishment of specific biomarkers for human exposure to MPs is necessary to address the real-world barriers to human toxicity studies of MPs. With specific biomarkers, the level of exposure to MPs in the livers of an individual or group can be precisely quantified, providing an objective basis for risk assessment. Without reliable biomarkers, it is challenging to establish a causal relationship between MP liver exposure dose and disease. In conclusion, the establishment of biomarkers for the exposure of humans to MPs will be key in revealing health risks and guiding public health interventions.
According to the results of animal experiments, the biomarkers of MPs are mainly associated with key mechanisms such as oxidative stress, as well as lysosomal and immune damage. Oxidative stress is the key mechanism by which MPs elicit adverse liver effects, with superoxide dismutase (SOD), CAT, GPx and glutathione S-transferase (GST) being the antioxidant enzymes most affected by MP exposure. Alterations in the activity of these enzymes can directly reflect the capacity of the liver to scavenge ROS, and they are significant biomarkers of MP-induced hepatic oxidative stress (122). The lipid peroxidation product MDA is a key indicator for assessing the extent of oxidative damage to hepatocyte membranes caused by MPs and can be quantified by the thiobarbituric acid reaction (123). The labeling of lysosomal and immune damage is centered on lysosomal membrane stability, whilst blood cell parameters such as total hematocrit and alterations in the granulocyte ratio serve as biomarkers of the hepatic immunosuppressive effects of MPs (124). Together, these markers reconfirm the role of MPs in interfering with hepatic oxidative homeostasis, disrupting hepatic organelle function and inhibiting hepatic detoxification capacity.
Existing studies have shown that specific MP polymers (such as polyurethane and polyester) detected in human sputum can be used as potential exposure markers and their concentrations are significantly correlated with smoking (125). However, human exposure testing samples are mainly sputum or feces, limiting the establishment of direct biomarkers. In addition, MPs often coexist with contaminants, such as plasticizers. This can lead to compound exposure interferences, making it difficult to distinguish between single effects (126). Future population cohort studies incorporating nanoscale detection technologies are required to validate the accuracy of these biomarkers. In conclusion, the establishment of biomarkers for human MPs requires expanding the range of human samples, breaking through technical bottlenecks and enhancing the integration of data from multiple exposure pathways.
Mechanism of effects of MPs on liver
Mitogen-activated protein kinase (MAPK) pathway
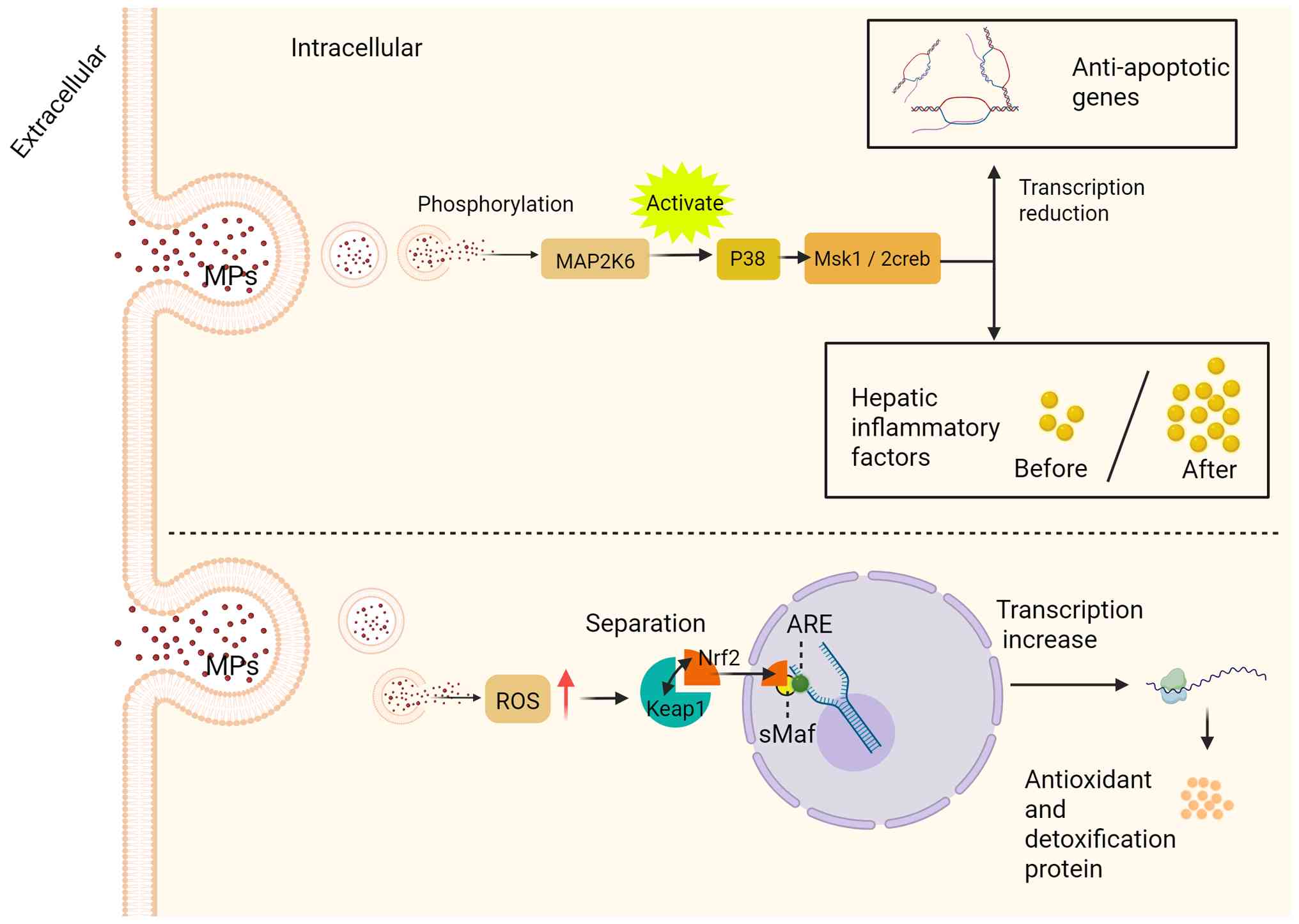
MAPK can be activated by different extracellular signaling molecules and plays an important role in transmitting cell surface signals into the nucleus. It can regulate various pathological and physiological processes, such as cell growth, differentiation, the stress response and inflammatory response (127), and the MAPK signaling pathway is implicated in the pathogenesis of hepatocellular carcinoma, fibrosis and hepatic injury (128-130). The main branching pathways of the MAPK pathway include JNK, ERK, ERK5 and p38 MAPK (121). Research has revealed that high concentrations of NPs can activate MAPK kinase 6 (MAP2K6) (131). Phosphorylation of MAP2K6 can lead to p38 MAPK signaling in response to inflammatory cytokine stimulation or environmental stress. In addition, NPs activate MAP2K6 to initiate the phosphorylation cascade MAP2K6-p38 MAPK-mitogen- and stress-activated protein kinase 1/2-cAMP response element-binding protein (a cascade reaction of the p38/MAPK signaling pathway in the cell). This cascade affects the transcription of anti-apoptotic genes in hepatocytes and triggers liver inflammation. In addition to activating the p38 MAPK branch pathway in the MAPK signaling pathway, NP treatment can also induce activation of the ERK branch pathway by promoting excessive ROS in hepatocytes and ultimately promoting lipid accumulation in hepatocytes (Fig. 5) (132,133). In summary, MPs can induce hepatocyte apoptosis, disrupt hepatocyte lipid homeostasis and lead to liver inflammation by activating the MAPK signaling pathway, initiating phosphorylation and multiple cascade reactions.
Nuclear factor erythroid 2-related factor 2 (Nrf2)-Kelch-like ECH-associated protein 1 (Keap1)-antioxidant response element (ARE) signaling pathway
The Nrf2-Keap1-ARE signaling pathway is a central defense mechanism against oxidative stress and exerts protective effects in the liver. This system comprises three core components: The transcription factor Nrf2, the sensor protein Keap1 and the ARE. By coordinately regulating downstream genes, this pathway controls the expression of key enzymes that counteract oxidative and toxic damage. Specifically, it enhances antioxidant capacity through rate-limiting enzymes in GSH synthesis (glutamate-cysteine ligase catalytic subunit/glutamate-cysteine ligase modifier subunit) and SOD, while promoting detoxification by modulating NAD(P)H quinone oxidoreductase 1 and GST. The pathway mediates anti-inflammatory repair via expression of heme oxygenase-1 and thioredoxin, and maintains proteostasis through autophagy-associated proteins p62 and heat shock protein 70. These coordinated responses preserve redox homeostasis, playing protective roles in neurodegenerative diseases and liver fibrosis (134-136). Notably, Keap1 mutations or Nrf2 hyperactivation in cancer contribute to chemotherapy resistance via drug efflux pumps such as multidrug resistance-associated protein and metabolic reprogramming. While moderate pathway activation alleviates chronic tissue injury, prolonged overactivation may exacerbate pathologies (134). These dynamic regulatory properties highlight its therapeutic potential in oxidative stress-related diseases. In the resting state, Keap1 binds to Nrf2 in the cytoplasm. Upon oxidant stimulation, Nrf2 first dissociates from Keap1 and relocates to the nucleus, where it then links to nuclear AREs to initiate the transcription of downstream antioxidant enzymes and detoxification proteins to protect against oxidative damage (135,136). It has been demonstrated that exposure to MPs causes overproduction of ROS within hepatocytes, which in turn activates the Nrf2-Keap1-ARE pathway, contributes to the dissociation of Nrf2 from the Keap1 complex and promotes Nrf2 phosphorylation. Phosphorylated Nrf2 subsequently forms a dimer with small maf proteins, binds ARE and upregulates antioxidant gene expression (137-139). Although this pathway initially mitigates oxidative stress, sustained or high-dose exposure to MPs may exceed its compensatory capacity, ultimately leading to lipid peroxidation and liver injury (Fig. 5). Thus, the Nrf2-Keap1-ARE pathway not only serves as a detoxification mechanism for hepatocytes to withstand oxidative damage from MPs, but also reveals a critical threshold for its protective effect.
Other mechanisms
In addition to the aforementioned mechanisms, there is a small body of literature that suggests other mechanisms by which MPs exert their effects on the liver. First, it has been hypothesized that MP-induced liver injury may be related to cell death. MPs were found to induce hepatocyte pyroptosis by inducing ROS accumulation and activating NLRP3 inflammatory vesicles. Hepatocyte pyrolysis promotes the release of IL-1β and IL-18, thereby exacerbating cellular inflammation. MPs were also able to reduce the expression of the iron storage protein ferritin heavy chain 1, while upregulating the expression of the iron uptake receptor transferrin receptor protein 1, which in turn triggered cellular iron death (29). Second, it has been demonstrated that MPs can increase the translation of fatty acid synthesis genes (such as FASN) through activation of the protein kinase r-like endoplasmic reticulum kinase-activating transcription factor 4 pathway, leading to the accumulation of hepatic lipid droplets (140). Of note, Fan et al (141) suggested that exposure to MPs can trigger the AMPK/unc-51 like autophagy activating kinase 1 pathway in hepatocytes, driving hepatocytes to produce autophagosomes to encapsulate intracellular lipid droplets. In addition, MP exposure damaged hepatocyte lysosomes. This dual action results in improper degradation of intracellular lipid droplets (141) and has prompted researchers to focus on the impact of multiple pathway interactions on the hepatic effects of MPs.
Factors affecting the impact of MPs on liver function
Size and concentration of MPs
The impact of MPs on liver function has been proven to be contingent upon their size and concentration (142-144). In 2021, Zhang et al (75) found that fish exposed to 200 µm PS-MPs resulted in alterations to hepatic adipocyte size and an increase in hepatic lipid content, which subsequently affected the function of hepatic lipid metabolism. However, exposure to 2 and 10 µM PS-MPs primarily caused liver inflammation and excessive deposition of extracellular collagen (liver fibrosis) in the liver. Furthermore, Li et al (104) discovered that PS-MPs with larger diameters could downregulate the translation of mitochondrial division-related genes (such as mitochondrial fission factor and dynamin-related protein 1). In addition, medium to high concentrations of PS-MPs inhibited mitochondrial outer membrane fusion, thereby affecting normal mitochondrial proliferation and material exchange processes. However, the environmental concentration of PS-MPs has the opposite effect (104,145). This suggests that the mitochondrial function of MPs may be reversed with alterations in their exposure concentration. In summary, various hepatic effects induced by MPs have been confirmed to be related to the size and concentration of MPs. Of note, the effects produced by MPs on livers also appears to change with different materials and chemical structures (146-149) (Table I).
Table IA brief summary analysis of typical research literature on the hepatotoxicity of MPs of different materials. |
Environmental pollutants
While the liver is exposed to MPs, it is also exposed to additives and contaminants carried on the surface of MPs. Several studies have shown that simultaneous exposure to plastic additives and environmental pollutants can exacerbate the hepatic effects of MPs (150-153). For instance, the co-exposure of MPs and the plasticizer di(2-ethylhexyl) phthalate exacerbated histopathological damage to the liver (154). Simultaneous exposure of the liver to MPs and common industrial pollutants, such as polybrominated diphenyl ethers (PBDEs), has been observed to induce liver atrophy and oxidative system dysfunction in zebrafish larvae (155). Concomitant exposure to perfluorooctanoic acid and MPs can disrupt immunoregulation within hepatocytes (156), suggesting that hepatocytes are undergoing immune and inflammatory responses. Of note, Mohsen et al (157) detected 20 heavy metals on the surface of six different types of plastics from various aquaculture areas in the Yellow and Bohai Seas of China. This hypothesis suggests that the human body may accumulate heavy metals attached to the surface of MPs through the ingestion of farmed fish. Of note, the influence of heavy metals on the hepatic effects of MPs is complex. Taking the metal cadmium as an example, studies have shown that exposure to MPs increases the accumulation of low-concentration cadmium in fish and enhances their liver toxicity, while reducing the accumulation of high-concentration cadmium and alleviating its toxicity (158). These and several other studies suggest that the co-exposure of MPs and heavy metals may have a more complex effect on the liver than previously considered (159-163).
Mechanisms by which MPs interact with organic pollutants and heavy metals to exacerbate liver effects include carrier effects, oxidative stress synergy and inflammatory activation. It was found that after the oxidation of MPs, the pores on the surface were enlarged and could adsorb a variety of pollutants, including cadmium and PBDEs (160). These contaminants can attach to MPs and enter the organism through ingestion or gills, thus significantly exacerbating the hepatotoxicity of MPs, and is termed the carrier effect. For instance, binding of NPs to tetrabromodiphenyl ether solutions increased their hepatic accumulation and exacerbated liver atrophy (155,160). In addition, combined exposure of MPs and pollutants can further amplify the role of MPs in inducing excessive ROS generation in the liver and inhibiting antioxidant enzyme activities (such as GSH, CAT and SOD), and ultimately exacerbates hepatic lipid peroxidation and DNA damage (154). For instance, exposure to NPs combined with arsenic markedly increased ROS levels and exacerbated lipid peroxidation in the liver compared with exposure to NPs alone (162). Combined exposure to MPs and pollutants also activates pathways such as NF-κB, upregulates TNF-α, IL-6 and other inflammatory factors, and triggers inflammatory infiltration in liver tissue (156,157). Of note, MPs are also capable of impairing liver detoxification of heavy metals to amplify their toxicity to the liver. For instance, MPs, when co-exposed with cadmium, inhibit the chelation of cadmium by metallothionein, a detoxifying protein in the liver, and promote heavy metal-induced hepatic steatosis (160). In conclusion, MPs can act as pollutant carriers and toxicity amplifiers, and their long-term compound exposure risk requires further exploration. Studies into the interaction between environmental pollutants and MPs have provided a new perspective on the hepatic effects of MPs.
Treatments of liver damage caused by MPs
MPs can act alone or in combination with other substances to cause liver damage in animals and humans. Therefore, research has been carried out to investigate therapeutic interventions that can be used to alleviate this damage. Of note, researchers studying fish have found that adding dietary antioxidants such as lycopene, microalgae and citric acid to aquaculture can partially or completely reverse the pathological changes in catfish liver tissue induced by MPs (76). The study also found that after astaxanthin (ASX) deprivation, the activity of SOD, a key antioxidant in fish liver, increased significantly with the increase in MP concentration, indicating that the fish liver was in a state of oxidative stress. However, after ASX supplementation, the level of SOD in the fish liver did not change significantly, and the level of the antioxidant GSH in the liver decreased significantly. This is an indication that ASX can improve the antioxidant defense status of fish exposed to MPs (164).
Studies have also found that melatonin can reduce the dysfunction of rat hepatocytes caused by environmental MPs (165). In addition, silymarin has been demonstrated to reduce the levels of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol and triglycerides in adult male albino rats exposed to PS-MPs (166). AST and ALT are established indicators for evaluating liver injury. Serum levels of these enzymes increase when the liver is injured and decrease when the injury is alleviated, and total cholesterol and triglycerides are important parameters to assess levels of blood lipids. The effects of silymarin administration on the levels of AST, ALT, total cholesterol and triglycerides demonstrated that silymarin could reduce the deleterious effects of MPs on the liver. A study has also demonstrated that silymarin administration can reduce the levels of MDA, a lipid peroxidation end product, in the serum of rats with MP-induced liver damage. This evidence suggests that silymarin may also have an anti-oxidative stress effect on MP-induced liver damage. In addition, the administration of tamarixetin had been found to reduce the impact of oxidative stress on the liver by regulating the Nrf2-Keap1-ARE pathway (167). This enables the basic recovery of MP-induced liver damage and histopathological changes. Furthermore, research has identified that lactobacillus rhamnosus and lactic acid bacteria may protect the liver by promoting intestinal homeostasis, improving intestinal barrier integrity and alleviating liver inflammation (168,169).
One study attempted to compare the existing strategies for the treatment of liver injury by MPs. It was found firstly that the mechanism of action and species specificity of ASX requires further verification. In addition, melatonin is a promising therapeutic strategy for MP-induced liver injury. Its mechanism of action may involve hepatic circadian rhythm regulation with a low risk of adverse effects. However, existing studies on melatonin treatment of MP-induced liver injury are largely based on cellular models, and the long-term efficacy in vivo has not yet been elucidated. Furthermore, silymarin combines both antioxidant and lipid-lowering effects, and it excels in multi-targeted interventions but is deficient in the modulation of inflammatory pathways. However, probiotics are slow acting and would perhaps be more suitable for chronic exposures. Finally, tamsulosin has a rapid onset of action, but the potential risk of Nrf2 overactivation must considered. In conclusion, silymarin and tamoxifen are more effective in MP-induced acute liver injury, whereas dietary antioxidants and probiotics are more suitable for long-term prevention. In the future, therapeutic strategies that combine multiple pathways require further exploration.
Cutting-edge perspectives
The effect of MPs on liver function is dependent upon the polymer type, thus the first step in assessing MP-driven hepatic function damage is to identify the polymer type. The MP polymer type can be investigated through spectroscopy or mass spectrometry, and Fourier transform-infrared spectroscopy (FT-IR) is the most commonly utilized technology in this field. FT-IR assigns a specific absorption value to a specific group by comparing it with the infrared reference spectrum library, thereby enabling the determination of the type of plastic. The instrument is capable of distinguishing particles on the basis of their chemical composition, as well as evaluating the aging of particles by observing the surface oxidation (170). However, this technique has low sensitivity, which precludes its ability to detect low concentrations of NPs (171). Similar to FT-IR, Raman spectroscopy can detect different vibrational modes in molecules and measure the change in scattering light frequency, and can be used to classify the components of MPs (172). Notably, the Raman spectrum offers superior size resolution in comparison with the FT-IR spectrum (173). However, the Raman spectrum is susceptible to interference from the fluorescence background emitted by the filter paper (172). Furthermore, novel detection methodologies, including dark-field hyperspectral microscopy, near-infrared aggregation-induced emission fluorophore labeling and surface-enhanced Raman scattering labeling have been employed to monitor the transport and accumulation of MPs in organisms (174,175). The advent of these novel detection technologies has enabled the quantification and visualization of the dynamic process of MP uptake and purification by organisms. In conclusion, although various detection technologies possess inherent advantages and disadvantages, they are indispensable tools for accurately identifying the different types of MPs and exploring their effects on liver function.
In addition to their effects on the liver, the effects of MPs on other organs and functions are also worthy of attention. For instance, research has shown that exposure to PS-MPs can inhibit the insulin signaling pathway in the liver of mice, thereby inducing insulin resistance in mice (176-179). Exposure to MPs can also disrupt the equilibrium of self-renewal and differentiation in the colonic epithelium, accelerating the development of colitis (180). Smaller NPs cause more severe damage to the intestinal mucosal layer than larger NPs, while larger NPs have a greater impact on the composition of the microbiota (181). Exposure to PE-MPs can induce gonadal pathological changes, while exposure to PS-MPs can increase the levels of sex hormones such as follicle-stimulating hormone, estradiol and testosterone in mice, leading to endocrine disorders (182-184). Of note, one study found that daily exposure to 600 µg of MPs significantly altered the typical structure of erythrocytes in animals (185). Furthermore, exposure to high levels of MPs also significantly reduced hematological parameters in fish, particularly the red blood cell count (RBC), haemoglobin (Hb) levels and erythrocyte pressure (Ht) (35,186). RBC, Hb and Ht levels are related to the number of RBCs in the circulation and the normal functioning of RBCs in transporting oxygen. The reduction of these parameters provides further evidence that exposure to MPs has detrimental effects on organisms, such as infection or inflammation (187). By systematically analyzing the biodistribution characteristics of MPs in different organ systems and their mechanisms of disruption of blood homeostasis, researchers can establish a molecular framework for the association of extrahepatic toxic effects with liver injury. This may provide important theoretical support for a more complete assessment regarding the systemic pathological consequences of MPs.
Conclusion
At present, there are still a number of limitations in the study of the hepatic effects of MPs. Firstly, it should be noted that the majority of MP research utilizes PS as the particle material. Although previous studies on PS have addressed the common hepatic effects of MPs, certain studies have indicated that the hepatic effects of MPs with different materials may vary, and the toxicological mechanisms may differ (24,82,66,187). Consequently, it is essential to expand the scope of material available for studying the hepatic effects of MPs. Secondly, the current basis of research on the effects of MPs on the liver is often acute or subacute exposure, which is inconsistent with the reality of the long-term exposure and intake of MPs that organisms undergo in the environment. In the majority of the research literature, the experimental animals employed are marine or freshwater fish. Although certain fish (such as zebrafish) are considered to be useful for studying the hepatic effects of MPs, it is important to consider the pathological differences between mammals and aquatic animals, human cells and aquatic animal cells. Furthermore, it should be noted that NPs exhibit a stronger tendency to cross the intestinal barrier compared with MPs based on their nanoscale structural features and enhanced biofilm permeability. However, there is a lack of studies on liver effects associated with NPs. Further research is needed to understand the mechanisms of action of NPs on the liver. It is also essential to investigate the dose-response relationship between MP/ NP-induced oxidative stress and liver metabolism disorders. Furthermore, the development of a composite exposure model of MPs/NPs and other environmental pollutants to simulate real environmental pollution is imperative. The integration of cutting-edge technologies such as single-cell sequencing and spatial metabolomics allows an in-depth analysis of the molecular network of MPs that interfere with hepatocyte homeostasis. This, in turn, provides new avenues for the study of the hepatic role of MPs. In conclusion, the present study systematically reviewed the available evidence and comprehensively elucidated the impact and potential mechanisms of MPs in animal and human livers. The present study aimed to provide a theoretical framework for the study of the hepatic impacts associated with MP exposure, and will assist in the formulation of precise risk management policies for MPs.
Availability of data and materials
Not applicable.
Authors' contributions
SY and TX were responsible for the conceptualization and study design. SY, SC and YZ performed literature search and data extraction and collation. SY and SC contributed to drafting the manuscript. BC and TX conducted critical revision and editing of the manuscript. All authors have read and agreed to the final published version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
The figures were created with BioRender (https://BioRender.com).
Funding
No funding was received.
References
|
Tang B, Zhang L, Salam M, Yang B, He Q, Yang Y and Li H: Revealing the environmental hazard posed by biodegradable microplastics in aquatic ecosystems: An investigation of polylactic acid's effects on microcystis aeruginosa. Environ Pollut. 344:1233472024. View Article : Google Scholar : PubMed/NCBI | |
|
Hirt N and Body-Malapel M: Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part Fibre Toxicol. 17:572020. View Article : Google Scholar : PubMed/NCBI | |
|
Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AWG, McGonigle D and Russell AE: Lost at sea: Where is all the plastic? Science. 304:8382004. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang N, Li YB, He HR, Zhang JF and Ma GS: You are what you eat: Microplastics in the feces of young men living in beijing. Sci Total Environ. 767:1443452021. View Article : Google Scholar : PubMed/NCBI | |
|
Horton AA, Walton A, Spurgeon DJ, Lahive E and Svendsen C: Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci Total Environ. 586:127–141. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Ren Z, Gui X, Xu X, Zhao L, Qiu H and Cao X: Microplastics in the soil-groundwater environment: aging, migration, and co-transport of contaminants - A critical review. J Hazard Mater. 419:1264552021. View Article : Google Scholar : PubMed/NCBI | |
|
Cózar A, Echevarría F, González-Gordillo JI, Irigoien X, Ubeda B, Hernández-León S, Palma AT, Navarro S, García-de-Lomas J, Ruiz A, et al: Plastic debris in the open ocean. Proc Natl Acad Sci USA. 111:10239–10244. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Kang S, Allen S, Allen D, Gao T and Sillanpää M: Atmospheric microplastics: A review on current status and perspectives. Earth-Sci Rev. 203:1031182020. View Article : Google Scholar | |
|
Navarro A, Luzardo OP, Gómez M, Acosta-Dacal A, Martínez I, Felipe de la Rosa J, Macías-Montes A, Suárez-Pérez A and Herrera A: Microplastics ingestion and chemical pollutants in seabirds of gran canaria (Canary Islands, Spain). Mar Pollut Bull. 186:1144342023. View Article : Google Scholar | |
|
Sambolino A, Iniguez E, Herrera I, Kaufmann M, Dinis A and Cordeiro N: Microplastic ingestion and plastic additive detection in pelagic squid and fish: Implications for bioindicators and plastic tracers in open oceanic food webs. Sci Total Environ. 894:1649522023. View Article : Google Scholar : PubMed/NCBI | |
|
Hollerova A, Hodkovicova N, Blahova J, Faldyna M, Franc A, Pavlokova S, Tichy F, Postulkova E, Mares J, Medkova D, et al: Polystyrene microparticles can affect the health status of freshwater fish - threat of oral microplastics intake. Sci Total Environ. 858(Pt 3): 1599762023. View Article : Google Scholar | |
|
Kim L, Cui R, Kwak JI and An YJ: Sub-acute exposure to nanoplastics via two-chain trophic transfer: From brine shrimp artemia franciscana to small yellow croaker larimichthys polyactis. Mar Pollut Bull. 175:1133142022. View Article : Google Scholar : PubMed/NCBI | |
|
Da Costa Araújo AP and Malafaia G: Microplastic ingestion induces behavioral disorders in mice: A preliminary study on the trophic transfer effects via tadpoles and fish. J Hazard Mater. 401:1232632021. View Article : Google Scholar | |
|
Schwabl P, Köppel S, Königshofer P, Bucsics T, Trauner M, Reiberger T and Liebmann B: Detection of various microplastics in human stool: A prospective case series. Ann Intern Med. 171:453–457. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Kernchen S, Schmalz H, Löder MGJ, Georgi C, Einhorn A, Greiner A, Nölscher AC, Laforsch C and Held A: Atmospheric deposition studies of microplastics in central germany. Air Qual. Atmosphere Health. 17:2247–2261. 2024. View Article : Google Scholar | |
|
Kosuth M, Mason SA and Wattenberg EV: Anthropogenic contamination of tap water, beer, and sea salt. PLoS One. 13:e01949702018. View Article : Google Scholar : PubMed/NCBI | |
|
Das BC, Ramanan P A, Gorakh SS, Pillai D and Vattiringal Jayadradhan RK: Sub-chronic exposure of oreochromis niloticus to environmentally relevant concentrations of smaller microplastics: Accumulation and toxico-physiological responses. J Hazard Mater. 458:1319162023. View Article : Google Scholar : PubMed/NCBI | |
|
Bobori DC, Dimitriadi A, Feidantsis K, Samiotaki A, Fafouti D, Sampsonidis I, Kalogiannis S, Kastrinaki G, Lambropoulou DA, Kyzas GZ, et al: Differentiation in the expression of toxic effects of polyethylene-microplastics on two freshwater fish species: Size matters. Sci Total Environ. 830:1546032022. View Article : Google Scholar : PubMed/NCBI | |
|
Ding Y, Zhang R, Li B, Du Y, Li J, Tong X, Wu Y, Ji X and Zhang Y: Tissue Distribution of polystyrene nanoplastics in mice and their entry, transport, and cytotoxicity to GES-1 cells. Environ Pollut. 280:1169742021. View Article : Google Scholar | |
|
Rotchell JM, Jenner LC, Chapman E, Bennett RT, Bolanle IO, Loubani M, Sadofsky L and Palmer TM: Detection of microplastics in human saphenous vein tissue using µFTIR: A pilot study. PLoS One. 18:e02805942023. View Article : Google Scholar | |
|
Zarus GM, Muianga C, Brenner S, Stallings K, Casillas G, Pohl HR, Mumtaz MM and Gehle K: Worker studies suggest unique liver carcinogenicity potential of polyvinyl chloride microplastics. Am J Ind Med. 66:1033–1047. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Horvatits T, Tamminga M, Liu B, Sebode M, Carambia A, Fischer L, Püschel K, Huber S and Fischer EK: Microplastics detected in cirrhotic liver tissue. eBioMedicine. 82:1041472022. View Article : Google Scholar : PubMed/NCBI | |
|
Hu JM, Zuo JE, Li JB, Zhang YY, Ai X, Gong DH, Zhang JW and Sun DM: Effects of microplastic exposure on crucian growth, liver damage, and gut microbiome composition. Huan Jing Ke Xue. 43:3664–3671. 2022.In Chinese. PubMed/NCBI | |
|
Iheanacho SC and Odo GE: Dietary exposure to polyvinyl chloride microparticles induced oxidative stress and hepatic damage in Clarias gariepinus (Burchell, 1822). Environ Sci Pollut Res. 27:21159–21173. 2020. View Article : Google Scholar | |
|
Yao FC, Gu Y, Jiang T, Wang PF, Song FB, Zhou Z, Sun JL and Luo J: The involvement of oxidative stress mediated endoplasmic reticulum pathway in apoptosis of golden pompano (Trachinotus Blochii) liver under PS-MPs stress. Ecotoxicol Environ Saf. 249:1144402023. View Article : Google Scholar | |
|
Yin K, Wang D, Zhang Y, Lu H, Hou L, Guo T, Zhao H and Xing M: Polystyrene Microplastics promote liver inflammation by inducing the formation of macrophages extracellular traps. J Hazard Mater. 452:1312362023. View Article : Google Scholar : PubMed/NCBI | |
|
Liu S, Wang Z, Xiang Q, Wu B, Lv W and Xu S: A Comparative study in healthy and diabetic mice followed the exposure of polystyrene microplastics: Differential lipid metabolism and inflammation reaction. Ecotoxicol Environ Saf. 244:1140312022. View Article : Google Scholar : PubMed/NCBI | |
|
Fu J, Zhang L, Xiang K, Zhang Y, Wang G and Chen L: Microplastic-contaminated antibiotics as an emerging threat to mammalian liver: Enhanced oxidative and inflammatory damages. Biomater Sci. 11:4298–4307. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Mu Y, Sun J, Li Z, Zhang W, Liu Z, Li C, Peng C, Cui G, Shao H and Du Z: Activation of pyroptosis and ferroptosis is involved in the hepatotoxicity induced by polystyrene microplastics in mice. Chemosphere. 291(Pt 2): 1329442022. View Article : Google Scholar | |
|
Wang Q, Wu Y, Zhang W, Shen T, Li H, Wu J, Zhang L, Qin L, Chen R, Gu W, et al: Lipidomics and transcriptomics insight into impacts of microplastics exposure on hepatic lipid metabolism in mice. Chemosphere. 308(Pt 3): 1365912022. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Z, Tian R, She Z, Cai J and Li H: Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. 152:116–141. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
World Health Organization (WHO): Global hepatitis report 2024: Action for access in low- and middle-income countries. WHO; Geneva: 2024 | |
|
Xia C, Xu Y, Li H, He S and Chen W: Benefits and harms of polygenic risk scores in organised cancer screening programmes: A cost-effectiveness analysis. Lancet Reg Health West Pac. 44:1010122024.PubMed/NCBI | |
|
Charlton M, Tonnu-Mihara I, Teng CC, Zhou Z, Asefaha F, Luthra R, Articolo A, Hoovler A and Uzoigwe C: Evaluating the burden of illness of metabolic dysfunction-associated steatohepatitis in a large managed care population: The ETHEREAL Study. J Manag Care Spec Pharm. 30:1414–1430. 2024.PubMed/NCBI | |
|
Choi JH and Kim JH: Toxic effects of sub-acute microplastic (Polyamide) exposure on the accumulation, hematological, and antioxidant responses in crucian carp, carassius carassius. Environ Toxicol Pharmacol. 102:1041992023. View Article : Google Scholar : PubMed/NCBI | |
|
Zou H, Qu H, Bian Y, Sun J, Wang T, Ma Y, Yuan Y, Gu J, Bian J and Liu Z: Polystyrene microplastics induce oxidative stress in mouse hepatocytes in relation to their size. Int J Mol Sci. 24:73822023. View Article : Google Scholar : PubMed/NCBI | |
|
Alibardi L: Regeneration among animals: An evolutionary hypothesis related to aquatic versus terrestrial environment. Dev. Biol. 501:74–80. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang W, Gao H, Jin S, Li R and Na G: The ecotoxicological effects of microplastics on aquatic food web, from primary producer to human: A review. Ecotoxicol Environ Saf. 173:110–117. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Yagi M, Ono Y and Kawaguchi T: Microplastic pollution in aquatic environments may facilitate misfeeding by fish. Environ Pollut. 315:1204572022. View Article : Google Scholar : PubMed/NCBI | |
|
Geng X, Wang J, Zhang Y and Jiang Y: How do microplastics affect the marine microbial loop? Predation of microplastics by microzooplankton. Sci Total Environ. 758:1440302021. View Article : Google Scholar | |
|
Wang T, Tong C, Wu F, Jiang S and Zhang S: Distribution characteristics of microplastics and corresponding feeding habits of the dominant shrimps in the rivers of chongming Island. Sci Total Environ. 888:1640412023. View Article : Google Scholar : PubMed/NCBI | |
|
Timilsina A, Adhikari K, Yadav AK, Joshi P, Ramena G and Bohara K: Effects of microplastics and nanoplastics in shrimp: Mechanisms of plastic particle and contaminant distribution and subsequent effects after uptake. Sci Total Environ. 894:1649992023. View Article : Google Scholar : PubMed/NCBI | |
|
Da Costa Araújo AP, de Melo NFS, de Oliveira Junior AG, Rodrigues FP, Fernandes T, de Andrade Vieira JE, Rocha TL and Malafaia G: How much are microplastics harmful to the health of amphibians? A study with pristine polyethylene microplastics and physalaemus cuvieri. J Hazard Mater. 382:1210662020. View Article : Google Scholar | |
|
Meng X, Yip Y and Valiyaveettil S: Understanding the aggregation, consumption, distribution and accumulation of nanoparticles of polyvinyl chloride and polymethyl methacrylate in ruditapes philippinarum. Sci Total Environ. 871:1619552023. View Article : Google Scholar : PubMed/NCBI | |
|
Helmberger MS, Miesel JR, Tiemann LK and Grieshop MJ: Soil invertebrates generate microplastics from polystyrene foam debris. J Insect Sci. 22:212022. View Article : Google Scholar : PubMed/NCBI | |
|
De Souza SS, Freitas ÍN, Gonçalves SO, Luz TMD, Araújo APDC, Rajagopal R, Balasubramani G, Rahman MM and Malafaia G: Toxicity induced via ingestion of naturally-aged polystyrene microplastics by a small-sized terrestrial bird and its potential role as vectors for the dispersion of these pollutants. J Hazard Mater. 434:1288142022. View Article : Google Scholar : PubMed/NCBI | |
|
Sucharitakul P, Wu WM, Zhang Y, Peng BY, Gao J, Wang L and Hou D: Exposure pathways and toxicity of microplastics in terrestrial insects. Environ Sci Technol. 58:11887–11900. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Tan YR, Shen SY, Shen HQ, Yi PF, Fu BD and Peng LY: The role of endoplasmic reticulum stress in regulation of intestinal barrier and inflammatory bowel disease. Exp Cell Res. 424:1134722023. View Article : Google Scholar : PubMed/NCBI | |
|
Peterson L and Artis D: Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat Rev Immunol. 14:141–153. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Vagner M, Boudry G, Courcot L, Vincent D, Dehaut A, Duflos G, Huvet A, Tallec K and Zambonino-Infante JL: Experimental evidence that polystyrene nanoplastics cross the intestinal barrier of european seabass. Environ. Int. 166:1073402022. View Article : Google Scholar : PubMed/NCBI | |
|
Gao Q, Wang Y, Ji Y, Zhao X, Zhang P and Chen L: Tracking of realistic nanoplastics in complicated matrices by iridium element labeling and inductively coupled plasma mass spectroscopy. J Hazard Mater. 424(Pt C): 1276282022. View Article : Google Scholar | |
|
Tsou TY, Lee SH, Kuo TH, Chien CC, Chen HC and Cheng TJ: Distribution and toxicity of submicron plastic particles in mice. Environ Toxicol Pharmacol. 97:1040382023. View Article : Google Scholar | |
|
Yang X, Jiang J, Wang Q, Duan J, Chen N, Wu D and Xia Y: Gender difference in hepatic AMPK pathway activated lipid metabolism induced by aged polystyrene microplastics exposure. Ecotoxicol Environ Saf. 245:1141052022. View Article : Google Scholar : PubMed/NCBI | |
|
Deng Y, Chen H, Huang Y, Zhang Y, Ren H, Fang M, Wang Q, Chen W, Hale RC, Galloway TS and Chen D: Long-term exposure to environmentally relevant doses of large polystyrene microplastics disturbs lipid homeostasis via bowel function interference. Environ Sci Technol. 56:15805–15817. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Brandts I, Cánovas M, Tvarijonaviciute A, Llorca M, Vega AS, Farré M, Pastor J, Roher N and Teles M: Nanoplastics are bioaccumulated in fish liver and muscle and cause DNA damage after a chronic exposure. Environ Res. 212(Pt A): 1134332022. View Article : Google Scholar : PubMed/NCBI | |
|
Choi YJ, Park JW, Lim Y, Seo S and Hwang DY: In vivo impact assessment of orally administered polystyrene nanoplastics: Biodistribution, toxicity, and inflammatory response in mice. Nanotoxicology. 15:1180–1198. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Yu YB, Choi JH, Choi CY, Kang JC and Kim JH: Toxic effects of microplastic (polyethylene) exposure: Bioaccumulation, hematological parameters and antioxidant responses in crucian carp, carassius carassius. Chemosphere. 332:1388012023. View Article : Google Scholar : PubMed/NCBI | |
|
Goodman KE, Hua T and Sang QA: Effects of polystyrene microplastics on human kidney and liver cell morphology, cellular proliferation, and metabolism. ACS Omega. 7:34136–34153. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Li Y, Li J, Song Z, Zhang C and Guan B: Toxicity of polystyrene nanoplastics to human embryonic kidney cells and human normal liver cells: Effect of particle size and Pb2+ enrichment. Chemosphere. 328:1385452023. View Article : Google Scholar | |
|
Florance I, Chandrasekaran N, Gopinath PM and Mukherjee A: Exposure to polystyrene nanoplastics impairs lipid metabolism in human and murine macrophages in vitro. Ecotoxicol Environ Saf. 238:1136122022. View Article : Google Scholar : PubMed/NCBI | |
|
Prietl B, Meindl C, Roblegg E, Pieber TR, Lanzer G and Fröhlich E: Nano-sized and micro-sized polystyrene particles affect phagocyte function. Cell Biol Toxicol. 30:1–16. 2014. View Article : Google Scholar | |
|
Banerjee A, Billey LO, McGarvey AM and Shelver WL: Effects of polystyrene micro/nanoplastics on liver cells based on particle size, surface functionalization, concentration and exposure period. Sci Total Environ. 836:1556212022. View Article : Google Scholar : PubMed/NCBI | |
|
He Y, Li J, Chen J, Miao X, Li G, He Q, Xu H, Li H and Wei Y: Cytotoxic effects of polystyrene nanoplastics with different surface functionalization on human HepG2 cells. Sci Total Environ. 723:1381802020. View Article : Google Scholar : PubMed/NCBI | |
|
Liu X, Liang C, Zhou M, Chang Z and Li L: Exposure of cyprinus carpio var. larvae to PVC microplastics reveals significant immunological alterations and irreversible histological organ damage. Ecotoxicol Environ Saf. 249:1143772023. View Article : Google Scholar | |
|
Zheng H, Wang J, Wei X, Chang L and Liu S: Proinflammatory properties and lipid disturbance of polystyrene microplastics in the livers of mice with acute colitis. Sci Total Environ. 750:1430852021. View Article : Google Scholar | |
|
Hodkovicova N, Hollerova A, Caloudova H, Blahova J, Franc A, Garajova M, Lenz J, Tichy F, Faldyna M, Kulich P, et al: Do food-borne polyethylene microparticles affect the health of rainbow trout (Oncorhynchus Mykiss)? Sci Total Environ. 793:1484902021. View Article : Google Scholar | |
|
Zhang Y, Yuan J and Mao T: Impact of microplastics exposure on liver health: A comprehensive meta-analysis. Comp Biochem Physiol C Toxicol Pharmacol. 288:1100802025. View Article : Google Scholar | |
|
Kwong S, Meyerson C, Zheng W, Kassardjian A, Stanzione N, Zhang K and Wang HL: Acute hepatitis and acute liver failure: Pathologic diagnosis and differential diagnosis. Semin Diagn Pathol. 36:404–414. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Fortea JI, Puente Á, Cuadrado A, Huelin P, Pellón R, González Sánchez FJ, Mayorga M, Cagigal ML, García Carrera I, Cobreros M, et al: Congestive hepatopathy. Int J Mol Sci. 21:94202020. View Article : Google Scholar : PubMed/NCBI | |
|
Sepehrinezhad A, Zarifkar A, Namvar G, Shahbazi A and Williams R: Astrocyte swelling in hepatic encephalopathy: Molecular perspective of cytotoxic edema. Metab Brain Dis. 35:559–578. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Ten Hove M, Pater L, Storm G, Weiskirchen S, Weiskirchen R, Lammers T and Bansal R: The hepatic lipidome: From basic science to clinical translation. Adv Drug Deliv Rev. 159:180–197. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Araújo APDC, Gomes AR and Malafaia G: Hepatotoxicity of pristine polyethylene microplastics in neotropical physalaemus cuvieri tadpoles (Fitzinger, 1826). J Hazard Mater. 386:1219922020. View Article : Google Scholar : PubMed/NCBI | |
|
Hamed M, Soliman HAM, Badrey AEA and Osman AGM: Microplastics induced histopathological lesions in some tissues of tilapia (Oreochromis Niloticus) early juveniles. Tissue Cell. 71:1015122021. View Article : Google Scholar : PubMed/NCBI | |
|
Liu M, Mu J, Wang M, Hu C, Ji J, Wen C and Zhang D: Impacts of polypropylene microplastics on lipid profiles of mouse liver uncovered by lipidomics analysis and raman spectroscopy. J Hazard Mater. 458:1319182023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang X, Wen K, Ding D, Liu J, Lei Z, Chen X, Ye G, Zhang J, Shen H, Yan C, et al: Size-dependent adverse effects of microplastics on intestinal microbiota and metabolic homeostasis in the marine medaka (Oryzias Melastigma). Environ Int. 151:1064522021. View Article : Google Scholar : PubMed/NCBI | |
|
Sayed AEH, Hana MN, Hamed M, Abdel-Latif HMR, Lee JS and Soliman HAM: Protective efficacy of dietary natural antioxidants on microplastic particles-induced histopathological lesions in African Catfish (Clarias Gariepinus). Environ Sci Pollut Res Int. 30:24424–24440. 2023. View Article : Google Scholar : | |
|
Liu Y, Jia X, Zhu H, Zhang Q, He Y, Shen Y, Xu X and Li J: The effects of exposure to microplastics on grass carp (Ctenopharyngodon Idella) at the physiological, biochemical, and transcriptomic levels. Chemosphere. 286(Pt 3): 1318312022. View Article : Google Scholar | |
|
Xia X, Sun M, Zhou M, Chang Z and Li L: Polyvinyl chloride microplastics induce growth inhibition and oxidative stress in cyprinus carpio var larvae. Sci Total Environ. 716:1364792020. View Article : Google Scholar | |
|
Schieber M and Chandel NS: ROS function in redox signaling and oxidative stress. Curr Biol. 24:R453–R462. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Allameh A, Niayesh-Mehr R, Aliarab A, Sebastiani G and Pantopoulos K: Oxidative stress in liver pathophysiology and disease. Antioxidants (Basel). 12:16532023. View Article : Google Scholar : PubMed/NCBI | |
|
Lai W, Xu D, Li J, Wang Z, Ding Y, Wang X, Li X, Xu N, Mai K and Ai Q: Dietary polystyrene nanoplastics exposure alters liver lipid metabolism and muscle nutritional quality in carnivorous marine fish large yellow croaker (Larimichthys Crocea). J Hazard Mater. 419:1264542021. View Article : Google Scholar : PubMed/NCBI | |
|
Banaei M, Forouzanfar M and Jafarinia M: Toxic effects of polyethylene microplastics on transcriptional changes, biochemical response, and oxidative stress in common carp (Cyprinus Carpio). Comp Biochem Physiol C Toxicol Pharmacol. 261:1094232022. View Article : Google Scholar : PubMed/NCBI | |
|
Ighodaro OM and Akinloye OA: First line defence antioxidants-superoxide dismutase (SOD), Catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex J Med. 54:287–293. 2018. | |
|
Gaweł S, Wardas M, Niedworok E and Wardas P: Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek. 57:453–455. 2004.In Polish. | |
|
Samal RR, Navani HS, Saha S, Kisan B and Subudhi U: Evidence of microplastics release from polythene and paper cups exposed to hot and cold: A case study on the compromised kinetics of catalase. J Hazard Mater. 454:1314962023. View Article : Google Scholar : PubMed/NCBI | |
|
Solomando A, Capó X, Alomar C, Compa M, Valencia JM, Sureda A and Deudero S: Assessment of the effect of long-term exposure to microplastics and depuration period in sparus aurata linnaeus, 1758: Liver and blood biomarkers. Sci Total Environ. 786:1474792021. View Article : Google Scholar : PubMed/NCBI | |
|
Megha KB, Joseph X, Akhil V and Mohanan PV: Cascade of Immune Mechanism and Consequences of Inflammatory Disorders. Phytomedicine. 91:1537122021. View Article : Google Scholar : PubMed/NCBI | |
|
Banerjee P, Gaddam N, Chandler V and Chakraborty S: Oxidative stress-induced liver damage and remodeling of the liver vasculature. Am J Pathol. 193:1400–1414. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Ahmed O, Robinson MW and O'Farrelly C: Inflammatory processes in the liver: Divergent roles in homeostasis and pathology. Cell Mol Immunol. 18:1375–1386. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Cui J, Zhang Y, Liu L, Zhang Q, Xu S and Guo MY: Polystyrene microplastics induced inflammation with activating the TLR2 signal by excessive accumulation of ROS in hepatopancreas of carp (Cyprinus Carpio). Ecotoxicol Environ Saf. 251:1145392023. View Article : Google Scholar : PubMed/NCBI | |
|
George L, Elizabeth H and George L: The Role of the Reticuloendothelial System in Natural Immunity. NeuroImmune Biology. Bertók L and Chow DA: 5. Elsevier; pp. 95–101. 2005, View Article : Google Scholar | |
|
Kubes P and Jenne C: Immune responses in the liver. Annu Rev Immunol. 36:247–277. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Deng J, Zeng X, Li J, Luo L, Yang Y and Luan T: Single-cell transcriptomic analysis reveals heterogeneity of the patterns of responsive genes and cell communications in liver cell populations of zebrafish exposed to polystyrene nanoplastics. Sci Total Environ. 889:1640822023. View Article : Google Scholar : PubMed/NCBI | |
|
Elizalde-Velázquez A, Crago J, Zhao X, Green MJ and Cañas-Carrell JE: In vivo effects on the immune function of fathead minnow (Pimephales Promelas) following ingestion and intraperitoneal injection of polystyrene nanoplastics. Sci Total Environ. 735:1394612020. View Article : Google Scholar : PubMed/NCBI | |
|
Cheng H, Duan Z, Wu Y, Wang Y, Zhang H, Shi Y, Zhang H, Wei Y and Sun H: Immunotoxicity responses to polystyrene nanoplastics and their related mechanisms in the liver of zebrafish (Danio Rerio) larvae. Environ Int. 161:1071282022. View Article : Google Scholar : PubMed/NCBI | |
|
Yoon H, Shaw JL, Haigis MC and Greka A: Lipid metabolism in sickness and in health: Emerging regulators of lipotoxicity. Mol Cell. 81:3708–3730. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Hsu SD, Huang HY, Chou CH, Sun YM, Hsu MT and Tsou AP: Integrated analyses to reconstruct microRNA-mediated regulatory networks in mouse liver using high-throughput profiling. BMC Genomics. 16(Suppl 2): S122015. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu C, Zhou W, Han M, Yang Y, Li Y, Jiang Q and Lv W: Dietary polystyrene nanoplastics exposure alters hepatic glycolipid metabolism, triggering inflammatory responses and apoptosis in monopterus albus. Sci Total Environ. 891:1644602023. View Article : Google Scholar : PubMed/NCBI | |
|
Yin K, Wang D, Zhang Y, Lu H, Wang Y and Xing M: Dose-effect of polystyrene microplastics on digestive toxicity in chickens (Gallus Gallus): Multi-omics reveals critical role of gut-liver axis. J Adv Res. 52:3–18. 2023. View Article : Google Scholar : | |
|
Long X, Zeng X, Tan F, Yi R, Pan Y, Zhou X, Mu J and Zhao X: Lactobacillus plantarum KFY04 prevents obesity in mice through the PPAR pathway and alleviates oxidative damage and inflammation. Food Funct. 11:5460–5472. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Feng S, Zeng Y, Cai Z, Wu J, Chan LL, Zhu J and Zhou J: Polystyrene microplastics alter the intestinal microbiota function and the hepatic metabolism status in marine medaka (Oryzias Melastigma). Sci Total Environ. 759:1435582021. View Article : Google Scholar | |
|
Lv W, Gu H, He D, Liu Z, Yao C, Huang W, Yuan Q and Zhou W: Polystyrene nanospheres-induced hepatotoxicity in swamp eel (Monopterus Albus): From biochemical, pathological and transcriptomic perspectives. Sci Total Environ. 893:1648442023. View Article : Google Scholar : PubMed/NCBI | |
|
Chwiałkowski W: Purification of the Used Palm Oil by Adsorption. PJCT. 10:19–21. 2008. View Article : Google Scholar | |
|
Li Y, Guo M, Niu S, Shang M, Chang X, Sun Z, Zhang R, Shen X and Xue Y: ROS and DRP1 interactions accelerate the mitochondrial injury induced by polystyrene nanoplastics in human liver HepG2 cells. Chem Biol Interact. 379:1105022023. View Article : Google Scholar : PubMed/NCBI | |
|
Diao L, Ding M, Sun H, Xu Y, Yin R and Chen H: Micro-algal astaxanthin ameliorates polystyrene microplastics-triggered necroptosis and inflammation by mediating mitochondrial Ca2+ homeostasis in carp's head kidney lymphocytes (Cyprinus carpio L.). Fish Shellfish Immunol. 143:1092052023. View Article : Google Scholar | |
|
Bai J, Xie N, Hou Y, Chen X, Hu Y, Zhang Y, Meng X, Wang X and Tang C: The enhanced mitochondrial dysfunction by cantleyoside confines inflammatory response and promotes apoptosis of human HFLS-RA cell line via AMPK/Sirt 1/NF-κB pathway activation. Biomed Pharmacother. 149:1128472022. View Article : Google Scholar | |
|
Appelqvist H, Wäster P, Kågedal K and Öllinger K: The lysosome: From waste bag to potential therapeutic target. J Mol Cell Biol. 5:214–226. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Deng J, Ibrahim MS, Tan LY, Yeo XY, Lee YA, Park SJ, Wüstefeld T, Park JW, Jung S and Cho NJ: Microplastics released from food containers can suppress lysosomal activity in mouse macrophages. J Hazard Mater. 435:1289802022. View Article : Google Scholar : PubMed/NCBI | |
|
Wang H, Shi X, Gao Y, Zhang X, Zhao H, Wang L, Zhang X and Chen R: Polystyrene nanoplastics induce profound metabolic shift in human cells as revealed by integrated proteomic and metabolomic analysis. Environ. Int. 166:1073492022. View Article : Google Scholar : PubMed/NCBI | |
|
Korolchuk V, Saiki S, Lichtenberg M, Siddiqi FH, Roberts EA, Imarisio S, Jahreiss L, Sarkar S, Futter M, Menzies FM, et al: Lysosomal positioning coordinates cellular nutrient responses. Nat Cell Biol. 13:453–460. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Huang J, Sun X, Wang Y, Su J, Li G, Wang X, Yang Y, Zhang Y, Li B, Zhang G, et al: Biological interactions of polystyrene nanoplastics: Their cytotoxic and immunotoxic effects on the hepatic and enteric systems. Ecotoxicol Environ Saf. 264:1154472023. View Article : Google Scholar : PubMed/NCBI | |
|
Djouina M, Waxin C, Dubuquoy L, Launay D, Vignal C and Body-Malapel M: Oral exposure to polyethylene microplastics induces inflammatory and metabolic changes and promotes fibrosis in mouse liver. Ecotoxicol Environ Saf. 264:1154172023. View Article : Google Scholar : PubMed/NCBI | |
|
Shen R, Yang K, Cheng X, Guo C, Xing X, Sun H, Liu D, Liu X and Wang D: Accumulation of polystyrene microplastics induces liver fibrosis by activating cGAS/STING pathway. Environ Pollut. 300:1189862022. View Article : Google Scholar : PubMed/NCBI | |
|
Li X, Feng L, Kuang Q, Wang X, Yang J, Niu X, Gao L, Huang L, Luo P and Li L: Microplastics cause hepatotoxicity in diabetic mice by disrupting glucolipid metabolism via PP2A/AMPK/ HNF4A and promoting fibrosis via the Wnt/β-catenin pathway. Environ Toxicol. 39:1018–1030. 2024. View Article : Google Scholar | |
|
Lu YY, Lu L, Ren HY, Hua W, Zheng N, Huang FY, Wang J, Tian M and Huang Q: The size-dependence and reversibility of polystyrene nanoplastics-induced lipid accumulation in mice: Possible roles of lysosomes. Environ Int. 185:1085322024. View Article : Google Scholar : PubMed/NCBI | |
|
Shen Q, Liu YJ, Qiu TT, Loon KS and Zhou D: Microplastic-induced NAFLD: Hepatoprotective effects of nanosized selenium. Ecotoxicol Environ Saf. 272:1158502024. View Article : Google Scholar : PubMed/NCBI | |
|
Xu JL, Lin X, Wang JJ and Gowen AA: A review of potential human health impacts of micro- and nanoplastics exposure. Sci Total Environ. 851(Pt 1): 1581112022. View Article : Google Scholar : PubMed/NCBI | |
|
Liu W, Li M, Guo H, Wei S, Xu W, Yan Y, Shi Y, Xu Z, Chang K, Wei G and Zhao S: Single-cell transcriptome analysis of liver immune microenvironment changes induced by microplastics in mice with non-alcoholic fatty liver. Sci Total Environ. 912:1683082024. View Article : Google Scholar | |
|
Wei J, Liu J, Wang H, Wen K, Ni X, Lin Y, Huang J, You X, Lei Z, Li J, et al: Nanoplastic propels diet-induced NAFL to NASH via ER-mitochondrial tether-controlled redox switch. J Hazard Mater. 465:1331422024. View Article : Google Scholar | |
|
Zha H, Han S, Tang R, Cao D, Chang K and Li L: Polylactic acid micro/nanoplastic-induced hepatotoxicity: Investigating food and air sources via multi-omics. Environ Sci Ecotechnol. 21:1004282024. View Article : Google Scholar : PubMed/NCBI | |
|
Sinkala M, Nkhoma P, Mulder N and Martin DP: Integrated Molecular characterisation of the MAPK pathways in human cancers reveals pharmacologically vulnerable mutations and gene dependencies. Commun Biol. 4:92021. View Article : Google Scholar : PubMed/NCBI | |
|
Köktürk M, Özgeriş FB, Atamanalp M, Uçar A, Özdemir S, Parlak V, Duyar HA and Alak G: Microplastic-induced oxidative stress response in turbot and potential intake by humans. Drug Chem Toxicol. 47:296–305. 2024. View Article : Google Scholar | |
|
Barboza LGA, Lopes C, Oliveira P, Bessa F, Otero V, Henriques B, Raimundo J, Caetano M, Vale C and Guilhermino L: Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci Total Environ. 717:1346252020. View Article : Google Scholar | |
|
Mkuye R, Gong S, Zhao L, Masanja F, Ndandala C, Bubelwa E, Yang C and Deng Y: Effects of microplastics on physiological performance of marine bivalves, potential impacts, and enlightening the future based on a comparative study. Sci Total Environ. 838(Pt 1): 1559332022. View Article : Google Scholar : PubMed/NCBI | |
|
Huang S, Huang X, Bi R, Guo Q, Yu X, Zeng Q, Huang Z, Liu T, Wu H, Chen Y, et al: Detection and analysis of microplastics in human sputum. Environ Sci Technol. 56:2476–2486. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Bhagat J, Nishimura N and Shimada Y: Toxicological interactions of microplastics/nanoplastics and environmental contaminants: Current knowledge and future perspectives. J Hazard Mater. 405:1239132021. View Article : Google Scholar | |
|
Cuevas BD, Abell AN and Johnson GL: Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene. 26:3159–3171. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Moon H and Ro SW: MAPK/ERK signaling pathway in hepatocellular carcinoma. Cancers (Basel). 13:30262021. View Article : Google Scholar : PubMed/NCBI | |
|
Yu B, Zhang Y, Wang T, Guo J, Kong C, Chen Z, Ma X and Qiu T: MAPK signaling pathways in hepatic ischemia/reperfusion injury. J Inflamm Res. 16:1405–1418. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Cai S, Wu L, Yuan S, Liu G, Wang Y, Fang L and Xu D: Carvacrol alleviates liver fibrosis by inhibiting TRPM7 and modulating the MAPK signaling pathway. Eur J Pharmacol. 898:1739822021. View Article : Google Scholar : PubMed/NCBI | |
|
Lv J, He Q, Yan Z, Xie Y, Wu Y, Li A, Zhang Y, Li J and Huang Z: Inhibitory impact of prenatal exposure to nano-polystyrene particles on the MAP2K6/p38 MAPK axis inducing embryonic developmental abnormalities in mice. Toxics. 12:3702024. View Article : Google Scholar : PubMed/NCBI | |
|
Yu P, Liu Z, Wu D, Chen M, Lv W and Zhao Y: Accumulation of polystyrene microplastics in juvenile eriocheir sinensis and oxidative stress effects in the liver. Aquat Toxicol. 200:28–36. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Xiong G, Zhang H, Shi H, Peng Y, Han M, Hu T, Liao X, Liu Y, Zhang J and Xu G: Enhanced hepatotoxicity in zebrafish due to co-exposure of microplastics and sulfamethoxazole: Insights into ROS-Mediated MAPK signaling pathway regulation. Ecotoxicol Environ Saf. 278:1164152024. View Article : Google Scholar : PubMed/NCBI | |
|
Ariffianto A, Deng L, Abe T, Matsui C, Ito M, Ryo A, Aly HH, Watashi K, Suzuki T, Mizokami M, et al: Oxidative stress sensor Keap1 recognizes HBx protein to activate the Nrf2/ARE signaling pathway, thereby inhibiting hepatitis B virus replication. J Virol. 97:e01287232023. View Article : Google Scholar : PubMed/NCBI | |
|
Bellezza I, Giambanco I, Minelli A and Donato R: Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 1865:721–733. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Ulasov AV, Rosenkranz AA, Georgiev GP and Sobolev AS: Nrf2/Keap1/ARE signaling: Towards specific regulation. Life Sci. 291:1201112022. View Article : Google Scholar | |
|
Li S, Shi M, Wang Y, Xiao Y, Cai D and Xiao F: Keap1-nrf2 pathway up-regulation via hydrogen sulfide mitigates polystyrene microplastics induced-hepatotoxic effects. J Hazard Mater. 402:1239332021. View Article : Google Scholar | |
|
Yin K, Wang D, Zhao H, Wang Y, Zhang Y, Liu Y, Li B and Xing M: Polystyrene microplastics up-regulates liver glutamine and glutamate synthesis and promotes autophagy-dependent ferroptosis and apoptosis in the cerebellum through the liver-brain axis. Environ Pollut. 307:1194492022. View Article : Google Scholar : PubMed/NCBI | |
|
Kamila S, Dey KK, Islam S and Chattopadhyay A: Arsenic and chromium induced hepatotoxicity in zebrafish (Danio Rerio) at environmentally relevant concentrations: Mixture effects and involvement of Nrf2-Keap1-ARE pathway. Sci Total Environ. 921:1712212024. View Article : Google Scholar : PubMed/NCBI | |
|
Yu Z, Fan X, Zhao X, He T, Li X, Du H, Zhao M, Zhu R, Li M, Zhang Z and Han F: Polystyrene nanoplastics induce lipid metabolism disorder by activating the PERK-ATF4 signaling pathway in mice. ACS Appl Mater Interfaces. 16:34524–34537. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Fan Z, Zhang Y, Fang Y, Zhong H, Wei T, Akhtar H, Zhang J, Yang M, Li Y, Zhou X, et al: Polystyrene nanoplastics induce lipophagy via the AMPK/ULK1 pathway and block lipophagic flux leading to lipid accumulation in hepatocytes. J Hazard Mater. 476:1348782024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Q, Lv Y, Liu J, Chang L, Chen Q, Zhu L, Wang B, Jiang J and Zhu W: Size matters either way: Differently-sized microplastics affect amphibian host and symbiotic microbiota discriminately. Environ Pollut. 328:1216342023. View Article : Google Scholar : PubMed/NCBI | |
|
Yu Z, Yan C, Qiu D, Zhang X, Wen C and Dong S: Accumulation and ecotoxicological effects induced by combined exposure of different sized polyethylene microplastics and oxytetracycline in zebrafish. Environ Pollut. 319:1209772023. View Article : Google Scholar : PubMed/NCBI | |
|
Ding J, Huang Y, Liu S, Zhang S, Zou H, Wang Z, Zhu W and Geng J: Toxicological effects of nano- and micro-polystyrene plastics on red tilapia: Are larger plastic particles more harmless? J Hazard Mater. 396:1226932020. View Article : Google Scholar : PubMed/NCBI | |
|
Guo T, Geng X, Zhang Y, Hou L, Lu H, Xing M and Wang Y: New insights into the spleen injury by mitochondrial dysfunction of chicken under polystyrene microplastics stress. Poult Sci. 103:1036742024. View Article : Google Scholar : PubMed/NCBI | |
|
Raza T, Rasool B, Asrar M, Manzoor M, Javed Z, Jabeen F and Younis T: Exploration of polyacrylamide microplastics and evaluation of their toxicity on multiple parameters of Oreochromis niloticus. Saudi J Biol Sci. 30:1035182023. View Article : Google Scholar | |
|
Brandts I, Barría C, Martins MA, Franco-Martínez L, Barreto A, Tvarijonaviciute A, Tort L, Oliveira M and Teles M: Waterborne exposure of gilthead seabream (Sparus aurata) to polymethylmethacrylate nanoplastics causes effects at cellular and molecular levels. J Hazard Mater. 403:1235902021. View Article : Google Scholar | |
|
Bobori DC, Feidantsis K, Dimitriadi A, Datsi N, Ripis P, Kalogiannis S, Sampsonidis I, Kastrinaki G, Ainali NM, Lambropoulou DA, et al: Dose-dependent cytotoxicity of polypropylene microplastics (PP-MPs) in two freshwater fishes. Int J Mol Sci. 23:138782022. View Article : Google Scholar : PubMed/NCBI | |
|
Wu X, Li M, Yang S, Dong J, Pan W, Ning Y, Hua X, Dong D and Liang D: Liver metabolic dysregulation induced by polypropylene nano- and microplastics in nile tilapia using internal extractive electrospray ionization mass spectrometry. Anal Chem. 95:7863–7871. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Zheng Q, Cui L, Liao H, Junaid M, Li Z, Liu S, Gao D, Zheng Y, Lu S, Qiu J and Wang J: Combined exposure to polystyrene nanoplastics and bisphenol A induces hepato- and intestinal-toxicity and disturbs gut microbiota in channel catfish (Ictalurus Punctatus). Sci Total Environ. 891:1643192023. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Li J, Zhai L and Lu K: Co-exposure of polycarbonate microplastics aggravated the toxic effects of imidacloprid on the liver and gut microbiota in mice. Environ. Toxicol Pharmacol. 101:1041942023. View Article : Google Scholar : PubMed/NCBI | |
|
Sun W, Yan S, Meng Z, Tian S, Jia M, Huang S, Wang Y, Zhou Z, Diao J and Zhu W: Combined ingestion of polystyrene microplastics and epoxiconazole increases health risk to mice: Based on their synergistic bioaccumulation in vivo. Environ Int. 166:1073912022. View Article : Google Scholar : PubMed/NCBI | |
|
Barboza LGA, Cunha SC, Monteiro C, Fernandes JO and Guilhermino L: Bisphenol A and its analogs in muscle and liver of fish from the North East Atlantic Ocean in relation to microplastic contamination. exposure and risk to human consumers. J Hazard. Mater. 393:1224192020. View Article : Google Scholar : PubMed/NCBI | |
|
Chen L, Qi M, Zhang L, Yu F, Tao D, Xu C and Xu S: Di(2-Ethylhexyl) phthalate and microplastics cause necroptosis and apoptosis in hepatocytes of mice by inducing oxidative stress. Environ Toxicol. 38:1226–1238. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Q, Chen G, Tian L, Kong C, Gao D, Chen Y, Junaid M and Wang J: Neuroand hepato-toxicity of polystyrene nanoplastics and polybrominated diphenyl ethers on early life stages of zebrafish. Sci Total Environ. 857(Pt 2): 1595672023. View Article : Google Scholar | |
|
Liu S, Yan L, Zhang Y, Junaid M and Wang J: Toxicological effects of polystyrene nanoplastics and perfluorooctanoic acid to gambusia affinis. Fish Shellfish Immunol. 127:1100–1112. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Mohsen M, Lin C, Tu C, Zhang C, Xu S and Yang H: Association of heavy metals with plastics used in aquaculture. Mar Pollut Bull. 174:1133122022. View Article : Google Scholar : PubMed/NCBI | |
|
Yang H, Zhu Z, Xie Y, Zheng C, Zhou Z, Zhu T and Zhang Y: Comparison of the combined toxicity of polystyrene microplastics and different concentrations of cadmium in zebrafish. Aquat Toxicol Amst Neth. 250:1062592022. View Article : Google Scholar | |
|
Kessabi K, Abbassi A, Lahmar S, Casado M, Banni M, Piña B and Messaoudi I: Combined toxic effects of cadmium and environmental microplastics in aphanius fasciatus(Pisces, Cyprinodontidae). Mar Environ Res. 189:1060712023. View Article : Google Scholar | |
|
Al Marshoudi M, Al Reasi HA, Al-Habsi A and Barry MJ: Additive effects of microplastics on accumulation and toxicity of cadmium in male zebrafish. Chemosphere. 334:1389692023. View Article : Google Scholar : PubMed/NCBI | |
|
Yang Z, Zhu L, Liu J, Cheng Y, Waiho K, Chen A and Wang Y: Polystyrene microplastics increase Pb bioaccumulation and health damage in the Chinese Mitten crab eriocheir sinensis. Sci Total Environ. 829:1545862022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhong G, Rao G, Tang L, Wu S, Tang Z, Huang R, Ruan Z and Hu L: Combined effect of arsenic and polystyrene-nanoplastics at environmentally relevant concentrations in mice liver: Activation of Apoptosis, pyroptosis and excessive autophagy. Chemosphere. 300:1345662022. View Article : Google Scholar : PubMed/NCBI | |
|
Clark NJ, Khan FR, Crowther C, Mitrano DM and Thompson RC: Uptake, distribution and elimination of palladium-doped polystyrene nanoplastics in rainbow trout (Oncorhynchus Mykiss) following dietary exposure. Sci Total Environ. 854:1587652023. View Article : Google Scholar | |
|
Huang JN, Wen B, Li XX, Xu L, Gao JZ and Chen ZZ: Astaxanthin mitigates oxidative stress caused by microplastics at the expense of reduced skin pigmentation in discus fish. Sci Total Environ. 874:1624942023. View Article : Google Scholar : PubMed/NCBI | |
|
Missawi O, Jeddou IB, Venditti M, Zitouni N, Zaouali MA, Abdennebi HB, Messaoudi I, Reiter RJ, Minucci S and Banni M: Environmental microplastic accumulation exacerbates liver ischemia-reperfusion injury in rat: protective effects of melatonin. Sci Total Environ. 860:1601552023. View Article : Google Scholar | |
|
Elsheikh AA, Alnasser SM, Shalaby AM, Alabiad MA, Abd-Almotaleb NA, Alorini M, Jaber FA and Khayal EE: Polystyrene microplastic particles induced hepatotoxic injury via pyroptosis, oxidative stress, and fibrotic changes in adult male albino rats; the therapeutic role of silymarin. Toxicol Mech Methods. 33:512–528. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Ijaz MU, Khalil M, Hamza A and Khatoon A: Attenuative effects of tamarixetin against polystyrene microplastics-induced hepatotoxicity in rats by regulation of Nrf-2/Keap-1 pathway. Cell Biochem Funct. 41:1451–1461. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Yu C, Xu Y, Wei Y, Guo Y, Wang Y, Song P and Yu J: Gut microbiota and liver metabolomics reveal the potential mechanism of lactobacillus rhamnosus GG modulating the liver toxicity caused by polystyrene microplastics in mice. Environ Sci Pollut Res Int. 31:6527–6542. 2024. View Article : Google Scholar | |
|
Dong R, Zhou C, Wang S, Yan Y and Jiang Q: Probiotics ameliorate polyethylene microplastics-induced liver injury by inhibition of oxidative stress in nile tilapia (Oreochromis Niloticus). Fish Shellfish Immunol. 130:261–272. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Silva AB, Bastos AS, Justino CIL, da Costa JP, Duarte AC and Rocha-Santos TAP: Microplastics in the environment: Challenges in analytical chemistry - A review. Anal Chim Acta. 1017:1–19. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Wright S, Levermore J and Ishikawa Y: Application of infrared and near-infrared microspectroscopy to microplastic human exposure measurements. Appl Spectrosc. 77:1105–1128. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Barbosa F, Adeyemi JA, Bocato MZ, Comas A and Campiglia A: A critical viewpoint on current issues, limitations, and future research needs on micro- and nanoplastic studies: From the detection to the toxicological assessment. environ. Res. 182:1090892020. View Article : Google Scholar : PubMed/NCBI | |
|
Cabernard L, Roscher L, Lorenz C, Gerdts G and Primpke S: Comparison of raman and fourier transform infrared spectroscopy for the quantification of microplastics in the aquatic environment. Environ Sci Technol. 52:13279–13288. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Nigamatzyanova L and Fakhrullin R: Dark-Field hyperspectral microscopy for label-free microplastics and nanoplastics detection and identification in vivo: A caenorhabditis elegans study. Environ Pollut. 271:1163372021. View Article : Google Scholar | |
|
Liang JL, Cao GX, Zheng FY, Li SX, Liu FJ, Lin LX, Huang XG, Zhang ZH, Zheng JY and Huang QY: Low-Toxic, fluorescent labeled and size-controlled graphene oxide quantum Dots@ polystyrene nanospheres as reference material for quantitative determination and in vivo tracing. Chemosphere. 307(Pt 4): 1360942022. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y, Wei Z, Xu K, Wang X, Gao X, Han Q, Wang S and Chen M: The effect and a mechanistic evaluation of polystyrene nanoplastics on a mouse model of type 2 diabetes. Food Chem Toxicol. 173:1136422023. View Article : Google Scholar : PubMed/NCBI | |
|
Shi C, Han X, Guo W, Wu Q, Yang X, Wang Y, Tang G, Wang S, Wang Z, Liu Y, et al: Disturbed gut-liver axis indicating oral exposure to polystyrene microplastic potentially increases the risk of insulin resistance. Environ Int. 164:1072732022. View Article : Google Scholar : PubMed/NCBI | |
|
Huang D, Zhang Y, Long J, Yang X, Bao L, Yang Z, Wu B, Si R, Zhao W, Peng C, et al: Polystyrene microplastic exposure induces insulin resistance in mice via dysbacteriosis and pro-inflammation. Sci Total Environ. 838(Pt 1): 1559372022. View Article : Google Scholar : PubMed/NCBI | |
|
Fan X, Wei X, Hu H, Zhang B, Yang D, Du H, Zhu R, Sun X, Oh Y and Gu N: Effects of oral administration of polystyrene nanoplastics on plasma glucose metabolism in mice. Chemosphere. 288(Pt 3): 1326072022. View Article : Google Scholar | |
|
Xie S, Zhang R, Li Z, Liu C, Chen Y and Yu Q: Microplastics perturb colonic epithelial homeostasis associated with intestinal overproliferation, exacerbating the severity of colitis. Environ Res. 217:1148612023. View Article : Google Scholar | |
|
Yin K, Wang Y, Zhao H, Wang D, Guo M, Mu M, Liu Y, Nie X, Li B, Li J and Xing M: A comparative review of microplastics and nanoplastics: Toxicity hazards on digestive, reproductive and nervous system. Sci Total Environ. 774:1457582021. View Article : Google Scholar | |
|
Xia X, Guo W, Ma X, Liang N, Duan X, Zhang P, Zhang Y, Chang Z and Zhang X: Reproductive toxicity and cross-generational effect of polyethylene microplastics in paramisgurnus dabryanus. Chemosphere. 313:1374402023. View Article : Google Scholar | |
|
Jin H, Yan M, Pan C, Liu Z, Sha X, Jiang C, Li L, Pan M, Li D, Han X and Ding J: Chronic exposure to polystyrene microplastics induced male reproductive toxicity and decreased testosterone levels via the LH-Mediated LHR/cAMP/PKA/StAR pathway. Part Fibre Toxicol. 19:132022. View Article : Google Scholar : PubMed/NCBI | |
|
Wei Z, Wang Y, Wang S, Xie J, Han Q and Chen M: Comparing the effects of polystyrene microplastics exposure on reproduction and fertility in male and female mice. Toxicology. 465:1530592022. View Article : Google Scholar | |
|
Abdel-Zaher S, Mohamed MS and Sayed AEH: Hemotoxic effects of polyethylene microplastics on mice. Front Physiol. 14:10727972023. View Article : Google Scholar : PubMed/NCBI | |
|
Lee JH, Kang JC and Kim JH: Toxic effects of microplastic (Polyethylene) on fish: Accumulation, hematological parameters and antioxidant responses in Korean Bullhead, Pseudobagrus fulvidraco. Sci Total Environ. 877:1628742023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang S, Chen L, Shi X, Wang Y and Xu S: Polystyrene microplastics-induced macrophage extracellular traps contributes to liver fibrotic injury by activating ROS/TGF-β/Smad2/3 signaling axis. Environ Pollut. 324:1213882023. View Article : Google Scholar |