Dulaglutide markedly prevents peritoneal fibrosis in a rodent model of chronic kidney disease: Insights into the pathogenesis
- Authors:
- Published online on: July 21, 2025 https://doi.org/10.3892/ijmm.2025.5592
- Article Number: 151
-
Copyright: © Yang et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Cardiovascular disease is the primary cause of morbidity and mortality in patients with end-stage renal disease (ESRD) undergoing long-term hemodialysis. This high risk is largely attributable to severe cardiovascular conditions and comorbidities, such as diabetes mellitus (DM), hypertension, advanced age and immunodeficiency present at the initiation of dialysis (1-8). Moreover, inadequate dialysis significantly contributes to high morbidity and mortality rates (8). Despite these challenges, renal replacement therapy (RRT) remains the most critical treatment for patients with ESRD worldwide. Specifically, peritoneal dialysis (PD), or ultrafiltration, is better suited for patients who retain some residual renal function (9). Currently, PD is an effective and affordable option for RRT, serving 9-11% of the global population being treated with dialysis (10).
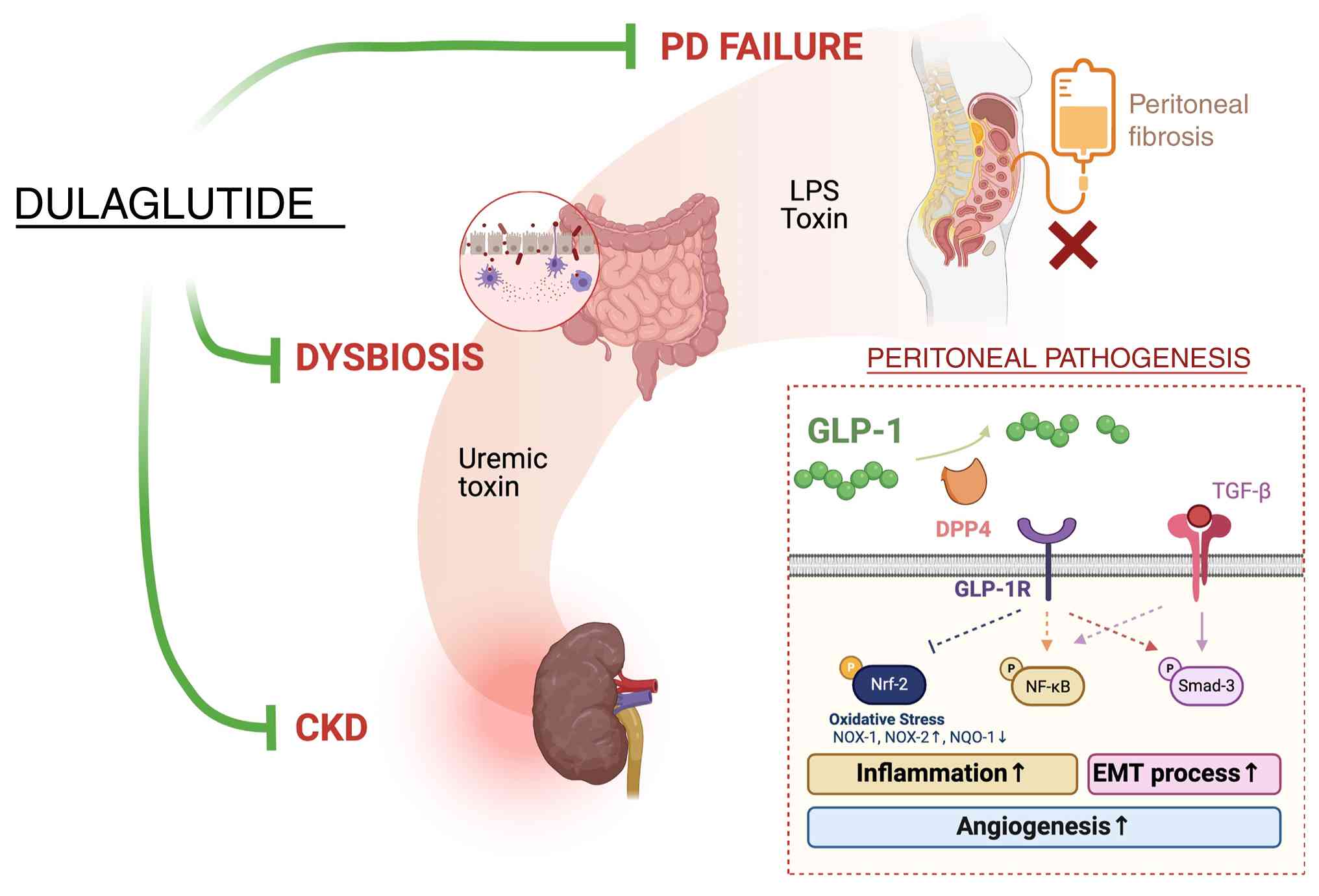
Hemodialysis (HD) through an arteriovenous shunt is recognized as the standard RRT method; however, it commonly leads to a rapid loss of residual renal function, potentially resulting in anuria even over short treatment intervals. Another limitation of this RRT method is the requirement for patients to remain in the HD room for restricted intervals, necessitating specialized nursing care, which can inconvenience patients and increase healthcare costs (11). By contrast, PD offers several advantages over HD: i) Patients can manage it themselves without technical challenges; ii) it can be performed at home; iii) it allows flexibility in timing; and iv) it is superior in preserving residual renal function. Nevertheless, 'dialysis inadequacy' remains a prevalent issue among patients with ESRD, particularly those with diabetes undergoing PD (12-15). Our previous study indicated that PD failure may primarily result from the activation of dipeptidyl peptidase 4 (DPP4) in the peritoneum following exposure to glucose or chlorhexidine gluconate (CG), leading to oxidative stress, inflammation, epithelial-mesenchymal transition (EMT) and peritoneal fibrosis (PF) (16). Inhibition of DPP4 using a DPP4 inhibitor or glucagon-like peptide 1 (GLP-1), both incretin-based therapies, has shown protective effects against fibrosis and dysfunction in rodents (16). Currently, there is a lack of effective methods to preserve or restore peritoneal function in patients undergoing PD, highlighting the importance of considering early prevention of PD failure.
The mesothelium is vital for peritoneal homeostasis and defending against infections; notably, it is well-documented that prolonged use of PD and recurrent episodes of peritonitis can lead to mesothelial denudation (17,18). Inflammation, inflammatory cell infiltration and the production of proinflammatory cytokines are initially critical in causing mesothelial cell injury and peritonitis, which can subsequently promote the local production of various inflammatory and fibrotic mediators, oxidative stress and PF, culminating in PD failure (19-22). This injury induces a transition of mesothelial cells to a mesenchymal phenotype through EMT (21,23). EMT involves the transformation of injured epithelial cells into sub-mesothelial myofibroblasts, which are extracellular matrix-secreting cells with contractile properties that are associated with fibrotic tissue development (24). Increased expression of TGF-β in mesothelial cells is key in translating injury signals into tissue changes, associated with fibrotic and angiogenic responses in patients undergoing long-term PD (19-22).
A previous study revealed that lipopolysaccharide (LPS) is commonly found in the peritoneal effluents of patients undergoing PD and is significantly elevated in those with peritonitis caused by Enterobacteriaceae (25). During bacterial peritonitis, LPS released by enterobacteria in the peritoneal cavity is crucial in promoting the inflammatory response (26). GLP-1 is known to impact the intestinal environment and can directly inhibit LPS-induced TNF-α production, providing anti-inflammatory effects (27). The potential benefits of GLP-1 against LPS-induced peritoneal injury include anti-inflammatory actions, alterations in gut microbiota and maintenance of mucosal integrity (28).
Dulaglutide, a novel, long-acting GLP-1 receptor (GLP-1R) agonist initially used to treat type 2 DM, acts as an incretin mimetic hormone or an analog of human GLP-1. The primary mechanism of dulaglutide is to increase insulin secretion when glucose levels are elevated, reduce glucagon secretion and delay gastric emptying to lower postprandial glucose levels (29). Notably, studies have shown that exendin-4, a GLP-1 receptor agonist, offers additional benefits in protecting tissues and organs from ischemic injuries beyond its blood sugar-lowering effects (30,31), suggesting the pleiotropic effects of GLP-1α. These effects are primarily mediated via its antioxidative and anti-inflammatory properties, and the upregulation of antioxidants (30,31). Our previous studies have demonstrated that exendin-4 therapy can protect the kidneys and preserve renal function in response to ischemia-reperfusion injury by upregulating antioxidants, and reducing inflammation, oxidative stress and fibrosis (30-34). Based on these findings, it may be hypothesized that dulaglutide therapy could prevent PD failure, particularly in diabetic patients with ESRD receiving PD.
Materials and methods
Mesothelial cell culture
The procedure and protocol for mesothelial cell culture have been detailed in our previous study (16). Met-5A mesothelial cells (cat. no. 65302), were obtained from Bioresource Collection and Research Center. This nonprofit organization, supported by the Taiwan government, upholds a strict quality control system, including sterility, mycoplasma contamination tests and short tandem repeat profiling analysis for each cell line. Met-5A cells were cultured in M199 medium (Gibco; Thermo Fisher Scientific, Inc.), supplemented with penicillin (100 U/ml) (Gibco; Thermo Fisher Scientific, Inc.), streptomycin (100 µg/ml) (Gibco; Thermo Fisher Scientific, Inc.) and 10% fetal bovine serum (Biowest), and were maintained at 37°C in a humidified atmosphere containing 95% air and 5% CO2.
Assessing the impact of dulaglutide on suppressing oxidative stress
To assess the therapeutic effects of dulaglutide on protecting Met-5A cells from injury induced by p-Cresol (a uremic compound that serves as an oxidative stress inducer), cells were co-cultured with stepwise increasing doses of dulaglutide (0, 20, 50 and 100 µM; cat. no. PS1434; Eli Lilly and Company) and 50 µM p-Cresol (cat. no. 805223; Sigma-Aldrich; Merck KGaA) for 24 h at 37°C. Human Met-5A pleural mesothelial cells were used in vitro and were divided into five groups: i) A1, Met-5A cells only; ii) A2, Met-5A cells + 50 µM p-Cresol, cultured for 24 h; iii) A3, Met-5A cells + 50 µM p-Cresol + 20 µM dulaglutide, co-cultured for 24 h; iv) A4, Met-5A cells + 50 µM -Cresol + 50 µM dulaglutide, co-cultured for 24 h; and v) A5, Met-5A cells + 50 µM p-Cresol + 100 µM dulaglutide, co-cultured for 24 h. The concentrations of p-Cresol (35) and dulaglutide (36) were selected based on our previous reports, with slight modifications: 50 µM p-Cresol, and 0, 20, 50 and 100 µM dulaglutide were used.
Assessing the safety of the optimal dulaglutide dosage in vitro
To determine whether a 100 µM dosage of dulaglutide adequately protected cell viability and prevented apoptosis under p-Cresol stimulation, the Met-5A cell line was divided into three groups: B1, Met-5A cells only; B2, Met-5A cells cultured with 50 µM p-Cresol; and B3, Met-5A cells co-cultured with 50 µM p-Cresol and 100 µM dulaglutide. Cells were collected at 24, 48 and 72 h for MTT assay. In addition, cells harvested at 72 h were subjected to flow cytometric analysis to evaluate intracellular reactive oxygen species (ROS) generation as well as early and late apoptotic events.
Assessing the impact of dulaglutide on suppressing the inflammatory reaction
To assess the therapeutic impact of dulaglutide on the expression of inflammatory markers by western blotting, Met-5A cells were used in vitro and were divided into the following groups: C1, Met-5A cells only; C2, Met-5A cells + 10 µg/ml LPS (cat. no. L2880; Sigma-Aldrich; Merck KGaA), cultured for 12 h; and C3, Met-5A cells pretreated with 100 µM dulaglutide for 12 h, followed by the addition of 10 µg/ml LPS and further culture for 12 h, totaling 24 h. Inflammatory responses were evaluated by detecting the expression levels of Toll-like receptor (TLR)-2, TLR-4, NF-κB, TNF-α and MMP-9 via western blot analysis, and CD11b+ cells were observed under a fluorescence microscope. The dosage of LPS (10 µg/ml) was based on our previous report (33).
Demonstrating the crucial roles of TGF-β and DPP4 in PF
To examine the roles of TGF-β and DPP4 in the development of EMT and PF, the Met-5A cell line was divided into the following groups: D1, Met-5A cells only; D2, Met-5A cells + 50 µM p-Cresol, cultured for 24 h; D3, double knockdown of TGF-β and DPP4 genes in Met-5A cells for 24 h; D4, Met-5A cells + double knockdown of TGF-β and DPP4 genes for 24 h, followed by treatment with 50 µM p-Cresol for an additional 24 h. For double knockdown of the TGF-β and DPP4 genes, the TGF-β, DPP4 and negative control small interfering (si)RNAs were used. The sequences were as follows: siRNA1-TGF-β, sense 5′-GGA GAG CCC UGG AUA CCA ATT-3′, antisense, 5′-UUG GUA UCC AGG GCU CUC CGG-3′ (cat. no. S133088; Thermo Fisher Scientific, Inc.); siRNA2-TGF-β, sense 5′-GCA ACA ACG CAA UCU AUG ATT-3′, antisense 5′-UCA UAG AUU GCG UUG UUG CGG-3′ (cat. no. S133090; Thermo Fisher Scientific, Inc.); siRNA1-DPP4, sense 5′-GCG UGA AUG AUA AAG GGC UTT-3′, antisense 5′-AGC CCU UUA UCA UUC ACG CTG-3′ (cat. no. S4254; Thermo Fisher Scientific, Inc.); siRNA2DPP4, sense 5′-GGU CAC CAG UGG GUC AUA ATT-3′, antisense 5′-UUA UGA CCC ACU GGU GAC CAT-3′ (cat. no. S4256; Thermo Fisher Scientific, Inc.). For efficient knockdown of the TGF-β and DPP4 genes, pooled siRNAs (siRNA1 and siRNA2) targeting each gene were used. For TGF-β knockdown, siRNA1-TGF-β and siRNA2-TGF-β were combined. Similarly, DPP4 knockdown was achieved using a combination of siRNA1-DPP4 and siRNA2-DPP4. The negative control siRNA was purchased from Santa Cruz Biotechnology, Inc. (scrambled siRNA control; cat. no. sc-37007). In the present study, the transient transfection of siRNAs was conducted using GenMute™ siRNA Transfection Reagent (cat. no. SL100568; SignaGen Laboratories) according to the manufacturer's instructions. Briefly, Met-5A cells at a density of ~1×106 cells were cultured to 50% confluence in 10-cm plates and were transfected with 5 µM siRNA-TGF-β, siRNA-DPP4 or negative control siRNA at 37°C for 24 h. Following 24 h of transfection, Met-5A cells were treated with 50 µM p-Cresol or harvested for further experiments.
MTT assay
For the MTT assay, 5,000 cells/well were plated in a 96-well plate. After an overnight incubation, the cells were treated with p-Cresol or dulaglutide for 1, 2 or 3 days. At the indicated time, the culture medium was removed, followed by the addition of 200 µl MTT reagent. After further incubation for 30 min, the reagent was removed, and the cells were solubilized with 100 µl DMSO. A spectrophotometer was used to record the optical density at 595 nm.
Evaluating the impact of dulaglutide on suppressing TGF-β and CG-induced EMT
Given that injury to peritoneal tissues can induce a transition of mesothelial cells to a mesenchymal phenotype through EMT, a cellular model was generated to investigate whether dulaglutide could inhibit this transition. The Met-5A cells were categorized into the following groups: E1, Met-5A cells only; E2, Met-5A cells + 3 ng/ml TGF-β1 (cat. no. 240-B; R&D Systems, Inc.) + 10 ng/ml epithelial growth factor (EGF; cat. no. AF-100-15; Peprotech; Thermo Fisher Scientific, Inc.), co-cultured for 5 days; E3, Met-5A cells + 5.0 µM CG (cat. no. GMP G-10846; Panion & BF Biotech Inc.), cultured for 5 days; E4, Met-5A cells + TGF-β1 + 10 ng/ml EGF + dulaglutide, co-cultured for 5 days; E5, Met-5A cells + CG + dulaglutide, co-cultured for 5 days. The dosages of TGF-β1, EGF and the culturing times were based on a previous report (16), with some modifications: 3 ng/ml TGF-β1 and 10 ng/ml EGF were used for 5 days at 37°C. TGF-β1 was used as a positive control for EMT. Cell morphology was subsequently observed under a fluorescence microscope (Olympus BX53; Olympus Corporation) at ×200 magnification.
Anesthesia protocol for animals
Prior to surgical procedures, an anesthesia box was pre-charged with 5% isoflurane for 5 min to fully saturate the atmosphere. Once the rat was placed inside, the concentration was reduced to 3% until the respiratory rate stabilized, then maintained at 2% throughout the surgical procedure. At the conclusion of the surgery, while preparing for suturing, the isoflurane level was reduced to 1%.
Animal model of CKD: Assessing the therapeutic impact of dulaglutide on peritoneal integrity and grouping
To determine whether dulaglutide therapy can protect rat PF against CG injury, the procedures and protocols from our recent report (16) were adapted, with the following modifications. Specifically, pathogen-free adult male Sprague-Dawley rats (Charles River Laboratories, Inc.), weighing 300-325 g and aged 70-75 days were used in the present study. All animal procedures were approved by the Institute of Animal Care and Use Committee at Kaohsiung Chang Gung Memorial Hospital (Affidavit of Approval of Animal Use Protocol No. 2021080501) and were performed in accordance with the Guide for the Care and Use of Laboratory Animals (37). Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care International-approved animal facility in the hospital with controlled humidity (55±10%), temperature (24°C) and light cycles (12-h light/dark cycle). Rats had free access to standard laboratory chow and autoclaved drinking water throughout the study. A total of 24 rats were randomly assigned into four groups (n=6/group) for experimental analysis. PF was induced through daily intraperitoneal injections of 0.1% CG in saline at a dose of 10 ml/kg body weight into the peritoneal cavity for 21 consecutive days. This experimental model of PF has been described in our previous report (16). The sham control (SC) group received an equal amount of 0.9% saline. Additionally, dulaglutide (10 µg/kg; cat. no. PS1434; Eli Lilly and Company) was administered subcutaneously to rats weekly for 3 weeks starting 1 week after the initial CG injection to evaluate early treatment effects aimed at preventing PF. The dosage of dulaglutide (10 µg/kg) was based on our previous studies (34,36) with some modifications-, dulaglutide (10 µg/kg) was used here as a GLP-1 receptor agonist.
The rats were divided into the following four groups: i) SC group, receiving only normal saline injections; ii) CKD group; iii) CKD + CG group, with PF induced by CG; and iv) CKD + CG + dulaglutide group, PF was induced by CG and rats received weekly subcutaneous injections of 10 µg/kg dulaglutide for 3 consecutive weeks, starting on day 21 after CKD induction. Notably, it is not possible to create an animal model of ESRD due to the high mortality and rapid progression to uremia, which compromise long-term survival and experimental control. Instead, a model of CKD was developed, which simulates conditions similar to ESRD. Moreover, PF induction was initiated on day 14 following CKD induction.
Experimental model of CKD induction
The procedure and protocol for CKD induction have been detailed in our previous report (38). Briefly, all rats were anesthetized with 2.0% isoflurane via inhalation, placed supine on a warming pad at 37°C and underwent midline laparotomies. The SC rats underwent laparotomy only, whereas CKD was induced in the CKD groups by right nephrectomy and arterial ligation of the upper two-thirds (upper and middle poles) of the blood supply to the left kidney, preserving only the lower third (lower pole) with normal blood supply. This model preserves a limited amount of functioning renal parenchyma to simulate CKD.
Serial collection of peripheral blood and peritoneal fluid: Evaluation of blood urea nitrogen (BUN), creatinine, DPP4 and GLP-1 levels at day 42
Blood samples (1 ml) were serially collected via the lateral tail vein before and after the CKD induction (i.e., prior to and at days 14, 35 and 42 before euthanasia) to evaluate the creation of the CKD model and the therapeutic impact of dulaglutide on protecting residual renal function and suppressing the PF process. Serum levels of creatinine and BUN, and circulating levels of DPP4 and GLP-1, as well as peritoneal fluid levels of GLP-1 and DPP4, were measured in duplicate using standard laboratory equipment. Immediately after sacrifice and prior to abdominal dissection, 10 ml sterile, pre-warmed PBS was gently injected into the peritoneal cavity using a sterile syringe. The abdomen was lightly massaged for 30 sec to distribute the fluid, after which, a second syringe was used to aspirate the peritoneal lavage fluid, typically recovering 5-8 ml/rat. The fluid was centrifuged at 200 × g for 10 min at 4°C to remove cells and debris, and the supernatant was collected and detecttored at −80 °C. Detection of DPP4 (cat. no. ab204722; Abcam) and GLP-1 (cat. no. EGLP-35K; MilliporeSigma) were measured in duplicate using ELISA kits according to the manufacturers' instructions.
Urine collection
The procedure for 24-h urine collection is described in our previous report (38). For this study, each rat was placed in a metabolic cage (DXL-D; dimensions: 190×290×550 mm3; Suzhou Fengshi Laboratory Animal Equipment Co., Ltd.) for 24 h with free access to food and water. Urine was collected over 24 h prior to and at days 14, 35 and 42 after CKD induction to determine the ratio of urine protein to creatinine. Urine samples were centrifuged at 380 × g for 10 min at 4°C to remove particulate matter, and the samples were aliquoted and stored at -80°C until biochemical analysis. Urinary protein levels were measured using a bicinchoninic acid (BCA) protein assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc.). Urinary creatinine was measured using the Creatinine Assay Kit-Colorimetric (cat. no. ab65340; Abcam). Assays were performed according to the manufacturers' instructions,
Flow cytometric analysis
To evaluate intracellular ROS generation, mitochondrial ROS levels and apoptotic cell death in Met-5A cells (groups B1, B2 and B3), cells were collected at 72 h, washed with PBS, and stained with DCFDA (cat. no. ab113851; Abcam) for 30 min, mitoSOX (cat. no. M36008; Invitrogen; Thermo Fisher Scientific, Inc.) for 15 min, and Annexin V-FITC and PI (cat. no. 556547; BD Pharmingen; BD Biosciences) for 30 min at 37°C. To identify and characterize neutrophils and myeloid-derived inflammatory cells in circulating blood and abdominal fluid, samples were collected from experimental animals and stained with CD11b/c-PE (cat. no. 554862; BD Pharmingen; BD Biosciences), Ly6g-FITC (cat. no. ab25024; Abcam) and intracellular myeloperoxidase (MPO)-FITC (cat. no. ab90812; Abcam) for 30 min at 4°C. Cell suspensions were washed with PBS and then analyzed on a flow cytometer. Flow cytometric analysis was performed at the Translational Research Center, Kaohsiung Chang Gung Memorial Hospital (Kaohsiung, Taiwan) using a BD FACSCanto™ II flow cytometer (BD Pharmingen; BD Biosciences). Data were acquired with BD FACSDiva™ Software, version 8.0.1 (BD Pharmingen; BD Biosciences) and analyzed using FlowJo™ Software, version 10.8.1 (BD Pharmingen; BD Biosciences).
Animal grouping to evaluate the impact of dulaglutide therapy on inflammation-induced peritoneal damage
To assess the impact of peritoneal inflammatory reactions on inducing mesothelial cell damage and subsequent PF, 18 additional Sprague-Dawley rats were divided into the following three groups (n=6/group): i) SC group, receiving normal saline; ii) LPS-induced peritonitis group, rats received a single intra-peritoneal injection of 3.8% glucose (2.0 ml) and peritonitis was induced through a single intraperitoneal administration of LPS (1.5 mg/kg); and iii) peritonitis + dulaglutide group, rats received glucose and peritonitis was induced through intraperitoneal administration of LPS, with a pre- and post-induction subcutaneous injection of 10 µg/kg dulaglutide 3 days prior to and 1 day after peritonitis induction.
The rats were euthanized on day 5, which was defined as the acute phase of peritonitis. Prior to euthanasia, a catheter was inserted into the jugular vein, and the abdomen was opened. Peritoneal fluid was aspirated and replaced with 1 ml 3.33 M FITC-dextran 10000 (Sigma-Aldrich; Merck KgaA). All rats were anesthetized with 2.0% isoflurane via inhalation and blood samples (1 ml) were drawn from the jugular vein at intervals of 10, 20 and 30 min to measure the concentration of FITC-dextran. After collecting the final blood sample, the rats were euthanized by exsanguination under deep anesthesia. The procedure and protocol for testing the leakage of FITC-dextran from circulation into the peritoneum were based on our previous study with the following modifications (39). Plasma samples collected at each time point were centrifuged at 1,000 × g for 10 min at 4°C to remove cellular debris. The supernatants were collected and transferred to a black 96-well plate to determine the concentration of FITC-dextran using a fluorescence microplate reader (SpectraMax; Molecular Devices, LLC) with an excitation wavelength of 485 nm and an emission wavelength of 530 nm. All measurements were performed in triplicate.
Method for animal euthanasia and histopathological assessment of kidney injury at day 42 post-CKD induction
At day 42 post-CKD induction, all rats-including those in SC, CKD, CKD + CG and CKD + CG + dulaglutide groups, were euthanized under the same conditions to ensure consistency in tissue sampling and data comparison. Rats were deeply anesthetized with 2.0% isoflurane, and euthanasia was performed by exsanguination via terminal blood collection (>10 ml) under anesthesia. This procedure typically required ~5 min, during which 2.0% isoflurane inhalation was maintained. Following respiratory arrest, the kidneys and peritoneum were harvested for histopathological analysis.
The histopathological scoring of kidney injury was conducted in a blinded fashion as described in our previous report (40). Briefly, left kidney specimens from all rats were fixed in 10% buffered formalin for 24 h at 25°C, embedded in paraffin, and sectioned at 4 µm. Sections were deparaffinized, rehydrated, and stained with hematoxylin for 5 min and eosin for 2 min. After dehydration and mounting, sections were evaluated by light microscopy evaluation. The injury was scored based on the grading of tubular necrosis, loss of brush border, cast formation and tubular dilatation across 10 randomly selected, non-overlapping fields (×200 magnification) for each animal as follows: 0 (none), 1 (≤10%), 2 (11-25%), 3 (26-45%), 4 (46-75%) and 5 (≥76%).
Western blot analysis of left kidney specimens and cultured cells
The procedure and protocol for western blotting have been described in our previous reports (41-46). Kidney tissues or cells were lysed in M-PER™ (cat. no. 78501; Thermo Fisher Scientific, Inc.) containing protease (cat. no. 539134; Sigma-Aldrich; Merck KGaA) and phosphatase inhibitor cocktails (cat. no. 524629; Sigma-Aldrich; Merck KGaA). Protein concentration was determined using the BCA assay (Pierce™ BCA Protein Assay Kit; cat. no. 23225; Thermo Fisher Scientific, Inc.), following the manufacturer's instructions, with bovine serum albumin (BSA) (cat. no. A9418; Sigma-Aldrich; Merck KgaA) as a standard. Equal amounts (50 µg) of protein extracts were loaded and separated by SDS-PAGE on 12% gels using acrylamide gradients. After electrophoresis, the proteins were transferred electrophoretically to polyvinylidene difluoride membranes (Amersham; Cytiva). Non-specific binding was blocked by incubating the membranes in blocking buffer (5% non-fat dry milk in TBS containing 0.05% Tween 20) overnight at 4°C. Primary antibodies used in the western blot analysis included: NOX-1 (1:1,000; cat. no. SAB4200097; Sigma-Aldrich; Merck KGaA), NOX-2 (1:1,000; cat. no. MABS2195; Sigma-Aldrich; Merck KGaA), TNF-α (1:1,000; cat. no. 3707; Cell Signaling Technology, Inc.), IL-1α (1:1,000; cat. no. 84618; Cell Signaling Technology, Inc.), MMP-9 (1:1,000; cat. no. ab76003; Abcam), DPP4 (1:1,000; cat. no. ARP63319_P050; Aviva Systems Biology), phosphorylated (p)-Smad3 (1:1,000; cat. no. 9520; Cell Signaling Technology, Inc.), Smad3 (1:1,000; cat. no. 9513; Cell Signaling Technology, Inc.), TGF-β (1:3,000; cat. no. ab215715; Abcam), GLP-1 (1:1,000; cat. no. ab108443; Abcam), GLP-1R (1:1,000; cat. no. ab218532; Abcam), nuclear factor erythroid 2-related factor 2 (Nrf2; 1:1,000; cat. no. ab62352; Abcam), NAD(P)H quinone oxidoreductase 1 (NQO-1; 1:1,000; cat. no. ab80588; Abcam), Snail (1:1,000; cat. no. 3879; Cell Signaling Technology, Inc.), von Willebrand factor (vWF; 1:1,000; cat. no. ab154193; Abcam), VEGF (1:1,000; cat. no. ab214424; Abcam), α-smooth muscle actin (α-SMA; 1:6,000; cat. no. A2547; Sigma-Aldrich; Merck KGaA), vimentin (1:1,000; cat. no. 5741; Cell Signaling Technology, Inc.), β-catenin (1:1,000; cat. no. 8480; Cell Signaling Technology, Inc.), fibronectin (1:1,000 cat. no. ab2413; Abcam), collagen I (1:1,000; cat. no. C2456; Sigma-Aldrich; Merck KGaA), N-cadherin (1:1,000; cat. no. 13116; Cell signaling Technology, Inc.), TLR-2 (1:4,000; cat. no. ab213676; Abcam), TLR-4 (1:4,000; cat. no. NB100-56566; Novus Biologicals, LLC; Bio-Techne), NF-κB (1:1,000; cat. no. 8242; Cell Signaling Technology, Inc.), p-NF-κB (1:1,000; cat. no. 3033; Cell Signaling Technology, Inc.), CD31 (1:1,000; cat. no. 77699; Cell Signaling Technology, Inc.) and β-actin (1:10,000; cat. no. A5441; MilliporeSigma). The membranes were then incubated with the indicated primary antibodies for 1 h at room temperature, followed by a horseradish peroxidase-conjugated anti-rabbit (1:3,000; cat. no. A0545; MilliporeSigma) or anti-mouse immunoglobulin IgG (1:3,000; cat. no. A5278; MilliporeSigma) for 1 h at room temperature. The washing procedure was repeated eight times within 1 h. Immunoreactive bands were visualized using enhanced chemiluminescence (Amersham; Cytiva) and exposed to Biomax L film (Kodak). For semi-quantification purposes, ECL signals were digitized using Labwork software (version 4.6; Analytik Jena AG).
Immunofluorescence (IF) analysis and Masson's trichrome staining
The procedures and protocols for IF studies were based on our previous reports (40-46). Kidney and peritoneum tissues were fixed in 10% neutral-buffered formalin at room temperature (25°C) for 24 h, embedded in paraffin and cut into 4-µm sections using a rotary microtome. Sections were then deparaffinized in xylene and rehydrated through a graded ethanol series. Citrate buffer (10 mM, pH 6.0) was used to perform antigen retrieval in a microwave for 15 min and sections were incubated in 5% BSA in PBS for 1 h at room temperature (22-25°C) to block non-specific binding. Sections were then incubated overnight at 4°C with rabbit anti-MMP-9 antibody (1:200; cat. no. MA5-14228; Invitrogen; Thermo Fisher Scientific, Inc.) or IgG Isotype Control (1:200, cat. no. 02-6102; Invitrogen; Thermo Fisher Scientific, Inc.). After washing with PBS, the sections were incubated with goat anti-rabbit IgG (H+L) Alexa Fluor® 488-conjugated secondary antibody (1:500; cat. no. A-11008; Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h at room temperature in the dark. Sections were counterstained with DAPI (1 µg/ml) for 5 min, rinsed and mounted with antifade mounting medium. For semi-quantification, three randomly selected high-power fields (HPFs) at ×200 magnification were evaluated in each section under a fluorescence microscope (Olympus BX53; Olympus Corporation). The mean number per HPF for each animal was determined by summing all counts and dividing by 9. Furthermore, tissue slides were reviewed by an expert in rodent pathology.
For IF staining of cultured cells, the cells were cultured on sterile glass coverslips in 12-well plates. After treatment, the cells were rinsed with PBS, fixed with 4% paraformaldehyde in PBS for 15 min at 22-25°C, then permeabilized with 0.1% Triton X-100 in PBS for 10 min at room temperature. Non-specific binding was blocked using 5% BSA in PBS for 1 h at room temperature. Cells were incubated overnight at 4°C with anti-CD11b (1:200; cat. no. 114-0118-82; Invitrogen; Thermo Fisher Scientific, Inc.), and after washing with PBS twice, they were incubated for 1 h at 4°C with Goat anti-mouse IgG (H+L), Alexa Fluor 488 (1:500; cat. no. A-11008; Invitrogen; Thermo Fisher Scientific, Inc.). For phalloidin staining, the cells were incubated 1 h at 4°C with Phalloidin-iFluor 488 (1:400; cat. no. ab176753; Abcam). Subsequently, the cells were counterstained with DAPI for 5 min, rinsed, mounted with antifade mounting medium and observed under a fluorescence microscope (Olympus BX53; Olympus Corporation).
For Masson's staining, paraffin-embedded peritoneal tissue sections (4 µm) were deparaffinized, rehydrated and subjected to Masson's trichrome staining using a standard protocol (Masson's Trichrome Stain Kit; cat. no. 87019; Thermo Fisher Scientific, Inc.). Briefly, sections were fixed in Bouin's solution at 56°C for 1 h to enhance staining, followed by staining with Weigert's iron hematoxylin for nuclei at room temperature for 10 min, Biebrich scarlet-acid fuchsin for cytoplasm and muscle fibers at room temperature for 10 min, and aniline blue for collagen at room temperature for 5 min. Slides were then differentiated in phosphomolybdic-phosphotungstic acid, dehydrated through graded ethanol, cleared in xylene and mounted. The extent of fibrosis was evaluated under a light microscope and was semi-quantified using ImageJ software (version 1.51; National Institutes of Health).
Statistical analysis
Quantitative data are presented as the mean ± SD. Statistical analyses were conducted using SAS statistical software, version 8.2 (SAS Institute, Inc.). One-way ANOVA followed by Bonferroni's multiple comparisons post hoc test was used to compare variables among groups. Additionally, kidney injury scores were presented as the median (interquartile range), and were analyzed using Kruskal-Wallis test and Dunn's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Protein expression levels of biomarkers of oxidative stress, inflammation, fibrosis and insulin regulation following p-Cresol and dulaglutide treatment
It is well-known that uremic toxic substances frequently induce oxidative stress and inflammatory reactions (47). To assess the impact of dulaglutide on suppressing these detrimental signaling pathways, the Met-5A cell line was split into five groups, and western blot analysis was conducted. The results showed that the protein expression levels of NOX-1 and NOX-2 (Fig. S1A, B and E), indicators of oxidative stress, and TNF-α, IL-1β and MMP-9 (Fig. S1A, F, H and I), markers of inflammation, were significantly higher in A2 compared with in A1. Notably, the levels of these markers were significantly and progressively reversed in groups A3-A5, suggesting that dulaglutide may effectively suppress uremic toxic substance-induced oxidative stress and inflammation, particularly at higher dosages.
Additionally, the protein expression levels of DPP4 (Fig. S1A and J), a marker of hyperglycemia and insulin sensitivity suppression, and p-Smad3/Smad3 and TGF-β (Fig. S1A, D and G), indicators of fibrosis, exhibited a similar pattern. Conversely, the protein expression levels of GLP-1 and GLP-1R (Fig. S1A, C and L), which promote insulin secretion displayed an opposite pattern to oxidative stress markers among the groups. The antioxidants Nrf2 and NQO-1 (Fig. S1A, K and M) were significantly higher in A5 and progressively decreased in groups A2-A4 compared with in A1.
Impact of dulaglutide therapy on regulating cell viability, and early and late apoptosis under p-Cresol stimulation
Next, the present study aimed to determine whether a 100 µM concentration of dulaglutide (i.e., the highest dose utilized in Fig. S1) was safe for the cell culture study. The cell line was divided into groups B1-B3, and the MTT assay and flow cytometric analysis were conducted. The results showed that cell viability was significantly lower in B2 cells compared with that in the B1 group, but that it was significantly reversed in the B3 group at 24, 48 and 72 h (Fig. S2A-C). Additionally, after 72 h, total intracellular (Fig. S2D and E) and mitochondrial (Fig. S2F and G) ROS levels, along with early (Annexin-V+/PI−) and late (Annexin-V+/PI+) apoptosis (Fig. S2H-J) in Met-5A cells, were significantly higher in the B2 group than in the B1 and B3 groups, but were significantly higher in the B3 group than in the B1 group. These findings suggested that 100 µM dulaglutide not only provided adequate cell protection against ROS levels and apoptosis but also was not harmful to the Met-5A cells. Accordingly, 100 µM dulaglutide was utilized in the subsequent in vitro study.
Impact of dulaglutide on inhibiting LPS-induced inflammation
Previous studies have demonstrated that GLP-1 antagonists protect renal function from inflammation and oxidative stress-induced kidney damage in settings of acute kidney injury (48,49) and CKD, mainly through the suppression of DPP4 activity (16). To verify the therapeutic impact of dulaglutide on attenuating the LPS-induced inflammatory reaction, mimicking the clinical setting of endotoxin-mediated peritonitis, Met-5A cells were used. The results of western blotting showed that the protein expression levels of TLR-2, TLR-4, NF-κB, TNF-α and MMP-9 were significantly higher in group C2 compared with those in group C1, but were significantly reversed in group C3 (Fig. S3A-F). Additionally, IF analysis revealed that the positively stained CD11b surface marker in Met-5A cells was markedly increased in group C2 compared with in group C1, consistent with enhanced inflammatory response (Fig. S3G-J), suggesting that dulaglutide may effectively suppress endotoxin-induced peritonitis in patients undergoing PD.
Successful gene transfection
To verify whether the knockdown of TGF-β and DPP4 genes was specific, western blotting was performed to examine protein levels. The results confirmed successful transfection, as the expression levels of TGF-β and DPP4 were markedly reduced in the knockdown group compared with those in the control and negative control groups. The results also showed that transfection alone did not alter the protein levels of EMT-, oxidative stress-, or inflammation-related biomarkers compared with those in the control and negative control groups (Fig. S4).
Unique roles of DPP4 and TGF-β in the EMT process and fibrosis
Our previous studies have established the association between the EMT process and lung and kidney fibrosis (16,41). The present study aimed to elucidate whether DPP4 and TGF-β serve crucial roles in regulating the EMT process and tissue fibrosis, which can result in PF and, ultimately, PD failure. An in vitro study was conducted using western blot analysis. The protein expression levels of TGF-β (Fig. S5A and G) and p-Smad3 (Fig. S5A and F), indicators of fibrosis; Snail (Fig. S5A and I), α-SMA (Fig. S5A and J), vimentin (Fig. S5A and C) and β-catenin (Fig. S5A and K), markers of EMT; NOX-1 (Fig. S5A and L) and NOX-2 (Fig. S5A and D), indicators of oxidative stress; and NF-κB (Fig. S5A and E) and TNF-α (Fig. S5A and M), markers of inflammation, were significantly higher in group D2 compared with those in D1. The efficiency of the double knockdown was confirmed by markedly reduced protein expression levels of both TGF-β and DPP4 (Fig. S4). Notably, the levels of these markers were significantly reversed in the D3 group, which underwent successful double silencing of TGF-β and DPP4, but were then upregulated in group D4. By contrast, the protein expression levels of GLP-1 (Fig. S5A and B) displayed an opposite trend to the aforementioned markers. These findings indicated that these two genes may have key roles in EMT and PF.
Expression levels of EMT biomarkers in Met-5A cells undergoing TGF-β and CG stimulation
Based on the aforementioned findings, the current study aimed to determine whether dulaglutide could suppress the upregulation of EMT biomarkers induced by TGF-β1 and CG in the Met-5A cell line. After culturing for 5 days, cells were collected for western blot analysis. The results showed that the protein expression levels of p-Smad3 (Fig. S6A and B), TGF-β (Fig. S6A and E), Snail (Fig. S6A and C), α-SMA (Fig. S6A and G), vimentin (Fig. S6A and D), β-catenin (Fig. S6A and F) and fibronectin (Fig. S6A and H), which are key EMT markers, were lowest in group E1, highest in group E2, and significantly higher in group E3 than in groups E4 and E5, with levels in E4 also being significantly higher than those in E5. These findings suggested that dulaglutide treatment may effectively inhibit the EMT process.
Identification of the morphological features of the Met-5A cell cytoskeleton
To elucidate the morphological features of the cytoskeleton in Met-5A cells, which can be used as indicators of the EMT process, the cells were divided into groups E1-E5, and IF staining was performed. The aim of this experiment was to determine whether dulaglutide could counteract CG-induced EMT-like cytoskeletal changes in Met-5A cells. The results demonstrated that the cytoskeleton (Fig. S7A-E) not only appeared more crowded in groups E2 and E3, but also exhibited a more typical mesenchymal cell morphology, with spindle-shaped features (Fig. S7G). Additionally, significant increases in cell length (Fig. S7F) and the mean fluorescence intensity of phalloidin staining (Fig. S7H) were observed in groups E2 and E3 compared with those in groups E1, E4 and E5. These elevated cell length and fluorescence intensity values were also significantly higher in groups E4 and E5 compared with those in group E1. This attenuation of EMT-associated cytoskeletal remodeling in the presence of dulaglutide further suggests that dulaglutide treatment significantly suppressed the EMT process in CG-treated renal tubular epithelial cells.
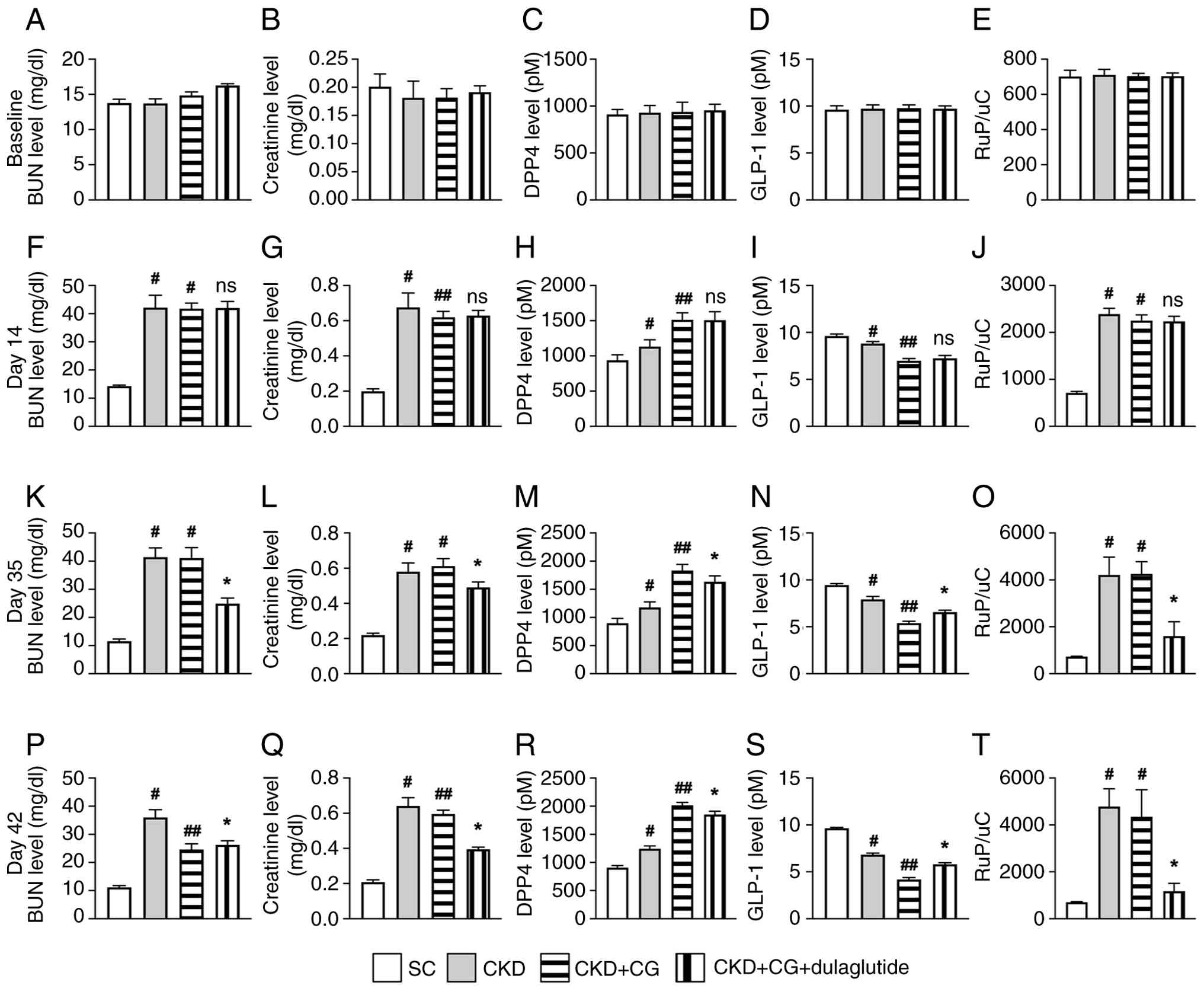
Changes in serum levels of BUN, creatinine, DPP4 and GLP-1, and the ratio of urine protein/urine creatinine (RUp/c)
To assess the protective effects of dulaglutide against CKD-induced kidney damage and its impact on DPP4 activity, time-course blood sampling, biochemical examinations and ELISA were conducted in an in vivo study (Figs. 1 and S8). The experimental groups were as follows: SC, CKD, CKD + CG and CKD + CG + dulaglutide. The baseline serum levels of BUN (Fig. 1A), creatinine (Fig. 1B), DPP4 (Fig. 1C), GLP-1 (Fig. 1D) and RUp/c (Fig. 1E) showed no significant differences among the groups. However, by day 14 post-CKD induction, the circulating levels of BUN (Fig. 1F), creatinine (Fig. 1G) and RUp/c (Fig. 1J) were markedly lower in the SC group than in groups CKD, CKD + CG and CKD + CG + dulaglutide, with no differences noted among the latter three groups. Additionally, ELISA results indicated that the circulating levels of DPP4 (Fig. 1H) were markedly lower in the SC group than those in groups CKD, CKD + CG and CKD + CG + dulaglutide, and l with no difference between groups CKD + CG and CKD + CG + dulaglutide. Conversely, the circulating levels of GLP-1 (Fig. 1I) exhibited an opposite pattern to DPP4 among the groups.
Post-CKD induction, the circulating levels of creatinine on day 35 (Fig. 1L) and DPP4 on days 35 and 42 (Fig. 1M and R) were highest in the CKD + CG groups, lowest in the SC group, and significantly lower in the CKD + CG + dulaglutide group than in the CKD group, whereas the circulatory level of GLP-1 displayed an opposite pattern to DPP4 among the groups (Fig. 1N and S). However, the circulating levels of creatinine on day 42 (Fig. 1Q) were highest in the CKD groups, lowest in the SC group, and significantly lower in the CKD + CG + dulaglutide group than in the CKD + CG group. Furthermore, on days 35 and 42 post-CKD induction, BUN (Fig. 1K and P) and RUp/c (Fig. 1O and T) were highest in the CKD group and lowest in the SC group.
Protein expression levels of EMT biomarkers in the peritoneum on day 42 after CKD induction
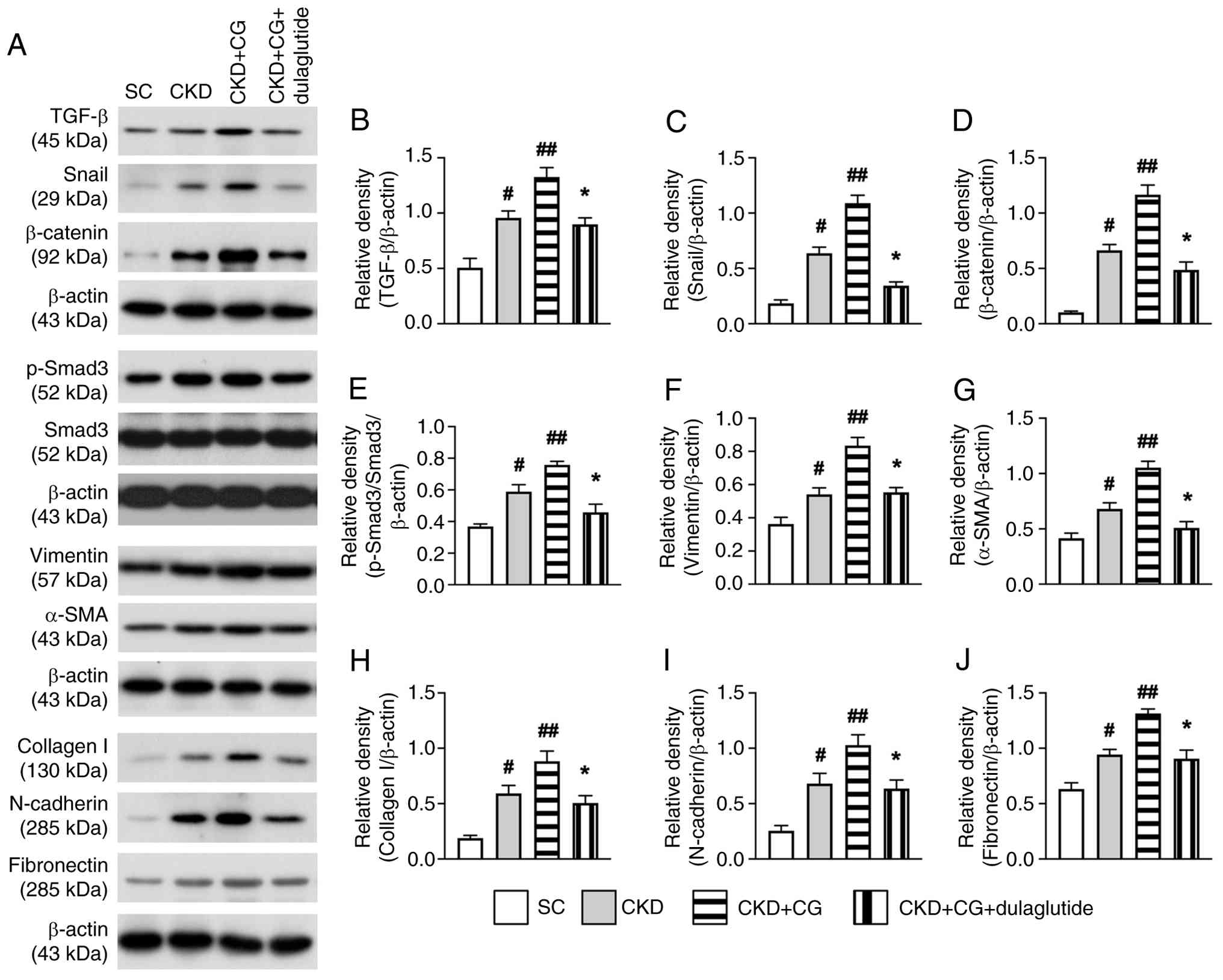
To explore the impact of PF on the upregulation of EMT biomarkers and the mitigating effects of dulaglutide treatment, western blot analysis was utilized in the present study. As anticipated, the protein expression levels of the EMT biomarkers TGF-β (Fig. 2A and B), Snail (Fig. 2A and C), β-catenin (Fig. 2A and D), p-Smad3/Smad3 (Fig. 2A and E), vimentin (Fig. 2A and F), α-SMA (Fig. 2A and G), collagen I (Fig. 2A and H), N-cadherin (Fig. 2A and I) and fibronectin (Fig. 2A and J) were highest in the CKD + CG group, lowest in the SC group, and significantly lower in the CKD + CG + dulaglutide group than in the CKD group. These findings suggested that PF augmented the expression levels of EMT biomarkers, which were markedly suppressed by dulaglutide treatment.
Protein expression levels of markers of oxidative stress, inflammation and angiogenesis, DPP4, GLP-1R and antioxidants in the peritoneum on day 42 after CKD induction
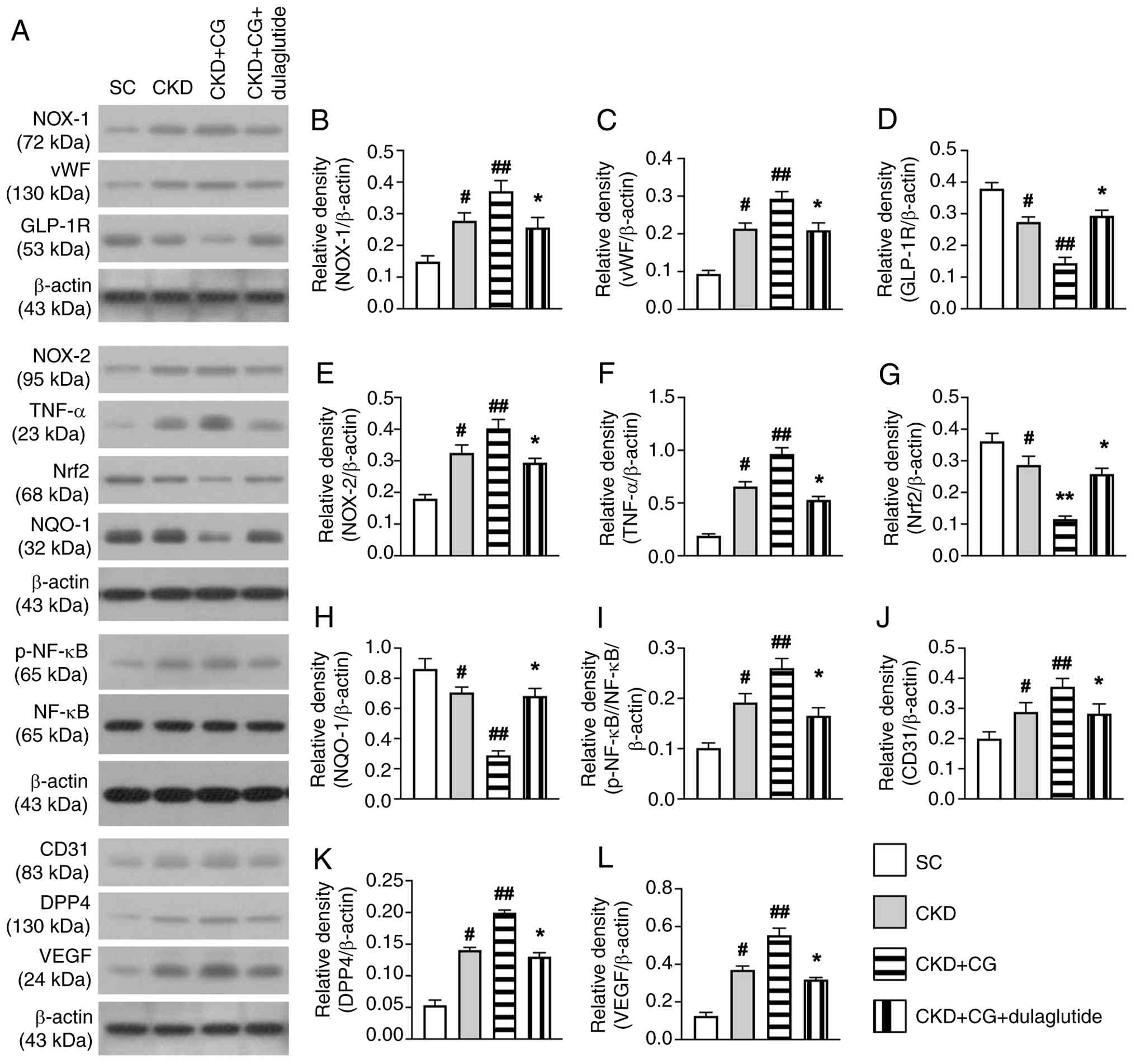
Subsequently, the present study aimed to examine the effects of PF and dulaglutide therapy on the regulation of molecular markers in the rat peritoneum using western blot analysis. The results indicated that the protein expression levels of NOX-1 (Fig. 3A and B) and NOX-2 (Fig. 3A and E), markers of oxidative stress; and p-NF-κB (Fig. 3A and I) and TNF-α (Fig. 3A and F), markers of inflammation; and VEGF (Fig. 3A and L), an angiogenic factor, were lowest in the SC group, highest in the the CKD + CG group, and significantly lower in the CKD + CG + dulaglutide group than in the CKD group. Similarly, the protein expression levels of CD31 (Fig. 3A and J) and vWF (Fig. 3A and C), which are indicators of angiogenesis and endothelial function, and DPP4 (Fig. 3A and K), which is involved in oxidative stress, exhibited similar trends.
By contrast, the protein expression levels of GLP-1R (Fig. 3A and D), Nrf2 (Fig. 3A and G) and NQO-1 (Fig. 3A and H), markers of an antioxidative response, exhibited a pattern opposite to that of oxidative stress markers among the groups. These findings suggested that dulaglutide therapy could markedly suppress oxidative stress and abnormal angio-genesis in the peritoneum.
Histopathological findings in the rat peritoneum on day 42 after CKD induction
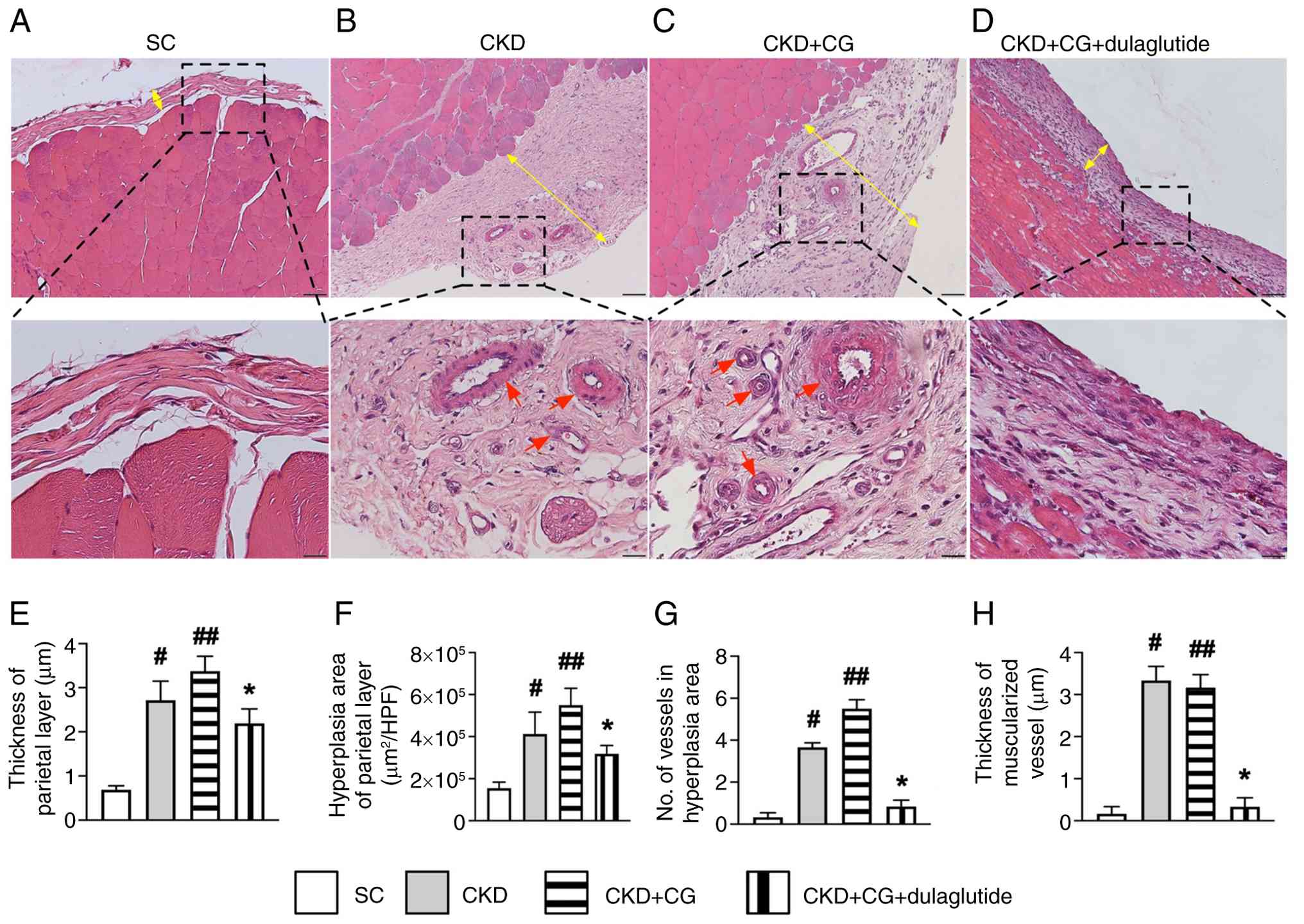
To explore the ultrastructural features of the peritoneum, microscopy was employed to identify histopathological changes in the rat peritoneum (Fig. 4A-D) following the induction of CKD, using hematoxylin and eosin staining. The results showed that the thickness (Fig. 4E) and hyperplasia (Fig. 4F) of the parietal layer of the peritoneum, both indicators of peritoneal proliferation, and the number of vessels (defined as ≤25 µm) (Fig. 4G) were highest in the CKD + CG group than in the other groups, higher in the CKD group than the SC and CKD + CG + dulaglutide groups, and were moderately higher in the CKD + CG + dulaglutide group than in the SC group. Furthermore, the number of muscularized vessels (Fig. 4H) in the parietal layer exhibited a similar pattern to that of small vessels among the groups, except it was similar between groups CKD and CKD + CG. The histopathological findings confirmed the successful creation of a PF model in CKD rats that mimics the clinical setting of PF in patients undergoing PD. These findings suggested that GLP-1 analogs may have potential clinical use in patients undergoing PD to prevent PD failure.
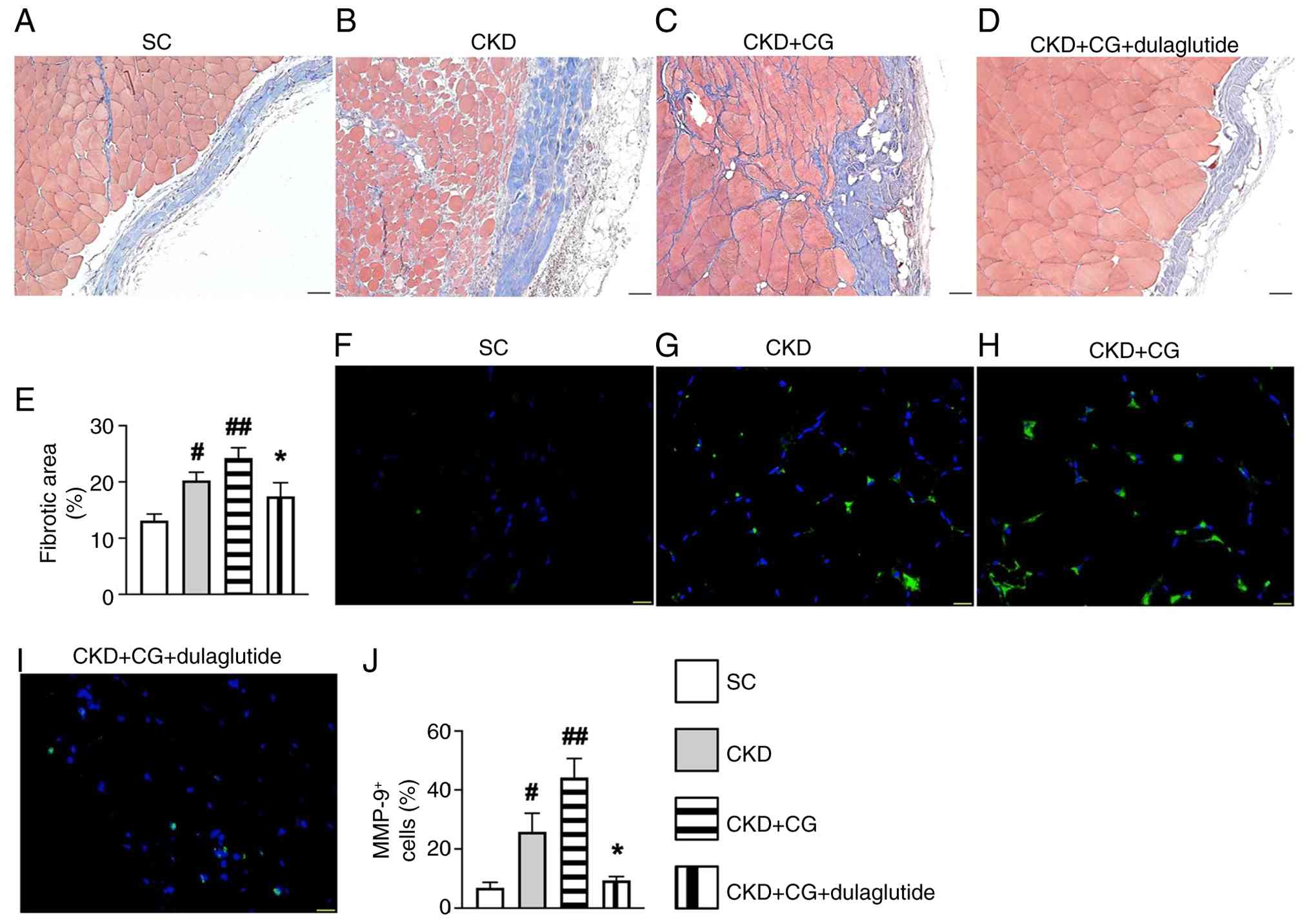
Fibrosis and inflammatory cell infiltration in the rat peritoneum on day 42 after CKD induction
Moreover, Masson's trichrome and IF staining were used to verify the changes in fibrosis and inflammatory cell infiltration in the peritoneum following CG stimulation and dulaglutide therapy. The results revealed that the fibrotic area (Fig. 5A-E) and MMP-9 cell infiltration (Fig. 5F-J), an index of inflammation, in the parietal layer of the peritoneum were lowest in the SC group 1, highest in the CKD + CG group, and significantly lower in the CKD + CG + dulaglutide group than in the CKD group. These findings indicated that dulaglutide therapy may be promising for clinical use in patients undergoing PD to prevent PF and inflammation.
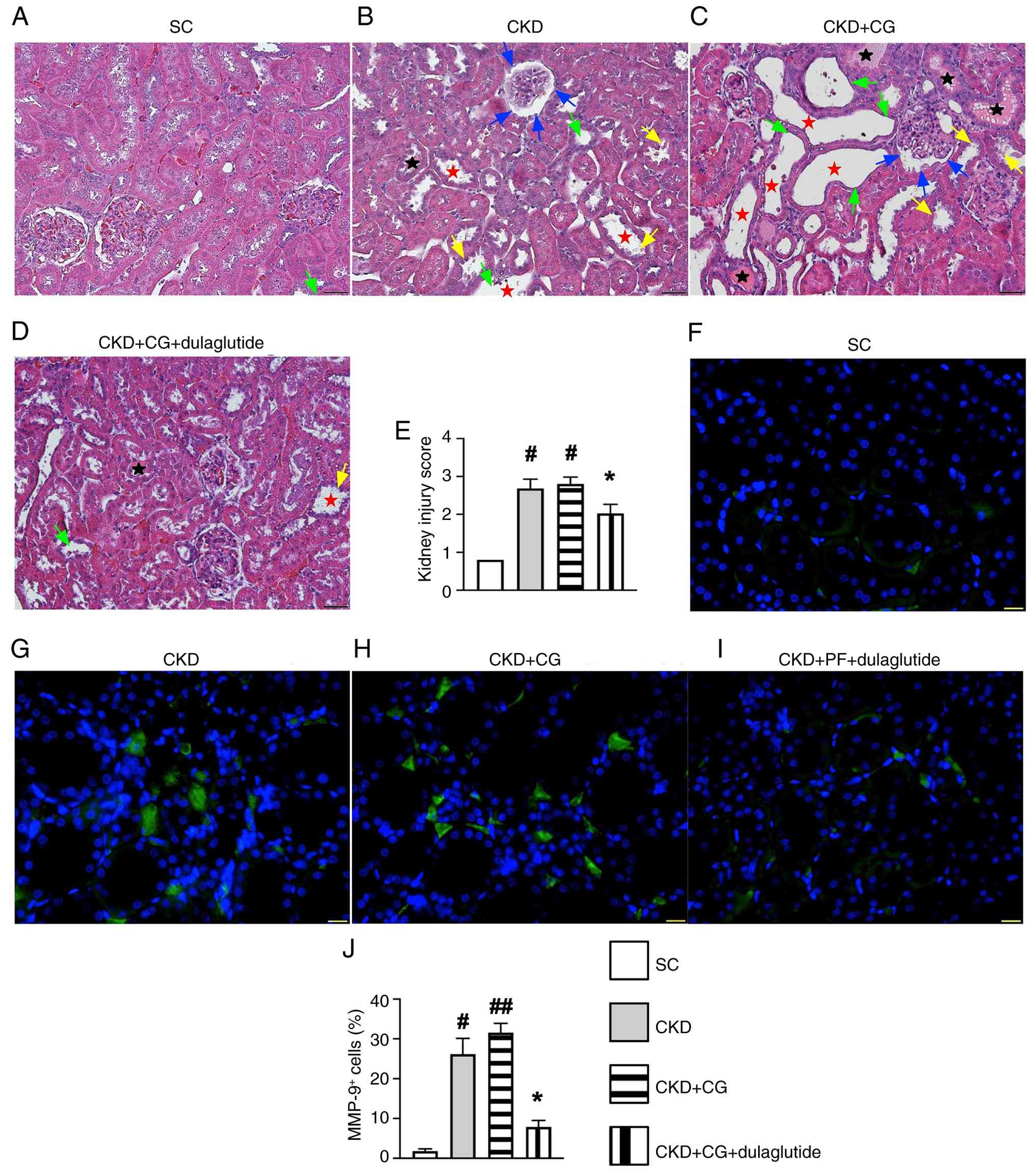
Kidney injury score and inflammatory cell infiltration in the rat peritoneum on day 42 after CKD induction
Additionally, light microscopy was used to evaluate the microstructural features of damaged kidney parenchyma and the morphology of the peritoneum in CKD rats in detail. The results showed that the kidney injury score (Fig. 6A-E) and the levels of MMP-9+ cells (Fig. 6F-J) in the kidney parenchyma were significantly increased in the CKD group and further increased in the CKD + CG group compared with those in the SC group, but were significantly reversed in the CKD + CG + dulaglutidegroup. These findings suggested that dulaglutide therapy not only mitigated inflammatory cell infiltration but also protected the kidney parenchyma against damage from CKD-related uremic toxins.
Impact of endotoxin on peritoneal damage
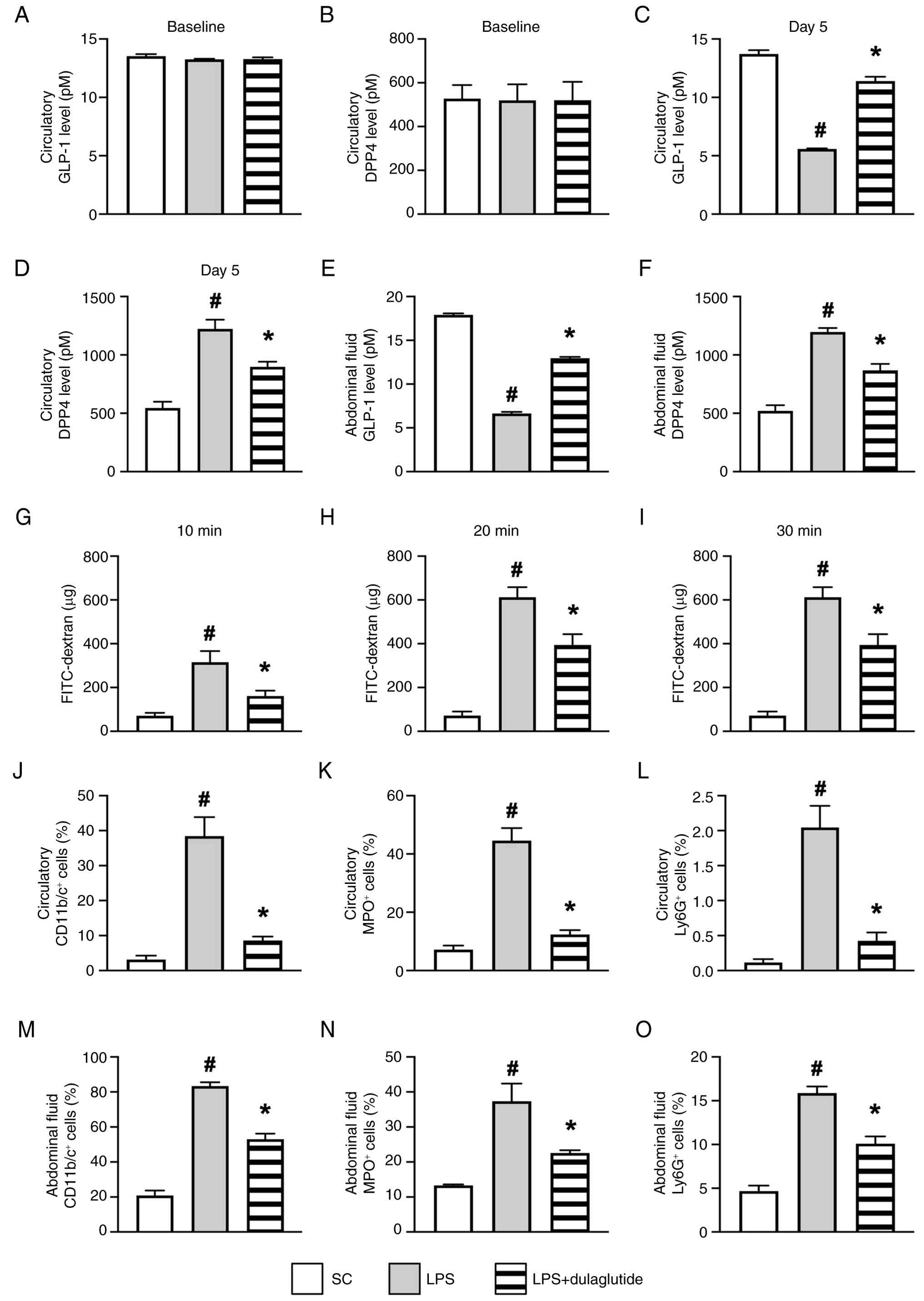
To determine whether inflammation serves a central role in peritoneal damage, an additional 24 animals were divided into the following groups: SC group, peritonitis induced by LPS group, and peritonitis induced by LPS + dulaglutide group, as aforementioned. As expected, the circulatory levels of GLP-1 (Fig. 7A) and DPP4 (Fig. 7B) at baseline did not differ among the three groups. However, by day 5 after the induction of peritonitis, ELISA showed that the circulating and abdominal fluid levels of DPP4 (Fig. 7D and F) were significantly higher in LPS than those in SC and LPS + dulaglutide, and were higher in LPS + dulaglutide than in SC, whereas the levels of GLP-1 (Fig. 7C and E) in circulation and in the abdominal fluid exhibited an opposite pattern to DPP4 among the groups. Additionally, blood samples from the jugular vein showed that the concentration of FITC-dextran at 10, 20 and 30 min, an indicator of peritoneal permeability, exhibited a pattern similar to that of DPP4 (Fig. 7G-I). These findings confirm that endotoxin (derived from LPS) significantly compromised the integrity of the peritoneum.
Furthermore, flow cytometric analysis demonstrated that the circulatory and abdominal-fluid levels of CD11b/c+ (Figs. 7J, 7M, S9A and S9D), MPO+ (Figs. 7K, 7N, S9B and S9E) and Ly6G+ cells (Figs. 7L, 7O, S9C and S9F), all indicators of inflammation, displayed a pattern identical to DPP4 among the groups.
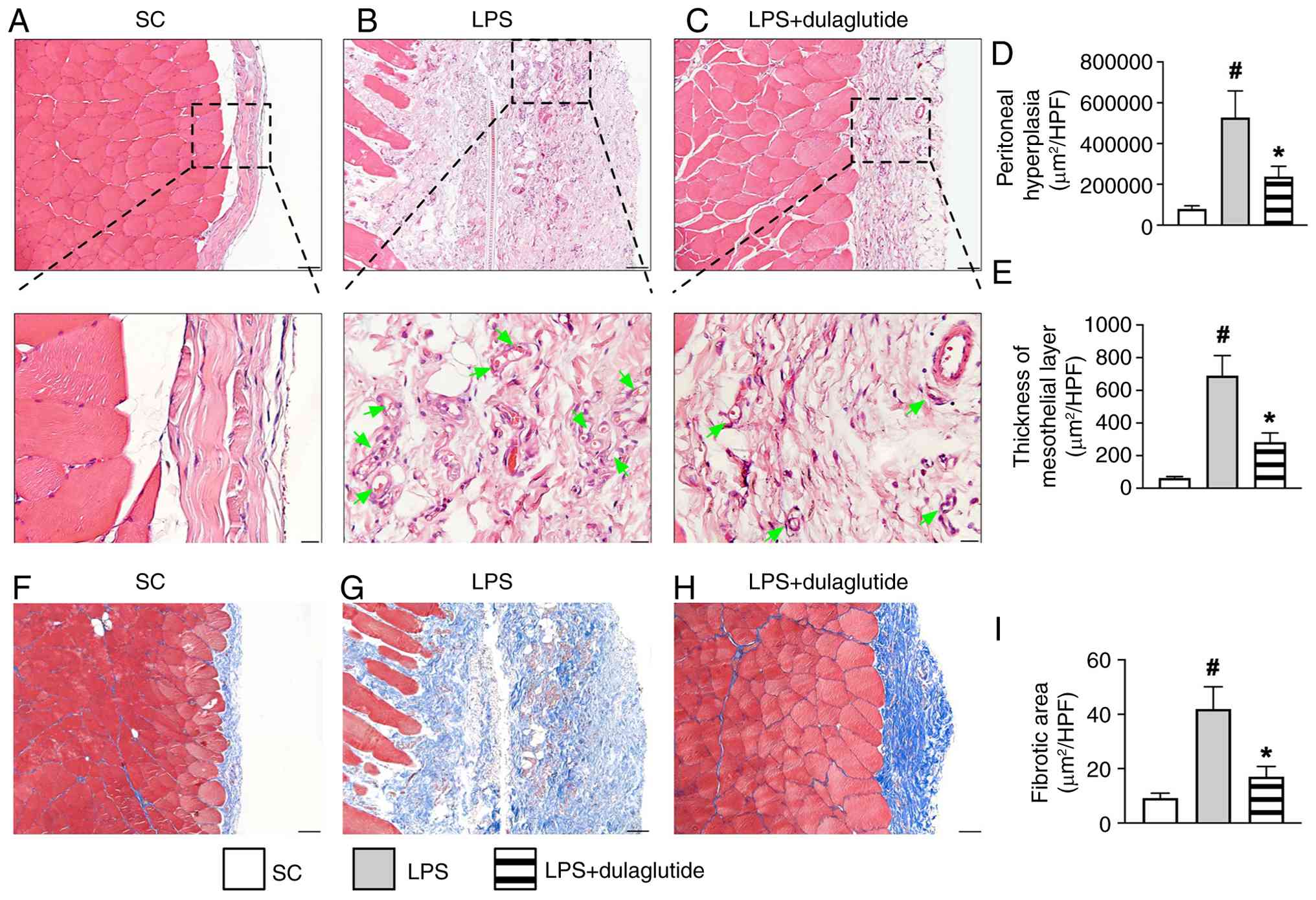
Light microscopic findings for identification of histopathological changes in the mesothelial layer of the peritoneum on day 5 after LPS treatment
As anticipated, the hyperplasia and thickness of the mesothelial layer of the peritoneum (Fig. 8A-E) were significantly greater in LPS group compared with those in SC group, and these changes were significantly reversed in LPS + dulaglutide group. Additionally, Masson's trichrome staining (Fig. 8F-I) revealed that the fibrotic area in the mesothelial layer of the peritoneum was significantly larger in LPS group than in SC group and LPS + dulaglutide, and also significantly larger in LPS + dulaglutide compared with in SC. These finding suggested that endotoxin, frequently released in the ESRD setting, may consistently cause damage to the mesothelial layer, potentially leading to PD failure.
Discussion
The present study investigated the therapeutic impact of dulaglutide on protecting the rat peritoneum against CG-induced damage and revealed several notable clinical implications. First, the histopathological findings confirmed the successful creation of a PF model in CKD rats that mimics the clinical setting of PF in patients undergoing PD, establishing a new platform for future translational medicine studies. Second, the results demonstrated that inflammation, DPP4 and fibrosis-induced EMT processes play a key role in PF, as evidenced by increased inflammatory infiltration and mesenchymal marker expression in the CKD + CG group, the levels of which were notably reduced by dulaglutide treatment in the CKD + CG + dulaglutide group. Finally, dulaglutide therapy could effectively suppress PF, primarily by inhibiting DPP4, EMT, oxidative stress and inflammation.
It is well-known that the failure of PD is multifactorial, involving inflammation, fibrotic mediators and oxidative stress (19-22). A notable finding of the present in vitro study was that the molecular-cellular perturbations associated with EMT activation, inflammatory responses and cytoskeletal remodeling (22) were substantially increased in the Met-5A cell line following stimulation with uremic toxins (i.e., p-Cresol), inflammatory substances (LPS) and fibrosis-inducing substances (i.e., CG, TGF-β). The results of the in vivo study mirrored these findings, aligning with previous research (19-22). Notably, these perturbations were significantly mitigated by dulaglutide therapy.
EMT has been recognized as having a fundamental role in tissue/organ fibrosis (16,21,23,41,42). An essential discovery in the present in vitro study was that EMT biomarkers (i.e., TGF-β, Snail, β-cadherin, vimentin, p-Smad3, α-SMA, collagen I, N-cadherin and fibronectin) were markedly upregulated in the Met-5A cell line treated with p-Cresol, LPS and TGF-β. Additionally, the current in vivo study showed that these biomarkers were significantly increased in the peritoneum of CKD rats and even more so in those treated with CG compared with those in the sham controls. The in vitro results were corroborated by the present in vivo findings and were supported by the results of previous studies (16,21,23,41,42). Particularly noteworthy was that these EMT biomarkers were substantially reduced in rats with CKD following treatment with dulaglutide, suggesting that GLP-1 analogs may hold significant potential for patients undergoing PD, especially patients with diabetes, in extending the durability of PD and preventing PD failure.
Clarifying the underlying mechanisms of PD failure was of utmost importance in the present study. A double knockdown of TGF-β and DPP4 genes was employed, and it was revealed that TGF-β/Smad3 and DPP4/oxidative stress signaling pathways may have crucial roles in PF and PD failure (Fig. 9).
Additionally, the administration of LPS (endotoxin) into the peritoneum of rats resulted in notable damage to the peritoneal mesothelial layer, increasing its permeability and suggesting that inflammatory signaling serves a key role in PD failure (Fig. 9). The in vitro and in vivo findings support the involvement of TGF-β/Smad3 and DPP4/oxidative stress, along with inflammatory signaling, in PF and PD failure in patients with ESRD.
Notably, our previous experimental study demonstrated that DPP4 activity is closely associated with the severity of peritoneal dysfunction, primarily through the upregulation of EMT (16). Treatment with sitagliptin and exendin-4 has been shown to significantly inhibit DPP4 activity, consequently reversing the EMT process, and inhibiting PF and PD failure (16). In the current study, it was observed that LPS-induced peritoneal inflammation led to an upregulation of DPP4 activity and oxidative stress, and a downregulation of GLP-1 levels in the peritoneum and circulation, resulting in increased peritoneal leakage (assessed using FITC-dextran permeability assay), PF (confirmed by Masson's trichrome staining) and PD failure. A particularly important finding was that dulaglutide therapy markedly attenuated peritoneal oxidative stress and permeability in CKD rodents with peritonitis and PF, highlighting the potential of GLP-1 analogs to prevent PF and PD failure in patients with ESRD.
The present findings may influence future research directions in exploring combination therapies involving dulaglutide and other agents targeting PF. PD failure is frequently associated with sepsis, leading to peritonitis, a common clinical issue that conventional therapy often fails to adequately address. The results of the present preclinical study may prompt consideration of combined dulaglutide and antibiotic therapy, offering additional benefits in preventing peritonitis caused by endotoxin, infection and sepsis. Particularly since off-label use of dulaglutide presents no ethical issues, meaning that the medication is used outside its approved indications under appropriate regulatory oversight, informed patient consent and clinical justification based on scientific evidence, this approach could be valuable in settings where existing treatments are insufficient, and emerging preclinical data support the therapeutic potential of dulaglutide beyond its original indications. Additionally, combining dulaglutide with a DPP4 inhibitor may synergistically prevent PF and PD failure.
Notably, the present study has several limitations. First, although the results are promising, the 42-day study period is relatively short. Consequently, the long-term effects of dulaglutide treatment on extending the durability of PD and suppressing PF in patients with ESRD remain unclear. Second, the study did not explore a stepwise increase in the concentrations of dulaglutide therapy administered to the animals; therefore, the optimal dose of dulaglutide that provides maximum efficacy in preventing PF has not been identified, nor have dose-dependent side effects of dulaglutide been evaluated. Third, the in vitro experiments were conducted using only one cell line rather than multiple, which may not fully represent the biological variability across different cell types. Finally, while the study extensively investigated the underlying mechanisms of PF and PD failure, the actual mechanisms may be more complex than those illustrated in the present model (Fig. 9).
In conclusion, the results of the present study indicated that TGF-β/Smad3-mediated EMT processes and DPP4/oxidative stress, along with inflammatory signaling, may serve pivotal roles in PF and PD failure, all of which could be significantly mitigated via dulaglutide treatment.
Supplementary Data
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
CCY and YY made substantial contributions to data acquisition, validation and interpretation. YTW, BCC, TWH and YLC contributed to the study design, and were involved in data acquisition and interpretation. JYC was involved in data acquisition and interpretation. YCL was responsible for project administration, and contributed to the study conception and design. HKY conceived and designed the study, supervised the experiments, interpreted the data, and was responsible for drafting and revising the manuscript for important intellectual content. CCY and HKY confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal procedures were approved by the Institute of Animal Care and Use Committee at Kaohsiung Chang Gung Memorial Hospital (Affidavit of Approval of Animal Use Protocol No. 2021080501) and performed in accordance with the Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Abbreviations:
|
EMT |
epithelial-mesenchymal transition |
|
CG |
chlorhexidine gluconate |
|
LPS |
lipopolysaccharide |
|
RRT |
renal replacement therapy |
|
PD |
peritoneal dialysis |
|
DM |
diabetes mellitus |
|
HD |
hemodialysis |
|
GLP-1R |
glucagon-like peptide 1 receptor |
|
CKD |
chronic kidney disease |
|
ROS |
reactive oxygen species |
|
vWF |
von Willebrand factor |
|
DPP4 |
dipeptidyl peptidase 4 |
|
BUN |
blood urea nitrogen |
|
Nrf2 |
nuclear factor erythroid 2-related factor 2 |
|
NQO-1 |
NAD(P)H quinone oxidoreductase 1 |
|
MPO |
myeloperoxidase |
|
α-SMA |
α-smooth muscle actin |
|
PF |
peritoneal fibrosis |
Acknowledgments
Not applicable.
Funding
The present study was funded by research grants from the Ministry of Science and Technology, Taiwan, Republic of China [grant nos. NMRPG8L6151 (1/3), NMRPG8L6152 (2/3), NMRPG8L6153 (3/3), CMRPG8K0531, CMRPG8L1411 and 110-2314-B-182A-112-MY3].
References
|
Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, Gu H, Hirth RA, Hutton D, Jin Y, et al: US renal data system 2019 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 75(Suppl): A6–A7. 2020. View Article : Google Scholar | |
|
McCullough KP, Morgenstern H, Saran R, Herman WH and Robinson BM: Projecting ESRD incidence and prevalence in the United States through 2030. J Am Soc Nephrol. 30:127–135. 2019. View Article : Google Scholar : | |
|
Pozzoni P, Del Vecchio L, Pontoriero G, Di Filippo S and Locatelli F: Long-term outcome in hemodialysis: Morbidity and mortality. J Nephrol. 17:S87–S95. 2004.PubMed/NCBI | |
|
Deb S, Wijeysundera HC, Ko DT, Tsubota H, Hill S and Fremes SE: Coronary artery bypass graft surgery vs percutaneous interventions in coronary revascularization: A systematic review. JAMA. 310:2086–2095. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Hori D, Yamaguchi A and Adachi H: Coronary artery bypass surgery in end-stage renal disease patients. Ann Vasc Dis. 10:79–87. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Pang PYK, Teow CKJ, Huang MJ, Naik MJ, Lim SL, Chao VTT, Tan TE, Chua YL and Sin YK: Long-term prognosis in patients with end-stage renal disease after coronary artery bypass grafting. J Thorac Dis. 12:6722–6730. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Serafinceanu C, Neculaescu C, Cimponeriu D, Timar R and Covic AC: Impact of gender and dialysis modality on early mortality risk in diabetic ESRD patients: Data from a large single center cohort. Int Urol Nephrol. 46:607–614. 2014. View Article : Google Scholar | |
|
Tsur N, Menashe I and Haviv YS: Risk factors before dialysis predominate as mortality predictors in diabetic maintenance dialysis patients. Sci Rep. 9:106332019. View Article : Google Scholar : PubMed/NCBI | |
|
Schena FP: Epidemiology of end-stage renal disease: International comparisons of renal replacement therapy. Kidney Int. 57:S39–S45. 2000. View Article : Google Scholar | |
|
Vaios V, Georgianos PI, Liakopoulos V and Agarwal R: Assessment and management of hypertension among patients on peritoneal dialysis. Clin J Am Soc Nephrol. 14:297–305. 2019. View Article : Google Scholar : | |
|
McKane W, Chandna SM, Tattersall JE, Greenwood RN and Farrington K: Identical decline of residual renal function in high-flux biocompatible hemodialysis and CAPD. Kidney Int. 61:256–265. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Coentrão L, Van Biesen W, Nistor I, Tordoir J, Gallieni M, Marti Monros A and Bolignano D: Preferred haemodialysis vascular access for diabetic chronic kidney disease patients: A systematic literature review. J Vasc Access. 16:259–264. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Almasri J, Alsawas M, Mainou M, Mustafa RA, Wang Z, Woo K, Cull DL and Murad MH: Outcomes of vascular access for hemodialysis: A systematic review and meta-analysis. J Vasc Surg. 64:236–243. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Arhuidese IJ, Orandi BJ, Nejim B and Malas M: Utilization, patency, and complications associated with vascular access for hemodialysis in the United States. J Vasc Surg. 68:1166–1174. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Arhuidese IJ, Purohit A, Elemuo C, Parkerson GR, Shames ML and Malas MB: Outcomes of autogenous fistulas and prosthetic grafts for hemodialysis access in diabetic and nondiabetic patients. J Vasc Surg. 72:2088–2096. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Li YC, Sung PH, Yang YH, Chiang JY, Yip HK and Yang CC: Dipeptidyl peptidase 4 promotes peritoneal fibrosis and its inhibitions prevent failure of peritoneal dialysis. Commun Biol. 4:1442021. View Article : Google Scholar : PubMed/NCBI | |
|
Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, Mackenzie RK and Williams GT: Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol. 13:470–479. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Williams JD, Craig KJ, von Ruhland C, Topley N and Williams GT; Biopsy Registry Study Group: The natural course of peritoneal membrane biology during peritoneal dialysis. Kidney Int. (Suppl): S43–S49. 2003. View Article : Google Scholar | |
|
Mutsaers SE, Birnie K, Lansley S, Herrick SE, Lim CB and Prêle CM: Mesothelial cells in tissue repair and fibrosis. Front Pharmacol. 6:1132015. View Article : Google Scholar : PubMed/NCBI | |
|
Di Paolo N and Sacchi G: Atlas of peritoneal histology. Perit Dial Int. 20:S5–S96. 2000.PubMed/NCBI | |
|
Yung S and Chan TM: Pathophysiological changes to the peritoneal membrane during PD-related peritonitis: The role of mesothelial cells. Mediators Inflamm. 2012:4841672012. View Article : Google Scholar : PubMed/NCBI | |
|
Terri M, Trionfetti F, Montaldo C, Cordani M, Tripodi M, Lopez-Cabrera M and Strippoli R: Mechanisms of peritoneal fibrosis: Focus on immune cells-peritoneal stroma interactions. Front Immunol. 12:6072042021. View Article : Google Scholar : PubMed/NCBI | |
|
Stone RC, Pastar I, Ojeh N, Chen V, Liu S, Garzon KI and Tomic-Canic M: Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 365:495–506. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Li M, Luan F, Zhao Y, Hao H, Zhou Y, Han W and Fu X: Epithelial-mesenchymal transition: An emerging target in tissue fibrosis. Exp Biol Med (Maywood). 241:1–13. 2016. View Article : Google Scholar | |
|
Milan Manani S, Virzi GM, Giuliani A, Baretta M, Corradi V, De Cal M, Biasi C, Crepaldi C and Ronco C: Lipopolysaccharide evaluation in peritoneal dialysis patients with peritonitis. Blood Purif. 49:434–439. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Knapp S, de Vos AF, Florquin S, Golenbock DT and van der Poll T: Lipopolysaccharide binding protein is an essential component of the innate immune response to escherichia coli peritonitis in mice. Infect Immun. 71:6747–6753. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Shen WR, Kimura K, Ishida M, Sugisawa H, Kishikawa A, Shima K, Ogawa S, Qi J and Kitaura H: The glucagon-like peptide-1 receptor agonist exendin-4 inhibits lipopolysaccharide-induced osteoclast formation and bone resorption via inhibition of TNF-α expression in macrophages. J Immunol Res. 2018:57836392018. View Article : Google Scholar | |
|
Mehdi SF, Pusapati S, Anwar MS, Lohana D, Kumar P, Nandula SA, Nawaz FK, Tracey K, Yang H, LeRoith D, et al: Glucagon-like peptide-1: A multi-faceted anti-inflammatory agent. Front Immunol. 14:11482092023. View Article : Google Scholar : PubMed/NCBI | |
|
Zheng Z, Zong Y, Ma Y, Tian Y, Pang Y, Zhang C and Gao J: Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct Target Ther. 9:2342024. View Article : Google Scholar : PubMed/NCBI | |
|
Chen YT, Tsai TH, Yang CC, Sun CK, Chang LT, Chen HH, Chang CL, Sung PH, Zhen YY, Leu S, et al: Exendin-4 and sitagliptin protect kidney from ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med. 11:2702013. View Article : Google Scholar : PubMed/NCBI | |
|
Yip HK, Yang CC, Chen KH, Huang TH, Chen YL, Zhen YY, Sung PH, Chiang HJ, Sheu JJ, Chang CL, et al: Combined melatonin and exendin-4 therapy preserves renal ultrastructural integrity after ischemia-reperfusion injury in the male rat. J Pineal Res. 59:434–447. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Chang YC, Hsu SY, Yang CC, Sung PH, Chen YL, Huang TH, Kao GS, Chen SY, Chen KH, Chiang HJ, et al: Enhanced protection against renal ischemia-reperfusion injury with combined melatonin and exendin-4 in a rodent model. Exp Biol Med (Maywood). 241:1588–1602. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Zhen YY, Yang CC, Hung CC, Lee CC, Lee CC, Wu CH, Chen YT, Chen WY, Chen KH, Yip HK and Ko SF: Extendin-4 protects kidney from acute ischemia-reperfusion injury through upregulation of NRF2 signaling. Am J Transl Res. 9:4756–4771. 2017.PubMed/NCBI | |
|
Sung PH, Chiang HJ, Wallace CG, Yang CC, Chen YT, Chen KH, Chen CH, Shao PL, Chen YL, Chua S, et al: Exendin-4-assisted adipose derived mesenchymal stem cell therapy protects renal function against co-existing acute kidney ischemia-reperfusion injury and severe sepsis syndrome in rat. Am J Transl Res. 9:3167–3183. 2017.PubMed/NCBI | |
|
Yang CC, Chen YT, Wallace CG, Chen KH, Cheng BC, Sung PH, Li YC, Ko SF, Chang HW and Yip HK: Early administration of empagliflozin preserved heart function in cardiorenal syndrome in rat. Biomed Pharmacother. 109:658–670. 2019. View Article : Google Scholar | |
|
Yin TC, Sung PH, Chen KH, Li YC, Luo CW, Huang CR, Sheu JJ, Chiang JY, Lee MS and Yip HK: Extracorporeal shock wave-assisted adipose-derived fresh stromal vascular fraction restores the blood flow of critical limb ischemia in rat. Vascul Pharmacol. 113:57–69. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals: Guide for the care and use of laboratory animals: Eighth edition. The National Academies Press; Washington, DC: 2011 | |
|
Huang TH, Chen YT, Sung PH, Chiang HJ, Chen YL, Chai HT, Chung SY, Tsai TH, Yang CC, Chen CH, et al: Peripheral blood-derived endothelial progenitor cell therapy prevented deterioration of chronic kidney disease in rats. Am J Transl Res. 7:804–824. 2015.PubMed/NCBI | |
|
Chang CL, Sung PH, Sun CK, Chen CH, Chiang HJ, Huang TH, Chen YL, Zhen YY, Chai HT, Chung SY, et al: Protective effect of melatonin-supported adipose-derived mesenchymal stem cells against small bowel ischemia-reperfusion injury in rat. J Pineal Res. 59:206–220. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Yue Y, Yeh JN, Chiang JY, Sung PH, Chen YL, Liu F and Yip HK: Intrarenal arterial administration of human umbilical cord-derived mesenchymal stem cells effectively preserved the residual renal function of diabetic kidney disease in rat. Stem Cell Res Ther. 13:1862022. View Article : Google Scholar : PubMed/NCBI | |
|
Lin KC, Yeh JN, Shao PL, Chiang JY, Sung PH, Huang CR, Chen YL, Yip HK and Guo J: Jagged/Notch proteins promote endothelial-mesenchymal transition-mediated pulmonary arterial hypertension via upregulation of the expression of GATAs. J Cell Mol Med. 27:1110–1130. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Sung PH, Cheng BC, Hsu TW, Chiang JY, Chiang HJ, Chen YL, Yang CC and Yip HK: Oxidized-LDL deteriorated the renal residual function and parenchyma in CKD rat through upregulating epithelial mesenchymal transition and extracellular matrix-mediated tubulointerstitial fibrosis-pharmacomodulation of rosuvastatin. Antioxidants (Basel). 11:24652022. View Article : Google Scholar : PubMed/NCBI | |
|
Sung PH, Sun CK, Ko SF, Chang LT, Sheu JJ, Lee FY, Wu CJ, Chua S and Yip HK: Impact of hyperglycemic control on left ventricular myocardium. A molecular and cellular basic study in a diabetic rat model. Int Heart J. 50:191–206. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Sheu JJ, Yang CC, Wallace CG, Chen KH, Shao PL, Sung PH, Li YC, Chu YC, Guo J and Yip HK: Uremic toxic substances are essential elements for enhancing carotid artery stenosis after balloon-induced endothelial denudation: Worsening role of the adventitial layer. Am J Transl Res. 12:7144–7159. 2020.PubMed/NCBI | |
|
Yip HK, Lee MS, Li YC, Shao PL, Chiang JY, Sung PH, Yang CH and Chen KH: Dipeptidyl peptidase-4 deficiency effectively protects the brain and neurological function in rodent after acute hemorrhagic stroke. Int J Biol Sci. 16:3116–3132. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Ko SF, Chen KH, Wallace CG, Yang CC, Sung PH, Shao PL, Li YC, Chen YT and Yip HK: Protective effect of combined therapy with hyperbaric oxygen and autologous adipose-derived mesenchymal stem cells on renal function in rodent after acute ischemia-reperfusion injury. Am J Transl Res. 12:3272–3287. 2020.PubMed/NCBI | |
|
Vanholder R, Pletinck A, Schepers E and Glorieux G: Biochemical and clinical impact of organic uremic retention solutes: A comprehensive update. Toxins (Basel). 10:332018. View Article : Google Scholar : PubMed/NCBI | |
|
Chen YT, Wallace CG, Yang CC, Chen CH, Chen KH, Sung PH, Chen YL, Chai HT, Chung SY, Chua S, et al: DPP-4 enzyme deficiency protects kidney from acute ischemia-reperfusion injury: Role for remote intermittent bowel ischemia-reperfusion preconditioning. Oncotarget. 8:54821–54837. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Chang MW, Chen CH, Chen YC, Wu YC, Zhen YY, Leu S, Tsai TH, Ko SF, Sung PH, Yang CC, et al: Sitagliptin protects rat kidneys from acute ischemia-reperfusion injury via upregulation of GLP-1 and GLP-1 receptors. Acta Pharmacol Sin. 36:119–130. 2015. View Article : Google Scholar : |