Chiasmatic syndrome caused by Rathke's cleft cyst: A case report
- Authors:
- Published online on: March 28, 2025 https://doi.org/10.3892/mco.2025.2842
- Article Number: 47
-
Copyright: © Lešták et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Rathke's cleft cysts (RCC) arise from the epithelial remnants of the Rathke cleft, which may manifest in adulthood between the pars anterior and pars posterior of the pituitary gland (1). RCCs are benign sellar and suprasellar lesions that peak in incidence between the ages of 30-50 years.
Lesions usually reach a diameter of between 10-20 mm. Cysts walls are generally thin and composed of fibrous connective tissue. A single layer of pseudostratified cuboidal or columnar epithelium forms the lining. Metaplastic squamous epithelium may also be present; the cyst content is usually mucin. More rarely, xanthogranulomatous changes can occur in the cyst wall (2-5).
Since RCCs are usually asymptomatic, a diagnosis of chiasmatic syndrome due to compression occurs relatively late. Although magnetic resonance imaging (MRI) can confirm cyst formation, it is difficult to distinguish RCCs from craniopharyngioma (CP) or other non-cancerous/non-infectious cysts. Another approach, often neglected in the era of imaging (optical coherence tomography), is a visual field examination.
A similar situation occurred in our patient, which motivated this case report. The diagnosis of chiasmatic syndrome and subsequent treatment was made relatively quickly, but the exact diagnosis was not made until the biopsy examination.
Case report
A 70-year-old woman presented to the ophthalmologist (Feb. 2022) for impaired vision in her left eye. Anamnestic history indicated that ‘something dropped’ into her right eye 2 weeks prior, which is when she first noticed the worsened vision in her left eye. On examination, Visual Acuity, Right Eye (VARE) was 0.4, and the Visual Acuity, Left Eye (VALE): see fingers at 1 m. Incipient cataracts were present in both eyes, more so on the right. Excavations were observed on the optic papillae (C/D=0.6-0.7 in both eyes). The macula was without a foveolar reflex. Intraocular pressure was 23/21 (R/L) mmHg. The central corneal thickness (CCT) was R/L, 563/561 um. The prior examination, performed in 2019, showed no ocular abnormalities.
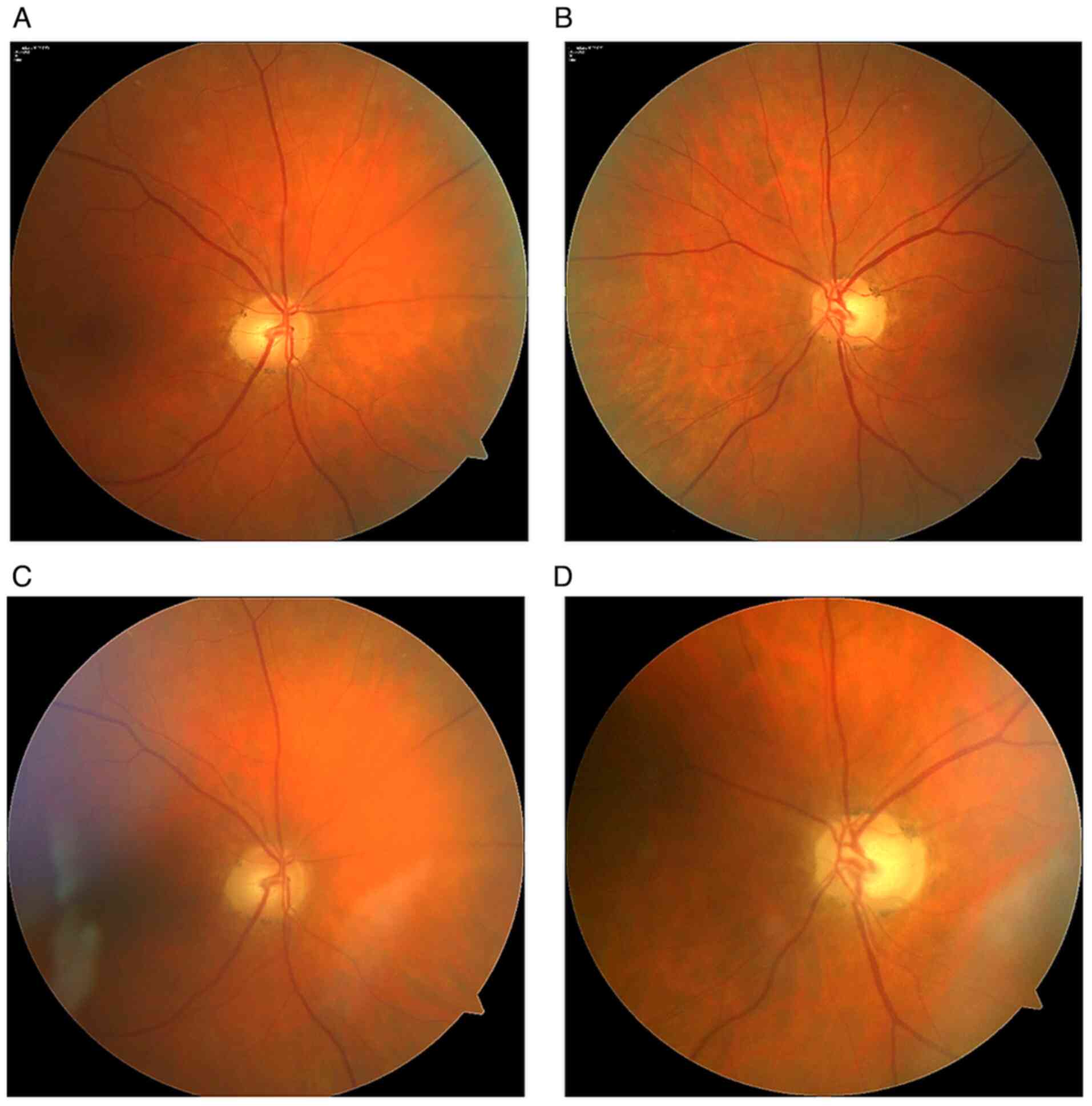
The following day, she sought another ophthalmologist, who completed a retinal nerve fiber layer examination but did not indicate a baseline perimetric examination. She visited our clinic (Ophthalmology Clinic JL, V Hůrkách 1296/10, Prague - clinical department of the Faculty of Biomedical Engineering, Czech Technical University in Prague) 1 month after the first examination (Mar. 2022). On examination, bulbs in medium position, free motility. Diplopia not detected. Pupils isocoric. VARE: 0.4, VALE: 0.16 excentric. Cataracts were present in both eyes that did not correspond to the decreased visual acuity. On examination, an excavation was found on the right papilla (C/D=0.6); the left papilla was atrophic (C/D=0.7). Otherwise, the fundus exam was normal (Fig. 1).
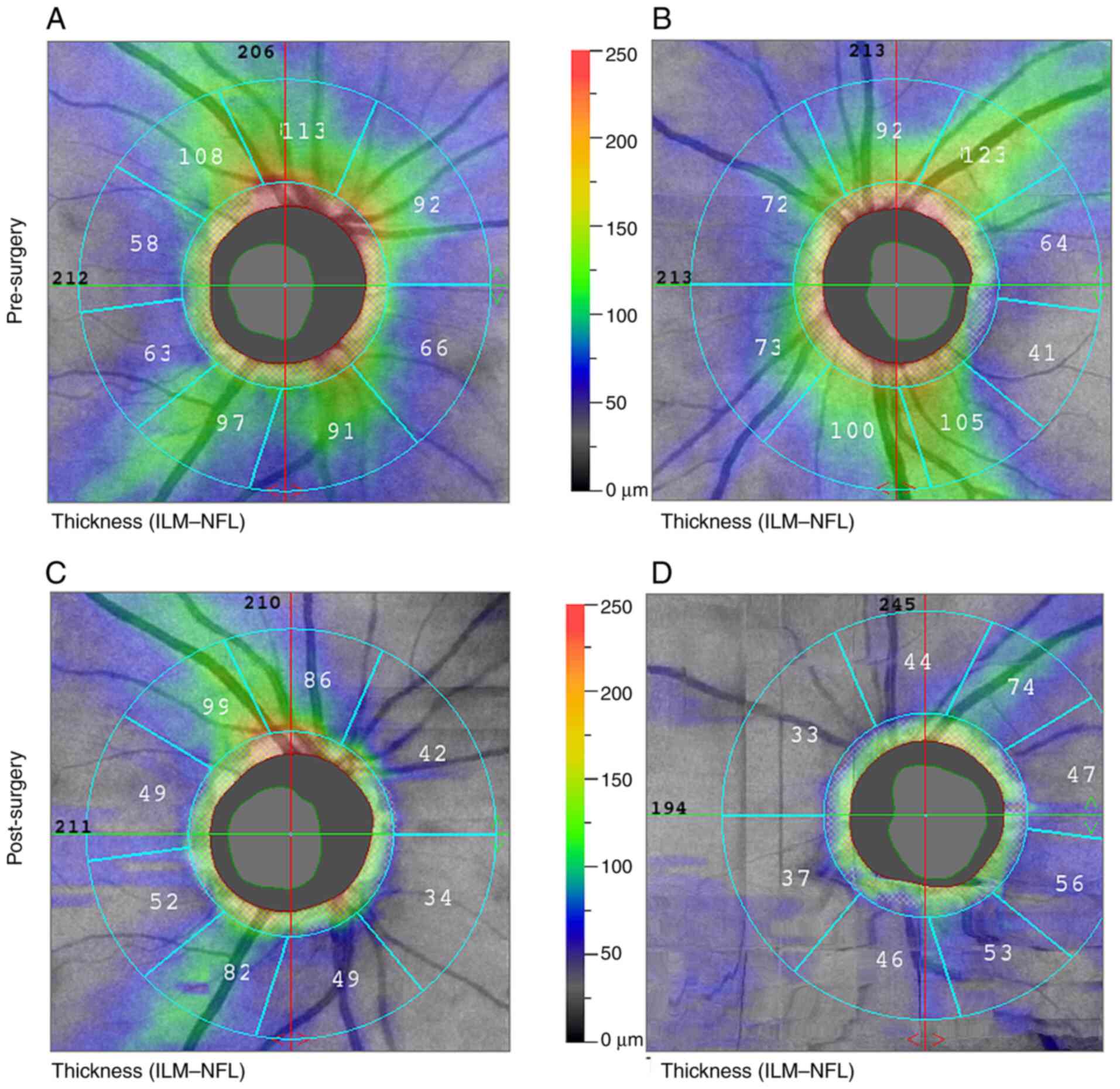
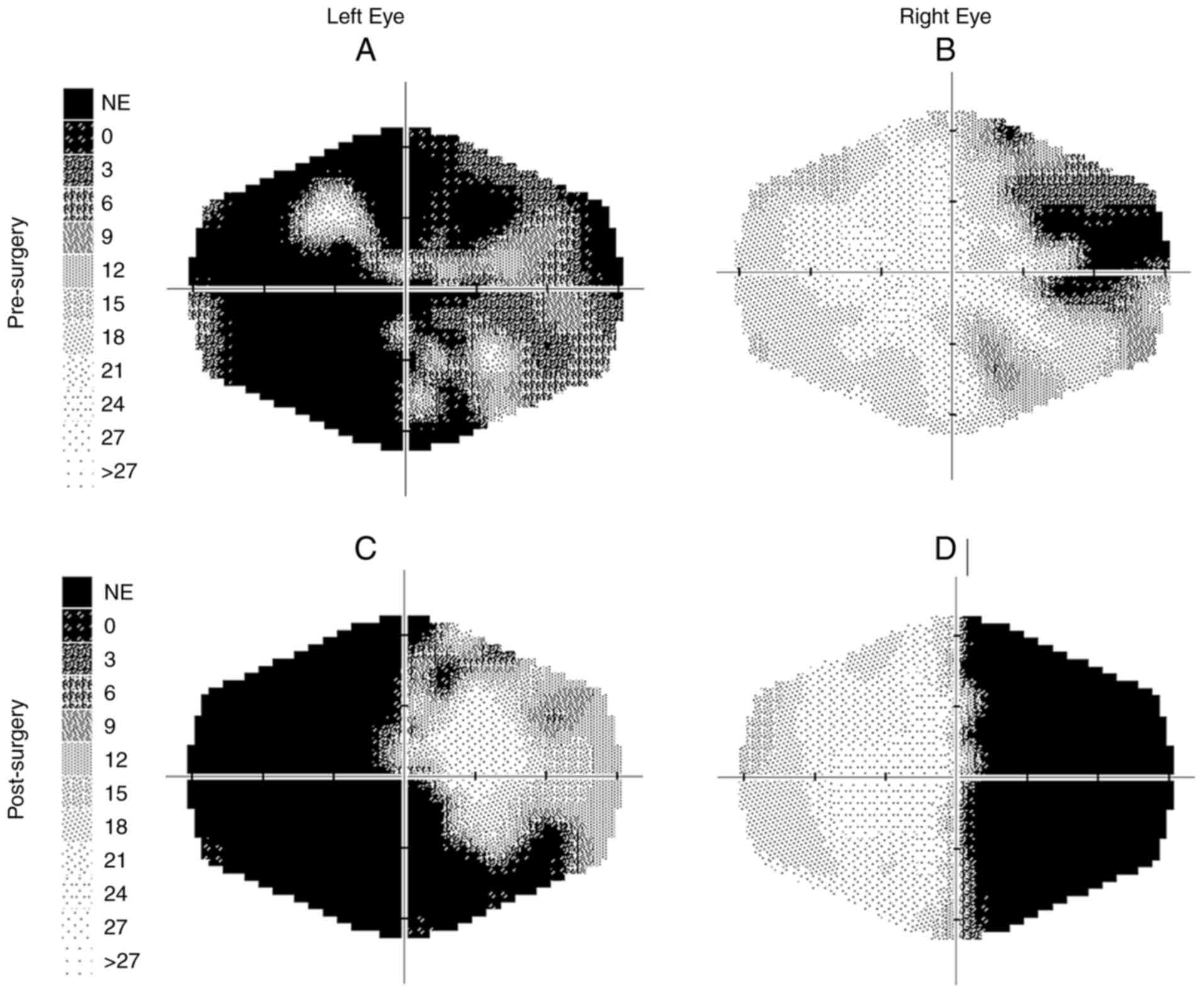
Retinal nerve fiber layer (RNFL) peripapillary was R/L, 86 and 83 µm. In the visual field (VF), incomplete superior temporal quadrantanopia was found on the right and temporal hemianopia extending into the central part on the left VF. The examination was performed using a Medmont M 700. She had not had a visual field examination until then.
Electroretinographic examination using pattern reversal stimulation showed borderline findings on amplitudes, with N95 and N95 latency prolongation to 114 and 105 ms, respectively. Pattern visual evoked potential showed a marked decrease in amplitudes on the right without prolongation of the P100 latency. On the left, there was a non-excitable response. The examination was performed using a Roland Consult (Germany) electrophysiological diagnostic system according to ISCEV methodology. The size of the stimulation field was 41x31 angular degrees.
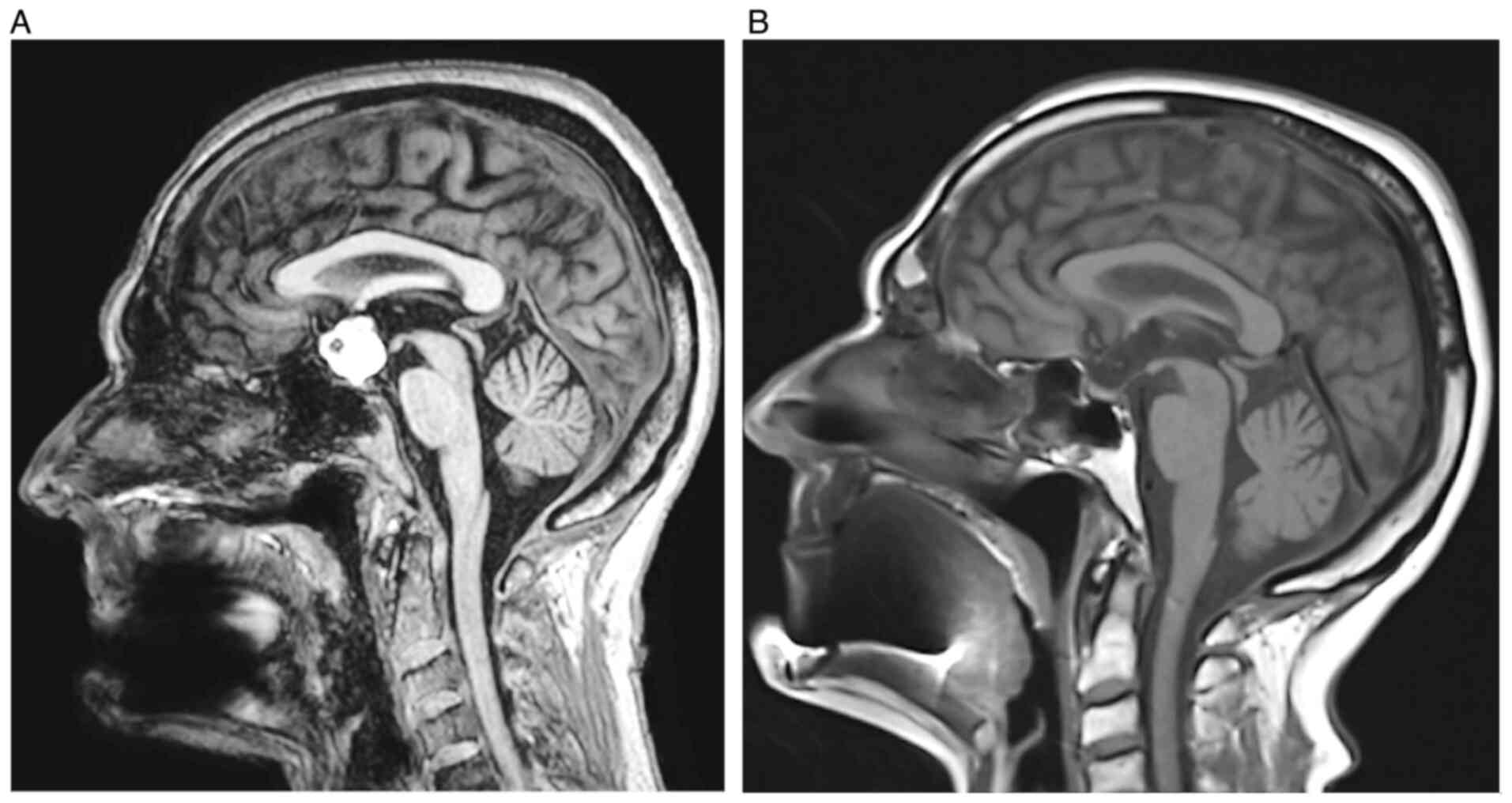
Due to suspicion of chiasmatic syndrome, the patient was referred for an MRI brain scan, which was performed the following week (3T machine; Ingenia, Philips) with the following findings: Suprasellar, predominantly cystic lesion with high protein content. The contours of the lesion were irregular, and the signal natively in the T1 image was high, except for a small 5 mm hyposignal portion ventrally. On T2, the lesion signal was inhomogeneous and somewhat lower. Etiologically, according to MR imaging, the differential diagnosis was a Rathke's cyst or, less likely, a craniopharyngioma. The optic nerve course and optic chiasm were elevated and atrophic.
The neurological examination, except for a visual disturbance, showed no abnormality. A neurosurgeon was consulted, and surgical treatment was recommended. The cystic tumor in the sellar region with suprasellar extension was removed 1 week after the MRI scan. A subfrontal approach was selected, utilizing a small right frontal craniotomy extending to the midline. The tumor was severely compressing the left optic nerve and optic chiasm and extended to the floor of the third ventricle. The firmly adherent tumor was carefully separated from the nerve structures and radically removed using microsurgical techniques. The continuity of the optic pathway and the pituitary stalk was preserved, and the resulting decompression was excellent.
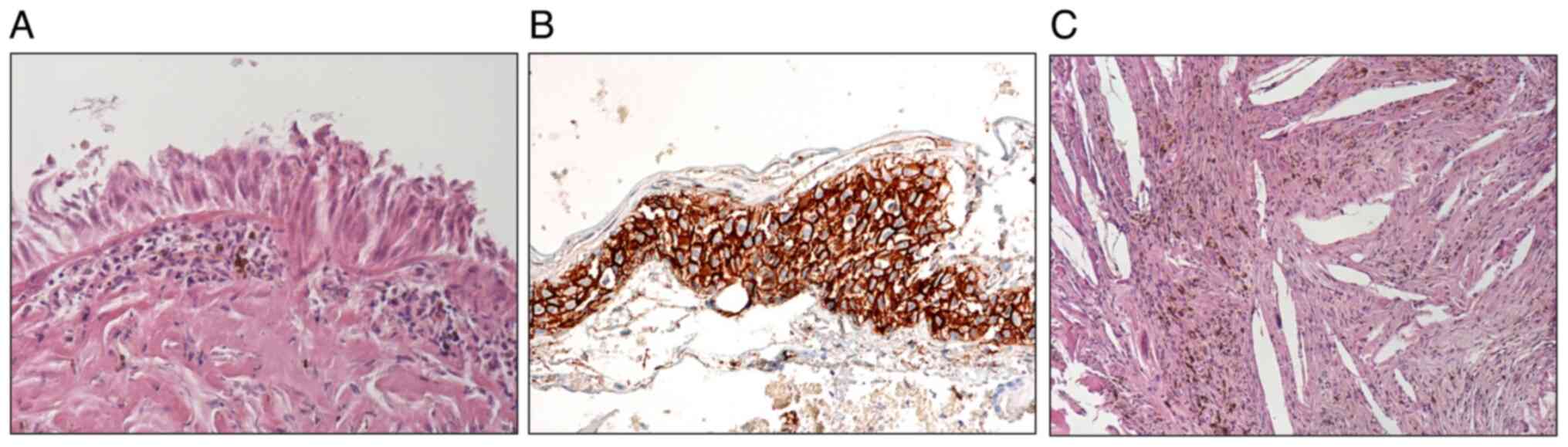
The biopsy examination revealed a cyst formed predominantly of fibrous connective tissue with extensive xanthogranulomatous changes containing cholesterol clefts with crystalophages, hemosiderin deposits, and small areas of metaplastic ossification. Foci of non-keratinizing squamous epithelium were immunohistologically positive for beta-catenin, and small areas of ciliated columnar and squamous epithelium were also present. These findings corresponded with the diagnosis of RCC with xanthogranulomatous changes (Fig. 2). Staining with H&E was performed in the Tissue-Tek Prisma Plus Automated Slide Stainer at the room temperature.
At the follow-up examination, 1 month after surgery (May 2022), VARE was 0.6 and VALE was 0.1, eccentrically. The examination showed partial papilla atrophy on the right (C/D=0.6); the left papilla was atrophic (C/D=0.7) (Figs. 3 and 4). After surgical removal of the expansion, no residual changes in the sellar region were evident on the MR image. No new lesions were observed in the brain compared to the preoperative examination (Fig. 5). Endocrinological examination showed central hypothyroidism, hypocortisolism and mild hyperprolactinemia. Otherwise, internal findings were normal.
Further follow-up (Sept. 2022) of the RNFL showed a peripapillary decrease from 86 to 59 µm in the right eye and, after surgery, from 83 to 47 µm in the left eye.
A total of 2 years after the first examination (Feb. 2024), VARE: 0.5 and VALE: 0.05. The visual field remained unchanged. The patient is under the care of a neurologist, endocrinologist and radiologist.
Discussion
RCCs are uncommon, predominantly intrasellar lesions, with significant suprasellar spread in ~1/3 of cases. The lining of RCCs usually consists of a single layer of ciliated cuboidal or ciliated columnar epithelium; in some cases, the lining may also consist of squamous epithelium. Although RCCs are usually an incidental finding in adults, functionally, these cysts are generally asymptomatic. Gradual cyst enlargement results in visual disturbances and endocrinopathy (6). More rarely, xanthogranulomatous changes can occur in the cyst wall, which was present in our case (3,4,7,8).
Fortunately, our patient received a quick diagnosis and timely treatment, even though the disease was advanced. Despite treatment, visual function did not improve with surgery, and the visual field deteriorated.
On MRI, RCC can be differentiated from cystic adenoma and craniopharyngioma by the presence of an intracystic nodule, T2 hypo-intensity, absence of a thick contrast wall, and absence of intralesional septa (9).
Kim et al (10) demonstrated high signal T2 hypo-intensity (72 cases), iso-intensity (39 cases) and hyperintensity (44 cases) on T1 images in a cohort of 115 RCC patients. In another large group of 35 patients, RCC showed hyperintensity on T1 images and hypo-intensity on T2 images (10).
Sharifi et al (11) reported that out of 27 patients, six (22.2%) had isointense signals on T2, one (3.7%) had a hypointense signal, and 20 (74%) had hyperintense signals. Our patient had a hyperintense signal on T1 imaging and a hypointense signal on T2 imaging. Although her treatment was not in the hands of the ophthalmologist, her current treatment options have been noted.
The endoscopic endonasal trans-sphenoidal approach remains the gold standard for RCC. Studies have demonstrated that this method allows for effective cyst drainage, symptom relief and reduced recovery times. However, RCCs with significant suprasellar extension, those firmly adhered to critical neurovascular structures, or in cases where a previous trans-sphenoidal approach was unsuccessful, transcranial approaches, such as subfrontal or pterional craniotomy, may be necessary. These approaches offer direct access to the suprasellar region, allowing for complete cyst removal. The choice of surgical technique should be guided by cyst size, anatomical location, surgeon expertise and individual patient factors to optimize outcomes and minimize recurrence risks (12-16).
Mukherjee et al (17) analyzed 12 patients who underwent surgery (10 trans-sphenoidal and two transcranial). A definitive histologic diagnosis could be made in eight patients; the diagnosis was presumptive in four. The median follow-up period was 30 months (1-168 months). Four patients (33%) underwent re-expansion at 3, 6 and 48 months after the first operation, three of whom were symptomatic and required re-operation. Two of these patients underwent post-operative external pituitary radiotherapy. The ideal treatment for these cysts is unclear, but aspiration followed by extensive cyst wall excision, if possible, appears to be the best initial option. For recurrent symptomatic tumors, surgical resection is the treatment of choice (17).
Because of possible recurrences, Ogawa et al (18) suggest performing follow-up MR imaging within 36 months of surgery, even if no re-accumulation was detected.
In their extensive study of 73 RCCs, Langlois et al (19) found recurrence rates of 25% within four years of surgery. Therefore, they recommend a minimum 5-year follow-up protocol. Qian et al (20) analyzed 44 studies containing 2,539 RCC cases. They observed the highest increase in recurrences in patients followed for >72 months. Therefore, they recommend a follow-up period of at least 6 years after surgery for patients with RCC.
Our patient was treated with a radical microsurgical procedure since it was not apparent beforehand whether it was RCC or CP. There have been two years of follow-up since the surgery, and no recurrence has been observed.
As Otradovec (21) states in his monograph, ‘Due to its high and irreplaceable topical value, examination of the visual field in neuroophthalmological diagnostics is by far the most important functional test. Nevertheless, it is often postponed, perhaps due to unfounded prejudice about its professional, time, and equipment requirements.
Similarly, Foroozan (22) notes, ‘Although many methods are used to assess visual function in patients with chiasma disorders, perimetry remains the most effective means of detecting and monitoring visual deficits.
Our patient visited two eye doctors in quick succession. However, neither performed a perimetric examination, which would have helped establish a diagnosis. There was no precise diagnosis. It was only after mutual consultation between the radiologist and the pathologist that RCC was concluded.
Management of RCC involves a multidisciplinary approach with endocrinologists, neurosurgeons, radiologists and ophthalmologists. Asymptomatic cysts are typically managed conservatively with regular imaging and hormonal follow-ups, while symptomatic cysts causing visual or hormonal dysfunction may require surgery. Post-treatment care focuses on long-term monitoring for recurrence, pituitary function, and visual recovery.
In conclusion, the authors present a case report of an older woman with significant unilateral vision loss due to RCC. From the ophthalmologist's point of view, the case emphasizes the importance of perimetric examinations, which are irreplaceable even in this era of modern diagnostic procedures. Another noteworthy point is the frequency of recurrences and the necessity for patient follow-up lasting 6 or more years after the intervention.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Charles University project Cooperatio Medical Diagnostics and Basic Medical Sciences.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
JL conceptualized and designed the study and is lead author of the manuscript. MK, PH, MF, VE and VM implemented clinical investigations and outcome assessment, and share co-authorship of the manuscript. All authors read and approved the final version of the manuscript. JL and MK confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The present case report was performed according to the Declaration of Helsinki and was approved (approval no. OKJL/02/2024) by the Internal Ethics Committee of the Ophthalmology Clinic JL (Faculty of Biomedical Engineering, Czech Technical University, Prague, Czech Republic). All data used were anonymized.
Patient consent for publication
All details, medical records, figures, medical history or test results were used with the written informed consent for publication from the patient.
Competing interests
The authors declare that they have no competing interests.
References
|
Fairburn B and Larkin IM: A cyst of Rathke's cleft. J Neurosurg. 21:223–225. 1964.PubMed/NCBI View Article : Google Scholar | |
|
Larkin S, Karavitaki N and Ansorge O: Rathke's cleft cyst. Handb Clin Neurol. 124:255–269. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Sprau A, Mahavadi A, Zhang M, Saste M, Deftos M and Singh H: Rathke's cleft cyst with xanthogranulomatous change: A case report and review of the literature. Surg Neurol Int. 11(246)2020.PubMed/NCBI View Article : Google Scholar | |
|
Poyuran R, Kalaparti VSVG, Thomas B, Kesavapisharady K and Narasimhaiah D: Nonneoplastic and noninfective cysts of the central nervous system: A histopathological study. Neuropathology. 43:221–232. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Donofrio CA, Bertazzoni G, Riccio L, Pinacoli A, Pianta L, Generali D, Ungari M, Servadei F, Roncaroli F and Fioravanti A: Intrasellar dermoid cyst: Case report of a rare lesion and systematic literature review comparing intrasellar, suprasellar, and parasellar locations. World Neurosurg. 182:83–90. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Jung JE, Jin J, Jung MK, Kwon A, Chae HW, Kim DH and Kim HS: Clinical manifestations of Rathke's cleft cysts and their natural progression during 2 years in children and adolescents. Ann Pediatr Endocrinol Metab. 22:164–169. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Byrd SE, Winter J, Takahashi M and Joyce P: Symptomatic Rathke's cleft cyst demonstrated on computed tomography. J Comput Assist Tomogr. 4:411–414. 1980.PubMed/NCBI View Article : Google Scholar | |
|
Rout D, Das L, Rao VR and Radhakrishnan VV: Symptomatic rathke's cleft cysts. Surg Neurol. 19:42–45. 1983.PubMed/NCBI View Article : Google Scholar | |
|
Altintas Taslicay C, Dervisoglu E, Cam I, Yaprak Bayrak B, Mese I, Anik I, Ceylan S and Anik Y: Differentiation of pure cystic sellar lesions on magnetic resonance paging. Neuroradiol J. 36:533–540. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Kim G, Moon JH, Kim SH and Kim EH: MRI-based classification of Rathke's cleft cyst and its clinical implication. Brain Tumor Res Treat. 11:59–65. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Sharifi G, Amin A, Lotfinia M, Hallajnejad M, Davoudi Z, Dilmaghani NA and Mirghaed OR: Rathke's cleft cysts: A single-center case series. Surg Neurol Int. 13(368)2022.PubMed/NCBI View Article : Google Scholar | |
|
Tang C, Wang P, Liu J, Jiang H, Zhang G and Wu N: Endoscopic endonasal transsphenoidal approach for symptomatic Rathke cleft cyst: A case series. Exp Ther Med. 24(713)2022.PubMed/NCBI View Article : Google Scholar | |
|
Ding Z, Lu X, Wang Q, Qian X, Lu H, Xu R and Zhu A: Endoscopic endonasal surgery of Rathke's cleft cysts-preoperative imaging evaluation, personalized removal and multilevel sellar floor reconstruction. Clin Neurol Neurosurg. 236(108111)2024.PubMed/NCBI View Article : Google Scholar | |
|
Alsavaf MB, Wu KC, Gosal JS, Finger G, Koch B, Abouammo MD, Prevedello LM, Carrau RL and Prevedello DM: Endoscopic endonasal marsupialization of rathke cleft cysts: Clinical outcomes and risk factors analysis of visual impairment, pituitary dysfunction, and CSF leak. Pituitary. 26:696–707. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Gaggero G, Vitulli F, Ramaglia A, Antico A, Canevari FR, Piatelli G and Criminelli Rossi D: Rathke's cyst with xanthogranulomatous change or chronic cystic craniopharingioma? A rare case. Childs Nerv Syst. 13:1311–1314. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Linsler S, Schon L, Fischer G, Senger S and Oertel J: Endonasal endoscopic or endoscopic-assisted transcranial surgery of Rathke's cleft cysts: Does the approach and surgical technique influence the radicality and recurrence rate? Neurosurg Rev. 47(403)2024.PubMed/NCBI View Article : Google Scholar | |
|
Mukherjee JJ, Islam N, Kaltsas G, Lowe DG, Charlesworth M, Afshar F, Trainer PJ, Monson JP, Besser GM and Grossman AB: Clinical, radiological and pathological features of patients with Rathke's cleft cysts: Tumors that may recur. J Clin Endocrinol Metab. 82:2357–2362. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Ogawa Y, Watanabe M and Tominaga T: Rathke's cleft cysts with significant squamous metaplasia-high risk of postoperative deterioration and close origins to craniopharyngioma. Acta Neurochir (Wien). 155:1069–1075. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Langlois F, Manea A, Lim DST, McCartney S, Yedinak CG, Cetas JS and Fleseriu M: High prevalence of adrenal insufficiency at diagnosis and headache recovery in surgically resected Rathke's cleft cysts-a large retrospective single center study. Endocrine. 63:463–469. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Qian A, Zhou J, Zhang X, Yu J and Wang X: Incidence and factors associated with the recurrence of Rathke's cleft cyst after surgery: A systematic review and meta-analysis. Front Surg. 9(1065316)2023.PubMed/NCBI View Article : Google Scholar | |
|
Otradovec J: Klinická Neurooftalmologie. Grada Publishing, 2003. | |
|
Foroozan R: Chiasmal syndromes. Curr Opin Ophthalmol. 14:325–331. 2003.PubMed/NCBI View Article : Google Scholar |