Enhanced efficacy of immune checkpoint inhibitors with OK‑432 in malignant pleural mesothelioma: A case report
- Authors:
- Published online on: July 3, 2025 https://doi.org/10.3892/mco.2025.2872
- Article Number: 77
Abstract
Introduction
Malignant pleural mesothelioma (MPM) is a rare, aggressive cancer with limited treatment options and presents significant management challenges. Immune checkpoint inhibitors (ICIs), such as ipilimumab (IPI) and nivolumab (NIVO), have shown therapeutic potential but with varying efficacy. In cases where treatment fails or disease progresses, patients may experience worsening pleural effusion or tumor burden, which can severely impact respiratory function and overall quality of life. Moreover, effective strategies for managing ICI resistance remain limited and represent an urgent clinical need. Despite initial responses, the disease may still progress-including the development of malignant pleural effusion. OK-432, an immunotherapeutic agent developed in Japan, consists of a penicillin-treated, freeze-dried powder derived from Streptococcus pyogenes (Group A, Type 3), and it has been effectively used for pleurodesis in managing malignant pleural effusions (1,2).
Herein, we report two cases of MPM in which OK-432 was administered intrathoracically, followed by successful ICI rechallenge. These cases suggest the therapeutic potential of combining OK-432 with ICIs, highlighting the need for further investigation into this therapeutic strategy.
Case report
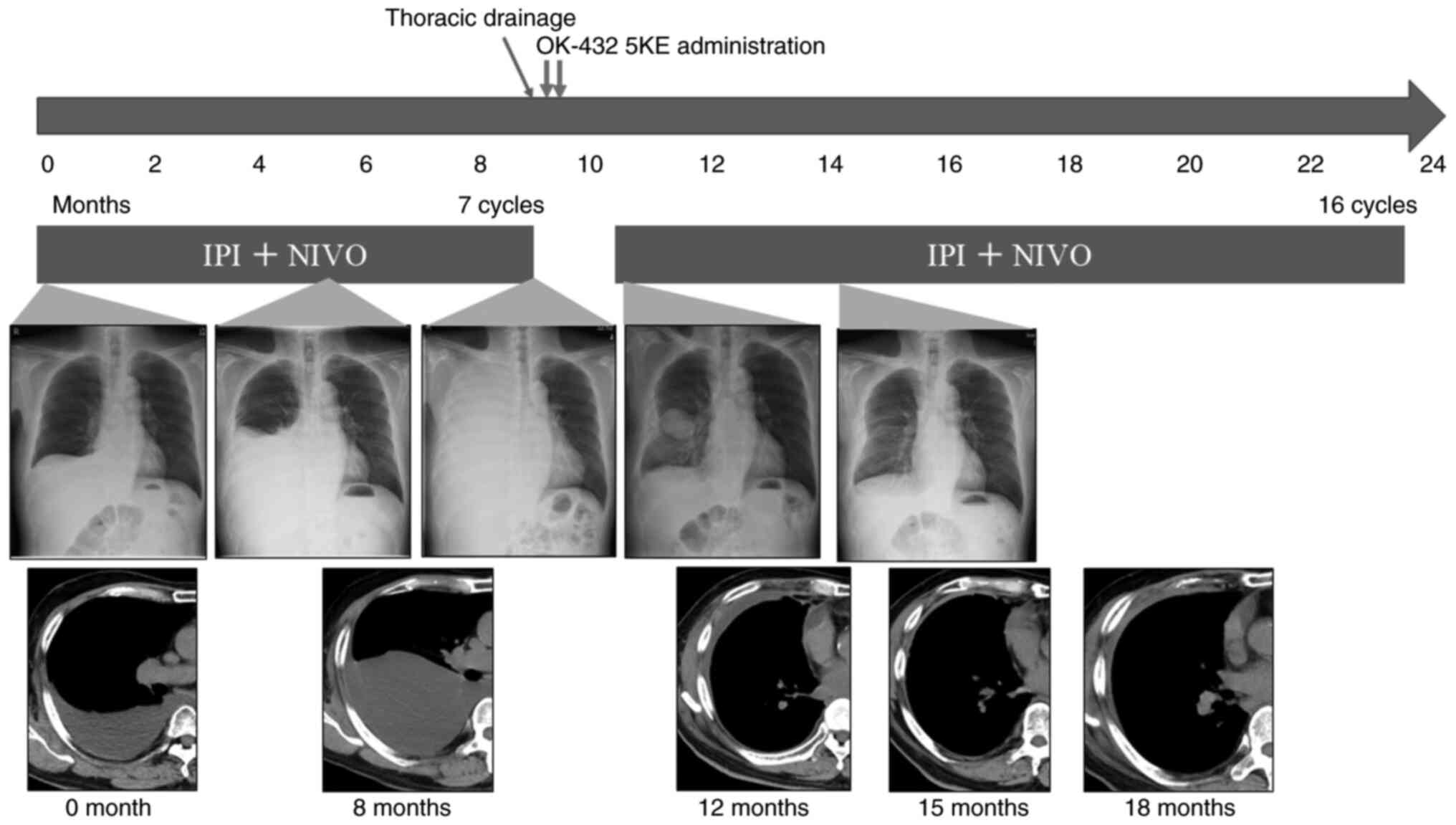
Patient A was a 77-year-old man with no history of asbestos exposure who presented with right pleural effusion. He had a history of hypertension, diabetes mellitus, dyslipidemia, and cerebral infarction, and a 20 pack-year tobacco history. Chest computed tomography (CT) revealed the presence of pleural effusion. He underwent thoracic drainage, and cytological analysis of the pleural effusion confirmed epithelioid mesothelioma. Imaging studies did not reveal any evidence of metastasis. The definitive diagnosis for this patient was clinical T2N0M0, stage IB MPM. As his performance status (PS) was 1, surgery was recommended; however, he declined due to his age. Therefore, chemotherapy was chosen as an alternative treatment, and treatment with IPI plus NIVO was initiated. We obtained written informed consent from the patient for the use of these agents, in accordance with our institutional ethical guidelines. Initially, treatment was effective and the pleural effusion was well-controlled, but after 9 months, it reaccumulated. Cytology revealed the presence of atypical cells, confirming disease progression (Fig. 1). A drainage tube was inserted, and OK-432 5KE was administered intrathoracically for pleurodesis. Despite the initial reaccumulation, the pleural effusion resolved following the rechallenge with IPI plus NIVO. The patient completed 2 years of treatment with no new side effects and remains in remission under observation.
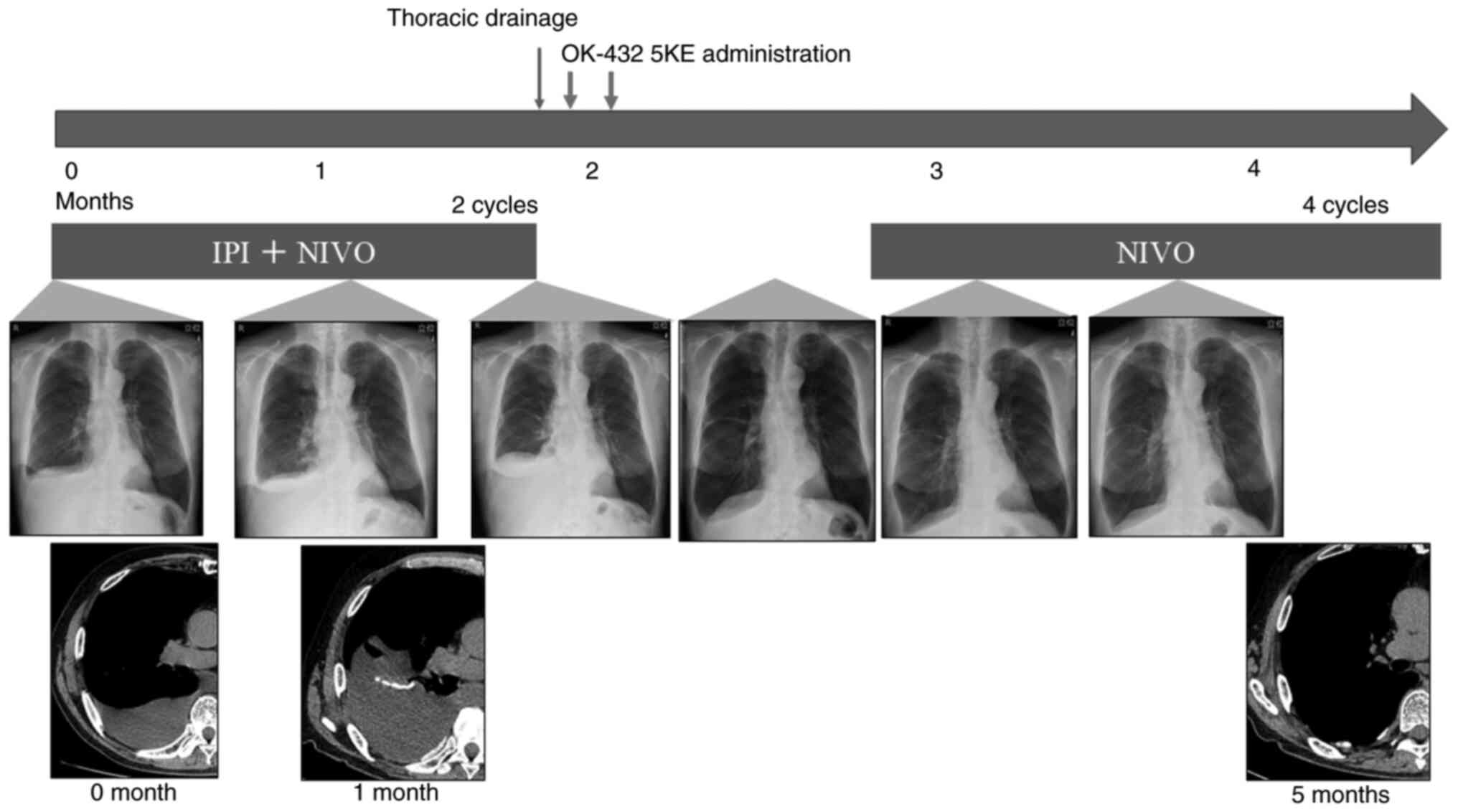
Patient B was a 74-year-old man with a history of asbestos exposure from construction work. He also presented with right pleural effusion on CT. He had a history of hypertension, hyperuricemia, and dyslipidemia and a 56 pack-year tobacco history. Thoracoscopic biopsy confirmed epithelioid mesothelioma. Imaging studies did not reveal any other metastases. The definitive diagnosis for this patient was clinical T1N0M0, stage IA MPM. His PS was 1, and surgery was considered; however, pulmonary function test results demonstrated a significant obstructive ventilatory defect, with a forced vital capacity (FVC) of 2.69 L (75.4% of predicted) and a forced expiratory volume in one second (FEV1) of 1.18 L (41.3% of predicted). Based on this reduced pulmonary function, surgical intervention was deemed high risk, and the patient opted for pharmacological treatment. As a result, he received first-line treatment with ipilimumab plus nivolumab. We obtained written informed consent from the patient for the use of these agents, in accordance with our institutional ethical guidelines. However, after 1 month, the pleural effusion worsened, and the presence of atypical cells in the cytology confirmed disease progression (Fig. 2). Blood tests indicated decreased levels of adrenocorticotropic hormones and cortisol, suggesting pituitary insufficiency caused by the combination therapy with IPI and NIVO. After draining the pleural fluid, OK-432 5KE was administered intrathoracically twice for pleurodesis. Subsequently, NIVO therapy was resumed, no pleural effusion reoccurred for 6 months, and no further adverse effects were reported.
Discussion
This report presents two cases in which rechallenging with combination therapy using IPI and NIVO was effective following intrathoracic administration of OK-432. To the best of our knowledge, this is the first report describing the reactivation of previously ineffective ICIs through OK-432 administration. In both cases, the interval between the initial ICI treatment and rechallenge was relatively short-approximately 1 month-and no new therapeutic agents were introduced aside from OK-432. Moreover, a reduction in pleural effusion was observed immediately following OK-432 administration. Based on these observations, we believe that OK-432 contributed to disease control and played a key role in restoring the efficacy of ICIs.
The effectiveness of ICI rechallenge after OK-432 administration may be attributed to three key mechanisms. First, OK-432 is known to induce a strong Th1-dominant immune response, primarily through the production of cytokines such as interleukin-12 (IL-12), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) (3-6). Th1 polarization enhances the activation of cytotoxic T cells, which are crucial for attacking tumor cells. This shift toward a Th1-dominant immune response not only amplifies the antitumor immune response but may also re-sensitize tumors to ICIs. Recent studies have highlighted the significance of Th1 responses in preventing the exhaustion of tumor-specific CD8+ T cells, thereby prolonging the therapeutic effect of PD-L1 blockade (7). OK-432's ability to induce a Th1 response may help restore immune sensitivity to ICIs and improve their therapeutic efficacy during rechallenge.
Second, OK-432 activates dendritic cells (DCs) via the Toll-like receptor (TLR) 4-MD2 signaling pathway (8). DCs are pivotal for initiating immune responses by presenting tumor antigens to T cells. OK-432 promotes DC maturation, thereby enhancing antigen presentation and overall immune activation. By improving antigen presentation and immune activation, OK-432 likely boosts the effectiveness of subsequent ICI therapy, enhancing the immune response to rechallenge.
Third, studies have demonstrated a synergistic effect between OK-432 and ICIs. For instance, in models of hepatocellular carcinoma and osteosarcoma, combining OK-432 with PD-1 inhibitors resulted in greater tumor volume reduction and prolonged survival compared to either treatment alone (9,10). These findings suggest that OK-432 not only enhances local immune responses but also elicit abscopal effects, wherein the systemic immune response is enhanced, resulting in distant tumor suppression. The ability of OK-432 to amplify the immune response through local and systemic effects likely contributes to its potential synergy with ICIs, increasing the therapeutic efficacy of ICIs during rechallenge.
Together, these mechanisms-Th1-dominant immune activation, dendritic cell maturation, and synergy with ICIs-provide a plausible explanation for the effectiveness of ICI rechallenge after OK-432 administration. By enhancing the immune system's ability to recognize and attack tumor cells, OK-432 may help overcome tumor resistance to ICIs, making rechallenge a viable and potentially effective strategy. This combination of immune-modulatory effects supports the clinical potential of OK-432 as an adjuvant to ICI therapy, particularly in cases where initial ICI therapy has been ineffective.
Furthermore, MPM is known for immune evasion mechanisms, such as upregulation of immunosuppressive cytokines and regulatory T cells, which contribute to resistance to ICIs (11,12). The immunomodulatory effects of OK-432 may help disrupt these suppressive pathways, thereby enhancing antitumor immune response. Given that pleural effusion is a common and often treatment-limiting complication in MPM, OK-432 not only aids in effusion control but also resensitizes tumors to immunotherapy. This dual benefit may ultimately contribute to improved long-term outcomes in patients with MPM.
However, as this report is based on only two cases, definitive conclusions regarding the efficacy of ICI rechallenge following OK-432 administration cannot be drawn at this time. Additional clinical cases are necessary to validate these observations and assess reproducibility in a broader patient population. Moreover, while the proposed mechanisms are biologically plausible, the precise immunological pathways and interactions underlying OK-432's role in modulating the tumor microenvironment remain incompletely understood. Another important limitation is the lack of direct experimental data to support these mechanistic hypotheses, such as analyses of immune cell subsets or cytokine level changes before and after OK-432 administration. Further experimental and clinical research is warranted to elucidate the detailed mechanisms of OK-432-mediated immune modulation and to optimize its integration into current therapeutic strategies for MPM and other cancers.
In conclusion, the two MPM cases responded to rechallenge with IPI plus NIVO following intrathoracic OK-432 administration. While the stabilization of pleural effusion following ICI rechallenge suggests that OK-432 promotes antitumor immune responses, these findings are based on only two cases. Further studies with larger cohorts are necessary to validate these findings and elucidate the precise mechanisms underlying the synergistic effects of OK-432 and ICIs. Continued investigation into these combination therapies may provide new therapeutic strategies for patients with MPM, potentially improving outcomes and prolonging survival.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
KY was involved in conceptualization, acquired funding and wrote the original draft. TU was involved in conceptualization, acquired funding, was involved in project administration and reviewed the manuscript. AM, TaT, IS, KI, YM, KF, SF, YK and ToT contributed to the conception and design of the study, participated in data interpretation and critical revision of the manuscript, approved the final version, and agreed to be accountable for all aspects of the work. YI, TO and AN contributed to the conception and design of the study, supervised the study, participated in data interpretation and critical revision of the manuscript, approved the final version, and agreed to be accountable for all aspects of the work. KY and TU confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Written informed consent for participation was obtained from the patients, although it was not required by the ethics committee of our research institution for this case report.
Patient consent for publication
Written informed consent for publication has been obtained from the patients.
Competing interests
The authors declare that they have no competing interests.
Use of artificial intelligence tools
During the preparation of this work, artificial intelligence tools (ChatGPT) were used to improve the readability and language of the manuscript or to generate images, and subsequently, the authors revised and edited the content produced by the artificial intelligence tools as necessary, taking full responsibility for the ultimate content of the present manuscript.
References
|
Okamoto H, Shoin S, Koshimura S and Shimizu R: Studies on the anticancer and streptolysin S-forming abilities of hemolytic streptococci. Jpn J Microbiol. 11:323–336. 1967.PubMed/NCBI View Article : Google Scholar | |
|
Reshad K, Inni K, Takeuchi Y, Takahashi Y and Hitomi S: Treatment of malignant pleural effusion. Chest. 88:393–397. 1985.PubMed/NCBI View Article : Google Scholar | |
|
Mori H, Itoh N, Yamada Y and Tamaya T: Induction of endogenous tumor necrosis factor by OK-432 in ovarian cancer patients with ascites. Biotherapy. 1:123–131. 1989.PubMed/NCBI View Article : Google Scholar | |
|
Fujimoto T, Duda RB, Szilvasi A, Chen X, Mai M and O'Donnell MA: Streptococcal preparation OK-432 is a potent inducer of IL-12 and a T helper cell 1 dominant state. J Immunol. 158:5619–5626. 1997.PubMed/NCBI | |
|
Katano M and Morisaki T: The past, the present and future of the OK-432 therapy for patients with malignant effusions. Anticancer Res. 18:3917–3925. 1998.PubMed/NCBI | |
|
Waldmann TA: Cytokines in cancer immunotherapy. Cold Spring Harb Perspect Biol. 10(a028472)2018.PubMed/NCBI View Article : Google Scholar | |
|
Xiao M, Xie L, Cao G, Lei S, Wang P, Wei Z, Luo Y, Fang J, Yang X, Huang Q, et al: CD4+ T-cell epitope-based heterologous prime-boost vaccination potentiates anti-tumor immunity and PD-1/PD-L1 immunotherapy. J Immunother Cancer. 10(e004022)2022.PubMed/NCBI View Article : Google Scholar | |
|
Ryoma Y, Moriya Y, Okamoto M, Kanaya I and Saito M: Biological effect of OK-432 (picibanil) and possible application to dendritic cell therapy. Anticancer Res. 24:3295–3301. 2004.PubMed/NCBI | |
|
Sun T, Sun B, Cao Y, Liu J, Chen J, Liang B, Zheng C and Kan X: Synergistic effect of OK-432 in combination with an anti-PD-1 antibody for residual tumors after radiofrequency ablation of hepatocellular carcinoma. Biomed Pharmacother. 166(115351)2023.PubMed/NCBI View Article : Google Scholar | |
|
Iwai T, Oebisu N, Hoshi M, Orita K, Yamamoto A, Hamamoto S, Kageyama K and Nakamura H: Promising abscopal effect of combination therapy with thermal tumour ablation and intratumoural OK-432 injection in the rat osteosarcoma model. Sci Rep. 10(9679)2020.PubMed/NCBI View Article : Google Scholar | |
|
Wong RM: Modulating immunosuppression in the intrapleural space of malignant pleural mesothelioma and predictive biomarkers to guide treatment decisions. J Thorac Oncol. 11:1602–1603. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Chu GJ, van Zandwijk N and Rasko JEJ: The immune microenvironment in mesothelioma: Mechanisms of resistance to immunotherapy. Front Oncol. 9(1366)2019.PubMed/NCBI View Article : Google Scholar |