Patient background and laboratory variables associated with the efficacy of nivolumab after chemoradiotherapy in esophageal squamous cell carcinoma
- Authors:
- Published online on: August 7, 2025 https://doi.org/10.3892/mco.2025.2885
- Article Number: 90
-
Copyright: © Sekino et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Esophageal cancer is the eighth most common cancer worldwide and the sixth leading cause of cancer-related mortality. Even in cases where the tumor is superficial, it exhibits a high metastatic potential with poor disease prognosis (1). Esophageal squamous cell carcinoma (ESCC) is the predominant type (2). ESCC remains refractory to multimodal treatment, with a 5-year survival rate of 20% in patients with stage IVb disease who are ineligible for surgery (3). Although neoadjuvant or definitive chemoradiotherapy (CRT) is the standard treatment for locally advanced ESCC, patient outcomes remain unsatisfactory, with recurrence rates as high as 30-50% (4). Recent studies have suggested a synergistic effect between CRT and immune checkpoint inhibitors (ICIs), highlighting the need for reliable biomarkers to predict the tumor response and survival outcomes (4).
Immunotherapy with ICIs has shown promise. However, the response rate to nivolumab, an anti-programmed cell death protein 1 (PD-1) antibody targeting the immunosuppressive auxiliary signal PD-1/PD-1 ligand 1 (PD-L1), in previously treatment-refractory ESCC was 19% in the ATTRACTION-3 trial (5). The CheckMate 648 trial (ClinicalTrials.gov, NCT03143153) (6) and KEYNOTE 590 trial (ClinicalTrials.gov, NCT03189719) (7) also demonstrated a certain degree of therapeutic efficacy, underscoring the urgent need to enhance immunotherapy response rates in ESCC.
Several studies have reported correlations between tumor immunity and inflammation, and the therapeutic effects of chemotherapy and immunotherapy. Nutrition-related factors, such as the prognostic nutritional index (PNI), which is based on serum albumin and lymphocytes (8,9), modified Glasgow prognostic score (mGPS), calculated from albumin and C-reactive protein (CRP) (10), CRP-to-albumin ratio (CAR) using albumin and lymphocytes (11), and controlling nutritional status (CONUT), which incorporates albumin, lymphocytes and total cholesterol (12,13), are widely used as prognostic indicators in cancers, including ESCC. These indices highlight the prognostic importance of albumin and C-reactive protein.
Predicting treatment effects is difficult; however, several reports have identified factors associated with treatment outcomes. Makino et al reported objective response and disease control rates of 17.0 and 47.9%, respectively, for nivolumab monotherapy in patients with unresectable advanced ESCC. They identified performance status, number of metastatic organs, CAR, neutrophil-lymphocyte ratio (NLR), and the psoas muscle index (PMI) as relevant influencing factors (14). Kosumi et al found that the waist circumference was significantly associated with the disease control rate at the initial evaluation and the PNI (15). These reports suggest that systemic factors, including the nutritional and cancer immunity status, influence the therapeutic efficacy of ICIs.
However, there are few reports on the factors that influence the therapeutic efficacy of nivolumab after definitive CRT. Given the availability of alternative therapies, such as paclitaxel, predicting nivolumab's efficacy is crucial, and a comprehensive evaluation, including assessment of systemic conditions and immune status, is needed.
Therefore, this study aimed to identify the factors influencing the therapeutic efficacy of nivolumab after CRT in ESCC and examine the prediction methods for treatment success.
Materials and methods
Patients
We retrospectively analyzed patients with ESCC who received CRT following anti-PD-1 therapy (nivolumab administration) at Chiba University Hospital between September 2019 and February 2023. For comparison, the non-nivolumab group included patients who underwent radical CRT between January and December 2018, followed by chemotherapy other than nivolumab.
Patients were eligible for this study if they met the following criteria: i) A pathological diagnosis of ESCC; ii) age between 20-90 years, with the capacity for self-determination; iii) treatment with 5-fluorouracil + cisplatin (FP) as the sole chemotherapy regimen prior to nivolumab administration; and iv) all patients underwent blood tests after a sufficient interval following the completion of CRT or chemotherapy. Blood tests were performed on the day of nivolumab administration in 21 patients, and 7 days prior to administration in one patient.
The data of 22 patients who received nivolumab and 14 patients who did not receive nivolumab were retrospectively analyzed after excluding those who did not meet the inclusion criteria. Informed consent was obtained from all participants. Stage classification was performed according to the 11th edition of the Japanese Classification of Esophageal Cancer (16).
Determination of treatment effectiveness
Initial treatment effectiveness was determined using computed tomography after two or four courses of nivolumab. Patients were classified into two groups: the response group with partial response (PR) or stable disease (SD) and the non-response group with progressive disease (PD). Factors correlating with the treatment response were identified and analyzed.
Blood test analysis
Results of blood tests performed on or immediately before the day of nivolumab administration were analyzed. Differences in the following parameters between the PR + SD group and PD group were examined using the unpaired Student's t-test: white blood cell count (WBC, /all), leukocyte fraction (neutrophil, eosinophil, basophil, monocyte, and lymphocyte, %), hemoglobin (HGB, g/dl), platelet count (PLT, x103/all), total protein (TP, g/dl), albumin (ALB, g/dl), aspartate aminotransferase (AST, U/l), alanine aminotransferase (ALT, U/l), lactate dehydrogenase (LDH, U/l), bilirubin (BIL, mg/dl), creatinine (CRE, mg/dl), estimated glomerular filtration rate (eGFR, ml/min/1.73 m2), creatinine clearance in Cockcroft-Gault (CoCr, ml/min), and C-reactive protein (CRP, mg/dl). NLR, PNI, and CAR were also calculated and analyzed.
Evaluation of categorical variables
The Youden index was used to determine the cut-off values for the six key variables [body weight (BW), WBC, basophil, PLT, ALB, and CRP]. The significance of these cut-off values was assessed using Fisher's exact test. Kaplan-Meier survival curves were examined for overall survival (OS) and compared using the log-rank test. A total score was calculated based on the number of favorable variables identified, and association of the total score with nivolumab efficacy and OS were examined. In addition, pairwise associations among the six dichotomized variables were assessed using Fisher's exact test to examine potential inter-variable relationships.
Statistical analyses
Associations between the pathological grade of chemotherapy and clinicopathological features (e.g., sex and tumor factors such as clinical T and clinical N factors) were evaluated using Fisher's exact test. Blood test results were evaluated using unpaired Student's t-test. The Youden index was used to establish cut-off values for the six key variables. All statistical analyses were conducted using JMP Pro version 18 software (SAS Institute Inc., Cary, NC, USA). P<0.050 was considered to indicate a statistically significant difference, while values P<0.100 were considered a trend.
Results
Patient background characteristics
The baseline characteristics of the 22 patients included in this study are shown in Table I. Of these, nine patients were in the PR + SD group, and 13 were in the PD group, with an overall response rate of 40.91%. There were no significant associations between age (P=0.6249), sex (P=0.0002), disease stage (tumor depth; P=1.0000, lymph node status; P=0.3602), and PD-L1 expression [combined positive score (CPS); P=1.0000, tumor proportion score (TPS); P=1.0000] in these two groups. PD-L1 expression was evaluated using both the CPS and TPS in 10 out of 22 patients. For the remaining 12 patients, PD-L1 data were not available. However, there were significant differences in weight (P<0.0001), height (P=0.0231), and body mass index (BMI) (P=0.0002), related to the body size. Additionally, the number of nivolumab courses administered was significantly higher in the PR + SD group (P=0.0015) (Table II). To investigate whether patient background characteristics differed by sex, we conducted the same analysis in only the 19 male patients (Table SI). The results showed a generally similar trend; however, the difference in body height was no longer statistically significant (P=0.1067). When assessing OS, no significant difference was found between the nivolumab-treated and non-nivolumab-treated groups (Fig. 1A, P=0.1333). In the nivolumab-treated group, there was a significant difference in prognosis when classified by the treatment effect, with a better prognosis in the PR + SD group and poorer prognosis in the PD group (Fig. 1B, P=0.0004 and 1C, P=0.0126, respectively).
Blood test analysis
The association between blood test parameters and treatment response is summarized in Table III. WBC (P=0.0067), basophil fraction (P0.0042), PLT (P=0.0229), ALB (P=0.0388), CRE (P=0.0196), eGFR (P=0.0470), and CRP (P=0.0939) showed significant differences or trends as determined by Student's t-test. Among the indices, CAR showed a trend toward a difference between the two groups (P=0.0926); however, NLR and PNI did not show significant differences. Since reference ranges for blood test parameters can differ by sex, we reanalyzed the data by including only male patients (Table SII). Although the results showed a similar trend, both ALB (P=0.0692) and CRP (P=0.1104) did not reach statistical significance and demonstrated only a tendency.
Classification and evaluation of BW and blood test results
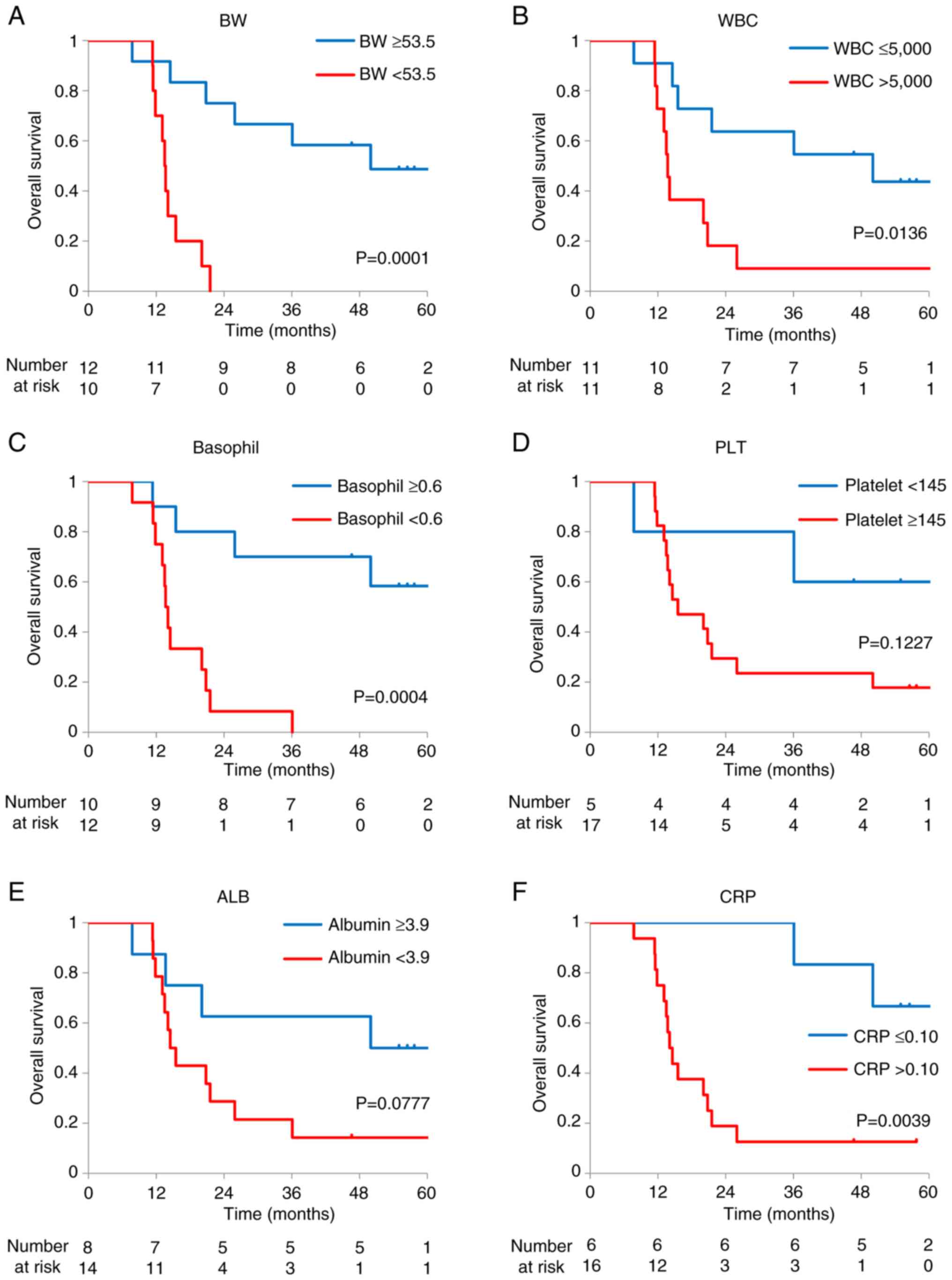
The classification and evaluation focused on variables showing significant differences: BW, WBC, basophil, PLT, ALB, and CRP. Given that no difference was observed in CoCr for renal function, CRE and eGFR were excluded from the evaluation because they were considered strongly influenced by the body size. The Youden index was used for determining the cut-off values for BW (53.5), WBC (5000), basophil (0.6), PLT (145), ALB (3.9), and CRP (0.10), dividing the patients into two groups. Fisher's exact test showed significant differences in BW (P=0.0005), WBC (P=0.0075), basophil (P=0.0048), PLT (P=0.0274), ALB (P=0.0260), and CRP (P=0.0011) (Table IV). Based on the results of Table SII, we also conducted this analysis in only male patients. In this subgroup, all six parameters showed statistically significant associations (Table SIII). Sex-based differences in these six variables were further examined (Table SIV). A significant difference was observed for BW (P=0.0015), whereas no significant differences were found for the other blood test parameters. When associations between these categories and prognosis were examined, favorable variables were associated with a better prognosis, with BW, WBC, basophil, and CRP demonstrating statistical significance (Fig. 2, log-rank: BW, P=0.0001; WBC, P=0.0136; basophil, P=0.0004; PLT, P=0.1227, ALB, P=0.0777; and CRP, P=0.0039).
Table IVAssociation of the classifications (BW, WBC, basophil, platelet, albumin, and CRP) and nivolumab efficacy. |
Association between overall evaluation and prognosis
The number of favorable variables for each patient was determined based on the identified cut-off values. The total score was calculated for each patient based on the number of favorable variables within the following thresholds: body weight ≥53.5 kg, WBC ≤5,000/mm³, basophil ≥0.6%, platelet <145x104/mm³, albumin ≥3.9 g/dl, and CRP ≤0.10 mg/dl. When assessing the associations among the categorized parameters (Table V), BW showed significant associations with the WBC (P=0.0300), basophil count (P=0.0427), platelet count (P=0.0396), and CRP (P=0.0152), but not with ALB. Additionally, WBC was significantly correlated with the basophil percentage (P=0.0351). No other notable associations were observed among the remaining parameters.
Table VP-values of the pairwise associations among the six variables used to calculate the total score. |
Among all patients, nine from the PR + SD group had at least three favorable variables, whereas 13 from the PD group had two or fewer favorable variables (Table VI, P<0.001). OS was significantly better in patients with three or more favorable variables than in those with two or fewer (Fig. 3, log-rank: P=0.0001).
Discussion
In this study, we examined the association of patient background characteristics and blood parameters with nivolumab efficacy after CRT. We identified BW, WBC, percentage of basophil fraction, PLT, ALB, and CRP as the significant factors influencing nivolumab treatment after CRT.
The overall response and disease control rates with nivolumab treatment were 22.72 and 40.91%, substantially similar to those observed in the ATTRACTION-3 trial (5). In recent years, an abscopal effect has been associated with the post-CRT status in gastrointestinal cancers, such as esophageal, gastric, pancreatic, hepatobiliary, and colorectal cancers, (17). These findings, together with the results of this study, suggest that ICIs may be more selective after radiation therapy, especially in patients meeting the conditions indicated in this study.
BW and ALB are important indicators of the nutritional status. Although the PNI did not show a significant difference in this study, our findings underscore the importance of nutritional status. Previous studies in patients with non-small cell lung cancer have demonstrated that a higher BMI correlates with better prognosis in patients treated with ICIs, with BMI categorized as <18.5, 18.5-22.9, 23-24.9, and ≥25(18). When considered alongside reports on waist circumference (15), it appears that body size, including body weight, is important in immunotherapy. However, additional studies are required to elucidate the underlying mechanism.
Interestingly, BW showed a significant association with several inflammation- and immunity-related parameters, including WBC, basophils, platelets, and CRP. These associations suggest that BW may reflect not only the long-term nutritional status but also systemic inflammatory and immune conditions. Given that WBC, basophils, and CRP are involved in tumor-associated immune modulation, BW could serve as an integrative marker of the host-tumor-immune axis.
Notably, in this analysis, BW did not show a significant association with ALB levels. While ALB is widely used as a nutritional marker, it is also a negative acute-phase reactant and can decrease rapidly in response to inflammation, due to cytokine-mediated suppression of hepatic synthesis (19). In contrast, BW may be less influenced by short-term inflammatory changes and could more accurately reflect the patient's baseline nutritional and immunological state. These findings warrant further investigation to determine whether BW functions as a surrogate marker or has a mechanistic role in the context of cancer-related inflammation and immunotherapy. WBC and CRP are direct inflammation-related factors. Cancers often induce both acute and chronic inflammation. In ESCC, high preoperative CRP levels have been associated with tumor progression and poor survival in patients (20). In environments with pre-existing systemic inflammation, tumor immunity may be impaired.
Among the WBC fractions, only basophils showed a significant difference; however, there are very few studies on the relationship between basophils and ICIs. In murine models, basophils have been shown to produce chemokines such as CCL3 and CCL4, thereby promoting the infiltration of CD8+ T cells into the tumor microenvironment (21). This suggests that basophil activation may enhance antitumor immunity by facilitating CD8+ T cell-mediated tumor rejection. Furthermore, an increase in CCL3 levels within the tumor microenvironment was shown to promote T cell infiltration and enhance the efficacy of PD-1 blockade therapy (22). Although the mechanism remains unclear, one study suggested that basophils affect the tumor microenvironment via M2 macrophages (23), warranting further investigation. In patients with melanoma treated with anti-PD1 immunotherapy with a combination of nivolumab and ipilimumab, the relative basophil count was reported to correlate with the treatment response, along with relative eosinophils, absolute monocytes, LDH, and NLR (24). Increased baseline basophil levels also predicted significantly improved progression-free survival (PFS) in patients with non-small cell lung carcinoma treated with nivolumab (25). In contrast, some studies have reported that patients with high basophil levels show poorer responses to ICI therapy. In gastric cancer, eosinophils have also been implicated in promoting M2 macrophage polarization, potentially contributing to an immunosuppressive microenvironment and resistance to immunotherapy (23). A recent mechanistic study revealed that IL-4 secreted by bone marrow-derived basophils drives the immunosuppressive phenotype of tumor-infiltrating monocyte-derived macrophages (26). This suggests that elevated basophil activity and IL-4 production may promote immune evasion and resistance to ICIs. These controversial results suggest that differences between cancer types and interactions with other factors may need to be considered for a comprehensive evaluation.
In the present study, the cutoff value for basophils was set at 0.6% based on the Youden Index. Given that the normal reference range for basophils is approximately 0-1%, this cutoff was considered appropriate for dividing patients into high and low groups. However, further studies are warranted to validate the utility of this parameter.
PLTs act as active immune-like cells that suppress T cell activity, promote cancer metastasis, and regulate the spliced mRNA content (27). Inoue et al reported that nivolumab treatment for recurrent esophageal cancer was significantly more effective in patients with a low platelet-lymphocyte ratio, in addition to low lymphocytes and CAR (28). Additionally, Zhang et al reported that a lower systemic immune inflammation index (SII=PLT x neutrophils/lymphocytes) was associated with a significantly higher resection rate after preoperative treatment, including radiotherapy in ESCC (29). Although PLTs were often evaluated in combination with other parameters in previous studies, they were also identified as a significant factor in this study. Thus, it is now considered appropriate to evaluate the systemic inflammatory status not only by assessing the WBC count and CRP but also by combining various factors such as the lymphocyte fractions and PLT, which was also supported by the findings in this study.
In this study, CRE was significantly elevated, whereas the eGFR was lower in the PR + SD group. Furthermore, nivolumab did not require dose adjustment based on renal function, as antibodies are too large to be excreted by the kidneys except in severe nonselective proteinuria (30). Given that body size parameters, such as height and weight, also showed significant differences, we evaluated renal function based on CoCr using the Cockcroft-Gault formula, which accounts for the BW. However, there was no significant difference in CoCr between the two groups, suggesting that the difference in creatinine clearance was due to differences in the body size.
In the present study, no significant association was found between PD-L1 expression and treatment efficacy. Moreover, data on other tumor-specific immunologic markers such as the tumor mutational burden were not available, and therefore not analyzed. These biomarkers are recognized as potentially important predictors of response to ICIs and should be investigated in future studies with a larger cohort.
The six variables identified in this study were considered valid when three or more variables were within the range in the PR + SD group. Based on this, we concluded that these variables may be useful for predicting the treatment efficacy.
However, this study has some limitations, including its small sample size and single-center design. The size of the cohort, consisting of 22 patients receiving nivolumab and 14 controls, limited the statistical power and may have introduced potential biases, thereby reducing the generalizability of the findings. Accordingly, the results should be interpreted with caution and regarded as hypothesis-generating rather than conclusive. Larger-scale, multicenter studies are needed to validate and extend these findings to broader clinical settings.
Although the scoring system using six variables demonstrated an apparent association with clinical outcomes, this should also be interpreted with caution given the exploratory nature of the study and limited sample size. Further validation in larger, independent cohorts will be required to confirm the predictive utility and clinical relevance of these parameters.
Moreover, according to the commonly used rule of thumb requiring at least 10 events per variable for logistic regression, including multiple variables in a multivariate model would have risked overfitting and unreliable estimates. Therefore, we limited our analysis to univariate methods. As the number of cases increases, the predictive value of these variables can be comprehensively examined, and the cut-off values will become more appropriate, enhancing the association between the number of applicable variables and treatment outcomes.
Finally, being a single-institution study, center-specific factors such as patient selection, treatment implementation, and healthcare infrastructure may have influenced the results. These findings should therefore be interpreted within the context of this limitation, and future multicenter investigations will be essential to establish broader applicability.
In conclusion, although this study was based on a small sample of 22 cases, the six identified variables (BW, WBC, percentage of basophil fraction, PLT, ALB, and CRP) may be valuable for predicting the efficacy of ICI treatment after CRT in ESCC. By accumulating more cases based on our study's methodology, more reliable prediction methods can be developed.
Supplementary Material
Relation between nivolumab efficacy and clinical characteristics in male patients.
Association between blood test results and nivolumab efficacy among male patients.
Association of the classifications (BW, WBC, basophil, platelet, albumin, and CRP) and nivolumab efficacy in male patients.
Association between sex and featured six variants.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Surgery II Scholarship Donation from the Department of Frontier Surgery, Graduate School of Medicine, Chiba University (grant no. J09KF00261).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
NS, TT, KM and HM contributed to the conceptualization of the study. NS, TT, KM, MU, AN, TS, KH, YM, YK, RO, SH and HH participated in data collection. NS, TT, KM, MU, AN and TS conducted project administration. NS and TT confirm the authenticity of all the raw data. NS wrote the manuscript under the supervision of TT, KM and HM. All authors provided critical feedback, and read and approved the final manuscript.
Ethics approval and consent to participate
All procedures followed were in accordance with the Ethics Committee of Graduate School of Medicine, Chiba University (approval no. 3043; Chiba, Japan) and with the Helsinki Declaration of 1964 and later versions. Written informed consent was obtained from all patients before inclusion in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Takeuchi H, Miyata H, Gotoh M, Kitagawa Y, Baba H, Kimura W, Tomita N, Nakagoe T, Shimada M, Sugihara K and Mori M: A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide Web-based database. Ann Surg. 260:259–266. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Pennathur A, Gibson MK, Jobe BA and Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Tachimori Y, Ozawa S, Numasaki H, Fujishiro M, Matsubara H, Oyama T, Shinoda M, Toh Y, Udagawa H and Uno T: Registration Committee for Esophageal Cancer of the Japan Esophageal Society. Comprehensive registry of esophageal cancer in Japan, 2009. Esophagus. 13:110–137. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Wang R, Liu S, Chen B and Xi M: Recent advances in combination of immunotherapy and chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. Cancers (Basel). 14(5168)2022.PubMed/NCBI View Article : Google Scholar | |
|
Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, et al: Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 20:1506–1517. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, et al: Nivolumab combination therapy in advanced esophageal Squamous-Cell carcinoma. N Engl J Med. 386:449–462. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Kato K, Kojima T, Hara H, Tsuji A, Yasui H, Muro K, Satoh T, Ogata T, Ishihara R, Goto M, et al: First-line pembrolizumab plus chemotherapy for advanced/metastatic esophageal cancer: 1-year extended follow-up in the Japanese subgroup of the phase 3 KEYNOTE-590 study. Esophagus. 21:306–318. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Xue Y, Zhou X, Xue L, Zhou R and Luo J: The role of pretreatment prognostic nutritional index in esophageal cancer: A meta-analysis. J Cell Physiol. 234:19655–19662. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Zhang H, Shang X, Ren P, Gong L, Ahmed A, Ma Z, Ma R, Wu X, Xiao X, Jiang H, et al: The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol. 234:1794–802. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Wang Y, Chen L, Wu Y, Li P and Che G: The prognostic value of modified Glasgow prognostic score in patients with esophageal squamous cell cancer: A Meta-analysis. Nutr Cancer. 72:1146–1154. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Sakai M, Sohda M, Saito H, Ubukata Y, Nakazawa N, Kuriyama K, Hara K, Sano A, Ogata K, Yokobori T, et al: Comparative analysis of immunoinflammatory and nutritional measures in surgically resected esophageal cancer: A Single-center retrospective study. In Vivo. 34:881–887. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, Rodríguez F and Fernández G: CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 20:38–45. 2005.PubMed/NCBI | |
|
Takagi K, Buettner S, Ijzermans JNM and Wijnhoven BPL: Systematic review on the controlling nutritional status (CONUT) score in patients undergoing esophagectomy for esophageal cancer. Anticancer Res. 40:5343–5349. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Makino T, Nakai S, Momose K, Yamashita K, Tanaka K, Miyata H, Yamamoto S, Motoori M, Kimura Y, Ushimaru Y, et al: Efficacy and survival of nivolumab treatment for recurrent/unresectable esophageal squamous-cell carcinoma: Real-world clinical data from a large Multi-institutional cohort. Esophagus. 21:319–327. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Kosumi K, Baba Y, Hara Y, Wang H, Nomoto D, Toihata T, Ohuchi M, Harada K, Eto K, Ogawa K, et al: Body composition and clinical outcomes in esophageal cancer patients treated with immune checkpoint inhibitors. Ann Surg Oncol. 31:3839–3849. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Kitagawa Y, Uno T, Oyama T, Kato K, Kato H, Kawakubo H, Kawamura O, Kusano M, Kuwano H, Takeuchi H, et al: Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: Part 1. Esophagus. 16:1–24. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Hino C, Lee EW and Yang GY: Harnessing the abscopal effect for gastrointestinal malignancies in the era of immunotherapy. J Gastrointest Oncol. 14:1613–1625. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Lee JH, Kang D, Ahn JS, Guallar E, Cho J and Lee HY: Obesity paradox in patients with Non-small cell lung cancer undergoing immune checkpoint inhibitor therapy. J Cachexia Sarcopenia Muscle. 14:2898–2907. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Tanaka T, Narazaki M and Kishimoto T: IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 6(a016295)2014.PubMed/NCBI View Article : Google Scholar | |
|
Shimada H, Nabeya Y, Okazumi S, Matsubara H, Shiratori T, Aoki T, Sugaya M, Miyazawa Y, Hayashi H, Miyazaki S and Ochiai T: Elevation of preoperative serum C-reactive protein level is related to poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol. 83:248–252. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Sektioglu IM, Carretero R, Bulbuc N, Bald T, Tüting T, Rudensky AY and Hämmerling GJ: Basophils promote tumor rejection via chemotaxis and infiltration of CD8+ T cells. Cancer Res. 77:291–302. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Kang TG, Park HJ, Moon J, Lee JH and Ha SJ: Enriching CCL3 in the tumor microenvironment facilitates T cell Responses and improves the efficacy of Anti-PD-1 therapy. Immune Netw. 21(e23)2021.PubMed/NCBI View Article : Google Scholar | |
|
Wu C, Qiu Y, Zhang R, Li X, Liang H, Wang M, Li F, Zhu M, Ye G, Liu H, et al: Association of peripheral basophils with tumor M2 macrophage infiltration and outcomes of the anti-PD-1 inhibitor plus chemotherapy combination in advanced gastric cancer. J Transl Med. 20(386)2022.PubMed/NCBI View Article : Google Scholar | |
|
Rosner S, Kwong E, Shoushtari AN, Friedman CF, Betof AS, Brady MS, Coit DG, Callahan MK, Wolchok JD, Chapman PB, et al: Peripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanoma. Cancer Med. 7:690–697. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Krizova L, Benesova I, Zemanova P, Spacek J, Strizova Z, Humlova Z, Mikulova V, Petruzelka L and Vocka M: Immunophenotyping of peripheral blood in NSCLC patients discriminates responders to immune checkpoint inhibitors. J Cancer Res Clin Oncol. 150(99)2024.PubMed/NCBI View Article : Google Scholar | |
|
LaMarche NM, Hegde S, Park MD, Maier BB, Troncoso L, Le Berichel J, Hamon P, Belabed M, Mattiuz R, Hennequin C, et al: An IL-4 signalling axis in bone marrow drives pro-tumorigenic myelopoiesis. Nature. 625:166–1674. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Muller M, Best MG, van der Noort V, Hiltermann TJN, Niemeijer AN, Post E, Sol N, In 't Veld SGJG, Nogarede T, Visser L, et al: Blood platelet RNA profiles do not enable for nivolumab response prediction at baseline in patients with Non-small cell lung cancer. Tumour Biol. 46 (Suppl):S327–S340. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Inoue H, Shiozaki A, Fujiwara H, Konishi H, Kiuchi J, Ohashi T, Shimizu H, Arita T, Yamamoto Y, Morimura R, et al: Absolute lymphocyte count and C-reactive protein-albumin ratio can predict prognosis and adverse events in patients with recurrent esophageal cancer treated with nivolumab therapy. Oncol Lett. 24(257)2022.PubMed/NCBI View Article : Google Scholar | |
|
Zhang H, Lin J, Huang Y and Chen Y: The systemic Immune-inflammation index as an independent predictor of survival in patients with locally advanced esophageal squamous cell carcinoma undergoing neoadjuvant radiotherapy. J Inflamm Res. 17:4575–4586. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Counsilman CE, Jol-van der Zijde CM, Stevens J, Cransberg K, Bredius RG and Sukhai RN: Pharmacokinetics of rituximab in a pediatric patient with therapy-resistant nephrotic syndrome. Pediatr Nephrol. 30:1367–1370. 2015.PubMed/NCBI View Article : Google Scholar |