Research advances in current drugs targeting hyperlipidemia (Review)
- Authors:
- Published online on: July 17, 2025 https://doi.org/10.3892/mmr.2025.13623
- Article Number: 258
-
Copyright: © Zhao et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Hyperlipidemia (HLP) is a common lipid metabolism disorder and dyslipidemia (1–4). Blood lipids refer to the total cholesterol, triglycerides (TG) and lipids in the serum. The main characteristics of HLP are elevated levels of total cholesterol, TG, and low-density lipoprotein cholesterol (LDL-C) in the blood, along with a decrease in high-density lipoprotein cholesterol (HDL-C) levels (5). Due to the fact that blood lipids are not soluble in water, they must combine with proteins called apolipoproteins (Apo) to form lipoproteins in order to dissolve in the blood and be transported to tissues for metabolism (6). Lipoproteins can be classified into chylomicrons (CM), very low-density lipoprotein (VLDL), intermediate-density lipoprotein, LDL, high-density lipoprotein (HDL) and lipoprotein(a) [Lp (a)] (7–17). The classification, main components, sources and functions of lipoproteins are shown in Table I. HLP also represents an abnormality of lipoproteins.
Cardiovascular disease (CVD) is the leading chronic non-communicable disease threatening human life and health in modern society (18,19), including atherosclerosis, stroke and myocardial infarction. Atherosclerosis is a chronic disease that primarily occurs in medium or large arteries, caused by the accumulation of lipids and complex carbohydrates in the vascular endothelium, forming hard structures known as plaques, which can lead to narrowing of the vascular lumen and ultimately result in deformation or even death of myocardial cells (20,21). HLP is one of the independent risk factors for atherosclerotic CVD (ASCVD) (21). A previous study demonstrated that the prevalence of dyslipidemia has markedly increased in low- and middle-income countries, especially in East and Southeast Asia (22). Since 2012 to 2018, the prevalence of dyslipidemia among adults in China has remained at high levels (23–26). In China, 26% of patients with ASCVD are classified as very high-risk (27). Preventing and treating HLP is an effective and commonly used method to reduce the incidence of CVD (28,29).
With the in-depth understanding and research of the molecular basis and genetic origins of dyslipidemia, the treatment options for dyslipidemia are continuously increasing. This present review summarizes the classification and pathogenesis of HLP, as well as the latest clinical research progress on statins, ezetimibe, proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibodies, volanesorsen targeting ApoC3, bile acid sequestrants and various other drugs including evinacumab that can lower LDL-C, along with newly launched and other emerging drugs, providing reference for the treatment of HLP and its associated diseases and drug research.
Classification of the causes of primary HLP
Primary HLP refers to lipid abnormalities caused by non-secondary factors (such as unhealthy diet, diseases and medications), usually resulting from mutations in a single gene or multiple genes, and is characterized by familial clustering and has a considerable genetic tendency, especially in cases of single gene mutations (Table II).
Table II.Classification of primary hyperlipidemia, pathogenic genes and their effects on blood lipids. |
Familial hypercholesterolemia (FH)
FH is an autosomal, single-gene hereditary disorder of cholesterol metabolism, usually inherited in a dominant manner, with recessive inheritance being relatively rare (30). It is one of the most common single-gene genetic disorders (31). Its characteristic is an increase in LDL-C (32), this is mainly caused by genetic defects in the LDL clearance pathway. The three most common explicit genetic factors are: LDL receptor (LDLR), ApoB and PCSK9, among which the most common is the LDLR gene mutation, accounting for ~90%. LDLR gene mutations leads to a decrease in LDLR activity (33). Secondly, pathogenic mutations of ApoB account for 5–10% and the proportion is higher among patients in China (34), this gene mutation impairs ligand-receptor mediated LDL binding (33); PCSK9 accounts for ~2% (35), PCSK9 can lead to the degradation of LDLR, therefore, gain-of-function mutations in the PCSK9 gene can also increase LDL by weakening LDLR activity (33). Mutations in the ApoB and PCSK9 genes rarely lead to hypercholesterolemia (36,37). Extremely rare recessive hypercholesterolemia is caused by homozygous mutations in the gene encoding LDLR adaptor protein 1 (36).
FH includes heterozygous FH (HeFH) and homozygous FH (HoFH), with HeFH being more common. A systematic review and meta-analysis reported that the overall prevalence of FH in the HeFH group is 1 in 200–250 individuals, while in the HoFH group it is 1 in 100,000-160,000 individuals (38). Due to the high cholesterol levels from birth, patients with FH have a markedly increased risk of cardiovascular disease, with severe atherosclerotic events occurring even in early childhood, leading to premature mortality (32,39–41). Although the majority of patients with FH have hypercholesterolemia caused by a single gene, there are still several patients with FH whose hypercholesterolemia is caused by polygenic, environmental or unknown single-gene factors (39). With the development of gene sequencing technology, more and more genes such as signaling transducer and activator of transcription 1, lysosomal acid lipase and apolipoprotein E (ApoE) have been considered potentially associate with the pathogenesis of FH (42).
Mixed familial HLP (FCHL)
FCHL is caused by polygenic inheritance with a complex genetic pattern, characterized by elevated levels of ApoB100 and TG in plasma (43–47). FCHL may be caused by a lipid disorder, where adipocytes have a poor ability to retain TGs, leading to an increased release of free fatty acids (48,49). The lipid genes located on chromosome 11 and genes associated with endoplasmic reticulum VLDL processing (such as uncoupling stimulus factor 1) exacerbate this phenotype (47). In addition, upstream transcription factor 1 is also associated with FCHL (50).
Familial dysbetalipoproteinemia (FD)
FD, also known as type III hyperlipoproteinemia, is a hereditary lipid disorder with low penetrance associated with ApoE mutations (51,52). ApoE can bind to LDLR and heparan sulfate proteoglycan receptor with high affinity (52,53), ApoE can inhibit the breakdown of lipoprotein lipase (LPL) and TG-rich lipoproteins (54), high ApoE levels are associated with cardiovascular and cancer mortality, while low ApoE levels are linked to mortality associated with dementia (51,55).
There are three common alleles of ApoE, namely ϵ2, ϵ3 and ϵ4, which can form six genotypes (ϵ2ϵ2, ϵ2ϵ3, ϵ2ϵ4, ϵ3ϵ3, ϵ3ϵ4 and ϵ4ϵ4). FD characteristics include mixed HLP, which is characterized by elevated cholesterol and TGs in the plasma and can also present primarily as hypercholesterolemia or hypertriglyceridemia (HTG), associated with an increased risk of ASCVD (51). The main genetic mutation of FD is the homozygosity of the ε2 allele (ε2ε2 genotype), but only 15% of patients with ε2ε2 will develop FD (52). Compared with ε3 homozygotes, the majority of ε2 allele carriers have lower LDL-C levels and higher TG levels, with ε2 homozygotes having even higher TG levels (52). The risk of coronary heart disease and stroke is reduced for ε2 carriers, but the risk for ε2 homozygotes is not reduced (56–58). The ε4 allele is associated with elevated LDL-C levels and the risk of coronary heart disease and stroke (56,57). In addition, the ε4 allele is also associated with Alzheimer's disease (59).
Familial chylomicronemia syndrome (FCS)
CM and VLDL are collectively referred to as triglyceride-rich lipoprotein (TRL), which is one of the CVD risk factors for certain special populations (8–12). TRL is cleared by LPL located in the capillaries of adipose tissue and muscle, and the hydrolyzed fatty acids are stored in these adipose tissues and capillaries or used for fuel. FCS, a rare autosomal recessive genetic disorder, is usually caused by homozygous or compound heterozygous mutations in the LPL gene that lead to loss of LPL function or reduced function (60,61), which can lead to the accumulation of CMs in the plasma and HTG. FCS caused by loss-of-function mutations in the ApoC2, ApoA5, GPIHBP1 and LMF1 genes is even rarer (60,61). Compared with patients with non-LPL mutations, those with LPL mutations often have increased TG levels. Due to lifelong persistent chylomicronemia, patients with FCS have an increased likelihood of developing acute pancreatitis (62).
Others
In addition to the aforementioned four common types of primary HLP, there are also Tangier disease (ApoA-I deficiency) (63), familial lecithin-cholesterol acyltransferase (LCAT) deficiency (64) and other types of primary HLP which are beyond the scope of the present review.
Secondary HLP
Secondary HLP is dyslipidemia caused by unhealthy diet, systemic diseases or medications. In this section, the effects of unhealthy diet, some systemic diseases and gut microbiota on blood lipids are briefly described.
Diet
Consuming a diet rich in saturated fatty acids (SFAs) and cholesterol can lead to an increase in cholesterol levels. Factors such as excessive intake of high carbohydrates and excessive alcohol consumption may also cause abnormal blood lipid levels. Animal fat is often rich in SFAs, which is the dietary factor with the greatest influence on LDL-C levels (65). Trans fatty acids (TFAs) are found in full-fat dairy products, butter and meat from ruminants such as cattle, sheep and goats, as well as in industrial production (66,67), TFAs can increase the level of LDL-C, reduce the level of HDL-C and promote the formation of atherosclerosis through sphingolipids, which increases the risk of ASCVD (68). ApoC3 expression induced by a high-sugar diet can reduce or reverse cholesterol transport, decrease LPL activity and affect TG hydrolysis in CM and VLDL (69,70). In addition, a high-fat diet may also affect the lipid metabolism of the body by interacting with intestinal microflora. For example, SFA can cause the increase of Gram-negative bacteria in intestinal microflora, increase serum endotoxin level, increase intestinal permeability and cause lipid metabolism disorders (71). By adjusting the dietary structure, reducing the intake of high-energy foods such as high oil and high sugar and reducing the intake of related lipids from exogenous pathways, it is beneficial to regulate blood lipid levels to a certain extent and reduce cardiovascular diseases caused by HLP.
Systemic disease
Systemic diseases include obesity, diabetes, hypothyroidism, kidney disease, liver disease and autoimmune diseases (such as systemic lupus erythematosus). A variety of diseases interact with each other, involving lipid metabolism, glucose metabolism, protein digestion and absorption, and amino acid metabolism in a variety of tissues and organs, forming a complex pathogenesis of dyslipidemia.
After the occurrence of obesity, lipid accumulation is obvious, the increase of adipose tissue mass leads to the increase of total free fatty acid (FFA) in the circulation system and organs (such as liver) will also receive different degrees of damage. Insulin can considerably inhibit the lipolysis process in adipose tissue by inhibiting the activity of hormone-sensitive lipase, the key enzyme of lipid metabolism in cells and control the production and secretion of FFA in plasma (72). Insulin also activates the degradation of ApoB48 and ApoB100 and inhibits secretion of CM and VLDL by stem cells (73). Insulin resistance leads to increased hepatic lipogenesis and fatty acid metabolism in adipose tissue, leading to dyslipidemia (74). In addition, insulin can also upregulate LPL and insulin resistance will reduce LPL activity and HDL-C levels, thereby affecting TG levels (75). Therefore, HTG and low HDL-C are common in patients with diabetes (76).
Nesfatin-1 is an 82-amino acid bioactive fragment derived from the cleavage of nucleobindin-2 (NUCB2) by PC enzymes and is currently the only known bioactive product of NUCB2 (77). Serum nesfatin-1 was demonstrated to be markedly decreased in patients with non-alcoholic fatty liver disease (78); in mice, nesfatin-1 was demonstrated to attenuate lipid accumulation in hepatocytes through a pathway mediated by AMP-activated protein kinase (79). Nesfatin-1-like peptide with high similarity to nesfatin-1 also reduced lipid accumulation in hepatocytes (80). PCSK9 mediates ApoB100 and TG synthesis as well as VLDL assembly pathways and upregulation of PCSK9 causes excess VLDL production in the liver (81). Visceral adipose tissue releases adipokines such as tumor necrosis factor-α and IL-6, which not only cause chronic inflammation but may also affect insulin signaling in hepatocytes and disrupt insulin sensitivity (82). Downregulation of adiponectin gene expression in adipocytes in metabolically associated dyslipidemia, circulating adiponectin levels are markedly reduced and inversely associated with the degree of visceral lipid accumulation (73), enhanced ApoA1 catabolism and decreased plasma HDL-C levels (83), which promote the accumulation of FFA in atherosclerosis and hepatocytes.
Relative to euthyroid individuals, patients with hypothyroidism have reduced thyroid hormone levels and are more likely to be obese, especially severely obese (84). Thyroid hormones exert biological effects by interacting with thyroid hormone receptor (TR). The TR has two isoforms, TRα and TRβ. The TRα mainly regulates thermogenesis, while TRβ mainly regulates cholesterol metabolism and lipogenesis (85). Synthetic TRβ agonists reduce cholesterol, TGs and obesity in rats and primates, suggesting a potential therapeutic mechanism (86,87). In addition, decreased thyroid hormone levels reduce the amount of LDLR in the liver and the synthesis of bile salts, increasing the absorption of cholesterol from the gastrointestinal tract. Decreased thyroid hormone levels also decrease the activities of cholesterol 7α-hydroxylase (CYP7A1) and ATP-binding cassette transporter G5/8 by reducing ATP-binding cassette transporter A1 and cholesteryl ester transfer protein (CETP) (88), and the production of dysfunctional HDL impairs cholesterol reverse transport, reduces the activity and amount of LPL and hepatic lipase and reduces cholesterol clearance (89).
Serum angiopoietin-like protein (ANGPTL3) levels are significantly positively associated with TG and cholesterol levels in patients with primary nephrotic syndrome. In addition, ANGPTL8 can promote ANGPTL3 cleavage and bind to the N-terminus of ANGPTL3 to form an N-terminal complex, which synergistically inhibits LPL activity and inhibits the clearance process of LDL and VLDL (90). In chronic kidney disease, ApoA1 and HDL levels are decreased, and LCAT deficiency and acyl-CoA cholesterol acyltransferase upregulation mediated by ApoA1 may lead to defective HDL maturation and impaired cholesterol reverse transport (91), thereby increasing TG levels.
In patients with lupus, LPL activity is compromised, leading to CM and VLDL accumulation followed by increased TG levels and decreased HDL levels (92,93). In addition, in patients with lupus, a notable proportion of HDL is dysfunctional and fails to inhibit LDL oxidation, a pro-inflammatory HDL that has been demonstrated to be an independent risk factor for carotid atherosclerosis (94,95).
Gut microbiota
Gut microbiota can regulate host metabolism and body weight through the gut-brain axis. The imbalance of intestinal flora can lead to the proliferation of potential pathogenic bacteria, activate innate immune recognition and initiate inflammatory responses, which in turn causes insulin resistance and fat accumulation, resulting in lipid metabolism disorders (96,97), it can also regulate lipid metabolic balance by changing the integrity of epithelial cells and the intestinal barrier, regulating cholesterol metabolism in the liver and lipid storage in adipose tissue (98).
Short-chain fatty acids (SCFAs), which are mainly composed of acetate, propionate and butyrate, are indirect nutrients produced by various intestinal microorganisms in the glycolysis of carbohydrates. In rodent administration studies, SCFAs have been reported to have effects on preventing weight gain and reducing weight gain (99). Although data in humans are limited, increased colonic propionate has been demonstrated to regulate appetite and prevent weight gain in overweight adults (100–103).
Bile acid is the main component of bile, which is released into the small intestine after eating. Bile acid can be broken down by intestinal microorganisms into secondary bile acids, including deoxycholic acid, lithocholic acid and ursodeoxycholic acid (secondary cholic acid in humans and primary cholic acid in rodents). Bile acids are considered to be key regulators of systemic metabolism, capable of regulating cholesterol metabolism and energy expenditure throughout the body (104). Gut microbes affect the metabolic reactions in which they participate by altering the bioavailability and biological activity of bile acids. In the small intestine, bile acid activation of faridoid X receptor (FXR) promotes fibroblast growth factor (FGF) 19/FGF15 release from intestinal epithelial cells and inhibits CYP7A1 transcriptional process, thereby increasing liver cholesterol levels (105). The activation of FXR is tissue-specific. The activation of FXR in the liver controls the process of adipogenesis, while the activation of FXR in the intestine reduces the absorption of lipids in the intestine and carries out a lipid-lowering role (106).
Tryptophan is an essential amino acid for protein synthesis and a key contributor to microbial influences on body weight and metabolism (107). The aryl hydrocarbon receptor (AhR) is a sensor of environmental and physiological signals and a potent regulator of intestinal barrier integrity, immunity and metabolism. Tryptophan catabolites, such as tryptamine, indole-3-acetic acid and 3-indole-propionic acid, are natural ligands for AhR (108). Reduced microbial production of AhR ligands leads to reduced secretion of glucagon-like peptide and defective mucosal barrier integrity, which may ultimately contribute to the development of metabolic syndrome, BMI, type 2 diabetes and hypertension (108–110).
In past studies, a variety of probiotics including Lactobacillus (L) plantarum, L. sakei, L. forementum, L. hamnosus, L. cidophilus, L. Paracasei, Pediococcus pentosaceus and bifidobacterium were reported to alleviate diet-induced lipid metabolism disorders by improving lipid and glucose metabolism, inhibiting metabolic inflammation and regulating the homeostasis and metabolites of gut microbiota (111–115). In a recent lipid-lowering activity screening study of 2,250 human gut strains, Blautia Producta demonstrated the strongest inhibition of cellular lipid accumulation and improvement of HLP in mice fed a high-fat diet. Its active metabolite, 12-methylmyristic acid can improve glucose metabolism by activating G protein-coupled receptor 120 to an exert anti-hyperglycemic effect (116). In addition, as a low-cost, high-safety functional food, probiotics have developed into an innovative strategy to prevent or improve diet-induced lipid metabolism disorders in the special context of rising medical costs for chronic diseases (111).
Clinical classification of HLP
In addition to the classification of HLP according to its cause, dyslipidemia can be divided into four types for practical use in clinical settings (Table III).
Lipid-lowering agents
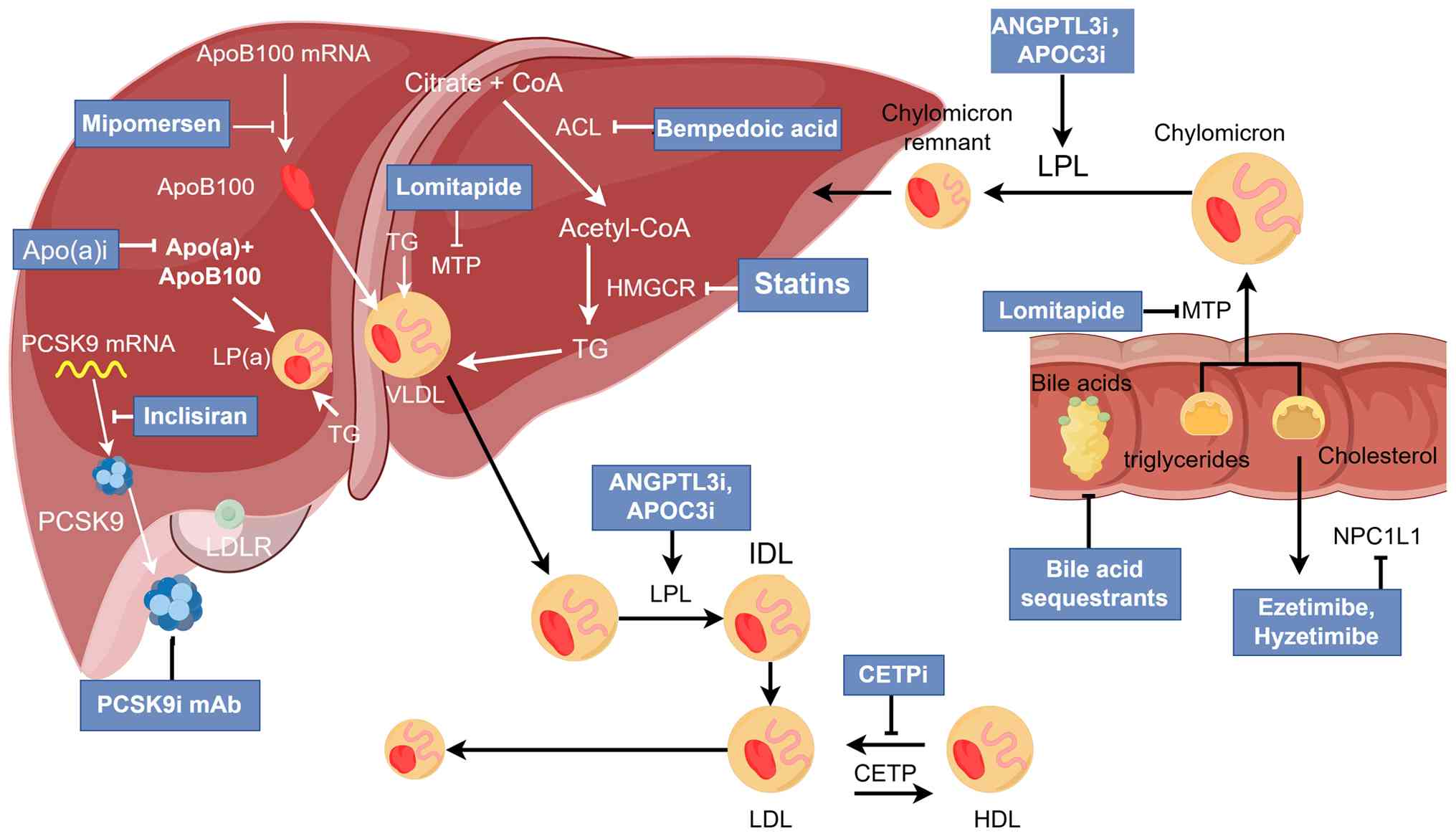
In lipid-lowering treatment, it is recommended to improve lifestyle to a healthy one, including reasonable diet, moderate exercise, weight control, reducing smoking and drinking (117,118). Regarding dietary changes, it is recommended to reduce the intake of high-energy foods such as high oil and sugar, limit the intake of SFAs and trans-fatty acids, and increase the intake of fruits, vegetables, dietary fiber and fish (118). Lipid-lowering drugs should be considered to control lipid levels when lifestyle is not effective or lipid lowering targets are achieved (118). The mechanism of action of lipid-lowering therapy (Fig. 1). Emerging lipid-lowering drugs are shown in Table IV.
Statins
Statins are inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. By competitively binding to the active site of HMG-CoA, statins prevent the conversion of HMG-CoA to mevalonate, reduce hepatic cholesterol synthesis and upregulate cellular LDLR. This increases LDL particle catabolism and decreases plasma LDL-C levels (119). In addition, statins also slightly reduce ApoB secretion (120), slightly reduce TG and increase HDL-C (121). Currently, there are seven types of statins used for treatment, including fluvastatin, lovastatin, simvastatin, pravastatin, atorvastatin, rosuvastatin and pitavastatin.
Multiple studies have reported pronounced ASCVD risk reductions with moderate-intensity statin therapy (122–126). ASCVD risk was further reduced with high-intensity statin therapy (123). However, due to statin intolerance, high-intensity statin therapy may be associated with more adverse events, such as myotoxicity, hepatotoxicity, nephrotoxicity and diabetes (127). Initiating a standard-dose or moderate-intensity statin is therefore recommended. In addition, the majority of patients who are intolerant to one statin can successfully switch to another (128).
Ezetimibe and hyzetimibe
Ezetimibe is a cholesterol absorption inhibitor that inhibits the absorption of cholesterol in the intestine by inhibiting Niemann-Pick C1-like protein 1 in the upper small intestine (129). Standard-measured ezetimibe reduces LDL-C levels by 18 to 25% (130). The combination of ezetimibe and statin resulted in a 70% reduction in LDL-C (130). The addition of ezetimibe to simvastatin can further reduce cardiovascular events in patients with ACS (131). Combination therapy also improves cardiovascular outcomes in patients with chronic kidney disease (132). Hyzetimibe is a cholesterol absorption inhibitor recently marketed in China (www.nmpa.gov.cn; H20210030, H20210031). Its mechanism of action, usage and lipid-lowering efficacy are similar to ezetimibe, which provides an additional treatment option for patients (133–136).
Bile acid chelator (BAS)
BAS are an alkaline anion exchange resin that can block the reabsorption of cholesterol from intestinal bile acids, reduce the inflow of bile acids into the liver and promote the synthesis of bile acids in the liver to deplete cholesterol in the body (137). Commonly used types of BAS in clinical practice are colestyramine, colestipol and colesevelam. BAS combined with statins can markedly improve lipid-lowering efficacy (138). BAS is insoluble in water and not easily absorbed by the human body. The common adverse effects of BAS include gastrointestinal discomfort and constipation, which can be relieved by adjusting the dose and increasing the intake of dietary fiber and water. In addition, it should be noted that BAS can elevate serum TG (139). Therefore, BAS drugs are contraindicated in patients with high TG.
PCSK9 inhibitors
PCSK9 is synthesized by the liver and can bind to LDLR and allow it to be transferred to lysosomes for degradation, thereby reducing the clearance of serum LDL-C by LDLR (140,141). At present, PCSK9 inhibitors mainly include PCSK9 monoclonal antibody and PCSK9 small interfering RNA. Currently, there are two major monoclonal antibodies against PCSK9, evolocumab and alirocumab, which are marketed in multiple countries. Both drugs reduced LDL-C and ASCVD events when combined with statins (142). Evolocumab induces regression and reversal of coronary plaques (143), evolocumab considerably reduced LDL-C and other atherogenic lipid components in patients with type 2 diabetes and HLP or mixed dyslipidemia in the setting of atorvastatin therapy and was well tolerated, with no marked effect on glycemic measures [national clinical trial (NCT) no. 02662569) (144). In long-term evolocumab studies, rates of serious adverse events, muscle related events, new-onset diabetes, hemorrhagic stroke and neurocognitive events were similar to placebo (NCT nos. 02867813 and 03080935) (145). In a randomized clinical trial, 615 high cardiovascular risk patients with HLP, were administered alirocumab or ezetimibe for 6 months, and LDL-C was reduced by 56 and 20.3%, respectively (146). In addition, evolocumab and alirocumab also reduced TG and Lp (a) levels and increased HDL-C levels (147–149). Evolocumab and alirocumab are effective in the vast majority of patients, including patients with HeFH and HoFH who have residual LDLR function and have a poor response in patients with LDLR receptor-deficient HoFH (150).
Recently, a new PCSK9 monoclonal antibody, tafolecimab, has been approved in China for the treatment of adult patients with primary HLP and mixed dyslipidemia who have not achieved LDL-C targets on moderate or higher doses of statins to reduce LDL-C, TC and ApoB levels (151). Its phase III clinical trials, CREDIT-2 (NCT no. 04179669) (152) and CREDIT-4 (NCT no. 04709536) (153), reported that at week 12 of tafolecimab treatment, compared with placebo, there was a ≥50% reduction in LDL-C levels in Chinese patients with HeFH or non-HeFH at high or very high cardiovascular risk, with comparable adverse events in both groups. This indicates that tafolecimab had robust short-term lipid-lowering efficacy and a good safety profile (152,153). In a trial evaluating the long-term efficacy and safety of talfolecimab, adverse events were similar in patients treated with tafolecimab vs. placebo after 48 weeks (NCT no. 04289285) (154). Tafolecimab may provide a novel treatment option with longer dosing intervals for Chinese patients with hypercholesterolemia.
Inclisiran is a small interfering (si)RNA targeting PCSK9 that binds to triantennary N-acetylgalactosamine (GalNac) and is an ultra-long-acting PCSK9 inhibitor approved for use in the European Union and Canada (www.ema.europa.eu, www.canada.ca; drug name, Leqvio). Studies have demonstrated that inclisiran can effectively reduce PCSK9 and LDL-C after a single injection for 6–12 months (155,156). In three randomized clinical trials involving a total of 3,660 patients, inclisiran reduced LDL-C by 51%, TC by 37%, non-HDL-C by 45% and ApoB by 41% compared with placebo. Inclisiran reduced the risk of major ASCVD and, in addition to a minor increase in injection-site reactions, reduced the risk of major ASCVD (157–159). Adverse effects did not differ between groups (159). Results from a phase 3 open-label study (ORION-8) demonstrated that inclisiran was consistently effective in lowering LDL-C and was well tolerated in patients at high cardiovascular risk, with a maximum exposure time of 6.8 years and a mean exposure time of 3.7 years. Treatment-related adverse events occurred at the injection site in 5.9% of patients and no new safety signals were identified (NCT no. 03814187) (160).
Probucol
Probucol is a synthetic antioxidant agent used in the treatment of patients with FH for the prevention and treatment of ASCVD and xanthelasoma, hepatic cholesterol 7 α-hydroxylase activity was increased by probucol, it lowers serum cholesterol mainly by increasing catabolic excretion of cholesterol into bile (161). While Probucol was discontinued in Western countries, as it caused a decrease in HDL-C levels and prolonged QT time (162), it is still used in Japan and China.
Bempedoic acid
Bempedoic acid inhibits hepatic cholesterol synthesis and is approved by the Food and Drug Administration (FDA) for lipid-lowering use. Bempedoic acid, which targets ATP citrate lyase upstream of HMG-CoA, inhibits hepatic cholesterol synthesis through the mevalonate pathway while upregulating LDLR to promote LDL particle catabolism (163). Bempedoic acid and its combination with ezetimibe can considerably reduce LDL-C levels in patients with high-intensity statin therapy and statin intolerance, and has a good safety profile (164,165). In addition, a recent meta-analysis demonstrated that bempedoic acid is a safe and effective alternative to statins and reduces the risk of adverse cardiovascular events in high-risk patients with statin intolerance (166).
CETP inhibitors
CETP is a plasma glycoprotein secreted by the liver, which mediates the bidirectional transport of cholesteryl ester and TG between HDL, VLDL and LDL particles. CETP activity results in a net mass transfer of cholesterol from HDL to VLDL and LDL, and CETP inhibition reduces these exchanges, resulting in increased HDL-C levels and lower concentrations of cholesterol (such as VLDL and LDL) in ApoB particles (167). Previously, several CETP inhibitors, including torcetrapib, evacetrapib and anacetrapib, have been discontinued due to reasons such as side effects and poor therapeutic efficacy (130,168). There are two other CETP inhibitors, dalcetrapib and obicetrapib. Dalcetrapib failed in the dal-OUTCOMES trial due to its lack of HDL-C-raising power, but studies of the genome have demonstrated that dalcetrapib may be effective in subjects with a specific genotype, such as those with the AA genotype (genes at the same locus on homologous chromosomes are completely identical) of rs1967309 in the ADCY9 gene (NCT no. 02525939) (168–170), and further relevant clinical trials are ongoing (NCT no. 05918861). Obicetrapib in ROSE2, combination therapy with ezetimibe (all in addition to high-intensity statin therapy) markedly reduced levels of LDL-C, non-HDL-C, ApoB, total LDL particles, small LDL particles, small dense LDL and Lp (a) and increased HDL-C levels (NCT no. 05266586) (171). Currently, obicetrapib is being studied in fixed-dose combinations of ezetimibe and obicetrapib in addition to maximally tolerated lipid-lowering therapy (NCT no. 06005597). There are a total of three relevant phase 3 clinical trials of obicetrapib in patients with HeFH and cardiovascular disease, including efficacy, safety and tolerability (NCT nos. 05142722, 05425745 and 05202509).
Mipomersen
Mipomersen, an antisense oligonucleotide targeting ApoB100 mRNA (172), has been approved by the FDA. A meta-analysis reported that mipomersen reduced LDL-C by a mean of 26% (173). However, mipomersen caused elevated liver enzymes, injection-site reactions and flu-like symptoms compared with placebo, and ≤20% of subjects discontinued the drug (173,174). Long-term follow-up data from a 2-year open-label extension study demonstrated similar adverse effects (175), the pathological examination of liver biopsy demonstrated that the majority of patients had hepatic steatosis (176), these safety concerns have limited the use of mipomersen (174). Currently, the use of mipomersen is limited to patients with HoFH (177).
Lomitapide
Lomitapide, an inhibitor of microsomal TG transporter, is approved by The European Medicines Agency and the US FDA for the treatment of patients with HoFH (177,178). Lomitapide is able to cause a large reduction in LDL-C, but it causes an increase in liver enzymes and hepatic steatosis (179). A total of two phase 3 extension studies of lomitapide now demonstrate the long-term safety and efficacy of lomitapide in lowering lipid levels in patients with HoFH (180,181).
Fibrates
Fibrates include gemfibrozil, fenofibrate, bezafibrate and ciprofibrate, which activate peroxisome proliferator-activated receptor α (PPARα) and LPL, downregulate ApoC3 expression and upregulate ApoA1. Thus, levels of serum TG were decreased and HDL-C levels were increased (182–184). Current fibrate drugs either target PPARα or activate all three PPAR isoforms and while the efficacy of fibrate drugs to lower TG is well established (182–184), their clinical benefit in reducing cardiovascular events is not convincing (185). Fibrates are used primarily to treat patients with severe HTG to reduce the risk for acute pancreatitis (186).
Pemafibrate, a novel PPARα agonist, is currently available in Japan (187). Pemafibrate is able to be metabolized and excreted into bile in the liver and therefore can be used in patients with renal insufficiency, as demonstrated in clinical trials (188,189). However, whether pemafibrate treatment markedly reduces cardiovascular events in patients with atherogenic dyslipidemia is unknown (185).
Niacin
Niacin can effectively reduce TG and LDL-C levels, increase HDL-C levels and reduce Lp (a) levels, but the mechanism is still unknown (190). The common adverse reactions of niacin are dizziness, skin flushing and pruritus, gastrointestinal discomfort, liver damage, hyperuricemia and deterioration of glucose tolerance. In two trials of niacin, there was no cardiovascular benefit and an increase in adverse effects (191,192).
ω-3 fatty acids
ω−3 fatty acids mainly include eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are mainly found in marine animals and plant oils. ω-3 fatty acids inhibit de novo lipogenesis by inhibiting the cholesterol regulatory element binding protein gene and reducing TG levels by increasing fatty acid oxidation and TG catabolism by non-specific activation of peroxisome proliferator gene family members (193). ω-3 fatty acids are mainly used to treat THG. Icosapent ethyl IPE (EPA only) is clinically beneficial for cardiovascular diseases (194), ω-3 fatty acids with EPA and DHA also reduced cardiovascular events, but to a lesser extent when compared with IPE (195). In addition, randomized trials evaluating the efficacy of statins combined with EPA for secondary prevention have suggested that EPA has a certain coronary vascular protective effect (196).
ANGPTL3 inhibitors
ANGPL3 is synthesized in the liver and regulates lipid metabolism mainly by inhibiting LPL and endothelial lipase (197). Loss-of-function variants in ANGPTL3 cause panhypolipidemia, and heterozygous carriers of ANGPTL3 loss-of-function mutations have a 41% lower risk of coronary artery disease when compared with non-carriers (198). These properties make ANGPTL3 a novel target for the treatment of hypercholesterolemia and severe HTG. Currently, ANGPTL3 inhibitors mainly include ANGPTL3 monoclonal antibody (evinacumab), antisense oligonucleotide (ASO) targeting ANGPTL3 mRNA (vupanorsen) and the small interfering RNA (siRNA) targeting ANGPTL3 mRNA (zodasiran or ARO-ANG3).
Evinacumab is approved for the treatment of HoFH in the European Union, the United Kingdom and the United States (www.ema.europa.eu; products.mhra.gov.uk; www.fda.gov; drug name, Evkeeza). A clinical trial demonstrated that evinacumab can further reduce LDL-C by nearly 50% in patients with HoFH beyond existing lipid-lowering therapies (NCT no. 03399786) (199). A study of evinacumab in 14 pediatric patients with HoFH who were treated with lipid-lowering therapy demonstrated rapid and durable reductions in LDL-C levels with evinacumab and only two reported adverse events (nausea and abdominal pain) considered to be related to treatment. evinacumab provides a new treatment for pediatric patients with HoFH that is poorly controlled despite lipid-lowering therapy (NCT no. 04233918) (200).
Another trial of long-term evinacumab therapy (median, 104.3 weeks) revealed that in a large cohort of patients with HoFH (116 patients), evinacumab was generally well tolerated and notably reduced LDL-C regardless of age and sex, and was associated with a marked reduction in LDL-C. Its efficacy and safety can be maintained over an extended period (NCT no. 03409744) (201). In addition, a recent phase 2 placebo-controlled randomized trial revealed that among three groups of patients with severe HTG (all with a history of acute pancreatitis), TG levels did not decrease in group I patients with FCS; in the second group of patients with multifactorial CM syndrome caused by heterozygous loss-of-function mutation of LPL, TG levels markedly decreased. In group 3, patients with multifactorial CM syndrome without LPL pathway mutations had greater decreases in TG levels (NCT no. 03452228) (202). These results also indicate that evinacumab can effectively reduce TG when LPL has a certain activity, and can be used to treat the majority of patients with severe HTG. However, a phase II trial in patients with severe HTG (history of acute pancreatitis) was stopped in 2023 because of unfavorable recruitment (203).
Vupanorsen is a GalNac-bound ASO that targets ANGPTL3 mRNA, thereby inhibiting ANGPTL3 synthesis. A phase 2b trial revealed that vupanorsen caused a dose-dependent increase in liver fat fraction (NCT no. 04516291) (204,205); this liver-related toxicity was not observed with evinacumab or in patients with ANGPTL3 loss-of-function mutations (206,207). Possibly due to its adverse reactions and the availability of better alternatives, the development of the drug was discontinued.
ARO-AGN3 (zodasiran) is an siRNA that targets ANGPTL3. In a phase 1 trial involving 52 healthy induviduals and 9 individuals with hepatic steatosis, ARO-AGN3 produced substantial and sustained reductions in serum ANGPTL3 concentrations of up to 92.7% from baseline in healthy individuals. Meanwhile, serum TG and atherogenic lipoproteins (LDL-C, non-HDL-C and VLDL-C) also decreased. ARO-AGN3 similarly reduced atherogenic lipoproteins in a small subset of participants with hepatic steatosis, and no increase in liver fat was observed with repeated dosing. In addition, ARO-ANG3 demonstrated durable pharmacological effects that lasted >3 months after the current administration. Injection-site reactions were the most common adverse events (NCT no. 03747224) (208). A double-blind, placebo-controlled, dose-ranging, phase 2b trial involving 204 patients with mixed HLP, almost all of whom had a background in statin therapy, revealed dose-dependent notable reductions of ≥50% in ANGPTL3 levels with Zodasiran at 24 weeks of treatment, as compared with baseline. TG levels were also decreased by ≥50% in a dose-dependent manner and LDL-C, non-HDL-C and ApoB levels were also decreased. Zodasiran also did not cause increases in liver fat content or serum aminotransferase levels, and symptoms of thrombocytopenia were not observed (NCT no. 04832971) (209). This suggests that the toxicity of vupanorsen is not caused by ANGPTL3 inhibition. A phase 3 clinical trial in Chinese patients with HoFH is currently planned to evaluate the efficacy and safety of zodasiran in patients with HoFH (NCT06712771).
ANGPTL3/8 complex inhibitors
ANGPTL8 is a cofactor for the efficacy of ANGPTL3, which can synergistically inhibit LPL activity by promoting the molecular cleavage of ANGPTL3 and forming an ANGPTL3/8 complex by binding to the N terminus of ANGPTL3, thereby increasing TG levels (90). The loss-of-function mutant of ANGPTL8 is associated with reduced TG and LDL-C levels and increased HDL-C levels compared with the ANGPTL3 loss-of-function mutant, but because this mutant is rare, there is insufficient evidence to assess its cardiovascular protective effects (203,210). The inhibition of LPL by the ANGPTL3/8 complex is 100-fold more potent than ANGPTL3 alone, making it a potential target for the treatment of hypercholesterolemia and HTG (211). A phase 1 clinical trial of a monoclonal antibody directed against the ANGPTL3/8 complex, LY3475766, has been completed, but the results have not yet been reported (NCT no. 04052594).
ApoC3 inhibitors
ApoC3 can increase plasma TG levels by reducing LPL activity, inhibiting the clearance of TG from the circulation and promoting the secretion of hepatic VLDL into plasma (212). Functional mutations in ApoC3 are associated with lower TG levels and reduced risk of ischemic CVD (213,214). Human genetic studies have confirmed ApoC3 as a therapeutic target for severe and mild-to-moderate HTG for the prevention of acute pancreatitis and ASCVD (215–220).
Volanesorsen, the first ASO RNA drug to be developed against ApoC3 (2′-O-methoxyethyl-modified ASO), is available in the European Union and the United Kingdom (www.ema.europa.eu; products.mhra.gov.uk; drug name, Waylivra). A 52-week phase III trial of Volanesorsen in 66 patients with FCS (APPROACH; NCT no. 02211209) (221), after treatment, mean TG levels decreased by 77%. Another phase 3 trial of volanesorsen was carried out in 114 patients with multifactorial severe HTG or FCS (COMPASS; NCT no. 02300233) (222), after 3 months of treatment, plasma TG levels were reduced by 71%. In both theses phase 3 trials, adverse events of thrombocytopenia were noted in a number of patients receiving volanesorsen, and platelet counts normalized after treatment discontinuation. This may be due to the chemical properties of the drug rather than a generic effect of all drugs that target ApoC3. A phase 3 long-term open-label treatment of volanesorsen revealed sustained reductions in plasma TG levels in patients with FCS, with common adverse events of injection-site reactions and reductions in platelet counts, which are consistent with previous studies (221–223).
Olezarsen, an ASO conjugated to GalNac, is another form of volanesorsen and is currently applying for marketing in the United States (www.fda.gov; drug name, Tryngolza). In the phase 2 clinical trial of Olezarsen, 114 patients with moderate HTG received olezarsen or corresponding placebo for 6–12 months, which markedly reduced the level of TG, ApoC3, non-HDL-C and ApoB. The most common adverse event was mild erythema at the injection site and no participants presented symptoms of thrombocytopenia (NCT no. 03385239) (224). The recent phase 2b trial of olezarsen showed that two doses (50 or 80 mg; administered subcutaneously every 4 weeks) of olezarsen or matching placebo were used to treat 154 patients with moderate HTG and elevated cardiovascular risk or severe HTG for 6–12 months. Olezarsen at both 50 and 80 mg reduced TG levels by 49 and 53%, respectively, as well as markedly reduced ApoC3, ApoB and non-HDL-C levels. Risks of adverse events and serious adverse events were similar among the three groups (NCT no. 05355402) (225). Currently, a number of clinical trials are ongoing or planned to evaluate the efficacy and safety of olezarsen in different types of patients (NCT nos. 04568434, 05130450, 05681351, 05185843, 05610280, 05552326 and 05079919).
Plozasiran (also known as ARO-APOC3) is a GalNac-conjugated siRNA targeting ApoC3 mRNA in hepatocytes (226). A phase 2b study of Plozasiran (SHASTA-2), in which 229 patients with severe HTG received two subcutaneous injections of plozasiran (10, 25 or 50 mg) or matching placebo at day 1 and week 12 and were followed up to week 48, revealed dose-dependent reductions in TG levels. An average reduction of 57% was observed and there was a dose-dependent increase in LDL-C levels, but not ApoB levels. In these patients, non-HDL-C levels decreased considerably at all doses (NCT no. 04720534) (227). In another 48-week clinical trial of 353 patients with mixed HLP (of which, ~60% patients also had diabetes), participants who received plozasiran had a marked reduction in fasting TG levels at week 24, with a mean reduction of ≥50% compared with placebo; in addition, ApoC3, ApoB and non-HDL-C were also notably decreased, while HDL-C was increased (NCT no. 04998201) (228). Multiple phase 3 trials of plozasiran are currently under way (NCT nos. 05089084, 06347133, 06347003 and 06347016).
Apo(a) inhibitors
Lp (a) is considered to be an independent risk factor for ASCVD and calcific aortic stenosis (16,17,229). Proinflammatory activation of circulating monocytes is a potential mechanism of Lp (a)-mediated CVD (230). Several inhibitors that inhibit Lp (a) formation are currently in clinical trials, such as pelacarsen, olpasiran, zerlasiran and muvalaplin.
Pelacarsen, also known as AKCEA-Apo(a)-LRx or TQJ230, is an ASO that binds GalNac to target APO(a) mRNA in hepatocytes (231). In the pelacarsen phase 2 trial of pelacarsen, which treated 286 patients with established CVD and high levels of Lp (a) or matching placebo, the pelacarsen group revealed a dose-dependent reduction in Lp (a) levels of ≤80%. Adverse events were similar when compared with placebo (NCT no. 03070782) (232). In addition, Pelacarsen decreased the expression of pro-inflammatory genes in monocytes from patients with CVD with elevated Lp (a). Although PCSK9ab treatment reduced Lp (a) by 16% and LDL-C by 65%, it did not alter monocyte transcriptomic or functional properties (233). These data support that substantial Lp (a) reduction produces beneficial effects in patients with elevated Lp (a). Multiple clinical trials of pelacarsen in the population, efficacy, safety, tolerability and pharmacokinetics are ongoing or planned (NCT nos. 04023552, 05305664, 05646381 and 06267560).
Olpasiran (AMG-890) is an siRNA that targets Apo(a) (234). The phase 2 OCEAN (a)-DOSE study of olpasiran revealed that 281 patients with confirmed ASCVD and high levels of Lp (a), the majority of whom had a background in lipid-lowering therapy, were treated with different doses of olpasiran or matching placebo. In the olpasiran group, Lp (a) decreased in a dose-dependent manner. At week 36, patients receiving ≥75 mg every 12 weeks had >95% reduction in Lp (a) levels. The incidence of adverse events was similar in olpasiran and placebo groups, and the most common adverse event was injection-site reaction (NCT no. 04270760) (235). A phase 3 clinical trial of olpasiran is currently underway (NCT no. 05581303).
Zerlasiran (SLN360) is also an siRNA targeting Apo(a). In a recent phase 2 trial of Zerlasiran, 178 patients with ASCVD with high Lp (a) levels who were treated with zerlasiran or matching placebo had a mean reduction in Lp (a) levels of >80% at week 36 in the zerlasiran group, as compared with the placebo group. The most common adverse events were mild injection site reactions (NCT no. 05537571) (236).
Muvalaplin (LY3473329) is the first small molecule drug in the world to enter phase 2 clinical trials, which can effectively lower Lp (a) (237). Lp (a) is generated by Apo(a) by first binding to the lysine residue of ApoB100 on LDL via the Kringle IV 7 and 8 (KIV8) domains, followed by the formation of a disulfide bond between Apo(a) and B100 (238–241). Muvalaplin is a trimeric molecule that binds three KIV8 domains simultaneously. In cynomolgus monkeys, muvalaplin reduced Lp (a) levels by 71% from baseline in a dose-dependent manner (237). A phase 2 trial of muvalaplin is currently under way (NCT no. 05563246).
Conclusions
Advancements in science and technology have allowed for a deeper understanding of the mechanisms underlying dyslipidemia. This allows for the development of new biotherapies specifically targeting molecules of core metabolic importance. Lipid-lowering therapies carry out a central role in the prevention of ASCVD. The main existing lipid-lowering medications include statins, ezetimibe, BAS, monoclonal antibodies that inhibit PSCK9, mipomersen, lomitapide and ω-3 fatty acids. Even when used in combination, they cannot meet current clinical needs due to various reasons such as intolerance, applicable populations and adverse reactions. Combining new therapeutic drugs, such as inclisiran, hyzetimibe, bempedoic acid, CETP inhibitors, ANGPTL3 inhibitors, ANGPTL3/8 complex inhibitors, ApoC3 inhibitors and Apo(a) inhibitors, with existing lipid-lowering medications, may help to meet the unmet clinical needs. Additionally, a reasonable dietary structure and appropriate exercise may help improve lipid levels and promote overall health, and the development of drugs targeting relevant points on the microbiome-gut-brain axis also demonstrates potential for therapeutic application.
Acknowledgements
Not applicable.
Funding
This work was supported by the Traditional Chinese Medicine Scientific Research Project of Heilongjiang Provincial Administration of Traditional Chinese Medicine (grant no. HY2024-242), the ‘National Administration of Traditional Chinese Medicine High-level Construction and Double First-Class’ Discipline Director Fund of Heilongjiang University of Chinese Medicine (grant no. GJJGSPZDXK31022) and the Self-financed Project of the Science and Technology Plan (grant no. 2023ZCZJNS088).
Availability of data and materials
Not applicable.
Authors' contributions
HZ drafted the original manuscript and prepared the figures. YW, YL and RC conducted the literature search and contributed to the writing of the paper. HZ and WC proposed the research design, conducted literature research and contributed to the writing of the paper. Additionally, WC provided professional advice and revisions. All authors critically reviewed the content. Data authentication not applicable. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Pirillo A, Casula M, Olmastroni E, Norata GD and Catapano AL: Global epidemiology of dyslipidaemias. Nat Rev Cardiol. 18:689–700. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Repositioning of the global epicentre of non-optimal cholesterol. Nature. 582:73–77. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Liu T, Zhao D and Qi Y: Global trends in the epidemiology and management of dyslipidemia. J Clin Med. 11:63772022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou XD, Targher G, Byrne CD, Somers V, Kim SU, Chahal CAA, Wong VW, Cai J, Shapiro MD, Eslam M, et al: An international multidisciplinary consensus statement on MAFLD and the risk of CVD. Hepatol Int. 17:773–791. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Yang L, Li Z, Song Y, Liu Y, Zhao H, Liu Y, Zhang T, Yuan Y, Cai X, Wang S, et al: Study on urine metabolic profiling and pathogenesis of hyperlipidemia. Clin Chim Acta. 495:365–373. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Corvilain B: Lipoprotein metabolism. Rev Med Brux. 18:3–9. 1997.(In French). PubMed/NCBI | |
|
Errico TL, Chen X, Martin Campos JM, Julve J, Escolà-Gil JC and Blanco-Vaca F: Basic mechanisms: Structure, function and metabolism of plasma lipoproteins. Clin Investig Arterioscler. 25:98–103. 2013.PubMed/NCBI | |
|
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al: 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur Heart J. 41:111–188. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Borén J, Catapano AL, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, et al: Triglyceride-rich lipoproteins and High-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: Evidence and guidance for management. Eur Heart J. 32:1345–1361. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Chait A, Ginsberg HN, Vaisar T, Heinecke JW, Goldberg IJ and Bornfeldt KE: Remnants of the triglyceride-rich lipoproteins, diabetes, and cardiovascular disease. Diabetes. 69:508–516. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Vallejo-Vaz AJ, Fayyad R, Boekholdt SM, Hovingh GK, Kastelein JJ, Melamed S, Barter P, Waters DD and Ray KK: Triglyceride-rich lipoprotein cholesterol and risk of cardiovascular events among patients receiving statin therapy in the TNT trial. Circulation. 138:770–781. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Raposeiras-Roubin S, Rosselló X, Oliva B, Fernández-Friera L, Mendiguren JM, Andrés V, Bueno H, Sanz J, Martínez de Vega V, Abu-Assi E, et al: Triglycerides and residual atherosclerotic risk. J Am Coll Cardiol. 77:3031–3041. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, Daemen MJ, Demer LL, Hegele RA, Nicholls SJ, et al: Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 41:2313–2330. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Gotto AM Jr and Brinton EA: Assessing low levels of High-density lipoprotein cholesterol as a risk factor in coronary heart disease: A working group report and update. J Am Coll Cardiol. 43:717–724. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Li JJ, Ma CS, Zhao D and Yan XW: Lipoprotein(a) and cardiovascular disease in Chinese population: A beijing heart society expert scientific statement. JACC Asia. 2:653–665. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Mehta A, Vasquez N, Ayers CR, Patel J, Hooda A, Khera A, Blumenthal RS, Shapiro MD, Rodriguez CJ, Tsai MY, et al: Independent association of lipoprotein(a) and coronary artery calcification with atherosclerotic cardiovascular risk. J Am Coll Cardiol. 79:757–768. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Ong KL, McClelland RL, Allison MA, Cushman M, Garg PK, Tsai MY, Rye KA and Tabet F: Lipoprotein (a) and coronary artery calcification: Prospective study assessing interactions with other risk factors. Metabolism. 116:1547062021. View Article : Google Scholar : PubMed/NCBI | |
|
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al: Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J Am Coll Cardiol. 76:2982–3021. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Unwin N and Alberti KG: Chronic non-communicable diseases. Annals of tropical medicine and parasitology. 100:455–464. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Lyu Y, Jiang X and Dai W: The roles of a novel inflammatory neopterin in subjects with coronary atherosclerotic heart disease. Int Immunopharmacol. 24:169–172. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Libby P, Ridker PM and Hansson GK: Progress and challenges in translating the biology of atherosclerosis. Nature. 473:317–325. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
NCD Risk Factor Collaboration (NCD-RisC), . Repositioning of the global epicentre of Non-optimal cholesterol. Nature. 582:73–77. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Song PK, Man QQ, Li H, Pang SJ, Jia SS, Li YQ, He L, Zhao WH and Zhang J: Trends in lipids level and dyslipidemia among chinese adults, 2002–2015. Biomed Environ Sci. 32:559–570. 2019.PubMed/NCBI | |
|
Pan L, Yang Z, Wu Y, Yin RX, Liao Y, Wang J, Gao B and Zhang L; China National Survey of Chronic Kidney Disease Working Group, : The prevalence, awareness, treatment and control of dyslipidemia among adults in China. Atherosclerosis. 248:2–9. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Li JJ: Chinese guideline for lipid management (2023): A new guideline rich in domestic elements for controlling dyslipidemia. J Geriatr Cardiol. 20:618–620. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Xia Q, Chen Y, Yu Z, Huang Z, Yang Y, Mao A and Qiu W: Prevalence, awareness, treatment, and control of dyslipidemia in Chinese adults: A systematic review and meta-analysis. Front Cardiovasc Med. 10:11863302023. View Article : Google Scholar : PubMed/NCBI | |
|
Li S, Liu HH, Guo YL, Zhu CG, Wu NQ, Xu RX, Dong Q and Li JJ: Improvement of evaluation in Chinese patients with atherosclerotic cardiovascular disease using the Very-high-risk refinement: A population-based study. Lancet Reg Health West Pac. 17:1002862021.PubMed/NCBI | |
|
Rygiel K: Hypertriglyceridemia-common causes, prevention and treatment strategies. Curr Cardiol Rev. 14:67–76. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Mc Namara K, Alzubaidi H and Jackson JK: Cardiovascular disease as a leading cause of death: How are pharmacists getting involved? Integr Pharm Res Pract. 8:1–11. 2019.PubMed/NCBI | |
|
Vaezi Z and Amini A: Familial Hypercholesterolemia. StatPearls. StatPearls Publishing Copyright © 2025, StatPearls Publishing LLC; Treasure Island (FL): ineligible companies. Disclosure: Afshin Amini declares no relevant financial relationships with ineligible companies. 2025 | |
|
Benn M, Watts GF, Tybjaerg-Hansen A and Nordestgaard BG: Mutations causative of familial hypercholesterolaemia: Screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. Eur Heart J. 37:1384–1394. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, et al: Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease: Consensus statement of the European atherosclerosis society. Eur Heart J. 34:3478–3490a. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Vrablik M, Tichy L, Freiberger T, Blaha V, Satny M and Hubacek JA: Genetics of familial hypercholesterolemia: New insights. Front Genet. 11:5744742020. View Article : Google Scholar : PubMed/NCBI | |
|
Sun D, Zhou BY, Li S, Sun NL, Hua Q, Wu SL, Cao YS, Guo YL, Wu NQ, Zhu CG, et al: Genetic basis of index patients with familial hypercholesterolemia in Chinese population: Mutation spectrum and Genotype-phenotype correlation. Lipids Health Dis. 17:2522018. View Article : Google Scholar : PubMed/NCBI | |
|
Benito-Vicente A, Uribe KB, Jebari S, Galicia-Garcia U, Ostolaza H and Martin C: Familial Hypercholesterolemia: The most frequent cholesterol metabolism disorder caused disease. Int J Mol Sci. 19:34262018. View Article : Google Scholar : PubMed/NCBI | |
|
Sawhney JPS and Madan K: Familial hypercholesterolemia. Indian Heart J. 76 (Suppl 1):S108–S112. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Hopkins PN, Toth PP, Ballantyne CM and Rader DJ; National Lipid Association Expert Panel on Familial Hypercholesterolemia, : Familial hypercholesterolemias: Prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 5 (3 Suppl):S9–S17. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Singh S and Bittner V: Familial hypercholesterolemia-epidemiology, diagnosis, and screening. Curr Atheroscler Rep. 17:4822015. View Article : Google Scholar : PubMed/NCBI | |
|
Choi D, Malick WA, Koenig W, Rader DJ and Rosenson RS: Familial Hypercholesterolemia: Challenges for a High-Risk Population: JACC Focus Seminar 1/3. J Am Coll Cardiol. 81:1621–1632. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Garg A and Radhakrishnan S: Pediatric hyperlipidemia. Indian Heart J. 76 (Suppl 1):S104–S107. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Hegele RA, Boren J, Ginsberg HN, Arca M, Averna M, Binder CJ, Calabresi L, Chapman MJ, Cuchel M, von Eckardstein A, et al: Rare dyslipidaemias, from phenotype to genotype to management: A European Atherosclerosis Society task force consensus statement. Lancet Diabetes Endocrinol. 8:50–67. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Cao YX, Sun D, Liu HH, Jin JL, Li S, Guo YL, Wu NQ, Zhu CG, Liu G, Dong Q, et al: Improvement of definite diagnosis of familial hypercholesterolemia using an expanding genetic analysis. JACC Asia. 1:82–89. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Gill PK and Hegele RA: Familial combined hyperlipidemia is a polygenic trait. Curr Opin Lipidol. 33:126–132. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Nawawi HM, Chua YA and Watts GF: The brave new world of genetic testing in the management of the dyslipidaemias. Curr Opin Cardiol. 35:226–233. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Taghizadeh E, Farahani N, Mardani R, Taheri F, Taghizadeh H and Gheibihayat SM: Genetics of familial combined hyperlipidemia (FCHL) disorder: An update. Biochem Genet. 60:453–481. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Trinder M, Vikulova D, Pimstone S, Mancini GBJ and Brunham LR: Polygenic architecture and cardiovascular risk of familial combined hyperlipidemia. Atherosclerosis. 340:35–43. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Wierzbicki AS, Kim EJ, Esan O and Ramachandran R: Hypertriglyceridaemia: An update. J Clin Pathol. 75:798–806. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Arner P, Bernard S, Salehpour M, Possnert G, Liebl J, Steier P, Buchholz BA, Eriksson M, Arner E, Hauner H, et al: Dynamics of human adipose lipid turnover in health and metabolic disease. Nature. 478:110–113. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Horswell SD, Fryer LG, Hutchison CE, Zindrou D, Speedy HE, Town MM, Duncan EJ, Sivapackianathan R, Patel HN, Jones EL, et al: CDKN2B expression in adipose tissue of familial combined hyperlipidemia patients. J Lipid Res. 54:3491–3505. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Naukkarinen J, Ehnholm C and Peltonen L: Genetics of familial combined hyperlipidemia. Curr Opin Lipidol. 17:285–290. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Heidemann BE, Koopal C, Baass A, Defesche JC, Zuurbier L, Mulder MT, Roeters van Lennep JE, Riksen NP, Boot C, Marais AD and Visseren FLJ: Establishing the relationship between familial dysbetalipoproteinemia and genetic variants in the APOE gene. Clin Genet. 102:253–261. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Koopal C, Marais AD and Visseren FL: Familial dysbetalipoproteinemia: An underdiagnosed lipid disorder. Curr Opin Endocrinol Diabetes Obes. 24:133–139. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Krauss RM, Lu JT, Higgins JJ, Clary CM and Tabibiazar R: VLDL receptor gene therapy for reducing atherogenic lipoproteins. Mol Metab. 69:1016852023. View Article : Google Scholar : PubMed/NCBI | |
|
Packard CJ and Shepherd J: Lipoprotein heterogeneity and apolipoprotein B metabolism. Arterioscler Thromb Vasc Biol. 17:3542–3556. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Rasmussen KL, Tybjaerg-Hansen A, Nordestgaard BG and Frikke-Schmidt R: Plasma levels of apolipoprotein E, APOE genotype, and All-cause and cause-specific mortality in 105 949 individuals from a white general population cohort. Eur Heart J. 40:2813–2824. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Bennet AM, Di Angelantonio E, Ye Z, Wensley F, Dahlin A, Ahlbom A, Keavney B, Collins R, Wiman B, de Faire U and Danesh J: Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 298:1300–1311. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Khan TA, Shah T, Prieto D, Zhang W, Price J, Fowkes GR, Cooper J, Talmud PJ, Humphries SE, Sundstrom J, et al: Apolipoprotein E genotype, cardiovascular biomarkers and risk of stroke: Systematic review and meta-analysis of 14,015 stroke cases and pooled analysis of primary biomarker data from up to 60,883 individuals. Int J Epidemiol. 42:475–492. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Koopal C, Geerlings MI, Muller M, de Borst GJ, Algra A, van der Graaf Y and Visseren FL; SMART Study Group, : The relation between apolipoprotein E (APOE) genotype and peripheral artery disease in patients at high risk for cardiovascular disease. Atherosclerosis. 246:187–192. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Mahley RW, Weisgraber KH and Huang Y: Apolipoprotein E4: A causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc Natl Acad Sci USA. 103:5644–5651. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Goldberg RB and Chait A: A comprehensive update on the chylomicronemia syndrome. Front Endocrinol (Lausanne). 11:5939312020. View Article : Google Scholar : PubMed/NCBI | |
|
Hegele RA, Berberich AJ, Ban MR, Wang J, Digenio A, Alexander VJ, D'Erasmo L, Arca M, Jones A, Bruckert E, et al: Clinical and biochemical features of different molecular etiologies of familial chylomicronemia. J Clin Lipidol. 12:920–927.e4. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Paquette M, Bernard S, Hegele RA and Baass A: Chylomicronemia: Differences between familial chylomicronemia syndrome and multifactorial chylomicronemia. Atherosclerosis. 283:137–142. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Koseki M, Yamashita S, Ogura M, Ishigaki Y, Ono K, Tsukamoto K, Hori M, Matsuki K, Yokoyama S and Harada-Shiba M: Current diagnosis and management of tangier disease. J Atheroscler Thromb. 28:802–810. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Vitali C, Bajaj A, Nguyen C, Schnall J, Chen J, Stylianou K, Rader DJ and Cuchel M: A systematic review of the natural history and biomarkers of primary lecithin: Cholesterol acyltransferase deficiency. J Lipid Res. 63:1001692022. View Article : Google Scholar : PubMed/NCBI | |
|
Mensink RP, Zock PL, Kester AD and Katan MB: Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A Meta-analysis of 60 controlled trials. Am J Clin Nutr. 77:1146–1155. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies, : 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. 290:140–205. 2019. View Article : Google Scholar | |
|
Trautwein EA and McKay S: The role of specific components of a Plant-based diet in management of dyslipidemia and the impact on cardiovascular risk. Nutrients. 12:26712020. View Article : Google Scholar : PubMed/NCBI | |
|
Gengatharan JM, Handzlik MK, Chih ZY, Ruchhoeft ML, Secrest P, Ashley EL, Green CR, Wallace M, Gordts PLSM and Metallo CM: Altered sphingolipid biosynthetic flux and lipoprotein trafficking contribute to trans-fat-induced atherosclerosis. Cell Metab. 37:274–290.e9. 2025. View Article : Google Scholar : PubMed/NCBI | |
|
Hieronimus B and Stanhope KL: Dietary fructose and dyslipidemia: New mechanisms involving apolipoprotein CIII. Curr Opin Lipidol. 31:20–26. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Yan H, Niimi M, Matsuhisa F, Zhou H, Kitajima S, Chen Y, Wang C, Yang X, Yao J, Yang D, et al: Apolipoprotein CIII deficiency protects against atherosclerosis in knockout rabbits. Arterioscler Thromb Vasc Biol. 40:2095–2107. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Rohr MW, Narasimhulu CA, Rudeski-Rohr TA and Parthasarathy S: Negative effects of a High-Fat diet on intestinal permeability: A review. Adv Nutr. 11:77–91. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Dimitriadis G, Mitrou P, Lambadiari V, Maratou E and Raptis SA: Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract. 93 (Suppl 1):S52–S59. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Su X, Cheng Y, Zhang G and Wang B: Novel insights into the pathological mechanisms of metabolic related dyslipidemia. Mol Biol Rep. 48:5675–5687. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Chen SC and Tseng CH: Dyslipidemia, kidney disease, and cardiovascular disease in diabetic patients. Rev Diabet Stud. 10:88–100. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Fukui T and Hirano T: High-density lipoprotein subspecies between patients with type 1 diabetes and type 2 diabetes without/with intensive insulin therapy. Endocr J. 59:561–569. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Hirano T: Pathophysiology of diabetic dyslipidemia. J Atheroscler Thromb. 25:771–782. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Cao X, Liu XM and Zhou LH: Recent progress in research on the distribution and function of NUCB2/nesfatin-1 in peripheral tissues. Endocr J. 60:1021–1027. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Basar O, Akbal E, Köklü S, Koçak E, Tuna Y, Ekiz F, Gültuna S, Yιlmaz FM and Aydoğan T: A novel appetite peptide, nesfatin-1 in patients with non-alcoholic fatty liver disease. Scand J Clin Lab Invest. 72:479–483. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Yin Y, Li Z, Gao L, Li Y, Zhao J and Zhang W: AMPK-dependent modulation of hepatic lipid metabolism by nesfatin-1. Mol Cell Endocrinol. 417:20–26. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Nasri A, Kowaluk M, Widenmaier SB and Unniappan S: Nesfatin-1 and nesfatin-1-like peptide attenuate hepatocyte lipid accumulation and nucleobindin-1 disruption modulates lipid metabolic pathways. Commun Biol. 7:6232024. View Article : Google Scholar : PubMed/NCBI | |
|
Ajoolabady A, Pratico D, Mazidi M, Davies IG, Lip GYH, Seidah N, Libby P, Kroemer G and Ren J: PCSK9 in metabolism and diseases. Metabolism. 163:1560642024. View Article : Google Scholar : PubMed/NCBI | |
|
Rosso C, Kazankov K, Younes R, Esmaili S, Marietti M, Sacco M, Carli F, Gaggini M, Salomone F, Møller HJ, et al: Crosstalk between adipose tissue insulin resistance and liver macrophages in Non-alcoholic fatty liver disease. J Hepatol. 71:1012–1021. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Verges B, Petit JM, Duvillard L, Dautin G, Florentin E, Galland F and Gambert P: Adiponectin is an important determinant of apoA-I catabolism. Arterioscler Thromb Vasc Biol. 26:1364–1369. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Biondi B: Thyroid and obesity: An intriguing relationship. J Clin Endocrinol Metab. 95:3614–3617. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Obregon MJ: Thyroid hormone and adipocyte differentiation. Thyroid. 18:185–195. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Grover GJ, Mellstrom K and Malm J: Therapeutic potential for thyroid hormone receptor-beta selective agonists for treating obesity, hyperlipidemia and diabetes. Curr Vasc Pharmacol. 5:141–154. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Lu C and Cheng SY: Thyroid hormone receptors regulate adipogenesis and carcinogenesis via crosstalk signaling with peroxisome proliferator-activated receptors. J Mol Endocrinol. 44:143–154. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Su X, Peng H, Chen X, Wu X and Wang B: Hyperlipidemia and hypothyroidism. Clin Chim Acta. 527:61–70. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Pearce EN: Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab. 97:326–333. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Chi X, Britt EC, Shows HW, Hjelmaas AJ, Shetty SK, Cushing EM, Li W, Dou A, Zhang R and Davies BSJ: ANGPTL8 promotes the ability of ANGPTL3 to bind and inhibit lipoprotein lipase. Mol Metab. 6:1137–1149. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Moradi H and Vaziri ND: Molecular mechanisms of disorders of lipid metabolism in chronic kidney disease. Front Biosci (Landmark Ed). 23:146–161. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Borba EF, Bonfá E, Vinagre CG, Ramires JA and Maranhão RC: Chylomicron metabolism is markedly altered in systemic lupus erythematosus. Arthritis Rheum. 43:1033–1040. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
de Carvalho JF, Bonfá E and Borba EF: Systemic lupus erythematosus and ‘lupus dyslipoproteinemia’. Autoimmun Rev. 7:246–250. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
McMahon M, Grossman J, Skaggs B, Fitzgerald J, Sahakian L, Ragavendra N, Charles-Schoeman C, Watson K, Wong WK, Volkmann E, et al: Dysfunctional proinflammatory High-density lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheum. 60:2428–2437. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Tselios K, Koumaras C, Gladman DD and Urowitz MB: Dyslipidemia in systemic lupus erythematosus: Just another comorbidity? Semin Arthritis Rheum. 45:604–610. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Cox AJ, West NP and Cripps AW: Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 3:207–215. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Fei N and Zhao L: An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 7:880–884. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Jia X, Xu W, Zhang L, Li X, Wang R and Wu S: Impact of gut microbiota and Microbiota-related metabolites on hyperlipidemia. Front Cell Infect Microbiol. 11:6347802021. View Article : Google Scholar : PubMed/NCBI | |
|
Blaak EE, Canfora EE, Theis S, Frost G, Groen AK, Mithieux G, Nauta A, Scott K, Stahl B, van Harsselaar J, et al: Short chain fatty acids in human gut and metabolic health. Benef Microbes. 11:411–455. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Chambers ES, Byrne CS, Morrison DJ, Murphy KG, Preston T, Tedford C, Garcia-Perez I, Fountana S, Serrano-Contreras JI, Holmes E, et al: Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: A randomised cross-over trial. Gut. 68:1430–1438. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Byrne CS, Chambers ES, Preston T, Tedford C, Brignardello J, Garcia-Perez I, Holmes E, Wallis GA, Morrison DJ and Frost GS: Effects of inulin propionate ester incorporated into palatable food products on appetite and resting energy expenditure: A randomised crossover study. Nutrients. 11:8612019. View Article : Google Scholar : PubMed/NCBI | |
|
van Deuren T, Blaak EE and Canfora EE: Butyrate to combat obesity and Obesity-associated metabolic disorders: Current status and future implications for therapeutic use. Obes Rev. 23:e134982022. View Article : Google Scholar : PubMed/NCBI | |
|
Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, MacDougall K, Preston T, Tedford C, Finlayson GS, et al: Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 64:1744–1754. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Tu J, Wang Y, Jin L and Huang W: Bile acids, gut microbiota and metabolic surgery. Front Endocrinol (Lausanne). 13:9295302022. View Article : Google Scholar : PubMed/NCBI | |
|
Xue R, Su L, Lai S, Wang Y, Zhao D, Fan J, Chen W, Hylemon PB and Zhou H: Bile acid receptors and the Gut-liver axis in nonalcoholic fatty liver disease. Cells. 10:28062021. View Article : Google Scholar : PubMed/NCBI | |
|
Clifford BL, Sedgeman LR, Williams KJ, Morand P, Cheng A, Jarrett KE, Chan AP, Brearley-Sholto MC, Wahlström A, Ashby JW, et al: FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab. 33:1671–1684.e4. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Gao K, Mu CL, Farzi A and Zhu WY: Tryptophan metabolism: A link between the gut microbiota and brain. Adv Nutr. 11:709–723. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Natividad JM, Agus A, Planchais J, Lamas B, Jarry AC, Martin R, Michel ML, Chong-Nguyen C, Roussel R, Straube M, et al: Impaired aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metab. 28:737–749.e4. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Postal BG, Ghezzal S, Aguanno D, André S, Garbin K, Genser L, Brot-Laroche E, Poitou C, Soula H, Leturque A, et al: AhR activation defends gut barrier integrity against damage occurring in obesity. Mol Metab. 39:1010072020. View Article : Google Scholar : PubMed/NCBI | |
|
Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D'Angelo C, Massi-Benedetti C, Fallarino F, et al: Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 39:372–385. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y, Ai Z, Xing X, Fan Y, Zhang Y, Nan B, Li X, Wang Y and Liu J: The ameliorative effect of probiotics on diet-induced lipid metabolism disorders: A review. Crit Rev Food Sci Nutr. 64:3556–3572. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Liang C, Zhou XH, Gong PM, Niu HY, Lyu LZ, Wu YF, Han X and Zhang LW: Lactiplantibacillus plantarum H-87 prevents high-fat diet-induced obesity by regulating bile acid metabolism in C57BL/6J mice. Food Funct. 12:4315–4324. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Oh N, Lee J, Kim H, Kwon M, Seo J and Roh S: Comparison of Cell-free extracts from three newly identified lactobacillus plantarum strains on the inhibitory effect of adipogenic differentiation and insulin resistance in 3T3-L1 adipocytes. Biomed Res Int. 2021:66765022021. View Article : Google Scholar : PubMed/NCBI | |
|
Rahman MS, Kang I, Lee Y, Habib MA, Choi BJ, Kang JS, Park DS and Kim YS: Bifidobacterium longum subsp. infantis YB0411 Inhibits Adipogenesis in 3T3-L1 Pre-adipocytes and reduces High-Fat-Diet-induced obesity in mice. J Agric Food Chem. 69:6032–6042. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
In Kim H, Kim JK, Kim JY, Jang SE, Han MJ and Kim DH: Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 simultaneously alleviate high-fat diet-induced colitis, endotoxemia, liver steatosis, and obesity in mice. Nutr Res. 67:78–89. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Xu W, Yu J, Yang Y, Li Z, Zhang Y, Zhang F, Wang Q, Xie Y, Zhao B and Wu C: Strain-level screening of human gut microbes identifies Blautia producta as a new anti-hyperlipidemic probiotic. Gut Microbes. 15:22280452023. View Article : Google Scholar : PubMed/NCBI | |
|
Lorkowski S: Long known and mostly unused: Lifestyle measures to support Lipid-lowering therapy. Dtsch Med Wochenschr. 147:796–806. 2022.(In German). PubMed/NCBI | |
|
National Clinical Guideline Centre (UK), . National Institute for Health and Clinical Excellence: Guidance: Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease. National Institute for Health and Care Excellence (UK), 2014. Copyright © National Clinical Guideline Centre; London: 2014 | |
|
Hirota T, Fujita Y and Ieiri I: An updated review of pharmacokinetic drug interactions and phar macogenetics of statins. Expert Opin Drug Metab Toxicol. 16:809–822. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Watts GF, Barrett PH, Ji J, Serone AP, Chan DC, Croft KD, Loehrer F and Johnson AG: Differential regulation of lipoprotein kinetics by atorvastatin and fenofibrate in subjects with the metabolic syndrome. Diabetes. 52:803–811. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang X, Xing L, Jia X, Pang X, Xiang Q, Zhao X, Ma L, Liu Z, Hu K, Wang Z and Cui Y: Comparative Lipid-Lowering/Increasing Efficacy of 7 statins in patients with dyslipidemia, cardiovascular diseases, or diabetes mellitus: Systematic review and network Meta-analyses of 50 randomized controlled trials. Cardiovasc Ther. 2020:39870652020. View Article : Google Scholar : PubMed/NCBI | |
|
Cannon CP, Steinberg BA, Murphy SA, Mega JL and Braunwald E: Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 48:438–445. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Cholesterol Treatment Trialists' (CTT) Collaboration, . Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, et al: Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 376:1670–1681. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Cholesterol Treatment Trialists' (CTT) Collaboration, . Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, et al: Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 385:1397–1405. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, et al: Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 359:2195–2207. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S and Heidenreich PA: Association between intensity of statin therapy and mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2:47–54. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Turner RM and Pirmohamed M: Statin-related myotoxicity: A comprehensive review of pharmacokinetic, pharmacogenomic and muscle components. J Clin Med. 9:222019. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang H, Plutzky J, Skentzos S, Morrison F, Mar P, Shubina M and Turchin A: Discontinuation of statins in routine care settings: A cohort study. Ann Intern Med. 158:526–534. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Feingold KR: Maximizing the benefits of cholesterol-lowering drugs. Curr Opin Lipidol. 30:388–394. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Berberich AJ and Hegele RA: A Modern approach to dyslipidemia. Endocr Rev. 43:611–653. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, et al: Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 372:2387–2397. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Sharp Collaborative Group, : Study of heart and renal protection (SHARP): Randomized trial to assess the effects of lowering Low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. Am Heart J. 160:785–794.e10. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Chen J, Lou H, Jiang B, Shao R, Ruan Z and Wang J: Simultaneous determination of hyzetimibe and its main active metabolite in plasma by LC-MS/MS and its application in PK study. Bioanalysis. 7:1857–1867. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Chen W, Ruan Z, Chen J, Yang D, Shao R, Lou H and Jiang B: Population pharmacokinetics and enterohepatic recirculation of hyzetimibe and its main metabolite in Chinese healthy subjects. Br J Clin Pharmacol. 88:3153–3161. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Liao J, Wang X, Li Z and Ouyang D: Pharmacokinetic study of Oral 14C-radiolabeled hyzetimibe, a new cholesterol absorption inhibitor. Front Pharmacol. 12:6653722021. View Article : Google Scholar : PubMed/NCBI | |
|
Ruan Z, Jiang B, Chen J, Zhang X, Lou H, Xiang M, Shao Q and Wang J: Pharmacokinetics, pharmacodynamics, safety, and tolerability of hyzetimibe (HS-25) in healthy Chinese subjects. J Clin Pharmacol. 54:1144–1152. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Knapp HH, Schrott H, Ma P, Knopp R, Chin B, Gaziano JM, Donovan JM, Burke SK and Davidson MH: Efficacy and safety of combination simvastatin and colesevelam in patients with primary hypercholesterolemia. Am J Med. 110:352–360. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Mazidi M, Rezaie P, Karimi E and Kengne AP: The effects of bile acid sequestrants on lipid profile and blood glucose concentrations: A systematic review and meta-analysis of randomized controlled trials. Int J Cardiol. 227:850–857. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Crouse JR III: Hypertriglyceridemia: A contraindication to the use of bile acid binding resins. Am J Med. 83:243–248. 1987. View Article : Google Scholar : PubMed/NCBI | |
|
Guo S, Xia XD, Gu HM and Zhang DW: Proprotein convertase Subtilisin/Kexin-type 9 and lipid Metabolism. Adv Exp Med Biol. 1276:137–156. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Rakipovski G, Hovingh GK and Nyberg M: Proprotein convertase Subtilisin/Kexin type 9 inhibition as the next statin? Curr Opin Lipidol. 31:340–346. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Lee S and Cannon CP: Combination Lipid-lowering therapies for the prevention of recurrent cardiovascular events. Curr Cardiol Rep. 20:552018. View Article : Google Scholar : PubMed/NCBI | |
|
Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, Koenig W, Somaratne R, Kassahun H, Yang J, et al: Effect of evolocumab on progression of coronary disease in Statin-treated patients: The GLAGOV randomized clinical trial. JAMA. 316:2373–2384. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Y, Yuan Z, Lu J, Eliaschewitz FG, Lorenzatti AJ, Monsalvo ML, Wang N, Hamer AW and Ge J: Randomized study of evolocumab in patients with type 2 diabetes and dyslipidaemia on background statin: Pre-specified analysis of the Chinese population from the BERSON clinical trial. Diabetes Obes Metab. 21:1464–1473. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
O'Donoghue ML, Giugliano RP, Wiviott SD, Atar D, Keech A, Kuder JF, Im K, Murphy SA, Flores-Arredondo JH, López JAG, et al: Long-term evolocumab in patients with established atherosclerotic cardiovascular disease. Circulation. 146:1109–1119. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Han Y, Chen J, Chopra VK, Zhang S, Su G, Ma C, Huang Z, Ma Y, Yao Z, Yuan Z, et al: ODYSSEY EAST: Alirocumab efficacy and safety vs ezetimibe in high cardiovascular risk patients with hypercholesterolemia and on maximally tolerated statin in China, India, and Thailand. J Clin Lipidol. 14:98–108. e1082020. View Article : Google Scholar : PubMed/NCBI | |
|
Cao YX, Liu HH, Li S and Li JJ: A Meta-analysis of the effect of PCSK9-monoclonal antibodies on circulating lipoprotein (a) levels. Am J Cardiovasc Drugs. 19:87–97. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, et al: Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 372:1489–1499. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Stein EA and Turner TA: Are the PCSK9 inhibitors the panacea of atherosclerosis treatment? Expert Rev Cardiovasc Ther. 15:491–494. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Thedrez A, Blom DJ, Ramin-Mangata S, Blanchard V, Croyal M, Chemello K, Nativel B, Pichelin M, Cariou B, Bourane S, et al: Homozygous familial hypercholesterolemia patients with identical mutations variably express the LDLR (Low-Density Lipoprotein Receptor): Implications for the efficacy of evolocumab. Arterioscler Thromb Vasc Biol. 38:592–598. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Keam SJ: Tafolecimab: First Approval. Drugs. 83:1545–1549. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Chai M, He Y, Zhao W, Han X, Zhao G, Ma X, Qiao P, Shi D, Liu Y, Han W, et al: Efficacy and safety of tafolecimab in Chinese patients with heterozygous familial hypercholesterolemia: A randomized, double-blind, placebo-controlled phase 3 trial (CREDIT-2). BMC Med. 21:772023. View Article : Google Scholar : PubMed/NCBI | |
|
Qi L, Liu D, Qu Y, Chen B, Meng H, Zhu L, Li L, Wang S, Liu C, Zheng G, et al: Tafolecimab in Chinese patients with hypercholesterolemia (CREDIT-4): A randomized, Double-Blind, Placebo-controlled phase 3 trial. JACC Asia. 3:636–645. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Huo Y, Chen B, Lian Q, Wang S, Liu L, Lu D, Qu Y, Zheng G, Li L, Ji Y, et al: Tafolecimab in Chinese patients with non-familial hypercholesterolemia (CREDIT-1): A 48-week randomized, double-blind, placebo-controlled phase 3 trial. Lancet Reg Health West Pac. 41:1009072023.PubMed/NCBI | |
|
Brandts J and Ray KK: Small interfering RNA to proprotein convertase subtilisin/kexin type 9: Transforming LDL-cholesterol-lowering strategies. Curr Opin Lipidol. 31:182–186. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Fitzgerald K, White S, Borodovsky A, Bettencourt BR, Strahs A, Clausen V, Wijngaard P, Horton JD, Taubel J, Brooks A, et al: A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 376:41–51. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Raal FJ, Kallend D, Ray KK, Turner T, Koenig W, Wright RS, Wijngaard PLJ, Curcio D, Jaros MJ, Leiter LA, et al: Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 382:1520–1530. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, Bisch JA, Richardson T, Jaros M, Wijngaard PLJ, et al: Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 382:1507–1519. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Khan SA, Naz A, Qamar Masood M and Shah R: Meta-Analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 134:69–73. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Wright RS, Raal FJ, Koenig W, Landmesser U, Leiter LA, Vikarunnessa S, Lesogor A, Maheux P, Talloczy Z, Zang X, et al: Inclisiran administration potently and durably lowers LDL-C over an extended-term follow-up: The ORION-8 trial. Cardiovasc Res. 120:1400–1410. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Tawara K, Tomikawa M and Abiko Y: Mode of action of probucol in reducing serum cholesterol in mice. Jpn J Pharmacol. 40:123–133. 1986. View Article : Google Scholar : PubMed/NCBI | |
|
Yamashita S, Masuda D and Matsuzawa Y: Did we abandon probucol too soon? Curr Opin Lipidol. 26:304–316. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Burke AC, Telford DE and Huff MW: Bempedoic acid: Effects on lipoprotein metabolism and atherosclerosis. Curr Opin Lipidol. 30:1–9. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Ballantyne CM, Laufs U, Ray KK, Leiter LA, Bays HE, Goldberg AC, Stroes ES, MacDougall D, Zhao X and Catapano AL: Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol. 27:593–603. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
De Filippo O, D'Ascenzo F, Iannaccone M, Bertaina M, Leone A, Borzillo I, Ravetti E, Solano A, Pagliassotto I, Nebiolo M, et al: Safety and efficacy of bempedoic acid: A systematic review and Meta-analysis of randomised controlled trials. Cardiovasc Diabetol. 22:3242023. View Article : Google Scholar : PubMed/NCBI | |
|
Hamayal M, Shahid W, Akhtar CH, Shekiba F, Iftikhar I, Tahir MD, Awwab M, Hussain S, Naeem S and Hafeez M: Risk of cardiovascular outcomes with bempedoic acid in High-risk statin intolerant patients: A systematic review and meta analysis. Future Cardiol. 20:639–650. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Xue H, Zhang M, Liu J, Wang J and Ren G: Structure-based mechanism and inhibition of cholesteryl ester transfer protein. Curr Atheroscler Rep. 25:155–166. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Doggrell SA: What have we learnt from the clinical outcomes trials with the cetrapibs? Curr Opin Lipidol. 29:327–332. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Tardif JC, Dube MP, Pfeffer MA, Waters DD, Koenig W, Maggioni AP, McMurray JJV, Mooser V, White HD, Heinonen T, et al: Study design of Dal-GenE, a pharmacogenetic trial targeting reduction of cardiovascular events with dalcetrapib. Am Heart J. 222:157–165. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Tardif JC, Pfeffer MA, Kouz S, Koenig W, Maggioni AP, McMurray JJV, Mooser V, Waters DD, Grégoire JC, L'Allier PL, et al: Pharmacogenetics-guided dalcetrapib therapy after an acute coronary syndrome: The dal-GenE trial. Eur Heart J. 43:3947–3956. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Ballantyne CM, Ditmarsch M, Kastelein JJ, Nelson AJ, Kling D, Hsieh A, Curcio DL, Maki KC, Davidson MH and Nicholls SJ: Obicetrapib plus ezetimibe as an adjunct to high-intensity statin therapy: A randomized phase 2 trial. J Clin Lipidol. 17:491–503. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Raal FJ, Santos RD, Blom DJ, Marais AD, Charng MJ, Cromwell WC, Lachmann RH, Gaudet D, Tan JL, Chasan-Taber S, et al: Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: A randomised, double-blind, placebo-controlled trial. Lancet. 375:998–1006. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Fogacci F, Ferri N, Toth PP, Ruscica M, Corsini A and Cicero AFG: Efficacy and safety of mipomersen: A systematic review and Meta-analysis of randomized clinical trials. Drugs. 79:751–766. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Reeskamp LF, Kastelein JJP, Moriarty PM, Duell PB, Catapano AL, Santos RD and Ballantyne CM: Safety and efficacy of mipomersen in patients with heterozygous familial hypercholesterolemia. Atherosclerosis. 280:109–117. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Santos RD, Duell PB, East C, Guyton JR, Moriarty PM, Chin W and Mittleman RS: Long-term efficacy and safety of mipomersen in patients with familial hypercholesterolaemia: 2-year interim results of an open-label extension. Eur Heart J. 36:566–575. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Hashemi N, Odze RD, McGowan MP, Santos RD, Stroes ESG and Cohen DE: Liver histology during Mipomersen therapy for severe hypercholesterolemia. J Clin Lipidol. 8:606–611. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Nurmohamed NS, Navar AM and Kastelein JJP: New and emerging therapies for reduction of LDL-cholesterol and apolipoprotein B: JACC focus seminar 1/4. J Am Coll Cardiol. 77:1564–1575. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Brunham LR and Hegele RA: Lomitapide for the treatment of homozygous familial hypercholesterolaemia in children. Lancet Diabetes Endocrinol. 12:866–867. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Cuchel M, Meagher EA, du Toit Theron H, Blom DJ, Marais AD, Hegele RA, Averna MR, Sirtori CR, Shah PK, Gaudet D, et al: Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: A single-arm, open-label, phase 3 study. Lancet. 381:40–46. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Blom DJ, Averna MR, Meagher EA, du Toit Theron H, Sirtori CR, Hegele RA, Shah PK, Gaudet D, Stefanutti C, Vigna GB, et al: Long-Term efficacy and safety of the microsomal triglyceride transfer protein inhibitor lomitapide in patients with homozygous familial hypercholesterolemia. Circulation. 136:332–335. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Nohara A, Otsubo Y, Yanagi K, Yoshida M, Ikewaki K, Harada-Shiba M and Jurecka A: Safety and efficacy of lomitapide in japanese patients with homozygous familial hypercholesterolemia (HoFH): Results from the AEGR-733-301 Long-term extension study. J Atheroscler Thromb. 26:368–377. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Jun M, Foote C, Lv J, Neal B, Patel A, Nicholls SJ, Grobbee DE, Cass A, Chalmers J and Perkovic V: Effects of fibrates on cardiovascular outcomes: A systematic review and meta-analysis. Lancet. 375:1875–1884. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, et al: Effects of Long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet. 366:1849–1861. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, et al: Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 341:410–418. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Fruchart JC, Hermans MP, Fruchart-Najib J and Kodama T: Selective peroxisome Proliferator-activated receptor alpha modulators (SPPARMα) in the metabolic syndrome: Is Pemafibrate light at the end of the tunnel? Curr Atheroscler Rep. 23:32021. View Article : Google Scholar : PubMed/NCBI | |
|
Diabetes Canada Clinical Practice Guidelines Expert Committee, . Mancini GBJ, Hegele RA and Leiter LA: Dyslipidemia. Can J Diabetes. 42 (Suppl 1):S178–S185. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Blair HA: Pemafibrate: First Global Approval. Drugs. 77:1805–1810. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Fruchart JC, Hermans MP and Fruchart-Najib J: Selective peroxisome proliferator-Activated receptor alpha modulators (SPPARMalpha): New opportunities to reduce residual cardiovascular risk in chronic kidney disease? Curr Atheroscler Rep. 22:432020. View Article : Google Scholar : PubMed/NCBI | |
|
Yokote K, Yamashita S, Arai H, Araki E, Suganami H and Ishibashi S; On Behalf Of The K-Study Group, : Long-term efficacy and safety of pemafibrate, a novel selective peroxisome Proliferator-Activated Receptor-α Modulator (SPPARMα), in dyslipidemic patients with renal impairment. Int J Mol Sci. 20:7062019. View Article : Google Scholar : PubMed/NCBI | |
|
Tuteja S: Activation of HCAR2 by niacin: Benefits beyond lipid lowering. Pharmacogenomics. 20:1143–1150. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
HPS2-THRIVE Collaborative Group, . Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, et al: Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 371:203–212. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Lavigne PM and Karas RH: The current state of niacin in cardiovascular disease prevention: A systematic review and Meta-regression. J Am Coll Cardiol. 61:440–446. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Liu QK: Triglyceride-lowering and anti-inflammatory mechanisms of omega-3 polyunsaturated fatty acids for atherosclerotic cardiovascular risk reduction. J Clin Lipidol. 15:556–568. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, et al: Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 380:11–22. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Khan SU, Lone AN, Khan MS, Virani SS, Blumenthal RS, Nasir K, Miller M, Michos ED, Ballantyne CM, Boden WE and Bhatt DL: Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. EClinicalMedicine. 38:1009972021. View Article : Google Scholar : PubMed/NCBI | |
|
Nishizaki Y, Miyauchi K, Iwata H, Inoue T, Hirayama A, Kimura K, Ozaki Y, Murohara T, Ueshima K, Kuwabara Y, et al: Study protocol and baseline characteristics of Randomized trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy-Statin and Eicosapentaenoic Acid: RESPECT-EPA, the combination of a randomized control trial and an observational biomarker study. Am Heart J. 257:1–8. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Ng DS: Evolving ANGPTL-based lipid-lowering strategies and beyond. Curr Opin Lipidol. 32:271–272. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Dewey FE, Gusarova V, Dunbar RL, O'Dushlaine C, Schurmann C, Gottesman O, McCarthy S, Van Hout CV, Bruse S, Dansky HM, et al: Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med. 377:211–221. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Raal FJ, Rosenson RS, Reeskamp LF, Hovingh GK, Kastelein JJP, Rubba P, Ali S, Banerjee P, Chan KC, Gipe DA, et al: Evinacumab for Homozygous Familial Hypercholesterolemia. N Engl J Med. 383:711–720. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Wiegman A, Greber-Platzer S, Ali S, Reijman MD, Brinton EA, Charng MJ, Srinivasan S, Baker-Smith C, Baum S, Brothers JA, et al: Evinacumab for pediatric patients with homozygous familial hypercholesterolemia. Circulation. 149:343–353. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Gaudet D, Greber-Platzer S, Reeskamp LF, Iannuzzo G, Rosenson RS, Saheb S, Stefanutti C, Stroes E, Wiegman A, Turner T, et al: Evinacumab in homozygous familial hypercholesterolaemia: Long-term safety and efficacy. Eur Heart J. 45:2422–2434. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Rosenson RS, Gaudet D, Ballantyne CM, Baum SJ, Bergeron J, Kershaw EE, Moriarty PM, Rubba P, Whitcomb DC, Banerjee P, et al: Evinacumab in severe hypertriglyceridemia with or without lipoprotein lipase pathway mutations: A phase 2 randomized trial. Nat Med. 29:729–737. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Gouni-Berthold I, Schwarz J and Berthold HK: Updates in drug treatment of severe hypertriglyceridemia. Curr Atheroscler Rep. 25:701–709. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Bergmark BA, Marston NA, Bramson CR, Curto M, Ramos V, Jevne A, Kuder JF, Park JG, Murphy SA, Verma S, et al: Effect of Vupanorsen on Non-high-density lipoprotein cholesterol levels in statin-treated patients with elevated cholesterol: TRANSLATE-TIMI 70. Circulation. 145:1377–1386. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Zimerman A, Wiviott SD, Park JG, Murphy SA, Ran X, Bramson CR, Curto M, Ramos V, Jevne A, Kuder JF, et al: Hepatic fat changes with antisense oligonucleotide therapy targeting ANGPTL3. J Clin Lipidol. 18:e261–e268. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Graham MJ, Lee RG, Brandt TA, Tai LJ, Fu W, Peralta R, Yu R, Hurh E, Paz E, McEvoy BW, et al: Cardiovascular and Metabolic Effects of ANGPTL3 Antisense Oligonucleotides. N Engl J Med. 377:222–232. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Minicocci I, Montali A, Robciuc MR, Quagliarini F, Censi V, Labbadia G, Gabiati C, Pigna G, Sepe ML, Pannozzo F, et al: Mutations in the ANGPTL3 gene and familial combined hypolipidemia: A clinical and biochemical characterization. J Clin Endocrinol Metab. 97:E1266–E1275. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Watts GF, Schwabe C, Scott R, Gladding PA, Sullivan D, Baker J, Clifton P, Hamilton J, Given B, Melquist S, et al: RNA interference targeting ANGPTL3 for triglyceride and cholesterol lowering: Phase 1 basket trial cohorts. Nat Med. 29:2216–2223. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Rosenson RS, Gaudet D, Hegele RA, Ballantyne CM, Nicholls SJ, Lucas KJ, San Martin J, Zhou R, Muhsin M, Chang T, et al: Zodasiran, an RNAi Therapeutic Targeting ANGPTL3, for Mixed hyperlipidemia. N Engl J Med. 391:913–925. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Peloso GM, Auer PL, Bis JC, Voorman A, Morrison AC, Stitziel NO, Brody JA, Khetarpal SA, Crosby JR, Fornage M, et al: Association of low-frequency and rare coding-sequence variants with blood lipids and coronary heart disease in 56,000 whites and blacks. Am J Hum Genet. 94:223–232. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Chen YQ, Pottanat TG, Siegel RW, Ehsani M, Qian YW, Zhen EY, Regmi A, Roell WC, Guo H, Luo MJ, et al: Angiopoietin-like protein 8 differentially regulates ANGPTL3 and ANGPTL4 during postprandial partitioning of fatty acids. J Lipid Res. 61:1203–1220. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Spagnuolo CM and Hegele RA: Recent advances in treating hypertriglyceridemia in patients at high risk of cardiovascular disease with apolipoprotein C-III inhibitors. Expert Opin Pharmacother. 24:1013–1020. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG and Tybjaerg-Hansen A: Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 371:32–41. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
TGHDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute, . Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, Lu Y, Tang ZZ, Zhang H, et al: Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 371:22–31. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Gill PK, Dron JS and Hegele RA: Genetics of hypertriglyceridemia and atherosclerosis. Curr Opin Cardiol. 36:264–271. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Nurmohamed NS, Dallinga-Thie GM and Stroes ESG: Targeting apoC-III and ANGPTL3 in the treatment of hypertriglyceridemia. Expert Rev Cardiovasc Ther. 18:355–361. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zheng C, Khoo C, Furtado J and Sacks FM: Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation. 121:1722–1734. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Hegele RA: APOC3 interference for familial chylomicronaemia syndrome. touchREV Endocrinol. 18:82–83. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Schmitz J and Gouni-Berthold I: APOC-III antisense oligonucleotides: A new option for the treatment of hypertriglyceridemia. Curr Med Chem. 25:1567–1576. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Giammanco A, Spina R, Cefalù AB and Averna M: APOC-III: A gatekeeper in controlling triglyceride metabolism. Curr Atheroscler Rep. 25:67–76. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Witztum JL, Gaudet D, Freedman SD, Alexander VJ, Digenio A, Williams KR, Yang Q, Hughes SG, Geary RS, Arca M, et al: Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. N Engl J Med. 381:531–542. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Gouni-Berthold I, Alexander VJ, Yang Q, Hurh E, Steinhagen-Thiessen E, Moriarty PM, Hughes SG, Gaudet D, Hegele RA, O'Dea LSL, et al: Efficacy and safety of volanesorsen in patients with multifactorial chylomicronaemia (COMPASS): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 9:264–275. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Witztum JL, Gaudet D, Arca M, Jones A, Soran H, Gouni-Berthold I, Stroes ESG, Alexander VJ, Jones R, Watts L, et al: Volanesorsen and triglyceride levels in familial chylomicronemia syndrome: Long-term efficacy and safety data from patients in an open-label extension trial. J Clin Lipidol. 17:342–355. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Tardif JC, Karwatowska-Prokopczuk E, Amour ES, Ballantyne CM, Shapiro MD, Moriarty PM, Baum SJ, Hurh E, Bartlett VJ, Kingsbury J, et al: Apolipoprotein C-III reduction in subjects with moderate hypertriglyceridaemia and at high cardiovascular risk. Eur Heart J. 43:1401–1412. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Bergmark BA, Marston NA, Prohaska TA, Alexander VJ, Zimerman A, Moura FA, Murphy SA, Goodrich EL, Zhang S, Gaudet D, et al: Olezarsen for Hypertriglyceridemia in patients at high cardiovascular risk. N Engl J Med. 390:1770–1780. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Gaudet D, Clifton P, Sullivan D, Baker J, Schwabe C, Thackwray S, Scott R, Hamilton J, Given B, Melquist S, et al: RNA interference therapy targeting apolipoprotein C-III in hypertriglyceridemia. NEJM Evid. 2:EVIDoa22003252023. View Article : Google Scholar : PubMed/NCBI | |
|
Gaudet D, Pall D, Watts GF, Nicholls SJ, Rosenson RS, Modesto K, San Martin J, Hellawell J and Ballantyne CM: Plozasiran (ARO-APOC3) for severe hypertriglyceridemia: The SHASTA-2 randomized clinical trial. JAMA Cardiol. 9:620–630. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Ballantyne CM, Vasas S, Azizad M, Clifton P, Rosenson RS, Chang T, Melquist S, Zhou R, Mushin M, Leeper NJ, et al: Plozasiran, an RNA interference agent targeting APOC3, for mixed hyperlipidemia. N Engl J Med. 391:899–912. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Li JJ, Ma CS, Zhao D, Yan XW, Beijing Heart S and Expert C: Lipoprotein(a) and cardiovascular disease in Chinese population: A beijing heart society expert scientific statement. JACC Asia. 2:653–665. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
van der Valk FM, Bekkering S, Kroon J, Yeang C, Van den Bossche J, van Buul JD, Ravandi A, Nederveen AJ, Verberne HJ, Scipione C, et al: Oxidized phospholipids on Lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 134:611–624. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Plakogiannis R, Sorbera M, Fischetti B and Chen M: The role of antisense therapies targeting Lipoprotein(a). J Cardiovasc Pharmacol. 78:e5–e11. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, Shapiro MD, Stroes ES, Moriarty PM, Nordestgaard BG, et al: Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 382:244–255. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Stiekema LCA, Prange KHM, Hoogeveen RM, Verweij SL, Kroon J, Schnitzler JG, Dzobo KE, Cupido AJ, Tsimikas S, Stroes ESG, et al: Potent lipoprotein(a) lowering following apolipoprotein(a) antisense treatment reduces the pro-inflammatory activation of circulating monocytes in patients with elevated lipoprotein(a). Eur Heart J. 41:2262–2271. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
O'Donoghue ML, JA GL, Knusel B, Gencer B, Wang H, Wu Y, Kassahun H and Sabatine MS: Study design and rationale for the Olpasiran trials of cardiovascular events And lipoproteiN(a) reduction-DOSE finding study (OCEAN(a)-DOSE). Am Heart J. 251:61–69. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
O'Donoghue ML, Rosenson RS, Gencer B, López JAG, Lepor NE, Baum SJ, Stout E, Gaudet D, Knusel B, Kuder JF, et al: Small interfering RNA to reduce Lipoprotein(a) in cardiovascular disease. N Engl J Med. 387:1855–1864. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Nissen SE, Wang Q, Nicholls SJ, Navar AM, Ray KK, Schwartz GG, Szarek M, Stroes ESG, Troquay R, Dorresteijn JAN, et al: Zerlasiran-A small-interfering RNA targeting Lipoprotein(a): A phase 2 randomized clinical trial. JAMA. 332:1992–2002. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Diaz N, Perez C, Escribano AM, Sanz G, Priego J, Lafuente C, Barberis M, Calle L, Espinosa JF, Priest BT, et al: Discovery of potent small-molecule inhibitors of lipoprotein(a) formation. Nature. 629:945–950. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Becker L, Cook PM and Koschinsky ML: Identification of sequences in apolipoprotein(a) that maintain its closed conformation: A novel role for apo(a) isoform size in determining the efficiency of covalent Lp(a) formation. Biochemistry. 43:9978–9988. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Becker L, Cook PM, Wright TG and Koschinsky ML: Quantitative evaluation of the contribution of weak lysine-binding sites present within apolipoprotein(a) kringle IV types 6–8 to lipoprotein(a) assembly. J Biol Chem. 279:2679–2688. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Brunner C, Kraft HG, Utermann G and Muller HJ: Cys4057 of apolipoprotein(a) is essential for lipoprotein(a) assembly. Proc Natl Acad Sci USA. 90:11643–11647. 1993. View Article : Google Scholar : PubMed/NCBI | |
|
Guevara J Jr, Spurlino J, Jan AY, Yang CY, Tulinsky A, Prasad BV, Gaubatz JW and Morrisett JD: Proposed mechanisms for binding of apo[a] kringle type 9 to apo B-100 in human lipoprotein[a]. Biophys J. 64:686–700. 1993. View Article : Google Scholar : PubMed/NCBI |