Reg3β promotes chondrocyte proliferation and ECM metabolism during acetabular roof remodeling in a rat model of DDH‑induced residual dysplasia
- Authors:
- Published online on: August 14, 2025 https://doi.org/10.3892/mmr.2025.13653
- Article Number: 288
-
Copyright: © Wang et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Early ultrasound screening can lead to favorable management of developmental dysplasia of the hip (DDH), one of the most common deformities in children (1–3). However, several complications, such as residual acetabular dysplasia (RAD) and avascular necrosis of the femoral head, are still challenging for pediatric orthopedists, and the underlying mechanism remains unclear (4–6).
To date, imaging morphological methods, such as the acetabulum index, center-edge angle Reimers index and center-head distance discrepancy, are commonly used for the identification of RAD (7,8). However, these parameters only indicate the existing condition of the affected hip and do not reveal the current state of cartilage at the molecular level. Although several risk factors affect the development of the affected hip and are used to predict the outcome of RAD (9,10), the optimal time for secondary surgery remains a challenge (11). Given this situation, several studies have focused on the application of magnetic resonance imaging (MRI) due to its advantages in cartilage imaging (12–16). Articular coverage is presumed to have greater sensitivity in identifying the condition of cartilage (17–21), yet the molecular function of the affected cartilage, which can be impacted prior to the presence of morphological changes, remains unclear. Accordingly, the identification of biomarkers to reflect the biological function of chondrocytes and to predict the outcome of RAD is urgently needed.
In addition to the MRI results of clinical patients, the pathological findings of animal experimental studies have shown that remodeling changes are associated with the thickness of the acetabular roof (11,22–24). Moreover, the remodeling potential is closely related to the risk of residual hip dysplasia. This evidence contributed to the hypothesis that the pathogenesis of DDH may be caused by delayed endochondral ossification of the acetabular roof (11). However, this hypothesis is limited. Cartilage is composed of corresponding chondrocytes, which form a complex and precise network to balance the internal environment for cartilage developmental remodeling, such as proliferation or apoptosis, along with anabolism and catabolism. Abnormal remodeling can occur if this balance is disrupted at any point (25).
A high-throughput microarray study revealed that regenerating islet-derived protein 3-β (Reg3β) was significantly upregulated in a DDH rat model (26). Recent studies have shown the potential of growth factors, cytokines and chemokines for tissue repair and regeneration of the liver and neurons (27–29). Moreover, this molecule was also shown to promote the repair of the infarcted myocardium in rats and improve the prognosis of patients with myocardial infarction (30), indicating broad potential for clinical application. However, it is unclear whether Reg3β is expressed in articular cartilage and affects the remodeling potential and prognosis of patients with RAD. Thus, in the present study, nearly a year was needed to complete the experiment (from January 2022 to February 2023), including establishing a RAD rat model with fixation removal from a 7-day-fixation neonatal rat DDH experimental model, and the expression of Reg3β in the acetabular roof and serum was determined by western blot (WB) analysis and enzyme-linked immunosorbent assays (ELISAs), respectively, while its function in chondrocytes in vitro and the underlying mechanism involved were also investigated.
Materials and methods
Animals and the DDH model
All animal experiments were performed according to the National Research Council's Guide for the Care and Use of Laboratory Animals. The study protocol was approved by the Ethics Committee of Jiaxing Women and Children's Hospital Affiliated with Wenzhou Medical University (approval no. 2023-016; Jiaxing, China) and the Ethics Committee of Soochow University (approval no. SUDA20211130A16; Suzhou, China). Newborn Sprague-Dawley rats, purchased from Zhaoyan (Suzhou) New Drug Research Center Co., Ltd., were randomly divided into CON (control) and DDH groups. Each group was evenly distributed by sex. DDH models were generated by fixing the hind limbs via hip adduction and extension as well as knee extension with a medical adhesive tape (3M) to simulate the swaddling position of humans. The tape was released for motion recovery 1 h per day. The RAD model was established by fixation removal as a preplanning time. The housing conditions, including room temperature maintained at 26°C along with a relative humidity of 45–65% and a 12-h light/dark cycle, met the requirements of SPF grade. Both the DDH model rats and control normal rats were fed freely by their mothers together in the same cage. Animal health and behavior were monitored every day. The specific criterion used to determine when the animals should be euthanized was the corresponding age of human developmental stage: i) Toddler (2 weeks) and ii) child (4 weeks). These selected time points are valuable for predicting the outcome of acetabular remodeling. A total of 150 newborn rats were used in the present study, including 100 for the evaluation of the dislocation ratio, 12 for gross morphology and Reg3β expression on different fixation days at 2 weeks by WB analysis, and 38 for Reg3β expression by WB analysis and ELISA from 1 week to 4 weeks after removal. However, during the WB analysis and ELISA procedures, 1 rat each from the CON and DDH groups at 1 week and one rat in the DDH group at 4 weeks succumbed unintentionally, with no obvious signs of infection. Moreover, a total of 10, 2-week-old rats were sacrificed for primary cellular culture and subsequent experiments. Finally, all the rats used in the experiments were euthanized with pentobarbital sodium to minimize suffering and distress at a dose of 200 mg/kg, and the hip was dissected in such a manner that the acetabulum and femoral head were completely isolated for evaluation and subsequent experiments.
Primary chondrocyte culture
The primary cell study protocol was approved by the Ethics Committee of Soochow University (approval no. SUDA20211130A16; Suzhou, China). The 2-week-old rats were sacrificed by overdose anesthesia, and the cartilage from the acetabular roof was dissected after disinfection with 75% ethanol under a clean bench, followed by washing with PBS solution three times. The cartilage was cut into 1-mm3 pieces with scissors and digested with 0.25% trypsin-EDTA for 30 min and 0.2% collagenase II (Worthington Biochemical Corporation) for 3 h at 37°C in a 5% CO2 incubator. The digestion was terminated with fresh DMEM (BasalMedia) supplemented with 12% fetal bovine serum (FBS) (Dongling) and 1% penicillin-streptomycin (Beyotime). The tissues were passed through a 100-µm molecular filter, and the resulting cell suspension was centrifuged at 300 g for 5 min at room temperature. The resulting pellet was subsequently resuspended in culture medium and seeded into a 25-cm2 vented cap flask with a final cell density of 5×106 cells/ml at 37°C with 5% CO2 overnight. The medium was replaced with fresh DMEM every 2 days. The cells were then identified via Alcian blue staining and Col2a1 immunostaining. Following fixation with 4% paraformaldehyde for 30 min and permeabilization with 0.2% Triton X-100 for 15 min at room temperature, the samples were either stained with 1% Alcian blue 8GX (cat. no. 801642-25, Macklin) for 2 h at room temperature for Alcian blue staining or processed for Col2a1 immunostaining by blocking with 1% bovine serum albumin (BSA) for 30 min, followed by incubation with a primary antibody against Col2a1 (1:250, cat. no. 28459-1-AP, Proteintech) overnight at 4°C, incubation with a secondary antibody conjugated to Cy3 (1:500, cat. no. abs20024, Absin) for 1 h at room temperature, and nuclear counterstaining with DAPI (cat. no. abs47047616, Absin). Passage 3 chondrocytes were used for subsequent studies.
WB analysis
Total protein from cartilage or chondrocytes was extracted with RIPA lysis buffer (Beyotime)supplemented with phenylmethanesulfonyl fluoride and a phosphatase cocktail. After being shaken for 5 min, the samples were incubated on ice for 30 min, followed by centrifugation at 13,500 g and 4°C for 20 min. The supernatant accompanied by the discarded precipitate was removed for concentration measurement by OneDrop1000plus Spectrophotometer (Service Card). RIPA buffer was used to balance the concentrations of all the samples. Finally, the samples were mixed with SDS 6X loading buffer and boiled at 95°C for 5 min. The samples (10 ug loaded per lane) were separated via 12.5% SDS polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% BSA (cat. no. B2064, Sigma-Aldrich) for 2 h at room temperature, followed by incubation with a primary antibody at 4°C overnight. The membranes were subsequently incubated with a secondary antibody conjugated to horseradish peroxidase (HRP) for 1 h at room temperature. Finally, the target bands were visualized by an Enhanced Chemiluminescence Detection Kit (cat. no. 32209, Thermo Fisher Scientific) and detected via AI600 imaging (GE Healthcare) and analyzed via ImageJ analysis software (version1.46r, National Institutes of Health). The primary and secondary antibodies used are listed in Table I.
ELISA
The serum concentration of Reg3β was determined with a commercially available ELISA kit cat. no. MB-7526A, Meibiao Biotechnology Co., Ltd) according to the manufacturer's instructions. The absorbance at 450 nm was determined with a microplate reader (Infinite M200; Tecan Group, Ltd.).
Cell Counting Kit-8 (CCK-8) assay
Following the manufacturer's instructions, the CCK-8 assay was used to evaluate the cell proliferation rates. The 96-well plates were seeded with cells at a density of 1×104 cells/well for the Reg3β knockdown experiment and at a density of 2×103 cells/well for the addition of recombinant Reg3β protein. For the gene knockdown experiments, CCK-8 was added after virus transfection for 48 h at 37°C; for the activation of recombinant Reg3β, CCK-8 was added after the chondrocytes were incubated for 24 h at 37°C. CCK-8 reagent (10 µl; cat. no. U10014A, UUBIO) was added to the culture for 2 h at 37°C. An auto-microplate reader (Infinite M200; Tecan Group, Ltd.) was used to measure the optical density at 450 nm.
Viral transfection and drug treatment in vitro
A third-generation lentiviral vector containing Reg3β short hairpin RNA was designed using a vector from IGEBio Biotech. 293T cells (IGEBio Biotech, China) were used for lentivirus packaging. When the 293T cells reached 80% confluence, the medium was changed to serum-free Opti-MEM (BasalMedia) containing a mixture of helper and shuttle plasmids. A total of 5 µg of lentiviral plasmid was used for transfection, following the protocol for Lipofectamine 2000 (cat. no. 11668-019, Thermo Fisher Scientific). The ratio of lentivirus, packaging, and envelope plasmids was 4:3:2. After 48 h of co-culture at 37°C in a 5% CO2 incubator, the viral-containing supernatant was harvested and concentrated for transfection into primary chondrocytes and subsequent experimental use. The viral titer was determined using the 50% tissue culture infective dose (TCID50) method. The optimal multiplicity of infection (MOI) was identified by determining the vector concentration (MOI=5). After 24 h of transduction at 37°C in a 5% CO2 incubator, the viral supernatant was replaced with complete culture medium containing puromycin (1:100, cat. no. A1113803, Thermo Fisher Scientific). Subsequent experiments were conducted 48 h post-transduction. The chondrocytes were randomly divided into the negative control (NC) group and the S1, S2 and S3 groups. The sequences of all the shRNA sequences, including those of the negative control, were as follows: S1 forward, 5′-TTGCCCTACGTCTGCAAATTT-3′ and reverse, 5′-AAATTTGCAGACGTAGGGCAA-3′; S2 forward, 5′-CTGGAAACAGCTACCAATATA-3′ and reverse, 5′-TATATTGGTAGCTGTTTCCAG-3′; S3 forward, 5′-TGGAGTAACAATGACATAATG-3′ and reverse, 5′-CATTATGTCATTGTTACTCCA-3′; and NC forward, 5′-CCTAAGGTTAAGTCGCCCT-3′ and reverse, 5′-CGAGGGCGACTTAACCTTAGG-3′. The most effective series was determined via WB analysis. The optimal concentration of recombinant Reg3b protein (cat.no.8288-RG, RD SYSTEMS) was determined via CCK-8 assays.
Colony forming assay
After 24 h, the transfected chondrocytes were digested and replated in 6-well plates at a density of 2×102 cells/well for 1 week. Subsequently, the chondrocytes were fixed with 4% paraformaldehyde for 30 min at room temperature and stained with Alcian blue for 2 h at room temperature. The colony formation efficiency was used to determine the proliferation of the test cells, and clones were counted manually (>50 cells, ranging in size from 0.3 to 1.0 mm).
Statistical analysis
The data are presented as the mean ± SD), and GraphPad Prism 8.0 software (GraphPad; Dotmatics) was used for statistical analysis. At least three technical and biological replicates were used for experiments. To compare 3 or more groups, a one-way analysis of variance followed by Dunnett's post hoc test was employed. For comparisons between two groups, an unpaired t-test was used. P<0.05 was considered to indicate a statistically significant difference.
Results
Gross morphology of residual dysplasia of the hip, and the expression of Reg3β is increased after removal of fixation
After 2 days of fixation, the hip samples from the sacrificed rats were dislocated, with an apparent shadow acetabulum and increased labrum thickness (Fig. 1A). Moreover, the longer the fixation continued, the greater the failure of reduction of the dislocated hip. After 7 days of fixation, the dislocated hip seldom showed a reduction compared with that after 3 days of fixation and 5 days of fixation (Fig. 1B). At 2 weeks, Reg3β was highly expressed in the acetabular roof after 7 days of fixation, whereas expression of Reg3β was barely detected after fixation for 3 or 5 days (Fig. 1B). Thus, 7 days of fixation was selected for further RAD experiments. With the removal of fixation, the expression of Reg3β in the acetabular roof of the RAD group was increased in the 1, 2 and 4-week observation periods, and the serum concentration of Reg3β transiently increased at week 2 and later returned to a normal level at week 4 (Fig. 1C).
Reg3β knockdown increases apoptosis and inhibits proliferation in primary chondrocytes
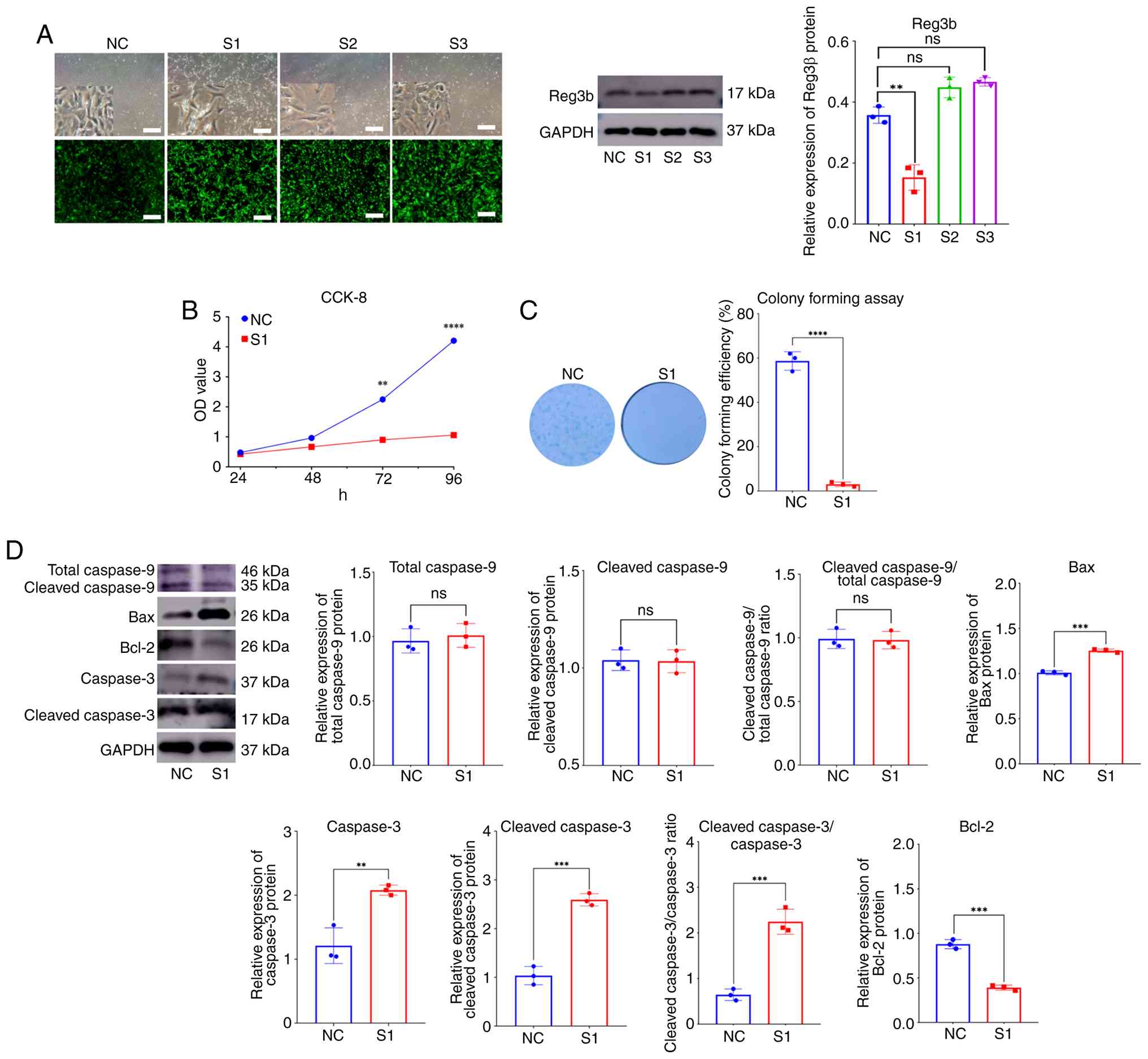
Previous results (Fig. 1) revealed high Reg3β expression in acetabular cartilage after fixation removal. The next objective was to explore the role of Reg3β in chondrocytes. A lentiviral vector containing three Reg3β shRNA sequences was constructed for the transfection of primary chondrocytes (Fig. 2A). The chondrocytes showed obvious cell death with the addition of the S1 sequence, which was identified as the most effective knockdown sequence via WB analysis (Fig. 2A). The S1 sequence was used for subsequent experiments. First, it was investigated whether Reg3β influences the proliferative capability of chondrocytes. CCK-8 cell proliferation was evidently inhibited after 72 h of transfection (Fig. 2B). Moreover, the colony formation assay results revealed that few colonies formed after 1 week of transfection (Fig. 2C). To confirm the effect of Reg3β knockdown on chondrocyte apoptosis, the expression levels of typical apoptotic proteins were measured via WB analyses. The results revealed that the expression of caspase-3 and cleaved caspase-3 was upregulated, whereas the expression of caspase-9 was not evidently altered. By contrast, Bcl-2 expression was significantly downregulated, whereas Bax expression was significantly upregulated (Fig. 2D).
Reg3β knockdown decreases the synthesis and degradation of the extracellular matrix (ECM)
After confirming the effects of Reg3β on the proliferation and apoptosis of chondrocytes in vitro, it was further investigated whether Reg3β affects the synthesis and metabolism of the ECM, which is another important component of articular cartilage (31). Anabolic and catabolic metabolism was assessed via WB analyses. The expression of Aggrecan, ADAM metallopeptidase with thrombospondin type 1 motif 5 (Adamts-5) and matrix metallopeptidase 13 (Mmp13) was significantly decreased, whereas the expression of collagen type II alpha 1 chain (Col2a1) remained unchanged (Fig. 3).
Reg3β knockdown inhibits the Jak2/Stat3/Socs3 axis signaling pathway
Both Jak2 and Stat3 were inhibited after Reg3β knockdown. The corresponding phosphorylated proteins were also significantly downregulated. Moreover, suppressor of cytokine signaling 3 (Socs3) expression was markedly downregulated. In addition, exostosin like glycosyltransferase 3 (Extl3), which is a receptor of Reg3β (32), was downregulated (Fig. 4).
Recombinant Reg3β promotes chondrocyte proliferation and ECM metabolism
Subsequently, the promotion of cellular proliferation was verified. A recombinant Reg3β protein was produced to assess its natural bioactivity. CCK-8 assays were performed to determine the optimal concentration for further experiments. The results showed that recombinant Reg3β promoted chondrocyte cell proliferation, and 10–160 nM recombinant Reg3β had a similar effect (Fig. 5A). However, a relatively higher dose of 320 nM had the opposite effect, inducing apparent cell death. Thus, a concentration of 10 nM was selected for the subsequent experiments. To confirm the optimal time for further experiments, the expression of recombinant Reg3β was evaluated; expression peaked at 48 h after the addition of recombinant Reg3β, which was consistent with the results of the CCK-8 assay (Fig. 5B). Further experiments confirmed that recombinant Reg3β can increase the expression of Aggrecan, Mmp-13 and Adamts-5, whereas Col2a1 exhibited no obvious changes following a transient downregulation at 24 h. Notably, the expression of Adamts-5 and Mmp13 was upregulated at 72 h (Fig. 5C). By contrast, Stat3 was activated by phosphorylation at 24 h, while Socs3 was upregulated after 24 h (Fig. 5D).
Discussion
The present study demonstrated that Reg3β expression in cartilage and serum is upregulated after fixation removal in a DDH rat model. Further experiments revealed that Reg3β may have a positive effect on chondrocyte proliferation and metabolism of the ECM, indicating its ability to remodel the acetabulum in RAD.
RAD is a common complication after closed or open reduction of the affected hip. A previous study confirmed that the affected hip may be remodeled after fixation removal in experimental models, which is similar to the results of closed or open reduction treatment in patients with clinical DDH (33). The results of hip remodeling are closely related to the fixation time. The shorter the fixation time of the model is, the greater the possibility of reduction being achieved (23,34,35). Thus, in the present study, the authors created a series of neonatal rat DDH models with different fixation times to identify which model would be optimal for simulating the pathological process of RAD. Compared with those in previous studies, including animal models and clinical imaging, the hip, especially the acetabular roof, in the neonatal rat model showed similar changes after removal of the fixator (23,24). By contrast, when the fixation time reached 7 days, almost no complete reduction was observed. Therefore, 7 days of fixation were selected as the RAD model for subsequent experiments. Notably, Reg3β expression in acetabular cartilage in the RAD group was significantly upregulated at 1, 2 and 4 weeks after fixation removal. Nevertheless, the serum concentration of Reg3β transiently increased in the RAD group at 2 weeks, after which it returned to the normal level, strongly indicating a close relationship between Reg3β and the early remodeling potential of RAD. However, at 2 weeks, Reg3β expression was greater in the 7-day fixation group than in the 3-day and 5-day fixation groups, which exhibited decreased Reg3β expression than did the normal cartilage group, whereas, conversely the 3-day fixation and 5-day fixation groups exhibited excellent remodeling. This appeared to be paradoxical and beyond the authors' initial hypothesis, in that it should be at least similar to the normal state. It was presumed that the expression pattern of Reg3β could be dynamically altered in response to the mechanical internal environment. With short-term fixation, the hip underwent reduction and remodeling, which alleviated the abnormal mechanical stress. On the other hand, the abnormal mechanical stress in the 7-day fixation group was still persistent due to incomplete reduction and remodeling, which resulted in increased expression of Reg3β. Previous studies have shown that the potential remodeling ability of the acetabulum varies and is observed in individuals ranging from 2–11 years of age (36–38). Moreover, 2-week-old rats may be equivalent to 4-year-old children. Some evidence suggests that secondary surgery is acceptable if the RAD persists at 4–5 years of age (39–42). Thus, Reg3b may be a potential biomarker for predicting the development and remodeling of the affected hip in patients with RAD.
Previous studies have revealed that Reg3b promotes cell proliferation and differentiation and has anti-inflammatory or antiapoptotic effects on cytokines, growth factors and chemokines (32,43–45). To explore the function of Reg3b, an Reg3b-knockdown vector was created for transfecting primary chondrocytes in vitro. As a result, the proliferation of chondrocytes was inhibited, accompanied by an increase in the number of apoptotic cells. Furthermore, caspase-3 and cleaved-caspase-3, key apoptotic proteins, were both significantly upregulated, indicating that the apoptotic signaling pathway, which is based on the caspase family, was activated (46). Moreover, the expression of Bax increased as the corresponding expression of Bcl-2 decreased, which indicated that the induction of chondrocyte apoptosis could occur via the mitochondrial signaling pathway. By contrast, when recombinant Reg3β protein was added to the culture, cell proliferation was increased. Overall, these results confirm that Reg3β plays an important positive role in the proliferation of chondrocytes.
Articular cartilage is composed of chondrocytes and the ECM, which is secreted by chondrocytes. Since Reg3β has a positive effect on chondrocyte proliferation, it was investigated whether Reg3β affects the metabolism of the ECM. The major components of the ECM are collagens and proteoglycans, which are produced by chondrocytes to acquire a phenotype and form a complex network to support the mechanical characteristics of articular cartilage during skeletal development, especially during endochondral ossification (47,48). By contrast, several types of degradative enzymes such as Mmps, Adamts-4/5 are secreted from chondrocytes or the synovium to maintain the internal environment of the articular cartilage. The dynamic balance of chondrocyte proliferation and apoptosis, as well as the synthesis and degradation of the ECM, promotes the development and remodeling of cartilage under beneficial and harmful mechanical stimuli, such as the process of RAD (23,24,49). In the present study, the findings revealed that Reg3β primarily regulated the anabolism and catabolism of Aggrecan rather than Col2a1. However, this finding appears paradoxical due to the simultaneous changes in the expression of Aggrecan and corresponding degradative enzymes after Reg3β intervention. Nevertheless, it is presumed that there may be a more complex regulatory mechanism involved in balancing the ECM.
Previous studies have confirmed that not only Stat3 but also Socs3, which is involved in the negative feedback to Stat3, are implicated in postnatal bone growth (50–52). Thus, the expression of relevant proteins in the signaling pathway was examined. After Reg3b was knocked down, the expression of Jak2/Stat3 was inhibited, and Socs3 was also inhibited. In addition, Extl3, presumed to be a receptor of Reg3β, was significantly inhibited. Conversely, after the addition of recombinant Reg3β protein, the phosphorylation of Stat3 and Socs3 was activated. Thus, these findings indicated that Reg3β affects chondrocyte phenotypes via the Jak2/Stat3/Socs3 signaling pathway. However, this molecule is a negative feedback regulator of the Jak2/Stat3 signaling pathway, and strategies to precisely regulate these phenotypes and maintain a dynamic remodeling balance are still required.
However, the present study has several limitations. First, our findings are derived from animal models, and it remains to be determined whether similar changes in Reg3b expression occur in children with residual acetabular dysplasia. This necessitates further validation in clinical settings. Moreover, the precise mechanisms through which Reg3b modulates chondrocyte proliferation and extracellular matrix synthesis via the Jak2/Stat3 signaling pathway are not fully understood and warrant additional investigation.
In conclusion, the findings of the present study confirmed that the affected hip of the RAD model can be remodeled after fixation removal. The present study revealed the upregulated expression of Reg3β in the acetabulum of the RAD model in the early stage, indicating that Reg3β could be a potent biomarker for predicting hip remodeling. The preliminary remodeling mechanism may be related to Reg3β-mediated promotion of chondrocyte proliferation and the activation of ECM metabolism.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Project of Jiangsu Province Key Research and Development Plan (grant no. BE2022732), the Project of Zhejiang Provincial Public Welfare Technology Application Research Plan (grant no. 2024KY1693), the National Natural Science Foundation of China (grant no. 82272441) and the Science and Technology Commission of Shanghai Municipality (CN) (grant no. 22Y11912200).
Availability of data and materials
The data generated in the present study are included in the figures and/or tables of this article.
Authors' contributions
PW, GS and XW conceptualized and designed the study. PW, GS, ML, WW, WS, WZ and QS designed the methodology. PW, ML and BN analyzed and interpreted data. PW wrote the manuscript. GS and BN reviewed and edited the manuscript. PW, GS and XW confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All animal experiments were performed according to the National Research Council's Guide for the Care and Use of Laboratory Animals. The study protocol was approved by the Ethics Committee of Jiaxing Women and Children's Hospital Affiliated with Wenzhou Medical University (approval no. 2023-016; Jiaxing, China) and the Ethics Committee of Soochow University (approval no. SUDA20211130A16; Suzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Kiani SN, Gornitzky AL, Matheney TH, Schaeffer EK, Mulpuri K, Shah HH, Yihua G, Upasani V, Aroojis A, Krishnamoorthy V, et al: A prospective, multicenter study of developmental dysplasia of the hip: What can patients expect after open reduction? J Pediatr Orthop. 43:279–285. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Håberg Ø, Bremnes T, Foss OA, Angenete O, Lian ØB and Holen KJ: Children treated for developmental dysplasia of the hip at birth and with normal acetabular index at 1 year: How many had residual dysplasia at 5 years? J Child Orthop. 16:183–190. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Bakti K, Lankinen V, Helminen M, Välipakka J, Laivuori H and Hyvärinen A: Clinical and sonographic improvement of developmental dysplasia of the hip: Analysis of 948 patients. J Orthop Surg. 17:5382022. View Article : Google Scholar : PubMed/NCBI | |
|
Dornacher D, Lutz B, Freitag T, Sgroi M, Taurman R and Reichel H: Residual dysplasia of the hip after successful ultrasound-monitored treatment: How does an infant's hip evolve? J Pediatr Orthop B. 31:524–531. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
de Courtivron B, Brulefert K, Portet A and Odent T: Residual acetabular dysplasia in congenital hip dysplasia. Orthop Traumatol Surg Res. 108:1031722022. View Article : Google Scholar : PubMed/NCBI | |
|
Tuhanioğlu Ü, Cicek H, Ogur HU, Seyfettinoglu F and Kapukaya A: Evaluation of late redislocation in patients who underwent open reduction and pelvic osteotomy as treament for developmental dysplasia of the hip. HIP Int. 28:309–314. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
McClincy MP, Wylie JD, Yen YM and Novais EN: Mild or borderline hip dysplasia: Are we characterizing hips with a lateral center-edge angle between 18° and 25° appropriately? Am J Sports Med. 47:112–122. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Barrera CA, Cohen SA, Sankar WN, Ho-Fung VM, Sze RW and Nguyen JC: Imaging of developmental dysplasia of the hip: Ultrasound, radiography and magnetic resonance imaging. Pediatr Radiol. 49:1652–1668. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Shin CH, Yang E, Lim C, Yoo WJ, Choi IH and Cho TJ: Which Acetabular landmarks are the most useful for measuring the acetabular index and Center-edge angle in developmental dysplasia of the hip? A comparison of two methods. Clin Orthop. 478:2120–2131. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Miyake T, Tetsunaga T, Endo H, Yamada K, Sanki T, Fujiwara K, Nakata E and Ozaki T: Predicting acetabular growth in developmental dysplasia of the hip following open reduction after walking age. J Orthop Sci. 24:326–331. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Liu X, Deng X, Ding R, Cheng X and Jia J: Chondrocyte suppression is mediated by miR-129-5p via GDF11/SMAD3 signaling in developmental dysplasia of the hip. J Orthop Res. 38:2559–2572. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Wakabayashi K, Wada I, Horiuchi O, Mizutani J, Tsuchiya D and Otsuka T: MRI Findings in Residual Hip Dysplasia. J Pediatr Orthop. 31:381–387. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Kim HT, Kim IB and Lee JS: MR-based parameters as a supplement to radiographs in managing developmental hip dysplasia. Clin Orthop Surg. 3:2022011. View Article : Google Scholar : PubMed/NCBI | |
|
Douira-Khomsi W, Smida M, Louati H, Hassine LB, Bouchoucha S, Saied W, Ladeb MF, Ghachem MB and Bellagha I: Magnetic resonance evaluation of acetabular residual dysplasia in developmental dysplasia of the hip: A preliminary study of 27 patients. J Pediatr Orthop. 30:37–43. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Schmaranzer F, Justo P, Kallini JR, Ferrer MG, Miller PE, Matheney T, Bixby SD and Novais EN: MRI hip morphology is abnormal in unilateral DDH and increased lateral limbus thickness is associated with residual DDH at minimum 10-year follow-up. J Child Orthop. 17:86–96. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Fu Z, Zhang Z, Deng S, Yang J, Li B, Zhang H and Liu J: MRI assessment of femoral head docking following closed reduction of developmental dysplasia of the hip. Bone Jt J. 105-B:140–147. 2023. View Article : Google Scholar | |
|
Johnson MA, Gohel S, Nguyen JC and Sankar WN: MRI predictors of residual dysplasia in developmental dysplasia of the hip following open and closed reduction. J Pediatr Orthop. 42:179–185. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Gather KS, Mavrev I, Gantz S, Dreher T, Hagmann S and Beckmann NA: Outcome prognostic factors in MRI during spica cast therapy treating developmental hip dysplasia with midterm follow-up. Children. 9:10102022. View Article : Google Scholar : PubMed/NCBI | |
|
Tetsunaga T, Tetsunaga T, Akazawa H, Yamada K, Furumatsu T and Ozaki T: Evaluation of the labrum on postoperative magnetic resonance images: A predictor of acetabular development in developmental dysplasia of the hip. HIP Int. 32:800–806. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Meng X, Yang J and Wang Z: Magnetic resonance imaging follow-up can screen for soft tissue changes and evaluate the short-term prognosis of patients with developmental dysplasia of the hip after closed reduction. BMC Pediatr. 21:1152021. View Article : Google Scholar : PubMed/NCBI | |
|
Kawamura Y, Tetsunaga T, Akazawa H, Yamada K, Sanki T, Sato Y, Nakata E and Ozaki T: Acetabular depth, an early predictive factor of acetabular development: MRI in patients with developmental dysplasia of the hip after open reduction. J Pediatr Orthop B. 30:509–514. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Ding R, Liu X, Zhang J, Yuan J, Zheng S, Cheng X and Jia J: Downregulation of miR-1-3p expression inhibits the hypertrophy and mineralization of chondrocytes in DDH. J Orthop Surg. 16:5122021. View Article : Google Scholar : PubMed/NCBI | |
|
Ning B, Jin R, Wan L and Wang D: Cellular and molecular changes to chondrocytes in an in vitro model of developmental dysplasia of the hip-an experimental model of DDH with swaddling position. Mol Med Rep. 18:3873–3881. 2018.PubMed/NCBI | |
|
Li TY and Ma RX: Increasing thickness and fibrosis of the cartilage in acetabular dysplasia: A rabbit model research. Chin Med J (Engl). 123:3061–3066. 2010.PubMed/NCBI | |
|
Fischer J, Knoch N, Sims T, Rosshirt N and Richter W: Time-dependent contribution of BMP, FGF, IGF, and HH signaling to the proliferation of mesenchymal stroma cells during chondrogenesis. J Cell Physiol. 233:8962–8970. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Ji X, Liu T, Zhao S, Li J, Li L and Wang E: WISP-2, an upregulated gene in hip cartilage from the DDH model rats, induces chondrocyte apoptosis through PPARγ in vitro. FASEB J. 34:4904–4917. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Otsuka N, Yoshioka M, Abe Y, Nakagawa Y, Uchinami H and Yamamoto Y: Reg3α and Reg3β expressions followed by JAK2/STAT3 activation play a pivotal role in the acceleration of liver hypertrophy in a rat ALPPS model. Int J Mol Sci. 21:40772020. View Article : Google Scholar : PubMed/NCBI | |
|
Namikawa K, Okamoto T, Suzuki A, Konishi H and Kiyama H: Pancreatitis-associated protein-III is a novel macrophage chemoattractant implicated in nerve regeneration. J Neurosci. 26:7460–7467. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Cao Y, Tian Y, Liu Y and Su Z: Reg3β: A potential therapeutic target for tissue injury and inflammation-associated disorders. Int Rev Immunol. 41:160–170. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Lindsey ML, Mouton AJ and Ma Y: Adding Reg3β to the acute coronary syndrome prognostic marker list. Int J Cardiol. 258:24–25. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Chijimatsu R and Saito T: Mechanisms of synovial joint and articular cartilage development. Cell Mol Life Sci. 76:3939–3952. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang H, Corredor ALG, Messina-Pacheco J, Li Q, Zogopoulos G, Kaddour N, Wang Y, Shi BY, Gregorieff A, Liu JL and Gao ZH: REG3A/REG3B promotes acinar to ductal metaplasia through binding to EXTL3 and activating the RAS-RAF-MEK-ERK signaling pathway. Commun Biol. 4:6882021. View Article : Google Scholar : PubMed/NCBI | |
|
Raab P, Löhr J and Krauspe R: Remodellierung des Azetabulum nach experimenteller Hüftgelenksdislokation-eine tierexperimentelle Studie an Kaninchen. Z Für Orthop Ihre Grenzgeb. 136:519–524. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Yamamoto N: Changes of the acetabular cartilage following experimental subluxation of the hip joint in rabbits. Nihon Seikeigeka Gakkai Zasshi. 57:1741–1753. 1983.(In Japanese). PubMed/NCBI | |
|
Ning B, Sun J, Yuan Y, Yao J, Wang P and Ma R: Early articular cartilage degeneration in a developmental dislocation of the hip model results from activation of β-catenin. Int J Clin Exp Pathol. 7:1369–1378. 2014.PubMed/NCBI | |
|
Brougham D, Broughton N, Cole W and Menelaus M: The predictability of acetabular development after closed reduction for congenital dislocation of the hip. J Bone Joint Surg Br. 70-B:733–736. 1988. View Article : Google Scholar : PubMed/NCBI | |
|
Bos CF, Bloem JL and Verbout AJ: Magnetic resonance imaging in acetabular residual dysplasia. Clin Orthop. 207–217. 1991. View Article : Google Scholar : PubMed/NCBI | |
|
Harris NH: Acetabular growth potential in congenital dislocation of the hip and some factors upon which it may depend. Clin Orthop. 99–106. 1976.PubMed/NCBI | |
|
Albinana J, Dolan LA, Spratt KF, Morcuende J, Meyer MD and Weinstein SL: Acetabular dysplasia after treatment for developmental dysplasia of the hip: Implications for secondary procedures. J Bone Joint Surg Br. 86:876–886. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Fu M, Liu J, Huang G, Huang Z, Zhang Z, Wu P, Wang B, Yang Z and Liao W: Impaired ossification coupled with accelerated cartilage degeneration in developmental dysplasia of the hip: Evidences from µCT arthrography in a rat model. BMC Musculoskelet Disord. 15:3392014. View Article : Google Scholar : PubMed/NCBI | |
|
Mansour E, Eid R, Romanos E and Ghanem I: The management of residual acetabular dysplasia: Updates and controversies. J Pediatr Orthop B. 26:344–349. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Morris WZ, Hinds S, Worrall H, Jo CH and Kim HKW: Secondary surgery and residual dysplasia following late closed or open reduction of developmental dysplasia of the hip. J Bone Jt Surg. 103:235–242. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Z, Huang Z, Xue H, Lin X, Chen R, Chen M and Jin R: REG3A promotes the proliferation, migration, and invasion of gastric cancer cells. Onco Targets Ther. 10:2017–2023. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Xu X, Fukui H, Ran Y, Wang X, Inoue Y, Ebisudani N, Nishimura H, Tomita T, Oshima T, Watari J, et al: The link between type III Reg and STAT3-associated cytokines in inflamed colonic tissues. Mediators Inflamm. 2019:78594602019. View Article : Google Scholar : PubMed/NCBI | |
|
Wang L, Quan Y, Zhu Y, Xie X, Wang Z, Wang L, Wei X and Che F: The regenerating protein 3A: A crucial molecular with dual roles in cancer. Mol Biol Rep. 49:1491–1500. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Guan M, Yu Q, Zhou G, Wang Y, Yu J, Yang W and Li Z: Mechanisms of chondrocyte cell death in osteoarthritis: Implications for disease progression and treatment. J Orthop Surg. 19:5502024. View Article : Google Scholar : PubMed/NCBI | |
|
Blumer MJF: Bone tissue and histological and molecular events during development of the long bones. Ann Anat. 235:1517042021. View Article : Google Scholar : PubMed/NCBI | |
|
Koosha E, Brenna CTA, Ashique AM, Jain N, Ovens K, Koike T, Kitagawa H and Eames BF: Proteoglycan inhibition of canonical BMP-dependent cartilage maturation delays endochondral ossification. Development. 151:dev2017162024. View Article : Google Scholar : PubMed/NCBI | |
|
Noritake K, Yoshihashi Y, Hattori T and Miura T: Acetabular development after closed reduction of congenital dislocation of the hip. J Bone Joint Surg Br. 75:737–743. 1993. View Article : Google Scholar : PubMed/NCBI | |
|
Sarkar A, Liu NQ, Magallanes J, Tassey J, Lee S, Shkhyan R, Lee Y, Lu J, Ouyang Y, Tang H, et al: STAT3 promotes a youthful epigenetic state in articular chondrocytes. Aging Cell. 22:e137732023. View Article : Google Scholar : PubMed/NCBI | |
|
Liu NQ, Lin Y, Li L, Lu J, Geng D, Zhang J, Jashashvili T, Buser Z, Magallanes J, Tassey J, et al: gp130/STAT3 signaling is required for homeostatic proliferation and anabolism in postnatal growth plate and articular chondrocytes. Commun Biol. 5:642022. View Article : Google Scholar : PubMed/NCBI | |
|
Liu X, D'Cruz AA, Hansen J, Croker BA, Lawlor KE, Sims NA and Wicks IP: Deleting suppressor of cytokine Signaling-3 in chondrocytes reduces bone growth by disrupting mitogen-activated protein kinase signaling. Osteoarthritis Cartilage. 27:1557–1563. 2019. View Article : Google Scholar : PubMed/NCBI |