Alectinib efficacy in advanced lung adenocarcinoma with coexistence of a novel ALK-MTUS2 and STRN3-ALK double fusion: A case report and literature review
- Authors:
- Published online on: July 7, 2025 https://doi.org/10.3892/ol.2025.15178
- Article Number: 432
-
Copyright: © Qiu et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Anaplastic lymphoma kinase (ALK) rearrangement occurs in an estimated 4–5% of patients diagnosed with non-small cell lung cancer (NSCLC), a characteristic associated with a younger age, non-smoking status and advanced disease stage at diagnosis (1–3). These patients also face a heightened risk of brain metastasis, affecting 50–60% of cases (4). Among them, 16.2% present with multiple ALK fusions, a prevalence that is rising due to the adoption of next-generation sequencing (NGS) in detecting these fusions (5). While the echinoderm microtubule-associated protein-like 4 (EML4)-ALK fusion is the most common rearrangement, non-canonical fusions and particularly dual ALK fusion variants are less frequently observed (6). A multicentre national registry by the French National Cancer Institute demonstrated that patients harbouring actionable genetic alterations, predominantly epidermal growth factor receptor (EGFR) mutations and ALK rearrangements, exhibited a 4.7-month extension in median overall survival time when treated with targeted therapeutics, underscoring the clinical utility of molecular profiling (1). Concurrently, real-world evidence highlights notable heterogeneity in first-line treatment duration of response (8.3–13.9 months) among ALK-rearranged cases, a variability strongly associated with the specific ALK fusion partner gene (2). Therefore, investigating the treatment response and its durability in patients with dual ALK fusion variants holds distinct clinical implications.
Alectinib, an effective ALK tyrosine kinase inhibitor (TKI), demonstrates promising results in treating advanced ALK-positive NSCLC and is particularly effective against brain metastases (4). Despite its recognized utility, research on the efficacy of alectinib in patients with NSCLC and concurrent brain metastasis harbouring rare dual fusion mutations remains scarce. To the best of our knowledge, the present study is the first to report a novel case of a patient with rare dual Striatin 3 (STRN3)-ALK and ALK-microtubule-associated tumour suppressor candidate 2 (MTUS2) fusions, who demonstrated a positive response to standard-dose alectinib therapy.
Case report
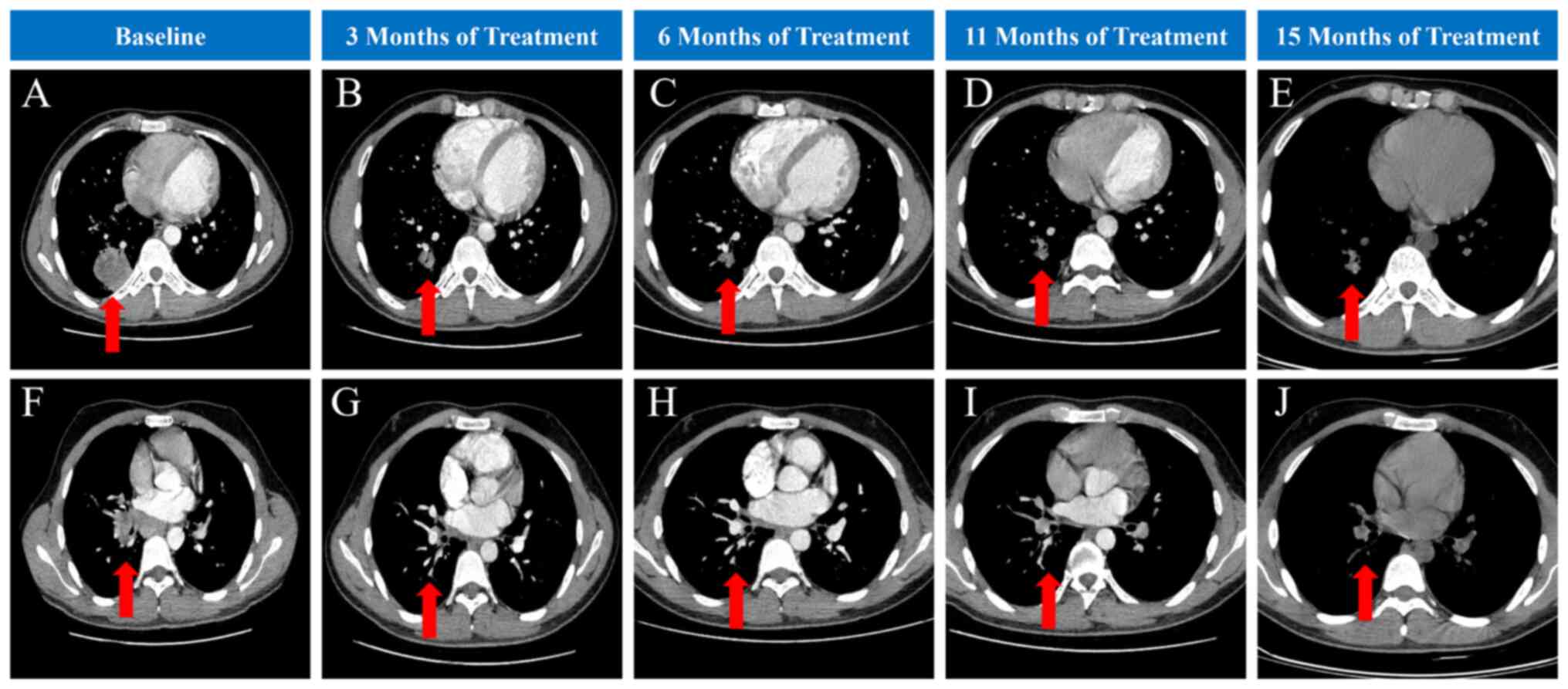
At initial presentation (September 2023), a 20-year-old man who had never smoked and had no significant medical history was initially admitted to the Affiliated Hospital of Nanjing University of Chinese Medicine (Nanjing, China) due to a persistent cough for 8 months. Chest computed tomography (CT) showed a soft tissue mass in the lower lobe of the right lung near the hilum and in the dorsal segment of the lower lobe, with enlarged lymph nodes in the mediastinum (Fig. 1A and F). Pulmonary function indicated moderate obstructive hypoventilation, and the bronchodilator test was negative. Post-bronchodilator spirometry showed: Forced expiratory volume in the first second (FEV1), 2.95 l; FEV1%, 63.9%; forced vital capacity (FVC), 3.85 l; FVC%, 70.1%; and FEV1/FVC, 76.53%. Cranial magnetic resonance imaging (MRI) revealed left cerebellar hemisphere and right frontal lobe enhancement nodules, which were considered to be metastatic tumours (Fig. S1A and E). Positron emission tomography/CT indicated hypermetabolic nodules in the posterior basal segment of the lower lobe of the right lung, suggesting lung cancer with multiple lymph node and bone metastases (Fig. S2). Adenocarcinoma was confirmed by bronchoscopic biopsy of the nodules in the basal segment of the lower lobe of the right lung.
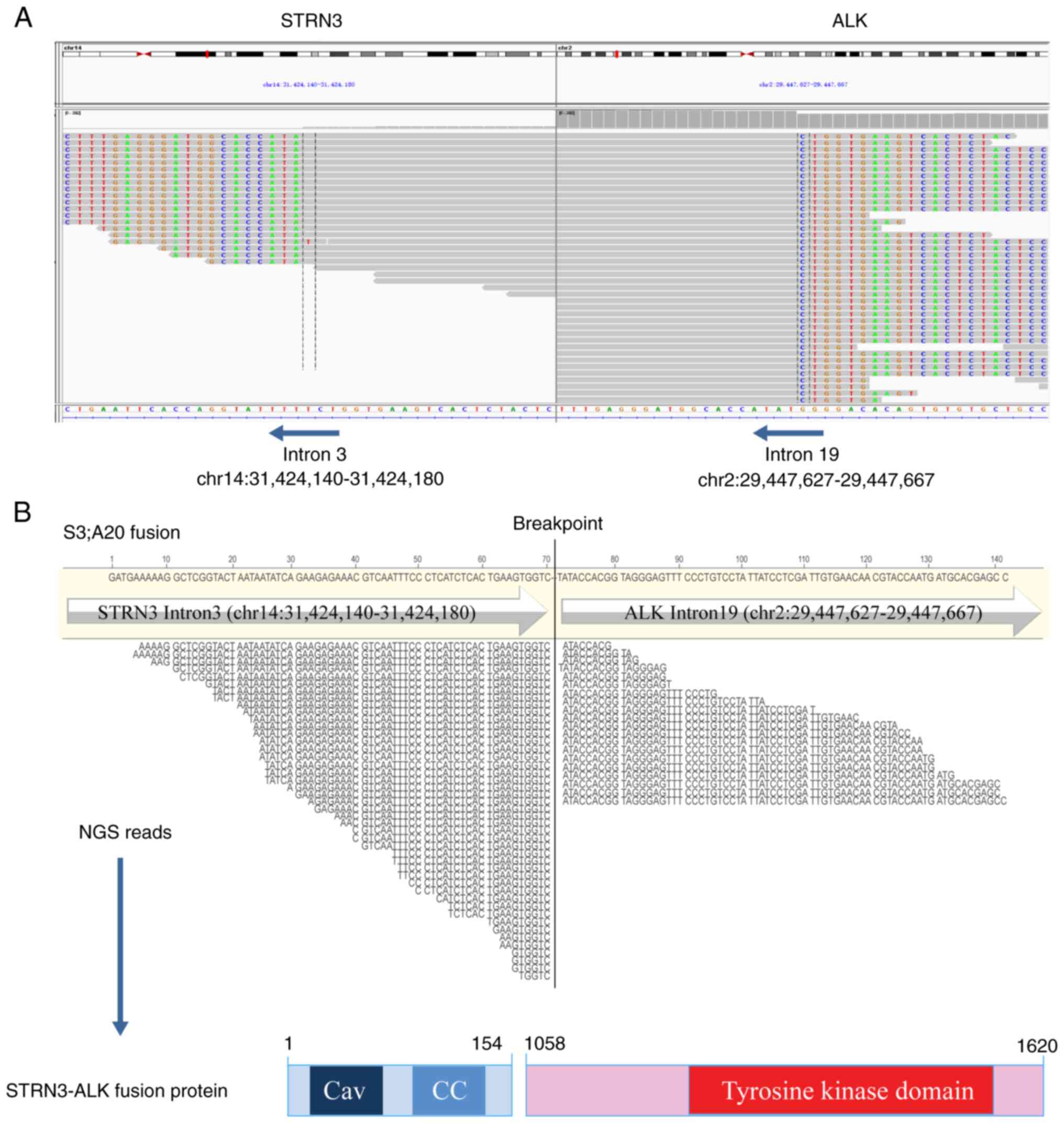
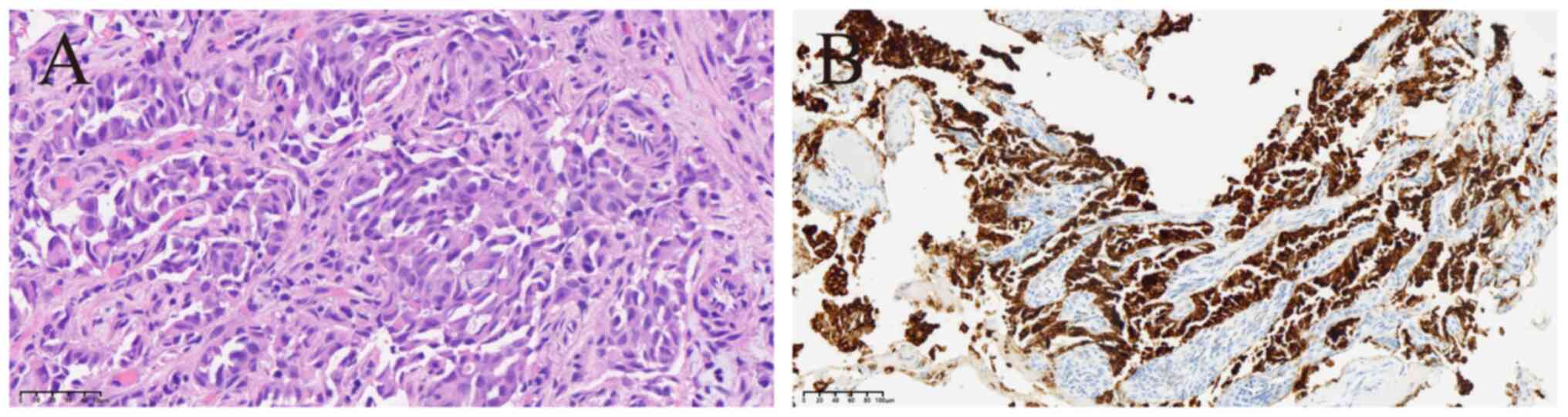
Subsequently, two rare STRN3-ALK and ALK-MTUS2 fusions as well as a heterozygous germline frameshift mutation in the ataxia-telangiectasia mutated (ATM) gene (p.M3011Dfs*6, in exon 63; Fig. S3) were identified through targeted NGS (Geneseeq Prime™; Nanjing Geneseeq Technologies, Inc.) technology (Table I) that covered exons, fusion-related introns and the microsatellite loci of 437 cancer-related genes with a target region of 1.53 Mb (Data S1). The STRN3-ALK fusion site involved exon 3 of STRN3 and exon 20 of ALK (Fig. 2). The sequence analysis of the ALK-MTUS2 cDNA showed that the 5′ untranslated region (5′UTR) of MTUS2 was fused to exon 19 of ALK (Fig. S4). To the best of our knowledge, this rare variant has not been previously reported. Haematoxylin-eosin staining showed histological differentiation typical of lung adenocarcinoma with alveolar and solid structures (Fig. 3A). Immunohistochemical analysis showed diffuse strong positivity for ALK (D5F3) (Fig. 3B) as well as diffuse positive expression of cytokeratin 7, thyroid transcription factor 1 and Napsin A in tumour cells; p53 showed ~80% positive expression, Ki67 exhibited ~15% positive expression, programmed death-ligand 1 (22C3) had a tumour proportion score of ~20% and p40 protein was negatively expressed (Fig. S5).
Table I.Targeted next-generation sequencing detected genetic alterations in both the blood and tumour samples from the patient. |
The final diagnosis was clinical stage IVB (cT2bN3bM1c) lung adenocarcinoma based on the eighth edition of the Tumour-Node-Metastasis classification (7). CT imaging revealed unresectable tumour invasion of major vessels and the trachea (Fig. 1A and F), and the patient presented with multiple distant metastases (bone metastasis and brain metastasis), assessed as clinical stage IV lung cancer, which was not amenable to surgical resection. Therefore, a decision was made to proceed with systemic therapy. The patient was treated with alectinib (600 mg orally twice a day) and zoledronic acid injection (5 mg intravenously once every 4 weeks) to treat the bone metastasis, after discussion with the Department of Respiratory Medicine and multidisciplinary team consultation. Following initiation of alectinib therapy (September 2023), the patient exhibited a rapid clinical response and the cough improved considerably after just 1 week of receiving alectinib. At present, the patient has maintained treatment for 15 months. Follow-up cranial MRI at 3 months post-treatment initiation (December 2023) demonstrated complete resolution of the brain metastases (Fig. S1B and F), with no recurrence observed during subsequent follow-up (Fig. S1C, D, G and H). CT imaging revealed significant lung tumour shrinkage 3 months after initial treatment (December 2023), and serial chest CT scans throughout the 15-month treatment (December 2024) and follow-up period showed sustained partial response without disease progression, and no adverse events were observed (Fig. 1).
Discussion
ALK, a receptor tyrosine kinase from the insulin receptor family, is prevalent in various cancer types, such as NSCLC, breast cancer and colorectal cancer (8). Typically, ALK resides on cell membranes and engages with growth factors. In NSCLC, the EML4-ALK fusion is the most frequent rearrangement (8). Recent advancements in genetic testing have identified additional fusion partners of the ALK gene (3,9). The present case study reports initial evidence of the potential efficacy of alectinib in treating two uncommon ALK fusions, ALK-MTUS2 and STRN3-ALK, in a patient diagnosed with lung adenocarcinoma.
The MTUS2 gene is located on chromosome 13q12.3 and has 21 exons. MTUS2 modulates the dynamics at the distal growing tip, or plus-end, of microtubules (10). In an NGS-based study of breast cancer genomics, MTUS2 emerged as a potential driver of the disease (11). Additionally, MTUS2 may be a potential therapeutic target for Alzheimer's disease (12). To date, and to the best of our knowledge, the ALK-MTUS2 fusion in NSCLC has been unreported. Notably, the patient described in the present study harboured an ALK-MTUS2 fusion, where the 5′UTR of MTUS2 was fused to ALK exon 19. Although the patient showed a clinical response to alectinib therapy, this fusion results in the loss of the tyrosine kinase domain of the ALK gene (ALK exon 20–29), which may may impair its kinase activity and potentially reduces its clinical significance as a driver mutation (6).
ATM gene mutation is a rare and harmful germline mutation (13). A study reported that 4.7% of patients with lung cancer carry pathogenic or suspected pathogenic germline variants, with the most common being in ATM, checkpoint kinase 2 and breast cancer susceptibility gene 2 (13). In a study of patients with NSCLC, 562 pathogenic mutations were identified in the ATM gene, 62.8% of which were missense mutations (14). In the patient reported in the present study, a heterozygous germline frameshift mutation was detected in exon 63 of the ATM gene (p.M3011Dfs*6), causing a deletion of bases 9031 to 9034 and a frameshift starting at amino acid 3011. This mutation led to a premature termination codon and a truncated protein that may disrupt critical functional domains. Previous literature has reported the pathogenicity of the STRN-ALK fusion in thyroid cancer, indicating that targeting STRN-ALK can inhibit tumour cell proliferation (15). Meanwhile, ATM, a gene commonly mutated in lung cancer (16), may synergistically promote tumour cell proliferation by regulating the cell cycle in conjunction with the STRN-ALK fusion. This may explain the mechanism behind the advanced lung cancer observed in the young patient reported in the present study.
The STRN3 gene product, a protein with a coiled-coil domain, mediates the constitutive activation of ALK through a dimerization-driven mechanism (17). The literature on STRN3-ALK fusions was reviewed to explore previous therapeutic interventions, with 12 published articles on patients with NSCLC harbouring this fusion identified. The findings are summarised in Table II, which outlines the characteristics and therapeutic approaches of the disease (6,17–27). Of the 12 patients, 10 were men and 8 were non-smokers, with ages ranging from 29 to 70 years old. Additionally, 11 patients identified were diagnosed with adenocarcinoma, and the histological type was not described for 1 patient (21). Only 2 patients had metastatic lung cancer (18,24), while the remaining patients were diagnosed with primary tumours. The majority of patients underwent NGS, mostly using tissue for testing, and the structural mutations were all characterized as STRN3-ALK (fusion breakpoint S3, A20). Various other mutations coexisted with STRN3-ALK, including ALK-DnaJ homolog subfamily C member 27 fusion, phosphoinositide-dependent kinase 1-ALK fusion, tumour protein p53 (TP53), EGFR and breast cancer susceptibility gene 1. Upon identifying the STRN3-ALK mutation, alectinib was administered in 2 cases (18,20), while crizotinib (17) and gefitinib (26,27) were each administered in 1 and 2 cases, respectively. Alectinib was administered as a first-line treatment to 4 out of 12 patients, with efficacy sustained for 6–19 months (19,22,23,25). In total, 2 patients achieved short-term partial response with alectinib but were switched to crizotinib due to disease progression, showing sustained partial response (18,23). One study suggests hepatocyte growth factor receptor amplification may confer alectinib resistance (18), while another implicates TP53 and phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit α isoform mutations (23). In summary, alectinib demonstrates therapeutic efficacy as first-line treatment for advanced STRN3-ALK fusion-positive adenocarcinoma. However, reported follow-up remains limited, and resistance mechanisms require further investigation.
Alectinib, a second-generation ALK-TKI, shows superior efficacy and reduced toxicity compared with crizotinib in the treatment of ALK-positive NSCLC, as evidenced by the ALEX (28) and ALESIA (29) studies. Additionally, a real-world study in a Chinese population confirmed its significant systemic and central nervous system benefits (30). As aforementioned, alectinib has shown promising clinical efficacy in treating patients with NSCLC that harbour the STRN-ALK fusion gene (19,25). Compared with previously reported cases of lung adenocarcinoma with a STRN3-ALK fusion, the patient described in the present is unique as being the youngest (20 years old), with no smoking history, no identifiable exposure to conventional risk factors and harbouring both a novel ALK-MTUS2 fusion and a rare STRN3-ALK fusion. The patient also presented with brain, bone and multiple lymph node metastases. After the 15 months of treatment (December 2024), the patient has responded well to alectinib treatment, with complete resolution of brain metastases and no recurrence observed. Considering the age and good baseline health of the patient, a full therapeutic dose of 600 mg alectinib twice daily was prescribed. Follow-up assessments at 3, 6 and 15 months revealed satisfactory therapeutic outcomes, with no adverse events observed.
The present study has certain limitations. First, it is a single case report, and the efficacy of alectinib in ALK dual fusions needs to be further validated through large-scale clinical trials. Second, the present study only involves the identification of the STRN3-ALK and ALK-MTUS2 dual fusion genes, without exploring the potential functional changes brought by these gene fusions, which requires further research and validation in the future. Third, the current follow-up time for the reported patient is relatively limited, and the specific efficacy of alectinib still requires longer-term follow-up observation.
In conclusion, the present study reports a lung adenocarcinoma case with an ALK-MTUS2 fusion and concurrent STRN3-ALK fusion, highlighting its innovation. Follow-up results confirmed the efficacy of the ALK TKI, alectinib, in NSCLC with this rare fusion type. The findings expand the spectrum of ALK rearrangements and explore the therapeutic strategies for lung adenocarcinoma.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the internal research project grant from the Jiangsu Province Hospital of Chinese Medicine (grant no. Y2023CX02) and Postgraduate Research & Practice Innovation Program of Jiangsu Province (grant no. SJCX25_0876).
Availability of data and materials
The high-throughput sequencing data generated in the present study may be found in the NCBI SRA database under accession number SRP584787 or at the following URL: https://www.ncbi.nlm.nih.gov/sra/?term=SRP584787. All other data generated in the present study may be requested from the corresponding author.
Authors' contributions
XQ contributed to conceptualization, writing the original draft and reviewing and editing the manuscript. YW and XW contributed to conceptualization, reviewing and editing the manuscript and obtaining MRI and PET/CT images. SX reviewed and edited the manuscript, obtained CT scan images and advised on patient treatment. YZ reviewed and edited the manuscript and carried out the high-throughput sequencing experiments. HH and XZ contributed to conceptualization, supervision, reviewing and editing the manuscript and advised on patient treatment. XQ, YW, XW, SX, YZ, HH and XZ confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Written informed consent was obtained from the patient, including consent to participate.
Patient consent for publication
Written informed consent was obtained from the patient, including consent for publication of the findings.
Competing interests
The authors declare that they have no competing interests.
References
|
Barlesi F, Mazieres J, Merlio JP, Debieuvre D, Mosser J, Lena H, Ouafik L, Besse B, Rouquette I, Westeel V, et al: Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French cooperative thoracic intergroup (IFCT). Lancet. 387:1415–1426. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Tian HX, Zhang XC, Yang JJ, Guo WB, Chen ZH, Wang Z and Wu YL: Clinical characteristics and sequence complexity of anaplastic lymphoma kinase gene fusions in Chinese lung cancer patients. Lung Cancer. 114:90–95. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Wu YL, Dziadziuszko R, Ahn JS, Barlesi F, Nishio M, Lee DH, Lee JS, Zhong W, Horinouchi H, Mao W, et al: Alectinib in resected ALK-positive non-small-cell lung cancer. N Engl J Med. 390:1265–1276. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Zou Z, Xing P, Hao X, Wang Y, Song X, Shan L, Zhang C, Liu Z, Ma K, Dong G and Li J: Intracranial efficacy of alectinib in ALK-positive NSCLC patients with CNS metastases-a multicenter retrospective study. BMC Med. 20:122022. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Zhang B, Zhang Y, Xu F, Zhang Z, Shao L, Yan C, Ulivi P, Denis MG, Christopoulos P, et al: Concomitant mutation status of ALK-rearranged non-small cell lung cancers and its prognostic impact on patients treated with crizotinib. Transl Lung Cancer Res. 10:1525–1535. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang L, Xiao P, Meng F and Zhong D: STRN-ALK fusion in lung adenocarcinoma with brain metastasis responded well to ensartinib: A case report. Curr Oncol. 29:6749–6753. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Detterbeck FC, Chansky K, Groome P, Bolejack V, Crowley J, Shemanski L, Kennedy C, Krasnik M, Peake M, Rami-Porta R, et al: The IASLC lung cancer staging project: Methodology and validation used in the development of proposals for revision of the stage classification of NSCLC in the forthcoming (eighth) edition of the TNM classification of lung cancer. J Thorac Oncol. 11:1433–1446. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Lin JJ, Riely GJ and Shaw AT. Targeting ALK: Precision medicine takes on drug resistance. Cancer Discov. 7:137–155. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Felip E, de Braud FG, Maur M, Loong HH, Shaw AT, Vansteenkiste JF, John T, Liu G, Lolkema MP, Selvaggi G, et al: Ceritinib plus nivolumab in patients with advanced ALK-rearranged non-small cell lung cancer: Results of an open-label, multicenter, phase 1b study. J Thorac Oncol. 15:392–403. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang K, Wang J, Liu J, Ward T, Wordeman L, Davidson A, Wang F and Yao X: TIP150 interacts with and targets MCAK at the microtubule plus ends. EMBO Rep. 10:857–865. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Pongor L, Kormos M, Hatzis C, Pusztai L, Szabó A and Győrffy B: A genome-wide approach to link genotype to clinical outcome by utilizing next generation sequencing and gene chip data of 6,697 breast cancer patients. Genome Med. 7:1042015. View Article : Google Scholar : PubMed/NCBI | |
|
Xicota L, Cosentino S, Vardarajan B, Mayeux R, Perls TT, Andersen SL, Zmuda JM, Thyagarajan B, Yashin A, Wojczynski MK, et al: Whole genome-wide sequence analysis of long-lived families (long-life family study) identifies MTUS2 gene associated with late-onset Alzheimer's disease. Alzheimers Dement. 20:2670–2679. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Liu M, Liu X, Suo P, Gong Y, Qu B, Peng X, Xiao W, Li Y, Chen Y, Zeng Z, et al: The contribution of hereditary cancer-related germline mutations to lung cancer susceptibility. Transl Lung Cancer Res. 9:646–658. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Ricciuti B, Elkrief A, Alessi J, Wang X, Li Y, Gupta H, Muldoon DM, Bertram AA, Pecci F, Lamberti G, et al: Clinicopathologic, genomic, and immunophenotypic landscape of ATM mutations in non-small cell lung cancer. Clin Cancer Res. 29:2540–2550. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Ferrari SM, Ragusa F, Elia G, Mazzi V, Balestri E, Botrini C, Rugani L, Patrizio A, Piaggi S, La Motta C, et al: Antineoplastic effect of ALK inhibitor crizotinib in primary human anaplastic thyroid cancer cells with STRN-ALK fusion in vitro. Int J Mol Sci. 25:67342024. View Article : Google Scholar : PubMed/NCBI | |
|
Lui K, Cheung KK, Ng WW, Wang Y, Au DWH and Cho WC: The impact of genetic mutations on the efficacy of immunotherapies in lung cancer. Int J Mol Sci. 25:119542024. View Article : Google Scholar : PubMed/NCBI | |
|
Ren H, Hou X, Eiken PW, Zhang J, Pierson KE, Nair AA, Davila JI, Kovarikova H, Jang JS, Johnson SH, et al: Identification and development of a lung adenocarcinoma PDX model with STRN-ALK fusion. Clin Lung Cancer. 20:e142–e147. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Li M, An Z, Tang Q, Ma Y, Yan J, Chen S and Wang Y: Mixed responses to first-line alectinib in non-small cell lung cancer patients with rare ALK gene fusions: A case series and literature review. J Cell Mol Med. 25:9476–9481. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Nagasaka M, Sarvadevabatla N, Iwata S, Ge Y, Sukari A, Klosowski C and Yanagihara R: STRN-ALK, A novel in-frame fusion with response to alectinib. JTO Clin Res Rep. 2:1001252020.PubMed/NCBI | |
|
Nakanishi Y, Masuda S, Iida Y, Takahashi N and Hashimoto S: Case report of non-small cell lung cancer with STRN-ALK translocation: A nonresponder to alectinib. J Thorac Oncol. 12:e202–e204. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Song GQ, Li YZ, Kong W and Hu GQ: Case Report: A rare case of non-small cell lung cancer with STRN-ALK fusion in a patient in very poor condition treated with first-line ensartinib. Front Oncol. 13:12356792023. View Article : Google Scholar : PubMed/NCBI | |
|
Su C, Jiang Y, Jiang W, Wang H, Liu S, Shao Y, Zhao W, Ning R and Yu Q: STRN-ALK fusion in lung adenocarcinoma with excellent response upon alectinib treatment: A case report and literature review. Onco Targets Ther. 13:12515–12519. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Sun K, Nie L, Nong L and Cheng Y: Primary resistance to alectinib in a patient with STRN-ALK-positive non-small cell lung cancer: A case report. Thorac Cancer. 12:1927–1930. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Yang Y, Qin SK, Zhu J, Wang R, Li YM, Xie ZY and Wu Q: A rare STRN-ALK fusion in lung adenocarcinoma identified using next-generation sequencing-based circulating tumor DNA profiling exhibits excellent response to crizotinib. Mayo Clin Proc Innov Qual Outcomes. 1:111–116. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Zeng H, Li Y, Wang Y, Huang M, Zhang Y, Tian P and Li W: Case report: Identification of two rare fusions, PDK1-ALK and STRN-ALK, that coexist in a lung adenocarcinoma patient and the response to alectinib. Front Oncol. 11:7228432021. View Article : Google Scholar : PubMed/NCBI | |
|
Zeng Q, Gao H, Zhang L, Qin S, Gu Y and Chen Q: Coexistence of a secondary STRN-ALK, EML4-ALK double-fusion variant in a lung adenocarcinoma patient with EGFR mutation: A case report. Anticancer Drugs. 32:890–893. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou C, Zeng L, Zhang Y and Yang N: Responder of gefitinib plus crizotinib in osimertinib failure EGFR-mutant NSCLC-resistant with newly identified STRN-ALK by next-generation sequencing. J Thorac Oncol. 14:e143–e144. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, Ou SI, Pérol M, Dziadziuszko R, Rosell R, et al: Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 377:829–838. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou C, Kim SW, Reungwetwattana T, Zhou J, Zhang Y, He J, Yang JJ, Cheng Y, Lee SH, Bu L, et al: Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): A randomised phase 3 study. Lancet Respir Med. 7:437–446. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Su C, Zhou J, Qiang H, Zhao J, Chang Q, Ji X, Li J, Xie M and Chu T: Special issue ‘The advance of solid tumor research in China’: Real-world clinical outcomes of alectinib for advanced nonsmall-cell lung cancer patients with ALK fusion in China. Int J Cancer. 152:15–23. 2023. View Article : Google Scholar : PubMed/NCBI |