Hepatic artery infusion chemotherapy combined with lenvatinib and PD‑1 inhibitors in the treatment of intermediate and advanced unresectable hepatocellular carcinoma
- Authors:
- Published online on: July 9, 2025 https://doi.org/10.3892/ol.2025.15183
- Article Number: 437
-
Copyright: © Jiang et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Liver cancer is a notable global health issue, characterized by high morbidity and mortality rates. According to the 2022 data from the International Agency for Research on Cancer (IARC), there were 865,269 newly diagnosed cases of liver cancer worldwide, representing 4.3% of all malignant tumor cases and ranking sixth in tumor incidence. Additionally, a total of 757,948 mortalities were attributed to liver cancer, representing 7.8% of all cancer-associated mortalities and placing it as the third leading cause of cancer mortalities (1). In China, liver cancer is notably prevalent, ranking fourth in incidence among malignant tumors and second in mortality (2). Most patients have hepatitis B virus (HBV) infection and liver cirrhosis. Upon seeking medical care, most present in the intermediate or advanced stages of the disease, characterized by notable hepatic tumor burden and a high incidence of portal vein tumor thrombus (PVTT), thereby losing the opportunity for surgical intervention (3).
Treatments for early-stage hepatocellular carcinoma (HCC) primarily include surgical resection, liver transplantation and ablation. For advanced HCC, the main therapeutic approaches consist of trans-arterial chemoembolization, systemic therapy and perioperative management to facilitate the surgical procedure (4,5). In previous years, there have been numerous advancements in the treatment of advanced HCC, particularly the integration of immunotherapy and targeted therapy, which has markedly prolonged patient survival. The IMbrave 150 trial evaluated the efficacy and safety of atezolizumab plus bevacizumab compared with sorafenib for the treatment of advanced or metastatic HCC. The results showed a median overall survival (mOS) of 19.2 months and an objective response rate (ORR) of 30% among patients receiving the combination of atezolizumab and bevacizumab (6,7).
Local treatments, such as vascular interventional embolization, ablation and radiotherapy, in combination with systemic drug therapy, have emerged as a novel approach for managing unresectable HCC. Hepatic arterial infusion chemotherapy (HAIC) represents a localized therapeutic approach for HCC, employing a catheter to administer chemotherapy agents directly and continuously to the tumor site within the liver. This technique not only enhances the local concentration of anticancer agents in the neoplastic tissue but also mitigates systemic adverse effects. A retrospective study performed in China demonstrated that the combination of HAIC, lenvatinib and PD-1 inhibitor was efficacious in managing advanced HCC characterized by Vp4-type portal vein tumor thrombus and/or liver invasion >50%. The ORR and disease control rate (DCR) were 76.7 and 92.2%, respectively, with a median progression-free survival (mPFS) of 9.6 months (95% CI, 8.5–10.8) and a mOS of 19.3 months (95% CI, 11.0–27.5). The study also indicated favorable safety profiles and manageable adverse events (AEs) (8).
The triple combination of hepatic arterial infusion chemotherapy (HAIC) with targeted immunotherapy has demonstrated preliminary notable efficacy in treating unresectable advanced HCC. To further validate these findings, the present retrospective comparative study was performed.
Materials and methods
Study population
The present retrospective study was performed on 63 patients with Barcelona Clinic Liver Cancer (BCLC) stage B or C HCC who received either a combination of HAIC, lenvatinib and a PD-1 inhibitor (triple therapy) or lenvatinib and a PD-1 inhibitor (dual therapy) as first-line treatment at Tengzhou Central People's Hospital Affiliated to Jining Medical College (Tengzhou, China) from January 2020 to December 2023. All patients were diagnosed with HCC based on non-invasive or biopsy criteria (9,10).
The inclusion criteria for the present study were as follows: i) Age of 18–75 years old; ii) Patients with Child-Pugh class A or B with ascites or hepatic encephalopathy albumin-bilirubin grade 1 or 2; iii) European Cooperative Oncology Group 0–2; iv) patients with BCLC stage B or C who were initially unresectable; v) ≥1 liver lesion was evaluable according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) (11–13); vi) adequate organ function, defined as an absolute neutrophil count >2.0×109/l, platelet count >50×109/l, total bilirubin <50 µmol/l, serum albumin >28 g/l, AST and ALT levels within 5 times the upper limit of normal (normal range, 0–40 U/l), creatinine clearance within 1.5 times the upper limit of normal (normal range, 80–120 ml/min) and a normal left ventricular ejection fraction (normal range, 50–70%); and vii) no prior antitumor therapy. Exclusion criteria were as follows: i) Multiple organ dysfunctions and immune disorders; ii) the combination of hepatobiliary system infection, fever and/or bleeding; and iii) allergic to the treatment drug used in the present study.
Treatment procedure
Patients voluntarily choose either triple or dual therapy and signed an informed consent form before the initiation of treatment. The triple therapy regimen was as follows: FOLFOX-HAIC (oxaliplatin, 85 mg/m2 continuous arterial infusion 2 h; leucovorin, 400 mg/m2 continuous arterial infusion 2 h; 5-fluorouracil, 2,400 mg/m2 continuous arterial infusion 46 h; every 4 weeks), lenvatinib (8 mg once daily), PD-1 inhibitors (camrelizumab 200 mg or sintilimab 200 mg, every 3 weeks). The dual therapy regimen was as follows: Lenvatinib [8 mg (body weight ≤60 kg) or 12 mg (body weight >60 kg) once daily], PD-1 inhibitors (camrelizumab 200 mg or sintilimab 200 mg, every 3 weeks).
The HAIC operational process was as follows: Under DSA guidance, the femoral artery was punctured using the Seldinger technique under local anesthesia. A 5F vascular sheath was subsequently inserted, followed by the use of a 5F RH catheter or Cobra catheter for selective angiography of the celiac axis and superior mesenteric artery to elucidate the blood supply to the liver tumor. A 2.7F microcatheter was introduced via the coaxial catheter technique, with the tip of the microcatheter positioned at the origin of the left or right hepatic artery supplying the liver lobe that harbors the tumor. If there were multiple liver invasions involving ≥2 liver lobes, the tip of the microcatheter traversed the gastroduodenal artery and was positioned in the proper hepatic artery. Subsequently, FOLFOX chemotherapy agents were continuously infused through the pre-established catheter, diffusing into the tumor tissue.
The choice of PD-1 inhibitors was mainly based on the availability of the drugs and the patients' willingness to use them. Commonly used PD-1 inhibitors include camrelizumab and sintilimab, both of which have demonstrated notable efficacy in multiple clinical trials for the treatment of HCC (14,15).
According to the Common Toxicity Criteria for AEs version 5.0 (CTCAE v5.0) (16) published by the National Cancer Institute of the United States, in conjunction with the specific characteristics of immune checkpoint inhibitor (ICI)-associated AEs. The present study systematically assessed and managed the AEs experienced by the enrolled patients (17,18). Grade 1 AEs did not influence the treatment course. For grade 2 AEs, the original treatment plan was temporarily suspended. Once symptoms had been adequately controlled through symptomatic therapy, patients resumed the original treatment plan. Grade 3 AEs required discontinuation of the initial treatment regimen and initiation of active symptomatic management. Once the AEs had been reduced to grade ≤1, the patient could resume the original treatment plan at a reduced dosage. Grade ≥4 adverse events would result in the termination of the initial treatment plan, with active management to bring patients out of danger.
Follow-up
All patients enrolled in this study were followed up every 3 months, either by telephone or through in-person visits at the outpatient clinic or ward. Contrast-enhanced CT or MRI scans were performed every 6–8 weeks to evaluate tumor changes and laboratory test results were collected throughout the treatment period. The efficacy assessment was performed according to the mRECIST, specifically by measuring the maximum diameter of the enhancing active region in each lesion.
The treatment response was assessed using the following criteria: Complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). The ORR was calculated as (CR + PR)/total number of patients ×100%, and the DCR was calculated as (CR + PR + SD)/total number of patients ×100%. Survival outcomes encompassed PFS and OS. AE assessment was performed using the CTCAE v5.0.
Statistical analysis
Statistical analyses were performed using R (RStudio, Inc.; version 4.2.1) and SPSS (IBM Corp.; version 26.0). Continuous numerical variables conforming to a normal distribution (Kolmogorov-Smirnov test) are represented as mean ± standard deviation, while those not adhering to a normal distribution are represented as median (interquartile range: Q1, Q3). Categorical variables are represented as the number of cases and their respective percentages. Continuous numerical variables between different groups were compared using the Student's t-test or Mann-Whitney U test, as appropriate. Categorical variables between different groups were compared using the χ2 test or Fisher's exact test, depending on the data characteristics. Survival analysis was performed to statistically evaluate the data. Survival curves were constructed using the Kaplan-Meier method and the Log-rank test was applied to assess univariate survival differences. Variables that exhibited statistical significance (P<0.05) in univariate analysis were subjected to the Schoenfeld residual test to evaluate whether the proportional hazards (PH) assumption was satisfied. If the PH assumption (P>0.05) was valid, the variables were subsequently included in the Cox proportional hazards regression model for multivariate analysis. Otherwise, the variables were stratified based on survival time and subsequently subjected to multivariate analysis or analyzed using time-dependent covariates (19–21).
Results
Patient baseline characteristics and treatment
A total of 63 patients who met the criteria were included in the present retrospective cohort study. The last follow-up date was June 2024, and the median follow-up time was 26.6 months. The baseline characteristics of the enrolled patients are summarized in Table I. Among the 63 patients, 33 were male and 30 were female, with a median age of 56 years (Q1, 46.5; Q3, 64.0). The majority of the patients were infected with HBV, and 43 HBsAg-positive patients accounted for 68.3% of the total population. The proportions of Child-Pugh A and B were 69.8 and 30.2%, respectively. Most patients were diagnosed at an advanced stage, with 45 patients (71.4%) classified as BCLC C stage, thereby losing the opportunity for surgical resection. Additionally, a notable number of patients exhibited PVTT. Among patients with PVTT, 38 were classified as type Vp3-4, accounting for 60.3%. Of these, 21 received triple therapy, while 17 received dual therapy. There were 20 patients with extrahepatic metastasis, accounting for 31.7%. Among them, 12 patients received dual therapy and 8 received triple therapy.
All baseline characteristics were balanced between the 33 patients receiving triple therapy and the 30 patients receiving dual therapy (P>0.05).
Tumor response
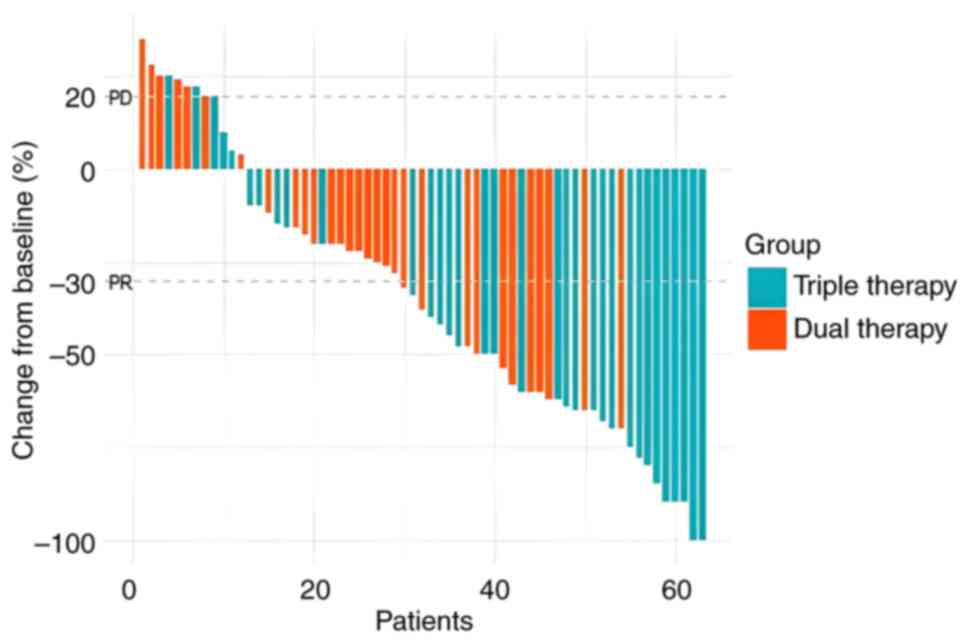
The evaluation of tumor response was performed according to mRECIST, specifically by measuring the maximum diameter of the enhancing region in each lesion (22). In the HAIC combined with targeted immunotherapy group, the CR, PR, SD and PD rates were 2 (6.1%), 21 (63.6%), 7 (21.2%) and 3 (9.1%), respectively. In the targeted immunotherapy group, the corresponding rates were 0 (0.0%), 11 (36.7%), 13 (43.3%) and 6 (20.0%), respectively. A waterfall chart presented in Fig. 1 illustrates the changes in tumor size for all 63 patients after treatment. The ORR of the triple therapy group was 69.7% (23 out of 33 patients) compared with 36.7% (11 out of 30 patients) in the dual therapy group (P=0.017), indicating a significant improvement in ORR for the triple therapy group. The DCR in the triple therapy group was 90.9% (30 out of 33 patients) compared with 80.0% (24 out of 30 patients) in the dual therapy group (P=0.381). No significant difference in the DCR was observed between the two groups, as detailed in Table II.
Survival
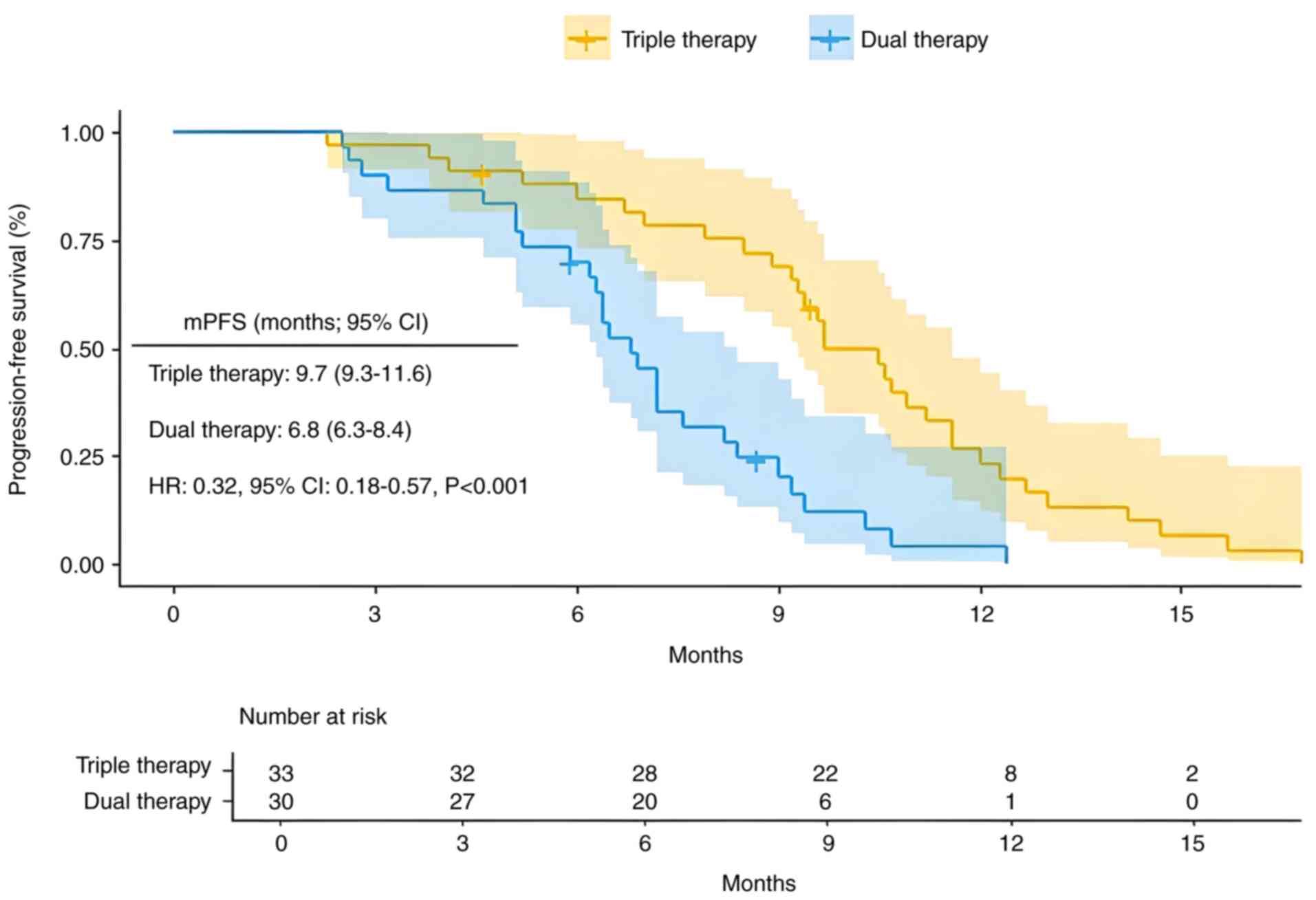
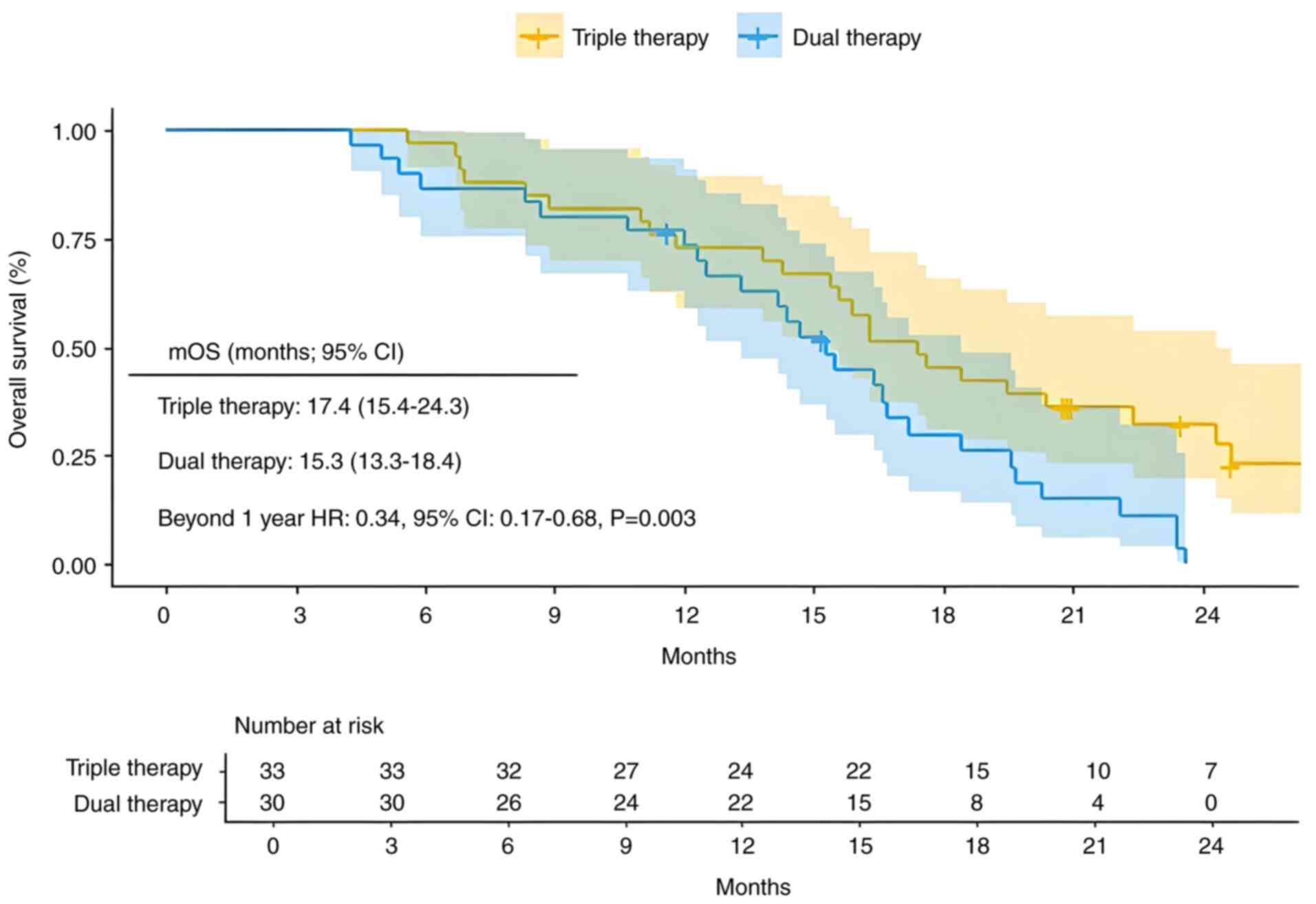
The mPFS for the triple therapy group was 9.7 months (95% CI, 9.3–11.6), with mPFS rates of 84.6% at 6 months and 23.2% at 12 months. The mPFS for the dual therapy group was 6.8 months (95% CI, 6.3–8.4), with PFS rates of 70.0% at 6 months and 4.1% at 12 months. Patients who received the triple therapy had a significantly lower risk of recurrence compared with those who received dual therapy [Fig. 2; hazard ratio (HR)=0.32; 95% CI,0.18–0.57; P<0.001]. The mOS in the triple therapy group was 17.4 months (95% CI, 15.4–24.3), with OS rates of 97.0, 72.7 and 45.5% at 6, 12 and 18 months, respectively. In the dual therapy group, the mOS was 15.3 months (95% CI, 13.3–18.4), with corresponding OS rates of 86.7, 73.2 and 29.9% at 6, 12 and 18 months, respectively. In the multivariate analysis of OS, the dual therapy group did not satisfy the PH assumption for OS during the first year. The survival curves of the dual therapy group showed no significant difference within the first year. However, after the first year, the survival rate of the triple therapy group was significantly higher compared with that of the dual therapy group, and the PH assumption was satisfied. Therefore, a stratified analysis of OS time was performed to assess the HR between the two groups post the first year (Fig. 3; HR=0.34; 95% CI, 0.17–0.68; P=0.003). Triple therapy showed a significant advantage over dual therapy in improving long-term survival rates >1 year for patients with HCC.
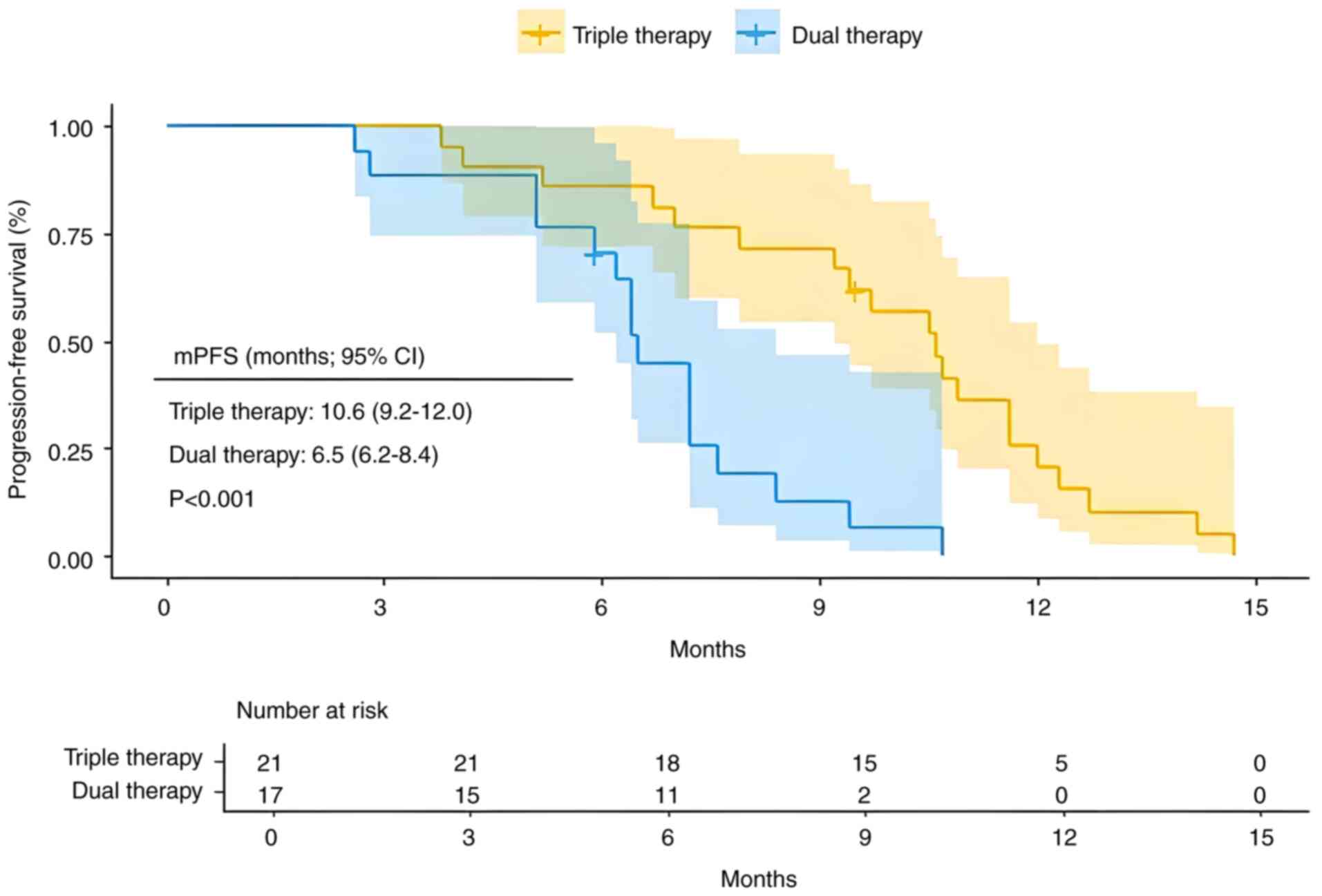
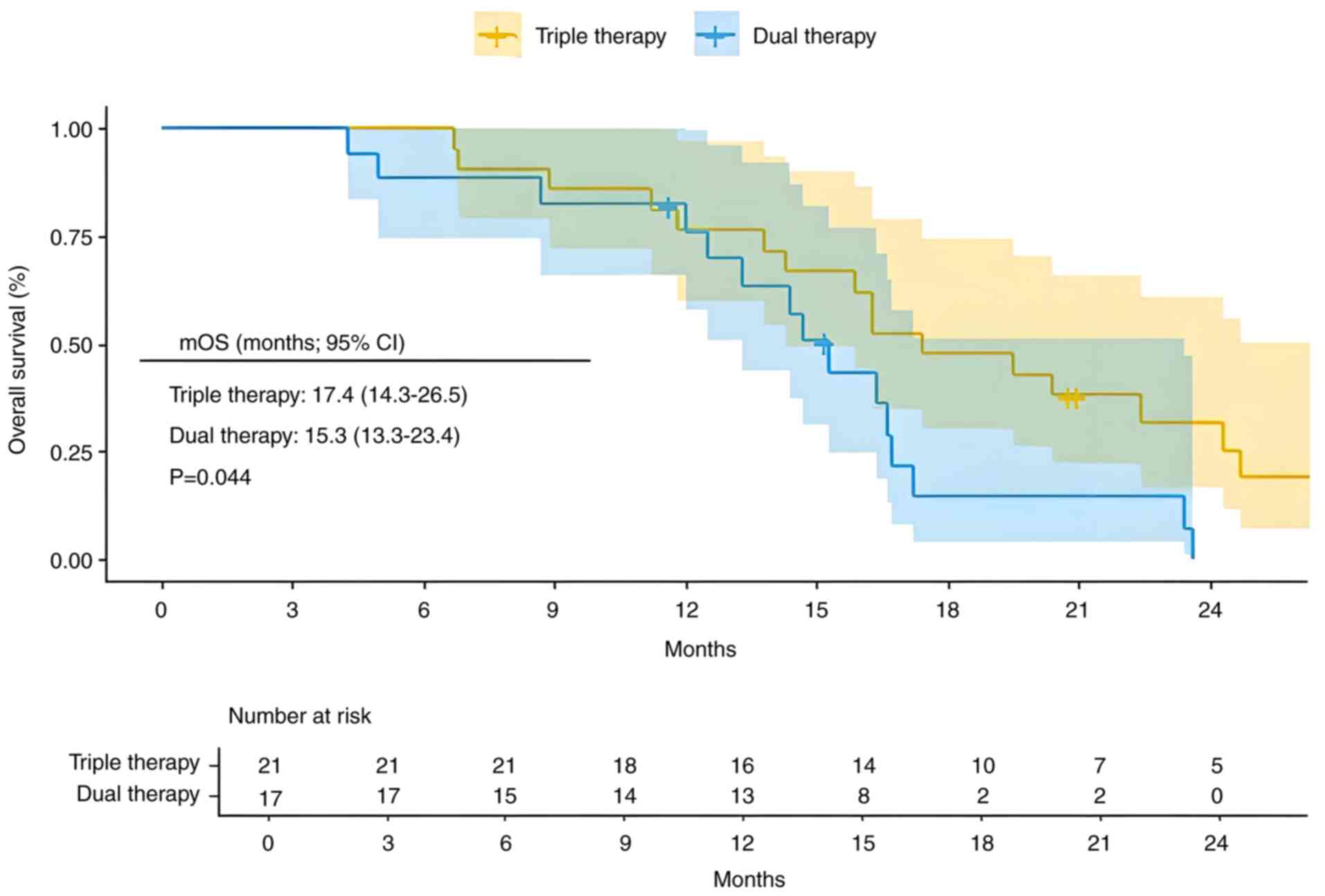
In the present cohort study, a total of 38 high-risk patients with advanced HCC characterized by Vp3-4 vascular invasion were recruited. Among these patients, 21 received triple therapy, while 17 received dual therapy. The triple therapy exhibited significantly improved clinical outcomes compared with the dual therapy, with mPFS of 10.6 months (95% CI, 9.2–12.0) vs. 6.5 months (95% CI, 6.2–8.4) (Fig. 4; P=0.00018). Additionally, mOS was 17.4 months (95% CI, 14.3–26.5) for triple therapy vs. 15.3 months (95% CI, 13.3–23.4) for dual therapy (Fig. 5; P=0.044).
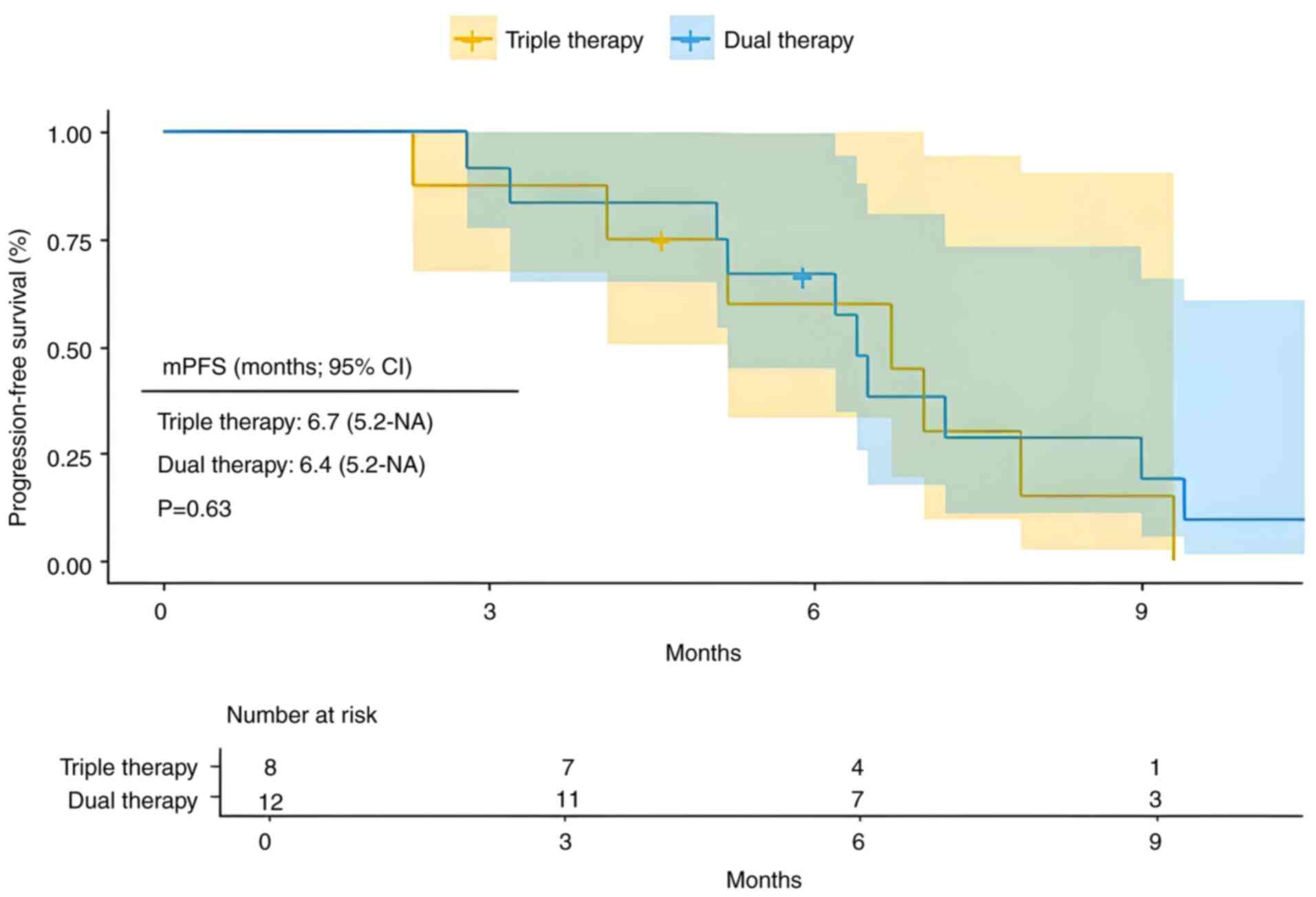
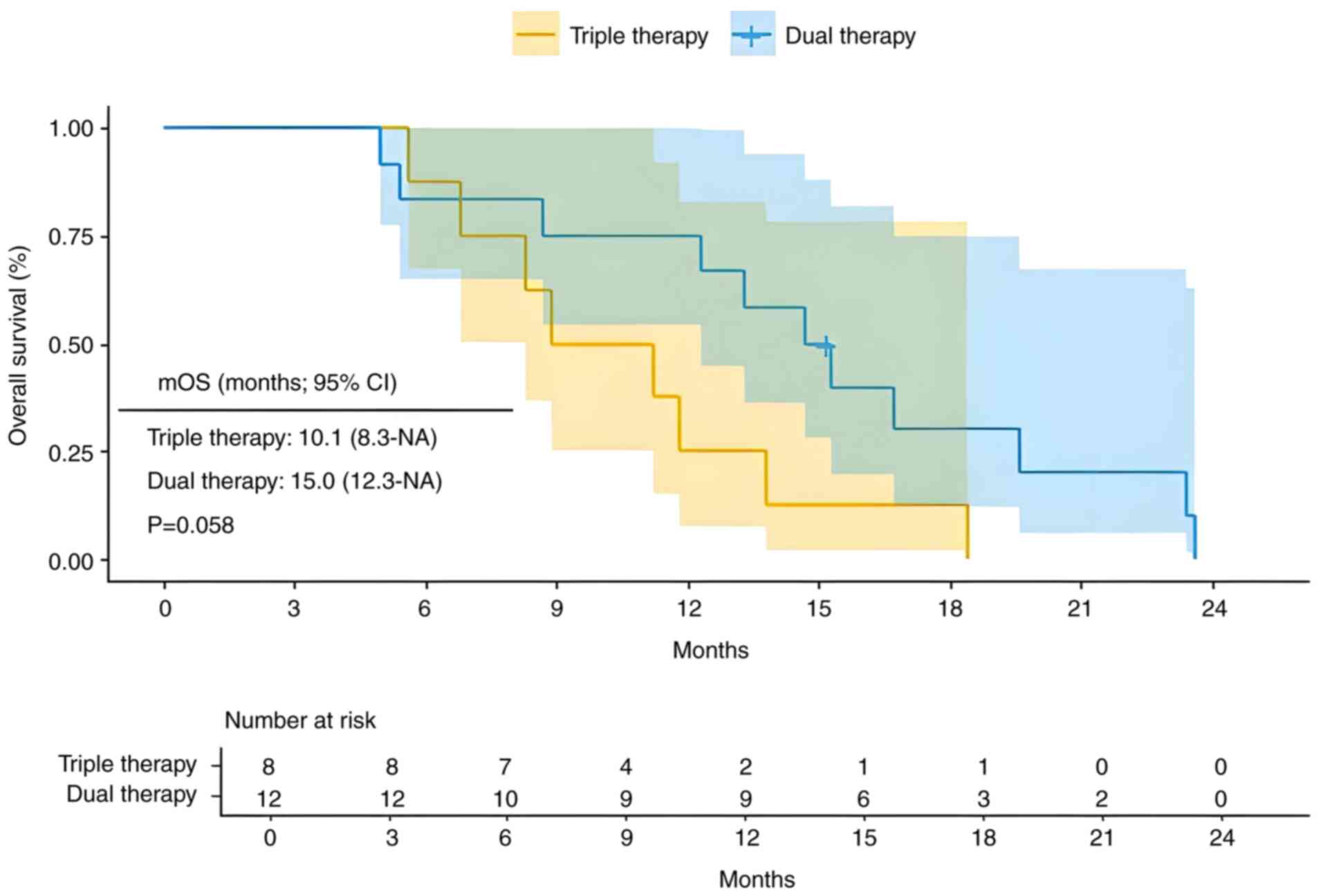
A total of 20 patients with extrahepatic metastasis were included in the present study, with 8 receiving triple therapy and 12 receiving dual therapy. Compared with dual therapy, triple therapy showed a mPFS of 6.7 months (95% CI, 5.2-NA) vs. 6.4 months (95% CI, 5.2-NA) (Fig. 6; P=0.63). Additionally, the mOS was 10.1 months (95% CI, 8.3-NA) for triple therapy compared with 15.0 months (95% CI, 12.3-NA) for dual therapy (Fig. 7; P=0.058). For advanced HCC with extrahepatic metastasis, the triple therapy demonstrated no significant difference in PFS or OS compared with the dual therapy.
Safety
Regarding safety, in the triple therapy group, the incidence of any grade AEs, listed from highest to lowest, was as follows: Abdominal pain (21 patients, 63.6%), increased aminotransferase (19 patients, 57.6%), nausea and vomiting (15 patients, 45.5%), fatigue (14 patients, 42.4%), hypertension (14 patients, 42.4%), hyperbilirubinemia (13 patients, 39.4%), leukopenia (12 patients, 36.4%), thrombocytopenia (12 patients, 36.4%) and pruritus (11 patients, 33.3%). In the dual therapy group, the incidence of any grade AEs was as follows: Fatigue (10 patients, 33.3%), hypertension (9 patients, 30.0%), pruritus (8 patients, 26.7%), increased aminotransferase (7 patients, 23.3%), proteinuria (6 patients, 20.0%) and leukopenia (5 patients, 16.7%). The incidence of any grade AEs was higher in the triple therapy group compared with the dual therapy group, specifically for abdominal pain (63.6 vs. 13.3%; P<0.001), increased aminotransferase (57.6 vs. 23.3%; P=0.006), nausea and vomiting (45.5 vs. 13.3%; P=0.006) and hyperbilirubinemia (39.4 vs. 13.3%; P=0.02). All these AEs were graded as mild (grade 1–2) and were well-tolerated by patients, with no significant impact on treatment progression.
The incidence of grade 3–4 AEs in the triple therapy group, ranked from highest to lowest, was as follows: Increased aminotransferase (5 patients, 15.2%), abdominal pain (3 patients, 9.1%), hypertension (3 patients, 9.1%), thrombocytopenia (3 patients, 9.1%), nausea and vomiting (2 patients, 6.1%) and hyperbilirubinemia (2 patients, 6.1%). In the dual therapy group, the incidence of grade 3–4 AEs was as follows: Increased aminotransferase (2 patients, 6.7%), hypertension (2 patients, 6.7%) and thrombocytopenia (2 patients, 6.7%). Grade 3–4 AEs in both groups were relatively infrequent, and no significant differences were observed between the two groups. No treatment-associated mortalities occurred in either the triple therapy group or the dual therapy group (Table III).
Discussion
In previous years, notable advancements have been made in systemic therapies for unresectable advanced HCC. With the evolving comprehensive treatment paradigm, extensive clinical evidence has demonstrated that the integration of local and systemic treatments can yield superior outcomes in terms of local control, long-term survival and surgical conversion rates for unresectable advanced HCC.
Systemic chemotherapy is rarely utilized in the treatment of HCC due to its limited efficacy and high incidence of AEs. Currently, the treatment of HCC primarily focuses on targeted therapy, immunotherapy, interventional therapy, surgical intervention and multimodal combination approaches. In 2007, sorafenib received approval for clinical use, marking a notable milestone in the targeted therapy for HCC. The SHARP study demonstrated that sorafenib extended the mOS to 10.7 months compared with 7.9 months with placebo (HR=0.69; 95% CI, 0.55–0.87; P<0.001); however, the ORR remained low at 2% (23). The REFLECT study compared the efficacy of lenvatinib and sorafenib as first-line treatments for unresectable HCC, and the results showed that lenvatinib was not superior to sorafenib in OS (HR=0.92; 95% CI, 0.79–1.06). The mOS for both groups were 13.6 vs. 12.3 months, respectively. The mPFS for both groups were 7.4 vs. 3.7 months, respectively (HR=0.66; 95% CI, 0.57–0.77; P<0.0001), indicating a significantly improved PFS for lenvatinib compared with sorafenib (24). The use of targeted therapy alone for advanced HCC exhibits limited clinical efficacy, with an ORR (based on RECIST 1.1 criteria) typically <20%, ~2% for sorafenib and 18% for lenvatinib, while the mOS is maintained at 10–13.5 months (25).
In order to improve the treatment outcomes for advanced HCC, a study from China aimed to evaluate the efficacy of sorafenib combined with HAIC vs. sorafenib alone for patients with HCC characterized by Vp3-4 PVTT. The results demonstrated that the mOS for the sorafenib plus HAIC group was 16.3 months (95% CI, 0.0–35.5), whereas it was 6.5 months (95% CI, 4.4–8.6) for the sorafenib-only group (HR=0.28; 95% CI, 0.15–0.53; P<0.001). Furthermore, the ORR was significantly higher in the sorafenib plus HAIC group (41 vs. 3%; P<0.001) and the mPFS was also prolonged (9.0 vs. 2.5 months; HR=0.26; 95% CI, 0.15–0.47; P<0.001) (26). Therefore, the combination therapy utilizing HAIC could markedly enhance the therapeutic efficacy for advanced HCC.
HAIC represents a locoregional therapeutic approach for HCC. Previous clinical studies have demonstrated that FOLFOX-HAIC and its combination therapies have exhibited promising efficacy in treating advanced HCC (27–31). Notably, the triple therapy regimen integrating HAIC with targeted and immunotherapy not only enhanced therapeutic outcomes but also maintained a favorable safety profile without a notable increase in treatment-related AEs (8,32–34). A previous study by Lai et al (35) reported 36 high-risk patients with advanced HCC, characterized by liver involvement >50% or Vp3-4 type PVTT, who received FOLFOX-HAIC combined with lenvatinib and PD-1 inhibitors. The results demonstrated an ORR of 66.7%, a DCR of 88.9%, an mPFS of 10.4 months (95% CI, 5.8–15.0) and an mOS of 17.9 months (95% CI, 14.5–21.3) (35). In the IMbrave150 study, the mOS for high-risk patients was only 7.6 months (95% CI, 6.6–12.8). Therefore, it can be considered that the triple therapy demonstrates improved performance in high-risk patients with HCC. The present study yielded comparable findings, enrolling a total of 38 patients with Vp3-4. Among these patients, 21 were treated with triple therapy, while 17 were treated with dual therapy. The results demonstrated that triple therapy was associated with significantly improved outcomes compared to dual therapy in terms of both PFS (10.6 vs. 6.5 months; P<0.001) and OS (17.4 vs. 15.3 months; P=0.044).
In the triple therapy regimen, HAIC continuously administers chemotherapeutic agents directly into the liver, achieving high drug concentrations within the tumor tissue. This facilitates the rapid and efficient eradication of tumor cells, thereby markedly reducing the tumor burden. Concurrently, it induces immunogenic cell death (ICD) in the tumor microenvironment, thereby potentiating the efficacy of immunotherapy (36–39). A 2021 study published by the Massachusetts Institute of Technology demonstrated that tumor cells with DNA damage induced by chemotherapy can activate T cells through a mechanism mediated by dendritic cells. In mouse melanoma models, the intra-tumoral injection of ex vivo chemotherapy-associated tumor cells can serve as an effective immune adjuvant, markedly enhancing the infiltration of dendritic cells and antigen-specific CD8+ T cells within the tumor microenvironment and synergistically potentiating the antitumor efficacy of immunotherapeutic agents (40). Systemic administration of chemotherapeutics may compromise the anticancer immune response by inducing systemic immunosuppression. By contrast, local administration of high-concentration chemotherapeutics or chemotherapeutics-induced immunogenic antigens have the potential to enhance the efficacy of immunotherapy. This enhancement is achieved through the induction of ICD in tumor cells, which promotes the release of tumor-associated antigens, tumor-specific antigens (TAMS) and damage-associated molecular patterns. Additionally, this approach can disrupt immunosuppressive microenvironments by reducing tumor burden (41). Chemoimmunotherapy represents an innovative cancer treatment modality that synergistically integrates the benefits of conventional chemotherapy with those of immunotherapy. This approach has demonstrated notable efficacy in treating several types of tumors (42). In the present study, the incorporation of HAIC markedly enhanced the long-term antitumor efficacy of immunotherapy agents. Specifically, the HR for OS beyond the first year (HR=0.34; 95% CI, 0.17–0.68; P=0.003) in the triple therapy group compared with the dual therapy group supports this conclusion.
VEGF serves as a crucial mediator in tumor angiogenesis and contributes to antitumor immune suppression. It inhibits the maturation of dendritic cells, resulting in the inactivation of CD8+ T cells. Additionally, VEGF potently induces the recruitment and expansion of Tregs, TAMs and myeloid-derived suppressor cells, fostering an immunosuppressive tumor microenvironment (43). Therefore, anti-angiogenic drugs not only inhibit the formation of new blood vessels in tumors to exert antitumor effects but also effectively alleviate VEGF-mediated immune suppression, reshape the tumor microenvironment and enhance the efficacy of immunotherapy (44–46). Therefore, the combination of HAIC-FOLFOX and targeted immunotherapy exhibits a synergistic effect, thereby compensating for the limitations of each approach and maximizing their combined antitumor efficacy (47).
The combination of HAIC and targeted immunotherapy demonstrated a favorable safety profile in the present study, with the majority of AEs classified as grade 1–2. Grade 3 events were rare, with <10% of cases exceeding this severity level. A study performed in 2021 analyzed the safety of tyrosine kinase inhibitors, ICIs, HAIC and HAIC combined therapies in treating advanced HCC and found that the incidence of grade 3–5 AEs was lowest with monotherapy using oxaliplatin-HAIC (48). Therefore, the combination of FOLFOX-HAIC with targeted immunotherapy does not seem to further elevate the incidence of severe AEs.
It is important to note that the present study was conducted as a single-center trial with a relatively small sample size, particularly among patients with extrahepatic metastasis. Furthermore, the subgroup analysis evaluating treatment benefit was conducted post hoc after all patients had been enrolled. Additionally, it should be acknowledged that prior studies have suggested that patients with extrahepatic metastases receiving triple therapy might achieve better therapeutic outcomes compared to those on dual therapy, which contradicts the conclusions of our study. Therefore, further large-scale, multi-center studies are warranted to validate and refine the findings of this investigation (49,50).
In conclusion, the integration of FOLFOX-HAIC with lenvatinib and PD-1 inhibitors, as compared with lenvatinib and PD-1 inhibitors alone, enhances both the efficacy and safety of treatment for unresectable advanced HCC, while maintaining manageable treatment-related AEs.
Acknowledgements
The authors would like to thank Professor Bin Liu and Professor Wujie Wang (Department of Interventional Medicine and Minimally Invasive Oncology at The Second Hospital of Shandong University, Jinan, China) for their guidance and assistance during the study design and manuscript preparation process.
Funding
The present study was supported by Young Elite Sponsorship Program of Shandong Provincial Medical Association (grant no. 2023_JC_0060).
Availability of data and materials
The data generated in the present study are included in the figures and/or tables of this article.
Authors' contributions
YG and HW conceived and designed the study, and confirmed the authenticity of all the raw data. PJ, LC and FY were responsible for data collection and analysis and co-drafted the manuscript. FL, YS, and ZJ contributed to the acquisition, analysis, and interpretation of data, critically revised the manuscript for important intellectual content, and provided final approval of the version to be published All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was reviewed and approved by the Ethics Committee of Tengzhou Central People's Hospital affiliated to Jining Medical College (grant no. 2024-Ethics Review-111). Written informed consent was obtained from all patients prior to the implementation of treatment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Zheng RS, Chen R, Han BF, Wang SM, Li L, Sun KX, Zeng HM, Wei WW and He J: Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi. 46:221–231. 2024.(In Chinese). PubMed/NCBI | |
|
Department of Medical Administration, National Health and Health Commission of the People's Republic of China, . Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition). Zhonghua Gan Zang Bing Za Zhi. 28:112–128. 2020.(In Chinese). PubMed/NCBI | |
|
Brown ZJ, Tsilimigras DI, Ruff SM, Mohseni A, Kamel IR, Cloyd JM and Pawlik TM: Management of hepatocellular carcinoma: A review. JAMA Surg. 158:410–420. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Haber PK, Puigvehí M, Castet F, Lourdusamy V, Montal R, Tabrizian P, Buckstein M, Kim E, Villanueva A, Schwartz M and Llovet JM: Evidence-based management of hepatocellular carcinoma: Systematic review and meta-analysis of randomized controlled trials (2002–2020). Gastroenterology. 161:879–898. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al: Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 382:1894–1905. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, et al: Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 76:862–873. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Chang X, Li X, Sun P, Li Z, Sun P and Ning S: HAIC Combined with lenvatinib plus PD-1 versus. lenvatinib Plus PD-1 in patients with high-risk advanced HCC: A real-world study. BMC Cancer. 24:4802024. View Article : Google Scholar : PubMed/NCBI | |
|
Xie D, Shi J, Zhou J, Fan J and Gao Q: Clinical practice guidelines and real-life practice in hepatocellular carcinoma: A Chinese perspective. Clin Mol Hepatol. 29:206–216. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Qiu G, Jin Z, Chen X and Huang J: Interpretation of guidelines for the diagnosis and treatment of primary liver cancer (2019 edition) in China. Glob Health Med. 2:306–311. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Mauro E and Forner A: Barcelona clinic liver cancer 2022 update: Linking prognosis prediction and evidence-based treatment recommendation with multidisciplinary clinical decision-making. Liver Int. 42:488–491. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, et al: BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 76:681–693. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Lencioni R and Llovet JM: Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Qin S, Chan SL, Gu S, Bai Y, Ren Z, Lin X, Chen Z, Jia W, Jin Y, Guo Y, et al: Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): A randomised, open-label, international phase 3 study. Lancet. 402:1133–1146. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, Li Q, Lu Y, Chen Y, Guo Y, et al: Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study. Lancet Oncol. 22:977–990. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Minasian LM, O'Mara A and Mitchell SA: Clinician and Patient Reporting of Symptomatic Adverse Events in Cancer Clinical Trials: Using CTCAE and PRO-CTCAE® to Provide Two Distinct and Complementary Perspectives. Patient Relat Outcome Meas. 13:249–258. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Postow MA, Sidlow R and Hellmann MD: Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 378:158–168. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Darnell EP, Mooradian MJ, Baruch EN, Yilmaz M and Reynolds KL: Immune-related adverse events (irAEs): Diagnosis, management, and clinical pearls. Curr Oncol Rep. 22:392020. View Article : Google Scholar : PubMed/NCBI | |
|
Stensrud MJ and Hernán MA: Why test for proportional hazards? JAMA. 323:1401–1402. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Sjölander A and Dickman PW: Why test for proportional hazards-or any other model assumptions? Am J Epidemiol. 193:926–927. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Jachno KM, Heritier S, Woods RL, Mahady S, Chan A, Tonkin A, Murray A, McNeil JJ and Wolfe R: Examining evidence of time-dependent treatment effects: an illustration using regression methods. Trials. 23:8572022. View Article : Google Scholar : PubMed/NCBI | |
|
Llovet JM and Lencioni R: mRECIST for HCC: Performance and novel refinements. J Hepatol. 72:288–306. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib versus. Sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
da Fonseca LG, Reig M and Bruix J: Tyrosine kinase inhibitors and hepatocellular carcinoma. Clin Liver Dis. 24:719–737. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zheng K, Zhu X, Fu S, Cao G, Li WQ, Xu L, Chen H, Wu D, Yang R, Wang K, et al: Sorafenib plus hepatic arterial infusion chemotherapy versus. sorafenib for hepatocellular carcinoma with major portal vein tumor thrombosis: A randomized trial. Radiology. 303:455–464. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
He M, Li Q, Zou R, Shen J, Fang W, Tan G, Zhou Y, Wu X, Xu L, Wei W, et al: Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs. sorafenib alone for hepatocellular carcinoma with portal vein invasion: A randomized clinical trial. JAMA Oncol. 5:953–960. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Long Y, Song X, Guan Y, Lan R, Huang Z, Li S and Zhang L: Sorafenib plus hepatic arterial infusion chemotherapy versus. sorafenib alone for advanced hepatocellular carcinoma: A systematic review and meta-analysis. J Gastroenterol Hepatol. 38:486–495. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang W, Zhang K, Liu C, Gao W, Si T, Zou Q, Guo Z, Yang X, Li M, Liu D, et al: Hepatic arterial infusion chemotherapy combined with anti-PD-1/PD-L1 immunotherapy and molecularly targeted agents for advanced hepatocellular carcinoma: A real world study. Front Immunol. 14:11273492023. View Article : Google Scholar : PubMed/NCBI | |
|
Cao YZ, Zheng GL, Zhang TQ, Shao HY, Pan JY, Huang ZL and Zuo MX: Hepatic arterial infusion chemotherapy with anti-angiogenesis agents and immune checkpoint inhibitors for unresectable hepatocellular carcinoma and meta-analysis. World J Gastroenterol. 30:318–331. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, Wei W, Zhang YJ, Guo Y, Guo RP, et al: Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus. Transarterial chemoembolization for large hepatocellular carcinoma: A randomized phase III trial. J Clin Oncol. 40:150–160. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang TQ, Geng ZJ, Zuo MX, Li JB, Huang JH, Huang ZL, Wu PH and Gu YK: Camrelizumab (a PD-1 inhibitor) plus apatinib (an VEGFR-2 inhibitor) and hepatic artery infusion chemotherapy for hepatocellular carcinoma in Barcelona clinic liver cancer stage C (TRIPLET): A phase II study. Signal Transduct Target Ther. 8:4132023. View Article : Google Scholar : PubMed/NCBI | |
|
He MK, Liang RB, Zhao Y, Xu YJ, Chen HW, Zhou YM, Lai ZC, Xu L, Wei W, Zhang YJ, et al: Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus. lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. 13:175883592110027202021. View Article : Google Scholar : PubMed/NCBI | |
|
Chen S, Shi F, Wu Z, Wang L, Cai H, Ma P, Zhou Y, Mai Q, Wang F, Tang S, et al: Hepatic arterial infusion chemotherapy plus lenvatinib and tislelizumab with or without transhepatic arterial embolization for unresectable hepatocellular carcinoma with portal vein tumor thrombus and high tumor Burden: A multicenter retrospective study. J Hepatocell Carcinoma. 10:1209–1222. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Lai Z, He M, Bu X, Xu Y, Huang Y, Wen D, Li Q, Xu L, Zhang Y, Wei W, et al: Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: A biomolecular exploratory, phase II trial. Eur J Cancer. 174:68–77. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Park SJ, Ye W, Xiao R, Silvin C, Padget M, Hodge JW, Van Waes C and Schmitt NC: Cisplatin and oxaliplatin induce similar immunogenic changes in preclinical models of head and neck cancer. Oral Oncol. 95:127–135. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, Altiok S, Celis E and Gabrilovich DI: Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 120:1111–1124. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Galluzzi L, Zitvogel L and Kroemer G: immunological mechanisms underneath the efficacy of cancer therapy. Cancer Immunol Res. 4:895–902. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Rabinovich GA, Gabrilovich D and Sotomayor EM: Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 25:267–296. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Sriram G, Milling LE, Chen JK, Kong YW, Joughin BA, Abraham W, Swartwout S, Handly ED, Irvine DJ and Yaffe MB: The injury response to DNA damage in live tumor cells promotes antitumor immunity. Sci Signal. 14:eabc47642021. View Article : Google Scholar : PubMed/NCBI | |
|
Li R, Hao Y, Roche K, Chen G, Pan W, Wang AZ and Min Y: Chemotherapy-induced nanovaccines implement immunogenicity equivalence for improving cancer chemoimmunotherapy. Biomaterials. 301:1222902023. View Article : Google Scholar : PubMed/NCBI | |
|
Sordo-Bahamonde C, Lorenzo-Herrero S, Gonzalez-Rodriguez AP, Martínez-Pérez A, Rodrigo JP, García-Pedrero JM and Gonzalez S: Chemo-immunotherapy: A new trend in cancer treatment. Cancers (Basel). 15:29122023. View Article : Google Scholar : PubMed/NCBI | |
|
Shigeta K, Datta M, Hato T, Kitahara S, Chen IX, Matsui A, Kikuchi H, Mamessier E, Aoki S, Ramjiawan RR, et al: Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology. 71:1247–1261. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
De Visser KE and Joyce JA: The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell. 41:374–403. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Franken A, Bila M, Mechels A, Kint S, Van Dessel J, Pomella V, Vanuytven S, Philips G, Bricard O, Xiong J, et al: CD4+ T cell activation distinguishes response to anti-PD-L1+anti CTLA4 therapy from anti-PD-L1 monotherapy. Immunity. 57:541–558.e7. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Liu X, Lu Y and Qin S: Atezolizumab and bevacizumab for hepatocellular carcinoma: Mechanism, pharmacokinetics and future treatment strategies. Future Oncol. 17:2243–2256. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu S, Zhang T, Zheng L, Liu H, Song W, Liu D, Li Z and Pan CX: Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol. 14:1562021. View Article : Google Scholar : PubMed/NCBI | |
|
Pan Y, Wang R, Hu D, Xie W, Fu Y, Hou J, Xu L, Zhang Y, Chen M and Zhou Z: Comparative safety and efficacy of molecular-targeted drugs, immune checkpoint inhibitors, hepatic arterial infusion chemotherapy and their combinations in advanced hepatocellular carcinoma: Findings from advances in landmark trials. Front Biosci (Landmark Ed). 26:873–881. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Guan R, Zhang N, Deng M, Lin Y, Huang G, Fu Y, Zheng Z, Wei W, Zhong C, Zhao H, et al: Patients with hepatocellular carcinoma extrahepatic metastases can benefit from hepatic arterial infusion chemotherapy combined with lenvatinib plus programmed death-1 inhibitors. Int J Surg. 110:4062–4073. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Chen S, Wang X, Yuan B, Peng J, Zhang Q, Yu W, Ge N, Weng Z, Huang J, Liu W, et al: Apatinib plus hepatic arterial infusion of oxaliplatin and raltitrexed for hepatocellular carcinoma with extrahepatic metastasis: Phase II trial. Nat Commun. 15:88572024. View Article : Google Scholar : PubMed/NCBI |