Prolonged survival of a case with three different malignancies - cutaneous malignant melanoma, gastrointestinal stromal tumor and thyroid papillary cancer: A case report
- Authors:
- Published online on: July 14, 2025 https://doi.org/10.3892/ol.2025.15185
- Article Number: 439
-
Copyright: © Odabaşi Bükün et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Gastrointestinal stromal tumor (GIST) is the most common malignant neoplasm of mesenchymal origin. The appropriate combination of targeted agents and surgical interventions significantly improves outcomes in patients with GIST. First-line treatment with imatinib leads to prolonged disease control (1). In nonmetastatic disease, surgical resection is the cornerstone of management, with imatinib used as adjuvant therapy in high-risk patients and as first-line treatment in the metastatic setting (2). Papillary thyroid carcinoma (PTC) is the most common histological subtype of differentiated thyroid cancer, characterized by early regional lymph metastasis. Despite this, it generally carries a favorable prognosis (3). Cutaneous malignant melanoma is a rare but aggressive form of skin cancer (3). While surgery remains the only curative treatment option for patients with early-stage disease, systemic therapies are recommended for those with metastatic melanoma. The presence of a BRAF-V600 mutation should be evaluated in malignant melanoma, as BRAF inhibitors and immunotherapeutic agents form the cornerstone of melanoma management in current clinical practice (4,5).
Cases of multiple primary malignancies have been reported in the literature, and ongoing research aims to elucidate the underlying etiological and genetic associations between coexistent malignancies. The current study presented a case involving three primary malignancies: GIST, PTC and melanoma.
Case report
A 58-year-old male patient with a history of hypertension, diabetes mellitus and coronary artery disease presented with anemia in March 2015 at Uludag University Hospital, affiliated with Uludag University (Bursa, Türkiye). Radiological imaging revealed a mass in the small intestine. The patient underwent surgical resection and pathological examinations confirmed GIST with omental metastasis. During the surgical resection, in addition to the primary GIST in the small intestine, locally advanced omental metastases were also resected. Due to multiple omental metastatic lesions, imatinib therapy was initiated, at a dose of 400 mg/day, orally. In April 2016, a partial thyroidectomy was performed for a suspicious thyroid nodule. Pathological examination revealed multifocal PTC and the patient was subsequently treated with 100 mCi of radioactive iodine, followed by total thyroidectomy.
In July 2017, while on imatinib treatment, the patient presented with a dark, irregular lesion on the left lateral side of the anterior chest. Pathological examination of the biopsy confirmed cutaneous metastatic malignant melanoma, with the tumor confined to the dermis and no involvement of the epidermis, suggesting local advancement metastasis. A sentinel lymph node dissection was performed during skin excision and histopathological examination of the excised specimen revealed no malignancy. The BRAF mutation, evaluated with VE1 antibody (cat. no. 760-5095; Roche Tissue Diagnostics) recognizing BRAF V600E mutant protein, was also negative. The staining was performed on formalin-fixed paraffin-embedded tissue sections, prepared at a thickness of 4 µm. In brief, slides were heated (60°C for 2 h), dewaxed with xylene, rehydrated using an alcohol gradient and blocked against non-specific staining with 3% hydrogen peroxide (room temperature for 4–6 h). VE1 antibody was applied at a dilution of 1:100 and the samples were incubated at 36°C, for 16 min. An OptiView DAB IHC Detection Kit (cat. no. 760-700; Roche Diagnostics) was used as a detection system and the slides were visualized using a DP-200 microscope (Roche Diagnostics). BRAF V600E-positive melanoma samples and negative controls were included for the analysis. Temozolomide (TMZ) was initiated in 2017, at a dose of 200 mg/m2 once daily for 5 days every 28 days and continued ~90 days, as part of the treatment for BRAF-negative metastatic melanoma. Surgery was not considered due to the metastatic nature of the disease. The pathology report indicated no epidermal involvement and dermal involvement malign melanoma was prioritized by the pathologist.
Approximately one year later, a millimetric nodule was detected in the lung via thoracic CT. The patient underwent wedge resection and the pathological examination confirmed metastatic malignant melanoma. In March 2018, the patient received ipilimumab, followed by nivolumab in August 2018 due to disease progression. While receiving ipilimumab 3 mg/kg every 3 weeks 4 doses and nivolumab 240 mg every 2 weeks for melanoma, the patient continued imatinib therapy for GIST. In January 2019, ipilimumab was discontinued due to toxicity (grade 3 immune-related hepatitis with 5 times higher enzyme levels) and the patient continued nivolumab treatment for approximately four years.
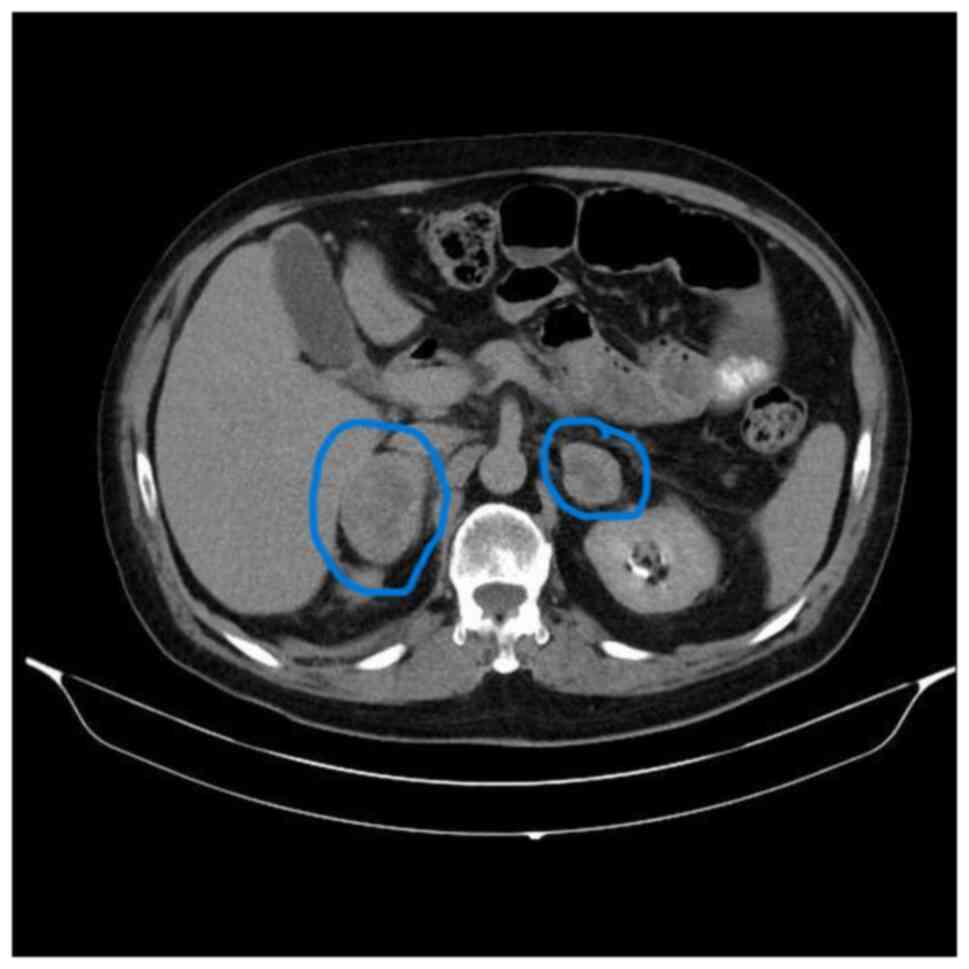
In August 2019, the patient was admitted to the emergency department of Uludag University Hospital (Bursa, Türkiye) with abdominal pain and diagnosed with acute pancreatitis. The patient was transferred to the intensive care unit and intubated due to acute respiratory distress syndrome. The patient was extubated and discharged two months later. At that time, positron emission tomography-computed tomography (PET-CT) showed local regression. However, during follow-up, in March 2021, CT revealed newly developed heterogeneous contrasting nodular lesions measuring 5×3 cm in the right and 4×4.5 cm in the left adrenal gland (Fig. 1). Due to multiple progressive lesions, the biopsied specimen was histologically processed. The tissue samples were fixed in 10% neutral-buffered formalin for 24 h and the tissue treatment for staining was followed as specified above. The H&E staining process involved incubating the sections in hematoxylin for 5 min, followed by eosin for 2 min. The sections were then assessed using a light microscope. The same histopathology protocol was used for all H&E stainings. Malignant melanoma metastasis (Figs. S1 and S2) was confirmed by immunohistochemical staining for anti-melanosome (HMB45; 1:100 dilution; cat. no. 790-4366; Roche Tissue Diagnostics). The same OptiView DAB IHC Detection Kit and visualization settings were used as specified earlier.
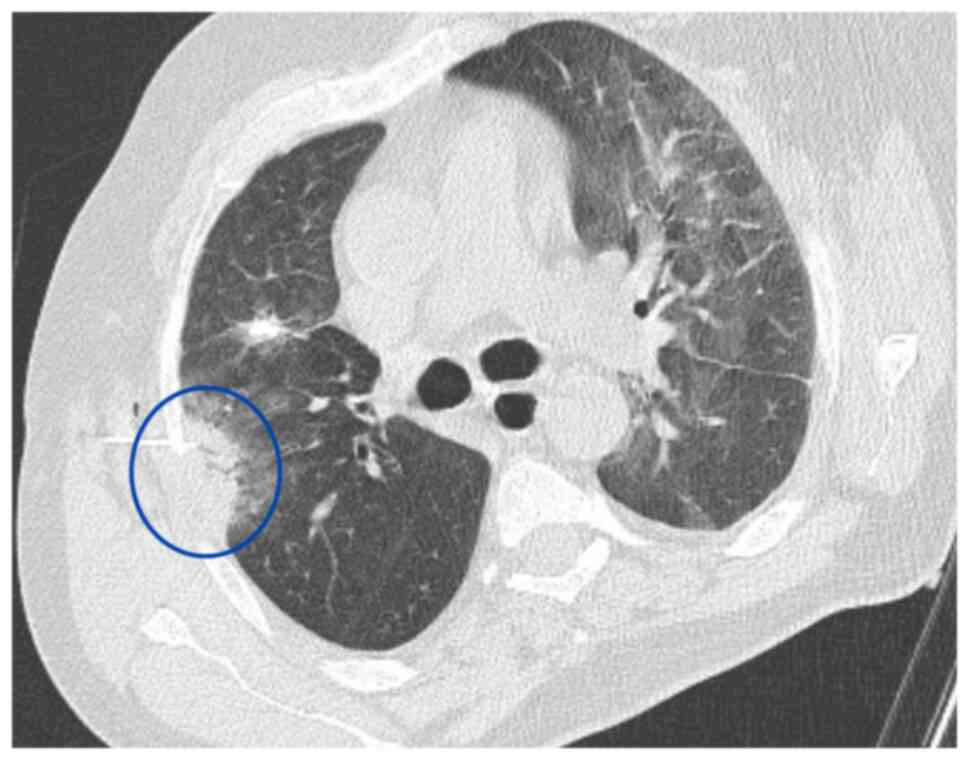
Subsequentlly, immunotherapy was discontinued and treatment with carboplatin plus paclitaxel was initiated. A Tru-Cut biopsy was performed on a newly developed ~3 cm solidified lesion in the right lung using thoracic CT (Fig. 2). Pathology results indicated a fibroinflammatory area. It was primarily evaluated as secondary to an infection.
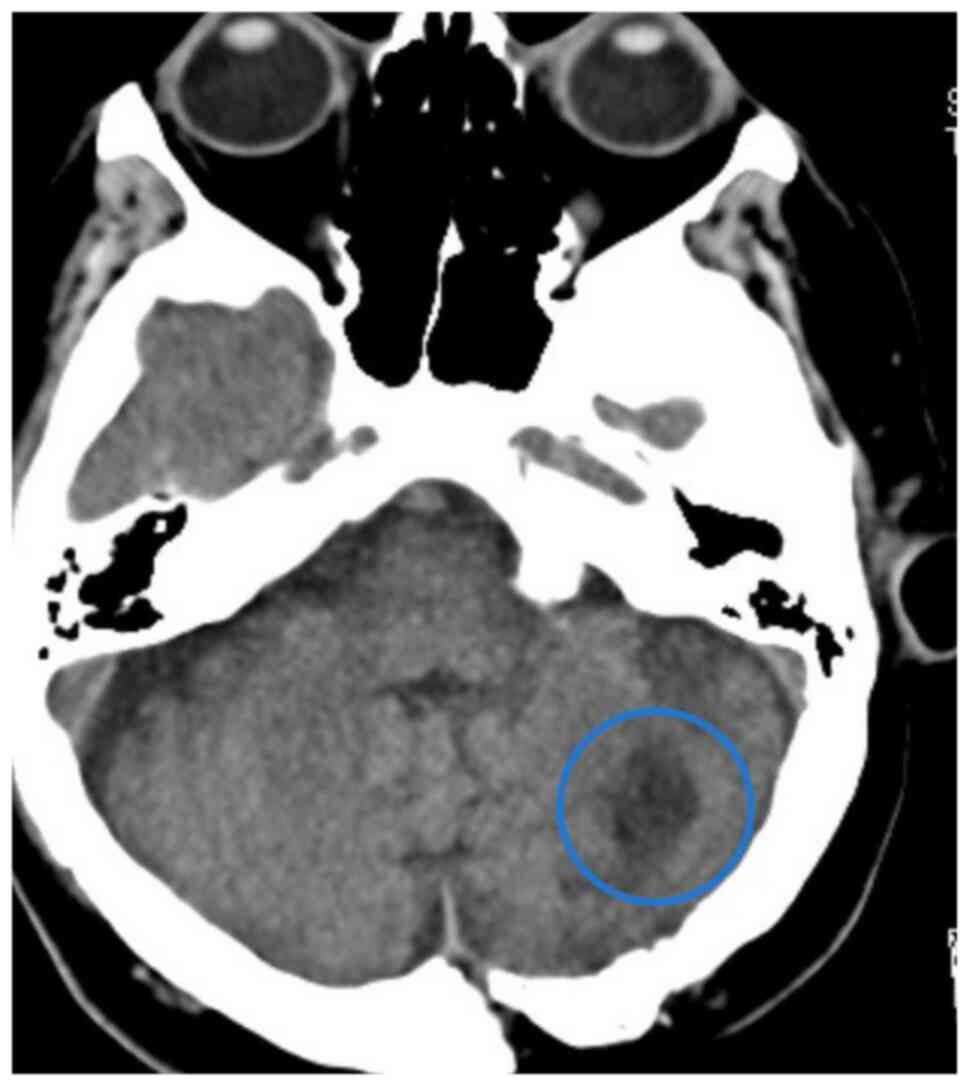
In February 2023, cranial CT revealed a 21-mm iso-hyperdense mass accompanied by edema extending to the fourth ventricle and right cerebellum (Fig. 3). Pathological examination of the excised cranial lesion confirmed melanoma metastasis (Fig. S3). The patient subsequently received postoperative cranial radiotherapy (10 days, 30 Gy). In April 2023, a lesion causing small bowel obstruction was resected following abdominal CT performed due to symptoms associated with ileus (absent gas and stool passage); evaluation for ileus and the pathological result were consistent with malignant melanoma metastasis. In addition, irregularly circumscribed hypodense areas, the largest measuring 36×30 mm in segment 7 of the liver, were detected. A biopsy of the suspected metastatic liver lesion confirmed malignant melanoma metastasis. Disease progression (including new metastatic solid nodules detected in both lungs) was observed in PET-CT scans and dacarbazine treatment was initiated in July 2023.
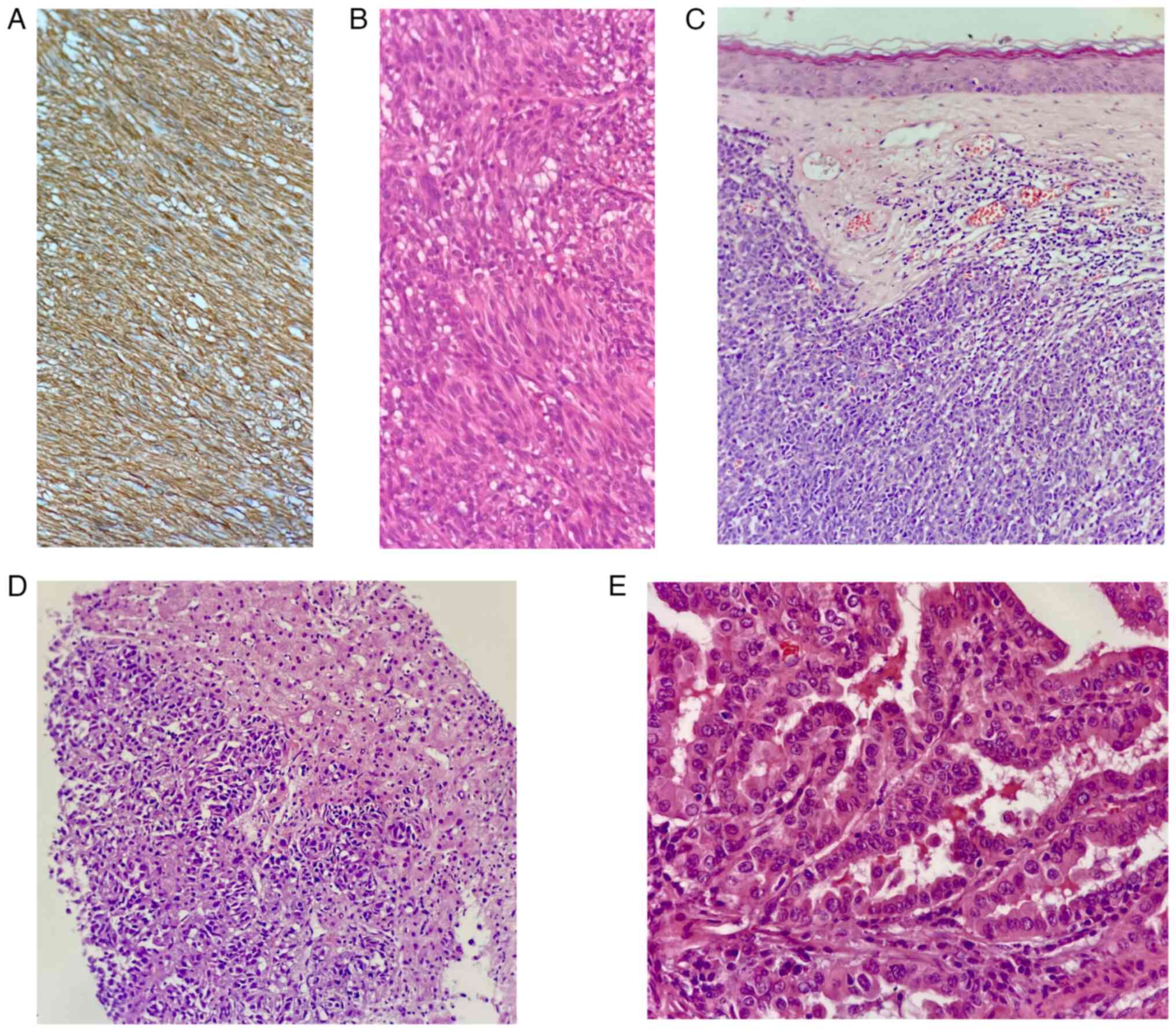
Histopathological examinations were perfomed postoperatively on samples obtained in July 2023, and the results are presented in Fig. 4. For gastrointestinal tumor immunohistochemistry, the streptavidin-perosidase method was performed strictly in accordance with the manufacturer's instructions. A positive control sample was set for each batch of dyeing and primary antibody was replaced with PBS for the negative control. After the application of the antibody, coloring was achieved with diaminobenzidine. A rabbit monoclonal discovered on GIST1 antibody (DOG-1; cat. no. 244, Cell Marque) was used at 1:100 dilution to confirm the diagnosis of GIST (Fig. 4A). The GIST tissue showed high-grade nuclear features, including nuclear pleomorphism and an elevated nuclear-cytoplasm ratio (Fig. 4B). The melanoma skin lesion exhibited intensive dermis invasion with irregularly arranged neoplastic cells (Fig. 4C), while liver metastasis demonstrated high-grade nuclear features and increased mitotic activity (Fig. 4D). The thyroid specimen showed complex papillary structures surrounded by follicular epithelial cells with characteristic nuclear features of PTC (Fig. 4E).
Regarding PTC, the thyroglobulin level remained <0.1 ng/ml (normal range, 3,68-64,15 ng/l), and no residual or recurrent thyroid tissue was detected on thyroid imaging. The patient continued long-term imatinib treatment for GIST. In terms of malignant melanoma, the disease progressed with widespread metastasis (e.g. adrenal glands, liver, small intestine and brain). The patient passed away in September 2023 due to melanoma progression.
Discussion
To the best of our knowledge, no other case report in the literature has documented the combination of these three malignancies (GIST, PTC and cutaneous malignant melanoma), highlighting the significance of the present case. Sharing such cases and accumulating data is crucial to better understand the reasons for these associations. Further research is needed to elucidate tumor pathogenesis, molecular pathways and common driver mutations involved in such cases.
Cases involving more than one malignancy are documented in the literature, with a notably high co-occurrence of GIST alongside other malignancies. While there are case reports of multiple primary malignancies, further studies are needed to clarify the relationship between certain cancers. The potential non-random and causal relationship between GIST and other neoplasms remains an area of ongoing investigation (6). In a study performed in Germany, spanning a 10-year period, 37 (43%) of 86 patients with GIST were found to have an additional malignancy. The associated malignancies included gastrointestinal (n=29; 69%), renal/urologic (n=5; 12%), hematologic (n=4; 9.5%), cutaneous (n=3; 7%) and thyroid (n=1; 2.5%) cancers (7). Malignant melanoma was observed in 2 patients, while thyroid cancer was observed in 1 patient (7). The simultaneous occurrence of GIST and other neoplasms is uncommon.
Reports in the literature document cases involving GIST alongside malignant melanoma and malignancies of other organs. Case reports have described the coexistence of oral malignant melanoma and GIST, as well as studies showing primary GIST of the liver in one patient with anorectal melanoma, and the co-occurrence of PTC and malignant melanoma in another case (8–11).
Additionally, there are numerous instances where immunotherapy combined with tyrosine kinase inhibitors (IO-TKI) is administered concurrently. For instance, IO-TKI therapy has become the first-line treatment for metastatic renal cell carcinoma (12). Another study demonstrated that anlotinib downregulates programmed death-ligand 1 and the combination of anlotinib with a programmed death-1 monoclonal antibody has shown benefits in the treatment of gastric cancer (13). These studies in the literature suggest that the patient of the present study may have benefited from combined IO-TKI treatment. In the management of metastatic melanoma, IO and TKI therapies are typically recommended separately, but the simultaneous use of both may have contributed to the patient's prolonged survival. Patients with metastatic melanoma harboring activating mutations in KIT, a receptor tyrosine kinase, are more likely to respond to imatinib, a TKI, compared to those without activating KIT mutations (14–16). Another study reviewed data emphasizing the critical role of KIT signaling in regulating antitumor immune responses and the potential benefits of combining selective KIT inhibitors with immune checkpoint inhibitors (17).
One limitation of the present case study is that the KIT mutation was not investigated due to a lack of resources, and there was no access to next-generation sequencing testing at the time. At our university hospital, C-KIT mutation testing was not available at that time, and it was not possible to send C-KIT samples for patients due to the limitations of the conditions during that period.
When reviewing this case, considering the patient's long-term survival despite disease progression under prolonged treatments, it is possible that the treatments targeted the same pathways involved in different tumors. In terms of GIST, the long-term use of TKIs, combined with the patient's extended survival despite extensive metastatic malignant melanoma, which was treated with immunotherapy for a certain period, suggests that the therapies may have influenced common tumor pathways shared across the malignancies. The TKI treatment administered to the patient for GIST may have also benefited metastatic malignant melanoma, particularly if KIT mutations were present. The patient may have further benefited from the concurrent administration of TKI while receiving IO therapy.
In conclusion, the long-term survival of the patient of the present study, despite disease progression under extensive treatment, suggests that the therapies administered may have targeted common pathways across the different tumors. However, this needs to be confirmed by further studies. In the case of GIST, the prolonged survival is likely attributable to long-term TKI therapy. In addition, TKI treatment for GIST may have provided benefits in the context of metastatic malignant melanoma, particularly in patients with KIT mutations. The concurrent administration of TKI and IO therapy may have further contributed to the patient's extended survival.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
HOB was involved in the study's conceptualization, methodology, validation, data curation and writing - original draft preparation. EC participated in the study's conceptualization and contributed by performing patient follow-up, writing - reviewing and editing and supervision. SBA was involved in data acqusition. ABS was involved in patient follow-up. All authors reviewed and approved the final version of the manuscript. HOB, EC, SBA and ABS confirm the authenticity of all raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for the publication of their data and images.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
GIST |
gastrointestinal stromal tumor |
|
PTC |
papillary thyroid carcinoma |
|
PET-CT |
positron emission tomography-computed tomography |
|
IO |
immunotherapy |
|
TKI |
tyrosine kinase inhibitor |
References
|
Schaefer IM, DeMatteo RP and Serrano C: The GIST of advances in treatment of advanced gastrointestinal stromal tumor. Am Soc Clin Oncol Educ Book. 42:1–15. 2022.PubMed/NCBI | |
|
Raut CP, Espat NJ, Maki RG, Araujo DM, Trent J, Williams TF, Purkayastha DD and DeMatteo RP: Efficacy and tolerability of 5-year adjuvant imatinib treatment for patients with resected intermediate- or high-risk primary gastrointestinal stromal tumor: The PERSIST-5 clinical trial. JAMA Oncol. 4:e1840602018. View Article : Google Scholar : PubMed/NCBI | |
|
Leonardi GC, Falzone L, Salemi R, Zanghì A, Spandidos DA, Mccubrey JA, Candido S and Libra M: Cutaneous melanoma: From pathogenesis to therapy (Review). Int J Oncol. 52:1071–1080. 2018.PubMed/NCBI | |
|
Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, et al: Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol. 40:127–137. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Sukniam K, Manaise HK, Popp K, Popp R and Gabriel E: Role of surgery in metastatic melanoma and review of melanoma molecular characteristics. Cells. 13:4652024. View Article : Google Scholar : PubMed/NCBI | |
|
Agaimy A, Wünsch PH, Sobin LH, Lasota J and Miettinen M: Occurrence of other malignancies in patients with gastrointestinal stromal tumors. Semin Diagn Pathol. 23:120–129. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Vassos N, Agaimy A, Hohenberger W and Croner RS: Coexistence of gastrointestinal stromal tumours (GIST) and malignant neoplasms of different origin: Prognostic implications. Int J Surg. 12:371–377. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Nagai K, Matsumura Y, Nomura J, Inui M and Tagawa T: A case of double cancer involving oral malignant melanoma and gastrointestinal stromal tumor (GIST). Int J Oral Maxillofac Surg. 34:328–330. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Linehan A, Harrold E, Pilson K and McCaffrey J: Recurrent vulvar melanoma in a patient with neurofibromatosis and gastrointestinal stromal tumour. BMJ Case Rep. 12:e2247442019. View Article : Google Scholar : PubMed/NCBI | |
|
Su YY, Chiang NJ, Wu CC and Chen LT: Primary gastrointestinal stromal tumor of the liver in an anorectal melanoma survivor: A case report. Oncol Lett. 10:2366–2370. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Kim YJ, Lee KA, Park TS, Baek HS and Jin HY: Multiple metastasis of follicular variant of papillary thyroid carcinoma coexistent with malignant melanoma. Korean J Intern Med. 33:634–637. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Chau V and Bilusic M: Pembrolizumab in combination with axitinib as first-line treatment for patients with renal cell carcinoma (RCC): Evidence to date. Cancer Manag Res. 12:7321–7330. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zheng W, Sun G, Li Z, Wu F, Sun G, Cao H, Zhou J and Ma Y: The effect of anlotinib combined with anti-PD-1 in the treatment of gastric cancer. Front Surg. 9:8959822022. View Article : Google Scholar : PubMed/NCBI | |
|
Guo J, Si L, Kong Y, Flaherty KT, Xu X, Zhu Y, Corless CL, Li L, Li H, Sheng X, et al: Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 29:2904–2909. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Carvajal RD, Antonescu CR, Wolchok JD, Chapman PB, Roman RA, Teitcher J, Panageas KS, Busam KJ, Chmielowski B, Lutzky J, et al: KIT as a therapeutic target in metastatic melanoma. JAMA. 305:2327–2334. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Hodi FS, Corless CL, Giobbie-Hurder A, Fletcher JA, Zhu M, Marino-Enriquez A, Friedlander P, Gonzalez R, Weber JS, Gajewski TF, et al: Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 31:3182–3190. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Stahl M, Gedrich R, Peck R, LaVallee T and Eder JP: Targeting KIT on innate immune cells to enhance the antitumor activity of checkpoint inhibitors. Immunotherapy. 8:767–774. 2016. View Article : Google Scholar : PubMed/NCBI |