Effect of TP53 mutation, expression and polymorphism on the survival, immune infiltration and ferroptosis in patients with prostate cancer
- Authors:
- Published online on: July 17, 2025 https://doi.org/10.3892/ol.2025.15191
- Article Number: 445
-
Copyright: © Wen et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Prostate cancer, an epithelial malignancy, which arises in the prostate gland, is the most common type of cancer among elderly men (1). Although the particular etiology remains unclear, the development of prostate cancer is affected by genetic, environmental and hormonal factors (2). The incidence of prostate cancer varies markedly by region and ethnicity, with a notable increase worldwide (3).
Tumor protein 53 (TP53), located on chromosome 17p13, encodes a protein that encompasses domains associated with transcriptional activation, DNA binding and oligomerization (4). As a key transcription factor, TP53 regulates several cellular processes, including cell cycle arrest, DNA repair, apoptosis, metabolism and ferroptosis (5,6). Over 4 decades of research have established TP53 as a tumor suppressor gene, whose inactivation serves a key role in tumorigenesis (7). Mutations in TP53, which occur in >50% of cancer cases, can convert TP53 from a tumor suppressor gene to an oncogene (8). A previous study reported that mice carrying prostate cells with TP53 mutations are more likely to develop prostate cancer compared with those without TP53 mutations or with TP53 deletions (9). Among the various mutation sites in TP53 gene, codon 72 polymorphism is the most studied (10).
Although extensive research has been conducted on this mutation in both Chinese and Western populations, the findings on the epidemiological and genomic differences between Chinese and Caucasian patients with prostate cancer remain elusive (11–13). Previous studies have reported that TP53 mutations are more prevalent in Chinese patients with prostate cancer, whereas TP53 expression is positively associated with higher pathological grading, which thus serves as a potential prognostic marker (14,15).
Current evidence highlights the multifaceted role of TP53 alterations in prostate cancer pathogenesis and progression. Inherited TP53 variants have been associated with increased prostate cancer risk, particularly in early-onset or familial cases (16). Analysis of ~7,000 prostate cancer cases revealed 38 TP53 mutation carriers, representing a ninefold increase compared with control populations (16). The mutations showed strong associations with more advanced tumor grades and earlier disease onset, highlighting TP53′s contribution to aggressive prostate cancer phenotypes (16). In Chinese populations, TP53 mutations are associated with aggressive disease features including higher Gleason scores and advanced clinical stages (17). Mechanistically, TP53 dysfunction contributes to immune evasion through altered tumor microenvironment (TME) interactions (18), while simultaneously creating metabolic vulnerabilities such as enhanced sensitivity to ferroptosis inducers (19). These findings collectively position TP53 mutation status as both a prognostic biomarker and a potential therapeutic determinant in prostate cancer management.
However, to the best of our knowledge, no previous large-scale multi-ethnic studies have systematically analyzed the comprehensive effects of TP53 mutation, expression profiles and polymorphism variations on survival outcomes, immune microenvironment infiltration and ferroptosis regulation in patients with prostate cancer. Current evidence is primarily derived from single-institution studies with limited sample sizes and ethnic diversity. Therefore, in the present study, bioinformatics analyses and meta-analysis were conducted to evaluate the effects of TP53 mutation and expression on prostate cancer. Furthermore, in vitro experiments were performed to validate the role of TP53 in ferroptosis.
Materials and methods
Bioinformatics analysis
Comprehensive Analysis on Multi-Omics of Immunotherapy in Pan-cancer (CAMOIP; http://www.camoip.net/) is a comprehensive tool designed to analyze expression and mutation data from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/) and immune checkpoint inhibitor-treated projects, using a standardized processing pipeline (20). CAMOIP offers several analytical functions, including survival, expression, mutational landscape, immune infiltration, immunogenicity and pathway enrichment analyses (20). Therefore, CAMOIP was utilized to analyze the survival of patients with prostate adenocarcinoma (PRAD) based on TP53 mutation or expression data from the TCGA-PRAD cohort (http://cancergenome.nih.gov/). The analysis was performed using the CAMOIP online tool by first selecting the TCGA cohort, followed by the prostate adenocarcinoma (PRAD) dataset (https://portal.gdc.cancer.gov/projects/TCGA-PRAD; accessed Jun 15th, 2025) and then specifying the TP53 gene from the gene list for subsequent investigation. The fragments per kilobase per million fragments mapped values were calculated for reference, while the normalization for differential expression analysis was performed using ‘edgeR’ (trimmed mean of M-values method). Kaplan-Meier analysis is a non-parametric statistical method used to estimate the survival probability over time in cohort studies. It generates a survival curve that depicts the proportion of subjects surviving at each time point and compares survival between TP53 mutant (MT; n=56) and wild-type (WT; n=422) groups, and TP53 low (n=241) and high (n=238) groups based on the median expression level of TP53. To explore the association between patient survival and TP53 expression or mutation, the Cox proportional hazards model was applied. Schoenfeld residual analysis was performed to evaluate whether the proportional hazards assumption of the Cox model holds for TP53 mutations and expression. Mutation frequencies were compared across patients with PRAD with different TP53 expression levels using Fisher's exact test. While the sample size imbalance between the TP53 mutant (MT; n=56) and wild-type (WT; n=422) groups was due to the natural distribution of TP53 mutations in the dataset, statistical corrections (such as weighted analyses or bootstrapping) were employed to mitigate potential biases. In addition, the immune cell infiltration scores were calculated using CIBERSORT algorithms, while the differences between patients with PRAD with varying TP53 expression levels or mutation statuses were compared using the Mann-Whitney U test. GSEA is a computational method that determines whether ‘Ribosome’, ‘Herpes simplex virus 1 infection’ and ‘Transcriptional misregulation in cancer’ pathways in prostate cancer show statistically significant, coordinated differences in expression between the TP53 mutant (MT; n=56) and wild-type (WT; n=422) groups. The R programming 3.3.4. packages ‘clusterProfiler’ and ‘fgsea’ (Posit Software, PBC) were used to perform GSEA analysis and enrichment plots were generated to visualize key contributors to each pathway. The R programming 3.3.4. packages ‘ggplot2’ and ‘pheatmap’ (Posit Software, PBC) were utilized to visualize the differential expression of the ferroptosis-related genes between patients with PRAD with different TP53 expression or mutation statuses.
Meta-analysis
For the meta-analysis, the PubMed (https://pubmed.ncbi.nlm.nih.gov/), EMBASE (https://www.embase.com/) and Cochrane Library databases (https://www.cochranelibrary.com/) were screened using the following terms: ‘p53’, ‘polymorphism’, ‘codon 72’ and ‘prostate cancer’. The inclusion criteria were as follows: i) Studies published in English, with the full-text available; ii) studies that employed a case-control or cohort design; and iii) studies that provided data that enabled the estimation of odds ratios (OR) for survival with 95% CIs. The review process was performed by two independent reviewers. Information, including the first author's name, number of cases and test methods used in each study, was summarized in Table I (21–44). Data extraction was performed by two authors and was subsequently reviewed and compared by different authors. To quantitatively aggregate the results, ORs and their corresponding 95% CIs were pooled to estimate the overall effect. A random effect model was used to calculate the pooled OR. The homogeneity of the present study results was assessed by Q and I2 statistics. All analyses were performed using Review Manager (version 5.3; The Cochrane Collaboration) for Windows.
Cell culture and erastin treatment
The prostate cancer cell lines, namely LnCAP (WT TP53), DU145 (TP53-MT) and PC3 (TP53 null), were obtained from the American Type Culture Collection. The cells were cultured in DMEM (HyClone; Cytiva) supplemented with 10% FBS (HyClone; Cytiva), 100 U/ml penicillin and 100 µg/ml streptomycin. To induce ferroptosis, cells were treated with 10 µM erastin (cat no. E7781; MilliporeSigma), a ferroptosis inducer, for 6 h prior to experimentation.
MTT assay
The Cytotoxicity Assay Kit was purchased from Beyotime Institute of Biotechnology. A 5 mg/ml MTT stock solution was prepared by dissolving 5 mg of MTT powder in 1 ml of sterile DMSO and vortexing for 1–2 min until completely dissolved, which was then aliquoted and stored at −20°C protected from light. A total of 1×103 cells per well were seeded into 96-well plates and allowed to adhere for 48 h at 37°C. Subsequently, each well was supplemented with 20 µl MTT solution and cells were incubated for 4 h at 37°C. To assess cell viability, the absorbance at a wavelength of 570 nm was measured using the TECAN microplate reader (Tecan Group, Ltd.).
Reactive oxygen species (ROS) detection
ROS levels in cells were measured using a ROS detection kit (Beyotime Institute of Biotechnology), according to the manufacturer's protocol. Cells were washed with PBS and incubated with 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA; dilution, 1:1,000) for 30 min at 37°C. Following incubation, ROS levels were analyzed by flow cytometry (Becton, Dickinson and Company).
Western blotting
Cellular proteins were extracted using the Total Protein Extraction Kit (Nanjing KeyGen Biotech Co., Ltd.) and measured using the BCA Protein Assay Kit (Nanjing KeyGen Biotech Co., Ltd.) according to the manufacture's instruction. Total protein extracts (30 µg per lane) were separated by 8% SDS-PAGE and were then transferred to nitrocellulose membranes (Beyotime Institute of Biotechnology). To block non-specific binding, the membranes were treated with 5% milk for 2 h at room temperature, followed by incubation with primary antibodies at 4°C overnight. The primary antibodies used were against TP53 (1:200; cat. no. sc-126; Santa Cruz Biotechnology, Inc.), solute carrier family 7 member 11 (SLC7A11; 1:100; cat. no. 98051; CST Biological Reagents Co., Ltd.), glutathione peroxidase 4 (GPX4; 1:100; cat. no. sc-166570; Santa Cruz Biotechnology, Inc.) and GAPDH (1:5,000; cat. no. sc-74512; Santa Cruz Biotechnology, Inc.). After 24 h, the membranes were incubated with horseradish peroxidase-coupled secondary antibodies including goat anti-mouse IgG (cat. no. A0216), goat anti-rabbit IgG (cat. no. A0208) or donkey anti-goat IgG (cat. no. A0181) antibodies at dilutions ranging from 1:1,000 to 1:2,000 (Beyotime Institute of Biotechnology) at room temperature for 2 h. The protein signals were detected using an enhanced chemiluminescence kit (Beyotime Institute of Biotechnology). The band intensities were quantified using ImageJ software version 1.8.0. (National Institutes of Health) by measuring the grayscale values. The grayscale value of the untreated control group was normalized to ‘1’ and the relative intensities of the bands from the treated groups were calculated accordingly.
Statistical analysis
Data are expressed as the mean ± SD from three independent experiments, each performed in triplicate. The statistical differences between two groups were compared using an unpaired, two-tailed Student's t-test. For comparisons among three or more groups, one-way ANOVA (for normally distributed data) or the Kruskal-Wallis test (for non-normal distributions) was applied, followed by appropriate post hoc tests where significant differences were detected. For datasets with multiple independent variables, two-way ANOVA with Tukey's or Bonferroni correction was used to account for multiple comparisons. P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using SPSS version 26 (IBM Corp.), with results presented along their corresponding significance levels, where applicable.
Results
Roles of TP53 mutation, polymorphism and expression in patients with prostate cancer
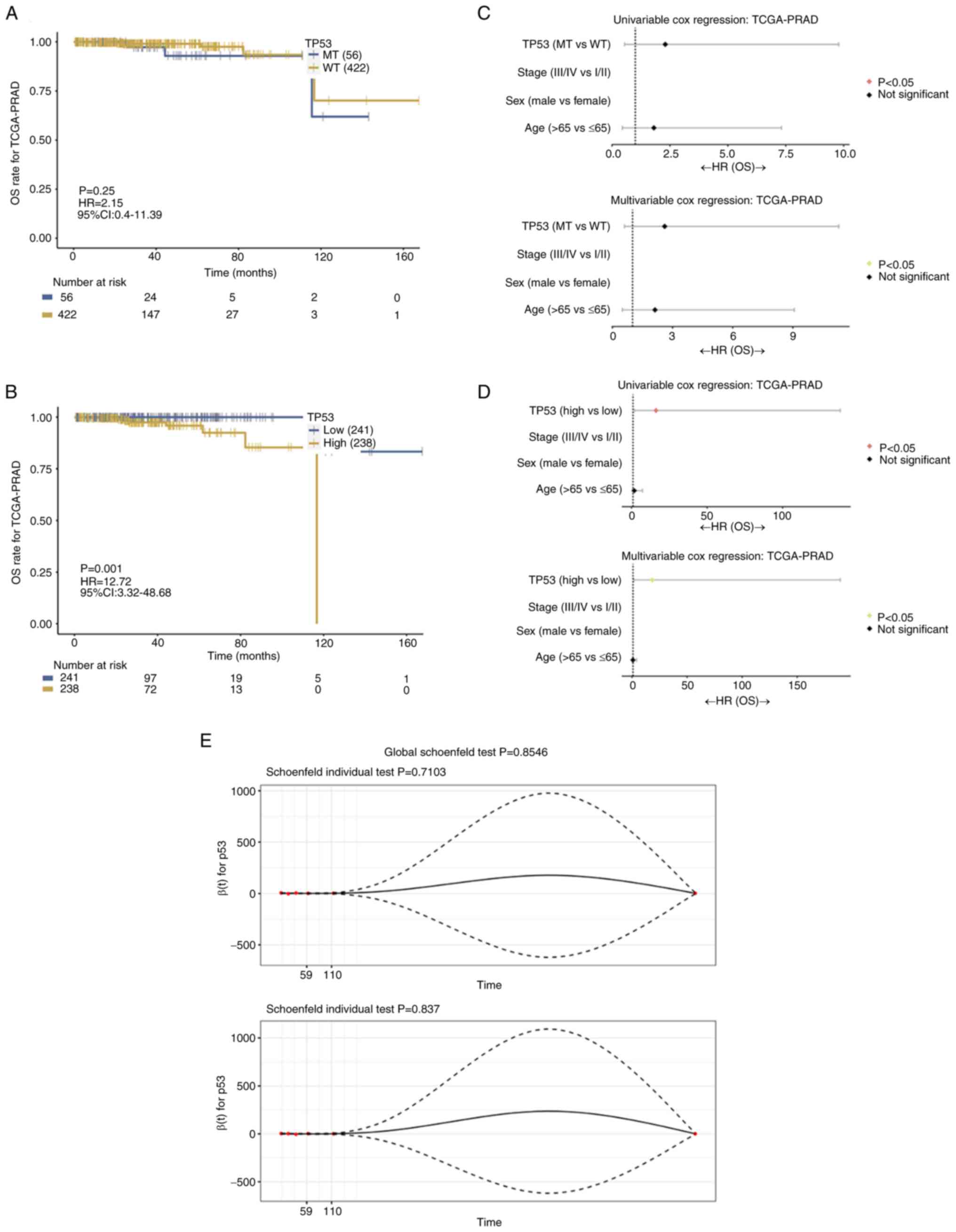
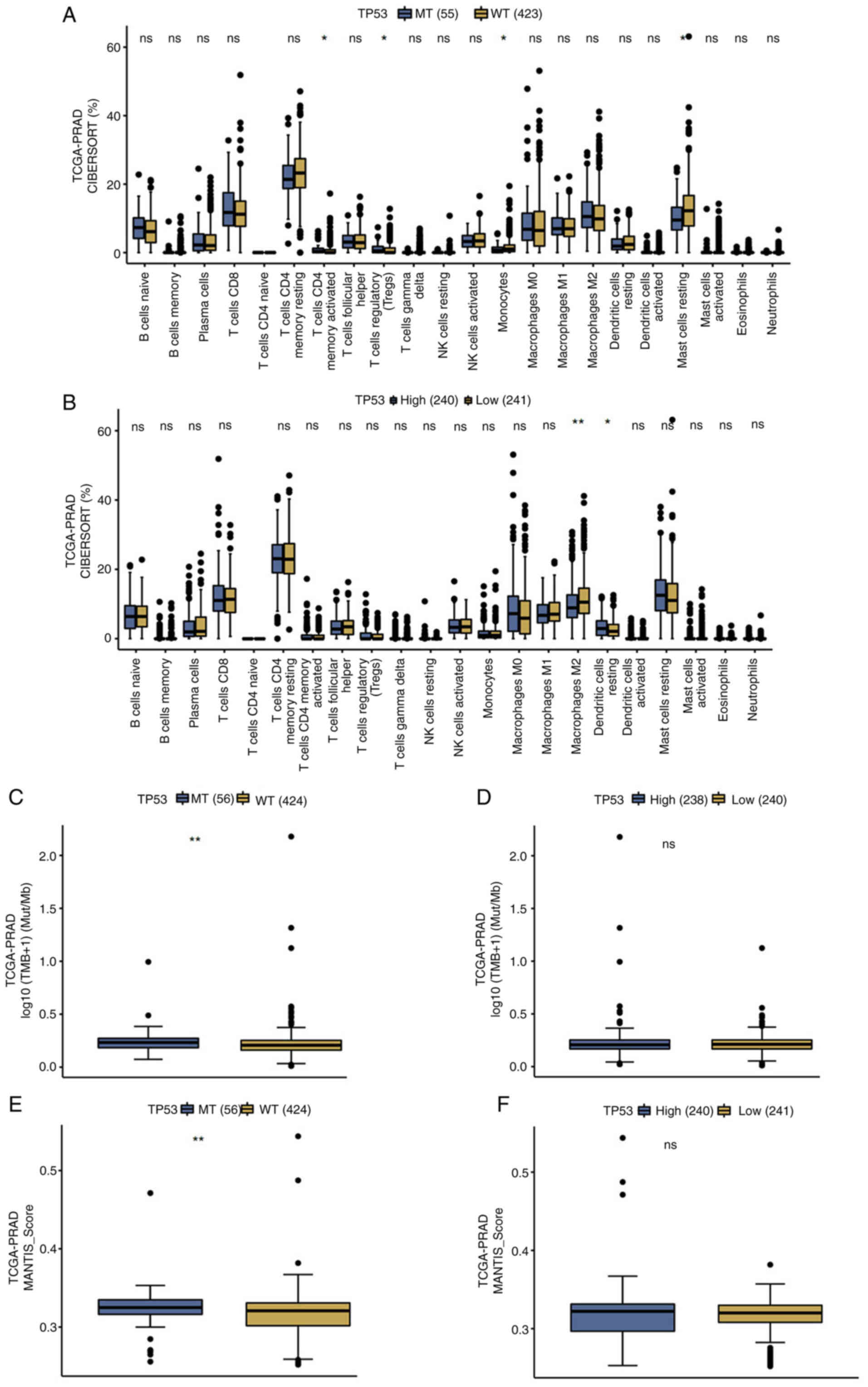
As shown in Fig. 1A, TP53 mutation did not affect the prognosis of patients with prostate cancer (P=0.25). However, high TP53 expression was significantly associated with poorer overall survival (OS) in these patients (P=0.001; Fig. 1B). Therefore, both univariable and multivariable Cox regression analyses identified TP53 expression levels (P>0.05; Fig. 1D), excluding TP53 mutation status (P<0.05; Fig. 1C), as a prognostic factor for OS in patients with prostate cancer. The proportional hazards for both TP53 mutation status and TP53 expression, as well as for the overall model did not indicate a statistically significant difference (P>0.05; Fig. 1E). In addition, CIBERSORT analysis demonstrated that TP53 mutation was significantly associated with a higher presence of activated memory CD4+ and regulatory T cells, fewer monocytes and resting mast cells, compared with WT TP53 tissues (P<0.05; Fig. 2A). Additionally, high TP53-expressing tissues were significantly associated with fewer M2 macrophages and more resting dendritic cells compared with the low TP53-expressing tissues (P<0.05; Fig. 2B). TP53 mutation was associated with higher tumor mutational burden (TMB), which measures the total number of mutations in DNA of prostate cancer and Microsatellite Analysis for Normal-Tumor InStability (MANTIS) scores, which evaluates microsatellite instability by comparing DNA at microsatellite loci, compared with WT TP53 patients (P<0.05; Fig. 2C and E). However, TP53 expression did not affect TMB or MANTIS scores in patients with prostate cancer (P>0.05; Fig. 2D and F).
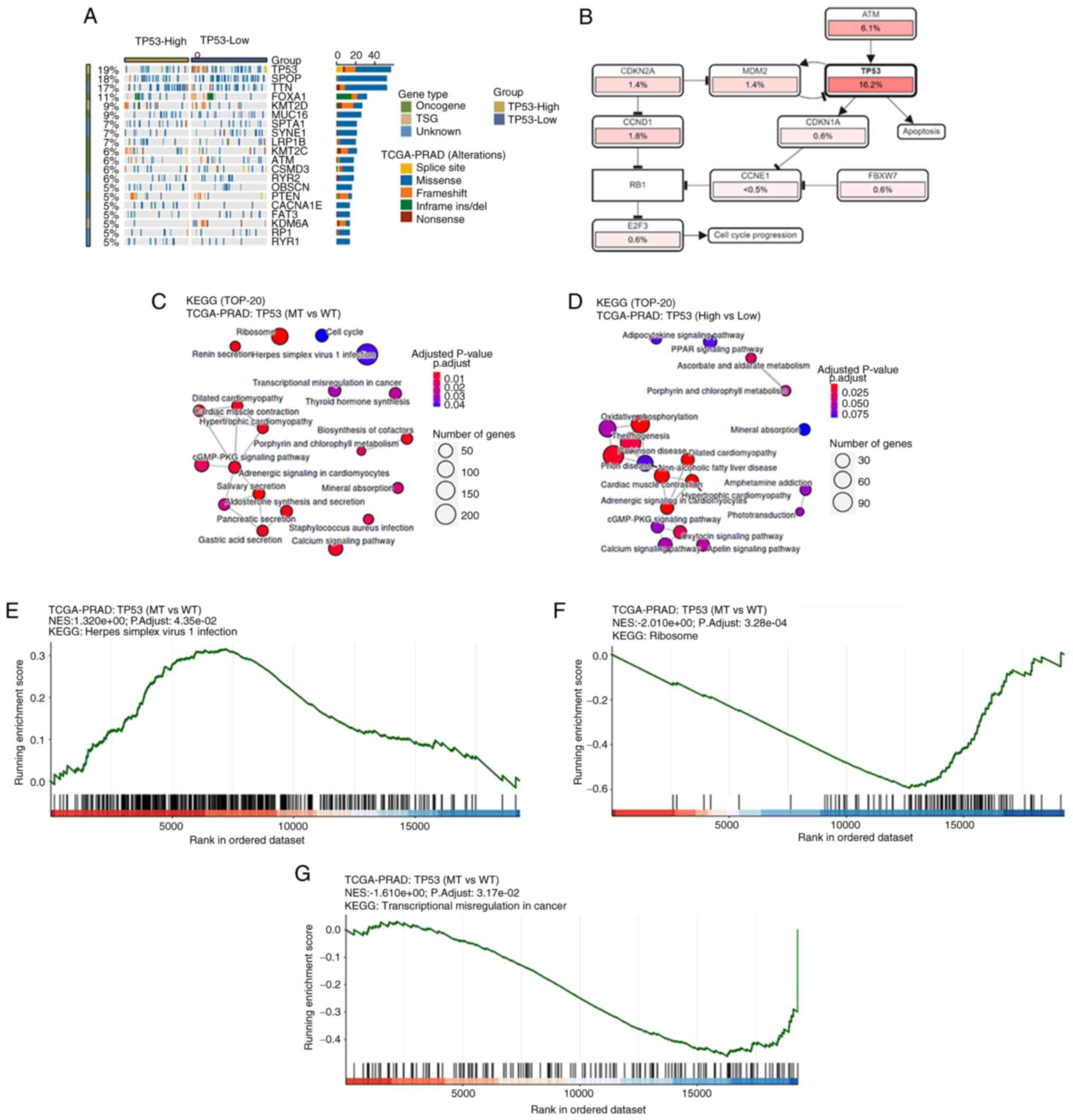
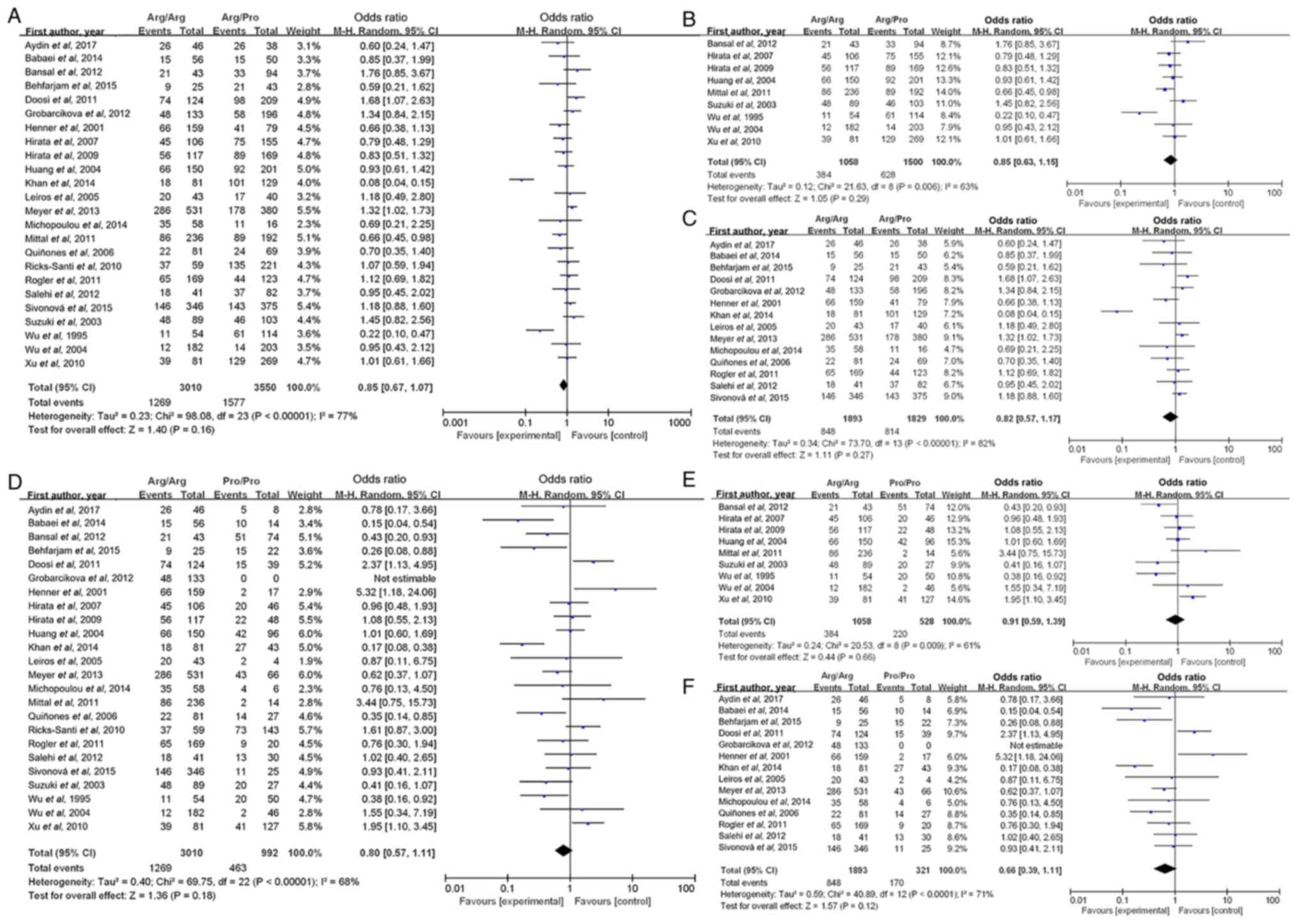
A waterfall plot of common tumor-related mutations in patients with prostate cancer demonstrated stratification based on TP53 expression levels (Fig. 3A). TP53 mutation was associated with altered cell cycle regulation and apoptosis (Fig. 3B). In addition, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis indicated that TP53 mutation status was significantly associated with the ‘cGMP-PKG signaling pathway’ and ‘calcium signaling pathway’ (Fig. 3C), while TP53 expression was associated with the ‘cGMP-PKG signaling pathway’, ‘oxytocin signaling pathway’ and ‘calcium signaling pathway’ (Fig. 3D). KEGG pathway enrichment analysis identified significantly enriched pathways including ‘Ribosome’ (Fig. 3F), ‘Herpes simplex virus 1 infection’ (Fig. 3E) and ‘Transcriptional misregulation in cancer’ (Fig. 3G), which were potentially associated with TP53 mutations or altered expression. Furthermore, the meta-analysis results indicated that the TP53 codon 72 polymorphism was not significantly associated with the risk of prostate cancer in the Arginine (Arg)/Arg vs. Arg/Proline (Pro) (OR, 0.85; 95% CI, 0.67–1.07; P=0.16; Fig. 4A) and Arg/Arg vs. Pro/Pro (OR, 0.80; 95% CI, 0.57–1.11; P=0.18; Fig. 4D) genetic models. The subgroup analyses by ethnicity further indicated that the TP53 codon 72 polymorphism was not significantly associated with prostate cancer risk in either Caucasian (Fig. 4C and F) or Asian patients (Fig. 4B and E).
Roles and mechanisms of TP53 in ferroptosis in prostate cancer cells
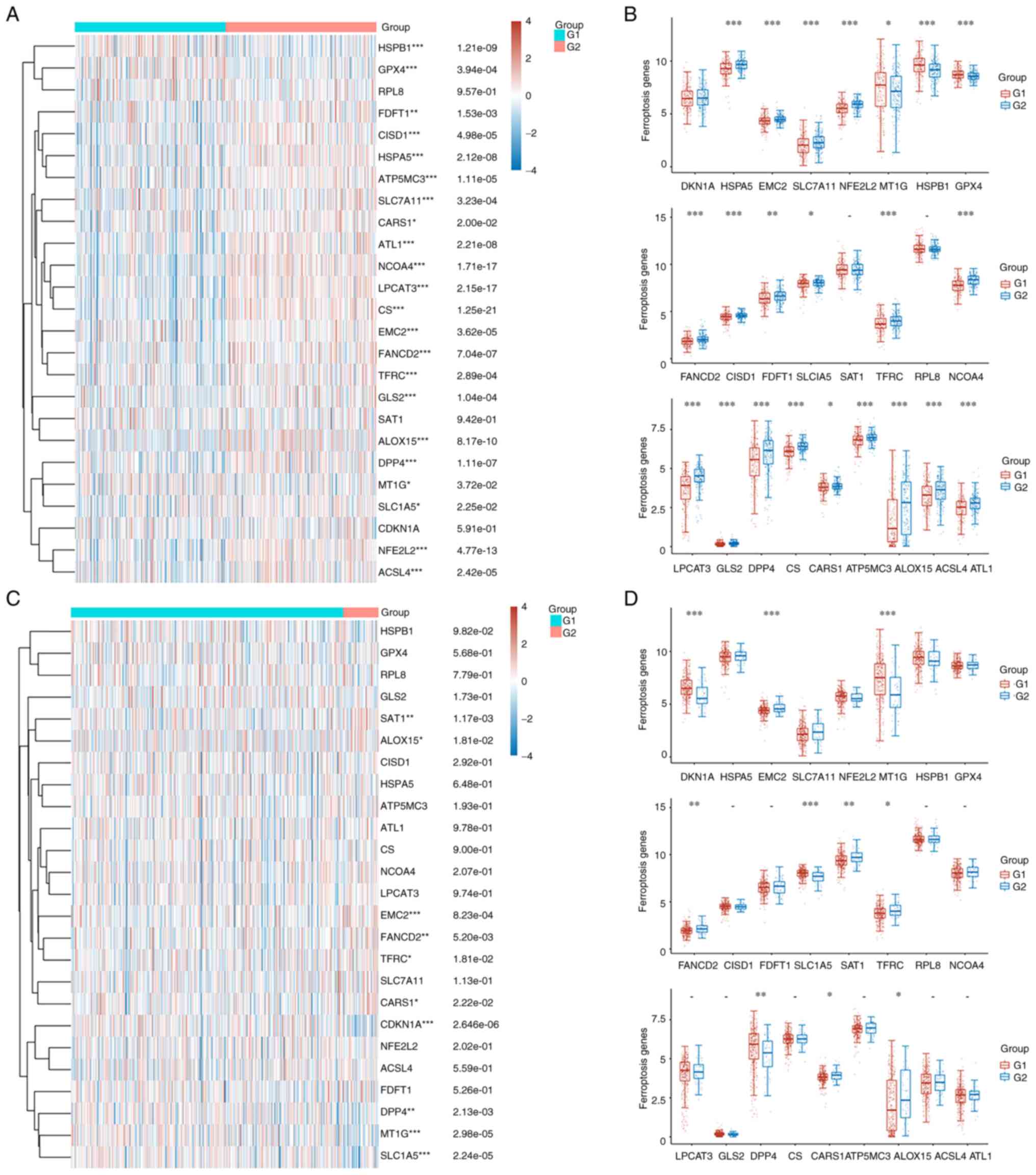
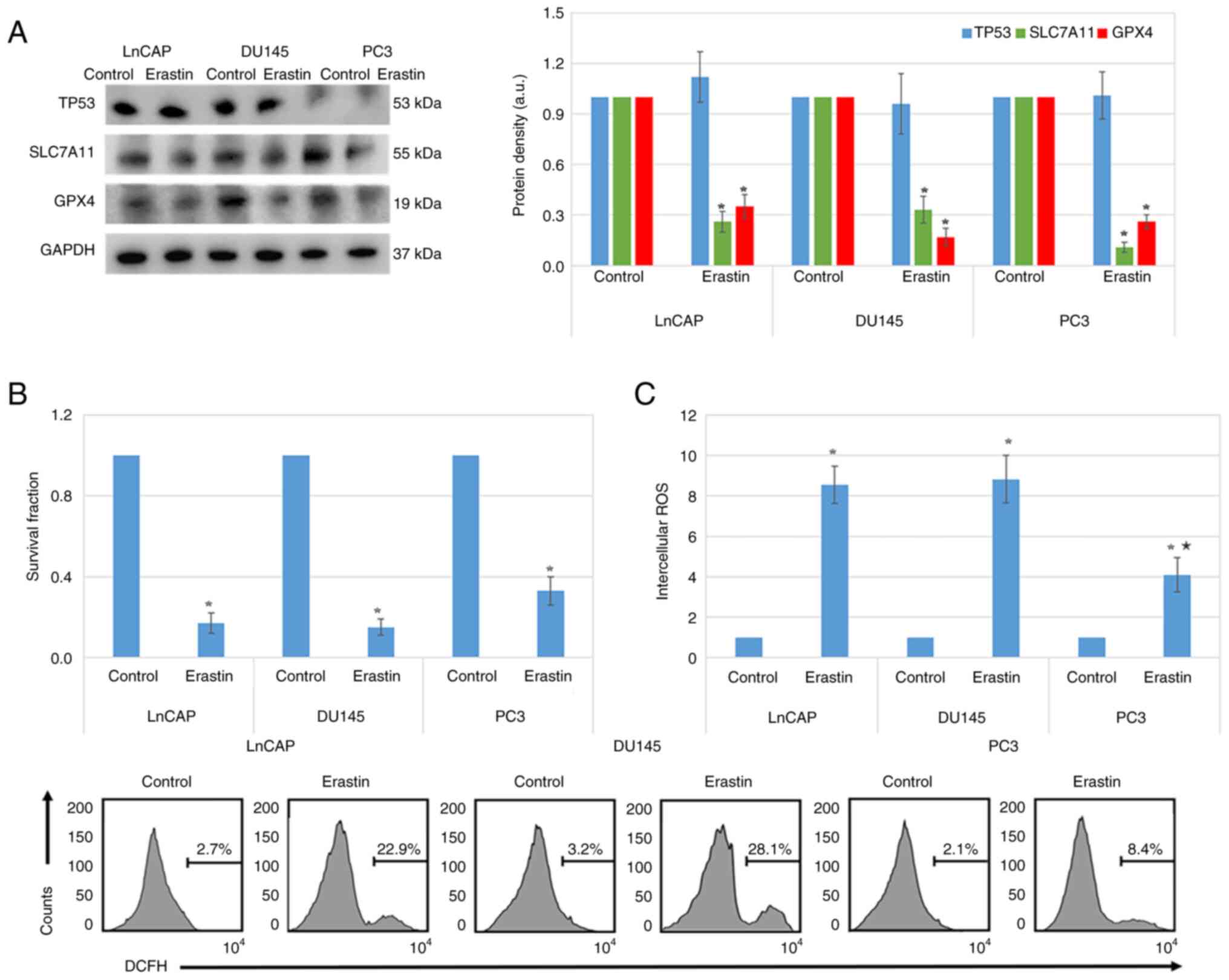
The heatmap in Fig. 5A illustrates the differences in the expression of ferroptosis-related genes between high and low TP53-expressing patients with prostate cancer. Statistically significant differences were observed in the expression of several genes, including HSPB1, GPX4, FDFT1, CISD1, HSPA5, ATP5MC3, SLC7A11, CARS1, ALT1, NCOA4, LPCAT3, CS, EMC2, FANCD2, TFRC, GLS2, ALOX15, DPP4, MT1G, SLC1A5, NFEF2L2 and ACSL4 (P<0.05; Fig. 5A). More particularly, genes such as FDFT1, CISD1, HSPA5, ATP5MC3, SLC7A11, ALT1, NCOA4, LPCAT3, CS, EMC2, FANCD2, GLS2, DPP4, SLC1A5, NFEF2L2 and ACSL4 were upregulated in high TP53-expressing patients compared with the low TP53-expressing patients (P<0.05; Fig. 5B). Conversely, HSPB1, GPX4, CARS1, TFRC, ALOX15 and MT1G were upregulated in the low TP53 expression group (P<0.05; Fig. 5B). In tissues with TP53 mutation, the expression levels of EMC2, FANCD2, SAT1, TFRC, CARS1 and ALOX15 were higher, while those of CDKN1A, MT1G, SLC1A5 and DPP4 were lower compared with WT TP53 prostate cancer tissues (P<0.05; Fig. 5C and D). Furthermore, western blotting verified that erastin treatment inhibited the expression of SLC7A11 and GPX4 in LnCAP, DU145 and PC3 cells (P<0.05; Fig. 6A). In addition, MTT assays demonstrated that erastin treatment significantly inhibited the proliferation of LnCAP, DU145 and PC3 cells (P<0.05; Fig. 6B). ROS levels were also significantly elevated in all three erastin-treated cell lines (P<0.05; Fig. 6C). However, the increase in ROS levels was less pronounced in PC3 cells compared with LnCAP and DU145 cells (P<0.05; Fig. 6C).
Discussion
TP53 serves a key role in cancer development via regulation of cell cycle arrest, DNA repair, aging and apoptosis (4,6). A previous study demonstrated that TP53 is upregulated in prostate cancer tissues, which serves as a marker of poor prognosis in patients with prostate cancer (20). Another previous study reported that TP53 is highly prone to mutation and mutated TP53 commonly results in more aggressive tumor behavior, which promotes cancer progression (8). In the present study, bioinformatics analysis indicated that WT and mutated TP53 expression, but not mutated TP53 expression alone, could be considered as a prognostic marker for patients with prostate cancer. TP53 mutations often result in a loss of tumor suppressor function, but their impact on survival may vary depending on the specific mutation type (for example, missense vs. nonsense mutations) (45,46). By contrast, high TP53 expression levels could be associated to the accumulation of MT p53 proteins, some of which exhibit gain-of-function oncogenic properties, which contribute to poor prognosis (47).
TP53 protein stability and activity are heavily regulated at multiple levels (45). High TP53 mRNA expression does not necessarily equate to functional WT p53 protein activity, especially in cancer types with altered mouse double minute 2 homolog (MDM2)/MDM4 regulation (48). However, TP53 mutation is associated with higher TMB and MANTIS scores (49). MANTIS score is used to assess microsatellite instability status, which has been associated with TP53 mutations in lung cancer as well (50). A previous study also demonstrated that MT TP53 can alter TME by affecting immune cell infiltration, such as that of CD8+ T cells and natural killer (NK) cells and the promotion of M2 macrophage polarization, which supports tumor growth (51,52). Another study also reported that TP53 activation can enhance T-cell infiltration in mouse tumor models (53). While TP53 mutations are associated with an increased number of CD8+ T cells and NK cells in several types of cancer, such breast cancer and lung adenocarcinoma (54,55), an opposite trend is observed in gastric cancer, colorectal cancer, and head and neck squamous carcinoma (56–58).
In the present study, distinct differences in immune cell infiltration were observed. More particularly, TP53-mutated tissues exhibited a higher proportion of activated memory CD4+ T cells, regulatory T cells, monocytes and resting mast cells. Additionally, high TP53-expressing tissues demonstrated an increased infiltration of M2 macrophages and resting dendritic cells. The present study findings suggested that both TP53 expression and mutation could modulate the TME in prostate cancer via recruitment of different immune cell populations.
MDM2 and TP53 are known to form a negative feedback loop that tightly regulates the protein expression levels of TP53 in normal cells (59). Therefore, the activation of TP53 can promote the transcription of MDM2, which in turn ubiquitinates and degrades TP53 (31). The results of the present study also demonstrated that TP53 was associated with MDM2 in prostate cancer, which supports the aforementioned regulatory mechanism. In the present KEGG pathway enrichment analysis, significant enrichment of pathways were observed such as ‘Ribosome’, ‘Herpes simplex virus 1 infection’ and ‘Transcriptional misregulation in cancer’. However, these pathways may not have an immediately apparent direct link to TP53 mutations or expression. TP53 serves a key role in transcriptional regulation and cellular response to viral infections, which could explain the enrichment of virus-related pathways (60). The antiviral function of p53 involves multiple mechanisms that enhance type I interferon production (61,62). p53 transcriptionally upregulates interferon regulatory factors including IRF5, IRF7 and IRF9 to amplify antiviral responses (61,62). This regulation forms a complex network of p53-mediated pathways that collectively strengthen innate immunity against viral infections (61,62). To further validate these findings, GSEA enrichment analysis was performed. These pathways (‘Ribosome’, ‘Herpes simplex virus 1 infection’ and ‘Transcriptional misregulation in cancer’) remained highly enriched within a more rigorous analytical framework. These findings suggested that TP53 mutations may influence multiple pathways through both direct and indirect mechanisms. The enrichment of viral infection-related pathways likely reflected the role of TP53 in antiviral immunity, such as the regulations of interferon signaling or apoptotic pathways (61,62). The association with ribosome pathways indicated that TP53 mutations may affect translational control via mTOR or ribosome biogenesis genes (63,64). Additionally, the enrichment of transcriptional misregulation pathways further confirmed the function of TP53 as a core transcription factor (65). The present study results highlighted the broad regulatory network of TP53 mutations, which warrants further experimental validation to elucidate the underlying mechanisms. Furthermore, a meta-analysis was performed to investigate the association between the TP53 codon 72 polymorphism and susceptibility to prostate cancer. Although previous individual studies demonstrated inconsistent or conflicting findings, the results of the present meta-analysis were consistent with that of previous meta-analyses, which verified that the TP53 codon 72 polymorphism was not significantly associated with prostate cancer risk in either Asian or Caucasian populations (11,66).
Another significant finding of the present study was the role of TP53 in the regulation of ferroptosis in prostate cancer cells. Ferroptosis is a type of non-apoptotic cell death, which is driven by oxidative damage, iron accumulation and lipid peroxidation (67). System xc− is a key antioxidant system composed of SLC7A11 and SLC 3 member 2 (3A2). Previous studies have reported that TP53 can promote ferroptosis via inhibition of SLC7A11 expression (68,69). In the present study, a set of differentially expressed ferroptosis-related genes were identified between high/low and MT/WT TP53-expressing tissues from the TCGA database. Notably, TP53 expression exhibited a more significant effect on the expression of ferroptosis-related genes compared with mutated TP53. To further investigate the role of TP53 in ferroptosis, ROS detection was conducted in three prostate cancer cell lines, namely LnCAP (WT TP53), DU145 (MT TP53) and PC3 (TP53 null). A previous study reported that erastin, a classic ferroptosis inducer, could induce ferroptosis in lung cancer cells via inhibition of System xc− and activation of TP53 (70). Consistent with previous studies (71,72), the results of the present study verified that erastin could inhibit cell proliferation and induce ROS production in prostate cancer cells, regardless of TP53 mutation status. However, ROS levels were significantly higher in TP53-expressing cells compared with TP53-null cells, which indicated that TP53 could partly regulate ROS production in prostate cancer cells. Mechanistically, prostate cancer cell treatment with erastin downregulated SLC7A11 and GPX4. However, the expression levels of TP53 itself did not change significantly.
TP53 regulates several biological processes, such as cell cycle arrest, apoptosis and DNA repair, which enhance tumor cell resistance to different therapies, including radiotherapy, chemotherapy and immunotherapy (65). Currently, the therapeutic potential of TP53 is gaining increasing attention in cancer treatment. In the present study, the effects of TP53 on immune microenvironment and ferroptosis in prostate cancer were assessed using bioinformatics analyses, meta-analysis and experimental validation. The results indicated that the expression levels of TP53 could serve as a poor prognostic marker for patients with prostate cancer, while mutated TP53 and TP53 expression could affect the tumor immune landscape. Therefore, the present study findings could provide novel insights into the development of potential therapeutic targets for prostate cancer in the future.
While the present study provides valuable insights, several limitations should be acknowledged. The number of at-risk patients substantially declined beyond 100 months of follow-up, which led to wider CIs in long-term survival estimates. This attrition is an inherent challenge in prolonged survival analyses, which potentially reduce statistical power and affect the precision of late-phase observations. Meta-analyses are susceptible to several biases that can affect validity, as follows: i) Studies with significant or positive results are more likely to be published; and ii) the restriction to English-only publications in the present meta-analysis introduced a potential language bias.
Further research is warranted to strengthen the present study findings and address current limitations. Firstly, validation in independent, multi-ethnic cohorts are essential to confirm the observed associations while mitigating potential biases from the imbalanced sample sizes between TP53-MT and WT groups. Additionally, large-scale population studies, which incorporate genetic, environmental and lifestyle data could elucidate factors that drive the elevated TP53 mutation prevalence in Chinese patients with prostate cancer. At the mechanistic level, pathway analysis with functional assays can be integrated to systematically investigate how TP53 regulates ferroptosis, which may potentially provide key insights into the molecular underpinnings of this interaction. These findings highlight the critical role of TP53 mutations in prostate cancer progression and their potential as predictive biomarkers for ferroptosis susceptibility, offering new avenues for precision therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Scientific Foundation of China (grant no. 81972784), Liaoning Province Science and Technology Joint Plan (grant no. 2023JH2/101700234) and Tianjin ‘131’ Innovative Talent Project.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
PX conceived, planned and supervised the entire project, performed the experiments and wrote the manuscript. GW and PX performed the experiments, data analysis and interpretation of the results. GW, DL and PX performed the bioinformatics analysis and generated the figures. ZZ and XZ performed the meta-analysis. GW and PX confirmed the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Siegel RL, Miller KD, Fuchs HE and Jemal A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.PubMed/NCBI | |
|
Milliron BJ, Bruneau M, Obeid E, Gross L, Bealin L, Smaltz C and Giri VN: Diet assessment among men undergoing genetic counseling and genetic testing for inherited prostate cancer: Exploring a teachable moment to support diet intervention. Prostate. 79:778–783. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Culp MB, Soerjomataram I, Efstathiou JA, Bray F and Jemal A: Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. 77:38–52. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Baker SJ and Vogelstein B: p53: A tumor suppressor hiding in plain sight. J Mol Cell Biol. 11:536–538. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Vousden KH and Prives C: Blinded by the light: The growing complexity of p53. Cell. 137:413–431. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Hassin O and Oren M: Drugging p53 in cancer: One protein, many targets. Nat Rev Drug Discov. 22:127–144. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Levine AJ: p53: 800 Million years of evolution and 40 years of discovery. Nat Rev Cancer. 20:471–480. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Olivier M, Hollstein M and Hainaut P: TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2:a0010082010. View Article : Google Scholar : PubMed/NCBI | |
|
He Y, Johnson DT, Yang JS, Wu H, You S, Yoon J, Lee DH, Kim WK, Aldahl J, Le V, et al: Loss of the tumor suppressor, Tp53, enhances the androgen receptor-mediated oncogenic transformation and tumor development in the mouse prostate. Oncogene. 38:6507–6520. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Lin HY, Huang CH, Wu WJ, Chang LC and Lung FW: TP53 codon 72 gene polymorphism paradox in associated with various carcinoma incidences, invasiveness and chemotherapy responses. Int J Biomed Sci. 4:248–254. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Lu Y, Liu Y, Zeng J, He Y, Peng Q, Deng Y, Wang J, Xie L, Li T, Qin X and Li S: Association of p53 codon 72 polymorphism with prostate cancer: An update meta-analysis. Tumour Biol. 35:3997–4005. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Han PZ, Cao DH, Zhang XL, Ren ZJ and Wei Q: Association between TP53 gene codon72 polymorphism and prostate cancer risk: A systematic review and meta-analysis. Medicine (Baltimore). 98:e161352019. View Article : Google Scholar : PubMed/NCBI | |
|
Fan S, Hao ZY, Zhang M and Liang CZ: Association between the rs1042522 polymorphism in TP53 and prostate cancer risk: An updated meta-analysis. Chronic Dis Transl Med. 3:95–104. 2017.PubMed/NCBI | |
|
Zhang TW, Wei Y, Pan J, Fang BW, Ye DW and Zhu Y: Clinical features and prognostic value of TP53 mutation in Chinese prostate cancer patients. Zhonghua Wai Ke Za Zhi. 59:897–901. 2021.(In Chinese). PubMed/NCBI | |
|
Stricker HJ, Jay JK, Linden MD, Tamboli P and Amin MB: Determining prognosis of clinically localized prostate cancer by immunohistochemical detection of mutant p53. Urology. 47:366–369. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Maxwell KN, Cheng HH, Powers J, Gulati R, Ledet EM, Morrison C, Le A, Hausler R, Stopfer J, Hyman S, et al: Inherited TP53 variants and risk of prostate cancer. Eur Urol. 81:243–250. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Z, Guo H, Zhu Y, Xia Y, Cui J, Shi K, Fan Y, Shi B and Chen S: TP53 alterations of hormone-naïve prostate cancer in the Chinese population. Prostate Cancer Prostatic Dis. 24:482–491. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Huang H, Tang Y, Li P, Ye X, Chen W, Xie H and Zheng Y: Significance of TP53 and immune-related genes to prostate cancer. Transl Androl Urol. 10:1754–1768. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y, Ma Y and Jiang K: The role of ferroptosis in prostate cancer: A novel therapeutic strategy. Prostate Cancer Prostatic Dis. 26:25–29. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Lin A, Qi C, Wei T, Li M, Cheng Q, Liu Z, Luo P and Zhang J: CAMOIP: A web server for comprehensive analysis on multi-omics of immunotherapy in pan-cancer. Brief Bioinform. 23:bbac1292022. View Article : Google Scholar : PubMed/NCBI | |
|
Aydin M, Bozkurt A, Cikman A, Gulhan B, Karabakan M, Gokce A, Alper M and Kara M: Lack of evidence of HPV etiology of prostate cancer following radical surgery and higher frequency of the Arg/Pro genotype in Turkish men with prostate cancer. Int Braz J Urol. 43:36–46. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Sivoňová MK, Vilčková M, Kliment J, Mahmood S, Jurečeková J, Dušenková S, Waczulíková I, Slezák P and Dobrota D: Association of p53 and p21 polymorphisms with prostate cancer. Biomed Rep. 3:707–714. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Behfarjam F, Rostamzadeh J, Zarei MA and Nikkhoo B: Association of two polymorphic codons in P53 and ABCC1 promoter with prostate cancer. Iran J Biotechnol. 13:49–54. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Babaei F, Ahmadi SA, Abiri R, Rezaei F, Naseri M, Mahmoudi M, Nategh R and Mokhtari Azad T: The TP53 codon 72 polymorphism and risk of sporadic prostate cancer among Iranian patients. Iran J Public Health. 43:453–459. 2014.PubMed/NCBI | |
|
Khan MH, Rashid H, Mansoor Q, Hameed A and Ismail M: Association of the rs1042522 polymorphism with increased risk of prostate adenocarcinoma in the Pakistani population and its HuGE review. Asian Pac J Cancer Prev. 15:3973–3980. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Michopoulou V, Derdas SP, Symvoulakis E, Mourmouras N, Nomikos A, Delakas D, Sourvinos G and Spandidos DA: Detection of human papillomavirus (HPV) DNA prevalence and p53 codon 72 (Arg72Pro) polymorphism in prostate cancer in a Greek group of patients. Tumour Biol. 35:12765–12773. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Meyer A, Coinac I, Bogdanova N, Dubrowinskaja N, Turmanov N, Haubold S, Schürmann P, Imkamp F, von Klot C, Merseburger AS, et al: Apoptosis gene polymorphisms and risk of prostate cancer: A hospital-based study of German patients treated with brachytherapy. Urol Oncol. 31:74–81. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Grobarcikova STR, Dusenka R, Kmetova Sivonova M, Dobrota D and Kliment J: The association of p53 gene polymorphism at codon 72 and prostate cancer risk: Case control study. Urology. 80 (Suppl 3A):S822012. | |
|
Bansal A, Soni A, Rao P, Singh LC, Mishra AK, Mohanty NK and Saxena S: Implication of DNA repair genes in prostate tumourigenesis in Indian males. Indian J Med Res. 136:622–632. 2012.PubMed/NCBI | |
|
Salehi Z and Hadavi M: Analysis of the codon 72 polymorphism of TP53 and human papillomavirus infection in Iranian patients with prostate cancer. J Med Virol. 84:1423–1427. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Mittal RD, George GP, Mishra J, Mittal T and Kapoor R: Role of functional polymorphisms of P53 and P73 genes with the risk of prostate cancer in a case-control study from Northern India. Arch Med Res. 42:122–127. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Rogler A, Rogenhofer M, Borchardt A, Lunz JC, Knoell A, Hofstaedter F, Tannapfel A, Wieland W, Hartmann A and Stoehr R: P53 codon 72 (Arg72Pro) polymorphism and prostate cancer risk: Association between disease onset and proline genotype. Pathobiology. 78:193–200. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Doosti A and Dehkordi PG: The p53 codon 72 polymorphism and association to prostate cancer in Iranian patients. Afr J Biotechnol. 10:12821–12825. 2011. View Article : Google Scholar | |
|
Ricks-Santi L, Mason T, Apprey V, Ahaghotu C, McLauchlin A, Josey D, Bonney G and Dunston GM: p53 Pro72Arg polymorphism and prostate cancer in men of African descent. Prostate. 70:1739–1745. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Xu B, Xu Z, Cheng G, Min ZC, Mi Y, Zhang ZZ, Tao J, Li PC, Wang ML, Tang JL, et al: Association between polymorphisms of TP53 and MDM2 and prostate cancer risk in southern Chinese. Cancer Genet Cytogenet. 202:76–81. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Hirata H, Hinoda Y, Kikuno N, Suehiro Y, Shahryari V, Ahmad AE, Tabatabai ZL, Igawa M and Dahiya R: Bcl2-938C/A polymorphism carries increased risk of biochemical recurrence after radical prostatectomy. J Urol. 181:1907–1912. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Hirata H, Hinoda Y, Kikuno N, Kawamoto K, Dahiya AV, Suehiro Y, Tanaka Y and Dahiya R: CXCL12 G801A polymorphism is a risk factor for sporadic prostate cancer susceptibility. Clin Cancer Res. 13:5056–5062. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Quiñones LA, Irarrázabal CE, Rojas CR, Orellana CE, Acevedo C, Huidobro C, Varela NE and Cáceres DD: Joint effect among p53, CYP1A1, GSTM1 polymorphism combinations and smoking on prostate cancer risk: An exploratory genotype-environment interaction study. Asian J Androl. 8:349–355. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Leiros GJ, Galliano SR, Sember ME, Kahn T, Schwarz E and Eiguchi K: Detection of human papillomavirus DNA and p53 codon 72 polymorphism in prostate carcinomas of patients from Argentina. BMC Urol. 5:152005. View Article : Google Scholar : PubMed/NCBI | |
|
Huang SP, Wu WJ, Chang WS, Wu MT, Chen YY, Chen YJ, Yu CC, Wu TT, Lee YH, Huang JK and Huang CH: p53 Codon 72 and p21 codon 31 polymorphisms in prostate cancer. Cancer Epidemiol Biomarkers Prev. 13:2217–2224. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Wu HC, Chang CH, Chen HY, Tsai FJ, Tsai JJP and Chen WC: p53 gene codon 72 polymorphism but not tumor necrosis factor-alpha gene is associated with prostate cancer. Urol Int. 73:41–46. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Suzuki K, Matsui H, Ohtake N, Nakata S, Takei T, Nakazato H, Okugi H, Koike H, Ono Y, Ito K, et al: A p53 codon 72 polymorphism associated with prostate cancer development and progression in Japanese. J Biomed Sci. 10:430–435. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Henner WD, Evans AJ, Hough KM, Harris EL, Lowe BA and Beer TM: Association of codon 72 polymorphism of p53 with lower prostate cancer risk. Prostate. 49:263–266. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Wu WJ, Kakehi Y, Habuchi T, Kinoshita H, Ogawa O, Terachi T, Huang CH, Chiang CP and Yoshida O: Allelic frequency of p53 gene codon 72 polymorphism in urologic cancers. Jpn J Cancer Res. 86:730–736. 1995. View Article : Google Scholar : PubMed/NCBI | |
|
Lam YK, Yu J, Huang H, Ding X, Wong AM, Leung HH, Chan AW, Ng KK, Xu M, Wang X and Wong N: TP53 R249S mutation in hepatic organoids captures the predisposing cancer risk. Hepatology. 78:727–740. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Raab M, Kostova I, Peña-Llopis S, Fietz D, Kressin M, Aberoumandi SM, Ullrich E, Becker S, Sanhaji M and Strebhardt K: Rescue of p53 functions by in vitro-transcribed mRNA impedes the growth of high-grade serous ovarian cancer. Cancer Commun (Lond). 44:101–126. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Shi W, Wang Y, Zhao Y, Kim JJ, Li H, Meng C, Chen F, Zhang J, Mak DH, Van V, et al: Immune checkpoint B7-H3 is a therapeutic vulnerability in prostate cancer harboring PTEN and TP53 deficiencies. Sci Transl Med. 15:eadf67242023. View Article : Google Scholar : PubMed/NCBI | |
|
Wei Q, Li C, Tang Y, Bai J, Li W, Liu J, Su Z and Cheng X: Mechanistic role of the Mdm2/MdmX Lid domain in regulating their interactions with p53. Biomolecules. 15:6422025. View Article : Google Scholar : PubMed/NCBI | |
|
Bonneville R, Krook MA, Kautto EA, Miya J, Wing MR, Chen HZ, Reeser JW, Yu L and Roychowdhury S: Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017.PO.17.00073. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Li H, Yang L, Wang Y, Wang L, Chen G, Zhang L and Wang D: Integrative analysis of TP53 mutations in lung adenocarcinoma for immunotherapies and prognosis. BMC Bioinformatics. 24:1552023. View Article : Google Scholar : PubMed/NCBI | |
|
Cooks T, Pateras IS, Jenkins LM, Patel KM, Robles AI, Morris J, Forshew T, Appella E, Gorgoulis VG and Harris CC: Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nat Commun. 9:7712018. View Article : Google Scholar : PubMed/NCBI | |
|
Kawashima H, Takatori H, Suzuki K, Iwata A, Yokota M, Suto A, Minamino T, Hirose K and Nakajima H: Tumor suppressor p53 inhibits systemic autoimmune diseases by inducing regulatory T cells. J Immunol. 191:3614–3623. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Klimovich B, Meyer L, Merle N, Neumann M, König AM, Ananikidis N, Keber CU, Elmshäuser S, Timofeev O and Stiewe T: Partial p53 reactivation is sufficient to induce cancer regression. J Exp Clin Cancer Res. 41:802022. View Article : Google Scholar : PubMed/NCBI | |
|
Uddin MB, Roy KR, Hill RA, Roy SC, Gu X, Li L, Zhang QJ, You Z and Liu YY: p53 missense mutant G242A subverts natural killer cells in sheltering mouse breast cancer cells against immune rejection. Exp Cell Res. 417:1132102022. View Article : Google Scholar : PubMed/NCBI | |
|
Kadara H, Choi M, Zhang J, Parra ER, Rodriguez-Canales J, Gaffney SG, Zhao Z, Behrens C, Fujimoto J, Chow C, et al: Whole-exome sequencing and immune profiling of early-stage lung adenocarcinoma with fully annotated clinical follow-up. Ann Oncol. 28:75–82. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Y, Sun Z, Chen W, Liu C, Chai R, Ding J, Liu W, Feng X, Zhou J, Shen X, et al: The immune subtypes and landscape of gastric cancer and to predict based on the whole-slide images using deep learning. Front Immunol. 12:6859922021. View Article : Google Scholar : PubMed/NCBI | |
|
Quandt J, Schlude C, Bartoschek M, Will R, Cid-Arregui A, Schölch S, Reissfelder C, Weitz J, Schneider M, Wiemann S, et al: Long-peptide vaccination with driver gene mutations in p53 and Kras induces cancer mutation-specific effector as well as regulatory T cell responses. Oncoimmunology. 7:e15006712018. View Article : Google Scholar : PubMed/NCBI | |
|
Kong W, Han Y, Gu H, Yang H and Zang Y: TP53 mutation-associated immune infiltration and a novel risk score model in HNSCC. Biochem Biophys Rep. 32:1013592022.PubMed/NCBI | |
|
Chao CC: Mechanisms of p53 degradation. Clin Chim Acta. 438:139–147. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Vieira VC, Leonard B, White EA, Starrett GJ, Temiz NA, Lorenz LD, Lee D, Soares MA, Lambert PF, Howley PM and Harris RS: Human papillomavirus E6 triggers upregulation of the antiviral and cancer genomic DNA deaminase APOBEC3B. mBio. 5:e02234–14. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Yuan L, Chen Z, Song S, Wang S, Tian C, Xing G, Chen X, Xiao ZX, He F and Zhang L: p53 degradation by a coronavirus papain-like protease suppresses type I interferon signaling. J Biol Chem. 290:3172–3182. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Muñoz-Fontela C, Macip S, Martínez-Sobrido L, Brown L, Ashour J, García-Sastre A, Lee SW and Aaronson SA: Transcriptional role of p53 in interferon-mediated antiviral immunity. J Exp Med. 205:1929–1938. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Zang Y, Ran X, Yuan J, Wu H, Wang Y, Li H, Teng H and Sun Z: Genomic hallmarks and therapeutic targets of ribosome biogenesis in cancer. Brief Bioinform. 25:bbae0232024. View Article : Google Scholar : PubMed/NCBI | |
|
Feng Z, Zhang H, Levine AJ and Jin S: The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci USA. 102:8204–8209. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Y, Su Z, Tavana O and Gu W: Understanding the complexity of p53 in a new era of tumor suppression. Cancer Cell. 42:946–967. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang L, Shao N, Yu Q, Hua L, Mi Y and Feng N: Association between p53 Pro72Arg polymorphism and prostate cancer risk: A meta-analysis. J Biomed Res. 25:25–32. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C and Li B: Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J Hematol Oncol. 12:342019. View Article : Google Scholar : PubMed/NCBI | |
|
Liu MR, Zhu WT and Pei DS: System Xc−: A key regulatory target of ferroptosis in cancer. Invest New Drugs. 39:1123–1131. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Liu DS, Duong CP, Haupt S, Montgomery KG, House CM, Azar WJ, Pearson HB, Fisher OM, Read M, Guerra GR, et al: Inhibiting the system xC-/glutathione axis selectively targets cancers with mutant-p53 accumulation. Nat Commun. 8:148442017. View Article : Google Scholar : PubMed/NCBI | |
|
Huang C, Yang M, Deng J, Li P, Su W and Jiang R: Upregulation and activation of p53 by erastin-induced reactive oxygen species contribute to cytotoxic and cytostatic effects in A549 lung cancer cells. Oncol Rep. 40:2363–2370. 2018.PubMed/NCBI | |
|
Sun Y, Deng R and Zhang C: Erastin induces apoptotic and ferroptotic cell death by inducing ROS accumulation by causing mitochondrial dysfunction in gastric cancer cell HGC-27. Mol Med Rep. 22:2826–2832. 2020.PubMed/NCBI | |
|
Wu X, Liu C, Li Z, Gai C, Ding D, Chen W, Hao F and Li W: Regulation of GSK3β/Nrf2 signaling pathway modulated erastin-induced ferroptosis in breast cancer. Mol Cell Biochem. 473:217–228. 2020. View Article : Google Scholar : PubMed/NCBI |