Colon adenocarcinoma hidden behind emphysematous pyelonephritis: A case report
- Authors:
- Published online on: August 4, 2025 https://doi.org/10.3892/ol.2025.15216
- Article Number: 470
-
Copyright: © Huang et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Emphysematous pyelonephritis (EPN) has always been a rare disease since it was first identified, The estimated proportion among all cases of pyelonephritis is between 0.8 and 1.4% (1). EPN is usually caused by gas-producing bacteria, the most common of which include Escherichia coli and Klebsiella pneumoniae. The main characteristic of EPN is the formation of gas in the kidneys or the surrounding area. This disease is generally associated with diabetes, followed by urinary tract obstruction, pregnancy and kidney transplantation (2,3). The clinical manifestations of EPN depend on the degree of infection, and may include fever, chills, vomiting, low back pain, abdominal pain and hematuria. In cases of severe sepsis, patients may have a disturbance of consciousness (4,5). EPN can rapidly develop into septic shock, or even death, if it is not identified early and treated promptly. In extremely rare cases, infection may spread to tissues near the kidneys, causing pneumoperitoneum and pneumatosis intestinalis (6,7). However, clinical cases of EPN derived from digestive tract malignant tumors are extremely unusual. In the present study, the case of a patient with EPN who had no previous medical history of diabetes or urinary tract obstruction is reported. After appropriate antibiotics, fluid resuscitation and drainage treatment, colon adenocarcinoma was finally diagnosed.
Case report
A 57-year-old woman was admitted to the Department of Urology of the Fifth Clinical College of Guangzhou University of Chinese Medicine (Guangdong, China) in February 2021 after presenting to the Emergency Department of the Fifth Clinical College of Guangzhou University of Chinese Medicine with severe right-sided lumbar and abdominal pain and fever. The patient reported a burning pain upon urination and multiple bouts of vomiting before the clinic visit, but had no chest tightness, chest pain, hematuria, diarrhea, constipation or any other discomfort. The patient had no previous medical history of chronic diseases such as diabetes and hypertension. At 6 months prior to admission, the patient underwent a laparoscopic appendectomy for acute appendicitis. No medications had been taken and there was no family history of disease.
On admission, a physical examination revealed a temperature of 42°C (normal range, 36.1–37°C), a heart rate of 105 beats/min (normal range, 60–100 beats/min), a blood pressure of 99/63 mmHg (normal range, 90–139/60-89 mmHg), a respiratory rate of 20 beats/min (normal range, 12–20 beats/min) and a blood oxygen level of 99% (normal range, >95%). The patient's generalized skin was moist and warm, the abdomen was soft and bowel sounds were not hyperactive. On palpation, there was tenderness and rebound tenderness in the right upper abdomen, tenderness in the right costochondral angle and marked percussive pain in the right renal region.
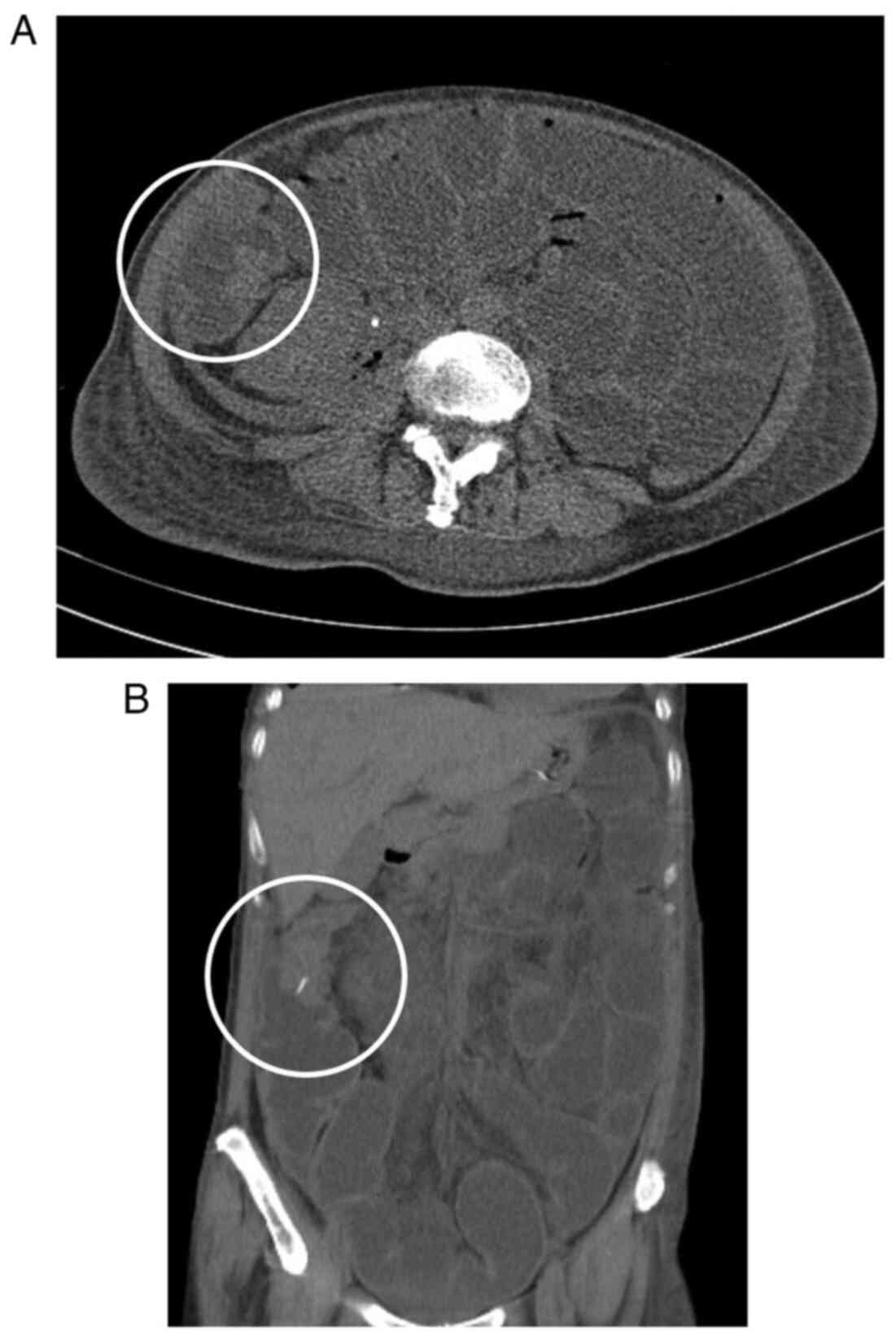
Blood and urine investigations presented some abnormal functional marker results. Laboratory tests showed leukocytosis of 9.75×109 cells/l (normal range, 3.50–9.50×10 cells/l), with 91.7% neutrophils (normal range, 40–75%) and hyperglycemia of 20.58 mmol/l (normal range, 3.9–6.1 mmol/l). Multiple follow-up visits within 33 days from hospitalization to discharge showed blood glucose fluctuations between 3.78 and 6.18 mmol/l. CRP was severely increased at 124.75 mg/l (normal range, 0–3 mg/l) and serum creatinine was decreased at 40.4 µmol/l (normal range, 41–81 µmol/l). The procalcitonin level was 1.31 ng/ml (normal value, <0.05 ng/ml). Routine urinalysis showed a leukocyte level of 15 cells/µl (normal range, 0–10 cells/µl), an extremely high erythrocyte level of 7,238 cells/µl (normal range, 0–10 cells/µl) and positivity for nitrites. Multiple urine culture results revealed an infection with E. coli, a common bacterium in urinary tract infections. This bacterium is resistant to first and second generation cephalosporins, and exhibits third generation ceftriaxone resistance, with susceptibility to cefotaxime, cefepime, enzyme inhibitors and carbapenems (8). During subsequent treatment, Candida albicans was also isolated in March 2021. It was hypothesized that this was due to dysbiosis caused by long-term antibiotic treatment (1.0 g imipenem, intravenous drip once every 8 h for 2 weeks). An abdominal computed tomography (CT) scan showed a diffusely expanded right kidney with reduced density. There was pneumatosis in the right renal collecting system and around the right kidney (Fig. 1). Based on these symptoms, the patient was diagnosed with EPN at the right kidney.
Due to the clear diagnosis of EPN, the patient was administered imipenem (1.0 g, intravenous drip once every 8 h for 2 weeks), a broad-spectrum antibiotic, for anti-infection and hypervolemic treatment. Meanwhile, the patient underwent ureteral stent placement and color-ultrasound guided drainage of the perinephric space with a pigtail catheter. After the aforementioned treatment, the patient's abdominal pain was relieved and levels of inflammatory markers gradually decreased as well. However, fecal water-like drainage fluid appeared in the drainage tube during treatment, and an intestinal obstruction occurred. The CT scan was re-reviewed and suggested a decrease in perirenal gas, but an abnormal mass was found in the hepatic flexure, considered to be a tumor (Fig. 2). To verify the diagnosis, a colonoscopy was performed, which revealed a tumor in the hepatic flexure of the colon. The cell blocks derived from the colonic hepatic flexure were fixed in 10% formalin at room temperature (18–28°C) for 24 h and subsequently processed through routine dehydration and paraffin embedding. Sections were cut at a thickness of 2–4 µm and stained with hematoxylin and eosin (H&E) at room temperature for 45 min. Finally, sections were examined under a light microscope. Specifically, the cells were arranged in clusters resembling glandular structures. These cells exhibited large and deeply stained nuclei, some showing vacuolation. The cells were markedly atypical and displayed visible mitotic figures, suggestive of adenocarcinoma (Fig. 3). Due to the limited quantity of pathological tissues acquired through colonoscopy, it was not feasible to accurately assess the extent of extramural dilation or the potential involvement of the urinary tract system. Finally, further positron emission tomography/CT was performed, suggesting uneven thickening of the intestinal wall at the hepatic flexure of the colon, along with increased metabolic activity. Colon cancer was suspected. The lesion had extended beyond the serosal layer, invading the adjacent peritoneum and infiltrating the right kidney. Additionally, multiple metastatic lesions were identified throughout the abdomen (Fig. 4). Since surgery was no longer possible, the patient was transferred to the Department of Oncology in April 2021 for further treatment and was treated with palliative mFOLFOX6 chemotherapy (14-day cycle). The specific plan was the use of oxaliplatin, leucovorin and a 5-FU bolus on day 1, with a 5-FU 46-h continuous infusion at the start of day 1, ending on day 3. The patient's body surface area is 1.47 m2, and the doses calculated based on BSA were 85 mg/m2 oxaliplatin, 400 mg/m2 leucovorin, 400 mg/m2 for the 5-FU bolus and 2,400 mg/m2 for the 5-FU continuous infusion. Chemotherapy was ineffective, however, and the patient's health declined. In September 2021, the patient passed away 8 months after the diagnosis of colon cancer.
Discussion
First reported in 1898 and named by Schultz and Klorfein in 1962 (9,10), EPN is a life-threatening acute necrotizing infection mainly caused by gas-producing urological pathogens that involve the renal parenchyma, collecting system or perirenal tissues. In total, >90% of EPN infection is associated with diabetes mellitus. EPN has high rates of renal loss and mortality, with the renal loss rate being 50% and the mortality rate being 40–90% (1,5,11–13). The most commonly reported clinical manifestations of EPN include fever, chills, lower back pain, vomiting, hematuria and renal percussion pain. However, these symptoms are not specific and cannot be used to distinguish EPN from typical pyelonephritis (14). At present, CT is the preferred imaging examination method for diagnosing and staging EPN (15). The main therapeutic goal for EPN is to control infection and improve the overall condition of the patient. Internal medical and surgical treatments can be used to control the patient's condition, and internal medical treatments such as broad-spectrum antibiotic therapy, venous transfusion, correction of electrolyte disturbances and glycemic control are preferred. In addition, drug therapy combined with percutaneous drainage can achieve a better therapeutic effect than drug therapy alone. In the worst-case scenario, surgical removal of the kidney may be required (16,17).
The treatment of EPN is mainly founded on the CT-based classification system described by Huang and Tseng (18), where EPN is classified into four categories. As described below, the classification system is divided into four categories: Class 1, gas is present only in the collecting system (i.e., emphysematous pyelitis); class 2, gas is present in the renal parenchyma without extending to the extrarenal space; class 3, extension into the extrarenal space (subclassified into class 3A, extension of gas or abscess to the perinephric space; and class 3B, extension of gas or abscess to the pararenal space); and class 4, bilateral EPN or a solitary functioning kidney with EPN. According to this system, the present patient could be categorized as Class 3A. This patient had already met the Systemic Inflammatory Response Syndrome criteria (19) when admitted to the hospital, with poor hemodynamic status and surgical tolerance. At the time of the present study, emergency nephrectomy had a higher mortality rate than drug therapy combined with percutaneous drainage, and multiple reports indicated that conservative treatment has a lower mortality rate compared with emergency nephrectomy (20–22). In addition, both the patient and their family were resistant to the use of surgical treatment, therefore the patient was treated with drainage and medication (14). The patient's condition was under control after prompt treatment. However, the patient had no history of chronic diseases, such as diabetes and hypertension, and there was no urinary tract obstruction at the time of treatment, leading to uncertainty when considering the cause of the patient's disease. The ultimate element that attracted attention was that the patient developed intestinal obstruction after resuming a normal diet. Furthermore, despite the reduction of pain symptoms in the patient's lower back and abdomen, and the gradual decrease of inflammatory markers, the amount of drainage fluid did not decrease. The subsequent appearance of fecal water-like drainage fluid led to reconsideration of the initial diagnosis, and all of the aforementioned symptoms suggested that the patient had issues in the digestive system. Therefore, a CT re-examination was conducted, focusing on the patient's gastrointestinal condition, and the colon tumor was discovered hidden behind the EPN. In addition to the laboratory tests, changes in clinical symptoms could also provide important information for a diagnosis. Hence, apart for laboratory tests, vigilance is required regarding changes in the clinical symptoms of affected patients.
Since the patient developed intestinal obstruction during treatment, oral contrast agents could not be used to assist diagnosis, and since the patient's family did not ultimately opt for an autopsy, it could only be assumed that the patient's EPN was caused by perforation of the colon tumor according to the drainage fluid. There are several studies and medical reports demonstrating the association between duodenal perforation and changes in the right perirenal hiatus, suggesting that both EPN and duodenal perforation are important causes for the presence of air in the right perirenal hiatus, and that the possibility of both disorders needs to be considered for diagnosis (12,23–25). However, with the appearance of this case, it is hypothesized that when air appears in the right perirenal hiatus, clinicians should be alerted not only to the possibility of duodenal perforation, but also to the possibility of colonic lesions. In addition, malignancy is a known risk factor for pyeloenteric fistulae (26), and it cannot be ruled out that this case was caused by the colon tumor, which caused pyeloenteric fistulae.
Since most malignant tumors progress slowly, the patient's medical record of an appendicitis surgery performed 6 months earlier was reviewed. The patient had definite metastatic right lower abdominal pain, a positive McBurney's sign and elevated inflammatory markers, and ultrasonic testing revealed acute septic appendicitis. The patient underwent laparoscopic appendectomy with a definitive postoperative pathological diagnosis of acute septic appendicitis. When the diagnosis of acute suppurative appendicitis was already clear, in order to reduce the patient's hospitalization costs and potential radiation exposure, CT scanning was not performed (27–29), which might have led to the underdiagnosis of the underlying colon tumor. Therefore, it is suggested that in patients with appendicitis, simultaneous abdominal CT testing is still required, with the purpose of ruling out underlying abdominal disease in addition to clarifying the diagnosis.
To the best of our knowledge, EPN secondary to digestive system diseases is extremely rare, and EPN secondary to colon lesions has not been reported yet. The current case report emphasizes that for patients with EPN of unknown causes, it is necessary to pay attention to changes in clinical symptoms while giving conventional therapy, and to further adjust the diagnosis and treatment if there are clinical changes. In addition, research has shown that the incidence of right kidney EPN (37.7%) is lower than that of left kidney (52.0%) (20) so when facing right kidney EPN, clinicians should also pay attention to whether it is accompanied by duodenal or colon lesions according to its anatomical characteristics.
In conclusion, EPN can be life-threatening and should be taken seriously. The present case reminds us that it is equally important to consider other diagnoses when EPN occurs. Clinicians should consider the possibility of concurrent colon lesions when a patient's condition changes.
Acknowledgements
Not applicable.
Funding
This study was supported by the Scientific Research Project of Traditional Chinese Medicine Research Project of Guangdong Provincial Bureau of Traditional Chinese Medicine (grant nos. 20232003 and 20252002).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
YYH, YSF and DZY conceived and designed the study. YYH, JXL, YSF, BLH, XPZ and DZY acquired, analyzed and interpreted the data. YYH, JXL and YSF drafted and revised the manuscript. XPZ and BLH reviewed the pathological specimens. All authors have read and approved the final manuscript. YYH and DZY confirm the authenticity of all the raw data.
Ethics approval and consent to participate
This study was conducted in accordance with the ethical standards of the Declaration of Helsinki and was deemed exempt from Ethics Review by the Ethics Committee of Guangdong Second Traditional Chinese Medicine Hospital (Guangzhou, China; opinion number: Z202411-003-01). The Fifth Clinical College of Guangzhou University of Chinese Medicine (Guangzhou, China) is the clinical teaching unit of Guangdong Second Traditional Chinese Medicine Hospital. The patient's husband provided written informed consent as approved by the Institutional Review Board.
Patient consent for publication
Written informed consent was obtained from the patient's family for the publication of this case report and any potentially identifiable images or data included in this article.
Competing interests
The authors declare that they have no competing interests.
References
|
Shokeir AA, El-Azab M, Mohsen T and El-Diasty T: Emphysematous pyelonephritis: A 15-year experience with 20 cases. Urology. 49:343–346. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Kaiser E and Fournier R: Emphysematous pyelonephritis: Diagnosis and treatment. Ann Urol (Paris). 39:49–60. 2005.(In French). View Article : Google Scholar : PubMed/NCBI | |
|
Gaither K, Ardite A and Mason TC: Pregnancy complicated by emphysematous pyonephrosis. J Natl Med Assoc. 97:1411–1413. 2005.PubMed/NCBI | |
|
Wu SY, Yang SS, Chang SJ and Hsu CK: Emphysematous pyelonephritis: Classification, management, and prognosis. Tzu Chi Med J. 34:297–302. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Tang HJ, Li CM, Yen MY, Chen YS, Wann SR, Lin HH, Lee SS and Liu YC: Clinical characteristics of emphysematous pyelonephritis. J Microbiol Immunol Infect. 34:125–130. 2001.PubMed/NCBI | |
|
Pandya B, Narang R and Kale S: Emphysematous pyelonephritis presenting with coexistent Pneumatosis intestinalis. IJHS. 2:15–19. 2015. | |
|
Strofilas A, Manouras A, Lagoudianakis EE, Kotzadimitriou A, Pappas A, Chrysikos I and Menenakos E: Emphysematous pyelonephritis, a rare cause of pneumoperitoneum: a case report and review of literature. Cases J. 1:912008. View Article : Google Scholar : PubMed/NCBI | |
|
Sujith S, Solomon AP and Rayappan JB: Comprehensive insights into UTIs: From pathophysiology to precision diagnosis and management. Front Cell Infect Microbiol. 14:14029412024. View Article : Google Scholar : PubMed/NCBI | |
|
Kelly HA and MacCallum WG: PNEUMATURIA. JAMA: The Journal of the American Medical Association. 31:375–381. 1898. View Article : Google Scholar | |
|
Schultz EJ and Klorfein EH: Emphysematous pyelonephritis. J Urol. 87:762–766. 1962. View Article : Google Scholar : PubMed/NCBI | |
|
Ciccarese F, Brandi N, Corcioni B, Golfieri R and Gaudiano C: Complicated pyelonephritis associated with chronic renal stone disease. Radiol Med. 126:505–516. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Ubee SS, Mcglynn L and Fordham M: Emphysematous pyelonephritis. BJU Int. 107:1474–1478. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Falagas ME, Alexiou VG, Giannopoulou KP and Siempos II: Risk factors for mortality in patients with emphysematous pyelonephritis: A meta-analysis. J Urol. 178:880–885. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Koch GE and Johnsen NV: The diagnosis and management of life-threatening urologic infections. Urology. 156:6–15. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Craig WD, Wagner BJ and Travis MD: Pyelonephritis: Radiologic-pathologic review. Radiographics. 28:255–277. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Misgar RA, Mubarik I, Wani AI, Bashir MI, Ramzan M and Laway BA: Emphysematous pyelonephritis: A 10-year experience with 26 cases. Indian J Endocrinol Metab. 20:475–480. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Stone SC, Mallon WK, Childs JM and Docherty SD: Emphysematous pyelonephritis: Clues to rapid diagnosis in the emergency department. J Emerg Med. 28:315–319. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Huang JJ and Tseng CC: Emphysematous pyelonephritis: Clinicoradiological classification, management, prognosis, and pathogenesis. Arch Intern Med. 160:797–805. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Beznoshchenko GB: Systemic inflammatory response syndrome in an obstetric clinic: resolved and unresolved problems. Rossiiskii Vestnik Akushera-ginekologa. 4:6–10. 2018.(In Russian). View Article : Google Scholar | |
|
Aboumarzouk OM, Hughes O, Narahari K, Coulthard R, Kynaston H, Chlosta P and Somani B: Emphysematous pyelonephritis: Time for a management plan with an evidence-based approach. Arab J Urol. 12:106–115. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Kapoor R, Muruganandham K, Gulia AK, Singla M, Agrawal S, Mandhani A, Ansari MS and Srivastava A: Predictive factors for mortality and need for nephrectomy in patients with emphysematous pyelonephritis. BJU Int. 105:986–989. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Ahlering TE, Boyd SD, Hamilton CL, Bragin SD, Chandrasoma PT, Lieskovsky G and Skinner DG: Emphysematous pyelonephritis: A 5-year experience with 13 patients. J Urol. 134:1086–1088. 1985. View Article : Google Scholar : PubMed/NCBI | |
|
Mehdi S, Singh V, Sinha RJ and Pandey S: Concealed diagnosis of duodenal perforation in a patient with emphysematous pyelonephritis: The dilemma of air in the right perirenal space. BMJ Case Rep. 12:e2286292019. View Article : Google Scholar : PubMed/NCBI | |
|
Yagan N, Auh YH and Fisher A: Extension of air into the right perirenal space after duodenal perforation: CT findings. Radiology. 250:740–748. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Grayson DE, Abbott RM, Levy AD and Sherman PM: Emphysematous infections of the abdomen and pelvis: A pictorial review. Radiographics. 22:543–561. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Gill HS: Diagnosis and surgical management of Uroenteric Fistula. Surg Clin North Am. 96:583–592. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Talan DA and Di Saverio S: Treatment of acute uncomplicated appendicitis. N Engl J Med. 385:1116–1123. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Di Saverio S, Podda M, De Simone B, Ceresoli M, Augustin G, Gori A, Boermeester M, Sartelli M, Coccolini F, Tarasconi A, et al: Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg. 15:272020. View Article : Google Scholar : PubMed/NCBI | |
|
Hwang ME: Sonography and computed tomography in diagnosing acute appendicitis. Radiol Technol. 89:224–237. 2018.PubMed/NCBI |