Novel biomarkers for prognosis in patients with localized prostate cancer
- Authors:
- Published online on: September 4, 2025 https://doi.org/10.3892/ol.2025.15258
- Article Number: 512
Abstract
Introduction
Prostate cancer (PCa) represents the second leading cause of cancer-related deaths among men despite the significant decrease of approximately 50% in PCa-related deaths since the early 1990s (1,2). The standard-of-care for patients with prostate gland-restricted disease has demonstrated clinical efficacy, though with no improvements regarding the prognosis for advanced PCa (1–3). A significant number of PCa patients with localized disease will experience recurrences, leading to advanced disease stages and mortality (4). Therefore, identifying reliable prognostic biomarkers in PCa is crucial for the design of appropriate therapeutic strategies to improve clinical outcomes and minimize side effects. While the existing clinicopathological parameters, such as prostate-specific antigen (PSA) values and Gleason score (GS), provide some level of risk stratification, they are still insufficient in precisely predicting clinical outcomes (5,6).
The vital importance of the immune system in cancer is universally acknowledged today. The ongoing, dynamic interaction between cancer cells and various immune cells significantly affects clinical outcomes (7,8). In addition, cancer progression involves changes in several crucial regulatory factors that downregulate antitumor immunity, allowing the escape of malignant cells from immune surveillance (9–12). We (13,14) and others (15) have identified increased serum levels of the HER-2/neu extracellular domain (HER-ECD) in patients with advanced breast and prostate cancer, which were correlated with increased immune suppression in the periphery. Studies have demonstrated that HER-ECD serum concentrations are elevated in individuals with prostate cancer and serve as an independent prognostic indicator linked to an increased likelihood of biochemical recurrence (15). Building on these observations, we measured plasma HER-ECD levels both at study entry and following the completion of our vaccine regimen in a phase I clinical trial (14). We observed a statistically significant, vaccine-induced reduction in circulating HER-ECD, which associated with more favorable clinical outcomes (14). Furthermore, HER-ECD levels in the serum have been widely investigated as a potential predictive biomarker in breast cancer patients undergoing trastuzumab therapy (16). A recent pooled analysis of seven first-line trastuzumab trials (with or without chemotherapy), including serial serum HER-ECD assessments, found that patients exhibiting minimal reductions in HER-ECD levels experienced less clinical benefit (17). In our previous study (13), we detected a strong positive association between the percentage down-regulation in T regulatory suppressor cell frequency and the percentage decrease in plasma HER-ECD during trastuzumab therapy.
TGFβ exerts diverse effects on adaptive immunity, notably modulating both effector and regulatory CD4+ T cell responses and predominantly serving as an immunosuppressive agent (18). In addition, TGFβ is a key regulator in carcinogenesis (19), and loss of TGFβ sensitivity in carcinoma cells is frequently accompanied by increased expression of TGFβ in the same cells (20). TGFβ levels are elevated in cancer cells relative to normal epithelial cells and rise even further in poorly differentiated tumors. In prostate cancer, heightened local TGFβ expression correlates with greater tumor grade, increased invasiveness, and progression to metastasis (21). In our previous phase I clinical trial, we demonstrated that PCa patients responding- both immunologically and clinically- to a modified HER-2/neu vaccine had decreased plasma levels of HER-ECD along with decreased levels of TGFβ (14). Retrospective analyses revealed that increased preexisting immunity to the vaccine and decreased plasma TGFβ levels correlated with delayed-type hypersensitivity reactions and overall survival (22). In addition, we have reported that vaccine responders had preexisting T-cell immunity to the vaccine, as well as for additional HER-2/neu and PSA peptides, including the peptide PSA(153–161) (23). Furthermore, we found an inverse association between circulating levels of interferon-gamma (IFNγ) and TGFβ in determining both immunological and clinical responses to the vaccine (17,23,24).
IL-8, a major pro-inflammatory chemokine, is upregulated in prostate cancer, as well as in breast, lung, and pancreatic cancers, where it drives tumor cell proliferation and migration (25–28). In our latest findings, prostate cancer patients who exhibited strong preexisting CD8+ T-cell responses against the HER-2/neu(780–788) peptide also displayed low circulating levels of TGFβ and IL8 and were characterized by longer survival. Conversely, those with elevated TGFβ and IL8 levels had also weak HER-2/neu(780–788)-specific CD8+ immunity, and poorer survival outcomes (24).
In the present study, we analyzed the prognostic role of HER-ECD, TGFβ, and IL-8 plasma levels along with the frequencies of total CD8+ and PSA(153–161)-specific T-cell subsets in patients with localized PCa (LPCa).
Materials and methods
Patients
A total of 139 patients diagnosed with LPCa at the Saint Savas Cancer Hospital (Athens, Greece) were enrolled in this study after the provision of written informed consent. The study received approval from the hospital's Institutional Review Board (IRB-ID6777/14-06-2017) as well as the Ethical Committee of the National and Kapodistrian University of Athens (IRBID1516015872/03-02-2016; Athens, Greece). All participants underwent standard medical treatment following their diagnosis. Detailed medical records, including prostate cancer characteristics and any prior therapeutic regimens, were documented. The median patient age at diagnosis was 72 years, with a range of 42–84 years. The patients were monitored for survival rates with clinical endpoints i) biochemical recurrence (BCR) post radical prostatectomy (RP) and ii) castration-resistance (CR) after the start period of hormonal treatment. The patients' clinicopathological data and prior treatment characteristics are presented in Table I.
Table I.Clinicopathological and treatment characteristics of the enrolled localized prostate cancer patients (n=139). |
Blood sample collection and isolation of peripheral blood mononuclear cells
This was performed as we recently described (24). Briefly, 20 ml of blood were collected at the time of enrollment, and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll (Biochrom, Holliston, MA, USA) gradient separation at room temperature (RT), washed twice with phosphate buffered saline (PBS), and counted in a Neubauer chamber (Poly-optik GmbH, Bad Blankenburg, Germany). The cell viability was always >95%. The cells were resuspended in RPMI-1640 with 20% fetal calf serum (FCS) (all from Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 10% dimethyl sulfoxide (DMSO; Applichem GmbH, Darmstadt, Germany) at a concentration of 10×106/ml, separated into 1 ml-containing cryovials (Thermo Fisher Scientific, Inc., Waltham, MA, USA), transferred at −80°C overnight and then stored in liquid nitrogen until use.
Flow cytometry
For the flow cytometry analysis, frozen PBMCs were thawed in pre-warmed culture medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA), and the number of cells was determined by microscopy. PBMCs were washed with PBS supplemented with 5% FCS and incubated with anti-CD14-BV510 (Clone: 63D3) for the exclusion of monocytes and anti-CD19-BV510 (Clone: H1B19) for the exclusion of B-cells (both from Biolegend, San Diego, CA, USA). The cells were also incubated with anti-CD3-PE/Cy7 (Clone: UCHT1), anti-CD8-APC/Cy7 (Clone: SK1), anti-CD28-PerCP/Cyanine5.5 (CD28.2), anti-CD45RA-Alexa Fluor 700 (Clone: HI100) and anti-CCR7-PE/Dazzle™ 594 (Clone: G043H7) (all from Biolegend) for 20 min in the dark at RT, for the identification of specific CD8+ T-cell (CD3+) subsets as follows: naïve (CD45RA+ CCR7+ CD28+), central memory (CM; CD45RA− CCR7+ CD28+), effector memory (EM; CD45RA− CCR7− CD28+), and terminal effector memory (TEMRA; CD45RA+ CCR7− CD28−). Staining of the PSA(153–161) peptide-specific (HLA-A24-restricted) CD8+ T-cells was performed using the dextramer A*02402- PSA(153–161) (CYASGWGSI)-FITC, as previously described (23). Finally, cells were washed twice and immediately analyzed (6×104 CD8+ T-cells per sample) by flow cytometry (FACSAria III, BD, Franklin Lakes, NJ, USA). Data analysis was performed using the Infinicyt 2.0.6 software (Cytognos S.L., Salamanca, Spain).
Quantification of TGFβ, IL-8, and HER-ECD
Plasma was isolated from patients' blood that was previously stored in BD Vacutainer™ K2E (EDTA) Plus Blood Collection Tubes (BD), after centrifugation at 2,000 × g for 10 min at RT, and was transferred to −20°C until use. IL-8 and TGFβ were measured by commercially available ELISA kits (for IL-8, Diaclone, Besancon Cedex, France; for TGFβ, R&D Systems, Minneapolis, MN, USA). HER-ECD was measured using the automated chemiluminescence immunoassay (CLIA) analyzer MAGLUMI 800 (Snibe Co., Ltd., Shenzhen, China).
Statistical analysis
The GraphPad Prism v.8.0 software was used for the statistical analysis of the data. Kaplan-Meier analysis with 95% confidence intervals (95% CIs) and the log-rank (Mantel-Cox) test were used for the evaluation of the association between either TGFβ, IL-8, and HER-ECD levels or low vs high GS and patients' survival. Statistically significant differences in cytokine and HER-ECD levels and in CD8+ T-cell subsets between different patient groups were identified by applying non-parametric Mann-Whitney unpaired tests. P<0.05 was considered to indicate a statistically significant difference.
Results
TGFβ, HER-ECD, and IL-8 levels in the peripheral blood are associated with the survival of LPCa patients
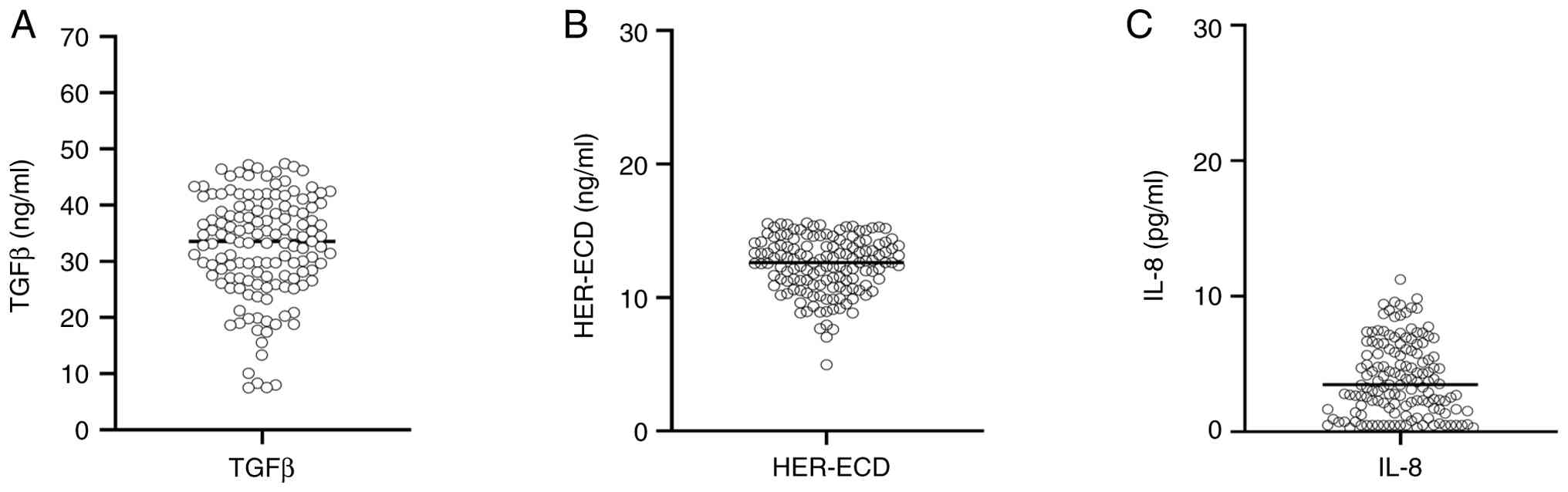
We analyzed TGFβ, HER-ECD, and IL-8 levels in the peripheral blood of patients with LPCa who had reached BCR and CR as clinical endpoints. TGFβ levels ranged between 7.5–47.39 ng/ml with a median of 33.51 ng/ml (Fig. 1A). The respective values for HER-ECD were 4.97–15.59 ng/ml, with a median of 12.63 ng/ml (Fig. 1B), and for IL-8, 1.3–11.25 pg/ml, and a median of 3.47 pg/ml (Fig. 1C).
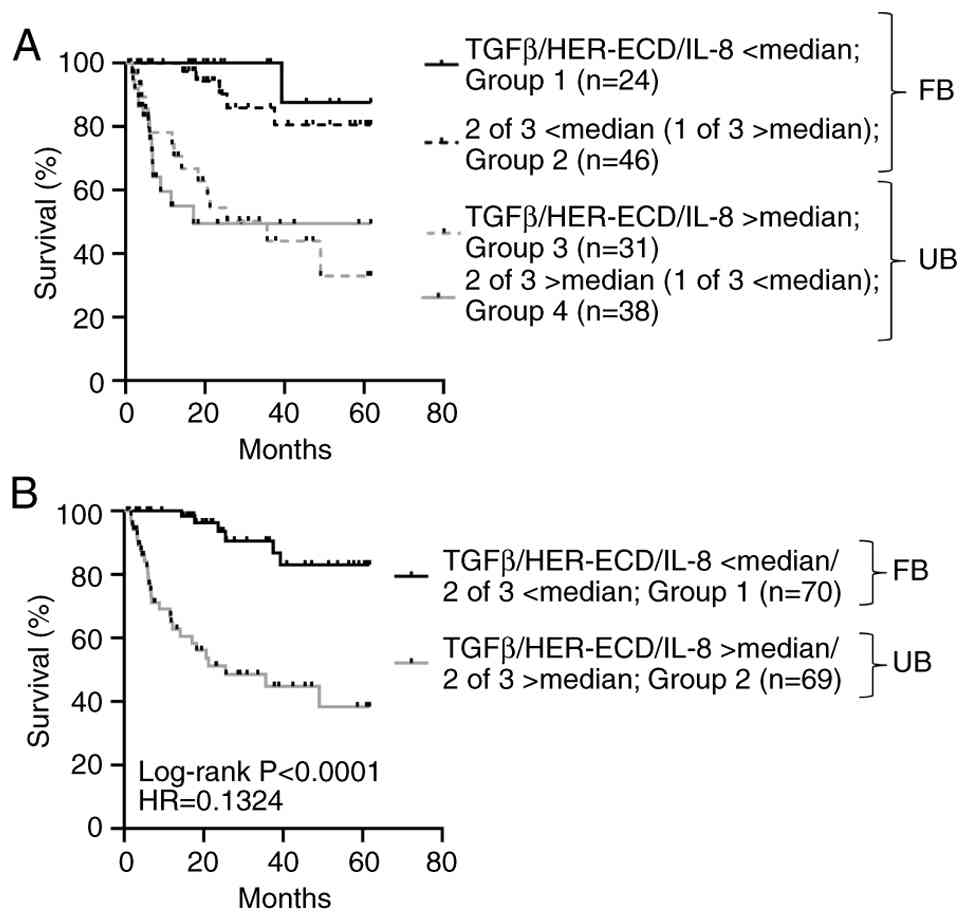
Progression-free survival (PFS), defined as time-to-progression from RP to BCR and from castration-sensitive disease to CR, was strictly dependent on the levels of these suppressive factors. Thus, survival was considerably higher in those patients having levels of all three factors below median (n=24) vs. patients with levels above median (n=31; P=0.0005) or vs. patients exhibiting levels of any two of the three factors above median (n=38; P=0.0006) (Fig. 2A). In addition, patients expressing even two of the three factors below median (n=46) had significantly higher survival compared to those having all three factors or any two of the three factors above median (P=0.0001 and P<0.0001, respectively) (Fig. 2A). Based on the above observations, we jointly analyzed patients with high survival rates and all three, or two of the three factors below median (patients with the FB) (n=70) against those with low survival rates and all three, or any two of the three factors above median (patients with the UB) (n=69). Survival curves for these two groups are shown in Fig. 2B (P<0.0001).
Circulating CD8+ T-cell subset densities in LPCa patients with low vs. high survival
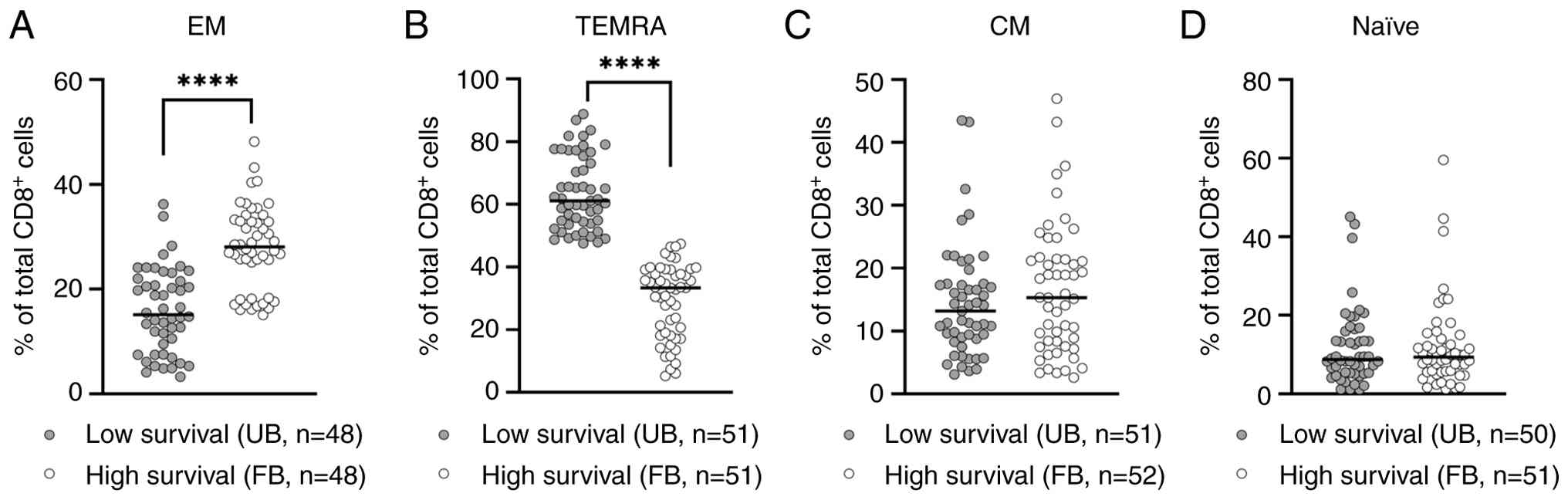
In our previous (14,17,23) and recent studies (24,29), we have demonstrated the essential role of CD8+ T-cell immunity in disease progression of PCa patients receiving immunotherapy or standard treatments. Thus, it was interesting to explore the association between CD8+ T-cell subsets' frequencies and survival rates in our LPCa patient groups with the FB or the UB, respectively. Initial gating of PBMCs was performed based on their FSC/SSC properties, followed by their subgating to CD3+ lymphocytes, which were further subgated to CD8+ T lymphocytes. Antigen-specific CD8+ T cells were detected using the peptide-MHC dextramer PSA153-161 and the identification of specific CD8+ T-cell (CD3+) subsets was performed as described above (Fig. S1). As depicted in Fig. 3A, the densities of CD8+ EM T-cells were exceedingly higher in the FB group of patients with high survival as compared to the group of patients with low survival rates (the UB group) (P<0.0001). Inversely, the densities of CD8+ TEMRA cells were at significantly higher (P<0.0001) levels in the patients belonging to the low survival group compared to those in the high survival group (Fig. 3B). No statistically significant differences in the densities of CD8+ central memory (CM) and CD8+ naïve (N) T-cells could be detected between these patient groups (Fig. 3C and D). Our results led us to the hypothesis that the less suppressive milieu in the peripheral blood of patients with the FB enabled a more potent antitumor immunity in the context of higher densities of EM in parallel with lower densities of TEMRA CD8+ T-cells, resulting in higher survival rates.
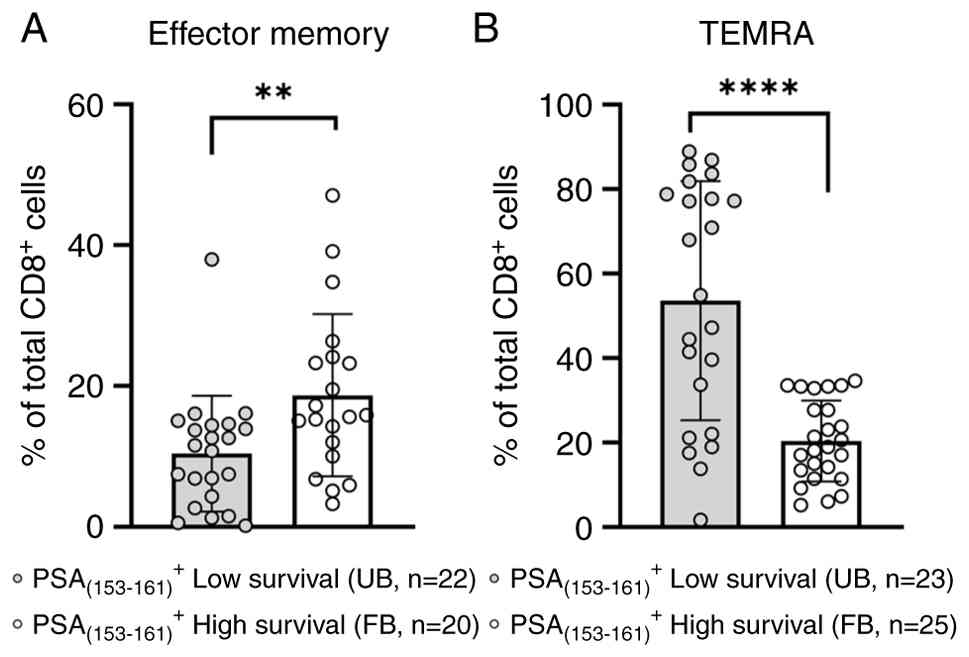
This hypothesis was supported by assessing densities of these CD8+ T-cell subsets that specifically recognize the immunogenic peptide PSA(153–161) (23). As shown in Fig. 4A, EM CD8+ T-cells in the group of patients with the FB and high survival recognized this peptide at much higher frequencies as compared to their counterparts in the group of patients with the UB and low survival (P=0.0058). On the other hand, the PSA(153–161)-specific CD8+ TEMRA T-cells were at significantly higher densities (P<0.0001) in the low survival patient group vs. those quantified among patients belonging to the high survival group (Fig. 4B).
Densities of EM and TEMRA CD8+ T-cell subsets and survival rates are independent of the GS in LPCa patients with the FB
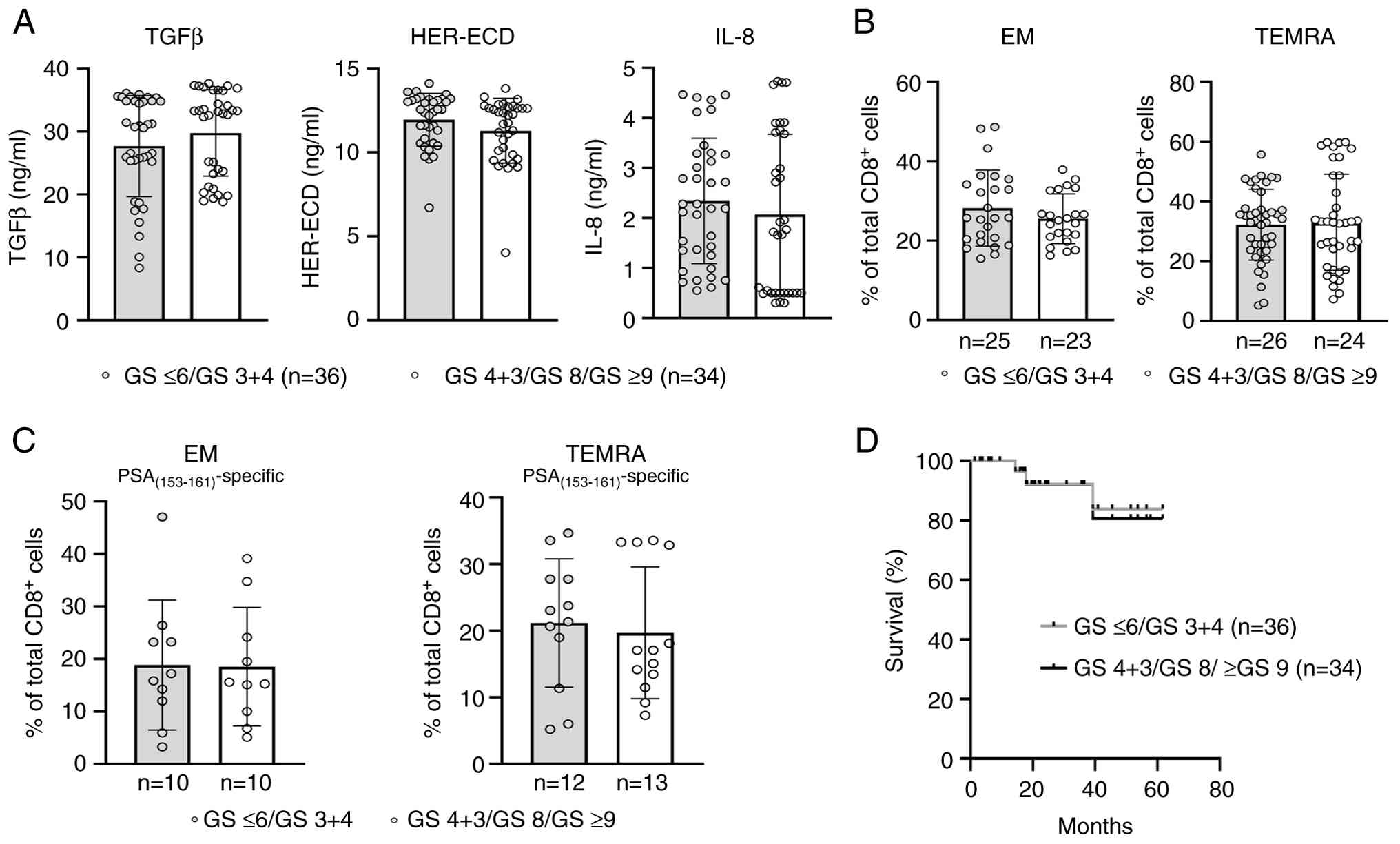
By reviewing the GS among our patients belonging to the FB group, we found that 36 patients had a low GS (i.e., GS 3+4 and GS ≤6), whereas 34 of them exhibited higher tumor grades (i.e., GS 4+3; GS 8 and GS ≥9). When analyzed separately, these two FB subgroups did not display any statistically significant differences in the levels of TGFβ, HER-ECD, or IL-8 (Fig. 5A). Moreover, the frequencies of total or PSA(153–161)-specific EM and TEMRA CD8+ T-cell subsets were detected at similarly high vs. low levels, respectively (Fig. 5B and C). Likewise, the survival rates were equally high between the FB/low GS and FB/high GS groups (Fig. 5D).
These data suggest that the association of the FB with a favorable antitumor CD8+ T-cell immunity results in improved survival rates irrespective of the GS. It should be noted that the majority of patients enrolled in this study had PSA values ≤20 ng/ml (105 of 139; 75.54%) (Table I). Similarly, the majority of patients belonging to the FB group (54 of 70; 77.14%) had PSA values ≤20 ng/ml and were almost equally distributed between patients with low GS (n=29) and high GS (n=25). In addition, 10 of the remaining 16 patients in this group with PSA values >20 ng/ml had a high GS, while the rest 6 patients had a low GS. A similar case was also noted when looking at the clinical T-stage distributions for the FB group. Of the 54 patients with PSA values ≤20 ng/ml, 30 patients (55.55%) had T1c and T2, and the remaining 24 patients (44.45%) were classified with T3a and T4. In addition, of the 16 patients with PSA values >20 ng/ml, 7 patients had T1c and T2 (43.75%), whereas 9 patients (56.25%) had T3a and T4. Such PSA and T-stage distributions among patients belonging to the FB group provided a significant obstacle in correlating these two clinical parameters with T-cell subset frequencies and survival.
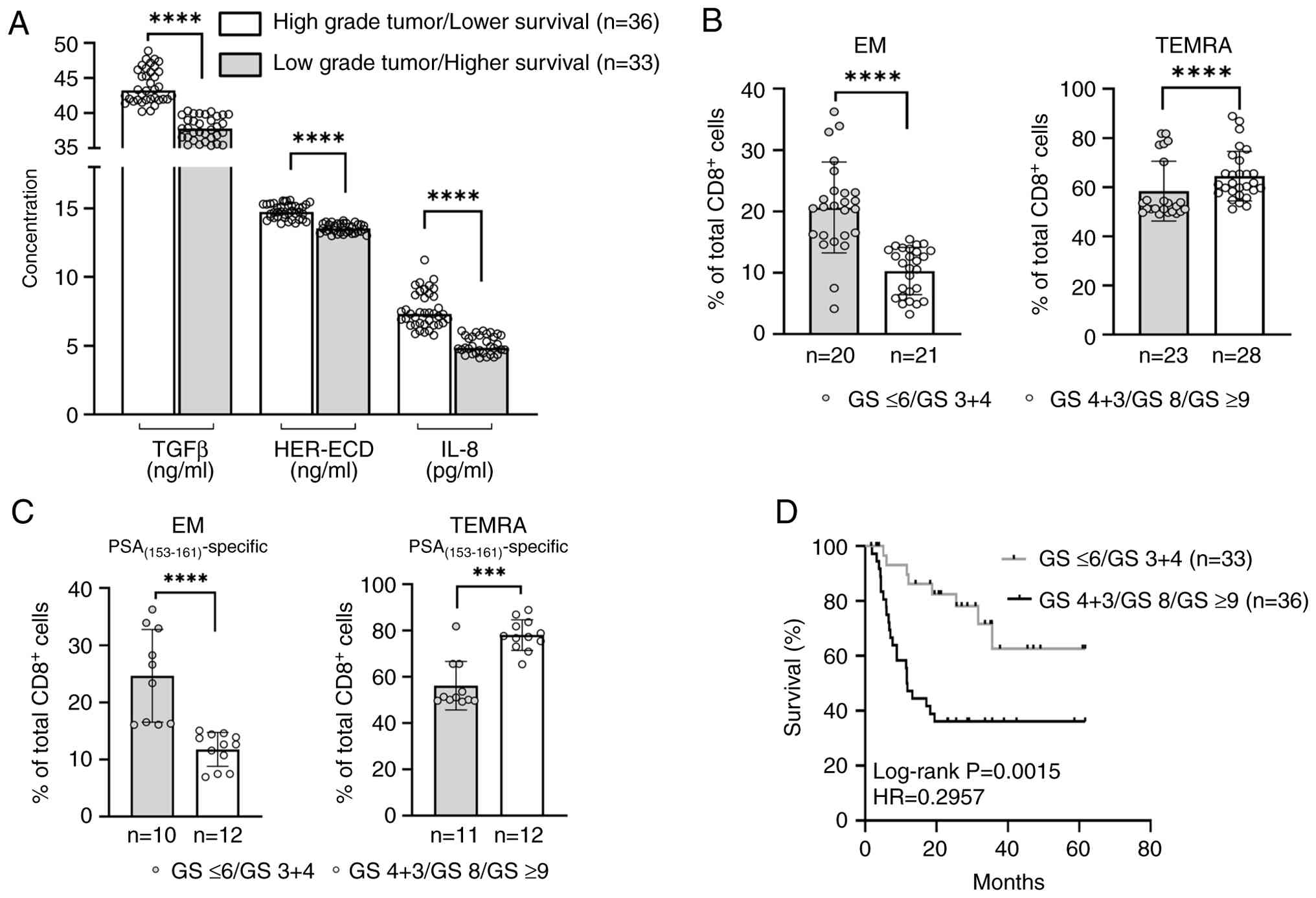
GS is associated with EM and TEMRA CD8+ T-cell subsets and survival in LPCa patients with the UB
Similar to the FB group, the UB group (n=69) included patients with high GS (n=36) and low GS (n=33). Patients with a low GS had significantly lower values of TGFβ, HER-ECD, or IL-8 as compared to those with a high GS (P<0.0001, Fig. 6A). There was also an association between GS and the densities of total or PSA(153–161)-specific TEMRA and EM CD8+ T-cell subsets among LPCa patients with the UB; patients with low GS had significantly higher frequencies of EM cells and lower frequencies of TEMRA cells, whereas EM cells were detected at much lower densities and TEMRA cells at substantially higher densities among patients with high GS (Fig. 6B and C). PSA and T-stage distributions among patients belonging to the UB group were close to those described above for the FB group, thus not allowing reliable associations with the densities of T-cell subsets and survival. However, in contrast to the FB group, the increased suppression in the periphery, possibly exerting a negative effect on the CD8+ T-cell-mediated antitumor immunity, allowed associations between GS and survival (Fig. 6D).
Discussion
Precision oncology could be greatly advanced through the identification of reliable prognostic and predictive biomarkers. These biomarkers would enable more personalized treatment modalities, aiming to prevent therapy-related issues such as excessive dosing, over-treatment, and adverse events. Ideally, biomarkers are determined within the tumor tissue (30,31), a challenging endeavor due to the difficulty of accessing sufficient malignant tissue in various cancers. On the other hand, liquid biopsies offer a significant advantage as non-invasive procedures that can be performed repeatedly with minimal discomfort to the patients during their treatment, making them preferable over traditional tissue biopsy analysis (32,33). In this study, we could identify prognostic biosignatures encompassing peripheral blood TGFβ, IL-8, and HER-ECD levels in LPCa patients. Low levels of TGFβ, IL-8, and HER-ECD (or low levels of any two of these factors) comprised the FB. LPCa patients with the FB had favorable survival rates and low vs. high frequencies of circulating total or PSA(153–161)-specific TEMRA and EM CD8+ T-cell subsets, respectively. The inverse situation was noted among LPCa patients with the UB (i.e., high levels for all three factors or at least for two of them). These patients had lower survival rates, high densities of circulating total or PSA(153–161)-specific TEMRA cells, and low frequencies of their EM counterparts.
Since CD8+ EM T-cells provide protective immunity by migrating to inflamed sites and exerting cytotoxic functions locally (6,34), it is plausible that the elevated frequencies of this CD8+ T-cell subset in LPCa patients with longer survival rates could indicate a functionally competent immune system, potentially serving as a biomarker for favorable prognosis. Recent human studies across multiple cancers highlight EM CD8+ T cells as powerful antitumor effectors. For example, Takahashi et al (35) analyzed 62 patients with head and neck squamous cell carcinoma and found that a higher fraction of circulating EM CD8+ T cells (CD45RO+CD62L−) was an independent favorable prognostic factor for survival. Similarly, Sun et al (36) showed that high densities of tumor-infiltrating CD8+CD45RO+ EM cells in 145 triple-negative breast cancer samples strongly predicted improved outcomes: effector-memory CD8+ T-cell density was independently associated with better overall and disease-free survival. In advanced hepatocellular carcinoma, single-cell analyses revealed that responders to anti-PD-L1/VEGF therapy had tumors enriched for CXCL10+ macrophages that attracted peripheral CXCR3+ CD8+ T EM cells (37). Overall, the clinical data show that higher effector-memory CD8+ T-cell infiltration or abundance is associated with superior tumor control and patient prognosis. Conversely, the higher percentages of CD8+ TEMRA T-cells observed in the LPCa patient group with shorter survival rates might correlate with a limited capacity for effective antitumor cytotoxic activity. In agreement with this, in a recent report, it was found that PD-1+ CD8+ T-cells in patients with advanced esophageal squamous cell carcinoma, therapeutically treated with radiotherapy combined with immunotherapy, included higher frequencies of functionally active EM cells than TEMRA cells (38). In addition, TEMRA cells, highly dysfunctional in their antitumor activities, were found at high frequencies in the peripheral blood of colorectal cancer patients with liver metastases (39).
To the best of our knowledge, the present study is the first to associate the circulating total and PSA peptide-specific CD8+ T-cell subsets and a biosignature consisting of the plasma cytokines TGFβ and IL-8, as well as the HER-ECD, with survival. As mentioned above (13–15,17,23,24), the association of these soluble factors with the induced suppression in the periphery suggests that their presence at low levels will allow the development of a more potent antitumor immunity, resulting in favorable clinical outcomes. In contrast, their presence at high levels in the blood circulation will be linked to tumor progression, metastasis, and immune evasion. In line with this, a meta-analysis of 14 cohort studies including 3,190 patients found that high IL-8 levels predicted poor outcomes on cancer immunotherapy. Patients with elevated IL-8 had significantly lower response rates and shorter overall survival and progression-free survival under checkpoint blockade (40). A review of metastatic renal cell carcinoma (RCC) trials showed that baseline IL-8 levels correlate with an immunosuppressive myeloid milieu and poor prognosis. In addition, elevated IL-8 has been associated with resistance to chemotherapy and targeted therapies, resulting in greater tumor burden and shorter survival in RCC and other types of cancer (41). Moreover, IL-8 has been shown to foster immune evasion and metastases by acting as a potent chemoattractant for neutrophils, myeloid-derived suppressor cells, and pro-tumoral N2 neutrophils into the tumor, all of which suppress T-cell immunity (41). High TGFβ levels have also been associated with poor clinical outcomes. In 49 gynecologic cancer patients treated with PD-1/CTLA-4 inhibitors, a high TGFβ signaling score predicted immunotherapy failure. Patients with elevated tumor TGFβ gene signatures had dramatically shorter progression-free survival and also showed abundant Tregs/M2 macrophages in the tumor (42). What is more, increased TGFβ serum levels correlate with advanced stage and poor survival in colorectal, pancreatic, and hepatocellular carcinomas (43). Mechanistically, TGFβ broadly suppresses anti-tumor immunity by inhibiting effector T-cell function and promoting Treg differentiation, M2 macrophage polarization, and extracellular matrix remodeling, all favoring immune escape. Clinically, TGFβ overactivity is implicated in immune checkpoint resistance and worse prognosis across cancers (43). Correlation of high HER-ECD with poorer clinical responses has been demonstrated in a Phase II trial of 58 metastatic breast cancer patients treated with paclitaxel plus doxorubicin. Patients with elevated pre-treatment HER-ECD had no complete response rate (vs. 26% in low-HER-ECD patients) and shorter duration of response (7.5 vs. 11 months), indicating HER-ECD-associated chemoresistance (44). In addition, in a prospective cohort of HER2+ breast cancer patients, high baseline sHER2-ECD levels were independently associated with worse disease-free, progression-free, and overall survival on trastuzumab-based regimens, consistent with a role for ECD shedding in therapeutic resistance (45). Our data is in line with this since we demonstrated an association between the FB and increased densities of total and PSA(153–161)-specific EM CD8+ T-cells in the context of low densities of their TEMRA counterparts in LPCa patients with high survival rates, which was independent of their tumor aggressiveness. To this end, we showed that patients of the FB group with low or high GS (i.e., GS 3+4 and GS ≤6 vs GS 4+3; GS 8 and GS ≥9, respectively), had similar densities of EM and TEMRA CD8+ T-cell subsets (both total and PSA(153–161)-specific) and survival rates. This is an important finding since it implies that by weaker suppression and unleashing of a potent antitumor immunity, tumor progression is hindered to similar levels independent of tumor grading. In this case, prostate cancer patients with low or high tumor grades have similarly increased disease-free intervals. Inversely, increased levels of TGFβ, IL-8, and HER-ECD (comprising the UB) were associated with decreased frequencies of total and PSA(153–161)-specific EM cells, along with high densities of the TEMRA subset and decreased survival rates. In this case, GS was directly associated with survival rates, suggesting that by ample suppression and decreased antitumor immunity, more aggressive tumors progress with higher growth rates.
In our previous study (23), we could show that the CD8+ T-cell-mediated antitumor immunity before vaccination (i.e., the endogenous or preexisting antitumor immunity) positively impacted clinical outcomes post-vaccination. We found that the PSA(153–161) peptide belonged to the most immunogenic ones based on the high frequencies of CD8+ T-cells at baseline, specifically recognizing this peptide, which were even further augmented during vaccinations. To unravel the possible predictive significance of preexisting immunity for clinical outcomes in our vaccinated prostate cancer patients, we associated the frequencies of CD8+ T-cells, specific for the PSA(153–161) peptide, at baseline and during vaccinations, with progression-free survival (PFS) and showed that high levels of preexisting immunity to PSA(153–161) were associated with significantly higher PFS in vaccinated patients (23). Thus, our previously published data, along with those from the present study, ascribe PSA(153–161) a dominant role in priming CD8+ T-cells to develop effector memory antitumor immunity, supporting its clinical use as a therapeutic vaccine in patients with LPCa.
This study provides novel information regarding the role of circulating TGFβ, IL-8, and HER-ECD as a biosignature in association with the frequencies of total and PSA(153–161) peptide-specific EM and TEMRA CD8+ T-cell subsets as surrogates for disease prognosis in LPCa patients. Our data showing similar densities of these subsets, and similar levels of TGFβ, IL-8, and HER-ECD in PCa patients with high vs. low GS in the FB group provide additional support for their role as indicators of disease progression and imply that their regular monitoring in the periphery could offer valuable insights into the prognosis and effectiveness of ongoing treatments. However, these results need to be confirmed in further studies involving a larger patient cohort in which the functional programs of the PSA(153–161)- specific EM and TEMRA T-cells will be analyzed.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This research has been co-financed by Eurobank and by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH-CREATE-INNOVATE (project code: T1EDK-01404, acronym: NEOVIOPRO).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
MG, CNB and SPF conceptualized and designed the study. SS and TA collected the patients' samples. MG, SS, TA, ADG, OET, CNB and SPF collected the data. MG, SS, ADG, CNB and SPF analyzed the data and performed the statistical analyses. MG, CNB and SPF drafted the manuscript. MG, SS, TA, ADG, OET, CNB and SPF edited the manuscript. MG, CNB and SPF confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The study was conducted in accordance with The Declaration of Helsinki and approved by the Institutional Review Board of Saint Savas Cancer Hospital (approval no. IRB-ID6777/ 14-06-2017) as well as the Ethical Committee of the National and Kapodistrian University of Athens (approval no. IRBID1516015872/03-02-2016). Written informed consent was obtained from all subjects involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Welch HG and Albertsen PC: Reconsidering prostate cancer mortality-The future of PSA screening. N Engl J Med. 382:1557–1563. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Siegel RL, Miller KD and Jemal A: Cancer statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI | |
|
Shelley M, Harrison C, Coles B, Staffurth J, Wilt TJ and Mason MD: Chemotherapy for hormone-refractory prostate cancer. Cochrane Database Syst Rev. 18:CD0052472006.PubMed/NCBI | |
|
Rebello RJ, Oing C, Knudsen KE, Loeb S, Johnson DC, Reiter RE, Gillessen S, Van der Kwast T and Bristow RG: Prostate cancer. Nat Rev Dis Primers. 7:92021. View Article : Google Scholar : PubMed/NCBI | |
|
Cooperberg MR, Carroll PR, Dall'Era MA, Davies BJ, Davis JW, Eggener SE, Feng FY, Lin DW, Morgan TM, Morgans AK, et al: The state of the science on prostate cancer biomarkers: The San Francisco Consensus statement. Eur Urol. 76:268–272. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Zelic R, Garmo H, Zugna D, Stattin P, Richiardi L, Akre O and Pettersson A: Predicting prostate cancer death with different pretreatment risk stratification tools: A head-to-head comparison in a nationwide cohort study. Eur Urol. 77:180–188. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Baxevanis CN, Fortis SP and Perez SA: The balance between breast cancer and the immune system: Challenges for prognosis and clinical benefit from immunotherapies. Semin Cancer Biol. 72:76–89. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Vesely MD and Schreiber RD: Cancer immunoediting: Antigens, mechanisms, and implications to cancer immunotherapy. Ann N Y Acad Sci. 1284:1–5. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Haen SP, Löffler MW, Rammensee HG and Brossart P: Towards new horizons: Characterization, classification and implications of the tumour antigenic repertoire. Nat Rev Clin Oncol. 17:595–610. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Mittal D, Gubin MM, Schreiber RD and Smyth MJ: New insights into cancer immunoediting and its three component phases-elimination, equilibrium and escape. Curr Opin Immunol. 27:16–25. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Baxevanis CN and Perez SA: Cancer dormancy: A regulatory role for endogenous immunity in establishing and maintaining the tumor dormant state. Vaccines (Basel). 3:597–619. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Baxevanis CN, Anastasopoulou EA, Voutsas IF, Papamichail M and Perez SA: Immune biomarkers: How well do they serve prognosis in human cancers? Expert Rev Mol Diagn. 15:49–59. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Perez SA, Karamouzis MV, Skarlos DV, Ardavanis A, Sotiriadou NN, Iliopoulou EG, Salagianni ML, Orphanos G, Baxevanis CN, Rigatos G and Papamichail M: CD4+CD25+ regulatory T-cell frequency in HER-2/neu (HER)-positive and HER-negative advanced-stage breast cancer patients. Clin Cancer Res. 13:2714–2721. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Perez SA, Kallinteris NL, Bisias S, Tzonis PK, Georgakopoulou K, Varla-Leftherioti M, Papamichail M, Thanos A, von Hofe E, Baxevanis CN, et al: Results from a phase I clinical study of the novel Ii-Key/HER-2/neu(776–790) hybrid peptide vaccine in patients with prostate cancer. Clin Cancer Res. 16:3495–3506. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Okegawa T, Kinjo M, Nutahara K and Higashihara E: Pretreatment serum level of HER2/nue as a prognostic factor in metastatic prostate cancer patients about to undergo endocrine therapy. Int J Urol. 13:1197–1201. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Köstler WJ, Schwab B, Singer CF, Neumann R, Rücklinger E, Brodowicz T, Tomek S, Niedermayr M, Hejna M, Steger GG, et al: Monitoring of serum Her-2/neu predicts response and progression-free survival to trastuzumab-based treatment in patients with metastatic breast cancer. Clin Cancer Res. 10:1618–1624. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Anastasopoulou EA, Voutsas IF, Keramitsoglou T, Gouttefangeas C, Kalbacher H, Thanos A, Papamichail M, Perez SA and Baxevanis CN: A pilot study in prostate cancer patients treated with the AE37 Ii-key-HER-2/neu polypeptide vaccine suggests that HLA-A*24 and HLA-DRB1*11 alleles may be prognostic and predictive biomarkers for clinical benefit. Cancer Immunol Immunother. 64:1123–1136. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Travis MA and Sheppard D: TGF-β activation and function in immunity. Annu Rev Immunol. 32:51–82. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Bierie B and Moses HL: TGFβ: The molecular Jekyll and hyde of cancer. Nat Rev Cancer. 6:506–520. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Steiner MS, Zhou ZZ, Tonb DC and Barrack ER: Expression of transforming growth factor-beta 1 in prostate cancer. Endocrinology. 135:2240–2247. 1994. View Article : Google Scholar : PubMed/NCBI | |
|
Barrack ER: TGF beta in prostate cancer: A growth inhibitor that can enhance tumorigenicity. Prostate. 31:61–70. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Perez SA, Anastasopoulou EA, Papamichail M and Baxevanis CN: AE37 peptide vaccination in prostate cancer: Identification of biomarkers in the context of prognosis and prediction. Cancer Immunol Immunother. 63:1141–1150. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Voutsas IF, Anastasopoulou EA, Tzonis P, Papamichail M, Perez SA and Baxevanis CN: Unraveling the role of preexisting immunity in prostate cancer patients vaccinated with a HER-2/neu hybrid peptide. J Immunother Cancer. 4:752016. View Article : Google Scholar : PubMed/NCBI | |
|
Goulielmaki M, Stokidis S, Anagnostou T, Voutsas IF, Gritzapis AD, Baxevanis CN and Fortis SP: Frequencies of an immunogenic HER-2/neu epitope of CD8+ T lymphocytes predict favorable clinical outcomes in prostate cancer. Int J Mol Sci. 24:59542023. View Article : Google Scholar : PubMed/NCBI | |
|
Dahal S, Chaudhary P, Jung YS and Kim JA: Megakaryocyte-Derived IL-8 acts as a paracrine factor for prostate cancer aggressiveness through CXCR2 activation and antagonistic AR downregulation. Biomol Ther (Seoul). 31:210–218. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Sunaga N, Kaira K, Tomizawa Y, Shimizu K, Imai H, Takahashi G, Kakegawa S, Ohtaki Y, Nagashima T, Kasahara N, et al: Clinicopathological and prognostic significance of interleukin-8 expression and its relationship to KRAS mutation in lung adenocarcinoma. Br J Cancer. 110:2047–2053. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Singh JK, Simões BM, Howell SJ, Farnie G and Clarke RB: Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res. 15:2102013. View Article : Google Scholar : PubMed/NCBI | |
|
Chen L, Fan J, Chen H, Meng Z, Chen Z, Wang P and Liu L: The IL-8/CXCR1 axis is associated with cancer stem cell-like properties and correlates with clinical prognosis in human pancreatic cancer cases. Sci Rep. 4:59112014. View Article : Google Scholar : PubMed/NCBI | |
|
Baxevanis CN, Stokidis S, Goulielmaki M, Gritzapis AD and Fortis SP: Peripheral blood CD8+ T-lymphocyte subsets are associated with prognosis in prostate cancer patients. Onco. 3:165–174. 2023. View Article : Google Scholar | |
|
Kim R, Kim S, Oh BB, Yu WS, Kim CW, Hur H, Son SY, Yang MJ, Cho DS, Ha T, et al: Clinical application of whole-genome sequencing of solid tumors for precision oncology. Exp Mol Med. 56:1856–1868. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Huang RJ, Huang YS, An N, Hu JJ, Wu CY, Chen YX, Chen JY, Zhao Q, Xu RH, Yuan SQ and Wang F: Pan-cancer analysis of heterogeneity of tumor mutational burden and genomic mutation under treatment pressure. ESMO Open. 9:1034942024. View Article : Google Scholar : PubMed/NCBI | |
|
Tan WY, Nagabhyrava S, Ang-Olson O, Das P, Ladel L, Sailo B, He L, Sharma A and Ahuja N: Translation of epigenetics in cell-free DNA liquid biopsy technology and precision oncology. Curr Issues Mol Biol. 46:6533–6565. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Koukourakis MI, Xanthopoulou E, Koukourakis IM, Fortis SP, Kesesidis N, Kakouratos C, Karakasiliotis I and Baxevanis CN: Next-generation sequencing analysis of mutations in circulating tumor DNA from the plasma of patients with head-Neck cancer undergoing chemo-radiotherapy using a pan-cancer cell-free assay. Curr Oncol. 30:8902–8915. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Lam N, Lee Y and Farber DL: A guide to adaptive immune memory. Nat Rev Immunol. 24:810–829. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Takahashi H, Sakakura K, Ida S, Kawabata-Iwakawa R, Matsuyama T, Tada H, Mito I and Chikamatsu K: Circulating naïve and effector memory T cells correlate with prognosis in head and neck squamous cell carcinoma. Cancer Sci. 113:53–64. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Sun X, Zhai J, Sun B, Parra ER, Jiang M, Ma W, Wang J, Kang AM, Kannan K, Pandurengan R, et al: Effector memory cytotoxic CD3+/CD8+/CD45RO+ T cells are predictive of good survival and a lower risk of recurrence in triple-negative breast cancer. Mod Pathol. 35:601–608. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Cappuyns S, Philips G, Vandecaveye V, Boeckx B, Schepers R, Van Brussel T, Arijs I, Mechels A, Bassez A, Lodi F, et al: PD-1− CD45RA+ effector-memory CD8 T cells and CXCL10+ macrophages are associated with response to atezolizumab plus bevacizumab in advanced hepatocellular carcinoma. Nat Commun. 14:78252023. View Article : Google Scholar : PubMed/NCBI | |
|
Wei H, Li Y, Guo Z, Ma X, Li Y, Wei X, Han D, Zhang T, Chen X, Yan C, et al: Comparison of dynamic changes in the peripheral CD8+ T cells function and differentiation in ESCC patients treated with radiotherapy combined with anti-PD-1 antibody or concurrent chemoradiotherapy. Front Immunol. 13:10606952022. View Article : Google Scholar : PubMed/NCBI | |
|
Bruni E, Cazzetta V, Donadon M, Cimino M, Torzilli G, Spata G, Leonardi G, Dieli F, Mikulak J and Mavilio D: Chemotherapy accelerates immune-senescence and functional impairments of Vδ2pos T cells in elderly patients affected by liver metastatic colorectal cancer. J Immunother Cancer. 7:3472019. View Article : Google Scholar : PubMed/NCBI | |
|
Zou D, Song A and Yong W: Prognostic role of IL-8 in cancer patients treated with immune checkpoint inhibitors: A system review and meta-analysis. Front Oncol. 13:11765742023. View Article : Google Scholar : PubMed/NCBI | |
|
Rizzo M, Varnier L, Pezzicoli G, Pirovano M, Cosmai L and Porta C: IL-8 and its role as a potential biomarker of resistance to anti-angiogenic agents and immune checkpoint inhibitors in metastatic renal cell carcinoma. Front Oncol. 12:9905682022. View Article : Google Scholar : PubMed/NCBI | |
|
Ni Y, Soliman A, Joehlin-Price A, Rose PG, Vlad A, Edwards RP and Mahdi H: High TGF-β signature predicts immunotherapy resistance in gynecologic cancer patients treated with immune checkpoint inhibition. NPJ Precis Oncol. 5:1012021. View Article : Google Scholar : PubMed/NCBI | |
|
Kim BG, Malek E, Choi SH, Ignatz-Hoover JJ and Driscoll JJ: Novel therapies emerging in oncology to target the TGF-β pathway. J Hematol Oncol. 14:552021. View Article : Google Scholar : PubMed/NCBI | |
|
Colomer R, Montero S, Lluch A, Ojeda B, Barnadas A, Casado A, Massutí B, Cortés-Funes H and Lloveras B: Circulating HER2 extracellular domain and resistance to chemotherapy in advanced breast cancer. Clin Cancer Res. 6:2356–2362. 2000.PubMed/NCBI | |
|
Reix N, Malina C, Chenard MP, Bellocq JP, Delpous S, Molière S, Sevrin A, Neuberger K, Tomasetto C and Mathelin C: A prospective study to assess the clinical utility of serum HER2 extracellular domain in breast cancer with HER2 overexpression. Breast Cancer Res Treat. 160:249–259. 2016. View Article : Google Scholar : PubMed/NCBI |