Peripheral blood tumor marker levels can indicate the location of lung cancer metastasis
- Authors:
- Published online on: September 23, 2025 https://doi.org/10.3892/ol.2025.15291
- Article Number: 545
-
Copyright: © Wang et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, with ~2.1 million new cases and 1.8 million deaths each year, accounting for ~12% of all cancer cases and 18% of cancer-related deaths (1). Lung cancer is mainly divided into two types: i) Small cell lung cancer (SCLC); and ii) non-SCLC (NSCLC). NSCLC consists of multiple histological subtypes, the most common of which are lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC), accounting for 75% of all lung cancer cases (2). It is estimated that by 2040, the number of lung cancer-related deaths will increase by 67% worldwide (3). The reduction of the mortality rate associated with lung cancer is one of the United Nations Sustainable Development Goals (4).
Despite progress in clinical treatment, tumor metastasis is the main cause of the high mortality associated with lung cancer. In total, ~90% of patients with lung cancer die from metastasis, as the cancer quickly spreads to the lymph nodes, bones, brain and liver (5). Brain metastasis will develop in 40–50% of patients with lung cancer, while 30–40% of patients with advanced lung cancer will develop bone metastasis and 20–40% of patients with NSCLC will develop liver metastasis (6–8). If a certain tumor marker in the peripheral blood of patients with lung cancer increases significantly, it may indicate metastasis and prompt doctors to conduct targeted examinations, which is of great significance for improving patient survival rates and reducing the economic and social burden of the disease.
Tumor markers are substances produced by the tumor or the body's microenvironment during tumor occurrence and development (9). Markers include proteins, hormones, oncogenes and other substances, and can play an important role in cancer screening, early diagnosis, prognosis, recurrence monitoring and judgment of efficacy (10). Omics analysis is a comprehensive, high-throughput bioinformatics technology that includes genomics, transcriptomics, proteomics and metabolomics, among others, and is widely used in the screening of tumor markers (11). Billatos et al (12) used transcriptomics and patient nasal epithelial samples to identify certain genes that are expressed differently between patients with lung cancer and those without lung cancer. Proteomics approaches have been used to identify potential biomarkers for lung cancer, and several potential protein markers (such as BAK1, CTSW, MICOS13, RELN, PTN, TPT1, SVEP1, PDGFD, LCN2 and GLG1) have been identified (13,14). Metabolites can reflect the biochemical state of healthy or unhealthy cells; therefore, the unique metabolic characteristics of tumors may help determine the cancer stage (15). An increasing number of lung cancer metabolomics experiments have provided new potential markers for the diagnosis of lung cancer, but few have been widely used in clinical practice as diagnostic markers for lung cancer (15–17). Currently, there are >20 tumor markers used in clinical practice, some of which are limited to a certain type of cancer, such as α-fetoprotein which is usually elevated only in liver cancer, while CEA is increased in both lung and colorectal cancer (18,19). In addition, most omics studies only use bioinformatics methods for prediction, and the conclusions drawn from the statistical methods used may deviate from the actual clinical situation. It is still necessary to use large-scale clinical data to verify their value in tumor diagnosis. Therefore, using tumor markers currently used in clinical practice to predict the target organs of lung cancer metastasis is an effective strategy.
At present, traditional tumor-associated antigen (TAA) biomarkers, such as cytokeratin 19 fragment antigen 21-1 (CYFRA21-1), carcinoembryonic antigen (CEA), squamous cell carcinoma (SCC) antigen, and carbohydrate antigen (CA)125, CA15-3 and CA19-9, are widely used as reference diagnostics for lung cancer (20). CYFRA21-1 is a fragment of cytokeratin 19 subunit that is expressed at high levels in SCC and can be used as a tumor biomarker in patients with cancer (21). CEA is a 200-kD glycoprotein found in embryonic endoderm epithelium. This protein is elevated in various malignancies, such as colorectal, gastric and lung cancer, and has no tumor specificity (22). SCC antigen is a subcomponent of TAAs associated with SCC and is widely used as a marker for SCCs of the head and neck, lung and esophagus, among others (23). CA15-3 belongs to mucin 1, which is a transmembrane protein expressed on the apical plasma membrane of normal secretory epithelial cells composed of two subunits. CA15-3 levels are markedly elevated in a number of cancer types, including lung cancer (24). CA19-9 is present in the glycolipid mucin of the cell membrane. The serum CA19-9 level is elevated in patients with cancer, especially in those with digestive system tumors, such as pancreatic, liver, colon and rectal cancer (25). CA125 is a repeating peptide epitope found in mucin 16, which can be recognized by the antibody OC125. CA125 also is the preferred biomarker for ovarian cancer and its level is also increased in lung and colorectal cancer (26). Although the aforementioned tumor markers are widely used in clinical diagnosis, their potential as prospective predictors of sites of tumor metastasis remains underexplored. In the present study, patients with one of three types of lung cancer, namely, LUAD, LUSC and SCLC, were selected and divided into groups based on the presence or absence of tumor metastasis and on which single tissue or organ site the metastasis occurred in, such as the lymph nodes, pleura, bones, liver and brain. The values of peripheral serum CEA, CA15-3, CA125, CA19-9 and SCC were recorded before treatment. One-way analysis of variance was used to preliminarily determine the tumor markers that were different in the non-metastatic group, and then the receiving operating characteristic (ROC) curve method was used to determine the efficacy of the marker in evaluating the metastasis to a certain organ. It is anticipated that the present study can use the existing common clinical tumor marker detection indicators, and through the abnormal changes in their content, it can indicate the possible metastatic organs. This should attract the attention of doctors and patients, and allow subsequent disease intervention and treatment to be performed in a timely manner.
Materials and methods
Patients
The present study included 631 patients (mean age, 62.9±3.7; range, 39–81 years) who were hospitalized in Chifeng Municipal Hospital (Chifeng, Inner Mongolia, China) and diagnosed with lung cancer between January and June 2024, including 357 males and 274 females (Table I). The inclusion criteria included: i) A lung cancer diagnosis confirmed by histopathology or cytology; ii) age ≥18 years; and iii) in cases of tissue or organ metastasis, patients with only single tissue or organ metastasis were selected. The exclusion criteria included: i) Pulmonary infection and other infectious diseases; and ii) tumor diseases in other parts of the body identified at the time of study inclusion. Based on the pathological type, patients were divided into three groups: i) LUAD (n=357); ii) LUSC (n=152); and iii) SCLC (n=122). Patients were divided into two categories: i) No metastasis at the time of presentation; and ii) distant metastasis at the time of presentation. If metastasis occurred, patients were further classified based on the site of metastasis: i) Lymph nodes; ii) pleura; iii) bone; iv) liver; and v) brain. For this retrospective analysis, all the clinical test data of the patients were collected before clinical medication. The current study was approved by the Chifeng Municipal Hospital Ethics Committee (approval no. CK2023027).
Detection of tumor markers
Fasting peripheral venous blood samples (4.0 ml) were collected from patients in non-anticoagulated tubes. All patients had samples collected once at the time of consultation and before treatment. Within 2 h of blood collection, the serum was separated by centrifugation at 1,000 × g for 15 min at room temperature. The fully automatic luminescent immunoassay analyzer ARCHITECT™ i2000 (Abbott) was used to detect CEA, CA15-3, CA125, CA19-9, SCC and CYFRA21-1 levels. Due to differences in the daily work habits of doctors in different wards, some patients may not have certain tumor markers tested. However, generally speaking, CEA, CA15-3, CA125, and CA19-9 are frequently tested items for patients with lung cancer.
ROC curve analysis
ROC curve refers to the line drawn from each point under specific stimulation conditions, with the false alarm probability P (y/N) obtained by the subject under different judgment criteria as the horizontal coordinate and the hit probability P (y/SN) as the vertical coordinate (27). Usually, the sensitivity represents the vertical coordinate and 1-specificity is the horizontal coordinate. The ROC curve is a probability index of a certain outcome of each sample calculated by the entire model, showing the diagnostic efficiency of the entire model. It is one of the most important evaluation indicators of the model prediction effect. Therefore, the ROC curve is often used to evaluate and compare various diagnostic models and prediction models. The ROC curve uses the size of the area under the curve (AUC) to evaluate the model. The value range of AUC is 0.5–1.0. The larger the AUC, i.e. the closer it is to 1, the better the diagnostic or prediction effect of the model. When the AUC is 0.5–0.7, the accuracy is low, when the AUC is 0.7–0.9, it has some accuracy, and when the AUC is >0.9, the accuracy is high. When the AUC is 0.5, the diagnostic method is considered completely ineffective and has no diagnostic value. An AUC value of <0.5 does not conform to the actual situation. The present study used an AUC value of >0.7 as the threshold for clinical diagnostic value.
Statistical analysis
Statistical analysis was performed using SPSS (version 21; IBM Corp.). Normally distributed data are presented as the mean ± SEM and the median (interquartile range). Tumor marker concentrations between metastatic (lymph nodes, pleura, bone, liver and brain) and non-metastatic groups were compared using Student's unpaired t-test and the Mann-Whitney U test. Multiple group comparisons were performed using ANOVA and the Kruskal-Wallis test, with Tukey's post hoc test used in the event of significant results. P<0.05 was considered to indicate a statistically significant difference, and markers with significant differences in both tests were considered to have potential diagnostic value. Binary logistic regression analysis was used to evaluate the predictive probability of lung cancer metastasis by tumor markers. ROC curves were drawn using the predicted probability as a variable, and the AUC was calculated to assess the statistical significance of each marker.
Results
Patient characteristics
When metastasis occurred, the lymph nodes and bones were the most common sites, with 181 and 120 patients, respectively, followed by 67 patients with brain metastasis. There were 286 patients with lung cancer (aged <60 years) and 345 patients with lung cancer (≥60 years), accounting for 45.3 and 54.7% of patients with lung cancer, respectively. The characteristics of the patients are shown in Table I.
Significance of single tumor marker in screening LUAD metastatic organs
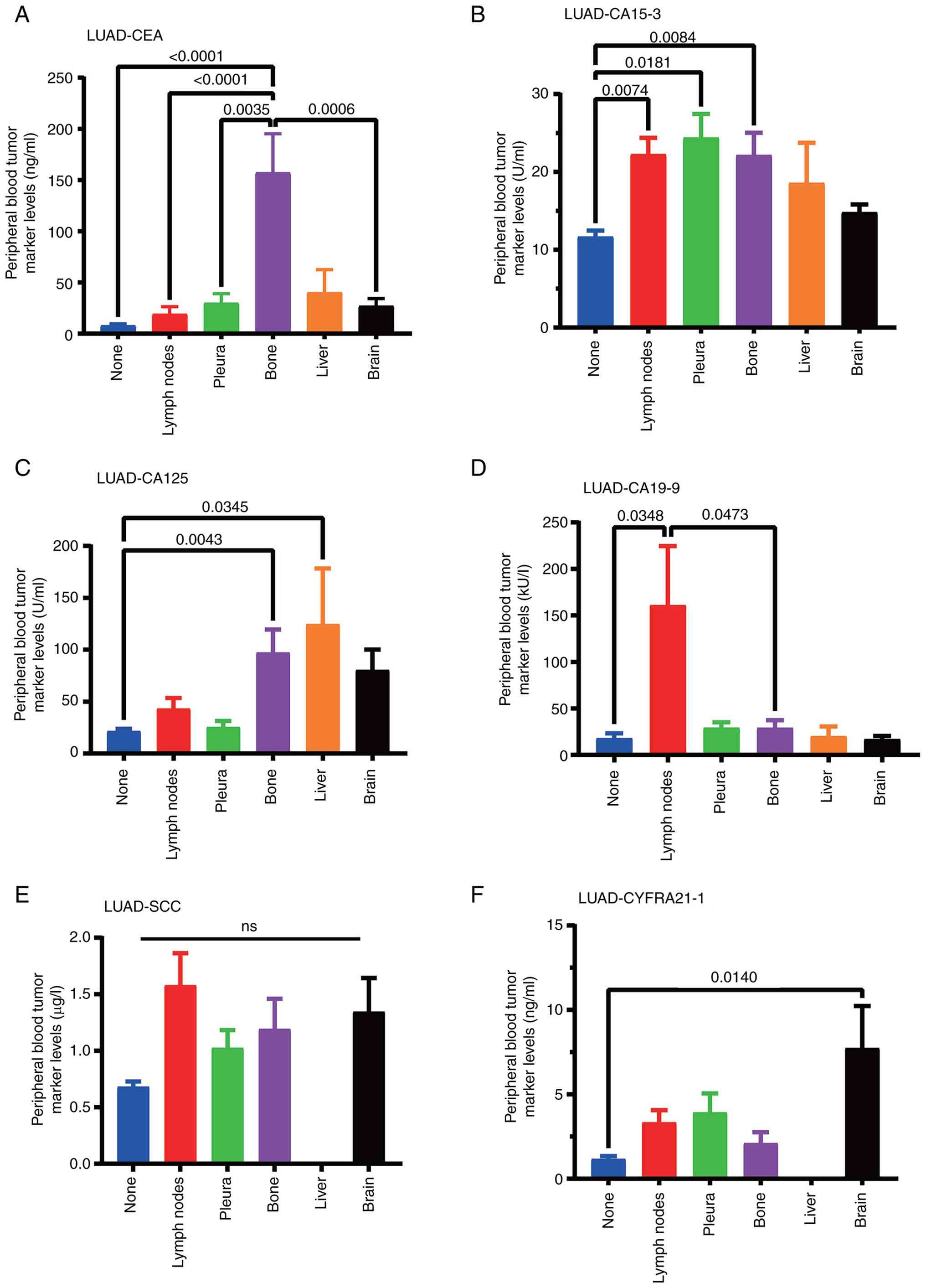
Data on the peripheral blood content of tumor markers in patients with LUAD and tissue and organ metastasis were collected. Compared with those in patients without metastasis, the levels of CA15-3 (P=0.0074) and CA19-9 (P=0.0348) significantly increased when lymph node metastasis occurred, the level of CA15-3 (P=0.0181) significantly increased when pleural metastasis occurred, the levels of CEA (P<0.001), CA15-3 (P=0.0084) and CA125 (P=0.0043) significantly increased when bone metastasis occurred, the level of CA125 (P=0.0345) significantly increased when liver metastasis occurred and the level of CYFRA21-1 (P=0.0140) significantly increased when brain metastasis occurred (Table II; Fig. 1). Inter-group comparisons were also conducted to observe the changes in certain indicators after metastasis to different organs and to better screen out differential markers. Results were considered significant only when P<0.05 for comparisons of both mean ± SEM and median (IQR) data.
In order to present the data more intuitively, bar graphs were used and statistical analysis was performed using the data of the different groups. CEA in bone metastasis, CA19-9 in lymph node metastasis and CYFRA21-1 in brain metastasis showed strong organ specificity, while SCC content only showed a metastatic trend (Fig. 1).
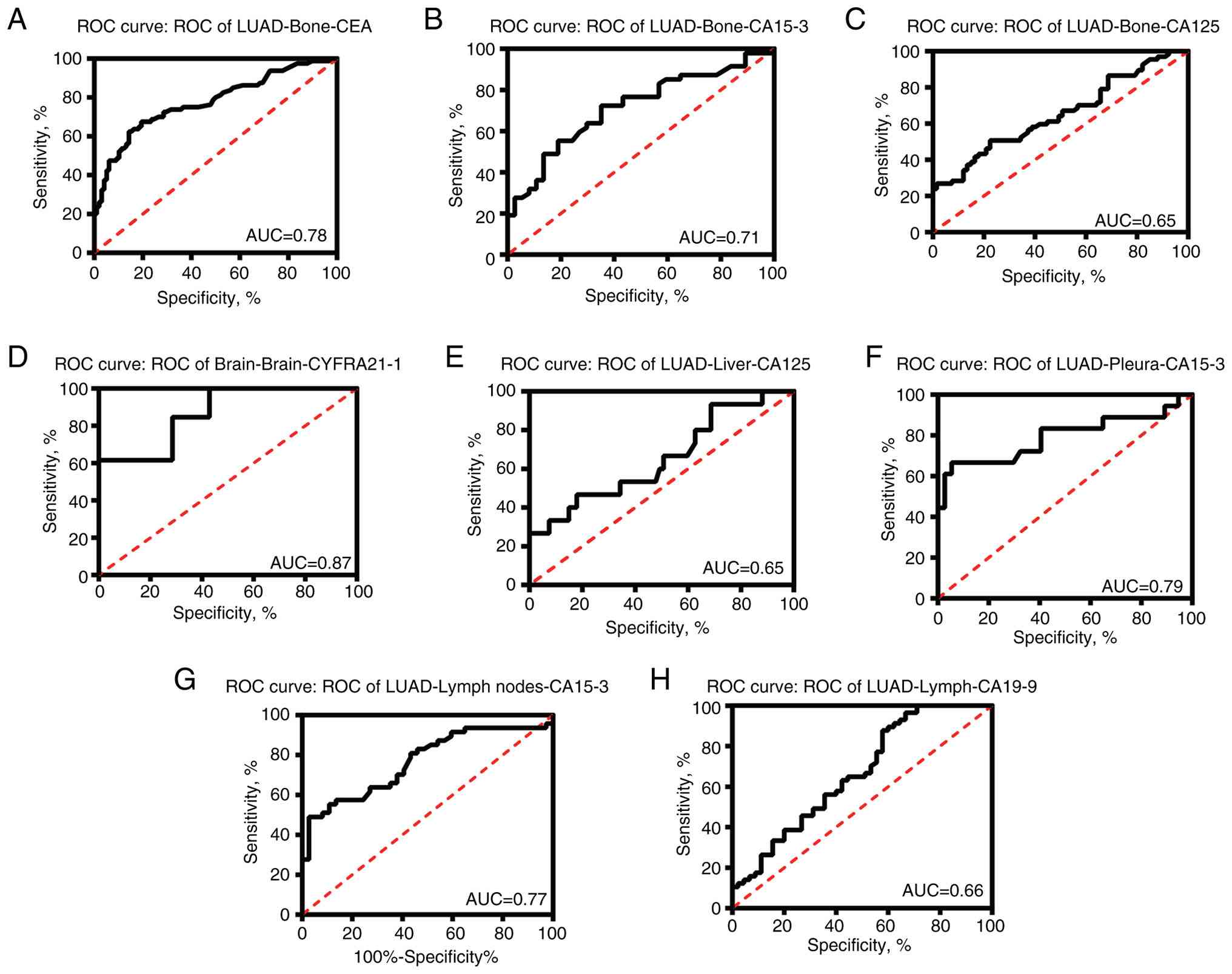
Compared with levels in lung cancer without metastasis, CEA (AUC, 0.78) and CA15-3 (AUC, 0.71) showed good diagnostic effects on bone metastasis screening. CYFRA21-1 (AUC, 0.87) also revealed a good diagnostic effect on brain metastasis screening. Although the tissue specificity was not high, the AUC values of CA15-3 in lymph node and pleural metastasis screening were 0.77 and 0.79, respectively, which also indicated good diagnostic value (Fig. 2).
Significance of single tumor marker in screening LUSC metastatic organs
LUSC and LUAD are both NSCLCs. Unlike in LUAD, in LUSC with tissue and organ metastasis, the peripheral blood levels of various tumor markers did not show significant differences, although CEA and CA15-3 levels showed a slight upward trend when liver metastasis occurred (Table III; Fig. S1). CEA, CA15-3 and SCC levels significantly decreased in patients with brain metastasis. CA15-3, CA125, CA19-9 and SCC levels also decreased significantly when pleural metastasis occurred (Table III; Fig. S1).
Significance of single tumor marker in screening SCLC metastatic organs
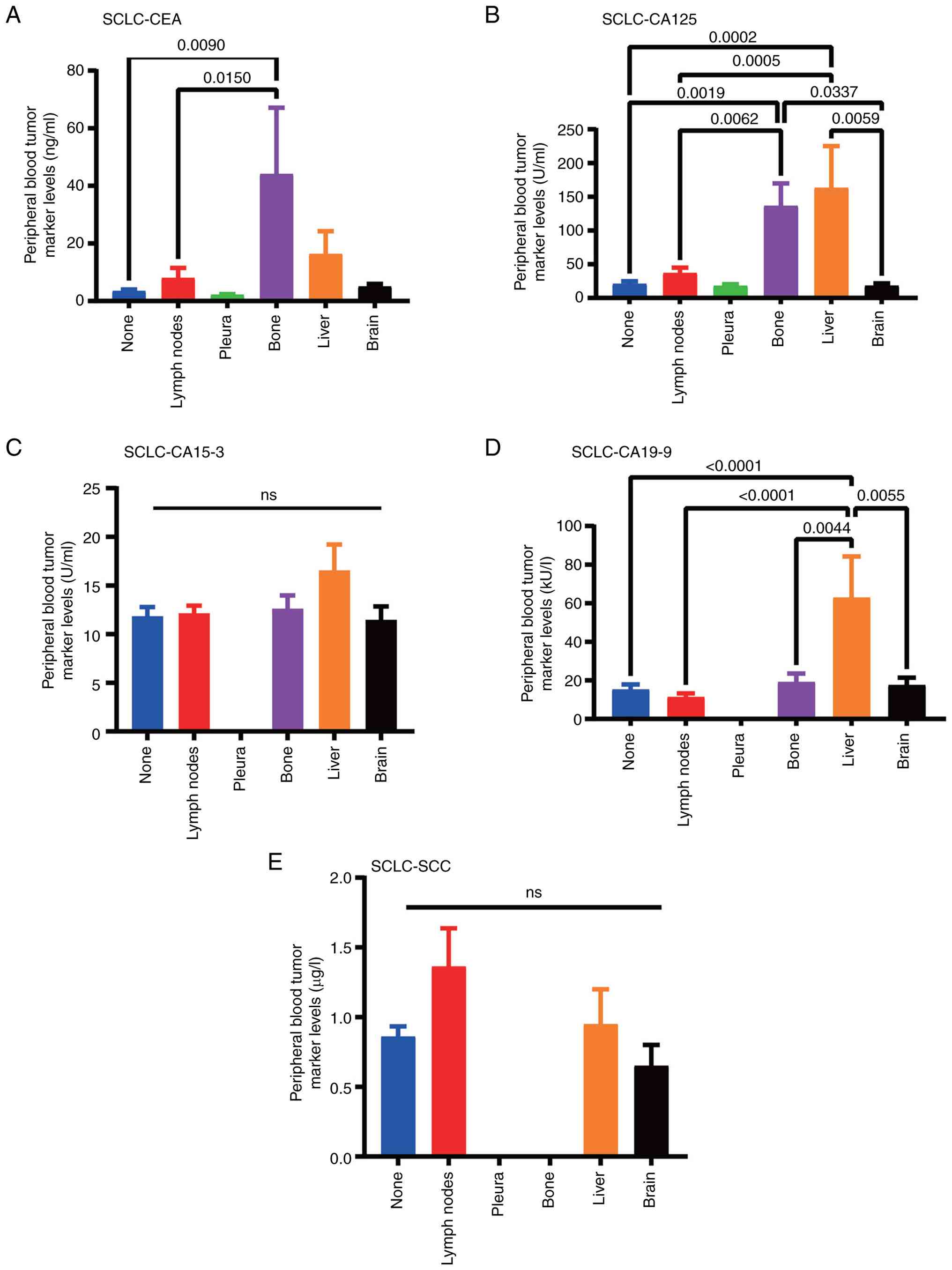
SCLC is considered one of the most aggressive cancers. Compared with the levels in the control group, the levels of CEA (P=0.0090) and CA125 (P=0.0019) were significantly increased in patients with bone metastasis. The increase of CEA indicates the possibility of bone metastasis. CA125 (P=0.0002) and CA19-9 (P<0.001) levels were significantly increased in liver metastasis, and the increase in CA19-9 suggests the possibility of liver metastasis. No significant changes were found in the content of SCC when metastasis occurred in various tissues and organs (Table IV; Fig. 3). Inter-group comparisons were also conducted to observe the changes in certain indicators after metastasis to different organs and to better screen out differential markers.
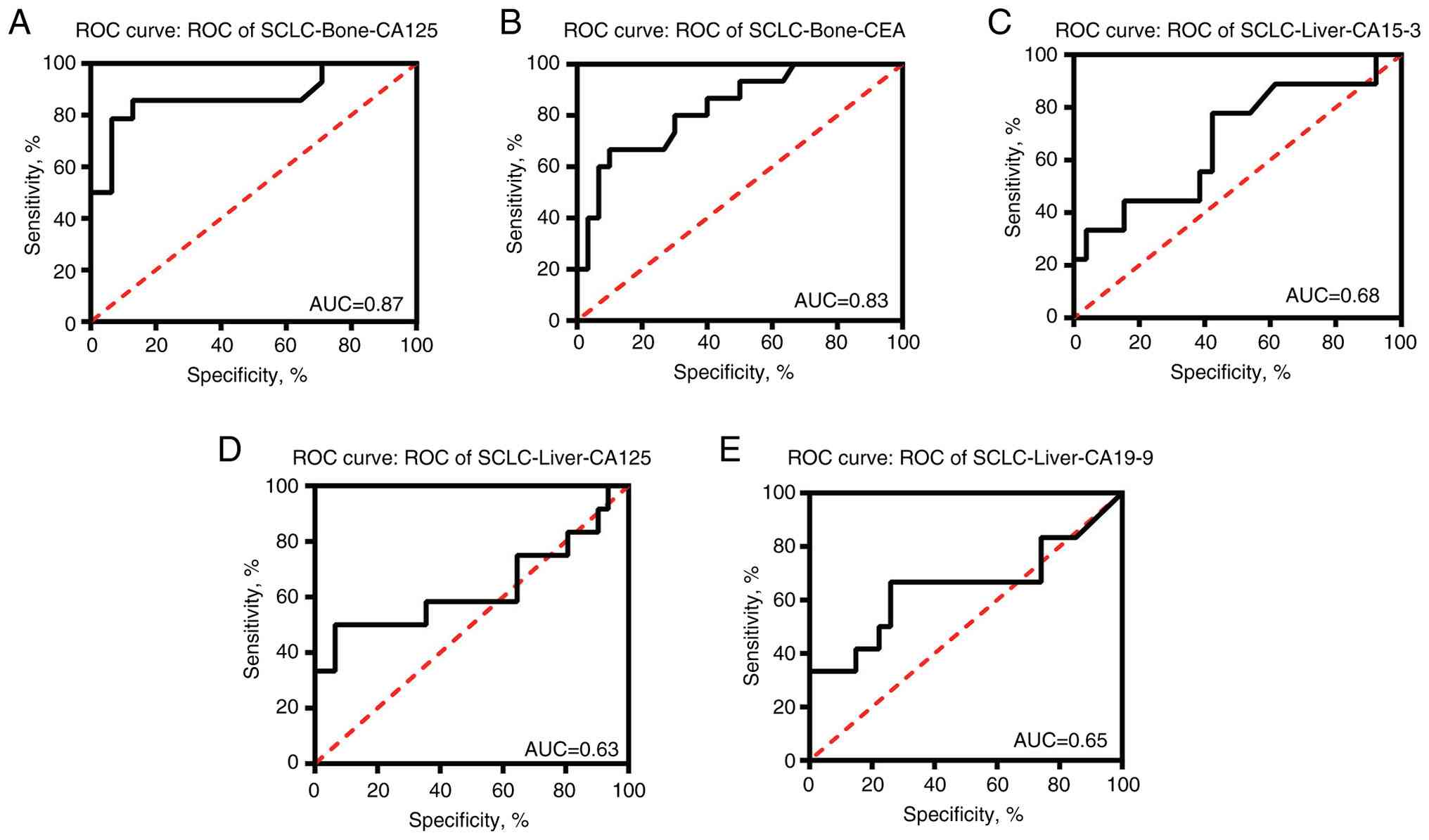
In SCLC tumors, CEA (AUC, 0.83) and CA125 (AUC, 0.87) exhibited promising diagnostic value for determining bone metastasis. CA125 (AUC, 0.63) and CA19-9 (AUC, 0.65) may have certain diagnostic value in liver metastasis (Fig. 4). Since the content of CA19-9 in liver metastasis was significantly higher than that in metastasis in other parts (Fig. 3), its diagnostic value was also analyzed and the AUC value was 0.65 (Fig. 4).
Discussion
Lung cancer is the most common malignant tumor in the world, with the highest incidence and mortality rates among malignant tumors. Tumor metastasis is the main cause of tumor-associated death, and early detection of tumor metastasis sites is crucial for subsequent treatment. The selection of lung cancer tumor markers has remained stable for a long time, although there have been some minor changes. The 2011 guidelines recommended the use of CEA, neuron-specific enolase (NSE), CYFRA21-1 and SCC (28). The main lung cancer markers recommended in the 2015 lung cancer guidelines include CEA, NSE, CYFRA21-1, pro-gastrin-releasing peptide and SCC (29). In the lung cancer guidelines from 2018 to date, the recommended tumor markers have not changed (30). However, which markers indicate metastasis to specific organs has not received attention from clinical workers. The present study discusses the predictive nature of various omics and bioinformatics in clinical practice, starting with the commonly used tumor markers CEA, CA15-3, CA125, CA19-9, SCC and CYFRA21-1, to explore whether these markers have certain clinical application value in indicating the occurrence of metastasis to specific organs. It was observed that the levels of CEA and CA15-3 in the peripheral blood of patients with LUAD metastasis were significantly increased when bone metastasis occurred, the level of CYFRA21-1 was significantly increased when brain metastasis occurred and the level of CA15-3 was significantly increased when lymph node and pleural metastasis occurred. The levels of CEA and CA125 in the peripheral blood of patients with SCLC and bone metastasis were significantly increased. When patients with LUSC develop metastasis to specific tissues and organs, the levels of the aforementioned five tumor markers do not increase significantly. Studies have shown that sex factors can affect the incidence of lung cancer. Fu et al (31) found that, in the United States between 2001 and 2019, men accounted for 53.3% of all lung cancer cases (32). The present study found that 57% of all collected cases were male, which is consistent with these findings. At the same time, Fu et al (31) found that ≤52.1% of patients in the United States were ≥70 years old. Histologically, SCLC and NSCLC account for 12.9 and 72.6% of all lung cancer cases, respectively (32). The current study found that in the present cohort from Chifeng, Inner Mongolia, people aged 51–70 years has the highest incidence of lung cancer, accounting for 70% of all lung cancer cases. In addition, NSCLC is the main lung cancer type, accounting for 81% of the total lung cancer cases, of which LUAD and LUSC account for 57 and 21% of cases, respectively (33). Smoking is the most important risk factor for lung cancer (34). Wang et al (35) found that the standardized smoking rate among residents ≥15 years in Inner Mongolia was 30.54%, and the smoking rate for both men and women was highest in the 45–59 years age group, which may to some extent explain the reason that the 51–70 age group has the highest incidence of lung cancer. The prognosis of patients with lung cancer and bone metastases is generally poor (36). CEA is usually a biomarker used for colorectal cancer screening and monitoring treatment response (37). In breast cancer and gastrointestinal tumors, CEA has good sensitivity and specificity for detecting the occurrence and development of bone metastasis, and can be used to evaluate the presence of bone metastasis (38). Clinical trials have found that elevated CEA levels in patients with breast cancer bone metastasis notably reduce the therapeutic effect (chemotherapy and radiation therapy) (39,40). A study by Duan et al (41) found that serum CEA can be used as a diagnostic biomarker for lung cancer bone metastasis. Ayan et al (42) also hypothesized that elevated CEA levels can be regarded as an early warning sign that requires precise imaging examinations as the risk of bone metastasis is higher. CA15-3 tumor markers have been widely used for the prognosis of bone metastasis in patients with breast cancer (43). A study by Fakhari et al (44) found that serum CA15-3 levels were higher in patients with bone metastasis from breast cancer, with a diagnostic threshold of >21.8 U/ml, and a sensitivity and specificity of 91.7 and 91.0%, respectively. Therefore, there is a view that CA15-3 can be regarded as a useful factor for the prognosis of bone metastasis in patients (45). However, there are few studies that can use CA15-3 to confirm bone metastasis in patients with lung cancer, and CA125 has a similar situation. Consistent with the present research conclusions, Zhou et al (46) found that CA-125 levels may be an independent risk factor for lung cancer bone metastasis (P=0.002), with a sensitivity and specificity of 32.1 and 80.8% for diagnosing bone metastasis, respectively. It was hypothesized that the incidence of bone metastasis in patients with lung cancer is high, and when patients are first diagnosed with lung cancer, their tissue pathological type and serum CA-125 concentration should be paid attention to in order to detect bone metastasis early.
Brain metastasis is the most common form of central nervous system malignancy, with ~50% of cases originating from lung cancer (47). Some studies have shown that CYFRA21-1 levels are notably associated with the overall survival time of patients with brain metastasis from lung cancer (48). Wang et al (49) found that the combined detection of CEA and CYFRA21-1 can be used as an early screening indicator for brain metastasis, especially for patients who are difficult to diagnose using cerebrospinal fluid histology and MRI examinations. In SCLC, Song et al (50) found that although CYFRA21-1 was elevated, it had no notable prognostic value. Ren et al (51) found that CYFRA21-1 was associated with metastasis in elderly patients with gonadal cancer, with a sensitivity of 70.5%, but without tissue specificity. There are few studies on related markers for lymph node and pleural metastasis of lung cancer. In a breast cancer study, Jiang et al (52) found that serum CA15-3 levels gradually increased with increasing pathological stage, and serum CA15-3 levels in patients with lymph node metastasis were markedly higher than those in patients without lymph node metastasis. Serum CA15-3 levels in patients with distal lymph node metastasis were markedly higher than those in patients with adjacent lymph node metastasis.
In complex multicellular organisms, different cells are specialized to perform different functions, often through differentiation (53). The functions of each type of cell are gradually restricted, eventually forming a specialized cell type. Tumor cells have two main characteristics: i) Unlimited proliferation; and ii) disordered differentiation (54). Taking LUAD and LUSC as an example, their treatment and prognosis are different, mainly since they originate from different cells, resulting in major differences in biological patterns (55). For example, activating mutations of epidermal growth factor receptor and mutations of ALK fusion protein often occur in LUAD, but not in LUSC (56). Traditionally, cytology and pathology are mainly used for the differential diagnosis of tumor pathological types. With the development of medicine, numerous tumors require additional molecular feature testing. Chen et al (57) used machine learning methods to find that CEA levels in LUSC were lower than those in LUAD, which is consistent with the results of the study by Zhang et al (58), which used immunohistochemical staining to distinguish LUAD and LUSC. Zengin et al (59) found that both LUAD and LUSC exhibit important genetic changes, such as SOX2 amplification in LUSC and PSMD4 amplification in LUAD. In short, the current research consensus is that NSCLC is highly heterogeneous, with different clinicopathological characteristics, serum biochemical markers and genomic profiles between LUAD and LUSC. In 2017, Imakita et al (60) first reported a patient with advanced NSCLC who was initially diagnosed with poorly differentiated carcinoma without EGFR gene mutations, which subsequently histologically transformed into SCLC after resistance to immunotherapy with nivolumab. In one study, 176 patients with SCLC tumors were analyzed, of whom 17 had NSCLC components. A total of 7 of these patients were diagnosed with NSCLC and received chemotherapy or radiotherapy, but were found to have mixed histological types at subsequent surgery or autopsy, and 2 patients were reported to have only adenocarcinoma (61). The reasons for the complexity of lung cancer remain to be studied. The ‘seed and soil hypothesis’ is an important hypothesis in cancer research (62), which holds that the success of tumor metastasis depends on the interaction between tumor cells (seeds) and their potential target organs (soil). It is now hypothesized that metastatic tumor cells need to gain a foothold in the new microenvironment, and the metastatic microenvironment may also be ready before the metastatic tumor cells actually reach the metastatic organ (63). Glycosylation is the most common post-translational modification of membrane-bound proteins, which participates in the composition of the tumor microenvironment and affects tumor metastasis (64). A number of intercellular adhesion molecules and molecules involved in intercellular communication are highly glycosylated, and they actively promote tumor progression; they can affect cell adhesion and intercellular recognition, as well as intracellular signaling. Abnormal sugar expression helps cells invade through the basement membrane, which constitutes a large part of the changes in tumor-related extracellular matrix, and the glycosylation pathway is severely dysregulated in advanced tumors (65–67). Wang et al (68) found that there is an N-glycosylation site at the Asn29 site of procollagen C proteinase enhancer protein, and cells that receive mutant forms of this glycosylation site show a phenotype of impaired migration and metastasis. CEA, CA15-3, CA125 and CA19-9 are all glycoproteins. Therefore, based on existing studies, it is hypothesized that tumors may release some tumor markers that are conducive to changing the pre-metastatic microenvironment, thereby leading to metastasis to specific organs. Glycoproteins have also become clinical markers for predicting possible metastatic target organs.
The current study also has some limitations. It is a retrospective, single cohort study based on currently limited data. The results may not be applicable to patient groups in different regions and need to be further studied by expanding the sample size through multicenter studies. Only six tumor markers were observed, and the potential of other markers as tumor organ specific remains to be studied. In particular, the present study did not explore other biomarkers that may improve diagnostic accuracy. Some patient groups were missing tumor markers, and the diagnostic ability of the marker was slightly flawed, which requires further research. Multiple markers were not analyzed together to evaluate their ability to distinguish organ metastasis. Although the patients underwent adequate physical examinations upon admission, diseases that may affect changes in tumor markers, such as inflammation, cannot be ruled out. Smoking status and certain treatment methods may affect biomarker levels. In the early stages of lung cancer, most metastases occur in a single organ, so the present study did not involve multiple organs. Future studies should also consider multiple organ metastases that are common in clinical practice.
In summary, in patients with LUAD metastasis, increased levels of peripheral blood CEA and CA15-3 can better predict the occurrence of bone metastasis, increased CYFRA21-1 is related to the occurrence of brain metastasis, and increased CA15-3 is related to lymph node and pleural metastasis. In patients with SCLC, increased levels of CEA and CA125 are associated with bone metastasis. No markers for predicting metastasis have been found in patients with LUSC. The levels of peripheral blood tumor markers CEA, CA15-3, CA125, CA19-9, SCC and CYFRA21-1 in patients with lung cancer have some clinical diagnostic value in specifically indicating the organs where lung cancer metastasizes.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Miss Rong Yu, (graduate student, Inner Mongolia Medical University, Chifeng, Inner Mongolia Autonomous Region, China), for their help and support with data collection.
Funding
This study was supported by the Public Hospital Research Joint Fund Science and Technology Project (grant no. 2024GLIH0990), the Natural Science Foundation of Inner Mongolia Autonomous Region (grant no. 2021MS08060), the Inner Mongolia Medical University Joint Project (grant no. YKD2022LH072) and the Chifeng Natural Science Foundation (grant no. SZR2023086).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
JW conceived the manuscript, checked the data, wrote the manuscript, participated in discussions, and critically commented on the research ideas. XW drafted the manuscript, analyzed the data, and revised it critically for important academic content. YM and HN participated in data acquisition and development. FZ entered and checked the data, participated in data analysis, and prepared tables and figures. DS and LW conceived, designed, and revised the article and confirm the authenticity of all original data. LW provided final review of the version to be published. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
This study was reviewed and approved by the Chifeng Municipal Hospital Ethics Committee (approval no. CK2023027). This study strictly adhered to the Declaration of Helsinki, and informed consent was obtained from all participants or their legal guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Regzedmaa O, Zhang H, Liu H and Chen J: Immune checkpoint inhibitors for small cell lung cancer: Opportunities and challenges. Onco Targets Ther. 12:4605–4620. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, Mariotto AB, Lowy DR and Feuer EJ: The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 383:640–649. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.PubMed/NCBI | |
|
Frieden TR, Cobb LK, Leidig RC, Mehta S and Kass D: Reducing premature mortality from cardiovascular and other non-communicable diseases by one third: Achieving sustainable development goal indicator 3.4.1. Glob Heart. 15:502020. View Article : Google Scholar : PubMed/NCBI | |
|
Ren Y, Cao L, Wang L, Zheng S, Zhang Q, Guo X, Li X, Chen M, Wu X, Furlong F, et al: Autophagic secretion of HMGB1 from cancer-associated fibroblasts promotes metastatic potential of non-small cell lung cancer cells via NFκB signaling. Cell Death Dis. 12:8582021. View Article : Google Scholar : PubMed/NCBI | |
|
Waqar SN, Samson PP, Robinson CG, Bradley J, Devarakonda S, Du L, Govindan R, Gao F, Puri V and Morgensztern D: Non-small-cell lung cancer with brain metastasis at presentation. Clin Lung Cancer. 19:e373–e379. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J and Hemminki K: Metastatic sites and survival in lung cancer. Lung Cancer. 86:78–84. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Mielgo A and Schmid MC: Liver tropism in cancer: The hepatic metastatic niche. Cold Spring Harb Perspect Med. 10:a0372592020. View Article : Google Scholar : PubMed/NCBI | |
|
Fanipakdel A, Seilanian Toussi M, Rezazadeh F, Mohamadian Roshan N and Javadinia SA: Overexpression of cancer-testis antigen melanoma-associated antigen A1 in lung cancer: A novel biomarker for prognosis, and a possible target for immunotherapy. J Cell Physiol. 234:12080–12086. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Guo H, Zhou X, Lu Y, Xie L, Chen Q, Keller ET, Liu Q, Zhou Q and Zhang J: Translational progress on tumor biomarkers. Thorac Cancer. 6:665–671. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Abu-Asab MS, Chaouchi M, Alesci S, Galli S, Laassri M, Cheema AK, Atouf F, VanMeter J and Amri H: Biomarkers in the age of omics: Time for a systems biology approach. OMICS. 15:105–112. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Billatos E, Vick JL, Lenburg ME and Spira AE: The airway transcriptome as a biomarker for early lung cancer detection. Clin Cancer Res. 24:2984–2992. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Fu F, Zhang Q, Li L, Liu H, Deng C, Xue Q, Zhao Y, Sun W, Han H, et al: Evolutionary proteogenomic landscape from pre-invasive to invasive lung adenocarcinoma. Cell Rep Med. 5:1013582024. View Article : Google Scholar : PubMed/NCBI | |
|
Liang H, Wang R, Cheng R, Ye Z, Zhao N, Zhao X, Huang Y, Jiang Z, Li W, Zheng J, et al: LcProt: Proteomics-based identification of plasma biomarkers for lung cancer multievent, a multicentre study. Clin Transl Med. 15:e701602025. View Article : Google Scholar : PubMed/NCBI | |
|
Liang S, Cao X, Wang Y, Leng P, Wen X, Xie G, Luo H and Yu R: Metabolomics analysis and diagnosis of lung cancer: Insights from diverse sample types. Int J Med Sci. 21:234–252. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Shi W, Cheng Y, Zhu H and Zhao L: Metabolomics and lipidomics in non-small cell lung cancer. Clin Chim Acta. 555:1178232024. View Article : Google Scholar : PubMed/NCBI | |
|
Zheng L, Hu F, Huang L, Lu J, Yang X, Xu J, Wang S, Shen Y, Zhong R, Chu T, et al: Association of metabolomics with PD-1 inhibitor plus chemotherapy outcomes in patients with advanced non-small-cell lung cancer. J Immunother Cancer. 12:e0081902024. View Article : Google Scholar : PubMed/NCBI | |
|
Li X, Li S, Zhang Z and Huang D: Association of multiple tumor markers with newly diagnosed gastric cancer patients: A retrospective study. PeerJ. 10:e134882022. View Article : Google Scholar : PubMed/NCBI | |
|
Wei Z, Zhang Y, Lu H, Ying J, Zhao H and Cai J: Serum alpha-fetoprotein as a predictive biomarker for tissue alpha-fetoprotein status and prognosis in patients with hepatocellular carcinoma. Transl Cancer Res. 11:669–677. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Shi JX, Qin JJ, Ye H, Wang P, Wang KJ and Zhang JY: Tumor associated antigens or anti-TAA autoantibodies as biomarkers in the diagnosis of ovarian cancer: A systematic review with meta-analysis. Expert Rev Mol Diagn. 15:829–852. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Fazilat-Panah D, Vakili Ahrari Roudi S, Keramati A, Fanipakdel A, Sadeghian MH, Homaei Shandiz F, Shahidsales S and Javadinia SA: Changes in cytokeratin 18 during neoadjuvant chemotherapy of breast cancer: A prospective study. Iran J Pathol. 15:117–126. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Pavlopoulou A and Scorilas A: A comprehensive phylogenetic and structural analysis of the carcinoembryonic antigen (CEA) gene family. Genome Biol Evol. 6:1314–1326. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Suzuki T, Yajima S, Okamura A, Yoshida N, Taniyama Y, Murakami K, Ohkura Y, Nakajima Y, Yagi K, Fukuda T, et al: Prognostic impact of serum SCC antigen in the 566 upfront surgery group of esophageal squamous cell carcinoma: A multi-institutional study of the Japan esophageal society. Ann Thorac Cardiovasc Surg. 30:24–00028. 2024. View Article : Google Scholar | |
|
Li X, Dai D, Chen B, Tang H, Xie X and Wei W: Clinicopathological and prognostic significance of cancer antigen 15-3 and carcinoembryonic antigen in breast cancer: A meta-analysis including 12,993 patients. Dis Markers. 2018:98630922018. View Article : Google Scholar : PubMed/NCBI | |
|
Wang R, Zuo CL, Zhang R and Zhu LM: Carcinoembryonic antigen, carbohydrate antigen 199 and carbohydrate antigen 724 in gastric cancer and their relationship with clinical prognosis. World J Gastrointest Oncol. 15:1475–1485. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Piatek S, Panek G, Lewandowski Z, Bidzinski M, Piatek D, Kosinski P and Wielgos M: Rising serum CA-125 levels within the normal range is strongly associated recurrence risk and survival of ovarian cancer. J Ovarian Res. 13:1022020. View Article : Google Scholar : PubMed/NCBI | |
|
Fischer S and Gillis J: Defining the extent of gene function using ROC curvature. Bioinformatics. 38:5390–5397. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
National Lung Screening Trial Research Team, . Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM and Sicks JD: Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 365:395–409. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Kazerooni EA, Armstrong MR, Amorosa JK, Hernandez D, Liebscher LA, Nath H, McNitt-Gray MF, Stern EJ and Wilcox PA: ACR CT accreditation program and the lung cancer screening program designation. J Am Coll Radiol. 13 (Suppl 2):R30–R34. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Wood DE, Kazerooni EA, Baum SL, Eapen GA, Ettinger DS, Hou L, Jackman DM, Klippenstein D, Kumar R, Lackner RP, et al: Lung cancer screening, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 16:412–441. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Fu Y, Liu J, Chen Y, Liu Z, Xia H and Xu H: Gender disparities in lung cancer incidence in the United States during 2001–2019. Sci Rep. 13:125812023. View Article : Google Scholar : PubMed/NCBI | |
|
Lewis DR, Check DP, Caporaso NE, Travis WD and Devesa SS: US lung cancer trends by histologic type. Cancer. 120:2883–2892. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.PubMed/NCBI | |
|
Li Y, Xiao X, Li J, Han Y, Cheng C, Fernandes GF, Slewitzke SE, Rosenberg SM, Zhu M, Byun J, et al: Lung cancer in ever- and never-smokers: Findings from multi-population GWAS studies. Cancer Epidemiol Biomarkers Prev. 33:389–399. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Wang X, Zhang T, Wu J, Yin S, Nan X, Du M, Liu A and Wang P: The association between socioeconomic status, smoking, and chronic disease in inner mongolia in northern China. Int J Environ Res Public Health. 16:1692019. View Article : Google Scholar : PubMed/NCBI | |
|
Arakil N, Akhund SA, Elaasser B and Mohammad KS: Intersecting paths: Unraveling the complex journey of cancer to bone metastasis. Biomedicines. 12:10752024. View Article : Google Scholar : PubMed/NCBI | |
|
Nozawa H, Yokota Y, Emoto S, Yokoyama Y, Sasaki K, Murono K, Abe S, Sonoda H, Shinagawa T and Ishihara S: Unexplained increases in serum carcinoembryonic antigen levels in colorectal cancer patients during the postoperative follow-up period: An analysis of its incidence and longitudinal pattern. Ann Med. 55:22469972023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao W, Li X, Wang W, Chen B, Wang L, Zhang N, Wang Z and Yang Q: Association of preoperative serum levels of CEA and CA15-3 with molecular subtypes of breast cancer. Dis Markers. 2021:55291062021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang L, Huang Y, Feng Z, Wang X, Li H, Song F, Liu L, Li J, Zheng H, Wang P, et al: Comparison of breast cancer risk factors among molecular subtypes: A case-only study. Cancer Med. 8:1882–1892. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Lee JS, Park S, Park JM, Cho JH, Kim SI and Park BW: Elevated levels of preoperative CA 15-3 and CEA serum levels have independently poor prognostic significance in breast cancer. Ann Oncol. 24:1225–1231. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Duan S, Cao H, Liu H, Miao L, Wang J, Zhou X, Wang W, Hu P, Qu L and Wu Y: Development of a machine learning-based multimode diagnosis system for lung cancer. Aging (Albany NY). 12:9840–9854. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Ayan AK, Erdemci B, Orsal E, Bayraktutan Z, Akpinar E, Topcu A, Turkeli M and Seven B: Is there any correlation between levels of serum ostepontin, CEA, and FDG uptake in lung cancer patients with bone metastasis? Rev Esp Med Nucl Imagen Mol. 35:102–106. 2016.PubMed/NCBI | |
|
Terävä J, Tiainen L, Lamminmäki U, Kellokumpu-Lehtinen PL, Pettersson K and Gidwani K: Lectin nanoparticle assays for detecting breast cancer-associated glycovariants of cancer antigen 15-3 (CA15-3) in human plasma. PLoS One. 14:e02194802019. View Article : Google Scholar : PubMed/NCBI | |
|
Fakhari A, Gharepapagh E, Dabiri S and Gilani N: Correlation of cancer antigen 15-3 (CA15-3) serum level and bony metastases in breast cancer patients. Med J Islam Repub Iran. 33:1422019.PubMed/NCBI | |
|
Pan Y, Lin Y and Mi C: Clinicopathological characteristics and prognostic risk factors of breast cancer patients with bone metastasis. Ann Transl Med. 9:13402021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou Y, Yu QF, Peng AF, Tong WL, Liu JM and Liu ZL: The risk factors of bone metastases in patients with lung cancer. Sci Rep. 7:89702017. View Article : Google Scholar : PubMed/NCBI | |
|
Gao S, Li N, Wang S, Zhang F, Wei W, Li N, Bi N, Wang Z and He J: Lung cancer in people's Republic of China. J Thorac Oncol. 15:1567–1576. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang L, Liu D, Li L, Pu D, Zhou P, Jing Y, Yu H, Wang Y, Zhu Y, He Y, et al: The important role of circulating CYFRA21-1 in metastasis diagnosis and prognostic value compared with carcinoembryonic antigen and neuron-specific enolase in lung cancer patients. BMC Cancer. 17:962017. View Article : Google Scholar : PubMed/NCBI | |
|
Wang P, Piao Y, Zhang X, Li W and Hao X: The concentration of CYFRA 21-1, NSE and CEA in cerebro-spinal fluid can be useful indicators for diagnosis of meningeal carcinomatosis of lung cancer. Cancer Biomark. 13:123–130. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Song B, Shi P, Xiao J, Song Y, Zeng M, Cao Y and Zhu X: Utility of red cell distribution width as a diagnostic and prognostic marker in non-small cell lung cancer. Sci Rep. 10:157172020. View Article : Google Scholar : PubMed/NCBI | |
|
Ren H, Hu Y, Xie T, Jin C, Hu Y and Yang B: Effect of gefitinib on serum EGFR and CYFRA21-1 in patients with advanced non-small cell lung cancer. Oncol Lett. 18:4167–4175. 2019.PubMed/NCBI | |
|
Jiang X, Guo D, Li W, Yu T, Zhou J and Gong J: Combination Twist1 and CA15-3 in axillary lymph nodes for breast cancer prognosis. Mol Med Rep. 15:1123–1134. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Gao Y, Park HJ, Traulsen A and Pichugin Y: Evolution of irreversible somatic differentiation. Elife. 10:e667112021. View Article : Google Scholar : PubMed/NCBI | |
|
Cheng Y, Liao S, Xu G, Hu J, Guo D, Du F, Contreras A, Cai KQ, Peri S, Wang Y, et al: NeuroD1 dictates tumor cell differentiation in medulloblastoma. Cell Rep. 31:1077822020. View Article : Google Scholar : PubMed/NCBI | |
|
Pan M, Huang P, Li L, Lei P, Fang L, Zhao L, Li Y, Huang S and Luo W: Comprehensive bioinformatics analysis on exportins in lung adenocarcinoma and lung squamous cell carcinoma. J Thorac Dis. 15:1872–1891. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Qin Z, Yue M, Tang S, Wu F, Sun H, Li Y, Zhang Y, Izumi H, Huang H, Wang W, et al: EML4-ALK fusions drive lung adeno-to-squamous transition through JAK-STAT activation. J Exp Med. 221:e202320282024. View Article : Google Scholar : PubMed/NCBI | |
|
Chen J, Zhang C, Xie J, Zheng X, Gu P, Liu S, Zhou Y, Wu J, Chen Y, Wang Y, et al: Automatic lung cancer subtyping using rapid on-site evaluation slides and serum biological markers. Respir Res. 25:3912024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang SL, Zhang CY, Chen YQ, Li YF, Xie Z, Zhang XC, Zhou Q, Zhong WZ, Huang J, Sun H, et al: Expression of EGFR-mutant proteins and genomic evolution in EGFR-mutant transformed small cell lung cancer. J Thorac Dis. 15:4620–4635. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Zengin T and Önal-Süzek T: Analysis of genomic and transcriptomic variations as prognostic signature for lung adenocarcinoma. BMC Bioinformatics. 21 (Suppl 14):3682020. View Article : Google Scholar : PubMed/NCBI | |
|
Imakita T, Fujita K, Kanai O, Terashima T and Mio T: Small cell lung cancer transformation during immunotherapy with nivolumab: A case report. Respir Med Case Rep. 21:52–55. 2017.PubMed/NCBI | |
|
Adelstein DJ, Tomashefski JF Jr, Snow NJ, Horrigan TP and Hines JD: Mixed small cell and non-small cell lung cancer. Chest. 89:699–704. 1986. View Article : Google Scholar : PubMed/NCBI | |
|
Ramakrishna R and Rostomily R: Seed, soil, and beyond: The basic biology of brain metastasis. Surg Neurol Int. 4 (Suppl 4):S256–S264. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Singh M, Manoranjan B, Mahendram S, McFarlane N, Venugopal C and Singh SK: Brain metastasis-initiating cells: Survival of the fittest. Int J Mol Sci. 15:9117–9133. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Peixoto A, Relvas-Santos M, Azevedo R, Santos LL and Ferreira JA: Protein glycosylation and tumor microenvironment alterations driving cancer hallmarks. Front Oncol. 9:3802019. View Article : Google Scholar : PubMed/NCBI | |
|
Hu M, Zhang R, Yang J, Zhao C, Liu W, Huang Y, Lyu H, Xiao S, Guo D, Zhou C and Tang J: The role of N-glycosylation modification in the pathogenesis of liver cancer. Cell Death Dis. 14:2222023. View Article : Google Scholar : PubMed/NCBI | |
|
Cao Y, Yi W and Zhu Q: Glycosylation in the tumor immune response: The bitter side of sweetness. Acta Biochim Biophys Sin (Shanghai). 56:1184–1198. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Ren X, Lin S, Guan F and Kang H: Glycosylation targeting: A paradigm shift in cancer immunotherapy. Int J Biol Sci. 20:2607–2621. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Wang S, Zhong L, Li Y, Xiao D, Zhang R, Liao D, Lv D, Wang X, Wang J, Xie X, et al: Up-regulation of PCOLCE by TWIST1 promotes metastasis in Osteosarcoma. Theranostics. 9:4342–4353. 2019. View Article : Google Scholar : PubMed/NCBI |