Pathogenic role and therapeutic targets of nuclear factor‑κB signaling pathway in cancer (Review)
- Authors:
- Published online on: October 2, 2025 https://doi.org/10.3892/ol.2025.15313
- Article Number: 567
-
Copyright: © Li et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Nuclear factor-κB (NF-κB) was first identified by Sen R and Baltimore D (1) in 1986 as a nuclear transcription factor that binds to the immunoglobulin κ light chain of activated B-cells. The mammalian NF-κB family consists of five subunits, including p50, p52, transcription factor p65 (RelA), transcription factor RelB and the proto-oncogene c-Rel. These proteins can combine through their Rel homologous domain to form various homo- and heterodimeric complexes with distinct DNA binding, dimerization and nuclear localization (2,3). The NF-κB signaling cascade represents a notable cellular regulatory system, which governs fundamental processes such as cell survival, proliferation, differentiation and apoptosis. As a master regulator of numerous physiological and pathological processes, the NF-κB signaling pathway has been implicated in the pathogenesis of various human diseases, particularly malignancies. The NF-κB signaling pathway consists of both classical and non-classical pathways and plays a fundamental role in multiple cancer processes, including cancer cell proliferation, apoptosis, autophagy, inflammation, tumor microenvironment interactions, therapy resistance, ion channel modulation, tumor heterogeneity and epithelial-mesenchymal transition (EMT)-mediated migration, invasion and metastasis. The present review systematically elaborates on the molecular mechanisms underpinning NF-κB signal transduction and discusses therapeutic methods for cancer treatment through NF-κB pathway modulation, which might offer potential directions for clinical translation.
NF-κB signaling pathways
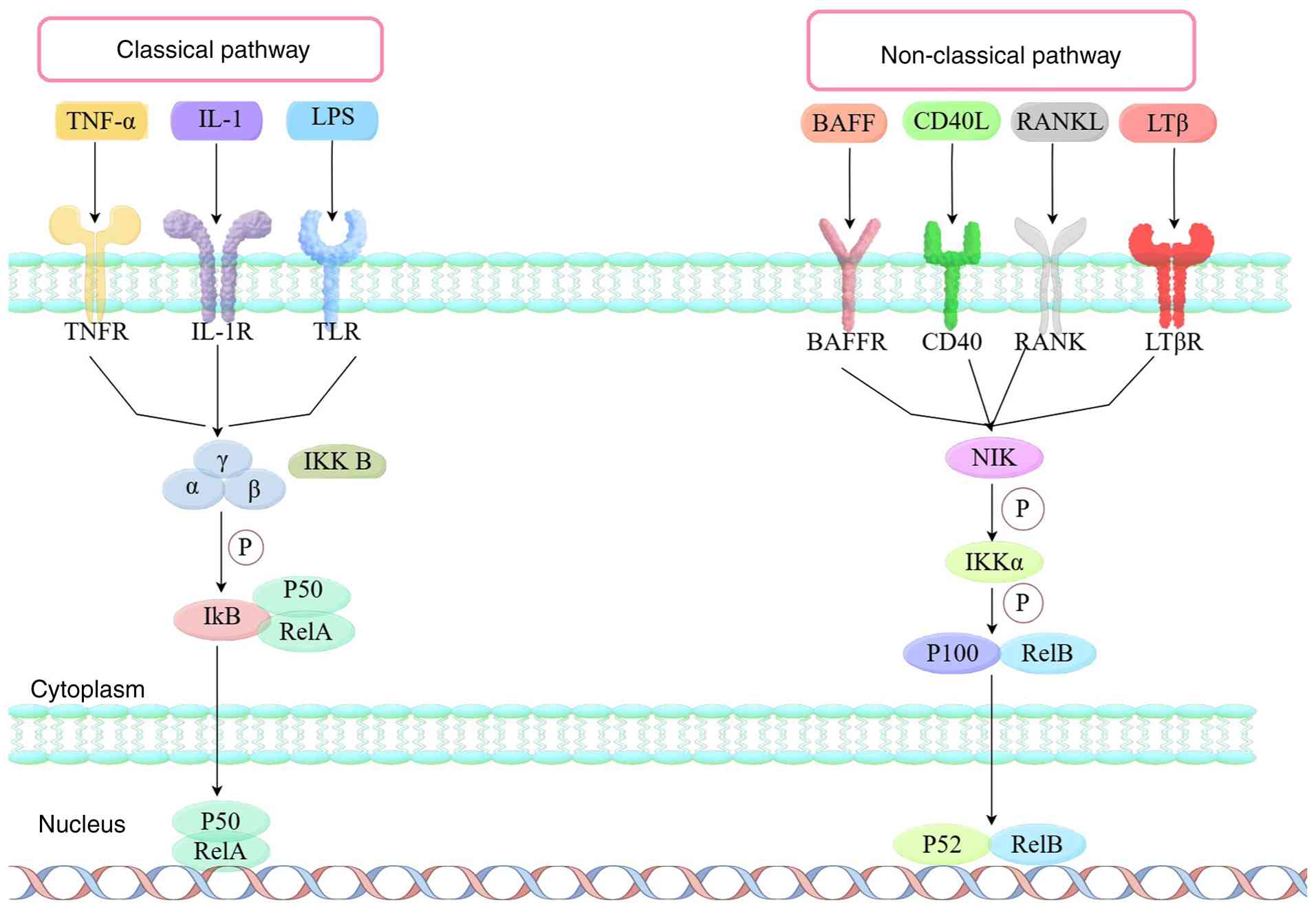
The NF-κB signaling pathway comprises both classical and non-classical signaling pathways (Fig. 1).
Classical signaling pathways: The interaction between upstream signaling molecules, such as inflammatory factors, tumor necrosis factor (TNF)α, interleukin 1 (IL-1), thrombin, lipopolysaccharide (LPS), and receptors such as the TNF receptor superfamily, T cell receptor, Toll like receptor (TLR) and B cell receptor, results in the activation of inhibitor of κ kinase (Iκ)B, which is composed of Iκα, Iκβ and Iκγ subunits. Phosphorylated IκB undergoes ubiquitination and is subsequently recognized and degraded by the 26S proteasome via the ubiquitin-proteasome pathway. Consequently, NF-κB can be released from the cytoplasmic NF-κB/IκB complex, activated and exposed to the nuclear localization domain, forming a p50/RelA dimer, which is then translocated from the cytoplasm into the nucleus. By binding to specific sites of the target gene, it initiates transcription and expression of the target genes (4–7).
The non-classical pathway is triggered by the activation of various receptors, including lymphotoxin β receptor (LTβR), B cell activating factor receptor (BAFFR), receptor activator of NF-κB (RANK) and CD40R. Upon activation of a receptor, NF-κB induced kinase (NIK) undergoes phosphorylation-mediated activation, subsequently initiating Iκα activation. The activated Iκα then promotes the phosphorylation and degradation of p100 (the precursor of p52), triggering its proteasomal processing into mature p52. After this processing, p52 binds to RelB in the cytoplasm and the resulting heterodimer translocases to the nucleus, where it modulates transcription of target genes (8,9).
NF-κB pathway-related biochemical processes
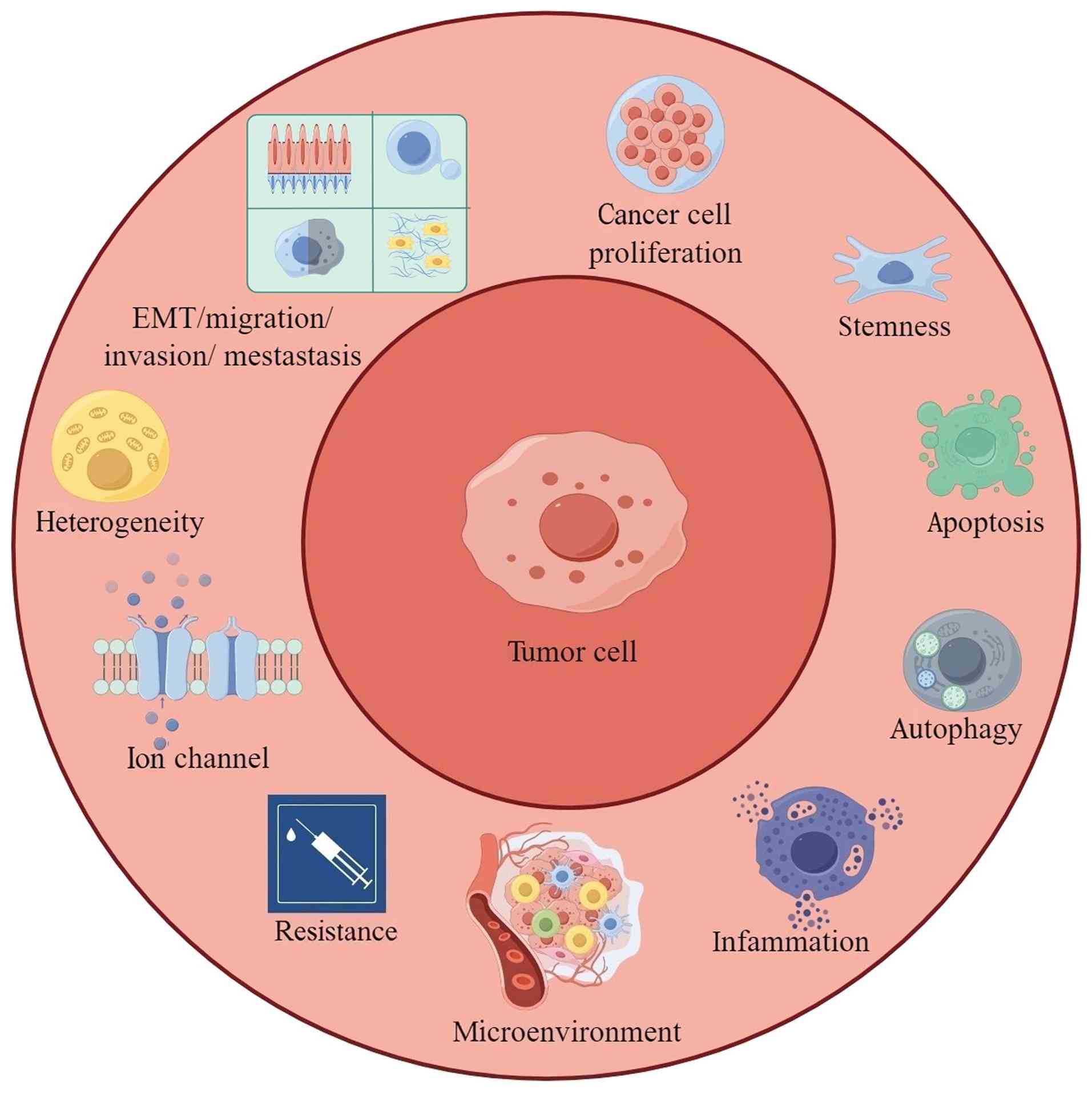
The NF-κB signaling pathway is implicated in multiple biochemical processes, such as cancer cell proliferation, stemness, apoptosis, autophagy, inflammation, microenvironment, drug resistance, ion channel regulation, tumor heterogeneity, and EMT-mediated migration, invasion and metastasis (Figs. 2 and 3).
Cancer cell proliferation
The NF-κB signaling pathway is critically involved in tumorigenesis and treatment via its regulation of genes controlling cell proliferation, migration and apoptosis (10). The tripartite motif (TRIM) protein family members are widely implicated in tumor cellular processes. TRIM52 is highly expressed in cancer and promotes the invasion, migration and proliferation of ovarian cancer cells. TRIM52 acts as a positive regulator of the NF-κB pathway, and its overexpression enhances TNF-α and IL-8 levels by activating the NF-κB signaling pathway (11). The phosphatase and tensin homolog (PTEN) is a notable tumor suppressor gene. Knockout of ubiquitin-specific protease 13 (USP13) downregulates PTEN, enhancing bladder cancer cell proliferation, invasion and migration. Furthermore, NF-κB signaling activation suppresses both USP13 and PTEN expression, thereby promoting tumorigenesis (12). Silencing Midkine (MDK) inhibits breast cancer cell proliferation and migration by reducing the expression of nuclear receptor subfamily 3 group C member 1 (NRS3C1) via inhibition of the NF-κB pathway and NF-κB nuclear distribution (13). Meis homeobox 2 (MEIS2), a crucial regulator of organ development and tumorigenesis is significantly downregulated in tumor tissues and cell lines compared with adjacent healthy counterparts. Research has found that MEIS2 inhibits the proliferation of thyroid cancer cells via regulation of the NF-κB pathway (14).
MicroRNAs (miRNAs/miRs) play a notable role in carcinogenesis, with bioinformatics analysis of cancer samples identifying notable downregulation of miR-139-5p and inhibition of gastric cancer cell proliferation by regulating peripheral myelin protein 22 (PMP22) via the NF-κB signaling pathway (15). Members of the let-7 family are abnormally expressed in human cancer, and homeobox B1 (HOXB1) is a potential target gene for the miRNA hsa-let-7. Downregulation of HOXB1 gene by hsa-let-7g activates the NF-κB pathway and promotes the development of osteosarcoma (16). In esophageal cancer cell lines, overexpression of transglutaminase 3 (TGM3) inhibits cell proliferation and induces apoptosis by activating the NF-κB signaling pathway (17). MiR-let-7a has been identified as an inhibitory factor for human tumor development and a positive regulator of ubiquitin-specific protease 35 (USP35) expression, which is closely associated with cancer development. USP35 regulates cell proliferation both in vivo (4–5 weeks of age athymic BALB/c nude mice xenograft model) and in vitro (prostate cancer cells, breast cancer cells, cervical cancer cells, lung cancer cells). USP35 promotes its de-ubiquitination to stabilize TNFAIP3-interacting protein 2 (ABIN-2) and inhibits NF-κB activation, thereby inhibiting cell proliferation (18). CXC chemokine ligand (CXCL) 16 is a membrane protein present in both transmembrane and soluble forms, which is associated with the pathogenesis of tumors. The silencing of CXCL16 not only reduces lung cancer cell proliferation and invasion, but also leads to the inactivation of the NF-κB pathway in tumor cells; thus, silencing CXCL16 regulates the NF-κB pathway to treat cancer (19).
Stemness
F-box and WD-repeat-containing protein 2 (FBXW2), as an E3 ligase, is crucial to tumorigenesis regulation. Studies have revealed that NF-κB-p65 is a novel substrate for FBXW2, and the FBXW2-p65 axis is a key regulatory factor for sex-determining region Y-box 2 (SOX2)-induced cancer-stem cells (CSCs). FBXW2 suppresses SOX2 by degrading p65, thereby promoting the stemness of breast cancer cells (20). Current cancer therapies often show limited efficacy due to chemotherapy-induced enrichment of CSCs. Metallothionein-1G (MT1G) is a cysteine-rich protein that is downregulated in CSCs. Activin A, a downstream target of NF-κB, is crucial for regulating self-renewal ability and stemness characteristics. Mechanically, MT1G inhibits NF-κB to limit the secretion of activin A, thereby suppressing pancreatic cancer cell stemness (21). TNF-α is a pro-inflammatory cytokine that is upregulated in cancer cells and is associated with cancer recurrence. Transcriptional co-activator with PDZ-binding motif (TAZ) is a transcription co-activator, and its high expression levels are associated with oncogenesis. A previous study demonstrated that TNF-α upregulates TAZ transcription via the non-classical NF-κB signaling pathway, thereby increasing breast cancer stemness (22). PTEN is a tumor suppressor gene, and it has been observed in multiple diverse types of cancer that the knockout of PTEN is closely related to tumorigenesis. CSCs are not only a renewable source of tumor cells, but also a source of antitumor drug resistance. PTEN deficiency, augmented by NF-κB signaling, promotes the generation of CSCs, indicating that the NF-κB signaling pathway has an important role in maintaining stemness in prostate cancer (23). A study has reported a positive correlation between inflammatory cytokines and CSCs. Research has determined that IL-32γ inhibits stemness and an inflammatory tumor microenvironment (TME) through inhibiting NF-κB signaling transduction, thus regulating the malignant behavior of skin carcinogenesis (24). MiRNAs have been implicated in a diverse range of biological activities, including CSC activity. Eps15 homologous domain-containing (EHD) 1 is a protein with a c-terminal comprising a member of the EHD family. A previous study has revealed that miR-590 downregulates EHD via the NF-κB signaling pathway and inhibits non-small cell lung cancer stem-like characteristics both in vitro (non-small cell lung cancer cells) and in vivo (male BALB/c nude mice xenograft model) (25).
Apoptosis
Aberrant activity of the NF-κB signaling pathway is implicated in the pathogenesis of multiple human malignant tumors by affecting cellular processes such as apoptosis. NF-κB collaborates with other transcription factors in cancer progression via subunit interactions or target gene regulation, with its activity is further modulated by crosstalk with kinases, including glycogen synthase kinase 3 β (GSK3β) (26). GSK3β is a phosphorylated glycogen synthase that is central to cell proliferation, cell apoptosis, cell migration and embryonic development. TNF-related apoptosis-inducing ligands (TRIM) can selectively target tumor cells for cell death. Experimental data has demonstrated that GSK3β exerts anti-apoptotic effects through the NF-κB signaling pathway, while TRIM21 can inhibit the activation of NF-κB by GSK3β to promote apoptosis of non-small cell lung cancer cells (27). Thioredoxin-dependent peroxide reductase (PRDX) 3 is a member of the peroxiredoxins family that is associated with the regulation of intracellular signaling pathways controlling cell apoptosis and proliferation. Silencing of overexpressed PRDX3 in cancer cells leads to ovarian cancer cell apoptosis, possibly by inhibiting the NF-κB signaling pathway (28). Previous evidence has revealed that maternally expressed gene 3 (MEG3) is a tumor suppressor gene and plays a key role in the development, progression and metastasis of cancer. The experimental data demonstrated that MEG3 is upregulated in cancer cells and promotes cell apoptosis, indicating that MEG3 is a tumor suppressor of cancer. Ectopic overexpression of MEG3 inhibits breast cancer cell growth and induces apoptosis of cancer cells by activating the NF-κB signal pathway (29).
Autophagy
Autophagy is a metabolic process that captures cellular waste for degradation in lysosomes. Autophagy primarily regulates cytoplasmic quality control by degrading proteins and organelles, while recycling intracellular components to maintain cellular homeostasis. Autophagy is upregulated in a majority of cancers to promote cell survival, proliferation and metastasis (30). During the development of cancer, apoptosis and autophagy can be triggered simultaneously, and in some cases autophagy can be suppressed by inducing apoptosis. Cells undergoing cancerous transformation are prone to utilizing any alterations in the process of apoptosis. Autophagy usually exerts an inhibitory role in the early stages of carcinogenicity and a promoting role in advanced stages of cancer (31). Autophagy is a double-edged sword. In the early stages/pre-cancerous stages, autophagy can selectively clear damaged organelles, reduce ROS production, maintain genomic stability and cellular homeostasis, thereby preventing normal cells from becoming malignant and inhibiting tumorigenesis. In the late stage/once a tumor has formed, autophagy recycles nutrients for energy, responds to hypoxia and nutrient deprivation, clears damage caused by treatment, and suppresses ROS generated by chemotherapy, thereby supporting tumor growth, enhancing adaptability, and producing treatment resistance (32). Exosomes (Exos) are membranous vesicles that fuse with targets to transfer miRNAs to the receptor cell membrane, thereby regulating cell proliferation, differentiation and apoptosis. Evidence indicates that Exos that transfer miR-1910-3p promote cancer proliferation and migration and induce autophagy. MiR-1910-3p downregulates myotubularin-related protein 3 and activates the NF-κB signaling pathway to promote breast cancer progression (33). TLRs activation of the NF-κB signaling pathway, while β-arrestin maintains the upregulated state by prolonging and altering the characteristics of NF-κB signaling. TLRs and β-arrestin can result in the development and progression of tumors by coordinating cellular signaling pathways, such as NF-κB signal transduction. β-arrestin 2 (ARRB2) is a multifunctional cellular adapter protein that regulates cellular signaling pathways. In a previous study, ARRB2 was identified to negatively regulate NF-κB activation and autophagy, thereby alleviating the progression of lung cancer induced by TLR3 and TLR4 (34). Long non-coding RNAs (lncRNAs) are typically upregulated in cancer. Highly upregulated in liver cancer (HULC) is an oncogenic lncRNA, and immunofluorescence staining further confirms that HULC may drive tumor development by promoting autophagy. HULC has been identified to be upregulated in human cancer and it was determined that the lncRNA can, to some extent, activate the NF-κB signaling pathway to drive malignant progression in an autophagy-dependent manner in liver cancer (35).
Inflammation
Inflammation is considered a hallmark of cancer and plays a notable role in the pathogenesis of multiple cancers (36). Inflammation plays a dual role in cancer progression, where it is capable of promoting or inhibiting tumor development, leading to divergent treatment outcomes. Chronic inflammation has been reported to promote tumor progression, while acute inflammation leads to antitumor immune responses (37). A study has described that dysregulation of the NF-κB pathway contributes to inflammation-related diseases as well as cancer. The pro-tumor functionality of NF-κB arises from several mechanisms that regulate diverse features of tumor progression (38). Primarily, ROS drives tumorigenesis through multiple mechanisms. Under physiological conditions, the high-mobility group box-1 (HMGB1) protein localizes to the nucleus but acts as a pro-inflammatory factor upon cellular damage. Beyond its classical role as an antioxidant enzyme mitigating lipid peroxidation, glutathione peroxidase 4 (GPX4) regulates cytokine signaling. Results of immunoprecipitation show that HMGB1 interacts with GPX4 in cancer cells, which leads to ROS accumulation and inflammation, contributing to the development of colon cancer. HMGB1 knockdown inhibits inflammation and ROS accumulation through the NF-κB pathway (39). Additionally, pro-inflammatory factors can also affect the proliferation of malignant cells. Spectrin β, non-erythrocytic 1 (SPTBN1) acts as a tumor suppressor, and its loss upregulates pro-inflammatory cytokines, such as IL-1α, IL-1β and IL-6, and enhances NF-κB transcriptional activity, driving hepatocellular carcinoma initiation and progression (40). Finally, the NF-κB signaling pathway also facilitates tumor invasion (such as VEGF, macrophage inflammatory protein-1 and CXC-chemokine ligand 8) by regulating the expression of pro-angiogenesis genes and inducing chemokines (38).
Microenvironment
The TME refers to the non-cancer cells and components present in tumors, including the molecules they produce and release. The sustained interaction between tumor cells and the TME plays a notable role in the occurrence, development, metastasis and treatment response of tumors (41). The NF-κB signaling pathway affects oncogenesis by interacting with the TME. As a crucial component of the TME, cancer-associated fibroblasts (CAFs) are a key determinant of cancer progression (42). Transforming growth factor-β (TGF-β) receptors are known tumor suppressors for cancer. In ovarian cancer cells, TGF-β increases the expression of versican in CAFs present in the TME by activating the NF-κB signaling pathway, thereby regulating the progression of ovarian cancer (43). A study has identified that the TME plays an important role in the progression of cancer. Activin B is a member of the TGF-β superfamily and is a dimeric structure containing the inhibin β subunit (INHBB). INHBB has been demonstrated to positively regulate the proliferation, migration and invasion of cancer cells in vitro (gastric cancer cells). In addition, activin B promotes the occurrence of cancer by reprogramming fibroblasts into CAFs. The overexpression of INHBB in gastric cancer cells results in activin B secretion, which activates the NF-κB signaling pathway of normal fibroblasts. The activated fibroblasts create a pro-tumor microenvironment, which can increase the tumor growth of gastric cancer through a positive feedback loop (44).
Chitinase-3 like protein 1 (CHI3L1) has been demonstrated to be upregulated in various types of tumors, which contributes to tumor progression. Experimental results indicated that CHI3L1 is upregulated in glioma cells and binds to actin α4 and NF-κB1, which result from the activation of the NF-κB signaling pathway within glioma cells and reprograms the TME to influence cancer progression; CHI3L1 was up-regulated in all disease stages of glioma, which was closely related with tumor survival, growth and invasion. Therefore, it serves as a promising therapeutic target for glioma (45). Stomatin-like protein 2 (STOML2) is a notable lipid raft component and a member of the stomatal protein superfamily. STOML2 is essential for the tumor inflammatory microenvironment, which induces angiogenesis and facilitates tumor immune escape simultaneously both in vitro and in vivo. Mechanistically, STOML2 regulates colorectal cancer proliferation, angiogenesis and immune escape through activated NF-κB signaling pathway via binding to tumor necrosis factor receptor-associated death domain protein, resulting in upregulation of Cyclin D1, VEGF and PD-L1 (46).
Tumor-associated macrophages, as one of the most important components of TME, play a dual role in promoting tumor growth and antitumor immunity (47). Evidence has revealed that tumor-derived Exos induce an immunosuppressive phenotype to promote tumor metastasis, and upregulate programmed cell death 1 ligand 1 (PD-L1) in macrophages by activating the NF-κB signaling pathway (48). PD-L1 is a biomarker on various cell surfaces, including tumor cells. The expression of PD-L1 in macrophages is associated with cancer development. Interferon-inducible 16 (IFI16), as a carcinogenic effect of PD-L1, activates a stimulator of interferon genes-(TANK-binding kinase-1) that mediate immune regulation, thereby activating the NF-κB pathway. In this manner, PD-L1 is reported to promote the progression of cervical cancer (49).
BRCA1 associated protein 1 (BAP1) is a hydrolytic enzyme. BAP1 mutations are shown to inhibit the NF-κB signaling pathway, and repress macrophage cytokine secretion and antigen presentation, thereby inducing an immunosuppressive TME and enhancing the malignant phenotype of uveal melanoma cells, ultimately promoting cell growth and metastasis (50). D2 dopamine receptor (DRD2) exerts tumor-suppressive effects by inhibiting the NF-κB signaling pathway. DRD2 also regulates the TME as it promotes M1 polarization of macrophages and triggers pyroptosis in breast cancer cells (51). Mesenchymal stem cells (MSCs) are a key component of TME and play a notable role in the development of tumors. Exos derived from tumor cells affect the biological characteristics of MSCs. For example, Exos derived from the gastric cancer cell line AGS affect the immune regulatory function of MSCs via the NF-κB signaling pathway, enhancing their ability to activate immune cells, maintaining an inflammatory environment and supporting tumor growth in gastric cancer cells (52). Rho-associated protein kinase (ROCK)-Myosin II activity and NF-κB establish a positive feedback loop initiated by ROCK regulation of IL-1α and perpetuated and amplified by IL-1α/IKKβ/NF-κB supporting amoeboid behavior in return. In addition, elevated activity of myosin II enhances cellular mechanical properties and signal secretion, thereby reshaping innate immune cell function and forming an immunosuppressive microenvironment, ultimately supporting tumor growth (53).
Resistance
Cancer remains the leading global cause of mortality. While chemotherapy is a primary treatment option, drug resistance poses a major challenge in oncology. The NF-κB signaling pathway has emerged as a key mediator of drug resistance, including chemotherapy, endocrine therapy and immune therapy resistance (54). F-box and WD-repeat-containing protein 2 (FBXW2) critically regulates tumor progression by directly binding to the p65 subunit, leading to its ubiquitination and degradation. Both in vitro (breast cancer cells) and in vivo (4-week-old female athymic nude mice xenograft model) experiments from a single study have revealed that FBXW2 overexpression eliminates p65-mediated paclitaxel resistance in breast cancer cells (20). Rac1b is an isomer of Rac1 in the Rho family of GTPases and is involved in cancer and oxaliplatin resistance. In one study, upon the upregulation of Rac1b, the NF-κB signaling pathway was activated, which promoted colorectal cancer cell proliferation and inhibited cell apoptosis. Furthermore, The NF-κB pathway ‘locks’ cancer cells in a state that is more susceptible to oxaliplatin-induced apoptosis by upregulating Rac1b (a pro-survival and anti-apoptotic protein), thereby increasing sensitivity. The core logic is a ‘pro-survival paradox’: Excessive activation of pro-survival signals paradoxically makes cells more sensitive to specific types of death (55).
TRIM47 is a potential diagnostic biomarker for tamoxifen resistance. NF-κB signaling pathway activation induces protein kinase C-ε and D3 interaction with TRIM47, forming a ternary complex. The formation of the TRIM47-PKC-ε-PKD3 complex accelerates division (proliferation) on the one hand and activates alternative pathways on the other, rendering endocrine therapy ineffective and leading to tumor expansion and drug resistance (56). Cancer progression leads to RelB upregulation, which transcriptionally activates Bcl-2, cyclin D1 and matrix metalloproteinase (MMP)1 to promote cancer cell proliferation, EMT and metastasis. A previous study suggested that the RelB-mediated non-classical NF-κB pathway is a key contributor to late-stage breast cancer progression and estrogen receptor (ER) functional impairment. In the early stages of breast cancer, the ER signaling pathway initiates tumorigenesis and promotes local tumor growth. Ultimately, in the absence of estrogen, activation of the non-canonical NF-κB signaling pathway primarily replaces ER function to maintain breast cancer metastasis (57). Research has demonstrated that epidermal growth factor receptor-tyrosine kinase inhibitor resistance develops via the NF-κB-induced upregulation of PD-L1, enabling immune escape in lung cancer (58).
The NF-κB pathway promotion of cancer cell survival and matrix fibrosis is driven by IL-1 receptor associated kinase-4 (IRAK4). IRAK4 deficiency eliminates NF-κB activity, multiple immunosuppressive factors, checkpoint ligands and hyaluronic acid synthase 2, resulting in T cell dysfunction in pancreatic ductal adenocarcinoma (59). CSCs represent promising therapeutic targets in oncology. Actin-related protein (ARP)-2/3 complexes are associated with cancer migration, invasion and differentiation. ARP subunit C1B activates the NF-κB and signal transducer and activator of transcription-3 signaling pathways by inhibiting the TRIM21-mediated degradation of IFI16 and Hu-antigen R in stem cells, thereby promoting mesenchymal phenotype maintenance and radiation resistance in glioma stem cells (60). δ-like canonical notch ligand 1 (Dll1+) cells represent quiescent CSCs. In a study on tumor initiation and progression, Dll1+ cells increased the sensitivity of tumor cells to chemotherapy and significantly eliminated tumor growth and metastasis through pharmacological inhibition of the NF-κB pathway in breast cancer (61). Heat shock protein β-1 (HSPB1) expression is upregulated in breast cancer tissues, which can promote the growth and metastasis of cancer both in vivo (4–6 weeks female BALB/c nude mice xenograft model) and in vitro (breast cancer cells). Mechanistically speaking, HSPB1 binds IκBα and promotes its ubiquitination-mediated degradation, resulting in increased nuclear translocation and activation of the NF-κB signaling pathway. Furthermore, HSPB1 overexpression leads to enhanced secretion of IL-6, which contributes further towards cancer progression. In addition, HSPB1 promotes doxorubicin resistance by protecting cancer cells from drug-induced iron-deprivation disease (62).
Ion channels
Aberrant calcium (Ca2+) signaling contributes to tumorigenesis. Mucolipin-2 (MCOLN2), as a Ca2+ ion channel, is upregulated in malignant tissues, where it promotes prostate cancer progression via IL-1β/NF-κB pathway activation (63). Previously, research has identified that chloride (Cl−) channels are pivotal in oncogenic progression. Cl− intracellular channel 1 (CLIC1) is a member of the Cl− ion channel (CLC) protein family, which are mainly expressed in the stomach, pancreas, liver and cervix. Knockout of CLIC1 significantly reduces the proliferation, migration and invasion of cervical cancer cells in vitro, as well as suppressing tumorigenesis in vivo (mouse xenograft models). At the molecular level, CLIC1 directly interacts with the IKK complex, a key kinase in the NF-κB pathway, and promotes its activation. Pyrrolidine dithiocarbamate, as an inhibitor of NF-κB, mitigates the tumor promoting effect of CLIC1 in cervical cancer cells (64). Acid-sensing ion channel 1 (ASIC1) is a non-selective cation channel that upregulates cell proliferation, apoptosis, angiogenesis and metastasis in tumor cells. For the acidic stimulation, pH 6.4 medium was prepared using lactic acid. MDA-MB-231 cells were seeded in 96-well plates and treated with control, pH 6.4, or control medium containing IL-8. Acidic treatment induced IL-8 expression in cancer cells; phosphorylation of NF-κB was induced by acidic treatment, and inhibition of NF-κB activation reduced acid-induced IL-8 expression. Acid stimulation increases the expression of ASIC1 and inhibition of the NF-κB pathway, and reduces acid-induced IL-8 expression, resulting in breast cancer progression (65).
The RNA of transient receptor potential cation channel subfamily M member 8 (TRPM8), a gene highly expressed in the prostate epithelium, triggers aseptic inflammatory states in healthy and prostate cancer cells via TLR3. Upon extracellular vesicles endocytosis, TRPM8 mRNA binds TLR3 in endosomes, thereby promoting NF-κB activation and release of pro-inflammatory signals. The NF-κB signaling pathway induces inflammation and promotes anticancer activity in cancer (66). The intermediate-conductance Ca2+-dependent potassium channel (KCa3.1) is highly expressed in the plasma membrane and in the inner mitochondrial membrane (mitoKCa3.1) of various cancer cells. By enhancing the production of mitochondrial ROS and activation of the NF-κB pathway, as well as downregulating Bcl-2 19 kDa-interacting protein, pharmacological targeting of mitoKCa3.1 significantly reduces cancer cell migration in melanoma, pancreatic ductal adenocarcinoma and breast cancer lines (67). CLC-3 is a member of the voltage-gated CLC family and is involved in the resistance of tumor cells to chemotherapy drugs (e.g., paclitaxel or doxorubicin). The upregulated expression of CLC-3 in cancer cells induces multidrug resistance by upregulating NF-κB-signal-dependent P-glycoprotein expression in lung adenocarcinoma and breast carcinoma cell lines (68).
Overexpressed voltage gated Ca2+ (CaV) channel subunit α2δ-1 is associated with the development and malignancy of various cancers. The CaV channel has been identified to exert a fundamental role in tumor biology. The activation of TLR4 upregulates the transcription and expression of α2δ-1 by modulating the NF-κB signaling pathway, thereby driving the proliferation and migration of human glioblastoma cells (69). The activation of the Ca2+ channel permits storage operation, initiation of the downstream NF-κB signaling pathway and phosphorylation of extracellular signal regulated kinase (ERK) 1/2, all of which promote the expression of the inflammatory gene cyclooxygenase (COX)-2 in gastric cancer cells (70). Ca2+ influx serves as a crucial regulator of cellular function and fate across diverse cell types. The transient receptor channel 3 (TRPC3) regulates Ca2+ flux through non-selective osmotic channels. In patients with colon cancer, knocking down TRPC3 significantly decreases the activation of the NF-κB pathway and impairs the ability of mesenchymal stem cell-transformed CAF cells to promote tumor migration and invasion (71). Extracellular pressure drives proliferation by increasing Ca2+ through the pressure-sensitive CaV3.3 channel. Extracellular pressure increases Ca2+ influx through Cav3.3, promotes proliferation through protein kinase C-β activation, and ultimately increases cell cycle protein D and proliferation through classic NF-κB signaling in colon, breast and prostate cancer cells (72).
Heterogeneity
EMT is a hallmark of cancer and is strongly correlated with cancer metastasis. The EMT status is frequently heterogeneous within a single tumor. Mechanistically, cyanidin-3-glucoside (C3G) induces EMT reversal, characterized by changes in epithelial markers and the related transcription factor Snail1. Research has found that NF-κB is attenuated by C3G in triple negative breast cancer cells, while Sirt1 is induced by C3G. Later evidence demonstrates that abrogation of Sirt1 with small interfering RNA transfection abolished NF-κB inhibition and EMT reversion by C3G. Subsequent experiment revealed that miR-138 inhibits Sirt1 through mRNA translation and is suppressed by C3G. Additionally, the inhibition of miR-138 contributes to the reactivation of Sirt1 and migration and invasive inhibition of TNBC by C3G (73). Tumor cells that have undergone EMT exhibit characteristics highly similar to those of CAFs and are capable of performing the tumor-promoting functions of CAFs. EMT has been recognized as a potential source of CAFs in solid tumors and a contributor to the heterogeneity of tumors, making it possible to initiate the invasion-metastasis cascade. EMT is reported to generate a unique phenotype of mesenchymal subpopulations that are resistant to traditional therapies, leading to cellular heterogeneity in head and neck squamous cell carcinoma and the abundance of E-cadherin being affected by NF-κB and Akt pathway (74). Non-glioblastomatous diffuse gliomas (non-GDGs) are a group of heterogeneous tumors. EMT has been verified as essential for tumor heterogeneity. Retinol binding protein 1 gene (RBP1) has been identified as one of the most upregulated genes in severe subtypes of non-GDGs. Upregulated RBP1 is implicated in the EMT process and participates in the NF-κB signaling pathway. Research has indicated that RBP1 induces phosphorylation of Iκα and enhances the expression of the NF-κB signaling pathway, thereby driving the invasion and migration characteristics of EMT, such as phenotype and cell proliferation, in non-GDG cells (75).
Low grade gliomas (LGGs) exhibit significant genetic heterogeneity. Fatty acid binding protein 5 (FABP5) is one of the most upregulated genes in malignant LGGs. EMT, which the NF-κB signaling pathway is involved in, is associated with the expression of FABP5 in LGGs, EMT. In addition, FABP5 induces Iκα phosphorylation, thereby activating the NF-κB signaling pathway. Furthermore, FABP5 enhances malignant tumors of LGG through classical activation of the NF-κB signaling pathway (76). Tumor heterogeneity arises not only from the tumor cells themselves, but also from different TMEs. The presence of nutrient-deficient hypoxic areas in the TME increases tumor occurrence and metastasis. IL-8 is a chemokine mainly produced by macrophages, neutrophils and cancer cells in the TME. TME is the main source of IL-8. The experiment found that IL-8 induces EMT in colorectal cancer cells by upregulating the phosphorylation of p65 (one of the five components of the NF-κB pathway), which is the core mechanism of TME driven cell metastasis (77). Research has suggested that CSCs exhibit heterogeneity. Annexin A3 (ANXA3) contributes to tumorigenesis across multiple cancer types, with its expression markedly upregulated in malignant tissues. Knockdown of ANXA3 has been shown to inhibit breast cancer cell invasion but promotes cell proliferation in both in vitro (breast cancer cells) and in vivo (6–8 weeks old female BALB/c nude mice xenograft model) assays and inhibits the NF-κB pathway by upregulating IκBα, leading to EMT and decreased heterogeneity of CSCs (78). In addition, cancer recurrence may stem from the chemoresistance of CSCs and their ability to reconstruct heterogeneous tumors, with the NF-κB signaling pathway involved in this process. TNF-like weak apoptosis inducer, a multifunctional cytokine important for tissue repair, has exhibited upregulation after chemotherapy to induce the activation of non-classical NF-κB, enhance the stem-like characteristics of CSCs and thus leading to ovarian cancer recurrence (79).
EMT-mediated migration, invasion and metastasis
MRG domain binding protein (MRGBP) plays a pro-cancer role in human cancers, and abnormally elevated MRGBP is considered to drive the occurrence of cancer. The expression of MRGBP is significantly upregulated in cancer tissues and is associated with EMT mediated by the Wnt/β-catenin and NF-κB/p65 pathways, which promotes colorectal cancer progression (80). DNA-damage-inducible transcript 4 (DDIT4) plays an important regulatory role in various cancer cell processes. DDIT4 overexpression has been significantly associated with lymph node metastasis in patients with cervical cancer. Knockout of DDIT4 mitigates the migratory and invasive activity of colorectal cells in vitro, and downregulates EMT related proteins and the NF-κB pathway in cancer cells. DDIT4 has also been demonstrated to promote tumor progression (81). Fermitin family member 1 (FERMT1) is broadly expressed in epithelial tissues and participates in diverse cellular processes. A study has demonstrated that FERMT1 was upregulated in nasopharyngeal carcinoma tissues. Both in vitro (cervical cancer cells) and in vivo (intraperitoneal injection mouse model) data demonstrated that knocking out FERMT1 significantly reduces cell proliferation, migration and invasion by inhibiting EMT and the NF-κB signaling pathway (82). Zipper interacting protein kinase (ZIPK) belongs to the death-related protein kinase family and has been identified as a tumor-suppressor factor. The upregulation of ZIPK promotes cell growth, migration, invasion, tumor formation and metastasis in gastric cancer nude mice. ZIPK activates Akt/IκBa/NF-κB signaling to promote EMT and metastasis in BGC-823, SGC-7901 and SNU-1 cells (83). Nucleotide-binding oligomerization domain-like receptors (NLRs) are anti-oncogenes in lung adenocarcinoma cells. NLRP2-silencing promotes cell proliferation and migration by promoting NF-κB signaling in the microenvironment, thereby inducing EMT phenotype and cytoskeletal reorganization (84).
N-myc downstream-regulated gene 1 (NDRG1) has been reported as a notable gene in cancer progression. The overexpression of NDRG1 inhibits migration, invasion and EMT in vitro (prostate cancer cells), and inhibits metastasis in vivo (5–6 weeks old male BALB/c nude mice xenograft model). In addition, miR-96-5p contributes to NDRG1 deficiency and promotes the migration and invasion of prostate cancer cells. Mechanistically, NDRG1 deficiency activates the NF-κB pathway, stimulates phosphorylation of p65 and IκBα, and induces EMT in cancer (85). S100 calcium-binding protein A14 (S100A14), is a functional regulatory factor and the expression of S100A14 in nasopharyngeal carcinoma cells and tissues has been reported to be significantly lower than in adjacent non-cancerous tissues. Overexpression of S100A14 inhibits cancer metastasis by inhibiting the NF-κB signaling pathway and reversing EMT (86). The absence of ATP-dependent DNA helicase Q4 (RECQL4), which is a member of the RECQ helicase family in cancer cells, has been shown to affect cell proliferation, invasion, apoptosis and tumorigenesis. Compared with adjacent non-tumor tissues, RECQL4 is highly expressed in esophageal squamous cell carcinoma tissues. RECQL4 activates Akt, ERK, and NF-κB, which are implicated in the proliferation and migration of esophageal squamous cell carcinoma cells (87). CAFs are key stromal cells that play a dominant role in tumor progression. Asporin, an extracellular matrix protein primarily expressed in CAFs across multiple tumor types, promotes tumor invasion and migration. Notably, it is also highly expressed in a variety of tumors (e.g., pancreatic ductal adenocarcinoma, gastric cancer and colorectal cancer), where it actively results in the invasion and migration of cancer cells. CD44 is a receptor involved in the invasion and migration of refractory cancer induced by asporin. Evidence has found that asporin interacts with CD44, activates the NF-κB/p65 signaling pathway and promotes pancreatic cancer invasion and migration by regulating EMT (88). Lipocalin2 (LCN2), as an upstream mediator of the NF-κB/snail signaling pathway, exerts a negative regulatory role in EMT, invasion and metastasis in colorectal cancer. LCN2 blocks cell proliferation, migration and invasion both in vitro (colorectal cancer cells) and in vivo (4–5 weeks old BALB/c nude mice xenograft model), and inhibits the translocation of NF-κB to the nucleus. LCN2 significantly inhibits EMT and metastasis induced by the NF-κB/snail pathway in vitro and in vivo by weakening the pathways promoter activity (89).
The inhibition of heat shock protein 90 (HSP 90) leads to EMT inhibition, motility and invasiveness by downregulating both HIF-1α and the NF-κB pathway in colorectal cancer (90). MiR-210-3p, a small endogenous non-coding RNA, is significantly elevated in prostate cancer tissues compared with the adjacent healthy tissues. Overexpression of miR-210-3p enhances EMT, invasion and migration of prostate cancer cells in vitro (prostate cancer cells) by activating the NF-κB signaling pathway (91). LncRNAs are considered to play a notable role in various cancer cell events. LncRNA MAFG antisense RNA 1 (MAFG-AS1) is significantly increased in ovarian cancer tissues and cells. MAFG-AS1 also upregulates the NF-κB signaling pathway and affects the EMT, invasion and migration of cancer cells (92).
TLRs play a crucial role in inflammation and innate immune responses by activating the NF-κB pathway. TLR2 levels are heightened in intrahepatic cholangiocarcinoma tissues and cell lines. TLR2 regulates the inflammatory response mediated by the NF-κB pathway, induces the expression of EMT markers, and promotes cancer cell migration and invasion (93). Ezrin is an important molecule involved in EMT. The expression of ezrin affects the nuclear translocation of p65 and phosphorylated IκBα in tumor cells. Ezrin affects the EMT, proliferation and migration of osteosarcoma cells through the NF-κB signaling pathway (94).
Mesencephalic astrocyte-derived neurotrophic factor (MANF) is a secreted protein induced by endoplasmic reticulum stress, which inhibits inflammation by interacting with a key subunit of p65 in NF-κB signaling pathway. MANF and albumin in liver cancer tissues are downregulated compared with the adjacent non-cancer tissues. MANF inhibits the migration and invasion of liver cancer cells by regulating the NF-κB signaling pathway (95).
The NF-κB interaction lncRNA (NKILA) has been verified to exert tumor suppressive effects in diverse cancer types (e.g., liver cancer, cervical cancer and esophageal squamous cell carcinoma). The expression level of NKILA is reduced in cervical cancer tissues and cell lines. NKILA inhibits the migration and invasion of cervical cancer cells by regulating the EMT process, which is related to NKILA-mediated inhibition of the NF-κB activation (96).
Double cortin-like kinase 1 (DCLK1), as an effective oncogene, drives malignant EMT features in colorectal cancer cells in an NF-κB dependent manner. During the EMT process of cancer cells, DCLK1 silencing in vivo (6-week-old female nude mice) inhibits the invasion and metastasis of CRC cells by regulating NF-κB signaling pathway activity (97). Sparc/osteonectin, cwcv and kazal-like domain proteoglycan 1 (SPOCK1) has been reported to act as an oncogene, participating in a cascade of metastasis reactions, including invasion, EMT and the formation of micro-metastasis. SPOCK1 has been demonstrated to be significantly elevated in pancreatic cancer tissues and is related to lymph node metastasis. SPOCK1 activates the NF-κB signaling pathway through direct interaction with IκBα, regulates the progress of EMT and promotes the migration and invasion of cancer cells (98).
Amphiregulin is a member of the epidermal growth factor (EGF) family and may play a key role in cell migration, invasion and EMT by activating the NF-κB signaling pathway in pancreatic cancer cells (99). RPB5-mediating protein (RMP) promotes the migration, invasion and progression of hepatocellular carcinoma and EMT in vitro (hepatocellular carcinoma cells), and promotes cancer metastasis in vivo (pulmonary metastases mouse model). Further research indicates that RMP activates the NF-κB pathway to inhibit Snail degradation and induce EMT and metastasis (100). T-cell immunoglobulin domain and mucin domain 4 (TIM-4) are novel growth-promoting factors in the progression of cancer. IL-6 relies on the NF-κB signaling pathway to promote TIM-4 expression in cancer cells. Both TIM-4 and IL-6 can promote the migration, invasion and EMT of non-small-cell lung cancer cells (101). N-myc downregulated gene-1 (NDRG1) plays an important role in the growth and metastasis of tumors. Knockout of NDRG1 promotes EMT in colorectal cancer cells through the NF-κB signaling pathway. The depletion of NDRG1 increases the phosphorylation level of NF-κB. NDRG1 may prevent EMT-induced metastasis via inhibition of the NF-κB signaling pathway in colorectal cancer (102).
CXCL8 is an autocrine growth factor that promotes tumor growth, invasion, angiogenesis, metastasis and resistance. Elevated CXCL8 expression in colon cancer cells is associated with enhanced metastatic potential and invasiveness. CXCL8 overexpression promotes the proliferation, migration and invasion of colon cancer cells, while promoting tumor growth in vivo (6–8 weeks BALB/c nude mice xenograft model). CXCL8 may facilitate these effects by inducing EMT by activating the phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt)/NF-κB signaling pathway (103). The expression of the oncogene Twist1 is associated with the development and metastasis of tumors. An in vitro (papillary thyroid carcinoma cells) study has shown that reducing the expression of Twist1 and reversing EMT through the NF-κB pathway can reduce the invasiveness and metastasis of papillary thyroid carcinoma cells (104). CD133 (also known as prominin-1) is considered a surface marker of CSCs in various malignant tumors (e.g., colorectal cancer, esophageal squamous cell carcinoma, and head and neck squamous cell carcinomas). CD133 induces EMT and increases in vitro (pancreatic cancer cells) invasion in pancreatic cancer cells, which is mediated by the activation of the NF-κB pathway (105). MiR-129 has been recognized as a tumor inhibitor or oncogene. As compared with normal tissues, the expression of miR-129 is notably reduced in colorectal cancer tissues and cells. Overexpression of miR-129 significantly inhibits cancer cell proliferation, migration, invasion and EMT, while inhibition of miR-129 shows the opposite effect. MiR-129 is reported to inhibit cancer cell proliferation, migration, invasion and EMT by activating the NF-κB signaling pathway in colorectal cancer cells (106).
Aquaporin 5 (AQP5), a transmembrane protein implicated in cancer progression, demonstrates elevated expression in malignant cell lines while inhibiting cancer cell invasion and metastasis both in vivo (4 to 6 weeks old male BALB/c nude mice) and in vitro (hepatocellular carcinoma cells). Downregulation of AQP5 attenuates EMT by regulating EMT-related molecules, with mechanistic investigation suggesting that AQP5 depletion partially inhibits hepatocellular carcinoma metastasis and EMT through the inactivation of the NF-κB signaling pathway (107). The upregulation of miR-940 inhibits IL-8-induced migration and invasion, while the silencing of CXC chemokine receptor type 2 (CXCR2) may mitigate this effect. The miR-940/CXCR2 system inhibits tongue squamous cell carcinoma cell migration and invasion through NF-κB signaling pathway-induced EMT processes (108). MiRNAs are notable regulators in human carcinogenesis. MiR-145-5p has been observed to be downregulated in esophageal squamous cell carcinoma tissues, where it exerts tumor suppressive effects by inhibiting cancer cell migration, invasion and EMT via the NF-κB signaling pathway (109).
Drugs and inhibitors
Development of agents/phytochemicals targeting the NF-κB pathway for cancer treatment
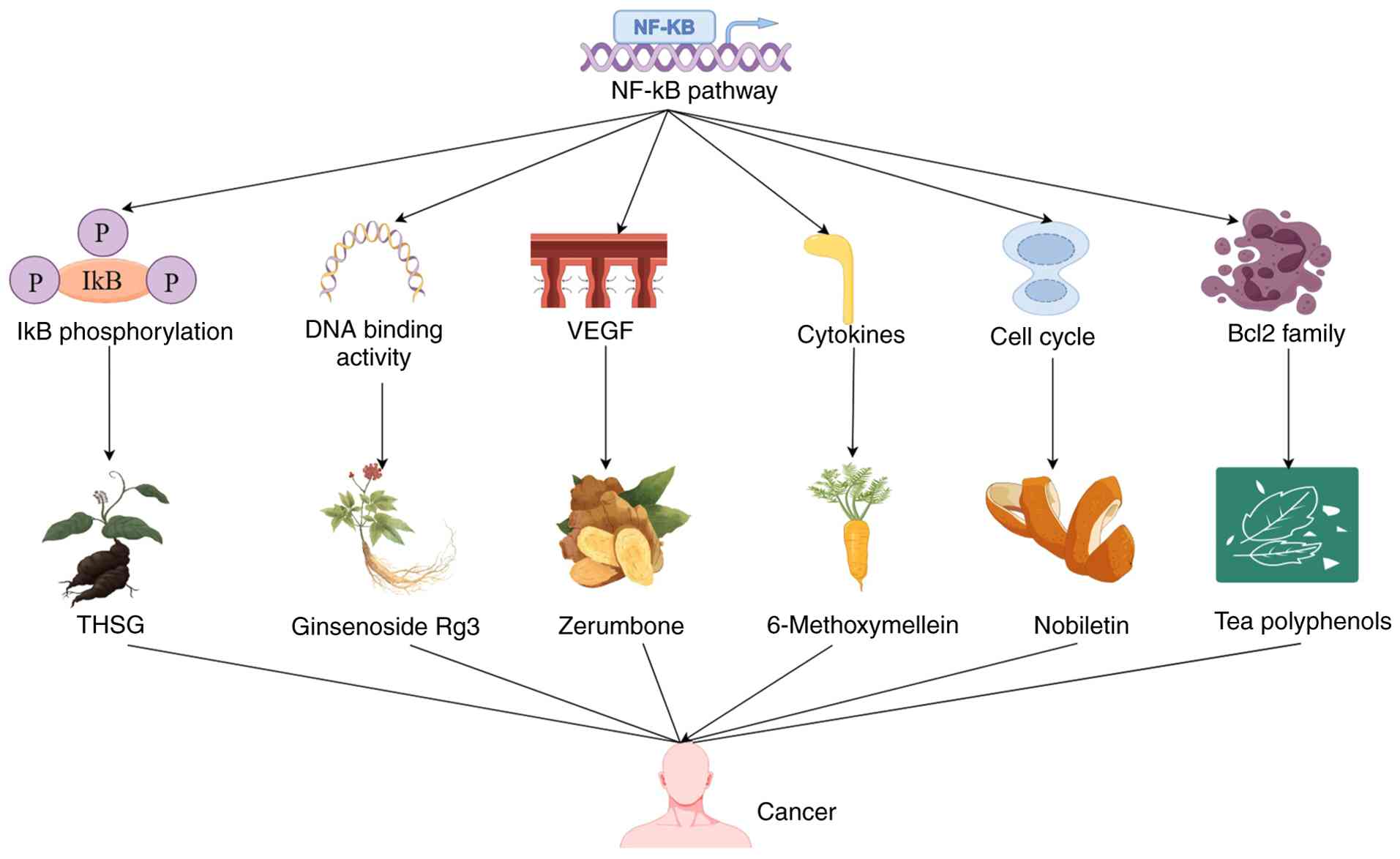
The main directions of NF-κB pathway therapy for cancer include inhibiting the phosphorylation of the IκBα protein; inhibiting the nuclear localization and DNA binding activity of NF-κB; inducing Bcl-2 family activity; downregulating the expression of vascular endothelial growth factor (VEGF); regulating the levels of cytokines that promote tumor growth (TNF, IL-6, IL-8); and inducing the cell cycle through regulation of cell cycle proteins (Table I) (110–139). Agents and phytochemicals targeting the NF-κB pathway for cancer treatment are shown in Fig. 4.
Phytochemicals can affect the progression of cancer by regulating the NF-κB signaling pathway. Curcumin is a natural non-toxic phenol isolated from turmeric. Substantial evidence suggests that curcumin has anticancer properties in various tumors. Curcumin and 5-fluorouracil have antiproliferative effects in cells, which are related to inhibiting the NF-κB pathways (140). Punicalagin is a dietary phytochemical that alters various cellular signaling pathways associated with apoptosis and proliferation. Punicalagin has demonstrated inhibition of the NF-κB signaling pathway to block cancer cell proliferation and stimulate cell apoptosis (141). Emodin was found to reduce the viability of cancer cells and induce apoptosis in a concentration-dependent manner. Emodin treatment increased the translocation of NF-κB from cytoplasm to nucleus, indicating that the NF-κB pathway is involved in emodin induced apoptosis of lung cancer cells (142). Beyond its application in the food and cosmetics industry due to its pleasant aroma, Osmanthus fragrans (OF) possesses well-documented anti-inflammatory and anticancer activities that have been applied in traditional Chinese medicine. Ethanol extract of OF has a significant antiproliferative effect on human colorectal cancer cells, and triterpenoids are important components of the ethanol component. Ethanol extract of OF also results in cell cycle arrest and apoptosis, accompanied by an increase in intracellular ROS and mitochondrial dysfunction. In addition, it inhibits the NF-κB signaling pathway, leading to a decrease in COX-2 expression (143). As the predominant polyphenol tea, epigallocatechin-3-gallate (EGCG) has demonstrated evident biological activities, functioning as an antioxidant, while offering protective benefits against cardiovascular disease and anticancer effects. Research has demonstrated that EGCG induces cancer cell apoptosis by activating caspase-8, −9, and −3, Bax, and Bcl-2. Additionally, EGCG downregulates the expression of the NF-κB and MMP-9 at both protein and mRNA levels to inhibit tumor proliferation (144). Quercetin is a flavonoid compound identified in diverse fruits and vegetables, which has demonstrated an ability to inhibit proliferation, induce apoptosis and prevent numerous types of human cancer, demonstrating its potential in cancer prevention and treatment. Quercetin also regulates the NF-κB signaling pathway and its target genes Bcl-2 and Bax to induce cell apoptosis (145).
Development of inhibiting molecules targeting the NF-κB pathway for cancer treatment
Certain biologics can regulate NF-κB signaling pathway (Table II) (146–189). For example, Hibiscus sabdariffa leaf extract has been verified to inhibit MMP-9 expression by suppressing the Akt/NF-κB signaling pathway, thereby decreasing the invasiveness of cancer cells (190). Baicalin promotes apoptosis by altering the Bax/Bcl-2 ratio. Furthermore, it regulates the NF-κB signaling pathway to induce autophagy and triggers mitotic cell cycle arrest by reducing the concentration of cyclin B1 (191). Alantolactone, an important sesquiterpene lactone derived from the Asteraceae family, enhances the expression of apoptosis regulatory proteins in cancer cells, prolongs the G2/M phase of the cell cycle and inhibits the expression of various cyclins. Furthermore, alantolactone has displayed a dose-dependent inhibition of the NF-κB signaling pathway (127). Baohuoside I is a flavonoid isolated from Epimedium koreanum Nakai and significantly inhibits the proliferation of cancer cells by inducing apoptosis and downregulating the NF-κB signaling pathway (192). Sulforaphene, a naturally occurring iso-thiocyanate identified in cruciferous vegetables, enhances the inhibitory effect of radiation on tumor growth within both in vitro (hepatocellular carcinoma cells) and in vivo (5 weeks old athymic BALB/c nude mice xenograft model) studies. Sulforaphene inhibits cell proliferation and induces apoptosis in cancer cells. It inhibits NF-κB activity and reduces downstream gene expressions of the NF-κB pathway to enhance the radiosensitivity (193). A bis-demethoxycurcumin-analog treatment has been shown to downregulate the levels of MMP-9, VEGF, TGF-β, IL-6 and IL-8, while upregulating tissue inhibitor of metalloproteinase 2 levels. By inhibiting the NF-κB pathway, it effectively inhibited key markers of invasion, angiogenesis and metastasis (194). The ethyl acetate extract of Annona muricata inhibits cancer cell proliferation, leading to cell cycle arrest and programmed cell death through activation of the mitochondrial-mediated apoptosis signaling pathway, with the involvement of the NF-κB signaling pathway (195).
Discussion
Following bone metastasis, tumor cells release various soluble mediators that stimulate osteoblasts to overexpress receptor activator of nuclear factor-κB ligand (RANKL) and inhibit osteoprotegerin (OPG) expression. As a result, the RANKL/OPG ratio is elevated, which leads to excessive osteoclast activation and bone destruction. The subsequent release of growth factors, such as transforming growth factor-β (TGF-β), further promotes tumor progression, forming a ‘vicious cycle’ (196). RANKL is a cytokine belonging to the TNF superfamily, whose main function is to activate the formation and function of osteoclasts. Most tumor cells have sustained activation of RANKL, and inhibiting RANKL can prevent bone destruction caused by tumor growth and metastasis. Therefore, RANKL is closely related to the occurrence and development of tumors. RANKL activates the non-classical NF-κB signaling pathway by binding to RANK, leading to the activation of NF-κB and playing a key role in the activation of osteoclasts in multiple myeloma. The RANKL/RANK/NF-κB pathway is the core signaling pathway regulating bone metabolism (197). P62 protein is a classic autophagy related protein, and its level is related to the degree of autophagy, thereby affecting the occurrence and development of cancer. A study has confirmed that the P62 protein, as a downstream adaptor protein of RANKL/RANK signaling, does not rely on autophagy to regulate the proliferation and invasion of giant cell tumors of bone by activating classical or non-classical NF-κB signaling pathways (198). In addition, a study has identified that the RANKL/RANK axis promotes the migration, invasion and metastasis of osteosarcoma by activating the NF-κB signaling pathway (199).
The NF-κB signaling pathway exhibits sustained abnormal activation, becoming a key driving factor for tumor occurrence and development. Hence, the NF-κB signaling pathway is considered a central target for cancer treatment. Despite NF-κB being an attractive therapeutic target, a number of NF-κB-targeted inhibitors cannot be widely applied in clinics due to detrimental side effects. A key challenge in targeting NF-κB depends on its dual role. While NF-κB activation promotes oncogenesis, its fundamental activity remains essential for normal cellular functions such as tissue homeostasis and immunity (200). Comprehensive inhibition of NF-κB can lead to side effects such as immune suppression. Furthermore, tumor heterogeneity and compensatory pathway activation often lead to drug resistance issues. In clinical applications, multiple myeloma drugs such as bortezomib indirectly inhibit NF-κB, but are subject to dose limiting toxicity and drug resistance.
To overcome the limitations of traditional inhibitors, researchers have developed an innovative method termed Highly Optimized Pol III-based Expression (HOPE), which utilizes the NF-κB-specific promoter dentin matrix acidic phosphoprotein to selectively activate the exogenous effector gene CRISPR-Cas13a in cancer cells, enabling the targeting of oncogenes such as telomerase reverse transcriptase, polo-like kinase 1 and kirsten rat sarcoma proto-oncogene at the mRNA level. Data have shown that HOPE induces cancer cell-specific death in vitro (cervical carcinoma cells, gastric cancer cells, non-small cell lung cancer cells, colorectal adenocarcinoma cells.) without affecting normal cells and significantly inhibits tumor growth without systemic toxicity in a colorectal cancer mouse model delivered by adeno-associated virus (200). Dual inhibition of NF-κB and programmed cell death protein 1/PD-L1 signaling revealed synergistic potential in overcoming tumor immune tolerance. In terms of targeted drug delivery systems, heparin bisphosphonates use targeted localization technology to reduce drug dosage to 1/50 of traditional therapies, while significantly reducing side effects.
Patient stratification is the core prerequisite for optimizing the efficacy of NF-κB inhibitors. Emerging biomarker candidates include the ubiquitin-conjugating enzyme E2S (UBE2S)/A-kinase-interacting protein 1 expression profile. In glioblastoma, UBE2S levels are positively correlated with NF-κB activity and demonstrate strong associations with tumor grading and the Ki67 proliferation index, which can serve as prognostic indicators and therapeutic response monitoring markers (201). Single cell signaling profiles reveal that HOPE treatment not only decreases the proportion of cancer cells but also significantly weakens their stemness characteristics. These phenotypic and molecular shifts might serve as early predictive biomarkers of treatment response (200).
NF-κB inhibitors retain notable potential in cancer treatment. However, traditional broad-spectrum inhibition strategies are limited by clinical challenges due to inevitable toxicity and drug resistance. Future breakthroughs in NF-κB-target management will require four key shifts: i) From systemic inhibition to tumor selective intervention; ii) from monotherapy to rational combination strategies; iii) from traditional drug delivery to intelligent delivery systems; and iv) from empirical medication to biomarker stratified precision medicine. However, these strategies demand large-scale clinical validation, particularly focusing on long-term safety profiles and drug resistance evolution issues. With a deeper understanding of the role of the NF-κB pathway in tumor heterogeneity and microenvironment regulation, and with advances in patient stratification techniques, NF-κB targeted therapies targeted therapies have potential in developing novel precision oncology treatments and ultimately improving the survival outcomes of patients with cancer.
Conclusions
The NF-κB transcription factor serves as a master regulator of cell survival, with dysregulated NF-κB signaling having been implicated in the pathogenesis of multiple human malignancies. The present review focused on the discussion of the pleiotropic roles of NF-κB in oncogenesis, particularly its involvement in diverse cellular processes, including cell proliferation, apoptosis, autophagy, invasion and metastatic potential. Dysregulated NF-κB signaling is a well-established driver of tumorigenesis, while pharmacological inhibition of this pathway has emerged as a promising therapeutic strategy. Although basic research and some clinical evidence confirm the target role of NF-κB in cancer, its safety and efficacy have not been fully confirmed. Therefore, optimal therapeutic effects must be balanced with potential safety factors.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
XL, LC and MZ drafted the manuscript. JD, FC, LY and MA reviewed the manuscript. All authors read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Sen R and Baltimore D: Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 46:705–716. 1986. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Q, Lenardo MJ and Baltimore D: 30 years of NF-κB: A blossoming of relevance to human pathobiology. Cell. 168:37–57. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Millar MW, Fazal F and Rahman A: Therapeutic targeting of NF-κB in acute lung injury: A double-edged sword. Cells. 11:33172022. View Article : Google Scholar : PubMed/NCBI | |
|
Ivanenkov YA, Balakin KV and Lavrovsky Y: Small molecule inhibitors of NF-κB and JAK/STAT signal transduction pathways as promising anti-inflammatory therapeutics. Mini Rev Med Chem. 11:55–78. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Hayden MS and Ghosh S: Shared principles in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Hoffmann A, Natoli G and Ghosh G: Transcriptional regulation via the NF-kappaB signaling module. Oncogene. 25:6706–6716. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Liu P, Li Y, Wang W, Bai Y, Jia H and Yuan Z: Role and mechanisms of the NF-ĸB signaling pathway in various developmental processes. Biomed Pharmacother. 153:1135132022. View Article : Google Scholar : PubMed/NCBI | |
|
Mulero MC, Huxford T and Ghosh G: NF-κB, IκB, and IKK: Integral components of immune system signaling. Adv Exp Med Biol. 1172:207–226. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Deka K and Li Y: Transcriptional regulation during aberrant activation of NF-κB signalling in cancer. Cells. 12:7882023. View Article : Google Scholar : PubMed/NCBI | |
|
Dolcet X, Llobet D, Pallares J and Matias-Guiu X: NF-κB in development and progression of human cancer. Virchows Arch. 446:475–482. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Yang W, Liu L, Li C, Luo N, Chen R, Li L, Yu F and Cheng Z: TRIM52 plays an oncogenic role in ovarian cancer associated with NF-κB pathway. Cell Death Dis. 9:9082018. View Article : Google Scholar : PubMed/NCBI | |
|
Man X, Piao C, Lin X, Kong C, Cui X and Jiang Y: USP13 functions as a tumor suppressor by blocking the NF-κB-mediated PTEN downregulation in human bladder cancer. J Exp Clin Cancer Res. 38:2592019. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang L, Song L, Xu Y, Xu Y, Zheng M, Zhang P and Wang Q: Midkine promotes breast cancer cell proliferation and migration by upregulating NR3C1 expression and activating the NF-κB pathway. Mol Biol Rep. 49:2953–2961. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Wen X, Liu M, Du J and Wang X: Meis homeobox 2 (MEIS2) inhibits the proliferation and promotes apoptosis of thyroid cancer cell and through the NF-κB signaling pathway. Bioengineered. 12:1766–1772. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Hou J, Zhuo H, Chen X, Cheng J, Zheng W, Zhong M and Cai J: MiR-139-5p negatively regulates PMP22 to repress cell proliferation by targeting the NF-κB signaling pathway in gastric cancer. Int J Biol Sci. 16:1218–1229. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou JL, Deng S, Fang HS, Yu G and Peng H: Hsa-let-7g promotes osteosarcoma by reducing HOXB1 to activate NF-κB pathway. Biomed Pharmacother. 109:2335–2341. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Li W, Zhang Z, Zhao W and Han N: Transglutaminase 3 protein modulates human esophageal cancer cell growth by targeting the NF-κB signaling pathway. Oncol Rep. 36:1723–1730. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Liu C, Wang L, Chen W, Zhao S, Yin C, Lin Y, Jiang A and Zhang P: USP35 activated by miR let-7a inhibits cell proliferation and NF-κB activation through stabilization of ABIN-2. Oncotarget. 6:27891–27906. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Liang K, Liu Y, Eer D, Liu J, Yang F and Hu K: High CXC chemokine ligand 16 (CXCL16) expression promotes proliferation and metastasis of lung cancer via regulating the NF-κB pathway. Med Sci Monit. 24:405–411. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Ren C, Han X, Lu C, Yang T, Qiao P, Sun Y and Yu Z: Ubiquitination of NF-κB p65 by FBXW2 suppresses breast cancer stemness, tumorigenesis, and paclitaxel resistance. Cell Death Differ. 29:381–392. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Li K, Zhang Z, Mei Y, Yang Q, Qiao S, Ni C, Yao Y, Li X, Li M, Wei D, et al: Metallothionein-1G suppresses pancreatic cancer cell stemness by limiting activin A secretion via NF-κB inhibition. Theranostics. 11:3196–3212. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Liu W, Lu X, Shi P, Yang G, Zhou Z, Li W, Mao X, Jiang D and Chen C: TNF-α increases breast cancer stem-like cells through up-regulating TAZ expression via the non-canonical NF-κB pathway. Sci Rep. 10:18042020. View Article : Google Scholar : PubMed/NCBI | |
|
Kim RJ, Bae E, Hong YK, Hong JY, Kim NK, Ahn HJ, Oh JJ and Park DS: PTEN loss-mediated Akt activation increases the properties of cancer stem-like cell populations in prostate cancer. Oncology. 87:270–279. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Lee YS, Lee CH, Bae JT, Nam KT, Moon DB, Hwang OK, Choi JS, Kim TH, Jun HO, Jung YS, et al: Inhibition of skin carcinogenesis by suppression of NF-κB dependent ITGAV and TIMP-1 expression in IL-32γ overexpressed condition. J Exp Clin Cancer Res. 37:2932018. View Article : Google Scholar : PubMed/NCBI | |
|
Wang X, Yin H, Zhang H, Hu J, Lu H, Li C, Cao M, Yan S and Cai L: NF-κB-driven improvement of EHD1 contributes to erlotinib resistance in EGFR-mutant lung cancers. Cell Death Dis. 9:4182018. View Article : Google Scholar : PubMed/NCBI | |
|
Hoesel B and Schmid JA: The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013. View Article : Google Scholar : PubMed/NCBI | |
|
Gao X, Xu F, Zhang HT, Chen M, Huang W, Zhang Q, Zeng Q and Liu L: PKCα-GSK3β-NF-κB signaling pathway and the possible involvement of TRIM21 in TRAIL-induced apoptosis. Biochem Cell Biol. 94:256–264. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Duan J, Lang Y, Song C, Xiong J, Wang Y and Yan Y: siRNA targeting of PRDX3 enhances cisplatin-induced apoptosis in ovarian cancer cells through the suppression of the NF-κB signaling pathway. Mol Med Rep. 7:1688–1694. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Wu J, Jing H, Huang G, Sun Z and Xu S: Long noncoding RNA MEG3 inhibits breast cancer growth via upregulating endoplasmic reticulum stress and activating NF-κB and p53. J Cell Biochem. 120:6789–6797. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Rangel M, Kong J, Bhatt V, Khayati K and Guo JY: Autophagy and tumorigenesis. FEBS J. 289:7177–7198. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Orlandi G, Roncucci L, Carnevale G and Sena P: Different roles of apoptosis and autophagy in the development of human colorectal cancer. Int J Mol Sci. 24:102012023. View Article : Google Scholar : PubMed/NCBI | |
|
Verzella D, Pescatore A, Capece D, Vecchiotti D, Ursini MV, Franzoso G, Alesse E and Zazzeroni F: Life, death, and autophagy in cancer: NF-κB turns up everywhere. Cell Death Dis. 11:2102020. View Article : Google Scholar : PubMed/NCBI | |
|
Wang B, Mao JH, Wang BY, Wang LX, Wen HY, Xu LJ, Fu JX and Yang H: Exosomal miR-1910-3p promotes proliferation, metastasis, and autophagy of breast cancer cells by targeting MTMR3 and activating the NF-κB signaling pathway. Cancer Lett. 489:87–99. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Kim JY, Shin JH, Kim MJ, Kang Y, Lee JS, Son J, Jeong SK, Kim D, Kim DH, Chun E and Lee KY: β-arrestin 2 negatively regulates lung cancer progression by inhibiting the TRAF6 signaling axis for NF-κB activation and autophagy induced by TLR3 and TLR4. Cell Death Dis. 14:4222023. View Article : Google Scholar : PubMed/NCBI | |
|
Liu S, Huttad L, He G, He W, Liu C, Cai D, Chen H and Qiu J: Long noncoding RNA HULC regulates the NF-κB pathway and represents a promising prognostic biomarker in liver cancer. Cancer Med. 12:5124–5136. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Taniguchi K and Karin M: NF-κB, inflammation, immunity and cancer: Coming of age. Nat Rev Immunol. 18:309–324. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y and Li Y: Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct Target Ther. 6:2632021. View Article : Google Scholar : PubMed/NCBI | |
|
Yu H, Lin L, Zhang Z, Zhang H and Hu H: Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct Target Ther. 5:2092020. View Article : Google Scholar : PubMed/NCBI | |
|
Yang Y, Yang L, Jiang S, Yang T, Lan J, Lei Y, Tan H and Pan K: HMGB1 mediates lipopolysaccharide-induced inflammation via interacting with GPX4 in colon cancer cells. Cancer Cell Int. 20:2052020. View Article : Google Scholar : PubMed/NCBI | |
|
Lin L, Chen S, Wang H, Gao B, Kallakury B, Bhuvaneshwar K, Cahn K, Gusev Y, Wang X, Wu Y, et al: SPTBN1 inhibits inflammatory responses and hepatocarcinogenesis via the stabilization of SOCS1 and downregulation of p65 in hepatocellular carcinoma. Theranostics. 11:4232–4250. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Xiao Y and Yu D: Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 221:1077532021. View Article : Google Scholar : PubMed/NCBI | |
|
Fang Z, Meng Q, Xu J, Wang W, Zhang B, Liu J, Liang C, Hua J, Zhao Y, Yu X and Shi S: Signaling pathways in cancer-associated fibroblasts: Recent advances and future perspectives. Cancer Commun (Lond). 43:3–41. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Yeung TL, Leung CS, Wong KK, Samimi G, Thompson MS, Liu J, Zaid TM, Ghosh S, Birrer MJ and Mok SC: TGF-β modulates ovarian cancer invasion by upregulating CAF-derived versican in the tumor microenvironment. Cancer Res. 73:5016–5028. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Jin Y, Cai Q, Wang L, Ji J, Sun Y, Jiang J, Wang C, Wu J, Zhang B, Zhao L, et al: Paracrine activin B-NF-κB signaling shapes an inflammatory tumor microenvironment in gastric cancer via fibroblast reprogramming. J Exp Clin Cancer Res. 42:2692023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao T, Zeng J, Xu Y, Su Z, Chong Y, Ling T, Xu H, Shi H, Zhu M, Mo Q, et al: Chitinase-3 like-protein-1 promotes glioma progression via the NF-κB signaling pathway and tumor microenvironment reprogramming. Theranostics. 12:6989–7008. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Gong H, Chen S, Liu S, Hu Q, Li Y, Li Y, Li G, Huang K, Li R and Fang L: Overexpressing lipid raft protein STOML2 modulates the tumor microenvironment via NF-κB signaling in colorectal cancer. Cell Mol Life Sci. 81:392024. View Article : Google Scholar : PubMed/NCBI | |
|
He R, He Y, Du R, Liu C, Chen Z, Zeng A and Song L: Revisiting of TAMs in tumor immune microenvironment: Insight from NF-κB signaling pathway. Biomed Pharmacother. 165:1150902023. View Article : Google Scholar : PubMed/NCBI | |
|
Morrissey SM, Zhang F, Ding C, Montoya-Durango DE, Hu X, Yang C, Wang Z, Yuan F, Fox M, Zhang HG, et al: Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. 33:2040–2058.e10. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Cai H, Yan L, Liu N, Xu M and Cai H: IFI16 promotes cervical cancer progression by upregulating PD-L1 in immunomicroenvironment through STING-TBK1-NF-κB pathway. Biomed Pharmacother. 123:1097902020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang C and Wu S: BAP1 mutations inhibit the NF-κB signaling pathway to induce an immunosuppressive microenvironment in uveal melanoma. Mol Med. 29:1262023. View Article : Google Scholar : PubMed/NCBI | |
|
Tan Y, Sun R, Liu L, Yang D, Xiang Q, Li L, Tang J, Qiu Z, Peng W, Wang Y, et al: Tumor suppressor DRD2 facilitates M1 macrophages and restricts NF-κB signaling to trigger pyroptosis in breast cancer. Theranostics. 11:5214–5231. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Shen Y, Xue C, Li X, Ba L, Gu J, Sun Z, Han Q and Zhao RC: Effects of gastric cancer cell-derived exosomes on the immune regulation of mesenchymal stem cells by the NF-κB signaling pathway. Stem Cells Dev. 28:464–476. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Georgouli M, Herraiz C, Crosas-Molist E, Fanshawe B, Maiques O, Perdrix A, Pandya P, Rodriguez-Hernandez I, Ilieva KM, Cantelli G, et al: Regional activation of myosin II in cancer cells drives tumor progression via a secretory cross-talk with the immune microenvironment. Cell. 176:757–774.e23. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Zhao B, Peng J, Tang H, Wang S, Peng S, Ye F, Wang J, Ouyang K, Li J, et al: Inhibition of NF-κB signaling unveils novel strategies to overcome drug resistance in cancers. Drug Resist Updat. 73:1010422024. View Article : Google Scholar : PubMed/NCBI | |
|
Goka ET, Chaturvedi P, Lopez DTM, Garza A and Lippman ME: RAC1b overexpression confers resistance to chemotherapy treatment in colorectal cancer. Mol Cancer Ther. 18:957–968. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Azuma K, Ikeda K, Suzuki T, Aogi K, Horie-Inoue K and Inoue S: TRIM47 activates NF-κB signaling via PKC-ε/PKD3 stabilization and contributes to endocrine therapy resistance in breast cancer. Proc Natl Acad Sci USA. 118:e21007841182021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang M, Zhang Y, Xu Z, Qian P, Sun W, Wang X, Jian Z, Xia T, Xu Y and Tang J: RelB sustains endocrine resistant malignancy: An insight of noncanonical NF-κB pathway into breast cancer progression. Cell Commun Signal. 18:1282020. View Article : Google Scholar : PubMed/NCBI | |
|
Peng S, Wang R, Zhang X, Ma Y, Zhong L, Li K, Nishiyama A, Arai S, Yano S and Wang W: EGFR-TKI resistance promotes immune escape in lung cancer via increased PD-L1 expression. Mol Cancer. 18:1652019. View Article : Google Scholar : PubMed/NCBI | |
|
Somani VK, Zhang D, Dodhiawala PB, Lander VE, Liu X, Kang LI, Chen HP, Knolhoff BL, Li L, Grierson PM, et al: IRAK4 signaling drives resistance to checkpoint immunotherapy in pancreatic ductal adenocarcinoma. Gastroenterology. 162:2047–2062. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Gao Z, Xu J, Fan Y, Zhang Z, Wang H, Qian M, Zhang P, Deng L, Shen J, Xue H, et al: ARPC1B promotes mesenchymal phenotype maintenance and radiotherapy resistance by blocking TRIM21-mediated degradation of IFI16 and HuR in glioma stem cells. J Exp Clin Cancer Res. 41:3232022. View Article : Google Scholar : PubMed/NCBI | |
|
Kumar S, Nandi A, Singh S, Regulapati R, Li N, Tobias JW, Siebel CW, Blanco MA, Klein-Szanto AJ, Lengner C, et al: Author correction: Dll1+ quiescent tumor stem cells drive chemoresistance in breast cancer through NF-κB survival pathway. Nat Commun. 13:39272022. View Article : Google Scholar : PubMed/NCBI | |
|
Liang Y, Wang Y, Zhang Y, Ye F, Luo D, Li Y, Jin Y, Han D, Wang Z, Chen B, et al: HSPB1 facilitates chemoresistance through inhibiting ferroptotic cancer cell death and regulating NF-κB signaling pathway in breast cancer. Cell Death Dis. 14:4342023. View Article : Google Scholar : PubMed/NCBI | |
|
Yu H, Xie M, Meng Z, Lo CY, Chan FL, Jiang L, Meng X and Yao X: Endolysosomal ion channel MCOLN2 (Mucolipin-2) promotes prostate cancer progression via IL-1β/NF-κB pathway. Br J Cancer. 125:1420–1431. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang W, Li X, Xu Y, Guo W, Yu H, Zhang L, Wang Y and Chen X: Acetylation-stabilized chloride intracellular channel 1 exerts a tumor-promoting effect on cervical cancer cells by activating NF-κB. Cell Oncol (Dordr). 44:557–568. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Nakanishi M, Korechika A, Yamakawa H, Kawabe N, Nakai K and Muragaki Y: Acidic microenvironment induction of interleukin-8 expression and matrix metalloproteinase-2/-9 activation via acid-sensing ion channel 1 promotes breast cancer cell progression. Oncol Rep. 45:1284–1294. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Alaimo A, Genovesi S, Annesi N, De Felice D, Subedi S, Macchia A, La Manna F, Ciani Y, Vannuccini F, Mugoni V, et al: Sterile inflammation via TRPM8 RNA-dependent TLR3-NF-κB/IRF3 activation promotes antitumor immunity in prostate cancer. EMBO J. 43:780–805. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Bachmann M, Rossa A, Varanita T, Fioretti B, Biasutto L, Milenkovic S, Checchetto V, Peruzzo R, Ahmad SA, Patel SH, et al: Pharmacological targeting of the mitochondrial calcium-dependent potassium channel KCa3.1 triggers cell death and reduces tumor growth and metastasis in vivo. Cell Death Dis. 13:10552024. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Q, Liu X, Luo Z, Wang S, Lin J, Xie Z, Li M, Li C, Cao H, Huang Q, et al: Chloride channel-3 mediates multidrug resistance of cancer by upregulating P-glycoprotein expression. J Cell Physiol. 234:6611–6623. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Fernández-Gallardo M, Corzo-Lopez A, Muñoz-Herrera D, Leyva-Leyva M, González-Ramírez R, Sandoval A, Delgado-Lezama R, Monjaraz E and Felix R: Role of the Ca2+ channel α2δ-1 auxiliary subunit in proliferation and migration of human glioblastoma cells. PLoS One. 17:e02791862022. View Article : Google Scholar : PubMed/NCBI | |
|
Wong JH, Ho KH, Nam S, Hsu WL, Lin CH, Chang CM, Wang JY and Chang WC: Store-operated Ca2+ entry facilitates the lipopolysaccharide-induced cyclooxygenase-2 expression in gastric cancer cells. Sci Rep. 7:128132017. View Article : Google Scholar : PubMed/NCBI | |
|
Xue C, Gao Y, Li X, Zhang M, Yang Y, Han Q, Sun Z, Bai C and Zhao RC: Mesenchymal stem cells derived from adipose accelerate the progression of colon cancer by inducing a MT-CAFs phenotype via TRPC3/NF-κB axis. Stem Cell Res Ther. 13:3352022. View Article : Google Scholar : PubMed/NCBI | |
|
Basson MD, Zeng B, Downey C, Sirivelu MP and Tepe JJ: Increased extracellular pressure stimulates tumor proliferation by a mechanosensitive calcium channel and PKC-β. Mol Oncol. 9:513–526. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Liang L, Liu X, He J, Shao Y, Liu J, Wang Z, Xia L, Han T and Wu P: Cyanidin-3-glucoside induces mesenchymal to epithelial transition via activating Sirt1 expression in triple negative breast cancer cells. Biochimie. 162:107–115. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Rasanen K, Sriswasdi S, Valiga A, Tang HY, Zhang G, Perego M, Somasundaram R, Li L, Speicher K, Klein-Szanto AJ, et al: Comparative secretome analysis of epithelial and mesenchymal subpopulations of head and neck squamous cell carcinoma identifies S100A4 as a potential therapeutic target. Mol Cell Proteomics. 12:3778–3792. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Wu W, Wang Y, Niu C, Wahafu A, Huo L, Guo X, Xiang J, Li X, Xie W, Bai X, et al: Retinol binding protein 1-dependent activation of NF- κB signaling enhances the malignancy of non-glioblastomatous diffuse gliomas. Cancer Sci. 113:517–528. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y, Wahafu A, Wu W, Xiang J, Huo L, Ma X, Wang N, Liu H, Bai X, Xu D, et al: FABP5 enhances malignancies of lower-grade gliomas via canonical activation of NF-κB signaling. J Cell Mol Med. 25:4487–4500. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Mi Y, Mu L, Huang K, Hu Y, Yan C, Zhao H, Ma C, Li X, Tao D and Qin J: Hypoxic colorectal cancer cells promote metastasis of normoxic cancer cells depending on IL-8/p65 signaling pathway. Cell Death Dis. 11:6102020. View Article : Google Scholar : PubMed/NCBI | |
|
Du R, Liu B, Zhou L, Wang D, He X, Xu X, Zhang L, Niu C and Liu S: Downregulation of annexin A3 inhibits tumor metastasis and decreases drug resistance in breast cancer. Cell Death Dis. 9:1262018. View Article : Google Scholar : PubMed/NCBI | |
|
Holmberg R, Robinson M, Gilbert SF, Lujano-Olazaba O, Waters JA, Kogan E, Velasquez CLR, Stevenson D, Cruz LS, Alexander LJ, et al: TWEAK-Fn14-RelB signaling cascade promotes stem cell-like features that contribute to post-chemotherapy ovarian cancer relapse. Mol Cancer Res. 21:170–186. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Long X, Hu Y, Duan S, Liu X, Huang W, Liu X, Xu Q, Song W and Zhou J: MRGBP promotes colorectal cancer metastasis via DKK1/Wnt/β-catenin and NF-κB/p65 pathways mediated EMT. Exp Cell Res. 421:1133752022. View Article : Google Scholar : PubMed/NCBI | |
|
Lin X, Yoshikawa N, Liu W, Matsukawa T, Nakamura K, Yoshihara M, Koya Y, Sugiyama M, Tamauchi S, Ikeda Y, et al: DDIT4 facilitates lymph node metastasis via the activation of NF-κB pathway and epithelial-mesenchymal transition. Reprod Sci. 30:2829–2841. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Li L, Li P, Zhang W, Zhou H, Guo E, Hu G and Zhang L: FERMT1 contributes to the migration and invasion of nasopharyngeal carcinoma through epithelial-mesenchymal transition and cell cycle arrest. Cancer Cell Int. 22:702022. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Deng Z, Wang Z, Wang D, Zhang L, Su Q, Lai Y, Li B, Luo Z, Chen X, et al: Zipper-interacting protein kinase promotes epithelial-mesenchymal transition, invasion and metastasis through AKT and NF-κB signaling and is associated with metastasis and poor prognosis in gastric cancer patients. Oncotarget. 6:8323–8338. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Li T, Li X, Mao R, Pan L, Que Y, Zhu C, Jin L and Li S: NLRP2 inhibits cell proliferation and migration by regulating EMT in lung adenocarcinoma cells. Cell Biol Int. 46:588–598. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Soror AA, Eshagh R, Fahim MR, Jamshidian A and Monfared GH: Prostate cancer invasion is promoted by the miR-96-5p-induced NDRG1 deficiency through NF-κB regulation. Klin Onkol. 38:95–101. 2024.PubMed/NCBI | |
|
Meng DF, Sun R, Liu GY, Peng LX, Zheng LS, Xie P, Lin ST, Mei Y, Qiang YY, Li CZ, et al: S100A14 suppresses metastasis of nasopharyngeal carcinoma by inhibition of NF-κB signaling through degradation of IRAK1. Oncogene. 39:5307–5322. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Lyu G, Su P, Hao X, Chen S, Ren S, Zhao Z, Gong Y, Liu Q and Shao C: RECQL4 regulates DNA damage response and redox homeostasis in esophageal cancer. Cancer Biol Med. 18:120–138. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang L, Wu H, Wang L, Zhang H, Lu J, Liang Z and Liu T: Asporin promotes pancreatic cancer cell invasion and migration by regulating the epithelial-to-mesenchymal transition (EMT) through both autocrine and paracrine mechanisms. Cancer Lett. 398:24–36. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Feng M, Feng J, Chen W, Wang W, Wu X, Zhang J, Xu F and Lai M: Lipocalin2 suppresses metastasis of colorectal cancer by attenuating NF-κB-dependent activation of snail and epithelial mesenchymal transition. Mol Cancer. 15:772016. View Article : Google Scholar : PubMed/NCBI | |
|
Nagaraju GP, Long TE, Park W, Landry JC, Taliaferro-Smith L, Farris AB, Diaz R and El-Rayes BF: Heat shock protein 90 promotes epithelial to mesenchymal transition, invasion, and migration in colorectal cancer. Mol Carcinog. 54:1147–1158. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Ren D, Yang Q, Dai Y, Guo W, Du H, Song L and Peng X: Oncogenic miR-210-3p promotes prostate cancer cell EMT and bone metastasis via NF-κB signaling pathway. Mol Cancer. 16:1172017. View Article : Google Scholar : PubMed/NCBI | |
|
Bai Y, Ren C, Wang B, Xue J, Li F, Liu J and Yang L: LncRNA MAFG-AS1 promotes the malignant phenotype of ovarian cancer by upregulating NFKB1-dependent IGF1. Cancer Gene Ther. 29:277–291. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Liu B, Yan S, Jia Y, Ma J, Wu S, Xu Y, Shang M and Mao A: TLR2 promotes human intrahepatic cholangiocarcinoma cell migration and invasion by modulating NF-κB pathway-mediated inflammatory responses. FEBS J. 283:3839–3850. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Liu P, Yang P, Zhang Z, Liu M and Hu S: Ezrin/NF-κB pathway regulates EGF-induced epithelial-mesenchymal transition (EMT), metastasis, and progression of osteosarcoma. Med Sci Monit. 24:2098–2108. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Liu J, Wu Z, Han D, Wei C, Liang Y, Jiang T, Chen L, Sha M, Cao Y, Huang F, et al: Mesencephalic astrocyte-derived neurotrophic factor inhibits liver cancer through small ubiquitin-related modifier (SUMO)ylation-related suppression of NF-κB/snail signaling pathway and epithelial-mesenchymal transition. Hepatology. 71:1262–1278. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Wang F, Jiang X and Wang P: NF-κB interaction long non-coding RNA inhibits migration, invasion and epithelial-mesenchymal transition of cervical cancer cells through inhibiting NF-κB signaling pathways. Exp Ther Med. 20:1039–1047. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Liu W, Wang S, Sun Q, Yang Z, Liu M and Tang H: DCLK1 promotes epithelial-mesenchymal transition via the PI3K/Akt/NF-κB pathway in colorectal cancer. Int J Cancer. 142:2068–2079. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Cui X, Wang Y, Lan W, Wang S, Cui Y, Zhang X, Lin Z and Piao J: SPOCK1 promotes metastasis in pancreatic cancer via NF-κB-dependent epithelial-mesenchymal transition by interacting with IκB-α. Cell Oncol (Dordr). 45:69–84. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Wang L, Wang L, Zhang H, Lu J, Zhang Z, Wu H and Liang Z: AREG mediates the epithelial-mesenchymal transition in pancreatic cancer cells via the EGFR/ERK/NF-κB signalling pathway. Oncol Rep. 43:1558–1568. 2020.PubMed/NCBI | |
|
Zhou W, Wang Q, Xu Y, Jiang J, Guo J, Yu H and Wei W: RMP promotes epithelial-mesenchymal transition through NF-κB/CSN2/snail pathway in hepatocellular carcinoma. Oncotarget. 8:40373–40388. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Liu W, Wang H, Bai F, Ding L, Huang Y, Lu C, Chen S, Li C, Yue X, Liang X, et al: IL-6 promotes metastasis of non-small-cell lung cancer by up-regulating TIM-4 via NF-κB. Cell Prolif. 53:e127762020. View Article : Google Scholar : PubMed/NCBI | |
|
Ma J, Gao Q, Zeng S and Shen H: Knockdown of NDRG1 promote epithelial-mesenchymal transition of colorectal cancer via NF-κB signaling. J Surg Oncol. 114:520–527. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Shen T, Yang Z, Cheng X, Xiao Y, Yu K, Cai X, Xia C and Li Y: CXCL8 induces epithelial-mesenchymal transition in colon cancer cells via the PI3K/Akt/NF-κB signaling pathway. Oncol Rep. 37:2095–2100. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Lv N, Shan Z, Gao Y, Guan H, Fan C, Wang H and Teng W: Twist1 regulates the epithelial-mesenchymal transition via the NF-κB pathway in papillary thyroid carcinoma. Endocrine. 51:469–477. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Nomura A, Banerjee S, Chugh R, Dudeja V, Yamamoto M, Vickers SM and Saluja AK: CD133 initiates tumors, induces epithelial-mesenchymal transition and increases metastasis in pancreatic cancer. Oncotarget. 6:8313–8322. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Z, Zhong T, Zhong J, Tang Y, Ling B and Wang L: MicroRNA-129 inhibits colorectal cancer cell proliferation, invasion and epithelial-to-mesenchymal transition by targeting SOX4. Oncol Rep. 45:612021. View Article : Google Scholar : PubMed/NCBI | |
|
He Z, Dong W, Hu J and Ren X: AQP5 promotes hepatocellular carcinoma metastasis via NF-κB-regulated epithelial-mesenchymal transition. Biochem Biophys Res Commun. 490:343–348. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Ma T, Zhao Z, Wang Z, Wang C and Zhang L: MiR-940 inhibits migration and invasion of tongue squamous cell carcinoma via regulatingCXCR2/NF-κB system-mediated epithelial-mesenchymal transition. Naunyn Schmiedebergs Arch Pharmacol. 392:1359–1369. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Mei LL, Wang WJ, Qiu YT, Xie XF, Bai J and Shi ZZ: miR-145-5p suppresses tumor cell migration, invasion and epithelial to mesenchymal transition by regulating the Sp1/NF-κB signaling pathway in esophageal squamous cell carcinoma. Int J Mol Sci. 18:18332017. View Article : Google Scholar : PubMed/NCBI | |
|
Tsuboi K, Matsuo Y, Shamoto T, Shibata T, Koide S, Morimoto M, Guha S, Sung B, Aggarwal BB, Takahashi H and Takeyama H: Zerumbone inhibits tumor angiogenesis via NF-κB in gastric cancer. Oncol Rep. 31:57–64. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Song L, Chen X, Mi L, Liu C, Zhu S, Yang T, Luo X, Zhang Q, Lu H and Liang X: Icariin-induced inhibition of SIRT6/NF-κB triggers redox mediated apoptosis and enhances anti-tumor immunity in triple-negative breast cancer. Cancer Sci. 111:4242–4256. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang T, Guo S, Zhu X, Qiu J, Deng G and Qiu C: Alpinetin inhibits breast cancer growth by ROS/NF-κB/HIF-1α axis. J Cell Mol Med. 24:8430–8440. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Sun X, Chang X, Wang Y, Xu B and Cao X: Oroxylin A suppresses the cell proliferation, migration, and EMT via NF-κB signaling pathway in human breast cancer cells. Biomed Res Int. 2019:92417692019. View Article : Google Scholar : PubMed/NCBI | |
|
Qiu J, Zhang T, Zhu X, Yang C, Wang Y, Zhou N, Ju B, Zhou T, Deng G and Qiu C: Hyperoside induces breast cancer cells apoptosis via ROS-mediated NF-κB signaling pathway. Int J Mol Sci. 21:1312019. View Article : Google Scholar : PubMed/NCBI | |
|
Yu X, Liu Y, Wang Y, Mao X, Zhang Y and Xia J: Baicalein induces cervical cancer apoptosis through the NF-κB signaling pathway. Mol Med Rep. 17:5088–5094. 2018.PubMed/NCBI | |
|
Junmin S, Hongxiang L, Zhen L, Chao Y and Chaojie W: Ginsenoside Rg3 inhibits colon cancer cell migration by suppressing nuclear factor kappa B activity. J Tradit Chin Med. 35:440–444. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Li W, Sun L, Lei J, Wu Z, Ma Q and Wang Z: Curcumin inhibits pancreatic cancer cell invasion and EMT by interfering with tumor-stromal crosstalk under hypoxic conditions via the IL-6/ERK/NF-κB axis. Oncol Rep. 44:382–392. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Kumar S, Singh R, Dutta D, Chandel S, Bhattacharya A, Ravichandiran V and Sukla S: In vitro anticancer activity of methanolic extract of Justicia adhatoda leaves with special emphasis on human breast cancer cell line. Molecules. 27:82222022. View Article : Google Scholar : PubMed/NCBI | |
|
De D, Chowdhury P, Panda SK and Ghosh U: Leaf extract and active fractions of Dillenia pentagyna Roxb. Reduce in vitro human cancer cell migration Via NF-κB pathway. Integr Cancer Ther. 21:153473542211288322022. View Article : Google Scholar : PubMed/NCBI | |
|
Jung EJ, Paramanantham A, Kim HJ, Shin SC, Kim GS, Jung JM, Hong SC, Chung KH, Kim CW and Lee WS: Identification of growth factors, cytokines and mediators regulated by Artemisia annua L. Polyphenols (pKAL) in HCT116 colorectal cancer cells: TGF-β1 and NGF-β attenuate pKAL-induced anticancer effects via NF-κB p65 upregulation. Int J Mol Sci. 23:15982022. View Article : Google Scholar : PubMed/NCBI | |
|
Jin J, Zhou M, Wang X, Liu M, Huang H, Yan F, Yu Z, Shu X, Huo X, Feng L, et al: Triptolidenol, isolated from Tripterygium wilfordii, disrupted NF-κB/COX-2 pathway by targeting ATP-binding sites of IKKβ in clear cell renal cell carcinoma. Fitoterapia. 148:1047792021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhen X, Choi HS, Kim JH, Kim SL, Liu R, Yun BS and Lee DS: Machilin D, a lignin derived from saururus chinensis, suppresses breast cancer stem cells and inhibits NF-κB signaling. Biomolecules. 10:2452020. View Article : Google Scholar : PubMed/NCBI | |
|
Wang X, Chen Z, Li X, Jiang ZK, Zhao YQ and Ping FF: Geraniin suppresses ovarian cancer growth through inhibition of NF-κB activation and downregulation of Mcl-1 expression. J Biochem Mol Toxicol. 31:2017. View Article : Google Scholar | |
|
Kong Y, Li F, Nian Y, Zhou Z, Yang R, Qiu MH and Chen C: KHF16 is a leading structure from cimicifuga foetida that suppresses breast cancer partially by inhibiting the NF-κB signaling pathway. Theranostics. 6:875–886. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Huang Y, Liu Z, Li L, Jiang M, Tang Y, Zhou L, Li J and Chen Y: Sesamin inhibits hypoxia-stimulated angiogenesis via the NF-κB p65/HIF-1α/VEGFA signaling pathway in human colorectal cancer. Food Funct. 13:8989–8997. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang H, Chen H, Jin C, Mo J and Wang H: Nobiletin flavone inhibits the growth and metastasis of human pancreatic cancer cells via induction of autophagy, G0/G1 cell cycle arrest and inhibition of NF-κB signalling pathway. J BUON. 25:1070–1075. 2020.PubMed/NCBI | |
|
Zhang Y, Zhao Y, Ran Y, Guo J, Cui H and Liu S: Alantolactone exhibits selective antitumor effects in HELA human cervical cancer cells by inhibiting cell migration and invasion, G2/M cell cycle arrest, mitochondrial mediated apoptosis and targeting Nf-κB signalling pathway. J BUON. 24:2310–2315. 2019.PubMed/NCBI | |
|
Zhang Y, Li G and Ji C: Inhibition of human cervical cancer cell growth by Salviolone is mediated via autophagy induction, cell migration and cell invasion suppression, G2/M cell cycle arrest and downregulation of Nf-κB/m-TOR/PI3K/AKT pathway. J BUON. 23:1739–1744. 2018.PubMed/NCBI | |
|
Chou YJ, Lin CC, Hsu YC, Syu JL, Tseng LM, Chiu JH, Lo JF, Lin CH and Fu SL: Andrographolide suppresses the malignancy of triple-negative breast cancer by reducing THOC1-promoted cancer stem cell characteristics. Biochem Pharmacol. 206:1153272022. View Article : Google Scholar : PubMed/NCBI | |
|
Kumar R, Bhardwaj P, Soni M, Singh R, Choudhary S, Virmani N, Asrani RK, Patial V, Sharma D, Gupta VK and Tripathi BN: Modulation of mammary tumour progression using murine model by ethanol root extract of Saussurea costus (falc.) lipsch. J Ethnopharmacol. 319:1173022024. View Article : Google Scholar : PubMed/NCBI | |
|
Rojo D, Madrid A, Martín SS, Párraga M, Silva Pinhal MA, Villena J and Valenzuela-Valderrama M: Resveratrol decreases the invasion potential of gastric cancer cells. Molecules. 27:30472022. View Article : Google Scholar : PubMed/NCBI | |
|
Chung SS, Wu Y, Okobi Q, Adekoya D, Atefi M, Clarke O, Dutta P and Vadgama JV: Proinflammatory cytokines IL-6 and TNF-α increased telomerase activity through NF-κB/STAT1/STAT3 activation, and Withaferin A inhibited the signaling in colorectal cancer cells. Mediators Inflamm. 2017:59584292017. View Article : Google Scholar : PubMed/NCBI | |
|
Han B, Jiang P, Liu W, Xu H, Li Y, Li Z, Ma H, Yu Y, Li X and Ye X: Role of daucosterol linoleate on breast cancer: Studies on apoptosis and metastasis. J Agric Food Chem. 66:6031–6041. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Paramanantham A, Kim MJ, Jung EJ, Nagappan A, Yun JW, Kim HJ, Shin SC, Kim GS and Lee WS: Pretreatment of anthocyanin from the fruit of vitis coignetiae pulliat acts as a potent inhibitor of TNF-α effect by inhibiting NF-κB-regulated genes in human breast cancer cells. Molecules. 25:23962020. View Article : Google Scholar : PubMed/NCBI | |
|
Lin CL, Hsieh SL, Leung W, Jeng JH, Huang GC, Lee CT, Lee CT and Wu CC: 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside suppresses human colorectal cancer cell metastasis through inhibiting NF-κB activation. Int J Oncol. 49:629–638. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao W, Jiang Y, Jia X, Wang X and Guo Y: Berbamine inhibits the biological activities of prostate cancer cells by modulating the ROS/NF-κB axis. Anticancer Agents Med Chem. 23:1626–1633. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Jia W, Luo S, Lai G, Li S, Huo S, Li M and Zeng X: Homogeneous polyporus polysaccharide inhibits bladder cancer by polarizing macrophages to M1 subtype in tumor microenvironment. BMC Complement Med Ther. 21:1502021. View Article : Google Scholar : PubMed/NCBI | |
|
Liu R, Choi HS, Kim SL, Kim JH, Yun BS and Lee DS: 6-Methoxymellein isolated from carrot (Daucus carota L.) targets breast cancer stem cells by regulating NF-κB signaling. Molecules. 25:43742020. View Article : Google Scholar : PubMed/NCBI | |
|
Chen X, Wang Y, Zhang L and Gao Y: Hydroxysafflor yellow A of Carthamus Tinctorius L., represses the malignant development of esophageal cancer cells via regulating NF-κB signaling pathway. Cell Biochem Biophys. 78:511–520. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Ghasemi F, Shafiee M, Banikazemi Z, Pourhanifeh MH, Khanbabaei H, Shamshirian A, Amiri Moghadam S, ArefNezhad R, Sahebkar A, Avan A and Mirzaei H: Curcumin inhibits NF-κB and Wnt/β-catenin pathways in cervical cancer cells. Pathol Res Pract. 215:1525562019. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang L, Chinnathambi A, Alharbi SA, Veeraraghavan VP, Mohan SK and Zhang G: Punicalagin promotes the apoptosis in human cervical cancer (ME-180) cells through mitochondrial pathway and by inhibiting the NF-κB signaling pathway. Saudi J Biol Sci. 27:1100–1106. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Su J, Yan Y, Qu J, Xue X, Liu Z and Cai H: Emodin induces apoptosis of lung cancer cells through ER stress and the TRIB3/NF-κB pathway. Oncol Rep. 37:1565–1572. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Han S, Lim SL, Kim H, Choi H, Lee MY, Shim SY, Le DD, Ha IJ, Lee M and Lee SG: Ethyl acetate fraction of Osmanthus fragrans var. aurantiacus and its triterpenoids suppress proliferation and survival of colorectal cancer cells by inhibiting NF-κB and COX2. J Ethnopharmacol. 319:1173622024. View Article : Google Scholar : PubMed/NCBI | |
|
Luo KW, Wei C, Lung WY, Wei XY, Cheng BH, Cai ZM and Huang WR: EGCG inhibited bladder cancer SW780 cell proliferation and migration both in vitro and in vivo via down-regulation of NF-κB and MMP-9. J Nutr Biochem. 41:56–64. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang W, Yin G, Dai J, Sun YU, Hoffman RM, Yang Z and Fan Y: Chemoprevention by quercetin of oral squamous cell carcinoma by suppression of the NF-κB signaling pathway in DMBA-treated hamsters. Anticancer Res. 37:4041–4049. 2017.PubMed/NCBI | |
|
Yu Z, Gao J, Zhang X, Peng Y, Wei W, Xu J, Li Z, Wang C, Zhou M, Tian X, et al: Characterization of a small-molecule inhibitor targeting NEMO/IKKβ to suppress colorectal cancer growth. Signal Transduct Target Ther. 7:712022. View Article : Google Scholar : PubMed/NCBI | |
|
Lawal B, Kuo YC, Wu AT and Huang HS: Therapeutic potential of EGFR/mTOR/Nf-kb targeting small molecule for the treatment of non-small cell lung cancer. Am J Cancer Res. 13:2598–2616. 2023.PubMed/NCBI | |
|
Véquaud E, Séveno C, Loussouarn D, Engelhart L, Campone M, Juin P and Barillé-Nion S: YM155 potently triggers cell death in breast cancer cells through an autophagy-NF-κB network. Oncotarget. 6:13476–13486. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Wong JH, Lui VW, Umezawa K, Ho Y, Wong EY, Ng MHL, Cheng SH, Tsang CM, Tsao SW and Chan AT: A small molecule inhibitor of NF-kappaB, dehydroxymethylepoxyquinomicin (DHMEQ), suppresses growth and invasion of nasopharyngeal carcinoma (NPC) cells. Cancer Lett. 287:23–32. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Z, Li MY, Mi C, Wang KS, Ma J and Jin X: Mollugin has an anti-cancer therapeutic effect by inhibiting TNF-α-induced NF-κB activation. Int J Mol Sci. 18:16192017. View Article : Google Scholar : PubMed/NCBI | |
|
Wu W, Chen F, Cui X, Yang L, Chen J, Zhao J, Huang D, Liu J, Yang L, Zeng J, et al: LncRNA NKILA suppresses TGF-β-induced epithelial-mesenchymal transition by blocking NF-κB signaling in breast cancer. Int J Cancer. 143:2213–2224. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang Z, Zhang J, Chen F and Sun Y: MiR-148b suppressed non-small cell lung cancer progression via inhibiting ALCAM through the NF-κB signaling pathway. Thorac Cancer. 11:415–425. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Ou J, Meng F, Liu J, Li D, Cao H and Sun B: Ovatodiolide exerts anticancer effects on human cervical cancer cells via mitotic catastrophe, apoptosis and inhibition of NF-κB pathway. J BUON. 25:87–92. 2020.PubMed/NCBI | |
|
Yin M, Ren X, Zhang X, Luo Y, Wang G, Huang K, Feng S, Bao X, Huang K, He X, et al: Selective killing of lung cancer cells by miRNA-506 molecule through inhibiting NF-κB p65 to evoke reactive oxygen species generation and p53 activation. Oncogene. 34:691–703. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Ginzburg S, Golovine KV, Makhov PB, Uzzo RG, Kutikov A and Kolenko VM: Piperlongumine inhibits NF-κB activity and attenuates aggressive growth characteristics of prostate cancer cells. Prostate. 74:177–186. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Minocha T, Das M, Rai V, Verma SS, Awasthee N, Gupta SC, Haldar C and Yadav SK: Melatonin induces apoptosis and cell cycle arrest in cervical cancer cells via inhibition of NF-κB pathway. Inflammopharmacology. 30:1411–1429. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Srivastava A, Mishra S, Avadhesh Shekher A, Rai V, Dhasmana A, Das J, Perenzoni D, Iori R and Gupta SC: Moringin, an isothiocyanate modulates multiple cellular signalling molecules in breast cancer cells. Cell Signal. 119:1111812024. View Article : Google Scholar : PubMed/NCBI | |
|
Chen X, Yuan XN, Zhang Z, Gong PJ, Yin WN, Jiang Q, Xu J, Xu XL, Gao Y, Chen WL, et al: Betulinic acid inhibits cell proliferation and migration in gastric cancer by targeting the NF-κB/VASP pathway. Eur J Pharmacol. 889:1734932020. View Article : Google Scholar : PubMed/NCBI | |
|
Lim JCW, Jeyaraj EJ, Sagineedu SR, Wong WSF and Stanslas J: SRS06, a new semisynthetic andrographolide derivative with improved anticancer potency and selectivity, inhibits nuclear factor-κB nuclear binding in the A549 non-small cell lung cancer cell line. Pharmacol. 95:70–77. 2015. View Article : Google Scholar | |
|
El-Deeb NM, Yassin AM, Al-Madboly LA and El-Hawiet A: A novel purified Lactobacillus acidophilus 20079 exopolysaccharide, LA-EPS-20079, molecularly regulates both apoptotic and NF-κB inflammatory pathways in human colon cancer. Microb Cell Fact. 17:292018. View Article : Google Scholar : PubMed/NCBI | |
|
Liu X, Gao Z, Wang X and Shen Y: Parthenolide targets NF-κB (P50) to inhibit HIF-1α-mediated metabolic reprogramming of HCC. Aging (Albany NY). 14:8346–8356. 2022.PubMed/NCBI | |
|
Leung HW, Wang Z, Yue GG, Zhao SM, Lee JK, Fung KP, Leung PC, Lau CB and Tan NH: Cyclopeptide RA-V inhibits cell adhesion and invasion in both estrogen receptor positive and negative breast cancer cells via PI3K/AKT and NF-κB signaling pathways. Biochim Biophys Acta. 1853:1827–1840. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Chen K, Pan Q, Gao Y, Yang X, Wang S, Peppelenbosch MP and Kong X: DMS triggers apoptosis associated with the inhibition of SPHK1/NF-κB activation and increase in intracellular Ca2+ concentration in human cancer cells. Int J Mol Med. 33:17–24. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Acuña UM, Mo S, Zi J, Orjala J and DE Blanco EJC: Hapalindole H induces apoptosis as an inhibitor of NF-ĸB and affects the intrinsic mitochondrial pathway in PC-3 androgen-insensitive prostate cancer cells. Anticancer Res. 38:3299–3307. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Al-Halabi R, Bou Chedid M, Abou Merhi R, El-Hajj H, Zahr H, Schneider-Stock R, Bazarbachi A and Gali-Muhtasib H: Gallotannin inhibits NFĸB signaling and growth of human colon cancer xenografts. Cancer Biol Ther. 12:59–68. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang T, Yu H, Dong G, Cai L and Bai Y: Chamaejasmine arrests cell cycle, induces apoptosis and inhibits nuclear NF-κB translocation in the human breast cancer cell line MDA-MB-231. Molecules. 18:845–858. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Annamalai G and Suresh K: [6]-Shogaol attenuates inflammation, cell proliferation via modulate NF-κB and AP-1 oncogenic signaling in 7,12-dimethylbenz[a]anthracene induced oral carcinogenesis. Biomed Pharmacother. 98:484–490. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Delma CR, Thirugnanasambandan S, Srinivasan GP, Raviprakash N, Manna SK, Natarajan M and Aravindan N: Fucoidan from marine brown algae attenuates pancreatic cancer progression by regulating p53-NFκB crosstalk. Phytochemistry. 167:1120782019. View Article : Google Scholar : PubMed/NCBI | |
|
Duan M, Hu F, Li D, Wu S and Peng N: Silencing KPNA2 inhibits IL-6-induced breast cancer exacerbation by blocking NF-κB signaling and c-Myc nuclear translocation in vitro. Life Sci. 253:1177362020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang L, Ruan J, Yan L, Li W, Wu Y, Tao L, Zhang F, Zheng S, Wang A and Lu Y: Xanthatin induces cell cycle arrest at G2/M checkpoint and apoptosis via disrupting NF-κB pathway in A549 non-small-cell lung cancer cells. Molecules. 17:3736–3750. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu J, Zhao J, Yu Z, Shrestha S, Song J, Liu W, Lan W, Xing J, Liu S, Chen C, et al: Epoxymicheliolide, a novelguaiane-type sesquiterpene lactone, inhibits NF-κB/COX-2 signaling pathways by targeting leucine 281 and leucine 25 in IKKβ in renal cell carcinoma. Int J Oncol. 53:987–1000. 2018.PubMed/NCBI | |
|
Wang R, Ma Y, Zhan S, Zhang G, Cao L, Zhang X, Shi T and Chen W: B7-H3 promotes colorectal cancer angiogenesis through activating the NF-κB pathway to induce VEGFA expression. Cell Death Dis. 11:552020. View Article : Google Scholar : PubMed/NCBI | |
|
Al-Saeedi FJ: Asiaticoside increases caspase-9 activity in MCF-7 cells and inhibits TNF-α and IL-6 expression in nude mouse xenografts via the NF-κB pathway. Molecules. 28:21012023. View Article : Google Scholar : PubMed/NCBI | |
|
Shiode Y, Kodama T, Shigeno S, Murai K, Tanaka S, Newberg JY, Kondo J, Kobayashi S, Yamada R, Hikita H, et al: TNF receptor-related factor 3 inactivation promotes the development of intrahepatic cholangiocarcinoma through NF-κB-inducing kinase-mediated hepatocyte transdifferentiation. Hepatology. 77:395–410. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang P, Xun W, Han T and Cheng Z: FAIM-S functions as a negative regulator of NF-κB pathway and blocks cell cycle progression in NSCLC cells. Cell Cycle. 19:3458–3467. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Chang H, Xu Q, Li J, Li M, Zhang Z, Ma H and Yang X: Lactate secreted by PKM2 upregulation promotes galectin-9-mediated immunosuppression via inhibiting NF-κB pathway in HNSCC. Cell Death Dis. 12:7252021. View Article : Google Scholar : PubMed/NCBI | |
|
Karelia DN, Kim S, M KP, Plano D, Amin S, Lu J and Sharma AK: Novel seleno-aspirinyl compound AS-10 induces apoptosis, G1 arrest of pancreatic ductal adenocarcinoma cells, inhibits their NF-κB signaling, and synergizes with gemcitabine cytotoxicity. Int J Mol Sci. 22:49662021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang X, Wang F, Zhang ZG, Yang XM and Zhang R: STK3 suppresses ovarian cancer progression by activating NF-κB signaling to recruit CD8+ T-cells. J Immunol Res. 2020:72636022020. View Article : Google Scholar : PubMed/NCBI | |
|
Yodkeeree S, Pompimon W and Limtrakul P: Crebanine, an aporphine alkaloid, sensitizes TNF-α-induced apoptosis and suppressed invasion of human lung adenocarcinoma cells A549 by blocking NF-κB-regulated gene products. Tumour Biol. 35:8615–8624. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Zhang ZC, Yuan XS, Tang SS, Wang T, Liu HF and Cao Y: TOPK affects autophagy of skin squamous cell carcinoma by regulating NF-KB pathway through HDAC1. Dis Markers. 2022:37717112022.PubMed/NCBI | |
|
Hai Ping P, Feng Bo T, Li L, Nan Hui Y and Hong Z: IL-1β/NF-kb signaling promotes colorectal cancer cell growth through miR-181a/PTEN axis. Arch Biochem Biophys. 604:20–26. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Fang X, Shi H and Sun F: The microRNA-520a-3p inhibits invasion and metastasis by targeting NF-kappaB signaling pathway in non-small cell lung cancer. Clin Transl Oncol. 24:1569–1579. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Sheng YH, He Y, Hasnain SZ, Wang R, Tong H, Clarke DT, Lourie R, Oancea I, Wong KY, Lumley JW, et al: MUC13 protects colorectal cancer cells from death by activating the NF-κB pathway and is a potential therapeutic target. Oncogene. 36:700–713. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Yong-Zheng X, Wan-Li M, Ji-Ming M and Xue-Qun R: Receptor for activated protein kinase C 1 suppresses gastric tumor progression through nuclear factor-κB pathway. Indian J Cancer. 52 (Suppl 3):E172–E175. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Barbie TU, Alexe G, Aref AR, Li S, Zhu Z, Zhang X, Imamura Y, Thai TC, Huang Y, Bowden M, et al: Targeting an IKBKE cytokine network impairs triple-negative breast cancer growth. J Clin Invest. 124:5411–5423. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Lim EY, Park J, Kim YT and Kim MJ: Imipramine inhibits migration and invasion in metastatic castration-resistant prostate cancer PC-3 cells via AKT-mediated NF-κB signaling pathway. Molecules. 25:46192020. View Article : Google Scholar : PubMed/NCBI | |
|
Chen MC, Lee NH, Hsu HH, Ho TJ, Tu CC, Chen RJ, Lin YM, Viswanadha VP, Kuo WW and Huang CY: Inhibition of NF-κB and metastasis in irinotecan (CPT-11)-resistant LoVo colon cancer cells by thymoquinone via JNK and p38. Environ Toxicol. 32:669–678. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Xu CY, Qin MB, Tan L, Liu SQ and Huang JA: NIBP impacts on the expression of E-cadherin, CD44 and vimentin in colon cancer via the NF-κB pathway. Mol Med Rep. 13:5379–5385. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang J, Zhang DL, Jiao XL and Dong Q: S100A4 regulates migration and invasion in hepatocellular carcinoma HepG2 cells via NF-κB-dependent MMP-9 signal. Eur Rev Med Pharmacol Sci. 17:2372–2382. 2013.PubMed/NCBI | |
|
Chiu CT, Chen JH, Chou FP and Lin HH: Hibiscus sabdariffa leaf extract inhibits human prostate cancer cell invasion via down-regulation of Akt/NF-κB/MMP-9 pathway. Nutrients. 7:5065–5087. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Yi S, Liu G, Wu Y, Liang Q and Li L: Baicalein suppresses the growth of the human thyroid cancer cells by inducing mitotic catastrophe, apoptosis and autophagy via NF-κB signalling pathway. J BUON. 25:389–394. 2020.PubMed/NCBI | |
|
Sun Y, Pang B, Wang Y, Xiao J and Jiang D: Baohuoside I inhibits the proliferation of hepatocellular carcinoma cells via apoptosis signaling and NF-κB pathway. Chem Biodivers. 18:e21000632021. View Article : Google Scholar : PubMed/NCBI | |
|
Ren K, Li Z, Li Y, Zhang W and Han X: Sulforaphene enhances radiosensitivity of hepatocellular carcinoma through suppression of the NF-κB pathway. J Biochem Mol Toxicol. 31:2017. View Article : Google Scholar | |
|
Mohankumar K, Francis AP, Pajaniradje S and Rajagopalan R: Synthetic curcumin analog: Inhibiting the invasion, angiogenesis, and metastasis in human laryngeal carcinoma cells via NF-κB pathway. Mol Biol Rep. 48:6065–6074. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Moghadamtousi SZ, Kadir HA, Paydar M, Rouhollahi E and Karimian H: Annona muricata leaves induced apoptosis in A549 cells through mitochondrial-mediated pathway and involvement of NF-κB. BMC Complement Altern Med. 14:2992014. View Article : Google Scholar : PubMed/NCBI | |
|
Mohammad KS and Guise TA: Breaking down barriers to chemoresistance: Role of chemotherapy-induced osteoblastic Jagged1. Cancer Cell. 32:717–718. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Giuliani N, Colla S and Rizzoli V: New insight in the mechanism of osteoclast activation and formation in multiple myeloma: Focus on the receptor activator of NF-kappaB ligand (RANKL). Exp Hematol. 32:685–691. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Liu S, Li DQ, Ye F and Zhang J: Research progress in mechanism of action of p62 protein in primary bone tumors. Medical Recapitulate. 26:2945–2950. 2020.(In Chinese). | |
|
Takeda T, Tsubaki M, Genno S, Tomita K and Nishida S: RANK/RANKL axis promotes migration, invasion, and metastasis of osteosarcoma via activating NF-κB pathway. Exp Cell Res. 436:1139782024. View Article : Google Scholar : PubMed/NCBI | |
|
Dai W, Wu J, Shui Y, Wu Q, Wang J and Xia X: NF-κB-activated oncogene inhibition strategy for cancer gene therapy. Cancer Gene Ther. 31:1632–1645. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Han Z, Xu L, Wang A, Wang B, Liu Q, Liu H, Liu Q, Gang Z, Yu S, Mu L, et al: UBE2S facilitates glioblastoma progression through activation of the NF-κB pathway via attenuating K11-linked ubiquitination of AKIP1. Int J Biol Macromol. 278:1344262024. View Article : Google Scholar : PubMed/NCBI |