Prognostic value of p53 expression in comparison with CEA, CA15‑3 and CA125 protein levels in patients with breast cancer
- Authors:
- Published online on: May 2, 2025 https://doi.org/10.3892/wasj.2025.348
- Article Number: 60
-
Copyright : © El‑Shenawy et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
Introduction
Breast cancer is a heterogeneous disease in which breast cells mutate, leading to the proliferation of cancerous cells that form tumors. This process typically begins with excessive growth in the milk ducts, which can progress to benign tumors or even metastatic carcinomas due to continuous exposure to various cancer-causing factors (1). Female breast cancer ranks second in incidence following lung cancer. It can affect females of any age following puberty, regardless of geographic location (2).
According to the World Health Organization (WHO), breast cancer is the most frequently diagnosed type of cancer globally, accounting for 11.7% of all cancer cases (1,3,4). Breast cancer is the leading cause of mortality among women. In 2020, there were 2.3 million new cases of breast cancer detected in women globally, resulting in 670,000 related deaths. This means that 1 in every 8 patients with cancer is affected by breast cancer, solidifying its status as the most prevalent type of cancer worldwide (5).
While developed regions report the highest rates of breast cancer incidence, countries in Asia and Africa accounted for 63% of total related deaths in 2020. The incidence and mortality rates of breast cancer have increased over time. In 60 out of 102 nations, including Afghanistan, The Philippines, Brazil and Argentina, the incidence of breast cancer has more than doubled. Similarly, in 43 out of 102 countries, mortality rates from the disease have also doubled, with notable increases observed in Yemen, Paraguay, Libya and Saudi Arabia. Current projections indicate that by 2030, the annual number of new breast cancer cases will reach 2.7 million, with 870,000 fatalities (6). Breast cancer is the most prevalent type of cancer among Egyptian women. The majority of patients experience poor outcomes as they present with a late stage of the disease by the time of diagnosis. It is expected that there will be almost 46,000 cases by the year 2050(7).
Breast cancer subtypes are commonly categorized into four groups as follows: Luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)-positive (HER2+) and triple-negative (TN). This classification is based on hormone receptors, including estrogen receptor-positive (ER+), progesterone receptor-positive (PR), HER2+ and the cell proliferation marker, Ki-67, determined using immunohistochemistry (8). Breast cancer can be classified into five types. The most common type begins in the milk ducts and is termed invasive ductal carcinoma (IDC), and spreads to surrounding tissue, accounting for ~80% of all breast cancer cases. Invasive lobular carcinoma is the second most common type, representing ~10% of cases. Ductal carcinoma in situ is a non-invasive type that begins in the milk ducts, but does not spread beyond them. Rare forms of breast cancer include inflammatory breast cancer and Paget's disease, which together account for <4% of all cases (9).
In addition to sex, age is a significant risk factor; women >50 years of age are at a higher risk of developing the disease. A family history of breast cancer also increases the risk, as ~15% of women diagnosed with breast cancer have a first-degree female relative who has been affected. Additionally, genetic factors play a role; mutations in genes, such as breast cancer gene (BRCA)1, BRCA2, tumor protein 53 (TP53), cadherin 1 (CDH1), phosphatase and tensin (PTEN) and serine threonine kinase (STK11) play a role in the development of the disease. These genes help regulate cell growth and prevent uncontrolled proliferation (10). Lifestyle choices can also influence the risk. A high alcohol consumption, particularly in women aged >55 years, can elevate estrogen levels and activate the estrogen receptor pathway. Increased dietary fat intake and the long-term use of hormone replacement therapies have also been associated with a higher risk of developing breast cancer. Additionally, reproductive factors such as early menarche, late menopause and an advanced age at first pregnancy can further increase the risk (11).
The emergence of novel biomarkers has become essential for breast cancer diagnosis and prognosis. Biomarkers, including proteins, hormones, genes, genetic mutations and other molecules, help determine the subtype and progression of breast cancer tumors (12). They enhance patient management and guide treatment decisions, particularly for patients with advanced-stage disease. Additionally, biomarkers assist doctors in assessing the likelihood of cancer responding to specific treatments and making decisions regarding post-surgical care (13).
The p53 transcription factor in breast cancer is vital in regulating various cellular processes. However, its tumor-suppressive activities are often impaired by the overexpression of the negative regulator, mouse double minute 2 (MDM2) or by mutation. Mutations in p53 are present in 30-35% of breast cancer cases (14). Furthermore, ~80% of TN tumors exhibit p53 mutations, which are associated with poor survival outcomes. Due to this high frequency, mutant p53 may be an effective therapeutic target and potential biomarker, particularly for patients with the TN subtype (15).
Some studies have also shown that p53 and Her2/neu have prognostic value in identifying more aggressive breast cancer behaviors and lower prognostic outcomes (16). Biomarkers, such as carbohydrate antigen 15-3 (CA15-3) and carcinoembryonic antigen (CEA) are valuable in monitoring therapy for patients with advanced-stage breast cancer (17). Biomarker levels are associated with unfavorable clinicopathological parameters in patients with breast cancer. Thus, combining the two markers could improve the sensitivity and accuracy of early breast cancer diagnosis (18).
Cancer antigen 125 (CA125) levels, along with CEA and CA15-3, are significantly higher in patients with breast cancer compared to controls. Moreover, patients with cancer have noticeably greater levels of CA15-3, CA125 and CEA tumor markers compared with patients in the early stages of the disease. In addition, higher levels of CA125 and CA15-3 are observed in patients with late-stage cancer and are associated with lymph node metastasis and tumors with larger diameters (19).
Due to the heterogeneity of tumor cells, using a single biomarker is not sufficient for the accurate diagnosis of cancer development and metastasis (20). Therefore, combining biomarkers is preferred and is more valuable in improving sensitivity (21,22).
Other recognized biomarkers, such as ER, PR and HER2 play crucial roles in selecting and managing patients for endocrine therapy and predicting the response to trastuzumab. ER is a negative indicator for chemotherapy, while HER2 is a positive indicator. It has been recognized that Ki67 is a poor prognostic factor (21). Early diagnosis is crucial for breast cancer, as it is metastatic type of cancer, which can spread to distant organs, such as the liver, brain, lung and bone; a better prognosis and higher survival rates result from the early diagnosis of the disease (23).
Patients and methods
Sample collection
The present study involved 86 female participants who had been clinically diagnosed with breast cancer, and 30 healthy individuals as the control group. According to the Declaration of Helsinki, the Ethics Committee of the National Research Centre (Cairo, Egypt) reviewed and approved the study under registration no. 09420924. Prior to participating, all individuals provided written informed consent.
Pre-operative neoadjuvant chemotherapy using paclitaxel or docetaxel was planned for 63 of the female patients, while 23 patients did not receive any treatment, as some of them did not complete the follow-up and others refused the treatment.
Participants were selected from the outpatient nuclear medicine and clinical oncology clinic at the Kasr Al-Ainy Centre of Cairo University (Cairo, Egypt). Clinicopathological data were obtained from the medical records of the patients. Following diagnosis, information regarding the presence of HER2, ER, PR, lymph node (LN) status, and tumor grades and sizes was collected. A pathologist confirmed the tumor grades and imaging techniques were used to validate the lymph node status before surgery clinically. Blood samples of 4 ml were collected from all patients. The serum was then isolated by centrifugation at 3,500 x g, 15˚C for 10 min and stored at -20˚C for further analysis.
Determination of CA 15-3, CEA and CA125 serum levels
The serum levels of CA15-3, CA125 and CEA tumor markers were determined using immunoradiometric assay (IRMA) kits as follows: Cat. no. KIP 0301 for CA 125. Cat. no. KIP0321 for CA 15-3 and cat. no. KIP0331 for CEA (DIAsource ImmunoAssays SA), which was prepared for use in compliance with the manufacturer's guidelines. First, the serum was placed in plastic tubes coated with the capture antibody Mab1, and room-temperature incubation was permitted for 90 min. After cleaning, the reaction tubes were shaken at room temperature for a further 90 min, followed by the addition of iodine-125-labeled anti-CA15-3 antibodies, CA125 antibodies and CEA antibodies (Mab2).
A gamma counter measured the bound radioactivity of each tube for 60 sec after cleaning. Computer-assisted data reduction simplified the calculations. The levels of CA15-3, CA125 and CEA in each serum sample were determined using a 5-parameter logistic function curve.
Determination of p53 tumor marker in patients with breast cancer
Using the sandwich-ELISA method, an ELISA kit (cat. no. E-El-H0910, Elabascience) was utilized to determine p53 levels in serum samples. This kit includes a micro-ELISA plate pre-coated with an antibody specific to human TP53. After adding standards or samples to the wells of the micro-ELISA plate, the appropriate antibody was introduced. Subsequently, a biotinylated detection antibody specific for human TP53 and an avidin-horseradish peroxidase (HRP) combination was added to each well prior to incubation.
Unbound components were washed away with washing buffer (provided with the kit), and a substrate solution was added to each well. Only wells containing human TP53, the biotinylated detection antibody, and the avidin-HRP conjugate will develop a blue color. The enzyme-substrate reaction was terminated by the addition of a stop solution (provided with the kit), which changes the color to yellow. Spectrophotometric measurements were performed using an ELISA plate reader (Statfax Chromate 4300, Awareness Technology, EAD Scientific) and the optical density (OD) was measured at 450 nm.
Statistical analysis
The Prism Graph Pad version 9 Graph (Pad Software, Inc.) was used to enter and code the data. Data are presented as the mean ± standard deviation (SD). Comparisons between two groups were performed using the Mann-Whitney test, while comparisons between more than two groups were performed using the Kruskal-Wallis test. The ROC curve was constructed with the area under curve (AUC) analyzed to detect the optimal cut-off value for p53, CEA, CA15-3 and CA125 in patients with breast cancer. Correlation analysis was performed using Spearman correlation analysis. P-values <0.05 were considered to indicate statistically significant differences.
Results
Clinical and pathological data of the patients
The study included 86 patients diagnosed with breast cancer; 63 patients had received adjuvant neoadjuvant chemotherapy (paclitaxel and docetaxel); 23 female patients did not receive treatment, and 30 healthy subjects served as the controls. IDC was the most common histological type. The characteristics of the patients are presented in Table I.
The age of the patients was 49±7.88 (mean ± SD) years. The findings revealed a non-significant difference in age among the patients with breast cancer and the p53 level (0.229). In addition, the expression levels of p53 and CA125 exhibited significant differences with metastasis (P=0.008 and P=0.0362, respectively). Furthermore, a significant association was observed between tumor grade and the levels of CEA (P=0.0322), CA15-3 (P=0.0012) and CA125 (P=0.0024).
Serum levels of p53, CEA, CA15 and CA125 in healthy controls, and patients with breast cancer
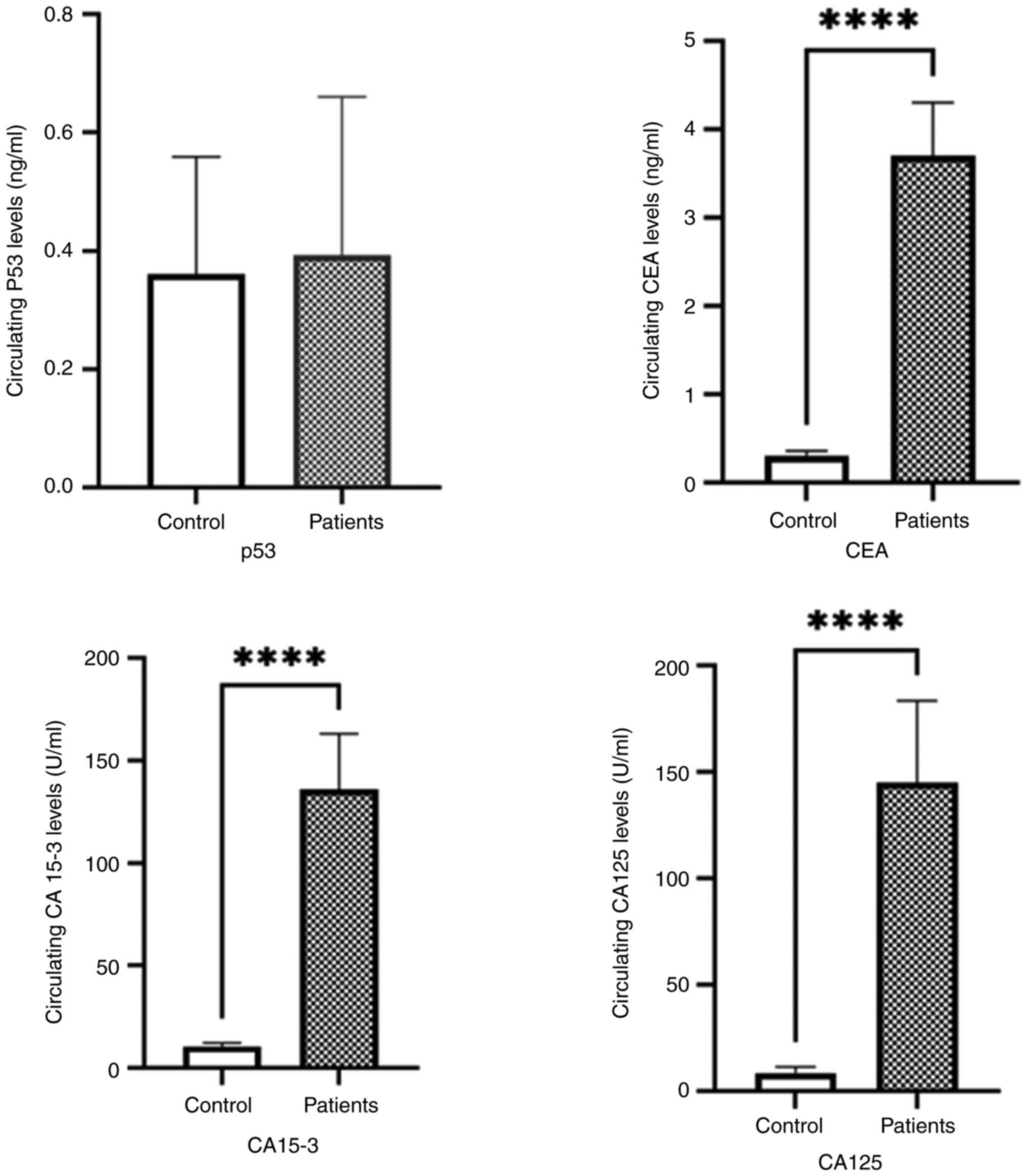
The CEA, CA15-3 and CA125 serum levels were measured in all samples using IRMA. The serum levels of CEA, CA15-3 and CA125 were significantly increased in the patients with breast cancer compared with the controls. The results indicated a significant difference between the mean levels of CEA in the patients with breast cancer compared with the healthy controls (Controls: CEA, 0.3010±0.13 ng/ml; CA15-3, 8.880±3.7 ng/ml; and CA125, 8.420±3.6 ng/ml; Patients: CEA, 5.56±15.63; CA15-3, 131±90.06; and CA125, 141.2±79.12 ng/ml; P<0.001) (Table II and Fig. 1).
Comparison of the diagnostic value of tumor markers in patients with breast cancer
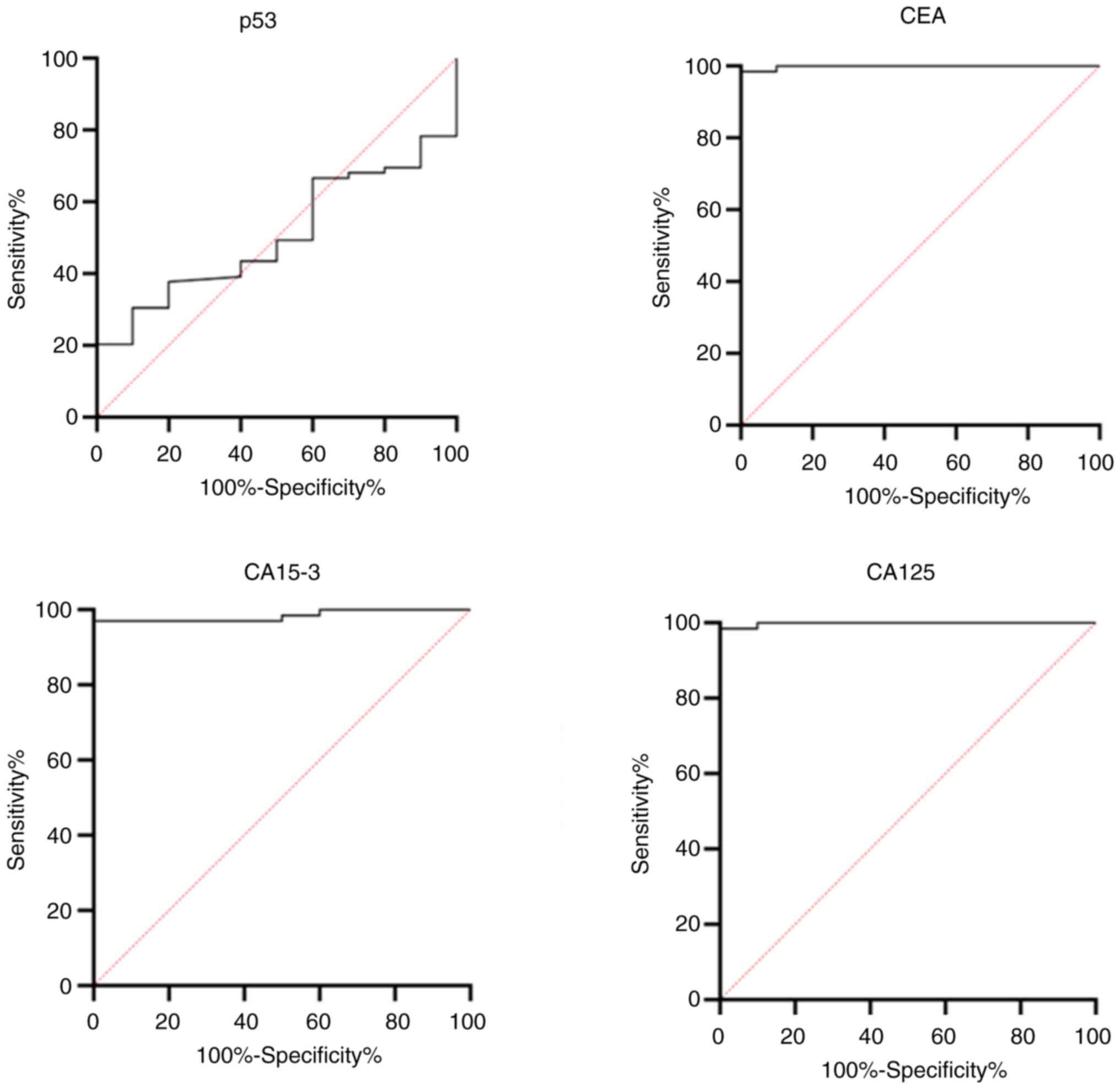
The cut-off values for the concentrations of the tumor markers used to predict breast cancer are presented in Table III and Fig. 2. The cut-off value for the predication breast cancer was estimated at 0.389 ng/ml for p53 with a sensitivity of 56.52%, specificity of 60%, and AUC of 0.5029 (P<0.6162). For CEA, the cut-off value was 0.91 ng/ml with a sensitivity of 98.55%, specificity of 100%, and AUC of 0.998 (P<0.0001). The cut-off values for CA15-3 and CA125 were >15.95 U/ml and >17.5 U/ml, respectively, with a sensitivity of 97.07 and 98.55%, and the AUCs of 0.9838 (P<0.0001) and 0.99 (P<0.0001), respectively, each having a specificity of 100%.
Table IIIArea under the ROC curves, sensitivity and specificity for serum tumor marker protein levels in patients with breast cancer. |
Measurement of tumor marker levels in patients with breast cancer according to tumor grade and stage
There was a variation in the measurements of the CEA and CA15-3 biomarker levels among the breast cancer patient groups classified by tumor grade (P<0.0322 and P<0.0396, respectively). However, there was no significant difference in the levels of p53 and CA125, as shown in Table IV. On the other hand, a significant difference was found between the p53 biomarker levels and the stages of breast cancer (P=0.030), as demonstrated in Table V.
Correlation between the expression of different tumor markers in patients with breast cancer
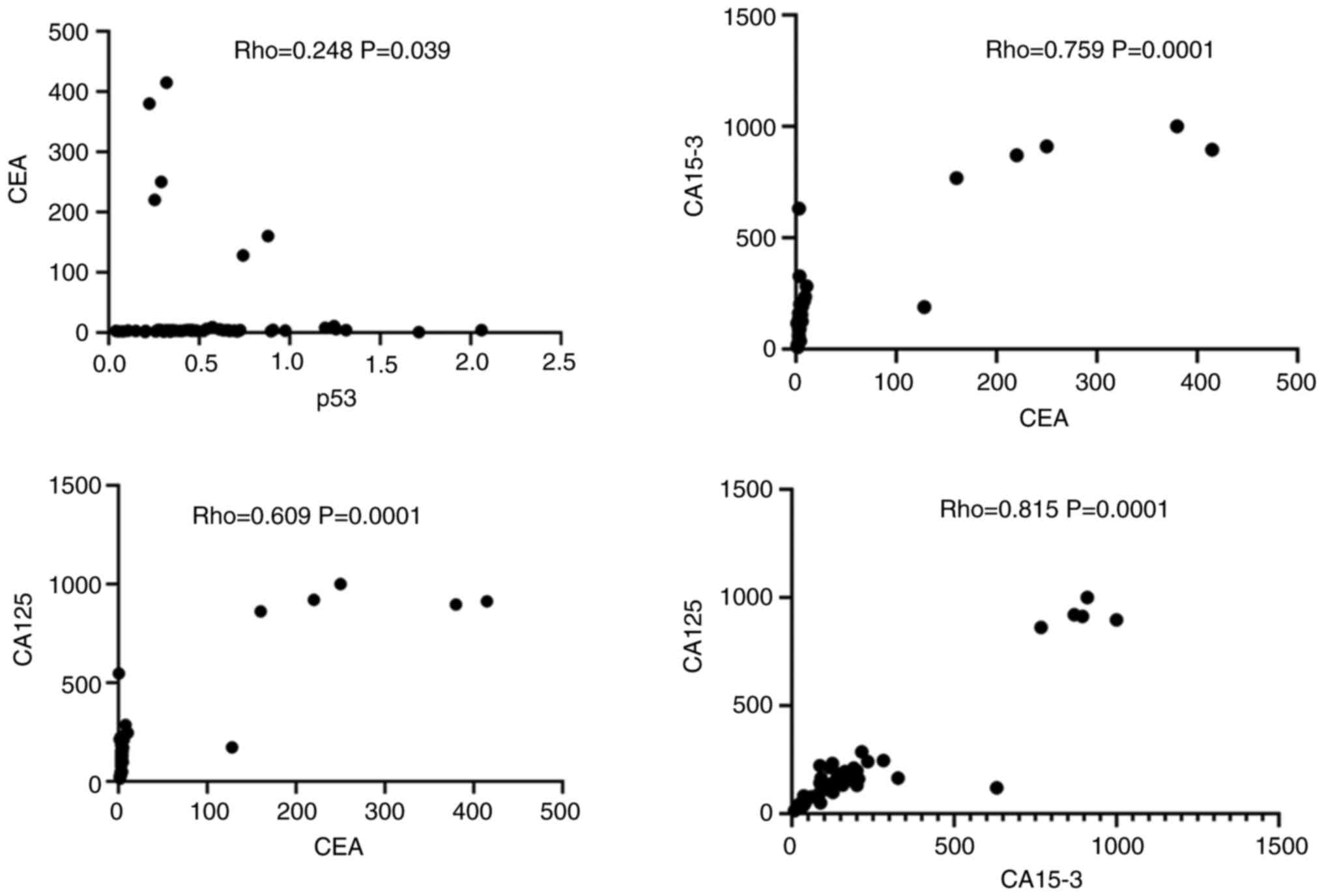
As illustrated in Fig. 3, there was a strong positive correlation between the tumor markers, CEA and CA15-3 (Rho=0.759, P=0.0001), and CEA and CA125 (Rho=0.609, P=0.0001). Similarly, a strong positive correlation between was found between the CA15-3 and CA125 levels in patients with breast cancer (Rho=0.8151, P=0.0001). A weak positive correlation was observed between the p53 and CEA levels (Rho=0.2485, P=0.0395).
Different levels of serum tumor markers in patients with breast cancer (pre- and post-chemotherapy)
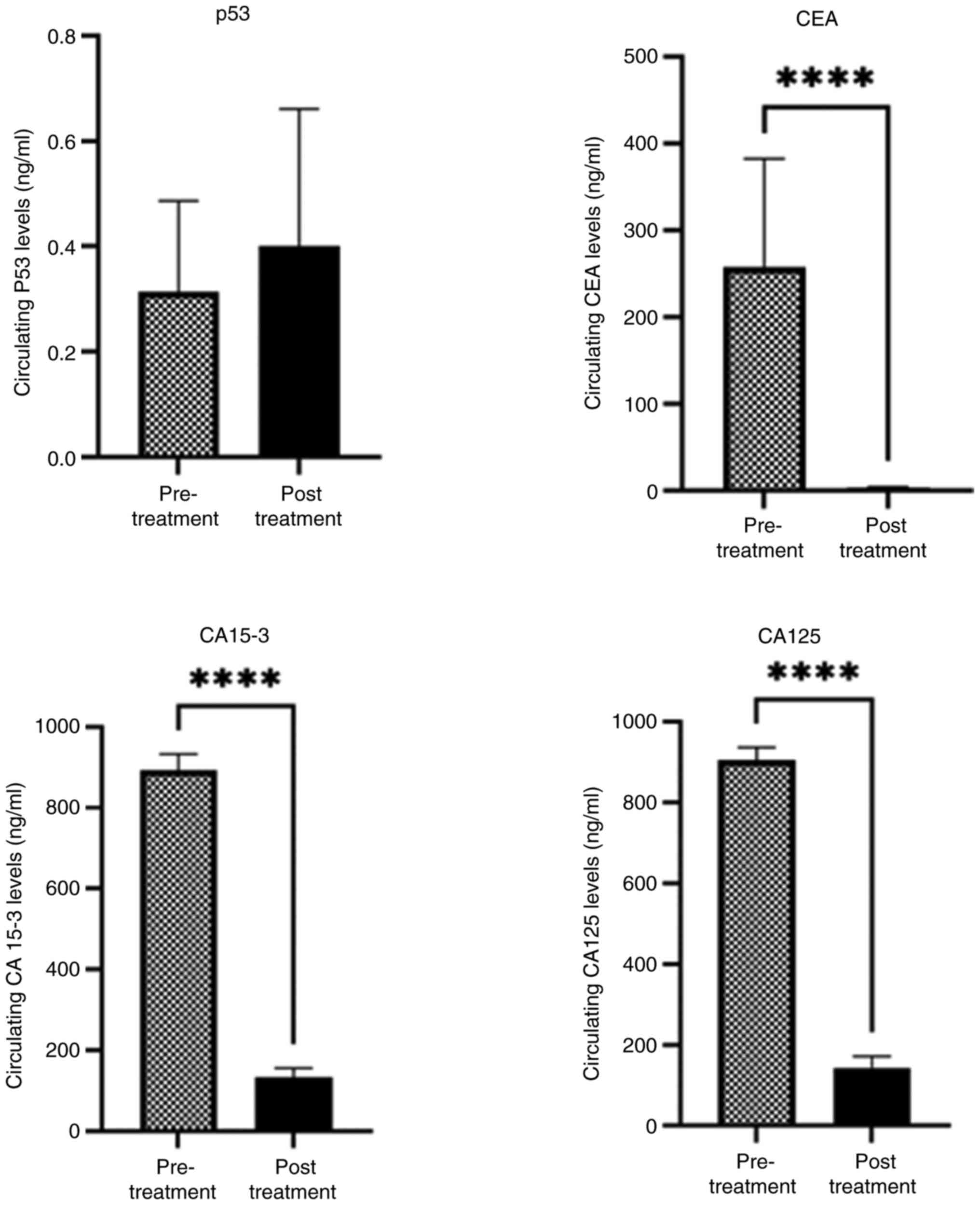
Significant increases were observed in the levels of CA15-3, CEA and CA125 (P=0.0001) in the pre-chemotherapy group compared to the post-chemotherapy group. Conversely, the expression level of p53 increased in the post-treatment group compared to the pre-treatment group (Fig. 4).
Discussion
Breast cancer is the most common malignancy among Egyptian women and has the second-highest mortality rate worldwide each year. The early detection of breast cancer can significantly reduce mortality, allowing for less aggressive treatment options and improving patient survival rates. Biomarkers are substances in biological systems that can indicate disease processes or provide clinical data for pharmacological responses to medication therapy. A single biomarker is insufficient for diagnosis; however, combining different diagnostic biomarkers is crucial for accurate diagnosis, prognosis, and treatment (20).
Serum tumor marker variations have been created as a non-invasive technique to evaluate the efficacy of treatment for malignancies in humans (19). Among the most commonly used tumor markers in breast cancer are CA125, CA15-3 and CEA. The present study assessed the significance of p53 as a potential prognostic marker compared to other tumor markers, including CEA, CA15-3 and CA125, by measuring the protein levels of these markers in patients with breast cancer.
The results revealed a significant increase in the levels of CEA, CA15-3 and CA125 in patients with breast cancer, with P-values of 0.0322, 0.0012 and 0.0024, respectively. The p53 tumor marker was the only marker that exhibited a significant difference with age, with a P-value of 0.0229. However, none of the markers examined in the present study exhibited a substantial difference with lymph node, PR, ER or HER2 status.
The results of the present study differed from those in the study by Ashour Byomy et al (24), who found that the levels of CEA and CA15-3 significantly increased with lymph node involvement and tumor size (P<0.05) (24). In another study, Zhao et al (24,25) identified an association between elevated levels of CEA, CA15-3 and CA125, and tumor size, with P-values of 0.0031, <0.0001 and 0.0296, respectively. The present study reported a significant difference in the p53 and CA125 expression levels between patients with breast cancer with metastasis, with P-values of 0.008 and 0.0362, respectively. Gaughran et al (26) noted that TN tumors often had elevated CA-125 levels associated with pleural metastases. Additionally, it has been documented that mutant p53 increases tumor aggressiveness and metastatic potential through various mechanisms (14). These results suggest that intensive follow-up and the use of alternative and appropriate chemotherapy are necessary. Additionally, combining two drugs has been shown to result in high response rates in metastatic disease. Measuring the levels of both serum markers may also be useful for the early diagnosis of metastases.
In the present study, the levels of the tumor markers, CEA, CA15-3 and CA125, were significantly elevated in patients with breast cancer compared with the healthy controls (P=0.0001). The mean of the elevated serum levels of CA15-3, CA125 and CEA were 131, 141.2 and 5.56 of breast cancer cases compared with the control group which were 0.3010, 8.880 and 8.420, respectively. These findings are in accordance with those the studies by Fang et al (19) and Shao et al (27), which reported that patients with breast cancer had significantly higher pre-operative serum levels of CEA, CA125 and CA15-3 than the control subjects. Additionally, significantly higher levels of CEA, CA125 and CA15-3 were observed in patients with late-stage cancer compared to those with early-stage disease (19,27). In the study by Hasan (28), CA15-3 exhibited a more significant increase than CEA, with both markers exhibiting significantly higher levels in patients with breast cancer at the time of diagnosis compared to the controls. These results suggest that combining tumor markers (CEA, CA15-3 and CA125) is critical in breast cancer, as they can accurately predict susceptibility to the disease and enhance the prognosis and detection of breast cancer (28). As regards p53, the levels of this tumor marker in healthy cells have a short half-life, making it difficult to detect. However, in stressed, mutated cells, p53 accumulates and has a longer half-life, allowing for its high levels to be detected immunohistochemically (29).
According to the findings of the present study, the cut-off value for breast cancer prediction was >0.389 ng/ml for p53, with a sensitivity value 56.52%, specificity value 60%, and an AUC of 0.5029 (P<0.6162). For CEA, the cut-off value was determined at 0.91 ng/ml, with a sensitivity of 98.55%, specificity of 100% and an AUC of 0.998 (P<0.0001). The cut-off values for CA15-3 and CA125 were >15.95 U/ml and >17.5 U/ml, respectively, with sensitivities of 97.07 and 98.55% and AUCs of 0.9838 (P<0.0001) and 0.99 (P<0.0001), both exhibiting 100% specificity. By contrast, El-Moneim Ebied et al (30) reported a cut-off value of 2.82 U/ml for serum p53, demonstrating a substantial AUC of 85.6% (P<0.05) with 80% sensitivity and 87% specificity (30). The results of the present study align with those of the study by Tang et al (31), who reported cut-off values of 16.78 U/ml for CA-125 and 63.175 U/ml for CA15-3, with sensitivities of 90 and 100%, specificities of 75.7 and 97.2%, and AUCs of 0.838 (P<0.001) and 0.984 (P<0.001), respectively.
In another study by Uygur and Gümüş (32), the significance of CEA and CA15-3 levels in predicting metastasis was assessed using ROC analysis. The cut-off values were 1.39 ng/ml for CEA and 14.54 U/ml for CA15-3. The sensitivity values for CEA and CA15-3 were 88.3 and 82.1%, respectively, with specificities of 46.2 and 47.3%. These findings highlight the critical role of these markers as tumor indicators for the early detection of metastases (32).
In another study by Rahemi et al (33), the AUC values for the three markers, CA15-3, CA125 and CEA, were 0.85, 0.85 and 0.75, respectively. The results indicated that the sensitivity levels for CA15-3, CA125 and CEA were 85, 85 and 75%, respectively. Additionally, when combining these three tumor markers to predict the risk of breast cancer, the AUC curve reached 0.93, demonstrating maximum specificity (91%) and sensitivity (90%). These findings suggest that the predictive value generated by combining these three tumor markers is more reliable and trustworthy (33). The present study revealed a significant difference in the levels of CEA and CA15-3 among patients classified according to breast cancer grade, with P-values <0.0322 and <0.0396, respectively. However, there was no discernible difference in the levels of the CA125 and p53 biomarkers. The CEA and CA15-3 levels were noticeably higher in patients with late-stage breast cancer than in those with early-stage disease (34).
On the other hand, the present study found a significant difference in p53 biomarker levels across different stages of breast cancer (P=0.030). This finding aligns with the results of the study by Khadhum et al (35), who reported a significant difference in p53 levels among all stages of breast cancer (P=0.01). Additionally, the level of CA15-3 was directly associated with advanced stages and recurrence, suggesting that it could serve as a reliable predictive biomarker (35). Similarly, in the study by Li et al (36), no significant differences were found in the levels of CA15-3, CA125 and CEA among patients with breast cancer, with P-values of 0.94, 0.39 and 0.69, respectively In the present study, there was a strong positive correlation observed between the CEA tumor markers and CA15-3 and CA125 levels, with correlation coefficients of Rho=0.759 (P=0.0001) and Rho=0.609 (P=0.0001), respectively. Similarly, A significant positive correlation was observed between the CA15-3 and CA125 levels in patients with breast cancer (Rho=0.8151, P=0.0001). A marginal positive association was discovered between p53 and CEA (Rho=0.2485, P=0.0395), while p53 did not correlate with CA15-3 or CA125.
The results of the present study indicated that significant increases were observed in the serum levels of CEA, CA15-3 and CA125 (P=0.0001) in the pre-chemotherapy group compared with the post-chemotherapy group. By contrast, the p53 levels increased in the post-treatment group compared with the pre-treatment group. This elevation in p53 may suggest that chemotherapy affects p53 protein levels, potentially extending its half-life. Additionally, the high levels of p53 following chemotherapy could indicate resistance to treatment, the presence of residual disease, or the need for further therapies or alternative treatment strategies.
In a previous study, Bae et al (2020), the expression of p53 was examined before and after chemotherapy (37). They found no change in p53 levels in TNBC patients before chemotherapy compared to those after treatment Similarly, Lee et al (38) reported that ~86.7% of patients did not exhibit a change in p53 levels following chemotherapy, while only 13.7% exhibited a change following neoadjuvant chemotherapy Some studies have reported that TP53 mutations may be associated with a good prognosis, while others have indicated that these are associated with a poor prognosis. These differing results may be attributed to the fact that the various types of tumors were used in the studies and involved different treatment protocols (39,40).
In the study by Anoop et al (40), the median CEA levels before and after treatment in the entire study population were 7.9 ng/ml (range, 1.8-40.7 ng/ml) and 4.39 ng/ml (range, 1.4-12.15 ng/ml), respectively (P=0.032). These findings suggest that elevated blood levels of CEA and CA15-3 in patients with breast cancer are valuable indicators for predicting aggressive behavior and the risk of recurrence (41). Serum biomarkers, particularly CEA, CA 15-3, and CA 125 can be highly effective in monitoring treatment efficacy and enabling the early detection of recurrence or metastasis in breast cancer, even before imaging techniques reveal tumor progression.
In conclusion, the combination of CEA, CA125 and CA15-3 is considered to be comprehensive marker for young patients with breast cancer and these markers are considered valuable tumor markers for the detection of metastases early diagnosis. The combination of the three markers CA15-3, CEA, and CA125 may provide a more effective biochemical diagnosis for breast cancer patients than using a single marker. This approach could be valuable for prognostic information and determining disease progression.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
RMES designed, supervised, wrote and prepared the draft of the manuscript. NBET and RMES performed the practical experiments. LMHK performed sample collection and prepared the clinical data sheets. SF was involved in preparing the draft of the manuscript, in statistical analysis and in the preparation of the figures. RMES and SF confirm the authenticity of all raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
In compliance with the Declaration of Helsinki, the present study was examined and approved by the Ethics Committee of the National Research Centre (Cairo, Egypt) under registration no. 09420924. Before participating in the study, all participants gave their written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Neves Rebello Alves L, Dummer Meira D, Poppe Merigueti L, Correia Casotti M, do Prado Ventorim D, Ferreira Figueiredo Almeida J, Pereira de Sousa V, Cindra Sant'Ana M, Gonçalves Coutinho da Cruz R, Santos Louro L, et al: Biomarkers in breast cancer: An old story with a new end. Genes (Basel). 14(1364)2023.PubMed/NCBI View Article : Google Scholar | |
|
Obeagu EI and Obeagu GU: Breast cancer: A review of risk factors and diagnosis. Medicine (Baltimore). 103(e36905)2024.PubMed/NCBI View Article : Google Scholar | |
|
Lei S, Zheng R, Zhang S, Wang S, Chen R, Sun K, Zeng H, Zhou J and Wei W: Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun (Lond). 41:1183–1194. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S and Soerjomataram I: Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. 66:15–23. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Lv L, Zhao B, Kang J, Li S and Wu H: Trend of disease burden and risk factors of breast cancer in developing countries and territories, from 1990 to 2019: Results from the global burden of disease study 2019. Front Public Health. 10(1078191)2023.PubMed/NCBI View Article : Google Scholar | |
|
Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R and Stanisławek A: Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers (Basel). 13(4287)2021.PubMed/NCBI View Article : Google Scholar | |
|
Azim HA, Elghazawy H, Ghazy RM, Abdelaziz AH, Abdelsalam M, Elzorkany A and Kassem L: Clinicopathologic features of breast cancer in Egypt-contemporary profile and future needs: A systematic review and meta-analysis. JCO Glob Oncol. 9(e2200387)2023.PubMed/NCBI View Article : Google Scholar | |
|
Rakha EA, Tse GM and Quinn CM: An update on the pathological classification of breast cancer. Histopathology. 82:5–16. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Rakha E, Toss M and Quinn C: Specific cell differentiation in breast cancer: A basis for histological classification. J Clin Pathol. 75:76–84. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ, Zhu ZY, Shi W, Jiang J, Yao PP and Zhu HP: Risk factors and preventions of breast cancer. Int J Biol Sci. 13:1387–1397. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Liu H, Shi S, Gao J, Guo J, Li M and Wang L: Analysis of risk factors associated with breast cancer in women: A systematic review and meta-analysis. Transl Cancer Res. 11:1344–1353. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Wu HJ and Chu PY: Recent discoveries of macromolecule- and cell-based biomarkers and therapeutic implications in breast cancer. Int J Mol Sci. 22(636)2021.PubMed/NCBI View Article : Google Scholar | |
|
Das S, Dey MK, Devireddy R and Gartia MR: Biomarkers in cancer detection, diagnosis, and prognosis. Sensors (Basel). 24(37)2023.PubMed/NCBI View Article : Google Scholar | |
|
Marvalim C, Datta A and Lee SC: Role of p53 in breast cancer progression: An insight into p53 targeted therapy. Theranostics. 13:1421–1442. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Duffy MJ, Synnott NC and Crown J: Mutant p53 in breast cancer: Potential as a therapeutic target and biomarker. Breast Cancer Res Treat. 170:213–219. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Gupta A, Patil BU and Gangane NM: Role of p53 and Her2/Neu as a prognostic biomarker in breast carcinoma. Med J Dr DY Patil Univ. 16:191–196. 2023. | |
|
Duffy MJ, Walsh S, McDermott EW and Crown J: Chapter one-biomarkers in breast cancer: Where are we and where are we going? In: Advances in Clinical Chemistry. Makowski GS (ed). Vol 71. Elsevier, Amsterdam, pp1-23, 2015. | |
|
Li H, Wang S, Li X, Cheng C, Shen X and Wang T: Dual-channel detection of breast cancer biomarkers CA15-3 and CEA in human serum using dialysis-silicon nanowire field effect transistor. Int J Nanomedicine. 17:6289–6299. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Fang C, Cao Y, Liu X, Zeng XT and Li Y: Serum CA125 is a predictive marker for breast cancer outcomes and correlates with molecular subtypes. Oncotarget. 8:63963–63970. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Afzal S, Hassan M, Ullah S, Abbas H, Tawakkal F and Khan MA: Breast cancer; discovery of novel diagnostic biomarkers, drug resistance, and therapeutic implications. Front Mol Biosci. 9(783450)2022.PubMed/NCBI View Article : Google Scholar | |
|
Zhang J, Wei Q, Dong D and Ren L: The role of TPS, CA125, CA15-3 and CEA in prediction of distant metastasis of breast cancer. Clin Chim Acta. 523:19–25. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Bader El Din NG, El-Shenawy R, Moustafa RI, Khairy A and Farouk S: Association between the expression level of miRNA-374a and TGF-β1 in patients with colorectal cancer. World Acad Sci J. 6(68)2024. | |
|
Taneja P, Maglic D, Kai F, Zhu S, Kendig RD, Fry EA and Inoue K: Classical and novel prognostic markers for breast cancer and their clinical significance. Clin Med Insights Oncol. 4:15–34. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Ashour Byomy LG, Zahraan F, Aref M and Ibrahim Mosa MF: Assessment of CEA and CA15-3 as potential prognostic markers for breast cancer in Egyptian females. Alfarama J Basic Appl Sci. 2:44–50. 2021. | |
|
Zhao W, Li X, Wang W, Chen B, Wang L, Zhang N, Wang Z and Yang Q: Association of preoperative serum levels of CEA and CA15-3 with molecular subtypes of breast cancer. Dis Markers. 2021(5529106)2021.PubMed/NCBI View Article : Google Scholar | |
|
Gaughran G, Aggarwal N, Shadbolt B and Stuart-Harris R: The utility of the tumor markers CA15.3, CEA, CA-125 and CA19.9 in metastatic breast cancer. Breast Cancer Manag. 9(BMT50)2020. | |
|
Shao Y, Sun X, He Y, Liu C and Liu H: Elevated levels of serum tumor markers CEA and CA15-3 are prognostic parameters for different molecular subtypes of breast cancer. PLoS One. 10(e0133830)2015.PubMed/NCBI View Article : Google Scholar | |
|
Hasan D: Diagnostic impact of CEA and CA 15-3 on chemotherapy monitoring of breast cancer patients. J Circ Biomark. 11:57–63. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Firouzabadi D, Rezvani A, Dehghanian A and Mahmoudi L: Association of ki67 and tumor marker p53 in locally advanced breast cancer patients and evaluation of response to neoadjuvant chemotherapy: A survey in South Iran. Cancer Manag Res. 11:6489–6497. 2019.PubMed/NCBI View Article : Google Scholar | |
|
El-Moneim Ebied SA, El-Moneim NAA, Hewala Tl, Anwar MM and Rabi SM: The diagnostic, prognostic and follow-up value of serum Bcl-2, Bax and p53 proteins in breast cancer patients: A comparison with serum CA 15-3. Middle East J Cancer. 4:51–62. 2013. | |
|
Tang J, Yan B, Li GF, Li QY, Liu WF, Liang RB, Ge QM and Shao Y: Carbohydrate antigen 125, carbohydrate antigen 15-3 and low-density lipoprotein as risk factors for intraocular metastases in postmenopausal breast cancer. Medicine (Baltimore). 100(e27693)2021.PubMed/NCBI View Article : Google Scholar | |
|
Uygur MM and Gümüş M: The utility of serum tumor markers CEA and CA 15-3 for breast cancer prognosis and their association with clinicopathological parameters. Cancer Treat Res Commun. 28(100402)2021.PubMed/NCBI View Article : Google Scholar | |
|
Rahemi Z, Javadi A, Kazeminejad B, Ebrahimi A, Vosough H, Taghavi A and Dabiri S: Diagnostic utility of combined CEA, CA15-3 and CA125 biomarkers and cytomorphology in suspicious and malignant serosal fluid. Iran J Pathol. 16:248–255. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Khushk M, Khan A, Rehman A, Sheraz S, Tunio YM, Rehman K, Rehman D, Ahmed M, Abbas K and Khan ME: The role of tumor markers: Carcinoembryonic antigen and cancer antigen 15-3 in patients with breast cancer. Cureus. 13(e16298)2021.PubMed/NCBI View Article : Google Scholar | |
|
Khadhum HS, Ameen AA and Mm T: Assessment of CA 15-3 and P53 biomarkers in diagnosis of breast cancer. BNIHS. 40:1343–4292. 2022. | |
|
Li J, Liu L, Feng Z, Wang X, Huang Y, Dai H, Zhang L, Song F, Wang D, Zhang P, et al: Tumor markers CA15-3, CA125, CEA and breast cancer survival by molecular subtype: A cohort study. Breast Cancer. 27:621–630. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Bae SY, Lee JH, Bae JW and Jung SP: Differences in prognosis by p53 expression after neoadjuvant chemotherapy in triple-negative breast cancer. Ann Surg Treat Res. 98:291–298. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Lee HC, Ko H, Seol H, Noh DY, Han W, Kim TY, Im SA and Park IA: Expression of immunohistochemical markers before and after neoadjuvant chemotherapy in breast carcinoma, and their use as predictors of response. J Breast Cancer. 16:395–403. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Varna M, Bousquet G, Plassa LF, Bertheau P and Janin A: TP53 status and response to treatment in breast cancers. J Biomed Biotechnol. 2011(284584)2011.PubMed/NCBI View Article : Google Scholar | |
|
Anoop TM, Joseph PR, Soman S, Chacko S and Mathew M: Significance of serum carcinoembryonic antigen in metastatic breast cancer patients: A prospective study. World J Clin Oncol. 13:529–539. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Varzaru VB, Eftenoiu AE, Vlad DC, Vlad CS, Moatar AE, Popescu R and Cobec IM: The influence of tumor-specific markers in breast cancer on other blood parameters. Life (Basel). 14(458)2024.PubMed/NCBI View Article : Google Scholar |