Vector‑borne infectious diseases in pregnancy in the era of climate change: A focus on mosquito‑ and tick‑borne pathogens (Review)
- Authors:
- Published online on: July 21, 2025 https://doi.org/10.3892/etm.2025.12924
- Article Number: 174
-
Copyright: © Georgakopoulou et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
1. Introduction
Vector-borne illnesses, including malaria, dengue fever, Zika virus (ZIKV), and Lyme disease, present significant health risks to pregnant women and their unborn children (1). Climate change alters temperature and precipitation patterns, directly impacting the distribution, abundance, and behavior of disease vectors such as mosquitoes and ticks, thereby increasing the risk of vector-borne infections during pregnancy (2). Rising temperatures and shifting rainfall patterns create favorable conditions for vector breeding and survival, facilitating the expansion of these diseases into new regions (3-6).
Pregnant women are particularly vulnerable to vector-borne diseases due to physiological changes and the compounding effects of socio-environmental factors, such as displacement from climate-related disasters and inadequate healthcare access in affected regions (7-12). Displacement and overcrowding in temporary shelters often lead to heightened exposure to vectors and increased disease transmission risks (10). Furthermore, sex inequalities and limited healthcare access exacerbate the vulnerability of displaced pregnant women, particularly in areas with poor infrastructure and high disease burdens (13).
Human-induced climate change, primarily driven by fossil fuel use and deforestation, has led to global temperature increases, altered precipitation regimes, and more frequent extreme weather events (14-17). These changes disrupt ecosystems, affecting the behaviors and interactions of species, and create cascading effects on public health (18-20). For example, vector-borne infections such as ZIKV and malaria have been linked to adverse pregnancy outcomes, including miscarriage, stillbirth, preterm birth, and congenital anomalies (21-23). The increasing geographic range of vectors due to climate change further underscores the need for integrated public health strategies and tailored healthcare interventions for pregnant women in at-risk regions (23).
This review explores the critical relationship between climate change, pregnancy, and vector-borne infectious diseases, focusing on diseases such as ZIKV, malaria, dengue fever, and Lyme disease. It aims to provide an overview of how climate-induced environmental changes alter disease epidemiology, with an emphasis on the specific risks and challenges faced by pregnant women.
2. Climate change and vector-borne infectious diseases
Impact of climate change on vector ecology, distribution, and behavior
Climate change alters vector ecology, affecting disease transmission dynamics. Temperature and precipitation changes impact the distribution, abundance, and behavior of vectors such as mosquitoes and ticks, influencing their survival, development, and reproduction, thus increasing the transmission rates of vector-borne diseases (24,25). A comprehensive review on the role of vector trait variation in vector-borne infection dynamics emphasized how traits such as biting rate, host preference, and longevity vary among individuals within vector populations (26). The study reviewed empirical evidence for variation in vector traits and their incorporation into mathematical models of vector-borne disease transmission, finding that traits such as biting rate, host preference, and longevity vary significantly across individual vectors and environmental conditions, impacting vector population dynamics and pathogen transmission rates (26). These variations, driven by genetic differences, phenotypic plasticity, and environmental factors such as temperature and humidity, result in non-linear effects on disease dynamics (26). The study emphasized that small variations within populations can have compounding effects on disease spread, with vectors exhibiting higher biting rates or longer lifespans disproportionately contributing to transmission (26).
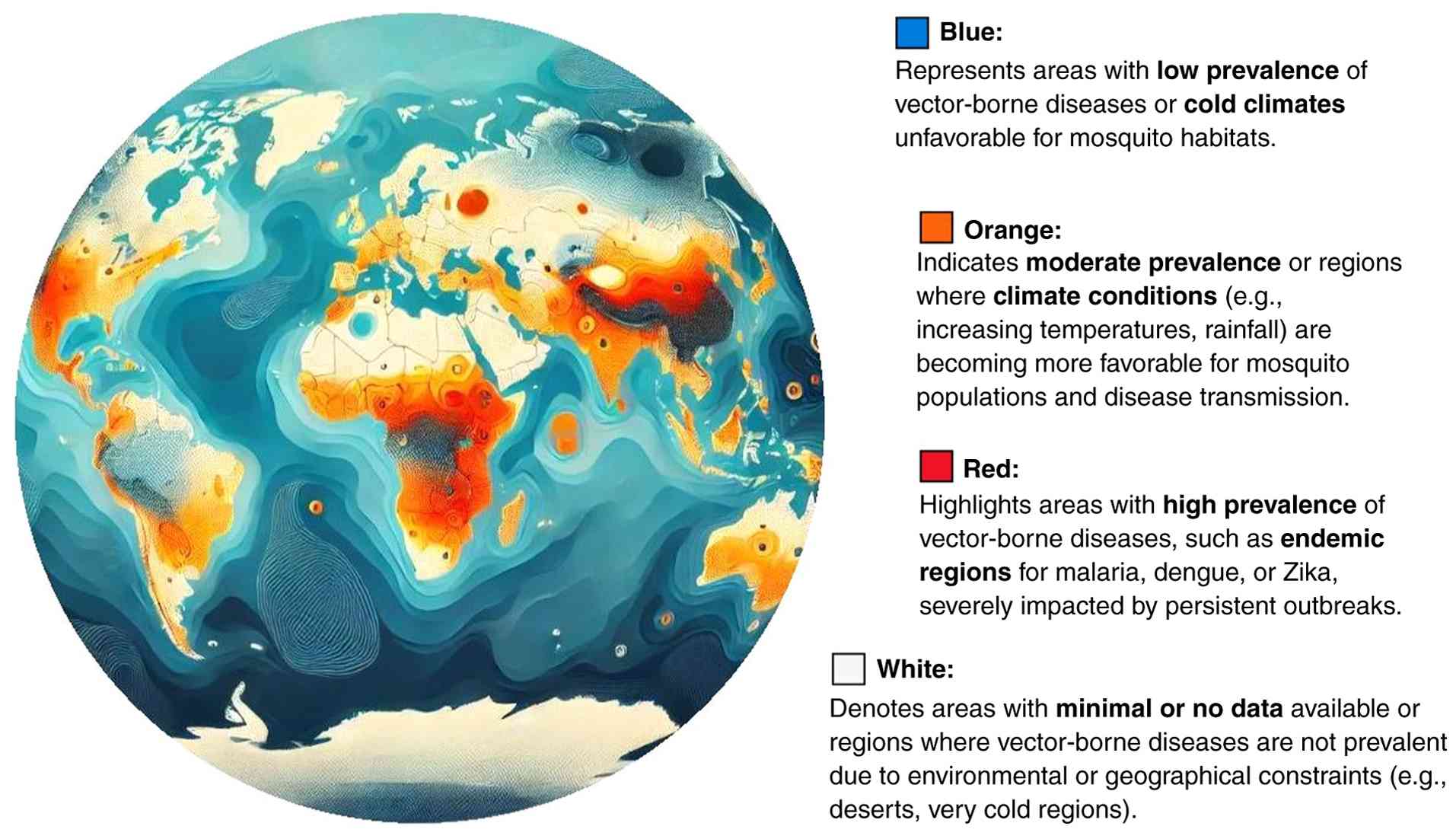
Changing climate conditions expands the geographic range and habitats of disease vectors. Warmer temperatures and altered precipitation patterns enable vectors to thrive in new regions, increasing the spread of diseases such as malaria, dengue, and Lyme disease in areas previously free from these illnesses (27-29). Changes in temperature and precipitation can also influence vector behavior, including feeding patterns, host-seeking behavior, and flight activity. Warmer temperatures may prolong the active season for vectors, increasing their biting rates and the likelihood of human-vector contact (30). Additionally, altered rainfall patterns can impact vector breeding sites, leading to changes in vector abundance and distribution (2).
Long-term projections based on climate models suggest substantial shifts in the distribution and seasonality of vector-borne diseases under various greenhouse gas emission scenarios. For example, under high-emission scenarios [Representative Concentration Pathway (RCP)8.5], the geographic range of malaria vectors is projected to expand into higher elevations and temperate regions by mid-century, with transmission suitability increasing in East Africa and parts of South Asia (31). Similarly, Aedes aegypti and Aedes albopictus, vectors of dengue and Zika, are expected to expand their habitat globally, affecting an estimated additional 1 billion individuals by 2080, particularly in Europe and North America (32). By contrast, lower-emission pathways (RCP2.6) could limit these expansions significantly, underscoring the importance of climate mitigation efforts in disease prevention. These projections not only highlight future hotspots of disease transmission but also stress the need to integrate climate modeling into vector surveillance systems and maternal health strategies.
Impact of climate change on mosquito vectors
A study by Baafi and Hurford (33) developed a stage-structured model incorporating laboratory data to assess how temperature and rainfall impact Anopheles mosquitoes, revealing that seasonal weather conditions cause significant variations in mosquito abundance across different African regions. The study focused on four distinct regions: Ain Mahbel in Algeria, Cape Town in South Africa, Nairobi in Kenya, and Kumasi in Ghana. The findings indicated that warmer regions with higher rainfall, such as Kumasi, exhibit higher mosquito abundance year-round. By contrast, cooler regions such as Ain Mahbel and Cape Town experience more pronounced seasonal peaks in mosquito populations (33). The model emphasizes the importance of considering seasonality, as neglecting it could lead to substantial overestimation or underestimation of mosquito populations, with different regions showing varying patterns of abundance peaks occurring between one to four times a year (33).
Further research conducted in the Canadian Prairies explored the influence of weather variables, temperature, precipitation, and humidity, on mosquito population densities, specifically focusing on four common mosquito vector species (34). Over 2 years, researchers collected >265,000 mosquitoes from 17 sites in Manitoba, identifying species-specific responses to climatic factors. Aedes vexans preferred high humidity, moderate temperatures, and low precipitation, while Culex tarsalis favored higher temperatures and had variable responses to precipitation. Coquillettidia perturbans thrived in high humidity and elevated minimum temperatures, and Ochlerotatus dorsalis showed increased numbers with higher precipitation levels (34).
In Hainan Island, China, a study examined the spatial and temporal dynamics of mosquito populations, revealing significant variations in density and community structures across different habitats and seasons (35). Using Centers for Disease Control and Prevention (CDC) light traps and Biogents (BG) Sentinel traps, researchers collected over 88,000 mosquitoes from urban, suburban, and rural areas between January and December 2018. The study identified nine species from four genera, with Culex quinquefasciatus being the most dominant, followed by Armigeres subalbatus and Anopheles sinensis. The study found clear seasonal variations in mosquito populations, with peak densities varying among species and sites. Danzhou had the highest mosquito biodiversity, while Haikou had the lowest. It also highlighted the importance of using complementary trapping methods, as CDC light traps were more effective for capturing Anopheles and Armigeres species, whereas BG Sentinel traps were more effective for Aedes mosquitoes (35).
Impact of climate change on tick vectors
Medlock et al (36) noted a significant expansion in the geographical distribution of the tick Ixodes ricinus (I. ricinus) in northern Europe, particularly in Sweden and Norway, attributable to climate change. The milder winters and extended vegetation periods resulting from increased temperatures and reduced snow cover have facilitated earlier questing activity in ticks (36). These climatic changes have made previously unsuitable areas habitable, leading to a northward shift in tick populations. This expansion is closely linked to warmer winters, which prevent ground temperatures from dropping below critical levels, thus enhancing tick survival and extending the period during which ticks can be active and reproduce (36). Consequently, these climatic shifts have significantly influenced the distribution and density of I. ricinus in northern Europe, with substantial implications for public health and ecological balance (36).
The climate change impacts on vector ecology are summarized in Table I, while the global distribution of vector-borne diseases in the context of climate change is illustrated in Fig. 1.
Influence of climate variability on vector-borne infectious diseases transmission dynamics
Climate variability, including natural climate oscillations such as the El Niño Southern Oscillation (ENSO) and the North Atlantic Oscillation (NAO), can also influence vector-borne infections transmission dynamics. These climate phenomena can affect temperature, precipitation, and atmospheric circulation patterns, leading to fluctuations in vector abundance and disease transmission rates. For example, during El Niño events, warmer and wetter conditions in some regions can create favorable breeding habitats for mosquitoes, increasing the risk of diseases such as dengue fever and ZIKV. Similarly, climate oscillations can influence the timing and intensity of seasonal outbreaks of vector-borne diseases. Changes in ENSO and NAO-related temperature and rainfall can have an impact on vector populations and the availability of favorable breeding grounds, which can cause changes in how diseases are spread (37-42).
Vector-borne infectious diseases affected by climate change
Numerous studies have documented the impacts of climate change on vector-borne infectious diseases worldwide, highlighting the complex interactions between climate, ecology, and human health.
For instance, researchers have linked the expansion of Lyme disease transmission areas in the United States to climate change, as warmer temperatures and altered precipitation patterns create more favorable conditions for tick survival and reproduction (43). Another study investigated the impact of climate change on the distribution of Lyme disease, a tick-borne illness, in North America (44). Using climate and ecological models, the researchers projected changes in the range of the black-legged tick, the primary vector of Lyme disease, under different climate scenarios (44). The study concluded that warming temperatures could lead to the expansion of tick habitats and an increase in Lyme disease cases in previously unaffected regions (44).
Similarly, in tropical regions, climate change has been associated with the increased transmission of diseases such as malaria and dengue fever, with rising temperatures extending the active season for mosquito vectors and expanding their geographical range (32,45). In a study of the effects of climate change on malaria transmission in Africa, the researchers utilized climate models to project future changes in temperature and rainfall patterns and their potential impacts on the distribution of malaria vectors. Their findings suggested that rising temperatures could expand the geographic range of malaria transmission, posing significant challenges for malaria control efforts (46). Another study examined the relationship between climate variability and dengue fever transmission in Southeast Asia. The authors found that fluctuations in temperature and precipitation influenced the breeding habitats of Aedes mosquitoes, the vectors responsible for transmitting the dengue virus (43). Changes in climate patterns were associated with shifts in the timing and intensity of dengue outbreaks, highlighting the role of climate variability in shaping disease dynamics (47).
Shifts in temperature and precipitation, which alter the distribution of disease vectors and amplify transmission risks, have implicated climate change in the resurgence of vector-borne diseases such as Chagas disease in South America. More specifically, research conducted in 2015 examined the potential effects of climate change on Chagas disease transmission in South America (48). Their findings indicated that climate change could lead to shifts in the distribution of Chagas disease, with implications for disease surveillance and control strategies (48).
In addition, another study investigated the influence of climate variability on the transmission of the West Nile virus (WNV) in the United States. The authors analyzed climate data alongside mosquito and bird abundance to assess the drivers of WNV transmission dynamics. The study found that warmer temperatures and changes in precipitation patterns influenced mosquito breeding and WNV transmission, highlighting the role of climate variability in shaping the risk of WNV outbreaks (49).
This section thoroughly examined how climate change alters vector behaviors, habitats, and disease dynamics, emphasizing how these changes increase the risks of vector-borne diseases such as malaria and dengue. Key mechanisms such as temperature shifts and precipitation changes were presented with case studies and models. While this section provided compelling evidence, it lacked granularity in addressing region-specific impacts and long-term projections under varied climate scenarios. Future studies should explore localized data to enhance predictive modeling for better adaptation strategies.
3. Vector-borne infectious diseases in pregnancy
One major concern is that higher temperatures and increased precipitation create more favorable conditions for vectors such as mosquitoes and ticks, which transmit diseases such as malaria, dengue fever, and ZIKV. These diseases pose serious health risks to pregnant women, potentially leading to complications such as preterm birth, stillbirth, and congenital anomalies. A report by the World Health Organization (WHO) emphasizes that pregnant women are particularly susceptible to the health impacts of climate change, including increased exposure to vector-borne diseases. The WHO notes that climate-related changes such as rising temperatures and increased rainfall can expand the habitats of vectors, increasing the incidence and geographical spread of diseases such as malaria and dengue fever, which disproportionately affect pregnant women (50).
Moreover, the CHAMNHA consortium highlighted the compounded risks faced by pregnant women due to climate change. The authors stated that the physiological changes during pregnancy make women more vulnerable to infections and illnesses. The report underscores the urgent need for tailored public health interventions to protect this demographic from the exacerbating effects of climate change on vector-borne diseases (51).
Global distribution and incidence of vector-borne diseases among pregnant women
The global distribution and incidence of vector-borne diseases among pregnant women have exhibited significant variations across regions and over time. In 2020, ~121.9 million pregnancies occurred in areas at risk of malaria transmission globally. The distribution of these pregnancies was as follows: 52.9 million in the WHO South-East Asia Region, 46.1 million in the African Region, 11.1 million in the Eastern Mediterranean Region, 6.7 million in the Americas Region, and 5.1 million in the Western Pacific Region. From 2007 to 2020, the incidence of pregnancies in areas of Plasmodium falciparum transmission decreased by 11.4% globally, despite an overall increase in the number of pregnancies in these regions (52).
Regarding dengue, the prevalence among pregnant women varies significantly by region. For example, a study conducted in Port Harcourt, Nigeria, found a seroprevalence of dengue IgG antibodies of only 2.1% among pregnant women. This contrasts sharply with higher prevalence rates in other regions, such as Malaysia, where a seroprevalence of 32.4% was reported among pregnant women (53).
Significant regional disparities exist in the burden of vector-borne diseases during pregnancy, largely reflecting differences in healthcare infrastructure, surveillance capacity, and environmental exposure. In sub-Saharan Africa, where over 10 million pregnancies occur annually in malaria-endemic areas, limited access to antenatal care and preventive tools such as insecticide-treated nets has been associated with high rates of placental malaria, stillbirth, and maternal mortality (54). By contrast, the 2015-2016 ZIKV outbreak in Brazil disproportionately affected the northeastern regions, where under-resourced health systems and delayed vector control responses contributed to elevated rates of congenital Zika syndrome, highlighting geographic inequalities even within a single country (51). Similarly, in Southeast Asia, recurrent dengue outbreaks in the densely populated urban slums of the Philippines and Thailand pose ongoing risks to pregnant women, exacerbated by poor sanitation, limited vector control, and insufficient maternal health services (55). These examples underscore the critical need for regionally tailored public health strategies that address both biological and structural vulnerabilities.
Impact of residence on vector-borne infections in pregnant women
Urbanization exacerbates the risk of vector-borne diseases for pregnant women. Poor urban infrastructure, inadequate waste management, and overcrowding create environments conducive to vector breeding, thereby increasing the risk of diseases such as dengue and malaria (56). Both urban and rural settings present unique challenges, necessitating tailored public health interventions (56). The burden of vector-borne diseases is often higher in urban settings due to these environmental conditions. For instance, urban areas with poor infrastructure and large slum populations experience higher rates of diseases such as dengue, as urban residents are more susceptible due to their proximity to breeding sites and limited access to adequate healthcare (54). In Cameroon, research revealed that mosquito-borne diseases such as dengue and malaria were significantly influenced by urbanization, with urban areas experiencing a higher disease burden compared with rural areas (57).
Conversely, rural areas face different challenges. Limited access to healthcare, poor housing, and closer proximity to natural vector habitats such as forests and water bodies contribute to high incidence rates of vector-borne diseases. In these regions, diseases such as malaria remain a critical concern due to the abundance of mosquito breeding sites and insufficient vector control measures. Pregnant women in rural areas are particularly vulnerable due to these factors, which are compounded by inadequate healthcare infrastructure (1).
The evolution of vector-borne infectious diseases in urban and rural settings is influenced by environmental changes, migration patterns, and socioeconomic factors. Urbanization, if well-planned, can reduce transmission through improved infrastructure and healthcare access. However, unplanned urbanization can lead to increased disease burdens among urban populations. Addressing these issues requires tailored interventions that consider the unique circumstances of both rural and urban settings to effectively mitigate the impact on pregnant women (56).
Physiological changes during pregnancy that may affect susceptibility to vector-borne diseases
Physiological and immunological changes during pregnancy, such as adaptations to tolerate the genetically distinct fetus, reduce the ability to combat infections, making pregnant women particularly vulnerable to vector-borne diseases. These changes include fewer lymphocytes such as CD4 and CD8, fewer cytotoxic T cells, and a shift from Th1 to Th2(57). Hormonal changes during pregnancy can elevate body temperature and alter the production of substances such as carbon dioxide, which renders pregnant women more attractive to biting insects such as mosquitoes. More specifically, progesterone is responsible for increasing body temperature by influencing the hypothalamus, and estrogen affects water and electrolyte balance by increasing renal sodium retention, which can lead to changes in blood volume and pressure (58,59). Furthermore, the expansion of the abdominal area can lead to changes in skin physiology, such as increased temperature and moisture, making the skin more attractive to biting insects (60). Pregnancy also restricts the use of certain medications due to potential risks to fetal health, complicating the management of vector-borne diseases during this period. The limited availability of safe therapeutic interventions during pregnancy makes disease management difficult (61).
Beyond physiological changes, a range of sociocultural and economic factors significantly heighten the vulnerability of pregnant women to vector-borne diseases, particularly in low- and middle-income countries. Women with limited access to education may lack critical knowledge about disease prevention strategies such as insecticide-treated net usage or early symptom recognition. Poor housing conditions, including inadequate sanitation and lack of protective barriers such as window screens, increase human-vector contact. Moreover, rural residency often corresponds with poor infrastructure, long distances to healthcare facilities, and inconsistent vector control programs, all of which impede timely diagnosis and treatment. Sex inequities may further constrain the autonomy of women in seeking care or participating in public health interventions, especially in patriarchal societies where health decisions are made by male household members (62). The intersection of poverty, social marginalization, and environmental exposure is particularly pronounced during extreme weather events, which disproportionately displace pregnant women and disrupt access to essential maternal services (51). These overlapping vulnerabilities underscore the need for intersectional, equity-based public health strategies tailored to the needs of at-risk pregnant populations.
Adverse pregnancy outcomes associated with vector-borne diseases
The effects of vector-borne diseases on pregnancy can vary depending on the stage of pregnancy, the type of pathogen, how the pathogen interacts with the immune system, and specific host factors.
ZIKV
ZIKV can cause numerous maternal and fetal complications during pregnancy, with the risk of these complications estimated to range from 5 to 42% (63). Pregnant women infected with ZIKV may experience symptoms such as mild fever, arthralgia, myalgia, a maculopapular rash, pruritus, conjunctivitis or conjunctival hyperemia, and edema of the extremities, typically manifesting in the first trimester. Rarely, severe neurological complications might occur (64). A systematic review demonstrated that complications associated with maternal ZIKV infection include a broad range of fetal and neonatal neurological and ocular abnormalities, fetal intrauterine growth restriction, stillbirth, and perinatal death. Notably, eight studies identified microcephaly as the primary neurological consequence, with an incidence of ~1% among newborns of ZIKV-infected women in one study (21).
Malaria
Malaria is one of the most common vector-borne infectious diseases among people living in the WHO African Region (54). Every year, 125 million women become pregnant in malaria-endemic areas (65). Pregnant women are three times more likely than non-pregnant women to experience severe illness due to malarial infection, with a mortality rate from severe disease approaching 50% (66). Women in their first pregnancy are most at risk (6). Pregnancy induces a state of immune modulation, necessary to tolerate the semi-allogeneic fetus, which involves a shift from a Th1 to a Th2 immune response, rendering pregnant women more susceptible to malaria and reducing their ability to effectively combat pathogens. This immune shift is more pronounced in first pregnancies as the maternal immune system encounters fetal antigens for the first time (67). Malaria infection in pregnancy is known to cause high rates of spontaneous abortion, preterm delivery, fetal intrauterine growth restriction, stillbirth, congenital infection, and neonatal mortality, contributing to an estimated 10,000 maternal deaths annually (68).
Dengue fever
Dengue fever infects an estimated 390 million individuals annually (69). It is a life-threatening illness with symptoms including fever, joint pain, myalgia, and cough in mild cases. Severe symptoms include dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS), the latter having a high mortality rate and requiring urgent medical treatment. Dengue appears to be more severe in pregnant women than in the general population. One study found that the risk of DHF/DSS increased 3.4 times during pregnancy and was higher in the third trimester. The overall death rate in this study was 7.4% (70). Complications such as hypertension, emergency caesarean sections due to fetal distress, obstetric hemorrhage, pre-eclampsia, and eclampsia have been reported (55). A systematic review and meta-analysis reported that preterm birth and low birth weight were also common adverse pregnancy outcomes following dengue infection (71).
Lyme disease
Lyme disease, transmitted by ticks, also poses serious risks during pregnancy. If untreated, it can lead to serious complications such as infection of the placenta, congenital heart defects, and stillbirth. However, with appropriate antibiotic treatment, these risks can be mitigated, and there is no increased risk of adverse birth outcomes (72). Early symptoms of Lyme disease, such as a bull's-eye rash, fatigue, and muscle aches, can occur within a month of being bitten by an infected tick. It is crucial for pregnant women who suspect they have been bitten to seek immediate medical attention to start treatment early and prevent complications. The standard treatment for Lyme disease during pregnancy involves antibiotics such as amoxicillin, as doxycycline is generally avoided due to its potential adverse effects on the fetus (73). Research on the long-term effects of Lyme disease on pregnancy and infant development is ongoing. A pilot study by Children's National Hospital (Washington, USA) aims to assess the neurodevelopmental impacts of utero exposure to Lyme disease, highlighting the need for further investigation into this under-researched area (74).
This section highlighted the amplified risks of vector-borne diseases during pregnancy, including adverse outcomes such as miscarriage, preterm birth, and congenital anomalies. It integrated physiological vulnerabilities of pregnant women and the effects of disease-specific pathogens such as Zika and malaria. The discussion is limited in addressing how sociocultural and economic factors exacerbate these risks, particularly in low-resource settings. Further research should investigate how intersecting vulnerabilities influence outcomes and how tailored public health interventions can be developed.
4. Interplay between climate change, pregnancy, and vector-borne infectious diseases
Heat exposure is one of the most obvious impacts of climate change on pregnancy. Exposure to extreme heat, especially during the third trimester, has been associated with an increased risk of preterm labor (75). Heatwaves during pregnancy can lead to a higher incidence of low birth weight in newborns (76). In addition, pregnant individuals need to stay particularly hydrated; excess heat can lead to dehydration, which can be harmful to both the mother and child (77).
Climate change can significantly worsen air quality due to increased emissions from forest fires and the use of fossil fuels. Poor air quality can lead to respiratory issues, affect the in utero development of the nervous system, and increase the risk of pregnancy-induced hypertension and gestational diabetes in pregnant individuals (78,79). Furthermore, changing climate patterns impact agriculture, with potential consequences for food availability and nutrition. Pregnant individuals require access to a healthy diet, and deficiencies in key nutrients can lead to adverse pregnancy outcomes such as anemia and growth restriction in the fetus (80). Additionally, the stress and anxiety resulting from extreme weather events disproportionately affect pregnant individuals. Natural disasters associated with climate change can disrupt healthcare infrastructure, making it difficult for pregnant individuals to receive necessary prenatal care (80).
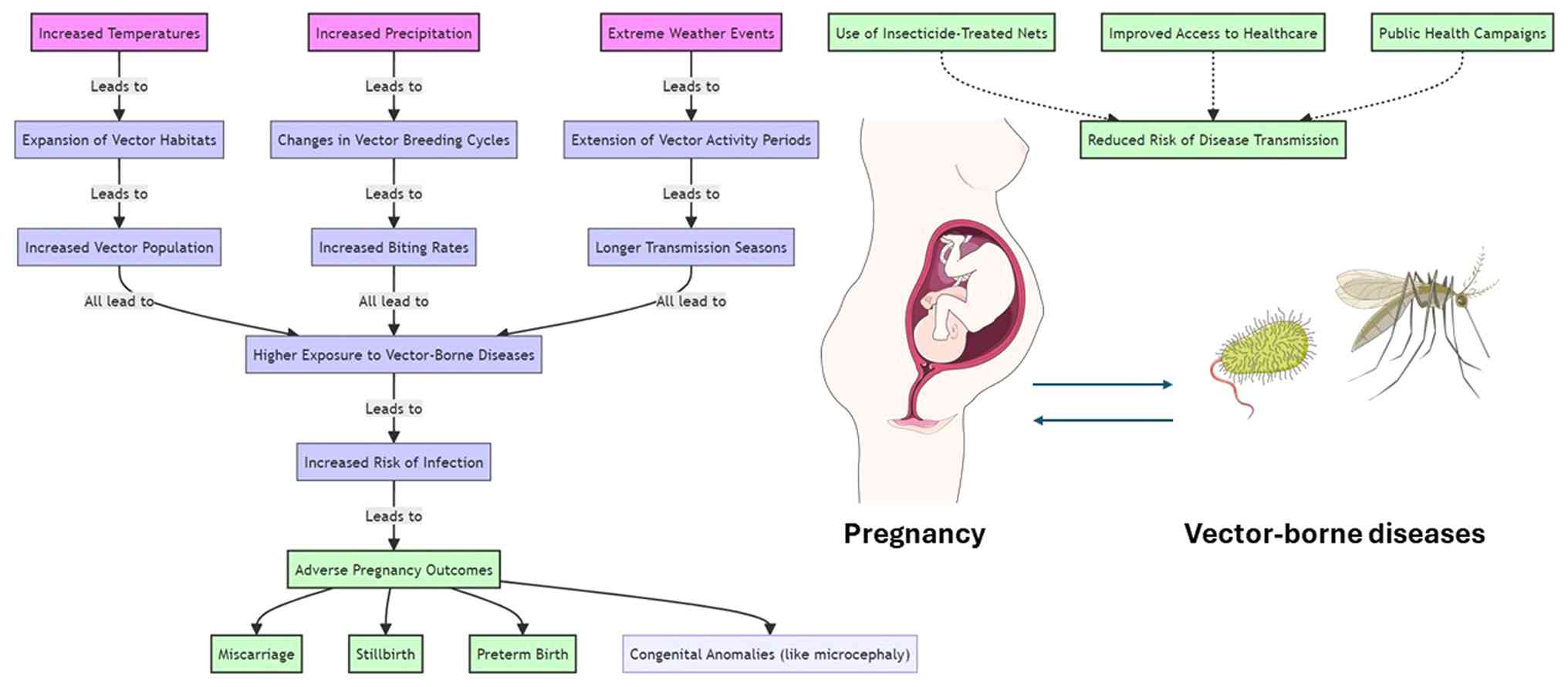
The effects of climate change are far-reaching, impacting not only the environment but also human health in a multitude of ways. These extreme weather events create hospitable environments for vectors, facilitating their reproduction, longevity, and geographic spread. Diseases such as Zika, dengue, malaria, and Lyme disease, traditionally concentrated in specific equatorial regions, are now expanding into broader territories due to this expansion, thereby increasing the risk to pregnant individuals (80).
Limited access to healthcare services, including antenatal care and disease prevention programs, increases the exposure and susceptibility of pregnant women to vector-borne diseases. Lower educational levels are linked to reduced knowledge of disease prevention and health-promoting practices. Poor housing quality, such as a lack of screens or bed nets, can facilitate contact with disease vectors. In addition, low socio-economic status is often associated with higher human-vector contact rates, due to factors such as outdoor occupations and inadequate vector control measures in residential areas (8). Sex inequalities may also limit the access of women to resources and autonomy in decision-making, impacting their ability to seek preventative care and treatment (81).
Rising temperatures and shifting rainfall patterns expand the habitats of disease-carrying vectors, changing transmission patterns. Unplanned urban growth can lead to increased breeding sites for vectors and a higher incidence of vector-borne diseases. Inadequate water management can create breeding grounds for disease vectors, such as stagnant water bodies. Deforestation and agricultural expansion can alter the ecosystem, sometimes increasing vector populations (82).
The primary challenge for healthcare systems lies in adapting to the increased demands posed by climate-related health issues, requiring robust strategies for surveillance, prevention, and management. Policymakers are tasked with creating resilient infrastructures and regulations to mitigate the impacts of climate change, ensuring that communities, especially vulnerable populations, are protected. For individuals, the challenge is to gain access to accurate information and resources that enable proactive measures against the health risks associated with climate change and vector-borne diseases. Overcoming these hurdles necessitates a collaborative effort that spans across disciplines and sectors, emphasizing the importance of integrated approaches in addressing the health ramifications of climate change (31,80).
Climate change poses significant threats to public health, particularly concerning pregnancy outcomes and the spread of vector-borne diseases. Adapting healthcare systems is crucial for managing the impacts of climate change on vector-borne diseases. Effective responses require training healthcare workers and developing robust surveillance systems to manage the rising incidences of vector-borne diseases and related pregnancy complications in a warming climate (83,84).
On the policymaking front, substantial investments in climate-resilient infrastructures are necessary. Ensuring that healthcare facilities can remain operational during extreme weather events is vital for continuous care, especially for pregnant populations who may require immediate and uninterrupted healthcare services. Moreover, enacting comprehensive public health policies that address the direct and indirect impacts of climate change is crucial. These policies should include vector control strategies, disease prevention, nutritional support programs, and educational initiatives aimed at raising awareness about the risks posed by climate change (85).
Individual actions also play a pivotal role in mitigating the adverse effects of climate change on health. Pregnant individuals and those planning pregnancy can significantly benefit from increased awareness and preparedness. This includes understanding the potential health risks posed by climate change and engaging in proactive measures such as emergency planning and adopting preventive practices against vector-borne diseases. Additionally, community engagement in environmental sustainability efforts, such as tree planting and waste reduction, can further aid in mitigating the impacts of climate change, thereby safeguarding individual and public health (86).
The section provided a comprehensive overview of how climate-induced environmental changes create synergistic risks for pregnant populations, with vectors expanding into new territories and vulnerable groups facing disproportionate impacts. Although the section discussed general trends, it lacked detailed examples of successful public health interventions or policies addressing these intersections. Future work should evaluate the effectiveness of existing adaptation programs to inform scalable solutions.
A flowchart illustrating the complex interactions between climate change, vector ecology, and pregnancy is provided in Fig. 2.
5. Public health implications and strategies
Public health responses to mitigate the impacts of vector-borne infectious diseases on pregnancy outcomes
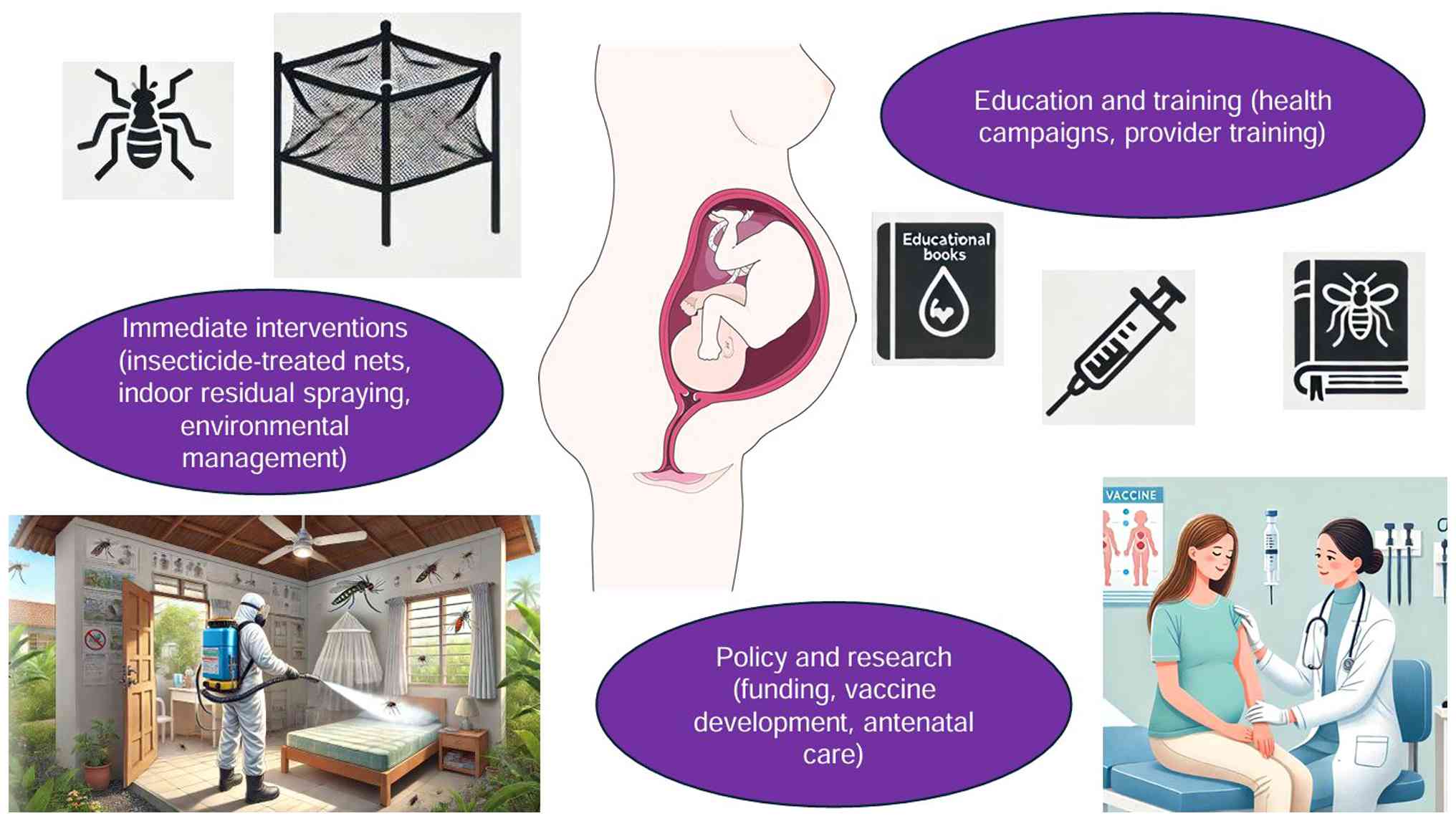
Malaria, ZIKV, and dengue fever significantly impact pregnancy outcomes, particularly in endemic regions, where they increase the risk of complications such as miscarriage, stillbirth, preterm birth, and congenital abnormalities (87). Public health strategies emphasize controlling vectors through measures such as the use of insecticide-treated nets, indoor residual spraying, and environmental management practices aimed at reducing mosquito breeding grounds (88). These measures are part of community-wide efforts not only to protect pregnant women but also to control disease transmission. Furthermore, health education is vital for informing pregnant women and healthcare providers about the risks and preventive measures associated with these diseases. Educational campaigns and provider training enhance community engagement, improve preventive practices, and ensure pregnant women have access to necessary prenatal care (89,90).
Policies and initiatives for preventing vector-borne infectious diseases in pregnant women
Effective prevention of vector-borne diseases in pregnant women requires multi-faceted policy initiatives that support vector control programs, healthcare access, and the strengthening of healthcare systems. This involves allocating funds for mosquito control, improving infrastructure for vector surveillance, and ensuring that pregnant women have access to comprehensive antenatal services. These services should include screenings and appropriate treatments for vector-borne diseases (91). Additionally, there is a strong emphasis on supporting research and development of targeted interventions such as vaccines, which are crucial for long-term prevention efforts (5,71).
Adapting healthcare systems to the changing landscape of vector-borne infectious diseases
Through enhanced surveillance and capacity building, healthcare systems must adapt to the changing epidemiology of vector-borne diseases due to climate change. Advanced data collection and analysis methods are necessary to detect shifts in disease patterns and vector distribution (3). Training for healthcare professionals is essential to recognize symptoms of emerging diseases and implement effective diagnostic and management protocols (92). Furthermore, integrating vector control and disease prevention into climate change adaptation strategies is crucial. Public health campaigns should raise awareness about the risks posed by a warming climate and promote preventive behaviors (93).
Examples of successful surveillance and vector control programs
Several successful case studies demonstrate how targeted interventions can effectively reduce the burden of vector-borne diseases among vulnerable populations, including pregnant women. In Sri Lanka, a sustained, multi-decade malaria elimination program combined indoor residual spraying, bed net distribution, and decentralized disease surveillance, resulting in zero indigenous cases since 2012 and WHO certification of malaria-free status in 2016(94). Similarly, the response of Brazil to the Zika outbreak incorporated real-time birth surveillance for microcephaly, integrated mosquito control efforts, and rapid prenatal screening in high-risk regions, which helped mitigate congenital Zika syndrome incidence during subsequent waves (95). In Vietnam, community-led dengue control campaigns focused on environmental management and water container cleaning reduced larval indices and dengue cases by over 50% in targeted provinces (96). These examples underscore the importance of integrated, context-sensitive approaches that combine public health infrastructure, community engagement, and sustained funding.
Future directions for research, surveillance, and capacity-building
Future research should prioritize understanding how climate change influences the transmission of vector-borne infectious diseases during pregnancy and assessing the long-term effects on maternal and neonatal health. Innovative surveillance tools and predictive modeling are needed to monitor these changes effectively (62,92). Longitudinal studies tracking the impact of vector-borne diseases on pregnancy outcomes can inform clinical guidelines and improve intervention strategies (93,97). Additionally, interdisciplinary collaboration among public health workers, midwives, and environmental health professionals is crucial for developing evidence-based strategies that address the interplay of environmental, social, and biological factors affecting pregnant women and their babies in the context of climate change (1,98). The public health strategies to mitigate the impact of vector-borne diseases on pregnant women is summarized in Table II. In addition, how climate change affects various infectious diseases and their specific impacts on pregnant women are presented in Table III. This section focused on actionable strategies such as vector control measures, surveillance systems, and antenatal care interventions. It underscored the importance of integrating climate adaptation into public health policies. While it presented numerous strategies, the lack of real-world case studies and quantitative evidence supporting the effectiveness of these measures is a limitation. Expanding the discussion with data-backed examples would strengthen the practical utility of the recommendations.
Table IIIDetailed overview of the effects of climate change on vector-borne diseases and associated risks to pregnant women. |
Comprehensive public health strategies to mitigate vector-borne diseases during pregnancy are illustrated in Fig. 3.
6. Conclusions
The interplay between climate change and vector-borne infectious diseases presents a pressing public health challenge, particularly concerning pregnancy outcomes. While substantial evidence links climate change to altered vector behaviors and increased disease risks, several gray areas persist. These include the precise mechanisms by which climate variability affects disease transmission during pregnancy and the long-term impacts on maternal and neonatal health. Additionally, data remain limited regarding the effective integration of climate adaptation strategies into maternal healthcare systems in vulnerable regions.
Addressing these uncertainties requires a multifaceted and equity-oriented approach. Immediate countermeasures should include bolstering vector surveillance systems, improving geographic access to antenatal care, and developing targeted educational campaigns to increase awareness of vector-borne disease risks among pregnant women. Policymakers should prioritize investments in climate-resilient healthcare infrastructure, including mobile maternal health units in remote and disaster-prone areas, and support the development and deployment of safe, pregnancy-appropriate vaccines and vector control tools.
Furthermore, national adaptation plans must explicitly incorporate maternal and reproductive health indicators, with a focus on high-risk groups such as women living in informal settlements, displacement settings, or rural communities. Support for community-based vector control programs, particularly those led by or tailored to women, can improve uptake and sustainability. These efforts should be accompanied by structural interventions to address underlying determinants such as sex inequality, inadequate housing, and poor sanitation, which exacerbate disease vulnerability.
Future research must continue to elucidate the complex interactions among environmental, biological, and social drivers of disease risk during pregnancy. Expanding predictive modeling efforts, fostering interdisciplinary collaboration, and evaluating the real-world effectiveness of adaptation strategies are essential for building resilient maternal health systems. By addressing these scientific and policy gaps, this review serves as a foundation for guiding targeted interventions and advancing public health protection in the face of a changing climate.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AB, CT and VEG conceptualized the study. AB, CT, VEG, AS, KG, DC and DAS made a substantial contribution to data interpretation and analysis of the literature and wrote and prepared the draft of the manuscript. AB and VEG interpreted the published data and provided critical revisions. All authors contributed to manuscript revision and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the editor-in-chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
Use of artificial intelligence tools
During the preparation of this work, AI tool Chat GPT was used to improve the readability and language of the manuscript, and subsequently, the authors revised and edited the content produced by the AI tool as necessary, taking full responsibility for the ultimate content of the present manuscript.
References
|
Papadiochou A, Diamanti A, Metallinou D, Georgakopoulou VE, Taskou C, Kagkouras I and Sarantaki A: Impact of climate change on reproductive health and pregnancy outcomes: A systematic review. Cureus. 16(e68221)2024.PubMed/NCBI View Article : Google Scholar | |
|
Rocklöv J and Dubrow R: Climate change: An enduring challenge for vector-borne disease prevention and control. Nat Immunol. 21:479–483. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Mojahed N, Mohammadkhani MA and Mohamadkhani A: Climate crises and developing vector-borne diseases: A narrative review. Iran J Public Health. 51:2664–2673. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Paz S: Climate change impacts on vector-borne diseases in Europe: Risks, predictions and actions. Lancet Reg Health Eur. 1(100017)2020.PubMed/NCBI View Article : Google Scholar | |
|
Ogden NH: Climate change and vector-borne diseases of public health significance. FEMS Microbiol Lett. 364(fnx186)2017.PubMed/NCBI View Article : Google Scholar | |
|
Schantz-Dunn J and Nour NM: Malaria and pregnancy: A global health perspective. Rev Obstet Gynecol. 2:186–192. 2009.PubMed/NCBI | |
|
Adepoju OA, Afinowi OA, Tauheed AM, Danazumi AU, Dibba LBS, Balogun JB, Flore G, Saidu U, Ibrahim B, Balogun OO and Balogun EO: Multisectoral perspectives on global warming and vector-borne diseases: A focus on Southern Europe. Curr Trop Med Rep. 10:47–70. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Thomson MC and Stanberry LR: Climate change and vectorborne diseases. N Engl J Med. 387:1969–1978. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Topluoğlu S, Taylan-Özkan A and Alp E: Impact of wars and natural disasters on emerging and re-emerging infectious diseases. Front Public Health. 11(1215929)2023.PubMed/NCBI View Article : Google Scholar | |
|
Bell JE, Brown CL, Conlon K, Herring S, Kunkel KE, Lawrimore J, Luber G, Schreck C, Smith A and Uejio C: Changes in extreme events and the potential impacts on human health. J Air Waste Manag Assoc. 68:265–287. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Murphy N, Rarama T, Atama A, Kauyaca I, Batibasaga K, Azzopardi P, Bowen KJ and Bohren MA: Changing climates, compounding challenges: A participatory study on how disasters affect the sexual and reproductive health and rights of young people in Fiji. BMJ Glob Health. 8 (Suppl 3)(e013299)2023.PubMed/NCBI View Article : Google Scholar | |
|
Rogerson SJ, Mwapasa V and Meshnick SR: Malaria in pregnancy: Linking immunity and pathogenesis to prevention. Am Soc Trop Med Hyg. 77 (6 Suppl):S14–S22. 2007.PubMed/NCBI | |
|
United Nations High Commissioner for Refugees (UNHCR). Gender, displacement and climate change. Available from: https://www.unhcr.org/news/stories/2022/3/622657fa4/costa-rican-asylum-seeking-women-together-save-cacao-plantation.html. Accessed 21 July, 2024. | |
|
Gahlawat IN and Lakra P: Global climate change and its effects. Integr J Soc Sci. 7:14–23. 2020. | |
|
Nema P, Nema S and Roy P: An overview of global climate changing in current scenario and mitigation action. Renew Sustain Energy Rev. 16:2329–2336. 2012. | |
|
AghaKouchak A, Chiang F, Huning LS, Love CA, Mallakpour I, Mazdiyasni O, Moftakhari H, Papalexiou SM, Ragno E and Sadegh M: Climate extremes and compound hazards in a warming world. Annu Rev Earth Planet Sci. 48:519–548. 2020. | |
|
Ingrao C, Strippoli R, Lagioia G and Huisingh D: Water scarcity in agriculture: An overview of causes, impacts and approaches for reducing the risks. Heliyon. 9(e18507)2023.PubMed/NCBI View Article : Google Scholar | |
|
Malhi GS, Kaur M and Kaushik P: Impact of climate change on agriculture and its mitigation strategies: A review. Sustainability. 13(1318)2021. | |
|
Weiskopf SR, Rubenstein MA, Crozier LG, Gaichas S, Griffis R, Halofsky JE, Hyde KJW, Morelli TL, Morisette JT, Muñoz RC, et al: Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci Total Environ. 733(137782)2020.PubMed/NCBI View Article : Google Scholar | |
|
Finch DM, Butler JL, Runyon JB, Fettig CJ, Kilkenny FF, Jose S, Frankel SJ, Cushman SA, Cobb RC, Dukes JS, et al: Effects of climate change on invasive species. In: Poland TM, Patel-Weynand T, Finch DM, Miniat CF, Hayes DC and Lopez VM (eds). Invasive Species in Forests and Rangelands of the United States. Springer, Cham, pp57-83, 2021. | |
|
Chibueze EC, Tirado V, Lopes KDS, Balogun OO, Takemoto Y, Swa T, Dagvadorj A, Nagata C, Morisaki N, Menendez C, et al: Zika virus infection in pregnancy: A systematic review of disease course and complications. Reprod Health. 14(28)2017.PubMed/NCBI View Article : Google Scholar | |
|
Lakos A and Solymosi N: Maternal Lyme borreliosis and pregnancy outcome. Int J Infect Dis. 14:e494–e498. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Chan EYY, Sham TST, Shahzada TS, Dubois C, Huang Z, Liu S, Hung KKC, Tse SLA, Kwok KO, Chung PH, et al: Narrative review on Health-EDRM primary prevention measures for vector-borne diseases. Int J Environ Res Public Health. 17(5981)2020.PubMed/NCBI View Article : Google Scholar | |
|
Ma J, Guo Y, Gao J, Tang H, Xu K, Liu Q and Xu L: Climate change drives the transmission and spread of vector-borne diseases: An ecological perspective. Biology (Basel). 11(1628)2022.PubMed/NCBI View Article : Google Scholar | |
|
Khezzani B, Baymakova M, Khechekhouche EA and Tsachev I: Global warming and mosquito-borne diseases in Africa: A narrative review. Pan Afr Med J. 44(70)2023.PubMed/NCBI View Article : Google Scholar | |
|
Cator LJ, Johnson LR, Mordecai EA, Moustaid FE, Smallwood TRC, LaDeau SL, Johansson MA, Hudson PJ, Boots M, Thomas MB, et al: The role of vector trait variation in vector-borne disease dynamics. Front Ecol Evol. 8(189)2020.PubMed/NCBI View Article : Google Scholar | |
|
Purse BV, Masante D, Golding N, Pigott D, Day JC, Ibañez-Bernal S, Kolb M and Jones L: How will climate change pathways and mitigation options alter incidence of vector-borne diseases? A framework for leishmaniasis in South and Meso-America. PLoS One. 12(e0183583)2017.PubMed/NCBI View Article : Google Scholar | |
|
Fouque F and Reeder JC: Impact of past and on-going changes on climate and weather on vector-borne diseases transmission: A look at the evidence. Infect Dis Poverty. 8(51)2019.PubMed/NCBI View Article : Google Scholar | |
|
Beugnet F and Chalvet-Monfray K: Impact of climate change in the epidemiology of vector-borne diseases in domestic carnivores. Comp Immunol Microbiol Infect Dis. 36:559–566. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Ogden NH and Lindsay LR: Effects of climate and climate change on vectors and vector-borne diseases: Ticks are different. Trends Parasitol. 32:646–656. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Ryan SJ, Carlson CJ, Mordecai EA and Johnson LR: Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl Trop Dis. 13(e0007213)2019.PubMed/NCBI View Article : Google Scholar | |
|
Caminade C, Kovats S, Rocklov J, Tompkins AM, Morse AP, Colón-González FJ, Stenlund H, Martens P and Lloyd SJ: Impact of climate change on global malaria distribution. Proc Natl Acad Sci USA. 111:3286–3291. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Baafi J and Hurford A: Modeling the impact of seasonality on mosquito population dynamics: Insights for vector control strategies. bioRxiv, 2024. | |
|
Baril C, Pilling BG, Mikkelsen MJ, Sparrow JM, Duncan CAM, Koloski CW, LaZerte SE and Cassone BJ: The influence of weather on the population dynamics of common mosquito vector species in the Canadian prairies. Parasit Vectors. 16(153)2023.PubMed/NCBI View Article : Google Scholar | |
|
Li Y, Zhou G, Zhong S, Wang X, Zhong D, Hemming-Schroeder E, Yi G, Fu F, Fu F, Cui L, et al: Spatial heterogeneity and temporal dynamics of mosquito population density and community structure in Hainan Island, China. Parasit Vectors. 13(444)2020.PubMed/NCBI View Article : Google Scholar | |
|
Medlock JM, Hansford KM, Bormane A, Derdakova M, Estrada-Peña A, George JC, Golovljova I, Jaenson TG, Jensen JK, Jensen PM, et al: Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 6(1)2013.PubMed/NCBI View Article : Google Scholar | |
|
Hales S, de Wet N, Maindonald J and Woodward A: Potential effect of population and climate changes on global distribution of dengue fever: An empirical model. Lancet. 360:830–834. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Anyamba A, Linthicum KJ, Mahoney R, Tucker CJ and Kelley PW: Mapping potential risk of Rift Valley fever outbreaks in African savannas using vegetation index time series data. Photogramm Eng Remote Sens. 68:137–145. 2002. | |
|
Rogers DJ and Randolph SE: The global spread of malaria in a future, warmer world. Science. 289:1763–1766. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Sutherst RW: Global change and human vulnerability to vector-borne diseases. Clin Microbiol Rev. 17:136–173. 2004.PubMed/NCBI View Article : Google Scholar | |
|
McMichael AJ, Woodruff RE and Hales S: Climate change and human health: Present and future risks. Lancet. 367:859–869. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Couper LI, MacDonald AJ and Mordecai EA: Impact of prior and projected climate change on US Lyme disease incidence. Glob Chang Biol. 27:738–754. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Ogden NH, Radojević M, Wu X, Duvvuri VR, Leighton PA and Wu J: Estimated effects of projected climate change on the basic reproductive number of the Lyme disease vector Ixodes scapularis. Environ Health Perspect. 122:631–638. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Colón-González FJ, Sewe MO, Tompkins AM, Sjödin H, Casallas A, Rocklöv J, Caminade C and Lowe R: Projecting the risk of mosquito-borne diseases in a warmer and more populated world: A multi-model, multi-scenario intercomparison modelling study. Lancet Planet Health. 5:e404–e414. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Wang Y, Zhao S, Wei Y, Li K, Jiang X, Li C, Ren C, Yin S, Ho J, Ran J, et al: Impact of climate change on dengue fever epidemics in South and Southeast Asian settings: A modelling study. Infect Dis Model. 8:645–655. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Morin CW, Comrie AC and Ernst K: Climate and dengue transmission: Evidence and implications. Environ Health Perspect. 121:1264–1272. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Medone P, Ceccarelli S, Parham PE, Figuera A and Rabinovich JE: The impact of climate change on the geographical distribution of two vectors of Chagas disease: Implications for the force of infection. Philos Trans R Soc Lond B Biol Sci. 370(20130560)2015.PubMed/NCBI View Article : Google Scholar | |
|
Paz S: Climate change impacts on West Nile virus transmission in a global context. Philos Trans R Soc Lond B Biol Sci. 370(20130561)2015.PubMed/NCBI View Article : Google Scholar | |
|
Mor G and Cardenas I: The immune system in pregnancy: A unique complexity. Am J Reprod Immunol. 63:425–433. 2010.PubMed/NCBI View Article : Google Scholar | |
|
World Health Organization (WHO): Experts warn of serious health impacts from climate change for pregnant women, children, and older people. WHO, Geneva, 2024. https://www.who.int/news/item/05-06-2024-experts-warn-of-serious-health-impacts-from-climate-change-for-pregnant-women--children--and-older-people. Accessed July 21, 2024. | |
|
Roos N, Kovats S, Hajat S, Filippi V, Chersich M, Luchters S, Scorgie F, Nakstad B and Stephansson O: CHAMNHA Consortium. Maternal and newborn health risks of climate change: A call for awareness and global action. Acta Obstet Gynecol Scand. 100:566–570. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Reddy V, Weiss DJ, Rozier J, Ter Kuile FO and Dellicour S: Global estimates of the number of pregnancies at risk of malaria from 2007 to 2020: A demographic study. Lancet Glob Health. 11:e40–e47. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Okonko IO, Innocent-Adiele HC, Njoku OV and Eugene EA: First serological prevalence of dengue virus IgG antibody among pregnant women in Port Harcourt, Nigeria. Sci Afr. 22:239–248. 2023. | |
|
World Health Organization (WHO): Malaria. WHO, Geneva, 2024. https://www.who.int/news-room/fact-sheets/detail/malaria#:~:text=The%20WHO%20African%20Region%20carries,malaria%20deaths%20in%20the%20Region. Accessed June 22, 2024. | |
|
Paixão ES, Teixeira MG, Costa MDCN and Rodrigues LC: Dengue during pregnancy and adverse fetal outcomes: A systematic review and meta-analysis. Lancet Infect Dis. 16:857–865. 2016.PubMed/NCBI View Article : Google Scholar | |
|
World Health Organization (WHO): Responding to malaria in urban areas: A new framework from WHO and UN-Habitat. WHO, Geneva, 2022. https://www.who.int/news-room/feature-stories/detail/responding-to-malaria-in-urban-areas-a-new-framework-from-who-and-un-habitat. Accessed July 21, 2024. | |
|
Alenou LD, Nwane P, Mbakop LR, Piameu M, Ekoko W, Mandeng S, Bikoy EN, Toto JC, Onguina H and Etang J: Burden of mosquito-borne diseases across rural versus urban areas in Cameroon between 2002 and 2021: Prospective for community-oriented vector management approaches. Parasit Vectors. 16(136)2023.PubMed/NCBI View Article : Google Scholar | |
|
Steward K and Raja A: Physiology, ovulation and basal body temperature. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. | |
|
Stachenfeld NS: Sex hormone effects on body fluid regulation. Exerc Sport Sci Rev. 36:152–159. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Konopka JK, Task D, Afify A, Raji J, Deibel K, Maguire S, Lawrence R and Potter CJ: Olfaction in Anopheles mosquitoes. Chem Senses. 46(bjab021)2021.PubMed/NCBI View Article : Google Scholar | |
|
Dathe K and Schaefer C: The use of medication in pregnancy. Dtsch Arztebl Int. 116:783–790. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Namasivayam A, Osuorah DC, Syed R and Antai D: The role of gender inequities in women's access to reproductive health care: A population-level study of Namibia, Kenya, Nepal, and India. Int J Womens Health. 4:351–364. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Brasil P, Pereira JP Jr, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai UA, Salles TS, et al: Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 375:2321–2334. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Pomar L, Musso D, Malinger G, Vouga M, Panchaud A and Baud D: Zika virus during pregnancy: From maternal exposure to congenital Zika virus syndrome. Prenat Diagn. 39:420–430. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Dellicour S, Tatem AJ, Guerra CA, Snow RW and ter Kuile FO: Quantifying the number of pregnancies at risk of malaria in 2007: A demographic study. PLoS Med. 7(e1000221)2010.PubMed/NCBI View Article : Google Scholar | |
|
World Health Organization. Guidelines for the treatment of malaria. Geneva: World Health Organization, 2006. | |
|
Brabin BJ and Rogerson SJ: The epidemiology and outcomes of maternal malaria. In: Malaria in Pregnancy. CRC Press, pp8-55, 2001. | |
|
Machado CR, Machado ES, Rohloff RD, Azevedo M, Campos DP, de Oliveira RB and Brasil P: Is pregnancy associated with severe dengue? A review of data from the Rio de Janeiro surveillance information system. PLoS Negl Trop Dis. 7(e2217)2013.PubMed/NCBI View Article : Google Scholar | |
|
World Health Organization (WHO): Dengue and severe dengue. WHO, Geneva, 2024. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. Accessed June 22, 2024. | |
|
Machain-Williams C, Raga E, Baak-Baak CM, Kiem S, Blitvich BJ and Ramos C: Maternal, fetal, and neonatal outcomes in pregnant dengue patients in Mexico. Biomed Res Int. 2018(9643083)2018.PubMed/NCBI View Article : Google Scholar | |
|
Kuehn L and McCormick S: Heat exposure and maternal health in the face of climate change. Int J Environ Res Public Health. 14(853)2017.PubMed/NCBI View Article : Google Scholar | |
|
Leavey K, MacKenzie RK, Faber S, Lloyd VK, Mao C, Wills MKB, Boucoiran I, Cates EC, Omar A, Marquez O and Darling EK: Lyme borreliosis in pregnancy and associations with parent and offspring health outcomes: An international cross-sectional survey. Front Med (Lausanne). 9(1022766)2022.PubMed/NCBI View Article : Google Scholar | |
|
Lyme Disease Association: CDC focus on maternal-fetal transmission of Lyme disease. https://lymediseaseassociation.org/lyme-tbd/pregnancy-and-lyme/cdc-includes-update-on-possible-maternal-fetal-transmission-of-lyme-disease/. Accessed June 22, 2024. | |
|
Children's National Hospital: First-of-its-kind study on impacts of Lyme disease in pregnancy and infant development. https://innovationdistrict.childrensnational.org/lyme-disease-in-pregnancy-and-infant-development/. Accessed June 22, 2024. | |
|
Díaz J, Arroyo V, Ortiz C, Carmona R and Linares C: Effect of environmental factors on low weight in non-premature births: A time series analysis. PLoS One. 11(e0164741)2016.PubMed/NCBI View Article : Google Scholar | |
|
Lusambili A and Nakstad B: Awareness and interventions to reduce dehydration in pregnant, postpartum women, and newborns in rural Kenya. Afr J Prim Health Care Fam Med. 15:e1–e3. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Grippo A, Zhang J, Chu L, Guo Y, Qiao L, Zhang J, Myneni AA and Mu L: Air pollution exposure during pregnancy and spontaneous abortion and stillbirth. Rev Environ Health. 33:247–264. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Xia B, Zhou Y, Zhu Q, Zhao Y, Wang Y, Ge W, Yang Q, Zhao Y, Wang P, Si J, et al: Personal exposure to PM2.5 constituents associated with gestational blood pressure and endothelial dysfunction. Environ Pollut. 250:346–356. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Ha S: The changing climate and pregnancy health. Curr Environ Health Rep. 9:263–275. 2022.PubMed/NCBI View Article : Google Scholar | |
|
de Souza WM and Weaver SC: Effects of climate change and human activities on vector-borne diseases. Nat Rev Microbiol. 22:476–491. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Campbell-Lendrum D, Neville T, Schweizer C and Neira M: Climate change and health: Three grand challenges. Nat Med. 29:1631–1638. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Hathaway J and Maibach EW: Health implications of climate change: A review of the literature about the perception of the public and health professionals. Curr Environ Health Rep. 5:197–204. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Davenport F, Grace K, Funk C and Shukla S: Child health outcomes in Sub-Saharan Africa: A comparison of changes in climate and socio-economic factors. Glob Environ Change. 46:72–87. 2017. | |
|
Salam A: Internet of Things for environmental sustainability and climate change. In: Internet of Things for Sustainable Community Development: Wireless Communications, Sensing, and Systems, pp33-69, 2020. | |
|
Chakhtoura N, Hazra R and Spong CY: Zika virus: A public health perspective. Curr Opin Obstet Gynecol. 30:116–122. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Lu HZ, Sui Y, Lobo NF, Fouque F, Gao C, Lu S, Lv S, Deng SQ and Wang DQ: Challenge and opportunity for vector control strategies on key mosquito-borne diseases during the COVID-19 pandemic. Front Public Health. 11(1207293)2023.PubMed/NCBI View Article : Google Scholar | |
|
U.S. Environmental Protection Agency: Climate change and the health of pregnant, breastfeeding, and postpartum women. https://www.epa.gov/climateimpacts/climate-change-and-health-pregnant-breastfeeding-and-postpartum-women. Accessed June 22, 2024. | |
|
Campbell-Lendrum D, Manga L, Bagayoko M and Sommerfeld J: Climate change and vector-borne diseases: What are the implications for public health research and policy? Philos Trans R Soc Lond B Biol Sci. 370(20130552)2015.PubMed/NCBI View Article : Google Scholar | |
|
Sorensen C, Murray V, Lemery J and Balbus J: Climate change and women's health: Impacts and policy directions. PLoS Med. 15(e1002603)2018.PubMed/NCBI View Article : Google Scholar | |
|
Ortu G and Williams O: Neglected tropical diseases: Exploring long term practical approaches to achieve sustainable disease elimination and beyond. Infect Dis Poverty. 6(147)2017.PubMed/NCBI View Article : Google Scholar | |
|
Fournet F, Jourdain F, Bonnet E, Degroote S and Ridde V: Effective surveillance systems for vector-borne diseases in urban settings and translation of the data into action: A scoping review. Infect Dis Poverty. 7(99)2018.PubMed/NCBI View Article : Google Scholar | |
|
Poursafa P, Keikha M and Kelishadi R: Systematic review on adverse birth outcomes of climate change. J Res Med Sci. 20:397–402. 2015.PubMed/NCBI | |
|
Rylander C, Odland JØ and Sandanger TM: Climate change and the potential effects on maternal and pregnancy outcomes: An assessment of the most vulnerable-the mother, fetus, and newborn child. Glob Health Action. 6(19538)2013.PubMed/NCBI View Article : Google Scholar | |
|
World Health Organization (WHO): Elimination of malaria and prevention of re-establishment in Sri Lanka. WHO, Geneva, 2024. https://www.who.int/publications/i/item/9789240087026 Accessed June 8, 2025. | |
|
de Oliveira WK, Carmo EH, Henriques CM, Coelho G, Vazquez E, Cortez-Escalante J, Molina J, Aldighieri S, Espinal MA and Dye C: Zika virus infection and associated neurologic disorders in Brazil. N Engl J Med. 376:1591–1593. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Kay BH, Nam VS, Tien TV, Yen NT, Phong TV, Diep VT, Ninh TU, Bektas A and Aaskov JG: Control of aedes vectors of dengue in three provinces of Vietnam by use of Mesocyclops (Copepoda) and community-based methods validated by entomologic, clinical, and serological surveillance. Am J Trop Med Hyg. 66:40–48. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Rosa WE, Catton H, Davidson PM, Hannaway CJ, Iro E, Klopper HC, Madigan EA, McConville FE, Stilwell B and Kurth AE: Nurses and midwives as global partners to achieve the sustainable development goals in the Anthropocene. J Nurs Scholarsh. 53:552–560. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Lu DH, Jiang H and Lian JQ: Hantavirus infection during pregnancy. Virol Sin. 36:345–353. 2021.PubMed/NCBI View Article : Google Scholar |