Exosome‑mediated crosstalk between the cardiovascular and musculoskeletal systems: Mechanisms and therapeutic potential (Review)
- Authors:
- Published online on: June 27, 2025 https://doi.org/10.3892/ijmm.2025.5570
- Article Number: 129
-
Copyright: © Li et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
The organ systems within the human body are closely interconnected and respond coordinately to both internal and external demands and disruptions, dynamically maintaining internal balance and normal physiological activities through the transmission of various signals. The cardiovascular and musculoskeletal systems are two major systems within the human body and their interactions and crosstalk form an essential foundation for vital activities and the maintenance of health. Exosomes are defined as extracellular vesicles (EVs) originating from the endosomal compartment and secreted through the multivesicular body (MVB) pathway (1). They carry diverse bioactive molecules including proteins, lipids and nucleic acids, which are essential for facilitating communication between cells and play significant roles in the pathophysiological mechanisms underlying multiple diseases (2). For instance, specific microRNAs (miRNA/miR) carried by exosomes can serve as biomarkers for myocardial or skeletal muscle injury and even as potential therapeutic targets (3,4). As a crucial medium of intercellular communication, exosomes have garnered growing attention within the biomedical sector, especially concerning diseases related to the cardiovascular and musculoskeletal systems.
Cardiovascular diseases (CVDs) remain a major worldwide health threat. In recent years, growing research has shown that crosstalk between the cardiovascular and musculoskeletal systems can markedly improve CVDs. For example, musculoskeletal-derived exosomes can promote cardiac repair and regeneration (5,6). In addition, this crosstalk also contributes to the pathophysiological progression of CVDs. During sarcopenia, skeletal muscle-derived exosomes impair cardiac recovery after myocardial infarction (MI) by facilitating cardiomyocytes (CMCs) apoptosis (7). Bone marrow-derived fibroblast progenitor cells release extracellular vesicles that exacerbate cardiac fibrosis through mediating signaling pathways involving miR-21-5p and integrin α-V (ITGAV) (8). On the other hand, the homeostasis and functionality of the musculoskeletal system are influenced by exosomes as well. For example, exosomes secreted by cardiac progenitor cells markedly increase muscle fiber number and alleviate inflammatory responses and fibrosis in the skeletal muscle of X-linked muscular dystrophy (mdx) mice (9). As research into the endocrine, autocrine and paracrine functions of musculoskeletal tissues deepens, understanding of musculoskeletal molecular homeostasis has improved and the crosstalk between musculoskeletal and cardiac molecular homeostasis has also garnered increasing attention. Exosomes markedly contribute to this process. By delivering bioactive molecules, they affect the homeostasis and function of the musculoskeletal system, thus generating crosstalk with the cardiovascular system.
The present review is based on a comprehensive search of major databases such as PubMed (https://pubmed.ncbi.nlm.nih.gov), Google Scholar (/scholar.google.com) and Web of Science (https://www.webofscience.com), as of March 2025, using keywords such as 'exosome', 'bone-derived exosomes AND cardiovascular', 'cardiovascular-derived exosomes AND bone', 'skeletal muscle-derived AND cardiovascular' and 'cardiovascular-derived exosomes AND skeletal muscle'. It provided an in-depth and detailed discussion of the molecular mechanisms and signaling pathways underlying exosomes-mediated crosstalk between the cardiovascular and musculoskeletal systems for the first time, to the best of the authors' knowledge. The present review introduced the processes involved in exosomes formation and the mechanisms governing their functional regulation. Moreover, it comprehensively reviewed the research progress on how bone- and skeletal muscle-derived exosomes mediate cardiac function and CVDs through the regulation of pathophysiological pathways. It also discussed the effects of cardiovascular-derived exosomes on bone and skeletal muscle under physiological and pathological conditions, along with their molecular mechanisms. Finally, it outlined the significant role of exosomes in clinical therapy, summarized current limitations and aimed to provide new perspectives and ideas for research in related fields.
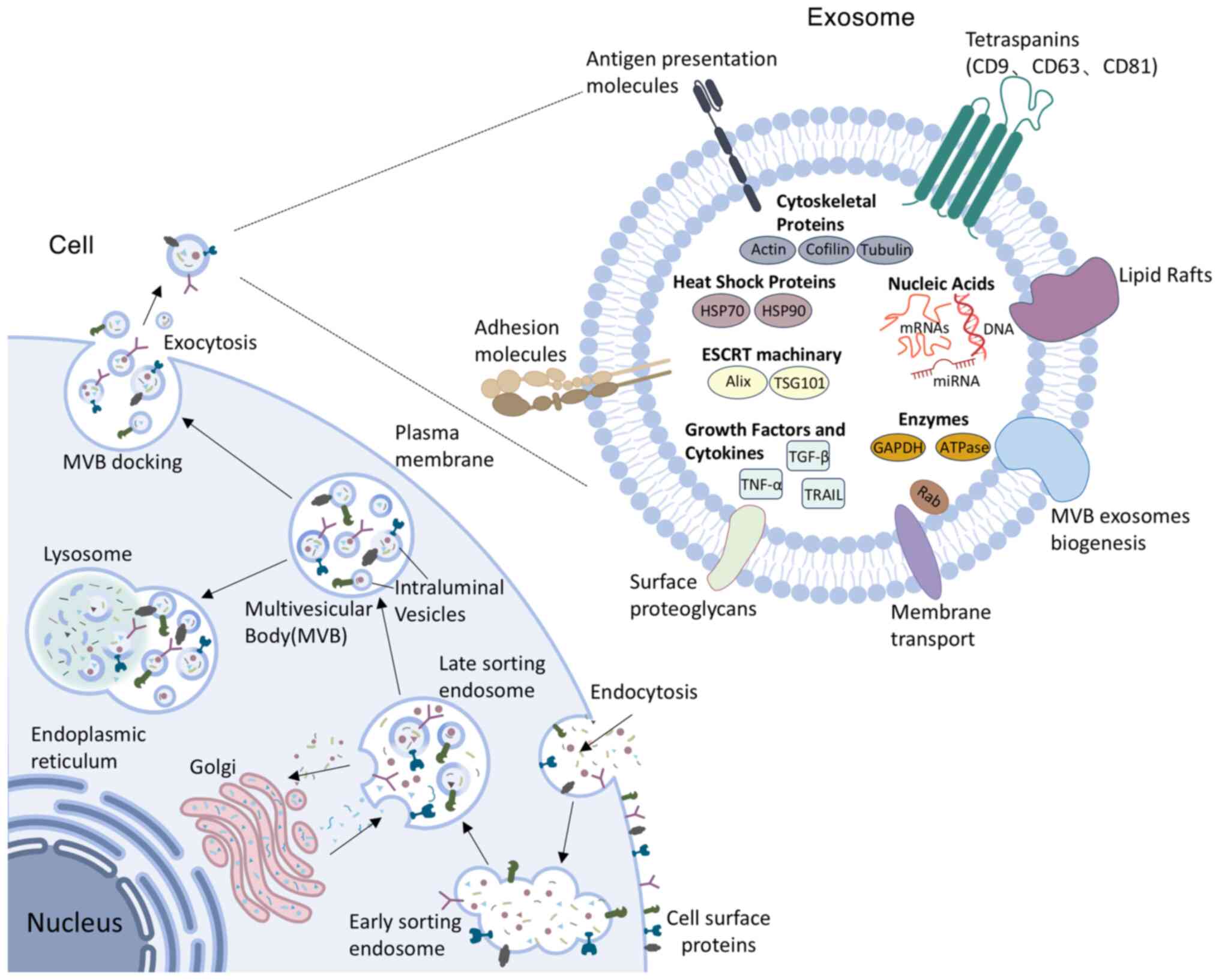
Biogenesis and functional regulation of exosomes
Exosomes, characterized by a lipid bilayer membrane and a diameter ranging from 30-150 nm, represent a subtype of small EVs (1,10). They are detectable in diverse biological fluids, such as plasma (11), lymph (12), urine (13), gastric fluid (14) and saliva (15). Exosomes originate from early endosomes generated through the inward budding of the plasma membrane. After early endosomes develop into late endosomes, the late endosomes invaginate to form MVBs, which sequester intraluminal vesicles (ILVs) within their lumen (16). Following maturation and fusion of MVBs with the plasma membrane, ILVs are secreted into the extracellular environment as exosomes (17), eventually entering circulation and exerting local paracrine or distant systemic influences. Thus, exosomes are instrumental in intercellular crosstalk and communication systems, effectively mediating the transmission of biological signals across diverse cell types and tissues. The formation of exosomes is co-regulated by membrane skeleton proteins, including apoptosis-linked gene 2-interacting protein X and the Rab family. Furthermore, transmembrane protein superfamily members, including cluster of differentiation (CD)9, CD63 and CD81, play crucial roles in the fusion of exosomes with recipient cells. These proteins are pivotal markers for the isolation and identification of exosomes (18).
The discovery of exosomes dates back to 1983, stemming from in vitro studies involving sheep reticulocytes (19). However, this finding did not gather considerable attention initially, as researchers regarded exosomes merely as a way for cells to eliminate unnecessary components (20). As research advanced, scholars gradually recognized that exosomes are essential for intercellular communication, immune responses, disease diagnosis and therapy, tissue repair and regeneration. Currently, it is unclear to what extent the delivery of exosomes is random rather than specifically targeted (21). Nevertheless, existing studies have confirmed that exosomes can transport their cargo to recipient cells by three mechanisms. Exosomes can interact with plasma membrane receptors through surface proteins (22). For example, cardiac endothelial cell (EC)-specific receptors, including kinase insert domain receptor, scavenger receptor class B type 1 and CD36, recognize exosomal ligands such as clusterin, heparan sulfate proteoglycan 2 and extracellular matrix proteins to initiate angiogenic signaling (23). In addition, exosomes can directly fuse with cell membranes, or be internalized through endocytic pathways involving phagocytosis, macropinocytosis and endocytic mechanisms dependent on lipid rafts, clathrin or caveolae (22).
Exosomes contain complex cargo comprising proteins, lipids and various nucleic acids such as DNA, mRNA, miRNA and long non-coding RNA (lncRNA), which facilitate intercellular exchange of materials and biological signals among diverse tissues and organs. Proteins in exosomes, including titin antisense RNA 1, Ras-related protein Rab-27B and synaptotagmin-7, can influence tumor growth through multiple mechanisms. They not only directly promote cancer cell proliferation and metastasis (24,25) but also indirectly facilitate tumor growth by modulating the tumor microenvironment (26). Lipid molecules in exosomes also exhibit therapeutic potential. For instance, eicosanoids in exosomes derived from different cell sources can serve as biomarkers for certain diseases and regulate distal immune responses (27). Exosomal DNA primarily originates from nuclear or mitochondrial DNA metabolic fragments or damage-induced DNA accumulation (28). These DNA molecules can circulate in the bloodstream and integrate into the genomes of specific immune cells, providing molecular markers for early cancer diagnosis and targeted therapy (29). As the key intermediary transferring genetic information from DNA to ribosomes, mRNA participates in various physiological and pathological processes by directing protein synthesis and has become an effective tool for treating diseases such as cancer. Yang et al (30) developed a cellular nanoporation technique to mass-produce exosomes loaded with therapeutic mRNA. These mRNA-loaded exosomes effectively restored tumor-suppressive functions in phosphatase and tensin homolog deleted on chromosome ten (PTEN)-deficient glioblastoma models, thereby inhibiting tumor progression and prolonging animal survival (30). Exosomal miRNAs modulate gene expression by binding target mRNAs and serve as key effector molecules mediating exosomal communication (31). In addition, exosomal lncRNAs demonstrate therapeutic effects in diverse diseases including cancer (32), metabolic disorders (33) and CVDs (34).
The composition and quantity of exosomal contents vary based on their cellular source and microenvironment and the number of exosomes released by a single cell can markedly fluctuate across various physiological and pathological states (35). Following acute myocardial infarction (AMI), damaged CMCs secrete a significant quantity of exosomes. Notably, under moderate hypoxic conditions for two hours, the secretion of exosomes from CMCs increases nearly threefold (36). In addition, exercise can increase the number of exosomal markers (such as TSG101, CD63 and CD81) as well as exosomal miRNAs (such as miR-125b-5p, miR-122-5p and miR-342-5p). These miRNAs mainly modulate the expression of mitogen-associated protein kinase (MAPK), nuclear factor-κB (NF-κB), vascular endothelial growth factor (VEGF) and cysteinyl aspartate specific proteinase (caspase), thereby contributing to cardiovascular protection (37) (Fig. 1).
The effects of bone-derived exosomes on the cardiovascular system
Bone-derived exosomes demonstrate multi-organ targeting capabilities through secretion into the extracellular environment and subsequent entry into bodily fluids or tissue interstitium, enabling their action on various organ systems including neural (38), tendinous (39) and cardiovascular systems (40). Consequently, bone-derived exosomes may similarly be internalized by target cells such as CMCs through membrane fusion or endocytosis, or alternatively regulate cardiac function via interactions between exosomal surface proteins and cardiac cell receptors. Bone marrow mesenchymal stem cells (BMSCs) are a type of non-hematopoietic stem cell originating from the bone marrow (BM) (41). They exhibit self-renewal capabilities and can extensively proliferate and differentiate into multiple cell types (42). Extensive research in recent years has demonstrated that exosomes released by BMSCs through paracrine mechanisms serve as critical membrane vesicles. These exosomes are crucial for mediating intercellular communication and exhibiting unique functions in disease diagnosis and treatment (43,44). BMSC-derived exosomes (BMSC-Exos) can regulate multiple signaling pathways in cells such as CMCs and macrophages. Through the synergistic action of various mechanisms, they exert therapeutic effects on CVDs, including MI, myocardial ischemia-reperfusion injury (MIRI), atherosclerosis (AS) and doxorubicin (DOX)-induced cardiomyopathy (DIC).
Amelioration of myocardial infarction
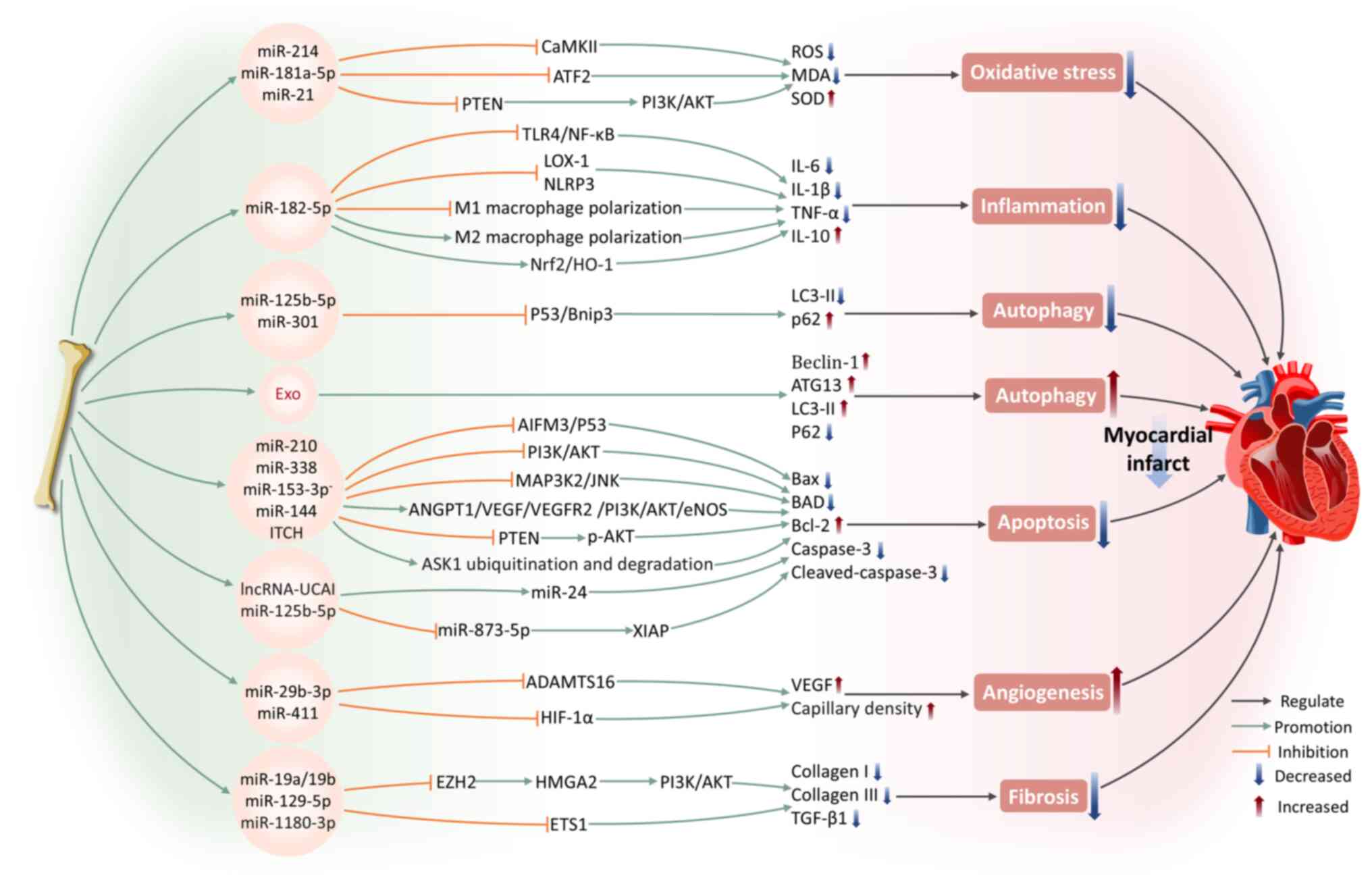
The mortality rate associated with MI is high (45). It is caused by the blockage of coronary arteries, resulting in abrupt interruption of blood flow and subsequent myocardial ischemia and hypoxia. It is often accompanied by severe oxidative stress and inflammatory responses, leading to extensive damage to cardiac tissue and a sharp decline in heart function (46). BMSC-Exos carry various bioactive molecules and can ameliorate MI through multiple mechanisms, including modulating oxidative stress, inflammation, autophagy, apoptosis, angiogenesis and fibrosis.
Oxidative stress
Oxidative stress arises from a disruption in the equilibrium between reactive oxygen species (ROS) generation and the antioxidant mechanisms of the body (47). Following MI, oxidative stress develops in both infarcted and non-infarcted regions of the myocardial tissues. Ischemic myocardium produces ROS, which, particularly following reperfusion, causes direct damage to cellular membranes, triggering cell death (48,49). ROS-induced oxidative stress is a major trigger for CMCs death following MI and contributes to the decline in cardiac contraction and relaxation function (47). Wang et al (40) found that BMSC-Exos can markedly reduce the apoptosis rate and ROS levels of cardiac stem cells (CSCs) following oxidative stress injury, inhibit malondialdehyde (MDA) levels and upregulate the expression of superoxide dismutase (SOD). Among these effects, miR-214, as a key functional molecule within BMSC-Exos, safeguards CSCs against oxidative stress by targeting and inhibiting calcium-calmodulin-dependent protein kinase II expression (40). Under lipopolysaccharide stimulation, BMSC-Exos exhibit a more significant inhibitory effect on oxidative stress (50). This is accompanied by an elevation in miR-181a-5p levels, which is transported to CMCs via exosomes, where miR-181a-5p targets and inhibits activating transcription factor 2 expression, leading to increased SOD1 and SOD2 production and suppressing oxidative stress in CMCs (50). Overexpression of PTEN inhibits the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) axis, which is a pathway widely acknowledged as a key mechanism driving cell apoptosis following oxidative stress (51). miR-21 in BMSC-Exos has been shown to effectively internalize into C-kit+ CSCs, inhibiting PTEN expression to activate PI3K/AKT pathway, thereby protecting CSCs from apoptosis under oxidative stress conditions (52).
Inflammation
MI facilitates the release of danger-associated molecular patterns from necrotic cells into the extracellular space, transmitting danger signals to surrounding CMCs via pattern recognition receptors, thus triggering strong inflammatory responses (53). The inflammatory response and oxidative stress following MI are key processes that contributing to myocardial injury, repair and remodeling (54). Research has shown that BMSC-Exos can effectively inhibit the levels of pro-inflammatory cytokines such as interleukin-6 (IL-6), interleukin-1 beta (IL-1β), tumor necrosis factor-α (TNF-α) and monocyte chemoattractant protein-1 in MI mouse heart tissue (55). This effect is mediated through the expression of miR-182-5p, which suppresses Toll-like receptor 4 (TLR4) expression, thus inhibiting the TLR4/NF-κB signaling pathway (55). Moreover, exosomes secreted by BMSCs with overexpressed miR-182-5p markedly enhance heart function recovery in MI mice, enhancing left ventricular ejection fraction while simultaneously reducing left ventricular end-diastolic pressure compared with normal exosomes (55). In addition, BMSC-Exos can reduce the activity of the nucleotide-binding domain and leucine-rich repeat pyrin domain-containing-3 (NLRP3) inflammasome in a left anterior descending coronary artery ligation mouse model, particularly in the peri-infarct region, by inhibiting inflammatory responses and pro-apoptotic mechanisms (56). This brings about a decrease in MI size as well as an enhancement of cardiac function (56).
Macrophages participate in the onset, progression and resolution of inflammation after MI. They are categorized into two principal types: Classically activated or pro-inflammatory macrophages (M1) and alternatively activated or anti-inflammatory macrophages (M2) (57). In the early stages following MI, M1 macrophages are recruited to the infarcted myocardial area, exhibiting potent phagocytic capabilities and pro-inflammatory effects. Subsequently, M2 macrophages gradually predominate and are instrumental in the process of resolving inflammation and repairing cardiac tissue (58). BMSC-Exos have been shown to promote M2 macrophage polarization while inhibiting M1 macrophage polarization, thereby alleviating myocardial injury and reducing inflammation after MI. The underlying mechanisms encompass the partial activation of the AKT1/AKT2 signaling pathway or direct inhibition of the NF-κB pathway (59,60). Furthermore, BMSCs treated with fibronectin type III domain-containing protein 5 effectively improve exosomal capacity to facilitate the transformation of M1 macrophages to the M2 phenotype, thereby alleviating inflammation following an infarction through the inhibition of the NF-κB pathway (60).
Autophagy
Autophagy can degrade proteins and organelles that are aged or damaged, converting them into amino acids and fatty acids which are utilized for energy production and recycling purposes (61). However, during severe myocardial ischemia, excessive autophagic flux promotes cell death and impairs cardiac function (62). The modulation of the autophagy process involves at least two signaling pathways: one centered on mammalian target of rapamycin (mTOR) and AMP-activated protein kinase (AMPK) (63) and the other involving tumor suppressor p53 and Bcl-2/adenovirus E1B 19-kDa interacting protein 3 (Bnip3) (64). Hypoxia leads to a marked upregulation of p53 and Bnip3 expression. In addition, overexpression of p53 further induces Bnip3 expression, increasing autophagic flux and inducing CMCs death (62). Xiao et al (62) revealed that miR-125b-5p within BMSC-Exos interferes with the p53/Bnip3 signaling, reducing the microtubule-associated protein 1 light chain 3-II (LC3-II) level while elevating the autophagy receptor P62 expression, thus providing cardioprotection. The authors showed that the benefits associated with BMSC transplantation following MI are partly due to the suppression of autophagy triggered by ischemic conditions (62). Overexpression of miR-301 in BMSC-Exos from MI rats markedly improve cardiac functional indicators, including an elevation in left ventricular ejection fraction and left ventricular fractional shortening, alongside a reduction in both the left ventricular end-diastolic and end-systolic diameters (65). Simultaneously, it reduces the LC3-II/LC3-I ratio in infarcted myocardial tissue and increases the relative expression of P62, effectively inhibiting autophagic processes in CMCs (65). Conversely, Zou et al (66) revealed that BMSC-Exos protect the myocardium by promoting autophagy. The authors showed that BMSC-Exos clearly upregulate autophagy-related protein 13 (ATG13) and Bcl-2-interacting protein 1 (Beclin-1) expression and that the autophagy inhibitor 3-methyladenine can reverse this protective effect (66). This indicates that the beneficial influence of BMSC-Exos on CMCs depends on their positive regulation of autophagic flux. A recent study demonstrated that pretreatment with Danshen decoction enhances the therapeutic effects of BMSC-Exos on MI, primarily by promoting CMCs autophagy to reduce infarcted areas and cardiac fibrosis (67). These findings further highlight the value of autophagy regulation in MI therapy.
Apoptosis
Apoptosis is a programmed cell death process. CMCs apoptosis initiates in the myocardial border zone affected by infarction within hours to days after AMI (68). It has been proved that miR-210 and miR-338 in BMSC-Exos can respectively regulate PI3K/AKT and the mitogen-activated protein kinase kinase kinase 2 (MAP3K2)/c-Jun N-terminal kinase (JNK) signaling pathways, reducing apoptosis-related genes expression, including Bcl-2 associated X protein (Bax), Bcl-xL/Bcl-2 associated death promoter and caspase-3, thus improving cardiac function following MI (69,70). Notably, inducing changes in gene expression of parental BMSCs can affect the apoptosis-suppressing capabilities of BMSC-Exos. For instance, exosomes released from GATA-binding protein-4 (GATA-4)-overexpressing BMSCs have been demonstrated to exhibit improved anti-apoptotic impacts on CMCs compared with control group, while also promoting BMSCs differentiation into CMC-like cells and increasing myocardial vascular density (71). According to Ning et al (72), BMSC-Exos with low expression of miR-153-3p enhance the phosphorylation of PI3K, AKT and endothelial nitric oxide synthase, thus reducing ECs and CMCs apoptosis and preventing hypoxic injury. miR-144 in BMSC-Exos is delivered to target cells, activating the PTEN/AKT pathway and exerts anti-apoptotic effects on hypoxic CMCs (73).
Ubiquitination, a crucial post-translational modification process of proteins, has also been shown to contribute to the cardiac protective mechanisms mediated by BMSC-Exos. For example, the itchy E3 ubiquitin ligase carried by BMSC-Exos can mediate the ubiquitination and degradation of apoptosis signal-regulated kinase-1, effectively inhibiting CMCs apoptosis and enhancing their viability, providing new insights for alleviating myocardial injury after MI (74).
Notably, pretreatment strategies further broaden the application prospects of BMSC-Exos in MI therapy. Hypoxic preconditioning of BMSC-Exos markedly upregulated miR-24 expression in myocardial tissue of rats with AMI and downregulated apoptosis-related proteins Bax, caspase-3 and cleaved-caspase-3, resulting in a reduction of the infarct size (75). Under conditions of hypoxic treatment for BMSCs, BMSC-Exos have been shown to reduce CMCs apoptosis by delivering miR-125b-5p and enhance CMCs viability via the miR-873-5p/X-linked inhibitor of apoptosis protein (XIAP) axis regulated by lncRNA-UCA1, thereby facilitating ischemic heart recovery and diminishing infarct size in an MI mouse model (6,76). Moreover, BMSC-Exos preconditioned with Danshen decoction showed superior efficacy in improving cardiac function, morphology, histopathology and CMCs ultrastructure compared with untreated groups (67). This effect involves the suppression of CMCs apoptosis, thus reducing the infarct area and cardiac fibrosis (67).
Angiogenesis
Angiogenesis refers to the biological mechanism through which new blood vessels originated from pre-existing vasculature. The occlusion of blood flow is the direct pathogenic mechanism of MI, whereas vascular reconstruction and the formation of new collateral circulation are critical steps in recovery following MI (77). In an AMI rat model, Teng et al (78) revealed the effects of BMSCs on both angiogenesis and anti-inflammatory responses through paracrine mechanisms. Specifically, BMSC-Exos enhance the angiogenic capacity of human umbilical vein endothelial cells (HUVECs), inhibit T-cell proliferation and, furthermore, decrease the size of infarcts, preserve cardiac contraction and relaxation performance and promote blood flow restoration (78). Zheng et al (79) showed that miR-29b-3p in BMSC-Exos targets a disintegrin-like metalloproteinase with thrombospondin motifs-16, reducing its expression levels and promoting myocardial angiogenesis and ventricular remodeling. This leads to a reduction in myocardial fibrosis and collagen volume fraction, upregulation of VEGF expression and increased capillary density, while effectively inhibiting CMCs apoptosis and alleviating MI symptoms in rats (79). A recent study found that hypoxia-inducible factor-1 (HIF-1α) expression is elevated in MI mice (80). BMSC-Exos preconditioned with Astragaloside IV can target and inhibit HIF-1α through miR-411, promoting neovascularization. This finding offers a new approach for MI treatment (80).
Fibrosis
Myocardial fibrosis, a significant pathophysiological process following MI, is mainly categorized into replacement fibrosis and reactive fibrosis (81). Replacement fibrosis occurs when CMCs in the infarcted area are replaced by a large number of fibrotic cells, forming scar tissue to prevent ventricular wall rupture (82). By contrast, reactive fibrosis involves the abnormal proliferation of connective tissue in the infarcted and distal myocardial regions, often leading to a decrease in ventricular compliance and increased stiffness (81). In addition, research indicates that BMSC-Exos can inhibit enhancer of zeste 2 polycomb repressive complex 2 subunit to activate high mobility group AT-hook 2 (HMGA2) (83). This action delays the epithelial-mesenchymal transition process and fibrosis progression in the myocardial tissue of MI rats (83). According to Wang et al (84), BMSC-Exos carrying miR-19a/19b reduce the fibrosis area in cardiac tissue of MI mouse models, leading to a reduction in collagen I and collagen III levels in the infarcted myocardium. Notably, BMSC-Exos that overexpress miR-129-5p enhance cardiac performance in MI mice, inhibit cell apoptosis and reduce fibrosis (85). They also decrease high mobility group box 1 (HMGB1) expression and inflammatory cytokines production, further suppressing inflammation (85). It has been demonstrated that BMSC-Exos pretreated with Vericiguat show superior efficacy in AMI therapy (86). Mechanistically, BMSC-Exos pretreated with Vericiguat are enriched with miR-1180-3p, which inhibits the ETS1 signaling, causing a marked suppression in the proliferation and migration of cardiac fibroblasts along with decreased collagen I and collagen III expression (86). Animal experiments have confirmed that Vericiguat-pretreated BMSC-Exos, compared with normal BMSC-Exos, more effectively improve cardiac contractile function and alleviates the extent of fibrosis (86).
To summarize, BMSC-Exos exhibit remarkable potential in the treatment of MI, improving cardiac function through multiple mechanisms, including modulation of oxidative stress, inflammatory response, autophagy, apoptosis, angiogenesis and fibrosis. BMSC-Exos carry various miRNAs, regulating key signaling pathways such asPI3K/AKT, TLR4/NF-κB, p53/Bnip3 and HIF-1α, to exert cardioprotective effects. Furthermore, preconditioning strategies, such as hypoxic preconditioning and drug induction, further enhance the cardiac repair function of BMSC-Exos (Fig. 2).
Alleviation of myocardial ischemia-reperfusion injury
MIRI refers to the phenomenon in which damage is exacerbated upon the restoration of blood flow after myocardial tissue ischemia, posing a serious threat to human health (87). During MI, a thrombus occludes a coronary artery, causing severe and extended myocardial ischemia, followed by local necrosis. In clinical interventions, such as thrombolytic treatment or percutaneous coronary intervention to restore blood circulation, ischemia/reperfusion (I/R) injury can occur (87,88). BMSC-Exos are crucial in alleviating MIRI and enhancing the survival of CMCs.
Oxidative stress
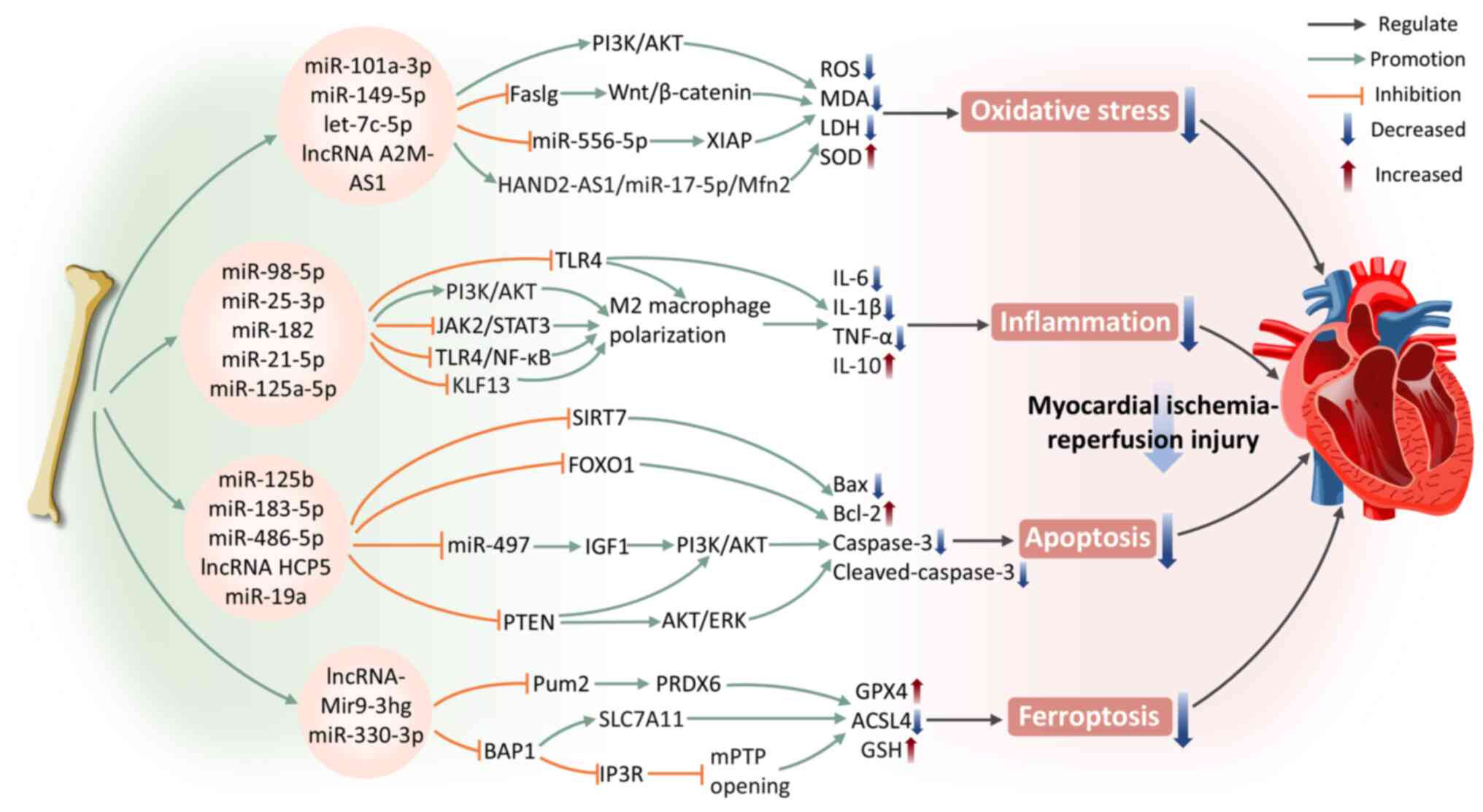
Oxidative stress is a vital pathological mechanism resulting in myocardial injury following reperfusion. It induces excessive ROS production, disrupts the redox balance and triggers CMCs apoptosis, autophagy, inflammation and mitochondrial dysfunction, ultimately resulting in irreversible myocardial damage and heart failure (89). HIF-1α is recognized as the central transcriptional regulator mediating the hypoxic adaptive response of tissue cells under ischemic conditions (90). A recent study uncovered that miR-101a-3p in BMSC-Exos activates the PI3K/AKT pathway, inhibits pathological autophagy in CMCs (91). It may also alleviate myocardial oxidative stress by inducing upregulation of HIF-1α, which subsequently enhances SOD production in CMCs and reduces MDA and ROS generation, thereby mitigating myocardial damage in I/R rats and potentiating cardioprotective effects (91). In addition, exosomes can deliver miR-149-5p, let-7c-5p and lncRNA A2M-AS1 molecules, which regulate the fas ligand gene, Wnt/β-catenin signaling pathway or miR-556-5p/XIAP signaling axis (92,93). This ameliorates oxidative stress in CMCs induced by hypoxia/reoxygenation (H/R) and markedly enhances the protective effects on CMCs. Another study demonstrated that BMSC-Exos reduce lactate dehydrogenase (LDH) and MDA levels in H/R CMCs and increase SOD levels (94). This protective effect results from modulating miR-17-5p expression in CMCs (94). The therapeutic effects of BMSC-Exos alone exhibits limitations, thus exploring methods to enhance their efficacy is crucial. Following pretreatment with Irisin, BMSC-Exos exhibit markedly stronger antioxidant effects compared with the control group (95). They reduce ROS production and inhibit oxidative stress in CMCs after H/R injury (95). This finding provides fresh perspectives for further optimizing cardioprotective strategies of BMSC-Exos.
Inflammation
During myocardial ischemia, CMCs death has already induced inflammation. However, when blood flow and oxygen supply are restored during reperfusion, inflammatory signaling pathways become further activated, exacerbating myocardial injury (87). Research has shown that hypoxia-induced BMSC-Exos with upregulated miR-98-5p reduce TLR4 expression in myocardial tissue and activate the PI3K/AKT signaling pathway (96). This mechanism effectively inhibits I/R injury, downregulates TNF-α, IL-1β and IL-6 expression, suppresses inflammatory responses and macrophage infiltration, thereby promoting cardiac function recovery (96). BMSC-Exos can also promote MIRI repair by facilitating M2 macrophage polarization. BMSC-Exos carrying specific miRNAs (such as miR-25-3p, miR-182, miR-21-5p and miR-125a-5p) attenuate MIRI by inhibiting the Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling pathway or targeting the TLR4/NF-κB/PI3K/Akt signaling cascade (97-100). These mechanisms induce the polarization of macrophages from the pro-inflammatory M1 state to the anti-inflammatory M2 state, reducing inflammation and promoting myocardial repair. Notably, the miR-125a-5p agomir treatment provides an efficient and safe strategy for utilizing stem cells and exosomes in the therapy of I/R (100).
Apoptosis
During MIRI, apoptosis serves as a critical event in the progression of myocardial injury. Apoptosis not only exacerbates myocardial tissue damage but also leads to a decline in cardiac function. Studies have shown that BMSC-Exos regulate apoptosis during I/R, providing diverse protective effects through the delivery of specific miRNAs. Specifically, BMSC-Exos carrying miR-125b negatively regulate sirtuin 7, downregulating the protein expression of Bax and caspase-3, while upregulating Bcl-2 levels, effectively alleviating MIRI (101). miR-183-5p in BMSC-Exos targets forkhead box transcription factor O1 (FOXO1) and reduces caspase-3 expression in the I/R rat model (102). Markedly, in vitro experiments demonstrated that overexpression of FOXO1 counteracted the protective role of miR-183-5p in CMCs induced by H/R, further confirming the importance of this regulatory pathway (102). A study using both an in vivo rat cardiac I/R model and in vitro H/R models of cardiac microvascular endothelial cells also demonstrated that BMSC-Exos restore heart function by inhibiting apoptosis (103). The authors revealed that BMSC-Exos promote microvascular regeneration during stress. They also protect cardiac microvascular endothelial cells from apoptosis and inhibit fibrosis by modulating platelet-derived growth factor receptor-β (103). The PI3K/AKT signaling pathway has a fundamental effect on the regulation of cell survival and apoptosis (104). miR-486-5p in BMSC-Exos inhibits PTEN expression, leading to the activation of the PI3K/AKT pathway, thus suppressing CMCs apoptosis and alleviating MIRI (105). In addition, lncRNA HCP5 inhibits insulin-like growth factor 1 expression via sponging miR-497, thereby activating PI3K/AKT pathway to enhance cell viability and contributing to the attenuation of MIRI (106). According to Yu et al (107), GATA-4-overexpressing BMSC-Exos deliver miR-19a to ischemic myocardium, activating the AKT and extracellular signal-regulated kinase (ERK) signaling pathways to mediate myocardial protection and promote in vivo ischemic myocardial repair.
Ferroptosis
Ferroptosis represents a type of programmed cell death induced by lipid peroxidation dependent on iron (108). In recent years, numerous studies have indicated that ferroptosis is another important form of CMCs death. Under myocardial ischemia, iron accumulates extensively within the heart tissue and the elevated iron levels in the myocardium exacerbate I/R injury, ultimately leading to CMCs ferroptosis (109,110). According to Zhang et al (109), BMSC-Exos clearly inhibit CMCs ferroptosis through the RNA-binding protein Pumilio2 (Pum2)/peroxiredoxin 6 (PRDX6) axis, mitigating cardiac injury caused by I/R and enhancing heart function. Specifically, lncRNA Mir9-3hg is enriched in mouse BMSC-Exos. It downregulates Pum2 expression which is promoting the activation of PRDX6 and demonstrates inhibition of ferroptosis marker proteins such as Acyl-CoA synthetase long-chain family member 4 (ACSL4) in H/R-induced HL-1 mouse cardiac tissue in in vivo and in vitro experiments (109). Kelch-like ECH-associated protein 1 (Keap1)/nuclear factor erythroid 2-related factor 2 (Nrf2) is considered a key regulatory pathway in ferroptosis (111). A recent study suggested that GATA-4 overexpression in BMSC-Exos can effectively inhibit H/R-induced CMCs ferroptosis through upregulating miR-330-3p (112). This process involves the regulation of the BRCA1-associated protein 1 (BAP1)/solute carrier family 7a member 11 (SLC7A11)/inositol 1,4,5-triphosphate receptor (IP3R) axis, along with the opening of the mitochondrial permeability transition pore (112). In addition, it may also be related to the H2S-mediated regulation of the Keap1 signaling pathway, wherein elevated H2S concentrations lead to reduced Keap1 levels and heightened Nrf2 expression. In in vitro experiments, BAP1 downregulates SLC7A11 expression, reversing the inhibitory effect of GATA-4-overexpressing BMSC-Exos on H/R-induced CMCs ferroptosis (112).
In conclusion, BMSC-Exos deliver specific miRNAs and lncRNAs, regulating vital signaling pathways such asPI3K/AKT and Wnt/β-catenin, effectively inhibiting oxidative stress, inflammatory responses, apoptosis and ferroptosis, thereby demonstrating significant protective effects against MIRI (Fig. 3).
Improvement of AS
AS is a progressive inflammatory disorder that markedly endangers cardiovascular health. Its pathological features primarily include lipid accumulation, fibrous proliferation and calcium deposition within the vascular structure (113). These changes may eventually lead to hemorrhage, plaque rupture and localized thrombosis, triggering CVDs (114). Regarding the complex pathogenesis of AS, growing evidence suggests that BMSC-Exos exhibit significant potential in mitigating AS progression.
Endothelial cells
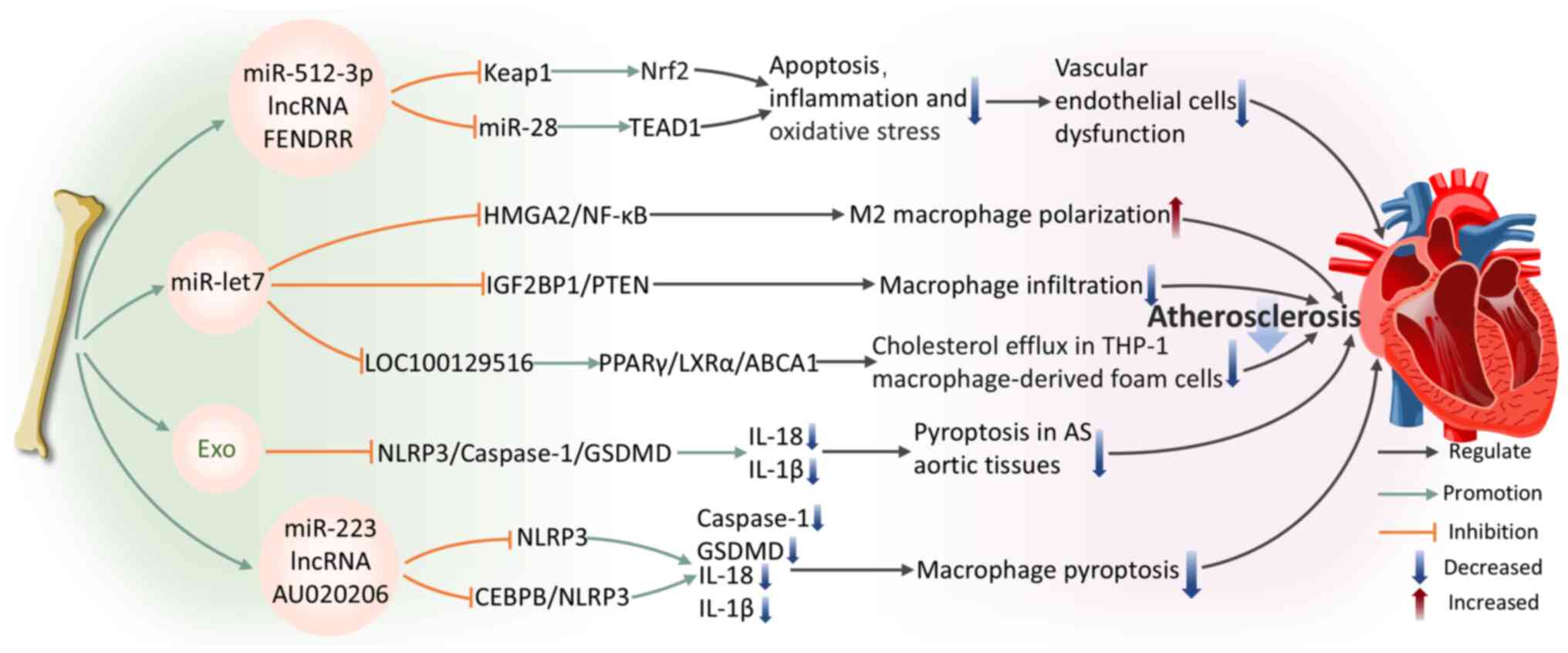
Oxidized low-density lipoprotein (ox-LDL) is a critical pathogenic factor in AS (115). It promotes the formation and progression of AS plaques through various mechanisms, including inducing EC activation and dysfunction, promoting foam cell formation in macrophages and the migration and proliferation of smooth muscle cells. (116). ECs dysfunction and damage are widely recognized as primary triggers for AS initiation and progression (117). A study confirmed that BMSC-Exos overexpressing miR-512-3p are internalized by ECs, markedly improving ox-LDL-induced ECs damage (117). miR-512-3p in ECs targets Keap1, inhibiting its expression and increasing Nrf2 protein levels, thereby reducing EC apoptosis and inflammatory responses (117). In addition, BMSC-Exos expressing the lncRNA FENDRR are internalized by HUVECs, regulating miR-28/TEA domain transcription factor 1 (TEAD1) expression to attenuate HUVECs damage and AS formation (118).
Macrophage function
Macrophages are of pivotal importance in the pathogenesis of AS. They modulate inflammatory signal integration and cell death pathways, particularly necrotic cell death, which aggravates inflammation and facilitates plaque rupture, causing acute cardiovascular incidents (119). BMSC-Exos regulate macrophage function through specific molecular pathways, highlighting their potential for the treatment of AS. Specifically, through the miR-let7/HMGA2/NF-kB pathway, BMSC-Exos facilitate the polarization of M2 macrophages (120). They also inhibit macrophage infiltration in plaques via the miR-let7/insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1)/PTEN pathway, ameliorating AS pathology in the apolipoprotein E-deficient mouse model (120). Sun et al (121) further reveal that the transport of small interfering RNA (siRNA/si)-LOC100129516 by BMSC-Exos effectively promotes cholesterol efflux while simultaneously inhibiting lipid deposition in foam cells derived from THP-1 macrophages. This effect is facilitated by the activation of the signaling pathway of peroxisome proliferator-activated receptor γ (PPARγ)/liver X receptor α (LXRα)/ATP-binding cassette transporter A1 (ABCA1), where PPARγ serves as a cholesterol sensor (122), enhancing cholesterol efflux through the induction of LXRα and ABCA1 transcription (123) and ultimately alleviating AS progression. These results underscore the essential contribution of BMSC-Exos to macrophage regulation and their value for therapeutic application in AS.
Pyroptosis
Pyroptosis is a type of inflammatory cell death that fundamentally relies on the activation of caspase-1 (124). This process involves the formation and activation of inflammasomes, which culminate in the rupture of the cell membrane and the release of cellular contents, triggering a strong inflammatory response (125). Pyroptosis is a key pathophysiological mechanism in AS, contributing to its initiation and progression (126). Therefore, inhibiting pyroptosis is a promising target for both the prevention and treatment of AS. Research indicates that BMSC-Exos alleviate inflammation by blocking the activation of the NLRP3/caspase-1/gasdermin-D (GSDMD) pathway in aortic tissue pyroptosis, thereby suppressing lipid buildup and inflammatory damage in aortic tissue affected by AS (127). The regulation of macrophage pyroptosis pathways in AS is gaining increasing attention. Lin et al (128) first demonstrated that BMSC-derived microvesicles can decrease the vulnerability index of atherosclerotic plaques and the thickness of the intimal layer, decreasing macrophage pyroptosis. The authors revealed that miR-223 carried by BMSC-derived microvesicles mediates this process by inhibiting NLRP3 expression (128). lncRNAs are becoming recognized as key modulators of macrophage pyroptosis pathways in AS. A recent study shows that BMSC-Exos deliver lncRNA AU020206, which obstructs the transcriptional activation of NLRP3 mediated by CCAAT/enhancer-binding protein beta (CEBPB), leading to a reduction in cleaved-caspase-1 and GSDMD expression (129). This process inhibits macrophage pyroptosis and alleviates AS symptom.
To summarize, BMSC-Exos exhibit multiple therapeutic mechanisms in alleviating AS pathological progression by regulating EC and macrophage function, inhibiting apoptosis, inflammation and pyroptosis. These findings not only enhance our comprehension of the function of BMSC-Exos in AS pathophysiology but also provide a foundation for developing novel exosome-based therapies for AS (Fig. 4).
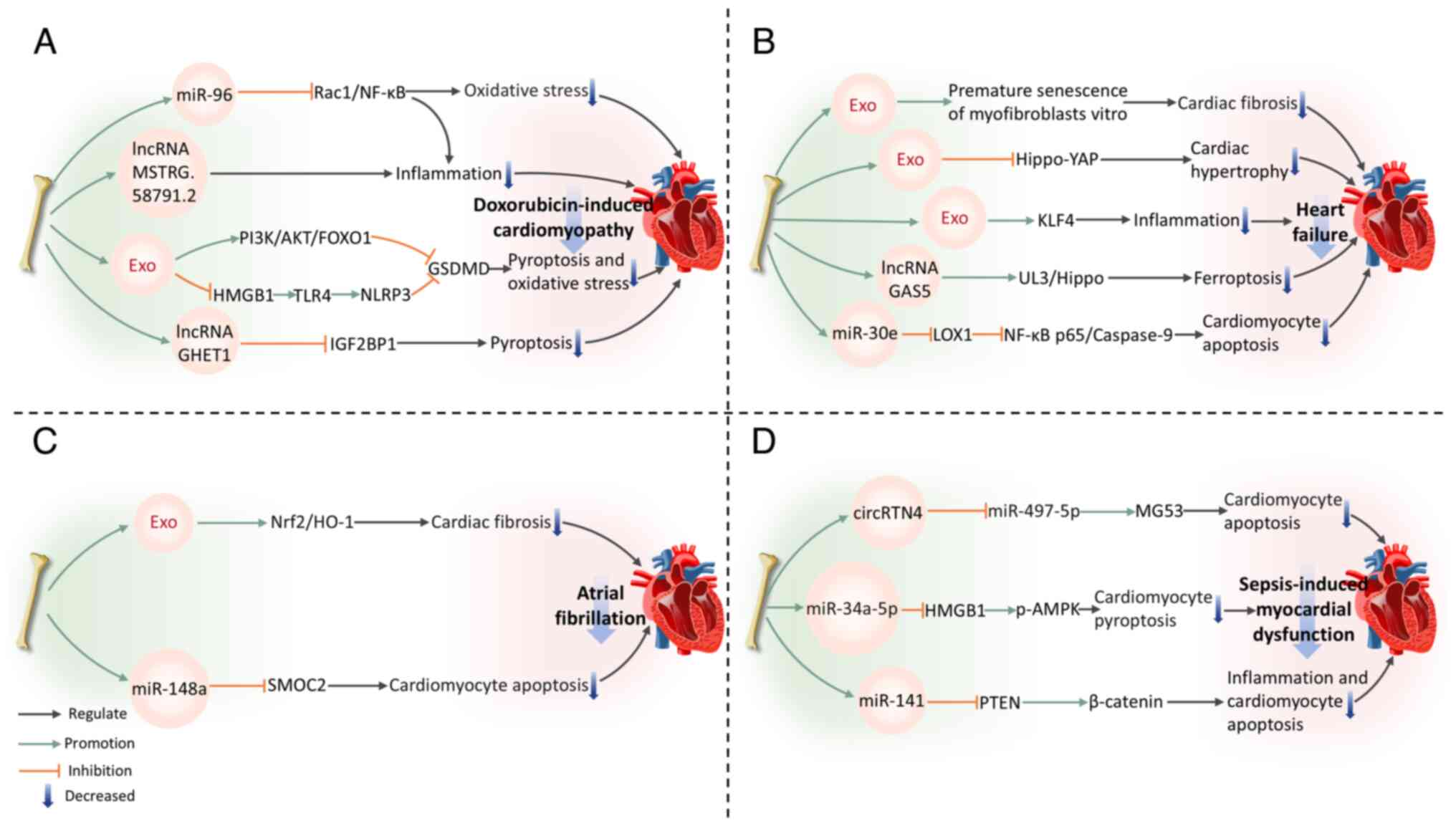
Mitigation of doxorubicin-induced cardiomyopathy
DOX, belonging to the anthracycline class of medications, is commonly employed in cancer treatment (130). Nonetheless, long-term DOX chemotherapy can induce cardiotoxicity, leading to various cardiac complications (131). The mechanism of DIC is complex, primarily involving inflammatory and pyroptosis pathways (132). BMSC-Exos can exert beneficial effects on DIC.
Inflammation
BMSC-Exos modulate the ras-related C3 botulinum toxin substrate 1 (Rac1)/NF-κB pathway by delivering miR-96 (133). The gene Rac1, which is a target of miR-96, is suppressed by this miRNA, resulting in the downregulation of NF-κB signaling transduction and protecting myocardium against DOX-induced toxicity (133). In addition, BMSC-Exos reduce pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) expression and collagen fiber deposition in the myocardium of DOX-treated rats. This suggests their potential to alleviate cardiac inflammation and fibrosis (133). Tian et al (134) constructed a DOX-induced CMCs damage model and performed transcriptome sequencing to analyze lncRNA expression in CMCs before and after co-culture with BMSCs. The results revealed that BMSC-Exos deliver multiple lncRNAs, thus inhibiting CMCs inflammatory responses and alleviating DIC. Among these, MSTRG.58791.2 was identified as a potential exosome-derived lncRNA with cardiotoxicity-ameliorating effects (134).
Pyroptosis
Inflammation and pyroptosis are closely interconnected. Pyroptosis, a type of programmed cell death driven primarily by Gasdermin proteins, especially the crucial protein GSDMD, can exacerbate the inflammatory response (119,135). In turn, the inflammatory response can further activate pyroptosis-related signaling pathways, promoting more cells to undergo pyroptosis, thereby creating a vicious cycle that aggravates tissue damage (136). BMSC-Exos phosphorylate FOXO1 and downregulate GSDMD expression by activating the PI3K-AKT pathway, which results in alleviating DOX-induced CMCs pyroptosis (137). Ali and Singla (138) also confirm that BMSC-Exos inhibit the NLRP3 inflammatory cascade mediated by the HMGB1/TLR4 axis, suppress GSDMD activation and reduce pyroptosis, thereby improving DIC. Notably, compared with exosomes originating from endothelial progenitor cells, BMSC-Exos exhibit superior efficacy in suppressing CMCs pyroptosis and improving DIC (139). This is partly due to the lncRNA GHET1 in BMSC-Exos, which binds to IGF2BP1, inhibiting its transcriptional activity and downregulating NLRP3 expression, thereby effectively improving DIC and pyroptosis (139).
In summary, the protective effects of BMSC-Exos in DIC are primarily achieved by regulating inflammation and pyroptosis-related signaling pathways. They effectively alleviate cardiac damage and provide novel strategies for treating DIC.
Amelioration of other cardiovascular diseases
Heart failure (HF) is a pathophysiological condition characterized by a reduced ability of the heart to pump effectively, causing insufficient cardiac output to fulfill metabolic requirements or systemic venous congestion (140). CMCs hypertrophy is a fundamental pathological feature of HF. According to Chen et al (141), BMSC-Exos are capable of alleviating CMCs hypertrophy, inhibiting CMCs apoptosis and reducing fibrosis in a mouse model of myocardial hypertrophy induced by pressure overload. These effects help preserve myocardial structure, delay cardiac remodeling and exert cardioprotective effects (141). In addition, BMSC-Exos reduce apoptosis and inflammation in hypertrophic CMCs, effectively inhibiting HF progression. This mechanism may involve the suppression of the Hippo-YAP pathway (142). Various lncRNAs play key roles in mediating the advantageous impacts of BMSC-Exos. For example, the upregulation of lncRNA GAS5 in BMSC-Exos contributes to alleviating HF by regulating the UL3/Hippo pathway and inhibiting ferroptosis (143). miR-30e in rat BMSC-Exos can markedly inhibit recombinant lectin-like oxidized low-density lipoprotein receptor 1 expression, downregulating NF-κB p65/caspase-9 signaling and improving HF post-MI in rats (144). A recent study indicated that BMSC-Exos alleviate inflammation in HF by increasing Krüppel-like factor 4 (KLF4) expression in CMCs (145). In a model of myocardial injury induced by DOX, BMSC-Exos treatment leads to a decrease in levels of inflammatory factors including TNF-α, IL-6 and IL-1β. KLF4 gene knockout experiments confirmed its role as a core molecular target for exosome-mediated anti-inflammatory effects, offering new avenues for HF treatment (145).
Atrial fibrillation (AF) is a prevalent arrhythmia, with the primary histological feature being atrial fibrosis, which is often caused by extracellular matrix metabolic dysregulation leading to excessive fibrinogen deposition (146,147). Xu et al (148) focused on the effect of Nrf2 in AF-specific atrial fibrosis, finding that BMSC-Exos derived from Nrf2-transfected cells effectively inhibit atrial fibrosis and excessive myofibroblast proliferation in AF rats. This mechanism involves the increased expression of Nrf2 and its target, heme oxygenase-1 (148). In another study, miR-148a overexpression in BMSC-Exos alleviates CMCs apoptosis in AF by targeting and suppressing secreted modular calcium-binding protein 2 (149).
Sepsis arises from a dysregulated host reaction to infection, leading to organ dysfunction marked by severe oxidative stress and inflammation (150,151). It can cause endothelial, coagulation and cellular dysfunction, often resulting in cardiovascular impairment (152,153). In this area, BMSC-Exos have also shown potential therapeutic benefits. BMSC-derived exosomal circular RNA RTN4 directly targets miR-497-5p, upregulating the expression of mitsugumin 53 in CMCs, enhancing cell survival rate and mitigating sepsis-induced myocardial injury (154). Moreover, downregulation of miR-141 in BMSC-Exos can reduce PTEN levels and increase β-catenin expression (155). This suggests that miR-141 may alleviate CMCs apoptosis and myocardial injury through the PTEN/β-catenin signaling cascade, thus reducing myocardial dysfunction in septic mice (155). Notably, a recent study has revealed the unique therapeutic value of Apelin-pretreated BMSC-Exos in sepsis-induced myocardial dysfunction (156). Mechanistically, Apelin-pretreated BMSC-Exos efficiently deliver miR-34a-5p to target and inhibit the HMGB1/AMPK signaling pathway, markedly reducing CMCs pyroptosis (156). This effect has been confirmed to effectively improve cardiac contractile function in mice with sepsis-induced myocardial dysfunction (Fig. 5).
Bone-derived exosomes impair the cardiovascular system
Under specific pathological conditions, bone-derived exosomes can also exert detrimental effects on the cardiovascular system. Pressure overload represents a pathophysiological state of the heart induced by sustained mechanical stress, such as hypertension or aortic stenosis, leading to compensatory myocardial hypertrophy and interstitial fibrotic remodeling (157). Initially manifesting as adaptive myocardial thickening, prolonged overload results in pathological cardiac remodeling characterized by CMCs apoptosis, excessive extracellular matrix deposition and aberrant activation of myofibroblasts, ultimately progressing to systolic dysfunction and HF (158). Ranjan et al (8) demonstrate that under pressure overload conditions, small EVs derived from inflammatory BM fibrogenic progenitors exacerbate cardiac fibrosis through the miR-21a-5p/ITGAV/collagen type I α 1 (Col1α) signaling pathway. The core mechanism involves miR-21a-5p-mediated upregulation of ITGAV expression, enhancing its interaction with Col1α and thereby irreversibly transforming fibroblasts into contractile myofibroblasts (8). Notably, inhibition of miR-21a-5p in small EVs markedly attenuates pressure overload-induced cardiac fibrosis and improves cardiac function (8). This finding suggests miR-21a-5p as a promising molecular target for developing miRNA-modified small EVs therapies to treat hypertrophic cardiac remodeling and HF.
In summary, bone-derived exosomes serve as vehicles for miRNAs, lncRNAs and other biomolecules that markedly influence the regulation of various CVDs by suppressing pathophysiological processes including oxidative stress, inflammation, apoptosis and pyroptosis. However, under specific pathological conditions, the exosomes may conversely induce detrimental effects on the cardiovascular system. Importantly, beyond BMSC-Exos, other bone-derived exosomes such as those from cortical bone stem cells exhibit distinct cardioprotective properties (159). They promote cardiac repair by inhibiting fibroblast activation and reducing scar formation (159). However, current understanding of the mechanisms underlying non-BMSC-Exos in the cardiovascular system remains limited, necessitating broader investigations to advance knowledge in this field. These discoveries not only uncover the complex regulatory mechanisms of bone-derived exosomes in the cardiovascular system but also offer novel strategies and perspectives for CVDs treatment.
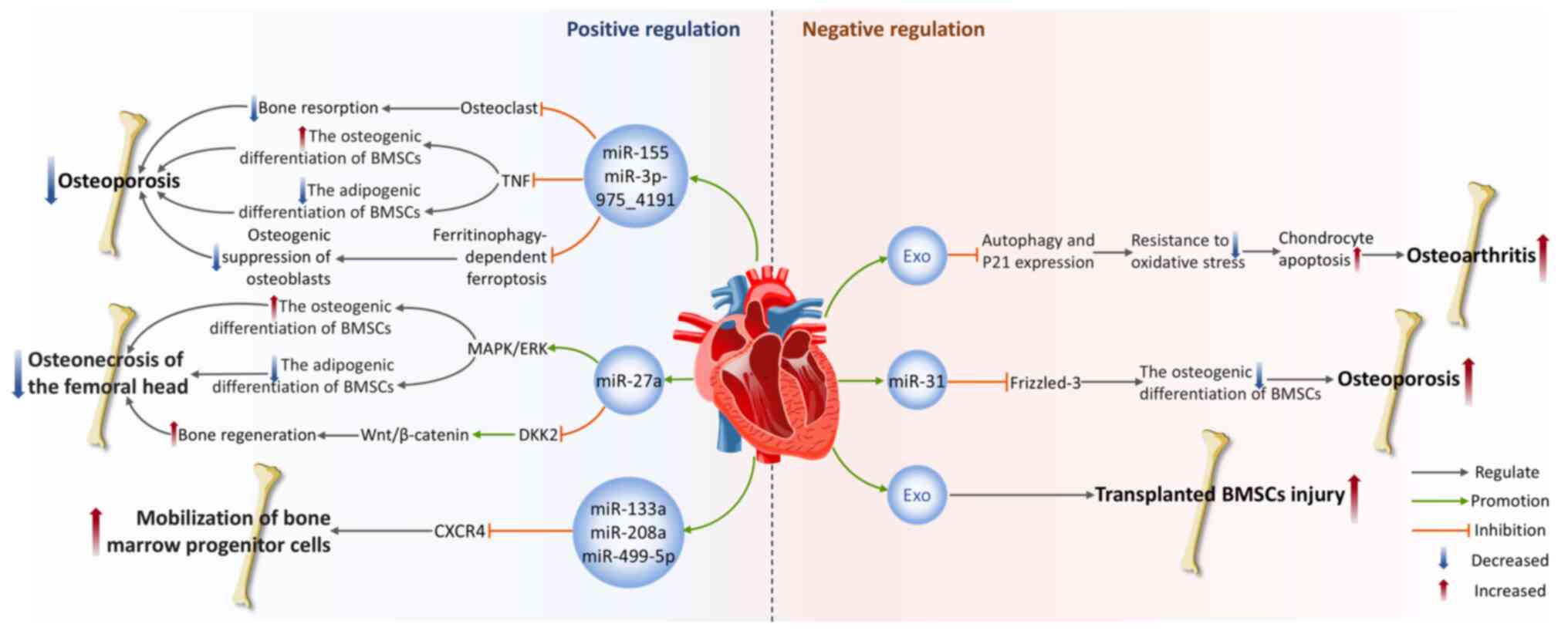
The effect of cardiovascular-derived exosomes on bone
Positive regulation of bone homeostasis
ECs are a layer of flat epithelial cells that cover the inner surfaces of the heart, blood vessels and lymphatic vessels (160), with functions such as regulating vascular tone, maintaining vascular structure and participating in inflammatory responses (161). During the last several years, the expanding body of literature has highlighted the important role of exosomes secreted by ECs (EC-Exos) in maintaining bone homeostasis.
Osteoporosis (OP) is a systemic skeletal disease marked by a decline in bone mass and deterioration of the bone microarchitecture, often causing increased bone fragility and an elevated risk of fractures (162). It is a primary clinical issue in aging populations. Research has demonstrated that EC-Exos possess effective bone-targeting capabilities and hold promise for treating OP (163). In vivo, EC-Exos precisely deliver miR-155 to macrophages derived from bone marrow, inhibiting osteoclast activity (163). This clearly slows the pathological progression of OP in ovariectomized mouse models, resulting in a marked increase in bone mineral density (BMD) and bone volume per total volume (BV/TV) compared with the control group (163). This finding not only validates the natural ability of EC-Exos to target bone tissue but also shows their capacity to impede osteoclastogenesis in vitro and decrease bone resorption in animal models, revealing their unique advantages over osteoblasts and BMSC-Exos. In a glucocorticoid dexamethasone-induced OP mouse model, researchers found that EC-Exos slow disease progression by preventing apoptosis and ferroptosis of osteoblasts induced by dexamethasone (164). This restores BMD and BV/TV in OP mice, although the specific exosomes components remain unidentified (164). A recent study also revealed the synergistic effect between EC-Exos and curcumin in preventing OP (165). Mechanistically, miR-3p-975_4191 targets tumor necrosis factor and directly regulates the differentiation of BMSCs, promoting osteogenic differentiation while inhibiting adipogenic differentiation (165). The combined intervention of EC-Exos and curcumin leads to enhanced therapeutic effects.
Osteonecrosis of the femoral head (ONFH) is a prevalent and refractory disorder in orthopedics, often leading to structural degradation and collapse of the femoral head (166). In a steroid-induced femoral head ischemic necrosis model, Wu et al (167) found that through the activation of the MAPK/ERK pathway, VEGF-modified vascular EC-Exos can promote the differentiation of osteoblasts while concurrently inhibiting adipogenic differentiation of BMSCs, thus accelerating bone tissue regeneration and repair. The Wnt signaling pathway is a vital regulatory mechanism in bone development. In the classical Wnt/β-catenin pathway, when Wnt ligands bind to receptors, they inhibit the activity of the β-catenin degradation complex (168). β-catenin levels rise and it translocates into the nucleus, where it binds with T-cell factors and lymphoid enhancer-binding factors to activate downstream gene transcription (168). However, in bone diseases, Wnt signaling is disrupted or blocked by various molecules such as the Dickkopf (DKK) protein family, Kremen and sclerostin (169). A study shows that EC-Exos overexpressing miR-27a can target DKK2 in osteoblasts and reduce its expression, relieving the suppressive effect of DKK2 on the Wnt pathway, thereby facilitating osteogenesis and improving glucocorticoid-induced ONFH (170).
Furthermore, Cheng et al (171) conducted a thorough analysis of how circulating exosomes mediate the function of miRNAs between ischemic hearts and BM in mice. After AMI, circulating exosomes become the main carriers of specific myocardial miRNAs, including miR-133a, miR-208a and miR-499-5p (171). These miRNAs selectively enter peripheral organs, especially BM, where they reduce the CXC motif chemokine receptor type 4 (CXCR4) expression in BM cells, mobilizing BM progenitor cells and promoting their proliferation, thus coordinating the body's systemic response to myocardial injury (171).
Negative regulation of bone homeostasis
However, some cardiovascular-derived exosomes also exhibit negative regulatory effects on bone homeostasis. Yang et al (172) discovered through in vivo and in vitro experiments that EC-Exos inhibit autophagy and the multifunctional cellular protein p21expression, weakening chondrocyte resistance to oxidative stress. This leads to a rise in ROS levels and induces apoptosis, thus aggravating the progression of osteoarthritis (OA) (172). A clinical study found that older adults and OP patients exhibited increased plasma levels of miR-31, with aging ECs being a potential source of secretion (173). miR-31 is secreted in microvesicles derived from aging ECs and absorbed by mesenchymal stem cells. It hampers osteogenic differentiation by downregulating the expression of its target gene, Frizzled-3 and enhances osteoclast activity, leading to bone loss and decreased BMD (173). Oxidative stress damage is a significant pathological feature of tissue injury following MI. Stem cell-based therapies offer a promising approach for repairing and regenerating the harmed cardiac tissue. Researchers collected exosomes released from hydrogen peroxide (H2O2)-treated CMCs and co-cultured them with BMSCs exposed to H2O2, to simulate the severe microenvironment encountered by CMCs or transplanted BMSCs after MI (174). The results showed that oxidative stress promoted CMCs to secrete exosomes and exosomes derived from H2O2-pretreated CMCs accelerated BMSCs damage and apoptosis under oxidative stress (174). This finding provides a possible strategy to improve the survival rate of implanted BMSCs after MI.
In summary, cardiovascular-derived exosomes exert multifaceted regulatory effects on bone homeostasis, with distinct functional effects mediated by their specific cargo compositions. Current evidence demonstrates that under skeletal disease conditions, certain cardiovascular-derived exosomes exert protective effects by precisely delivering miRNAs to regulate cellular differentiation, indicating significant therapeutic potential for OP and ONFH. However, under sustained pathological microenvironments such as aging or oxidative stress, cardiovascular-derived exosomes may negatively regulate bone homeostasis by suppressing osteogenesis while enhancing osteoclast activity, thereby exacerbating OA progression. Other cardiac cell types (such as fibroblasts and inflammatory cells) also secrete substantial amounts of exosomes, yet their specific effects on the skeletal system remain unexplored. Consequently, future investigations should elucidate the potential regulatory roles of these cell-specific exosomes in bone homeostasis (Fig. 6).
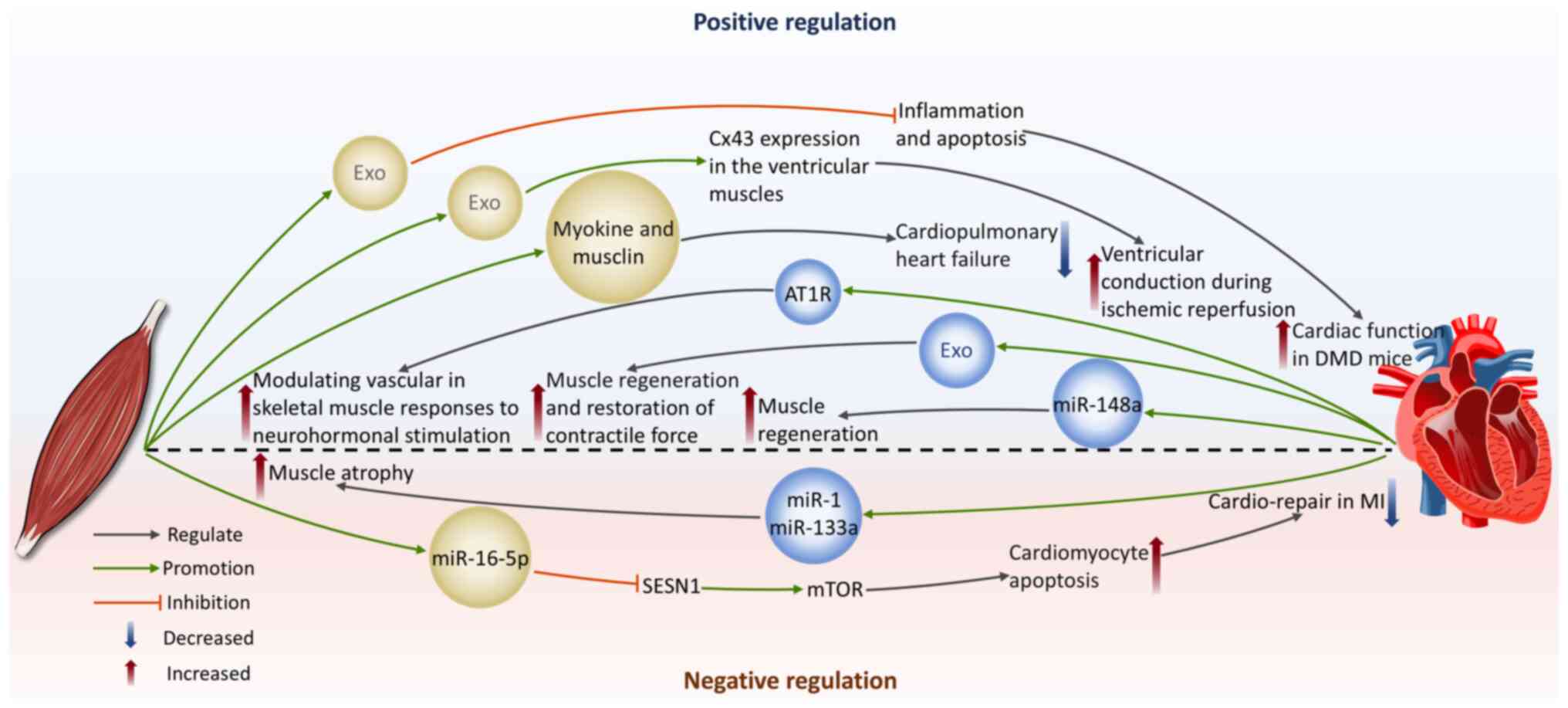
The effect of skeletal muscle-derived exosomes on the cardiovascular system
Protection of cardiomyocytes
Duchenne muscular dystrophy (DMD) is an X-linked recessive genetic disease resulting from mutations in the dystrophin gene (175), often affecting the heart. Common cardiovascular complications include dilated cardiomyopathy, arrhythmias and congestive heart failure (176,177). Studies by Su et al (178-180) reveal the capability of exosomes derived from myogenic progenitor cells (MPC-Exos) in cardiac protection in DMD. MPC-Exos carry mRNA encoding dystrophin. Transplantation of MPC-Exos can enhance dystrophin gene expression in the cardiac tissue of mdx mice, improving heart function (178). They also show significant anti-apoptotic and anti-inflammatory properties by downregulating MAPK8 and IL-6 expression levels and upregulating Bcl-2 levels, providing new insights into the cardiac protection mechanisms in DMD (180).
In addition, the positive effects of remote ischemic conditioning (RIC) during HF have also garnered attention. Myocardial ischemia is a frequent contributor to HF. The core mechanism of RIC therapy is that it activates the body's endogenous protective pathways through brief, repeated I/R stimuli applied to distant limbs, thus providing systemic protection to the heart (181). Homme et al (5) induced cardiopulmonary heart failure in mice by constructing an aorta-vena-cava fistula and used hind-limb RIC as a treatment method. The authors found that RIC induced the release of skeletal muscle-derived exosomes containing musclin and myokines. These exosomes can alleviate HF in mice and protect CMCs (5). This provides further evidence of the skeletal endocrine function mediated by exosomes in regulating heart function. Exosomes derived from skeletal myoblasts pretreated with hypoxia may play a role in conveying protective signals produced by RIC in distant limbs to the targeted tissues. These exosomes promote connexin 43 expression in ventricular muscles, improving ventricular myocardial conduction and reducing reperfusion arrhythmias under the condition of hypothermic I/R (182).
Promotion of cardiomyocyte apoptosis
In some cases, skeletal muscle-derived exosomes may have detrimental effects on the heart. Sarcopenia is a disease characterized by the gradual decline of skeletal muscle, causing a decrease in both muscle mass and functionality (183). It is closely associated with negative consequences, including increased fall risk, functional decline, frailty and higher mortality rates (184). Sarcopenia-induced cardiac cachexia has been noted to be connected to poor cardiac prognosis (7). In MI patients, concomitant HF often delays the transition from bed rest to early activity, causing a decline in life quality and reduction in skeletal muscle mass, eventually inducing sarcopenia (7,185). Sarcopenia has significant adverse effects on heart disease patients with skeletal muscle loss, which can lead to cardiac cachexia. This process is often accompanied by disturbances in nutrition and metabolic functions, thereby forming a malignant cycle that results in continuous deterioration of heart function (186-188). Hayasaka et al (7) induced skeletal muscle atrophy in mice after I/R by continuous tail suspension and further revealed the potential mechanisms of impaired cardiac repair associated with sarcopenia. The authors found that sarcopenia following I/R interfered with cardiac recovery and increased miR-16-5p levels in circulating exosomes secreted by the atrophied limbs and hearts of continuously tail-suspended mice (7). miR-16-5p directly inhibits sestrin 1 transcription, activating mTOR signaling, which subsequently promotes CMCs apoptosis and exacerbates impaired cardiac repair (7). This suggests that the pro-apoptotic effect of miR-16-5p derived from sarcopenia might contribute to the deterioration of MI.
Collectively, skeletal muscle-derived exosomes exhibit a dual role in cardiac health regulation. They can promote cardiac protection and functional recovery, promote angiogenesis and optimize cardiac blood supply by delivering therapeutic miRNAs and other active molecules to regulate the relevant signaling pathways. However, they can also induce CMCs apoptosis, exacerbating impaired cardiac repair. Therefore, precise regulation and rational use of skeletal muscle-derived exosomes are essential in CVDs treatment.
The effect of cardiovascular-derived exosomes on skeletal muscle
Improvement of skeletal muscle injury and promotion of regeneration
Cardiovascular-derived exosomes can be delivered to skeletal muscle tissue via peripheral circulation, where they interact with skeletal muscle cells, thereby regulating skeletal muscle physiological functions. Cardiac-derived progenitor cells (CDCs) possess properties that are anti-inflammatory, antioxidant, anti-fibrotic and cardiogenic (189). Rogers et al (190) found that CDCs can improve the functionality of both cardiac and skeletal muscles in DMD mice and enhance skeletal muscle regenerative capacity. Further research showed that CDCs secrete exosomes that deliver miR-148a to skeletal muscle, promoting muscle regeneration (190). In another DMD animal study, researchers directly injected CDC-derived exosomes into the soleus muscle of mdx mice and observed a rise in the total quantity of muscle fibers with a shift in size distribution toward a smaller diameter and this phenomenon was not influenced by the insulin-like growth factor 1 receptor. Simultaneously, with enhanced muscle regeneration, CDC-derived exosomes injection reduced skeletal muscle inflammation and fibrosis and increased contractile force (9). On the other hand, research has demonstrated that mechanical stress, under both in vitro hypotonic conditions and in vivo pressure overload, induces CMCs to release exosomes carrying angiotensin II type 1 receptor (AT1R). Skeletal muscle cells are one of the target cells, thus regulating the vascular response to neurohormonal stimulation (191). This finding provides strong evidence for the crosstalk between the cardiovascular system and skeletal muscle.
Induction of skeletal muscle atrophy
A class of miRNAs specifically expressed in muscle tissue is called myomiRs (192). The majority of myomiRs, such as miR-1, miR-133a and miR-499, are present in both skeletal muscle and myocardium. By contrast, miR-206 is exclusive to skeletal muscle and miR-208a is exclusive to myocardium (192,193). In HF-related studies, a series of specific miRNAs frequently appear (194), including miR-1, miR-21, miR-24, miR-29b, miR-133a and b, miR-199, miR-208, miR-214 and miR-499. Notably, most of these miRNAs belong to the sequences of myomiRs and they change in the cardiac tissue of HF patients with various causes and severity. This not only reveals the complexity of the pathological mechanisms of HF but also strongly suggests that these molecules could serve as important mediators of communication between the cardiovascular and skeletal muscle through the release of miRNA-rich exosomes during the progression of HF. In instances of overload-induced hypertrophy in skeletal muscles, reduced expression levels of miR-1 and miR-133a have been observed (195). Therefore, the increased circulation of miR-1 and miR-133a caused by HF (196) are hypothesized to be important promoting factors of skeletal muscle atrophy after the onset of CVDs (193).
Taken together, cardiovascular-derived exosomes can promote skeletal muscle function improvement and regeneration by delivering beneficial molecules, but they may also transmit HF-related miRNAs that exacerbate skeletal muscle atrophy. These findings reveal the complex effects of cardiovascular-derived exosomes in the pathological progression of skeletal muscle (Fig. 7).
Clinical applications of exosome therapy
Diagnostic biomarkers
The ubiquitous distribution of exosomes in biological fluids provides new avenues for diagnosing musculoskeletal diseases and CVDs. Research reveals that compared with men, female OA patients have more differentially regulated miRNAs in their synovial fluid, such as miR-16-2-3p, miR-26a-5p, miR-146a-5p and miR-6821-5p (197). The exosome miRNAs specifically expressed in the synovial fluid of women with OA react to estrogen and influence the TLR signaling pathway. These exosome miRNAs can serve as sex-specific biomarkers for diagnosing OA (197). In addition, Zhao et al (198) mention the potential of lncRNAs in exosomes as OA biomarkers. The authors conducted a comparative analysis of exosome lncRNAs from synovial fluid and plasma to evaluate the progression of OA. The findings indicate that there are no notable discrepancies in the expression of plasma exosome lncRNAs, while the levels of synovial fluid exosome lncRNAs (HOTAIR, PCGEM1 and GAS5) are markedly elevated in both early and late OA patients compared with healthy controls. Notably, the expression of exosome lncRNA PCGEM1 was greater in individuals with late-stage OA relative to those with early-stage OA. For CVDs, exosomal lncRNAs also show potential diagnostic value. Specifically, plasma levels of exosomal lncRNA UCA1 are elevated in AMI patients compared with healthy individuals, suggesting that circulating exosomal lncRNA UCA1 could function as an effective biomarker for diagnosing AMI (76). To develop more efficient biomarkers for effectively monitoring disease onset and progression, it is critical to investigate the sorting mechanisms of exosome contents and their secretions and to clarify their stability levels under different physiological and pathological conditions (199).
Biologically engineered drug delivery vectors
Exosomes have a nanometer-scale size and a lipid bilayer membrane structure that protects bioactive molecules. They can cross cell membranes rapidly and have the advantages of low immunogenicity and toxicity as well as organ-targeting properties, demonstrating tremendous potential as drug delivery vehicles (200). Exosomes can be modified with the specific ligand, such as hyaluronic acid, enabling the delivery of Forsythoside A-loaded exosomes to recipient cells and forming a targeted ligand-receptor interaction with the cell surface adhesion molecule CD44. This approach enhances the hepatoprotective effect of Forsythoside A against fibrosis (201). Exosomes released by macrophages carrying paclitaxel are capable of capable of specifically targeting cancer cells to deliver the drug, suppressing their proliferation and addressing issues related to multidrug resistance (202). Through bioengineering modifications, the targeting ability and therapeutic efficiency of exosomes are further enhanced. For example, after the modification of surfaces using anti-CD20 monoclonal antibodies, exosomes loaded with zinc oxide nanocrystals can specifically recognize and target lymphoma cells, showing higher cytotoxicity (203). Meanwhile, adipose-derived mesenchymal stem cells (ADSCs), owing to their easy accessibility and unique capacities for self-renewal and differentiation, have been widely applied in targeted therapy for musculoskeletal disorders (including fractures, OP, OA and tendon injuries) through their secreted exosomes serving as nanoscale drug carriers (204). Hydrogels, as ideal delivery vehicles, have been demonstrated to effectively enhance the in vivo stability and targeted distribution of MSC-Exos, thereby showing promising applications in regenerative medicine for age-related musculoskeletal diseases (205). In addition, the combined use of exosomes with biological scaffolds can more effectively utilize their unique advantages in drug delivery and tissue repair and reconstruction. A minimally invasive exosome spray composite material integrating MSC-Exos into a fibrin scaffold was designed by Yao et al (206). It promotes the retention of MSC-Exos within cardiac tissue, enhancing heart function and angiogenesis in MI mouse models. Subsequent experiments in rat and pig models confirmed that exosomes effectively reduce surgical stress and inflammation, verifying their clinical translation feasibility (206). Chen et al (207) designed an extracellular matrix/gelatin methacrylate scaffold to deliver exosomes. Experimental data showed that transplanting exosome-combined scaffolds into a rabbit osteochondral defects model markedly improved cartilage repair efficiency.
Novel therapeutic approaches for tissue repair and regeneration post-injury
Within the domain of regenerative medicine, exosomes have attracted significant attention for their remarkable tissue repair-promoting abilities, as well as their low-risk and high-treatment efficacy advantages compared with traditional stem cell transplantation therapies. Compared with stem cell therapy, exosomes can precisely deliver bioactive molecules to target tissues, improving treatment targeting and efficiency and effectively avoiding immune rejection issues that may be induced by stem cell transplantation, providing novel therapeutic approaches for repair and regeneration following tissue damage. Specifically, BMSC-Exos overexpressing miR-486-5 have been demonstrated to activate the matrix metalloproteinase MMP19 and VEGF signaling pathways, facilitating angiogenesis and decreasing infarct area in a MI monkey model (208). In addition, as mentioned earlier, MSCs and their exosomes can regulate macrophage polarization. With the capacity to swiftly alter their phenotype and functional traits, macrophages are essential in the process of tissue regeneration (209). For example, in the MI pig model, CDC-derived exosomes reduce infarct myocardial area, which is mediated by the exosomal transfer of miR-181b to macrophages to regulate their polarization (210). In musculoskeletal diseases, amniotic fluid stem cells-derived exosomes promote cartilage formation and inhibit inflammation by mediating M2 macrophage polarization (211). An emerging finding indicates that synovial MSC-Exos preconditioned with growth differentiation factor 5 enhances chondrogenesis through activation of the lysine-specific demethylase 2A/SRY-box transcription factor 2 pathway (212). When combined with digitally light-processed bio-printed scaffolds composed of glycyrrhizic acid/methacrylated-acylated hyaluronic acid, this system achieves sustained-release delivery and promotes articular cartilage regeneration in rat models (212). Tendinopathy ranks among the most prevalent musculoskeletal disorders globally (213). Song et al (214) used a rat model with the tendon defect, discovering that exosomes derived from tendon-derived stem cells containing miR-144-3p promote the proliferation and migration of tendon cells by targeting AT-rich interaction domain 1A. Exosomes, owing to their minute size and lipid bilayer structure, can also traverse the blood-brain barrier, target specific cells and regulate pathological processes in neural tissue. It has been reported that BMSC-Exos can stimulate the proliferation of hippocampal neurons and decrease apoptosis in rats through the upregulation of miR-26a, improving symptoms in a depression model (215).
Multiple factors influence the therapeutic efficacy of exosomes
The therapeutic effects of exosomes are highly dependent on their cellular origin, administration dosage, frequency and recipient species. In diabetic ulcer mouse models, EVs derived from BMSCs and ADSCs exhibit distinct therapeutic effects on wound healing (216). BMSC-EVs primarily stimulate cell proliferation, whereas ADSC-EVs accelerate wound closure through pro-angiogenic mechanisms (216). A study demonstrated dose-dependent neuroprotective effects of BMSC-Exos in cerebral ischemia models (217). Compared with controls and low-dose groups (80 μg/rat), medium (100 μg/rat) and high-dose (120 μg/rat) BMSC-Exos administration markedly reduces cerebral infarction area and alleviates brain edema, with the highest dose showing most pronounced effects (217). Zhou et al (218) found that ADSC-Exos administered three times daily promoted skin wound healing more effectively than single or twice-daily dosing, suggesting increased administration frequency enhances tissue repair. Species-matched exosomes demonstrate superior therapeutic efficacy for ischemic stroke (219). In middle cerebral artery occlusion mouse models, murine cerebral EC-derived exosomes more effectively improve mitochondrial function in recipient cells, reduce infarct volume and decrease neurological deficit scores compared with human-derived exosomes (219). This finding supports using species-matched exosomes in preclinical studies to optimize outcomes. Notably, current exosomal research remains predominantly animal-based, with limited clinical trials. In existing clinical studies, human-derived exosomes (from adipose, BM, or umbilical cord MSCs) and plant-derived exosomes represent the two most commonly used types (199). Adipose tissue has become a preferred MSC-Exos source due to its accessibility, with applications in seven clinical trials (220). Common administration routes include intravenous infusion, inhalation and local delivery. However, dosage standards vary depending on disease type and administration method, with some studies quantifying exosomes by weight (mg) while others use particle counts or parental cell equivalents as metrics (220).
Conclusions
As soluble mediators of intercellular communication, exosomes can load and transport mRNAs, lipids, proteins, miRNAs, lncRNAs and other bioactive molecules to adjacent or distant cells, playing an essential part in intercellular communication. Substantial evidence demonstrates that exosomes play pivotal roles in bone-muscle crosstalk, not only promoting skeletal muscle regeneration and repair (221,222) but also critically regulating bone metabolic homeostasis and remodeling (223,224). However, the mechanisms underlying exosomes-mediated inter-organ communication between the cardiovascular and musculoskeletal systems remain poorly investigated. The present review provided the first comprehensive synthesis of the complex bidirectional regulatory networks mediated by exosomes across these two major systems, encompassing both beneficial effects and potential adverse impacts. BMSC-Exos and other bone-derived exosomes show significant promise for therapeutic applications in CVDs such as MI, I/R, AS and DIC. They provide strong support for cardiovascular repair and regeneration through mechanisms such as modulating oxidative stress, inflammation, autophagy, apoptosis, fibrosis, ferroptosis, pyroptosis and promoting angiogenesis. Nevertheless, under specific pathological conditions, such as pressure overload-induced pathological cardiac remodeling, bone-derived exosomes may also exert detrimental effects on the cardiovascular system. Meanwhile, cardiovascular-derived exosomes also have beneficial effects on bone diseases such as OP and ONFH. However, some exosomes may have negative effects on bones, such as inhibiting osteogenic differentiation or reducing bone mineral density. On the other hand, skeletal muscle-derived exosomes provide new therapeutic perspectives for CVDs recovery through mechanisms such as promoting angiogenesis. However, these exosomes also have adverse effects, such as promoting CMCs apoptosis. In addition, cardiovascular-derived exosomes exhibit positive effects on skeletal muscle regeneration and repair, but under pathological conditions such as HF, some exosomes may induce skeletal muscle atrophy, leading to adverse consequences.
Although exosome-based therapies have broad prospects, several challenges still need to be overcome. First, the extraction, purification and identification techniques for exosomes still need further improvement. There is a need for rapid and inexpensive methods, as well as straightforward, standardized processes for separation and purification, to achieve high yields of exosomes with high purity. Second, the specific molecular mechanisms by which exosomes implement their reparative and protective functions remain inadequately elucidated and their long-term therapeutic effects are still in the exploratory stage. Exosomes derived from various tissues contain different bioactive molecules, thus generating varying degrees of reparative effects in different disease injuries. Finally, issues related to the stability, targeting ability and biosafety of exosomes in vivo need to be thoroughly investigated. These challenges may be addressed through tissue engineering approaches to functionally optimize exosomes by modifying their surface properties and drug-loading capacity, thereby enhancing their therapeutic efficacy and expanding potential clinical applications. Currently, the complete understanding of exosomal biodistribution, metabolism and immunogenicity remains lacking and appropriate animal models for evaluating the required medicine concentration and duration of its effectiveness for large-scale manufacturing are unavailable. Future research should encompass a broader spectrum of in vivo studies and clinical trials, with the objective of evaluating the safety and efficacy of drug formulations based on exosomes.
In conclusion, exosomes serve significant functions that contribute to the crosstalk between the cardiovascular and musculoskeletal systems, showing great potential and application prospects in addressing CVDs and musculoskeletal-related diseases. As investigations into exosomes deepens, those released from cultured cells are anticipated to emerge as novel, efficient and versatile drug carriers, driving innovative development in drug delivery systems, tissue engineering, as well as regenerative therapies. This will further unveil their roles in intercellular communication and disease mechanisms, contributing to human health.
Availability of data and materials
Not applicable.
Authors' contributions
QL was responsible for conceptualization, investigation, methodology, writing the original draft and illustration. HG was responsible for conceptualization, investigation, software, writing the original draft, illustration, reviewing and editing. XM was responsible for writing the original draft, reviewing and editing. ZW was responsible for illustration, reviewing and editing. LZ was responsible for conceptualization, reviewing and editing. WX was responsible for funding, supervision, visualization, reviewing and editing. Data authentication is not applicable. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Use of artificial intelligence tools
During the preparation of this work, the authors used ChatGPT to improve readability and language quality. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Acknowledgements
Not applicable.
Funding
The present study was sponsored by the National Natural Science Foundation of China (grant no. 32371185); the Shanghai Science and Technology Plan Project (grant no. 23010504200); the Shanghai Oriental Talents Program (Youth Project); the Key Lab of Exercise and Health Sciences of Ministry of Education (Shanghai University of Sport) (grant no. 2025KF002) and the Shanghai Key Lab of Human Performance (Shanghai University of Sport) (grant no. 11DZ2261100) and the College Students' Innovation and Entrepreneurship Training Program (grant no. STYK20250415).
References
|
Welsh JA, Goberdhan DCI, O'Driscoll L, Buzas EI, Blenkiron C, Bussolati B, Cai H, Di Vizio D, Driedonks TAP, Erdbrügger U, et al: Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. 13:e124042024. View Article : Google Scholar : PubMed/NCBI | |
|
Patil M, Henderson J, Luong H, Annamalai D, Sreejit G and Krishnamurthy P: The art of intercellular wireless communications: Exosomes in heart disease and therapy. Front Cell Dev Biol. 7:3152019. View Article : Google Scholar : PubMed/NCBI | |
|
Deddens JC, Vrijsen KR, Colijn JM, Oerlemans MI, Metz CHG, van der Vlist EJ, Nolte-'t Hoen ENM, den Ouden K, Jansen Of Lorkeers SJ, van der Spoel TI, et al: Circulating extracellular vesicles contain miRNAs and are released as early biomarkers for cardiac injury. J Cardiovasc Transl Res. 9:291–301. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Tian Y, Wang TS, Bu H, Shao G, Zhang W and Zhang L: Role of exosomal miR-223 in chronic skeletal muscle inflammation. Orthop Surg. 14:644–651. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Homme RP, Zheng Y, Smolenkova I, Singh M and Tyagi SC: Remote hind-limb ischemia mechanism of preserved ejection fraction during heart failure. Front Physiol. 12:7453282021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu LP, Tian T, Wang JY, He JN, Chen T, Pan M, Xu L, Zhang HX, Qiu XT, Li CC, et al: Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 8:6163–6177. 2018. View Article : Google Scholar | |
|
Hayasaka T, Takehara N, Aonuma T, Kano K, Horiuchi K, Nakagawa N, Tanaka H, Kawabe JI and Hasebe N: Sarcopenia-derived exosomal micro-RNA 16-5p disturbs cardio-repair via a pro-apoptotic mechanism in myocardial infarction in mice. Sci Rep. 11:191632021. View Article : Google Scholar : PubMed/NCBI | |
|
Ranjan P, Dutta RK, Colin K, Li J, Zhang Q, Lal H, Qin G and Verma SK: Bone marrow-fibroblast progenitor cell-derived small extracellular vesicles promote cardiac fibrosis via miR-21-5p and integrin subunit αV signalling. J Extracell Biol. 3:e1522024. View Article : Google Scholar | |
|
Aminzadeh MA, Rogers RG, Fournier M, Tobin RE, Guan X, Childers MK, Andres AM, Taylor DJ, Ibrahim A, Ding X, et al: Exosome-mediated benefits of cell therapy in mouse and human models of duchenne muscular dystrophy. Stem Cell Reports. 10:942–955. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou C, Zhang B, Yang Y, Jiang Q, Li T, Gong J, Tang H and Zhang Q: Stem cell-derived exosomes: Emerging therapeutic opportunities for wound healing. Stem Cell Res Ther. 14:1072023. View Article : Google Scholar : PubMed/NCBI | |
|
Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G and Bonnerot C: Exosomal-like vesicles are present in human blood plasma. Int Immunol. 17:879–887. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Milasan A, Tessandier N, Tan S, Brisson A, Boilard E and Martel C: Extracellular vesicles are present in mouse lymph and their level differs in atherosclerosis. J Extracell Vesicles. 5:314272016. View Article : Google Scholar : PubMed/NCBI | |
|
Pisitkun T, Shen RF and Knepper MA: Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 101:13368–13373. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Kagota S, Taniguchi K, Lee SW, Ito Y, Kuranaga Y, Hashiguchi Y, Inomata Y, Imai Y, Tanaka R, Tashiro K, et al: Analysis of extracellular vesicles in gastric juice from gastric cancer patients. Int J Mol Sci. 20:9532019. View Article : Google Scholar : PubMed/NCBI | |
|
Zlotogorski-Hurvitz A, Dayan D, Chaushu G, Korvala J, Salo T, Sormunen R and Vered M: Human saliva-derived exosomes: Comparing methods of isolation. J Histochem Cytochem. 63:181–189. 2015. View Article : Google Scholar : | |
|
McAndrews KM and Kalluri R: Mechanisms associated with biogenesis of exosomes in cancer. Mol Cancer. 18:522019. View Article : Google Scholar : PubMed/NCBI | |
|
Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G, Chaput N, Chatterjee D, Court FA, Del Portillo HA, et al: Applying extracellular vesicles based therapeutics in clinical trials-an ISEV position paper. J Extracell Vesicles. 4:300872015. View Article : Google Scholar | |
|
Roucourt B, Meeussen S, Bao J, Zimmermann P and David G: Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res. 25:412–428. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Pan BT and Johnstone RM: Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell. 33:967–978. 1983. View Article : Google Scholar : PubMed/NCBI | |
|
Johnstone RM, Adam M, Hammond JR, Orr L and Turbide C: Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 262:9412–9420. 1987. View Article : Google Scholar : PubMed/NCBI | |
|
Mulcahy LA, Pink RC and Carter DR: Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 3:2014. View Article : Google Scholar : PubMed/NCBI | |
|
Krylova SV and Feng D: The machinery of exosomes: Biogenesis, release and uptake. Int J Mol Sci. 24:13372023. View Article : Google Scholar | |
|
Li X, Lian Y, Wu Y, Ye Z, Feng J, Zhao Y, Guo X and Kang J: Neonatal plasma exosomes contribute to endothelial cell-mediated angiogenesis and cardiac repair after acute myocardial infarction. Int J Mol Sci. 24:31962023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y, Jiang R, Zhao H, Li F, Li Y and Zhu M: TTN-AS1 delivered by gastric cancer cell-derived exosome induces gastric cancer progression through in vivo and in vitro studies. Cell Biol Toxicol. 39:557–571. 2023. View Article : Google Scholar | |
|
Nambara S, Masuda T, Hirose K, Hu Q, Tobo T, Ozato Y, Kurashige J, Hiraki Y, Hisamatsu Y, Iguchi T, et al: Rab27b, a regulator of exosome secretion, is associated with peritoneal metastases in gastric cancer. Cancer Genomics Proteomics. 20:30–39. 2023. View Article : Google Scholar : | |
|
Liu X, Li R, Chen X, Yao J, Wang Q, Zhang J, Jiang Y and Qu Y: SYT7 is a key player in increasing exosome secretion and promoting angiogenesis in non-small-cell lung cancer. Cancer Lett. 577:2164002023. View Article : Google Scholar : PubMed/NCBI | |
|
Boilard E: Extracellular vesicles and their content in bioactive lipid mediators: More than a sack of microRNA. J Lipid Res. 59:2037–2046. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Sharma A and Johnson A: Exosome DNA: Critical regulator of tumor immunity and a diagnostic biomarker. J Cell Physiol. 235:1921–1932. 2020. View Article : Google Scholar | |
|
Chennakrishnaiah S, Meehan B, D'Asti E, Montermini L, Lee TH, Karatzas N, Buchanan M, Tawil N, Choi D, Divangahi M, et al: Leukocytes as a reservoir of circulating oncogenic DNA and regulatory targets of tumor-derived extracellular vesicles. J Thromb Haemost. 16:1800–1813. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Yang Z, Shi J, Xie J, Wang Y, Sun J, Liu T, Zhao Y, Zhao X, Wang X, Ma Y, et al: Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng. 4:69–83. 2020. View Article : Google Scholar : | |
|
Zheng D, Huo M, Li B, Wang W, Piao H, Wang Y, Zhu Z, Li D, Wang T and Liu K: The role of exosomes and exosomal MicroRNA in cardiovascular disease. Front Cell Dev Biol. 8:6161612021. View Article : Google Scholar : PubMed/NCBI | |
|
Poulet C, Njock MS, Moermans C, Louis E, Louis R, Malaise M and Guiot J: Exosomal long non-coding RNAs in lung diseases. Int J Mol Sci. 21:35802020. View Article : Google Scholar : PubMed/NCBI | |
|
Cao X, Xue LD, Di Y, Li T, Tian YJ and Song Y: MSC-derived exosomal lncRNA SNHG7 suppresses endothelial-mesenchymal transition and tube formation in diabetic retinopathy via miR-34a-5p/XBP1 axis. Life Sci. 272:1192322021. View Article : Google Scholar : PubMed/NCBI | |
|
Shyu KG, Wang BW, Fang WJ, Pan CM and Lin CM: Hyperbaric oxygen-induced long non-coding RNA MALAT1 exosomes suppress MicroRNA-92a expression in a rat model of acute myocardial infarction. J Cell Mol Med. 24:12945–12954. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Bei Y, Das S, Rodosthenous RS, Holvoet P, Vanhaverbeke M, Monteiro MC, Monteiro VVS, Radosinska J, Bartekova M, Jansen F, et al: Extracellular vesicles in cardiovascular theranostics. Theranostics. 7:4168–4182. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Gupta S and Knowlton AA: HSP60 trafficking in adult cardiac myocytes: Role of the exosomal pathway. Am J Physiol Heart Circ Physiol. 292:H3052–H3056. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Lai Z, Liang J, Zhang J, Mao Y, Zheng X, Shen X, Lin W and Xu G: Exosomes as a delivery tool of exercise-induced beneficial factors for the prevention and treatment of cardiovascular disease: A systematic review and meta-analysis. Front Physiol. 14:11900952023. View Article : Google Scholar : PubMed/NCBI | |
|
Li C, Li X, Shi Z, Wu P, Fu J, Tang J and Qing L: Exosomes from LPS-preconditioned bone marrow MSCs accelerated peripheral nerve regeneration via M2 macrophage polarization: Involvement of TSG-6/NF-κB/NLRP3 signaling pathway. Exp Neurol. 356:1141392022. View Article : Google Scholar | |
|
Yu H, Cheng J, Shi W, Ren B, Zhao F, Shi Y, Yang P, Duan X, Zhang J, Fu X, et al: Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 106:328–341. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y, Zhao R, Liu D, Deng W, Xu G, Liu W, Rong J, Long X, Ge J and Shi B: Exosomes derived from miR-214-enriched bone marrow-derived mesenchymal stem cells regulate oxidative damage in cardiac stem cells by targeting CaMKII. Oxid Med Cell Longev. 2018:49712612018. View Article : Google Scholar : PubMed/NCBI | |
|
Wu J, Ji C, Cao F, Lui H, Xia B and Wang L: Bone marrow mesenchymal stem cells inhibit dendritic cells differentiation and maturation by microRNA-23b. Biosci Rep. 37:BSR201604362017. View Article : Google Scholar : PubMed/NCBI | |
|
Liu R, Chang W, Wei H and Zhang K: Comparison of the biological characteristics of mesenchymal stem cells derived from bone marrow and skin. Stem Cells Int. 2016:36587982016. View Article : Google Scholar : PubMed/NCBI | |
|
Théry C, Zitvogel L and Amigorena S: Exosomes: Composition, biogenesis and function. Nat Rev Immunol. 2:569–579. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Raposo G and Stoorvogel W: Extracellular vesicles: Exosomes, microvesicles and friends. J Cell Biol. 200:373–383. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Thygesen K, Alpert JS and White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction: Universal definition of myocardial infarction. Eur Heart J. 28:2525–2538. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Frangogiannis NG: Pathophysiology of myocardial infarction. Compr Physiol. 5:1841–1875. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Shen Y, Liu X, Shi J and Wu X: Involvement of Nrf2 in myocardial ischemia and reperfusion injury. Int J Biol Macromol. 125:496–502. 2019. View Article : Google Scholar | |
|
Hori M and Nishida K: Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res. 81:457–464. 2009. View Article : Google Scholar | |
|
Sun Y: Oxidative stress and cardiac repair/remodeling following infarction. Am J Med Sci. 334:197–205. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Liu HY, Yu LF, Zhou TG, Wang YD, Sun DH, Chen HR and Hou YF: Lipopolysaccharide-stimulated bone marrow mesenchymal stem cells-derived exosomes inhibit H2O2-induced cardiomyocyte inflammation and oxidative stress via regulating miR-181a-5p/ATF2 axis. Eur Rev Med Pharmacol Sci. 24:10069–10077. 2020.PubMed/NCBI | |
|
Feng J, Yang F, Wu H, Xing C, Xue H, Zhang L, Zhang C, Hu G and Cao H: Selenium protects against cadmium-induced cardiac injury by attenuating programmed cell death via PI3K/AKT/PTEN signaling. Environ Toxicol. 37:1185–1197. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Shi B, Wang Y, Zhao R, Long X, Deng W and Wang Z: Bone marrow mesenchymal stem cell-derived exosomal miR-21 protects C-kit+ cardiac stem cells from oxidative injury through the PTEN/PI3K/Akt axis. PLoS One. 13:e01916162018. View Article : Google Scholar : PubMed/NCBI | |
|
Chen GY and Nuñez G: Sterile inflammation: Sensing and reacting to damage. Nat Rev Immunol. 10:826–837. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Pan W, Zhu Y, Meng X, Zhang C, Yang Y and Bei Y: Immunomodulation by exosomes in myocardial infarction. J Cardiovasc Transl Res. 12:28–36. 2019. View Article : Google Scholar | |
|
Sun C, Li W, Li Y, Chen J, An H, Zeng G, Wang T, Guo Y and Wang C: MiR-182-5p mediated by exosomes derived from bone marrow mesenchymal stem cell attenuates inflammatory responses by targeting TLR4 in a mouse model of myocardial infraction. Immune Netw. 22:e492022. View Article : Google Scholar | |
|
Kore RA, Wang X, Ding Z, Griffin RJ, Tackett AJ and Mehta JL: MSC exosome-mediated cardioprotection in ischemic mouse heart comparative proteomics of infarct and peri-infarct areas. Mol Cell Biochem. 476:1691–1704. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
de Couto G, Liu W, Tseliou E, Sun B, Makkar N, Kanazawa H, Arditi M and Marbán E: Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. J Clin Invest. 125:3147–3162. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Ben-Mordechai T, Palevski D, Glucksam-Galnoy Y, Elron-Gross I, Margalit R and Leor J: Targeting macrophage subsets for infarct repair. J Cardiovasc Pharmacol Ther. 20:36–51. 2015. View Article : Google Scholar | |
|
Xu R, Zhang F, Chai R, Zhou W, Hu M, Liu B, Chen X, Liu M, Xu Q, Liu N and Liu S: Exosomes derived from pro-inflammatory bone marrow-derived mesenchymal stem cells reduce inflammation and myocardial injury via mediating macrophage polarization. J Cell Mol Med. 23:7617–7631. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Ning H, Chen H, Deng J, Xiao C, Xu M, Shan L, Yang C and Zhang Z: Exosomes secreted by FNDC5-BMMSCs protect myocardial infarction by anti-inflammation and macrophage polarization via NF-κB signaling pathway and Nrf2/HO-1 axis. Stem Cell Res Ther. 12:5192021. View Article : Google Scholar | |
|
Cătană CS, Atanasov AG and Berindan-Neagoe I: Natural products with anti-aging potential: Affected targets and molecular mechanisms. Biotechnol Adv. 36:1649–1656. 2018. View Article : Google Scholar | |
|
Xiao C, Wang K, Xu Y, Hu H, Zhang N, Wang Y, Zhong Z, Zhao J, Li Q, Zhu D, et al: Transplanted mesenchymal stem cells reduce autophagic flux in infarcted hearts via the exosomal transfer of miR-125b. Circ Res. 123:564–578. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Kim J, Kundu M, Viollet B and Guan KL: AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 13:132–141. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Lavandero S, Troncoso R, Rothermel BA, Martinet W, Sadoshima J and Hill JA: Cardiovascular autophagy: Concepts, controversies and perspectives. Autophagy. 9:1455–1466. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Yang R, Guo B, Zhang H, Zhang H, Liu S and Li Y: Exosomal miR-301 derived from mesenchymal stem cells protects myocardial infarction by inhibiting myocardial autophagy. Biochem Biophys Res Commun. 514:323–328. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Zou L, Ma X, Lin S, Wu B, Chen Y and Peng C: Bone marrow mesenchymal stem cell-derived exosomes protect against myocardial infarction by promoting autophagy. Exp Ther Med. 18:2574–2582. 2019.PubMed/NCBI | |
|
Yang Q, Zhong QM, Song MQ, Tong LG and Bai CZ: Exosomes derived from Danshen decoction-pretreated bone marrow mesenchymal stem cells alleviate myocardial infarction via anti-apoptosis and up-regulation of autophagy. Heliyon. 10:e380342024. View Article : Google Scholar : PubMed/NCBI | |
|
Saraste A, Pulkki K, Kallajoki M, Henriksen K, Parvinen M and Voipio-Pulkki LM: Apoptosis in human acute myocardial infarction. Circulation. 95:320–323. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Cheng H, Chang S, Xu R, Chen L, Song X, Wu J, Qian J, Zou Y and Ma J: Hypoxia-challenged MSC-derived exosomes deliver miR-210 to attenuate post-infarction cardiac apoptosis. Stem Cell Res Ther. 11:2242020. View Article : Google Scholar : PubMed/NCBI | |
|
Fu DL, Jiang H, Li CY, Gao T, Liu MR and Li HW: MicroRNA-338 in MSCs-derived exosomes inhibits cardiomyocyte apoptosis in myocardial infarction. Eur Rev Med Pharmacol Sci. 24:10107–10117. 2020.PubMed/NCBI | |
|
He JG, Li HR, Han JX, Li BB, Yan D, Li HY, Wang P and Luo Y: GATA-4-expressing mouse bone marrow mesenchymal stem cells improve cardiac function after myocardial infarction via secreted exosomes. Sci Rep. 8:90472018. View Article : Google Scholar : PubMed/NCBI | |
|
Ning W, Li S, Yang W, Yang B, Xin C, Ping X, Huang C, Gu Y and Guo L: Blocking exosomal miRNA-153-3p derived from bone marrow mesenchymal stem cells ameliorates hypoxia-induced myocardial and microvascular damage by targeting the ANGPT1-mediated VEGF/PI3k/Akt/eNOS pathway. Cell Signal. 77:1098122021. View Article : Google Scholar | |
|
Wen Z, Mai Z, Zhu X, Wu T, Chen Y, Geng D and Wang J: Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 11:362020. View Article : Google Scholar : PubMed/NCBI | |
|
Li X, Hu X, Chen Q and Jiang T: Bone marrow mesenchymal stem cell-derived exosomes carrying E3 ubiquitin ligase ITCH attenuated cardiomyocyte apoptosis by mediating apoptosis signal-regulated kinase-1. Pharmacogenet Genomics. 33:117–125. 2023.PubMed/NCBI | |
|
Zhang CS, Shao K, Liu CW, Li CJ and Yu BT: Hypoxic preconditioning BMSCs-exosomes inhibit cardiomyocyte apoptosis after acute myocardial infarction by upregulating microRNA-24. Eur Rev Med Pharmacol Sci. 23:6691–6699. 2019.PubMed/NCBI | |
|
Sun L, Zhu W, Zhao P, Wang Q, Fan B, Zhu Y, Lu Y, Chen Q, Zhang J and Zhang F: Long noncoding RNA UCA1 from hypoxia-conditioned hMSC-derived exosomes: A novel molecular target for cardioprotection through miR-873-5p/XIAP axis. Cell Death Dis. 11:6962020. View Article : Google Scholar : PubMed/NCBI | |
|
Cochain C, Channon KM and Silvestre JS: Angiogenesis in the infarcted myocardium. Antioxid Redox Signal. 18:1100–1113. 2013. View Article : Google Scholar : | |
|
Teng X, Chen L, Chen W, Yang J, Yang Z and Shen Z: Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem. 37:2415–2424. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Zheng J, Zhang X, Cai W, Yang Y, Guo T, Li J and Dai H: Bone marrow mesenchymal stem cell-derived exosomal microRNA-29b-3p promotes angiogenesis and ventricular remodeling in rats with myocardial infarction by targeting ADAMTS16. Cardiovasc Toxicol. 22:689–700. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Yang L, Liu N and Yang Y: Astragaloside IV-induced BMSC exosomes promote neovascularization and protect cardiac function in myocardial infarction mice via the miR-411/HIF-1α axis. J Liposome Res. 34:452–463. 2024. View Article : Google Scholar | |
|
Talman V and Ruskoaho H: Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 365:563–581. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
van den Borne SWM, Diez J, Blankesteijn WM, Verjans J, Hofstra L and Narula J: Myocardial remodeling after infarction: The role of myofibroblasts. Nat Rev Cardiol. 7:30–37. 2010. View Article : Google Scholar | |
|
Jiao W, Hao J, Xie Y, Meng M and Gao W: EZH2 mitigates the cardioprotective effects of mesenchymal stem cell-secreted exosomes against infarction via HMGA2-mediated PI3K/AKT signaling. BMC Cardiovasc Disord. 22:952022. View Article : Google Scholar : PubMed/NCBI | |
|
Wang S, Li L, Liu T, Jiang W and Hu X: miR-19a/19b-loaded exosomes in combination with mesenchymal stem cell transplantation in a preclinical model of myocardial infarction. Regen Med. 15:1749–1759. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Wang S, Dong J, Li L, Wu R, Xu L, Ren Y and Hu X: Exosomes derived from miR-129-5p modified bone marrow mesenchymal stem cells represses ventricular remolding of mice with myocardial infarction. J Tissue Eng Regen Med. 16:177–187. 2022. View Article : Google Scholar | |
|
Li C, Zheng C, Pu Y, Zhou H, Li Y, Wang W, Chen X, Zhang C and Chen Y: Vericiguat enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction through microRNA-1180-3p/ETS1 pathway. Cell Signal. 125:1115122025. View Article : Google Scholar | |
|
Algoet M, Janssens S, Himmelreich U, Gsell W, Pusovnik M, Van den Eynde J and Oosterlinck W: Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc Med. 33:357–366. 2023. View Article : Google Scholar | |
|
Hausenloy DJ and Yellon DM: Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J Clin Invest. 123:92–100. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Xiang M, Lu Y, Xin L, Gao J, Shang C, Jiang Z, Lin H, Fang X, Qu Y, Wang Y, et al: Role of oxidative stress in reperfusion following myocardial ischemia and its treatments. Oxid Med Cell Longev. 2021:66140092021. View Article : Google Scholar : PubMed/NCBI | |
|
Sousa Fialho MDL, Abd Jamil AH, Stannard GA and Heather LC: Hypoxia-inducible factor 1 signalling, metabolism and its therapeutic potential in cardiovascular disease. Biochim Biophys Acta Mol Basis Dis. 1865:831–843. 2019. View Article : Google Scholar | |
|
Zhao D, Bu Y, Shao H, Wang J, Li W and Li Q: Protective effect of exosomes derived from bone marrow mesenchymal stem cells on hypoxia reperfusion injury of cardiomyocytes. Cell Mol Biol (Noisy-le-grand). 70:73–80. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Zou L, Ma X, Wu B, Chen Y, Xie D and Peng C: Protective effect of bone marrow mesenchymal stem cell-derived exosomes on cardiomyoblast hypoxia-reperfusion injury through the miR-149/let-7c/Faslg axis. Free Radic Res. 54:722–731. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Yu H, Pan Y, Dai M, Wang X and Chen H: Mesenchymal stem cell-originated exosomal Lnc A2M-AS1 alleviates hypoxia/reperfusion-induced apoptosis and oxidative stress in cardiomyocytes. Cardiovasc Drugs Ther. 37:891–904. 2023. View Article : Google Scholar | |
|
Li Q, Bu Y, Shao H, Li W, Zhao D and Wang J: Protective effect of bone marrow mesenchymal stem cell-derived exosomes on cardiomyoblast hypoxia-reperfusion injury through the HAND2-AS1/miR-17-5p/Mfn2 axis. BMC Cardiovasc Disord. 23:1142023. View Article : Google Scholar : PubMed/NCBI | |
|
Deng J, Zhang T, Li M, Cao G, Wei H, Zhang Z and Hu T: Irisin-pretreated BMMSCs Secrete exosomes to alleviate cardiomyocytes pyroptosis and oxidative stress to hypoxia/reoxygenation injury. Curr Stem Cell Res Ther. 18:843–852. 2023. View Article : Google Scholar | |
|
Zhang L, Wei Q, Liu X, Zhang T, Wang S, Zhou L, Zou L, Fan F, Chi H, Sun J and Wang D: Exosomal microRNA-98-5p from hypoxic bone marrow mesenchymal stem cells inhibits myocardial ischemia-reperfusion injury by reducing TLR4 and activating the PI3K/Akt signaling pathway. Int Immunopharmacol. 101:1075922021. View Article : Google Scholar : PubMed/NCBI | |
|
Du J, Dong Y, Song J, Shui H, Xiao C, Hu Y, Zhou S and Wang S: BMSC-derived exosome-mediated miR-25-3p delivery protects against myocardial ischemia/reperfusion injury by constraining M1-like macrophage polarization. Mol Med Rep. 30:1422024. View Article : Google Scholar | |
|
Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, Gao L, Xie J and Xu B: Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res. 115:1205–1216. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Shen D and He Z: Mesenchymal stem cell-derived exosomes regulate the polarization and inflammatory response of macrophages via miR-21-5p to promote repair after myocardial reperfusion injury. Ann Transl Med. 9:13232021. View Article : Google Scholar : PubMed/NCBI | |
|
Gao L, Qiu F, Cao H, Li H, Dai G, Ma T, Gong Y, Luo W, Zhu D, Qiu Z, et al: Therapeutic delivery of microRNA-125a-5p oligonucleotides improves recovery from myocardial ischemia/reperfusion injury in mice and swine. Theranostics. 13:685–703. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Q, Liu Y, Ding X, Li Q, Qiu F, Wang M, Shen Z, Zheng H and Fu G: Bone marrow mesenchymal stem cell-secreted exosomes carrying microRNA-125b protect against myocardial ischemia reperfusion injury via targeting SIRT7. Mol Cell Biochem. 465:103–114. 2020. View Article : Google Scholar : | |
|
Mao S, Zhao J, Zhang ZJ and Zhao Q: MiR-183-5p overexpression in bone mesenchymal stem cell-derived exosomes protects against myocardial ischemia/reperfusion injury by targeting FOXO1. Immunobiology. 227:1522042022. View Article : Google Scholar : PubMed/NCBI | |
|
Wang X, Bai L, Liu X, Shen W, Tian H, Liu W and Yu B: Cardiac microvascular functions improved by MSC-derived exosomes attenuate cardiac fibrosis after ischemia-reperfusion via PDGFR-β modulation. Int J Cardiol. 344:13–24. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Sun XH, Wang X, Zhang Y and Hui J: Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb Res. 177:23–32. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Li KS, Bai Y, Li J, Li SL, Pan J, Cheng YQ, Li K, Wang ZG, Ji WJ, Zhou Q and Wang DJ: LncRNA HCP5 in hBMSC-derived exosomes alleviates myocardial ischemia reperfusion injury by sponging miR-497 to activate IGF1/PI3K/AKT pathway. Int J Cardiol. 342:72–81. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y, Ashraf M and Xu M: Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol. 182:349–360. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang X, Stockwell BR and Conrad M: Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang JK, Zhang Z, Guo ZA, Fu Y, Chen XJ, Chen WJ, Wu HF and Cui XJ: The BMSC-derived exosomal lncRNA Mir9-3hg suppresses cardiomyocyte ferroptosis in ischemia-reperfusion mice via the Pum2/PRDX6 axis. Nutr Metab Cardiovasc Dis. 32:515–527. 2022. View Article : Google Scholar | |
|
Strzyz P: Iron expulsion by exosomes drives ferroptosis resistance. Nat Rev Mol Cell Biol. 21:4–5. 2020. View Article : Google Scholar | |
|
Shen K, Wang X, Wang Y, Jia Y, Zhang Y, Wang K, Luo L, Cai W, Li J, Li S, et al: miR-125b-5p in adipose derived stem cells exosome alleviates pulmonary microvascular endothelial cells ferroptosis via Keap1/Nrf2/GPX4 in sepsis lung injury. Redox Biol. 62:1026552023. View Article : Google Scholar : PubMed/NCBI | |
|
Xiao Z, Li S, Wu X, Chen X, Yan D and He J: GATA-4 overexpressing BMSC-derived exosomes suppress H/R-induced cardiomyocyte ferroptosis. iScience. 27:1107842024. View Article : Google Scholar : PubMed/NCBI | |
|
Lusis AJ: Atherosclerosis. Nature. 407:233–241. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Falk E: Pathogenesis of atherosclerosis. J Am Coll Cardiol. 47(Suppl 8): C7–C12. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Hang L, Peng Y, Xiang R, Li X and Li Z: Ox-LDL causes endothelial cell injury through ASK1/NLRP3-mediated inflammasome activation via endoplasmic reticulum stress. Drug Des Devel Ther. 14:731–744. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Pirillo A, Norata GD and Catapano AL: LOX-1, OxLDL and atherosclerosis. Mediators Inflamm. 2013:1527862013. View Article : Google Scholar | |
|
Chen S, Zhou H, Zhang B and Hu Q: Exosomal miR-512-3p derived from mesenchymal stem cells inhibits oxidized low-density lipoprotein-induced vascular endothelial cells dysfunction via regulating Keap1. J Biochem Mol Toxicol. 35:1–11. 2021. View Article : Google Scholar | |
|
Zhang N, Luo Y, Zhang H, Zhang F, Gao X and Shao J: Exosomes derived from mesenchymal stem cells ameliorate the progression of atherosclerosis in ApoE−/− mice via FENDRR. Cardiovasc Toxicol. 22:528–544. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Wei Y, Lan B, Zheng T, Yang L, Zhang X, Cheng L, Tuerhongjiang G, Yuan Z and Wu Y: GSDME-mediated pyroptosis promotes the progression and associated inflammation of atherosclerosis. Nat Commun. 14:9292023. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Xue H, Li T, Chu X, Xin D, Xiong Y, Qiu W, Gao X, Qian M, Xu J, et al: Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE−/− mice via miR-let7 mediated infiltration and polarization of M2 macrophage. Biochem Biophys Res Commun. 510:565–572. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Sun L, He X, Zhang T, Han Y and Tao G: Knockdown of mesenchymal stem cell-derived exosomal LOC100129516 suppresses the symptoms of atherosclerosis via upregulation of the PPARγ/LXRα/ABCA1 signaling pathway. Int J Mol Med. 48:2082021. View Article : Google Scholar | |
|
Zhao R, Feng J and He G: miR-613 regulates cholesterol efflux by targeting LXRα and ABCA1 in PPARγ activated THP-1 macrophages. Biochem Biophys Res Commun. 448:329–334. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Xu Y, Lai F, Xu Y, Wu Y, Liu Q, Li N, Wei Y, Feng T, Zheng Z, Jiang W, et al: Mycophenolic acid induces ATP-binding cassette transporter A1 (ABCA1) expression through the PPARγ-LXRα-ABCA1 pathway. Biochem Biophys Res Commun. 414:779–782. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Bergsbaken T, Fink SL and Cookson BT: Pyroptosis: Host cell death and inflammation. Nat Rev Microbiol. 7:99–109. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Shi J, Gao W and Shao F: Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 42:245–254. 2017. View Article : Google Scholar | |
|
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, et al: Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 25:486–541. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Bai Z, Hu H, Hu F, Ji J and Ji Z: Bone marrow mesenchymal stem cellsderived exosomes stabilize atherosclerosis through inhibiting pyroptosis. BMC Cardiovasc Disord. 23:4412023. View Article : Google Scholar : PubMed/NCBI | |
|
Lin Y, Liu M, Chen E, Jiang W, Shi W and Wang Z: Bone marrow-derived mesenchymal stem cells microvesicles stabilize atherosclerotic plaques by inhibiting NLRP3-mediated macrophage pyroptosis. Cell Biol Int. 45:820–830. 2021. View Article : Google Scholar | |
|
Zhang N, Luo Y, Shao J, Sun H, Ma K and Gao X: Exosomal long non-coding RNA AU020206 alleviates macrophage pyroptosis in atherosclerosis by suppressing CEBPB-mediated NLRP3 transcription. Exp Cell Res. 438:1140542024. View Article : Google Scholar : PubMed/NCBI | |
|
Damiani RM, Moura DJ, Viau CM, Caceres RA, Henriques JAP and Saffi J: Pathways of cardiac toxicity: Comparison between chemotherapeutic drugs doxorubicin and mitoxantrone. Arch Toxicol. 90:2063–2076. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Matusik K, Kamińska K, Sobiborowicz-Sadowska A, Borzuta H, Buczma K and Cudnoch-Jędrzejewska A: The significance of the apelinergic system in doxorubicin-induced cardiotoxicity. Heart Fail Rev. 29:969–988. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Renu K, V G A, P B TP and Arunachalam S: Molecular mechanism of doxorubicin-induced cardiomyopathy-an update. Eur J Pharmacol. 818:241–253. 2018. View Article : Google Scholar | |
|
Lei B, Wu X, Xia K, Sun H and Wang J: Exosomal Micro-RNA-96 derived from bone marrow mesenchymal stem cells inhibits doxorubicin-induced myocardial toxicity by inhibiting the Rac1/nuclear factor-κB signaling pathway. J Am Heart Assoc. 10:e0205892021. View Article : Google Scholar | |
|
Tian C, Yang Y, Li B, Liu M, He X, Zhao L, Song X, Yu T and Chu XM: Doxorubicin-induced cardiotoxicity may be alleviated by bone marrow mesenchymal stem cell-derived exosomal lncRNA via inhibiting inflammation. J Inflamm Res. 15:4467–4486. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 526:660–665. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Li YJ and Zhu ZQ: To re-examine the intersection of microglial activation and neuroinflammation in neurodegenerative diseases from the perspective of pyroptosis. Front Aging Neurosci. 15:12842142023. View Article : Google Scholar : PubMed/NCBI | |
|
Zeng H, Yang Y, Tou F, Zhan Y, Liu S, Zou P, Chen Y and Shao L: Bone marrow stromal cell-derived exosomes improve oxidative stress and pyroptosis in doxorubicin-induced myocardial injury in vitro by regulating the transcription of GSDMD through the PI3K-AKT-Foxo1 pathway. Immun Inflamm Dis. 11:e8102023. View Article : Google Scholar : PubMed/NCBI | |
|
Ali SA and Singla DK: Mesenchymal stem cell-derived exosomes ameliorate doxorubicin-induced cardiotoxicity. Pharmaceuticals (Basel). 17:932024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhai X, Zhou J, Huang X, Weng J, Lin H, Sun S, Chi J and Meng L: LncRNA GHET1 from bone mesenchymal stem cell-derived exosomes improves doxorubicin-induced pyroptosis of cardiomyocytes by mediating NLRP3. Sci Rep. 14:190782024. View Article : Google Scholar | |
|
Tanai E and Frantz S: Pathophysiology of heart failure. Compr Physiol. 6:187–214. 2015. View Article : Google Scholar | |
|
Chen F, Li X, Zhao J, Geng J, Xie J and Xu B: Bone marrow mesenchymal stem cell-derived exosomes attenuate cardiac hypertrophy and fibrosis in pressure overload induced remodeling. In Vitro Cell Dev Biol Anim. 56:567–576. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Ren Y, Wu Y, He W, Tian Y and Zhao X: Exosomes secreted from bone marrow mesenchymal stem cells suppress cardiomyocyte hypertrophy through Hippo-YAP pathway in heart failure. Genet Mol Biol. 46:e202202212023. View Article : Google Scholar : PubMed/NCBI | |
|
Ren Y and Zhao X: Bone marrow mesenchymal stem cells-derived exosomal lncRNA GAS5 mitigates heart failure by inhibiting UL3/Hippo pathway-mediated ferroptosis. Eur J Med Res. 29:3032024. View Article : Google Scholar : PubMed/NCBI | |
|
Pu L, Kong X, Li H and He X: Exosomes released from mesenchymal stem cells overexpressing microRNA-30e ameliorate heart failure in rats with myocardial infarction. Am J Transl Res. 13:4007–4025. 2021.PubMed/NCBI | |
|
Han Y, Bi Y, Zhang D and Liu Y: The exosomes derived from bone marrow mesenchymal stem cells alleviate inflammatory injury in heart failure disease by enhancing the expression of KLF4. Immun Inflamm Dis. 13:e701612025. View Article : Google Scholar : PubMed/NCBI | |
|
Beyer C, Tokarska L, Stühlinger M, Feuchtner G, Hintringer F, Honold S, Fiedler L, Schönbauer MS, Schönbauer R and Plank F: Structural cardiac remodeling in atrial fibrillation. JACC Cardiovasc Imaging. 14:2199–2208. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Paliwal N, Ali RL, Salvador M, O'Hara R, Yu R, Daimee UA, Akhtar T, Pandey P, Spragg DD, Calkins H and Trayanova NA: Presence of left atrial fibrosis may contribute to aberrant hemodynamics and increased risk of stroke in atrial fibrillation patients. Front Physiol. 12:6574522021. View Article : Google Scholar : PubMed/NCBI | |
|
Xu L, Fan Y, Wu L, Zhang C, Chu M, Wang Y and Zhuang W: Exosomes from bone marrow mesenchymal stem cells with overexpressed Nrf2 inhibit cardiac fibrosis in rats with atrial fibrillation. Cardiovasc Ther. 2022:26878072022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang W, Man Y and Chen Z: microRNA-148a in exosomes derived from bone marrow mesenchymal stem cells alleviates cardiomyocyte apoptosis in atrial fibrillation by inhibiting SMOC2. Mol Biotechnol. 64:1076–1087. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Martensson J and Bellomo R: Sepsis-induced acute kidney injury. Crit Care Clin. 31:649–660. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, Goldstein SL, Cerda J and Chawla LS: Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 14:607–625. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Huang M, Cai S and Su J: The pathogenesis of sepsis and potential therapeutic targets. Int J Mol Sci. 20:53762019. View Article : Google Scholar : PubMed/NCBI | |
|
van der Poll T, van de Veerdonk FL, Scicluna BP and Netea MG: The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 17:407–420. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Jiang R, Hou Y and Lin A: Mesenchymal stem cells-derived exosomes prevent sepsis-induced myocardial injury by a CircRTN4/miR-497-5p/MG53 pathway. Biochem Biophys Res Commun. 618:133–140. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Pei Y, Xie S, Li J and Jia B: Bone marrow-mesenchymal stem cell-derived exosomal microRNA-141 targets PTEN and activates β-catenin to alleviate myocardial injury in septic mice. Immunopharmacol Immunotoxicol. 43:584–593. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Li T, Zhao Y, Cao Z, Shen Y, Chen J, Huang X, Shao Z, Zeng Y, Chen Q, Yan X, et al: Exosomes derived from apelin-pretreated mesenchymal stem cells ameliorate sepsis-induced myocardial dysfunction by alleviating cardiomyocyte pyroptosis via delivery of miR-34a-5p. Int J Nanomedicine. 20:687–703. 2025. View Article : Google Scholar : PubMed/NCBI | |
|
Pan L, Huang C, Jin X, Wu J, Jin K, Lin J, Wang Y, Li J, Yin C, Wang X, et al: Cardiac secreted HSP90α exacerbates pressure overload myocardial hypertrophy and heart failure. Redox Biol. 79:1034662025. View Article : Google Scholar | |
|
Schirone L, Forte M, Palmerio S, Yee D, Nocella C, Angelini F, Pagano F, Schiavon S, Bordin A, Carrizzo A, et al: A review of the molecular mechanisms underlying the development and progression of cardiac remodeling. Oxid Med Cell Longev. 2017:39201952017. View Article : Google Scholar : PubMed/NCBI | |
|
Schena GJ, Murray EK, Hildebrand AN, Headrick AL, Yang Y, Koch KA, Kubo H, Eaton D, Johnson J, Berretta R, et al: Cortical bone stem cell-derived exosomes' therapeutic effect on myocardial ischemia-reperfusion and cardiac remodeling. Am J Physiol Heart Circ Physiol. 321:H1014–H1029. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Gao H, Zhang L, Wang Z, Yan K, Zhao L and Xiao W: Research progress on transorgan regulation of the cardiovascular and motor system through cardiogenic exosomes. Int J Mol Sci. 23:57652022. View Article : Google Scholar : PubMed/NCBI | |
|
Deanfield JE, Halcox JP and Rabelink TJ: Endothelial function and dysfunction: testing and clinical relevance. Circulation. 115:1285–1295. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Subarajan P, Arceo-Mendoza RM and Camacho PM: Postmenopausal osteoporosis: A review of latest guidelines. Endocrinol Metab Clin North Am. 53:497–512. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Song H, Li X, Zhao Z, Qian J, Wang Y, Cui J, Weng W, Cao L, Chen X, Hu Y and Su J: Reversal of osteoporotic activity by endothelial cell-secreted bone targeting and biocompatible exosomes. Nano Lett. 19:3040–3048. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Yang RZ, Xu WN, Zheng HL, Zheng XF, Li B, Jiang LS and Jiang SD: Exosomes derived from vascular endothelial cells antagonize glucocorticoid-induced osteoporosis by inhibiting ferritinophagy with resultant limited ferroptosis of osteoblasts. J Cell Physiol. 236:6691–6705. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang J, Xie X, Li H, Zheng Q, Chen Y, Chen W, Chen Y, He J and Lu Q: Vascular endothelial cells-derived exosomes synergize with curcumin to prevent osteoporosis development. iScience. 27:1096082024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao D, Zhang F, Wang B, Liu B, Li L, Kim SY, Goodman SB, Hernigou P, Cui Q, Lineaweaver WC, et al: Guidelines for clinical diagnosis and treatment of osteonecrosis of the femoral head in adults (2019 version). J Orthop Translat. 21:100–110. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Wu H, Chen G, Zhang G, Lv Q, Gu D and Dai M: Mechanism of vascular endothelial cell-derived exosomes modified with vascular endothelial growth factor in steroid-induced femoral head necrosis. Biomed Mater. 18:0250172023. View Article : Google Scholar | |
|
Maeda K, Kobayashi Y, Koide M, Uehara S, Okamoto M, Ishihara A, Kayama T, Saito M and Marumo K: The regulation of bone metabolism and disorders by Wnt signaling. Int J Mol Sci. 20:55252019. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou B, Peng K, Wang G, Chen W, Liu P, Chen F and Kang Y: miR-483-3p promotes the osteogenesis of human osteoblasts by targeting Dikkopf 2 (DKK2) and the Wnt signaling pathway. Int J Mol Med. 46:1571–1581. 2020.PubMed/NCBI | |
|
Zhang G, Liu R, Dang X, Liu J and Jiao H: Experimental study on improvement of osteonecrosis of femoral head with exosomes derived from miR-27a-overexpressing vascular endothelial cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 35:356–365. 2021.In Chinese. PubMed/NCBI | |
|
Cheng M, Yang J, Zhao X, Zhang E, Zeng Q, Yu Y, Yang L, Wu B, Yi G, Mao X, et al: Circulating myocardial microRNAs from infarcted hearts are carried in exosomes and mobilise bone marrow progenitor cells. Nat Commun. 10:9592019. View Article : Google Scholar : PubMed/NCBI | |
|
Yang RZ, Zheng HL, Xu WN, Zheng XF, Li B, Jiang LS and Jiang SD: Vascular endothelial cell-secreted exosomes facilitate osteoarthritis pathogenesis by promoting chondrocyte apoptosis. Aging (Albany NY). 13:4647–4662. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Weilner S, Schraml E, Wieser M, Messner P, Schneider K, Wassermann K, Micutkova L, Fortschegger K, Maier AB, Westendorp R, et al: Secreted microvesicular miR-31 inhibits osteogenic differentiation of mesenchymal stem cells. Aging Cell. 15:744–754. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Hu M, Guo G, Huang Q, Cheng C, Xu R, Li A, Liu N and Liu S: The harsh microenvironment in infarcted heart accelerates transplanted bone marrow mesenchymal stem cells injury: The role of injured cardiomyocytes-derived exosomes. Cell Death Dis. 9:3572018. View Article : Google Scholar : PubMed/NCBI | |
|
Duan D, Goemans N, Takeda S, Mercuri E and Aartsma-Rus A: Duchenne muscular dystrophy. Nat Rev Dis Primers. 7:132021. View Article : Google Scholar : PubMed/NCBI | |
|
Kamdar F and Garry DJ: Dystrophin-deficient cardiomyopathy. J Am Coll Cardiol. 67:2533–2546. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Z, Su X, Ashraf M, Kim IM, Weintraub NL, Jiang M and Tang Y: Regenerative Therapy for cardiomyopathies. J Cardiovasc Transl Res. 11:357–365. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Su X, Shen Y, Jin Y, Jiang M, Weintraub N and Tang Y: Purification and transplantation of myogenic progenitor cell derived exosomes to improve cardiac function in duchenne muscular dystrophic mice. J Vis Exp. View Article : Google Scholar : 2019. | |
|
Su X, Jin Y, Shen Y, Ju C, Cai J, Liu Y, Kim IM, Wang Y, Yu H, Weintraub NL, et al: Exosome-derived dystrophin from allograft myogenic progenitors improves cardiac function in duchenne muscular dystrophic mice. J Cardiovasc Transl Res. 11:412–419. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Su X, Shen Y, Jin Y, Weintraub NL and Tang YL: Identification of critical molecular pathways involved in exosome-mediated improvement of cardiac function in a mouse model of muscular dystrophy. Acta Pharmacol Sin. 42:529–535. 2021. View Article : Google Scholar : | |
|
Heusch G, Bøtker HE, Przyklenk K, Redington A and Yellon D: Remote ischemic conditioning. J Am Coll Cardiol. 65:177–195. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Hu T, Duan R, Gao H, Bai X, Huang X, Yan X, An L, Ma Y, Chen R, Hong S and Gan M: Exosomes from myoblasts induced by hypoxic preconditioning improved ventricular conduction by increasing Cx43 expression in hypothermia ischemia reperfusion hearts. Cytotechnology. 76:533–546. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Cruz-Jentoft AJ and Sayer AA: Sarcopenia. Lancet. 393:2636–2646. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, et al: Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 48:16–31. 2019. View Article : Google Scholar : | |
|
Bekfani T, Pellicori P, Morris DA, Ebner N, Valentova M, Steinbeck L, Wachter R, Elsner S, Sliziuk V, Schefold JC, et al: Sarcopenia in patients with heart failure with preserved ejection fraction: Impact on muscle strength, exercise capacity and quality of life. Int J Cardiol. 222:41–46. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Anker SD and Sharma R: The syndrome of cardiac cachexia. Int J Cardiol. 85:51–66. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Coats AJ: Research on cachexia, sarcopenia and skeletal muscle in cardiology. J Cachexia Sarcopenia Muscle. 3:219–223. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Loncar G, Springer J, Anker M, Doehner W and Lainscak M: Cardiac cachexia: hic et nunc. J Cachexia Sarcopenia Muscle. 7:246–260. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Marbán E: A mechanistic roadmap for the clinical application of cardiac cell therapies. Nat Biomed Eng. 2:353–361. 2018. View Article : Google Scholar | |
|
Rogers RG, Fournier M, Sanchez L, Ibrahim AG, Aminzadeh MA, Lewis MI and Marbán E: Disease-modifying bioactivity of intravenous cardiosphere-derived cells and exosomes in mdx mice. JCI Insight. 4:e1257542019. View Article : Google Scholar : PubMed/NCBI | |
|
Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W, Hanada K, Mao L, Watson LJ and Rockman HA: Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation. 131:2120–2130. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Wang XH: MicroRNA in myogenesis and muscle atrophy. Curr Opin Clin Nutr Metab Care. 16:258–266. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Murach KA and McCarthy JJ: MicroRNAs, heart failure and aging: Potential interactions with skeletal muscle. Heart Fail Rev. 22:209–218. 2017. View Article : Google Scholar : | |
|
Melman YF, Shah R and Das S: MicroRNAs in heart failure: Is the picture becoming less miRky? Circ Heart Fail. 7:203–214. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
McCarthy JJ and Esser KA: MicroRNA-1 and microRNA-133a expression are decreased during skeletal muscle hypertrophy. J Appl Physiol (1985). 102:306–313. 2007. View Article : Google Scholar | |
|
Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M, Watanabe S, Baba O, Kojima Y, Shizuta S, et al: Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet. 4:446–454. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Kolhe R, Hunter M, Liu S, Jadeja RN, Pundkar C, Mondal AK, Mendhe B, Drewry M, Rojiani MV, Liu Y, et al: Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci Rep. 7:20292017. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao Y and Xu J: Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int Orthop. 42:2865–2872. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Rezaie J, Feghhi M and Etemadi T: A review on exosomes application in clinical trials: Perspective, questions and challenges. Cell Commun Signal. 20:1452022. View Article : Google Scholar | |
|
Tai YL, Chen KC, Hsieh JT and Shen TL: Exosomes in cancer development and clinical applications. Cancer Sci. 109:2364–2374. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Gong L, Zhou H, Zhang S, Wang C, Fu K, Ma C, Zhang Y, Peng C and Li Y: CD44-targeting drug delivery system of exosomes loading forsythiaside A combats liver fibrosis via regulating NLRP3-mediated pyroptosis. Adv Healthc Mater. 12:e22022282023. View Article : Google Scholar : PubMed/NCBI | |
|
Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, et al: Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 12:655–664. 2016. View Article : Google Scholar | |
|
Dumontel B, Susa F, Limongi T, Vighetto V, Debellis D, Canta M and Cauda V: Nanotechnological engineering of extracellular vesicles for the development of actively targeted hybrid nanodevices. Cell Biosci. 12:612022. View Article : Google Scholar : PubMed/NCBI | |
|
Tang A, Shu Q, Jia S, Lai Z and Tian J: Adipose mesenchymal stem cell-derived exosomes as nanocarriers for treating musculoskeletal disorders. Int J Nanomedicine. 19:13547–13562. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Liu L, Chen S, Song Y, Cui L, Chen Y, Xia J, Fan Y and Yang L and Yang L: Hydrogels empowered mesenchymal stem cells and the derived exosomes for regenerative medicine in age-related musculoskeletal diseases. Pharmacol Res. 213:1076182025. View Article : Google Scholar : PubMed/NCBI | |
|
Yao J, Huang K, Zhu D, Chen T, Jiang Y, Zhang J, Mi L, Xuan H, Hu S, Li J, et al: A minimally invasive exosome spray repairs heart after myocardial infarction. ACS Nano. 15:11099–11111. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Chen P, Zheng L, Wang Y, Tao M, Xie Z, Xia C, Gu C, Chen J, Qiu P, Mei S, et al: Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 9:2439–2459. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Li Q, Xu Y, Lv K, Wang Y, Zhong Z, Xiao C, Zhu K, Ni C, Wang K, Kong M, et al: Small extracellular vesicles containing miR-486-5p promote angiogenesis after myocardial infarction in mice and nonhuman primates. Sci Transl Med. 13:eabb02022021. View Article : Google Scholar : PubMed/NCBI | |
|
Spiller KL and Koh TJ: Macrophage-based therapeutic strategies in regenerative medicine. Adv Drug Deliv Rev. 122:74–83. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
de Couto G, Gallet R, Cambier L, Jaghatspanyan E, Makkar N, Dawkins JF, Berman BP and Marbán E: Exosomal MicroRNA transfer into macrophages mediates cellular postconditioning. Circulation. 136:200–214. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Zavatti M, Beretti F, Casciaro F, Bertucci E and Maraldi T: Comparison of the therapeutic effect of amniotic fluid stem cells and their exosomes on monoiodoacetate-induced animal model of osteoarthritis. Biofactors. 46:106–117. 2020. View Article : Google Scholar | |
|
Zheng Y, Fu L, Zhang Z, Wu J, Yuan X, Ding Z, Ning C, Sui X, Liu S and Guo Q: Three-dimensional bioprinting of growth differentiation factor 5-preconditioned mesenchymal stem cell-derived exosomes facilitates articular cartilage endogenous regeneration. ACS Nano. 19:15281–15301. 2025. View Article : Google Scholar : PubMed/NCBI | |
|
Maffulli N, Wong J and Almekinders LC: Types and epidemiology of tendinopathy. Clin Sports Med. 22:675–692. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Song K, Jiang T, Pan P, Yao Y and Jiang Q: Exosomes from tendon derived stem cells promote tendon repair through miR-144-3p-regulated tenocyte proliferation and migration. Stem Cell Res Ther. 13:802022. View Article : Google Scholar : PubMed/NCBI | |
|
Guo H, Huang B, Wang Y, Zhang Y, Ma Q and Ren Y: Bone marrow mesenchymal stem cells-derived exosomes improve injury of hippocampal neurons in rats with depression by upregulating microRNA-26a expression. Int Immunopharmacol. 82:1062852020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI | |
|
Pomatto M, Gai C, Negro F, Cedrino M, Grange C, Ceccotti E, Togliatto G, Collino F, Tapparo M, Figliolini F, et al: Differential therapeutic effect of extracellular vesicles derived by bone marrow and adipose mesenchymal stem cells on wound healing of diabetic ulcers and correlation to their cargoes. Int J Mol Sci. 22:38512021. View Article : Google Scholar : PubMed/NCBI | |
|
Liu X, Zhang M, Liu H, Zhu R, He H, Zhou Y, Zhang Y, Li C, Liang D, Zeng Q and Huang G: Bone marrow mesenchymal stem cell-derived exosomes attenuate cerebral ischemia-reperfusion injury-induced neuroinflammation and pyroptosis by modulating microglia M1/M2 phenotypes. Exp Neurol. 341:1137002021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou Y, Zhao B, Zhang XL, Lu YJ, Lu ST, Cheng J, Fu Y, Lin L, Zhang NY, Li PX, et al: Combined topical and systemic administration with human adipose-derived mesenchymal stem cells (hADSC) and hADSC-derived exosomes markedly promoted cutaneous wound healing and regeneration. Stem Cell Res Ther. 12:2572021. View Article : Google Scholar : PubMed/NCBI | |
|
Dave KM, Venna VR, Rao KS, Stolz DB, Brady B, Quaicoe VA, Maniskas ME, Hildebrand EE, Green D, Chen M, et al: Mitochondria-containing extracellular vesicles from mouse vs human brain endothelial cells for ischemic stroke therapy. J Control Release. 373:803–822. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Lotfy A, AboQuella NM and Wang H: Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res Ther. 14:662023. View Article : Google Scholar : PubMed/NCBI | |
|
Shin DI, Jin YJ, Noh S, Yun HW, Park DY and Min BH: Exosomes secreted during myogenic differentiation of human fetal cartilage-derived progenitor cells promote skeletal muscle regeneration through miR-145-5p. Tissue Eng Regen Med. 21:487–497. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Guo R, Wu Z, Liu A, Li Q, Han T and Shen C: Hypoxic preconditioning-engineered bone marrow mesenchymal stem cell-derived exosomes promote muscle satellite cell activation and skeletal muscle regeneration via the miR-210-3p/KLF7 mechanism. Int Immunopharmacol. 142:1131432024. View Article : Google Scholar : PubMed/NCBI | |
|
Xu N, Cui G, Zhao S, Li Y, Liu Q, Liu X, Zhao C, Feng R, Kuang M and Han S: Therapeutic effects of mechanical stress-induced C2C12-derived exosomes on glucocorticoid-induced osteoporosis through miR-92a-3p/PTEN/AKT signaling pathway. Int J Nanomedicine. 18:7583–7603. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Chen W, Zheng H, Liao Q, Zeng S, Bai R, Shi J, Jiang Y, Wang T, Jia H, Liang W, et al: Zhuang-Gu-Fang promotes osteoblast differentiation via myoblasts and myoblast-derived exosomal miRNAs:miR-5100, miR-126a-3p, miR-450b-5p, and miR-669a-5p. Phytomedicine. 130:1557182024. View Article : Google Scholar : PubMed/NCBI |