Microbiota metabolites affect sleep as drivers of brain‑gut communication (Review)
- Authors:
- Published online on: June 30, 2025 https://doi.org/10.3892/ijmm.2025.5571
- Article Number: 130
-
Copyright: © Cheng et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Sleep is crucial for humans and is closely linked to overall health. In modern life, an increasing number of individuals face sleep disorders. In 2010, a cross-sectional online survey of 1,125 students (aged 17-24) at a Midwestern urban university found that >60% were poor sleepers based on the Pittsburgh Sleep Quality Index (1). Previous studies have shown that adequate sleep promotes metabolism, alleviates fatigue, delays the aging process and enhances the immune system (2). Conversely, prolonged insomnia leads to compromised immune function, dysregulation of the autonomic nervous system, cardiovascular and endocrine disorders, and contributes to anxiety and depression, thus affecting overall physical and mental health. Common sleep disorders include insomnia, circadian rhythm disruptions, hypersomnia, restless leg syndrome and obstructive sleep apnea. These disorders are associated with dysfunction in multiple body systems, including the endocrine, immune and nervous systems (2-7).
There are various treatments for sleep issues, including medication, therapy, physical treatments and cognitive behavioral therapy for insomnia. Previously, a study demonstrated a close connection between gut microbiota metabolites and the brain (8). Therefore, taking prebiotics and probiotics has become a popular way to help solve sleep problems (9). Their effects are influenced by multiple factors such as tissue type and metabolic state, dietary environment, and circulating levels of metabolites (10). Bacterial metabolites include several types, including neuroregulators (11,12), pro-inflammatory and anti-inflammatory mediators (13,14), uremic toxins, and molecules that provide energy for host cell metabolism (15). Certain metabolites also participate in brain neurodevelopment and blood-brain barrier (BBB) integrity, regulating brain neuroinflammation (16). A previous study showed that diet-induced gut microbiota metabolites act as crucial mediators in host-microbiota interactions (17). Additionally, it has been revealed that gut microbiota metabolites are closely linked to diet. For example, short-chain fatty acids (SCFAs) from dietary fiber, indole from amino acid metabolism, ergothioneine and trimethylamine formed from choline, betaine and carnitine metabolism are all dietary nutrients (18). These diet-related metabolites may guide the diet for patients with sleep disorders.
Early research on sleep disorders mainly focused on how the central nervous system (CNS) controls the sleep-wake cycle. However, how peripheral systems such as the gut affect sleep regulation and disorders remains to be elucidated. Despite previous research focused on some sleep mechanisms, research into the impact of gut microbiota on sleep is still in its nascent stages, particularly concerning the mechanisms through which gut microbiota metabolites impact sleep patterns.
Brain-gut axis
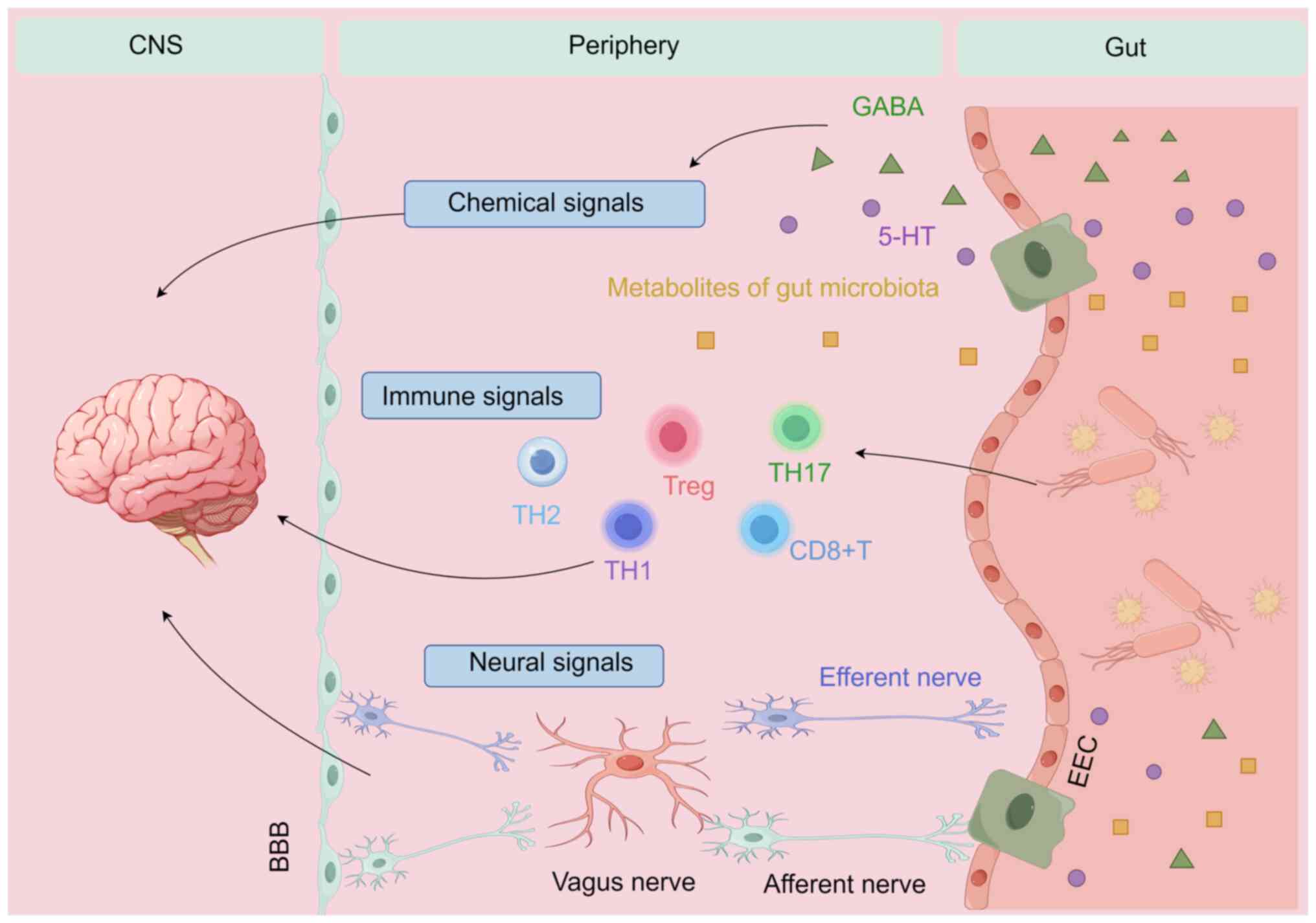
Currently, the brain-gut-microbiota concept goes beyond just an axis; it represents a complex system involving the brain, gut and microbiota, along with their interactions through the CNS, chemical signals, immune regulation and barrier functions in both the brain and gut (19-21). The vagus nerve (VN) serves as a vital conduit, sensing gut microbiota metabolites and relaying this message to the CNS (22). Furthermore, the migration of immune cells in the immune pathway is one of the key mechanisms connecting the gut and the brain. Cytotoxic CD8+ T, CD4 effector [helper T (Th) 1 cells, Th2 and Th17 cells] and regulatory T (Treg) cells not only play local roles in the intestine but can also migrate to the brain to exert their immune functions. They are key players in the gut-brain microbiome connection for maintaining balance during disease (23-27). Previous research into chemical signaling has deepened the present understanding of the brain-gut-microbiota system (28,29). These chemical signals, such as metabolites of gut microbiota, serotonin (5-HT) and γ-aminobutyric acid, which influence both gut and brain functions, may impact various pathways (30) (Fig. 1).
Possible mechanisms by which microbiota metabolites affect sleep
Tryptophan (TPH) and SCFA
Research on the role of the amino acid TPH is receiving increasing attention. TPH is found in protein-rich foods such as egg, milk, oats, cheese, nuts and seeds. The majority of TPH is absorbed within the small intestine, but excessive intake of protein and amino acids (6-18 g/day) can make some protein reach the colon (31). SCFAs are the major metabolites produced by microbial fermentation of dietary fiber. They are vital in maintaining metabolism, neurological function and immune systems (32). Dietary fiber is consumed from the diet, and it cannot be digested and absorbed directly by the gastrointestinal system. Instead, it escapes digestion in the stomach and small intestine, and undergoes fermentation by anaerobic microbes in the cecum and colon. This fermentation process produces SCFAs, mainly including acetate, propionate and butyrate salts (33,34). SCFAs are readily absorbed from the gut into the circulation and directly reach the liver. Previous research has shown that injecting butyrate via portal vein results in a 70% increase in non-rapid eye movement sleep in mice (35,36). This finding supports the connection between gut microbiota and related metabolites with sleep disorders (37). These metabolites can be absorbed and utilized by the host's gut and are measurable in the host's circulation. However, some microbial metabolites can promote health, while others can be toxic and detrimental to health. A previous study linked insufficient sleep to reduced SCFAs production by the gut microbiome. Another study found a positive association between sleep duration and the concentrations of total SCFAs, acetate and propionate in stool (38). These results suggest that shorter sleep duration is associated with lower SCFA production, providing some evidence that SCFAs may influence sleep. Furthermore, studies have indicated that diet may influence sleep through melatonin and its biosynthesis from TPH (39).
5-HT
TPH is the only substance needed to make 5-HT, and in germ-free mice, 5-HT levels were 2.8-fold lower. This reduction can partly be due to microbial processes (40). 5-HT, a neurochemical molecule that exhibits diurnal variations, is associated with the hypothalamic pathways that promote sleep and regulate glucose homeostasis (41). Both gut microbiota and enterochromaffin cells can influence 5-HT synthesis by controlling the rate-limiting enzyme TPH hydroxylase (42). Subsequently, 5-HT is converted by different enzymes into either melatonin or 5-hydroxyindoleacetic acid, with melatonin being the primary substance responsible for regulating sleep initiation and circadian rhythms (43). Certain specific bacterial strains, including Lactobacillus, Lactococcus, Prevotella, Streptococcus thermophilus, Escherichia coli, K-12, Morganella morganii, Klebsiella pneumoniae and Staphylococcus aureus, can produce 5-HT and other biogenic amines from TPH (44). This impacts gut motility and secretion, and can also affect 5-HT levels in the brain, potentially influencing mood and cognitive functions (45).
In addition, SCFAs can influence the serotonergic system. 5-HT activity is primarily influenced by the extracellular availability of 5-HT, which is regulated by the 5-HT transporter (SERT) (46). A previous study found that SCFAs affected the activity of intestinal SERT (47). In this study, the activity and expression levels of SERT were decreased by propionate and acetate, concurrently elevating the levels of specific 5-HT receptors that amplified 5-HT signaling. By contrast, butyrate enhanced both the activity and expression levels of SERT, and upregulated anti-inflammatory molecules such as interleukin (IL)-10 (47). Additionally, it was observed that low levels of acetate, propionate and butyrate has an impact on SERT activity, whereas increased concentrations did not influence SERT functionality. Although butyrate appeared to have no effect on receptor expression, propionate and acetate both notably increased the mRNA levels of 5-HTR1A, 2B and 7 (48). The 5-HTR1A receptor, which is expressed in intestinal epithelial cells and enteric neurons, can activate the release of 5-HT from enterochromaffin cells (49).
In conclusion, dietary intake of TPH is crucial for 5-HT synthesis, and influences emotions and cognitive functions through the actions of gut microbiota, highlighting the importance of the gut-brain axis in maintaining overall health.
VN and enteroendocrine cells (EECs)
The VN serves as the principal conduit for transmitting internal organ information to the CNS. Earlier research indicated that the VN may be involved in conveying information from the gut microbiota to the brain (50). Stimuli from chemicals or microbes in the gastrointestinal tract can activate Trpa1+ EECs, which subsequently transmit signals to the vagal ganglia. Previous research shows that intestinal epithelial cells have developed specialized EECs, specifically enterochromaffin cells (51). EECs are distributed throughout the whole gastrointestinal tract and respond to various luminal stimuli by secreting hormones or neurotransmitters in a calcium-dependent mechanism (52). Therefore, the link between EECs and neurons establishes a direct pathway through which the intestinal epithelium can convey sensory information to the brain. Recent research has shown that bacterial TPH degradation metabolites, such as indole or Indole-3-acetaldehyde (IAld), activate the vagal ganglia through the EEC Trpa1 signaling pathway. Additionally, by measuring fresh tissue slices from the intestines of humans and mice exposed to indole, it was observed that indole significantly induced the secretion of 5-HT in both human and mouse intestines, while a Trpa1 inhibitor blocked this effect (53) (Fig. 1).
Inhibition of the activation of the Toll-like receptor 4 (TLR4)/NF-κB signaling pathway
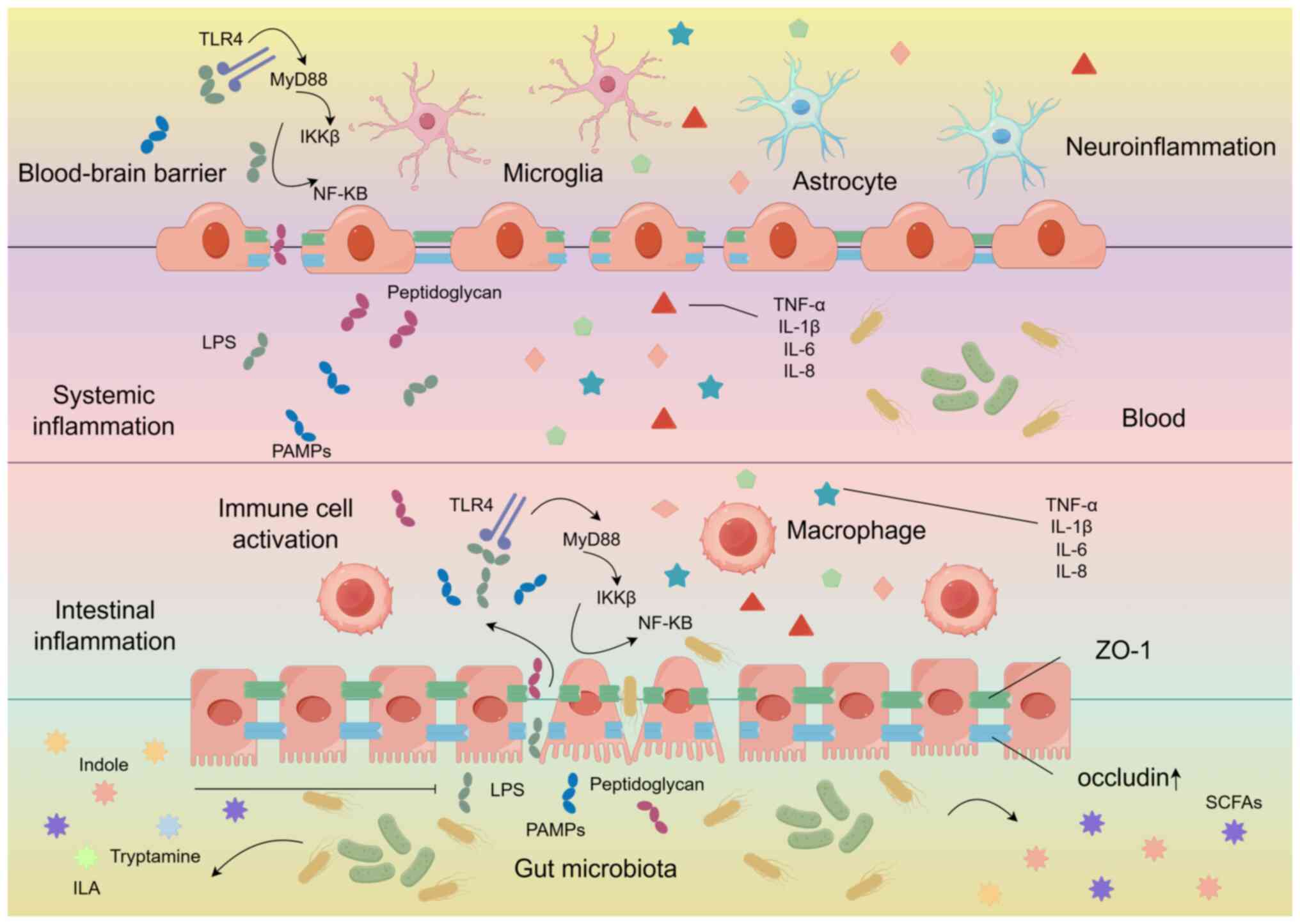
Previous research has shown that sleep deprivation (SD) may impair the function of the intestinal barrier (54), triggering oxidative stress and causing damage to the intestinal mucosa (55). The expression of tight junction proteins (occludin and ZO-1) in the intestinal tissue decreases, leading to an increase in intestinal permeability. After the disruption of the intestinal barrier, TLR4 recognizes lipopolysaccharide (LPS), it binds to myeloid differentiation factor 88, ultimately activating the IκB kinase complex. This leads to the degradation of IκB, the release of NF-κB, and the initiation of transcription of inflammatory genes, resulting in the transcription of pro-inflammatory factors IL-6, IL-1β and TNF-α (56), leading to an increase in their levels in both the brain and the intestine (57). IL-1β, TNF-α and IL-6 are the most extensively studied pro-sleep and pro-inflammatory cytokines that regulate sleep (24,58), and poor sleep quality is also positively associated with an increase in IL-8 (59).
In a previous study, the antioxidant fullerene nano-antioxidants (FNAO) improved the sleep of sleep-deprived zebrafish by regulating the redox balance in the intestine. In addition, in mice that received oral administration of FNAO, the levels of the tight junction protein occludin in the intestine increased, while the levels of the pro-inflammatory cytokines IL-6, IL-1β and TNF-α in the intestine decreased, significantly improving sleep in mice (57). All these findings indicate that pro-inflammatory immune response and oxidative stress in the intestine are closely related to sleep. Continuous disruption of the intestinal barrier leading to an increase in intestinal permeability; microbes, LPS, peptidoglycans and pathogen-associated molecular patterns can be recognized by peripheral macrophages; and this recognition promotes the release of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) into the bloodstream, thus triggering systemic inflammation (60,61). Moreover, These molecules then cross the intestinal barrier (IB) and BBB to reach the brain parenchyma, where they are exposed to microglia. This is an example of microbiome-derived microbial-associated molecular patterns. Microglia and astrocytes are activated through the TLR4-myeloid differentiation factor 88-NF-κB signaling pathway, thereby generating neuroinflammation that affects sleep (62-64). The suprachiasmatic nucleus (SCN) in the hypothalamus is the central regulator of human's circadian rhythm and is crucial for controlling the metabolic rhythm in mice (65). Extensive evidence shows that pro-inflammatory mediators inhibit the expression of clock genes and their targets in both the SCN and peripheral tissues, affecting the biological clock and thus disrupting the circadian rhythm. In addition, microglia and astrocytes are the brain's primary immune and inflammatory cells, respectively, playing key roles in antigen presentation and the production of both pro-inflammatory and anti-inflammatory factors (66,67). Chronic SD activates these cells, indicating that they could serve as potential targets for reducing neuroinflammation and oxidative stress in the brain following SD (68). In a previous study reporting both the melatonin supplementation and butyrate supplementation groups reversed the changes in neuroinflammation and cell apoptosis induced by SD (69). The levels of Iba1-positive cells, as well as IL-6 and TNF-α in the CA1, CA3 and dentate gyrus regions of the hippocampus were significantly lower compared to those in the sleep deprivation group. This is likely related to the inhibition of the activation of NF-κB in intestinal cells, which partially suppresses the production of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6, as well as the inhibition of microglial activation (70-72).
In addition, various TPH metabolites produced by the gut microbiota, such as indole and indole-3-acetic acid can significantly weaken the activation of NF-κB in macrophages induced by LPS. It can also significantly reduce the increase of pro-inflammatory cytokines in intestinal epithelial cells induced by TNF-α and LPS, as well as decrease the activation of IL-6 and IL-1β in cells stimulated by LPS (Fig. 2) (73).
Influencing CNS inflammation via signaling of aromatic hydrocarbon receptors
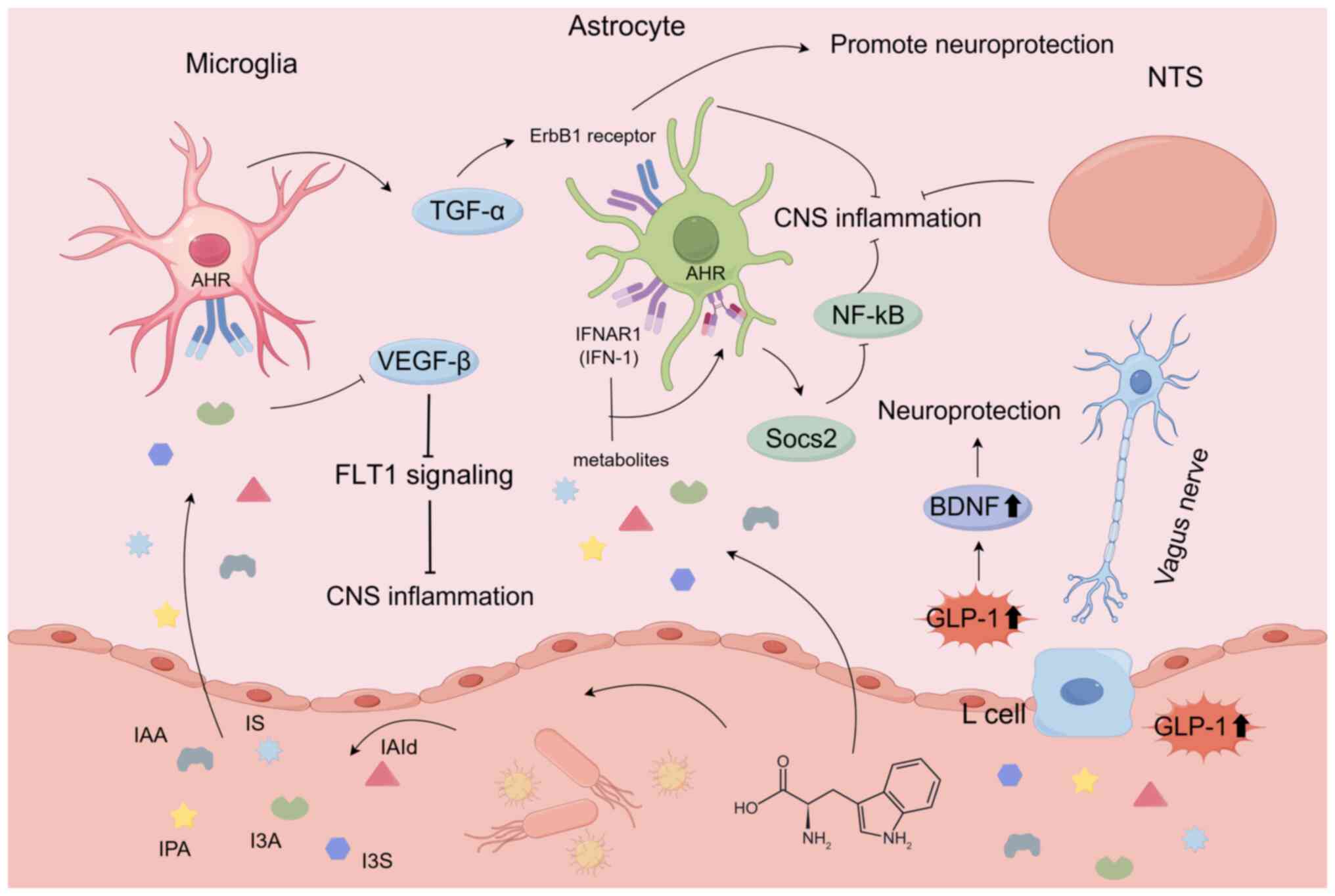
Aromatic hydrocarbon receptors (AHR) act as a key mediator in brain signaling pathways for TPH metabolites. In recent years, bacterial TPH metabolites have been extensively studied as ligands for AHR, a transcription factor that is commonly found in immune system cells. A study has indicated that activated AHR can modulate innate and adaptive immune responses in a ligand-specific manner (74). It is associated with various chronic diseases, particularly inflammatory conditions. TPH metabolites derived from the gut microbiota, such as indole-3-sulfonic acid, indole-3-acetic acid (IAA), indoxyl sulfate (IS), indole-3-propionic acid (IPA), indole-3-aldehyde (I3A) and IAld, can activate AHR (75). TPH metabolites, including IAA, IS, IPA, I3A and IAld, transmit signals through astrocytic AHR, activating TGF-α or inhibiting vascular endothelial growth factor (VEGF)-β (76,77). TGF-α derived from microglia exerts neuroprotective effects through the epidermal growth factor (ErbB1) receptor in astrocytes. On the other hand, VEGF-B produced by microglia and other sources, when activating Flt-1 in astrocytes, can promote CNS inflammation (78). Type-I interferon (IFN-I) signaling in astrocytes collaborates with TPH microbial metabolites to activate AHR (79). Activated AHR subsequently suppresses the activation of NF-κB by inducing the expression of suppressor of cytokine signaling 2 (Socs2) through cytokine signaling (80). In addition, AHR plays a key role in blocking the inflammatory and neurotoxic effects of IFN-α receptor 1 (IFNAR-1) (79). Therefore, the IFN-I-AHR-Socs2-NF-κB pathway indicates that targets interferon α and β receptor subunit 1 signaling could potentially be used to treat CNS inflammation (Fig. 3).
Bidirectional regulation of glucagon-like peptide-1 (GLP-1)
Microbial-derived indole metabolites can bidirectionally regulate the release of the appetite hormone GLP-1 (81). GLP-1, when present in the CNS, shows potential neuroprotective effects and helps improve neuronal survival. Specifically, when exposed to physiological levels of indole, GLP-1 secretion from colonic enteroendocrine L cells increases (82). GLP-1 can enhance brain-derived neurotrophic factor (BDNF) in the brain (83). A previous study investigated the role of BDNF in cognition, inflammation and neurodegenerative diseases (84). The role of GLP-1 in inhibiting CNS cell apoptosis, promoting neuronal growth, reducing cell proliferation and decreasing oxidative damage has been revealed (85). Indole interacts with enteroendocrine L cells, leading to the release of GLP-1. Before being released by the intestine, GLP-1 is rapidly deactivated by dipeptidyl peptidase-4. It has been speculated that GLP-1 acts locally on the terminals of the vagal afferent nerves (86). Based on the input from the VN, GLP-1 then signals to brain circuits and the nucleus tractus solitarius (NTS). The NTS can project to multiple sleep-regulating brain regions impact sleep (Fig. 3) (87).
Affects circadian rhythm
The biological clock is a key timer in the interaction between the host and microbiota. It is a 24 h biological oscillator that coordinates changes in behavior and physiology to anticipate environmental fluctuations within the 24 h day-night cycle. The biological clock is primarily controlled by the SCN in the hypothalamus, which is influenced by light-dark cycles, sleep-wake patterns and feeding rhythms. The SCN, in turn, coordinates peripheral clocks, regulating tissue-specific clock-controlled genes. The gut microbiome's daily rhythms synchronize with those of distant tissues and organs, including the gastrointestinal tract and liver, as well as key physiological processes. By integrating both nutritional and hormonal elements, this process modulates gene expression and regulates the biological rhythm of the host (88,89).
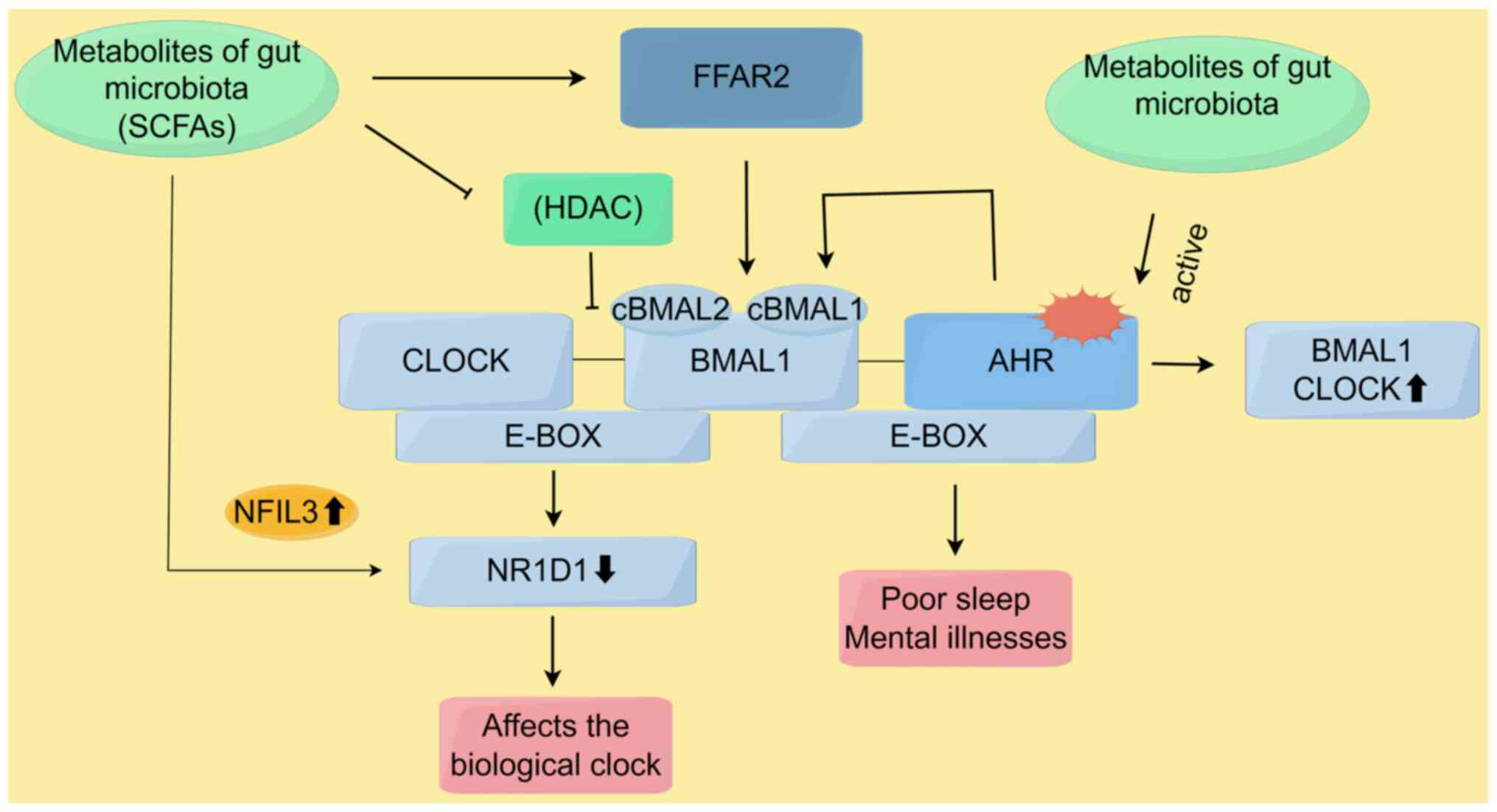
In birds, the circadian rhythm regulation system includes positive clock genes such as circadian locomotor output cycles kaput (CLOCK) and cBMAL1 (cBMAL1 and cBMAL2), as well as negative clock genes such as CPER (CPER2 and CPER3) and cCRY (cCRY1 and cCRY2) (90). Disturbances in the gut microbiome's circadian rhythms and its metabolites may influence the central biological clock, as well as the rhythmic expression of the aforementioned genes in the liver and gastrointestinal tract (91). The regulation of host biological clock gene expression is primarily mediated by the gut microbiome through its derived metabolites, including SCFAs and their receptor genes, such as free fatty acid receptor 2 (FFAR2) and FFAR3 (92).
Previous research showed that an intermittent light cycle enhanced the circadian rhythms of cBMAL1, cBMAL2, cCRY1 and cCRY2 in the hypothalamus, increased the expression of cCLOCK, cBMAL1 and cCRY2 in the liver, and upregulated the expression of seven clock genes (including cBMAL1 and FFAR2) in the cecal wall. Such intermittent light cycle also significantly altered the composition and metabolic function of the cecal microbiome via the melatonin pathway. Under intermittent light cycle treatment, the concentrations of SCFAs and the abundance of SCFA-producing genera, such as Odoribacter splanchnicus, were significantly increased. Correlation analysis indicated a positive correlation between the presence of Enterococcus and the expression of cBMAL1 and FFAR2 in the cecal wall, as well as cBMAL2 expression in the hypothalamus. cBMAL2 expression in the hypothalamus showed a clear circadian rhythm under an intermittent light cycle (93). It was therefore hypothesized that SCFAs may further feedback and enhance peripheral and central rhythms by activating SCFA receptor genes (such as FFAR2) in the cecal wall.
In addition, in the positive feedback loop of the circadian clock, CLOCK and BMAL1 proteins promote the activation of downstream genes by acetylating histones. Acetylation loosens chromatin, making the genes more accessible to transcription factors and RNA polymerase, which helps initiate transcription (94,95). Deacetylation is often carried out by histone deacetylases (HDACs), and cryptochrome (CRY) proteins maintain histone acetylation by inhibiting HDAC activity, thereby enhancing transcriptional activity. On the other hand, increased HDAC activity suppresses the expression of clock genes. Similarly, SCFAs such as butyrate, propionate and isovalerate strongly inhibit HDAC activity, increasing histone acetylation levels. After oral administration of SCFAs, the circadian rhythm phase in the peripheral tissues of mice changes (96). Using oscillation experiments with LUC-type intestinal cells over 72 h, a study revealed that acetate, butyrate, isovalerate and propionate induces phase delays. The expression of intestinal BMAL1-ELuc showed significant cyclical changes, which closely resembled the phase delay pattern induced by HDAC inhibitors such as trichostatin A and suberoylanilide hydroxamic acid (97). This study supported the idea that SCFAs and microbial metabolites alter the host's clock through HDAC inhibition.
Nuclear receptor subfamily 1 group D member 1 (NR1D1), also known as Rev-erb, is a core component of the molecular circadian clock, regulating the cellular circadian rhythm. A previous study found that, in mice and human submandibular gland cells treated with butyrate, the number of NR1D1-positive cells and the expression level of NR1D1 decreased. As a result, NFIL3, which is negatively regulated by NR1D1, showed increased expression levels under NR1D1 inhibition. Notably, the expression level of nuclear factor, interleukin 3 regulated (NFIL3) was elevated in butyrate-treated cells (98).
Indole can act as an agonist to induce the activation of AHR. Depending on their molecular structure, TPH metabolites can function as either agonists or antagonists to influence AHR (99). The gut microbiota serves as a source of different AHR signals, and evidence suggests that the gut microbiome can influence the circadian rhythm of the host (45). The genes involved in the circadian rhythm are known as 'clock genes', including BMAL1 CLOCK, neuronal PAS domain protein 2 and NR1D1. The interaction between CLOCK/BMAL1 heterodimer and period homolog and CRY proteins is the main feedback loop driving the circadian rhythm. AHR is a PAS domain protein, and BMAL1/CLOCK proteins can form heterodimers with AHR (100). This interaction can disrupt the oligomerization of core clock proteins and impair their transcriptional activation, leading to a disruption of the circadian rhythm (101). Evidence suggests that activation of AHR signaling inhibits the expression of circadian clock genes and, therefore, impairs the circadian rhythm in various experimental models. These studies demonstrate that AHR can function as an inhibitory factor of the circadian rhythm in central and peripheral clocks (Fig. 4) (102).
Impact on BBB integrity and permeability
A previous study found that the BBB is essential for maintaining the homeostasis of nutrients, ions and other molecules in the brain. Its permeability is regulated by SD and can independently influence sleep changes (103). TPH can be broken down via the 5-HT and kynurenine pathways, and is closely associated with the CNS. TPH can enter the CNS through L-type amino acid transporter 1 to cross the BBB (104). In addition, the positive impact of SCFA-producing gut microbiota on BBB integrity has been demonstrated in germ-free mouse models and in mice treated with antibiotics that alter the abundance of specific bacterial families. In mice treated with five non-absorbable antibiotics, the mRNA levels of occludin and ZO-1 in brain microvessels were reduced, while BBB permeability increased. These antibiotics reduced the relative abundance of SCFA-producing bacteria in the gut. However, after fecal microbiota transplantation from pathogen-free gut microbiota to antibiotic-treated mice, the mice restored tight junction protein expression and BBB integrity (105). Another study also showed that, in mice treated with an antibiotic mixture, the levels of acetate, propionate and butyrate in the colon were reduced, along with a decline in object recognition memory. Additionally, the mRNA expression levels of claudin-5 and occludin in the amygdala and hippocampus decreased (106). Similar results were obtained in mice undergoing anesthesia/surgery treated with an antibiotic mixture. Administration of a Lactobacillus bacterial mixture restored the expression levels of claudin-5, occludin and ZO-1 in the hippocampus, and improved BBB permeability (107). These experiments suggest that SCFAs produced by the microbiome play a key role in restoring and maintaining BBB integrity.
Effects of other microbial metabolites on sleep
Trimethylamine N-oxide (TMAO)
TMAO is a colorless, odorless, naturally occurring osmolyte classified as an amine oxide, and is considered an important gut microbiome-derived metabolite. Trimethylamine (TMA) is produced by gut microbes through the metabolism of dietary nutrients such as choline, betaine and carnitine. Once formed, TMA is oxidized to TMAO by the action of TMA N-oxidase, an enzyme expressed in various gut microbiota (108). The intake of dietary sources such as eggs, dairy products, red meat, mushrooms, beans, almonds, milk and saltwater fish, which are rich in substrates such as choline, carnitine and betaine, has been positively linked to elevated circulating levels of TMAO (109).
TMAO is involved in various physiological and biochemical functions. For instance, it protects intracellular constituents from osmotic stress, hyperammonemia and glutamate-induced neurotoxicity, while also mitigating endoplasmic reticulum stress (110). Additionally, TMAO can potentially impair the BBB by diminishing the levels of tight junction proteins such as claudin-5 and occludin (111). However, the exact mechanism by which TMAO crosses the BBB remains unclear. Moreover, TMAO also mediates neuroinflammation. A previous study showed that, compared to young adult mice (6 months old), aged mice (27 months old) with elevated TMAO levels exhibited increased levels of pro-inflammatory cytokines and markers of astrocyte activation. Obstructive sleep apnea (OSA) is a chronic and highly prevalent condition characterized by the repeated partial or complete obstruction of the upper airway during sleep, leading to intermittent hypoxia (IH). Previous research has found that changes in the gut microbiome promote increases TMAO levels in IH-fecal microbiota transplantation mice (112). However, there is a lack of more direct evidence linking TMAO with OSA.
Ergothioneine
Ergothioneine is a sulfur-containing histidine derivative synthesized by numerous bacteria and the majority of fungi. It can also be absorbed from specific dietary sources into human tissues (113). Mushrooms, particularly Lentinula edodes (shiitake) and Boletus edulis (porcini), are the richest dietary sources of ergothioneine. Other relatively good sources include animal liver, legumes, oats and certain seafood (114). Previous research has suggested that ergothioneine, a product of Lactobacillus rogosae metabolism, may be a prevalent component within the microbiota-gut-brain pathway, potentially preventing stress-induced sleep disorders, particularly those associated with depression (115). Ergothioneine accumulates in considerable quantities (100 μM-2 mM) within most cells and tissues in mammals, including the nervous system, the key transporter for its accumulation in cells and tissues is the carnitine/organic cation transporter 1 (OCTN1), which is encoded by the SLC22A4 gene (116). Since the ileum expresses OCTN1 most abundantly, dietary ergothioneine is considered to be primarily absorbed in the ileum and then crosses the BBB to enter the brain (115). In an experiment using a 14-day social defeat stress (SDS) depression rat model, it was found that SDS induced significant sleep abnormalities, such as increased rapid eye movement (REM) sleep duration, shortened REM sleep latency and increased sleep fragmentation. L-ergothioneine administered orally demonstrated a substantial alleviation of REM sleep irregularities, with the exception of REM latency duration. Additionally, in non-rapid eye movement (NREM) sleep, SDS significantly shortened its duration and increased the number of fragments, which was also improved by L-ergothioneine treatment (117). The mechanism by which L-ergothioneine improves sleep may be related to its anti-inflammatory and antioxidant effects.
Hydrogen sulfide (H2S)
H2S, a substance generated by both the host's cells and the gut microbiota, is primarily produced in the colon by sulfate-reducing microbes and bacteria that degrade cysteine, with the latter being more prevalent in the microbiome (118). A previous study demonstrated that SD leads to changes in hippocampal synaptic and membrane excitability, indicating that SD affects hippocampal damage, resulting in cognitive impairment (119). Previous research found that sodium hydrosulfide (a donor of H2S) alleviated SD-generated hippocampal oxidative stress, and H2S mitigated SD-induced hippocampal injury through the enhancement of Sirt1 expression in the hippocampus, and suppressed neuronal apoptosis in rats exposed to homocysteine (120). Additionally, H2S has been found to exert an antagonistic effect on SD-induced depressive-like behaviors through the mediation of Sirt1 (121).
Bile acid (BA) metabolites
Bile acids (BAs) are cholesterol-derived steroids that can affect the CNS both directly and indirectly. Two primary BAs, cholic acid and chenodeoxycholic acid are synthesized in the liver, where they are conjugated with glycine or taurine before being secreted into bile. After eating, BAs are released into the small intestine, where the majority (95%) are reabsorbed. A small portion of BAs that are not reabsorbed reach the colon, where they are metabolized by microbes into secondary BAs such as deoxycholic acid (DCA) and ursodeoxycholic acid (122). Previous research has shown that chronic insomnia may significantly affect the gut microbiota-BA axis. It has been demonstrated that repeated sleep disruption in mice leads to changes in the gut microbiota's BA metabolism. Gene analysis suggested a decrease in the abundance of microbial bile salt hydrolase (BSH) genes in the microbiome of sleep-disrupted mice. BSH is a key enzyme that catalyzes the first step in microbial BA metabolism. Reduced microbial BSH levels, resulting in a decreased fecal BA pool, may contribute to host inflammation and metabolic dysregulation, ultimately impacting sleep (123). A recent study showed that DCA may improve the gut microbiota homeostasis disrupted by SD through its effective antibacterial activity, as well as its synergistic effect with antibiotics on bacteria. However, the combination of DCA and ciprofloxacin downregulated the expression of genes such as metB, malY and cysK, which are responsible for catalyzing the production of H2S in E. coli. This reduced the bacteria's ability to produce H2S (124). These factors may contribute to the mechanism by which DCA affects sleep.
Polyamines
A study indicated that nitric oxide (NO), neuronal nitric oxide synthase, inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase can increase sleep (125). A microdialysis experiment indicated that SD increases iNOS and NO levels in the frontal cortex and basal forebrain of rats (126). Mice deficient in iNOS exhibit reduced spontaneous NREM sleep (127). These findings illustrate the connection between sleep and the nitrogen cycle.
Previous research has shown that SD increases the adenosine levels in the basal forebrain of rats, which is considered one of the mechanisms regulating sleep homeostasis (128). A previous study found that the adenosine levels in the basal forebrain were elevated during SD in control and hyperammonemic animals, with a more significant increase in hyperammonemic rats. These rats exhibited shorter and more fragmented recovery sleep responses (129). Foods rich in amino acids, such as meat, fish, poultry and grains, can help generate higher levels of polyamines, including putrescine and spermidine. These essential metabolites are derived from amino acids such as ornithine and methionine. The gut microbiota produces polyamines in the intestinal lumen, particularly in the colon, where they can be absorbed by intestinal epithelial cells (130). Although polyamines have limited transport across the BBB, spermidine has been shown to cross the BBB and improve cognition in mice by enhancing mitochondrial function in the hippocampus (131). Polyamine synthesis requires a large quantity of nitrogen. A previous study showed that increased polyamine levels, particularly putrescine, promoted sleep in control fruit flies, possibly by triggering nitrogen stress and promoting nitrogen homeostasis (132).
Conclusions and future perspectives
The present review has explored the connection between gut microbiota metabolites and sleep disorders, with a particular focus on the effects of TPH-based metabolites and SCFAs on sleep. AHR has a dual role in circadian regulation, which may be due to the fact that the activation of downstream pathways by AHR is influenced by ligand affinity, cell type and other environmental factors. The dose-dependent pattern of SCFAs implies a delicate balance in the regulation of serotonin transporter activity, highlighting the complexity of the effects of SCFAs on sleep. The mechanisms by which gut microbiota affects sleep are complex, and, since sleep disorders are subjective, it is difficult to clearly measure sleep improvement using objective indicators. Future research should provide more insights into sleep behavior. There is an ongoing debate about the source of brain-affecting gut microbiota metabolites, as the host body can also produce some of these metabolites. It is difficult to determine whether these metabolites derive from the gut microbiota or from the host itself, and future research should use improved methods to study this. Gut microbiota metabolites are closely related to the diet, with numerous metabolites deriving from nutrients in food, such as TPH, dietary fiber, betaine, sulfur-containing histidine and cholesterol. It is necessary to expand research on the association between gut microbiota metabolites and sleep disorders to provide improved dietary guidance for patients with sleep problems. The brain-gut axis theory suggests that gut microbiota may be a potential treatment target for sleep disorders. However, no approved drugs currently exist that can correct sleep and wakefulness issues caused by gut microbiota imbalances. Additionally, due to the various side effects of prescription sleeping pills, there is still a high demand for new natural products that improve sleep. Certain phytochemicals may have sedative and hypnotic effects through the brain-gut axis, and could be useful for treating sleep disorders (Table I).
However, research on gut microbiota metabolites and sleep disorders is still in its early stages, possibly due to difficulties in obtaining certain metabolites. In recent years, with increased studies on circadian rhythms and clock genes, the diurnal variations in the abundance and function of gut microbiota and their metabolites have drawn important attention, offering new approaches for treating patients with circadian rhythm disorders. Expanding the current knowledge on the gut microbiota metabolite-brain-sleep connection is essential for developing gut microbiota-based interventions for sleep disorders and providing dietary guidance to patients.
Availability of data and materials
Not applicable.
Authors' contributions
HC, WY and HuX were responsible for the overall conceptualization and writing of the study. ZW, AG and YX were tasked with drafting key content. SL and HoX oversaw the critical review of key content and coordinated the study. All authors read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
Not applicable.
Funding
This study was funded by Southwest Medical University Technology Program (grant no. 2023XGZX011), Peng Zhou People's Hospital-Southwest Medical University Cooperation Program (grant no. 2023PZXNYD09), People's Government of Luzhou City-Southwest Medical University Science and Technology Strategic Cooperation Project (grant no. 2021LZXNYD-D14) and Administration of Traditional Chinese Medicine of Sichuan Province (grant no. 2024zd032).
References
|
Lund HG, Reider BD, Whiting AB and Prichard JR: Sleep patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Health. 46:124–132. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Bishir M, Bhat A, Essa MM, Ekpo O, Ihunwo AO, Veeraraghavan VP, Mohan SK, Mahalakshmi AM, Ray B, Tuladhar S, et al: Sleep deprivation and neurological disorders. Biomed Res Int. 2020:57640172020. View Article : Google Scholar : | |
|
Palagini L, Hertenstein E, Riemann D and Nissen C: Sleep, insomnia and mental health. J Sleep Res. 31:e136282022. View Article : Google Scholar : PubMed/NCBI | |
|
Chang L, Wei Y and Hashimoto K: Brain-gut-microbiota axis in depression: A historical overview and future directions. Brain Res Bull. 182:44–56. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Gossard TR, Trotti LM, Videnovic A and St Louis EK: Restless legs syndrome: Contemporary diagnosis and treatment. Neurotherapeutic. 18:140–155. 2021. View Article : Google Scholar | |
|
Sun SY and Chen GH: Treatment of circadian rhythm sleep-wake disorders. Curr Neuropharmacol. 20:1022–1034. 2022. View Article : Google Scholar | |
|
Arnulf I, Thomas R, Roy A and Dauvilliers Y: Update on the treatment of idiopathic hypersomnia: Progress, challenges, and expert opinion. Sleep Med Rev. 69:1017662023. View Article : Google Scholar : PubMed/NCBI | |
|
You M, Chen N, Yang Y, Cheng L, He H, Cai Y, Liu Y, Liu H and Hong G: The gut microbiota-brain axis in neurological disorders. MedComm (2020). 5:e6562024. View Article : Google Scholar : PubMed/NCBI | |
|
Haarhuis JE, Kardinaal A and Kortman GAM: Probiotics, prebiotics and postbiotics for better sleep quality: A narrative review. Benef Microbes. 13:169–182. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Singh V, Yeoh BS, Chassaing B, Xiao X, Saha P, Aguilera Olvera R, Lapek JD Jr, Zhang L, Wang WB, Hao S, et al: Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer. Cell. 175:679–694.e22. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Barrett E, Ross RP, O'Toole PW, Fitzgerald GF and Stanton C: γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 113:411–417. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Chen H, Nwe PK, Yang Y, Rosen CE, Bielecka AA, Kuchroo M, Cline GW, Kruse AC, Ring AM, Crawford JM and Palm NW: A forward chemical genetic screen reveals gut microbiota metabolites that modulate host physiology. Cell. 177:1217–1231.e18. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Ren Z, Zhang R, Li Y, Li Y, Yang Z and Yang H: Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int J Mol Med. 40:1444–1456. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Verzelloni E, Pellacani C, Tagliazucchi D, Tagliaferri S, Calani L, Costa LG, Brighenti F, Borges G, Crozier A, Conte A and Del Rio D: Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol Nutr Food Res. 55(Suppl 1): S35–S43. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Mao JH, Kim YM, Zhou YX, Hu D, Zhong C, Chang H, Brislawn CJ, Fansler S, Langley S, Wang Y, et al: Genetic and metabolic links between the murine microbiome and memory. Microbiome. 8:532020. View Article : Google Scholar : PubMed/NCBI | |
|
Ahmed H, Leyrolle Q, Koistinen V, Kärkkäinen O, Layé S, Delzenne N and Hanhineva K: Microbiota-derived metabolites as drivers of gut-brain communication. Gut Microbes. 14:21028782022. View Article : Google Scholar : PubMed/NCBI | |
|
Mann ER, Lam YK and Uhlig HH: Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat Rev Immunol. 24:577–595. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Agus A, Clément K and Sokol H: Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 70:1174–1182. 2021. View Article : Google Scholar | |
|
Ganz J: Revealing the complexity of the gut's brain. Nat Neurosci. 24:1–2. 2021. View Article : Google Scholar | |
|
Margolis KG, Cryan JF and Mayer EA: The microbiota-gut-brain axis: From motility to mood. Gastroenterology. 160:1486–1501. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Niesler B, Kuerten S, Demir IE and Schäfer KH: Disorders of the enteric nervous system-a holistic view. Nat Rev Gastroenterol Hepatol. 18:393–410. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Bonaz B, Bazin T and Pellissier S: The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 12:492018. View Article : Google Scholar : PubMed/NCBI | |
|
Agirman G, Yu KB and Hsiao EY: Signaling inflammation across the gut-brain axis. Science. 374:1087–1092. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Zielinski MR and Gibbons AJ: Neuroinflammation, sleep, and circadian rhythms. Front Cell Infect Microbiol. 12:8530962022. View Article : Google Scholar : PubMed/NCBI | |
|
Grigg JB and Sonnenberg GF: Host-microbiota interactions shape local and systemic inflammatory diseases. J Immunol. 198:564–571. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Salvo-Romero E, Stokes P and Gareau MG: Microbiota-immune interactions: From gut to brain. Lymphosign J. 7:1–23. 2020. View Article : Google Scholar | |
|
Zheng D, Liwinski T and Elinav E: Interaction between microbiota and immunity in health and disease. Cell Res. 30:492–506. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Averina OV, Zorkina YA, Yunes RA, Kovtun AS, Ushakova VM, Morozova AY, Kostyuk GP, Danilenko VN and Chekhonin VP: Bacterial metabolites of human gut microbiota correlating with depression. Int J Mol Sci. 21:92342020. View Article : Google Scholar : PubMed/NCBI | |
|
Parker A, Fonseca S and Carding SR: Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes. 11:135–157. 2020. View Article : Google Scholar : | |
|
Dalile B, Van Oudenhove L, Vervliet B and Verbeke K: The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 16:461–478. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Gibson JA, Sladen GE and Dawson AM: Protein absorption and ammonia production: The effects of dietary protein and removal of the colon. Br J Nutr. 35:61–65. 1976. View Article : Google Scholar : PubMed/NCBI | |
|
Tan JK, Macia L and Mackay CR: Dietary fiber and SCFAs in the regulation of mucosal immunity. J Allergy Clin Immunol. 151:361–370. 2023. View Article : Google Scholar | |
|
Fock E and Parnova R: Mechanisms of blood-brain barrier protection by microbiota-derived short-chain fatty acids. Cells. 12:6572023. View Article : Google Scholar : PubMed/NCBI | |
|
Ikeda T, Nishida A, Yamano M and Kimura I: Short-chain fatty acid receptors and gut microbiota as therapeutic targets in metabolic, immune, and neurological diseases. Pharmacol Ther. 239:1082732022. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Shao L, Mou Y, Zhang Y and Ping Y: Sleep, circadian rhythm and gut microbiota: Alterations in Alzheimer's disease and their potential links in the pathogenesis. Gut Microbes. 13:19574072021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu R, Fang Y, Li H, Liu Y, Wei J, Zhang S, Wang L, Fan R, Wang L, Li S and Chen T: Psychobiotic Lactobacillus plantarum JYLP-326 relieves anxiety, depression, and insomnia symptoms in test anxious college via modulating the gut microbiota and its metabolism. Front Immunol. 14:11581372023. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Zhang B, Zhou Y, Wang D, Liu X, Li L, Wang T, Zhang Y, Jiang M, Tang H, et al: Gut microbiota changes and their relationship with inflammation in patients with acute and chronic insomnia. Nat Sci Sleep. 12:895–905. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Shimizu Y, Yamamura R, Yokoi Y, Ayabe T, Ukawa S and Nakamura K, Okada E, Imae A, Nakagawa T, Tamakoshi A and Nakamura K: Shorter sleep time relates to lower human defensin 5 secretion and compositional disturbance of the intestinal microbiota accompanied by decreased short-chain fatty acid production. Gut Microbes. 15:21903062023. View Article : Google Scholar : PubMed/NCBI | |
|
Zuraikat FM, Wood RA, Barragán R and St-Onge MP: Sleep and diet: Mounting evidence of a cyclical relationship. Annu Rev Nutr. 41:309–332. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC and Siuzdak G: Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 106:3698–3703. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Dicks LMT: Gut bacteria and neurotransmitters. Microorganisms. 10:18382022. View Article : Google Scholar : PubMed/NCBI | |
|
Liu N, Sun S, Wang P, Sun Y, Hu Q and Wang X: The mechanism of secretion and metabolism of gut-derived 5-hydroxytryptamine. Int J Mol Sci. 22:79312021. View Article : Google Scholar : PubMed/NCBI | |
|
Gao K, Mu CL, Farzi A and Zhu WY: Tryptophan metabolism: A link between the gut microbiota and brain. Adv Nutr. 11:709–723. 2020. View Article : Google Scholar : | |
|
Xie Y, Wang C, Zhao D, Wang C and Li C: Dietary proteins regulate serotonin biosynthesis and catabolism by specific gut microbes. J Agric Food Chem. 68:5880–5890. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Parkar SG, Kalsbeek A and Cheeseman JF: Potential role for the gut microbiota in modulating host circadian rhythms and metabolic health. Microorganisms. 7:412019. View Article : Google Scholar : PubMed/NCBI | |
|
Gershon MD and Tack J: The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology. 132:397–414. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Latorre E, Mendoza C, Matheus N, Castro M, Grasa L, Mesonero JE and Alcalde AI: IL-10 modulates serotonin transporter activity and molecular expression in intestinal epithelial cells. Cytokine. 61:778–784. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Buey B, Forcén A, Grasa L, Layunta E, Mesonero JE and Latorre E: Gut microbiota-derived short-chain fatty acids: Novel regulators of intestinal serotonin transporter. Life (Basel). 13:10852023.PubMed/NCBI | |
|
Cai J, Cheung J, Cheung SWM, Chin KTC, Leung RWK, Lam RST, Sharma R, Yiu JHC and Woo CW: Butyrate acts as a positive allosteric modulator of the 5-HT transporter to decrease availability of 5-HT in the ileum. Br J Pharmacol. 181:1654–1670. 2024. View Article : Google Scholar | |
|
Dicks LMT: Our mental health is determined by an intrinsic interplay between the central nervous system, enteric nerves, and gut microbiota. Int J Mol Sci. 25:382023. View Article : Google Scholar | |
|
Wei L, Singh R and Ghoshal UC: Enterochromaffin cells-gut microbiota crosstalk: Underpinning the symptoms, pathogenesis, and pharmacotherapy in disorders of gut-brain interaction. J Neurogastroenterol Motil. 28:357–375. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Guo X, Yin C, Yang F, Zhang Y, Huang H, Wang J, Deng B, Cai T, Rao Y and Xi R: The cellular diversity and transcription factor code of Drosophila enteroendocrine cells. Cell Rep. 29:4172–4185.e5. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Ye L, Bae M, Cassilly CD, Jabba SV, Thorpe DW, Martin AM, Lu HY, Wang J, Thompson JD, Lickwar CR, et al: Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe. 29:179–196.e9. 2021. View Article : Google Scholar : | |
|
Gao T, Wang Z, Cao J, Dong Y and Chen Y: Melatonin alleviates oxidative stress in sleep deprived mice: Involvement of small intestinal mucosa injury. Int Immunopharmacol. 78:1060412020. View Article : Google Scholar | |
|
Gao T, Wang Z, Dong Y, Cao J, Lin R, Wang X, Yu Z and Chen Y: Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J Pineal Res. 67:e125742019. View Article : Google Scholar : PubMed/NCBI | |
|
Yu H, Lin L, Zhang Z, Zhang H and Hu H: Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct Target Ther. 5:2092020. View Article : Google Scholar | |
|
Wu Z, Liu L, Li L, Cao X, Jia W, Liao X, Zhao Z, Qi H, Fan G, Lu H, et al: Oral nano-antioxidants improve sleep by restoring intestinal barrier integrity and preventing systemic inflammation. Natl Sci Rev. 10:nwad3092023. View Article : Google Scholar | |
|
Veler H: Sleep and inflammation: Bidirectional relationship. Sleep Med Clin. 18:213–218. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Yang Y, Gu K, Meng C, Li J, Lu Q, Zhou X, Yan D, Li D, Pei C, Lu Y, et al: Relationship between sleep and serum inflammatory factors in patients with major depressive disorder. Psychiatry Res. 329:1155282023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Z, Chen WH, Li SX, He ZM, Zhu WL, Ji YB, Wang Z, Zhu XM, Yuan K, Bao YP, et al: Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol Psychiatry. 26:6277–6292. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Su H, Zhang C, Zou X, Lu F, Zeng Y, Guan H, Ren Y, Yuan F, Xu L, Zhang M and Dong H: Jiao-tai-wan inhibits inflammation of the gut-brain-axis and attenuates cognitive impairment in insomnic rats. J Ethnopharmacol. 250:1124782020. View Article : Google Scholar | |
|
Hergenhan S, Holtkamp S and Scheiermann C: Molecular interactions between components of the circadian clock and the immune system. J Mol Biol. 432:3700–3713. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao Z, Ning J, Bao XQ, Shang M, Ma J, Li G and Zhang D: Fecal microbiota transplantation protects rotenone-induced Parkinson's disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome. 9:2262021. View Article : Google Scholar : PubMed/NCBI | |
|
McCuaig B and Goto Y: Immunostimulating commensal bacteria and their potential use as therapeutics. Int J Mol Sci. 24:156442023. View Article : Google Scholar : PubMed/NCBI | |
|
Mohawk JA, Green CB and Takahashi JS: Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 35:445–462. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Prinz M, Jung S and Priller J: Microglia biology: One century of evolving concepts. Cell. 179:292–311. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Horng S, Therattil A, Moyon S, Gordon A, Kim K, Argaw AT, Hara Y, Mariani JN, Sawai S, Flodby P, et al: Astrocytic tight junctions control inflammatory CNS lesion pathogenesis. J Clin Invest. 127:3136–3151. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Gudkov SV, Burmistrov DE, Kondakova EV, Sarimov RM, Yarkov RS, Franceschi C and Vedunova MV: An emerging role of astrocytes in aging/neuroinflammation and gut-brain axis with consequences on sleep and sleep disorders. Ageing Res Rev. 83:1017752023. View Article : Google Scholar | |
|
Wang X, Wang Z, Cao J, Dong Y and Chen Y: Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome. 11:172023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang L, Ko CY and Zeng YM: Immunoregulatory effect of short-chain fatty acids from gut microbiota on obstructive sleep apnea-associated hypertension. Nat Sci Sleep. 14:393–405. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Mowat AM and Agace WW: Regional specialization within the intestinal immune system. Nat Rev Immunol. 14:667–685. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Nastasi C, Candela M, Bonefeld CM, Geisler C, Hansen M, Krejsgaard T, Biagi E, Andersen MH, Brigidi P, Ødum N, et al: The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci Rep. 5:161482015. View Article : Google Scholar : PubMed/NCBI | |
|
Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T and Yin Y: Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol. 8:132018. View Article : Google Scholar : PubMed/NCBI | |
|
Szelest M, Walczak K and Plech T: A new insight into the potential role of tryptophan-derived AhR ligands in skin physiological and pathological processes. Int J Mol Sci. 22:11042021. View Article : Google Scholar : PubMed/NCBI | |
|
Sun M, Ma N, He T, Johnston LJ and Ma X: Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon receptor (AhR). Crit Rev Food Sci Nutr. 60:1760–1768. 2020. View Article : Google Scholar | |
|
Nicolas GR and Chang PV: Deciphering the chemical lexicon of host-gut microbiota interactions. Trends Pharmacol Sci. 40:430–445. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Xie L, Wu Q, Li K, Khan MAS, Zhang A, Sinha B, Li S, Chang SL, Brody DL, Grinstaff MW, et al: Tryptophan metabolism in Alzheimer's disease with the involvement of microglia and astrocyte crosstalk and gut-brain axis. Aging Dis. 15:2168–2190. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, de Lima KA, Gutiérrez-Vázquez C, Hewson P, Staszewski O, et al: Microglial control of astrocytes in response to microbial metabolites. Nature. 557:724–728. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao CC, Patel B, Yan R, Blain M, et al: Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 22:586–597. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Marsland BJ: Regulating inflammation with microbial metabolites. Nat Med. 22:581–583. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Wang P, Sun H, Maitiabula G, Zhang L, Yang J, Zhang Y, Gao X, Li J, Xue B, Li CJ and Wang X: Total parenteral nutrition impairs glucose metabolism by modifying the gut microbiome. Nat Metab. 5:331–348. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Chimerel C, Emery E, Summers DK, Keyser U, Gribble FM and Reimann F: Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 9:1202–1208. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Taati M, Barzegar PEF and Raisi A: Exercise improves spatial learning and memory performance through the central GLP-1 receptors. Behav Neurol. 2022:29006282022. View Article : Google Scholar : PubMed/NCBI | |
|
Budni J, Bellettini-Santos T, Mina F, Garcez ML and Zugno AI: The involvement of BDNF, NGF and GDNF in aging and Alzheimer's disease. Aging Dis. 6:331–341. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Athauda D and Foltynie T: Protective effects of the GLP-1 mimetic exendin-4 in Parkinson's disease. Neuropharmacology. 136:260–270. 2018. View Article : Google Scholar | |
|
van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG and Diamant M: Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol. 221:T1–T16. 2014. View Article : Google Scholar | |
|
Mir FA and Jha SK: The Kir channel in the nucleus tractus solitarius integrates the chemosensory system with REM sleep executive machinery for homeostatic balance. Sci Rep. 14:216512024. View Article : Google Scholar : PubMed/NCBI | |
|
Zheng D, Ratiner K and Elinav E: Circadian influences of diet on the microbiome and immunity. Trends Immunol. 41:512–530. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Kuang Z, Wang Y, Li Y, Ye C, Ruhn KA, Behrendt CL, Olson EN and Hooper LV: The intestinal microbiota programs diurnal rhythms in host metabolism through histone deacetylase 3. Science. 365:1428–1434. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Ni Y, Ma L, Wu T, Lim AL, Zhang W, Yang L, Nakao Y and Fu Z: The involvement of sympathetic nervous system in essence of chicken-facilitated physiological adaption and circadian resetting. Life Sci. 201:54–62. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Nobs SP, Tuganbaev T and Elinav E: Microbiome diurnal rhythmicity and its impact on host physiology and disease risk. EMBO Rep. 20:e471292019. View Article : Google Scholar : PubMed/NCBI | |
|
Thaiss CA, Levy M, Korem T, Dohnalová L, Shapiro H, Jaitin DA, David E, Winter DR, Gury-BenAri M, Tatirovsky E, et al: Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell. 167:1495–1510.e12. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Li Y, Yuan Y, Wang J, Zhang S, Zhu R, Wang Y, Wu Y, Liao X and Mi J: Reducing light exposure enhances the circadian rhythm of the biological clock through interactions with the gut microbiota. Sci Total Environ. 858:1600412023. View Article : Google Scholar | |
|
Singh K, Jha NK and Thakur A: Spatiotemporal chromatin dynamics-A telltale of circadian epigenetic gene regulation. Life Sci. 221:377–391. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Xiong L, Zhou W and Mas P: Illuminating the Arabidopsis circadian epigenome: Dynamics of histone acetylation and deacetylation. Curr Opin Plant Biol. 69:1022682022. View Article : Google Scholar : PubMed/NCBI | |
|
Tahara Y, Yamazaki M, Sukigara H, Motohashi H, Sasaki H, Miyakawa H, Haraguchi A, Ikeda Y, Fukuda S and Shibata S: Gut microbiota-derived short chain fatty acids induce circadian clock entrainment in mouse peripheral tissue. Sci Rep. 8:13952018. View Article : Google Scholar : PubMed/NCBI | |
|
Fawad JA, Luzader DH, Hanson GF, Moutinho TJ Jr, McKinney CA, Mitchell PG, Brown-Steinke K, Kumar A, Park M, Lee S, et al: Histone deacetylase inhibition by gut microbe-generated short-chain fatty acids entrains intestinal epithelial circadian rhythms. Gastroenterology. 163:1377–1390.e11. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Kim DS, Woo JS, Min HK, Choi JW, Moon JH, Park MJ, Kwok SK, Park SH and Cho ML: Short-chain fatty acid butyrate induces IL-10-producing B cells by regulating circadian-clock-related genes to ameliorate Sjögren's syndrome. J Autoimmun. 119:1026112021. View Article : Google Scholar | |
|
Jin UH, Lee SO, Sridharan G, Lee K, Davidson LA, Jayaraman A, Chapkin RS, Alaniz R and Safe S: Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol. 85:777–788. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Tischkau SA: Mechanisms of circadian clock interactions with aryl hydrocarbon receptor signalling. Eur J of Neurosci. 51:379–395. 2020. View Article : Google Scholar | |
|
Salminen A: Aryl hydrocarbon receptor (AhR) impairs circadian regulation: Impact on the aging process. Ageing Res Rev. 87:1019282023. View Article : Google Scholar : PubMed/NCBI | |
|
Petrus P, Cervantes M, Samad M, Sato T, Chao A, Sato S, Koronowski KB, Park G, Alam Y, Mejhert N, et al: Tryptophan metabolism is a physiological integrator regulating circadian rhythms. Mol Metab. 64:1015562022. View Article : Google Scholar : PubMed/NCBI | |
|
Axelrod S, Li X, Sun Y, Lincoln S, Terceros A, O'Neil J, Wang Z, Nguyen A, Vora A, Spicer C, et al: The Drosophila blood-brain barrier regulates sleep via Moody G protein-coupled receptor signaling. Proc Natl Acad Sci USA. 120:e23093311202023. View Article : Google Scholar : PubMed/NCBI | |
|
Pardridge WM and Fierer G: Transport of tryptophan into brain from the circulating, albumin-bound pool in rats and in rabbits. J Neurochem. 54:971–976. 1990. View Article : Google Scholar : PubMed/NCBI | |
|
Sun N, Hu H, Wang F, Li L, Zhu W, Shen Y, Xiu J and Xu Q: Antibiotic-induced microbiome depletion in adult mice disrupts blood-brain barrier and facilitates brain infiltration of monocytes after bone-marrow transplantation. Brain Behav Immun. 92:102–114. 2021. View Article : Google Scholar | |
|
Fröhlich EE, Farzi A, Mayerhofer R, Reichmann F, Jačan A, Wagner B, Zinser E, Bordag N, Magnes C, Fröhlich E, et al: Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav Immun. 56:1402016. View Article : Google Scholar : PubMed/NCBI | |
|
Wen J, Ding Y, Wang L and Xiao Y: Gut microbiome improves postoperative cognitive function by decreasing permeability of the blood-brain barrier in aged mice. Brain Res Bull. 164:249–256. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Praveenraj SS, Sonali S, Anand N, Tousif HA, Vichitra C, Kalyan M, Kanna PV, Chandana KA, Shasthara P, Mahalakshmi AM, et al: The role of a gut microbial-derived metabolite, trimethylamine N-oxide (TMAO), in neurological disorders. Mol Neurobiol. 59:6684–6700. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Wallace TC, Blusztajn JK, Caudill MA, Klatt KC, Natker E, Zeisel SH and Zelman KM: Choline: The underconsumed and underappreciated essential nutrient. Nutr Today. 53:240–253. 2018. View Article : Google Scholar | |
|
Janeiro MH, Ramírez MJ, Milagro FI, Martínez JA and Solas M: Implication of trimethylamine N-Oxide (TMAO) in disease: Potential biomarker or new therapeutic target. Nutrients. 10:13982018. View Article : Google Scholar : PubMed/NCBI | |
|
Hoyles L, Pontifex MG, Rodriguez-Ramiro I, Anis-Alavi MA, Jelane KS, Snelling T, Solito E, Fonseca S, Carvalho AL, Carding SR, et al: Regulation of blood-brain barrier integrity by microbiome-associated methylamines and cognition by trimethylamine N-oxide. Microbiome. 9:2352021. View Article : Google Scholar : PubMed/NCBI | |
|
Badran M, Khalyfa A, Ericsson AC, Puech C, McAdams Z, Bender SB and Gozal D: Gut microbiota mediate vascular dysfunction in a murine model of sleep apnoea: Effect of probiotics. Eur Respir J. 61:22000022023. View Article : Google Scholar | |
|
Gamage AM, Liao C, Cheah IK, Chen Y, Lim DRX, Ku JWK, Chee RSL, Seebeck MGFP, Halliwell B and Gan YH: The proteobacterial species Burkholderia pseudomallei produces ergothioneine, which enhances virulence in mammalian infection. FASEB J. 32:6395–6409. 2018. View Article : Google Scholar | |
|
Kalaras MD, Richie JP, Calcagnotto A and Beelman RB: Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 233:429–433. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Vallianatou T, Lin W, Bèchet NB, Correia MS, Shanbhag NC, Lundgaard I and Globisch D: Differential regulation of oxidative stress, microbiota-derived, and energy metabolites in the mouse brain during sleep. J Cereb Blood Flow Metab. 41:3324–3338. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Cheah IK and Halliwell B: Ergothioneine, recent developments. Redox Biol. 42:1018682021. View Article : Google Scholar : PubMed/NCBI | |
|
Matsuda Y, Ozawa N, Shinozaki T, Wakabayashi KI, Suzuki K, Kawano Y, Ohtsu I and Tatebayashi Y: Ergothioneine, a metabolite of the gut bacterium Lactobacillus reuteri, protects against stress-induced sleep disturbances. Transl Psychiatry. 10:1702020. View Article : Google Scholar : PubMed/NCBI | |
|
Buret AG, Allain T, Motta JP and Wallace JL: Effects of hydrogen sulfide on the microbiome: From toxicity to therapy. Antioxid Redox Signal. 36:211–219. 2022. View Article : Google Scholar : | |
|
Tudor JC, Davis EJ, Peixoto L, Wimmer ME, van Tilborg E, Park AJ, Poplawski SG, Chung CW, Havekes R, Huang J, et al: Sleep deprivation impairs memory by attenuating mTORC1-dependent protein synthesis. Sci Signal. 9:ra412016. View Article : Google Scholar : PubMed/NCBI | |
|
Wei HJ, Xu JH, Li MH, Tang JP, Zou W, Zhang P, Wang L, Wang CY and Tang XQ: Hydrogen sulfide inhibits homocysteine-induced endoplasmic reticulum stress and neuronal apoptosis in rat hippocampus via upregulation of the BDNF-TrkB pathway. Acta Pharmacol Sin. 35:707–715. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Kang X, Jiang L, Lan F, Tang YY, Zhang P, Zou W, Chen YJ and Tang XQ: Hydrogen sulfide antagonizes sleep deprivation-induced depression- and anxiety-like behaviors by inhibiting neuroinflammation in a hippocampal Sirt1-dependent manner. Brain Res Bull. 177:194–202. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Caspani G and Swann J: Small talk: Microbial metabolites involved in the signaling from microbiota to brain. Curr Opinion Pharmacol. 48:99–106. 2019. View Article : Google Scholar | |
|
Bowers SJ, Vargas F, González A, He S, Jiang P, Dorrestein PC, Knight R, Wright KP Jr, Lowry CA, Fleshner M, et al: Repeated sleep disruption in mice leads to persistent shifts in the fecal microbiome and metabolome. PLoS One. 15:e02290012020. View Article : Google Scholar : PubMed/NCBI | |
|
Fang D, Xu T, Sun J, Shi J, Li F, Yin Y, Wang Z and Liu Y: Nicotinamide mononucleotide ameliorates sleep deprivation-induced gut microbiota dysbiosis and restores colonization resistance against intestinal infections. Adv Sci (Weinh). 10:22071702023. View Article : Google Scholar : PubMed/NCBI | |
|
Zielinski MR, McKenna JT and McCarley RW: Functions and mechanisms of sleep. AIMS Neurosci. 3:67–104. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Kalinchuk AV, McCarley RW, Porkka-Heiskanen T and Basheer R: The time course of adenosine, nitric oxide (NO) and inducible NO synthase changes in the brain with sleep loss and their role in the non-rapid eye movement sleep homeostatic cascade. J Neurochem. 116:260–272. 2011. View Article : Google Scholar | |
|
Chen L, Majde JA and Krueger JM: Spontaneous sleep in mice with targeted disruptions of neuronal or inducible nitric oxide synthase genes. Brain Res. 973:214–222. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjørkum AA, Greene RW and McCarley RW: Adenosine: A mediator of the sleep-inducing effects of prolonged wakefulness. Science. 276:1265–1268. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Marini S, Santangeli O, Saarelainen P, Middleton B, Chowdhury N, Skene DJ, Costa R, Porkka-Heiskanen T and Montagnese S: Abnormalities in the polysomnographic, adenosine and metabolic response to sleep deprivation in an animal model of hyperammonemia. Front Physiol. 8:6362017. View Article : Google Scholar : PubMed/NCBI | |
|
Aburto MR and Cryan JF: Gastrointestinal and brain barriers: Unlocking gates of communication across the microbiota-gut-brain axis. Nat Rev Gastroenterol Hepatol. 21:222–247. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Schroeder S, Hofer SJ, Zimmermann A, Pechlaner R, Dammbrueck C, Pendl T, Marcello GM, Pogatschnigg V, Bergmann M, Müller M, et al: Dietary spermidine improves cognitive function. Cell Rep. 35:1089852021. View Article : Google Scholar : PubMed/NCBI | |
|
Bedont JL, Kolesnik A, Pivarshev P, Malik D, Hsu CT, Weljie A and Sehgal A: Chronic sleep loss sensitizes Drosophila melanogaster to nitrogen stress. Curr Biol. 33:1613–1623.e5. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Ren H, Kong X, Zhang Y, Deng F, Li J, Zhao F, Li P, Pei K, Tan J, Cheng Y, et al: The therapeutic potential of Ziziphi Spinosae Semen and Polygalae Radix in insomnia management: Insights from gut microbiota and serum metabolomics techniques. J Ethnopharmacol. 330:1182552024. View Article : Google Scholar : PubMed/NCBI | |
|
Sun Y, Chen S, Wei R, Xie X, Wang C, Fan S, Zhang X, Su J, Liu J, Jia W and Wang X: Metabolome and gut microbiota variation with long-term intake of Panax ginseng extracts on rats. Food Funct. 9:3547–3556. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Qiao T, Wang Y, Liang K, Zheng B, Ma J, Li F, Liu C, Zhu M and Song M: Effects of the Radix Ginseng and Semen Ziziphi Spinosae drug pair on the GLU/GABA-GLN metabolic cycle and the intestinal microflora of insomniac rats based on the brain-gut axis. Front Pharmacol. 13:10945072022. View Article : Google Scholar | |
|
Hao KX, Shen CY and Jiang JG: Sedative and hypnotic effects of Polygala tenuifolia willd. Saponins on insomnia mice and their targets. J Ethnopharmacol. 323:1176182024. View Article : Google Scholar | |
|
Fasina OB, Wang J, Mo J, Osada H, Ohno H, Pan W, Xiang L and Qi J: Gastrodin from gastrodia elata enhances cognitive function and neuroprotection of AD mice via the regulation of gut microbiota composition and inhibition of neuron inflammation. Front Pharmacol. 13:8142712022. View Article : Google Scholar : PubMed/NCBI | |
|
Liu B, Li F, Xu Y, Wu Q and Shi J: Gastrodin improves cognitive dysfunction in REM sleep-deprived rats by regulating TLR4/NF-κB and Wnt/β-catenin signaling pathways. Brain Sci. 13:1792023. View Article : Google Scholar | |
|
Zhu C, Zhang Z, Wang S and Sun Z: Study on the mechanism of Gastrodiae Rhizoma, Lycii Fructus, and Ziziphi Spinosae Semen in sedation and tranquillising mind. Mol Divers. 28:3279–3294. 2024. View Article : Google Scholar | |
|
Chang HH, Yi PL, Cheng CH, Lu CY, Hsiao YT, Tsai YF, Li CL and Chang FC: Biphasic effects of baicalin, an active constituent of Scutellaria baicalensis Georgi, in the spontaneous sleep-wake regulation. J Ethnopharmacol. 135:359–368. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Wan S, Wang L, Hao Z, Zhu L, Mao X, Li H, Sun P, Yin W, Fan K, Zhang H, et al: Baicalin ameliorates the gut barrier function and intestinal microbiota of broiler chickens. Acta Biochim Biophys Sin (Shanghai). 56:634–644. 2024.PubMed/NCBI | |
|
Yao C, Wang Z, Jiang H, Yan R, Huang Q, Wang Y, Xie H, Zou Y, Yu Y and Lv L: Ganoderma lucidum promotes sleep through a gut microbiota-dependent and serotonin-involved pathway in mice. Sci Rep. 11:136602021. View Article : Google Scholar : PubMed/NCBI |