Microbiota metabolites affect sleep as drivers of brain‑gut communication (Review)
- Authors:
- Hanxing Cheng
- Wanying Yang
- Huaiyi Xu
- Wenwen Zhu
- Ailin Gong
- Xuemei Yang
- Sen Li
- Houping Xu
-
Affiliations: Geriatric Department, The Affiliated Traditional Chinese Medicine Hospital, Southwest Medical University, Luzhou, Sichuan 646000, P.R. China, Department of Orthopedic Surgery, Division of Spine Surgery, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, Jiangsu 210000, P.R. China - Published online on: June 30, 2025 https://doi.org/10.3892/ijmm.2025.5571
- Article Number: 130
-
Copyright: © Cheng et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
This article is mentioned in:
Abstract
 |
 |
 |
 |
|
Lund HG, Reider BD, Whiting AB and Prichard JR: Sleep patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Health. 46:124–132. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Bishir M, Bhat A, Essa MM, Ekpo O, Ihunwo AO, Veeraraghavan VP, Mohan SK, Mahalakshmi AM, Ray B, Tuladhar S, et al: Sleep deprivation and neurological disorders. Biomed Res Int. 2020:57640172020. View Article : Google Scholar : | |
|
Palagini L, Hertenstein E, Riemann D and Nissen C: Sleep, insomnia and mental health. J Sleep Res. 31:e136282022. View Article : Google Scholar : PubMed/NCBI | |
|
Chang L, Wei Y and Hashimoto K: Brain-gut-microbiota axis in depression: A historical overview and future directions. Brain Res Bull. 182:44–56. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Gossard TR, Trotti LM, Videnovic A and St Louis EK: Restless legs syndrome: Contemporary diagnosis and treatment. Neurotherapeutic. 18:140–155. 2021. View Article : Google Scholar | |
|
Sun SY and Chen GH: Treatment of circadian rhythm sleep-wake disorders. Curr Neuropharmacol. 20:1022–1034. 2022. View Article : Google Scholar | |
|
Arnulf I, Thomas R, Roy A and Dauvilliers Y: Update on the treatment of idiopathic hypersomnia: Progress, challenges, and expert opinion. Sleep Med Rev. 69:1017662023. View Article : Google Scholar : PubMed/NCBI | |
|
You M, Chen N, Yang Y, Cheng L, He H, Cai Y, Liu Y, Liu H and Hong G: The gut microbiota-brain axis in neurological disorders. MedComm (2020). 5:e6562024. View Article : Google Scholar : PubMed/NCBI | |
|
Haarhuis JE, Kardinaal A and Kortman GAM: Probiotics, prebiotics and postbiotics for better sleep quality: A narrative review. Benef Microbes. 13:169–182. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Singh V, Yeoh BS, Chassaing B, Xiao X, Saha P, Aguilera Olvera R, Lapek JD Jr, Zhang L, Wang WB, Hao S, et al: Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer. Cell. 175:679–694.e22. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Barrett E, Ross RP, O'Toole PW, Fitzgerald GF and Stanton C: γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 113:411–417. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Chen H, Nwe PK, Yang Y, Rosen CE, Bielecka AA, Kuchroo M, Cline GW, Kruse AC, Ring AM, Crawford JM and Palm NW: A forward chemical genetic screen reveals gut microbiota metabolites that modulate host physiology. Cell. 177:1217–1231.e18. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Ren Z, Zhang R, Li Y, Li Y, Yang Z and Yang H: Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int J Mol Med. 40:1444–1456. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Verzelloni E, Pellacani C, Tagliazucchi D, Tagliaferri S, Calani L, Costa LG, Brighenti F, Borges G, Crozier A, Conte A and Del Rio D: Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol Nutr Food Res. 55(Suppl 1): S35–S43. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Mao JH, Kim YM, Zhou YX, Hu D, Zhong C, Chang H, Brislawn CJ, Fansler S, Langley S, Wang Y, et al: Genetic and metabolic links between the murine microbiome and memory. Microbiome. 8:532020. View Article : Google Scholar : PubMed/NCBI | |
|
Ahmed H, Leyrolle Q, Koistinen V, Kärkkäinen O, Layé S, Delzenne N and Hanhineva K: Microbiota-derived metabolites as drivers of gut-brain communication. Gut Microbes. 14:21028782022. View Article : Google Scholar : PubMed/NCBI | |
|
Mann ER, Lam YK and Uhlig HH: Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat Rev Immunol. 24:577–595. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Agus A, Clément K and Sokol H: Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 70:1174–1182. 2021. View Article : Google Scholar | |
|
Ganz J: Revealing the complexity of the gut's brain. Nat Neurosci. 24:1–2. 2021. View Article : Google Scholar | |
|
Margolis KG, Cryan JF and Mayer EA: The microbiota-gut-brain axis: From motility to mood. Gastroenterology. 160:1486–1501. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Niesler B, Kuerten S, Demir IE and Schäfer KH: Disorders of the enteric nervous system-a holistic view. Nat Rev Gastroenterol Hepatol. 18:393–410. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
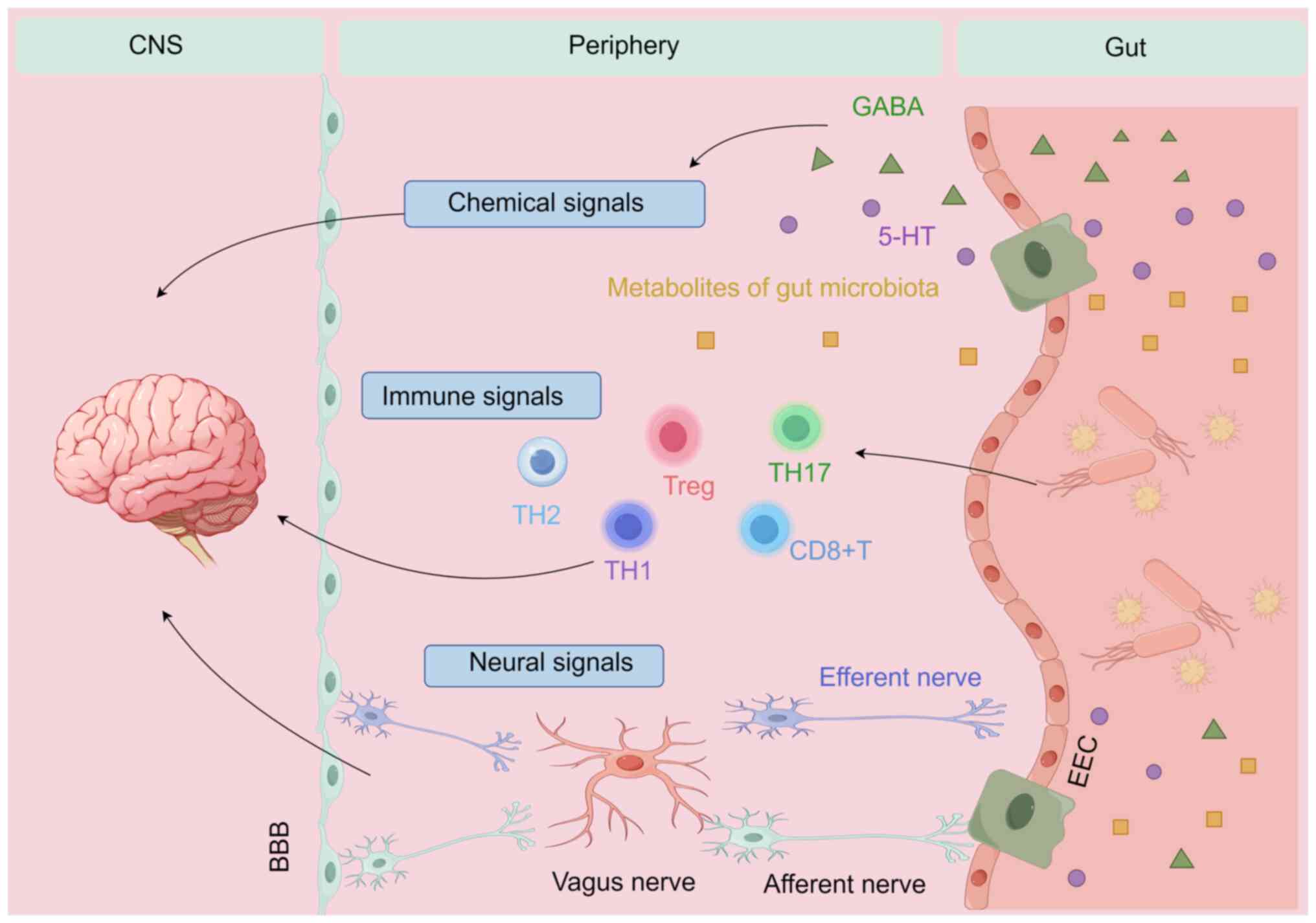
Bonaz B, Bazin T and Pellissier S: The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 12:492018. View Article : Google Scholar : PubMed/NCBI | |
|
Agirman G, Yu KB and Hsiao EY: Signaling inflammation across the gut-brain axis. Science. 374:1087–1092. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Zielinski MR and Gibbons AJ: Neuroinflammation, sleep, and circadian rhythms. Front Cell Infect Microbiol. 12:8530962022. View Article : Google Scholar : PubMed/NCBI | |
|
Grigg JB and Sonnenberg GF: Host-microbiota interactions shape local and systemic inflammatory diseases. J Immunol. 198:564–571. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Salvo-Romero E, Stokes P and Gareau MG: Microbiota-immune interactions: From gut to brain. Lymphosign J. 7:1–23. 2020. View Article : Google Scholar | |
|
Zheng D, Liwinski T and Elinav E: Interaction between microbiota and immunity in health and disease. Cell Res. 30:492–506. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Averina OV, Zorkina YA, Yunes RA, Kovtun AS, Ushakova VM, Morozova AY, Kostyuk GP, Danilenko VN and Chekhonin VP: Bacterial metabolites of human gut microbiota correlating with depression. Int J Mol Sci. 21:92342020. View Article : Google Scholar : PubMed/NCBI | |
|
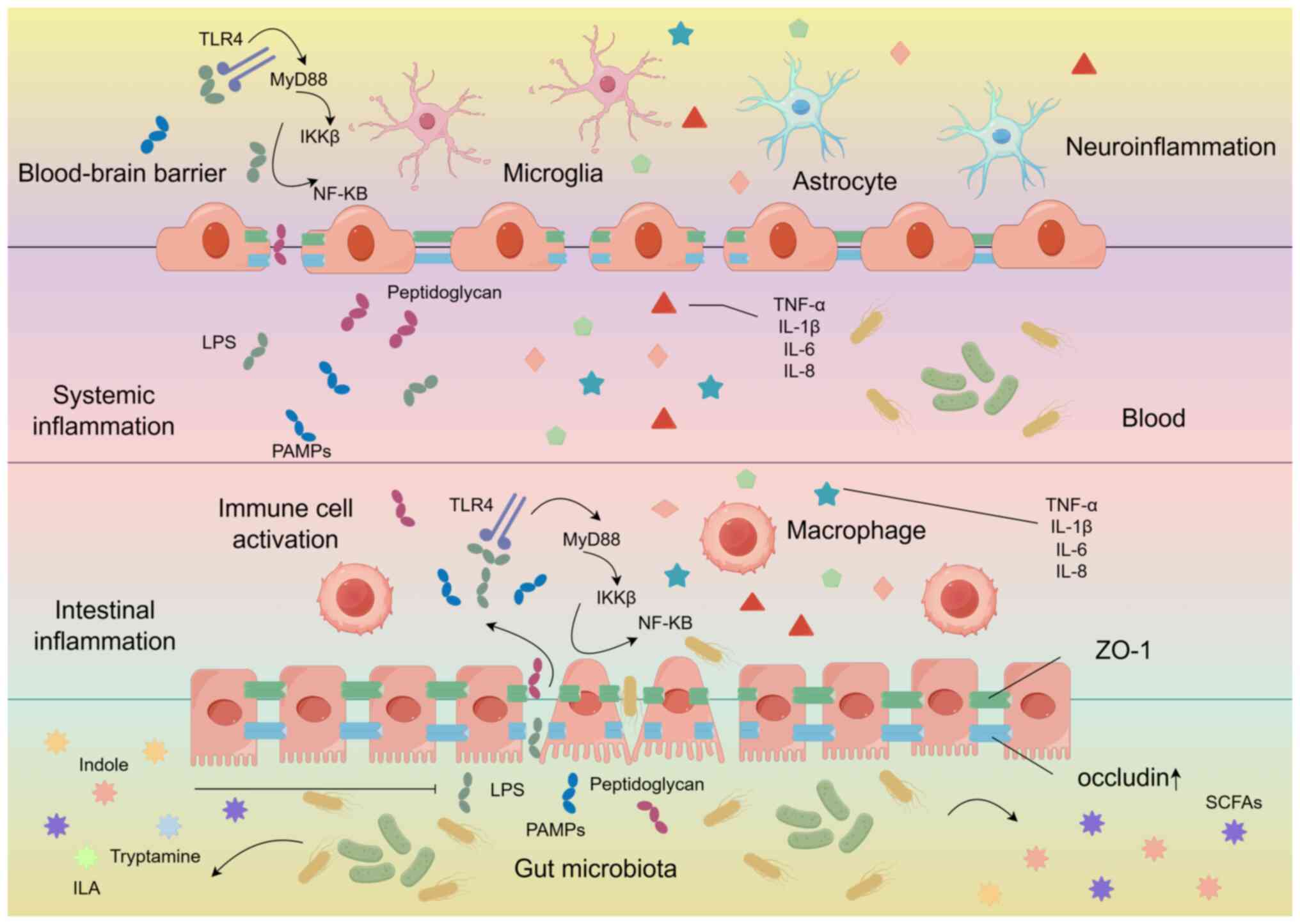
Parker A, Fonseca S and Carding SR: Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes. 11:135–157. 2020. View Article : Google Scholar : | |
|
Dalile B, Van Oudenhove L, Vervliet B and Verbeke K: The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 16:461–478. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Gibson JA, Sladen GE and Dawson AM: Protein absorption and ammonia production: The effects of dietary protein and removal of the colon. Br J Nutr. 35:61–65. 1976. View Article : Google Scholar : PubMed/NCBI | |
|
Tan JK, Macia L and Mackay CR: Dietary fiber and SCFAs in the regulation of mucosal immunity. J Allergy Clin Immunol. 151:361–370. 2023. View Article : Google Scholar | |
|
Fock E and Parnova R: Mechanisms of blood-brain barrier protection by microbiota-derived short-chain fatty acids. Cells. 12:6572023. View Article : Google Scholar : PubMed/NCBI | |
|
Ikeda T, Nishida A, Yamano M and Kimura I: Short-chain fatty acid receptors and gut microbiota as therapeutic targets in metabolic, immune, and neurological diseases. Pharmacol Ther. 239:1082732022. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Shao L, Mou Y, Zhang Y and Ping Y: Sleep, circadian rhythm and gut microbiota: Alterations in Alzheimer's disease and their potential links in the pathogenesis. Gut Microbes. 13:19574072021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu R, Fang Y, Li H, Liu Y, Wei J, Zhang S, Wang L, Fan R, Wang L, Li S and Chen T: Psychobiotic Lactobacillus plantarum JYLP-326 relieves anxiety, depression, and insomnia symptoms in test anxious college via modulating the gut microbiota and its metabolism. Front Immunol. 14:11581372023. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Zhang B, Zhou Y, Wang D, Liu X, Li L, Wang T, Zhang Y, Jiang M, Tang H, et al: Gut microbiota changes and their relationship with inflammation in patients with acute and chronic insomnia. Nat Sci Sleep. 12:895–905. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Shimizu Y, Yamamura R, Yokoi Y, Ayabe T, Ukawa S and Nakamura K, Okada E, Imae A, Nakagawa T, Tamakoshi A and Nakamura K: Shorter sleep time relates to lower human defensin 5 secretion and compositional disturbance of the intestinal microbiota accompanied by decreased short-chain fatty acid production. Gut Microbes. 15:21903062023. View Article : Google Scholar : PubMed/NCBI | |
|
Zuraikat FM, Wood RA, Barragán R and St-Onge MP: Sleep and diet: Mounting evidence of a cyclical relationship. Annu Rev Nutr. 41:309–332. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC and Siuzdak G: Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 106:3698–3703. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Dicks LMT: Gut bacteria and neurotransmitters. Microorganisms. 10:18382022. View Article : Google Scholar : PubMed/NCBI | |
|
Liu N, Sun S, Wang P, Sun Y, Hu Q and Wang X: The mechanism of secretion and metabolism of gut-derived 5-hydroxytryptamine. Int J Mol Sci. 22:79312021. View Article : Google Scholar : PubMed/NCBI | |
|
Gao K, Mu CL, Farzi A and Zhu WY: Tryptophan metabolism: A link between the gut microbiota and brain. Adv Nutr. 11:709–723. 2020. View Article : Google Scholar : | |
|
Xie Y, Wang C, Zhao D, Wang C and Li C: Dietary proteins regulate serotonin biosynthesis and catabolism by specific gut microbes. J Agric Food Chem. 68:5880–5890. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Parkar SG, Kalsbeek A and Cheeseman JF: Potential role for the gut microbiota in modulating host circadian rhythms and metabolic health. Microorganisms. 7:412019. View Article : Google Scholar : PubMed/NCBI | |
|
Gershon MD and Tack J: The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology. 132:397–414. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Latorre E, Mendoza C, Matheus N, Castro M, Grasa L, Mesonero JE and Alcalde AI: IL-10 modulates serotonin transporter activity and molecular expression in intestinal epithelial cells. Cytokine. 61:778–784. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Buey B, Forcén A, Grasa L, Layunta E, Mesonero JE and Latorre E: Gut microbiota-derived short-chain fatty acids: Novel regulators of intestinal serotonin transporter. Life (Basel). 13:10852023.PubMed/NCBI | |
|
Cai J, Cheung J, Cheung SWM, Chin KTC, Leung RWK, Lam RST, Sharma R, Yiu JHC and Woo CW: Butyrate acts as a positive allosteric modulator of the 5-HT transporter to decrease availability of 5-HT in the ileum. Br J Pharmacol. 181:1654–1670. 2024. View Article : Google Scholar | |
|
Dicks LMT: Our mental health is determined by an intrinsic interplay between the central nervous system, enteric nerves, and gut microbiota. Int J Mol Sci. 25:382023. View Article : Google Scholar | |
|
Wei L, Singh R and Ghoshal UC: Enterochromaffin cells-gut microbiota crosstalk: Underpinning the symptoms, pathogenesis, and pharmacotherapy in disorders of gut-brain interaction. J Neurogastroenterol Motil. 28:357–375. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Guo X, Yin C, Yang F, Zhang Y, Huang H, Wang J, Deng B, Cai T, Rao Y and Xi R: The cellular diversity and transcription factor code of Drosophila enteroendocrine cells. Cell Rep. 29:4172–4185.e5. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Ye L, Bae M, Cassilly CD, Jabba SV, Thorpe DW, Martin AM, Lu HY, Wang J, Thompson JD, Lickwar CR, et al: Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe. 29:179–196.e9. 2021. View Article : Google Scholar : | |
|
Gao T, Wang Z, Cao J, Dong Y and Chen Y: Melatonin alleviates oxidative stress in sleep deprived mice: Involvement of small intestinal mucosa injury. Int Immunopharmacol. 78:1060412020. View Article : Google Scholar | |
|
Gao T, Wang Z, Dong Y, Cao J, Lin R, Wang X, Yu Z and Chen Y: Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J Pineal Res. 67:e125742019. View Article : Google Scholar : PubMed/NCBI | |
|
Yu H, Lin L, Zhang Z, Zhang H and Hu H: Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct Target Ther. 5:2092020. View Article : Google Scholar | |
|
Wu Z, Liu L, Li L, Cao X, Jia W, Liao X, Zhao Z, Qi H, Fan G, Lu H, et al: Oral nano-antioxidants improve sleep by restoring intestinal barrier integrity and preventing systemic inflammation. Natl Sci Rev. 10:nwad3092023. View Article : Google Scholar | |
|
Veler H: Sleep and inflammation: Bidirectional relationship. Sleep Med Clin. 18:213–218. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Yang Y, Gu K, Meng C, Li J, Lu Q, Zhou X, Yan D, Li D, Pei C, Lu Y, et al: Relationship between sleep and serum inflammatory factors in patients with major depressive disorder. Psychiatry Res. 329:1155282023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Z, Chen WH, Li SX, He ZM, Zhu WL, Ji YB, Wang Z, Zhu XM, Yuan K, Bao YP, et al: Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol Psychiatry. 26:6277–6292. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Su H, Zhang C, Zou X, Lu F, Zeng Y, Guan H, Ren Y, Yuan F, Xu L, Zhang M and Dong H: Jiao-tai-wan inhibits inflammation of the gut-brain-axis and attenuates cognitive impairment in insomnic rats. J Ethnopharmacol. 250:1124782020. View Article : Google Scholar | |
|
Hergenhan S, Holtkamp S and Scheiermann C: Molecular interactions between components of the circadian clock and the immune system. J Mol Biol. 432:3700–3713. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao Z, Ning J, Bao XQ, Shang M, Ma J, Li G and Zhang D: Fecal microbiota transplantation protects rotenone-induced Parkinson's disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome. 9:2262021. View Article : Google Scholar : PubMed/NCBI | |
|
McCuaig B and Goto Y: Immunostimulating commensal bacteria and their potential use as therapeutics. Int J Mol Sci. 24:156442023. View Article : Google Scholar : PubMed/NCBI | |
|
Mohawk JA, Green CB and Takahashi JS: Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 35:445–462. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Prinz M, Jung S and Priller J: Microglia biology: One century of evolving concepts. Cell. 179:292–311. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Horng S, Therattil A, Moyon S, Gordon A, Kim K, Argaw AT, Hara Y, Mariani JN, Sawai S, Flodby P, et al: Astrocytic tight junctions control inflammatory CNS lesion pathogenesis. J Clin Invest. 127:3136–3151. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Gudkov SV, Burmistrov DE, Kondakova EV, Sarimov RM, Yarkov RS, Franceschi C and Vedunova MV: An emerging role of astrocytes in aging/neuroinflammation and gut-brain axis with consequences on sleep and sleep disorders. Ageing Res Rev. 83:1017752023. View Article : Google Scholar | |
|
Wang X, Wang Z, Cao J, Dong Y and Chen Y: Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome. 11:172023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang L, Ko CY and Zeng YM: Immunoregulatory effect of short-chain fatty acids from gut microbiota on obstructive sleep apnea-associated hypertension. Nat Sci Sleep. 14:393–405. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Mowat AM and Agace WW: Regional specialization within the intestinal immune system. Nat Rev Immunol. 14:667–685. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Nastasi C, Candela M, Bonefeld CM, Geisler C, Hansen M, Krejsgaard T, Biagi E, Andersen MH, Brigidi P, Ødum N, et al: The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci Rep. 5:161482015. View Article : Google Scholar : PubMed/NCBI | |
|
Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T and Yin Y: Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol. 8:132018. View Article : Google Scholar : PubMed/NCBI | |
|
Szelest M, Walczak K and Plech T: A new insight into the potential role of tryptophan-derived AhR ligands in skin physiological and pathological processes. Int J Mol Sci. 22:11042021. View Article : Google Scholar : PubMed/NCBI | |
|
Sun M, Ma N, He T, Johnston LJ and Ma X: Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon receptor (AhR). Crit Rev Food Sci Nutr. 60:1760–1768. 2020. View Article : Google Scholar | |
|
Nicolas GR and Chang PV: Deciphering the chemical lexicon of host-gut microbiota interactions. Trends Pharmacol Sci. 40:430–445. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Xie L, Wu Q, Li K, Khan MAS, Zhang A, Sinha B, Li S, Chang SL, Brody DL, Grinstaff MW, et al: Tryptophan metabolism in Alzheimer's disease with the involvement of microglia and astrocyte crosstalk and gut-brain axis. Aging Dis. 15:2168–2190. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
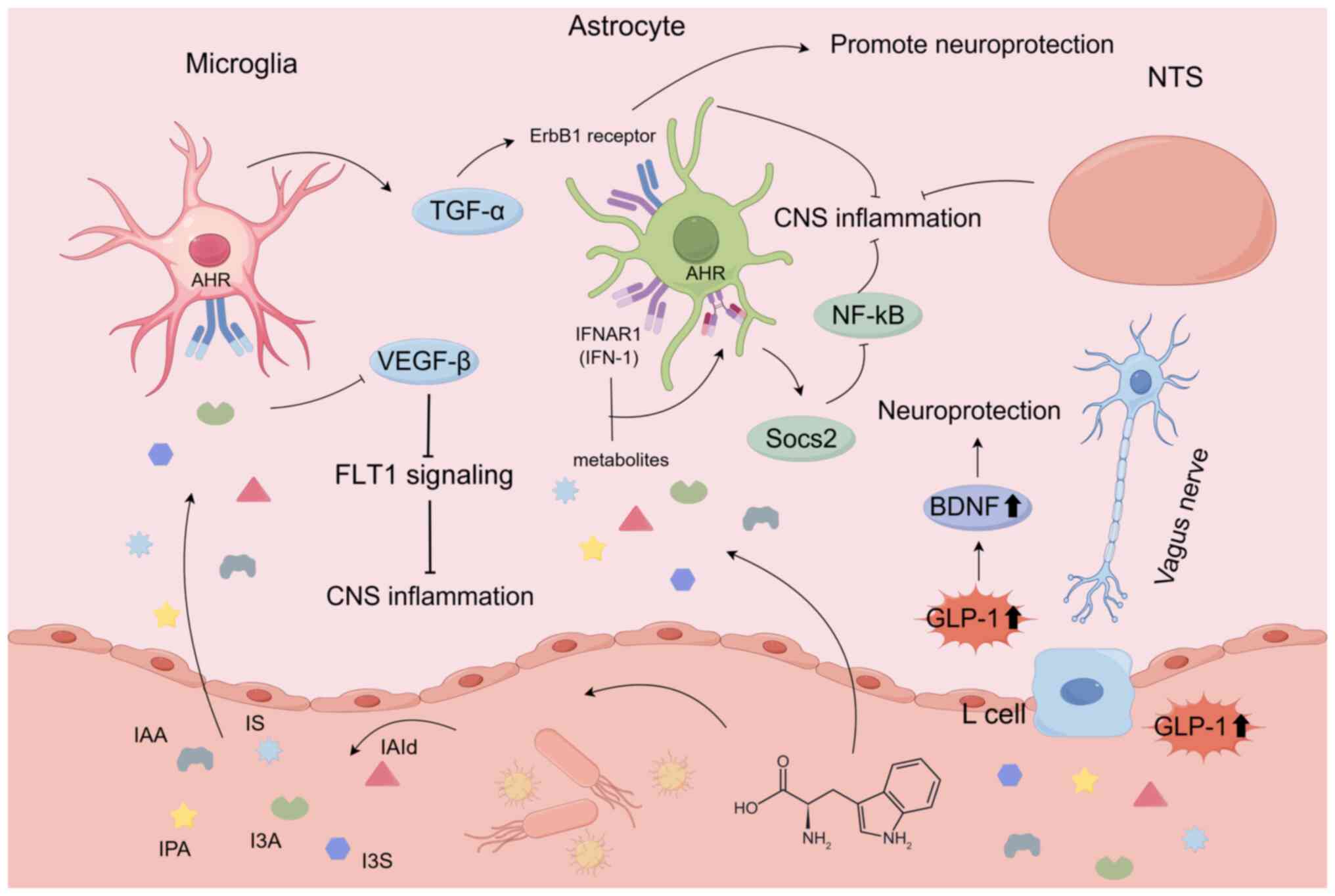
Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, de Lima KA, Gutiérrez-Vázquez C, Hewson P, Staszewski O, et al: Microglial control of astrocytes in response to microbial metabolites. Nature. 557:724–728. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao CC, Patel B, Yan R, Blain M, et al: Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 22:586–597. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Marsland BJ: Regulating inflammation with microbial metabolites. Nat Med. 22:581–583. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Wang P, Sun H, Maitiabula G, Zhang L, Yang J, Zhang Y, Gao X, Li J, Xue B, Li CJ and Wang X: Total parenteral nutrition impairs glucose metabolism by modifying the gut microbiome. Nat Metab. 5:331–348. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Chimerel C, Emery E, Summers DK, Keyser U, Gribble FM and Reimann F: Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 9:1202–1208. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Taati M, Barzegar PEF and Raisi A: Exercise improves spatial learning and memory performance through the central GLP-1 receptors. Behav Neurol. 2022:29006282022. View Article : Google Scholar : PubMed/NCBI | |
|
Budni J, Bellettini-Santos T, Mina F, Garcez ML and Zugno AI: The involvement of BDNF, NGF and GDNF in aging and Alzheimer's disease. Aging Dis. 6:331–341. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Athauda D and Foltynie T: Protective effects of the GLP-1 mimetic exendin-4 in Parkinson's disease. Neuropharmacology. 136:260–270. 2018. View Article : Google Scholar | |
|
van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG and Diamant M: Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol. 221:T1–T16. 2014. View Article : Google Scholar | |
|
Mir FA and Jha SK: The Kir channel in the nucleus tractus solitarius integrates the chemosensory system with REM sleep executive machinery for homeostatic balance. Sci Rep. 14:216512024. View Article : Google Scholar : PubMed/NCBI | |
|
Zheng D, Ratiner K and Elinav E: Circadian influences of diet on the microbiome and immunity. Trends Immunol. 41:512–530. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
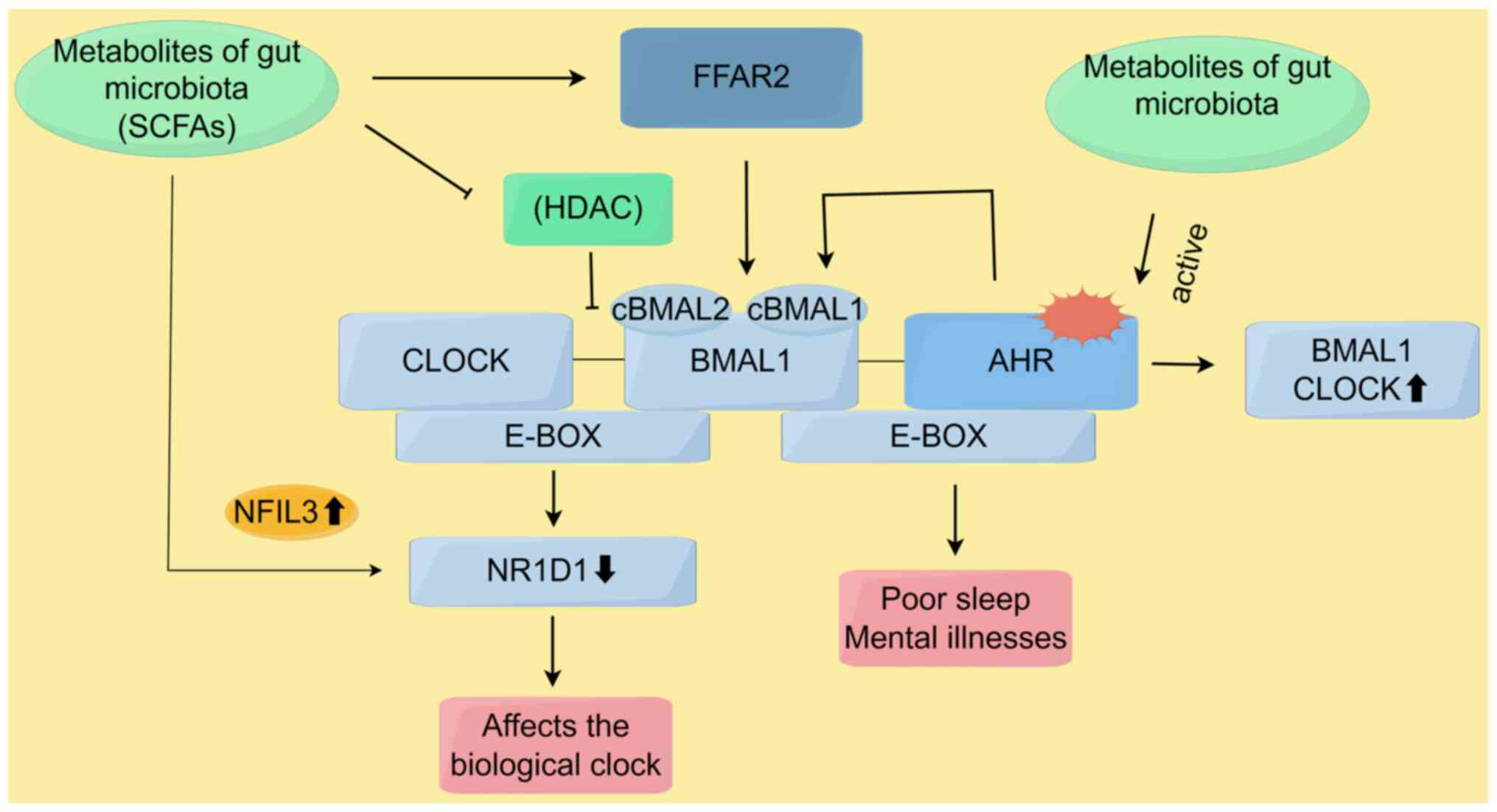
Kuang Z, Wang Y, Li Y, Ye C, Ruhn KA, Behrendt CL, Olson EN and Hooper LV: The intestinal microbiota programs diurnal rhythms in host metabolism through histone deacetylase 3. Science. 365:1428–1434. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Ni Y, Ma L, Wu T, Lim AL, Zhang W, Yang L, Nakao Y and Fu Z: The involvement of sympathetic nervous system in essence of chicken-facilitated physiological adaption and circadian resetting. Life Sci. 201:54–62. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Nobs SP, Tuganbaev T and Elinav E: Microbiome diurnal rhythmicity and its impact on host physiology and disease risk. EMBO Rep. 20:e471292019. View Article : Google Scholar : PubMed/NCBI | |
|
Thaiss CA, Levy M, Korem T, Dohnalová L, Shapiro H, Jaitin DA, David E, Winter DR, Gury-BenAri M, Tatirovsky E, et al: Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell. 167:1495–1510.e12. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Li Y, Yuan Y, Wang J, Zhang S, Zhu R, Wang Y, Wu Y, Liao X and Mi J: Reducing light exposure enhances the circadian rhythm of the biological clock through interactions with the gut microbiota. Sci Total Environ. 858:1600412023. View Article : Google Scholar | |
|
Singh K, Jha NK and Thakur A: Spatiotemporal chromatin dynamics-A telltale of circadian epigenetic gene regulation. Life Sci. 221:377–391. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Xiong L, Zhou W and Mas P: Illuminating the Arabidopsis circadian epigenome: Dynamics of histone acetylation and deacetylation. Curr Opin Plant Biol. 69:1022682022. View Article : Google Scholar : PubMed/NCBI | |
|
Tahara Y, Yamazaki M, Sukigara H, Motohashi H, Sasaki H, Miyakawa H, Haraguchi A, Ikeda Y, Fukuda S and Shibata S: Gut microbiota-derived short chain fatty acids induce circadian clock entrainment in mouse peripheral tissue. Sci Rep. 8:13952018. View Article : Google Scholar : PubMed/NCBI | |
|
Fawad JA, Luzader DH, Hanson GF, Moutinho TJ Jr, McKinney CA, Mitchell PG, Brown-Steinke K, Kumar A, Park M, Lee S, et al: Histone deacetylase inhibition by gut microbe-generated short-chain fatty acids entrains intestinal epithelial circadian rhythms. Gastroenterology. 163:1377–1390.e11. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Kim DS, Woo JS, Min HK, Choi JW, Moon JH, Park MJ, Kwok SK, Park SH and Cho ML: Short-chain fatty acid butyrate induces IL-10-producing B cells by regulating circadian-clock-related genes to ameliorate Sjögren's syndrome. J Autoimmun. 119:1026112021. View Article : Google Scholar | |
|
Jin UH, Lee SO, Sridharan G, Lee K, Davidson LA, Jayaraman A, Chapkin RS, Alaniz R and Safe S: Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol. 85:777–788. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Tischkau SA: Mechanisms of circadian clock interactions with aryl hydrocarbon receptor signalling. Eur J of Neurosci. 51:379–395. 2020. View Article : Google Scholar | |
|
Salminen A: Aryl hydrocarbon receptor (AhR) impairs circadian regulation: Impact on the aging process. Ageing Res Rev. 87:1019282023. View Article : Google Scholar : PubMed/NCBI | |
|
Petrus P, Cervantes M, Samad M, Sato T, Chao A, Sato S, Koronowski KB, Park G, Alam Y, Mejhert N, et al: Tryptophan metabolism is a physiological integrator regulating circadian rhythms. Mol Metab. 64:1015562022. View Article : Google Scholar : PubMed/NCBI | |
|
Axelrod S, Li X, Sun Y, Lincoln S, Terceros A, O'Neil J, Wang Z, Nguyen A, Vora A, Spicer C, et al: The Drosophila blood-brain barrier regulates sleep via Moody G protein-coupled receptor signaling. Proc Natl Acad Sci USA. 120:e23093311202023. View Article : Google Scholar : PubMed/NCBI | |
|
Pardridge WM and Fierer G: Transport of tryptophan into brain from the circulating, albumin-bound pool in rats and in rabbits. J Neurochem. 54:971–976. 1990. View Article : Google Scholar : PubMed/NCBI | |
|
Sun N, Hu H, Wang F, Li L, Zhu W, Shen Y, Xiu J and Xu Q: Antibiotic-induced microbiome depletion in adult mice disrupts blood-brain barrier and facilitates brain infiltration of monocytes after bone-marrow transplantation. Brain Behav Immun. 92:102–114. 2021. View Article : Google Scholar | |
|
Fröhlich EE, Farzi A, Mayerhofer R, Reichmann F, Jačan A, Wagner B, Zinser E, Bordag N, Magnes C, Fröhlich E, et al: Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav Immun. 56:1402016. View Article : Google Scholar : PubMed/NCBI | |
|
Wen J, Ding Y, Wang L and Xiao Y: Gut microbiome improves postoperative cognitive function by decreasing permeability of the blood-brain barrier in aged mice. Brain Res Bull. 164:249–256. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Praveenraj SS, Sonali S, Anand N, Tousif HA, Vichitra C, Kalyan M, Kanna PV, Chandana KA, Shasthara P, Mahalakshmi AM, et al: The role of a gut microbial-derived metabolite, trimethylamine N-oxide (TMAO), in neurological disorders. Mol Neurobiol. 59:6684–6700. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Wallace TC, Blusztajn JK, Caudill MA, Klatt KC, Natker E, Zeisel SH and Zelman KM: Choline: The underconsumed and underappreciated essential nutrient. Nutr Today. 53:240–253. 2018. View Article : Google Scholar | |
|
Janeiro MH, Ramírez MJ, Milagro FI, Martínez JA and Solas M: Implication of trimethylamine N-Oxide (TMAO) in disease: Potential biomarker or new therapeutic target. Nutrients. 10:13982018. View Article : Google Scholar : PubMed/NCBI | |
|
Hoyles L, Pontifex MG, Rodriguez-Ramiro I, Anis-Alavi MA, Jelane KS, Snelling T, Solito E, Fonseca S, Carvalho AL, Carding SR, et al: Regulation of blood-brain barrier integrity by microbiome-associated methylamines and cognition by trimethylamine N-oxide. Microbiome. 9:2352021. View Article : Google Scholar : PubMed/NCBI | |
|
Badran M, Khalyfa A, Ericsson AC, Puech C, McAdams Z, Bender SB and Gozal D: Gut microbiota mediate vascular dysfunction in a murine model of sleep apnoea: Effect of probiotics. Eur Respir J. 61:22000022023. View Article : Google Scholar | |
|
Gamage AM, Liao C, Cheah IK, Chen Y, Lim DRX, Ku JWK, Chee RSL, Seebeck MGFP, Halliwell B and Gan YH: The proteobacterial species Burkholderia pseudomallei produces ergothioneine, which enhances virulence in mammalian infection. FASEB J. 32:6395–6409. 2018. View Article : Google Scholar | |
|
Kalaras MD, Richie JP, Calcagnotto A and Beelman RB: Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 233:429–433. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Vallianatou T, Lin W, Bèchet NB, Correia MS, Shanbhag NC, Lundgaard I and Globisch D: Differential regulation of oxidative stress, microbiota-derived, and energy metabolites in the mouse brain during sleep. J Cereb Blood Flow Metab. 41:3324–3338. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Cheah IK and Halliwell B: Ergothioneine, recent developments. Redox Biol. 42:1018682021. View Article : Google Scholar : PubMed/NCBI | |
|
Matsuda Y, Ozawa N, Shinozaki T, Wakabayashi KI, Suzuki K, Kawano Y, Ohtsu I and Tatebayashi Y: Ergothioneine, a metabolite of the gut bacterium Lactobacillus reuteri, protects against stress-induced sleep disturbances. Transl Psychiatry. 10:1702020. View Article : Google Scholar : PubMed/NCBI | |
|
Buret AG, Allain T, Motta JP and Wallace JL: Effects of hydrogen sulfide on the microbiome: From toxicity to therapy. Antioxid Redox Signal. 36:211–219. 2022. View Article : Google Scholar : | |
|
Tudor JC, Davis EJ, Peixoto L, Wimmer ME, van Tilborg E, Park AJ, Poplawski SG, Chung CW, Havekes R, Huang J, et al: Sleep deprivation impairs memory by attenuating mTORC1-dependent protein synthesis. Sci Signal. 9:ra412016. View Article : Google Scholar : PubMed/NCBI | |
|
Wei HJ, Xu JH, Li MH, Tang JP, Zou W, Zhang P, Wang L, Wang CY and Tang XQ: Hydrogen sulfide inhibits homocysteine-induced endoplasmic reticulum stress and neuronal apoptosis in rat hippocampus via upregulation of the BDNF-TrkB pathway. Acta Pharmacol Sin. 35:707–715. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Kang X, Jiang L, Lan F, Tang YY, Zhang P, Zou W, Chen YJ and Tang XQ: Hydrogen sulfide antagonizes sleep deprivation-induced depression- and anxiety-like behaviors by inhibiting neuroinflammation in a hippocampal Sirt1-dependent manner. Brain Res Bull. 177:194–202. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Caspani G and Swann J: Small talk: Microbial metabolites involved in the signaling from microbiota to brain. Curr Opinion Pharmacol. 48:99–106. 2019. View Article : Google Scholar | |
|
Bowers SJ, Vargas F, González A, He S, Jiang P, Dorrestein PC, Knight R, Wright KP Jr, Lowry CA, Fleshner M, et al: Repeated sleep disruption in mice leads to persistent shifts in the fecal microbiome and metabolome. PLoS One. 15:e02290012020. View Article : Google Scholar : PubMed/NCBI | |
|
Fang D, Xu T, Sun J, Shi J, Li F, Yin Y, Wang Z and Liu Y: Nicotinamide mononucleotide ameliorates sleep deprivation-induced gut microbiota dysbiosis and restores colonization resistance against intestinal infections. Adv Sci (Weinh). 10:22071702023. View Article : Google Scholar : PubMed/NCBI | |
|
Zielinski MR, McKenna JT and McCarley RW: Functions and mechanisms of sleep. AIMS Neurosci. 3:67–104. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Kalinchuk AV, McCarley RW, Porkka-Heiskanen T and Basheer R: The time course of adenosine, nitric oxide (NO) and inducible NO synthase changes in the brain with sleep loss and their role in the non-rapid eye movement sleep homeostatic cascade. J Neurochem. 116:260–272. 2011. View Article : Google Scholar | |
|
Chen L, Majde JA and Krueger JM: Spontaneous sleep in mice with targeted disruptions of neuronal or inducible nitric oxide synthase genes. Brain Res. 973:214–222. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjørkum AA, Greene RW and McCarley RW: Adenosine: A mediator of the sleep-inducing effects of prolonged wakefulness. Science. 276:1265–1268. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Marini S, Santangeli O, Saarelainen P, Middleton B, Chowdhury N, Skene DJ, Costa R, Porkka-Heiskanen T and Montagnese S: Abnormalities in the polysomnographic, adenosine and metabolic response to sleep deprivation in an animal model of hyperammonemia. Front Physiol. 8:6362017. View Article : Google Scholar : PubMed/NCBI | |
|
Aburto MR and Cryan JF: Gastrointestinal and brain barriers: Unlocking gates of communication across the microbiota-gut-brain axis. Nat Rev Gastroenterol Hepatol. 21:222–247. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Schroeder S, Hofer SJ, Zimmermann A, Pechlaner R, Dammbrueck C, Pendl T, Marcello GM, Pogatschnigg V, Bergmann M, Müller M, et al: Dietary spermidine improves cognitive function. Cell Rep. 35:1089852021. View Article : Google Scholar : PubMed/NCBI | |
|
Bedont JL, Kolesnik A, Pivarshev P, Malik D, Hsu CT, Weljie A and Sehgal A: Chronic sleep loss sensitizes Drosophila melanogaster to nitrogen stress. Curr Biol. 33:1613–1623.e5. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Ren H, Kong X, Zhang Y, Deng F, Li J, Zhao F, Li P, Pei K, Tan J, Cheng Y, et al: The therapeutic potential of Ziziphi Spinosae Semen and Polygalae Radix in insomnia management: Insights from gut microbiota and serum metabolomics techniques. J Ethnopharmacol. 330:1182552024. View Article : Google Scholar : PubMed/NCBI | |
|
Sun Y, Chen S, Wei R, Xie X, Wang C, Fan S, Zhang X, Su J, Liu J, Jia W and Wang X: Metabolome and gut microbiota variation with long-term intake of Panax ginseng extracts on rats. Food Funct. 9:3547–3556. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Qiao T, Wang Y, Liang K, Zheng B, Ma J, Li F, Liu C, Zhu M and Song M: Effects of the Radix Ginseng and Semen Ziziphi Spinosae drug pair on the GLU/GABA-GLN metabolic cycle and the intestinal microflora of insomniac rats based on the brain-gut axis. Front Pharmacol. 13:10945072022. View Article : Google Scholar | |
|
Hao KX, Shen CY and Jiang JG: Sedative and hypnotic effects of Polygala tenuifolia willd. Saponins on insomnia mice and their targets. J Ethnopharmacol. 323:1176182024. View Article : Google Scholar | |
|
Fasina OB, Wang J, Mo J, Osada H, Ohno H, Pan W, Xiang L and Qi J: Gastrodin from gastrodia elata enhances cognitive function and neuroprotection of AD mice via the regulation of gut microbiota composition and inhibition of neuron inflammation. Front Pharmacol. 13:8142712022. View Article : Google Scholar : PubMed/NCBI | |
|
Liu B, Li F, Xu Y, Wu Q and Shi J: Gastrodin improves cognitive dysfunction in REM sleep-deprived rats by regulating TLR4/NF-κB and Wnt/β-catenin signaling pathways. Brain Sci. 13:1792023. View Article : Google Scholar | |
|
Zhu C, Zhang Z, Wang S and Sun Z: Study on the mechanism of Gastrodiae Rhizoma, Lycii Fructus, and Ziziphi Spinosae Semen in sedation and tranquillising mind. Mol Divers. 28:3279–3294. 2024. View Article : Google Scholar | |
|
Chang HH, Yi PL, Cheng CH, Lu CY, Hsiao YT, Tsai YF, Li CL and Chang FC: Biphasic effects of baicalin, an active constituent of Scutellaria baicalensis Georgi, in the spontaneous sleep-wake regulation. J Ethnopharmacol. 135:359–368. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Wan S, Wang L, Hao Z, Zhu L, Mao X, Li H, Sun P, Yin W, Fan K, Zhang H, et al: Baicalin ameliorates the gut barrier function and intestinal microbiota of broiler chickens. Acta Biochim Biophys Sin (Shanghai). 56:634–644. 2024.PubMed/NCBI | |
|
Yao C, Wang Z, Jiang H, Yan R, Huang Q, Wang Y, Xie H, Zou Y, Yu Y and Lv L: Ganoderma lucidum promotes sleep through a gut microbiota-dependent and serotonin-involved pathway in mice. Sci Rep. 11:136602021. View Article : Google Scholar : PubMed/NCBI |










