Effects of anti‑VEGF on peripapillary retinal nerve fiber layer and papillary/peripapillary blood circulation in retinopathies (Review)
- Authors:
- Published online on: July 2, 2025 https://doi.org/10.3892/ijmm.2025.5574
- Article Number: 133
-
Copyright: © Wang et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Vascular endothelial growth factor (VEGF), a secreted 46 kDa glycoprotein, is an endothelial-cell-specific angiogenic factor (1). Although initially considered to be endothelial-cell-specific, VEGF is involved in the development and maturation of neural tissue, including the retina (2). Retinal pigment epithelial (RPE) cells and astrocytes in the retinal ganglion cell layer can secrete VEGF (3). In the normal adult retina, VEGF is expressed in the absence of active neovascularization and plays a role in the function of adult retina neuronal cells (4). Upregulation of VEGF occurs in several retinopathies associated with ischemia in the retina. Upregulated intraocular VEGF expression leads to vitreous hemorrhage, neovascular glaucoma and even retinal detachment, with subsequent loss of vision (5). Intravitreal injections of anti-VEGFs are widely used in the treatment of retinopathies to reduce angiogenesis and macular edema. Hypothetically, repeated treatment with anti-VEGFs for retinopathies should interfere with the neuroprotective function of VEGF (6). Additionally, long-term studies have suggested that, besides the short-term intraocular pressure (IOP) elevation, anti-VEGF injections can also lead to long-term elevations in IOP (7,8) and repeated episodes of IOP spikes can lead to glaucoma-like progression (9). Given that multiple anti-VEGF injections are often required due to the chronic course of retinopathies, the cumulative long-term effect of IOP elevation may affect the optic nerve (7).
Damage to the optic nerve leads to changes in the peripapillary and intrapapillary regions of the optic nerve head (known as the papillary region or optic disc) (10). In patients with optic nerve damage, peripapillary retinal nerve fiber layer (p-RNFL) thinning occurs earlier prior to other manifestations, such as loss of vision or functional visual field defects (11). Therefore, measurement of p-RNFL thickness is a more sensitive method for detecting early optic nerve damage. Anti-VEGFs are effective not only for resolving macular edema, but also for vasoconstriction (12). Thus, it can be hypothesized that the therapeutic anti-VEGFs may induce vasoconstriction in the papillary/peripapillary vessels, resulting in a subsequent decrease in ocular perfusion. The restrained blood flow induces alterations to the extracellular matrix in the optic nerve and retina, which leads to progressive optic nerve degeneration and loss of vision (13). In the present review, two indicators that show optic nerve damage, p-RNFL thickness and papillary/peripapillary blood circulation, are discussed in detail.
Multiple databases were searched and no reviews were found on this topic. Previous reviews have focused on the resolution of macular edema and the changes in the macular RNFL by anti-VEGFs. The novelty of the present review lay in its comprehensive discussion of the effects of anti-VEGF on p-RNFL and papillary/peripapillary blood circulation to explore whether the anti-VEGFs cause damage to the optic nerve.
Pathogenicity of VEGF upregulation in retinopathies
Retinal ischemia is a fundamental pathological factor associated with retinopathies, such as retinal vein occlusion (RVO), diabetic retinopathy (DR), age-related macular degeneration (AMD) and diabetic macular edema (DME). Upregulation of VEGF secreted by human RPE cells is a fundamental cause of abnormal angiogenesis (14) and is also considered to be one of the pathogenic causes of numerous retinopathies.
Pathogenicity of oxidative stress/reactive oxygen species (ROS)-mediated imbalances between VEGF and pigment epithelium-derived factor (PEDF)
Reduction/oxidation (redox) imbalances, which are caused by imbalances in pro-oxidant/antioxidant reactions increase oxidative stress (OS), resulting in retinopathies; ROS are one of the most important oxidants (15). RPE cells secrete a variety of cytokines, including VEGF (an angiogenic stimulator) and PEDF (an angiogenic inhibitor) (14,16). Physiologically, in the retina, there is a balance between PEDF and VEGF, which is essential for retinal health. VEGF-induced angiogenesis is normally inhibited by PEDF (17). An increased VEGF/PEDF ratio can lead to retinopathies (18). In a model of ischemia-induced retinal neovascularization, the retina shows a 5-fold increase in VEGF and a 2-fold decrease in PEDF, compared with the age-matched controls (16). Advanced glycation end product (AGE), a senescent macro-protein derivative, formation of which is accelerated in patients with diabetes, elicits OS and induces vascular inflammation and is therefore involved in retinopathies (19,20). PEDF inhibits AGE-induced retinal vascular hyperpermeability and angiogenesis by blocking the generation of ROS and subsequent ROS-mediated upregulation of VEGF (21). These findings suggest that an imbalance between angiogenic stimulators and inhibitors contributes to retinopathies.
Pathogenicity of VEGF-mediated inflammation
Inflammation is a critical factor in the pathogenesis of certain retinopathies (14). Endoplasmic reticulum (ER) stress is an inducer of the inflammatory response, which can cause AMD via the interaction of C-reactive protein (CRP) and serum amyloid P protein (14). CRP is an inflammatory biomarker and risk factor for AMD (22). VEGF contributes to the inflammatory process following laser-induced retinopathy (23). In human RPE cells, CRP can induce VEGF and interleukin-8 (IL-8) expression (24). The increased level of inflammation occurs in proliferative DR eyes with larger non-perfusion areas and the expression of IL-8 positively correlates with the non-perfusion area (25). Inflammatory mediators [such as VEGF, hypoxia-inducible factor (HIF)-1α, IL-8 and others] are markedly increased (4-10-fold) compared with the control retina in laser-induced retinopathy (23). Additionally, there is upregulation of VEGF and IFN-γ-inducible protein-10 (IP-10), characteristic inflammatory cytokines present in the aqueous humor of patients with AMD (26,27). These indicate that the VEGF pathway is involved in retinal inflammation and damage and plays a role in the inflammation-induced pathogenesis of retinopathies.
VEGF upregulation and hypoxia/ischemia
DR and RVO are the two most common ischemic retinopathies and upregulation of VEGF further aggravates retinal ischemia and hypoxia, commonly leading to loss of vision (28). Noninvasive perfusion measurement data have suggested that the decreased choroidal circulation and subsequent hypoxia/ischemia can aggravate the pathogenesis of AMD (29-31). In human RPE cells, ER stress upregulates the expression of VEGF via the actions of activating transcription factor (ATF) (32,33). Furthermore, the excessive proteasomal inhibition generates ER stress and induces the expression of VEGF, HIF-1α and angiopoietin-2 to support neovascularization in AMD (34). Hypoxia is also an efficient inducer of VEGF expression (35). VEGF, as an HIF-1α-regulated angiogenic mediator, and HIF-1α are upregulated in the retinal tissue of DR (36).
VEGF upregulation and its association with other factors
VEGF upregulation in retinopathies is also associated with other factors that collectively affect the pathological process, leading to increased VEGF levels. The missense mutation in R345W of fibulin-3 protein causes an accumulation of a misfolded protein within the ER and the retention of the protein in the ER stimulates ER stress, resulting in upregulation of VEGF in human RPE cells (37). Alternatively, increased levels of homocysteine (an ER stressor) are involved in wet AMD (w-AMD) (38). Homocysteine is a toxic metabolite that induces ER stress in the liver in patients who abuse alcohol (39). Notably, excessive alcohol consumption is also considered to be a risk factor for AMD (40). Homocysteine can activate the protein kinase R-like ER kinase (PERK)/ATF4 pathway, which is an important component of ER stress, inducing upregulation of VEGF expression in human RPE cells (32). OS induces the upregulation of ATF4 via arsenite (an oxidative stressor) in human RPE cells (14). Upregulation of ATF4 is sufficient to activate the VEGF promoter (32). These findings suggest that the homocysteine/arsenite induces the expression of VEGF via an ATF4-dependent mechanism.
Types of anti-VEGF and ocular side effects
Types of anti-VEGFs
The commonality among w-AMD, DME, DR and RVO is the vascular permeability and pathological neovascularization induced by VEGF. Intravitreal injections of anti-VEGFs can resolve macular edema, subretinal fluid and neovascularization (12). Although anti-VEGFs were initially developed as a cancer treatment, bevacizumab was first approved by U.S. Food and Drug Administration (FDA) in 2004 for colon cancer treatment (41); however, they are now widely used in various retinopathies, including RVO, DR, AMD and DME. Bevacizumab, a humanized monoclonal antibody with a molecular weight of 149 kDa, has been used in ophthalmology since 2005 as an off-label therapy (42). Pegaptanib, an anti-VEGF drug used in certain retinopathies (w-AMD), was approved by the FDA in 2004 (41). Ranibizumab, approved by the FDA in 2006 for w-AMD, contains the Fab fragment of a humanized IgG1 κ isotype murine monoclonal antibody and has a molecular weight of 48 kDa, almost three times smaller than bevacizumab (43). Aflibercept, with a molecular weight of 115 kDa, was approved by the FDA in 2011 (42).
Recently introduced anti-VEGF drugs for retinopathies include brolucizumab and faricimab. Brolucizumab was approved by the FDA in 2019. Brolucizumab is a single-chain humanized antibody fragment, designed to bind VEGF-A (43,44). Faricimab, a bispecific heterodimeric monoclonal human antibody, has a greater molecular weight than bevacizumab and was approved by the FDA for DME and w-AMD in 2022 (45). Due to their shorter presence on the market, they have not yet been extensively used and studied.
Ocular side effects of anti-VEGF treatments
Intraocular inflammation is a primary ocular adverse effect associated with anti-VEGF therapies (46). The incidence of significant ocular inflammation following ranibizumab injection for AMD ranges from 1.4-2.9%, whereas the incidence following bevacizumab injection is comparatively lower, between 0.09-0.4% (47). An acute increase in IOP is relatively common immediately after an anti-VEGF injection, with most studies reporting spontaneous normalization of IOP within several hours (42,48,49). The incidence of rhegmatogenous retinal detachment post-anti-VEGF injections is low, ranging from 0-0.67% (47,50). Of note, there was no statistically significant difference in the occurrence of rhegmatogenous retinal detachment and retinal tear between eyes treated with anti-VEGF treatments and the control group (46).
Following bevacizumab injection, rare ocular side effects include anterior ischemic optic neuropathy in w-AMD and angioid streaks in pseudoxanthoma elasticum (51,52), central retinal artery occlusion (RAO) and RVO in central RVO, AMD and proliferative DR (53) and hemorrhagic macular infarction in central RVO (54), acute retinal ischemic changes in diabetic rubeosis (55) and sixth nerve palsy (56). Among 4,069 injections (ranibizumab or aflibercept) administered for the treatment of DME (464 cases), w-AMD (187 cases) or RVO (156 cases), only 18 cases (0.44%) exhibited transient central RAO, including four severe cases (ranibizumab: two cases, aflibercept: two cases) (57).
Methods of literature retrieval and summary of retrieved literature
Methods of literature retrieval
The literature search was conducted on January 1, 2025, using PubMed, Clinicalkey (Elsevier), SpringerLink, Cochrane Library and CNKI data-bases. The search used combinations of the following search terms: (anti-VEGF OR aflibercept OR bevacizumab OR ranibizumab OR brolucizumab OR faricimab) AND (papillary OR circumpapillary OR juxtapapillary OR intrapapillary OR optic disk OR optic nerve head OR optic papilla) AND (p-RNFL OR blood flow OR blood vessel OR capillary OR microvascular OR microcirculation). Filters applied included human studies and articles were initially screened based on the titles or abstracts. The inclusion criteria were: i) Original research articles and ii) participants included were aged over 18 years. Case reports were excluded.
Summary of retrieved literature
Studies that assessed p-RNFL thickness using optical coherence tomography angiography (OCTA) and blood circulation parameters using laser speckle flowgraphy (LSFG) [measuring mean blur rate (MBR), relative flow volume (RFV) and blowout score], Canon laser blood flowmeter (CLBF; measuring vessel diameter), scanning laser Doppler flowmetry (SLDF; measuring blood flow) and OCTA [measuring vessel density (VD), vessel length density (VLD), capillary volume (CV), perfusion density (PD) and perfusion index (PI, or flux index)] were included. All studies included in the present review involved patients with RVO, DR, AMD or DME. Previous studies and clinical trials examining the effects of anti-VEGF treatments on p-RNFL and papillary/peripapillary blood circulation are summarized in Tables I and II, respectively. The studies included in the present review encompass an analysis of 3,077 affected eyes treated with anti-VEGF treatments for retinopathies, as detailed in Tables I and II. The underlying mechanisms for the effects of anti-VEGF treatments on p-RNFL and papillary/peripapillary blood circulation are illustrated in Figs. 1 and 2, respectively.
Table IA summary of studies targeting effects of anti-VEGF on p-RNFL in patients with retinopathies. |
Table IISummary of studies targeting effects of anti-VEGF on papillary/peripapillary blood circulation in patients with retinopathies. |
Effects of anti-VEGF on p-RNFL in w-AMD
With increasing life expectancy, the prevalence of patients with AMD is increasing. By 2030, it is projected that 243.4 million individuals will be affected by AMD (58). In the early stages of development of AMD, patients with w-AMD typically exhibit a thicker p-RNFL compared with healthy eyes (59), probably due to the potential fluid shift from macular edema to the p-RNFL (60).
Effects of ranibizumab monotherapy on p-RNFL
Martinez de-la-Casa et al (61) reported significant p-RNFL thinning in patients treated with ranibizumab over 12 months, compared with an untreated control group. Although IOP elevation was observed, no statistical correlations were found between changes in the outer macular ring and changes in p-RNFL (61). It appears unlikely that post-injection p-RNFL thinning is secondary to changes in reduced macular edema possibly extending to the peripapillary region. IOP fluctuations induced by anti-VEGF treatments could potentially damage the p-RNFL (62). Subsequent continuation of this study revealed the long-term effects (8-year follow-up) of ranibizumab on p-RNFL, with statistically significant thinning observed in both the treated and control groups (63). Of note, IOP remained unchanged throughout the study (63). Parlak et al (64) also observed significant thinning in the affected eyes treated with ranibizumab, with no significant change in IOP over 12 months. The p-RNFL thickness decreased with age at a rate of -0.21 µm/year (65). Advanced age in patients with w-AMD is associated with a significant decrease in p-RNFL thickness (66) and higher stages of w-AMD are associated with a thinner p-RNFL (67). These studies suggest that aging and the natural progression of w-AMD may contribute to p-RNFL thinning. Additionally, unexpected p-RNFL thinning in the untreated fellow eyes was reported by Valverde-Megías et al (63) and Parlak et al (64), indicating a potential systemic effect of anti-VEGF on untreated fellow eyes. Another study indicated a significant decrease in p-RNFL thickness in treated eyes over 12 months, with no significant difference in the fellow eyes (68). The authors attributed the thinning of p-RNFL thickness to the anatomical improvement of macular lesions, although a direct effect of ranibizumab on decreased p-RNFL could not be excluded.
Given that ranibizumab may affect fellow eyes of in patients with w-AMD via systemic circulation, certain studies included age-matched healthy subjects as controls. Long-term follow-up revealed no statistically significant differences in p-RNFL thickness (69,70) and IOP (70) between treated, fellow and healthy eyes. Therefore, p-RNFL thinning in fellow eyes may not be associated with a systemic effect of ranibizumab on untreated fellow eyes. The absence of p-RNFL thinning due to ranibizumab has also been reported by Zucchiatti et al (71) over 12 months, El-Ashry et al (72) over 1 month and Sobacı et al (73) over a mean of 14 months. Notably, Horsley et al (74) reported an increase of 5.88 µm in the p-RNFL thickness during a follow-up of 20.1±3.6 months despite no statistical difference (P=0.35) and there were no sustained IOP elevations for any of the patients with w-AMD. This suggests that ranibizumab may not affect p-RNFL in w-AMD.
Effects of aflibercept monotherapy on p-RNFL
The safety of aflibercept in relation to p-RNFL thickness has been evaluated in both long-term and short-term studies. Following aflibercept injections, a significant reduction in p-RNFL thickness was observed in treated eyes over a 12-month period, with no significant changes in intraocular pressure (IOP) (68). Additionally, no significant differences in p-RNFL thickness and IOP were noted in the fellow eyes (68), suggesting that aflibercept does not affect fellow eyes through systemic circulation. Within 2-5 min post-injection, aflibercept induces immediate p-RNFL thinning, particularly in the nasal region and increases IOP, with a significant negative correlation between increased IOP and p-RNFL thinning (75). The IOP spike immediately following anti-VEGF injections may compromise the integrity of the p-RNFL (71), with the nasal p-RNFL being particularly susceptible to IOP fluctuations. Furthermore, the nasal sector p-RNFL is less affected by macular edema in w-AMD prior to anti-VEGF injection (75), potentially making it a more accurate indicator of damage caused by anti-VEGF injections leading to increased IOP, thereby justifying the significant thinning of p-RNFL in the nasal sector. In contrast to the aforementioned studies, Gunay et al (6) reported no significant changes in p-RNFL thickness in patients with w-AMD with macular edema over 12 months and no significant change in IOP was observed. Thus, the p-RNFL thinning attributed to the reduction of macular edema and increased IOP following aflibercept injections contradicts the findings of Gunay et al (6).
Effects of bevacizumab monotherapy on p-RNFL
Regarding the effects of bevacizumab monotherapy on p-RNFL, Entezari et al (76) found a significant reduction in p-RNFL thickness in patients with w-AMD at 12 weeks, which returned values that were comparable to baseline values at 24 weeks. Furthermore, IOP elevation returned to baseline values within 30-60 min in most patients receiving two injections of bevacizumab (76), indicating that fewer bevacizumab injections and short-term IOP increases were probably insufficient to cause p-RNFL damage. However, another prospective study involving 135 patients with w-AMD found no statistical differences in p-RNFL thickness after 12.4±2.4 bevacizumab injections over 24 months (77). Similarly, Sobacı et al (73) observed no changes in p-RNFL after 5.1±1.3 bevacizumab injections over a mean period of 14 months, although IOP was statistically different compared with baseline values. These studies suggest that a greater number of bevacizumab injections do not result in significant p-RNFL damage.
AMD and DME are among the most common causes of blindness in adults. Several macular diseases are prevalent in the elderly and thus, conditions such as AMD and DME can occur concurrently (78). The standard treatment for DME and w-AMD involves anti-VEGF injections (79). Due to the chronic nature of w-AMD and DME, standard treatment regimens typically recommend multiple injections during the first year of treatment (80). Additionally, the duration of effectiveness of anti-VEGF agents is limited (81), necessitating repeated injections to maintain therapeutic effects. A transient and acute volume-related IOP increase is commonly observed following an anti-VEGF injection (82). Although the incidence of delayed and sustained IOP is low after a single or multiple anti-VEGF injections (83), an acute increase in IOP may cause loss of p-RNFL (81). Prophylactic anterior chamber paracentesis (ACP) combined with anti-VEGF treatments offers an effective alternative for preventing the acute rise in IOP (84). Thus, it is important to determine whether an anti-VEGF injection and the transient elevation in IOP adversely affect p-RNFL thickness in patients with w-AMD and DME.
In two short-term studies conducted by Khodabande et al (80) and Soheilian et al (81), patients with w-AMD or DME were randomly assigned to one of two groups: A group that was not treated with prophylactic ACP and the other which was treated with prophylactic ACP. The two studies consistently found that IOP was markedly higher in patients without ACP compared with those with prophylactic ACP following bevacizumab injections and no significant p-RNFL thinning was observed in patients with prophylactic ACP. However, the studies diverged in their conclusions regarding p-RNFL thickness changes in patients without ACP. Soheilian et al (81) reported significant p-RNFL thinning in patients without ACP, whereas Khodabande et al (80) found no significant thinning in this group. This discrepancy may be attributed to the older age and thinner baseline p-RNFL in the study by Soheilian et al (81) compared with those in the study by Khodabande et al (80) (66.4±4.9 vs. 62.9±10 years; 85.3±5.6 vs. 110±19 µm, respectively), potentially rendering them more susceptible to IOP fluctuations. Overall, ACP appears to mitigate acute IOP elevation and offers a certain degree of protection to the p-RNFL.
Effects of a combination of anti-VEGFs on p-RNFL
Regarding the effects of a combination of anti-VEGF treatments on p-RNFL, no statistically significant differences in p-RNFL thickness were observed among patients with w-AMD treated with ranibizumab alone (over 20.1±3.6 months; 13.4±3.6 injections), a combination of ranibizumab and bevacizumab (over 27.1±4.2 months; 17.7±3.7 injections), or a combination of ranibizumab, bevacizumab and pegaptanib (over 27.0±9.7 months; 16.0±5.5 injections) (74). Recent investigations have explored the correlation between the number of anti-VEGF injections and p-RNFL thickness. No significant effect on p-RNFL was observed in patients with w-AMD following a combination of anti-VEGF treatments (5-50 injections) (66,85). However, a study by Wang et al (86) with an extended follow-up period (46.8 ±42.0 months) suggested a dose-response relationship between the number of injections (a combination of bevacizumab, ranibizumab and aflibercept; 3-138 injections) and p-RNFL thinning, which became more pronounced after ~30 injections and 50 months of treatment. These findings indicate that prolonged treatment duration and a higher number of injections may be required to detect damage to the p-RNFL caused by active w-AMD.
Progressive optic neuropathy, characterized by a deficit in p-RNFL and corresponding thinning of the neuroretinal rim tissue, can lead to progressive visual field defects (87,88). Thinning of both p-RNFL and Bruch's membrane opening minimum rim width (BMO-MRW) occurs in early progressive optic neuropathy (89). Moreover, a decrease in the minimum rim area precedes reductions in p-RNFL and visual field loss (90). Theoretically, BMO-MRW serves as a sensitive marker for evaluating the effects of anti-VEGF injections on the optic nerve head. BMO-MRW markedly decreases with an increasing number of anti-VEGF injections, while no significant effect on p-RNFL is observed (66). It appears that BMO-MRW is affected earlier than p-RNFL following long-term anti-VEGF injections, suggesting that BMO-MRW may be a more sensitive marker than p-RNFL. Given the relative lack of literature on this topic, further studies with larger sample sizes are required to draw more concrete conclusions. BMO-MRW declines with age at a rate of -1.34 µm/year and the association with age is stronger with BMO-MRW than with p-RNFL thickness (65). Therefore, the decrease in BMO-MRW after anti-VEGF injections may not necessarily be drug-induced, but is instead likely age-induced.
A significant yet transient elevation in IOP immediately following anti-VEGF administration is relatively common. Typically, IOP returns to baseline levels within 30 min to several hours post-injection (42,48,49,91). The prevalence of sustained IOP increase ranges from 3.4-11% (92). ACP offers a less painful and effective alternative to mitigate the acute rise in IOP following anti-VEGF administration (84). Theoretically, regular administration of ACP may reduce IOP spikes post-anti-VEGF administration, thereby preventing repeated stress on the p-RNFL. In patients with w-AMD who do not receive ACP, the loss in p-RNFL thickness (-2.16±3.60 µm) is markedly greater than in those who receive ACP regularly (93). The p-RNFL thickness declines with age at a rate of -0.21 µm/year (65). Patients with w-AMD who do not receive ACP experience a loss of -0.86 µm/year in p-RNFL thickness after an average of 2.5 years (93). This indicates a greater loss of p-RNFL thickness in long-term anti-VEGF injections for w-AMD than the age-related p-RNFL loss. Furthermore, there is a negative correlation between temporal p-RNFL and IOP following a combination of anti-VEGF injections (94). This suggests that higher IOP measurements are associated with a greater decrease in p-RNFL thickness, indicating that p-RNFL thickness loss is due to IOP spikes rather than age. In patients with w-AMD or DME treated with more than six ranibizumab or aflibercept injections, a significant acute and transient IOP increase is observed 5 min post-injection and significant p-RNFL thinning is observed by the third month (92). Additionally, similar immediate post-injection changes, such as IOP increases, are observed after 1 year (92). This suggests that repeated anti-VEGF injections could lead to irreversible changes in the optic nerve head structures. It appears that IOP elevation may be associated with p-RNFL thinning. The p-RNFL thickness may also be influenced by other factors, such as macular edema, in patients with w-AMD or DME treated with a combination of anti-VEGF treatments. The p-RNFL thickness in these retinopathies affecting the macula is greater than in age-matched control eyes (59). Although changes in p-RNFL thickness are markedly associated with changes in central macular thickness in patients with DME, no such association exists in patients with AMD (60). Whether changes in macular edema in patients with w-AMD or DME affect p-RNFL thickness following a combination of anti-VEGF injections and to what extent, remains unclear.
Stereotactic radiotherapy (SRT) is a novel adjuvant approach for treating w-AMD (95). The mean number of anti-VEGF injections decreases by nearly 50% during the 12 months following SRT compared with the preceding year, while visual acuity improves (96). A single dose of SRT markedly reduces the need for anti-VEGF injections over 2 years without compromising visual acuity (97). Morphological changes only affect the outer retinal layers following SRT (96). It is necessary to investigate whether SRT, in conjunction with decreased anti-VEGF injections, affects p-RNFL thickness. It has been demonstrated that SRT in conjunction with ranibizumab and aflibercept does not lead to significant changes in p-RNFL thickness over 12 months in patients with w-AMD (94). Radiation of SRT can induce microvascular changes; however, only 1% of affected eyes exhibit a worsening of vision (97). Therefore, SRT may be a potential first step in preventing a worsening of vision as a result of a decrease in anti-VEGF injections. A combination of anti-VEGFs and SRT is thus a novel direction for the treatment of w-AMD.
Radial peripapillary capillaries (RPC) constitute the vascular network located within the RNFL (98). The relationship between RPC density and p-RNFL thickness has been extensively investigated in both healthy individuals (99) and those with pathological peripapillary retinas (100). A study suggested that there was a positive correlation between RPC-VD and p-RNFL thickness (101). However, a recent study by Zhuang et al (102) reported significant negative associations between transient reductions in RPC-VD and increases in p-RNFL thickness, primarily in the nasal regions, 1 week after treatment with a combination of conbercept, aflibercept and ranibizumab. These increases in p-RNFL thickness may be attributed to nerve fiber layer edema. Given that both RPC-VD and p-RNFL thickness returned to baseline values within 1-3 months post-injection, it appears that nerve fiber layer edema contributes to these negative correlations.
Brolucizumab has recently been approved for the treatment of w-AMD (103). It offers the potential for greater tissue penetration compared with earlier anti-VEGF agents and can deliver higher molar doses than larger molecules (104,105). Patients with refractory w-AMD who exhibit poor responses to other anti-VEGF treatments are often transitioned to brolucizumab. No significant changes in p-RNFL thickness have been observed within 3 months following a single brolucizumab injection, which is subsequently followed by a combination of aflibercept, bevacizumab and ranibizumab (3-37 injections) (62). However, a significant decrease in temporal p-RNFL thickness is noted 1 month after the brolucizumab injection, with this reduction dissipating after 3 months as macular edema increases (62). Therefore, the decrease in temporal p-RNFL thickness following brolucizumab injection in patients with w-AMD may be associated with improvements in macular edema. A single brolucizumab injection appears to be safe concerning p-RNFL damage in patients with intractable w-AMD.
Effects of anti-VEGF on p-RNFL in w-AMD concomitant with primary open-angle glaucoma (POAG)
POAG is a chronic o ptic neuropathy characterized by the thinning of p-RNFL (106). Elevated IOP is associated with an increased risk of POAG (107). Regular measurements of p-RNFL thickness are crucial for patients with POAG. Patients with POAG concomitant with w-AMD or DME are at risk of vision loss. Consequently, certain studies have investigated whether anti-VEGF treatments lead to further decreases in p-RNFL thickness and elevated IOP in these patients. Following a combination of aflibercept and ranibizumab, patients with w-AMD concomitant with POAG exhibited no changes in IOP; however, a statistically significant thinning of p-RNFL in the temporal quadrant was observed (108). This thinning of p-RNFL was attributed to the reduction of macular edema. A decrease in p-RNFL thickness was also observed in patients with w-AMD and POAG, as well as those with DME and POAG, following bevacizumab injections over 12 months (109). A more pronounced reduction in p-RNFL thickness was observed in patients with DME and POAG (109). Generally, p-RNFL thickness increases in patients with DME and this increment is associated with the degree of macular edema (110). In patients with DME and POAG, the p-RNFL is often thicker than in those with w-AMD and POAG. This increased thickness may account for the more pronounced post-injection changes in p-RNFL thickness. Consequently, ranibizumab, aflibercept and bevacizumab are considered safe therapeutic options for patients with w-AMD concomitant with POAG. Given that patients with POAG typically exhibit a compromised p-RNFL and that patients with w-AMD often present with a thinner p-RNFL, the potential alterations in p-RNFL thickness induced by anti-VEGF treatments warrant attention. Therefore, it is prudent to closely monitor p-RNFL thickness during anti-VEGF injections in patients with w-AMD, particularly those with DME and POAG.
Effects of anti-VEGF on p-RNFL in treated eyes with w-AMD and in the fellow eyes of patients with dry AMD
AMD is categorized into two types: W-AMD and dry AMD (d-AMD). While no effective treatment has been identified for d-AMD, w-AMD is successfully managed with anti-VEGF therapies (109). To compare p-RNFL thickness in treated w-AMD eyes with that in fellow d-AMD eyes, several studies have been conducted. However, the results of these studies vary. Wichrowska et al (58) reported that post-injection p-RNFL thickness in treated w-AMD eyes was 6.16 µm thinner (not statistically significant) compared with untreated fellow d-AMD eyes, with an increase in p-RNFL thickness observed in the nasal quadrant of the papillary region. Conversely, a study by Yau et al (111) indicated that post-injection p-RNFL thickness in treated w-AMD eyes was markedly greater than in non-treated fellow d-AMD eyes, with a notable increase in the temporal RNFL in the treated group. The authors attributed this to a fluid shift, spreading to the p-RNFL, due to macular thickening. Post-injection changes in p-RNFL thickness in the temporal quadrant markedly correlate with changes in central macular thickness (60). In patients with DR with macular edema, the p-RNFL was significantly thicker compared with a control group (temporal quadrant: 117.1±43.3 vs. 81.2±13.8 µm; nasal quadrant: 87.6±22.1 vs. 70.8±11.2 µm) (112). Furthermore, macular edema predominantly affected temporal and nasal p-RNFL thicknesses in patients with DME (110). The fluid shift associated with macular edema may extend to the temporal and nasal p-RNFL, explaining the observed thickening in different p-RNFL quadrants.
Effects of anti-VEGF on p-RNFL in DR
DME is the leading cause of visual impairment in patients with diabetes mellitus (113). DME and macular ischemia are primary contributors to DR-associated visual loss (114). Previously, focal/grid laser photocoagulation was the standard treatment (115), but its use is now primarily restricted to non-center-involved DME cases (116). Current treatment options for DME include anti-VEGF injections or dexamethasone implants (114). The p-RNFL thickness is generally greater in patients with DME than in age-matched controls and this increase markedly correlates with the degree of macular edema and peripapillary retinal thickness (59,60,112). Therefore, p-RNFL thickness measurements may be influenced by these conditions following anti-VEGF treatment in DR.
The post-injection p-RNFL thickness in patients with DR and macular edema is markedly reduced (110,112), with the most pronounced reduction observed in the temporal sector (110). This thinning of the p-RNFL may be attributed to changes in macular tomography following the resolution of macular edema (110). Given that p-RNFL thickness is strongly influenced by macular edema, the observed changes may not accurately reflect the actual gain or loss of p-RNFL in patients with DR with macular edema. However, Viggiano et al (114) reported no statistically significant changes in p-RNFL thickness and IOP fluctuations in patients with DR treated with anti-VEGF and dexamethasone. However, the authors did not specify whether macular edema was resolved post-anti-VEGF treatment, leaving it unclear whether the unchanged p-RNFL thickness and IOP were due to unresolved macular edema.
While panretinal photocoagulation (PRP) remains the standard treatment for proliferative DR, anti-VEGF injections have gained popularity as an adjunctive treatment for proliferative DR. PRP and anti-VEGF injections have both been associated with p-RNFL thinning in several studies (61,102). The effect of DME on p-RNFL thickness has been noted, with patients exhibiting a thicker p-RNFL, indicative of inner retinal edema (110,112). Consequently, the thinning of p-RNFL following anti-VEGF treatment could be due to the resolution of inner retinal edema. Significant p-RNFL thinning after proliferative DR treatment may be attributed to axonal loss secondary to PRP treatment (117). Studies have reported on the combined effects of PRP and anti-VEGF on p-RNFL. Unlike patients treated solely with PRP, where p-RNFL thickness is markedly greater than baseline, no significant p-RNFL changes were observed in patients with DR treated with both PRP and anti-VEGF injections (118). The thickening of p-RNFL could be associated with PRP-induced intra-retinal inflammation, which increases capillary permeability and results in axonal edema due to cytokine release (117). Elevated levels of inflammatory factors are detected in the proliferative DR eyes with larger non-perfusion areas (25). These findings suggest that anti-VEGF injections may mitigate the effects of PRP and protect p-RNFL. Proliferative DR eyes treated with anti-VEGFs exhibit greater p-RNFL thinning than those treated with PRP after 2 years (119). Although diabetic retinal thickening is typically attributed to outer retinal edema, the inner retina, including p-RNFL, may also become edematous (110,112). Therefore, a reduction in nerve fiber layer edema (inner retina) could contribute to p-RNFL thinning in patients treated with anti-VEGFs (119). The p-RNFL consists of the axons of retinal ganglion cells that form nerve fiber bundles converging upon the optic nerve head (120). There is a weak negative association between p-RNFL thickness and visual field in DR patients treated with anti-VEGFs (thinner retina, less field loss), whereas a positive association (thinner retina, more field loss) is observed in patients treated with PRP. (119). Thus, a loss of axons may be responsible for the p-RNFL thinning in patients treated with PRP. In patients treated with anti-VEGF injections, it is also possible that the damage to the axons is not sufficient to influence the visual field function. Therefore, it is not possible to rule out that the p-RNFL thinning could be associated with the loss of axons after anti-VEGF treatment.
Effects of anti-VEGF on p-RNFL in RVO
RVO is the second most common retinal vascular disorder, after DR (121). RVO impairs vision and induces macular edema (122) and is categorized into branch and central RVOs based on the location of the vascular obstruction (123). Although various factors contribute to vision loss in RVO, macular edema secondary to RVO is the predominant cause (124). The p-RNFL thickness in eyes affected by RVO is associated with central macular thickness (125). Consequently, retinal edema is typically observed in the early stages of RVO, leading to an increase in p-RNFL thickness in the affected eyes (126). Studies examining the relationship between p-RNFL thickness and retinal/macular edema in patients with RVO have demonstrated that both p-RNFL and central macular thickness are elevated following the acute event in both branch and central RVO due to the ensuing acute edema, with significant reductions observed after anti-VEGF injections (127). Pre- and post-injection p-RNFL thickness is notably greater in patients with central RVO compared with those with branch RVO (127). Patients with central RVO-associated macular edema generally experience more severe vision loss than those with branch RVO, with deterioration often persisting despite treatment (128). Therefore, central RVO is likely to result in more severe and extensive edema than branch RVO, leading to a markedly thicker p-RNFL in patients with central RVO compared with those with branch RVO, both before and after anti-VEGF treatment.
Risk factors for RVO include systemic diseases that cause vascular abnormalities, such as hypertension and diabetes (125,126). Increased attention has been directed towards the unaffected fellow eye in patients with RVO. The reduction in p-RNFL thickness is markedly greater in the fellow eyes of patients with RVO than in healthy individuals over time, following an initial increase due to retinal edema (120,129). Age and concurrent systemic comorbidities, such as hypertension, should be considered as factors contributing to the decrease in p-RNFL thickness over time in the fellow eyes of patients with RVO (126). Additionally, p-RNFL thickness is markedly thinner in the fellow eyes of patients with central RVO compared with those of patients with branch RVO and healthy individuals over time, suggesting that the fellow eyes of patients with central RVO are more susceptible to p-RNFL damage (125). These findings indicate that the p-RNFL of the fellow eyes of patients with RVO is prone to damage, necessitating increased attention to the fellow eye when administering anti-VEGF treatments.
Effects of anti-VEGF on papillary/peripapillary blood circulation in w-AMD
In patients with w-AMD, the VD values decrease in both affected and unaffected eyes compared with healthy individuals (130). A number of studies have documented changes in blood circulation parameters in the papillary/peripapillary regions of patients with w-AMD following anti-VEGF treatment, including reductions in peripapillary VD (102), peripapillary arterial diameter (131), peripapillary pulsatility index (PI) (85), MBR (132-134), peripapillary arteriolar blood flow and blood flow velocity (131) and peripapillary PD (85).
A significant association has been identified between peripapillary VD and p-RNFL thickness in healthy individuals, while a negative association between age and peripapillary VD is observed in the peripapillary region (135). Due to their distinct pattern and distribution, RPC may be more susceptible to IOP than other retinal capillaries (99). Consequently, several studies have been conducted to examine changes in p-RNFL and RPC following anti-VEGF injections. A notable reduction in peripapillary VD in RPC and an increase in p-RNFL thickness, particularly in the nasal regions, are observed 1 week after anti-VEGF injections for w-AMD. Subsequently, over 1-3 months, peripapillary VD and p-RNFL thickness generally returned to baseline values (102). w-AMD is considered not only an exudative vascular event but also a chronic inflammatory disease of the retina (136). The overexpression of VEGF and IP-10 characterizes the inflammatory cytokine profile in the aqueous humor of patients with w-AMD (26,27). IP-10 is implicated in the pathogenesis of w-AMD (137) and functions as a pro-inflammatory cytokine (138). Following anti-VEGF treatment, IP-10 levels increase (26). A transient p-RNFL edema is observed post-anti-VEGF treatment for w-AMD (102), potentially representing an early manifestation of inflammation. Given the strong association between a decrease in PRC-VD and an increase in p-RNFL thickness, the p-RNFL edema may be a response to the reduction in PRC-VD (102).
A number of studies have examined alterations in MBR following anti-VEGF injections for w-AMD. Post-injection, the MBR of the optic nerve head in w-AMD patients is markedly reduced after 30 min (132), 45 min (134) and 1 week (133), with these reductions persisting throughout a 3-month follow-up period (133). In the peripapillary retinal region, the arteriolar diameter markedly decreases in patients with w-AMD; however, arterial blood flow does not exhibit significant changes (131). It remains unclear whether the measurement of a non-significant change in blood flow, despite a significant effect on arteriolar diameter, is attributable to the technical limitations of CLBF and SLDF. With the marked post-injection decreases in MBR, IOP notably increased in the treated eyes and return to baseline values after 30 min; however, the MBR at the optic nerve head and the region of large vessels was markedly reduced for up to 60 min (134). Conversely, other studies have reported that significant decreases in MBR in the treated eyes were accompanied by a stable IOP (102) and the peripapillary PD and PI were not associated with post-injection IOP (85). Furthermore, considering that the optic nerve head can autoregulate its blood flow in response to experimental changes in ocular perfusion pressure induced by IOP elevations (132), the changes in papillary/peripapillary blood circulation parameters in patients with w-AMD could reflect the direct pharmacologic effects of anti-VEGFs on the vasculature of the papillary/peripapillary regions. These effects could potentially disrupt the normal morphological and functional characteristics of blood vessels in these regions (102). These findings warrant further investigation into the potentially adverse effects of anti-VEGF treatment on papillary/peripapillary microcirculation, particularly in vulnerable eyes. No changes in MBR have been observed in the untreated fellow eyes of patients with w-AMD following anti-VEGF injection (133,134), suggesting no potential effect of anti-VEGF on the untreated fellow eye via the systemic route. Additionally, the peripapillary blood circulation parameters are not associated with the number of anti-VEGF injections (85) or the types of anti-VEGF treatments (132).
Effects of anti-VEGF on papillary/peripapillary blood circulation in DR
Anti-VEGFs are short-acting drugs and a single anti-VEGF injection is generally not sufficient for the elimination of retinopathies (139). An acute rise of IOP appears to be relatively common immediately after anti-VEGF injection, with most studies indicating the spontaneous normalization of IOP within 30 min to several hours (42,48,49,91). Short-term IOP spikes have been reported to range from 49 mmHg (7) to 72 mmHg (140), but can be as high as ≥90 mmHg (8) after an anti-VEGF injection. Given the need for frequent injections, particularly in patients with DR, there are reports of long-term IOP increases. During a follow-up of >12 months, anti-VEGF injections are associated with sustained IOP elevations and this is likely due to the administration of multiple injections (141). The repeated episodes of IOP spikes might lead to glaucoma-like progression (9). An acute increase in IOP and a reduction in peripapillary perfusion density occur immediately after an anti-VEGF injection in patients with DR (140). With the increase in IOP (a maximum of 49 mmHg), it is not common to see changes in VLD and VD during a short-term follow-up (7). Furthermore, IOP spikes in patients who have received multiple anti-VEGF injections may resolve after a day (7). This suggests that multiple anti-VEGF injections can lead to persistent increased IOP. However, the question of whether the post-injection acute IOP increase is associated with the decreased peripapillary perfusion remains to be causally determined. Therefore, an elevated IOP due to anti-VEGF injections remains a concern, despite its generally favorable safety profile (8).
A transient but significant decrease in peripapillary CV is observed in patients with DR 1-2 weeks after anti-VEGF injections, suggesting that anti-VEGF treatments have a transient inhibitory effect on the peripapillary distribution, which gradually diminishes over time (142). Although anti-VEGF treatments reduce angiogenic drive and neovascularization (1), they do not cause a reorganization of the retinal microcirculation distribution. This may explain a key limitation of current anti-VEGF treatments: Their short-lived effects (139). In a rabbit model, anti-VEGF treatments suppressed vascular leakage for 8-10 weeks, before recurrence of leakage to pre-treatment levels (143). Symptoms frequently recur as soon as the effect of anti-VEGF treatments wears off. This leads to the need for frequent anti-VEGF injections. Furthermore, no correlation is observed between the decreases in the peripapillary CV distribution and the improvements in the best-corrected visual acuity 3 months post-injection (142). This suggests that anti-VEGF treatment improves visual acuity primarily by promoting the resolution of macular edema rather than changing peripapillary CV distribution.
PRP has been established for >40 years as an effective treatment for high-risk proliferative DR (144). However, PRP can cause several complications, such as visual field defects, decreased visual acuity (145), epiretinal membrane formation, macular edema (146), retinal detachment and vitreous hemorrhage (144). Anti-VEGF treatments were found to be superior to PRP after a 2-year follow-up (144). An RPC-VD increase is observed in patients with proliferative DR treated with anti-VEGF and a decrease was found in those treated with PRP (147). Moreover, the visual acuity gain was higher in patients treated with anti-VEGF than in those treated with PRP (147). Thus, anti-VEGF markedly improves proliferative DR compared with the negative effect of PRP on RPC-VD.
Effects of anti-VEGF on papillary/peripapillary blood circulation in RVO
VD of RPC in the fellow and affected eyes in patients with RVO is markedly lower than that in the normal subjects (148). In different retinal layers, eyes affected by RVO compared with the unaffected eyes and the unaffected fellow eyes as compared with the eyes of the healthy subjects, showed a lower VD (149). Macular edema secondary to branch RVO is a common retinal vascular condition that causes severe visual loss. Several therapies have been introduced to treat edema, such as anti-VEGF treatments (127), laser photocoagulation (150), intravitreal or sub-Tenon's injection of triamcinolone acetonide (151) and pars plana vitrectomy (152). At present, anti-VEGF treatments are the strongest evidence-based therapy, reaching evidence level I (153).
Prior to treatment, the RPC-VD in patients with branch RVO is lower than that in the fellow eyes (154-156) and the RPC-VD in the fellow eyes is lower than in normal subjects (155,156). Additionally, before treatment, the p-RNFL thickness in patients with branch RVO is greater than that in the fellow eyes (154-156), while the p-RNFL thickness in the fellow eyes is reduced compared with normal subjects (154,156). This suggests that the microvasculature of the unaffected fellow eyes also exhibits ischemic changes and alterations in the p-RNFL are already present. Consequently, greater attention should be directed towards the prevention of complications in the unaffected fellow eye and the management of systemic diseases. However, there are instances where no significant difference in p-RNFL thickness is observed between the fellow eyes and those of normal subjects (155), indicating that macular edema may not yet have affected the p-RNFL. Following anti-VEGF injections, a significant decrease in p-RNFL thickness is observed in the treated eyes, accompanied by an increase in RPC-VD at 3 months (155), 4 months (154) and 6 months (156). The initial thickening of the p-RNFL before treatment, followed by thinning post-anti-VEGF injections, is attributed to the regression of edema. Regarding the fellow eyes, Liu et al (154) reported no significant differences in p-RNFL thickness and RPC-VD before and after treatment. Similarly, Nicolai et al (157) found that RPC-VD in the fellow eyes was not statistically significant, while post-injection RPC-VD was markedly elevated in the affected eyes with central RVO; however, the RPC-VD of the affected eye remained lower than that of the fellow eyes. The authors attributed the gradual increase following anti-VEGF therapy to a sudden reduction in RPC-VD after an RVO event (157). The absence of changes in the fellow eyes indicates that there is no potential systemic effect of anti-VEGF on the untreated fellow eye.
In a study examining branch RVO with macular edema, changes in the vessel MBR were investigated. The findings indicated that the MBR values at 1, 3 and 6 months were 38.3±7.3, 37.4±9.4 and 41.2±7.3%, respectively (158). When compared with the baseline level of 40.2±8.5%, the slight increase observed at 6 months in the treated eyes was comparable to that in the unaffected fellow eyes, which was 41.0±8.3% (158). By contrast, Nagasato et al (159) reported a slight decrease in MBR in eyes affected by central RVO, with values of 34.8±11.2% at baseline, 32.0±10.3% at 1 month, 31.6±10.9% at 3 months and 32.9±12.2% at 6 months, although these changes did not reach statistical significance. The discrepancies between the two studies may be attributed to measurement errors caused by the dilation or blood stasis of papillary capillary vessels, which obscure the boundary between the vessel and tissue in LSFG images. Furthermore, Nagasato et al (159) also observed that best-corrected visual acuity and retinal sensitivity at 1, 3 and 6 months were markedly improved compared with baseline. Similarly, another study indicated that visual acuity markedly improved and macular thickness markedly decreased during a 2-month follow-up, with a notable reduction in MBR and RFV following anti-VEGF treatment in branch RVO eyes (160). These findings suggest that anti-VEGF treatment induces transient vasoconstriction of the papillary/peripapillary vessels, leading to a reduction in blood flow and velocity. The alterations in papillary/peripapillary microcirculation may not affect vision and retinal sensitivity.
Effects of anti-VEGF on papillary/peripapillary blood circulation in DME
In patients with DME, the MBR in the papillary and peripapillary retinal vessels markedly decreases at 1 week (12), 2 weeks (161) and 1 month (162) post-injection. This reduction is associated with a decrease in macular edema and an improvement in best-corrected visual acuity. No significant differences in MBR reduction were observed between different types of anti-VEGF agents, such as aflibercept and faricimab (162). During anti-VEGF treatment, there is a notable reduction in vascular calibers, an indicator of vascular integrity, in the retina of DME patients and macular edema is markedly reduced (163). It is established that VEGF induces vasodilation of retinal vessels, increasing retinal blood flow and velocity, likely due to elevated nitric oxide levels (161,164). Anti-VEGF agents are effective not only in resolving macular edema but also in inducing vasoconstriction (12). Furthermore, post-injection regression of macular edema was not correlated with a reduction in vascular caliber in the retinal vessels of patients with DME (163). This suggests that anti-VEGF therapy reduces macular edema and improves vision, potentially, also diminishing papillary and peripapillary microcirculation and disrupting vascular integrity due to vasoconstriction.
A meta-analysis of 21 randomized clinical trials involving 9,557 patients with DME reported that the frequency of systemic side effects following anti-VEGF treatment was not high in patients with retinal diseases (165). In a study by Fung et al (166), which was similar to the aforementioned meta-analysis, an internet-based survey of 5,228 patients with retinopathies was conducted to evaluate systemic complications from anti-VEGF therapy, concluding that none of the adverse event rates exceeded 0.21%. Another review of 10 trials, encompassing >4,000 patients with DME, suggested that the rates of ocular and systemic adverse events following anti-VEGF injections were comparable to those of the controls (167). Thus, there remains controversy regarding the systemic circulation of anti-VEGF agents. Sugimoto et al (12) evaluated the effects of anti-VEGF therapy on the papillary circulation of the fellow eyes in patients with DME and found that, unlike the treated eyes, significant changes in MBR were not observed in the untreated fellow eyes. This suggests that anti-VEGF agents may not enter the systemic circulation at concentrations sufficient to affect the circulation of the fellow eyes. However, the precise mechanisms causing systemic complications in patients with retinopathies treated with anti-VEGF agents remain unclear, necessitating further research. Additionally, no significant short-term changes are observed in RPC-VD, IOP and p-RNFL thickness in DME eyes treated with anti-VEGF agents (114). This indicates no short-term effects of anti-VEGF therapy on peripapillary microcirculation; however, further prospective studies with longer follow-up periods are required to confirm these findings.
Discussions on effects of anti-VEGF on p-RNFL in retinopathies
Thinning p-RNFL in the affected eyes
In examining the long-term effects (≥12 months), a significant thinning of the p-RNFL was observed in patients with w-AMD from 12 months to 8 years post-injection (61,63,68,86,92-94) and in patients with DR after 24 months (119). Regarding short-term effects (<12 months), significant p-RNFL thinning was noted in patients with w-AMD from 2 min to 4 months post-injection (60,75,76,81,108,109,168), in patients with DR from 1-6 months post-injection (110,112) and in RVO patients from 3-9 months post-injection (127,154-156).
Thickening p-RNFL in the affected eyes
Significant p-RNFL thickening was observed in patients with w-AMD from 3-47.9 months post-injection (58,102,111). A slight increase in p-RNFL thickness post-injection was found in patients with w-AMD (74) or non-proliferative DR concomitant with DME (114), although this increase did not reach statistical significance.
No changes in p-RNFL thickness in the affected eyes
No changes in p-RNFL thickness have been reported in several studies, indicating no significant alterations in p-RNFL thickness in patients with w-AMD during a follow-up period of 1-96 months post-injection (6,69,70-73,77,80). Of note, repeated anti-VEGF injections appeared to have no detrimental effects on p-RNFL.
Factors contributing to discrepancies in p-RNFL thickness
Contradictory results have been described in the effects of anti-VEGF on p-RNFL in retinopathies, such as p-RNFL thinning, p-RNFL thickening and no change. The present study systematically categorize factors contributing to discrepancies as IOP, edema, normal course of retinopathies, aging and co-morbidities. For p-RNFL thinning, studies are divided into long-term and short-term based on study duration.
Role of IOP spikes
For long-term p-RNFL thinning, data are mixed regarding the relationship between post-injection IOP increase and p-RNFL thinning. Certain studies suggest that p-RNFL thickness loss could be attributed to IOP spikes rather than age, with repeated anti-VEGF injections potentially leading to changes in optic nerve head structures (92-94). However, other findings indicate no changes in IOP accompanying p-RNFL thinning (63,68). Thus, the involvement of post-injection IOP elevation in long-term p-RNFL thinning remains contested, necessitating further investigation. For short-term p-RNFL thinning, the relationship between elevated IOP and p-RNFL thinning is also debated. A significant negative correlation between increased IOP and p-RNFL thinning, with IOP spikes immediately after anti-VEGF injection potentially affecting p-RNFL integrity, has been observed (71,75). Conversely, several studies have reported that short-term increased IOP may not be severe enough to cause p-RNFL damage (76,108). In cases where no changes in p-RNFL thickness were observed, sustained IOP was not detected (6,71-73,77).
Effect of edema
The long-term thinning of the p-RNFL is probably attributable to the anatomical improvement of macular lesions. By contrast, the short-term thinning of p-RNFL may be explained by the regression of retinal/macular edema following anti-VEGF injections (119,156). Another potential explanation is that in patients newly diagnosed with w-AMD, baseline p-RNFL values may have been elevated due to edema spreading from the macula, rather than actual thinning of the p-RNFL (111). Regarding the thickening of p-RNFL, there are two perspectives on the effect of edema. One view suggests that anti-VEGF injections may transiently affect relatively normal retinal vessels, leading to decreased blood flow and subsequent nerve fiber edema (102). Alternatively, the thickened p-RNFL may result from fluid spreading from the thickened central retina in patients with w-AMD (111). In studies reporting no changes in p-RNFL thickness, there is no mention of changes in patient edema (6,71-73,77).
Normal course of retinopathies
The normal progression of retinopathies may also contribute to the long-term thinning of p-RNFL, as observed in diabetic retinas (119), suggesting that the thinning could be a natural course rather than an effect of anti-VEGF treatment.
Aging
Aging is another factor, with p-RNFL thickness declining by 0.21 µm per year (65). Advanced age in patients with w-AMD is markedly associated with reduced p-RNFL thickness (66) and higher stages of w-AMD are associated with a thinner p-RNFL (67). Additionally, in patients with unilateral w-AMD, the fellow eyes exhibited thinner p-RNFL over a 12-month study period (42), indicating that aging may be linked to thinning.
Co-morbidities
Co-morbidities also play a role in the long-term thinning of p-RNFL. Hypertensive retinopathy, for example, involves high blood pressure damaging both retinal microcirculation and RNFL (169). Diabetes mellitus is another example, with a thinner p-RNFL observed in patients without diabetic retinopathy (170). Abnormalities in blood vessels by diabetes mellitus and hypertension (systemic diseases) give rise to RVO and glaucomatous optic neuropathy (125). Glaucoma is more prevalent in patients with RVO compared with healthy individuals (126). In patients with glaucoma, RNFL declines by 0.76±0.85 µm per year during 3.7±1.5 years (171). Visual field damage in glaucoma, corresponding with RNFL thinning, is observed in the fellow eyes of certain patients with unilateral RVO (172). These findings suggest that RVO and glaucoma may share systemic risk factors, reflecting a common pathogenic mechanism (172). Consequently, the long-term thinning of p-RNFL in patients with retinopathy may be attributed to coexisting glaucoma.
Effects of anti-VEGF on the unaffected fellow eyes
No changes in the p-RNFL thickness have been observed in the fellow eyes, accompanying the post-injection thinning in the affected eyes after 1 year, indicating no effects via the systemic route (68). However, significant thinning was detected in both the fellow and affected eyes in an 8-year follow-up (63). The effect should have been cumulative over aging, although the systemic route was not excluded.
Correlation between the number of anti-VEGF injections and p-RNFL thickness in the affected eyes
Studies have reported that there are no associations between the number of injections (from 3-50 injections) and the p-RNFL thickness (64,66,68-70,85). However, other studies have suggested that there were associations and the injection number ranged from 11.27±9.31 (58), 19.29±6.92 (94) and ≥30 (86). Additionally, no associations have been reported between the types of anti-VEGF treatments and the p-RNFL thickness (132).
Discussions on effects of anti-VEGF on papillary/peripapillary blood circulation in retinopathies
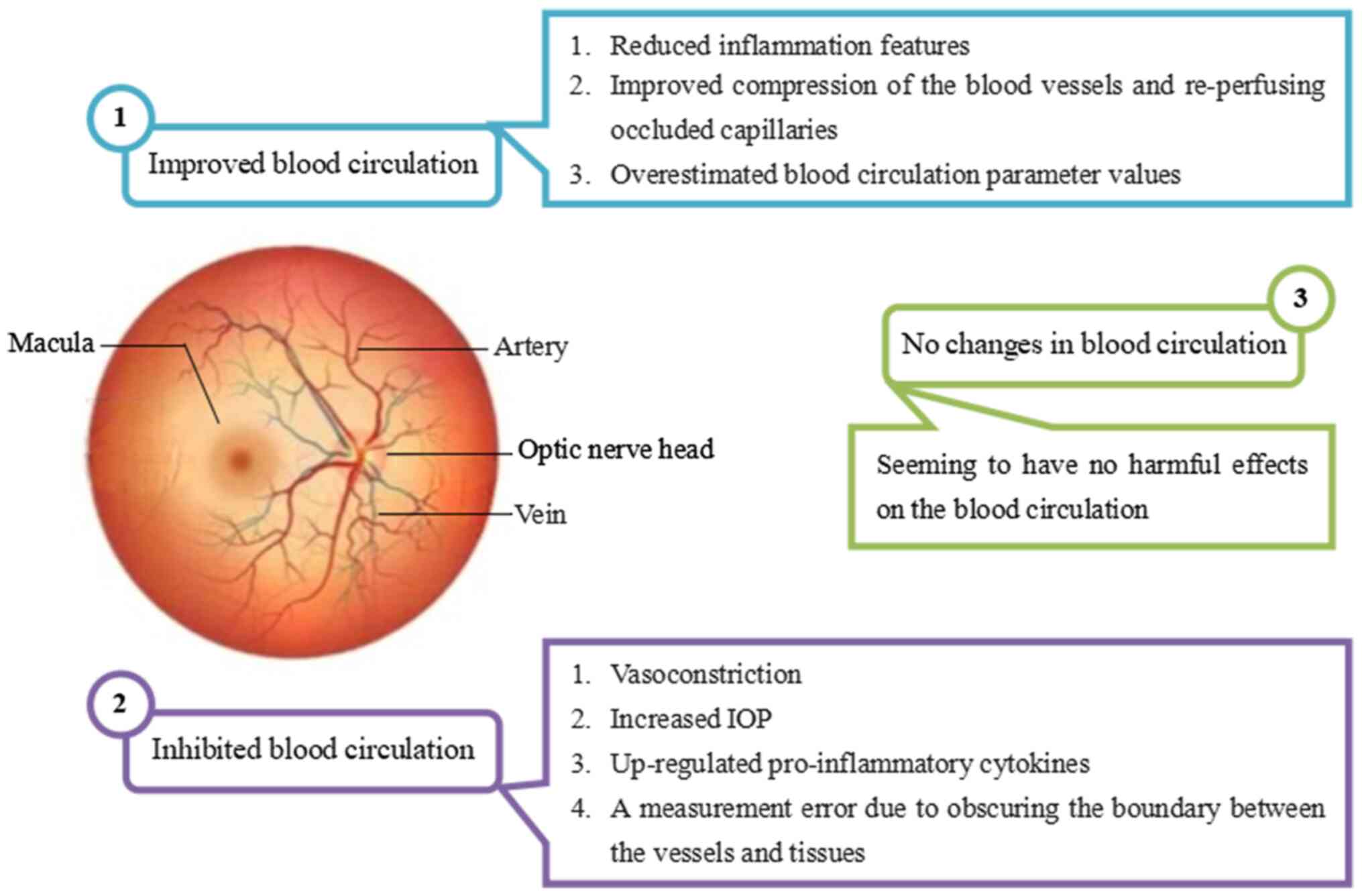
Improvements in blood circulation in the affected eyes
An increase in VD was observed in patients with branch RVO (154-156), central RVO (157) and DR (147) following anti-VEGF treatment. There are three potential explanations for the observed improvement in blood circulation: i) The anti-VEGF treatment reduces inflammatory features in macular edema (156,173-175); ii) the treatment enhances the compression of blood vessels and re-perfuses occluded capillaries (154); or iii) there may be an overestimation of blood circulation parameter values. Prior to anti-VEGF injection, these values might be underestimated due to macular edema, which causes a masking effect or retinal vessel displacement (157) and the movement rate of red blood cells may fall below the detection limit of OCTA due to increased blood viscosity and a decreased blood flow velocity in patients with retinopathies (154). Consequently, post-injection values may be overestimated, suggesting that a statistically significant increase after anti-VEGF injections may not reflect a genuine statistical gain.
Inhibited blood circulation in the affected eyes
Inhibited blood circulation has been observed in the affected eyes. Post-injection decreases in MBR were observed in patients with w-AMD within 30 min-3 months (102,132-134). A slight reduction in MBR post-injection was also noted in patients with central RVO (159) and branch RVO (160). Several studies have reported a decrease in post-injection MBR in patients with DME within 1-4 weeks (12,161,162). Additionally, post-injection decreases in VD (102), flux index (85), arterial diameter (131), perfusion density (140), vascular caliber (163) and CV (142) have been documented.
Potential reasons for the inhibited blood circulation due to anti-VEGF treatments are: i) Vasoconstriction, a possible pharmacological effect of anti-VEGF treatments accompanied by regression of macular edema and improvement in visual acuity (12,161,162) and retinal sensitivity (159); ii) increased IOP; certain studies have suggested a significant post-injection increase in IOP, accompanied by a significant reduction in blood circulation parameters (140). However, other findings indicate no correlation between post-injection changes in IOP and blood circulation parameters (85,102,132). Thus, the involvement of acute increased IOP after anti-VEGF injections in the reduction of peripapillary perfusion remains contested; iii) upregulation of pro-inflammatory cytokines in patients with w-AMD due to anti-VEGF treatments. In certain patients, IP-10 and IL-6 levels increased following anti-VEGF injections (26). The up-regulation of adhesion molecule levels during inflammation caused leukocyte arrest, potentially leading to capillary lumen obstruction (156); iv) measurement error due to obscuring the border between tissue and vessel on LSFG images, caused by blood stasis or capillary vessel dilation (159).
Lack of changes in blood circulation in the affected eyes
Several short-term studies have reported no significant alterations in blood circulation parameters following an anti-VEGF injection (7,114). While these findings suggest an absence of short-term effects, further research employing prospective designs and extended follow-up periods are necessary to validate these results.
Potential mechanistic discussions of the effects of anti-VEGF on the papillary/peripapillary microcirculation in retinopathies
The potential mechanisms underlying the effects of anti-VEGF on papillary and peripapillary microcirculation in retinopathies warrant discussion. VEGF-induced vasodilation may enhance blood flow to neuronal tissues, potentially contributing to neuroprotection in the retina, thereby indicating the neuroprotective role of VEGF (176). Ischemia is one of the important causes of retinopathy. The pathological mechanisms of retinal ischemia-reperfusion injury are complex, involving oxidative stress (OS), inflammation and neuronal apoptosis (177). OS-induced inflammatory cytokines, such as intercellular adhesion molecule-1, are implicated in VEGF transcription, with increased VEGF expression further exacerbating inflammation (178).
The results suggest that anti-VEGF injections mitigate certain types of damage induced by ischemia-reperfusion injury, such as increased VD in patients with branch RVO post-anti-VEGF injections (154-156), potentially due to reduced expression of inflammatory factors (156), decreased retinal cell apoptosis with significant downregulation of cleaved caspase-3 (177) and inhibition of oxidative stress via activation of the Akt/Nrf2 pathway by the anti-VEGF treatment and N-Acetylserotonin (177). However, potential reasons for reduced blood circulation by anti-VEGF treatments include vasoconstriction (161), upregulation of IL-6, a pro-inflammatory cytokine (26) and increased IOP (140). Although vasoconstriction may reduce edema (162), decreased volumetric blood flow to neuronal tissues could adversely affect the optic nerve. IL-6 induces phosphorylation (S47) of Sirtuin 1 through activation of PI3K/AKT/mTOR signaling, thereby inhibiting Sirtuin 1 activity and increasing acetylation of E2F transcription factor 1, consequently elevating apoptosis in RPE cells under OS (27). Additionally, elevated adhesion molecule levels during inflammation can cause leukocyte arrest, potentially obstructing the capillary lumen (156) and resulting in decreased blood flow to neuronal tissue. The volume of anti-VEGF injections could induce ischemia mediated by elevated IOP, possibly leading to decreased blood flow to neuronal tissue (8). However, the increase in IOP due to the volume of anti-VEGF injections typically returns to baseline over time. Therefore, further investigation is required to determine whether anti-VEGF affects the optic nerve due to decreased volumetric blood flow to neuronal tissues caused by increased IOP and if so, whether retinal neuronal cell damage is related to recurrent vascular defects, or whether retinal blood vessels become more susceptible to damage from repeated elevated IOP in patients with retinopathy undergoing multiple anti-VEGF injections remains to be determined, particularly in those with pre-existing vasculopathy or glaucoma.
Effects of anti-VEGF on the unaffected fellow eyes
In patients with retinopathies post-injection, no statistically significant changes in the blood circulation parameters were observed in the fellow eye, accompanying the changes in the affected eyes (12,133,134,154,157). It is possible that anti-VEGF treatments do not enter the systemic circulation in a concentration at a high enough level to affect the circulation of the fellow eyes.
Correlations between the number of anti-VEGF injections and blood circulation in the affected eyes
No correlations have been observed between blood circulation parameters and the different types of anti-VEGF treatments (132,162) or the number of anti-VEGF injections (85).
Conclusions
Present data are mixed on the effects of anti-VEGF on p-RNFL in patients with retinopathies. Researchers commonly attribute the p-RNFL thinning to edema regression, ageing and the physiological course of retinopathies. It is still contested whether the p-RNFL thinning is due to an elevated IOP. The effects of anti-VEGF on papillary/peripapillary blood circulation in retinopathies have been evaluated in numerous studies; however, the results remain inconclusive. Researchers commonly attribute the reduced blood circulation to vasoconstriction, upregulated pro-inflammatory cytokines and measurement errors following anti-VEGF injections. It is still contested whether the reduced blood circulation is due to an elevated IOP.
In summary, these discrepancies across studies indicate the complex responses to anti-VEGF treatments on p-RNFL and papillary/peripapillary blood circulation in patients with retinopathies. No direct evidence of optic nerve damage associated with anti-VEGF treatment has been reported. Studies with large cohorts and long follow-up times are required to explore whether anti-VEGF treatment has adverse effects on p-RNFL and papillary/peripapillary blood circulation, which subsequently leads to damage of the optic nerve in patients with retinopathies.
Availability of data and materials
Not applicable.
Authors' contributions
HW conceived and designed the entire review and wrote the manuscript and created the images. RD and WJ collected the literature. SL, YW, JM and YC proofread the manuscript. PS and MS were fully responsible for drafting and finalizing the review. All authors contributed to the article. Data authentication is not applicable. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Scientific Research Project of Jingjiang People's Hospital Affiliated to Yangzhou University (grant no. JRY-KY-2023-020).
References
|
Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J and Connolly DT: Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 246:1309–1312. 1989. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA and Jin K: Vascular endothelial growth factor overexpression delays neurodegeneration and prolongs survival in amyotrophic lateral sclerosis mice. J Neurosci. 27:304–307. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Du Y, Chen Q, Huang L, Wang S, Yin X, Zhou L, Ye Z, Ren X, Cai Y, Ding X, et al: VEGFR2 and VEGF-C suppresses the epithelial-mesenchymal transition via YAP in retinal pigment epithelial cells. Curr Mol Med. 18:273–286. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Saint-Geniez M, Maharaj AS, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, Darland DC, Young MJ and D'Amore PA: Endogenous VEGF is required for visual function: evidence for a survival role on müller cells and photoreceptors. PLoS One. 3:e35542008. View Article : Google Scholar | |
|
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA and Park JE: Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 331:1480–1487. 1994. View Article : Google Scholar : PubMed/NCBI | |
|
Gunay BO and Esenulku CM: Retinal nerve fibre layer and ganglion cell layer thickness changes following intravitreal aflibercept for age-related macular degeneration. Cutan Ocul Toxicol. 41:91–97. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Arumuganathan N, Wiest MRJ, Toro MD, Hamann T, Fasler K and Zweifel SA: Acute and subacute macular and peripapillary angiographic changes in choroidal and retinal blood flow post-intravitreal injections. Sci Rep. 11:19381"2021. View Article : Google Scholar : PubMed/NCBI | |
|
Cheung CMG, Teo KYC, Tun SBB, Busoy JM, Veluchamy AB and Spaide RF: Differential reperfusion patterns in retinal vascular plexuses following increase in intraocular pressure an OCT angiography study. Sci Rep. 10:165052020. View Article : Google Scholar : PubMed/NCBI | |
|
Gómez-Mariscal M, Muñoz-Negrete FJ and Rebolleda Fernández G: Effects of intravitreal anti-VEGF therapy on glaucoma-like progression in susceptible eyes. J Glaucoma. 29:e54–e55. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Jonas JB, Budde WM and Panda-Jonas S: Ophthalmoscopic evaluation of the optic nerve head. Surv Ophthalmol. 43:293–320. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Alasil T, Wang K, Yu F, Field MG, Lee H, Baniasadi N, de Boer JF, Coleman AL and Chen TC: Correlation of retinal nerve fiber layer thickness and visual fields in glaucoma: A broken stick model. Am J Ophthalmol. 157:953–959. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Sugimoto M, Nunome T, Sakamoto R, Kobayashi M and Kondo M: Effect of intravitreal ranibizumab on the ocular circulation of the untreated fellow eye. Graefes Arch Clin Exp Ophthalmol. 255:1543–1550. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Reinhard J, Renner M, Wiemann S, Shakoor DA, Stute G, Dick HB, Faissner A and Joachim SC: Ischemic injury leads to extracellular matrix alterations in retina and optic nerve. Sci Rep. 7:434702017. View Article : Google Scholar : PubMed/NCBI | |
|
Salminen A, Kauppinen A, Hyttinen JM, Toropainen E and Kaarniranta K: Endoplasmic reticulum stress in age-related macular degeneration: Trigger for neovascularization. Mol Med. 16:535–542. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Kruk J, Kubasik-Kladna K and Aboul-Enein HY: The role oxidative stress in the pathogenesis of eye diseases: Current status and a dual role of physical activity. Mini Rev Med Chem. 16:241–257. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Gao G, Li Y, Zhang D, Gee S, Crosson C and Ma J: Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Lett. 489:270–276. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Angayarkanni N, Selvi R, Pukhraj R, Biswas J, Bhavesh SJ and Tombran-Tink J: Ratio of the vitreous vascular endothelial growth factor and pigment epithelial-derived factor in Eales disease. J Ocul Biol Dis Infor. 2:20–28. 2009. View Article : Google Scholar | |
|
Chen JF, Luo QH, Huang C, Liu WT, Zeng W, Gao Q, Chen P, Chen B and Chen ZL: Expression of VEGF and PEDF in early-stage retinopathy in diabetic Macaca mulatta. Nan Fang Yi Ke Da Xue Xue Bao. 37:1217–1221. 2017.In Chinese. PubMed/NCBI | |
|
Yamagishi S and Imaizumi T: Diabetic vascular complications: Pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des. 11:2279–2299. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Yamagishi S, Matsui T, Nakamura K, Takeuchi M and Imaizumi T: Pigment epithelium-derived factor (PEDF) prevents diabetes- or advanced glycation end products (AGE)-elicited retinal leukostasis. Microvasc Res. 72:86–90. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Yamagishi S, Nakamura K, Matsui T, Inagaki Y, Takenaka K, Jinnouchi Y, Yoshida Y, Matsuura T, Narama I, Motomiya Y, et al: Pigment epithelium-derived factor inhibits advanced glycation end product-induced retinal vascular hyperpermeability by blocking reactive oxygen species-mediated vascular endothelial growth factor expression. J Biol Chem. 281:20213–20220. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Seddon JM, Gensler G, Milton RC, Klein ML and Rifai N: Association between C-reactive protein and age-related macular degeneration. JAMA. 291:704–710. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Hachana S, Fontaine O, Sapieha P, Lesk M, Couture R and Vaucher E: The effects of anti-VEGF and kinin B (1) receptor blockade on retinal inflammation in laser-induced choroidal neovascularization. Br J Pharmacol. 177:1949–1966. 2020. View Article : Google Scholar : | |
|
Wang Y, Bian ZM, Yu WZ, Yan Z, Chen WC and Li XX: Induction of interleukin-8 gene expression and protein secretion by C-reactive protein in ARPE-19 cells. Exp Eye Res. 91:135–142. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Lee MY, Park S, Song JY, Ra H, Baek JU and Baek J: Inflammatory cytokines and retinal nonperfusion area in quiescent proliferative diabetic retinopathy. Cytokine. 154:1557742022. View Article : Google Scholar : PubMed/NCBI | |
|
Sato T, Takeuchi M, Karasawa Y, Enoki T and Ito M: Intraocular inflammatory cytokines in patients with neovascular age-related macular degeneration before and after initiation of intravitreal injection of anti-VEGF inhibitor. Sci Rep. 8:10982018. View Article : Google Scholar : PubMed/NCBI | |
|
Gong C, Qiao L, Feng R, Xu Q, Zhang Y, Fang Z, Shen J and Li S: IL-6-induced acetylation of E2F1 aggravates oxidative damage of retinal pigment epithelial cell line. Exp Eye Res. 200:1082192020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu ZY, Meng YA, Yan B and Luo J: Effect of anti-VEGF treatment on nonperfusion areas in ischemic retinopathy. Int J Ophthalmol. 14:1647–1652. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Metelitsina TI, Grunwald JE, DuPont JC, Ying GS, Brucker AJ and Dunaief JL: Foveolar choroidal circulation and choroidal neovascularization in age-related macular degeneration. Invest Ophthalmol Vis Sci. 49:358–363. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Arjamaa O, Nikinmaa M, Salminen A and Kaarniranta K: Regulatory role of HIF-1alpha in the pathogenesis of age-related macular degeneration (AMD). Ageing Res Rev. 8:349–358. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Feigl B: Age-related maculopathy-linking aetiology and pathophysiological changes to the ischaemia hypothesis. Prog Retin Eye Res. 28:63–86. 2009. View Article : Google Scholar | |
|
Roybal CN, Yang S, Sun CW, Hurtado D, Jagt DL, Townes TM and Abcouwer SF: Homocysteine increases the expression of vascular endothelial growth factor by a mechanism involving endoplasmic reticulum stress and transcription factor ATF4. J Biol Chem. 279:14844–14852. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Roybal CN, Hunsaker LA, Barbash O, Jagt DL and Abcouwer SF: The oxidative stressor arsenite activates vascular endothelial growth factor mRNA transcription by an ATF4-dependent mechanism. J Biol Chem. 280:20331–20339. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Fernandes AF, Guo W, Zhang X, Gallagher M, Ivan M, Taylor A, Pereira P and Shang F: Proteasome-dependent regulation of signal transduction in retinal pigment epithelial cells. Exp Eye Res. 83:1472–1481. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Moenner M, Pluquet O, Bouchecareilh M and Chevet E: Integrated endoplasmic reticulum stress responses in cancer. Cancer Res. 67:10631–10634. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Feng Y, Wang C and Wang G: Inhibition of KCTD10 affects diabetic retinopathy progression by reducing VEGF and affecting angiogenesis. Genet Res (Camb). 2022:41123072022. View Article : Google Scholar : PubMed/NCBI | |
|
Roybal CN, Marmorstein LY, Jagt DL and Abcouwer SF: Aberrant accumulation of fibulin-3 in the endoplasmic reticulum leads to activation of the unfolded protein response and VEGF expression. Invest Ophthalmol Vis Sci. 46:3973–3979. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Axer-Siegel R, Bourla D, Ehrlich R, Dotan G, Benjamini Y, Gavendo S, Weinberger D and Sela BA: Association of neovascular age-related macular degeneration and hyperhomocysteinemia. Am J Ophthalmol. 137:84–89. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Ji C: Dissection of endoplasmic reticulum stress signaling in alcoholic and non-alcoholic liver injury. J Gastroenterol Hepatol. 23(Suppl 1): S16–S24. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Chong EW, Kreis AJ, Wong TY, Simpson JA and Guymer RH: Alcohol consumption and the risk of age-related macular degeneration: A systematic review and meta-analysis. Am J Ophthalmol. 145:707–715. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Kim LA and D'Amore PA: A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am J Pathol. 181:376–379. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Wichrowska M, Goździewska E and Kocięcki J: The safety of Anti-VEGF treatment, in the context of the retinal nerve fibre layer, in patients with wet age-related macular degeneration: A review. Front Biosci (Landmark Ed). 28:2222023. View Article : Google Scholar : PubMed/NCBI | |
|
Fleckenstein M, Keenan TDL, Guymer RH, Chakravarthy U, Schmitz-Valckenberg S, Klaver CC, Wong WT and Chew EY: Age-related macular degeneration. Nat Rev Dis Primers. 7:312021. View Article : Google Scholar : PubMed/NCBI | |
|
Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, Gomes AV, Warburton J, Weichselberger A and Holz FG; HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 127:72–84. 2020. View Article : Google Scholar | |
|
Liberski S, Wichrowska M and Kocięcki J: Aflibercept versus faricimab in the treatment of neovascular age-related macular degeneration and diabetic macular edema: A review. Int J Mol Sci. 23:94242022. View Article : Google Scholar : PubMed/NCBI | |
|
Falavarjani KG and Nguyen QD: Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye (Lond). 27:787–794. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Tolentino M: Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Surv Ophthalmol. 56:95–113. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Bakri SJ, Pulido JS, McCannel CA, Hodge DO, Diehl N and Hillemeier J: Immediate intraocular pressure changes following intravitreal injections of triamcinolone, pegaptanib, and bevacizumab. Eye (Lond). 23:181–185. 2009. View Article : Google Scholar | |
|
Gismondi M, Salati C, Salvetat ML, Zeppieri M and Brusini P: Short-term effect of intravitreal injection of Ranibizumab (Lucentis) on intraocular pressure. J Glaucoma. 18:658–661. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Meyer CH, Michels S, Rodrigues EB, Hager A, Mennel S, Schmidt JC, Helb HM and Farah ME: Incidence of rhegmatogenous retinal detachments after intravitreal antivascular endothelial factor injections. Acta Ophthalmol. 89:70–75. 2011. View Article : Google Scholar | |
|
Ganssauge M, Wilhelm H, Bartz-Schmidt KU and Aisenbrey S: Non-arteritic anterior ischemic optic neuropathy (NA-AION) after intravitreal injection of bevacizumab (Avastin) for treatment of angoid streaks in pseudoxanthoma elasticum. Graefes Arch Clin Exp Ophthalmol. 247:1707–1710. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Hosseini H and Razeghinejad MR: Anterior ischemic optic neuropathy after intravitreal injection of bevacizumab. J Neuroophthalmol. 29:160–161. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Mansour AM, Bynoe LA, Welch JC, Pesavento R, Mahendradas P, Ziemssen F and Pai SA: Retinal vascular events after intravitreal bevacizumab. Acta Ophthalmol. 88:730–735. 2010. View Article : Google Scholar | |
|
Furino C, Boscia F, Cardascia N, Alessio G and Sborgia C: Hemorrhagic macular infarction after intravitreal bevacizumab for central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 9:1–2. 2010. View Article : Google Scholar | |
|
Huang ZL, Lin KH, Lee YC, Sheu MM and Tsai RK: Acute vision loss after intravitreal injection of bevacizumab (avastin) associated with ocular ischemic syndrome. Ophthalmologica. 224:86–89. 2010. View Article : Google Scholar | |
|
Cakmak HB, Toklu Y, Yorgun MA and Simşek S: Isolated sixth nerve palsy after intravitreal bevacizumab injection. Strabismus. 18:18–20. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Damasceno NA, Yannuzzi NA, Maia M, Farah ME, Flynn HW Jr and Damasceno EF: Transient central retina artery occlusion in patients undergoing intravitreal anti vegf injections. Eur J Ophthalmol. 32:2819–2823. 2022. View Article : Google Scholar | |
|
Wichrowska M, Wichrowski P and Kocięcki J: Morphological and functional assessment of the optic nerve head and retinal ganglion cells in dry vs chronically treated wet age-related macular degeneration. Clin Ophthalmol. 16:2373–2384. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Lim HB, Sung JY, Ahn SI, Jo YJ and Kim JY: Retinal nerve fiber layer thickness in various retinal diseases. Optom Vis Sci. 95:247–255. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Dikmetas O, Gungor G, Kapucu Y, Kocabeyoglu S, Kadayıfcılar S, Eldem B, Karahan S and Cankaya AB: Short-term effect of macular edema on the peripapillary retinal nerve fiber layer in patients with wet age-related macular degeneration and diabetic macular edema: A comparative study. Photodiagnosis Photodyn Ther. 42:1036022023. View Article : Google Scholar : PubMed/NCBI | |
|
Martinez-de-la-Casa JM, Ruiz-Calvo A, Saenz-Frances F, Reche-Frutos J, Calvo-Gonzalez C, Donate-Lopez J and Garcia-Feijoo J: Retinal nerve fiber layer thickness changes in patients with age-related macular degeneration treated with intravitreal ranibizumab. Invest Ophthalmol Vis Sci. 53:6214–6218. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Jun SY and Hwang DD: Short-term effect of intravitreal brolucizumab injections in patients with neovascular age-related macular degeneration on retinal nerve fiber layer thickness. Sci Rep. 13:66852023. View Article : Google Scholar : PubMed/NCBI | |
|
Valverde-Megías A, Ruiz-Calvo A, Murciano-Cespedosa A, Hernández-Ruiz S, Martínez-de-la-Casa JM and García-Feijoo J: Long-term effect of intravitreal ranibizumab therapy on retinal nerve fiber layer in eyes with exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 257:1459–1466. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Parlak M, Oner FH and Saatci AO: The long-term effect of intravitreal ranibizumab on retinal nerve fiber layer thickness in exudative age-related macular degeneration. Int Ophthalmol. 35:473–480. 2015. View Article : Google Scholar | |
|
Chauhan BC, Danthurebandara VM, Sharpe GP, Demirel S, Girkin CA, Mardin CY, Scheuerle AF and Burgoyne CF: Bruch's membrane opening minimum rim width and retinal nerve fiber layer thickness in a normal white population: A multicenter study. Ophthalmology. 122:1786–1794. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Boltz A, Spöttl T, Huf W, Weingessel B and Vécsei-Marlovits VP: Effect of intravitreal injections due to neovascular age-related macular degeneration on retinal nerve fiber layer thickness and minimum rim width: A cross sectional study. BMC Ophthalmol. 24:1852024. View Article : Google Scholar : PubMed/NCBI | |
|
Law SK, Small KW and Caprioli J: Peripapillary retinal nerve fiber measurement with spectral-domain optical coherence tomography in age-related macular degeneration. Vision (Basel). 1:262017. View Article : Google Scholar : PubMed/NCBI | |
|
Ahn J, Jang K, Sohn J, Park JI and Hwang DD: Effect of intravitreal ranibizumab and aflibercept injections on retinal nerve fiber layer thickness. Sci Rep. 11:50102021. View Article : Google Scholar : PubMed/NCBI | |
|
Bitirgen G, Belviranli S, Malik RA, Kerimoglu H, Satirtav G and Zengin N: Assessment of corneal sensation, innervation and retinal nerve fiber layer in patients treated with multiple intravitreal ranibizumab injections. PLoS One. 12:e01702712017. View Article : Google Scholar : PubMed/NCBI | |
|
Demirel S, Batioğlu F, Özmert E and Erenler F: The effect of multiple injections of ranibizumab on retinal nerve fiber layer thickness in patients with age-related macular degeneration. Curr Eye Res. 40:87–92. 2015. View Article : Google Scholar | |
|
Zucchiatti I, Cicinelli MV, Parodi MB, Pierro L, Gagliardi M, Accardo A and Bandello F: Effect of intravitreal ranibizumab on ganglion cell complex and peripapillary retinal nerve fiber layer in neovascular age-related macular degeneration using spectral domain optical coherence tomography. Retina. 37:1314–1319. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
El-Ashry MF, Lascaratos G and Dhillon B: Evaluation of the effect of intravitreal ranibizumab injections in patients with neovascular age related macular degeneration on retinal nerve fiber layer thickness using optical coherence tomography. Clin Ophthalmol. 9:1269–1274. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Sobacı G, Güngör R and Ozge G: Effects of multiple intravitreal anti-VEGF injections on retinal nerve fiber layer and intraocular pressure: A comparative clinical study. Int J Ophthalmol. 6:211–215. 2013. | |
|
Horsley MB, Mandava N, Maycotte MA and Kahook MY: Retinal nerve fiber layer thickness in patients receiving chronic anti-vascular endothelial growth factor therapy. Am J Ophthalmol. 150558–561. (e551)2010. View Article : Google Scholar : PubMed/NCBI | |
|
Viggiano P, Buonamassa R, Grassi MO, Boscia G, Borrelli E, Landini L, Evangelista F, Malerba MG, Alessio G and Boscia F: Immediate effect of anti-VEGF injections on optic nerve head: Correlation between intraocular pressure and anatomical peripapillary changes. Eur J Ophthalmol. 34:1174–1182. 2024. View Article : Google Scholar | |
|
Entezari M, Ramezani A and Yaseri M: Changes in retinal nerve fiber layer thickness after two intravitreal bevacizumab injections for wet type age-related macular degeneration. J Ophthalmic Vis Res. 9:449–452. 2014. View Article : Google Scholar | |
|
Zivkovic M, Radosavljevic A, Zlatanovic M, Jaksic V, Davidovic S, Stamenkovic M, Todorovic I and Jaksic J: Influence of multiple Anti-VEGF injections on retinal nerve fiber layer and ganglion cell-inner plexiform layer thickness in patients with exudative age-related macular degeneration. Medicina (Kaunas). 59:1382023. View Article : Google Scholar : PubMed/NCBI | |
|
Klein BE, Klein R and Jensen SC: Open-angle glaucoma and older-onset diabetes. The beaver dam eye study Ophthalmology. 101:1173–1177. 1994. | |
|
Hoguet A, Chen PP, Junk AK, Mruthyunjaya P, Nouri-Mahdavi K, Radhakrishnan S, Takusagawa HL and Chen TC: The effect of anti-vascular endothelial growth factor agents on intraocular pressure and glaucoma: A report by the american academy of ophthalmology. Ophthalmology. 126:611–622. 2019. View Article : Google Scholar | |
|
Khodabande A, Zarei M, Khojasteh H, Mohammadi M, Khameneh EA, Torkashvand A and Davoodabadi M: The effect of acute rises in intraocular pressure after intravitreal bevacizumab injection on the peripapillary retinal nerve fiber layer thickness and the role of anterior chamber paracentesis. J Curr Ophthalmol. 33:12–16. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Soheilian M, Karimi S, Montahae T, Nikkhah H and Mosavi SA: Effects of intravitreal injection of bevacizumab with or without anterior chamber paracentesis on intraocular pressure and peripapillary retinal nerve fiber layer thickness: A prospective study. Graefes Arch Clin Exp Ophthalmol. 255:1705–1712. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Levin AM, Chaya CJ, Kahook MY and Wirostko BM: Intraocular pressure elevation following intravitreal Anti-VEGF injections: Short- and long-term considerations. J Glaucoma. 30:1019–1026. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Mansoori T, Agraharam SG, Manwani S and Balakrishna N: Intraocular pressure changes after intravitreal bevacizumab or ranibizumab injection: A retrospective study. J Curr Ophthalmol. 33:6–11. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Karakahya RH: Anterior chamber paracentesis offers a less painful experience during intravitreal anti-vascular endothelial growth factor administration: An intraindividual study. Cureus. 13:e200512021. | |
|
Türksever C, Hoffmann L and Hatz K: Peripapillary and macular microvasculature in neovascular age-related macular degeneration in long-term and recently started anti-VEGF therapy versus healthy controls. Front. Med (Lausanne). 9:10800522023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang L, Swaminathan SS, Yang J, Barikian A, Shi Y, Shen M, Jiang X, Feuer W, Gregori G and Rosenfeld PJ: Dose-Response relationship between intravitreal injections and retinal nerve fiber layer thinning in age-related macular degeneration. Ophthalmol Retina. 5:648–654. 2021. View Article : Google Scholar | |
|
Weinreb RN and Khaw PT: Primary open-angle glaucoma. Lancet. 363:1711–1720. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Sommer A, Miller NR, Pollack I, Maumenee AE and George T: The nerve fiber layer in the diagnosis of glaucoma. Arch Ophthalmol. 95:2149–2156. 1977. View Article : Google Scholar : PubMed/NCBI | |
|
Cho HK and Kee C: Rate of change in Bruch's membrane opening-minimum rim width and peripapillary RNFL in early normal tension glaucoma. J Clin Med. 9:23212020. View Article : Google Scholar : PubMed/NCBI | |
|
Choi HS, Joo CW, Park SP and Na KI: A decrease in Bruch's membrane opening-minimum rim area precedes decreased retinal nerve fiber layer thickness and visual field loss in glaucoma. J Glaucoma. 30:1033–1038. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
LoBue SA, Gindina S, Saba NJ, Chang T, Davis MJ and Fish S: Clinical features associated with acute elevated intraocular pressure after intravitreal anti-VEGF injections. Clin Ophthalmol. 17:1683–1690. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Gómez-Mariscal M, Puerto B, Muñoz-Negrete FJ, de Juan V and Rebolleda G: Acute and chronic optic nerve head biomechanics and intraocular pressure changes in patients receiving multiple intravitreal injections of anti-VEGF. Graefes Arch Clin Exp Ophthalmol. 257:2221–2231. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Enders P, Sitnilska V, Altay L, Schaub F, Muether PS and Fauser S: Retinal nerve fiber loss in anti-VEGF therapy for age-related macular degeneration can be decreased by anterior chamber paracentesis. Ophthalmologica. 237:111–118. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Ranjbar M, Kurz M, Holzhey A, Rades D and Grisanti S: Changes in peripapillary nerve fiber layer thickness after adjuvant stereotactic radiotherapy in patients with neovascular age-related macular degeneration. Curr Eye Res. 42:1698–1706. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Moshfeghi DM, Kaiser PK and Gertner M: Stereotactic low-voltage x-ray irradiation for age-related macular degeneration. Br J Ophthalmol. 95:185–188. 2011. View Article : Google Scholar | |
|
Ranjbar M, Kurz M, Holzhey A, Melchert C, Rades D and Grisanti S: Stereotactic radiotherapy in neovascular age-related macular degeneration: Real-life efficacy and morphological evaluation of the outer retina-choroid complex. Medicine (Baltimore). 95:e57292016. View Article : Google Scholar : PubMed/NCBI | |
|
Jackson TL, Chakravarthy U, Slakter JS, Muldrew A, Shusterman EM, O'Shaughnessy D, Arnoldussen M, Gertner ME, Danielson L and Moshfeghi DM; INTREPID Study Group: Stereotactic radiotherapy for neovascular age-related macular degeneration: Year 2 results of the INTREPID study. Ophthalmology. 122:138–145. 2015. View Article : Google Scholar | |
|
Chan G, Balaratnasingam C, Xu J, Mammo Z, Han S, Mackenzie P, Merkur A, Kirker A, Albiani D, Sarunic MV and Yu DY: In vivo optical imaging of human retinal capillary networks using speckle variance optical coherence tomography with quantitative clinico-histological correlation. Microvasc Res. 100:32–39. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Mansoori T, Sivaswamy J, Gamalapati JS, Agraharam SG and Balakrishna N: Measurement of radial peripapillary capillary density in the normal human retina using optical coherence tomography angiography. J Glaucoma. 26:241–246. 2017. View Article : Google Scholar | |
|
Jo YH, Sung KR and Yun SC: The relationship between peripapillary vascular density and visual field sensitivity in primary open-angle and angle-closure glaucoma. Invest Ophthalmol Vis Sci. 59:5862–5867. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Liu G, Wang Y and Gao P: Distributions of radial peripapillary capillary density and correlations with retinal nerve fiber layer thickness in normal subjects. Med Sci Monit. 27:e9336012021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhuang X, Su Y, Li M, Zhang L, Mi L, Ji Y, Deng F, Xiao O, Zhang X, Zhou L, et al: A prospective observation of influence of anti-VEGF on optic disc vasculature in nAMD patients. Photodiagnosis Photodyn Ther. 45:1038632024. View Article : Google Scholar | |
|
Dugel PU, Singh RP, Koh A, Ogura Y, Weissgerber G, Gedif K, Jaffe GJ, Tadayoni R, Schmidt-Erfurth U and Holz FG: HAWK and HARRIER: Ninety-Six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 128:89–99. 2021. View Article : Google Scholar | |
|
Holz FG, Dugel PU, Weissgerber G, Hamilton R, Silva R, Bandello F, Larsen M, Weichselberger A, Wenzel A, Schmidt A, et al: Single-Chain antibody fragment VEGF inhibitor RTH258 for neovascular age-related macular degeneration: A randomized controlled study. Ophthalmology. 123:1080–1089. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Dugel PU, Jaffe GJ, Sallstig P, Warburton J, Weichselberger A, Wieland M and Singerman L: Brolucizumab versus aflibercept in participants with neovascular age-related macular degeneration: A randomized trial. Ophthalmology. 124:1296–1304. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Lee K, Bae HW, Lee SY, Seong GJ and Kim CY: Hierarchical cluster analysis of peripapillary retinal nerve fiber layer damage and macular ganglion cell loss in open angle glaucoma. Korean J Ophthalmol. 34:56–66. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Hollands H, Johnson D, Hollands S, Simel DL, Jinapriya D and Sharma S: Do findings on routine examination identify patients at risk for primary open-angle glaucoma? The rational clinical examination systematic review. JAMA. 309:2035–2042. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Rud'ko AS, Budzinskaya MV, Andreeva IV, Karpilova MA and Smirnova TV: Effect of intravitreal injections of ranibizumab and aflibercept on the retinal nerve fiber layer in patients with concomitant neovascular age-related macular degeneration and glaucoma. Vestn Oftalmol. 135:177–183. 2019.In Russian. View Article : Google Scholar | |
|
Kopić A, Biuk D, Barać J, Vinković M, Benašić T and Kopić V: Retinal nerve fiber layer thickness in glaucoma patients treated with multiple intravitreal anti-Vegf (Bevacizumab) injections. Acta Clin Croat. 56:406–414. 2017. | |
|
Hwang DJ, Lee EJ, Lee SY, Park KH and Woo SJ: Effect of diabetic macular edema on peripapillary retinal nerve fiber layer thickness profiles. Invest Ophthalmol Vis Sci. 55:4213–4219. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Yau GL, Campbell RJ, Li C and Sharma S: Peripapillary RNFL thickness in nonexudative versus chronically treated exudative age-related macular degeneration. Can J Ophthalmol. 50:345–349. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Yang HS, Woo JE, Kim MH, Kim DY and Yoon YH: Co-Evaluation of peripapillary RNFL thickness and retinal thickness in patients with diabetic macular edema: RNFL misinterpretation and its adjustment. PLoS One. 12:e01703412017. View Article : Google Scholar : PubMed/NCBI | |
|
Fong DS, Ferris FL III, Davis MD and Chew EY: Causes of severe visual loss in the early treatment diabetic retinopathy study: ETDRS report no. 24. Early treatment diabetic retinopathy study research group. Am J Ophthalmol. 127:137–141. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Viggiano P, Grassi MO, Bisceglia G, Boscia G, Borrelli E, Malerba MG, Fracchiolla A, Alessio G and Boscia F: Short-term peripapillary structural and vascular changes following anti-VEGF vs. Dexamethasone intravitreal therapy in patients with DME. Eur J Ophthalmol. 33:2236–2242. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Photocoagulation for diabetic macular edema. Early treatment diabetic retinopathy study report number 1. Early treatment diabetic retinopathy study research group. Arch Ophthalmol. 103:1796–1806. 1985. View Article : Google Scholar : PubMed/NCBI | |
|
Scott IU, Danis RP, Bressler SB, Bressler NM, Browning DJ and Qin H; Diabetic Retinopathy Clinical Research Network: Effect of focal/grid photocoagulation on visual acuity and retinal thickening in eyes with non-center-involved diabetic macular edema. Retina. 29:613–617. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Eren S, Ozturk T, Yaman A, Oner H and A OS: Retinal nerve fiber layer alterations after photocoagulation: A prospective spectral-domain OCT study. Open Ophthalmol J. 8:82–86. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Roohipour R, Sharifian E, Moghimi S, Fard MA, Ghassemi F, Zarei M, Davoodi S, Bazvand F and Modjtahedi BS: The effect of panretinal photocoagulation (PRP) versus intravitreal bevacizumab (IVB) plus PRP on peripapillary retinal nerve fiber layer (RNFL) thickness analyzed by optical coherence tomography in patients with proliferative diabetic retinopathy. J Ophthalmic Vis Res. 14:157–163. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Jampol LM, Odia I, Glassman AR, Baker CW, Bhorade AM, Han DP, Jaffe GJ, Melia M, Bressler NM and Tanna AP; Diabetic Retinopathy Clinical Research Network: Panretinal photocoagulation versus ranibizumab for proliferative diabetic retinopathy: Comparison of peripapillary retinal nerve fiber layer thickness in a randomized clinical trial. Retina. 39:69–78. 2019. View Article : Google Scholar : | |
|
Shin YI, Lim HB, Koo H, Lee WH and Kim JY: Longitudinal changes in the peripapillary retinal nerve fiber layer thickness in the fellow eyes of unilateral retinal vein occlusion. Sci Rep. 10:77082020. View Article : Google Scholar : PubMed/NCBI | |
|
Klein R, Klein BE, Moss SE and Meuer SM: The epidemiology of retinal vein occlusion: The beaver dam eye study. Trans Am Ophthalmol Soc. 98:133–141. 2000. | |
|
Hahn P and Fekrat S: Best practices for treatment of retinal vein occlusion. Curr Opin Ophthalmol. 23:175–181. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Pulido JS, Flaxel CJ, Adelman RA, Hyman L, Folk JC and Olsen TW: Retinal vein occlusions preferred practice pattern (®) guidelines. Ophthalmology. 123:P182–P208. 2016. View Article : Google Scholar | |
|
Zhang M, Liu Y, Song M, Yu Y, Ruan S, Zheng K, Wang F and Sun X: Intravitreal dexamethasone implant has better retinal perfusion than anti-vascular endothelial growth factor treatment for macular edema secondary to retinal vein occlusion: A five-year real-world study. Ophthalmic Res. 66:247–258. 2023. View Article : Google Scholar | |
|
Ahn J and Hwang DD: Peripapillary retinal nerve fiber layer thickness in patients with unilateral retinal vein occlusion. Sci Rep. 11:181152021. View Article : Google Scholar : PubMed/NCBI | |
|
Goda I, Saliem EA, Mostafa SM, Amin AM, Omran MY, Eltantawy B, Soliman HB, Abu El-Wafa EG, Abdelgbar AA, Osman HO, et al: Longitudinal changes in peri-papillary retinal nerve fiber layer thickness in patients with unilateral branch retinal vein occlusion. Med Hypothesis Discov Innov Ophthalmol. 12:62–69. 2023. View Article : Google Scholar | |
|
Moleiro AF, Godinho G, Madeira C, Pereira AF, Brandão E, Falcão-Reis F, Beato JN and Penas S: Peripapillary and subfoveal choroidal thickness in retinal vein occlusions. Clin Ophthalmol. 16:3775–3783. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Munk MR, Ceklic L, Stillenmunkes R, Chaudhary V, Waheed N, Chhablani J, de Smet MD and Tillmann A: Integrated assessment of OCT, multimodal imaging, and cytokine markers for predicting treatment responses in retinal vein occlusion associated macular edema: A comparative review of Anti-VEGF and steroid therapies. Diagnostics (Basel). 14:19832024. View Article : Google Scholar : PubMed/NCBI | |
|
Shin YI, Nam KY, Lee SE, Lim HB, Lee MW, Jo YJ and Kim JY: Changes in peripapillary microvasculature and retinal thickness in the fellow eyes of patients with unilateral retinal vein occlusion: An OCTA study. Invest Ophthalmol Vis Sci. 60:823–829. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Qiu Y, Sun T, Xu F, Gao P, Tang G and Peng Q: Correlation of vascular change and cognitive impairment in age-related macular degeneration patients. Am J Transl Res. 13:336–348. 2021.PubMed/NCBI | |
|
Fontaine O, Olivier S, Descovich D, Cordahi G, Vaucher E and Lesk MR: The effect of intravitreal injection of bevacizumab on retinal circulation in patients with neovascular macular degeneration. Invest Ophthalmol Vis Sci. 52:7400–7405. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Kato N, Haruta M, Furushima K, Arai R, Matsuo Y and Yoshida S: Decrease in ocular blood flow thirty minutes after intravitreal injections of brolucizumab and aflibercept for neovascular age-related macular degeneration. Clin Ophthalmol. 17:1187–1192. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Mursch-Edlmayr AS, Luft N, Podkowinski D, Ring M, Schmetterer L and Bolz M: Effects of three intravitreal injections of aflibercept on the ocular circulation in eyes with age-related maculopathy. Br J Ophthalmol. 104:53–57. 2020. View Article : Google Scholar | |
|
Mursch-Edlmayr AS, Luft N, Podkowinski D, Ring M, Schmetterer L and Bolz M: Short-term effect on the ocular circulation induced by unilateral intravitreal injection of aflibercept in age-related maculopathy. Acta Ophthalmol. 97:e927–e932. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Ding X, Lu L, Yang J, Chen Y and Ma J: The peripapillary retinal capillary density is highly correlated with its nerve fibre layer in normal population. Clin Hemorheol Microcirc. 74:231–239. 2020. View Article : Google Scholar | |
|
Jager RD, Mieler WF and Miller JW: Age-related macular degeneration. N Engl J Med. 358:2606–2617. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Sakurada Y, Nakamura Y, Yoneyama S, Mabuchi F, Gotoh T, Tateno Y, Sugiyama A, Kubota T and Iijima H: Aqueous humor cytokine levels in patients with polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Ophthalmic Res. 53:2–7. 2015. View Article : Google Scholar | |
|
Obeng-Aboagye E, Frimpong A, Amponsah JA, Danso SE, Owusu EDA and Ofori MF: Inflammatory cytokines as potential biomarkers for early diagnosis of severe malaria in children in Ghana. Malar J. 22:2202023. View Article : Google Scholar : PubMed/NCBI | |
|
Pessoa B, Melo-Beirão J, Meireles A and Menéres P: Challenging clinical cases-a walk through supplemental therapy with intravitreal ranibizumab therapy following treatment of diabetic macular edema with the 0.19 mg fluocinolone acetonide implant (ILUVIEN®. Int Med Case Rep J. 13:437–448. 2020. | |
|
Barash A, Chui TYP, Garcia P and Rosen RB: Acute macular and peripapillary angiographic changes with intravitreal injections. Retina. 40:648–656. 2020. View Article : Google Scholar | |
|
Baek SU, Park IW and Suh W: Long-term intraocular pressure changes after intravitreal injection of bevacizumab. Cutan Ocul Toxicol. 35:310–314. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Zeng L, Liu X, Chen S and Ma J: Quantitative analysis of peripapillary capillary volume using dense B-scan OCT angiography in normal and diabetic retina. Eye Vis (Lond). 11:342024. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Busoy JM, Zaman BAA, Tan QSW, Tan GSW, Barathi VA, Cheung N, Wei JJ, Hunziker W, Hong W, et al: A novel model of persistent retinal neovascularization for the development of sustained anti-VEGF therapies. Exp Eye Res. 174:98–106. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, Antoszyk AN, Baker CW, Berger BB, Bressler NM, Browning D, et al: Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. JAMA. 314:2137–2146. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Reddy SV and Husain D: Panretinal photocoagulation: A review of complications. Semin Ophthalmol. 33:83–88. 2018. View Article : Google Scholar | |
|
Soman M, Ganekal S, Nair U and Nair K: Effect of panretinal photocoagulation on macular morphology and thickness in eyes with proliferative diabetic retinopathy without clinically significant macular edema. Clin Ophthalmol. 6:2013–2017. 2012.PubMed/NCBI | |
|
Helmy AMR, Rashad MA, Gharieb HM, Gomaa WA and Zaki RGE: Optic nerve head perfusion changes in eyes with proliferative diabetic retinopathy treated with intravitreal ranibizumab or photocoagulation: A randomized controlled trial. Med Hypothesis Discov Innov Ophthalmol. 11:144–150. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Fan L, Zhu Y, Sun X, Yu J and Yan H: Patients with unilateral retinal vein occlusion show reduced radial peripapillary capillary density in their fellow eyes. BMC Ophthalmol. 21:4482021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Q, Chan SY, Yan Y, Yang J, Zhou W, Jonas JB and Wei WB: Optical coherence tomography angiography in retinal vein occlusions. Graefes Arch Clin Exp Ophthalmol. 256:1615–1622. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Finkelstein D: Argon laser photocoagulation for macular edema in branch vein occlusion. Ophthalmology. 93:975–977. 1986. View Article : Google Scholar : PubMed/NCBI | |
|
Hayashi K and Hayashi H: Intravitreal versus retrobulbar injections of triamcinolone for macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 139:972–982. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Kumagai K, Furukawa M, Ogino N, Uemura A and Larson E: Long-term outcomes of vitrectomy with or without arteriovenous sheathotomy in branch retinal vein occlusion. Retina. 27:49–54. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Ehlers JP, Kim SJ, Yeh S, Thorne JE, Mruthyunjaya P, Schoenberger SD and Bakri SJ: Therapies for macular edema associated with branch retinal vein occlusion: A report by the american academy of ophthalmology. Ophthalmology. 124:1412–1423. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Liu S: Effect of anti-VEGF therapy on optic disc microcirculation in patients with macular edema secondary to branch retinal vein occlusion based on OCTA. MA thesis. Gannan Medical College; Gannan: pp. 16–21. 2022, In Chinese. | |
|
Sun J: The changes of radial peripapillary capillary density before and after anti-VEGF treatments in patients with branch retinal vein occlusion complicated with macular edema were evaluated by OCTA. MA thesis. Chengde Medical College; Chengde: pp. 17–22. 2022, In Chinese. | |
|
Tan C: Detection and correlation analysis of microvessel density and nerve fiber layer thickness in optic disc before and after anti-vascular endothelial growth factor treatment in patients with retinal vein occlusion. MA thesis. Bengbu Medical College; Bengbu: pp. 25–30. 2021, In Chinese. | |
|
Nicolai M, Franceschi A, Turris S, Rosati A, Pirani V and Mariotti C: Papillary vessel density changes after intravitreal Anti-VEGF injections in hypertensive patients with central retinal vein occlusion: An angio-OCT study. J Clin Med. 8:16362019. View Article : Google Scholar : PubMed/NCBI | |
|
Asano T, Kunikata H, Yasuda M, Nishiguchi KM, Abe T and Nakazawa T: Ocular microcirculation changes, measured with laser speckle flowgraphy and optical coherence tomography angiography, in branch retinal vein occlusion with macular edema treated by ranibizumab. Int Ophthalmol. 41:151–162. 2021. View Article : Google Scholar | |
|
Nagasato D, Mitamura Y, Semba K, Akaiwa K, Nagasawa T, Yoshizumi Y, Tabuchi H and Kiuchi Y: Correlation between optic nerve head circulation and visual function before and after anti-VEGF therapy for central retinal vein occlusion: Prospective, interventional case series. BMC Ophthalmol. 16:362016. View Article : Google Scholar : PubMed/NCBI | |
|
Fukami M, Iwase T, Yamamoto K, Kaneko H, Yasuda S and Terasaki H: Changes in retinal microcirculation after intravitreal ranibizumab injection in eyes with macular edema secondary to branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 58:1246–1255. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Toto L, Evangelista F, Viggiano P, Erroi E, D'Onofrio G, Libertini D, Porreca A, D'Aloisio R, Mariacristina P, Di Antonio L, et al: Changes in ocular blood flow after ranibizumab intravitreal injection for diabetic macular edema measured using laser speckle flowgraphy. Biomed Res Int. 2020:94962422020. View Article : Google Scholar : PubMed/NCBI | |
|
Mizukami T, Mizumoto S, Ishibashi T, Ueno S, Toyonishi T, Tachibana K, Mishima S and Shimomura Y: Changes in ocular blood flow after intravitreal injection for diabetic macular edema between aflibercept and faricimab. Clin Ophthalmol. 18:2407–2416. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Consigli A, Papanastasiou A, Roquelaure D, Wuarin R, Roy S, Thumann G and Chronopoulos A: Changes in retinal vascular caliber after intravitreal aflibercept treatment for diabetic macular oedema. Klin Monbl Augenheilkd. 236:1318–1324. 2019.In German. | |
|
Förstermann U and Sessa WC: Nitric oxide synthases: Regulation and function. Eur Heart J. 33:829–837. 2012. View Article : Google Scholar : | |
|
Thulliez M, Angoulvant D, Lez ML, Jonville-Bera AP, Pisella PJ, Gueyffier F and Bejan-Angoulvant T: Cardiovascular events and bleeding risk associated with intravitreal antivascular endothelial growth factor monoclonal antibodies: Systematic review and meta-analysis. JAMA Ophthalmol. 132:1317–1326. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Fung AE, Rosenfeld PJ and Reichel E: The international intravitreal bevacizumab safety survey: Using the internet to assess drug safety worldwide. Br J Ophthalmol. 90:1344–1349. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Kitchens JW, Do DV, Boyer DS, Thompson D, Gibson A, Saroj N, Vitti R, Berliner AJ and Kaiser PK: Comprehensive review of ocular and systemic safety events with intravitreal aflibercept injection in randomized controlled trials. Ophthalmology. 123:1511–1520. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Buyukavsar C, Sonmez M, Sagdic SK and Unal MH: Relationship between ganglion cell complex thickness and vision in age-related macular degeneration treated with aflibercept. Eur J Ophthalmol. 33:1672–1682. 2023. View Article : Google Scholar | |
|
Dziedziak J, Zaleska-Żmijewska A, Szaflik JP and Cudnoch-Jędrzejewska A: Impact of arterial hypertension on the eye: A review of the pathogenesis, diagnostic methods, and treatment of hypertensive retinopathy. Med Sci Monit. 28:e9351352022. View Article : Google Scholar : PubMed/NCBI | |
|
Kiyat P and Karti O: Comparison of choroidal vascularity index, retinal, and optic nerve changes in diabetes mellitus patients without diabetic retinopathy. Beyoglu Eye J. 9:228–234. 2024. View Article : Google Scholar | |
|
Swaminathan SS, Jammal AA, Berchuck SI and Medeiros FA: Rapid initial OCT RNFL thinning is predictive of faster visual field loss during extended follow-up in glaucoma. Am J Ophthalmol. 229:100–107. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Kim MJ, Woo SJ, Park KH and Kim TW: Retinal nerve fiber layer thickness is decreased in the fellow eyes of patients with unilateral retinal vein occlusion. Ophthalmology. 118:706–710. 2011. View Article : Google Scholar | |
|
Qin HF, Shi FJ, Zhang CY, Luo DW, Qin SY, Wu J, Xie H, Zhang JT, Qiu QH, Liu K, et al: Anti-VEGF reduces inflammatory features in macular edema secondary to retinal vein occlusion. Int J Ophthalmol. 15:1296–1304. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Imazeki M, Noma H, Yasuda K, Motohashi R, Goto H and Shimura M: Anti-VEGF therapy reduces inflammation in diabetic macular edema. Ophthalmic Res. 64:43–49. 2021. View Article : Google Scholar | |
|
Nakao S, Arima M, Ishikawa K, Kohno R, Kawahara S, Miyazaki M, Yoshida S, Enaida H, Hafezi-Moghadam A, Kono T and Ishibashi T: Intravitreal anti-VEGF therapy blocks inflammatory cell infiltration and re-entry into the circulation in retinal angiogenesis. Invest Ophthalmol Vis Sci. 53:4323–4328. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Nishijima K, Ng YS, Zhong L, Bradley J, Schubert W, Jo N, Akita J, Samuelsson SJ, Robinson GS, Adamis AP and Shima DT: Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 171:53–67. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang J, Zhang Z, Jiang L, He S, Long X and Zheng X: Combination therapy with N-Acetylserotonin and aflibercept activated the Akt/Nrf2 pathway to inhibit apoptosis and oxidative stress in rats with retinal ischemia-reperfusion injury. Curr Eye Res. 4:280–287. 2024. View Article : Google Scholar | |
|
Rani EA, Janani R, Chonche MJ and Vallikannan B: Lactucaxanthin regulates the cascade of retinal oxidative stress, endoplasmic reticulum stress and inflammatory signaling in diabetic rats. Ocul Immunol Inflamm. 31:320–328. 2023. View Article : Google Scholar |