Mechanism of action and therapeutic potential of S100A8/A9 in neuroinflammation and cognitive impairment: From molecular target to clinical application (Review)
- Authors:
- Published online on: July 16, 2025 https://doi.org/10.3892/ijmm.2025.5588
- Article Number: 147
-
Copyright: © Guan et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
The incidence of cognitive disorders in modern society has risen, making these conditions prevail among the most significant neurological diseases impacting individuals' quality of life, mental health and well-being (1,2). Cognitive disorders often result in long-lasting negative effects (3-5). A prominent pathogenic factor contributing to cognitive impairment is neuroinflammation, which plays a crucial role in the etiology of central nervous system (CNS) diseases, involving a complex array of biological changes (6-15). For instance, neuroinflammation is closely associated with Alzheimer's disease (AD), the most common form of dementia (1). The gravity of this issue is underscored by the estimated 50 million individuals worldwide affected by cognitive impairment (1). Approximately one-third of stroke survivors experience significant cognitive impairment within the first 3 months post-stroke, and elevated expression of S100A8/A9 in brain tissue following a stroke is similarly correlated with cognitive decline (1,6,16-18). Furthermore, cognitive impairment is associated with increased levels of S100A8/A9 in the blood, being present in 38% of patients with systemic lupus erythematosus (SLE). Patients with sepsis are also vulnerable to sepsis-associated encephalopathy (SAE), which has a 70% mortality rate and results in long-term cognitive damage (19,20).
Given its involvement in various neurodegenerative diseases and its capacity to exacerbate neuroinflammation and cognitive impairments, S100A8/A9 may play a pervasive role in cognitive disorders (21,22). The understanding of the pathogenic mechanisms underlying cognitive impairment is advancing alongside the scientific community's interest in the condition. Nevertheless, effective treatments for cognitive impairment remain limited, posing significant challenges. Notably, elevated levels of S100A8/A9 have been observed in patients' blood and cerebrospinal fluid (CSF) (23,24), indicating its potential as a therapeutic target for conditions such as sepsis, AD, stroke and SLE. Neuroinflammation, a major contributor to the development of CNS diseases, is associated with various cognitive domains, including emotion regulation, memory, learning, aging and sleep disturbances (25,26). Numerous CNS disorders exhibit increased expression of S100A8/A9, which is strongly associated with pathogenic processes such as neuroinflammation, oxidative stress and neuronal death (6,27). It may also induce inflammation and damage the nervous system by interacting with Toll-like receptor 4 (TLR4) and receptor for advanced glycation end-products (RAGE) receptors (28). Targeting S100A8/A9 and its associated signaling pathways may offer therapeutic potential for CNS disorders. Future research should further investigate the precise role of S100A8/A9 in neuroinflammation to develop more effective therapeutic strategies.
S100A8/A9 is a Ca2+-binding 93/114-amino acid protein with respective molecular weights of 10.8/13.2 kDa (6,29). Its molecular structure consists of an EF-hand type Ca2+-binding domain, which forms an S100A8/A9 tetramer or heterodimer. It is this structure that allows S100A8/A9 to exert its multifunctional extracellular and intracellular activities.
S100A8/A9 expression, predominantly on immune cells, is upregulated following inflammatory stimuli, which further induces its expression in and secretion from other cell types (30). Intracellularly, it is involved in phagocyte motility, where it is involved in cytoskeletal rearrangement and microtubule stabilization (6,18). Furthermore, it contains antimicrobial qualities that chelate metal ions to prevent the development of bacteria (6). The significance of S100A8/A9 in inflammation and immunological responses is supported by these structural characteristics, which also provide fresh insights into the protein's potential as a therapeutic target. Future research should investigate the processes behind the effects of S100A8/A9 in various illnesses and how altering its structure and function can lead to the development of novel treatment approaches.
The purpose of the present review was to examine how the S100A8/A9 protein contributes to neuroinflammation and how it relates to cognitive impairment. The structure and function of S100A8/A9 are described, along with its involvement in neuroinflammatory signaling networks and its relevance in certain illnesses that impact cognitive processes. The potential and challenges of S100A8/A9 as a therapeutic target are discussed to provide novel perspectives and strategies for the treatment of cognitive disorders associated with neuroinflammation.
It is impossible to ignore the importance of S100A8/A9 in cognitive disorders. In addition to improving the understanding of the pathophysiology of CNS disorders, research on this protein may lead to the development of new treatment strategies.
Function and mechanism of S100A8/A9 heterodimerization
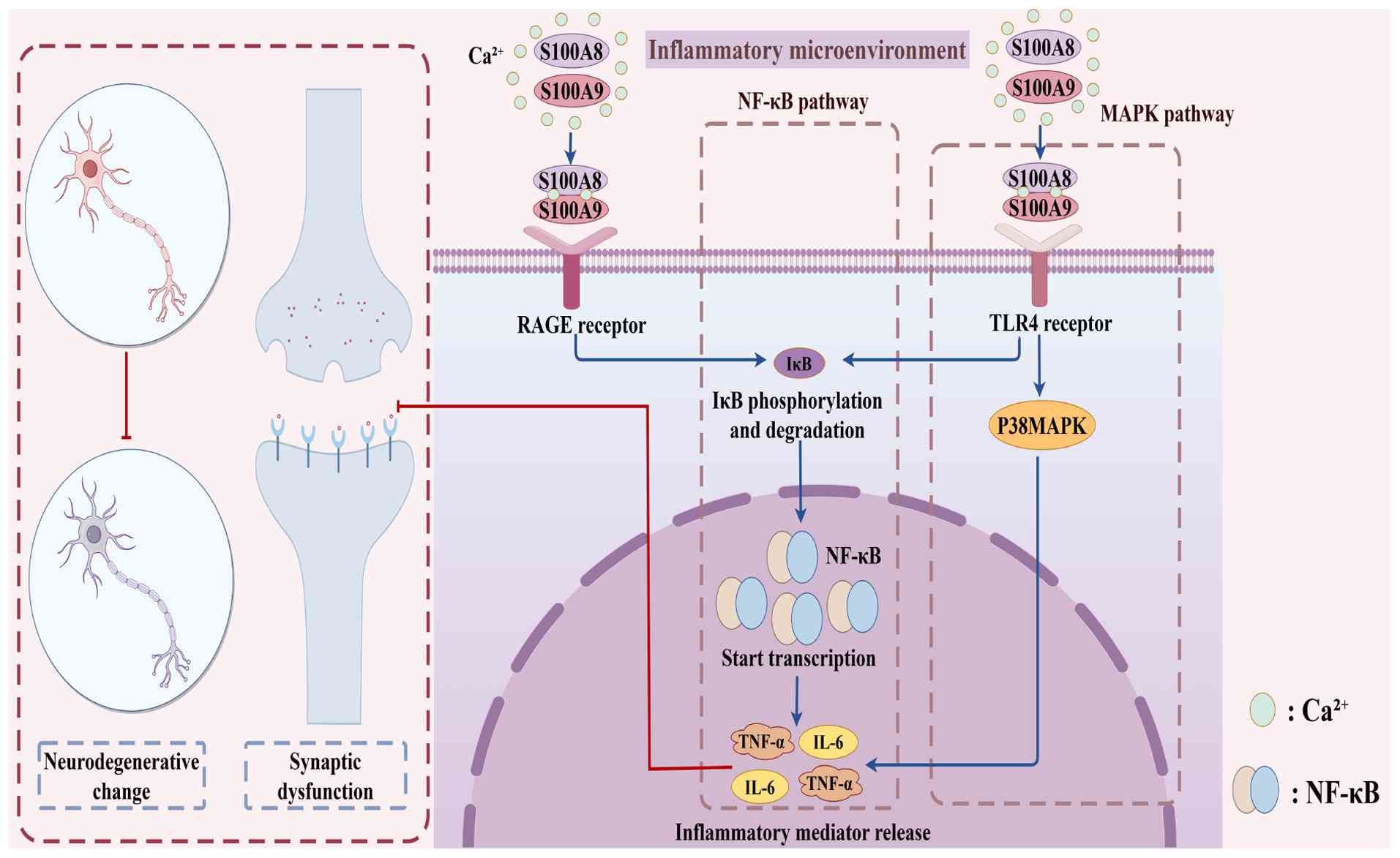
The heterodimer S100A8/A9 undergoes a significant conformational change upon binding with Ca2+ ions, which enhances its ability to bind target molecules (31). The interaction of the S100A8/A9 heterodimer with cell-surface receptors, specifically RAGE and TLR4, is crucial for modulating inflammatory responses and the chemotaxis of immune cells, involving both immunological and inflammatory processes (28,32). During an inflammatory response, S100A8/A9 promotes the production and release of inflammatory mediators, such as TNF-α and IL-6, by activating the NF-κB and MAPK signaling pathways (33). This activation not only intensifies the local inflammatory response but also exacerbates inflammation by altering immune cell activation states and inducing phenotypic shifts, such as the transition from anti-inflammatory M2-type macrophages to pro-inflammatory M1-type macrophages (34-36). Furthermore, S100A8/A9 enhances the production of reactive oxygen species (ROS) and reactive nitrogen species through the activation of NADPH oxidase 2, thereby aggravating the inflammatory condition (37-39).
S100A8/A9 serves as a significant neuroinflammatory marker, regulating the initiation and progression of neuroinflammation through interactions with RAGE and TLR4 receptors (40,41). RAGE is expressed by various brain cells, including astrocytes, neurons and microglial cells. The binding of S100A8/A9 to RAGE activates downstream signaling pathways, leading to the formation of neurodegenerative lesions and the production of inflammatory mediators (41,42). In addition, by activating the TLR4 receptor, S100A8/A9 acts as a damage-associated molecular pattern (DAMP), further initiating the NF-κB and MAPK signaling pathways and exacerbating neuroinflammation (8,33,43). The phosphorylation and degradation of IκB by S100A8/A9 via RAGE or TLR4 lead to the release of NF-κB into the nucleus, initiating the transcription of inflammation-related genes (44).
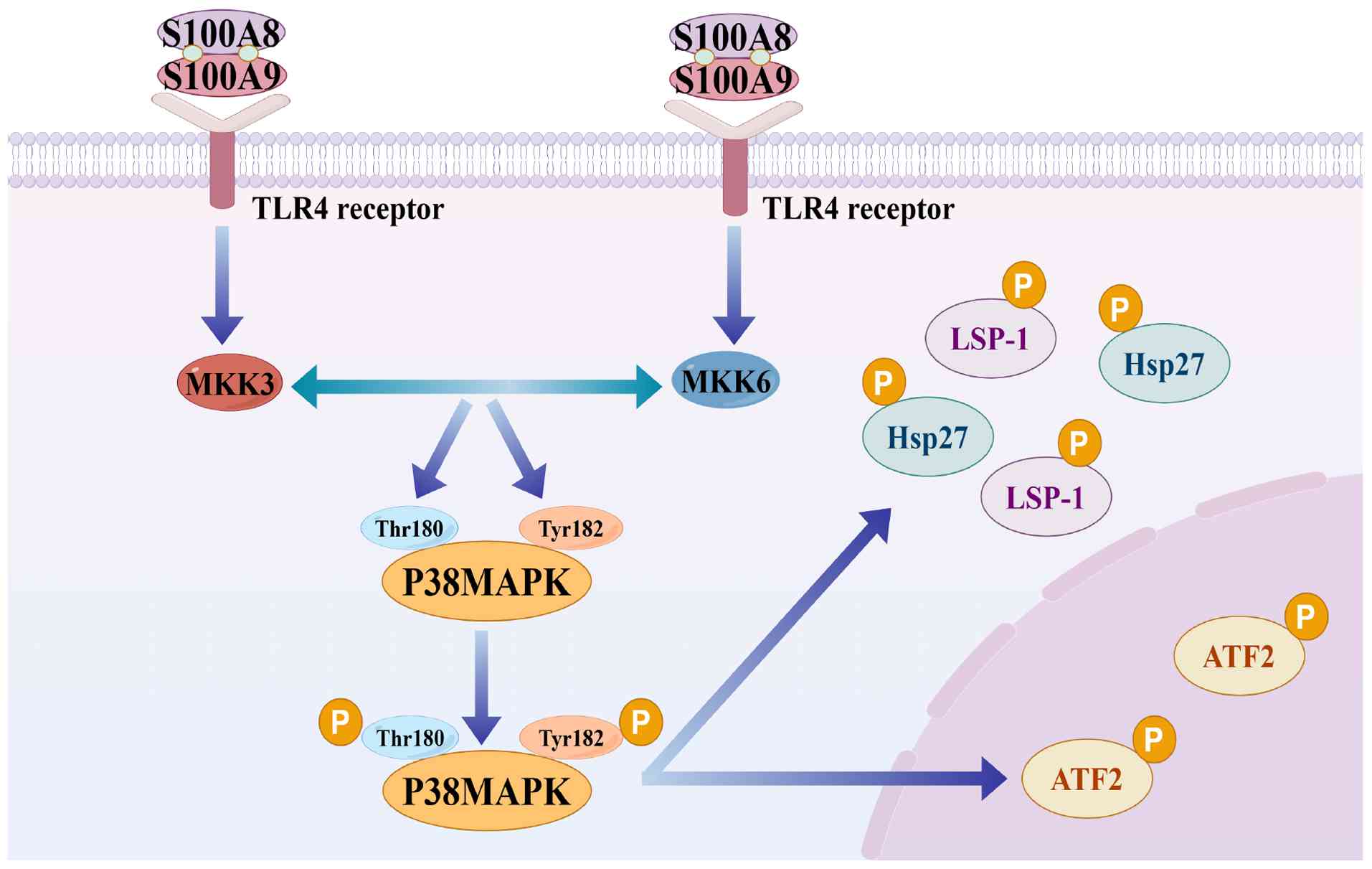
In the MAPK pathway, S100A8/A9 activates p38 MAPK via TLR4, thereby influencing inflammation, pain perception and disease onset (45). Specifically, p38 MAPK is phosphorylated by MKK3 and MKK6, the primary MAP2Ks. The Thr180 and Tyr182 residues in the activation loop of p38 MAPK are phosphorylated to activate it. This dual phosphorylation stabilizes the activation loop in an open conformation, promoting substrate recognition and kinase activity. Once activated, p38 MAPK regulates inflammation and cytoskeletal alterations by phosphorylating various substrates, including heat shock protein 27 (Hsp27), a type of small molecular heat shock protein, with a molecular chaperone function, activating transcription factor 2 (ATF-2), a transcription factor that can regulate the expression of multiple genes and is closely related to cell proliferation, differentiation and stress response, and lymphocyte-specific protein 1 (LSP-1), a protein related to the cytoskeleton, mainly involved in cell migration and signal transduction processes (45-47) (Fig. 1). Due to its direct impact on cell activity and cytokine production, p38 MAPK activation is particularly significant in the pathophysiology of neuroinflammation (Fig. 2) (45).
Given the pivotal role of S100A8/A9 in triggering neuroinflammation, personalized therapeutic strategies targeting its specific mechanisms are anticipated to yield novel breakthroughs in the intervention of cognitive disorders. Future research should focus on developing specific drugs targeting S100A8/A9 itself or up/downstream members of the signaling pathways that trigger neuroinflammation, to regulate the inflammatory response more precisely. This will necessitate not only an in-depth investigation of their mechanisms of action in cognitive disorders but also clinical trials to validate efficacy and safety. As research progresses, the therapeutic prospects in this area will become clearer, providing more effective treatment options for patients with cognitive disorders.
In conclusion, S100A8/A9 regulates the release of inflammatory mediators and the start of neuroinflammation by interacting with RAGE and TLR4 to activate the NF-κB and MAPK signaling pathways. This biological process offers possibilities for innovative treatment methods for cognitive disorders in addition to providing a critical basis for comprehending the role of S100A8/A9 in neuroinflammation. The involvement of S100A8/A9 in neuroinflammation and its potential as a therapeutic target may become clearer as future studies further unravel its role in these pathways.
Role of S100A8/A9 in cognitive impairment-related disorders
Cognitive impairment is directly related to the development of AD, stroke, sepsis and neuropsychiatric lupus in SLE (NPSLE). Neuroinflammation is a common feature in these illnesses, with S100A8/A9 serving as critical inflammatory markers involved in their pathophysiological processes. As DAMPs, S100A8/A9 exacerbate neuroinflammation and cognitive deficits by activating immune cells and releasing inflammatory mediators, which is their mode of action in these conditions. Future research may focus on elucidating the precise mechanisms of S100A8/A9 in various diseases and developing innovative therapeutic strategies to improve the prognosis of patients with cognitive impairments by regulating its expression or inhibiting the signaling pathways it is involved in.
AD
AD is a neurodegenerative disorder characterized by progressive cognitive decline, with clinical features including the formation of amyloid β (Aβ) plaques and intracellular hyperphosphorylated tau protein tangles (48). Studies have demonstrated elevated levels of S100A8/A9 in plasma-derived extracellular vesicles (EVs) of individuals with AD, as well as increased expression in the blood of patients with AD and those with mild cognitive impairment. These findings suggest a role for S100A8/A9 in AD pathogenesis, potentially serving as a valuable biomarker for the disease (27,49,50). Furthermore, S100A8/A9 has been detected in the hippocampus of AD model mice, induced by increased expression in Aβ42-stimulated glial cells (51), underscoring its critical role in AD.
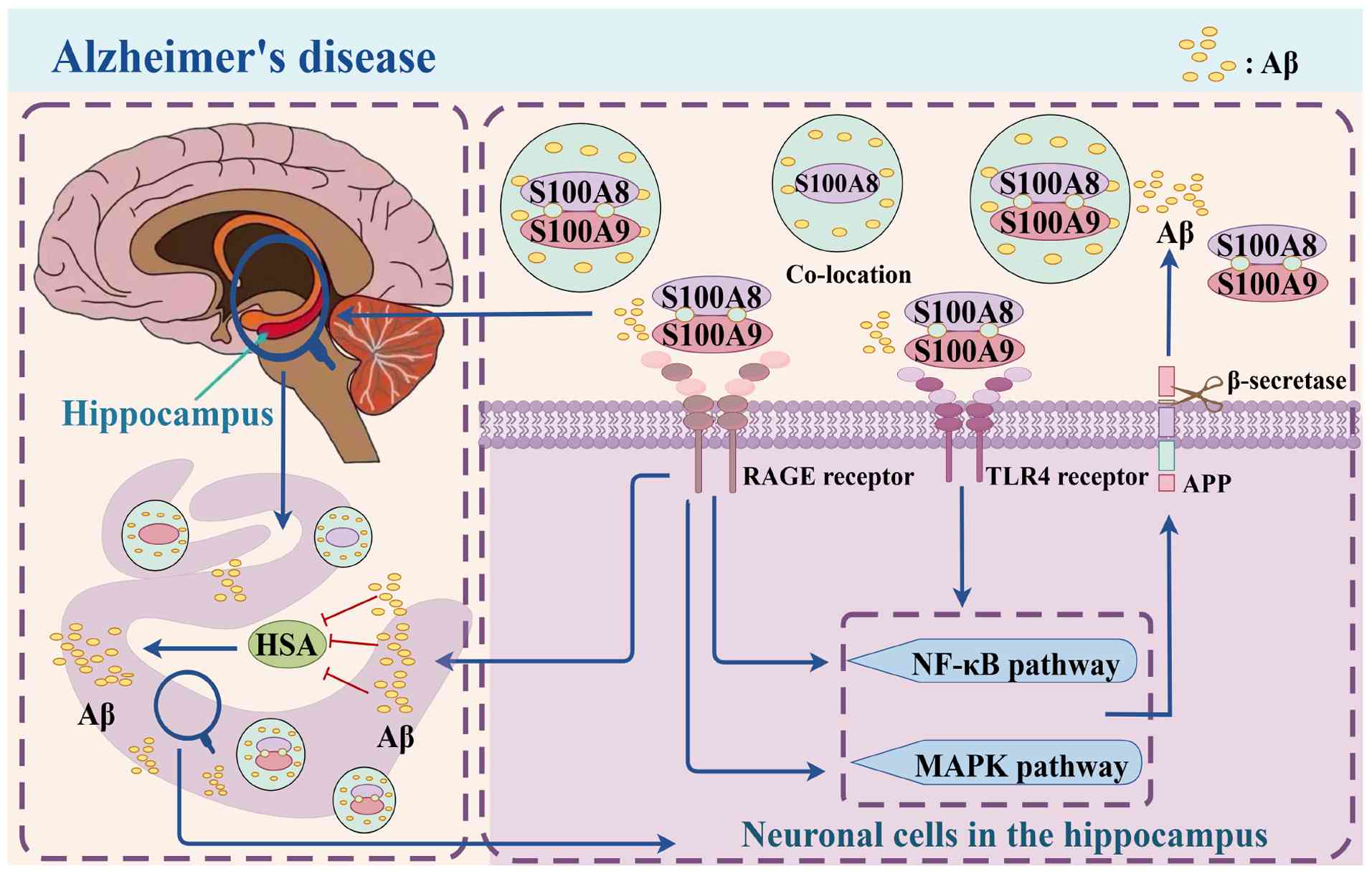
S100A8/A9 influences Aβ accumulation and associated degenerative processes through various pathways. It binds to the RAGE receptor, preventing human serum albumin (HSA) from binding to Aβ monomers, thereby accelerating Aβ plaque formation by directly promoting Aβ peptide aggregation in the brain. Additionally, S100A8/A9 binds to TLR4 and RAGE receptors, activating downstream NF-κB and MAPK signaling pathways (27,52). The neuroinflammatory response is exacerbated by the release of pro-inflammatory cytokines TNF-α and IL-6 upon NF-κB activation (39,53). Furthermore, activation of the MAPK signaling pathway is closely associated with the processing of the Aβ precursor protein, potentially leading to increased Aβ42 synthesis. Notably, S100A8/A9 exhibits a specific binding affinity for Aβ monomers, with S100A8 showing a greater affinity for Aβ42 compared to Aβ40. This specific binding facilitates Aβ aggregation and fibrosis, ultimately resulting in Aβ plaque development (25,54,55). A positive feedback loop between Aβ and S100A8/A9 is crucial to AD pathophysiology. S100A8 aggregation precedes Aβ deposition, and once Aβ is deposited, it further promotes S100A8/A9 production, creating a vicious cycle that exacerbates neuroinflammation and cognitive decline. S100A8/A9 expression is significantly elevated in the central and peripheral regions of Aβ plaques in the AD model. Its deposition and aggregation not only worsen localized neuroinflammation but may also interfere with normal neuronal function, potentially impairing cognitive function (55). The positive feedback loop between S100A8/A9 and Aβ exacerbates the pathogenic progression of AD.
Additionally, by enhancing β-secretase activity and increasing the production of Aβ peptides, S100A8/A9 contributes to the processing of amyloid precursor protein (APP), thereby promoting the pathological progression of AD (56). Another experimental finding demonstrated that inhibition of S100A8/A9 reduced Aβ aggregation in Aβ1-42-induced SH-SY5Y cells (27), suggesting that S100A8/A9 may regulate Aβ aggregation through EVs, offering novel insights for potential AD treatment strategies. Furthermore, upregulated S100A8/A9 in the brains of patients with AD co-localized with Aβ plaques, indicating a strong association with the disease, which may be related to astrocyte activation and further cognitive impairment (51,57). These findings imply that, due to its strong association with amyloid plaques, S100A8/A9 could serve as a potential therapeutic target in AD. Increased expression of S100A8/A9 has been linked to microglia, the primary immune cells in the CNS, and their aberrant activation is consistent with the neuroinflammation and neuronal damage associated with AD (54,58-60). A notable similarity between the development of tauopathies and accelerated aging is the presence of S100A8/A9-positive microglia, which may be associated with subsequent brain dysfunction (61,62). These studies underscore the central role of S100A8/A9 in AD pathology, suggesting it is a key driver of AD-related cognitive impairment. Future research should investigate the precise regulatory mechanisms of S100A8/A9 and develop specific inhibitors or signaling pathway interventions to provide novel strategies for AD treatment. Additionally, its interaction with other pathological mechanisms should be explored to establish a foundation for combination therapy. S100A8/A9 plays a role in AD and its expression level is closely associated with both neuroinflammation and Aβ deposition. It stimulates the release of inflammatory mediators, exacerbates neuroinflammation and impairs cognitive function by binding to RAGE and TLR4 receptors and activating the NF-κB and MAPK signaling pathways. Furthermore, plasma-derived EVs from patients with AD exhibited increased expression of S100A8/A9, indicating their potential use as early diagnostic markers (27,33,52). Future research could explore methods to disrupt its positive feedback loop with Aβ42 to mitigate the progression of the disease and its application in early AD diagnosis. To reduce neuroinflammation, further investigation into the role of S100A8/A9 in microglia activation is warranted. Its association with tauopathy and accelerated aging suggests its involvement in broader neurodegenerative processes, highlighting novel avenues for research into its role in other neurodegenerative diseases (61). Additionally, the mechanism by which S100A8/A9 contributes to the processing of APPs and stimulates the synthesis of Aβ peptide to promote AD pathology implies that future development of medications targeting β-secretase inhibition may delay the onset and progression of cognitive deficits in patients with AD, which would be highly beneficial (Fig. 3).
Stroke
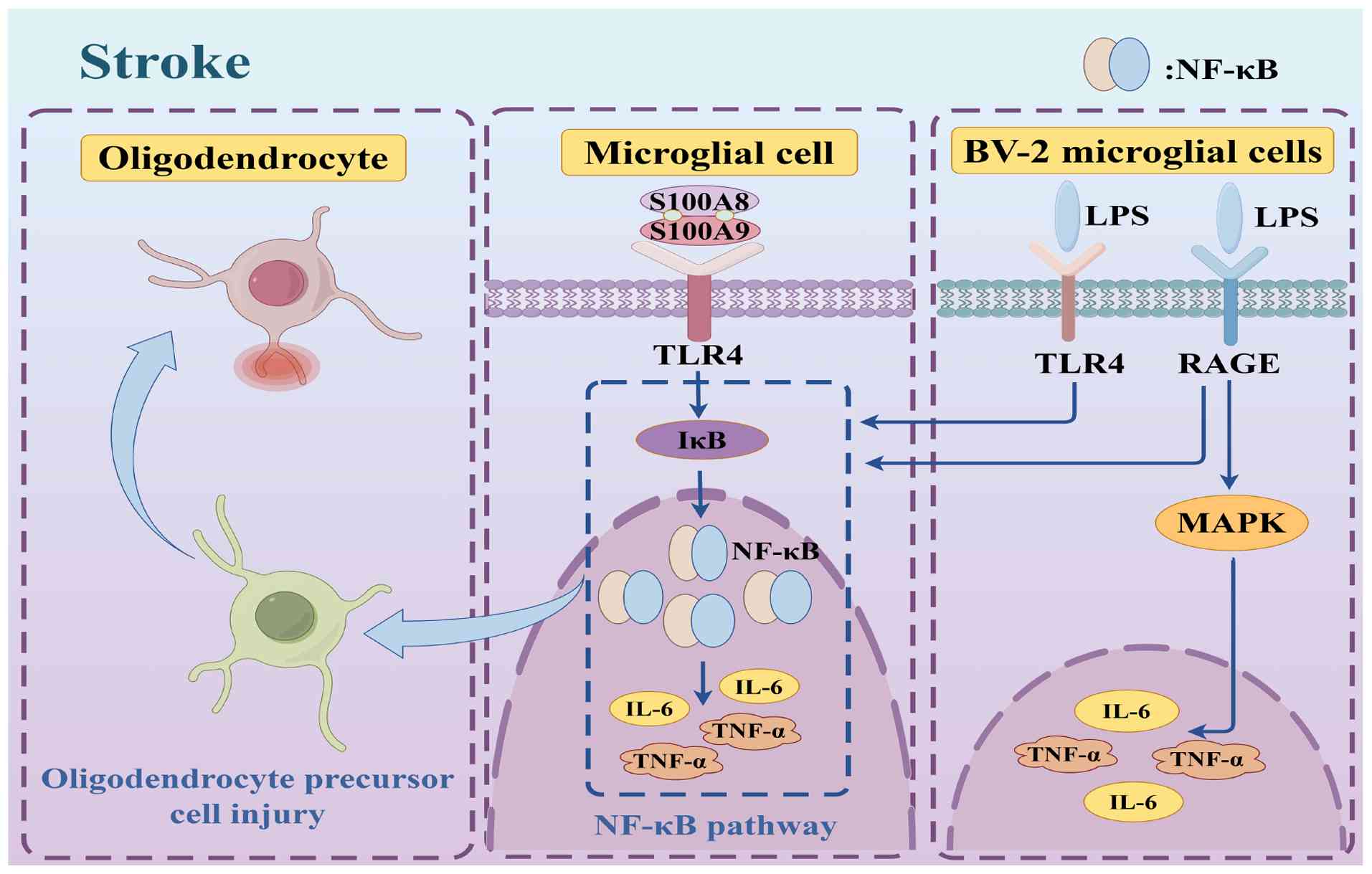
Stroke is a leading cause of adult disability and is the second most common cause of cognitive impairment and dementia (63). A prevalent cognitive condition that significantly impacts daily functioning is vascular dementia, which occurs following a stroke. Approximately 80% of stroke cases in China are ischemic strokes (IS), which have become much more common in recent decades (63,64). IS is caused by vascular occlusion, which results in localized interruption of blood flow to brain tissues, which, in turn, deprives it of needed oxygen and ultimately triggers softening and necrosis of brain tissues (65). The pathogenesis of IS is significantly influenced by inflammation, as persistent inflammation encourages the development of atherosclerotic plaques, which gradually become unstable and eventually produce blood clots that obstruct cerebral blood flow and cause stroke (66). In the pathophysiology of stroke, S100A8/A9 primarily increases thrombosis and pro-inflammatory signaling pathways, which worsen cerebral impairment. S100A8/A9 has been shown to bind to TLR4 and trigger the downstream NF-κB signaling cascade, which induces the release of pro-inflammatory cytokines, such as TNF-α and IL-6. These cytokines induce platelet aggregation and the development of thrombi in addition to intensifying the local inflammatory response (67,68). Furthermore, in patients who have experienced an ischemic stroke, the plasma and thrombotic tissue show markedly higher S100A8/A9 levels, which were closely linked to a worse prognosis (69). Through the regulation of neutrophil activity, S100A8/A9 can also impact tissue healing and the inflammatory response following a stroke. The quick and reversible release of S100A8/A9 in neutrophils is reliant on Gasdermin D and Caspase 1 activity during inflammation. Through the regulation of neutrophil chemotaxis and activity, this system plays a crucial role in the inflammatory response following stroke and may affect the severity and length of the inflammatory response (70). Additionally, S100A8/A9 is concentrated in microglia and microvascular endothelial cells in the ischemic hemisphere, and its expression is markedly increased in the post-stroke brain; increased neuroinflammation and cognitive impairment are linked to this upregulation. In a mouse model, S100A8/A9 knockdown reduced neuroinflammation and enhanced stroke-related neurological recovery (70). The release of DAMPs from brain cells initiates the inflammatory process during a stroke, and microglia are then activated by TLR (67,68). Through TLR4 and RAGE, S100A8/A9 promotes the release of proinflammatory cytokines from BV-2 microglia stimulated by lipopolysaccharides and exacerbates damage to oligodendrocyte precursor cells by activating the NF-κB signaling cascade (53,56). These processes may eventually lead to cognitive impairment in addition to inducing a localized inflammatory response (71,72). Patients with acute stroke have elevated levels of the innate immune mediator S100A8/A9, which exhibits inflammatory properties. This finding is supported by recent studies indicating that S100A8/A9 plays a role in inflammatory responses (73). Consequently, atherosclerotic plaques burst more frequently, speeding up the onset of stroke. A worse prognosis for IS has also been associated with elevated levels of S100A8/A9 (74). One of the primary inflammatory elements in the pathways involved in neuroinflammation and cognitive impairment brought on by IS is S100A8/A9. Specifically, a model of middle cerebral artery occlusion showed increased expression levels of S100A8/A9, a feature that is directly linked to the generation of inflammatory responses and worse patient outcomes (75). A previous study by Chen et al (76) investigated whether there was any association between a calmodulin gene polymorphism and IS susceptibility among southern Chinese individuals by employing a novel multi-temperature ligase test for an extensive reactive genotype study. There is ample evidence showing the involvement of the S100A8/A9 gene as a potential biomarker for predicting the risk of IS and delivering individualized care, as specific mutations of the gene were shown to be strongly associated with an elevated risk of stroke resulting from IS in humans (63-76).
In conclusion, S100A8/A9 is involved in neuroinflammation and cognitive impairment following a stroke. It not only induces neuroinflammation but is also significantly correlated with poststroke neuroinflammation and cognitive impairment. Studies show not only the role of S100A8/A9 in the pathophysiology of stroke but also offer novel insights for the development of therapeutic and preventative approaches. Monitoring and modulating S100A8/A9 may be a novel strategy for preventing and treating cognitive impairment following a stroke (Fig. 4).
NPSLE
SLE is a chronic autoimmune disorder characterized by a widespread inflammatory response, affecting multiple organs, including the CNS. Among the prevalent diffuse CNS NPSLE disorders, cognitive impairment significantly hinders a patient's memory, processing speed, attention and planning abilities. These symptoms profoundly impact the quality of life and daily functioning. NPSLE manifests through a spectrum of neurological and psychiatric symptoms (20,77,78), with its high prevalence in patients correlating with disease activity. Notably, the expression of S100A8/A9 is strongly associated with the severity and activity of NPSLE and decreases following the administration of immunosuppressive therapies (79-83). This suggests that S100A8/A9 may serve as a biomarker for SLE disease activity and may be linked to the inflammatory processes in SLE.
In NPSLE, S100A8/A9 facilitates the activation of downstream NF-κB and MAPK signaling pathways through interactions with TLR4 and RAGE receptors, leading to the production of inflammatory mediators such as TNF-α and IL-6 (49,50). These inflammatory factors not only accelerate SLE progression but also exacerbate cognitive impairment (49,50,84-87). Furthermore, patients with NPSLE exhibit significantly elevated serum levels of S100A8/A9, which correlate with the severity of cognitive impairment and disease activity. These proteins induce chronic neuroinflammation by persistently activating astrocytes and microglia, thereby affecting neuronal survival and function (88). As a DAMP, S100A8/A9 exacerbates the inflammatory response and tissue damage by promoting the generation of autoantibodies and the formation of immune complexes in SLE (19). Additionally, prolonged S100A8/A9 production may lead to neuronal dysfunction, potentially impacting synaptic transmission (19). The continuous production of S100A8/A9 further exacerbates cognitive impairment by activating microglia and producing neurotoxic pro-inflammatory cytokines (89). It has been proposed that S100A8/A9 may be the most effective blood biomarker for identifying NPSLE-associated cognitive issues in adults (90-95), and elevated blood levels of this protein may be associated with the degree of fatigue and neuropsychiatric involvement in SLE (96). These findings provide critical insights for developing therapeutic strategies aimed at enhancing cognitive function in patients with SLE and suggest a potential role for S100A8/A9 in the neuropsychiatric pathogenesis of NPSLE (19).
The upregulation of S100A8/A9 is closely associated with the pathogenic stage of SLE and influences cognitive function through multiple pathways. Consequently, it is not only a biomarker of SLE activity but also a potential target for treating cognitive impairment associated with SLE. Future research should further investigate the specific mechanisms of S100A8/A9 in SLE and explore strategies to modulate its expression to improve cognitive function in patients with SLE, as current research in this area remains insufficient.
SAE
A dysregulated host response to infection causes sepsis, a potentially lethal organ failure disease that is often associated with both acute and chronic brain dysfunction (97). In particular, >70% of patients with sepsis suffer from SAE, a common side effect of sepsis that is strongly associated with a higher mortality rate and a poor long-term prognosis (24,98). It is not possible to exclude the contribution of S100A8/A9 in the pathogenesis of the disease due to the onset and progression of SAE being closely related to increased levels of this putative biomarker (23), and increased expression of S100A8/A9 under sepsis is associated with an increased risk of brain injury and mortality (99). The persistent expression of S100A8/A9 in the brain during sepsis is linked to the activation of microglia and the infiltration of peripherally derived immune cells (70). This expression may correlate with an increased likelihood of cognitive dysfunction, and its overexpression has been implicated in the mechanisms underlying cognitive impairment (23).
In the context of sepsis-induced systemic inflammatory response, elevated levels of S100A8/A9 exacerbate neuroinflammation, thereby affecting cognitive function through the induction of microglia and the production of inflammatory mediators. Beyond initiating an inflammatory response, the sustained expression and activity of S100A8/A9 may also serve a protective role in the infected environment (70). This mechanism modifies the function of microglia and macrophages, leading to persistent post-sepsis neuroinflammation through multiple pathways, with S100A8/A9 production being crucial for neutrophil chemotaxis (70,100). Although not constitutively activated, post-sepsis resident brain myeloid cells are stimulated to release ROS and TNF-a in sepsis-surviving mice following secondary stimulation; this effect is dependent on S100A8/A9 (70,101). Additionally, S100A9 has been shown to induce M2-type polarization in microglia and enhance TGF-β1 production; this polarization process may contribute to the pathogenesis of SAE (102,103). The expression of S100A8/A9 is not merely incidental to the inflammatory process in sepsis but plays a significant role (70). Although sepsis is not traditionally considered a primary trigger of cognitive impairment, it is prevalent in critical care settings and poses a significant risk to patients. Sepsis can induce a range of neuropsychiatric symptoms, including executive dysfunction, memory loss and reduced attention span. These symptoms not only significantly diminish a patient's quality of life but also increase the cost of medical and social care, imposing a substantial burden on families and society. The medical community has shown growing interest in sepsis-related cognitive impairment as research in this area has advanced. Future studies must explore specific strategies for treating sepsis-related cognitive impairment and develop targeted therapies that address oxidative stress and neuroinflammation. In the interim, early interventions are being investigated to improve patient outcomes based on the long-term impact of cognitive impairment in sepsis. For instance, novel approaches to treating sepsis-related cognitive impairment may involve modulating gut microbiota or inhibiting exosomes produced by intestinal epithelial cells. Furthermore, examining the potential connection between sepsis-related cognitive impairment and other neurodegenerative diseases provides a theoretical basis for combination therapy approaches that could enhance neurological recovery and quality of life of patients with sepsis. The significance of S100A8/A9 as a key biomarker and potential therapeutic target in sepsis-related neuroinflammation and cognitive deficits cannot be overlooked. Future research must elucidate the precise mechanism of action of S100A8/A9 and investigate how modulating its expression could improve the cognitive prognosis of patients with sepsis.
Effects of S100A8/A9 on cognitive function
A study by Lu et al (104) highlighted the involvement of S100A8/A9 in postoperative cognitive impairment (POCD). After tibial fracture surgery, TLR4 activation by S100A8/A9 induced the migration of mononuclear macrophages to the hippocampus, where it promoted microglia expansion and exacerbated neuroinflammation, both of which are associated with the occurrence of POCD. The positive feedback S100A8/A9-TLR4-myeloid differentiation primary response 8 (MyD88; a key adaptor protein in TLR signaling pathways, is involved in mediating downstream inflammatory responses) signaling pathway is a vital catalytic link in the onset of inflammatory POCD. Up- and downstream of TLR, S100A8/A9 expression diminished post-surgery. S100A8 injection in MyD88 knockout rats increased TLR4 expression in microglia, supporting the hypothesis that neutralization of S100A8/A9 may inhibit neuroinflammation and improve POCD. Such studies further broaden the insight into the impact of S100A8/A9 on cognitive performance as well as highlight novel aspects and avenues for research and the treatment of cognitive disorders. Future research related to S100A8/A9 should focus on the development of novel therapeutic regimens to facilitate or reverse the cognitive decline in aging, insomnia and other affective illnesses.
Sleep
The relation between cognitive disturbances and chronic sleep deprivation (CSD) is well established. Activation of autophagy and apoptotic pathways results in CSD, causing cognitive impairment and neuronal cell death in the hippocampus, and also affects mood, learning and memory, among other functions (105). Additionally, several neurotoxic compounds, including oxidative stress products and inflammatory factors, are produced as a result of CSD. These substances may also contribute to cognitive dysfunction by interfering with the physiological function of nerve cells (106,107). As resident phagocytes in the CNS, microglia are crucial to this process (17); however, their hyperactivity aggravates oxidative stress and neuroinflammation, which worsens the cognitive impairments brought on by CSD (9,108). Cognitive abnormalities caused by CSD have been revealed to be significantly regulated by the microglia-expressed protein S100A8. Upregulated expression of S100A8 may modify the microglial response mechanisms and contribute to cognitive abnormalities induced by CSD (25). In mice with CSD, elevated expression of S100A8 in the hippocampal area has been associated with cognitive deficiencies; these deficits are significantly alleviated when S100A8 expression is reduced (25). Of note, reduced expression of S100A8 considerably improved cognitive abnormalities caused by CSD (25). The PI3K/AKT signaling pathway may be altered by S100A8 knockdown, and this knockdown had a neuroprotective effect on cognitive impairment by reducing excessive autophagy and apoptosis in the hippocampal cells of sleep-deprived mice. The increase in expression of pro-apoptotic proteins, such as Bax and Caspase-3, and the decrease in anti-apoptotic protein Bcl-2 induced by CSD, were reversed at the cellular level by S100A8 knockdown (25). These findings emphasize the critical role of S100A8/A9 in CSD. Future studies should focus on developing specific interventions targeting S100A8/A9 to alleviate CSD-associated neuroinflammation and cognitive deficits, and to provide novel therapeutic strategies to improve cognitive function in patients with sleep deprivation.
Senescence
Changes in the immune system throughout aging are strongly associated with low-grade inflammation, which can weaken immunological responses and make an individual more vulnerable to several illnesses (109). Research indicates that serum S100A8/A9 levels are lower in older individuals (66), suggesting a potential link between S100A8/A9 and age-related inflammation. However, other studies have reported an increase in S100A8/A9 levels with aging. For instance, Swindell et al (110) observed an age-related increase in S100A8/A9 across various tissues, with brain biopsies from patients with tauopathies and AD revealing S100A8/A9-positive microglia. This finding suggests an association connection between S100A8/A9 and the progression of neurodegenerative diseases and accelerated aging (61). Additionally, a subset of S100A8+IBA1+ microglia was identified in human brain tissue, further confirming the involvement of S100A8/A9 in brain aging (61). These results imply that tau pathology and accelerated aging are characterized by upregulated S100A8 and S100A9, and that tau protein accumulation may indirectly influence brain aging by promoting earlier S100A8/A9 expression (61). Furthermore, the brains of elderly individuals exhibit higher S100A8/A9 expression than those of younger individuals, and overexpression of these proteins in the brains of 6-month-old P301L + K18 mice aligns with molecular markers of brain aging (110), suggesting a role for S100A8/A9 in brain aging. The discrepancy between these findings may be attributed to different sample types, as serum levels may not reflect tissue-specific expression. Furthermore, the complex roles of S100A8/A9 in various biological processes may lead to distinct regulatory mechanisms across tissues and age groups.
S100A8/A9 is the only co-expressed differential gene upregulated in the cerebral cortex, hippocampus and cerebellum among aging-related molecular markers in these brain regions, and it is strongly associated with neuroinflammation and inflammation-related molecular changes (26). The distribution of S100A8/A9 in the brain is linked to neurodegenerative disorders such as dementia, Parkinson's disease (PD) and AD, as well as aging-related cognitive decline. Their expression is also closely associated with inflammatory responses (26). Elevated levels of S100A8/A9 in the prefrontal cortex, hippocampus and cerebellum of older rats are crucial for regulating inflammation. In the CNS, neurodegenerative and neuroinflammatory diseases are closely associated with Ca2+ signaling dysfunction (111). S100 protein expression is ubiquitous in several brain regions, with S100A8/A9 exhibiting the highest staining intensity. Most S100A8/A9 proteins are located near activated microglia and amyloid plaques (112), which may explain the frequent occurrence of neuroinflammation and amyloid accumulation in the aged brain (113). Aging is a natural and inevitable process that significantly contributes to cognitive impairment. However, various strategies can be employed to delay the impact of aging on cognitive performance. Increased expression of S100A8/A9 is closely linked to neuroinflammation and cognitive decline, playing a significant role in aging-associated cognitive impairment. Future research should investigate the mechanisms of S100A8/A9 and develop targeted interventions, such as modulating its signaling pathway or creating specific inhibitors to mitigate neuroinflammation, thereby slowing cognitive decline. In addition, an integrated strategy combining lifestyle interventions (e.g., diet and exercise) as well as pharmacotherapy may be more effective in improving cognitive function and quality of life for older adults.
Learning, memory and emotions
Several mechanisms through which S100A8/A9 play crucial roles in learning/memory and emotion control have been identified, and these are largely dependent on regulation and alteration of neuroinflammatory and cell signaling mechanisms. For instance, it can activate TLR4, RAGE receptors, etc., induce the production of inflammatory factors and affect neuronal function and neural network activity. It can also interfere with neural signal transmission and synaptic plasticity by regulating factors such as microglial cell activity, oxidative stress levels and neurotransmitter systems (42,43). Increased expression of S100A8/A9 in Aβ42-induced glia and in the hippocampus from Tg2576 and TgAPParptic mice highlights its functions (51). Regarding mood regulation, upregulation of S100A8/0A9 improves cognitive function in patients with PD, suppresses pro-inflammatory responses in microglia and encourages autophagic α-synuclein degradation, suggesting that S100A8/A9 may affect inflammatory responses and apoptosis to control mood (114). Through the PI3K/AKT and MAPK pathways, which control autophagy and apoptosis in the early phases of a heart attack, S100A8/A9 also significantly contributes to mood regulation (115), and mood control is also associated with these signaling pathways. Thus, the role of the S100A8/A9 protein in learning, memory and the regulation of emotions should be taken into account. These results also provide a scientific basis for possible S100A8/A9-targeting therapeutic strategies.
S100A8/A9 as a therapeutic target
S100A8/A9 as a potential target for the treatment of cognitive disorders
In AD, elevated expression of the S100A8/A9 protein is closely linked to the formation of Aβ plaques and neuroinflammatory responses (116). Furthermore, increased levels of S100A8/A9 have been directly associated with cognitive impairment in patients with SLE (19). In a model of middle cerebral artery occlusion in the context of IS, elevated S100A8/A9 expression is significantly associated with the induction of an inflammatory response and deterioration of patient prognosis (61). The involvement of S100A8/A9 extends beyond the aforementioned conditions. As individuals age, S100A8/A9 is associated with a persistent low-grade inflammatory state that compromises immunity and increases susceptibility to various diseases (109). Cognitive dysfunction has been shown to be closely linked to CSD, with S100A8/A9 serving as a crucial regulator of this process (1). Through neuroinflammatory and cell signaling pathways, S100A8/A9 influences learning, memory and emotion, playing a vital role in the pathophysiology of neurodegenerative diseases such as PD and AD (2). These diseases share the commonality of affecting cognitive function through several mechanisms, potentially leading to cognitive impairment. Consequently, these findings suggest the potential of S100A8/A9 as a therapeutic target for cognitive disorders. The involvement of S100A8/A9 in neuroinflammation and cognitive disorders is undeniable, and research on this protein not only enhances a comprehensive understanding of the pathological mechanisms underlying CNS diseases but also paves the way for the development of novel therapeutic strategies. Therapeutic approaches targeting S100A8/A9 may offer new perspectives and strategies for managing cognitive disorders associated with neuroinflammation.
Recent advances in drug development and clinical trials of S100A8/A9
Recent advances in drug development and clinical trials of S100A8/A9 have shown progress. For instance, small molecule inhibitors such as Tasquinimod and Paquinimod have been demonstrated to suppress the inflammatory response by preventing S100A8/A9 from interacting with TLR4 (8,117). Additionally, researchers are working on developing small-molecule inhibitors that specifically target S100A8/A9 to mitigate its role in the inflammatory process. These inhibitors, based on a study by Bonora et al (118), aim to block the binding of S100A8/A9 to RAGE. Regarding natural products, several plant-derived compounds, including flavonoids like kaempferol and glycyrrhizic acid (glycyrrhizin), have been shown to inhibit S100A8/A9 (119) and exhibit anti-inflammatory properties in animal models, further supporting the findings of Zheng et al (8) and Chen and Di (117). Despite the limited number of direct clinical studies on S100A8/A9, there is already evidence of significantly elevated levels of S100A8/A9 in the brain tissue and CSF of individuals with PD and AD, suggesting their potential as biomarkers (8,114). Furthermore, the correlation between S100A8/A9 levels and disease severity provides a scientific basis for the development of future therapeutic strategies targeting S100A8/A9 (117).
Potential and challenges of S100A8 as a biomarker and therapeutic target
S100A8/A9 has demonstrated considerable potential as a target for both diagnosis and treatment, given its variable expression across a range of pathological conditions. Alterations in S100A8/A9 levels present a promising biomarker for monitoring the activity of inflammatory disorders such as AD, SLE and IS (79,81-83). Additionally, fluctuations in S100A8/A9 levels may enhance the accuracy of autoimmune disease diagnosis, including SLE (19). In terms of prognostic evaluation, S100A8/A9 expression levels are strongly correlated with disease prognosis and assist in predicting patients' responses to treatment and the progression of their illness. Due to its pivotal role in the inflammatory response, S100A8/A9 is a viable target for anti-inflammatory therapy, as its activity can be mitigated through inhibition (49,50). Furthermore, S100A8/A9 may contribute to neuroprotection and the enhancement of cognitive function, as evidenced by its involvement in neuroinflammation and cognitive disorders (25). The role of S100A8/A9 in immune cell activation and immunological responses supports its potential as a target for immunomodulation therapy (31).
Blood-brain barrier (BBB) penetration strategies
To enhance the delivery of therapeutic agents to the CNS, several innovative approaches are being investigated to overcome the BBB. One potential strategy involves the use of nanocarriers. Nanoparticles can be engineered to transport therapeutic agents across the BBB either through passive diffusion or by interacting with specific receptors on the BBB endothelial cells (120). For instance, carbon dots and gold nanoparticles smaller than 4 nm can traverse the BBB via passive diffusion (120). Furthermore, ligand-functionalized nanoparticles can facilitate BBB crossing by exploiting receptor-mediated transcytosis (120). Another promising technique is focused ultrasound (FUS) combined with microbubbles (121). FUS is a non-invasive method that induces microbubbles to oscillate, exerting mechanical forces on endothelial cells and temporarily disrupting the BBB. This facilitates the penetration of medications into the brain parenchyma. The technique is a safe and controlled method for drug delivery, offering precise targeting and reversibility (121,122). Additionally, intranasal delivery provides a non-invasive approach to targeting the CNS. This method enables direct delivery of therapeutic agents to the brain by bypassing the BBB via the olfactory and trigeminal pathways (123,124). These strategies offer novel approaches to improving drug delivery to the brain and may enhance the management of various neurological conditions.
Potential risks and limitations
The translational application of S100A8/A9 as a therapeutic target and biomarker poses several challenges. Primarily, these proteins are integral to the immune response and their complete suppression may compromise immunity, thereby increasing the risk of infection. Furthermore, inhibiting S100A8/A9 may have unforeseen effects, as they are also involved in normal physiological functions such as cell migration and microtubule stability. Clinically, targeting S100A8/A9 alone, which may be beneficial for all patients, is complicated by the substantial variability in disease processes and progression among patients (high heterogeneity).
Controlling the activity of these proteins poses significant challenges due to their operation through numerous interconnected signaling pathways with complex feedback mechanisms. The delivery of medication is particularly challenging in conditions affecting the CNS, where the BBB impedes drug entry, necessitating the development of specialized delivery systems capable of crossing this barrier (49,50). The temporal aspect is also crucial, as S100A8/A9 expression levels vary over time, necessitating the identification of the optimal intervention window. Furthermore, there are challenges in the research and development phase, as there is currently limited information on the long-term safety and efficacy of inhibitors, requiring extensive clinical trials to fully ascertain their true effects and potential risks. To fully realize precision medicine and enhance therapeutic efficacy, the development of treatments targeting S100A8/A9 must consider these risks and limitations. To thoroughly evaluate the potential of S100A8/A9 as a therapeutic target, future research must meticulously investigate its precise mechanism of action in various diseases and its interactions with other biomarkers. Comprehensive clinical investigations are also necessary to confirm the validity and reliability of S100A8/A9 as a biomarker. To harness the therapeutic potential of S100A8/A9, novel compounds or biologics targeting the protein must be developed, and their safety and efficacy must be assessed (71,73,74,125).
Finally, the significant role of S100A8/A9 in various diseases, including inflammatory and neurodegenerative disorders, provides a robust foundation for its use as a target for diagnosis and treatment. Challenges such as medication specificity, delivery strategies and clinical validation must be addressed to realize this potential. Future studies will be essential to fully comprehend the potential of S100A8/A9 in the treatment and management of diseases.
Conclusions and future directions
As the population continues to age, cognitive impairment has emerged as a significant health concern. The S100A8/A9 protein has also emerged as a major neuroinflammatory regulator and, as such, has seen increased research attention. By interacting with RAGE and TLR4 receptors to activate NF-κB and MAPK signaling pathways, S100A8/A9 plays a key role in neuroinflammation and cognitive impairment. By encouraging the development of a pathological positive feedback loop promoting Aβ plaque aggregation and the neuroinflammatory response in AD, it is strongly associated with the release of inflammatory mediators and a poor cognitive prognosis in stroke models. It also exhibits long-lasting pro-inflammatory effects in SLE and SAE. These results suggest that S100A8/A9 is a significant biomarker and targeting it may have therapeutic potential. However, there are still gaps in our understanding, particularly regarding the relationship between S100A8/A9 and PD. Current research is limited and future studies should investigate the specific mechanisms through which S100A8/A9 contributes to neuroinflammation and neurodegeneration in PD. This could involve exploring its interaction with α-synuclein aggregates and its role in activating microglia and astrocytes in the PD brain. Additionally, the development of targeted therapies for PD based on S100A8/A9 inhibition or modulation warrants further exploration. The tissue-specific expression of S100A8/A9 in various brain regions necessitates the development of a precise and targeted drug delivery system, and its intricate signaling pathway interactions require further elucidation. Additionally, the BBB penetration and long-term safety assessment remain significant bottlenecks in the clinical translation process. In order to precisely release the S100A8/A9 inhibitor at the site of the lesion, future research should concentrate on the development of improved drug delivery systems in conjunction with nanotechnology and brain-targeted peptide design. Nevertheless, to precisely suppress the aberrant production of S100A8/A9 and preserve its physiological function, gene editing and epigenetic regulatory technologies should be employed. Additionally, by building a model of the S100A8/A9-mediated neuroinflammation network, the integrated analysis of multi-omics data will optimize therapeutic options and offer a scientific foundation for individualized treatment. In conclusion, as S100A8/A9 acts as the junction between neuroinflammation and cognitive impairment, targeting it may have therapeutic value. S100A8/A9 targeting may overcome the limitations of current technologies by fusing state-of-the-art biotechnology and systems medicine concepts, opening up novel avenues for precision medicine in the future.
Availability of data and materials
Not applicable.
Authors' contributions
FH and JX provided supervision and assisted with writing and editing. XG conducted the literature search and drafted and compiled the manuscript. LZ revised the article and created the figures. XZ, XR, XH, YX and YG provided constructive suggestions for the article and contributed to its design and structuring. MZ, DZ and JW arranged and adjusted the article, contributing to its revision. QT and XW modified and adjusted the figures, ensuring their accuracy and clarity. All authors have read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
Not applicable.
Funding
This work was supported by grants from the Science Foundation of Yingtan City (grant no. 20244-390331) Natural Science Foundation of Jiangxi Province (grant nos. 20242BAB26133, 20243BCE51077) and Jiangxi Province Key Laboratory of Anesthesiology (grant no. 2024SSY06161).
References
|
Qin F, Luo M, Xiong Y, Zhang N, Dai Y, Kuang W and Cen X: Prevalence and associated factors of cognitive impairment among the elderly population: A nationwide cross-sectional study in China. Front Public Health. 10:10326662022. View Article : Google Scholar : PubMed/NCBI | |
|
Feng X, Zhan F, Luo D, Hu J, Wei G, Hua F and Xu G: LncRNA 4344 promotes NLRP3-related neuroinflammation and cognitive impairment by targeting miR-138-5p. Brain Behav Immun. 98:283–298. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang X, Luo L, Zhao J, Guo X, Tao L, Zhang F, Liu X, Gao B and Luo Y: Associations between sleep duration trajectories and cognitive decline: A longitudinal cohort study in China. Arch Gerontol Geriatr. 124:1054452024. View Article : Google Scholar : PubMed/NCBI | |
|
Sharifi-Rad J, Quispe C, Shaheen S, El Haouari M, Azzini E, Butnariu M, Sarac I, Pentea M, Ramírez-Alarcón K, Martorell M, et al: Flavonoids as potential anti-platelet aggregation agents: From biochemistry to health promoting abilities. Crit Rev Food Sci Nutr. 62:8045–8058. 2022. View Article : Google Scholar | |
|
Zhao C, Noble JM, Marder K, Hartman JS, Gu Y and Scarmeas N: Dietary patterns, physical activity, sleep, and risk for dementia and cognitive decline. Curr Nutr Rep. 7:335–345. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Tian Q, Li Z, Yan Z, Jiang S, Zhao X, Wang L and Li M: Inflammatory role of S100A8/A9 in the central nervous system non-neoplastic diseases. Brain Res Bull. 218:1111002024. View Article : Google Scholar : PubMed/NCBI | |
|
Tampé JF, Monni E, Palma-Tortosa S, Brogårdh E, Böiers C, Lindgren AG and Kokaia Z: Human monocyte subtype expression of neuroinflammation- and regeneration-related genes is linked to age and sex. PLoS One. 19:e03009462024. View Article : Google Scholar : PubMed/NCBI | |
|
Chen B and Di B: Endogenous ligands of TLR4 in microglia: Potential targets for related neurological diseases. Curr Drug Targets. 25:953–970. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Cheng Y, Kim WK, Wellman LL, Sanford LD and Guo ML: Short-term sleep fragmentation dysregulates autophagy in a brain Region-specific manner. Life (Basel). 11:10982021.PubMed/NCBI | |
|
Zera KA and Buckwalter MS: The local and peripheral immune responses to stroke: Implications for therapeutic development. Neurotherapeutics. 17:414–435. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Stephenson J, Nutma E, van der Valk P and Amor S: Inflammation in CNS neurodegenerative diseases. Immunology. 154:204–219. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Bowman GL, Dayon L, Kirkland R, Wojcik J, Peyratout G, Severin IC, Henry H, Oikonomidi A, Migliavacca E, Bacher M and Popp J: Blood-brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimers Dement. 14:1640–1650. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Wang R, Hou L, Lu H, Zhang Y, Guo T, Zhou B, Zhao H and Xing M: Unveiling the interplay of MAPK/NF-κB/MLKL axis in brain health: Omega-3 as a promising candidates against copper neurotoxicity. J Environ Manage. 370:1227912024. View Article : Google Scholar | |
|
Wang D, Yin K, Zhang Y, Lu H, Hou L, Zhao H and Xing M: Fluoride induces neutrophil extracellular traps and aggravates brain inflammation by disrupting neutrophil calcium homeostasis and causing ferroptosis. Environ Pollut. 331:1218472023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y, Zhao H, Yang X, Mu M, Zong H, Luo L and Xing M: Excessive Cu2+ deteriorates arsenite-induced apoptosis in chicken brain and resulting in immunosuppression, not in homeostasis. Chemosphere. 239:1247582020. View Article : Google Scholar | |
|
Tao Q, Qiu X, Li C, Zhou J, Gu L, Zhang L, Pang J, Zhang L, Yin S, Jiang Y and Peng J: S100A8 regulates autophagy-dependent ferroptosis in microglia after experimental subarachnoid hemorrhage. Exp Neurol. 357:1141712022. View Article : Google Scholar : PubMed/NCBI | |
|
Woodburn SC, Bollinger JL and Wohleb ES: The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J Neuroinflammation. 18:2582021. View Article : Google Scholar : PubMed/NCBI | |
|
Cerón JJ, Ortín-Bustillo A, López-Martínez MJ, Martínez-Subiela S, Eckersall PD, Tecles F, Tvarijonaviciute A and Muñoz-Prieto A: S-100 proteins: Basics and applications as biomarkers in animals with special focus on calgranulins (S100A8, A9, and A12). Biology (Basel). 12:8812023.PubMed/NCBI | |
|
Muñoz-Grajales C, Barraclough ML, Diaz-Martinez JP, Su J, Bingham K, Kakvan M, Kretzmann RP, Tartaglia MC, Ruttan L, Choi MY, et al: Serum S100A8/A9 and MMP-9 levels are elevated in systemic lupus erythematosus patients with cognitive impairment. Front Immunol. 14:13267512023. View Article : Google Scholar | |
|
Rayes HA, Tani C, Kwan A, Marzouk S, Colosimo K, Medina-Rosas J, Mustafa A, Su J, Lambiris P, Mosca M and Touma Z: What is the prevalence of cognitive impairment in lupus and which instruments are used to measure it? A systematic review and meta-analysis. Semin Arthritis Rheum. 48:240–255. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Zheng F and Xie W: High-sensitivity C-reactive protein and cognitive decline: The English Longitudinal study of ageing. Psychol Med. 48:1381–1389. 2018. View Article : Google Scholar | |
|
Iadecola C: The pathobiology of vascular dementia. Neuron. 80:844–866. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Dong J, Wang S, Hu Z and Gong L: Extracellular proteins as potential biomarkers in Sepsis-related cerebral injury. Front Immunol. 14:11284762023. View Article : Google Scholar : PubMed/NCBI | |
|
Gofton TE and Young GB: Sepsis-associated encephalopathy. Nat Rev Neurol. 8:557–566. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Xiong Y, Liang W, Wang X, Zhu H, Yi P, Wei G, Liu H, Lin Y, Zhang L, Ying J and Hua F: S100A8 knockdown activates the PI3K/AKT signaling pathway to inhibit microglial autophagy and improve cognitive impairment mediated by chronic sleep deprivation. Int Immunopharmacol. 143:1133752024. View Article : Google Scholar : PubMed/NCBI | |
|
Su X, Xie L, Li J, Tian X, Lin B and Chen M: Exploring molecular signatures related to the mechanism of aging in different brain regions by integrated bioinformatics. Front Mol Neurosci. 16:11331062023. View Article : Google Scholar : PubMed/NCBI | |
|
Shen L, Liao L, Chen C, Guo Y, Song D, Wang Y, Chen Y, Zhang K, Ying M, Li S, et al: Proteomics analysis of blood serums from Alzheimer's disease patients using iTRAQ labeling technology. J Alzheimers Dis. 56:361–378. 2017. View Article : Google Scholar | |
|
Chen Y, Ouyang Y, Li Z, Wang X and Ma J: S100A8 and S100A9 in cancer. Biochim Biophys Acta Rev Cancer. 1878:1888912023. View Article : Google Scholar : PubMed/NCBI | |
|
Shabani F, Farasat A, Mahdavi M and Gheibi N: Calprotectin (S100A8/S100A9): A key protein between inflammation and cancer. Inflamm Res. 67:801–812. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Mondet J, Chevalier S and Mossuz P: Pathogenic roles of S100A8 and S100A9 proteins in acute myeloid and lymphoid leukemia: Clinical and therapeutic impacts. Molecules. 26:13232021. View Article : Google Scholar : PubMed/NCBI | |
|
Garcia V, Perera YR and Chazin WJ: A structural perspective on calprotectin as a ligand of receptors mediating inflammation and potential drug target. Biomolecules. 12:1592022. View Article : Google Scholar | |
|
Pan S, Hu Y, Hu M, Xu Y, Chen M, Du C, Cui J, Zheng P, Lai J, Zhang Y, et al: S100A8 facilitates cholangiocarcinoma metastasis via upregulation of VEGF through TLR4/NF-κB pathway activation. Int J Oncol. 56:101–112. 2020. | |
|
Mondet J, Laurin D, Lo Presti C, Jacob MC, Meunier M, Giraudon E, Lefebvre C, Berthier S, Leer AM, Park S and Mossuz P: Increased S100A8 expression in bone marrow plasma by monocytic cells from acute myeloid leukemia patients. Hematol Oncol. 38:114–118. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Gomes LH, Raftery MJ, Yan WX, Goyette JD, Thomas PS and Geczy CL: S100A8 and S100A9-oxidant scavengers in inflammation. Free Radic Biol Med. 58:170–186. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Kwon MS: Advanced therapeutic strategies targeting microglia: Beyond neuroinflammation. Arch Pharm Res. 45:618–630. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Orihuela R, McPherson CA and Harry GJ: Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 173:649–665. 2016. View Article : Google Scholar : | |
|
Blom AB, van den Bosch MH, Blaney Davidson EN, Roth J, Vogl T, van de Loo FA, Koenders M, van der Kraan PM, Geven EJ and van Lent PL: The alarmins S100A8 and S100A9 mediate acute pain in experimental synovitis. Arthritis Res Ther. 22:1992020. View Article : Google Scholar : PubMed/NCBI | |
|
Bach M, Moon J, Moore R, Pan T, Nelson JL and Lood C: A neutrophil activation biomarker panel in prognosis and monitoring of patients with rheumatoid arthritis. Arthritis Rheumatol. 72:47–56. 2020. View Article : Google Scholar | |
|
Schenten V, Melchior C, Steinckwich N, Tschirhart EJ and Bréchard S: Sphingosine kinases regulate NOX2 activity via p38 MAPK-dependent translocation of S100A8/A9. J Leukoc Biol. 89:587–596. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Park IH, Yeon SI, Youn JH, Choi JE, Sasaki N, Choi IH and Shin JS: Expression of a novel secreted splice variant of the receptor for advanced glycation end products (RAGE) in human brain astrocytes and peripheral blood mononuclear cells. Mol Immunol. 40:1203–1211. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Yan SF, Yan SD, Ramasamy R and Schmidt AM: Tempering the wrath of RAGE: An emerging therapeutic strategy against diabetic complications, neurodegeneration, and inflammation. Ann Med. 41:408–422. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Fang F, Lue LF, Yan S, Xu H, Luddy JS, Chen D, Walker DG, Stern DM, Yan S, Schmidt AM, et al: RAGE-dependent signaling in microglia contributes to neuroinflammation, Abeta accumulation, and impaired learning/memory in a mouse model of Alzheimer's disease. FASEB J. 24:1043–1055. 2010. View Article : Google Scholar | |
|
Zhu G, Cheng Z, Lin C, Hoffman RM, Huang Y, Singh SR, Zheng W, Yang S and Ye J: MyD88 regulates LPS-induced NF-ĸB/MAPK cytokines and promotes inflammation and malignancy in colorectal cancer cells. Cancer Genomics Proteomics. 16:409–419. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Lawrence T: The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 1:a0016512009. View Article : Google Scholar | |
|
Zhu K, Zhu X, Sun S, Yang W, Liu S, Tang Z, Zhang R, Li J, Shen T and Hei M: Inhibition of TLR4 prevents hippocampal hypoxic-ischemic injury by regulating ferroptosis in neonatal rats. Exp Neurol. 345:1138282021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou H, Zhao C, Shao R, Xu Y and Zhao W: The functions and regulatory pathways of S100A8/A9 and its receptors in cancers. Front Pharmacol. 14:11877412023. View Article : Google Scholar : PubMed/NCBI | |
|
Sun Y, Xu H, Gao W, Deng J, Song X, Li J and Liu X: S100a8/A9 proteins: Critical regulators of inflammation in cardiovascular diseases. Front Cardiovasc Med. 11:13941372024. View Article : Google Scholar : PubMed/NCBI | |
|
Flemmig J, Zámocký M and Alia A: Amyloid β and free heme: Bloody new insights into the pathogenesis of Alzheimer's disease. Neural Regen Res. 13:1170–1174. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Wang S, Song R, Wang Z, Jing Z, Wang S and Ma J: S100A8/A9 in inflammation. Front Immunol. 9:12982018. View Article : Google Scholar : PubMed/NCBI | |
|
Pruenster M, Vogl T, Roth J and Sperandio M: S100A8/A9: From basic science to clinical application. Pharmacol Ther. 167:120–131. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Lodeiro M, Puerta E, Ismail MA, Rodriguez-Rodriguez P, Rönnbäck A, Codita A, Parrado-Fernandez C, Maioli S, Gil-Bea F, Merino-Serrais P and Cedazo-Minguez A: Aggregation of the inflammatory S100A8 precedes Aβ plaque formation in transgenic APP mice: Positive feedback for S100A8 and Aβ productions. J Gerontol A Biol Sci Med Sci. 72:319–328. 2017. | |
|
Zheng J, Wang J, Liu H, Chen F, Wang H, Chen S, Xie J, Zheng Z and Li Z: Alarmins S100A8/A9 Promote intervertebral disc degeneration and inflammation-related pain in a rat model through toll-like receptor-4 and activation of the NF-κB signaling pathway. Osteoarthritis Cartilage. 30:998–1011. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Wu M, Xu L, Wang Y, Zhou N, Zhen F, Zhang Y, Qu X, Fan H, Liu S, Chen Y and Yao R: S100A8/A9 induces microglia activation and promotes the apoptosis of oligodendrocyte precursor cells by activating the NF-κB signaling pathway. Brain Res Bull. 143:234–245. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Barbosa-Silva MC, Lima MN, Battaglini D, Robba C, Pelosi P, Rocco PRM and Maron-Gutierrez T: Infectious disease-associated encephalopathies. Crit Care. 25:2362021. View Article : Google Scholar : PubMed/NCBI | |
|
Litus EA, Shevelyova MP, Vologzhannikova AA, Deryusheva EI, Machulin AV, Nemashkalova EL, Permyakova ME, Sokolov AS, Alikova VD, Uversky VN and Permyakov SE: Binding of Pro-inflammatory proteins S100A8 or S100A9 to Amyloid-β peptide suppresses its fibrillation. Biomolecules. 15:4312025. View Article : Google Scholar | |
|
Stephan JR, Yu F, Costello RM, Bleier BS and Nolan EM: Oxidative Post-translational modifications accelerate proteolytic degradation of calprotectin. J Am Chem Soc. 140:17444–17455. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Hou Z, Sun A, Li Y, Song X, Liu S, Hu X, Luan Y, Guan H, He C, Sun Y and Chen J: What are the reliable plasma biomarkers for mild cognitive impairment? A clinical 4D proteomics study and validation. Mediators Inflamm. 2024:77092772024. View Article : Google Scholar : PubMed/NCBI | |
|
Gratuze M, Chen Y, Parhizkar S, Jain N, Strickland MR, Serrano JR, Colonna M, Ulrich JD and Holtzman DM: Activated microglia mitigate Aβ-associated tau seeding and spreading. J Exp Med. 218:e202105422021. View Article : Google Scholar | |
|
Mancuso R, Fryatt G, Cleal M, Obst J, Pipi E, Monzón-Sandoval J, Ribe E, Winchester L, Webber C, Nevado A, et al: CSF1R inhibitor JNJ-40346527 attenuates microglial proliferation and neurodegeneration in P301S mice. Brain. 142:3243–3264. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Bhaskar K, Konerth M, Kokiko-Cochran ON, Cardona A, Ransohoff RM and Lamb BT: Regulation of tau pathology by the microglial fractalkine receptor. Neuron. 68:19–31. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Gruel R, Bijnens B, Van Den Daele J, Thys S, Willems R, Wuyts D, Van Dam D, Verstraelen P, Verboven R, Roels J, et al: S100A8-enriched microglia populate the brain of tau-seeded and accelerated aging mice. Aging Cell. 23:e141202024. View Article : Google Scholar : PubMed/NCBI | |
|
Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, et al: RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 9:907–913. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Santiago JA, Bottero V and Potashkin JA: Transcriptomic and network analysis identifies shared and unique pathways across dementia spectrum disorders. Int J Mol Sci. 21:20502020. View Article : Google Scholar : PubMed/NCBI | |
|
Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al: Heart disease and stroke statistics-2012 update: A report from the American Heart Association. Circulation. 125:e2–e220. 2012. | |
|
Ge X, Zheng M, Hu M, Fang X, Geng D, Liu S, Wang L, Zhang J, Guan L, Zheng P, et al: Butyrate ameliorates quinolinic acid-induced cognitive decline in obesity models. J Clin Invest. 133:e1546122023. View Article : Google Scholar : PubMed/NCBI | |
|
Metcalf TU, Wilkinson PA, Cameron MJ, Ghneim K, Chiang C, Wertheimer AM, Hiscott JB, Nikolich-Zugich J and Haddad EK: Human monocyte subsets are transcriptionally and functionally altered in aging in response to pattern recognition receptor agonists. J Immunol. 199:1405–1417. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Gülke E, Gelderblom M and Magnus T: Danger signals in stroke and their role on microglia activation after ischemia. Ther Adv Neurol Disord. 11:17562864187742542018. View Article : Google Scholar : PubMed/NCBI | |
|
Shichita T, Ito M and Yoshimura A: Post-ischemic inflammation regulates neural damage and protection. Front Cell Neurosci. 8:3192014. View Article : Google Scholar : PubMed/NCBI | |
|
Marta-Enguita J, Navarro-Oviedo M, Rubio-Baines I, Aymerich N, Herrera M, Zandio B, Mayor S, Rodriguez JA, Páramo JA, Toledo E, et al: Association of calprotectin with other inflammatory parameters in the prediction of mortality for ischemic stroke. J Neuroinflammation. 18:32021. View Article : Google Scholar : PubMed/NCBI | |
|
Denstaedt SJ, Spencer-Segal JL, Newstead MW, Laborc K, Zhao AP, Hjelmaas A, Zeng X, Akil H, Standiford TJ and Singer BH: S100A8/A9 drives neuroinflammatory priming and protects against Anxiety-like behavior after sepsis. J Immunol. 200:3188–3200. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Ge R, Tornero D, Hirota M, Monni E, Laterza C, Lindvall O and Kokaia Z: Choroid plexus-cerebrospinal fluid route for monocyte-derived macrophages after stroke. J Neuroinflammation. 14:1532017. View Article : Google Scholar : PubMed/NCBI | |
|
Wattananit S, Tornero D, Graubardt N, Memanishvili T, Monni E, Tatarishvili J, Miskinyte G, Ge R, Ahlenius H, Lindvall O, et al: Monocyte-derived macrophages contribute to spontaneous Long-term functional recovery after stroke in mice. J Neurosci. 36:4182–4195. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Nacken W, Roth J, Sorg C and Kerkhoff C: S100A9/S100A8: Myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc Res Tech. 60:569–580. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Guo D, Zhu Z, Xu T, Zhong C, Wang A, Xie X, Peng Y, Peng H, Li Q, Ju Z, et al: Plasma S100A8/A9 concentrations and clinical outcomes of ischemic stroke in 2 independent multicenter cohorts. Clin Chem. 66:706–717. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Li L, Dong L, Xiao Z, He W, Zhao J, Pan H, Chu B, Cheng J and Wang H: Integrated analysis of the proteome and transcriptome in a MCAO mouse model revealed the molecular landscape during stroke progression. J Adv Res. 24:13–27. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Chen L, Chen X, Wang Y, Li S, Huang S, Wu Z, He J, Chen S, Deng F, Zhu P, et al: Polymorphisms of calgranulin genes and ischemic stroke in a Chinese population. J Inflamm Res. 15:3355–3368. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Carrión-Barberà I, Salman-Monte TC, Vílchez-Oya F and Monfort J: Neuropsychiatric involvement in systemic lupus erythematosus: A review. Autoimmun Rev. 20:1027802021. View Article : Google Scholar : PubMed/NCBI | |
|
Hanly JG: Diagnosis and management of neuropsychiatric SLE. Nat Rev Rheumatol. 10:338–347. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Wakiya R, Kameda T, Ueeda K, Nakashima S, Shimada H, Mansour MF, Kato M, Miyagi T, Miyatake N, Kadowaki N and Dobashi H: Hydroxychloroquine modulates elevated expression of S100 proteins in systemic lupus erythematosus. Lupus. 28:826–833. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Šumová B, Cerezo LA, Szczuková L, Nekvindová L, Uher M, Hulejová H, Moravcová R, Grigorian M, Pavelka K, Vencovský J, et al: Circulating S100 proteins effectively discriminate SLE patients from healthy controls: A cross-sectional study. Rheumatol Int. 39:469–478. 2019. View Article : Google Scholar | |
|
Tydén H, Lood C, Gullstrand B, Jönsen A, Ivars F, Leanderson T and Bengtsson AA: Pro-inflammatory S100 proteins are associated with glomerulonephritis and anti-dsDNA antibodies in systemic lupus erythematosus. Lupus. 26:139–149. 2017. View Article : Google Scholar | |
|
Tydén H, Lood C, Gullstrand B, Jönsen A, Nived O, Sturfelt G, Truedsson L, Ivars F, Leanderson T and Bengtsson AA: Increased serum levels of S100A8/A9 and S100A12 are associated with cardiovascular disease in patients with inactive systemic lupus erythematosus. Rheumatology (Oxford). 52:2048–2055. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Soyfoo MS, Roth J, Vogl T, Pochet R and Decaux G: Phagocyte-specific S100A8/A9 protein levels during disease exacerbations and infections in systemic lupus erythematosus. J Rheumatol. 36:2190–2194. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Wu CY, Bawa KK, Ouk M, Leung N, Yu D, Lanctôt KL, Herrmann N, Pakosh M and Swardfager W: Neutrophil activation in Alzheimer's disease and mild cognitive impairment: A systematic review and meta-analysis of protein markers in blood and cerebrospinal fluid. Ageing Res Rev. 62:1011302020. View Article : Google Scholar : PubMed/NCBI | |
|
Bracko O, Njiru BN, Swallow M, Ali M, Haft-Javaherian M and Schaffer CB: Increasing cerebral blood flow improves cognition into late stages in Alzheimer's disease mice. J Cereb Blood Flow Metab. 40:1441–1452. 2020. View Article : Google Scholar | |
|
Volkman R, Ben-Zur T, Kahana A, Garty BZ and Offen D: Myeloperoxidase Deficiency inhibits cognitive decline in the 5XFAD mouse model of Alzheimer's disease. Front Neurosci. 13:9902019. View Article : Google Scholar : PubMed/NCBI | |
|
Cruz Hernández JC, Bracko O, Kersbergen CJ, Muse V, Haft-Javaherian M, Berg M, Park L, Vinarcsik LK, Ivasyk I, Rivera DA, et al: Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer's disease mouse models. Nat Neurosci. 22:413–420. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Jönsen A, Bengtsson AA, Nived O, Ryberg B and Sturfelt G: Outcome of neuropsychiatric systemic lupus erythematosus within a defined Swedish population: Increased morbidity but low mortality. Rheumatology (Oxford). 41:1308–1312. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Walker DG, Link J, Lue LF, Dalsing-Hernandez JE and Boyes BE: Gene expression changes by amyloid beta peptide-stimulated human postmortem brain microglia identify activation of multiple inflammatory processes. J Leukoc Biol. 79:596–610. 2006. View Article : Google Scholar | |
|
Stanek A, Brożyna-Tkaczyk K and Myśliński W: Oxidative stress markers among obstructive sleep apnea patients. Oxid Med Cell Longev. 2021:96815952021. View Article : Google Scholar : PubMed/NCBI | |
|
Ha JS, Choi HR, Kim IS, Kim EA, Cho SW and Yang SJ: Hypoxia-induced S100A8 expression activates microglial inflammation and promotes neuronal apoptosis. Int J Mol Sci. 22:12052021. View Article : Google Scholar : PubMed/NCBI | |
|
Fei W, Jiao W, Feng X, Chen X and Wang Y: Intermittent hypoxia mimicking obstructive sleep apnea aggravates early brain injury following ICH via neuroinflammation and apoptosis. Mol Med Rep. 24:8242021. View Article : Google Scholar : PubMed/NCBI | |
|
Zeng X, Guo R, Dong M, Zheng J, Lin H and Lu H: Contribution of TLR4 signaling in intermittent hypoxia-mediated atherosclerosis progression. J Transl Med. 16:1062018. View Article : Google Scholar : PubMed/NCBI | |
|
Akinnusi M, Jaoude P, Kufel T and El-Solh AA: Toll-like receptor activity in patients with obstructive sleep apnea. Sleep Breath. 17:1009–1016. 2013. View Article : Google Scholar | |
|
Chaput JP, McHill AW, Cox RC, Broussard JL, Dutil C, da Costa BGG, Sampasa-Kanyinga H and Wright KP: The role of insufficient sleep and circadian misalignment in obesity. Nat Rev Endocrinol. 19:82–97. 2023. View Article : Google Scholar | |
|
Korte SM and Straub RH: Fatigue in inflammatory rheumatic disorders: Pathophysiological mechanisms. Rheumatology (Oxford). 58(Suppl 5): v35–v50. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Singer BH, Dickson RP, Denstaedt SJ, Newstead MW, Kim K, Falkowski NR, Erb-Downward JR, Schmidt TM, Huffnagle GB and Standiford TJ: Bacterial dissemination to the brain in sepsis. Am J Respir Crit Care Med. 197:747–756. 2018. View Article : Google Scholar : | |
|
Lamar CD, Hurley RA and Taber KH: Sepsis-associated encephalopathy: Review of the neuropsychiatric manifestations and cognitive outcome. J Neuropsychiatry Clin Neurosci. 23:237–241. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Lamers KJ, Vos P, Verbeek MM, Rosmalen F, van Geel WJ and van Engelen BG: Protein S-100B, neuron-specific enolase (NSE), myelin basic protein (MBP) and glial fibrillary acidic protein (GFAP) in cerebrospinal fluid (CSF) and blood of neurological patients. Brain Res Bull. 61:261–264. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Ghavami S, Eshragi M, Ande SR, Chazin WJ, Klonisch T, Halayko AJ, McNeill KD, Hashemi M, Kerkhoff C and Los M: S100A8/A9 induces autophagy and apoptosis via ROS-mediated cross-talk between mitochondria and lysosomes that involves BNIP3. Cell Res. 20:314–331. 2010. View Article : Google Scholar | |
|
Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C and Roth J: Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 13:1042–1049. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Lv J, Wang Z, Wang B, Deng C, Wang W and Sun L: S100A9 induces macrophage M2 polarization and immunomodulatory role in the lesion site after spinal cord injury in rats. Mol Neurobiol. 61:5525–5540. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Huang N, Tang J, Yi X, Zhang M, Li B, Cheng Y and Chen J: Glioma-derived S100A9 polarizes M2 microglia to inhibit CD8+T lymphocytes for immunosuppression via αvβ3 integrin/AKT1/TGFβ1. Biochim Biophys Acta Mol Cell Res. 1871:1196192024. View Article : Google Scholar | |
|
Lu SM, Yu CJ, Liu YH, Dong HQ, Zhang X, Zhang SS, Hu LQ, Zhang F, Qian YN and Gui B: S100A8 contributes to postoperative cognitive dysfunction in mice undergoing tibial fracture surgery by activating the TLR4/MyD88 pathway. Brain Behav Immun. 44:221–234. 2015. View Article : Google Scholar | |
|
Cao Y, Yang Y, Wu H, Lu Y, Wu S, Liu L, Wang C, Huang F, Shi H, Zhang B, et al: Stem-leaf saponins from Panax notoginseng counteract aberrant autophagy and apoptosis in hippocampal neurons of mice with cognitive impairment induced by sleep deprivation. J Ginseng Res. 44:442–452. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Uddin MS, Tewari D, Mamun AA, Kabir MT, Niaz K, Wahed MII, Barreto GE and Ashraf GM: Circadian and sleep dysfunction in Alzheimer's disease. Ageing Res Rev. 60:1010462020. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Y, Zhang T, Meng D, Sun L, Yang G, He Y and Zhang C: Involvement of CX3CL1/CX3CR1 in depression and cognitive impairment induced by chronic unpredictable stress and relevant underlying mechanism. Behav Brain Res. 381:1123712020. View Article : Google Scholar | |
|
Quick JD, Silva C, Wong JH, Lim KL, Reynolds R, Barron AM, Zeng J and Lo CH: Lysosomal acidification dysfunction in microglia: An emerging pathogenic mechanism of neuroinflammation and neurodegeneration. J Neuroinflammation. 20:1852023. View Article : Google Scholar : PubMed/NCBI | |
|
Franceschi C, Garagnani P, Parini P, Giuliani C and Santoro A: Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 14:576–590. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Swindell WR, Johnston A, Xing X, Little A, Robichaud P, Voorhees JJ, Fisher G and Gudjonsson JE: Robust shifts in S100a9 expression with aging: A novel mechanism for chronic inflammation. Sci Rep. 3:12152013. View Article : Google Scholar : PubMed/NCBI | |
|
Hamasaki MY, Severino P, Puga RD, Koike MK, Hernandes C, Barbeiro HV, Barbeiro DF, Machado MCC, Reis EM and Pinheiro da Silva F: Short-term effects of sepsis and the impact of aging on the transcriptional profile of different brain regions. Inflammation. 42:1023–1031. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Hoyaux D, Decaestecker C, Heizmann CW, Vogl T, Schäfer BW, Salmon I, Kiss R and Pochet R: S100 proteins in Corpora amylacea from normal human brain. Brain Res. 867:280–288. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang K, Mizuma H, Zhang X, Takahashi K, Jin C, Song F, Gao Y, Kanayama Y, Wu Y, Li Y, et al: PET imaging of neural activity, β-amyloid, and tau in normal brain aging. Eur J Nucl Med Mol Imaging. 48:3859–3871. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Ding L, Lu L, Zheng S, Zhang Z, Huang X, Ma R, Zhang M, Xu Z, Chen M, Guo Z, et al: Usp14 deficiency removes α-synuclein by regulating S100A8/A9 in Parkinson's disease. Cell Mol Life Sci. 81:2322024. View Article : Google Scholar | |
|
Yi W, Zhu R, Hou X, Wu F and Feng R: Integrated analysis reveals S100a8/a9 Regulates autophagy and apoptosis through the MAPK and PI3K-AKT signaling pathway in the early stage of myocardial infarction. Cells. 11:19112022. View Article : Google Scholar : PubMed/NCBI | |
|
Wang C, Klechikov AG, Gharibyan AL, Wärmländer SK, Jarvet J, Zhao L, Jia X, Narayana VK, Shankar SK, Olofsson A, et al: The role of pro-inflammatory S100A9 in Alzheimer's disease amyloid-neuroinflammatory cascade. Acta Neuropathol. 127:507–522. 2014. View Article : Google Scholar | |
|
Zheng X, Wang M, Liu S, Chen H, Li Y, Yuan F, Yang L, Qiu S, Wang H, Xie Z and Xiang M: A lncRNA-encoded mitochondrial micropeptide exacerbates microglia-mediated neuroinflammation in retinal ischemia/reperfusion injury. Cell Death Dis. 14:1262023. View Article : Google Scholar : PubMed/NCBI | |
|
Bonora BM, Palano MT, Testa G, Fadini GP, Sangalli E, Madotto F, Persico G, Casciaro F, Vono R, Colpani O, et al: Hematopoietic progenitor cell liabilities and alarmins S100A8/A9-related inflammaging associate with frailty and predict poor cardiovascular outcomes in older adults. Aging Cell. 21:e135452022. View Article : Google Scholar : PubMed/NCBI | |
|
Gong X, Shen H, Guo L, Huang C, Su T, Wang H, Feng S, Yang S, Huo F, Liu H, et al: Glycyrrhizic acid inhibits myeloid differentiation of hematopoietic stem cells by binding S100 calcium binding protein A8 to improve cognition in aged mice. Immun Ageing. 20:122023. View Article : Google Scholar : PubMed/NCBI | |
|
Ding S, Khan AI, Cai X, Song Y, Lyu Z, Du D, Dutta P and Lin Y: Overcoming blood-brain barrier transport: Advances in nanoparticle-based drug delivery strategies. Mater Today (Kidlington). 37:112–125. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Wu SK, Tsai CL, Huang Y and Hynynen K: Focused ultrasound and Microbubbles-mediated drug delivery to brain tumor. Pharmaceutics. 13:152021. View Article : Google Scholar : | |
|
Song KH, Harvey BK and Borden MA: State-of-the-art of microbubble-assisted blood-brain barrier disruption. Theranostics. 8:4393–4408. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Keller LA, Merkel O and Popp A: Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv Transl Res. 12:735–757. 2022. View Article : Google Scholar | |
|
Shah P, Lalan M and Barve K: Intranasal delivery: An attractive route for the administration of nucleic acid based therapeutics for CNS disorders. Front Pharmacol. 13:9746662022. View Article : Google Scholar : PubMed/NCBI | |
|
Ma L, Sun P, Zhang JC, Zhang Q and Yao SL: Proinflammatory effects of S100A8/A9 via TLR4 and RAGE signaling pathways in BV-2 microglial cells. Int J Mol Med. 40:31–38. 2017. View Article : Google Scholar : PubMed/NCBI |