Brain‑heart axis: Neurostimulation techniques in ischemic heart disease (Review)
- Authors:
- Published online on: July 17, 2025 https://doi.org/10.3892/ijmm.2025.5589
- Article Number: 148
-
Copyright: © Liu et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Ischemic heart disease (IHD) is the predominant cause of mortality worldwide, accounting for roughly one-third of all fatalities in those aged >35 (1). This condition manifests primarily as coronary artery disease (CAD) and coronary microvascular disease (CMD). CAD involves the narrowing or obstruction of large coronary arteries caused by atherosclerosis, leading to reduced blood flow to the myocardium. By contrast, CMD affects the small coronary vessels, resulting in impaired myocardial perfusion even without major artery disease. CAD and CMD both contribute to reduced blood supply to the heart and can lead to similar acute clinical outcomes, including angina, myocardial infarction, arrhythmias and sudden cardiac mortality (2-4). The economic burden of CAD is substantial, with the first-year treatment costs for newly diagnosed cases estimated at $5.54 billion in the United States in 1995 (5).
Although CAD and CMD differ in their pathophysiological mechanisms, they share common risk factors and clinical outcomes. Major risk factors include decreased high-density lipoprotein cholesterol, elevated low-density lipoprotein (LDL) cholesterol, diabetes, hypertension, smoking, obesity and a family history of cardiovascular disease. These factors contribute to endothelial dysfunction, inflammation and lipid accumulation within the vascular wall, processes that is central to both CAD and CMD (6-11). Age and sex further influence susceptibility, with men being more prone to IHD after the age of 45 and women experiencing a heightened risk post-menopause due to the decline in the cardioprotective effects of estrogen (12,13). The incidence of IHD increases with advancing age for both sexes, but consistently remains more prevalent among men (14).
In CAD, atherosclerosis is the primary pathological process, characterized by the accumulation of lipid-rich plaques in the major coronary arteries, which impedes myocardial blood flow (15,16). The process typically begins with endothelial injury, leading to inflammation, lipid accumulation and plaque formation, contributing to functional ischemia and a heightened atherosclerotic burden in patients with CAD (17,18). By contrast, CMD involves microvascular dysfunction, which is characterized by endothelial injury, microvascular rarefaction (reduced density of small vessels) and reduced coronary reserve (impaired capacity of the coronary vessels to dilate in response to enhanced metabolic demand). These lead to inadequate myocardial blood flow despite the absence of significant obstruction in the larger coronary arteries. CAD and CMD can both lead to similar clinical outcomes, including angina, myocardial infarction, arrhythmias and even sudden cardiac mortality. In advanced stages of CAD, interventions such as angioplasty and coronary artery bypass grafting (CABG) are often required to reestablish myocardial perfusion (19,20). However, the effectiveness of these treatments remains suboptimal in a number of cases, particularly when microvascular dysfunction is also present, as in CMD (21). This underscores the necessity for more inclusive therapeutic strategies that tackle both the macrovascular and microvascular dimensions of the disease.
The brain-heart axis serves as a vital communication pathway linking the central nervous system (CNS) to the cardiovascular system, primarily through the autonomic nervous system (ANS) (22). The ANS is comprised of three divisions: The sympathetic nervous system (SNS), the para-sympathetic nervous system (PNS) and the enteric nervous system (23). The SNS and PNS are both crucial for the regulation of heart rate, blood pressure and vascular tone (24). During periods of stress or physical exertion, the SNS elevates heart rate, myocardial contractility and oxygen demand, while the PNS, primarily through the vagus nerve, slows the heart rate and promotes relaxation (25,26). SNS overactivity and PNS underactivity often occur together and are associated with heightened cardiovascular risk (27). This autonomic imbalance is reflected in heart rate variability (HRV), with elevated low-frequency power often indicating increased sympathetic or decreased parasympathetic activity (28). This imbalance can lead to increased inflammation, vascular constriction and impaired myocardial perfusion, all of which contribute to the onset and progression of various cardiovascular diseases (29,30). Emerging evidence also supports the association between autonomic dysfunction and IHD, where SNS overactivity exacerbates CAD and microvascular dysfunction (31,32). Given the strong links between the ANS and these cardiovascular conditions, therapies aimed at restoring autonomic balance, such as vagus nerve stimulation (VNS), are increasingly considered promising for enhancing patient outcomes in IHD (33).
Advances in understanding the brain-heart axis have paved the way for innovative therapeutic strategies, including VNS, spinal cord stimulation (SCS) and brain stimulation. These neural modulation techniques aim to re-establish balance between the sympathetic and parasympathetic divisions of the ANS (34). VNS, for example, has been demonstrated to enhance parasympathetic activity, decrease sympathetic overactivation and improve cardiovascular outcomes by modulating heart rate, blood pressure and inflammatory responses (35). Similarly, SCS, though primarily used for pain management (36), has demonstrated potential in improving autonomic function and may benefit patients with arrhythmias and heart failure by positively influencing the ANS (37,38). In addition to VNS and SCS, brain stimulation techniques, such as deep brain stimulation (DBS) (39), transcranial direct current stimulation (tDCS) (40) and transcranial magnetic stimulation (TMS) (41), are gaining increasing attention as potential interventions for cardiovascular health. These techniques modulate brain activity to restore autonomic balance, which can positively affect cardiovascular function. Neural modulation techniques are increasingly recognized as effective interventions for IHD (42). Clinical studies suggest that VNS, SCS and brain stimulation therapies may not only alleviate symptoms but also improve overall cardiovascular health (33,43,44). Some evidence indicates these therapies could help reduce hospitalizations, improve quality of life and decrease the risk of cardiovascular events (34,45). Nonetheless, further research is necessary to fully comprehend their long-term impact and their potential role in mitigating or halting the progression of IHD.
Ischemic heart disease
IHD is an overarching term that encompasses several conditions, including CAD and CMD, both of which can manifest as clinical symptoms such as angina pectoris and myocardial infarction (MI) (46). CAD is primarily characterized by the obstruction of large coronary arteries resulting from the accumulation of atherosclerotic plaques, which impedes blood flow to the myocardium and may cause plaque rupture and thrombosis, leading to acute coronary events (47). By contrast, CMD involves dysfunction of the smaller coronary vessels, which leads to impaired myocardial perfusion despite no significant obstruction in the large vessels (48). CMD arises from several mechanisms, including microvascular dysfunction, endothelial dysfunction and metabolic abnormalities such as insulin resistance and dyslipidemia, which impair myocardial blood flow (49). Although atherosclerosis is a primary contributor to CAD, CMD can occur through a variety of mechanisms, including microvascular dysfunction, endothelial dysfunction and metabolic abnormalities, which can impair myocardial blood flow despite the absence of large-vessel disease (50,51).
Structure and function of coronary circulation
The coronary arteries originate from the root of the aorta and are divided into two primary branches: The left main coronary artery (LMCA) and the right coronary artery (RCA) (52) (Fig. 1). The LMCA further branches into the left anterior descending (LAD) artery and the left circumflex (LCx) artery, while the RCA travels along the right atrioventricular groove (53). Coronary dominance refers to the origin of the posterior descending artery (PDA), which supplies the posterior inferior wall of the left ventricle (LV). There are three types of dominance: Right dominance (70%), where the RCA gives rise to the PDA; left dominance (10%), where the PDA is supplied by the LCx; and co-dominance (20%), where both the RCA and LCx contribute to the PDA (52). The RCA supplies the right atrium, right ventricle, sinoatrial node, atrioventricular node and parts of the posterior wall of the LV. By contrast, the LMCA delivers blood to the anterior wall and left side of the heart through the LAD and the posterior-lateral wall of the LV and left atrium (LA) via the LCx (52).
In coronary circulation, each vessel category uniquely supports myocardial perfusion (Fig. 1). Epicardial arteries, with diameters >400 µm, primarily function as conduits for blood flow to the myocardium, contributing only ~5% to the total vascular resistance (54,55). Pre-arterioles, ranging from 100-400 µm, are crucial for metabolic control and regulating the coronary blood flow, accounting for 20% of the vascular resistance. These vessels participate in the autoregulation of coronary flow, adjusting blood supply according to myocardial oxygen demand (56). Arterioles, with diameters between 40-100 µm, are the primary regulators of coronary blood flow, making up 60% of the vascular resistance (57). These small vessels are integral in local blood distribution and metabolic processes and are also a target of therapeutic interventions in conditions such as angina and myocardial ischemia (58). Capillaries, <10 µm in diameter, are vital for nutrient and gas exchange, contributing ~15% to vascular resistance (59). While they contribute to resistance, their fundamental function is facilitating the exchange of oxygen, nutrients and waste products at the cellular level (60). The capillary network's extensive surface area is essential for the diffusion of oxygen and nutrients to the heart muscle.
The myocardium, with its high metabolic demands, consumes ~5% of cardiac output, or 50-120 ml/100 g of myocardial tissue, with an oxygen extraction ratio of 60-70% (61,62). This high extraction ratio reflects the limited capacity of the myocardium to increase oxygen uptake under stress, highlighting the importance of efficient coronary blood flow (CBF) to meet elevated oxygen demands. As myocardial oxygen demand rises during physical activity or stress, CBF must adjust to ensure sufficient oxygen delivery to the heart (63,64). CBF is primarily diastolic, with 75% of the flow through the LMCA and 50% through the RCA occurring during diastole (65,66). During systole, left ventricular blood flow decreases due to the compression of coronary vessels by increased chamber pressure, while right ventricular flow is less affected by systolic pressure (67,68). To meet the increasing oxygen demand, CBF is tightly regulated by several mechanisms working in concert. Metabolic regulation plays a crucial role: carbon dioxide, adenosine, lactate and potassium ions are released by myocardial cells in response to elevated oxygen demand (69-74). In parallel, smooth muscle cells (SMCs) in the coronary arteries show a myogenic response to variations in blood pressure, modulating vascular tone to ensure consistent perfusion, even in the presence of fluctuations in systemic blood pressure (75,76). Additionally, the ANS further modulates CBF through its sympathetic and parasympathetic branches. Sympathetic activation, primarily through β-receptors, raises heart rate and myocardial contractility, increasing myocardial oxygen demand and triggers coronary vasodilation to meet these needs. Conversely, parasympathetic activity (via muscarinic receptors) promotes vasodilation by stimulating the release of nitric oxide (NO), especially during restful periods when myocardial oxygen demand is lower (77-79). The combined actions of metabolic signals, smooth muscle regulation and autonomic modulation ensure that the coronary circulation adjusts dynamically to meet the myocardial oxygen requirements, both at rest and during stress.
Pathological mechanisms of IHD
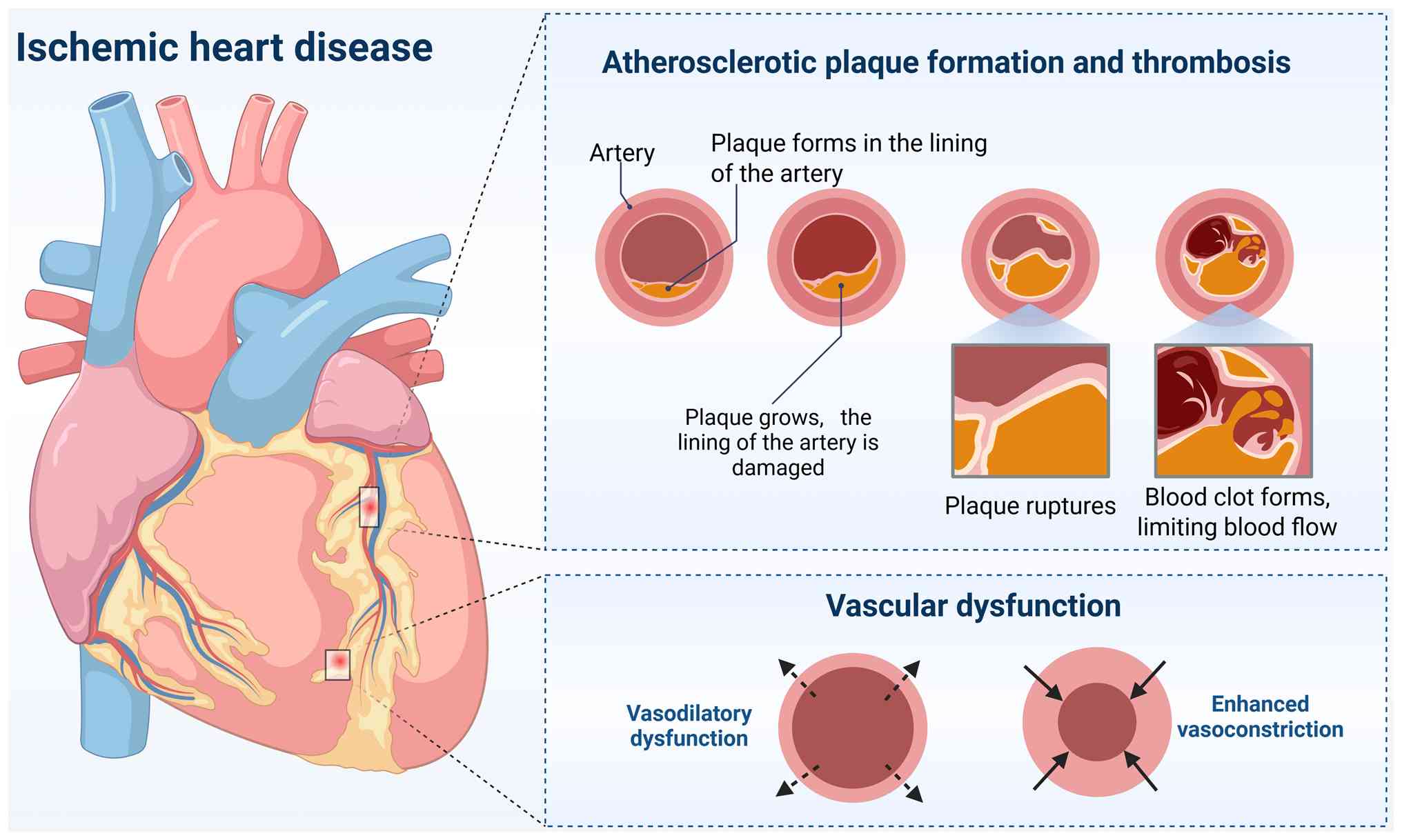
Atherosclerotic plaque formation and thrombosis
The formation of atherosclerotic plaque is a key pathological feature of IHD (Fig. 2). The process begins with endothelial injury, often induced by risk factors such as hyperlipidemia, hypertension and smoking (80-82). This damage leads to the deposition of LDL particles in the subendothelial space, where they become oxidized (83). Oxidized LDL triggers an inflammatory response that attracts monocytes, which differentiate into macrophages (84). These macrophages subsequently phagocytize the oxidized LDL and transform into foam cells, a process facilitated by receptors such as CD36 that bind oxidized lipids, promote their uptake and inhibit macrophage migration (85). Further analyses of human and mouse plaques have identified distinct macrophage subpopulations, including inflammatory, resident-like and TREM2hi macrophages. Notably, pathway analysis of TREM2hi macrophages reveals enrichment in lipid metabolism, oxidative stress responses, cholesterol efflux regulation and catabolic processes, which are integral to intracellular lipid accumulation and the formation of foam cells (86).
As atherosclerotic plaques mature, SMCs from the media of the artery migrate to the intima, where they proliferate and develop into a fibrous cap covering over the lipid-rich core (87). Co-staining of human coronary artery sections has shown that ~40% of CD68+ cells found in advanced coronary atherosclerosis originate from SMCs (88). This fibrous cap provides structural integrity and helps stabilize the plaque. However, in response to ongoing inflammation and lipid accumulation, some SMCs take on characteristics of foam cells by taking up oxidized LDL, displaying increased expression of macrophage markers such as CD68, galectin-3 and the foam cell marker ATP binding cassette subfamily A member 1 (89). The continuous activation of macrophages and other immune cells within the plaque triggers the release of pro-inflammatory cytokines such as TNF-α and IL-1β, which promote further plaque necrosis and contribute to the thinning of the fibrous cap (90-92). As the plaque becomes unstable, it may evolve into a thin-cap fibroatheroma, a particularly vulnerable form of plaque characterized by a thin fibrous cap. This thinning makes the plaque more susceptible to rupture, exposing thrombogenic materials in the core to the bloodstream (93,94).
When a plaque ruptures, it exposes thrombogenic substances to the bloodstream, triggering platelet aggregation and thrombus formation (95). Upon rupture, platelets adhere to exposed collagen and tissue factor in the plaque, becoming activated. These activated platelets then release thromboxane A2, serotonin and ADP, which recruit and activate additional platelets. The aggregated platelets form a platelet plug, serving as the foundation for thrombus formation. Subsequently, fibrinogen is converted into fibrin by thrombin, stabilizing the thrombus and trapping red blood cells and additional platelets, forming a mature thrombus (96-98). As the thrombus enlarges, it progressively occludes the coronary artery, potentially causing complete arterial obstruction. This blockage prevents blood flow to the downstream myocardium, causing myocardial ischemia. If left unresolved, this can result in MI, causing irreversible damage to heart tissue (99,100). While plaque rupture often leads to acute coronary events, it may not always present with immediate symptoms. In some cases, plaque rupture and the subsequent thrombosis develop subclinically, increasing plaque burden and causing gradual narrowing of the coronary lumen over time (101,102). Thrombus formation may partially occlude the coronary artery, leading to unstable angina or non-ST elevation myocardial infarction. These conditions are related to a higher risk of progression to full occlusion and ST-elevation myocardial infarction (STEMI) (103). This subclinical progression can contribute to chronic ischemia, which may initially be asymptomatic but eventually lead to angina or heart failure. Even without an immediate rupture, ongoing plaque growth and thrombosis can worsen coronary occlusion, further impairing myocardial perfusion (104,105).
CMD
CMD is increasingly identified as a critical factor in the development of IHD, often manifesting clinically as angina in patients without significant coronary artery blockages on angiography (49,106). CMD results from the inability of the coronary microvasculature to properly regulate blood flow, leading to an imbalance between myocardial oxygen supply and demand, causing myocardial ischemia (107). Structural alterations in the microvasculature, including microvascular remodeling, capillary rarefaction and microvascular obstruction, contribute to CMD (108). These changes are frequently accompanied by an increase in left ventricular mass and vascular stiffness, further impairing myocardial perfusion (109). This can contribute to both chronic ischemia and heart failure, making it a key target for both diagnosis and therapy in IHD (110).
A key factor in the progression of CMD is endothelial dysfunction (107). The vascular endothelium normally regulates smooth muscle function by releasing vasodilators such as NO, which facilitates coronary vasodilation during periods of heightened oxygen demand (111). In CMD, the reduced ability of the endothelium to release these vasodilators, combined with increased endothelin-1 and other vasoconstrictors, leads to impaired vasodilation and reduced coronary reserve, exacerbating the supply-demand mismatch and contributing to myocardial ischemia (112-116). This endothelial dysfunction also contributes to vascular stiffness, further impairing coronary blood flow.
In addition to endothelial dysfunction, coronary vasospasm markedly contributes to CMD and IHD (117). These spasms, which can occur independently or alongside atherosclerotic lesions, cause temporary constrictions of both large and small coronary arteries, leading to transient reductions in blood flow (118,119). This can result in ischemic symptoms such as chest pain, especially during stress or after exposure to triggers such as cold or certain medications (120-123). Coronary vasospasm exacerbates CMD by further hindering the coronary vessels' ability to properly dilate in response to increased myocardial oxygen demand.
Treatment of IHD
The management of IHD requires a multidisciplinary approach that integrates pharmacological, surgical and lifestyle interventions to alleviate symptoms, reduce risks and prevent disease progression (124). The primary objective is to address the underlying pathophysiological mechanisms while optimizing patient outcomes.
Pharmacological treatments
Antiplatelet agents. Aspirin remains pivotal in preventing thrombus formation and lowering the risk of MI in patients with CAD and CMD (125). Due to the significant overlap between CAD and CMD, low-dose aspirin (or alternative antiplatelet agents) for those with aspirin intolerance are essential in these patients.
Lipid-lowering agents. Statins are the cornerstone of CAD management, as they lower LDL cholesterol and reduce MI risk, as well as slow atherosclerotic plaque progression (126). Statins also offer pleiotropic effects in CMD by improving endothelial function, reducing vascular inflammation and potentially improving microvascular function, which is particularly beneficial in patients with microvascular ischemia.
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). ACE inhibitors and angiotensin receptor blockers ARBs improve coronary microvascular function by inhibiting angiotensin II, which causes vasoconstriction and microvascular dysfunction in CMD. These agents reduce vascular tone, improve endothelial function and enhance coronary reserve, which is essential in patients with extensive myocardial ischemia or flow-limiting CAD (127). These agents also lower blood pressure and decrease myocardial workload by inhibiting the renin-angiotensin-aldosterone system, often activated in CAD and CMD.
Anti-anginal therapies. The combination of beta-blockers and calcium antagonists is frequently employed to manage angina symptoms. Beta-blockers reduce heart rate and blood pressure, decreasing myocardial workload and ischemia risk (128,129). Calcium antagonists relax coronary vessels, enhance coronary flow and alleviate symptoms, particularly in cases where vasomotor tone or spasm is the primary cause of symptoms (130). Long-acting nitrates also play a role by improving coronary blood flow and reducing preload (131).
Surgical and interventional treatments
When pharmacological treatments are insufficient, surgical interventions become necessary. Percutaneous coronary intervention (PCI), commonly known as angioplasty, is used to dilate narrowed coronary arteries and is often combined with stent placement to keep the arteries open (132). CABG improves myocardial perfusion by grafting healthy vessels to bypass obstructed coronary arteries (133). For patients with severe, medication-resistant angina, coronary endarterectomy may be performed to excise atherosclerotic plaques from the coronary vessels (134).
While PCI is beneficial for restoring blood flow, it can also lead to reperfusion injury, where oxygen-rich blood reintroduced into ischemic tissues triggers oxidative stress and inflammation, leading to tissue damage (135,136). Additionally, refractory angina pectoris, which is characterized by persistent myocardial ischemia despite optimal medical treatment and procedures such as angioplasty or CABG, underscores the limitations of current treatments when microvascular dysfunction is present.
Restenosis, or the recurrence of arterial narrowing, is another challenge that arises after angioplasty, occurring in 30-50% of patients within 6 months to a year due to continued atherosclerosis progression or incomplete plaque removal (137). Although CABG can provide long-term relief by bypassing obstructed coronary arteries, graft failure and progression of disease in native coronary vessels often occur over time (138). Furthermore, in patients with mixed CAD and CMD, grafts may not bypass microvascular dysfunction, limiting their effectiveness (139).
However, while these procedures effectively address macrovascular disease, they may not fully resolve CMD. Despite these interventions, microvascular dysfunction can persist, leading to inadequate coronary perfusion and ongoing ischemia (139). This results in persistent angina and heart failure symptoms. In CMD, microvascular rarefaction and abnormal vasomotion remain unaddressed, as traditional treatments such as angioplasty and CABG primarily target macrovascular disease (140,141). Since CMD typically does not involve significant stenosis in large coronary arteries, it may not be detected by traditional diagnostic techniques such as coronary angiography, further complicating treatment (142). Consequently, treatments focusing solely on macrovascular disease may fail to address the underlying causes of ischemia, leading to suboptimal outcomes.
Additionally, therapies targeting neurovascular coupling and sympathetic inhibition are being explored to specifically address microvascular dysfunction (143). By modulating neurotransmitter levels at cardiac nerve terminals, these newer pharmacological approaches may help restore normal vascular tone and improve microvascular reactivity in patients with CMD (144). These emerging interventions may provide valuable adjuncts to PCI and CABG, particularly in patients with mixed CAD and CMD, where traditional treatments often fail to resolve the underlying microvascular pathology (145,146).
Nervous system modulation in IHD
ANS in the heart
The ANS is essential in in regulating cardiac function, with both extrinsic and intrinsic components working together to control heart rate, contractility and coronary blood flow (147). The extrinsic component consists of neural fibers that connect the heart to the CNS, enabling the integration of systemic physiological responses. The intrinsic component includes autonomic nerve fibers within the heart, primarily those that traverse the pericardial sac, which release key neurotransmitters such as norepinephrine (NE) and acetylcholine (ACh) to modulate cardiac activity (148)
SNS
The SNS plays a vital role in modulating cardiac function and coronary blood flow (149). Originating from the thoracolumbar region of the spinal cord (T1-L2), sympathetic preganglionic neurons synapse in the cervicothoracic ganglia (commonly the C8-T1 stellate ganglion) and send postganglionic fibers that innervate the heart through the coronary arteries (150). These postganglionic sympathetic fibers release NE, which activates various adrenergic receptors present on cardiac myocytes and vascular SMCs.
β1 adrenergic receptors are primarily responsible for increasing heart rate and contractility, thereby elevating myocardial oxygen demand, a key factor in IHD. α1 receptors, located at the coronary vasculature, mediate vasoconstriction, which can worsen myocardial ischemia by restricting coronary blood flow. By contrast, α2 receptors play a protective role by regulating calcium handling in cardiomyocytes, helping to mitigate the effects of excessive sympathetic stimulation during IHD (151). The SNS, through norepinephrine and epinephrine release, increases heart rate and myocardial contractility via β1-adrenergic receptors, which increases myocardial oxygen demand (152). Additionally, SNS activation through α1-adrenergic receptors causes vasoconstriction, restricting coronary blood flow, particularly under conditions of heightened myocardial oxygen demand.
The right-sided sympathetic fibers predominantly influence chronotropism (heart rate), primarily by modulating the sinus node, while the left-sided fibers primarily affect the atrioventricular node, contributing to the regulation of cardiac rhythm (153). The asymmetry in SNS innervation may have implications for arrhythmias in IHD, particularly in atrial arrhythmias.
In IHD, a hallmark feature is sympathetic hyperactivity, which results in elevated myocardial oxygen demand, ischemic events and arrhythmias. Chronic SNS activation exacerbates ischemia, promotes vasoconstriction and contributes to the progression of heart failure.
PNS
The PNS regulates cardiac function through vagal innervation, with pre-ganglionic neurons originating from the medulla oblongata, specifically within the dorsal vagal motor nucleus and the nucleus ambiguus (150). These nuclei are essential for regulating nodal and ventricular tissue, respectively (154). The axons of these neurons travel along the vagus nerve and synapse within the intrinsic ganglionic plexuses, which are situated on the posterior atrial surface and the superior regions of the ventricles, embedded in the epicardial fat pads. Postganglionic parasympathetic neurons primarily release ACh, which interacts with muscarinic (M) receptors, particularly M2 receptors, to modulate heart rate and myocardial relaxation (155). ACh staining studies have demonstrated widespread cardiac cholinergic innervation throughout all chambers of the heart (156).
M2 receptors, classified as Gi-coupled G protein-coupled receptors, mediate their effects by reducing cyclic adenosine monophosphate (cAMP) levels. This cAMP reduction leads to negative chronotropy by inhibiting calcium influx via L-type calcium channels, slowing the pacemaker activity in the sinus node (90). This results in a slower heart rate (157). Additionally, M2 receptor activation promotes negative inotropy (reduced contractility) through hyperpolarization of pacemaker cells, primarily mediated by ACh-sensitive potassium channels (91).
In addition to ACh, the parasympathetic system also releases NO. It enhances ACh release from presynaptic neurons, further increasing parasympathetic tone. Additionally, NO inhibits the release of NE from sympathetic nerve terminals, reducing sympathetic drive to the heart, thus contributing to cardioprotection during stress and ischemia (92).
Intrinsic cardiac autonomic nervous system (ICANS)
The ICANS is a sophisticated neural network embedded within the heart tissue that autonomously regulates heart rate and myocardial contractility, independent of central nervous system input (158). It consists of parasympathetic postganglionic neurons, preganglionic endings, interneurons, afferent neurons and efferent neurons (159). These components collectively form the intrinsic cardiac ganglia, which are organized into ganglionated plexi (GP) located on the atrial and ventricular surfaces within epicardial fat pads (160). The GP within the ICANS contain various types of neurons, including unipolar, bipolar and multipolar neurons, each contributing to the modulation of cardiac autonomic tone (161). These neurons are morphologically classified into spherical and straight ganglia, with spherical ganglia containing a higher density of neurons, potentially enhancing autonomic integration (158,162). These ganglia act as integration centers, coordinating sympathetic and parasympathetic signals and facilitating autonomous cardiac regulation (158). These elements collectively establish the intrinsic cardiac ganglia or ICNS, which includes sensitive neurons, regulatory interneurons and neurons that release a range of neuropeptides, such as norepinephrine (159,163). In IHD, ischemic injury can lead to pathological alterations in the ICANS, which disrupt the heart's electrophysiological stability. These disruptions, altering the balance between sympathetic and parasympathetic inputs, can impair signal transmission, increasing the susceptibility to arrhythmias, particularly ventricular arrhythmias, by altering the heart's response to acute stress (164,165).
Autonomic imbalance in ischemic heart disease
The disruption of the nervous system in IHD was first documented by Hopkins et al (166), who observed structural abnormalities, including inclusions, vacuoles and degenerative changes, in the posterior atrial ganglia of patients with right coronary artery disease. Subsequent studies in guinea pigs following MI revealed altered neuronal excitability, impaired synaptic function and neurochemical changes in the affected neurons (167). These observations support the idea that ischemic injury induces pathological remodeling of the ICANS. Vaseghi et al (168) further advanced this understanding by showing that, despite ACh levels remaining stable in the border zones and remote myocardium post-MI, there were significant changes in parasympathetic neuronal firing frequencies. Neurons normally activated by VNS exhibited reduced firing rates, while neurons typically suppressed by vagal tone showed increased firing rates. These changes suggest that although the parasympathetic network remains anatomically intact post-MI, its functional integrity is compromised. Research indicates that parasympathetic dysfunction, often assessed through abnormal baroreflex sensitivity and HRV, is frequently observed in patients with cardiomyopathy and stroke, who may experience increased arrhythmia and heart failure risks due to disrupted neural signaling (169-173). In IHD, there is a critical imbalance between SNS overactivity and PNS dysfunction, impairing the ability of the body to effectively modulate the heightened sympathetic response (174).
Normally, the PNS counteracts SNS-induced vasoconstriction by promoting vasodilation (175). However, in IHD, endothelial dysfunction due to atherosclerosis impairs the vasodilatory effects of ACh. Under normal conditions, ACh interacts with muscarinic receptors on endothelial cells, triggering NO release, which contributes to smooth muscle relaxation and vasodilation (176). In IHD, reduced para-sympathetic activity fails to adequately counterbalance the SNS-driven increase in oxygen demand, exacerbating ischemic conditions (177), leading to enhanced vasoconstriction of the coronary arteries and reduced dilation capacity (178) This sympathetic dominance increases vascular resistance, which in turn raises myocardial workload and worsens the ischemic condition (179). Chronic SNS activation exacerbates myocardial oxygen demand and workload, aggravating ischemia and accelerating heart failure progression (180,181). This exacerbates the oxygen supply-demand mismatch during IHD and contributes to myocardial ischemia (182).
The SNS overactivity is critical for exacerbating ischemia and chronic inflammation in IHD (182,183). Upon binding to β-adrenergic receptors, particularly β2 receptors present on immune cells such as macrophages and T cells, NE or epinephrine stimulates adenylyl cyclase, resulting in an elevation of intracellular cAMP levels. Subsequently, the heightened cAMP concentrations activate protein kinase A, typically suppressing the secretion of pro-inflammatory cytokines, including TNF-α and IL-1β. However, chronic β2-AR activation can lead to receptor desensitization, reducing its anti-inflammatory effects over time. This desensitization is particularly relevant in chronic inflammatory diseases such as IHD, where prolonged receptor activation occurs (184). Additionally, NE binding to α1-adrenergic receptors on immune cells activates the phospholipase C pathway, which increases intracellular calcium levels and activates protein kinase C. This cascade amplifies pro-inflammatory responses by triggering the release of additional cytokines such as TNF-α, IL-6 and IL-1β (185,186). Moreover, α1-adrenergic activation promotes the upregulation of matrix metalloproteinases (MMPs) in macrophages, enzymes responsible for the degradation of extracellular matrix. MMPs weaken the fibrous cap covering atherosclerotic plaques, making them more susceptible to rupture and increasing the risk of thrombosis (187,188). In healthy conditions, ACh released from parasympathetic nerve terminals suppresses immune cell activation and cytokine production, thereby decreasing systemic inflammation and stabilizing plaques (189-191). The anti-inflammatory actions of ACh are predominantly mediated through M2 and nicotinic receptors, including the α7 nicotinic acetylcholine receptor on macrophages, which is integral to the cholinergic anti-inflammatory pathway. However, in IHD, parasympathetic dysfunction impairs this protective mechanism, exacerbating inflammation and increasing plaque instability (192,193).
In summary, the imbalance between SNS overactivity and PNS dysfunction in IHD exacerbates ischemic conditions and arrhythmia risks. Restoring parasympathetic tone may offer potential therapeutic benefits by counteracting sympathetic overactivity, improving autonomic balance and lowering the risk of cardiac events in patients with IHD.
Neurostimulation techniques in IHD
Neurostimulation techniques, such as VNS and SCS, target the ANS to restore balance between the sympathetic and parasympathetic branches. These therapies help improve heart rate variability and enhance parasympathetic function, presenting a promising avenue for alleviating symptoms and slowing disease progression in IHD. By adjusting the neural signals in the heart, these therapies provide a more dynamic and adaptive solution to the cardiovascular challenges posed by IHD (194) (Fig. 3).
Spinal cord stimulation
SCS involves the surgical implantation of a pulse generator, typically placed in the abdomen, chest, or buttocks. This device, which functions similarly to cardiac pacemakers, delivers low-intensity electrical impulses that modulate neural activity near the spinal cord (195). Originally developed for chronic pain management (196), SCS has gained traction as a viable therapeutic option for cardiovascular conditions, including IHD. This application is based on its dual impact on pain sensation and autonomic heart regulation (197,198). In Europe, refractory angina, a persistent, severe type of chest pain associated with IHD, is a recognized indication for SCS therapy (195). SCS therapy has demonstrated efficacy in reducing the frequency and intensity of angina, enhancing exercise capacity and improving the overall quality of life, especially in patients for whom surgical interventions are not viable options (199-201). Evidence from randomized trials and comprehensive registry data has substantiated the clinical efficacy of SCS, demonstrating significant symptom relief in patients with refractory angina compared to those receiving no stimulation or conventional therapy alone (Table I) (199-211).
Clinical evidence supports the significant role of SCS in improving myocardial perfusion and autonomic function, critical in the management of IHD. SCS has been found to effectively enhance myocardial blood flow distribution and reducing myocardial oxygen consumption. This adjustment effectively addresses the critical imbalance between oxygen supply and demand commonly observed in ischemic regions (203). Even short-term application of SCS has been shown to bolster myocardial ischemia tolerance and enhance myocardial perfusion reserve (MPR), essential for managing refractory angina (211). Positron emission tomography studies have demonstrated this improvement at the microvascular level (212).
The underlying mechanisms for these benefits include a marked reduction in SNS activity and the normalization of intrinsic cardiac sympathetic tone (213). SCS also reduces neural synchrony between the dorsal horn (DH) and intermediolateral column (IML) of the spinal cord, stabilizing cardiac function during ischemia by mitigating excessive sympathetic excitation and arrhythmias (214). SCS mitigates the hyperactivity of the IML, which is responsible for sympathetic outflow to the heart (215). By decreasing the synchrony between the DH and IML neurons, SCS helps to lower excessive sympathetic excitation (215). During myocardial ischemia, ischemia-sensitive cardiac afferent neurons convey excitatory signals to the dorsal horn of the spinal cord, which triggers the spinal neural network (215). This activation results in heightened activity in the IML preganglionic sympathetic neurons, which then transmit excitatory signals to post-ganglionic neurons in the stellate and middle cervical ganglia (215,216). The subsequent increase in sympathetic activity directed to the heart can exacerbate myocardial injury, contributing to malignant ventricular tachyarrhythmias and raising the risk of sudden cardiac mortality (217). In cases of chronic MI, significant neural remodeling at various points along the cardiac neuraxis alters the pattern of sympathetic innervation, thereby amplifying the arrhythmogenic potential of the heart (218). Additionally, SCS has been shown to influence the ability of cardiac sensory neurons to transmit ischemic signals from the myocardium to spinal cord neurons, thus modulating the spinal sympathetic efferent pathways to the heart (215,219). Research also indicates that SCS is associated with improved HRV, a key marker of autonomic balance (220). Particularly in patients with IHD, SCS has demonstrated notable improvements in left ventricular ejection fraction and overall cardiac output, crucial metrics of heart function (221). Overall, SCS offers substantial therapeutic potential in CAD, particularly in enhancing myocardial perfusion and autonomic regulation. However, the precise mechanism by which SCS alleviates refractory angina remains unclear (212,222) and further large-scale studies with long-term follow-up are necessary to explore its full therapeutic potential and underlying mechanisms.
Vagus nerve stimulation
Initially developed for treating conditions such as epilepsy and depression (223,224), VNS is now increasingly recognized for its utility in managing IHD through targeting the brain-heart axis (225). Electrodes from this device are positioned around the vagus nerve in the neck (226), where controlled electrical impulses modulate vagal nerve activity, a critical component of the ANS that regulates essential functions including heart rate (34).
VNS is available in two modalities: open-loop and closed-loop systems (227). The open-loop system delivers constant electrical pulses and is traditionally used in the management of epilepsy (228). By contrast, the closed-loop system is designed to adjust the intensity of stimulation in response to real-time physiological feedback, such as fluctuations in heart rate, thus tailoring the therapy to individual patient needs (229). This adaptive feature of closed-loop VNS is especially advantageous in the treatment of IHD, as it dynamically responds to variations in cardiac demand (230). Closed-loop VNS represents an advanced form of therapy for cardiovascular diseases, offering real-time adjustments to stimulation parameters based on physiological feedback (230). This approach tailors treatment by fine-tuning stimulation intensity and timing to the changing needs of the patient, improving autonomic regulation and heart function (194). By integrating real-time monitoring of key health indicators such as HRV, breathing patterns and neural activity, closed-loop systems optimize therapeutic benefits while minimizing adverse effects (194).
Preclinical studies in animals have shown that VNS can enhance myocardial blood supply during ischemia, attenuate reperfusion injury and improve ventricular remodeling (Table II) (231-237). Clinical trials have also explored its potential (Table II) (238-240). One notable study investigated non-invasive low-level tragus stimulation in patients with STEMI, evaluating its effect on myocardial ischemia and reperfusion injury (240). The results demonstrated that VNS effectively reduced sympathetic tone, improved HRV and alleviated cardiac stress. In patients with IHD, vagal activation was also shown to improve cardiac blood supply and enhance left ventricular contractility (239).
Additionally, VNS can activate the cholinergic anti-inflammatory pathway (33,241). VNS stimulates enhances the release of acetylcholine activation of α7 nicotinic acetylcholine receptors on macrophages. This activation is essential for mediating the anti-inflammatory effects of VNS, which helps to inhibit the production of pro-inflammatory cytokines, such as TNF-α and IL-6 (242,243). This reduction in inflammatory markers is critical for preventing chronic inflammation that contributes to atherosclerosis and plaque instability. VNS has been shown to mitigate the accumulation of reactive oxygen species (ROS) and enhance mitochondrial energy metabolism during myocardial ischemia-reperfusion events. This protective effect is mediated through modulation of muscarinic receptors, specifically via the M2AChR/OGDHL/ROS axis, which plays a crucial role in reducing pyroptosis (244,245). Research also indicates that VNS promotes angiogenesis and improves coronary blood flow and cardiac function in hearts affected by ischemia. This is achieved by activating VEGF-A/B, which are essential for maintaining cardiac function and are intimately linked with coronary microvasculature and myocardial energy metabolism (246).
Brain stimulation
In addition to VNS and SCS, DBS is an invasive neuromodulation technique that implants electrodes into specific brain areas to deliver continuous electrical impulses (247). Initially developed for treating movement disorders such as Parkinson's disease and essential tremor (248), DBS has expanded into potential applications for managing IHD (249). DBS specifically targets brain areas involved in autonomic regulation, such as the subthalamic nucleus and the pedunculopontine nucleus (250). Stimulation of these areas has been shown to increase parasympathetic activity, contributing to improved HRV and overall cardiac function, which are particularly concerning for patients with IHD (254). Functional mapping along the rostrocaudal axis of the posterior insular cortex has identified distinct regions responsible for sympathoinhibitory and sympathoexcitatory control, aligning with past findings (252). This autonomic control extends beyond the insular cortex to involve the amygdala, hippocampus, hypothalamus, bed nucleus of the stria terminalis, ventrolateral medulla, parabrachial region and periaqueductal gray. Higher cortical regions, including the orbitofrontal cortex and dorsal cingulate cortex, receive and process afferent information from peripheral organs and modulate autonomic output to fine-tune cardiovascular functions (253). The capacity of DBS to alter these signaling pathways is crucial for balancing autonomic function, enhancing parasympathetic activity and alleviating sympathetic overactivation (249,254). DBS has been proved to decrease hemorrhagic and cardiovascular-related events in patients with Parkinson's disease with cardiovascular disease, including IHD (255). While research on DBS for IHD remains in its early stages, preliminary results show promise.
Limitation and future perspectives
Despite the promising potential of neural modulation in the treatment of IHD, several significant limitations must be addressed to fully realize its clinical applications.
Technological complexity and cost-effectiveness
Techniques such as VNS and SCS require advanced equipment and skilled personnel, which may not be available in all healthcare settings. The considerable expenses associated with their surgical implantation and maintenance pose significant barriers to their widespread adoption, especially in cost-sensitive healthcare environments. For instance, although high-frequency SCS may reduce long-term healthcare costs, the initial outlay is substantial, with average device cost of ~$42,937 (256). These factors limit the broader application of these therapies, particularly in resource-limited areas (257). High costs make invasive VNS accessible mostly in well-resourced settings (258,259). Economic evaluations such as the incremental cost-effectiveness ratio help determine if the benefits of new technologies, measured in quality-adjusted life years, justify their costs (260,261). Such economic assessments are crucial for determining the feasibility of integrating neuromodulation techniques into standard CAD care. A thorough cost-benefit analysis is required to validate the choice of neuromodulation over established CAD management strategies, ensuring they provide value in improving patient outcomes.
Invasiveness and potential side effects
Techniques such as DBS and SCS are invasive, necessitating surgical procedures that come with inherent risks including infection, bleeding and long-term complications. DBS, for example, may lead to intracranial hemorrhage, implant site infections and hardware issues such as electrode migration or skin erosion (262-264). SCS carries risks such as epidural hematomas, dural punctures and infections (265-267). The invasive nature of these interventions might deter patients, particularly when non-invasive alternatives exist. Surveys indicate patient concerns regarding the invasiveness, cost and potential side effects of DBS, which may discourage their use despite potential benefits (268,269). The clinical application of these procedures necessitates a meticulous assessment of the associated risks vs. the potential therapeutic gains. The short-term benefits of neural modulation are well-documented; however, the long-term effects remain less clear. Extended use could result in adverse outcomes, necessitating ongoing monitoring and research to evaluate safety and effectiveness. The ability to modify neural activity raises concerns about informed consent, potential neurological changes and the long-term implications of neuromodulation (270,271). For instance, tDCS is found to induce neurostructural changes in targeted brain areas; the long-term implications and potential unintended effects of these alterations require further study (272). DBS has demonstrated sustained efficacy in treating resistant depression over several years but may cause neuropsychiatric side effects, such as mood swings and cognitive impairments (273). A comprehensive understanding of these long-term side effects is essential for ensuring the ongoing safety and effectiveness of these therapies. Magnetic stimulation is a non-invasive technique that uses magnetic fields to generate electrical currents within targeted brain regions, thereby modulating cortical excitability (274). This method markedly influences the ANS, presenting substantial therapeutic potential for cardiovascular diseases (41). Neuro-cardiac-guided TMS, which tailors stimulation sites based on individual physiological responses, has been effective in regulating heart rate and promoting autonomic balance (275,276). The incorporation of TMS into cardiac rehabilitation programs offers a comprehensive approach by addressing both the physiological and psychological factors that affect the progression and management of IDH. Acupuncture, particularly through auricular vagus nerve stimulation (aVNS), has come to be recognized as a promising minimally invasive therapy for modulating the nervous system to alleviate symptoms and enhance cardiac function in IHD. As a non-invasive therapy, aVNS offers a patient-friendly alternative to more invasive procedures. Its ease of application makes it suitable for outpatient settings, providing an accessible treatment option for patients with IHD (277).
Sustainability and practicality of devices
The sustainability and practicality of VNS systems depend on improving device longevity and user-friendliness. Current research focuses on extending battery life, enhancing durability and refining user interfaces to ensure effective, consistent treatment. For example, advances in wirelessly powered and batteryless VNS devices have the potential to eliminate frequent battery replacements, reducing the need for reoperations and improving long-term usability (278,289). These systems also aim to reduce device size while maintaining efficiency, making them more practical for patients (278,289). Additionally, user-friendly designs, such as portable and discreet transcutaneous VNS devices, allow patients to incorporate therapy into their daily routines with minimal disruption (258). These innovations are crucial for making VNS a more practical and effective long-term solution for managing CAD. The development of closed-loop VNS systems, which modify stimulation parameters in response to real-time physiological feedback, marks a significant advancement. However, challenges remain, particularly with motion artifacts during patient movement that can compromise the accuracy of physiological data (280). Research is focused on refining signal processing techniques for an improved differentiation between relevant physiological signals and noise, improving the reliability of these systems (194,281).
Variability and personalized medicine
Neural modulation therapies often yield variable outcomes due to individual differences in anatomy and physiology, challenging the standardization of treatment protocols. Factors such as brain structure, baseline neuronal state, age, genetics and neurotransmitter levels markedly influence the effectiveness of these techniques (282-284). Cognitive strategies and functional connectivity patterns also contribute to individual differences (282,285). The movement towards personalized medicine in cardiovascular care is increasingly being driven by the integration of genetic, biochemical and physiological data into treatment strategies (286). In personalized medicine, advancements such as improved anatomical understanding of the vagus nerve have refined VNS precision (287). By identifying specific fiber types responsible for distinct physiological responses, it is now possible to selectively stimulate beneficial fibers while avoiding those that might cause adverse effects (288). Notable innovations include respiratory-gated auricular vagal afferent nerve stimulation, which synchronizes VNS with the respiratory cycle, delivering stimulation bursts during exhalation to enhance efficacy in conditions such as hypertension and depression (289,290). Another innovation, myoelectric-triggered auricular VNS, activates stimulation based on muscle movements, such as orofacial activity during rehabilitation exercises (291). This closed-loop design ensures precise timing, enhancing neuroplasticity and functional recovery in motor rehabilitation (292,293). Machine learning (ML) is revolutionizing CAD management by enhancing diagnostic accuracy, enabling early detection and improving risk stratification (294-296). Looking ahead, the fusion of neurotechnology and ML will drive innovations in CAD management (296), with future research focusing on refining VNS protocols (297,298), exploring neural-immune interactions in cardiovascular contexts (299) and conducting large-scale clinical trials. This integrated approach promises to markedly improve patient outcomes and longevity.
Availability of data and materials
Not applicable.
Authors' contributions
YL, HY, JX and YW reviewed literature and wrote the manuscript. CY, QZ and FL collected the data. Data authentication is not applicable. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
Not applicable.
Funding
The present study was funded by National Natural Science Foundation of China Regional Innovation and Development Joint Fund (grant no. U21A20404).
References
|
Ralapanawa U and Sivakanesan R: Epidemiology and the magnitude of coronary artery disease and acute coronary syndrome: A narrative review. J Epidemiol Glob Health. 11:169–177. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Pagliaro BR, Cannata F, Stefanini GG and Bolognese L: Myocardial ischemia and coronary disease in heart failure. Heart Fail Rev. 25:53–65. 2020. View Article : Google Scholar | |
|
Horowitz LN, Harken AH, Josephson ME and Kastor JA: Surgical treatment of ventricular arrhythmias in coronary artery disease. Ann Intern Med. 95:88–97. 1981. View Article : Google Scholar : PubMed/NCBI | |
|
Sara JD, Eleid MF, Gulati R and Holmes DR Jr: Sudden cardiac death from the perspective of coronary artery disease. Mayo Clin Proc. 89:1685–1698. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Russell MW, Huse DM, Drowns S, Hamel EC and Hartz SC: Direct medical costs of coronary artery disease in the United States. Am J Cardiol. 81:1110–1115. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Ference BA, Yoo W, Alesh I, Mahajan N, Mirowska KK, Mewada A, Kahn J, Afonso L, Williams KA Sr and Flack JM: Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: A Mendelian randomization analysis. J Am Coll Cardiol. 60:2631–2639. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Toth PP: High-density lipoprotein and cardiovascular risk. Circulation. 109:1809–1812. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Gallo G, Volpe M and Savoia C: Endothelial dysfunction in hypertension: Current concepts and clinical implications. Front Med (Lausanne). 8:7989582022. View Article : Google Scholar : PubMed/NCBI | |
|
Yang DR, Wang MY, Zhang CL and Wang Y: Endothelial dysfunction in vascular complications of diabetes: A comprehensive review of mechanisms and implications. Front Endocrinol (Lausanne). 15:13592552024. View Article : Google Scholar : PubMed/NCBI | |
|
Alpert JS: New coronary heart disease risk factors. Am J Med. 136:331–332. 2023. View Article : Google Scholar | |
|
Malakar AK, Choudhury D, Halder B, Paul P, Uddin A and Chakraborty S: A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. 234:16812–16823. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Nettleship J, Jones R, Channer K and Jones T: Testosterone and coronary artery disease. Front Horm Res. 37:91–107. 2009. View Article : Google Scholar | |
|
Bauersachs R, Zeymer U, Brière JB, Marre C, Bowrin K and Huelsebeck M: Burden of coronary artery disease and peripheral artery disease: A literature review. Cardiovasc Ther. 2019:82950542019. View Article : Google Scholar | |
|
Lee YTH, Fang J, Schieb L, Park S, Casper M and Gillespie C: Prevalence and trends of coronary heart disease in the United States, 2011 to 2018. JAMA Cardiol. 7:459–462. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Libby P and Theroux P: Pathophysiology of coronary artery disease. Circulation. 111:3481–3488. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Weber C and Noels H: Atherosclerosis: Current pathogenesis and therapeutic options. Nat Med. 17:1410–1422. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Attiq A, Afzal S, Ahmad W and Kandeel M: Hegemony of inflammation in atherosclerosis and coronary artery disease. Eur J Pharmacol. 966:1763382024. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Y, Yu Y, Zou W, Zhang M, Wang Y and Gu Y: Association between cardiac autonomic nervous dysfunction and the severity of coronary lesions in patients with stable coronary artery disease. J Int Med Res. 46:3729–3740. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Parisi AF, Folland ED and Hartigan P: A comparison of angioplasty with medical therapy in the treatment of single-vessel coronary artery disease. Veterans affairs ACME investigators. N Engl J Med. 326:10–16. 1992. View Article : Google Scholar : PubMed/NCBI | |
|
Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, Michler RE, Bonow RO, Doenst T, Petrie MC, et al: Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 374:1511–1520. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Del Buono MG, Montone RA, Camilli M, Carbone S, Narula J, Lavie CJ, Niccoli G and Crea F: Coronary microvascular dysfunction across the spectrum of cardiovascular diseases: JACC state-of-the-art review. J Am Coll Cardiol. 78:1352–1371. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Silvani A, Calandra-Buonaura G, Dampney RAL and Cortelli P: Brain-heart interactions: Physiology and clinical implications. Philos Trans A Math Phys Eng Sci. 374:201501812016.PubMed/NCBI | |
|
Wehrwein EA, Orer HS and Barman SM: Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system. Compr Physiol. 6:1239–1278. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Silva LEV, Silva CAA, Salgado HC and Fazan R Jr: The role of sympathetic and vagal cardiac control on complexity of heart rate dynamics. Am J Physiol Heart Circ Physiol. 312:H469–H477. 2017. View Article : Google Scholar | |
|
Charkoudian N and Rabbitts JA: Sympathetic neural mechanisms in human cardiovascular health and disease. Mayo Clin Proc. 84:822–830. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Kasahara Y, Yoshida C, Saito M and Kimura Y: Assessments of heart rate and sympathetic and parasympathetic nervous activities of normal mouse fetuses at different stages of fetal development using fetal electrocardiography. Front Physiol. 12:6528282021. View Article : Google Scholar : PubMed/NCBI | |
|
Curtis BM and O'Keefe JH Jr: Autonomic tone as a cardiovascular risk factor: The dangers of chronic fight or flight. Mayo Clin Proc. 77:45–54. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Brunner-La Rocca HP, Esler MD, Jennings GL and Kaye DM: Effect of cardiac sympathetic nervous activity on mode of death in congestive heart failure. Eur Heart J. 22:1136–1143. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Hadaya J and Ardell JL: Autonomic modulation for cardiovascular disease. Front Physiol. 11:6174592020. View Article : Google Scholar | |
|
Shen MJ and Zipes DP: Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 114:1004–1021. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Malpas SC: Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 90:513–557. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Grassi G and Drager LF: Sympathetic overactivity, hypertension and cardiovascular disease: State of the art. Curr Med Res Opin. 40(Suppl 1): S5–S13. 2024. View Article : Google Scholar | |
|
Bazoukis G, Stavrakis S and Armoundas AA: Vagus nerve stimulation and inflammation in cardiovascular disease: A state-of-the-art review. J Am Heart Assoc. 12:e0305392023. View Article : Google Scholar : PubMed/NCBI | |
|
Capilupi MJ, Kerath SM and Becker LB: Vagus nerve stimulation and the cardiovascular system. Cold Spring Harb Perspect Med. 10:a0341732020. View Article : Google Scholar | |
|
De Ferrari GM and Schwartz PJ: Vagus nerve stimulation: from pre-clinical to clinical application: Challenges and future directions. Heart Fail Rev. 16:195–203. 2011. View Article : Google Scholar | |
|
Deer TR, Levy RM, Kramer J, Poree L, Amirdelfan K, Grigsby E, Staats P, Burton AW, Burgher AH, Obray J, et al: Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: A randomized comparative trial. Pain. 158:669–681. 2017. View Article : Google Scholar : | |
|
Bernstein SA, Wong B, Vasquez C, Rosenberg SP, Rooke R, Kuznekoff LM, Lader JM, Mahoney VM, Budylin T, Älvstrand M, et al: Spinal cord stimulation protects against atrial fibrillation induced by tachypacing. Heart Rhythm. 9:1426–1433.e3. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Torre-Amione G, Alo K, Estep JD, Valderrabano M, Khalil N, Farazi TG, Rosenberg SP, Ness L and Gill J: Spinal cord stimulation is safe and feasible in patients with advanced heart failure: Early clinical experience. Eur J Heart Fail. 16:788–795. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Cucinotta F, Swinnen B, Makovac E, Hirschbichler S, Pereira E, Little S, Morgante F and Ricciardi L: Short term cardiovascular symptoms improvement after deep brain stimulation in patients with Parkinson's disease: A systematic review. J Neurol. 271:3764–3776. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Clancy JA, Johnson R, Raw R, Deuchars SA and Deuchars J: Anodal transcranial direct current stimulation (tDCS) over the motor cortex increases sympathetic nerve activity. Brain Stimul. 7:97–104. 2014. View Article : Google Scholar | |
|
Lee H, Lee JH, Hwang MH and Kang N: Repetitive transcranial magnetic stimulation improves cardiovascular autonomic nervous system control: A meta-analysis. J Affect Disord. 339:443–453. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
De Decker K, Beese U, Staal MJ and Dejongste MJL: Electrical neuromodulation for patients with cardiac diseases. Neth Heart J. 21:91–94. 2013. View Article : Google Scholar : | |
|
Zipes DP, Neuzil P, Theres H, Caraway D, Mann DL, Mannheimer C, Van Buren P, Linde C, Linderoth B, Kueffer F, et al: Determining the feasibility of spinal cord neuromodulation for the treatment of chronic systolic heart failure: The DEFEAT-HF study. JACC Heart Fail. 4:129–136. 2016. View Article : Google Scholar | |
|
Rodrigues B, Barboza CA, Moura EG, Ministro G, Ferreira-Melo SE, Castaño JB, Nunes WMS, Mostarda C, Coca A, Vianna LC and Moreno-Junior H: Acute and short-term autonomic and hemodynamic responses to transcranial direct current stimulation in patients with resistant hypertension. Front Cardiovasc Med. 9:8534272022. View Article : Google Scholar : PubMed/NCBI | |
|
Imran TF, Malapero R, Qavi AH, Hasan Z, de la Torre B, Patel YR, Yong RJ, Djousse L, Gaziano JM and Gerhard-Herman MD: Efficacy of spinal cord stimulation as an adjunct therapy for chronic refractory angina pectoris. Int J Cardiol. 227:535–542. 2017. View Article : Google Scholar | |
|
Palasubramaniam J, Wang X and Peter K: Myocardial infarction-from atherosclerosis to thrombosis. Arterioscler Thromb Vasc Biol. 39:e176–e185. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Libby P: Inflammation in atherosclerosis. Nature. 420:868–874. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Bradley C and Berry C: Definition and epidemiology of coronary microvascular disease. J Nucl Cardiol. 29:1763–1775. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Marinescu MA, Löffler AI, Ouellette M, Smith L, Kramer CM and Bourque JM: Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC Cardiovasc Imaging. 8:210–220. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Gutiérrez E, Flammer AJ, Lerman LO, Elízaga J, Lerman A and Fernández-Avilés F: Endothelial dysfunction over the course of coronary artery disease. Eur Heart J. 34:3175–3181. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Bentzon JF, Otsuka F, Virmani R and Falk E: Mechanisms of plaque formation and rupture. Circ Res. 114:1852–1866. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Villa AD, Sammut E, Nair A, Rajani R, Bonamini R and Chiribiri A: Coronary artery anomalies overview: The normal and the abnormal. World J Radiol. 8:537–555. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Chen M, Wu X and Xu C: The 'hands' teaching method in coronary artery anatomy. Asian J Surg. 47:3183–3184. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Chilian WM and Marcus ML: Phasic coronary blood flow velocity in intramural and epicardial coronary arteries. Circ Res. 50:775–781. 1982. View Article : Google Scholar : PubMed/NCBI | |
|
De Bruyne B, Hersbach F, Pijls NH, Bartunek J, Bech JW, Heyndrickx GR, Gould KL and Wijns W: Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but 'normal' coronary angiography. Circulation. 104:2401–2406. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Camici PG and Rimoldi OE: The clinical value of myocardial blood flow measurement. J Nucl Med. 50:1076–1087. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Duncker DJ, Koller A, Merkus D and Canty JM Jr: Regulation of coronary blood flow in health and ischemic heart disease. Prog Cardiovasc Dis. 57:409–422. 2015. View Article : Google Scholar | |
|
Dedkov EI, Christensen LP, Weiss RM and Tomanek RJ: Reduction of heart rate by chronic beta1-adrenoceptor blockade promotes growth of arterioles and preserves coronary perfusion reserve in postinfarcted heart. Am J Physiol Heart Circ Physiol. 288:H2684–H2693. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Pruthi S, Siddiqui E and Smilowitz NR: Beyond coronary artery disease: Assessing the microcirculation. Interv Cardiol Clin. 12:119–129. 2023. | |
|
Palade GE: Blood capillaries of the heart and other organs. Circulation. 24:368–388. 1961. View Article : Google Scholar : PubMed/NCBI | |
|
Wolff CB: Normal cardiac output, oxygen delivery and oxygen extraction. Adv Exp Med Biol. 599:169–182. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Gandoy-Fieiras N, Gonzalez-Juanatey JR and Eiras S: Myocardium metabolism in physiological and pathophysiological states: Implications of epicardial adipose tissue and potential therapeutic targets. Int J Mol Sci. 21:26412020. View Article : Google Scholar : PubMed/NCBI | |
|
Hollenberg M and Tager IB: Oxygen uptake efficiency slope: An index of exercise performance and cardiopulmonary reserve requiring only submaximal exercise. J Am Coll Cardiol. 36:194–201. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Downey JM: Myocardial contractile force as a function of coronary blood flow. Am J Physiol. 230:1–6. 1976. View Article : Google Scholar : PubMed/NCBI | |
|
Heusch G: Heart rate in the pathophysiology of coronary blood flow and myocardial ischaemia: Benefit from selective bradycardic agents. Br J Pharmacol. 153:1589–1601. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Seligman H, Nijjer SS, van de Hoef TP, de Waard GA, Mejía-Rentería H, Echavarria-Pinto M, Shun-Shin MJ, Howard JP, Cook CM, Warisawa T, et al: Phasic flow patterns of right versus left coronary arteries in patients undergoing clinical physiological assessment. EuroIntervention. 17:1260–1270. 2022. View Article : Google Scholar | |
|
Comunale G, Peruzzo P, Castaldi B, Razzolini R, Di Salvo G, Padalino MA and Susin FM: Understanding and recognition of the right ventricular function and dysfunction via a numerical study. Sci Rep. 11:37092021. View Article : Google Scholar : PubMed/NCBI | |
|
Nikorowitsch J, Bei der Kellen R, Haack A, Magnussen C, Prochaska J, Wild PS, Dörr M, Twerenbold R, Schnabel RB, Kirchhof P, et al: Correlation of systolic and diastolic blood pressure with echocardiographic phenotypes of cardiac structure and function from three German population-based studies. Sci Rep. 13:145252023. View Article : Google Scholar : PubMed/NCBI | |
|
Yang HJ, Dey D, Sykes J, Klein M, Butler J, Kovacs MS, Sobczyk O, Sharif B, Bi X, Kali A, et al: Arterial CO2 as a potent coronary vasodilator: A preclinical PET/MR validation study with implications for cardiac stress testing. J Nucl Med. 58:953–960. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Shryock JC, Snowdy S, Baraldi PG, Cacciari B, Spalluto G, Monopoli A, Ongini E, Baker SP and Belardinelli L: A2A-adenosine receptor reserve for coronary vasodilation. Circulation. 98:711–718. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Mori K, Nakaya Y, Sakamoto S, Hayabuchi Y, Matsuoka S and Kuroda Y: Lactate-induced vascular relaxation in porcine coronary arteries is mediated by Ca2+-activated K+ channels. J Mol Cell Cardiol. 30:349–356. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Tarnow J, Brückner JB, Eberlein HJ, Gethmann JW, Hess W, Patschke D and Wilde J: Blood pH and PaCO2 as chemical factors in myocardial blood flow control. Basic Res Cardiol. 70:685–696. 1975. View Article : Google Scholar : PubMed/NCBI | |
|
Ishizaka H and Kuo L: Acidosis-induced coronary arteriolar dilation is mediated by ATP-sensitive potassium channels in vascular smooth muscle. Circ Res. 78:50–57. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Knot HJ, Zimmermann PA and Nelson MT: Extracellular K(+)-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K(+) channels. J Physiol. 492:419–430. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Dora KA, Borysova L, Ye X, Powell C, Beleznai TZ, Stanley CP, Bruno VD, Starborg T, Johnson E, Pielach A, et al: Human coronary microvascular contractile dysfunction associates with viable synthetic smooth muscle cells. Cardiovasc Res. 118:1978–1992. 2022. View Article : Google Scholar : | |
|
Zhuge Y, Zhang J, Qian F, Wen Z, Niu C, Xu K, Ji H, Rong X, Chu M and Jia C: Role of smooth muscle cells in cardiovascular disease. Int J Biol Sci. 16:2741–2751. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Young MA, Knight DR and Vatner SF: Autonomic control of large coronary arteries and resistance vessels. Prog Cardiovasc Dis. 30:211–234. 1987. View Article : Google Scholar : PubMed/NCBI | |
|
Seddon M, Melikian N, Dworakowski R, Shabeeh H, Jiang B, Byrne J, Casadei B, Chowienczyk P and Shah AM: Effects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivo. Circulation. 119:2656–2662. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Schrör K: Possible role of prostaglandins in the regulation of coronary blood flow. Basic Res Cardiol. 76:239–249. 1981. View Article : Google Scholar : PubMed/NCBI | |
|
Dharmashankar K and Widlansky ME: Vascular endothelial function and hypertension: Insights and directions. Curr Hypertens Rep. 12:448–455. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Lu Y, Cui X, Zhang L, Wang X, Xu Y, Qin Z, Liu G, Wang Q, Tian K, Lim KS, et al: The functional role of lipoproteins in atherosclerosis: Novel directions for diagnosis and targeting therapy. Aging Dis. 13:491–520. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Kobayashi Y, Sakai C, Ishida T, Nagata M, Nakano Y and Ishida M: Mitochondrial DNA is a key driver in cigarette smoke extract-induced IL-6 expression. Hypertens Res. 47:88–101. 2024. View Article : Google Scholar | |
|
Mundi S, Massaro M, Scoditti E, Carluccio MA, van Hinsbergh VWM, Iruela-Arispe ML and De Caterina R: Endothelial permeability, LDL deposition, and cardiovascular risk factors-a review. Cardiovasc Res. 114:35–52. 2018. View Article : Google Scholar | |
|
Falk E: Pathogenesis of atherosclerosis. J Am Coll Cardiol. 47(8 Suppl): C7–C12. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Aviram M: Macrophage foam cell formation during early atherogenesis is determined by the balance between pro-oxidants and anti-oxidants in arterial cells and blood lipoproteins. Antioxid Redox Signal. 1:585–594. 1999. View Article : Google Scholar | |
|
Willemsen L and de Winther MP: Macrophage subsets in atherosclerosis as defined by single-cell technologies. J Pathol. 250:705–714. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Newby AC and Zaltsman AB: Fibrous cap formation or destruction-the critical importance of vascular smooth muscle cell proliferation, migration and matrix formation. Cardiovasc Res. 41:345–360. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Allahverdian S, Chehroudi AC, McManus BM, Abraham T and Francis GA: Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation. 129:1551–1559. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Rong JX, Shapiro M, Trogan E and Fisher EA: Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci USA. 100:13531–13536. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Ajoolabady A, Pratico D, Lin L, Mantzoros CS, Bahijri S, Tuomilehto J and Ren J: Inflammation in atherosclerosis: Pathophysiology and mechanisms. Cell Death Dis. 15:8172024. View Article : Google Scholar : PubMed/NCBI | |
|
Song B, Bie Y, Feng H, Xie B, Liu M and Zhao F: Inflammatory factors driving atherosclerotic plaque progression new insights. J Transl Int Med. 10:36–47. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Francisco J and Del Re DP: Inflammation in myocardial ischemia/reperfusion injury: Underlying mechanisms and therapeutic potential. Antioxidants (Basel). 12:19442023. View Article : Google Scholar : PubMed/NCBI | |
|
Bennett MR, Sinha S and Owens GK: Vascular smooth muscle cells in atherosclerosis. Circ Res. 118:692–702. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Otsuka F, Kramer MCA, Woudstra P, Yahagi K, Ladich E, Finn AV, de Winter RJ, Kolodgie FD, Wight TN, Davis HR, et al: Natural progression of atherosclerosis from pathologic intimal thickening to late fibroatheroma in human coronary arteries: A pathology study. Atherosclerosis. 241:772–782. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Badimon L and Vilahur G: Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. 276:618–632. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Kumar A, Kar S and Fay WP: Thrombosis, physical activity, and acute coronary syndromes. J Appl Physiol (1985). 111:599–605. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Ha EJ, Kim Y, Cheung JY and Shim SS: Coronary artery disease in asymptomatic young adults: Its prevalence according to coronary artery disease risk stratification and the CT characteristics. Korean J Radiol. 11:425–432. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Dzaye O, Razavi AC, Blaha MJ and Mortensen MB: Evaluation of coronary stenosis versus plaque burden for atherosclerotic cardiovascular disease risk assessment and management. Curr Opin Cardiol. 36:769–775. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Kragel AH, Reddy SG, Wittes JT and Roberts WC: Morphometric analysis of the composition of atherosclerotic plaques in the four major epicardial coronary arteries in acute myocardial infarction and in sudden coronary death. Circulation. 80:1747–1756. 1989. View Article : Google Scholar : PubMed/NCBI | |
|
Servoss SJ, Januzzi JL and Muller JE: Triggers of acute coronary syndromes. Prog Cardiovasc Dis. 44:369–380. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Burke AP, Kolodgie FD, Farb A, Weber DK, Malcom GT, Smialek J and Virmani R: Healed plaque ruptures and sudden coronary death: Evidence that subclinical rupture has a role in plaque progression. Circulation. 103:934–940. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Amabile N and Veugeois A: Ruptured and healed atherosclerotic plaques: Breaking bad? EuroIntervention. 15:e742–e744. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Rittersma SZH, van der Wal AC, Koch KT, Piek JJ, Henriques JP, Mulder KJ, Ploegmakers JP, Meesterman M and de Winter RJ: Plaque instability frequently occurs days or weeks before occlusive coronary thrombosis: A pathological thrombectomy study in primary percutaneous coronary intervention. Circulation. 111:1160–1165. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Fernández-Ortiz A, Badimon JJ, Falk E, Fuster V, Meyer B, Mailhac A, Weng D, Shah PK and Badimon L: Characterization of the relative thrombogenicity of atherosclerotic plaque components: Implications for consequences of plaque rupture. J Am Coll Cardiol. 23:1562–1569. 1994. View Article : Google Scholar : PubMed/NCBI | |
|
Silvain J, Collet JP, Nagaswami C, Beygui F, Edmondson KE, Bellemain-Appaix A, Cayla G, Pena A, Brugier D, Barthelemy O, et al: Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol. 57:1359–1367. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Mohammed AQ, Abdu FA, Liu L, Yin G, Mareai RM, Mohammed AA, Xu Y and Che W: Coronary microvascular dysfunction and myocardial infarction with non-obstructive coronary arteries: Where do we stand? Eur J Intern Med. 117:8–20. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Herrmann J, Kaski JC and Lerman A: Coronary microvascular dysfunction in the clinical setting: From mystery to reality. Eur Heart J. 33:2771–2782b. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Sinha A, Rahman H and Perera D: Coronary microvascular disease: Current concepts of pathophysiology, diagnosis and management. Cardiovasc Endocrinol Metab. 10:22–30. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Rezaeian P, Shufelt CL, Wei J, Pacheco C, Cook-Wiens G, Berman D, Tamarappoo B, Thomson LE, Nelson MD, Anderson RD, et al: Arterial stiffness assessment in coronary microvascular dysfunction and heart failure with preserved ejection fraction: An initial report from the WISE-CVD continuation study. Am Heart J Plus. 41:1003902024.PubMed/NCBI | |
|
Singh A, Ashraf S, Irfan H, Venjhraj F, Verma A, Shaukat A, Tariq MD and Hamza HM: Heart failure and microvascular dysfunction: An in-depth review of mechanisms, diagnostic strategies, and innovative therapies. Ann Med Surg (Lond). 87:616–626. 2024. View Article : Google Scholar | |
|
Li M, Qian M, Kyler K and Xu J: Endothelial-vascular smooth muscle cells interactions in atherosclerosis. Front Cardiovasc Med. 5:1512018. View Article : Google Scholar : PubMed/NCBI | |
|
Medina-Leyte DJ, Zepeda-García O, Domínguez-Pérez M, González-Garrido A, Villarreal-Molina T and Jacobo-Albavera L: Endothelial dysfunction, inflammation and coronary artery disease: potential biomarkers and promising therapeutical approaches. Int J Mol Sci. 22:38502021. View Article : Google Scholar : PubMed/NCBI | |
|
Fleissner F and Thum T: Critical role of the nitric oxide/reactive oxygen species balance in endothelial progenitor dysfunction. Antioxid Redox Signal. 15:933–948. 2011. View Article : Google Scholar : | |
|
Goodwill AG, Dick GM, Kiel AM and Tune JD: Regulation of coronary blood flow. Compr Physiol. 7:321–382. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, Patel MR, Sandhu A, Valle J, Magid DJ, et al: Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 312:1754–1763. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Heusch G: Myocardial ischemia: Lack of coronary blood flow or myocardial oxygen supply/demand imbalance? Circ Res. 119:194–196. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Pasupathy S, Tavella R and Beltrame JF: Myocardial infarction with nonobstructive coronary arteries (MINOCA): The past, present, and future management. Circulation. 135:1490–1493. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Ford TJ, Rocchiccioli P, Good R, McEntegart M, Eteiba H, Watkins S, Shaukat A, Lindsay M, Robertson K, Hood S, et al: Systemic microvascular dysfunction in microvascular and vasospastic angina. Eur Heart J. 39:4086–4097. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Mohri M, Koyanagi M, Egashira K, Tagawa H, Ichiki T, Shimokawa H and Takeshita A: Angina pectoris caused by coronary microvascular spasm. Lancet. 351:1165–1169. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Mehta PK, Thobani A and Vaccarino V: Coronary artery spasm, coronary reactivity, and their psychological context. Psychosom Med. 81:233–236. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Hung MJ, Hu P and Hung MY: Coronary artery spasm: Review and update. Int J Med Sci. 11:1161–1171. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Igarashi Y, Yamazoe M and Shibata A: Effect of direct intracoronary administration of methylergonovine in patients with and without variant angina. Am Heart J. 121:1094–1100. 1991. View Article : Google Scholar : PubMed/NCBI | |
|
Frantz RP, Lerman A, Edwards BS, Olson LJ, Higano ST, Schwartz RS, Daly RC, McGregor CG and Rodeheffer RJ: Methylergonovine-induced diffuse coronary spasm in a patient with exercise-induced coronary spasm after heart transplantation. J Heart Lung Transplant. 13:834–839. 1994.PubMed/NCBI | |
|
Doenst T, Thiele H, Haasenritter J, Wahlers T, Massberg S and Haverich A: The treatment of coronary artery disease. Dtsch Arztebl Int. 119:716–723. 2022.PubMed/NCBI | |
|
Hennekens CH: Aspirin in the treatment and prevention of cardiovascular disease. Annu Rev Public Health. 18:37–49. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Sabatine MS, Wiviott SD, Im K, Murphy SA and Giugliano RP: Efficacy and safety of further lowering of low-density lipoprotein cholesterol in patients starting with very low levels: A meta-analysis. JAMA Cardiol. 3:823–828. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Manikandan A, Moharil P, Sathishkumar M, Muñoz-Garay C and Sivakumar A: Therapeutic investigations of novel indoxyl-based indolines: A drug target validation and structure-activity relationship of angiotensin-converting enzyme inhibitors with cardiovascular regulation and thrombolytic potential. Eur J Med Chem. 141:417–426. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Godoy LC, Farkouh ME, Austin PC, Shah BR, Qiu F, Jackevicius CA, Wijeysundera HC, Krumholz HM and Ko DT: Association of beta-blocker therapy with cardiovascular outcomes in patients with stable ischemic heart disease. J Am Coll Cardiol. 81:2299–2311. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Yan Y, An W, Mei S, Zhu Q, Li C, Yang L, Zhao Z and Huo J: Real-world research on beta-blocker usage trends in China and safety exploration based on the FDA adverse event reporting system (FAERS). BMC Pharmacol Toxicol. 25:862024. View Article : Google Scholar : PubMed/NCBI | |
|
Elliott WJ and Ram CVS: Calcium channel blockers. J Clin Hypertens (Greenwich). 13:687–689. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Kalinowski L, Dobrucki LW, Szczepanska-Konkel M, Jankowski M, Martyniec L, Angielski S and Malinski T: Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: A novel mechanism for antihypertensive action. Circulation. 107:2747–2752. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, et al: 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: Executive summary: A report of the American college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation. 145:e4–e17. 2022. | |
|
Head SJ, Kieser TM, Falk V, Huysmans HA and Kappetein AP: Coronary artery bypass grafting: Part 1-the evolution over the first 50 years. Eur Heart J. 34:2862–2872. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Costa MACD, Betero AL, Okamoto J, Schafranski M, Reis ESD and Gomes RZ: Coronary endarterectomy: A case control study and evaluation of early patency rate of endarterectomized arteries. Braz J Cardiovasc Surg. 35:9–15. 2020.PubMed/NCBI | |
|
González-Montero J, Brito R, Gajardo AI and Rodrigo R: Myocardial reperfusion injury and oxidative stress: Therapeutic opportunities. World J Cardiol. 10:74–86. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Hausenloy DJ and Yellon DM: Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J Clin Invest. 123:92–100. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
McBride W, Lange RA and Hillis LD: Restenosis after successful coronary angioplasty. Pathophysiology and prevention. N Engl J Med. 318:1734–1737. 1988. View Article : Google Scholar : PubMed/NCBI | |
|
Smart NA, Dieberg G and King N: Long-term outcomes of on-versus off-pump coronary artery bypass grafting. J Am Coll Cardiol. 71:983–991. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Camici PG and Crea F: Coronary microvascular dysfunction. N Engl J Med. 356:830–840. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Roffi M, Meier B and Gallino A: Fifty years of percutaneous transluminal angioplasty. Eur Heart J. 45:1779–1780. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Ruel M and Chikwe J: Coronary artery bypass grafting: Past and future. Circulation. 150:1067–1069. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Rocco E, Grimaldi MC, Maino A, Cappannoli L, Pedicino D, Liuzzo G and Biasucci LM: Advances and challenges in biomarkers use for coronary microvascular dysfunction: From bench to clinical practice. J Clin Med. 11:20552022. View Article : Google Scholar : PubMed/NCBI | |
|
Soleymani M, Masoudkabir F, Shabani M, Vasheghani-Farahani A, Behnoush AH and Khalaji A: Updates on pharmacologic management of microvascular angina. Cardiovasc Ther. 2022:60802582022. View Article : Google Scholar : PubMed/NCBI | |
|
Yang L, Zhao W, Kan Y, Ren C and Ji X: From mechanisms to medicine: Neurovascular coupling in the diagnosis and treatment of cerebrovascular disorders: A narrative review. Cells. 14:162024. View Article : Google Scholar | |
|
Bairey Merz CN, Pepine CJ, Shimokawa H and Berry C: Treatment of coronary microvascular dysfunction. Cardiovasc Res. 116:856–870. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Fatima M, Bazarbaev A, Rana A, Khurshid R, Effiom V, Bajwa NK, Nasir A, Candelario K, Tabraiz SA, Colon S, et al: Neuroprotective strategies in coronary artery disease interventions. J Cardiovasc Dev Dis. 12:1432025.PubMed/NCBI | |
|
Manolis AA, Manolis TA, Apostolopoulos EJ, Apostolaki NE, Melita H and Manolis AS: The role of the autonomic nervous system in cardiac arrhythmias: The neuro-cardiac axis, more foe than friend? Trends Cardiovasc Med. 31:290–302. 2021. View Article : Google Scholar | |
|
Armour JA: Functional anatomy of intrathoracic neurons innervating the atria and ventricles. Heart Rhythm. 7:994–996. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Brodde OE, Bruck H, Leineweber K and Seyfarth T: Presence, distribution and physiological function of adrenergic and muscarinic receptor subtypes in the human heart. Basic Res Cardiol. 96:528–538. 2001. View Article : Google Scholar | |
|
Kawashima T: The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat Embryol (Berl). 209:425–438. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Burnstock G: Autonomic neurotransmission: 60 Years since sir henry dale. Annu Rev Pharmacol Toxicol. 49:1–30. 2009. View Article : Google Scholar | |
|
Nikolaidis LA, Trumble D, Hentosz T, Doverspike A, Huerbin R, Mathier MA, Shen YT and Shannon RP: Catecholamines restore myocardial contractility in dilated cardiomyopathy at the expense of increased coronary blood flow and myocardial oxygen consumption (MvO2 cost of catecholamines in heart failure). Eur J Heart Fail. 6:409–419. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Rajendran PS, Challis RC, Fowlkes CC, Hanna P, Tompkins JD, Jordan MC, Hiyari S, Gabris-Weber BA, Greenbaum A, Chan KY, et al: Identification of peripheral neural circuits that regulate heart rate using optogenetic and viral vector strategies. Nat Commun. 10:19442019. View Article : Google Scholar : PubMed/NCBI | |
|
Machhada A, Hosford PS, Dyson A, Ackland GL, Mastitskaya S and Gourine AV: Optogenetic stimulation of vagal efferent activity preserves left ventricular function in experimental heart failure. JACC Basic Transl Sci. 5:799–810. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Finlay M, Harmer SC and Tinker A: The control of cardiac ventricular excitability by autonomic pathways. Pharmacol Ther. 174:97–111. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Kawano H, Okada R and Yano K: Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. 18:32–39. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Liu X, Yu Y, Zhang H, Zhang M and Liu Y: The role of muscarinic acetylcholine receptor M3 in cardiovascular diseases. Int J Mol Sci. 25:75602024. View Article : Google Scholar | |
|
Giannino G, Braia V, Griffith Brookles C, Giacobbe F, D'Ascenzo F, Angelini F, Saglietto A, De Ferrari GM and Dusi V: The intrinsic cardiac nervous system: From pathophysiology to therapeutic implications. Biology (Basel). 13:1052024.PubMed/NCBI | |
|
Armour JA, Murphy DA, Yuan BX, Macdonald S and Hopkins DA: Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 247:289–298. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Wake E and Brack K: Characterization of the intrinsic cardiac nervous system. Auton Neurosci. 199:3–16. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Fedele L and Brand T: The intrinsic cardiac nervous system and its role in cardiac pacemaking and conduction. J Cardiovasc Dev Dis. 7:542020.PubMed/NCBI | |
|
Rysevaite K, Saburkina I, Pauziene N, Noujaim SF, Jalife J and Pauza DH: Morphologic pattern of the intrinsic ganglionated nerve plexus in mouse heart. Heart Rhythm. 8:448–454. 2011. View Article : Google Scholar | |
|
Kimura K, Ieda M and Fukuda K: Development, maturation, and transdifferentiation of cardiac sympathetic nerves. Circ Res. 110:325–336. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Rajendran PS, Nakamura K, Ajijola OA, Vaseghi M, Armour JA, Ardell JL and Shivkumar K: Myocardial infarction induces structural and functional remodelling of the intrinsic cardiac nervous system. J Physiol. 594:321–341. 2016. View Article : Google Scholar : | |
|
Gardner RT, Ripplinger CM, Myles RC and Habecker BA: Molecular mechanisms of sympathetic remodeling and arrhythmias. Circ Arrhythm Electrophysiol. 9:e0013592016. View Article : Google Scholar : PubMed/NCBI | |
|
Hopkins DA, Macdonald SE, Murphy DA and Armour JA: Pathology of intrinsic cardiac neurons from ischemic human hearts. Anat Rec. 259:424–436. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Hardwick JC, Ryan SE, Beaumont E, Ardell JL and Southerland EM: Dynamic remodeling of the guinea pig intrinsic cardiac plexus induced by chronic myocardial infarction. Auton Neurosci. 181:4–12. 2014. View Article : Google Scholar : | |
|
Vaseghi M, Salavatian S, Rajendran PS, Yagishita D, Woodward WR, Hamon D, Yamakawa K, Irie T, Habecker BA and Shivkumar K: Parasympathetic dysfunction and antiarrhythmic effect of vagal nerve stimulation following myocardial infarction. JCI Insight. 2:e867152017. View Article : Google Scholar : PubMed/NCBI | |
|
Yperzeele L, van Hooff RJ, Nagels G, De Smedt A, De Keyser J and Brouns R: Heart rate variability and baroreceptor sensitivity in acute stroke: A systematic review. Int J Stroke. 10:796–800. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Grilletti JVF, Scapini KB, Bernardes N, Spadari J, Bigongiari A, Mazuchi FAES, Caperuto EC, Sanches IC, Rodrigues B and De Angelis K: Impaired baroreflex sensitivity and increased systolic blood pressure variability in chronic post-ischemic stroke. Clinics (Sao Paulo). 73:e2532018. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Z, Venkat P, Seyfried D, Chopp M, Yan T and Chen J: Brain-heart interaction: Cardiac complications after stroke. Circ Res. 121:451–468. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Tang S, Xiong L, Fan Y, Mok VCT, Wong KS and Leung TW: Stroke outcome prediction by blood pressure variability, heart rate variability, and baroreflex sensitivity. Stroke. 51:1317–1320. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Aftyka J, Staszewski J, Dębiec A, Pogoda-Wesołowska A and Żebrowski J: Heart rate variability as a predictor of stroke course, functional outcome, and medical complications: A systematic review. Front Physiol. 14:11151642023. View Article : Google Scholar : PubMed/NCBI | |
|
Giunta S, Xia S, Pelliccioni G and Olivieri F: Autonomic nervous system imbalance during aging contributes to impair endogenous anti-inflammaging strategies. Geroscience. 46:113–127. 2024. View Article : Google Scholar : | |
|
Bruno RM, Ghiadoni L, Seravalle G, Dell'oro R, Taddei S and Grassi G: Sympathetic regulation of vascular function in health and disease. Front Physiol. 3:2842012. View Article : Google Scholar : PubMed/NCBI | |
|
Amiya E, Watanabe M and Komuro I: The relationship between vascular function and the autonomic nervous system. Ann Vasc Dis. 7:109–119. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
He Z: The control mechanisms of heart rate dynamics in a new heart rate nonlinear time series model. Sci Rep. 10:48142020. View Article : Google Scholar : PubMed/NCBI | |
|
Valensi P: Autonomic nervous system activity changes in patients with hypertension and overweight: Role and therapeutic implications. Cardiovasc Diabetol. 20:1702021. View Article : Google Scholar : PubMed/NCBI | |
|
Monahan KD, Feehan RP, Sinoway LI and Gao Z: Contribution of sympathetic activation to coronary vasodilatation during the cold pressor test in healthy men: Effect of ageing. J Physiol. 591:2937–2947. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Hoffman JIE and Buckberg GD: The myocardial oxygen supply: Demand index revisited. J Am Heart Assoc. 3:e0002852014. View Article : Google Scholar | |
|
Hartupee J and Mann DL: Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol. 14:30–38. 2017. View Article : Google Scholar : | |
|
Remme WJ: The sympathetic nervous system and ischaemic heart disease. Eur Heart J. 19(Suppl F): F62–F71. 1998.PubMed/NCBI | |
|
Szczepanska-Sadowska E: Neuromodulation of cardiac ischemic pain: Role of the autonomic nervous system and vasopressin. J Integr Neurosci. 23:492024. View Article : Google Scholar : PubMed/NCBI | |
|
Wu L, Tai Y, Hu S, Zhang M, Wang R, Zhou W, Tao J, Han Y, Wang Q and Wei W: Bidirectional role of β2-adrenergic receptor in autoimmune diseases. Front Pharmacol. 9:13132018. View Article : Google Scholar | |
|
Grisanti LA, Perez DM and Porter JE: Modulation of immune cell function by α(1)-adrenergic receptor activation. Curr Top Membr. 67:113–138. 2011. View Article : Google Scholar | |
|
Perez DM: α1-Adrenergic receptors: insights into potential therapeutic opportunities for COVID-19, heart failure, and Alzheimer's disease. Int J Mol Sci. 24:41882023. View Article : Google Scholar | |
|
Kinugawa T, Kato M, Ogino K, Osaki S, Tomikura Y, Igawa O, Hisatome I and Shigemasa C: Interleukin-6 and tumor necrosis factor-alpha levels increase in response to maximal exercise in patients with chronic heart failure. Int J Cardiol. 87:83–90. 2003. View Article : Google Scholar | |
|
Li M, Yao W, Li S and Xi J: Norepinephrine induces the expression of interleukin-6 via β-adrenoreceptor-NAD(P)H oxidase system-NF-κB dependent signal pathway in U937 macrophages. Biochem Biophys Res Commun. 460:1029–1034. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Al-Sharea A, Lee MKS, Whillas A, Michell DL, Shihata WA, Nicholls AJ, Cooney OD, Kraakman MJ, Veiga CB, Jefferis AM, et al: Chronic sympathetic driven hypertension promotes atherosclerosis by enhancing hematopoiesis. Haematologica. 104:456–467. 2019. View Article : Google Scholar : | |
|
Stone PH, Libby P and Boden WE: Fundamental pathobiology of coronary atherosclerosis and clinical implications for chronic ischemic heart disease management-the plaque hypothesis: A narrative review. JAMA Cardiol. 8:192–201. 2023. View Article : Google Scholar | |
|
Wang Y, Anesi J, Maier MC, Myers MA, Oqueli E, Sobey CG, Drummond GR and Denton KM: Sympathetic nervous system and atherosclerosis. Int J Mol Sci. 24:131322023. View Article : Google Scholar : PubMed/NCBI | |
|
Chen YC, Smith M, Ying YL, Makridakis M, Noonan J, Kanellakis P, Rai A, Salim A, Murphy A, Bobik A, et al: Quantitative proteomic landscape of unstable atherosclerosis identifies molecular signatures and therapeutic targets for plaque stabilization. Commun Biol. 6:2652023. View Article : Google Scholar : PubMed/NCBI | |
|
Apolloni S and D'Ambrosi N: Inflammation in the CNS and PNS: From molecular basis to therapy. Int J Mol Sci. 24:94172023. View Article : Google Scholar : PubMed/NCBI | |
|
Zanos S: Closed-loop neuromodulation in physiological and translational research. Cold Spring Harb Perspect Med. 9:a0343142019. View Article : Google Scholar | |
|
Ali R and Schwalb JM: History and future of spinal cord stimulation. Neurosurgery. 94:20–28. 2024. | |
|
Ferraro MC, Gibson W, Rice ASC, Vase L, Coyle D and O'Connell NE: Spinal cord stimulation for chronic pain. Lancet Neurol. 21:4052022. View Article : Google Scholar : PubMed/NCBI | |
|
Augustinsson LE, Linderoth B, Mannheimer C and Eliasson T: Spinal cord stimulation in cardiovascular disease. Neurosurg Clin N Am. 6:157–165. 1995. View Article : Google Scholar : PubMed/NCBI | |
|
Theofilis P, Oikonomou E, Sagris M, Papageorgiou N, Tsioufis K and Tousoulis D: Novel concepts in the management of angina in coronary artery disease. Curr Pharm Des. 29:1825–1834. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Greco S, Auriti A, Fiume D, Gazzeri G, Gentilucci G, Antonini L and Santini M: Spinal cord stimulation for the treatment of refractory angina pectoris: A two-year follow-up. Pacing Clin Electrophysiol. 22:26–32. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Jessurun GA, DeJongste MJ, Hautvast RW, Tio RA, Brouwer J, van Lelieveld S and Crijns HJ: Clinical follow-up after cessation of chronic electrical neuromodulation in patients with severe coronary artery disease: A prospective randomized controlled study on putative involvement of sympathetic activity. Pacing Clin Electrophysiol. 22:1432–1439. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Eddicks S, Maier-Hauff K, Schenk M, Müller A, Baumann G and Theres H: Thoracic spinal cord stimulation improves functional status and relieves symptoms in patients with refractory angina pectoris: The first placebo-controlled randomised study. Heart. 93:585–590. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
de Jongste MJ, Hautvast RW, Hillege HL and Lie KI: Efficacy of spinal cord stimulation as adjuvant therapy for intractable angina pectoris: A prospective, randomized clinical study. Working group on neurocardiology. J Am Coll Cardiol. 23:1592–1597. 1994. View Article : Google Scholar : PubMed/NCBI | |
|
Hautvast RW, Blanksma PK, DeJongste MJ, Pruim J, van der Wall EE, Vaalburg W and Lie KI: Effect of spinal cord stimulation on myocardial blood flow assessed by positron emission tomography in patients with refractory angina pectoris. Am J Cardiol. 77:462–467. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Hautvast RW, DeJongste MJ, Staal MJ, van Gilst WH and Lie KI: Spinal cord stimulation in chronic intractable angina pectoris: A randomized, controlled efficacy study. Am Heart J. 136:1114–1120. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Vulink NC, Overgaauw DM, Jessurun GA, Tenvaarwerk IA, Kropmans TJ, van der Schans CP, Middel B, Staal MJ and Dejongste MJ: The effects of spinal cord stimulation on quality of life in patients with therapeutically chronic refractory angina pectoris. Neuromodulation. 2:33–40. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
McNab D, Khan SN, Sharples LD, Ryan JY, Freeman C, Caine N, Tait S, Hardy I and Schofield PM: An open label, single-centre, randomized trial of spinal cord stimulation vs percutaneous myocardial laser revascularization in patients with refractory angina pectoris: The SPiRiT trial. Eur Heart J. 27:1048–1053. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Dyer MT, Goldsmith KA, Khan SN, Sharples LD, Freeman C, Hardy I, Buxton MJ and Schofield PM: Clinical and cost-effectiveness analysis of an open label, single-centre, randomised trial of spinal cord stimulation (SCS) versus percutaneous myocardial laser revascularisation (PMR) in patients with refractory angina pectoris: The SPiRiT trial. Trials. 9:402008. View Article : Google Scholar : PubMed/NCBI | |
|
Bondesson S, Pettersson T, Erdling A, Hallberg IR, Wackenfors A and Edvinsson L: Comparison of patients undergoing enhanced external counterpulsation and spinal cord stimulation for refractory angina pectoris. Coron Artery Dis. 19:627–634. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Andréll P, Yu W, Gersbach P, Gillberg L, Pehrsson K, Hardy I, Ståhle A, Andersen C and Mannheimer C: Long-term effects of spinal cord stimulation on angina symptoms and quality of life in patients with refractory angina pectoris-results from the European angina registry link study (EARL). Heart. 96:1132–1136. 2010. View Article : Google Scholar | |
|
Lanza GA, Grimaldi R, Greco S, Ghio S, Sarullo F, Zuin G, De Luca A, Allegri M, Di Pede F, Castagno D, et al: Spinal cord stimulation for the treatment of refractory angina pectoris: A multicenter randomized single-blind study (the SCS-ITA trial). Pain. 152:45–52. 2011. View Article : Google Scholar | |
|
Saraste A, Ukkonen H, Varis A, Vasankari T, Tunturi S, Taittonen M, Rautakorpi P, Luotolahti M, Airaksinen KE and Knuuti J: Effect of spinal cord stimulation on myocardial perfusion reserve in patients with refractory angina pectoris. Eur Heart J Cardiovasc Imaging. 16:449–455. 2015. View Article : Google Scholar | |
|
Latif OA, Nedeljkovic SS and Stevenson LW: Spinal cord stimulation for chronic intractable angina pectoris: A unified theory on its mechanism. Clin Cardiol. 24:533–541. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Southerland EM, Milhorn DM, Foreman RD, Linderoth B, DeJongste MJ, Armour JA, Subramanian V, Singh M, Singh K and Ardell JL: Preemptive, but not reactive, spinal cord stimulation mitigates transient ischemia-induced myocardial infarction via cardiac adrenergic neurons. Am J Physiol Heart Circ Physio. 292:H311–H327. 2007. View Article : Google Scholar | |
|
Dale EA, Kipke J, Kubo Y, Sunshine MD, Castro PA, Ardell JL and Mahajan A: Spinal cord neural network interactions: Implications for sympathetic control of the porcine heart. Am J Physiol Heart Circ Physiol. 318:H830–H839. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Salavatian S, Kuwabara Y, Wong B, Fritz JR, Howard-Quijano K, Foreman RD, Armour JA, Ardell JL and Mahajan A: Spinal neuromodulation mitigates myocardial ischemia-induced sympathoexcitation by suppressing the intermediolateral nucleus hyperactivity and spinal neural synchrony. Front Neurosci. 17:11802942023. View Article : Google Scholar : PubMed/NCBI | |
|
Saddic LA, Howard-Quijano K, Kipke J, Kubo Y, Dale EA, Hoover D, Shivkumar K, Eghbali M and Mahajan A: Progression of myocardial ischemia leads to unique changes in immediate-early gene expression in the spinal cord dorsal horn. Am J Physiol Heart Circ Physiol. 315:H1592–H1601. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Minisi AJ and Thames MD: Activation of cardiac sympathetic afferents during coronary occlusion. Evidence for reflex activation of sympathetic nervous system during transmural myocardial ischemia in the dog. Circulation. 84:357–367. 1991. View Article : Google Scholar : PubMed/NCBI | |
|
Ajijola OA and Shivkumar K: Neural remodeling and myocardial infarction: The stellate ganglion as a double agent. J Am Coll Cardiol. 59:962–964. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Ding X, Ardell JL, Hua F, McAuley RJ, Sutherly K, Daniel JJ and Williams CA: Modulation of cardiac ischemia-sensitive afferent neuron signaling by preemptive C2 spinal cord stimulation: Effect on substance P release from rat spinal cord. Am J Physiol Regul Integr Comp Physiol. 294:R93–R101. 2008. View Article : Google Scholar | |
|
Law M, Sachdeva R, Darrow D and Krassioukov A: Cardiovascular effects of spinal cord stimulation: The highs, the lows, and the don't knows. Neuromodulation. 27:1164–1176. 2024. View Article : Google Scholar | |
|
Tse HF, Turner S, Sanders P, Okuyama Y, Fujiu K, Cheung CW, Russo M, Green MDS, Yiu KH, Chen P, et al: Thoracic spinal cord stimulation for heart failure as a restorative treatment (SCS HEART study): First-in-man experience. Heart Rhythm. 12:588–595. 2015. View Article : Google Scholar | |
|
Wu M, Linderoth B and Foreman RD: Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: A review of experimental studies. Auton Neurosci. 138:9–23. 2008. View Article : Google Scholar | |
|
Gouveia FV, Warsi NM, Suresh H, Matin R and Ibrahim GM: Neurostimulation treatments for epilepsy: Deep brain stimulation, responsive neurostimulation and vagus nerve stimulation. Neurotherapeutics. 21:e003082024. View Article : Google Scholar : PubMed/NCBI | |
|
Austelle CW, O'Leary GH, Thompson S, Gruber E, Kahn A, Manett AJ, Short B and Badran BW: A comprehensive review of vagus nerve stimulation for depression. Neuromodulation. 25:309–315. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Gidron Y, Kupper N, Kwaijtaal M, Winter J and Denollet J: Vagus-brain communication in atherosclerosis-related inflammation: A neuroimmunomodulation perspective of CAD. Atherosclerosis. 195:e1–e9. 2007. View Article : Google Scholar | |
|
Giordano F, Zicca A, Barba C, Guerrini R and Genitori L: Vagus nerve stimulation: Surgical technique of implantation and revision and related morbidity. Epilepsia. 58(Suppl 1): S85–S90. 2017. View Article : Google Scholar | |
|
Winston GM, Guadix S, Lavieri MT, Uribe-Cardenas R, Kocharian G, Williams N, Sholle E, Grinspan Z and Hoffman CE: Closed-loop vagal nerve stimulation for intractable epilepsy: A single-center experience. Seizure. 88:95–101. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Skarpaas TL and Morrell MJ: Intracranial stimulation therapy for epilepsy. Neurotherapeutics. 6:238–243. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Sun FT and Morrell MJ: Closed-loop neurostimulation: The clinical experience. Neurotherapeutics. 11:553–563. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Ottaviani MM, Vallone F, Micera S and Recchia FA: Closed-loop vagus nerve stimulation for the treatment of cardiovascular diseases: State of the art and future directions. Front Cardiovasc Med. 9:8669572022. View Article : Google Scholar : PubMed/NCBI | |
|
Vilaine JP, Berdeaux A and Giudicelli JF: Effects of vagal stimulation on regional myocardial flows and ischemic injury in dogs. Eur J Pharmacol. 66:243–247. 1980. View Article : Google Scholar : PubMed/NCBI | |
|
Zuanetti G, De Ferrari GM, Priori SG and Schwartz PJ: Protective effect of vagal stimulation on reperfusion arrhythmias in cats. Circ Res. 61:429–435. 1987. View Article : Google Scholar : PubMed/NCBI | |
|
Rosenshtraukh L, Danilo P Jr, Anyukhovsky EP, Steinberg SF, Rybin V, Brittain-Valenti K, Molina-Viamonte V and Rosen MR: Mechanisms for vagal modulation of ventricular repolarization and of coronary occlusion-induced lethal arrhythmias in cats. Circ Res. 75:722–732. 1994. View Article : Google Scholar : PubMed/NCBI | |
|
Ando M, Katare RG, Kakinuma Y, Zhang D, Yamasaki F, Muramoto K and Sato T: Efferent vagal nerve stimulation protects heart against ischemia-induced arrhythmias by preserving connexin43 protein. Circulation. 112:164–170. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Uemura K, Li M, Tsutsumi T, Yamazaki T, Kawada T, Kamiya A, Inagaki M, Sunagawa K and Sugimachi M: Efferent vagal nerve stimulation induces tissue inhibitor of metalloproteinase-1 in myocardial ischemia-reperfusion injury in rabbit. Am J Physiol Heart Circ Physiol. 293:H2254–H2261. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Del Rio CL, Dawson TA, Clymer BD, Paterson DJ and Billman GE: Effects of acute vagal nerve stimulation on the early passive electrical changes induced by myocardial ischaemia in dogs: Heart rate-mediated attenuation. Exp Physiol. 93:931–944. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Beaumont E, Southerland EM, Hardwick JC, Wright GL, Ryan S, Li Y, KenKnight BH, Armour JA and Ardell JL: Vagus nerve stimulation mitigates intrinsic cardiac neuronal and adverse myocyte remodeling postmyocardial infarction. Am J Physiol Heart Circ Physiol. 309:H1198–H1206. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Zamotrinsky A, Afanasiev S, Karpov RS and Cherniavsky A: Effects of electrostimulation of the vagus afferent endings in patients with coronary artery disease. Coron Artery Dis. 8:551–557. 1997.PubMed/NCBI | |
|
Zamotrinsky AV, Kondratiev B and de Jong JW: Vagal neurostimulation in patients with coronary artery disease. Auton Neurosci. 88:109–116. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Yu L, Huang B, Po SS, Tan T, Wang M, Zhou L, Meng G, Yuan S, Zhou X, Li X, et al: Low-level tragus stimulation for the treatment of ischemia and reperfusion injury in patients with ST-segment elevation myocardial infarction: A proof-of-concept study. JACC Cardiovasc Interv. 10:1511–1520. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Bonaz B, Sinniger V and Pellissier S: Anti-inflammatory properties of the vagus nerve: Potential therapeutic implications of vagus nerve stimulation. J Physiol. 594:5781–5790. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Li S, Qi D, Li JN, Deng XY and Wang DX: Vagus nerve stimulation enhances the cholinergic anti-inflammatory pathway to reduce lung injury in acute respiratory distress syndrome via STAT3. Cell Death Discovery. 7:632021. View Article : Google Scholar : PubMed/NCBI | |
|
Hachuła M, Kosowski M, Basiak M and Okopień B: Influence of dulaglutide on serum biomarkers of atherosclerotic plaque instability: An interventional analysis of cytokine profiles in diabetic subjects-a pilot study. Medicina (Kaunas). 60:9082024. View Article : Google Scholar | |
|
Shinlapawittayatorn K, Chinda K, Palee S, Surinkaew S, Thunsiri K, Weerateerangkul P, Chattipakorn S, KenKnight BH and Chattipakorn N: Low-amplitude, left vagus nerve stimulation significantly attenuates ventricular dysfunction and infarct size through prevention of mitochondrial dysfunction during acute ischemia-reperfusion injury. Heart Rhythm. 10:1700–1707. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Lu Y, Chen K, Zhao W, Hua Y, Bao S, Zhang J, Wu T, Ge G, Yu Y, Sun J and Zhang F: Magnetic vagus nerve stimulation alleviates myocardial ischemia-reperfusion injury by the inhibition of pyroptosis through the M2AChR/OGDHL/ROS axis in rats. J Nanobiotechnology. 21:4212023. View Article : Google Scholar | |
|
Luo B, Wu Y, Liu SL, Li XY, Zhu HR, Zhang L, Zheng F, Liu XY, Guo LY, Wang L, et al: Vagus nerve stimulation optimized cardiomyocyte phenotype, sarcomere organization and energy metabolism in infarcted heart through FoxO3A-VEGF signaling. Cell Death Dis. 11:9712020. View Article : Google Scholar : PubMed/NCBI | |
|
Munhoz RP and Albuainain G: Deep brain stimulation: New programming algorithms and teleprogramming. Expert Rev Neurother. 23:467–478. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Lee DJ, Lozano CS, Dallapiazza RF and Lozano AM: Current and future directions of deep brain stimulation for neurological and psychiatric disorders. J Neurosurg. 131:333–342. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Basiago A and Binder DK: Effects of deep brain stimulation on autonomic function. Brain Sci. 6:332016. View Article : Google Scholar : PubMed/NCBI | |
|
Fontes MAP, Dos Santos Machado LR, Viana ACR, Cruz MH, Nogueira ÍS, Oliveira MGL, Neves CB, Godoy ACV, Henderson LA and Macefield VG: The insular cortex, autonomic asymmetry and cardiovascular control: Looking at the right side of stroke. Clin Auton Res. 34:549–560. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Hyam JA, Kringelbach ML, Silburn PA, Aziz TZ and Green AL: The autonomic effects of deep brain stimulation-a therapeutic opportunity. Nat Rev Neurol. 8:391–400. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Marins FR, Limborço-Filho M, Xavier CH, Biancardi VC, Vaz GC, Stern JE, Oppenheimer SM and Fontes MA: Functional topography of cardiovascular regulation along the rostrocaudal axis of the rat posterior insular cortex. Clin Exp Pharmacol Physiol. 43:484–493. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Shivkumar K, Ajijola OA, Anand I, Armour JA, Chen PS, Esler M, De Ferrari GM, Fishbein MC, Goldberger JJ, Harper RM, et al: Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J Physiol. 594:3911–3954. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Sumi K, Katayama Y, Otaka T, Obuchi T, Kano T, Kobayashi K, Oshima H, Fukaya C, Yamamoto T, Ogawa Y and Iwasaki K: Effect of subthalamic nucleus deep brain stimulation on the autonomic nervous system in Parkinson's disease patients assessed by spectral analyses of R-R interval variability and blood pressure variability. Stereotact Funct Neurosurg. 90:248–254. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang C, Xu J, Wu B, Ling Y, Guo Q, Wang S, Liu L, Jiang N, Chen L and Liu J: Subthalamic nucleus deep brain stimulation treats Parkinson's disease patients with cardiovascular disease comorbidity: A retrospective study of a single center experience. Brain Sci. 13:702022. View Article : Google Scholar | |
|
Rajkumar S, Venkatraman V, Yang LZ, Parente B, Lee HJ and Lad SP: Short-term health care costs of high-frequency spinal cord stimulation for the treatment of postsurgical persistent spinal pain syndrome. Neuromodulation. 26:1450–1458. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Kumar K, Caraway DL, Rizvi S and Bishop S: Current challenges in spinal cord stimulation. Neuromodulation. 17(Suppl 1): S22–S35. 2014. View Article : Google Scholar | |
|
Yap JYY, Keatch C, Lambert E, Woods W, Stoddart PR and Kameneva T: Critical review of transcutaneous vagus nerve stimulation: Challenges for translation to clinical practice. Front Neurosci. 14:2842020. View Article : Google Scholar : PubMed/NCBI | |
|
Austelle CW, Cox SS, Wills KE and Badran BW: Vagus nerve stimulation (VNS): Recent advances and future directions. Clin Auton Res. 34:529–547. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Javan-Noughabi J, Rezapour A, Hajahmadi M and Alipour V: Economic evaluation of single-photon emission-computed tomography versus stress echocardiography in stable chest pain patients. Sci Rep. 12:152232022. View Article : Google Scholar : PubMed/NCBI | |
|
Hijazi W, Vandenberk B, Rennert-May E, Quinn A, Sumner G and Chew DS: Economic evaluation in cardiac electrophysiology: Determining the value of emerging technologies. Front Cardiovasc Med. 10:11424292023. View Article : Google Scholar : PubMed/NCBI | |
|
Tabaja H, Yuen J, Tai DBG, Campioli CC, Chesdachai S, DeSimone DC, Hassan A, Klassen BT, Miller KJ, Lee KH and Mahmood M: Deep brain stimulator device infection: The mayo clinic rochester experience. Open Forum Infect Dis. 10:ofac6312022. View Article : Google Scholar | |
|
Jung IH, Chang KW, Park SH, Chang WS, Jung HH and Chang JW: Complications After deep brain stimulation: A 21-year experience in 426 patients. Front Aging Neurosci. 14:8197302022. View Article : Google Scholar : PubMed/NCBI | |
|
Olson MC, Shill H, Ponce F and Aslam S: Deep brain stimulation in PD: Risk of complications, morbidity, and hospitalizations: A systematic review. Front Aging Neurosci. 15:12581902023. View Article : Google Scholar : PubMed/NCBI | |
|
Lee JM, Lee D, Christiansen S, Hagedorn JM, Chen Z and Deer T: Spinal cord stimulation in special populations: Best practices from the american society of pain and neuroscience to improve safety and efficacy. J Pain Res. 15:3263–3273. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Ki YM, Park HJ, Yi SH, Sim WS and Lee JY: Latent infection after spinal cord stimulation device implantation for complex regional pain syndrome: A case report. Medicine (Baltimore). 102:e337502023. View Article : Google Scholar : PubMed/NCBI | |
|
Vu PD, Pinkhasova D, Sarwary ZB, Rita Markaryan A, Mousa B, Viswanath O, Robinson CL, Varrassi G, Orhurhu V, Urits I and Hasoon J: Biologic complications associated with cylindrical lead spinal cord stimulator implants: A narrative review. Orthop Rev (Pavia). 16:1234432024. View Article : Google Scholar : PubMed/NCBI | |
|
Das S, Matias CM, Ramesh S, Velagapudi L, Barbera JP, Katz S, Baldassari MP, Rasool M, Kremens D, Ratliff J, et al: Capturing initial understanding and impressions of surgical therapy for Parkinson's disease. Front Neurol. 12:6059592021. View Article : Google Scholar : PubMed/NCBI | |
|
Salinas M, Yazdani U, Oblack A, McDaniels B, Ahmed N, Haque B, Pouratian N and Chitnis S: Know DBS: Patient perceptions and knowledge of deep brain stimulation in Parkinson's disease. Ther Adv Neurol Disord. 17:175628642412330382024. View Article : Google Scholar : PubMed/NCBI | |
|
Müller O and Rotter S: Neurotechnology: Current developments and ethical issues. Front Syst Neurosci. 11:932017. View Article : Google Scholar | |
|
Faltus T, Freise J, Fluck C and Zillmann H: Ethics and regulation of neuronal optogenetics in the European Union. Pflugers Arch. 475:1505–1517. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Jog MA, Anderson C, Kubicki A, Boucher M, Leaver A, Hellemann G, Iacoboni M, Woods R and Narr K: Transcranial direct current stimulation (tDCS) in depression induces structural plasticity. Sci Rep. 13:28412023. View Article : Google Scholar : PubMed/NCBI | |
|
Lapa JDS, Duarte JFS, Campos ACP, Davidson B, Nestor SM, Rabin JS, Giacobbe P, Lipsman N and Hamani C: Adverse effects of deep brain stimulation for treatment-resistant depression: A scoping review. Neurosurgery. 95:509–516. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Siebner HR, Funke K, Aberra AS, Antal A, Bestmann S, Chen R, Classen J, Davare M, Di Lazzaro V, Fox PT, et al: Transcranial magnetic stimulation of the brain: What is stimulated?-A consensus and critical position paper. Clin Neurophysiol. 140:59–97. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Iseger TA, van Bueren NER, Kenemans JL, Gevirtz R and Arns M: A frontal-vagal network theory for major depressive disorder: Implications for optimizing neuromodulation techniques. Brain Stimul. 13:1–9. 2020. View Article : Google Scholar | |
|
Jiao Y, Cheng C, Jia M, Chu Z, Song X, Zhang M, Xu H, Zeng X, Sun JB, Qin W and Yang XJ: Neuro-cardiac-guided transcranial magnetic stimulation: Unveiling the modulatory effects of low-frequency and high-frequency stimulation on heart rate. Psychophysiology. 61:e146312024. View Article : Google Scholar : PubMed/NCBI | |
|
Zou N, Zhou Q, Zhang Y, Xin C, Wang Y, Claire-Marie R, Rong P, Gao G and Li S: Transcutaneous auricular vagus nerve stimulation as a novel therapy connecting the central and peripheral systems: A review. Int J Surg. 110:4993–5006. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Afra P, Adamolekun B, Aydemir S and Watson GDR: Evolution of the vagus nerve stimulation (VNS) therapy system technology for drug-resistant epilepsy. Front Med Technol. 3:6965432021. View Article : Google Scholar | |
|
Habibagahi I, Omidbeigi M, Hadaya J, Lyu H, Jang J, Ardell JL, Bari AA and Babakhani A: Vagus nerve stimulation using a miniaturized wirelessly powered stimulator in pigs. Sci Rep. 12:81842022. View Article : Google Scholar : PubMed/NCBI | |
|
Mirza KB, Golden CT, Nikolic K and Toumazou C: Closed-loop implantable therapeutic neuromodulation systems based on neurochemical monitoring. Front Neurosci. 13:8082019. View Article : Google Scholar : PubMed/NCBI | |
|
de Faria GM, Lopes EG, Tobaldini E, Montano N, Cunha TS, Casali KR and de Amorim HA: Advances in non-invasive neuromodulation: Designing closed-loop devices for respiratory-controlled transcutaneous vagus nerve stimulation. Healthcare (Basel). 12:312023. View Article : Google Scholar | |
|
Van Horn JD, Grafton ST and Miller MB: Individual variability in brain activity: A nuisance or an opportunity? Brain Imaging Behav. 2:327–334. 2008. View Article : Google Scholar | |
|
Li LM, Uehara K and Hanakawa T: The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Front Cell Neurosci. 9:1812015. View Article : Google Scholar : PubMed/NCBI | |
|
Seghier ML and Price CJ: Interpreting and utilising intersubject variability in brain function. Trends Cogn Sci. 22:517–530. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Johnson MD, Lim HH, Netoff TI, Connolly AT, Johnson N, Roy A, Holt A, Lim KO, Carey JR, Vitek JL and He B: Neuromodulation for brain disorders: Challenges and opportunities. IEEE Trans Biomed Eng. 60:610–624. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Völzke H, Schmidt CO, Baumeister SE, Ittermann T, Fung G, Krafczyk-Korth J, Hoffmann W, Schwab M, Meyer zu Schwabedissen HE, Dörr M, et al: Personalized cardiovascular medicine: Concepts and methodological considerations. Nat Rev Cardiol. 10:308–316. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Thompson N, Mastitskaya S and Holder D: Avoiding off-target effects in electrical stimulation of the cervical vagus nerve: Neuroanatomical tracing techniques to study fascicular anatomy of the vagus nerve. J Neurosci Methods. 325:1083252019. View Article : Google Scholar : PubMed/NCBI | |
|
Fitchett A, Mastitskaya S and Aristovich K: Selective neuromodulation of the vagus nerve. Front Neurosci. 15:6858722021. View Article : Google Scholar : PubMed/NCBI | |
|
Sclocco R, Garcia RG, Gabriel A, Kettner NW, Napadow V and Barbieri R: Respiratory-gated auricular vagal afferent nerve stimulation (RAVANS) effects on autonomic outflow in hypertension. Annu Int Conf IEEE Eng Med Biol Soc. 2017:3130–3133. 2017.PubMed/NCBI | |
|
Garcia RG, Cohen JE, Stanford AD, Gabriel A, Stowell J, Aizley H, Barbieri R, Gitlin D, Napadow V and Goldstein JM: Respiratory-gated auricular vagal afferent nerve stimulation (RAVANS) modulates brain response to stress in major depression. J Psychiatr Res. 142:188–197. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Cook DN, Thompson S, Stomberg-Firestein S, Bikson M, George MS, Jenkins DD and Badran BW: Design and validation of a closed-loop, motor-activated auricular vagus nerve stimulation (MAAVNS) system for neurorehabilitation. Brain Stimul. 13:800–803. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Badran BW, Peng X, Baker-Vogel B, Hutchison S, Finetto P, Rishe K, Fortune A, Kitchens E, O'Leary GH, Short A, et al: Motor activated auricular vagus nerve stimulation as a potential neuromodulation approach for post-stroke motor rehabilitation: A pilot study. Neurorehabil Neural Repair. 37:374–383. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Yu Y, Ling J, Yu L, Liu P and Jiang M: Closed-loop transcutaneous auricular vagal nerve stimulation: Current situation and future possibilities. Front Hum Neurosci. 15:7856202022. View Article : Google Scholar : PubMed/NCBI | |
|
Forrest IS, Petrazzini BO, Duffy Á, Park JK, Marquez-Luna C, Jordan DM, Rocheleau G, Cho JH, Rosenson RS, Narula J, et al: Machine learning-based marker for coronary artery disease: Derivation and validation in two longitudinal cohorts. Lancet. 401:215–225. 2023. View Article : Google Scholar : | |
|
Upton R, Mumith A, Beqiri A, Parker A, Hawkes W, Gao S, Porumb M, Sarwar R, Marques P, Markham D, et al: Automated echocardiographic detection of severe coronary artery disease using artificial intelligence. JACC Cardiovasc Imaging. 15:715–727. 2022. View Article : Google Scholar | |
|
Alizadehsani R, Abdar M, Roshanzamir M, Khosravi A, Kebria PM, Khozeimeh F, Nahavandi S, Sarrafzadegan N and Acharya UR: Machine learning-based coronary artery disease diagnosis: A comprehensive review. Comput Biol Med. 111:1033462019. View Article : Google Scholar : PubMed/NCBI | |
|
Obst MA, Al-Zubaidi A, Heldmann M, Nolde JM, Blümel N, Kannenberg S and Münte TF: Five weeks of intermittent transcutaneous vagus nerve stimulation shape neural networks: A machine learning approach. Brain Imaging Behav. 16:1217–1233. 2022. View Article : Google Scholar : | |
|
Tarasenko A, Guazzotti S, Minot T, Oganesyan M and Vysokov N: Determination of the effects of transcutaneous auricular vagus nerve stimulation on the heart rate variability using a machine learning pipeline. Bioelectricity. 4:168–177. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Chen H, Lai H, Chi H, Fan W, Huang J, Zhang S, Jiang C, Jiang L, Hu Q, Yan X, et al: Multi-modal transcriptomics: integrating machine learning and convolutional neural networks to identify immune biomarkers in atherosclerosis. Front Cardiovasc Med. 11:13974072024. View Article : Google Scholar : PubMed/NCBI |