Nitric oxide‑mediated S‑Nitrosylation contributes to signaling transduction in human physiological and pathological status (Review)
- Authors:
- Published online on: July 21, 2025 https://doi.org/10.3892/ijmm.2025.5593
- Article Number: 152
-
Copyright: © Xu et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Protein post-translational modifications (PTMs) increase the functional diversity of the proteome by controlling protein activity, localization, expression and interactions with other cellular components through the covalent addition or removal of chemical groups at amino acid residues. These changes ultimately influence cellular phenotype and biogenesis (1). At present, nearly all proteins are subject to some form of PTM, which markedly expands the range of proteome functions (2).
Protein S-nitrosylation refers to the oxidative modification of cysteine residues by nitric oxide (NO), leading to the formation of protein S-nitrosothiols (SNOs). This modification mediates redox-dependent signal transduction and reflects how NO regulates various cellular processes (3). As a widespread PTM, S-nitrosylation transmits the effects of NO throughout the cell and is involved in multiple biological activities. Under physiological conditions, S-nitrosylation governs protein structure, stability, subcellular distribution and protein-protein interactions, allowing NO to function beyond the classical cyclic guanosine monophosphate (cGMP)-dependent signaling pathway (4). Under pathological conditions, S-nitrosylation targets diverse substrates, including transcription factors, enzymes, receptors and signaling molecules, in an effort to restore disrupted cellular balance. An increasing body of evidence suggests that abnormal NO levels can interfere with the regulation of S-nitrosylation, ultimately affecting the physiological and pathological processes central to life (2).
The first author conducted a thorough literature search in databases such as PubMed, Web of Science and ScienceDirect using the terms 'post-translational modification', 'nitric oxide', 'S-nitrosylation', 'S-nitrosothiols' and 'biotin switch assay' to explore the association between S-nitrosylation and disease. Articles were initially screened by reviewing titles and abstracts and 119 relevant publications were selected for detailed examination.
NO generation and signaling
NO synthase
NO is generated through the oxidation of L-arginine by NO synthase (NOS) and is involved in key physiological functions, including vasodilatation, platelet aggregation, immune defense, cell proliferation and mitochondrial respiration (5-7). As NO is a gas with a short half-life in the body, its activity is concentration-dependent, often confining its action to local target sites (7). Identifying NOS-specific inhibitors can contribute to understanding NO-driven signaling pathways and may provide a basis for developing therapies for conditions linked to abnormal NO levels.
In mammals, NOS exists in three major isoforms: inducible (iNOS/NOS2), neural (nNOS/NOS1) and endothelial (eNOS/NOS3), each showing distinct expression patterns and biological functions across various tissues and cell types (8,9). Under resting conditions, iNOS is generally not expressed. Its expression is triggered by stimuli such as inflammatory mediators, endotoxins and hypoxic environments (9). Pro-inflammatory cytokines including tumor necrosis factor-α, interleukin-1, interferon-γ and lipopolysaccharide bind to membrane receptors, activating intracellular kinases that phosphorylate target proteins and initiate specific transcription factors. These transcription factors then translocate into the nucleus, bind promoter sequences and induce iNOS gene expression, leading to sustained, high-output NO production (10,11).
By contrast, nNOS and eNOS are constitutively expressed and their enzymatic activities are fine-tuned by phosphorylation, S-nitrosylation, protein-protein interactions and calcium signaling (9). eNOS is primarily located in endothelial cells and is the predominant isoform involved in regulating vascular tone and function (12). NO derived from endothelial cells inhibits platelet aggregation, prevents thrombus formation and regulates the expression of genes associated with atherosclerosis (AS), thereby blocking leukocyte adhesion and migration to the endothelium, which helps prevent AS progression (13,14). nNOS is mainly found in neurons and is regulated by Ca2+ and calmodulin, playing essential roles in learning, memory and neurogenesis (15). Under physiological conditions, nNOS rapidly produces small amounts of NO to maintain internal stability and contribute to neuroprotection (16).
NO-mediated signaling
NO signaling functions through both classical and non-classical mechanisms to regulate diverse cellular activities (17). In the classical pathway, NO binds to the heme moiety of soluble guanylate cyclase, facilitating the enzymatic conversion of guanosine triphosphate into cGMP (18). Acting as a second messenger, cGMP activates cGMP-dependent protein kinase G, which in turn regulates downstream targets, including cGMP-regulated kinases, phosphodiesterases and ion channels, thereby regulating mitochondrial dynamics and biogenesis.
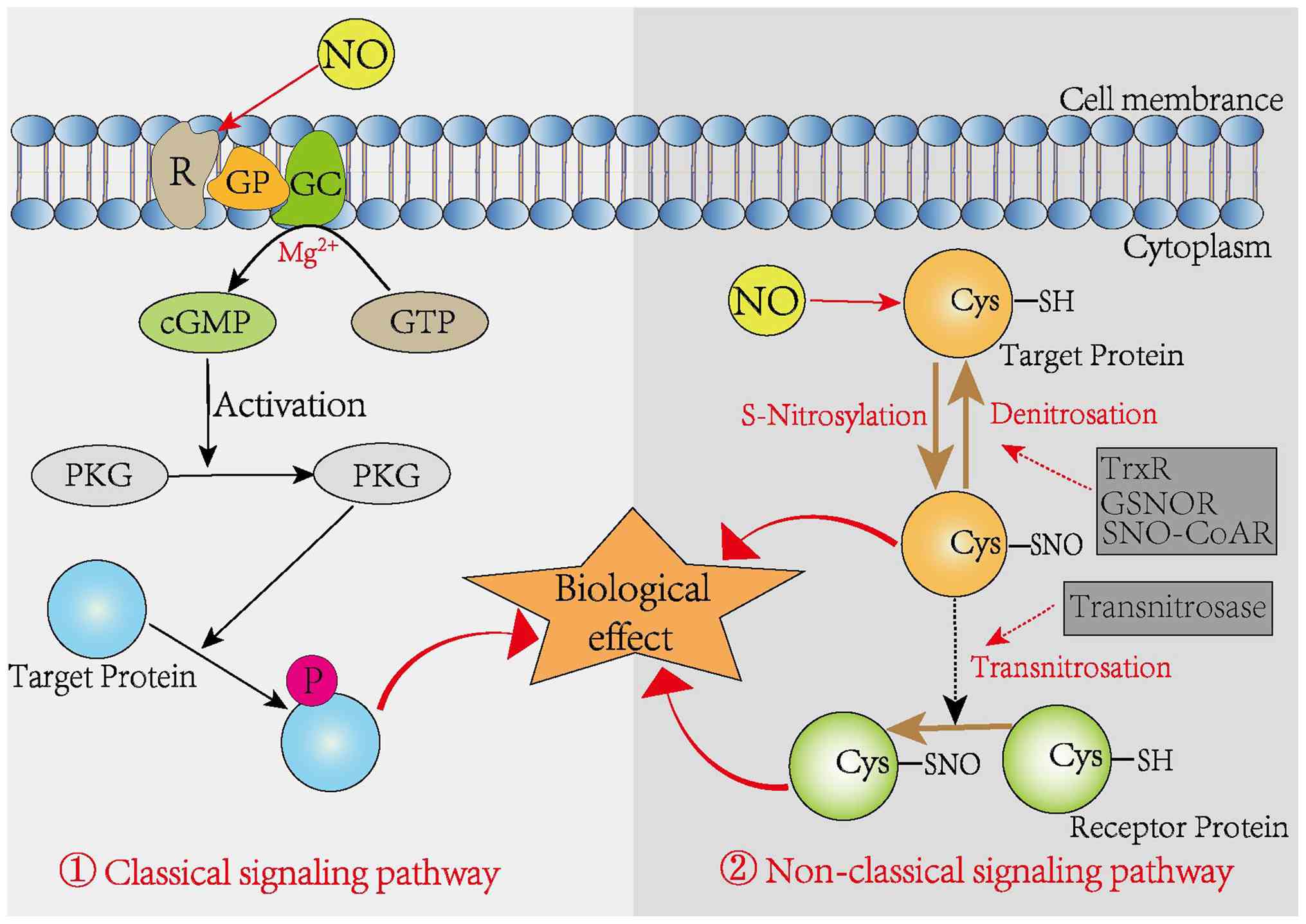
By contrast, the non-classical signaling pathway involves the covalent post-translational modification of biomolecules by NO and its reactive derivatives. This mode of action, primarily through S-nitrosylation, plays a regulatory role across various tissues and organ systems (19,20). S-nitrosylation has emerged as a critical form of redox-based signaling that enables cells to manage oxidative stress and limit reactive oxygen species levels (21,22). Importantly, S-nitrosylation is a selective and reversible modification encompassing nitrosylation, transnitrosylation and denitrosylation processes (23). Two principal classes of denitrosylating enzymes have been identified: The Trx system and low-molecular-weight SNO-cleaving enzymes, which include S-nitrosoglutathione reductase (GSNOR) and S-nitroso-coenzyme A reductase (24). Enzymes such as GSNOR and thioredoxin reductase (TrxR) catalyze the denitrosylation of SNOs, restoring free sulfhydryl groups. In parallel, SNOs can donate NO groups to the cysteine thiols of target proteins, a process facilitated by transnitrosylases (Fig. 1). Recent findings indicate that NO-driven S-nitrosylation markedly affects essential cellular processes including transcription, DNA repair, cell growth, differentiation and apoptosis (17,25).
Protein S-nitrosylation
The formation of protein S-nitrosylation
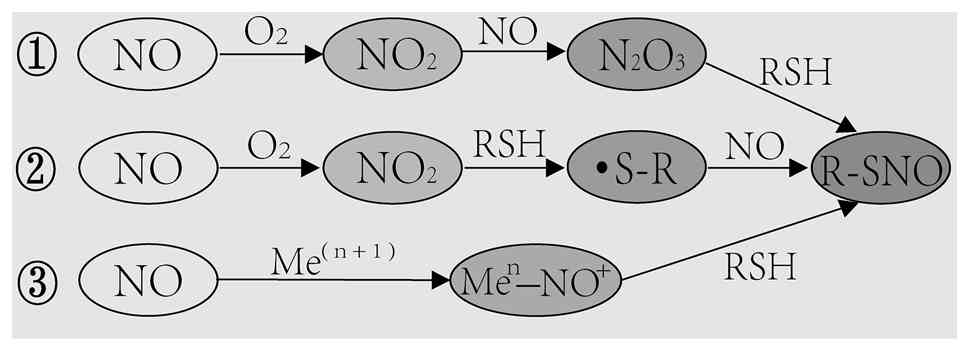
In 1992, Stamler et al (26) first identified SNO, a product formed when NO reacts with bovine serum albumin. Protein S-nitrosylation, a covalent modification involving the attachment of NO to reactive cysteine residues, has since been recognized as a key mechanism by which NO mediates its biological functions (27). As NO itself is not an oxidant and does not efficiently interact with protein thiols directly (17), most S-nitrosylation events proceed through an intermediate oxidation process. Initially, NO reacts with O2 to form NO2, which then combines with another NO molecule to produce N2O3, a more reactive intermediate that reacts with protein thiols to yield nitrosothiols (28). Additionally, NO2 can oxidize thiols to generate thiol radicals, which subsequently react with NO to form nitrosothiols (17). Other pathways also exist. During the auto-nitrosylation of hemoglobin or in the formation of GSNO by cytochrome c, NO is oxidized by a transition metal such as Fe3+ or Cu2+ within a metalloenzyme, forming a nitrosonium ion (18). This positively charged intermediate then reacts with a nearby thiol group close to the catalytic center, producing a stable nitrosothiol (18) (Fig. 2).
Specificity of the S-nitrosylation reaction
S-nitrosylation does not occur randomly across all cysteine residues; it is selective and occurs only on specific thiols within proteins (29). The target cysteine typically needs to be spatially close to the NO source for direct recognition by NOS. It is often located in a hydrophobic environment shaped by the tertiary protein structure or membranes, which avoids steric hindrance and increases reaction probability (29,30). Moreover, certain motifs, such as I/L-X-C-X2-D/E, are preferentially targeted by NOS. These motifs often lie within α-helices that present a broad surface area, making them more accessible for S-nitrosylation (31). Proteomic analyses have revealed that the likelihood of S-nitrosylation is determined not only by the surrounding amino acid sequence but also by the pKa of the cysteine thiol, the local pH, the electrophilic nature of nitrosothiols, NOS enzymatic activity and the concentration of external NO (22,27,32-34).
Regulation of S-nitrosylation levels
Intracellular SNO levels are shaped not only by the cellular redox environment but also by the action of specific nitrosylases (35,36). Among the most abundant endogenous SNOs, GSNO acts as a key NO donor for protein modification in vivo (37). GSNO is metabolized by GSNOR, a member of the alcohol dehydrogenase family, which catalyzes its breakdown into oxidized glutathione and ammonia (38). In addition to GSNOR, the Trx enzyme family, primarily located in the cytoplasm, forms a central part of the denitrosylation system (39). Unlike the GSNOR system, which specifically targets GSNO, TrxR enables denitrosylation across a broader range of nitrosylated proteins. Changes in the expression or function of reductases such as GSNOR and TrxR can disturb S-nitrosylation dynamics and contribute to disease development (4,40). S-nitrosylation is also regulated through transnitrosylation, a process in which the NO group is transferred from low molecular weight nitrosothiols such as GSNO to cysteine thiols on target proteins (41). GSNO can transfer its S-nitroso group to various substrates, affecting cellular physiology or contributing to disease progression through protein S-nitrosylation (17,41). Together, nitrosylation, denitrosylation and transnitrosylation work in concert to maintain SNO homeostasis in vivo, playing essential roles in normal physiology. Disruptions in these processes can lead to imbalances in SNO levels, resulting in functional disturbances.
S-nitrosylation detection method
As interest in protein S-nitrosylation continues to grow, understanding its physiological functions has become a major research focus. Although significant advances have been made in exploring the role of S-nitrosylation in health and disease, detecting protein's SNO levels within complex biological systems remains difficult due to their low abundance and instability under physiological conditions. The present review outlined the main techniques currently available for identifying and analyzing protein S-nitrosylation (Table I). Common approaches include iodide/copper-based reduction methods and immunohistochemistry (42,43). However, the S-NO bond in nitrosylated proteins is inherently unstable and prone to breakdown under reducing agents or UV exposure, complicating direct detection efforts (43).
Table 1Different methods for detection and characterization of protein S-nitrosylation have respective advantages and drawbacks. |
Liu et al (44) proposed a label-free detection strategy using resonance Raman spectroscopy of ferrous cytochrome c. In this method, light releases NO from S-nitrosylated proteins, which then reacts with the heme center of ferrous cytochrome c. Shifts in the resonance Raman spectra allow for quantitative measurement of S-nitrosylated protein levels.
Currently, the biotinylation-based method is the most widely applied technique for detecting protein S-nitrosylation. In 2001, Jaffrey et al (45) introduced a protocol in which biotin is covalently linked to the sulfhydryl groups of nitrosylated proteins, stabilizing otherwise labile nitroso groups. This method permits reliable analysis of S-nitrosylated protein expression via relative quantification of biotin-labeled proteins. It also allows detection of S-nitrosylation at specific thiol sites within protein mixtures. The procedure involves three main steps: Alkylation to block free thiols, selective reduction of nitrosothiols to free sulfhydryls and labeling of these with biotin. Biotinylated proteins are then purified and analyzed to reflect the S-nitrosylation status of target cysteine residues. Later, Forrester et al (46) showed that the biotin switch assay had become a widely used and foundational tool for identifying S-nitrosylated proteins in complex samples. They also addressed potential technical limitations of the method and proposed updated versions, highlighting directions for further development in the expanding field of S-nitrosylation research.
With the development of molecular and cellular biology techniques, refined protocols such as the idoTMT transformation method (47), the Cys-Boost technique (48) and surface plasmon resonance (49) have improved both the sensitivity and precision of protein nitrosylation detection. Direct identification of protein S-nitrosylation through mass spectrometry has made high-throughput analysis possible, allowing for the detection of previously unknown post-translational modifications, pinpointing modification sites and quantifying expression or modification levels at the proteome scale (48). Qin et al (50) introduced a fluorine affinity tag-switch chemical proteomics approach to enrich and detect mercapto-nitrosylated peptide fragments. This technique enabled accurate quantitative analysis of mercapto-nitrosylated groups in plants and revealed their key involvement in regulating endoplasmic reticulum stress. However, the instability of the S-NO bond on cysteine poses challenges for mass spectrometry. Accurate identification of S-nitrosylation sites requires careful experimental planning and targeted analysis to distinguish these modifications from other thiol-based changes, minimizing the risk of both false positives and false negatives (51). Standardized operating procedures, along with strict quality control systems, are necessary to maintain consistency and reliability across different sample batches.
Regulatory functions of S-nitrosylation in cellular life activities
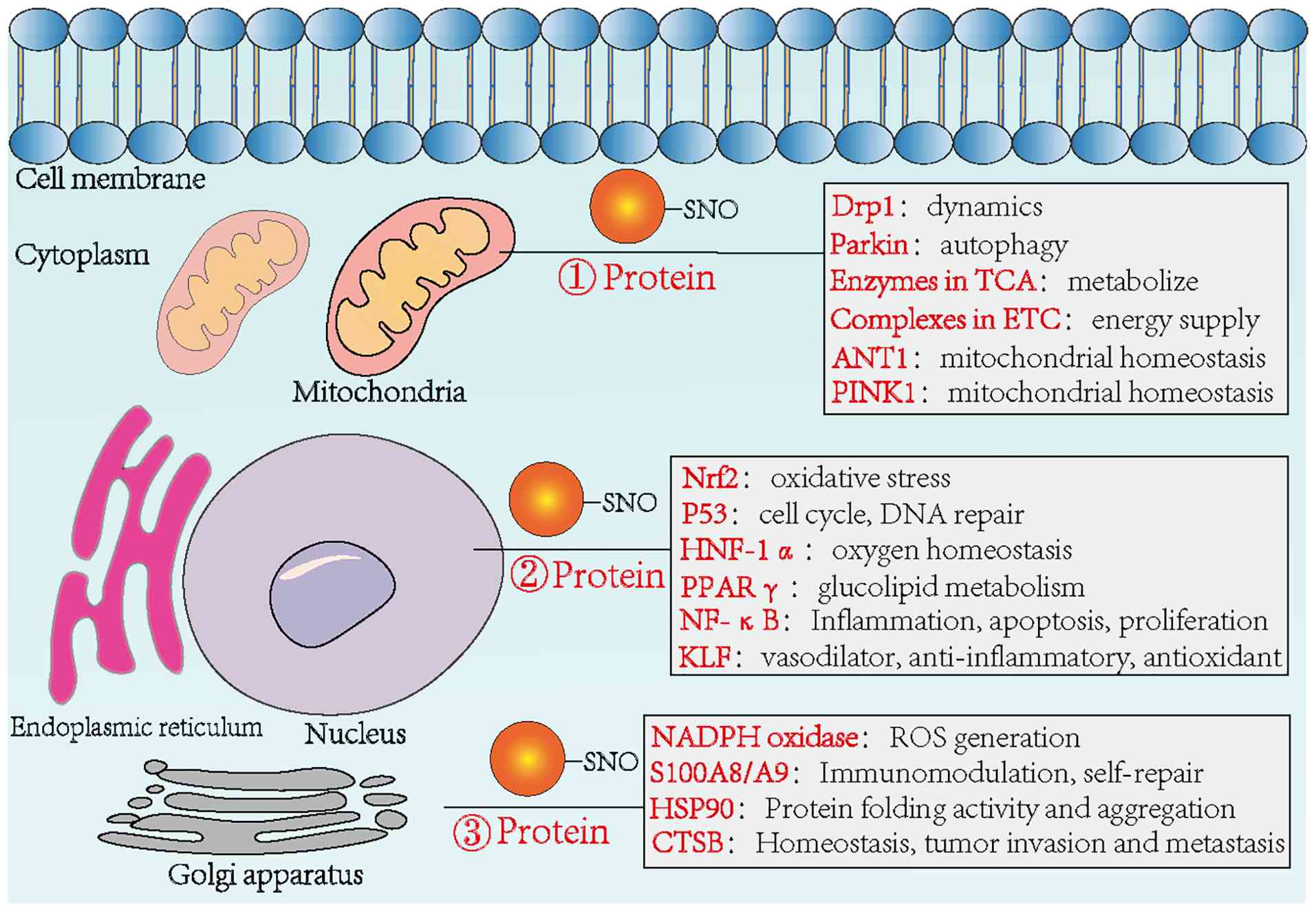
S-nitrosylation plays an essential role in both physiological and pathological conditions (9). S-nitrosylated proteins are found throughout the cell, including in membrane domains, the cytosol, mitochondria, nucleus and extracellular matrix, where they affect numerous signaling pathways. These include synaptic transmission, transcription factor regulation, mitochondrial dynamics and receptor or ion channel activity, all of which help maintain tissue homeostasis (42). Moreover, nitroso groups can be regulated through transnitrosylation or secretion, influencing broader tissue-level processes. The sections below outline the regulatory roles of S-nitrosylation in three fundamental cellular activities (Fig. 3).
Regulating mitochondrial function
Mitochondria, central to cell growth and cycle regulation, function not only as the primary source of cellular ATP but also participate in signal transduction, differentiation and apoptosis. Murray et al (43) identified several proteins modified by S-nitrosylation in rat heart tissue following co-incubation with the NO donor GSNO. These proteins were mainly involved in mitochondrial quality control, energy metabolism and mitochondria-mediated apoptosis. This suggests that S-nitrosylation-mediated control of mitochondrial biogenesis and dynamics may offer a potential strategy for addressing diseases linked to mitochondrial dysfunction (52,53). In plant mitochondria, protein S-nitrosylation has been shown to participate in multiple metabolic pathways, including photorespiration (54), the tricarboxylic acid cycle (55-67), oxidative phosphorylation (54,58), protein processing and turnover (59) and other core metabolic processes (60). Beyond metabolic regulation, S-nitrosylation also influences mitochondrial autophagy. Parkin, a ubiquitin E3 ligase of the RING-between-RING (RBR) family, is a key player in mitochondrial quality control. Ozawa et al (61) reported that S-nitrosylation levels of Parkin increased markedly in GSNO-treated HeLa cells, which increased Parkin's E3 ligase activity and promoted mitochondrial degradation. In addition to its role in regulating mitochondrial pathways, Tang et al (38) demonstrated that mitochondrial GSNOR is critical for maintaining mitochondrial homeostasis. Through the denitrosylation of ANT1, GSNOR contributes to preserving mitochondrial integrity and improving mitochondrial performance.
Regulating transcription factors
S-nitrosylation not only affects mitochondrial function but also modifies key transcription factors and their associated regulatory proteins, including NF-κB, p53, hypoxia-inducible factor (HIF) and nuclear respiratory factor 2 (52,62-64). NF-κB is a major transcriptional regulator involved in immune and inflammatory responses, development, cell proliferation and apoptosis. It activates transcription by binding specific DNA sequences within promoter regions. S-nitrosylation of the NF-κB p50 subunit at Cys62 and the p65 subunit at Cys38 impairs this DNA-binding ability, thereby reducing transcriptional activity (65,66). Matthews et al (62) showed that in A549 human lung cancer cells, treatment with the NO donor GSNO led to S-nitrosylation of NF-κB, which suppressed its transcriptional activity and increased the cells' sensitivity to tumor necrosis factor-α-induced apoptosis. Additionally, under excitotoxic conditions triggered by glutamate, eNOS caused S-nitrosylation of the NF-κB p65 subunit. This modification decreased NF-κB activity and offered neuroprotection, improving neuronal survival (67).
S-nitrosylation also influences p53. Specifically, modification at Cys124 within its DNA-binding domain increases the regulation of downstream targets such as superoxide dismutase and catalytic subunits, helping reduce oxidative stress (50). Beyond NF-κB and p53, S-nitrosylation of HIF-1α stabilizes the protein, increasing its protective function in hypoxic environments (68).
Regulating protein homeostasis
S-nitrosylation, as part of redox-based signaling, influences protein function by affecting enzyme activity, subcellular localization and protein-protein interactions. Under conditions of proliferative stress, hematopoietic stem cells show increased levels of NO and SNO, which are associated with the accumulation of misfolded or aggregated proteins. Yi et al (69) identified a mechanistic connection between GSNOR-regulated protein S-nitrosylation and the CHOP-mediated unfolded protein response, which plays a role in maintaining hematopoietic stem cell activity. Their findings suggest that adjusting S-nitrosylation levels could support the expansion of functional hematopoietic stem cells without compromising viability. Lin et al (70) reported that S-nitrosylated recombinant cathepsin B (CTSB) regulates its mRNA reprogramming through the ADD1/MATR3/ADAR1 signaling pathway, affecting the steady-state expression of CTSB. S-nitrosylation may either promote or suppress protein activity depending on the cellular context, timing and location (71). This highlights the need to evaluate how SNO-modified proteins influence downstream signaling processes under specific conditions.
Regulatory functions of S-nitrosylation in human diseases
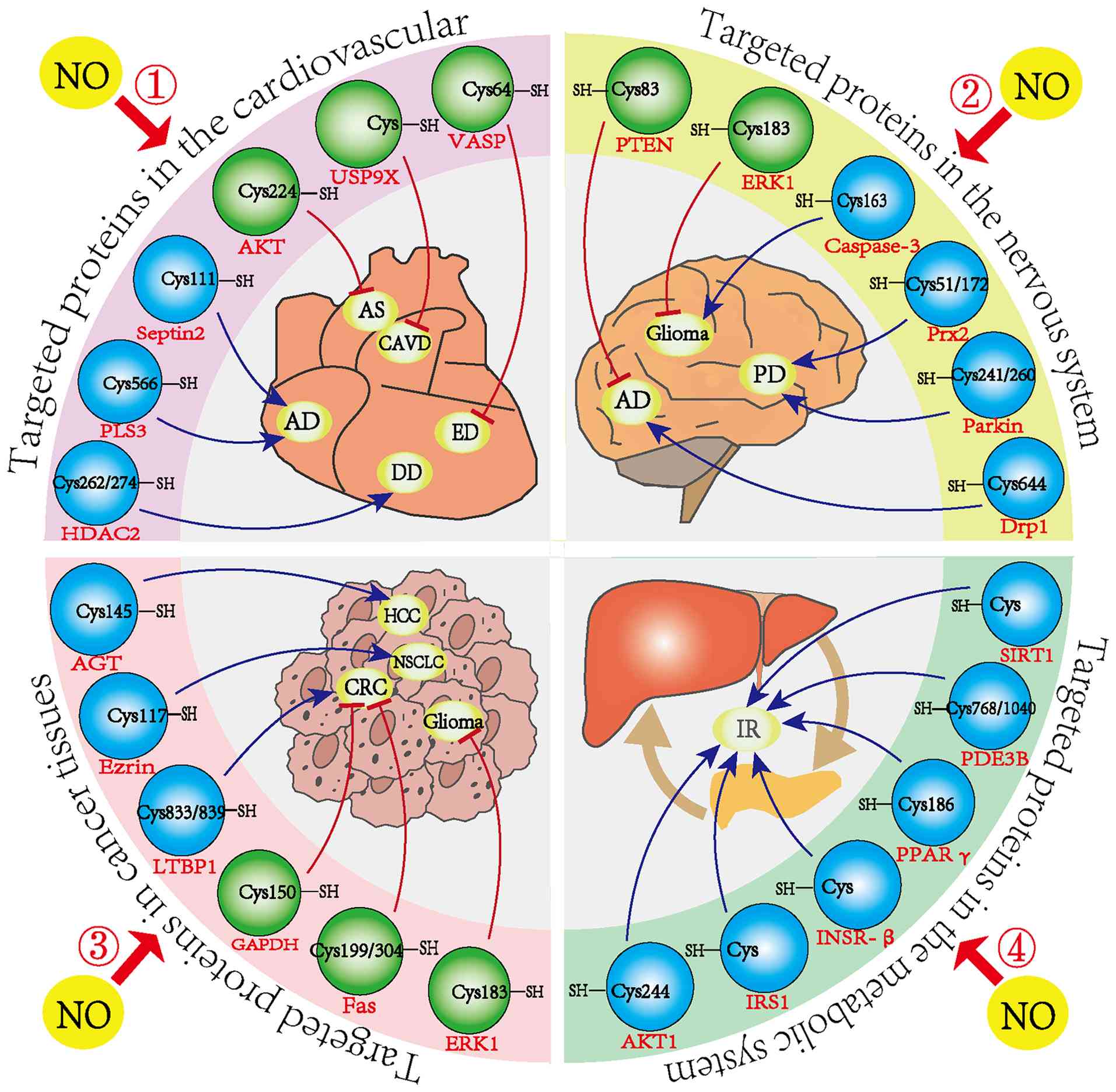
S-nitrosylation has emerged as a central mechanism for transmitting NO signals within cells and tissues, functioning similarly to phosphorylation (4). Unlike irreversible post-translational modifications that permanently alter protein regulation, S-nitrosylation of cysteine residues is reversible, enabling dynamic control over numerous biological processes in both healthy and diseased states (72). Under normal physiological conditions, protein S-nitrosylation is tightly controlled to preserve redox balance (73,74). However, disrupted regulation of S-nitrosylation in pathological settings can affect key proteins, contributing to the development of diseases such as AS, Parkinson's disease (PD) and cancer. These changes may represent promising targets for therapeutic intervention (Fig. 4).
Cardiovascular system
In the complex development of cardiovascular diseases, protein S-nitrosylation is gaining recognition for its distinct role in regulating inflammation, apoptosis, angiogenesis, myocardial contractility and electrophysiological signaling transduction (Table II). Yoon et al (75) showed that increased nNOS activity in hearts with diastolic dysfunction (DD) raised NO levels in vivo, leading to S-nitrosylation of histone deacetylase 2 and worsening clinical symptoms in patients with DD. This study identified protein S-nitrosylation as a contributing factor in DD pathogenesis and proposed it as a potential therapeutic target for treatment-resistant heart failure.
Table IIS-nitrosylation targeted proteins and SNO sites for common diseases in cardiovascular system. |
As mentioned earlier, mitochondrial GSNOR is critical for maintaining mitochondrial homeostasis via denitrosylation of ANT1. A decline in mitochondrial GSNOR expression during heart failure increases S-nitrosylation of ANT1 at Cys160. In line with this, overexpression of either mitochondrial GSNOR or a non-nitrosylated mutant, ANT1 Cys160A, markedly improved mitochondrial function, offering a new potential target for heart failure therapy (38). Aortic aneurysm and coarctation, life-threatening conditions that can lead to aortic dissection and sudden death, have also been associated with increased SNO levels. Aortic tissues from patients with coarctation showed elevated S-nitrosylation compared to healthy controls (76). Pan et al (77) identified Plastin 3, modified at Cys566, as a key protein affected by S-nitrosylation in this disease. Additionally, Zhang et al (78) found that the S-nitrosylation level of Septin2 was markedly increased in diseased vascular tissues and peripheral blood mononuclear cells of patients with aortic coarctation. This modification altered the TIAM1/RAC1/NF-κB signaling cascade, contributing to macrophage-driven vascular inflammation and aortic pathology. Two compounds, NSC23766 and R-ketoglutarate, targeting this pathway, were found effective in preventing and treating the condition.
In conclusion, the levels of a number of protein SNOs are upregulated in various cardiovascular diseases. De-nitrosylation can play a role in protecting the heart and alleviating vascular damage. The research on SNO modification in cardiovascular physiology and pathology has made certain progress, suggesting that S-nitrosylation of proteins plays an important role in regulating cardiovascular diseases. These findings suggest that detailed exploration of the molecular role of S-nitrosylation in cardiovascular physiology and disease may guide new drug development and help identify novel therapeutic strategies. In addition, continuously promoting the research on the targets and mechanisms between SNO and cardiovascular diseases and integrating them with clinical treatment, will bring good news to more patients with cardiovascular diseases.
Nervous system
Studies have shown that NO is involved in a wide range of neural processes, including development, neurotransmitter release and synaptic plasticity (7). However, abnormal S-nitrosylation driven by excessive NO can contribute to neuronal damage in various neurodegenerative diseases (79) (Table III). Nakamura et al (29) reported that under normal conditions, nitrosylated NMDA receptors remain inactive, helping maintain physiological NO levels and supporting normal neuronal function. By contrast, under pathological conditions, overactivation of the extrasynaptic NMDA-nNOS pathway leads to excess NO production, driving the formation of abnormal nitrosylated proteins and accelerating disease progression. In Alzheimer's disease (AD), NO targets the Cys644 residue of guanosine triphosphate-bound Drp1, forming SNO-Drp1, which overactivates the protein and triggers mitochondrial fragmentation. This results in insufficient energy for neurotransmission and contributes to synaptic failure and cognitive decline (80). S-nitrosylation of Drp1 also promotes mitochondrial overactivation and fission (81,82). Wang et al (82) found that S-nitrosylation of Drp1 was present in all cases of sporadic AD. Inhibiting this modification prevented Aβ-induced mitochondrial fragmentation, synaptic dysfunction and neuronal death, suggesting that SNO-Drp1 could serve as a potential therapeutic target for preserving neuronal and synaptic integrity in AD. Furthermore, Nakamura et al (83) demonstrated, using in vitro and in vivo AD models and postmortem brain samples from AD patients, that enzymes from various biochemical pathways can undergo a cascade of S-nitrosylation events, ultimately leading to synapse loss.
In PD, both animal models and human tissue studies have shown that S-nitrosylation of the parkin protein disrupts its neuroprotective function by impairing substrate ubiquitination and suppressing its ubiquitin ligase activity (84). Tsang et al (85) further confirmed that nitrosative stress contributes to PD progression by impairing prosurvival proteins such as parkin and X-linked inhibitor of apoptosis protein via distinct pathways, indicating that abnormal S-nitrosylation is a key factor in neurodegeneration. In glioma cells, S-nitrosylation of extracellular signal-regulated kinase 1/2 (ERK1/2) within the MAPK pathway suppresses its phosphorylation, promoting apoptosis (86). On the other hand, S-nitrosylation of caspase-3 in glioma cells inhibits apoptosis and facilitates tumor progression (87). Altogether, aberrant S-nitrosylation plays a critical role in the pathogenesis of AD, PD and gliomas.
In the clinic, neurodegenerative diseases can be prevented or treated by controlling NO production through drug delivery or the modulation of NOS activity (88). In addition, by inhibiting the excessive release of α-synuclein, which oligomerizes, aggregates and deposits in the cytoplasm, Parkin SNO can be reduced and cells can be protected from extracellular α-synuclein oligomer-induced toxicity, thus treating diseases (89). Therefore, gaining a deeper understanding of how dysregulated S-nitrosylation pathways contribute to neurological disorders will improve insight into their molecular basis and support the search for therapeutic targets.
Tumors
S-nitrosylation plays dual roles in cancer, acting as both a tumor suppressor and promoter and is involved across multiple stages of tumor progression. Abnormal S-nitrosylation patterns have been identified in various cancers, including pancreatic, lung, liver and prostate, where they influence tumor behavior in both positive and negative directions (Table IV). In pancreatic ductal adenocarcinoma (PDAC), dysregulated NO production by iNOS and eNOS contributes to tumor development and correlates with poor prognosis, although the detailed mechanisms remain unclear (90,91). Tan et al (92) used site-specific proteomics to identify 585 S-nitrosylation sites on 434 proteins in PDAC tissues and PANC-1 cell lines, reporting markedly more S-nitrosylated proteins in tumor samples than in adjacent normal tissue. Deng et al (93) demonstrated that tumor necrosis factor-related apoptosis-inducing ligand triggered apoptosis in human thyroid carcinoma FRO cells by increasing NO levels, which led to S-nitrosylation of GAPDH and its accumulation in the nucleus. In another study, increased S-nitrosylation of the parkin protein, driven by iNOS and GSNOR deficiency, was found to contribute to hepatocellular carcinoma formation in mice (94). Similarly, Wei et al (95) identified S-nitrosylation of O6-alkylguanine-DNA alkyltransferase (AGT) at Cys145 in both in vivo and in vitro models. This modification promoted AGT degradation through the proteasome and led to the accumulation of mutagenic alkylamines, accelerating liver tumor progression.
In the context of non-small cell carcinoma (NSCLC), Zhang et al (96) found that inflammatory conditions caused S-nitrosylation of the cytoskeletal regulator ezrin, promoting mechanical signaling between the cytoskeleton and membrane and increasing cell invasiveness and metastatic capacity. This provides a new perspective on NSCLC progression and potential therapeutic approaches. S-nitrosylation has also been shown to influence the function of epigenetic regulators. DNA methyltransferases (DNMTs), which control DNA methylation patterns in mammals, are key epigenetic enzymes. Okuda et al (97) discovered that S-nitrosylation of DNMT suppressed its enzymatic activity and promoted tumor formation. They also identified DBIC, a compound that blocks S-nitrosylation of DNMT3B at low concentrations (IC50 ≤100 nM), without interfering with its catalytic function. DBIC markedly reduced tumor cell proliferation and malignant transformation. Taken together, these findings show that disruptions in S-nitrosylation contribute to cancer development.
Although SNO modification accounts for a relatively low proportion of overall protein PTMs, it still plays an important role in tumor development and inhibition (98). Increasing the level of SNO modification in certain proteins by developing NO donors, inhibiting GSNOR and denitrification and enhancing the targeting of SNO modification and protein active sites with nanomaterials may provide a new approach for tumor cell therapy. Conversely, reducing the SNO levels of certain cancer-promoting proteins can promote their ubiquitination and degradation and reverse inhibit tumor growth. This can be achieved by using NO scavengers or NOS inhibitors to reduce the activity of certain pro-cancer proteins and using exogenous GSNOR to enhance cell denitrification and inhibit tumor growth. Investigating the role of S-nitrosylation in tumorigenesis can help identify potential targets for early intervention. For patients already diagnosed with cancer, targeting S-nitrosylation-modified proteins may provide new therapeutic options and help delay the onset of drug resistance.
Metabolic diseases
Diabetes mellitus is closely linked to disrupted S-nitrosylation, which affects all stages of insulin activity, from its synthesis and secretion to signaling and degradation in target tissues (99). This regulation plays an important role in insulin-related metabolic disorders. In type 2 diabetes, insulin resistance (IR) is influenced by S-nitrosylation through three key mechanisms: i) S-nitrosylation of insulin receptor-β, insulin receptor substrate 1 and AKT1 either suppresses their activity or accelerates their degradation, thereby impairing insulin signaling. ii) S-nitrosylation of PPARγ and PDE3B reduces their activity, weakening adipocyte function and contributing to adipocyte IR. iii) S-nitrosylation of SIRT1 increases acetylation of NF-κB p65, boosting NF-κB's transcriptional activity, which triggers pro-inflammatory gene expression and worsens IR.
More recently, attention has turned to the role of S-nitrosylation in cellular metabolism, particularly in relation to type 2 diabetes and insulin resistance. Zhou et al (100) demonstrated that insulin signaling is directly tied to protein S-nitrosylation, where NO covalently binds to cysteine residues to form SNOs, serving as functional effectors of insulin action. This finding highlights the relevance of S-nitrosylation in metabolic regulation and supports its growing importance in diabetes research.
Zhou et al (99) identified a new enzyme, S-nitro-coenzyme A-assisted nitrotransferase (SCAN), which uses SNO-CoA as a cofactor to S-nitrosylate various target proteins. SCAN has been shown to regulate insulin signaling under normal conditions, while its dysregulation may contribute to the development of diabetes. This discovery advances the understanding of islet signaling pathways and suggests new possibilities for treating diseases such as obesity and diabetes. In addition, a number of individuals with diabetes develop neuropathy. Li et al (101) found that in the hippocampus of diabetic rats, defective autophagy caused by S-nitrosylation of the ATG4B protein plays a significant role in CNS neuropathy. This finding reveals a novel mechanism involved in diabetes-related CNS complications.
Carvalho-filho et al (102) indicate that aspirin treatment not only reduces iNOS protein levels but also S-nitrosylation of IRβ, IRS-1 and Akt. These changes are associated with improved insulin resistance and signaling, suggesting a novel mechanism of insulin sensitization evoked by aspirin treatment. These findings also suggest that inhibition of iNOS might be a major mediator of the insulin-sensitizing effects of IKKβ inhibition. In conclusion, targeting S-nitrosylation presents an opportunity for novel or refined drug development that could provide a novel and more precise approach for the management of metabolic diseases.
Fig. 4 systematically summarized the aforementioned 24 studies related to 'S-nitrosylation modification and disease occurrence and development'. Among them, 16 studies have shown that NO-mediated S-nitrosylation modification of target proteins has a promoting effect on the occurrence of diseases. For example, in a study where the protein S-nitrosylation of HDAC2 plays a key role in the development of DD, NO reduction and protein denitrosylation markedly improved the mitral early wave/mitral annulus movement ratio and the effect on movement time in DD mice, but it did not markedly affect the ejection fraction (75). The results indicated that the denitrosylation of HDAC2 induced by antioxidants could alleviate DD. Furthermore, in another study to determine whether the level of SNO-Prx2 in the brains of Patients with PD has pathophysiological significance, the researchers found that the ratio of SNO-Prx2 to total Prx2 was comparable to the ratio of cell death in PD cell-based Models (103). The results indicated that there were pathophysiological-related amounts of SNO-Prx2 in the human brain of patients with PD and the production of SNO-Prx2, interfering with the normal antioxidant system, might lead to neuronal cell death. Conversely, eight studies have shown that S-nitrosylation modification of target proteins has an inhibitory effect on the occurrence of diseases. For instance, Microcystins-LR induces apoptosis of human colon cancer cells (SW480) through the SNO-GAPDH-Siah1 signaling pathway (with significant differences between the treatment group and the control group) (104). This study provides further understanding of the in vitro molecular mechanism of Microcystins-LR-induced colorectal toxicity. Finally, it is worth noting that in metabolic system diseases, all six studies revealed that S-nitrosylation modification of target proteins can aggravate insulin resistance. Therefore, targeted inhibition of S-nitrosylated proteins may be an effective strategy for treating metabolic system diseases.
Conclusions and future perspectives
Protein S-nitrosylation, a covalent modification involving the addition of NO to reactive cysteine thiols, plays a critical role in redox-based signaling and contributes to altered signaling under pathological conditions. This modification changes protein structure and function, influencing bioactivity, stability, subcellular distribution and interactions with other proteins, thereby affecting a broad range of physiological and pathological processes (25). The cellular level of S-nitrosylation is controlled by the dynamic balance between S-nitrosylation and denitrosylation in both space and time. Disruption of this balance is linked to the development of multiple diseases.
At present, there is an ongoing debate over the dual roles of S-nitrosylation in disease, where it may act as either a protective or pathogenic factor depending on context. For instance, the same protein may be beneficial in one disease and harmful in another when modified by S-nitrosylation (105). Moreover, technical limitations in current detection methods raise concerns. The biotin switch assay, for example, may generate false positives due to the nonspecific reduction of disulfide bonds by ascorbic acid (46). There are also significant gaps in understanding, such as the inability to distinguish the functional impact of SNOs at different cysteine residues within the same protein (75). Most studies capture only static snapshots of nitrosylation, failing to reflect real-time fluctuations. The inherent instability of SNOs and the absence of reliable biomarkers further complicate clinical translation. Furthermore, the molecular reaction mechanism of denitrosylation, its role and its relationship with nitrosylation remain unclear. Meanwhile, the level of nitrosylation in vivo and different tissues determines the different physiological roles it plays. Improving our understanding of the protein nitrosylation network requires a comprehensive and systematic analysis using an interdisciplinary approach that integrates insights from biochemistry, molecular biology and pharmacology, aiming to provide new solutions and directions for the clinical control and treatment of various diseases caused by the imbalance of protein nitrosylation levels.
To date, no theranostic strategies have been reported for addressing dysregulated SNO in disease models. Given the challenges associated with current detection technologies, limited sensitivity, poor stability, interference issues and cumbersome protocols, future research should aim to develop a highly specific, real-time detection platform. Integrating nanomaterials, microfluidics and AI-driven analysis may provide tools capable of meeting the demands of environmental monitoring, drug safety testing and clinical diagnostics. Ye et al (106) developed a theranostic SNO probe, P-EHC, capable of detecting SNO level changes in cells under oxygen-glucose deprivation/reperfusion (OGD/R) conditions while simultaneously enhancing cell viability. To the best of the authors' knowledge, this is the first report of such a concept. P-EHC serves as a powerful tool for analyzing SNO level dynamics in OGD/R cells and offers new perspectives for the treatment of ischemia-reperfusion diseases.
Due to the limitations of animal models (such as species differences and inability to simulate the dynamic changes of the microenvironment) as well as the bottlenecks in clinical sample analysis (such as poor sample stability and lack of standardized detection procedures), there are obstacles between disease models and clinical translation. Further work should also address the dynamic nature of nitrosylation, the mismatch between site-specificity and disease complexity and how these influence downstream biochemical responses. Only through cross-disciplinary and multi-scale collaboration can we uncover how S-nitrosylation initiates, drives and worsens disease processes, ultimately enabling the development of targeted, effective therapeutic interventions.
Availability of data and materials
Not applicable.
Authors' contributions
YX performed the literature search and drafted the manuscript. XW, XZ, LP, JY, YZ and NW retrieved the relevant literature. JY were major contributors to revision of the manuscript. Data authentication is not applicable. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Abbreviations:
|
AD |
Alzheimer's disease |
|
AGT |
angiotensinogen |
|
ANT1 |
adenine nucleotide translocator 1 |
|
ATP |
adenosine triphosphate |
|
cGMP |
cyclic guanosine monophosphate |
|
CTSB |
recombinant cathepsin B |
|
DD |
diastolic dysfunction |
|
Drp1 |
dynamin-related protein 1 |
|
ED |
endothelial dysfunction |
|
GSNOR |
S-Nitrosoglutathione Reductase |
|
IR |
insulin resistance |
|
NF-κB |
nuclear factor kappa-B |
|
NO |
nitric oxide |
|
NOS |
nitric oxide synthase |
|
NSCLC |
non-small cell carcinoma |
|
PD |
Parkinson's disease |
|
PDAC |
pancreatic ductal adenocarcinoma |
|
PTM |
post-translational modification |
|
SCAN |
S-nitro-coenzyme A-assisted nitrotransferase |
|
SNO |
S-nitrosothiol |
|
TRX |
thioredoxin reductase |
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China, (grant no. 32060232); the Natural Science Foundation of Jiangxi Province, (grant no. 20212BAB206075); the Foundation of Jiangxi Provincial Key Laboratory of Tissue Engineering (grant no. 2024SSY06291); the Foundation of Technology Innovation Team of First Affiliated Hospital of Gannan Medical University (grant no. 2021CXTD-08); the Science and Technology Project of Ganzhou (grant no. 202101034530), First Affiliated Hospital of Gannan Medical University, Doctor Start-up Fund (grant no. QD076) and the Stem Cell Clinical Research Bi-Filing Project of First Affiliated Hospital of Gannan Medical University (grant no. SC-BiFR-001).
References
|
Fan S, Kong C, Zhou R, Zheng X, Ren D and Yin Z: Protein post-translational modifications based on proteomics: A potential regulatory role in animal science. J Agric Food Chem. 72:6077–6088. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Nakamura T and Lipton SA: Nitrosative stress in the nervous system: Guidelines for designing experimental strategies to study protein S-Nitrosylation. Neurochem Res. 41:510–514. 2016. View Article : Google Scholar | |
|
Zhao Q, Ma J, Xie F, Wang Y, Zhang Y, Li H, Sun Y, Wang L, Guo M and Han K: Recent advances in predicting protein S-nitrosylation sites. Biomed Res Int. 2021:55422242021. View Article : Google Scholar : PubMed/NCBI | |
|
Kaya E, Zinnuroglu M and Tugcu I: Kinesio taping compared to physical therapy modalities for the treatment of shoulder impingement syndrome. Clin Rheumatol. 30:201–207. 2011. View Article : Google Scholar | |
|
Yu B, Ichinose F, Bloch DB and Zapol WM: Inhaled nitric oxide. Br J Pharmacol. 176:246–255. 2019. View Article : Google Scholar | |
|
Lundberg JO and Weitzberg E: Nitric oxide signaling in health and disease. Cell. 185:2853–2878. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Andrabi SM, Sharma NS, Karan A, Shahriar SMS, Cordon B, Ma B and Xie J: Nitric oxide: Physiological functions, delivery, and biomedical applications. Adv Sci (Weinh). 10:e23032592023. View Article : Google Scholar : PubMed/NCBI | |
|
Alderton WK, Cooper CE and Knowles RG: Nitric oxide synthases: Structure, function and inhibition. Biochemical J. 357:593–615. 2001. View Article : Google Scholar | |
|
Förstermann U and Sessa WC: Nitric oxide synthases: Regulation and function. Eur Heart J. 33:829–837. 2012. View Article : Google Scholar : | |
|
Guo Y, Wen J, He A, Qu C, Peng Y, Luo S and Wang X: iNOS contributes to heart failure with preserved ejection fraction through mitochondrial dysfunction and Akt S-nitrosylation. J Adv Res. 43:175–186. 2023. View Article : Google Scholar : | |
|
Anavi S and Tirosh O: iNOS as a metabolic enzyme under stress conditions. Free Radical Biol Med. 146:16–35. 2020. View Article : Google Scholar | |
|
Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS and Sessa WC: Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 101:731–736. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Radomski MW, Palmer RM and Moncada S: The antiaggregating properties of vascular endothelium: Interactions between prostacyclin and nitric oxide. Br J Pharmacol. 92:639–646. 1987. View Article : Google Scholar : PubMed/NCBI | |
|
Kubes P, Suzuki M and Granger DN: Nitric oxide: An endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 88:4651–4655. 1991. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou L and Zhu DY: Neuronal nitric oxide synthase: Structure, subcellular localization, regulation, and clinical implications. Nitric Oxide. 20:223–230. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Schwarz PM, Kleinert H and Förstermann U: Potential functional significance of brain-type and muscle-type nitric oxide synthase I expressed in adventitia and media of rat aorta. Arterioscler Thromb Vasc Biol. 19:2584–2590. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Fernando V, Zheng X, Walia Y, Sharma V, Letson J and Furuta S: S-Nitrosylation: An emerging paradigm of redox signaling. Antioxidants (Basel). 8:4042019. View Article : Google Scholar : PubMed/NCBI | |
|
Martínez-Ruiz A, Araújo IM, Izquierdo-Álvarez A, Hernansanz-Agustín P, Lamas S and Serrador J: Specificity in S-nitrosylation: A short-range mechanism for NO signaling? Antioxid Redox Signal. 19:1220–1235. 2013. View Article : Google Scholar : | |
|
Tegeder I: Nitric oxide mediated redox regulation of protein homeostasis. Cell Signal. 53:348–356. 2019. View Article : Google Scholar | |
|
Bradley SA and Steinert JR: Nitric oxide-mediated posttranslational modifications: Impacts at the synapse. Oxid Med Cell Longev. 2016:56810362016. View Article : Google Scholar | |
|
Penna C, Sorge M, Femminò S, Pagliaro P and Brancaccio M: Redox aspects of chaperones in cardiac function. Front Physiol. 9:2162018. View Article : Google Scholar : PubMed/NCBI | |
|
Sun J, Steenbergen C and Murphy E: S-nitrosylation: NO-related redox signaling to protect against oxidative stress. Antioxid Redox Signal. 8:1693–1705. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Sharma V, Fernando V, Letson J, Walia Y, Zheng X, Fackelman D and Furuta S: S-Nitrosylation in tumor microenvironment. Int J Mol Sci. 22:46002021. View Article : Google Scholar : PubMed/NCBI | |
|
Anand P, Hausladen A, Wang YJ, Zhang GF, Stomberski C, Brunengraber H, Hess DT and Stamler JS: Identification of S-nitroso-CoA reductases that regulate protein S-nitrosylation. Proc Natl Acad Sci USA. 111:18572–18577. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Hess DT and Stamler JS: Regulation by S-nitrosylation of protein post-translational modification. J Biol Chem. 287:4411–4418. 2012. View Article : Google Scholar : | |
|
Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, Singel DJ and Loscalzo J: S-nitrosylation of proteins with nitric oxide: Synthesis and characterization of biologically active compounds. Proc Natl Acad Sci USA. 89:444–448. 1992. View Article : Google Scholar : PubMed/NCBI | |
|
Hess DT, Matsumoto A, Kim SO, Marshall HE and Stamler JS: Protein S-nitrosylation: Purview and parameters. Nat Rev Mol Cell Biol. 6:150–166. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Lancaster JR Jr: Nitric oxide: A brief overview of chemical and physical properties relevant to therapeutic applications. Future Sci OA. 1:Fso592015. View Article : Google Scholar : PubMed/NCBI | |
|
Nakamura T and Lipton SA: Protein S-Nitrosylation as a therapeutic target for neurodegenerative diseases. Trends Pharmacol Sci. 37:73–84. 2016. View Article : Google Scholar : | |
|
Möller MN, Li Q, Vitturi DA, Robinson JM, Lancaster JR Jr and Denicola A: Membrane 'lens' effect: Focusing the formation of reactive nitrogen oxides from the *NO/O2 reaction. Chem Res Toxicol. 20:709–714. 2007. View Article : Google Scholar | |
|
Jia J, Arif A, Terenzi F, Willard B, Plow EF, Hazen SL and Fox PL: Target-selective protein S-nitrosylation by sequence motif recognition. Cell. 159:623–634. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Cheng S, Shi T, Wang XL, Liang J, Wu H, Xie L, Li Y and Zhao YL: Features of S-nitrosylation based on statistical analysis and molecular dynamics simulation: Cysteine acidity, surrounding basicity, steric hindrance and local flexibility. Mol Biosyst. 10:2597–2606. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Pérez-Mato I, Castro C, Ruiz FA, Corrales FJ and Mato JM: Methionine adenosyltransferase S-nitrosylation is regulated by the basic and acidic amino acids surrounding the target thiol. J Biol Chem. 274:17075–17079. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Doulias PT, Greene JL, Greco TM, Tenopoulou M, Seeholzer SH, Dunbrack RL and Ischiropoulos H: Structural profiling of endogenous S-nitrosocysteine residues reveals unique features that accommodate diverse mechanisms for protein S-nitrosylation. Proc Natl Acad Sci USA. 107:16958–16963. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Beltrán B, Orsi A, Clementi E and Moncada S: Oxidative stress and S-nitrosylation of proteins in cells. Br J Pharmacol. 129:953–960. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Treuer AV and Gonzalez DR: Nitric oxide synthases, S-nitrosylation and cardiovascular health: From molecular mechanisms to therapeutic opportunities (review). Mol Med Rep. 11:1555–1565. 2015. View Article : Google Scholar | |
|
Rizza S and Filomeni G: Chronicles of a reductase: Biochemistry, genetics and physio-pathological role of GSNOR. Free Radic Biol Med. 110:19–30. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Tang X, Zhao S, Liu J, Liu X, Sha X, Huang C, Hu L, Sun S, Gao Y, Chen H, et al: Mitochondrial GSNOR alleviates cardiac dysfunction via ANT1 denitrosylation. Circ Res. 133:220–236. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Sengupta R, Ryter SW, Zuckerbraun BS, Tzeng E, Billiar TR and Stoyanovsky DA: Thioredoxin catalyzes the denitrosation of low-molecular mass and protein S-nitrosothiols. Biochemistry. 46:8472–8483. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Kalinina E and Novichkova M: Glutathione in protein redox modulation through S-Glutathionylation and S-Nitrosylation. Molecules. 26:4352021. View Article : Google Scholar : PubMed/NCBI | |
|
Chen L, Wu R, Feng J, Feng T, Wang C, Hu J, Zhan N, Li Y, Ma X, Ren B, et al: Transnitrosylation mediated by the non-canonical catalase ROG1 regulates nitric oxide signaling in plants. Dev Cell. 53:444–457. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Furuta S: Basal S-Nitrosylation is the guardian of tissue homeostasis. Trends Cancer. 3:744–748. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Murray CI, Uhrigshardt H, O'meally RN, Cole RN and Van Eyk JE: Identification and quantification of S-nitrosylation by cysteine reactive tandem mass tag switch assay. Mol Cell Proteomics. 11:M111.0134412012. View Article : Google Scholar : | |
|
Liu LS, Ma H, Zhu JY, Han XX and Zhao B: Quantification of protein S-nitrosylation probed by resonance Raman spectroscopy. Spectroscopy and Spectral Analysis. 40:141–142. 2020.In Chinese. | |
|
Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P and Snyder SH: Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat Cell Biol. 3:193–197. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Forrester MT, Foster MW, Benhar M and Stamler JS: Detection of protein S-nitrosylation with the biotin-switch technique. Free Radic Biol Med. 46:119–126. 2009. View Article : Google Scholar | |
|
Qu Z, Meng F, Bomgarden RD, Viner RI, Li J, Rogers JC, Cheng J, Greenlief CM, Cui J, Lubahn DB, et al: Proteomic quantification and site-mapping of S-nitrosylated proteins using isobaric iodoTMT reagents. J Proteome Res. 13:3200–3211. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Mnatsakanyan R, Markoutsa S, Walbrunn K, Roos A, Verhelst SHL and Zahedi RP: Proteome-wide detection of S-nitrosylation targets and motifs using bioorthogonal cleavable-linker-based enrichment and switch technique. Nat Commun. 10:21952019. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang L, Shang P, Chen C, Zhou J and Zhu S: Surface plasmon resonance spectroscopy for detection of S-nitrosylated proteins. Methods Mol Biol. 1747:103–111. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Qin G, Qu M, Jia B, Wang W, Luo Z, Song CP, Tao WA and Wang P: FAT-switch-based quantitative S-nitrosoproteomics reveals a key role of GSNOR1 in regulating ER functions. Nat Commun. 14:32682023. View Article : Google Scholar : PubMed/NCBI | |
|
Chen YJ, Ching WC, Lin YP and Chen Y: Methods for detection and characterization of protein S-nitrosylation. Methods. 62:138–150. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K and Yamamoto M: Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 26:221–229. 2006. View Article : Google Scholar : | |
|
Morris G, Walder K, Carvalho AF, Tye SJ, Lucas K, Berk M and Maes M: The role of hypernitrosylation in the pathogenesis and pathophysiology of neuroprogressive diseases. Neurosci Biobehav Rev. 84:453–469. 2018. View Article : Google Scholar | |
|
Palmieri MC, Lindermayr C, Bauwe H, Steinhauser C and Durner J: Regulation of plant glycine decarboxylase by s-nitrosylation and glutathionylation. Plant Physiol. 152:1514–1528. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Gietler M, Nykiel M, Orzechowski S, Fettke J and Zagdańska B: Proteomic analysis of S-nitrosylated and S-glutathionylated proteins in wheat seedlings with different dehydration tolerances. Plant Physiol Biochem. 108:507–518. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Lin A, Wang Y, Tang J, Xue P, Li C, Liu L, Hu B, Yang F, Loake GJ and Chu C: Nitric oxide and protein S-nitrosylation are integral to hydrogen peroxide-induced leaf cell death in rice. Plant Physiol. 158:451–464. 2012. View Article : Google Scholar : | |
|
Ortega-Galisteo AP, Rodríguez-Serrano M, Pazmiño DM, Gupta DK, Sandalio LM and Romero-Puertas MC: S-Nitrosylated proteins in pea (Pisum sativum L.) leaf peroxisomes: Changes under abiotic stress. J Exp Bot. 63:2089–2103. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Montrichard F, Alkhalfioui F, Yano H, Vensel WH, Hurkman WJ and Buchanan B: Thioredoxin targets in plants: The first 30 years. J Proteomics. 72:452–474. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Wang W, Vinocur B, Shoseyov O and Altman A: Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 9:244–252. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Balmant KM, Parker J, Yoo MJ, Zhu N, Dufresne C and Chen S: Redox proteomics of tomato in response to Pseudomonas syringae infection. Hortic Res. 2:150432015. View Article : Google Scholar : PubMed/NCBI | |
|
Ozawa K, Komatsubara AT, Nishimura Y, Sawada T, Kawafune H, Tsumoto H, Tsuji Y, Zhao J, Kyotani Y, Tanaka T, et al: S-nitrosylation regulates mitochondrial quality control via activation of parkin. Sci Rep. 3:22022013. View Article : Google Scholar : PubMed/NCBI | |
|
Matthews JR, Botting CH, Panico M, Morris HR and Hay RT: Inhibition of NF-kappaB DNA binding by nitric oxide. Nucleic Acids Res. 24:2236–2242. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, Vujaskovic Z, Dewhirst MW and Li CY: Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell. 26:63–74. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Fourquet S, Guerois R, Biard D and Toledano MB: Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J Biol Chem. 285:8463–8471. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Kim SO, Merchant K, Nudelman R, Beyer WF Jr, Keng T, DeAngelo J, Hausladen A and Stamler J: OxyR: A molecular code for redox-related signaling. Cell. 109:383–396. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Barrett DM, Black SM, Todor H, Schmidt-Ullrich RK, Dawson KS and Mikkelsen RB: Inhibition of protein-tyrosine phosphatases by mild oxidative stresses is dependent on S-nitrosylation. J Biol Chem. 280:14453–14461. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Caviedes A, Maturana B, Corvalán K, Engler A, Gordillo F, Varas-Godoy M, Smalla KH, Batiz LF, Lafourcade C, Kaehne T and Wyneken U: eNOS-dependent S-nitrosylation of the NF-κB subunit p65 has neuroprotective effects. Cell Death Dis. 12:42021. View Article : Google Scholar | |
|
Sanhueza C, Bennett JC, Valenzuela-Valderrama M, Contreras P, Lobos-González L, Campos A, Wehinger S, Lladser Á, Kiessling R, Leyton L and Quest AFG: Caveolin-1-mediated tumor suppression is linked to reduced HIF1α S-Nitrosylation and transcriptional activity in hypoxia. Cancers (Basel). 12:23492020. View Article : Google Scholar | |
|
Yi W, Zhang Y, Liu B, Zhou Y, Liao D, Qiao X, Gao D, Xie T, Yao Q, Zhang Y, et al: Protein S-nitrosylation regulates proteostasis and viability of hematopoietic stem cell during regeneration. Cell Rep. 34:1089222021. View Article : Google Scholar : PubMed/NCBI | |
|
Lin Z, Zhao S, Li X, Miao Z, Cao J, Chen Y, Shi Z, Zhang J, Wang D, Chen S, et al: Cathepsin B S-nitrosylation promotes ADAR1-mediated editing of its own mRNA transcript via an ADD1/MATR3 regulatory axis. Cell Res. 33:546–561. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Ryan SD, Dolatabadi N, Chan SF, Zhang X, Akhtar MW, Parker J, Soldner F, Sunico CR, Nagar S, Talantova M, et al: Isogenic human iPSC Parkinson's model shows nitrosative stress-induced dysfunction in MEF2-PGC1α transcription. Cell. 155:1351–1364. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou HL, Zhang R, Anand P, Stomberski CT, Qian Z, Hausladen A, Wang L, Rhee EP, Parikh SM, Karumanchi SA and Stamler JS: Metabolic reprogramming by the S-nitroso-CoA reductase system protects against kidney injury. Nature. 565:96–100. 2019. View Article : Google Scholar | |
|
Foster MW, Mcmahon TJ and Stamler JS: S-nitrosylation in health and disease. Trends Mol Med. 9:160–168. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Foster MW, Hess DT and Stamler JS: Protein S-nitrosylation in health and disease: A current perspective. Trends Mol Med. 15:391–404. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Yoon S, Kim M, Lee H, Kang G, Bedi K, Margulies KB, Jain R, Nam KI, Kook H and Eom GH: S-Nitrosylation of histone deacetylase 2 by neuronal nitric oxide synthase as a mechanism of diastolic dysfunction. Circulation. 143:1912–1925. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Nogi M, Satoh K, Sunamura S, Kikuchi N, Satoh T, Kurosawa R, Omura J, Elias-Al-Mamun M, Siddique MA, Numano K, et al: Small GTP-binding protein GDP dissociation stimulator prevents thoracic aortic aneurysm formation and rupture by phenotypic preservation of aortic smooth muscle cells. Circulation. 138:2413–2433. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Pan L, Lin Z, Tang X, Tian J, Zheng Q, Jing J, Xie L, Chen H, Lu Q, Wang H, et al: S-Nitrosylation of plastin-3 exacerbates thoracic aortic dissection formation via endothelial barrier dysfunction. Arterioscler Thromb Vasc Biol. 40:175–188. 2020. View Article : Google Scholar | |
|
Zhang Y, Zhang H, Zhao S, Qi Z, He Y, Zhang X, Wu W, Yan K, Hu L, Sun S, et al: S-Nitrosylation of Septin2 exacerbates aortic aneurysm and dissection by coupling the TIAM1-RAC1 axis in macrophages. Circulation. 149:1903–1920. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Nakamura T, Tu S, Akhtar MW, Sunico CR, Okamoto S and Lipton SA: Aberrant protein s-nitrosylation in neurodegenerative diseases. Neuron. 78:596–614. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Okamoto S, Nakamura T, Cieplak P, Chan SF, Kalashnikova E, Liao L, Saleem S, Han X, Clemente A, Nutter A, et al: S-nitrosylation-mediated redox transcriptional switch modulates neurogenesis and neuronal cell death. Cell Rep. 8:217–228. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z and Lipton SA: S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 324:102–105. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Wang X, Su B, Lee HG, Li X, Perry G, Smith MA and Zhu X: Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci. 29:9090–9103. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Nakamura T, Oh CK, Liao L, Zhang X, Lopez KM, Gibbs D, Deal AK, Scott HR, Spencer B, Masliah E, et al: Noncanonical transnitrosylation network contributes to synapse loss in Alzheimer's disease. Science. 371:eaaw08432021. View Article : Google Scholar | |
|
Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL and Dawson TM: S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 304:1328–1331. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Tsang AH, Lee YI, Ko HS, Savitt JM, Pletnikova O, Troncoso JC, Dawson VL, Dawson TM and Chung KK: S-nitrosylation of XIAP compromises neuronal survival in Parkinson's disease. Proc Natl Acad Sci USA. 106:4900–4905. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Jin L, Cao Y, Zhang T, Wang P, Ji D, Liu X, Shi H, Hua L, Yu R and Gao S: Effects of ERK1/2 S-nitrosylation on ERK1/2 phosphorylation and cell survival in glioma cells. Int J Mol Med. 41:1339–1348. 2018. | |
|
Shen X, Burguillos MA, Osman AM, Frijhoff J, Carrillo-Jiménez A, Kanatani S, Augsten M, Saidi D, Rodhe J, Kavanagh E, et al: Glioma-induced inhibition of caspase-3 in microglia promotes a tumor-supportive phenotype. Nat Immunol. 17:1282–1290. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Qiu F, Liu Y and Liu Z: The role of protein S-nitrosylation in mitochondrial quality control in central nervous system Diseases. Aging Dis. 25: View Article : Google Scholar : 2024. | |
|
Yao D, Gu Z, Nakamura T, Shi ZQ, Ma Y, Gaston B, Palmer LA, Rockenstein EM, Zhang Z, Masliah E, et al: Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci USA. 101:10810–10814. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Wang J, Yang S, He P, Schetter AJ, Gaedcke J, Ghadimi BM, Ried T, Yfantis HG, Lee DH, Gaida MM, et al: Endothelial nitric oxide synthase traffic inducer (NOSTRIN) is a negative regulator of disease aggressiveness in pancreatic cancer. Clin Cancer Res. 22:5992–6001. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Wang J, He P, Gaida M, Yang S, Schetter AJ, Gaedcke J, Ghadimi BM, Ried T, Yfantis H, Lee D, et al: Inducible nitric oxide synthase enhances disease aggressiveness in pancreatic cancer. Oncotarget. 7:52993–53004. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Tan C, Li Y, Huang X, Wei M, Huang Y, Tang Z, Huang H, Zhou W, Wang Y and Hu J: Extensive protein S-nitrosylation associated with human pancreatic ductal adenocarcinoma pathogenesis. Cell Death Dis. 10:9142019. View Article : Google Scholar : PubMed/NCBI | |
|
Deng WW, Zhou ZK, Zhang HY, Du ZX and Wang HQ: Effect of NO on apoptosis of human thyroid cancer cells induced by tumor necrosis factor-related apoptosis-inducing ligand. Chin J Cancer Prev Treat. 15:1691–1694. 2008.In Chinese. | |
|
Tang CH, Wei W, Hanes MA and Liu L: Hepatocarcinogenesis driven by GSNOR deficiency is prevented by iNOS inhibition. Cancer Res. 73:2897–2904. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Wei W, Yang Z, Tang CH and Liu L: Targeted deletion of GSNOR in hepatocytes of mice causes nitrosative inactivation of O6-alkylguanine-DNA alkyltransferase and increased sensitivity to genotoxic diethylnitrosamine. Carcinogenesis. 32:973–977. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang X, Li G, Guo Y, Song Y, Chen L, Ruan Q, Wang Y, Sun L, Hu Y, Zhou J, et al: Regulation of ezrin tension by S-nitrosylation mediates non-small cell lung cancer invasion and metastasis. Theranostics. 9:2555–2571. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Okuda K, Nakahara K, Ito A, Iijima Y, Nomura R, Kumar A, Fujikawa K, Adachi K, Shimada Y, Fujio S, et al: Pivotal role for S-nitrosylation of DNA methyltransferase 3B in epigenetic regulation of tumorigenesis. Nat Commun. 14:6212023. View Article : Google Scholar : PubMed/NCBI | |
|
Liang F, Wang M, Li J and Guo J: The evolution of S-nitrosylation detection methodology and the role of protein S-nitrosylation in various cancers. Cancer Cell Int. 24:4082024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou HL, Grimmett ZW, Venetos NM, Stomberski CT, Qian Z, McLaughlin PJ, Bansal PK, Zhang R, Reynolds JD, Premont RT and Stamler JS: An enzyme that selectively S-nitrosylates proteins to regulate insulin signaling. Cell. 186:5812–5825.e21. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou HL, Premont RT and Stamler JS: The manifold roles of protein S-nitrosylation in the life of insulin. Nat Rev Endocrinol. 18:111–128. 2022. View Article : Google Scholar : | |
|
Li Y, Zhang Y, Wang L, Wang P, Xue Y, Li X, Qiao X, Zhang X, Xu T, Liu G, et al: Autophagy impairment mediated by S-nitrosation of ATG4B leads to neurotoxicity in response to hyperglycemia. Autophagy. 13:1145–1160. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Carvalho-Filho MA, Ropelle ER, Pauli RJ, Cintra DE, Tsukumo DM, Silveira LR, Curi R, Carvalheira JB, Velloso LA and Saad MJ: Aspirin attenuates insulin resistance in muscle of diet-induced obese rats by inhibiting inducible nitric oxide synthase production and S-nitrosylation of IRbeta/IRS-1 and Akt. Diabetologia. 52:2425–2434. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Fang J, Nakamura T, Cho DH, Gu Z and Lipton SA: S-nitrosylation of peroxiredoxin 2 promotes oxidative stress-induced neuronal cell death in Parkinson's disease. Proc Natl Acad Sci USA. 104:18742–18747. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Li K, Huang M, Xu P, Wang M, Ye S, Wang Q, Zeng S, Chen X, Gao W, Chen J, et al: Microcystins-LR induced apoptosis via S-nitrosylation of GAPDH in colorectal cancer cells. Ecotoxicol Environ Saf. 190:1100962020. View Article : Google Scholar : PubMed/NCBI | |
|
Wang W, Wang D, Kong C, Li S, Xie L, Lin Z, Zheng Y, Zhou J, Han Y and Ji Y: eNOS S-nitrosylation mediated OxLDL-induced endothelial dysfunction via increasing the interaction of eNOS with β-catenin. Biochim Biophys Acta Mol Basic Dis. 1865:1793–1801. 2019. View Article : Google Scholar | |
|
Ye H, Zhang C, Li L, Li C, Yu J, Ji D, Liang Z, Wu J and Huang Z: A fluorescent probe for imaging and treating S-nitrosation stress in OGD/R cells. Antioxidants (Basel). 14:3112025. View Article : Google Scholar : PubMed/NCBI | |
|
Marley R, Feelisch M, Holt S and Moore K: A chemiluminescense-based assay for S-nitrosoalbumin and other plasma S-nitrosothiols. Free Radic Res. 32:1–9. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Gow AJ, Chen Q, Hess DT, Day BJ, Ischiropoulos H and Stamler JS: Basal and stimulated protein S-nitrosylation in multiple cell types and tissues. J Biol Chem. 277:9637–9640. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Forrester MT, Thompson JW, Foster MW, Nogueira L, Moseley MA and Stamler JS: Proteomic analysis of S-nitrosylation and denitrosylation by resin-assisted capture. Nat Biotechnol. 27:557–559. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Seneviratne U, Nott A, Bhat VB, Ravindra KC, Wishnok JS, Tsai LH and Tannenbaum SR: S-nitrosation of proteins relevant to Alzheimer's disease during early stages of neurodegeneration. Proc Natl Acad Sci USA. 113:4152–4157. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Liu J: Effects and mechanisms of S-nitrosylated ANT1 on pathological cardiac hypertrophy. Nanjing Medical University; Master's thesis. 2017, In Chinese. | |
|
Zamorano P, Marín N, Córdova F, Aguilar A, Meininger C, Boric MP, Golenhofen N, Contreras JE, Sarmiento J, Durán WN and Sánchez FA: S-nitrosylation of VASP at cysteine 64 mediates the inflammation-stimulated increase in microvascular permeability. Am J Physiol Heart Circ Physiol. 313:H66–H71. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Zhang Y, Zhang Y, Lü S, Miao Y, Yang J, Huang S, Ma X, Han L, Deng J, et al: GSNOR modulates hyperhomocysteinemia-induced T cell activation and atherosclerosis by switching Akt S-nitrosylation to phosphorylation. Redox Biol. 17:386–399. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Majumdar U, Manivannan S, Basu M, Ueyama Y, Blaser MC, Cameron E, McDermott MR, Lincoln J, Cole SE, Wood S, et al: Nitric oxide prevents aortic valve calcification by S-nitrosylation of USP9X to activate NOTCH signaling. Sci Adv. 7:eabe37062021. View Article : Google Scholar : PubMed/NCBI | |
|
Chao ML, Luo S, Zhang C, Zhou X, Zhou M, Wang J, Kong C, Chen J, Lin Z, Tang X, et al: S-nitrosylation-mediated coupling of G-protein alpha-2 with CXCR5 induces Hippo/YAP-dependent diabetes-accelerated atherosclerosis. Nat Commun. 12:44522021. View Article : Google Scholar : PubMed/NCBI | |
|
Martínez-Ruiz A, Villanueva L, González De Orduña C, López-Ferrer D, Higueras MA, Tarín C, Rodríguez-Crespo I, Vázquez J and Lamas S: S-nitrosylation of Hsp90 promotes the inhibition of its ATPase and endothelial nitric oxide synthase regulatory activities. Proc Natl Acad Sci USA. 102:8525–8530. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Hara MR, Thomas B, Cascio MB, Bae BI, Hester LD, Dawson VL, Dawson TM, Sawa A and Snyder SH: Neuroprotection by pharmacologic blockade of the GAPDH death cascade. Proc Natl Acad Sci USA. 103:3887–3889. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Kwak YD, Ma T, Diao S, Zhang X, Chen Y, Hsu J, Lipton SA, Masliah E, Xu H and Liao FF: NO signaling and S-nitrosylation regulate PTEN inhibition in neurodegeneration. Mol Neurodegener. 5:492010. View Article : Google Scholar : PubMed/NCBI | |
|
Nakamura T, Wang L, Wong CC, Scott FL, Eckelman BP, Han X, Tzitzilonis C, Meng F, Gu Z, Holland EA, et al: Transnitrosylation of XIAP regulates caspase-dependent neuronal cell death. Mol Cell. 39:184–195. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Abrams AJ, Farooq A and Wang G: S-nitrosylation of ApoE in Alzheimer's disease. Biochemistry. 50:3405–3407. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Qu J, Nakamura T, Cao G, Holland EA, McKercher SR and Lipton SA: S-Nitrosylation activates Cdk5 and contributes to synaptic spine loss induced by beta-amyloid peptide. Proc Natl Acad Sci USA. 108:14330–143305. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Kornberg MD, Sen N, Hara MR, Juluri KR, Nguyen JV, Snowman AM, Law L, Hester LD and Snyder SH: GAPDH mediates nitrosylation of nuclear proteins. Nat Cell Biol. 12:1094–1100. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Leon-Bollotte L, Subramaniam S, Cauvard O, Plenchette-Colas S, Paul C, Godard C, Martinez-Ruiz A, Legembre P, Jeannin JF and Bettaieb A: S-nitrosylation of the death receptor fas promotes fas ligand-mediated apoptosis in cancer cells. Gastroenterology. 140:2009–2018. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao Q, Zheng K, Ma C, Li J, Zhuo L, Huang W, Chen T and Jiang Y: PTPS facilitates compartmentalized LTBP1 S-nitrosylation and promotes tumor growth under hypoxia. Mol Cell. 77:95–107.e5. 2020. View Article : Google Scholar |