Mechanisms of action of retinal microglia in diabetic retinopathy (Review)
- Authors:
- Published online on: September 22, 2025 https://doi.org/10.3892/ijmm.2025.5643
- Article Number: 202
-
Copyright : © Bai et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
Introduction
The prevalence of diabetes mellitus (DM), a global chronic metabolic disorder, is rapidly increasing in developing countries, with >400 million current global cases; approximately one-third of individuals with DM develop diabetic retinopathy (DR) (1-4). DR is characterized by progressive neurovascular damage in the retina and is clinically classified into non-proliferative DR (NPDR) and proliferative DR (PDR); the latter leads to irreversible vision loss through complications such as pathological neovascularization and vitreous haemorrhage, with global PDR cases projected to surpass 100 million by 2045 (5-9). Previous studies have reported the dual role of retinal microglia, the resident immune sentinels of the central nervous system (CNS), in DR pathogenesis (2,10). While maintaining homeostasis through metabolic waste clearance and neuroprotection, the hyperglycaemia-induced aberrant activation of microglia exacerbates inflammatory cascades and vascular injury (Fig. 1) (11-14). The application of single-cell sequencing technologies has further elucidated microglial heterogeneity and their interactive networks with retinal endothelial cells and astrocytes, providing novel insights into the molecular mechanisms underlying DR (14).
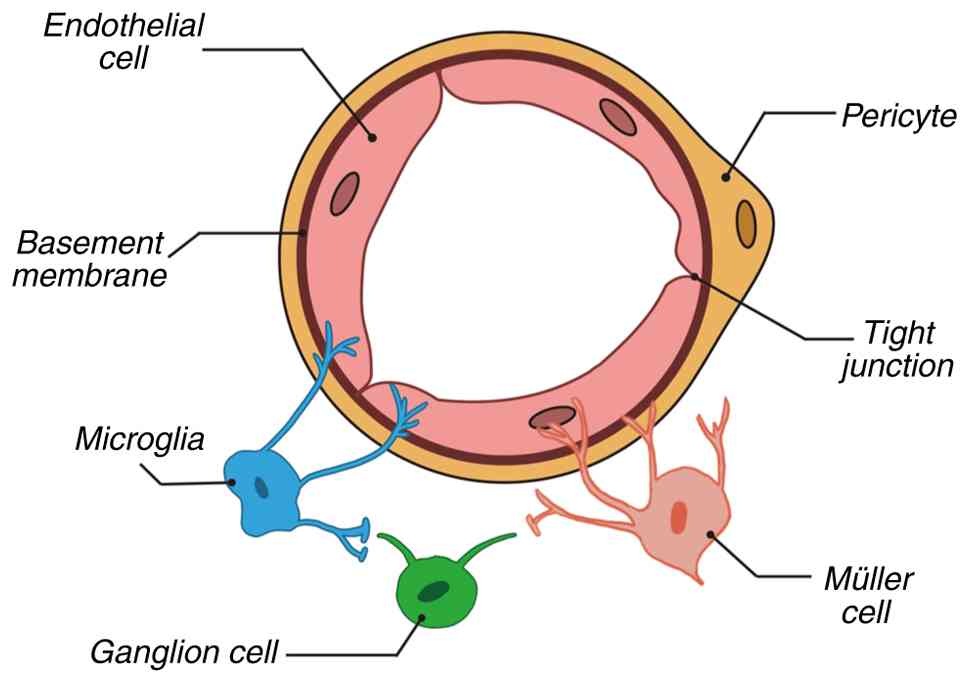
Microglia, the resident immune cells of the CNS, originate from erythromyeloid progenitors in the yolk sac during embryogenesis. These cells possess unique self-renewal capabilities and maintain homeostatic density through localized proliferation throughout their lifespan (13). In 1919, Pio del Rio-Hortega first defined microglia as a distinct population separate from neurons and other glial cells, confirming their presence in the retina (13). As members of the mononuclear phagocyte system, microglia continuously perform immune surveillance in adulthood, stabilizing the retinal microenvironment by clearing cellular debris, pathogens and abnormal proteins (15,16). A previous study reported that the functional states of microglia are closely linked to metabolic regulation and interactions with the neurovascular unit (NVU), resulting in significant dynamic alterations in pathological contexts such as DR (17). The NVU is a multicellular network of endothelial cells, pericytes, glia, neurons and the extracellular matrix that regulates blood flow and molecular exchange (Fig. 2) (18).
As a critical component of the mononuclear phagocyte system, retinal microglia colonize the retina during embryogenesis and participate in the regulation of vascular development (15,19,20). Postnatally, microglia undergo dynamic migration to establish stable spatial distribution patterns. In adulthood, microglia are widely distributed across inner retinal layers (for example, the ganglion cell layer) and outer layers (such as the outer nuclear layer), with notably reduced density in perivascular regions (21-24). This distribution is closely associated with neuronal functional activity and metabolic demands, as microglia in areas with high neuronal density predominantly exhibit a ramified morphology and maintain tissue homeostasis through continuous microenvironmental surveillance (16,25).
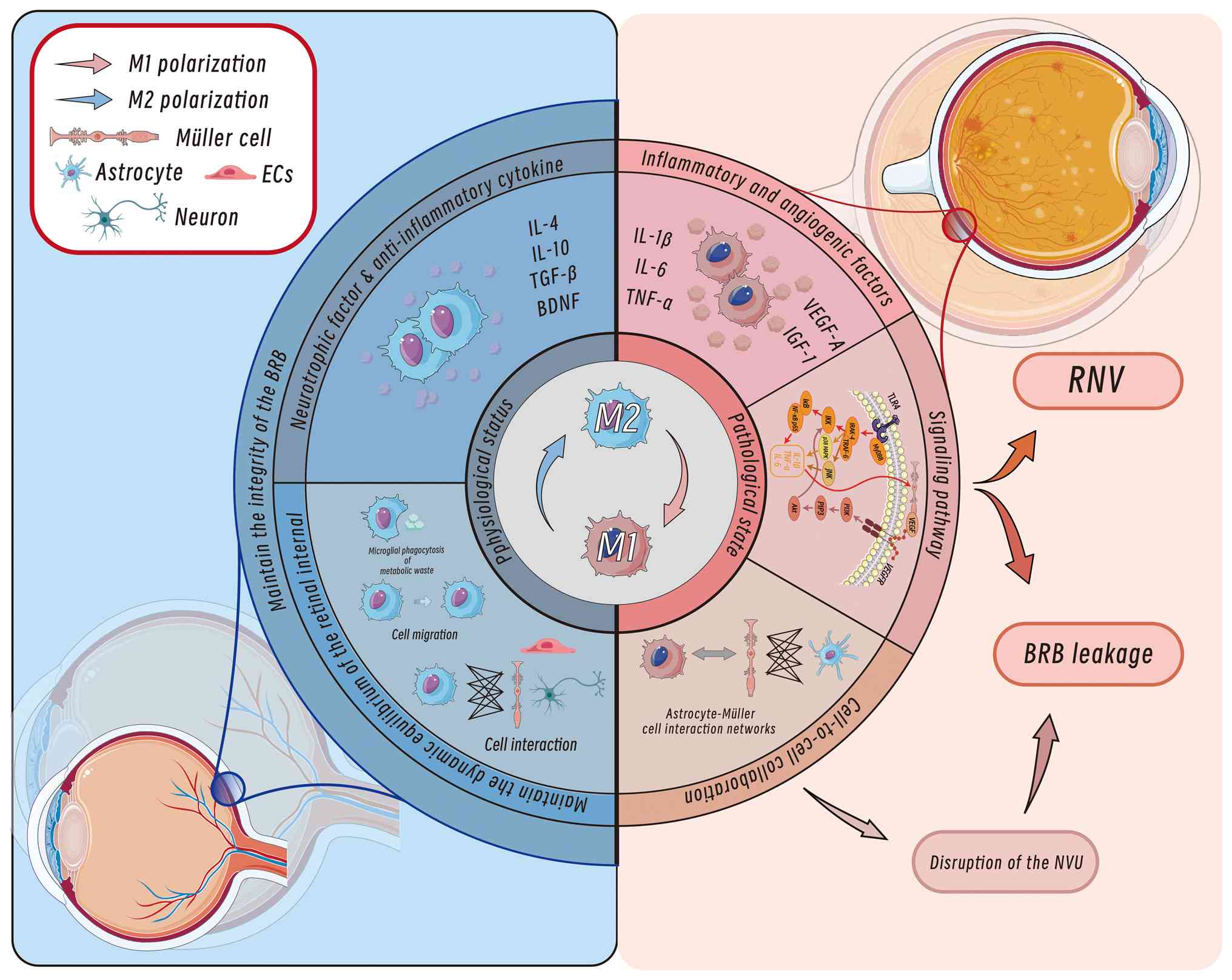
Under physiological conditions, retinal microglia sustain a dynamic equilibrium by forming a functional interaction network with neurons, vascular endothelial cells and Müller cells. The roles of retinal microglia include phagocytosing pathogens, clearing cellular debris and modulating synaptic plasticity (16,24,26). Notably, the distribution and activation states of retinal microglia are highly context dependent. For example, in pathological contexts such as DR, microglia display marked abnormalities in quantity and spatial positioning, characterized by perivascular infiltrative clustering and proinflammatory phenotype activation. These alterations suggest that disruption of microglial homeostasis may drive disease progression through mechanisms such as vascular permeability regulation and neuroinflammatory cascades (17). Given that microglial interactions with endothelial cells are integral to vascular integrity, their dysregulation may directly compromise the structural and functional stability of the blood-retinal barrier (BRB) (27).
The BRB, comprising the inner (iBRB) and outer barriers, safeguards retinal homeostasis through its NVU (18,28-30). In DR, hyperglycaemia-driven oxidative stress disrupts the iBRB, leading to pathological neovascularization characterized by fragile, leaky vessels that exacerbate macular oedema, haemorrhage and tractional retinal detachment (31,32). Central to this process are retinal microglia, the resident retinal immune sentinels, which serve dual roles in DR progression. Under physiological conditions, microglia maintain BRB integrity by modulating ocular immune privilege, phagocytosing debris and stabilizing the vascular tone through dynamic NVU interactions (33-35). However, in DR, chronic hyperglycaemia activates microglia in a proinflammatory state, triggering innate immune responses that amplify neurovascular injury, disrupt BRB function and recruit systemic immune mediators (36-39). Paradoxically, microglia also retain protective plasticity, migrating to subretinal spaces to clear cytotoxic debris, supporting retinal pigment epithelium (RPE) integrity and mitigating vascular leakage (40,41). This duality positions microglia as key regulators of retinal immune-metabolic crosstalk, balancing destructive inflammation with reparative functions and making them pivotal therapeutic targets in DR pathogenesis.
Phenotypic polarization of microglia in DR
Microglia can be classified into multiple subtypes based on morphological features, gene expression profiles, anatomical localization and functional states. The classical categorization distinguishes between M1-like and M2-like phenotypes (42). M1-polarized microglia, characterized by the expression of markers such as inducible nitric oxide synthase, CD16 and CD32, represent a 'classically activated' proinflammatory state. M1-polarized microglia secrete high levels of IL-1β, IL-6 and TNF-α. By contrast, M2-polarized microglia express markers such as CD206, arginase-1 and chitinase-like 3, exhibiting an 'alternatively activated' anti-inflammatory and tissue-reparative phenotype through the release of IL-4, IL-10 and TGF-β (43-45). In DR, microglial activation becomes imbalanced, with hyperactivated M1-like microglia predominating and driving retinal inflammation and injury (17). Consequently, modulating microglial activation may represent a key therapeutic strategy for DR. Using streptozotocin (STZ)-induced type 1 diabetes models and spontaneous type 2 diabetes models, studies have found that drugs such as minocycline and genistein can effectively inhibit microglial activation, thereby alleviating the symptoms of diabetic retinopathy (46-48). Additionally, certain pharmacological agents, such as minocycline and pioglitazone, have been shown to have retinal protective effects by regulating microglial activation states (49). These findings underscore the importance of investigating microglial activation mechanisms for developing novel DR therapies.
Advances in single-cell RNA sequencing have improved the understanding of microglial transcriptional and metabolic dynamics (50). For example, homeostatic microglia exhibit a unique transcriptional signature involving genes such as the purinergic receptor P2Y12 (P2RY12) and C-X3-C motif chemokine receptor 1 (51,52). In addition to these known factors, previous studies have identified the spalt-like transcription factor 1 as a key regulator essential for maintaining microglial identity and function, potentially restricting the transition of microglia into the disease-associated microglia (DAM) state by repressing the expression of certain DAM marker genes, such as Spp1/Osteopontin (53,54).
Although individual microglia have a finite lifespan, their self-renewal capacity compensates for cellular attrition, preserving region-specific population densities (13). Given their morphological plasticity and subtype diversity, identifying highly specific markers is crucial for precise characterization. The established microglial markers include transmembrane protein 119 (TMEM119) (55), P2RY12 (55), hexosaminidase subunit b (56,57) and ionized calcium-binding adapter molecule 1 (58). Microglia undergo adaptive morphological changes under varying conditions; healthy retinal microglia display a ramified morphology, whereas activated microglia in retinal injury adopt an amoeboid shape (59).
Mechanisms of microglial involvement in DR
Impact of a hyperglycaemic microenvironment on microglial activation
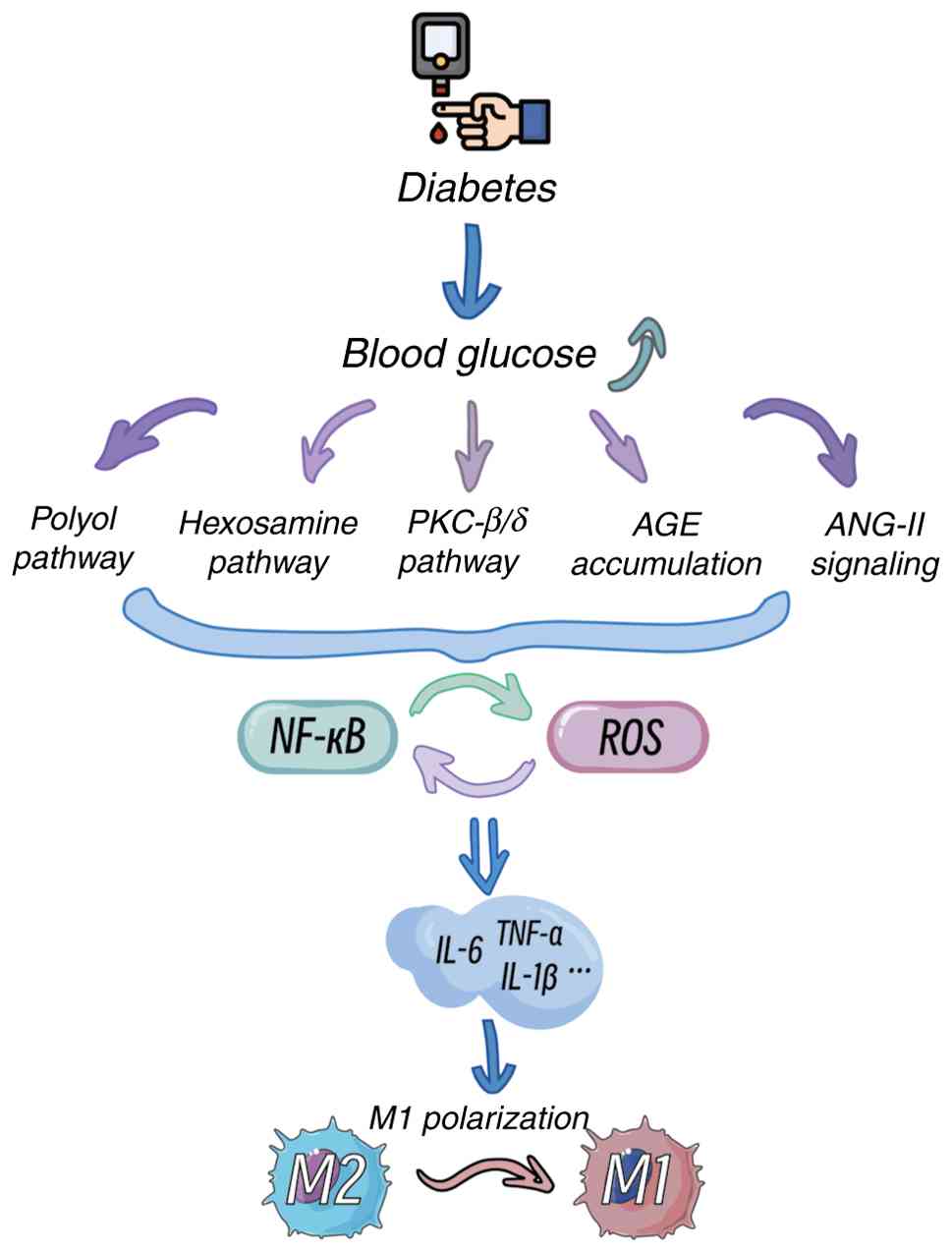
The pathogenesis of DR is closely associated with chronic hyperglycaemia (2). Hyperglycaemia induces oxidative stress and inflammatory responses through multiple pathways, including the polyol pathway, hexosamine pathway, protein kinase C (PKC) activation, angiotensin II (ANG-II) signalling and advanced glycation end product (AGE) accumulation, thereby driving microglial activation (Fig. 3) (60-63). Among these pathways, the polyol pathway increases the intracellular osmotic pressure, leading to cellular oedema and rupture, whereas activated microglia participate in inflammatory processes by inhibiting the tricarboxylic acid cycle (61). Activation of the hexosamine pathway significantly elevates reactive oxygen species (ROS) levels, enhances the proinflammatory phenotype of microglia and exacerbates damage to the retinal NVU (63). Additionally, hyperglycaemia activates the PKC-β/δ pathway, triggering p38/MAPK-mediated oxidative stress and caspase-dependent apoptotic signalling, resulting in pericyte loss and microaneurysm formation (60,64,65). ANG-II and AGEs further amplify inflammatory cascades by activating the NF-κB pathway and binding to the receptor for AGEs (RAGE), respectively, promoting VEGF release and increased vascular permeability (64,66-69).
Clinical studies have indicated that under hyperglycaemic conditions, microglia rapidly activate and release various inflammatory cytokines, such as TNF-α, IL-1β and IL-6, forming a proinflammatory cycle (70-72). In diabetic patients with poor glycaemic control, retinal microglia exhibit significantly elevated activation levels, predominantly displaying the M1 proinflammatory phenotype during early DR stages, which exacerbates local inflammation. As the disease progresses, the capacity for M2 reparative phenotypic transformation diminishes, leading to impaired retinal repair functions (73,74).
Retinal microglial effects on retinal vasculature
Hyperglycaemia-induced microglial activation serves as a central link connecting metabolic dysregulation and retinal vascular injury. Activated M1-like microglia disrupt BRB function and promote pathological neovascularization through the release of inflammatory mediators and the modulation of key signalling pathways (75,76). This process involves the destabilization of NVU homeostasis, endothelial cell dysfunction and immune microenvironment dysregulation, ultimately leading to the characteristic vascular pathologies of DR (77) Thus, microglia serve dual roles in both maintaining retinal homeostasis and driving BRB breakdown.
The dual regulatory role of retinal microglia in BRB homeostasis is central to vascular pathology in DR. While maintaining ocular immune privilege through selective molecular transport regulation (34,35), activated microglia exhibit paradoxical effects. Under hyperglycaemic conditions, M1-like microglia disrupt BRB integrity via Toll-like receptor 4 (TLR4)/myeloid differentiation primary response 88 (MyD88)/NF-κB signalling, promoting IL-1β secretion and subsequent downregulation of endothelial tight junction proteins (ZO-1 and claudin-5) (78-80). By contrast, quiescent microglia enhance BRB stability by upregulating these junctional complexes (80). This functional dichotomy extends to cellular interactions; microglial phagocytosis of endothelial cells and complement-mediated activation of RPE cells exacerbate vascular leakage, while their debris clearance and NVU maintenance functions provide protective effects (41,81-86). Pharmacological interventions, such as asiatic acid, demonstrate therapeutic potential by modulating the balance of microglial polarization in DR (79).
These microglial-mediated vascular barrier alterations create a proangiogenic microenvironment that synergistically promotes the pathological neovascularization processes. In retinal diseases, microglial activation promotes inflammatory factor production and BRB damage, leading to a hypoxic retinal microenvironment. This hypoxia further drives aberrant neovascularization, exacerbating disease progression (87).
Microglia exert both direct and indirect effects on pathological neovascularization in DR. A previous study demonstrated that microglia cultured under hypoxic conditions in vitro exhibit significantly increased expression of proangiogenic factors, particularly VEGF and insulin-like growth factor 1 (IGF-1) (88). This finding highlights that hypoxia induces microglia to upregulate these key factors, thereby promoting retinal neovascularization (90). These observations suggest that in PDR, microglia not only mediate retinal neovascularization but also amplify this process by exacerbating hypoxia (89,90).
Microglia regulate pathological neovascularization through the release of inflammatory mediators. VEGFR1 expression increases with M1-like microglial activation, promoting pathological angiogenesis (89). Under pathological conditions, VEGFR1 activation further upregulates VEGF-A, TNF-α and placental growth factor (PGF), forming a positive feedback loop that perpetuates neovascularization (91). As microglia accumulate around nascent vessels, they release additional angiogenic factors through distinct subtypes. Activated microglia engaging in receptor-interacting protein 1 and 3 (RIP-1 and -3) signalling undergo necroptosis, releasing fibroblast growth factor 2 to stimulate retinal neovascularization (92). Colony-stimulating factor 1 receptor (CSF1R)-positive microglia secrete TNF-α to promote angiogenesis (93). Basigin 2-enriched microglia clustered around sprouting vessels increase IGF-1 secretion, driving neovascular growth (94). Galectin 3 binding protein-overexpressing microglia increase the levels of hypoxia-inducible factor 1α (HIF-1α), VEGF-A and matrix metalloproteinases (MMP-2/MMP-9), facilitating pathological angiogenesis (95).
Certain microglial subtypes mitigate neovascularization. Fas ligand-positive (FasL+) microglia induce the apoptosis of Fas+ endothelial cells and phagocytose them (96), whereas thrombospondin-1-positive (Trp-1+) microglia suppress endothelial proliferation and migration via microRNA-enriched exosomes and SMAD3 signalling in endothelial cells (97).
Microglia also regulate retinal neovascularization through specific signalling pathways. Enhanced CD200R-CD200 (on microglia and endothelial cells, respectively) interactions critically drive angiogenesis (98). Neuropilin-1 (NRP-1) expression in microglia indirectly supports neovascularization, as NRP-1 deficiency reduces perivascular microglial recruitment and angiogenesis (99,100). Secreted phosphoprotein 1 mediates microglia-endothelial crosstalk to promote endothelial proliferation (101). Galectin-3 binds jagged-1 to inhibit Notch signalling, thereby enhancing endothelial cell proliferation (102).
These findings underscore the dual roles of microglia in retinal vascular pathology. The phenotypic heterogeneity of microglia and their interactions with hypoxic microenvironments exacerbate vascular lesions, suggesting that targeting microglial polarization or key signalling pathways may offer novel therapeutic strategies for DR.
Dual roles of microglia in neuroprotection and neurotoxicity in DR
As resident immune cells of the retina, microglia serve critical roles in maintaining retinal homeostasis and responding to injury. Under physiological conditions, microglia exert neuroprotective effects by releasing anti-inflammatory factors, such as IL-10 and TGF-β, to suppress inflammatory responses and safeguard retinal neurons (103). In early DR stages, microglia further protect retinal ganglion cells from hyperglycaemia-induced damage by activating the PI3K/Akt signalling pathway to secrete neurotrophic factors, such as brain-derived neurotrophic factor, thereby promoting the repair and regeneration of injured neurons (104).
During DR progression, however, microglia shift towards a proinflammatory phenotype, contributing to neural injury. Under hyperglycaemic conditions, activated microglia release excessive proinflammatory cytokines, including TNF-α and IL-1β, which disrupt synaptic connectivity and impair neuronal signalling, exacerbating retinal inflammation and neurodegeneration (16). The number of activated microglia increases significantly in DR retinas, where they sustain inflammatory factor secretion, which not only directly damages neurons but also compromises BRB integrity, facilitating peripheral immune cell infiltration. These processes establish a self-perpetuating 'inflammation-injury' cycle, ultimately accelerating degenerative changes in retinal neural tissue (105).
In the systemic hyperglycaemic milieu, retinal microglia generate excessive ROS, which directly damage neuronal cell membranes and mitochondria (106). Additionally, hyperglycaemia-activated microglia release nitric oxide, which induces neuronal apoptosis by mediating glutamate excitotoxicity and promoting the nuclear accumulation of GAPDH (74).
In summary, microglia exhibit dual mechanisms in DR, balancing neuroprotection and neurotoxicity. A deeper understanding of these mechanisms may inform novel therapeutic strategies to delay DR progression.
Altered microglial intercellular interactions in DR
During retinal vascular development, macroglia, such as astrocytes, form scaffold-like structures with loose ensheathment around nascent vessels, whereas Müller cells collaborate with astrocytes to maintain BRB integrity (107-109). Microglia further support vascular growth and stabilization through dynamic interactions with these macroglial cells (110).
Microglial-Müller cell interactions
Müller cells respond to microglial activation at the molecular and functional levels, enhancing microglial activation and migration through bidirectional signalling between the two cell types (111). Additionally, Müller cells amplify inflammatory responses across retinal layers via chemotactic and adhesive cell-cell contacts, mobilizing further microglial migration (111). Activated microglia release inflammatory factors such as TNF-α, which in turn activate Müller cells, triggering their proliferation and additional cytokine release. This mutual activation forms a vicious cycle that exacerbates retinal inflammation. Activated Müller cells further release ATP, promoting microglial activation via the P2X7 receptor pathway (112). These reciprocal interactions not only disrupt the BRB, increasing vascular permeability and retinal oedema, but also impair neural signalling and worsen neurodegeneration (113). Moreover, inflammatory factors (e.g., TNF-α and IL-1β) released through microglial-Müller cell crosstalk directly damage retinal neurons, accelerating DR progression (114).
Microglia-astrocyte interactions
In the early DR stages, activated retinal microglia release inflammatory mediators that stimulate astrocyte activation, driving their proliferation and further cytokine secretion (115). Hypoxia-stressed astrocytes in DR also rapidly activate and secrete VEGF and inflammatory factors to promote pathological neovascularization (115). Concurrently, microglial activation synergistically enhances angiogenesis, hastening DR progression. Mutual activation of microglia and astrocytes contributes to BRB disruption (116). Microglia indirectly regulate vascular development by modulating the spatial patterning of astrocytes (117). Activated astrocytes adjacent to vascular endothelial cells release vasoactive substances, such as VEGF, increasing vascular permeability and exacerbating retinal oedema (115).
Signalling pathways of microglia in DR
TLR4/MyD88/NF-κB p65 signalling pathway
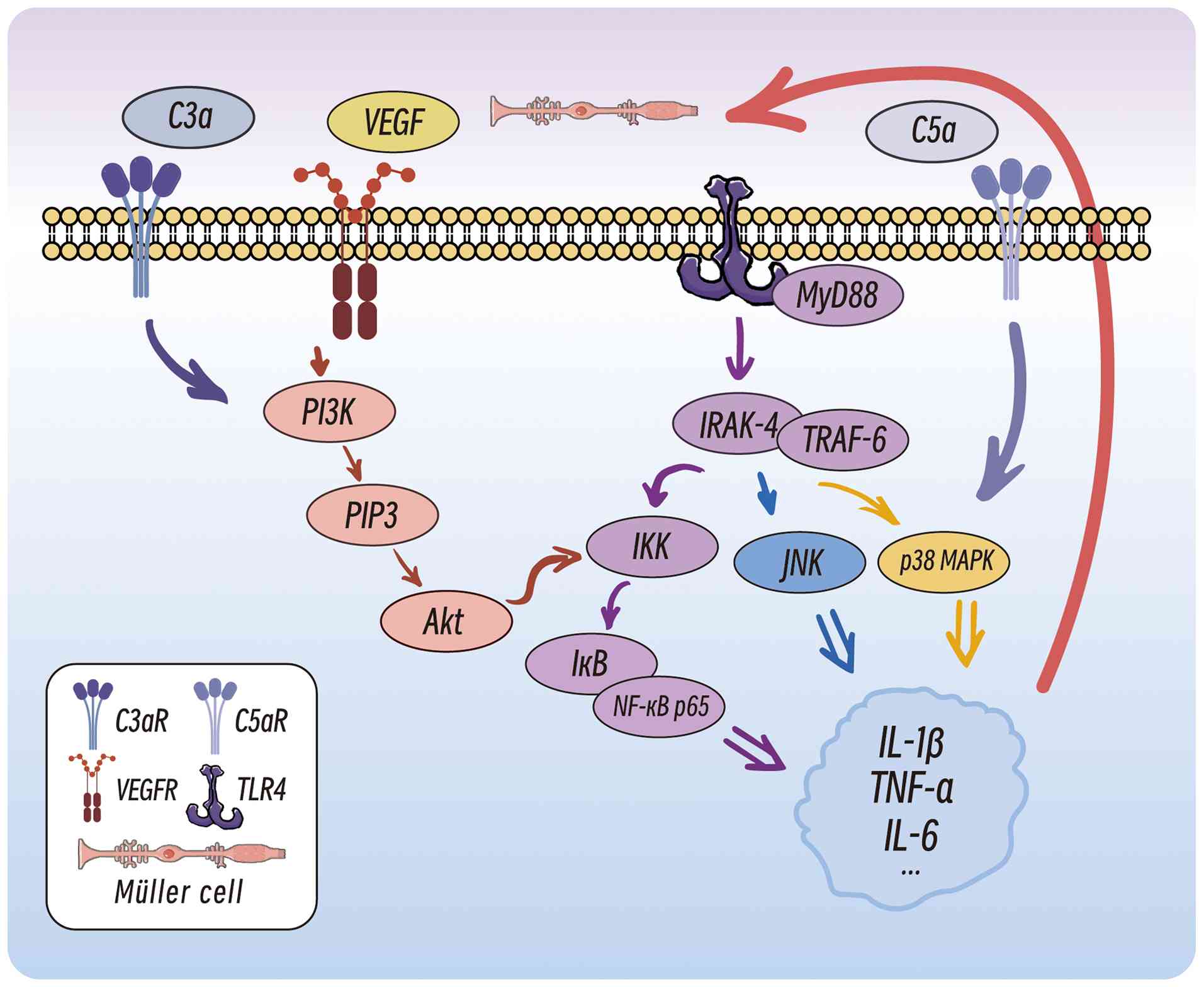
The activation of retinal microglia involves multiple signalling pathways, with the TLR4/MyD88/NF-κB p65 pathway serving a pivotal role (Fig. 4) (118). M1-polarized microglia recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) through TLRs, particularly TLR4. MyD88 binds to TLR4 via its Toll/interleukin-1 receptor domain to initiate downstream signalling, which recruits interleukin-1 receptor-associated kinases (IRAK), such as IRAK-4. This leads to IRAK phosphorylation and activation, followed by interaction with TNF receptor-associated factor 6 (TRAF6), which activates the IκB kinase (IKK) complex. IKKβ phosphorylates IκBα, a cytoplasmic inhibitor of NF-κB, resulting in the release and nuclear translocation of NF-κB. Nuclear NF-κB binds to promoters of proinflammatory genes (including IL-1β, IL-6 and TNF-α), driving cytokine production (119). These cytokines further activate the TLR4/MyD88/NF-κB p65 pathway, creating a self-amplifying inflammatory loop (120).
MAPK signalling pathway
The MAPK pathway also plays a critical role in microglial activation. In DR, hyperglycemia-induced oxidative stress and AGEs first trigger Toll-like/IL-1 receptors on retinal microglia. Receptor engagement leads to phosphorylation of IRAK-1/4, which forms a complex with TRAF6 and initiates downstream MAPK cascades, sequentially activating the MKK3/6-p38 MAPK and MKK4/7-JNK pathways (121). These cascades drive sustained synthesis of pro-inflammatory and pro-angiogenic factors (TNF-α, IL-1β, VEGF), disrupt the blood-retinal barrier, and accelerate neovascularization, positioning them at the core of DR pathogenesis (83,122).
Interactions with Müller cells and endothelial cells
Proinflammatory IL-1β binds to IL-1 receptors (IL-1Rs) on Müller cells, enhancing VEGF transcription and secretion (78). VEGF binds to VEGFR2 on endothelial cells, recruiting PI3K to generate phosphatidylinositol (3,4,5)-trisphosphate (PIP3) and activate Akt (123). Akt phosphorylates the IKK complex, accelerating IκB degradation and NF-κB nuclear translocation (123). Additionally, VEGF disrupts the BRB by activating the MAPK1 and p38 pathways, which downregulates tight junction proteins (ZO-1 and claudin-5) in endothelial cells (124,125).
Complement system activation
The C3a and C5a complement components activate microglia by binding their respective receptors, inducing the secretion of inflammatory mediators (126). As a key component of innate immunity, the complement system regulates tissue homeostasis and angiogenesis (127). The activation of complement receptors on microglia further stimulates the NF-κB and MAPK pathways, forming a positive feedback loop that exacerbates microglial activation and inflammation (128). The terminal stage of complement activation generates the membrane attack complex (MAC), which inserts into cell membranes, causing pore formation and cell death. Increased MAC levels are observed in the retinas of patients with DR and a STZ-induced diabetic rat model, directly damaging neuronal membranes and impairing function (129).
Hyperglycaemia-specific signalling features
Under hyperglycaemic conditions, microglia upregulate TLR4 expression, which increases their sensitivity to PAMPs and DAMPs, thereby sustaining chronic inflammation (119). Concurrently, high glucose activates oxidative stress pathways (such as NADPH oxidase) in microglia, leading to excessive ROS production that amplifies inflammatory responses (130,131).
Studies have demonstrated that inhibition of the NF-κB pathway significantly reduces microglial activation and inflammation in diabetic retinas (83,132-134). Thus, targeting NF-κB and MAPK signalling or blocking complement activation may represent novel therapeutic strategies for DR.
Potential therapeutic applications of microglia in DR
Current standard treatments for PDR include panretinal photocoagulation (PRP), intravitreal anti-VEGF agents (used alone or combined with surgery), angiopoietin inhibitors and vitrectomy (135,136). While these approaches provide clinical benefits, they fail to address the underlying disease mechanisms (137,138). Furthermore, therapeutic efficacy varies among patients, with suboptimal responses observed in certain cases. PRP, while effective in eliminating neovascularization, may stimulate neuroglial hyperplasia, which damages surrounding healthy retinal tissue and causing peripheral vision loss or permanent retinal scarring. PRP should be administered with caution in patients who still rely on peripheral vision or in those with extensive retinal scarring or severely impaired retinal function (139). Anti-VEGF therapy, despite its effectiveness, carries risks of exacerbating inflammation and accelerating cataract formation (140). For patients with tractional retinal changes or vitreoretinal traction, anti-VEGF therapy may exacerbate the traction and increase the risk of tractional retinal detachment (135). These limitations underscore the need for improved treatment strategies (141,142).
Abnormal aggregation and polarization of microglia serve pivotal roles in DR pathogenesis (143). Targeting microglial activation may thus serve as a promising adjunctive therapy for PDR, offering a novel approach that is complementary to existing treatments.
Reducing activated microglial populations
Previous studies have demonstrated that certain anti-VEGF therapies influence microglial activity (89,144,145). For example, Arias et al (145) reported that the anti VEGF agent aflibercept modulates microglial activity and increases the proportion of quiescent microglia, thereby promoting the regression of retinal neovascularization in oxygen-induced retinopathy models. Ranibizumab is a recombinant humanized monoclonal antibody fragment (Fab fragment) that binds with high affinity to and inhibits all biologically active forms of VEGF-A, and has also been shown to inhibit the expansion of activated microglia (146).
In addition to anti-VEGF therapies, various novel approaches have demonstrated the potential to suppress or reduce activated microglia, thereby attenuating pathological retinal angiogenesis. These approaches include melatonin (147), TGF-β-activated kinase 1 inhibitors (148), diphtheria toxin (149), CSF1R antagonists (149), microglial replacement strategies (clearing diseased microglia and repopulating with healthy donor-derived cells) (150), cyanidin-3-O-glucoside (151), honokiol (152), KC7F2 antagonists and HIF1α antagonists (153). These therapeutic interventions target the activation state of microglia, offering diverse strategies and options for DR treatment.
Promoting M1-to-M2 phenotype shift
M2-polarized microglia exhibit inhibitory effects on DR progression, making the conversion of M1 microglia to M2 microglia a potential therapeutic strategy to suppress DR pathogenesis (154). Sun et al (155) suggested that ferulic acid induces this phenotypic shift, although its clinical application in patients with DR remains unexplored. However, excessive M2 polarization may paradoxically drive a proangiogenic shift, promoting retinal neovascularization (156,157). Thus, therapeutic strategies should aim to balance M1/M2 polarization by maintaining a specific ratio, although the optimal range requires further investigation.
Arginase-1, an enzyme involved in the urea cycle and immune regulation, serves a key role in regulating microglial polarization (158-160). While preliminary evidence indicates the influence of arginase-1 on microglial phenotypes, the precise molecular mechanisms and functional outcomes need further exploration (43). Additionally, melatonin has been shown to promote M1-to-M2 conversion via regulatory T-cell-mediated pathways, facilitating tissue repair (35).
Inhibiting proangiogenic cytokine secretion
In addition to TNF-α and IL-1β, VEGF is a key proangiogenic biomarker secreted by microglia that promotes retinal neovascularization through direct or indirect pathways (87,89). Anti-VEGF therapies, including monoclonal antibodies (e.g., bevacizumab and ranibizumab), fusion proteins (e.g., aflibercept), tyrosine kinase inhibitors (e.g., sunitinib, sorafenib and pazopanib), receptor-targeting antibodies (e.g., ramucirumab) and aptamers (e.g., pegaptanib), block angiogenesis-related cytokines via diverse mechanisms to inhibit endothelial cell proliferation and pathological neovascularization (161). Additionally, several pharmacological and biological agents, such as intravitreal triamcinolone acetonide-conjugated dendritic nanoparticles (162), the FAD286 aldosterone synthase inhibitor (163), ω-3 polyunsaturated fatty acids (ω-3 PUFAs) (93,164), the NLY01 glucagon-like peptide-1 receptor agonist (96), chlorogenic acid (165), erianin (166) and celastrol (167), have demonstrated efficacy in suppressing DR progression to PDR by inhibiting microglial secretion of proangiogenic factors or modulating their interactions with the retinal vasculature.
Risks and challenges in microglial-targeted therapies
Although microglial-targeted therapies show promising potential for DR treatment, significant risks and challenges are faced. Under physiological conditions, microglia perform critical functions, such as immune surveillance and maintenance of retinal microenvironment homeostasis (168). However, pathological overactivation or excessive suppression of microglia may lead to adverse outcomes (169).
The activation states and functional regulation of microglia are highly complex. Single intervention approaches often fail to precisely steer the polarization of microglia towards a therapeutically beneficial phenotype, limiting treatment efficacy (170). In addition, excessive suppression of microglial activation and potential reduction of inflammation may compromise the normal immune surveillance capacity of microglia, increasing susceptibility to infections (171). Excessively suppressing microglial activation may also impair the role of microglia in retinal microenvironment maintenance and repair, potentially triggering secondary complications (52).
Furthermore, the long-term efficacy and safety of microglial-targeted therapies require further validation. Current studies predominantly focus on short-term outcomes, with limited experimental or clinical data addressing critical issues such as long-term functional changes in microglia, delayed adverse effects or persistent impacts on retinal physiology. This includes research demonstrating the significant therapeutic potential of the NOX1/4 inhibitor GKT137831 (Setanaxib) in diabetic retinopathy models, which effectively reduces Iba1-positive microglia population, lowers their production of reactive oxygen species, and decreases expression of inflammatory factors such as VEGF, IL-6 and TNF-α (74,170,172,173,174).
Advancements in localized drug delivery technologies, however, offer new avenues to address these challenges. For example, nanoparticle-based retinal targeting systems are rapidly evolving. By engineering nanocarriers for precise intraretinal drug delivery and sustained release, these systems increase the drug concentration at lesion sites while minimizing systemic side effects (175). Such innovations may mitigate the risks associated with microglial modulation, improving both therapeutic efficacy and safety, thereby strengthening the foundation for the clinical translation of microglial-targeted strategies in DR (176).
Conclusions and perspectives
In DR, microglia serve dual roles; microglia migrate and proliferate to participate in ocular immune privilege, vascular debris clearance and retinal protection/repair, and simultaneously contribute to BRB disruption via activation of the TLR4/MyD88/NF-κB p65 signalling pathway. Furthermore, activated microglia promote NVU dysfunction and pathological neovascularization through the release of inflammatory mediators and the modulation of signalling pathways.
Given this dual functionality, microglial-targeted therapies, such as reducing activated microglial populations, promoting M1-M2 phenotypic shifts and inhibiting proangiogenic cytokine secretion, offer a novel approach to DR management. These strategies may complement existing treatments, such as PRP and intravitreal anti-VEGF injections, while mitigating their side effects. However, as microglia are also present in the CNS, therapeutic interventions must be localized to the retina to avoid systemic impacts. The development of precise microglial-targeted therapies remains challenging owing to an incomplete understanding of the exact contributions of microglia to DR progression.
Future research should prioritize comprehensive investigations into microglial subtypes, their functional dynamics in the diabetic retina and their interactions with other glial cells. This includes developing strategies to precisely regulate microglial activity without off-target effects and exploring microglia-derived biomarkers for early DR detection and intervention. Advances in these areas will provide transformative therapeutic solutions for this high-incidence disease that severely impacts quality of life.
Availability of data and materials
Not applicable.
Authors' contributions
YB wrote the manuscript and drew the figures. XZ performed the literature search and drafted the manuscript. XW revised the manuscript. FQ and GZ reviewed and edited the manuscript. All authors read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
Not applicable.
Funding
Supported by the Natural Science Foundation of Ningxia (grant no. 2024AAC02067).
References
|
World Health Organization (WHO): Global report on diabetes. WHO; Geneva: pp. 1–88. 2016 | |
|
Altmann C and Schmidt MHH: The role of microglia in diabetic retinopathy: Inflammation, microvasculature defects and neurodegeneration. Int J Mol Sci. 19:1102018. View Article : Google Scholar : PubMed/NCBI | |
|
Obrosova IG and Kador PF: Aldose reductase/polyol inhibitors for diabetic retinopathy. Curr Pharm Biotechnol. 12:373–385. 2011. View Article : Google Scholar | |
|
Cheung N, Mitchell P and Wong TY: Diabetic retinopathy. Lancet. 376:124–136. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, Bikbov MM, Wang YX, Tang Y, Lu Y, et al: Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology. 128:1580–1591. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Yang QH, Zhang Y, Zhang XM and Li XR: Prevalence of diabetic retinopathy, proliferative diabetic retinopathy and non-proliferative diabetic retinopathy in Asian T2DM patients: A systematic review and meta-analysis. Int J Ophthalmol. 12:302–311. 2019.PubMed/NCBI | |
|
Cheyne CP, Burgess PI, Broadbent DM, García-Fiñana M, Stratton IM, Criddle T, Wang A, Alshukri A, Rahni MM, Vazquez-Arango P, et al: Incidence of sight-threatening diabetic retinopathy in an established urban screening programme: An 11-year cohort study. Diabet Med. 38:e145832021. View Article : Google Scholar : PubMed/NCBI | |
|
Das A, Stroud S, Mehta A and Rangasamy S: New treatments for diabetic retinopathy. Diabetes Obes Metab. 17:219–230. 2015. View Article : Google Scholar | |
|
Wei L, Sun X, Fan C, Li R, Zhou S and Yu H: The pathophysiological mechanisms underlying diabetic retinopathy. Front Cell Dev Biol. 10:9636152022. View Article : Google Scholar : PubMed/NCBI | |
|
Arroba AI, Alcalde-Estevez E, García-Ramírez M, Cazzoni D, de la Villa P, Sánchez-Fernández EM, Mellet CO, García Fernández JM, Hernández C, Simó R and Valverde ÁM: Modulation of microglia polarization dynamics during diabetic retinopathy in db/db mice. Biochim Biophys Acta. 1862:1663–1674. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Kinuthia UM, Wolf A and Langmann T: Microglia and inflammatory responses in diabetic retinopathy. Front Immunol. 11:5640772020. View Article : Google Scholar : PubMed/NCBI | |
|
Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK and Ting JPY: TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 4:1116–1122. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Prinz M, Jung S and Priller J: Microglia biology: One century of evolving concepts. Cell. 179:292–311. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Yang S, Zhang J and Chen L: The cells involved in the pathological process of diabetic retinopathy. Biomed Pharmacother. 132:1108182020. View Article : Google Scholar : PubMed/NCBI | |
|
Van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG and Langevoort HL: The mononuclear phagocyte system: A new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 46:845–852. 1972.PubMed/NCBI | |
|
Rao B, Liu X, Xiao J, Wu X, He F, Yang Q, Zhao W, Lin X and Zhang J: Microglia heterogeneity during neuroinflammation and neurodegeneration in the mouse retina. Brain Struct Funct. 230:192024. View Article : Google Scholar : PubMed/NCBI | |
|
Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM and Langmann T: Retinal microglia: Just bystander or target for therapy? Prog Retin Eye Res. 45:30–57. 2015. View Article : Google Scholar | |
|
Usui Y: Elucidation of pathophysiology and novel treatment for diabetic macular edema derived from the concept of neurovascular unit. JMA J. 3:201–207. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Hughes S, Yang H and Chan-Ling T: Vascularization of the human fetal retina: Roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci. 41:1217–1228. 2000. | |
|
Frost JL and Schafer DP: Microglia: Architects of the developing nervous system. Trends Cell Biol. 26:587–597. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Rymo SF, Gerhardt H, Wolfhagen Sand F, Lang R, Uv A and Betsholtz C: A two-way communication between microglial cells and angiogenic sprouts regulates angiogenesis in aortic ring cultures. PLoS One. 6:e158462011. View Article : Google Scholar : PubMed/NCBI | |
|
Haupt F, Krishnasamy K, Napp LC, Augustynik M, Limbourg A, Gamrekelashvili J, Bauersachs J, Haller H and Limbourg FP: Retinal myeloid cells regulate tip cell selection and vascular branching morphogenesis via Notch ligand Delta-like 1. Sci Rep. 9:97982019. View Article : Google Scholar : PubMed/NCBI | |
|
Endo Y, Asanuma D, Namiki S, Sugihara K, Hirose K, Uemura A, Kubota Y and Miura T: Quantitative modeling of regular retinal microglia distribution. Sci Rep. 11:226712021. View Article : Google Scholar : PubMed/NCBI | |
|
Asare-Bediako B, Adu-Agyeiwaah Y, Abad A, Li Calzi S, Floyd JL, Prasad R, DuPont M, Asare-Bediako R, Bustelo XR and Grant MB: Hematopoietic cells influence vascular development in the retina. Cells. 11:32072022. View Article : Google Scholar : PubMed/NCBI | |
|
Perochon T, Krsnik Z, Massimo M, Ruchiy Y, Romero AL, Mohammadi E, Li X, Long KR, Parkkinen L, Blomgren K, et al: Unraveling microglial spatial organization in the developing human brain with DeepCellMap, a deep learning approach coupled with spatial statistics. Nat Commun. 16:15772025. View Article : Google Scholar : PubMed/NCBI | |
|
Li F, Jiang D and Samuel MA: Microglia in the developing retina. Neural Dev. 14:122019. View Article : Google Scholar | |
|
Zhou LY, Liu ZG, Sun YQ, Li YZ, Teng ZQ and Liu CM: Preserving blood-retinal barrier integrity: A path to retinal ganglion cell protection in glaucoma and traumatic optic neuropathy. Cell Regen. 14:132025. View Article : Google Scholar : PubMed/NCBI | |
|
Klaassen I, Van Noorden CJF and Schlingemann RO: Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res. 34:19–48. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Guymer RH, Bird AC and Hageman GS: Cytoarchitecture of choroidal capillary endothelial cells. Invest Ophthalmol Vis Sci. 45:1660–1666. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Hormel TT, Jia Y, Jian Y, Hwang TS, Bailey ST, Pennesi ME, Wilson DJ, Morrison JC and Huang D: Plexus-specific retinal vascular anatomy and pathologies as seen by projection-resolved optical coherence tomographic angiography. Prog Retin Eye Res. 80:1008782021. View Article : Google Scholar : | |
|
Amoaku WM, Ghanchi F, Bailey C, Banerjee S, Banerjee S, Downey L, Gale R, Hamilton R, Khunti K, Posner E, et al: Diabetic retinopathy and diabetic macular oedema pathways and management: UK consensus working group. Eye (Lond). 34(Suppl 1): S1–S51. 2020. View Article : Google Scholar | |
|
Opdenakker G and Abu El-Asrar A: Metalloproteinases mediate diabetes-induced retinal neuropathy and vasculopathy. Cell Mol Life Sci. 76:3157–3166. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
O'Leary F and Campbell M: The blood-retina barrier in health and disease. FEBS J. 290:878–891. 2023. View Article : Google Scholar | |
|
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA and Stevens B: Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 74:691–705. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu X, Hong J and Zhou X: Biological immune mechanism of retina. Front Biosci (Landmark Ed). 28:3632023. View Article : Google Scholar | |
|
Kumar A, Kumar A, Kumar J, Bai G, Jeewnani R, Dembra M, Kanwal K, Qadeer U, Khawar MH, Yaseen Khan I, et al: Comparative efficacy of anti-vascular endothelial growth factor (anti-VEGF) agents and corticosteroids in managing diabetic retinopathy-associated diabetic macular edema: A meta-analysis and comprehensive systematic review. Cureus. 16:e519102024.PubMed/NCBI | |
|
Lee WJ, Kang MH, Seong M and Cho HY: Comparison of aqueous concentrations of angiogenic and inflammatory cytokines in diabetic macular oedema and macular oedema due to branch retinal vein occlusion. Br J Ophthalmol. 96:1426–1430. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Meng C, Gu C, He S, Su T, Lhamo T, Draga D and Qiu Q: Pyroptosis in the retinal neurovascular unit: New insights into diabetic retinopathy. Front Immunol. 12:7630922021. View Article : Google Scholar : PubMed/NCBI | |
|
Romero-Aroca P, Baget-Bernaldiz M, Pareja-Rios A, Lopez-Galvez M, Navarro-Gil R and Verges R: Diabetic macular edema pathophysiology: Vasogenic versus inflammatory. J Diabetes Res. 2016:21562732016. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Zhao L, Wang X, Ma W, Lazere A, Qian HH, Zhang J, Abu-Asab M, Fariss RN, Roger JE and Wong WT: Repopulating retinal microglia restore endogenous organization and function under CX3CL1-CX3CR1 regulation. Sci Adv. 4:eaap84922018. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao L, Zabel MK, Wang X, Ma W, Shah P, Fariss RN, Qian H, Parkhurst CN, Gan WB and Wong WT: Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol Med. 7:1179–1197. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Wang J, He W and Zhang J: A richer and more diverse future for microglia phenotypes. Heliyon. 9:e147132023. View Article : Google Scholar : PubMed/NCBI | |
|
Fouda AY, Xu Z, Suwanpradid J, Rojas M, Shosha E, Lemtalsi T, Patel C, Xing J, Zaidi SA, Zhi W, et al: Targeting proliferative retinopathy: Arginase 1 limits vitreoretinal neovascularization and promotes angiogenic repair. Cell Death Dis. 13:7452022. View Article : Google Scholar : PubMed/NCBI | |
|
Li X, Yu ZW, Li HY, Yuan Y, Gao XY and Kuang HY: Retinal microglia polarization in diabetic retinopathy. Vis Neurosci. 38:E0062021. View Article : Google Scholar : PubMed/NCBI | |
|
Ikeda T, Nakamura K, Kida T and Oku H: Possible roles of anti-type II collagen antibody and innate immunity in the development and progression of diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 260:387–403. 2022. View Article : Google Scholar : | |
|
Ibrahim AS, El-Shishtawy MM, Peña A Jr and Liou GI: Genistein attenuates retinal inflammation associated with diabetes by targeting of microglial activation. Mol Vis. 16:2033–2042. 2010.PubMed/NCBI | |
|
Cukras CA, Petrou P, Chew EY, Meyerle CB and Wong WT: Oral minocycline for the treatment of diabetic macular edema (DME): Results of a phase I/II clinical study. Invest Ophthalmol Vis Sci. 53:3865–3874. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Madeira MH, Boia R, Santos PF, Ambrósio AF and Santiago AR: Contribution of microglia-mediated neuroinflammation to retinal degenerative diseases. Mediators Inflamm. 2015:6730902015. View Article : Google Scholar : PubMed/NCBI | |
|
Grigsby JG, Cardona SM, Pouw CE, Muniz A, Mendiola AS, Tsin ATC, Allen DM and Cardona AE: The role of microglia in diabetic retinopathy. J Ophthalmol. 2014:7057832014. View Article : Google Scholar : PubMed/NCBI | |
|
Paolicelli RC, Sierra A, Stevens B, Tremblay ME, Aguzzi A, Ajami B, Amit I, Audinat E, Bechmann I, Bennett M, et al: Microglia states and nomenclature: A field at its crossroads. Neuron. 110:3458–3483. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Sun N, Victor MB, Park YP, Xiong X, Scannail AN, Leary N, Prosper S, Viswanathan S, Luna X, Boix CA, et al: Human microglial state dynamics in Alzheimer's disease progression. Cell. 186:4386–4403.e29. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
He J, Fu Y, Ge L, Dai J, Fang Y, Li Y, Gu X, Tao Z, Zou T, Li M, et al: Disease-associated microglial activation prevents photoreceptor degeneration by suppressing the accumulation of cell debris and neutrophils in degenerating rat retinas. Theranostics. 12:2687–2706. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Shemer A, Grozovski J, Tay TL, Tao J, Volaski A, Süß P, Ardura-Fabregat A, Gross-Vered M, Kim JS, David E, et al: Engrafted parenchymal brain macrophages differ from microglia in transcriptome, chromatin landscape and response to challenge. Nat Commun. 9:52062018. View Article : Google Scholar : PubMed/NCBI | |
|
Buttgereit A, Lelios I, Yu X, Vrohlings M, Krakoski NR, Gautier EL, Nishinakamura R, Becher B and Greter M: Sall1 is a transcriptional regulator defining microglia identity and function. Nat Immunol. 17:1397–1406. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Jurga AM, Paleczna M and Kuter KZ: Overview of general and discriminating markers of differential microglia phenotypes. Front Cell Neurosci. 14:1982020. View Article : Google Scholar : PubMed/NCBI | |
|
Wolf J, Boneva S, Rosmus DD, Agostini H, Schlunck G, Wieghofer P, Schlecht A and Lange C: In-depth molecular profiling specifies human retinal microglia identity. Front Immunol. 13:8631582022. View Article : Google Scholar : PubMed/NCBI | |
|
Masuda T, Amann L, Sankowski R, Staszewski O, Lenz M, D'Errico P, Snaidero N, Costa Jordão MJ, Böttcher C, Kierdorf K, et al: Novel Hexb-based tools for studying microglia in the CNS. Nat Immunol. 21:802–815. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Kenkhuis B, Somarakis A, Kleindouwel LRT, Van Roon-Mom WMC, Höllt T and Van Der Weerd L: Co-expression patterns of microglia markers Iba1, TMEM119 and P2RY12 in Alzheimer's disease. Neurobiol Dis. 167:1056842022. View Article : Google Scholar : PubMed/NCBI | |
|
Qin Z, He S, Yang C, Yung JSY, Chen C, Leung CKS, Liu K and Qu JY: Adaptive optics two-photon microscopy enables near-diffraction-limited and functional retinal imaging in vivo. Light Sci Appl. 9:792020. View Article : Google Scholar : PubMed/NCBI | |
|
Wu MY, Yiang GT, Lai TT and Li CJ: The oxidative stress and mitochondrial dysfunction during the pathogenesis of diabetic retinopathy. Oxidative Med Cell Longev. 2018:34201872018. View Article : Google Scholar | |
|
Lv K, Ying H, Hu G, Hu J, Jian Q and Zhang F: Integrated multi-omics reveals the activated retinal microglia with intracellular metabolic reprogramming contributes to inflammation in STZ-induced early diabetic retinopathy. Front Immunol. 13:9427682022. View Article : Google Scholar : PubMed/NCBI | |
|
Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J and Brownlee M: Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci USA. 97:12222–12226. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Du X, Matsumura T, Edelstein D, Rossetti L, Zsengellér Z, Szabó C and Brownlee M: Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Investig. 112:1049–1057. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Kowluru RA: Effect of advanced glycation end products on accelerated apoptosis of retinal capillary cells under in vitro conditions. Life Sci. 76:1051–1060. 2005. View Article : Google Scholar | |
|
Glomb MA and Monnier VM: Mechanism of protein modification by glyoxal and glycolaldehyde, reactive intermediates of the maillard reaction. J Biol Chem. 270:10017–10026. 1995. View Article : Google Scholar : PubMed/NCBI | |
|
Funatsu H and Yamashita H: Pathogenesis of diabetic retinopathy and the renin-angiotensin system. Ophthalmic Physiol Opt. 23:495–501. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Cai Y, Li W, Tu H, Chen N, Zhong Z, Yan P and Dong J: Curcumolide reduces diabetic retinal vascular leukostasis and leakage partly via inhibition of the p38MAPK/NF-κB signaling. Bioorg Med Chem Lett. 27:1835–1839. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Stitt AW: The role of advanced glycation in the pathogenesis of diabetic retinopathy. Exp Mol Pathol. 75:95–108. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Yamagishi SI and Matsui T: Advanced glycation end products (AGEs), oxidative stress and diabetic retinopathy. Curr Pharm Biotechnol. 12:362–368. 2011. View Article : Google Scholar | |
|
Song J and Lee JE: ASK1 modulates the expression of microRNA Let7A in microglia under high glucose in vitro condition. Front Cell Neurosci. 9:1982015. View Article : Google Scholar : PubMed/NCBI | |
|
Du A, Xie Y, Ouyang H, Lu B, Jia W, Xu H and Ji L: Si-miao-yong-an decoction for diabetic retinopathy: A combined network pharmacological and in vivo approach. Front Pharmacol. 12:7631632021. View Article : Google Scholar : PubMed/NCBI | |
|
Vargas-Soria M, García-Alloza M and Corraliza-Gómez M: Effects of diabetes on microglial physiology: A systematic review of in vitro, preclinical and clinical studies. J Neuroinflammation. 20:572023. View Article : Google Scholar : PubMed/NCBI | |
|
Quiriconi P, Hristov V, Aburaya M, Greferath U, Jobling AI and Fletcher EL: The role of microglia in the development of diabetic retinopathy. NPJ Metab Health Dis. 2:72024. View Article : Google Scholar : PubMed/NCBI | |
|
Cai L, Xia M and Zhang F: Redox regulation of immunometabolism in microglia underpinning diabetic retinopathy. Antioxidants (Basel). 13:4232024. View Article : Google Scholar : PubMed/NCBI | |
|
Yamaguchi M, Nakao S, Arima M, Little K, Singh A, Wada I, Kaizu Y, Zandi S, Garweg JG, Matoba T, et al: Heterotypic macrophages/microglia differentially contribute to retinal ischaemia and neovascularisation. Diabetologia. 67:2329–2345. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Wang J, Liu X, Wei W, Yang J, Li Q, Chu S, Liu P, Zhang J and He W: Regulation of oxygen-glucose deprivation/reperfusion-induced inflammatory responses and M1-M2 phenotype switch of BV2 microglia by lobetyolin. Metab Brain Dis. 38:2627–2644. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Nian S, Lo ACY, Mi Y, Ren K and Yang D: Neurovascular unit in diabetic retinopathy: Pathophysiological roles and potential therapeutical targets. Eye Vis (Lond Engl). 8:152021. View Article : Google Scholar | |
|
Inada M, Xu H, Takeuchi M, Ito M and Chen M: Microglia increase tight-junction permeability in coordination with Müller cells under hypoxic condition in an in vitro model of inner blood-retinal barrier. Exp Eye Res. 205:1084902021. View Article : Google Scholar | |
|
Fang M, Wan W, Li Q, Wan W, Long Y, Liu H and Yang X: Asiatic acid attenuates diabetic retinopathy through TLR4/MyD88/NF-κB p65 mediated modulation of microglia polarization. Life Sci. 277:1195672021. View Article : Google Scholar | |
|
Mehrabadi AR, Korolainen MA, Odero G, Miller DW and Kauppinen TM: Poly(ADP-ribose) polymerase-1 regulates microglia mediated decrease of endothelial tight junction integrity. Neurochem Int. 108:266–271. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Usui-Ouchi A, Usui Y, Kurihara T, Aguilar E, Dorrell MI, Ideguchi Y, Sakimoto S, Bravo S and Friedlander M: Retinal microglia are critical for subretinal neovascular formation. JCI Insight. 5:e1373172020. View Article : Google Scholar : PubMed/NCBI | |
|
Xie H, Zhang C, Liu D, Yang Q, Tang L, Wang T, Tian H, Lu L, Xu JY, Gao F, et al: Erythropoietin protects the inner blood-retinal barrier by inhibiting microglia phagocytosis via Src/Akt/cofilin signalling in experimental diabetic retinopathy. Diabetologia. 64:211–225. 2021. View Article : Google Scholar | |
|
Zhang T, Ouyang H, Mei X, Lu B, Yu Z, Chen K, Wang Z and Ji L: Erianin alleviates diabetic retinopathy by reducing retinal inflammation initiated by microglial cells via inhibiting hyperglycemia-mediated ERK1/2-NF-κB signaling pathway. FASEB J. 33:11776–11790. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Behnke V, Wolf A and Langmann T: The role of lymphocytes and phagocytes in age-related macular degeneration (AMD). Cell Mol Life Sci. 77:781–788. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Ogura S, Baldeosingh R, Bhutto IA, Kambhampati SP, McLeod DS, Edwards MM, Rais R, Schubert W and Lutty GA: A role for mast cells in geographic atrophy. FASEB J. 34:10117–10131. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Lueck K, Busch M, Moss SE, Greenwood J, Kasper M, Lommatzsch A, Pauleikhoff D and Wasmuth S: Complement stimulates retinal pigment epithelial cells to undergo pro-inflammatory changes. Ophthalmic Res. 54:195–203. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Fu X, Feng S, Qin H, Yan L, Zheng C and Yao K: Microglia: The breakthrough to treat neovascularization and repair blood-retinal barrier in retinopathy. Front Mol Neurosci. 16:11002542023. View Article : Google Scholar : PubMed/NCBI | |
|
Ding X, Gu R, Zhang M, Ren H, Shu Q, Xu G and Wu H: Microglia enhanced the angiogenesis, migration and proliferation of co-cultured RMECs. BMC Ophthalmol. 18:2492018. View Article : Google Scholar : PubMed/NCBI | |
|
Hu A, Schmidt MHH and Heinig N: Microglia in retinal angiogenesis and diabetic retinopathy. Angiogenesis. 27:311–331. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Tang L, Xu GT and Zhang JF: Inflammation in diabetic retinopathy: Possible roles in pathogenesis and potential implications for therapy. Neural Regen Res. 18:976–982. 2023. View Article : Google Scholar : | |
|
Ogura S, Kurata K, Hattori Y, Takase H, Ishiguro-Oonuma T, Hwang Y, Ahn S, Park I, Ikeda W, Kusuhara S, et al: Sustained inflammation after pericyte depletion induces irreversible blood-retina barrier breakdown. JCI Insight. 2:e909052017. View Article : Google Scholar : PubMed/NCBI | |
|
He C, Liu Y, Huang Z, Yang Z, Zhou T, Liu S, Hao Z, Wang J, Feng Q, Liu Y, et al: A specific RIP3+ subpopulation of microglia promotes retinopathy through a hypoxia-triggered necroptotic mechanism. Proc Natl Acad Sci USA. 118:e20232901182021. View Article : Google Scholar | |
|
Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, et al: Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 13:868–873. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Yin J, Xu WQ, Ye MX, Zhang Y, Wang HY, Zhang J, Li Y and Wang YS: Up-regulated basigin-2 in microglia induced by hypoxia promotes retinal angiogenesis. J Cell Mol Med. 21:3467–3480. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao C, Liu Y, Meng J, Wang X, Liu X, Li W, Zhou Q, Xiang J, Li N and Hou S: LGALS3BP in microglia promotes retinal angiogenesis through PI3K/AKT pathway during hypoxia. Invest Ophthalmol Vis Sci. 63:252022. View Article : Google Scholar : PubMed/NCBI | |
|
Murinello S, Usui Y, Sakimoto S, Kitano M, Aguilar E, Friedlander HM, Schricker A, Wittgrove C, Wakabayashi Y, Dorrell MI, et al: miR-30a-5p inhibition promotes interaction of Fas+ endothelial cells and FasL+ microglia to decrease pathological neovascularization and promote physiological angiogenesis. Glia. 67:332–344. 2019. View Article : Google Scholar | |
|
Luo Q, Jiang Z, Jiang J, Wan L, Li Y, Huang Y, Qiu J, Yu K and Zhuang J: Tsp-1+ microglia attenuate retinal neovascularization by maintaining the expression of Smad3 in endothelial cells through exosomes with decreased miR-27a-5p. Theranostics. 13:3689–3706. 2023. View Article : Google Scholar : | |
|
Hu Y, Wei T, Gao S and Cheng Q: Anti-angiogenic and anti-inflammatory effects of CD200-CD200R1 axis in oxygen-induced retinopathy mice model. Inflamm Res. 68:945–955. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Dejda A, Mawambo G, Daudelin JF, Miloudi K, Akla N, Patel C, Andriessen EMMA, Labrecque N, Sennlaub F and Sapieha P: Neuropilin-1-expressing microglia are associated with nascent retinal vasculature yet dispensable for developmental angiogenesis. Invest Ophthalmol Vis Sci. 57:1530–1536. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Dejda A, Mawambo G, Cerani A, Miloudi K, Shao Z, Daudelin JF, Boulet S, Oubaha M, Beaudoin F, Akla N, et al: Neuropilin-1 mediates myeloid cell chemoattraction and influences retinal neuroimmune crosstalk. J Clin Investig. 124:4807–4822. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Bai Q, Wang X, Yan H, Wen L, Zhou Z, Ye Y, Jing Y, Niu Y, Wang L, Zhang Z, et al: Microglia-derived Spp1 promotes pathological retinal neovascularization via activating endothelial Kit/Akt/mTOR signaling. J Pers Med. 13:1462023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou ZY, Chang TF, Lin ZB, Jing YT, Wen LS, Niu YL, Bai Q, Guo CM, Sun JX, Wang YS and Dou GR: Microglial Galectin3 enhances endothelial metabolism and promotes pathological angiogenesis via notch inhibition by competitively binding to Jag1. Cell Death Dis. 14:3802023. View Article : Google Scholar : PubMed/NCBI | |
|
Li L, Sun B, Harris OA and Luo J: TGF-β signaling in microglia: A key regulator of development, homeostasis and reactivity. Biomedicines. 12:24682024. View Article : Google Scholar | |
|
Wiens KR, Wasti N, Ulloa OO and Klegeris A: Diversity of microglia-derived molecules with neurotrophic properties that support neurons in the central nervous system and other tissues. Molecules. 29:55252024. View Article : Google Scholar : PubMed/NCBI | |
|
Liu W, Tong B, Xiong J, Zhu Y, Lu H, Xu H, Yang X, Wang F, Yu P and Hu Y: Identification of macrophage polarisation and mitochondria-related biomarkers in diabetic retinopathy. J Transl Med. 23:232025. View Article : Google Scholar : PubMed/NCBI | |
|
Amankwa CE, Acha LG, Dibas A, Chavala SH, Roth S, Mathew B and Acharya S: Neuroprotective and anti-inflammatory activities of hybrid small-molecule SA-10 in ischemia/reperfusion-induced retinal neuronal injury models. Cells. 13:3962024. View Article : Google Scholar : PubMed/NCBI | |
|
Paisley CE and Kay JN: Seeing stars: Development and function of retinal astrocytes. Dev Biol. 478:144–154. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Chan-Ling T: Development of the retinal vasculature. Encyclopedia of the eye. D'Amore P: Academic Press; Cambridge: pp. 22–33. 2010, View Article : Google Scholar | |
|
Gnanaguru G, Tabor SJ, Bonilla GM, Sadreyev R, Yuda K, Köhl J and Connor KM: Microglia refine developing retinal astrocytic and vascular networks through the complement C3/C3aR axis. Development. 150:dev2010472023. View Article : Google Scholar : PubMed/NCBI | |
|
Checchin D, Sennlaub F, Levavasseur E, Leduc M and Chemtob S: Potential role of microglia in retinal blood vessel formation. Invest Ophthalmol Vis Sci. 47:3595–3602. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Wang M, Ma W, Zhao L, Fariss RN and Wong WT: Adaptive Müller cell responses to microglial activation mediate neuroprotection and coordinate inflammation in the retina. J Neuroinflammation. 8:1732011. View Article : Google Scholar | |
|
Tewari M, Michalski S and Egan TM: Modulation of microglial function by ATP-gated P2X7 receptors: Studies in rat, mice and human. Cells. 13:1612024. View Article : Google Scholar : PubMed/NCBI | |
|
Kong H, Zhao H, Chen T, Song Y and Cui Y: Targeted P2X7/NLRP3 signaling pathway against inflammation, apoptosis, and pyroptosis of retinal endothelial cells in diabetic retinopathy. Cell Death Dis. 13:3362022. View Article : Google Scholar : PubMed/NCBI | |
|
Antonetti DA, Silva PS and Stitt AW: Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat Rev Endocrinol. 17:195–206. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang X, Ye J and Liu W: GPER-mediated inhibition of astrocyte activation mitigates retinal neovascularization in oxygen-induced retinopathy mice. J Army Med Univ. 46:1369–1377. 2024. | |
|
Kaur C, Foulds W and Ling E: Blood-retinal barrier in hypoxic ischaemic conditions: Basic concepts, clinical features and management. Prog Retin Eye Res. 27:622–647. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Puebla M, Tapia PJ and Espinoza H: Key role of astrocytes in postnatal brain and retinal angiogenesis. Int J Mol Sci. 23:26462022. View Article : Google Scholar : PubMed/NCBI | |
|
Navarro HI, Daly AE, Rodriguez B, Wu S, Ngo KA, Fraser A, Schiffman A, Liu Y, Smale ST, Chia JJ and Hoffmann A: NF-κB RelB suppresses the inflammatory gene expression programs of dendritic cells by competing with RelA for binding to target gene promoters. Cell Discov. 11:132025. View Article : Google Scholar | |
|
Acioglu C and Elkabes S: Innate immune sensors and regulators at the blood brain barrier: Focus on toll-like receptors and inflammasomes as mediators of neuro-immune crosstalk and inflammation. J Neuroinflammation. 22:392025. View Article : Google Scholar : PubMed/NCBI | |
|
Song Y, Dou H, Gong W, Liu X, Yu Z, Li E, Tan R and Hou Y: Bis-N-norgliovictin, a small-molecule compound from marine fungus, inhibits LPS-induced inflammation in macrophages and improves survival in sepsis. Eur J Pharmacol. 705:49–60. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Abcouwer SF: Neural inflammation and the microglial response in diabetic retinopathy. J Ocul Biol Dis Infor. 4:25–33. 2012. View Article : Google Scholar : | |
|
Sun H, Ma X, Ma H, Li S, Xia Y, Yao L, Wang Y, Pang X, Zhong J, Yao G, et al: High glucose levels accelerate atherosclerosis via NLRP3-IL/MAPK/NF-κB-related inflammation pathways. Biochem Biophys Res Commun. 704:1497022024. View Article : Google Scholar | |
|
He Y, Sun MM, Zhang GG, Yang J, Chen KS, Xu WW and Li B: Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther. 6:4252021. View Article : Google Scholar : PubMed/NCBI | |
|
Chidiac R, Zhang Y, Tessier S, Faubert D, Delisle C and Gratton JP: Comparative phosphoproteomics analysis of VEGF and angiopoietin-1 signaling reveals ZO-1 as a critical regulator of endothelial cell proliferation. Mol Cell Proteom. 15:1511–1525. 2016. View Article : Google Scholar | |
|
Greene C, Hanley N and Campbell M: Claudin-5: Gatekeeper of neurological function. Fluids Barriers CNS. 16:32019. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao H, Lv Y, Xu J, Song X, Wang Q, Zhai X, Ma X, Qiu J, Cui L and Sun Y: The activation of microglia by the complement system in neurodegenerative diseases. Ageing Res Rev. 104:1026362025. View Article : Google Scholar | |
|
Ricklin D, Hajishengallis G, Yang K and Lambris JD: Complement: A key system for immune surveillance and homeostasis. Nat Immunol. 11:785–797. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Ayyubova G and Madhu LN: Microglial NLRP3 inflammasomes in Alzheimer's disease pathogenesis: From interaction with autophagy/mitophagy to therapeutics. Mol Neurobiol. 62:7124–7143. 2025. View Article : Google Scholar : PubMed/NCBI | |
|
Yanai R, Thanos A and Connor KM: Complement involvement in neovascular ocular diseases. Adv Exp Med Biol. 946:161–183. 2012. View Article : Google Scholar | |
|
Padmakumar L, Menon RN, Gopala S and Vilanilam GC: MTH1 in the disorders of the central nervous system: Scope beyond brain tumors and challenges. Acta Neurol Belg. Feb 17–2025.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI | |
|
Chung J, Jernigan J, Menees KB and Lee JK: RGS10 mitigates high glucose-induced microglial inflammation via the reactive oxidative stress pathway and enhances synuclein clearance in microglia. Front Cell Neurosci. 18:13742982024. View Article : Google Scholar : PubMed/NCBI | |
|
Homme RP, Sandhu HS, George AK, Tyagi SC and Singh M: Sustained inhibition of NF-κB activity mitigates retinal vasculopathy in diabetes. Am J Pathol. 191:947–964. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Park SS: Retinal glia and NF-κB in diabetic retinopathy pathogenesis. Ann Transl Med. 11:3072023. View Article : Google Scholar | |
|
Chen W, Wu Z, Cheng Z, Zhang Y, Luo Q and Yin M: HO-1 represses NF-κB signaling pathway to mediate microglia polarization and phagocytosis in intracerebral hemorrhage. Neuroscience. 566:17–27. 2025. View Article : Google Scholar | |
|
Gonzalez-Cortes JH, Martinez-Pacheco VA, Gonzalez-Cantu JE, Bilgic A, de Ribot FM, Sudhalkar A, Mohamed-Hamsho J, Kodjikian L and Mathis T: Current treatments and innovations in diabetic retinopathy and diabetic macular edema. Pharmaceutics. 15:1222022. View Article : Google Scholar | |
|
Mounirou BAM, Adam ND, Yakoura AKH, Aminou MSM, Liu YT and Tan LY: Diabetic retinopathy: An overview of treatments. Indian J Endocrinol Metab. 26:111–118. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Arrigo A, Aragona E and Bandello F: VEGF-targeting drugs for the treatment of retinal neovascularization in diabetic retinopathy. Ann Med. 54:1089–1111. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang W, Geng J and Sang A: Effectiveness of panretinal photocoagulation plus intravitreal anti-VEGF treatment against PRP alone for diabetic retinopathy: A systematic review with meta-analysis. Front Endocrinol (Lausanne). 13:8076872022. View Article : Google Scholar : PubMed/NCBI | |
|
Shukla UV and Tripathy K: Diabetic retinopathy. StatPearls [Internet]. StatPearls Publishing; Treasure Island, FL: 2025 | |
|
Vergmann AS, Nguyen TT, Lee Torp T, Kawasaki R, Wong TY, Peto T and Grauslund J: Efficacy and side effects of individualized panretinal photocoagulation. Ophthalmol Retina. 4:642–644. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Xu W, Cheng W, Cui X and Xu G: Therapeutic effect against retinal neovascularization in a mouse model of oxygen-induced retinopathy: Bone marrow-derived mesenchymal stem cells versus Conbercept. BMC Ophthalmol. 20:72020. View Article : Google Scholar : PubMed/NCBI | |
|
Martinez-Alejo JM, Baiza-Duran LM and Quintana-Hau JDD: Novel therapies for proliferative retinopathies. Ther Adv Chronic Dis. 13:204062232211403952022. View Article : Google Scholar : PubMed/NCBI | |
|
Xu W, Hu Z, Lv Y, Dou G, Zhang Z, Wang H and Wang Y: Microglial density determines the appearance of pathological neovascular tufts in oxygen-induced retinopathy. Cell Tissue Res. 374:25–38. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Qin S, Zhang C, Qin H, Xie H, Luo D, Qiu Q, Liu K and Zhang J, Xu G and Zhang J: Hyperreflective foci and subretinal fluid are potential imaging biomarkers to evaluate anti-VEGF effect in diabetic macular edema. Front Physiol. 12:7914422021. View Article : Google Scholar | |
|
Rojo Arias JE, Englmaier VE and Jászai J: VEGF-trap modulates retinal inflammation in the murine oxygen-induced retinopathy (OIR) model. Biomedicines. 10:2012022. View Article : Google Scholar : PubMed/NCBI | |
|
Palmhof M, Lohmann S, Schulte D, Stute G, Wagner N, Dick HB and Joachim SC: Fewer functional deficits and reduced cell death after ranibizumab treatment in a retinal ischemia model. Int J Mol Sci. 19:16362018. View Article : Google Scholar : PubMed/NCBI | |
|
Xu Y, Lu X, Hu Y, Yang B, Tsui CK, Yu S, Lu L and Liang X: Melatonin attenuated retinal neovascularization and neuroglial dysfunction by inhibition of HIF-1α-VEGF pathway in oxygen-induced retinopathy mice. J Pineal Res. 64:e124732018. View Article : Google Scholar | |
|
Wang JH, Lin FL, Chen J, Zhu L, Chuang YF, Tu L, Ma C, Ling D, Hewitt AW, Tseng CL, et al: TAK1 blockade as a therapy for retinal neovascularization. Pharmacol Res. 187:1066172023. View Article : Google Scholar | |
|
Church KA, Rodriguez D, Mendiola AS, Vanegas D, Gutierrez IL, Tamayo I, Amadu A, Velazquez P, Cardona SM, Gyoneva S, et al: Pharmacological depletion of microglia alleviates neuronal and vascular damage in the diabetic CX3CR1-WT retina but not in CX3CR1-KO or hCX3CR1I249/M280-expressing retina. Front Immunol. 14:11307352023. View Article : Google Scholar | |
|
Church KA, Rodriguez D, Vanegas D, Gutierrez IL, Cardona SM, Madrigal JLM, Kaur T and Cardona AE: Models of microglia depletion and replenishment elicit protective effects to alleviate vascular and neuronal damage in the diabetic murine retina. J Neuroinflammation. 19:3002022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao F, Gao X, Ge X, Cui J and Liu X: Cyanidin-3-o-glucoside (C3G) inhibits vascular leakage regulated by microglial activation in early diabetic retinopathy and neovascularization in advanced diabetic retinopathy. Bioengineered. 12:9266–9278. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Yang B, Xu Y, Yu S, Huang Y, Lu L and Liang X: Anti-angiogenic and anti-inflammatory effect of Magnolol in the oxygen-induced retinopathy model. Inflamm Res. 65:81–93. 2016. View Article : Google Scholar | |
|
Tang X, Cui K, Lu X, Wu P, Yu S, Yang B, Xu Y and Liang X: A novel hypoxia-inducible factor 1α inhibitor KC7F2 attenuates oxygen-induced retinal neovascularization. Invest Ophthalmol Vis Sci. 63:132022. View Article : Google Scholar | |
|
Song GJ and Suk K: Pharmacological modulation of functional phenotypes of microglia in neurodegenerative diseases. Front Aging Neurosci. 9:1392017. View Article : Google Scholar : PubMed/NCBI | |
|
Sun X, Ma L, Li X, Wang J, Li Y and Huang Z: Ferulic acid alleviates retinal neovascularization by modulating microglia/macrophage polarization through the ROS/NF-κB axis. Front Immunol. 13:9767292022. View Article : Google Scholar | |
|
Wang Y, Chang T, Wu T, Xu W, Dou G, Wang Y and Guo C: M2 macrophages promote vasculogenesis during retinal neovascularization by regulating bone marrow-derived cells via SDF-1/VEGF. Cell Tissue Res. 380:469–486. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou Y, Yoshida S, Nakao S, Yoshimura T, Kobayashi Y, Nakama T, Kubo Y, Miyawaki K, Yamaguchi M, Ishikawa K, et al: M2 macrophages enhance pathological neovascularization in the mouse model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 56:4767–4777. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Caldwell RW, Rodriguez PC, Toque HA, Narayanan SP and Caldwell RB: Arginase: A multifaceted enzyme important in health and disease. Physiol Rev. 98:641–665. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Monticelli LA, Buck MD, Flamar AL, Saenz SA, Tait Wojno ED, Yudanin NA, Osborne LC, Hepworth MR, Tran SV, Rodewald HR, et al: Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nat Immunol. 17:656–665. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Chen T, Huang X, Zhao YX, Zhou ZW and Zhou WS: NEAT1 inhibits the angiogenic activity of cerebral arterial endothelial cells by inducing the M1 polarization of microglia through the AMPK signaling pathway. Cell Mol Biol Lett. 29:622024. View Article : Google Scholar : PubMed/NCBI | |
|
Niu G and Chen X: Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr Drug Targets. 11:1000–1017. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Cho H, Kambhampati SP, Lai MJ, Zhou L, Lee G, Xie Y, Hui Q, Kannan RM and Duh EJ: Dendrimer-triamcinolone acetonide reduces neuroinflammation, pathological angiogenesis, and neuroretinal dysfunction in ischemic retinopathy. Adv Ther (Weinh). 4:20001812021. View Article : Google Scholar : PubMed/NCBI | |
|
Fan W, Huang W, Chen J, Li N, Mao L and Hou S: Retinal microglia: Functions and diseases. Immunology. 166:268–286. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Wang S, Zhang J, Chen J, Tang L, Ke M, Xue Y, He Y, Gong Y and Li Z: ω-3PUFAs inhibit hypoxia-induced retinal neovascularization via regulating microglial pyroptosis through METTL14-mediated m6A modification of IFNB1 mRNA. Appl Biochem Biotechnol. 196:5936–5952. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Mei X, Zhou L, Zhang T, Lu B, Sheng Y and Ji L: Chlorogenic acid attenuates diabetic retinopathy by reducing VEGF expression and inhibiting VEGF-mediated retinal neoangiogenesis. Vasc Pharmacol. 101:29–37. 2018. View Article : Google Scholar | |
|
Yu Z, Zhang T, Gong C, Sheng Y, Lu B, Zhou L, Ji L and Wang Z: Erianin inhibits high glucose-induced retinal angiogenesis via blocking ERK1/2-regulated HIF-1α-VEGF/VEGFR2 signaling pathway. Sci Rep. 6:343062016. View Article : Google Scholar | |
|
Zhao K, Jiang Y, Zhang J, Shi J, Zheng P, Yang C and Chen Y: Celastrol inhibits pathologic neovascularization in oxygen-induced retinopathy by targeting the miR-17-5p/HIF-1α/VEGF pathway. Cell Cycle. 21:2091–2108. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Guo L, Choi S, Bikkannavar P and Cordeiro MF: Microglia: Key players in retinal ageing and neurodegeneration. Front Cell Neurosci. 16:8047822022. View Article : Google Scholar : PubMed/NCBI | |
|
Shao F, Wang X, Wu H, Wu Q and Zhang J: Microglia and neuroinflammation: Crucial pathological mechanisms in traumatic brain injury-induced neurodegeneration. Front Aging Neurosci. 14:8250862022. View Article : Google Scholar : PubMed/NCBI | |
|
Gao C, Jiang J, Tan Y and Chen S: Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct Target Ther. 8:3592023. View Article : Google Scholar : PubMed/NCBI | |
|
Yang I, Han SJ, Kaur G, Crane C and Parsa AT: The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci. 17:6–10. 2010. View Article : Google Scholar | |
|
Masuda T, Sankowski R, Staszewski O and Prinz M: Microglia heterogeneity in the single-cell era. Cell Rep. 30:1271–1281. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Jiao W, Ji J, Li F, Guo J, Zheng Y, Li S and Xu W: Activation of the Notch-Nox4-reactive oxygen species signaling pathway induces cell death in high glucose-treated human retinal endothelial cells. Mol Med Rep. 19:667–677. 2019. | |
|
Seo H, Park SJ and Song M: Diabetic retinopathy (DR): Mechanisms, current therapies, and emerging strategies. Cells. 14:3762025. View Article : Google Scholar : PubMed/NCBI | |
|
Radwan SES, El-Kamel A, Zaki EI, Burgalassi S, Zucchetti E and El-Moslemany RM: Hyaluronic-coated albumin nanoparticles for the non-invasive delivery of apatinib in diabetic retinopathy. Int J Nanomedicine. 16:4481–4494. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang S, Yang H, Zheng J, Tong A, Mu S, Wang D, Zhao M and Li J: Recent advances and prospects of nanoparticle-based drug delivery for diabetic ocular complications. Theranostics. 15:3551–3570. 2025. View Article : Google Scholar : PubMed/NCBI |