The role of abnormal epigenetic regulation of small GTPases in glioma (Review)
- Authors:
- Published online on: July 2, 2025 https://doi.org/10.3892/ijo.2025.5769
- Article Number: 63
-
Copyright: © Zhang et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Brain tumors are one of the most severe types of malignant tumors, due to their poor prognosis, and serious impact on quality of life and cognitive function. Glioma accounts for ~30% of all primary brain tumors and 80% of malignant brain tumors (1,2). Meanwhile, glioblastoma (GBM) is the most aggressive malignant glioma and constitute ~49% of malignant brain tumors (3,4). The main treatment methods for glioma are surgical resection combined with adjuvant radiotherapy and chemotherapy (5). Although clinicians and researchers have explored new treatment approaches, patient survival has not yet been markedly improved (6). Glioma has obvious heterogeneity due to the fact that it can originate from different precursor cells, which complicates treatment (7,8). Based on the similarity of tissue morphology to that of normal brain cells, glioma is divided into different types, including astrocytoma, oligodendroglioma, oligoastrocytoma, ependymoma, and neuronal and mixed neuronal-glial tumors (8,9). However, there are two major disadvantages of the histological classification system; one is the obvious interobserver variability and the other is the ability to accurately predict prognosis in patients with glioma (9). To overcome these shortcomings, the World Health Organization (WHO) classification criteria of tumors of the central nervous system (CNS) underwent revisions in 2016 (10). For the first time, the WHO classification criteria used a combination of histological and molecular features to diagnose CNS tumors (10). In addition, with the development of new diagnostic technologies, an increasing number of molecular markers [including TERT promoter mutations and epidermal growth factor receptor (EGFR) amplification] are being introduced into diagnostic criteria, which are beneficial for more precise classification (11). These molecular markers are conducive to the accurate prediction of patient prognosis and personalized treatment. With in-depth research on the pathogenesis of glioma, novel molecular markers have emerged, including certain members of the small guanosine triphosphate (GTP)ases (12-14).
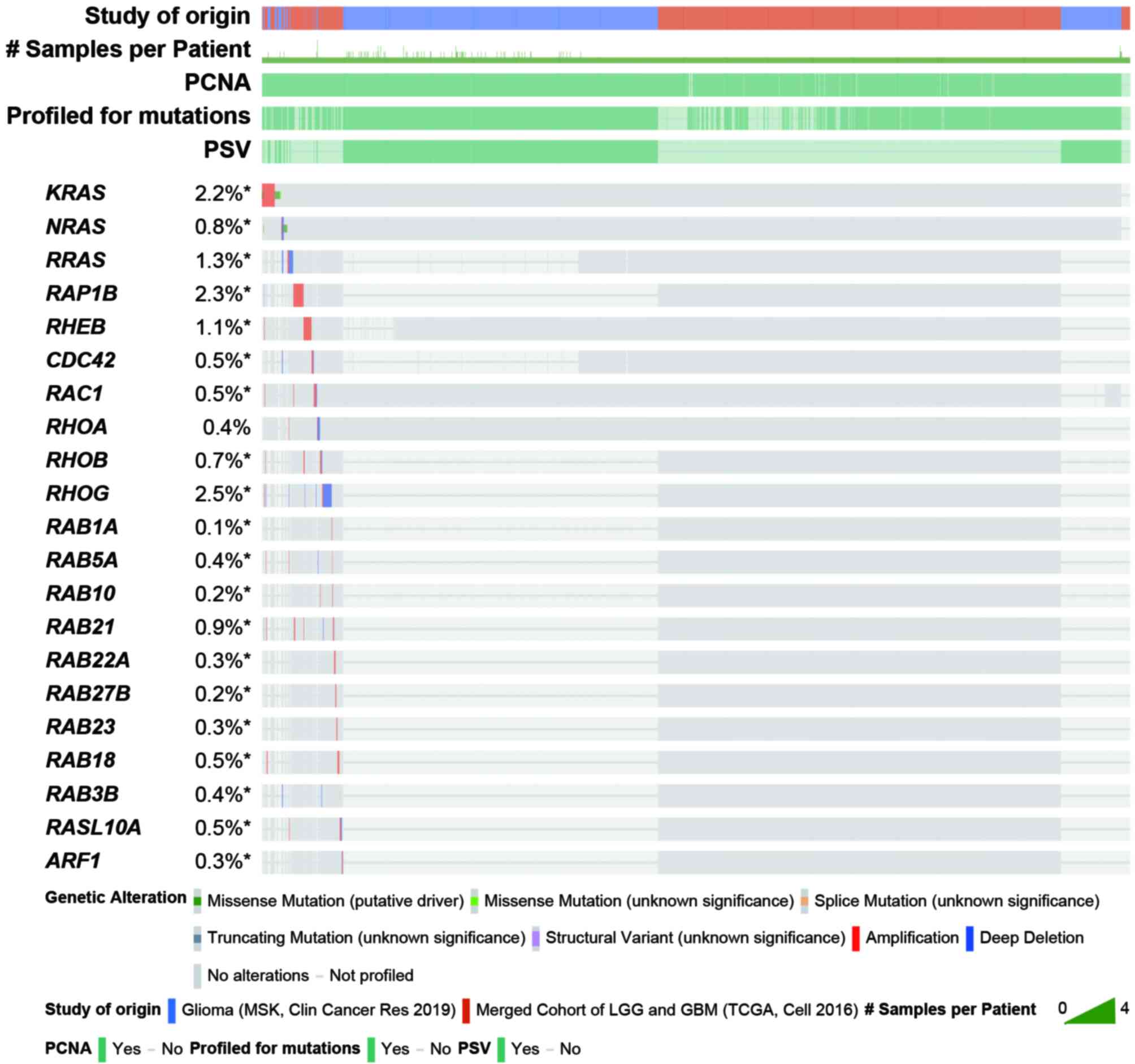
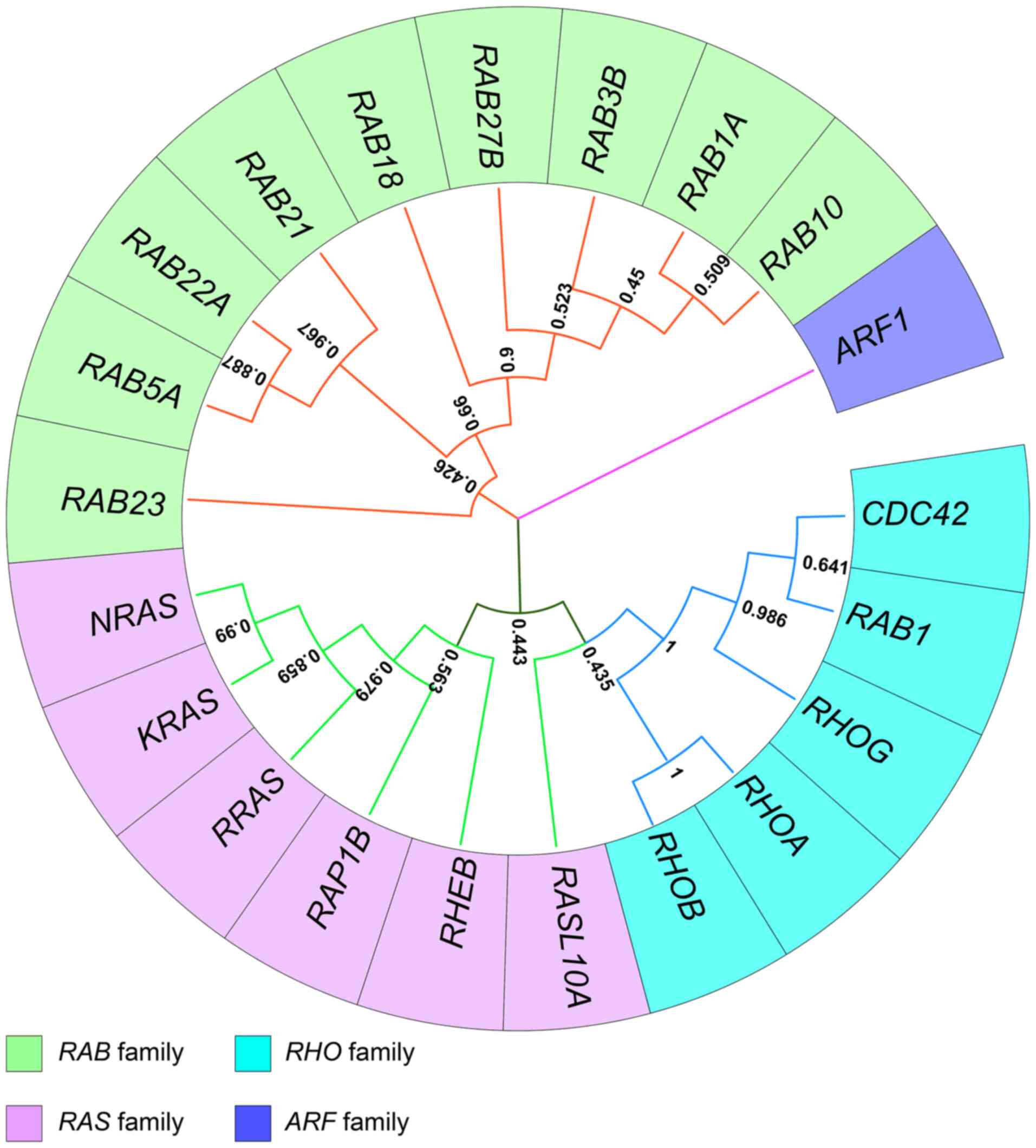
The small GTPases, also known as the small G proteins or RAS superfamily, is a large protein family, which can be roughly divided into five subfamilies, namely the RAS subfamily, RHO subfamily, RAB subfamily, ADP-ribosylation factor (ARF) subfamily and RAN subfamily (15), which have similar modes of action. They are binary molecular switches that can cycle between an active GTP-bound conformation and an inactive guanosine diphosphate (GDP)-bound conformation (16). This process is strictly controlled by guanine nucleotide exchange factors that facilitate GDP dissociation, GTPase-activating proteins that stimulate GTP hydrolysis, and guanine nucleotide dissociation inhibitors that form soluble complexes with small GTPases by shielding their lipid (17,18). These small GTPases participate in most cellular biological processes, including differentiation, proliferation, cell migration, apoptosis, vesicle and organelle dynamics and transport, nuclear dynamics and regulation of the cytoskeleton (15,19). The mutation and abnormal expression of genes encoding small GTPases serve important roles in multiple diseases, including glioma (12,20-32). To investigate structural alterations in small GTPase genes in patients with glioma, a comprehensive analysis was performed utilizing data from Glioma (MSK, Clin Cancer Res 2019) and Merged Cohort of LGG and GBM (TCGA, Cell 2016) in cBioPortal (https://www.cbioportal.org) (33). The results demonstrated that the incidence of changes in small GTPase gene structure is considered to be low in glioma (Fig. 1) (33). Several studies have shown that the abnormal expression of genes encoding small GTPases is often influenced by epigenetic regulation in glioma (34-66) (Tables I and II). To elucidate the evolutionary relationships among subfamily members, phylogenetic analysis was performed using MEGA11 (67) (Fig. 2).
Table ICharacteristics of studies, including patient samples, cell line-based models in vitro and xenograft models in vivo. |
Epigenetic regulation is defined as an inheritable change in gene activity without significant alterations in genomic sequences (68), and it is a dynamic and reversible process. Epigenetic modifications are important for normal tissue growth, and the development and regulation of temporal and spatial expression of genes (69). The abnormal epigenetic regulation of genes participates in the initiation, development and maintenance of various diseases, including neoplasms (70-77). Epigenetic modifications include DNA methylation, histone or chromatin post-translational modifications (PTMs) and non-coding RNA (ncRNA) regulations (78).
DNA methylation is the most quintessential epigenetic modification, which is able to regulate gene expression, genomic stability and chromatin structure (69,79). DNA methylation mainly occurs on CpG sites, and higher frequency CpG sites are known as CpG islands, which are usually unmethylated (80-82). In addition, the abnormal hypermethylation of promoter CpG islands is able to silence tumor suppressor genes that contribute to tumorigenesis (83). DNA methyltransferases (DNMTs) and ten-eleven translocation proteins serve important roles in CpG methylation sites (84,85). Nucleosomes are the most basic units of chromatin, which are formed by DNA strands wrapping around histone octamers (86). DNA methylation and histone PTMs synergistically regulate chromatin structure and gene regulation (87,88). The majority of histone PTMs occur on the N- or C-terminal tails of histones that extend away from the nucleosome core particle, including methylation, acetylation, ubiquitylation, phosphorylation, SUMOylation, ADP ribosylation, citrullination and biotinylation (78,89). For histone PTMs, most studies have focused on acetylation, and the methylation of lysine residues on H3 and H4 (78,90-92). Different histone PTMs have different biological functions (93). For example, abnormal H3K27me3 can lead to the inactivation of tumor suppressor genes (such as SOX7 and KLF6) (90). Conversely, the dysregulation of H3K27ac can increase the expression levels of oncogenes (such as BCL6 and BCL11A) (91,92).
ncRNAs also serve an important role in epigenetic regulation. The vast majority of the genome cannot encode proteins and is transcribed into RNA termed ncRNA (94). ncRNAs can be divided into two categories based on the number of bases: Small ncRNAs and long ncRNAs (lncRNAs) (95). Small ncRNAs include Piwi-interacting RNAs, small interfering RNAs, microRNAs (miRNAs/miRs) and some bacterial regulatory RNAs (96). lncRNAs comprise linear lncRNAs and circular RNAs (circRNAs) (97). The most extensively studied small ncRNAs are miRNAs, which regulate 60% of protein-coding genes through complementarily binding to the 3′UTR of target mRNAs leading to their degradation or translational repression (98,99). miRNAs can exert both anticancer and pro-cancer functions depending on its target genes and the cell context. lncRNAs measure >200 nucleotides in length and have a low sequence conservation among species (100,101); however, they are more tissue-specific than mRNAs (102). lncRNAs can biologically function as chromatin regulators, enhancers, ncRNA sponges and molecular scaffolds, among others, in the nucleus or cytoplasm (78). circRNAs are produced through the back-splicing of linear transcripts and have circular structures that make them insensitive to exonuclease; they also have a higher stability than linear RNAs (103,104). The similarity between circRNAs and lncRNAs lies in their obvious tissue specificity, with the difference being that circRNAs are relatively conserved in evolution. The forms in which circRNAs function are diverse. For example, they serve as adsorption sponges for miRNAs (105), and protein scaffolds or templates for translation, among others (106). The abnormal expression of lncRNAs and circRNAs can disrupt their biological functions leading to oncogenesis (107).
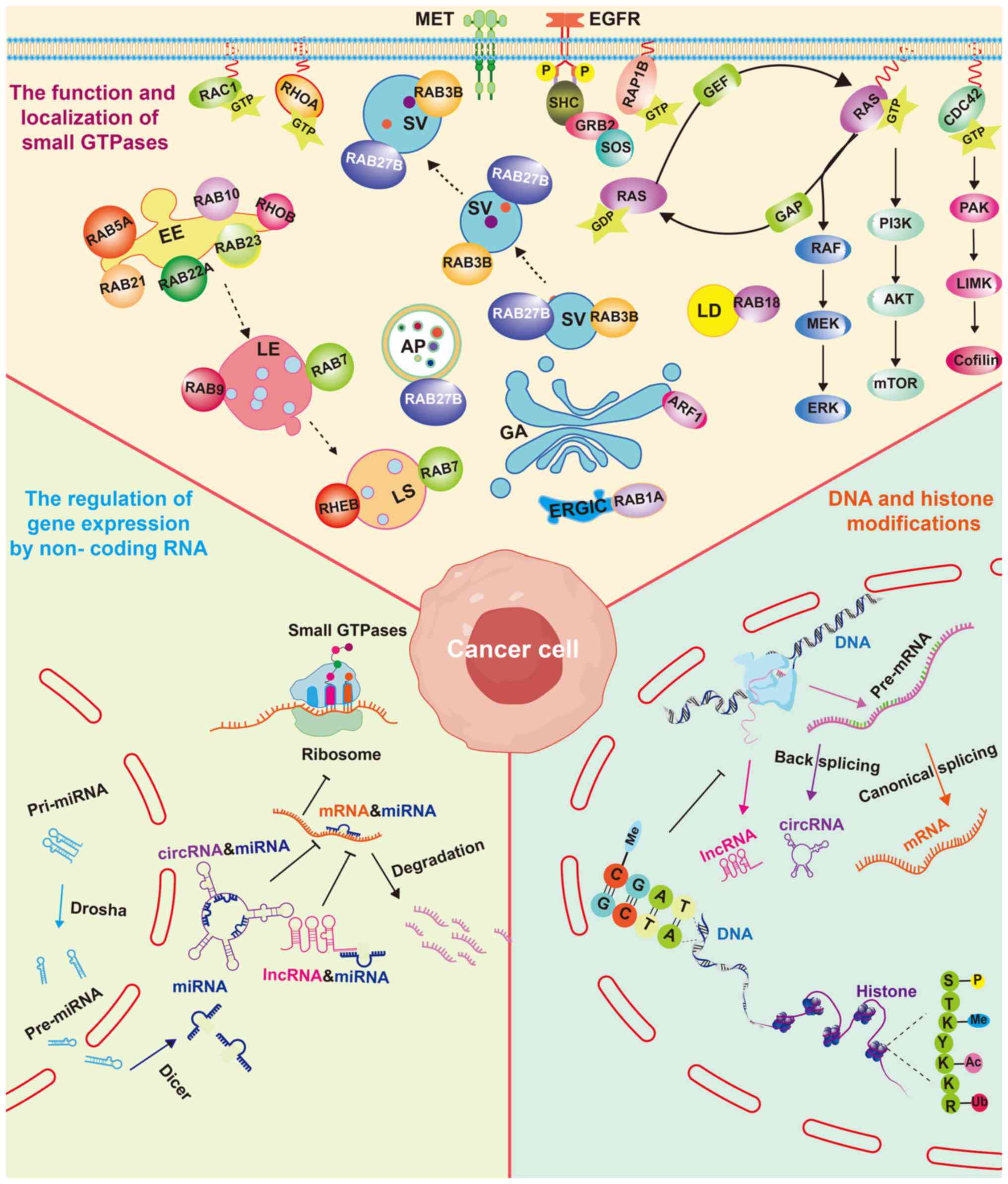
Small GTPases, as important biomolecules, serve vital roles in the development of glioma; however, they rarely undergo changes in gene structure in glioma, which indicates that epigenetic regulation may be involved in regulating their abnormal expression. Due to the dynamic and reversible nature of epigenetic regulation, they have the potential to become therapeutic targets. In addition, ncRNAs can be released from tumors into the blood and urine, and thus may serve a role as diagnostic and prognostic biomarkers, since these samples can be obtained through non-invasive means. The aforementioned advantages have led to extensive research being conducted to elucidate the mechanism underlying the epigenetic regulation of small GTPases in glioma (34-66,108-111) (Tables I and II; Fig. 3). In order to promote clinical translation and offer references for follow-up studies, these studies were reviewed herein.
RAS family
The RAS subfamily contains 35 members, six of which are mentioned in the current review, namely KRAS, NRAS, RRAS, RAS-associated protein-1B (RAP1B), RHEB and RAS like protein family member 10A (RASL10A) (Fig. 2) (34-40,42-46,112).
There are three classic RAS genes, HRAS, NRAS and KRAS, which encode four highly homologous protein isoforms (HRAS, NRAS, KRAS4A and KRAS4B) (113). It is well known that RAS genes are proto-oncogenes, and ~19% of patients with cancer carry RAS mutations (114). Although RAS signaling pathways are often abnormally activated in patients with GBM, the RAS mutation frequency is very low (115). As is widely recognized, RAS protein is the most critical downstream effector of EGFR, which can directly activate RAS via GRB2/SOS recruitment (116). Subsequently, the RAF-MEK-ERK1/2 signaling cascade is activated, which can regulate cell proliferation, differentiation, motility, and survival (117). Abnormal EGFR often leads to aberrant RAS activation (118). Studies have demonstrated that 57.4% of patients with GBM carry EGFR amplifications or mutations, and EGFR variant III (EGFRvIII) has been reported in 25-33% of patients with GBM (119,120). EGFRvIII exhibits ligand-independent constitutive activation and can directly activate RAS, which results in aberrant RAS activation (120). Some studies have also shown that the upregulation of wild-type RAS can promote oncogenesis, including in glioma (121,122). This implies that combined therapy targeting mutant EGFR and inhibiting RAS expression could potentially improve glioma treatment outcomes.
Certain studies have shown that downregulated miRNAs, which are negative regulatory factors of RAS genes, may contribute to overactivated RAS signaling pathways in glioma (34-38). Wang et al (34) reported that miR-181d is downregulated and KRAS is upregulated in glioma tissues compared with those in normal tissues. In addition, this previous study confirmed that miR-181d can directly target KRAS to restrain RAS/RAF/MEK/ERK and RAS/PI3K/PTEN/AKT signaling pathways, which promote apoptosis and cell cycle arrest, and restrain the proliferation of glioma cells. Furthermore, Wang et al (35) showed that the expression of miRNA let-7a is lower in high-grade glioma compared with that in low-grade glioma, and patients with a low level of miRNA let-7a have a poor prognosis. In addition, the results of this study suggested a negative association between miRNA let-7a and KRAS expression in glioma. Furthermore, it was reported that miRNA let-7a may directly target KRAS to suppress downstream signaling pathways, which affect proliferation, apoptosis, migration and invasion in glioma cells independent of their PTEN mutation status (35).
RRAS is a close relative of the classic RAS protein (123); however, unlike the classic RAS gene, the RRAS gene rarely undergoes mutations in human cancer (123). Certain studies have reported that the aberrant expression of RRAS contributes to tumors of the human CNS (124,125). Shi et al (36) reported that the expression levels of miR-124 are clearly reduced in glioma compared with those in normal tissues, and a negative correlation has been observed between the expression of miR-124 and that of RRAS/NRAS. This previous study also revealed that RRAS and NRAS are the direct targets of miR-124, and the re-expression of miR-124 can block the PI3K/AKT and RAF/ERK1/2 pathways, which are major downstream effectors of RRAS and NRAS molecules (36). These results ultimately resulted in the inhibition of cell proliferation, invasion and angiogenesis, and increased the chemosensitivity of glioma cells. In addition, it was revealed that RRAS and NRAS can synergistically regulate vascular endothelial growth factor transcriptional activation (36). Zhang et al (37) reported that activated receptor tyrosine kinases, mesenchymal to epithelial transition factor (MET), EGFR and platelet-derived growth factor receptors might be capable of decreasing the expression levels of miR-134 in glioma. Furthermore, it was confirmed that MET could suppress the expression of miR-134 via mitogen-activated protein kinase and Krüppel-like factor 4. A reduction in the expression levels of miR-134 was shown to lead to upregulation of KRAS and the transcription factor signal transducer and activator of transcription 5B, as targets of miR-134 (37). These aforementioned processes may promote GBM cell proliferation and GBM stem cell (GSC) neuro-sphere formation, and block the differentiation of GSCs (37). Zhao et al (38) also revealed the antitumor effects of miR-134 by targeting KRAS in glioma.
RAP1 also belongs to the RAS subfamily and is involved in regulating cell adhesion, cell-cell junctions, migration and polarization (126). RAP1 possesses two isoforms: RAP1A and RAP1B; RAP1A mainly participates in maintaining cell-cell junctions, and RAP1B mainly regulates dynamic changes in cell-cell junctions (127,128). Previous studies have shown that the upregulation of RAP1B can contribute to the development of various types of cancer (129,130). She et al (39,40) reported that miR-181 subunits miR-128 and miR-149 are lowly expressed, and RAP1B is highly expressed in glioma, as a target of miR-181 subunits (miR-128 and miR-149). miR-181 subunits miR-128 and miR-149 have been shown to suppress cell proliferation and invasion, and affect the cytoskeleton remodeling of glioma cells by controlling RAP1B (39,40). Notably, miR-181 subunits miR-128 and miR-149 may enhance the chemosensitivity of glioma cells for temozolomide by targeting RAP1B (39,40). Li et al (41) indicated that the expression levels of lncRNA MALAT1 and RAP1B are increased, and those of miR-101 are decreased in glioma cell lines, compared with those in normal human astrocytes (NHAs). Furthermore, this previous study elucidated that lncRNA MALAT1 serves as a sponge of miR-101 to decrease miR-101 expression, and then increases RAP1B expression, which can promote the proliferation and block the apoptosis of glioma cell lines (41). Wan et al (42) identified that transient receptor potential cation channel, subfamily M, member 7 facilitates the proliferation and invasion of glioma cells by suppressing miR-28-5p targeting RAP1B.
RHEB is found in species from yeast to humans, and has only a single effector, the target of rapamycin (TOR) Ser/Thr kinase (15,131). Aberrantly activated RHEB/mammalian TOR complex 1 (mTORC1) signaling is associated with proliferative disorders and tumorigenesis (132). RHEB upregulation is rarely observed in glioma cell lines compared with in normal brain cells (133); however, the aberrantly activated RTK/PI3K/AKT/mTOR signaling pathway serves a critical role in the development of glioma (134,135). RHEB is a key activator of TOR, and targeting RHEB may therefore suppress the progression of glioma by blocking mTOR signaling (15). Besse et al (43) demonstrated that miR-338-5p, which is a brain-specific miRNA, may partially exert antitumor effects by suppressing RHEB in glioma. Kalhori et al (44,45) reported that miR-579, miR-548x and miR-4698 are able to simultaneously target AKT1 and RHEB to inhibit proliferation, migration and process of the cell cycle, and promote apoptosis in glioma. However, these studies are limited to cell lines and lack clinical validation.
RHO family
The RHO subfamily has 20 members and can be divided into six groups: RHO subfamily (RHOA, RHOB and RHOC), RAC family small GTPase (RAC) subfamily (RAC1, RAC2, RAC3 and RHOG), cell division cycle 42 (CDC42) subfamily (CDC42, WRCH1, TC10, CHP and TCL), RND subfamily (RND1, RND2 and RND3), RHO BTB subfamily (RHOBTB1, RHOBTB2 and RHOBTB3) and MIRO subfamily (MIRO1 and MIRO2) (136). Their biological functions mainly include the regulation of cytoskeletal rearrangement, cell motility, cell polarity, axon guidance, vesicle trafficking and the cell cycle process (137). Dysfunctional RHO GTPase has been reported to be involved in the development of various diseases, including cancer, immunological disorders and neurological abnormalities (136). The present review mainly focuses on the abnormal epigenetic regulation of RHOA, RHOB, RHOG, RAC1 and CDC42 in glioma (47-52).
The most well-known function of RHOA is to regulate actin-myosin contractility and stress fiber formation, which drive cell motility and invasion, suggesting that RHOA signaling may be implicated in tumor invasion and metastasis (138). In addition, studies have established that the RHOA/ROCK signaling pathway can regulate the migration and invasion of glioma (139-141). Several research groups have concentrated on elucidating the mechanisms by which ncRNAs control RHOA expression in glioma. Tang et al (50) reported that leucine-rich repeat (LRR), a brain-specific gene, is able to upregulate miR-185, and indicated that the expression levels of LRR and miR-185 are lower in glioma tissues than those in normal tissues. This previous study also elucidated that miR-185 can suppress the invasion of glioma cells and the expression of vascular endothelial growth factor A by directly targeting CDC42 and RHOA (50). Notably, a significantly positive association has been reported between the expression of LRR and miR-185, and patient prognosis (50). RHOB regulates actin organization and vesicle transport, which is similar to that of RHOA and RHOC (142); however, RHOB mainly exerts anticancer function in several types of cancer (143-145). RHOA, RHOB and RHOC have homologous effector domains and the effectors of RHOA can interact with RHOB in a specific environment (146). RHOB may block the tumor-promoting function of RHOA and RHOC through competitive binding (146). The abnormal downregulation of RHOB has been shown to contribute to the progression of certain types of cancer, including glioma (25,51,147,148). Chen et al (51) reported that miR-19a expression is increased in glioma tissues, and glioma cell proliferation and invasion are promoted by miR-19a. Furthermore, it has been revealed that miR-19a performs cancer-promoting functions by directly targeting RHOB (51).
The RAC subfamily contains four members, namely RAC1, RAC2, RAC3 and RHOG (149). The RAC subfamily regulates membrane ruffling and lamellipodia formation by interacting with effector proteins (150). RAC1 is one of the most studied members, and it is involved in several types of cellular processes, including cell cytoskeletal reorganization, cell transformation, DNA synthesis, superoxide generation, axonal guidance and cell migration (151). Numerous studies have shown that RAC1 participates in cell transformation and tumor progression (152-154). Sun et al (48) reported that miR-137 expression is reduced in glioma tissues and that it is negatively correlated with RAC1 in glioma cell lines. It has also been reported that miR-137 hinders human glioma cell proliferation by directly targeting RAC1 (48). Subsequently, Qin et al (49) revealed that miR-142 can suppress the migration and invasion of glioma by targeting RAC1. A previous study also indicated that RHOG is involved in regulating the development of glioma (155). Cai et al (52) demonstrated that there is a negative correlation between the expression levels of miR-124-3p and RHOG in patients with glioma. It was also revealed that miR-124-3p can block the viability and motility of GBM by directly targeting RHOG (52).
As members of a RHO subfamily, CDC42 regulate the actin cytoskeletal architecture (156). CDC42 has also been shown to be involved in the establishment of cell polarity (157). CDC42 is upregulated in various types of cancer, and is closely associated with oncogenic transformation, invasion and tumorigenesis (22,158,159). Shi et al (47) revealed that the expression levels of miR-29a/b/c are reduced with an increase in tumor grade and indicated that they have a good prognostic value. They also showed that miR-29a/b/c directly targets CDC42, and there is a negative correlation between the expression of miR-29a/b/c and that of CDC42 in patients with glioma (47). In addition, miR-29a/b/c could suppress glioma invasion by blocking the CDC42-PAK pathway (47).
RASL10A, also known as Ras-related protein on chromosome 22 (46), is member of the more distal branches of the RAS family and was designated as a 'distal-RAS' by Bernal Astrain et al (112). However, phylogenetic analysis revealed its closer affinity to the RHO subfamily; therefore, the present study has elaborated on this (Fig. 2). The RASL10A gene is located on a region of chromosome 22, which frequently undergoes loss of heterozygosity in human cancer and RASL10A is considered a tumor suppressor gene (160). Schmidt et al (46) reported that the mRNA levels of RASL10A are reduced in most glioma tissues compared with those in normal tissues, with few RASL10A mutations and allelic deletions observed (46). This finding is consistent with the previously mentioned results, which revealed that the incidence of changes in small GTPase gene structure is considered to be low in glioma (Fig. 1). In addition, it has been reported that the hypermethylation of the RASL10A 5′-CpG island and hypoacetylation of H3 and H4 contribute to a low RASL10A mRNA level (46). Notably, RASL10A mRNA level can serve as a prognostic marker in glioma (46).
RAB family
The RAB family is the largest subgroup of the RAS superfamily, which comprises ~70 members that function as controllers of vesicle traffic, membrane tethering and fusion (161-163). Each RAB has its own specific membrane localization, which is beneficial for controlling the specificity and directionality of membrane trafficking pathways (161). The abnormal expression and activity of RABs are associated with the occurrence and progression of various tumors (164-168). RAB1A, RAB3B, RAB5A, RAB10, RAB21, RAB22A, RAB27B, RAB23 and RAB18 are mentioned in the present review (53-65).
RAB1 is able to regulate dynamic membrane trafficking between the endoplasmic reticulum (ER) and Golgi apparatus, and evidence has shown that it is also involved in nutrient sensing and signaling, cell migration and the presentation of cell-surface receptors (169). RAB1 contains two isoforms, RAB1A and RAB1B, which share 92% amino-acid sequence homology and are functionally interchangeable (170,171). RAB1A and RAB1B are mainly located at the ER and the Golgi apparatus membrane, and are also observed in lipid rafts and autophagosomes (172). The dysregulation of RAB1A and RAB1B are associated with the initiation and development of multiple types of cancer, including glioma (173-176). Quan et al (53) reported that miR-1202 is decreased in glioma, and the expression levels of miR-1202 are negatively correlated with RAB1A. Furthermore, it was revealed that that miR-1202 can suppress the proliferation, and induce ER stress and apoptosis in glioma by directly targeting RAB1A (53). Subsequently, Xu et al (54) demonstrated that lncRNA DANCR is increased in glioma compared with that in normal tissues. It was also revealed that lncRNA DANCR functions as competing endogenous RNA by directly targeting miR-634, and there is an inverse correlation between the expression levels of lncRNA DANCR and miR-634 in glioma tissues (54). This previous study also verified that lncRNA DANCR promotes glioma cell proliferation through the lncRNA DANCR/miR-634/RAB1A axis (54).
RAB5 is able to mediate intracellular trafficking, both at the level of receptor endocytosis and endosomal dynamics (177). RAB5 comprises RAB5A, RAB5B and RAB5C, which can be separately recognized by diverse kinases, but there are no differences in their function in endocytosis (178). Previous studies have indicated that RAB5A is also implicated in the regulation of autophagy (179,180). The abnormal expression of RAB5A has been shown to be associated with the initiation and development of various types of cancer, including glioma (27,167,181,182). Fu et al (55) confirmed that lncRNA MALAT1 is elevated in glioma tissues. Notably, lncRNA MALAT1 enhances the proliferation of glioma cells by activating autophagy (55) and it partially achieves the aforementioned functions through the lncRNA MALAT1/miR-101/RAB5A axis (55). Similarly, Gao et al (56) revealed that another lncRNA TP53 target 1 (TP53TG1) is clearly increased in glioma tissues and strengthens the radioresistance of glioma cells by enhancing autophagy through the lncRNA TP53TG1/miR-524-5p/RAB5A axis. Wu et al (58) demonstrated that lncRNA cancer susceptibility 19 is clearly increased in glioma tissues, and promotes the proliferation, migration and invasion of glioma cells through the miR-454-3p/RAB5A axis. Zhang et al (57) showed that propofol hinders glioma cell proliferation and metastasis by regulating the circRNA non-SMC condensin I complex subunit G/miR-200a-3p/RAB5A axis.
RAB10 is mainly located at the ER and Golgi/Trans-Golgi network (183-185). Moreover, it is associated with endosomes/phagosomes and primary cilia (186,187). RAB10 is mainly involved in regulating ER dynamics and morphology, polarized trafficking, establishment of basement membrane polarity and tubular endosome formation (183,188-190). Dysregulation of RAB10 has been shown to be associated with the initiation and progress of various types of cancer (191,192). Zhang et al (60) verified that circ-PTN expression is increased and that of miR-432-5p is decreased in glioma. In addition, it was reported that circ-PTN enhances the proliferation, invasion and glycolysis of glioma cells via the circ-PTN/miR-432-5p/RAB10 axis (60). By analyzing public databases, Shen et al (59) revealed that lncRNA PSMB8-AS1 is increased in glioma and is upregulated in glioma cells via NHA. Subsequently, it was indicated that lncRNA PSMB8-AS1 activated by the ETS transcription factor ELK1 enhances glioma cell proliferation by mediating the miR-574-5p/RAB10 axis (59). Peng et al (61) revealed that LINC00152, an oncogene, is increased in glioma and is a valuable prognostic factor. It was also reported that LINC00152 accelerates glioma cell proliferation and invasion through the miR-107/RAB10 axis (61).
Eukaryotic cells internalize fractions of cytomembrane, cell surface receptors and diverse soluble molecules from the extracellular fluid through the endocytosis pathway (193). These are vital to a series of cellular functions, including nutrient absorption, signaling receptor downregulation and antigen processing (194). RAB5, RAB21 and RAB22 all reside in the early endosome and regulate early endosome dynamics (194,195). RAB23 is also involved in mediating early endosome dynamics and is a negative regulator of hedgehog signaling (196). Previous studies have revealed that the dysregulation of these genes is able to disturb endosome dynamics, which lead to the occurrence of cancer (197-200). Song et al (62) reported that circ_0030018 abundance is higher in glioma cell lines compared with that in NHAs. It was subsequently suggested that circ_0030018 accelerates cell proliferation and metastasis, and blocks apoptosis and cell cycle arrest in glioma by regulating the miR-1297/RAB21 axis (62). Similarly, RAB22A is regulated by ncRNA in glioma. Xia et al (63) reported that miR-204-5p expression is reduced in glioma tissues compared with that in normal tissues, and is negatively associated with pathology classification. It has also been shown that miR-204-5p inhibits glioma cell proliferation, migration and invasion by directly targeting RAB22A (63). Notably, miR-200b can suppress the progression of glioma by targeting a set of RABs (65). Liu et al (65) demonstrated that miR-200b, a tumor suppressor, is able to directly target RAB3B, RAB18, RAB21 and RAB23. Notably, their expression levels have a good prognostic value (65). RAB21 and RAB23 can regulate endosome dynamics; RAB3 serves a critical role in the regulation of exocytosis and contains two isoform, RAB3A and RAB3B (201); RAB18 is a lipid droplet-associated small GTPase, and its abnormal expression disturbs the storage and mobilization of lipids (202). It is also involved in ER structure maintenance and ER-Golgi trafficking (203,204). These findings indicate that miR-200b inhibits cancer progression by regulating multiple biological processes.
RAB27 is an important regulator of secretory pathways and comprises two isoforms, RAB27A and RAB27B (205). Notably, it has been reported that RAB27A and RAB27B serve different roles in specific types of secretion by interacting with various effectors (205). Ostrowski et al (206) reported that RAB27A and RAB27B are involved in multivesicular endosomes docking at the plasma membrane, but both serve different roles in the exosome secretion pathway via their effectors SLP4 and SLAC2B, respectively (206). The aberrant regulation of RAB27 is associated with the initiation and progression of cancer (207). Wang et al (64) reported that the RAB27B promoter region is hypomethylated in high-grade glioma compared with that in low-grade glioma, and identified a negative association between RAB27B abundance and RAB27B methylation level. Furthermore, it was revealed that RAB27B enhances glioma invasion by activating matrix metalloproteinase-9 (64). Notably, the RAB27B methylation level is a valuable prognostic factor of glioma (64).
ARF family
The ARF family mainly controls membrane traffic and organelle structure, and is divided into three classes: Class I (ARF1, ARF2 and ARF3), Class II (ARF4 and ARF5) and Class III (ARF6) (208). Class I ARFs exist in all eukaryotic organisms and are highly conserved (208). They are able to mediate the fabrication of various types of 'coat' complexes onto budding vesicles along the secretory pathway, and activate lipid-modifying enzymes (209). The present review focusses on ARF1.
ARF1 is the most studied mammalian ARF protein, primarily located at the Golgi apparatus (210). ARF1 mainly regulates the recruitment of effectors, coat protein complex I, adaptor protein 1 and Golgi-associated, γ-adaptin homologs, ARF-interacting proteins on the Golgi apparatus (211). Several studies have shown that the abnormal expression and activation of ARF1 are involved in the occurrence and progression of cancer (212-214). In a previous study, 40-50% of patients with GBM carried EGFR amplification (215). López-Ginés et al (66) explored the correlation between EGFR amplification and the promoter methylation status of 10 genes relevant to GBM. The results revealed that the ARF1 promoter methylation level was lower in the EGFR amplification group compared with that in the group without EGFR amplification; however, the mRNA expression levels of ARF1 were higher in the EGFR amplification group (66). ARF1 overexpression was also shown to cause metabolic reprogramming, which may promote the progression of glioma (66).
Clinical application prospects
Gliomas account for the vast majority of primary malignant brain tumors (2). Although there are a number of novel treatment methods for glioma, most are in the preclinical experimental stage (216). The current treatment methods for glioma are still limited, and include surgery, radiotherapy and chemotherapy (217). The efficacy of the treatment methods is unsatisfactory, and patients often exhibit drug resistance, relapse and metastasis (218). It is urgent to explore new diagnostic and therapeutic methods for glioma. Due to the diversity and importance of the biological functions of small GTPases, an increasing number of studies have focused on them; however, the incidence of changes in small GTPase gene structure is low in glioma (Fig. 1). Numerous studies have focused on exploring the epigenetic regulation of small GTPase genes in glioma. Epigenetic regulation is a dynamic and reversible process, which implies that the reversion of abnormal epigenetic modifications is a good treatment strategy for glioma. These studies not only provide new therapeutic targets and prognostic markers, but also supply new clues for the treatment of glioma.
DNMT inhibitors (DNMTIs)
DNA methylation is more stable than other epigenetic modifications (219). The hypomethylation of oncogenes and hypermethylation of tumor suppressor genes can disrupt normal gene expression, which promotes the occurrence and development of cancer (220). However, the treatment methods for the dysregulation of DNA mainly focuses on the high methylation of tumor suppressor genes. DNMTIs, include 5-azacytidine and 5-aza-2′-deoxycytidine, the most important therapeutic drugs for abnormal DNA methylation (221). These drugs have achieved good therapeutic effects in the treatment of hematological tumors (222). Isocitrate dehydrogenase (IDH) mutations in glioma cause genome-wide DNA hypermethylation (9). A small sample size study previously demonstrated that 5-azacitidine treatment is suitable for a subset of patients with IDH1/2-mutated gliomas, particularly those without prior bevacizumab treatment (223). This previous study also revealed that 5-azacitidine treatment induces adverse effects, with grade 3-4 neutropenia being the most frequently observed, which led to dose reduction and some patients having to receive concomitant granulocyte colony stimulating factor injections during the following cycles (223). Although this study had a small sample size, its findings still offer valuable clinical insights. Schmidt et al (46) demonstrated that the mRNA levels of RASL10A were reduced in most glioma tissues compared with in normal tissues, and that the hypermethylation of the RASL10A 5′-CpG island and hypoacetylation of H3 and H4 contributed to a low RASL10A mRNA level. These findings indicated that patients with glioma carrying both IDH1/2 mutations and RASL10A hypermethylation, which enhance the progression of glioma, are more likely to benefit from 5-azacitidine treatment. Complementary to conventional methylation inhibitor therapy, emerging gene editing approaches are being investigated as precision tools against epigenetically aberrant tumors. He et al (224) reported that the dCas9-TET1 fusion system can successfully remove methyl groups at CpG islands in the ZNF154 promoter region, and inhibit the proliferation and migration of esophageal squamous carcinoma cells. However, their clinical applicability, particularly for glioma treatment, requires further investigation.
Histone deacetylase inhibitors (HDACIs)
DNA methylation and histone PTMs synergistically regulate chromatin structure and gene regulation (225). Histone methylation and acetylation are the most extensively studied epigenetic modifications (225). To date, there are no US Food and Drug Administration (FDA)-approved drugs that directly target histone methylation (226). However, accumulating studies have demonstrated the therapeutic potential of HDACIs against various malignancies, including glioma (227-230). Belinostat is a HDACI that can cross the blood-brain barrier. Xu et al (231) reported on the possibility of combining belinostat with standard-of-care therapy in GBM. Panobinostat represents the first HDACI approved by the FDA for the treatment of relapsed multiple myeloma (232). Monje et al (229) explored the tolerability of systemically administered panobinostat in children with diffuse intrinsic pontine glioma (DIPG)/diffuse midline glioma. This study revealed constrained tolerability of panobinostat in pediatric patients with DIPG, and indicated that panobinostat did not improve the OS and PFS of patients with glioma (229). In addition, Mueller et al (230) assessed the safety and efficacy of repeated delivery of aqueous panobinostat via convection-enhanced delivery in patients with newly diagnosed DIPG. By contrast, their results demonstrated that patients with DIPG may benefit from this treatment method (230). But some treatment-related adverse events need to be taken seriously, including muscle weakness, vagus nerve dysfunction and neutropenia (230). In this previous study, patients with DIPG underwent both postoperative recovery and drug infusion, with temporal overlap between these procedures. Both interventions carry the potential to induce certain adverse effects (such as vagus nerve dysfunction), making it clinically challenging to determine the predominant causative factor. Given these considerations, only grade ≥3 neutropenia was attributed to aqueous panobinostat (230). Patients with grade ≥3 neutropenia may need to receive concomitant granulocyte colony stimulating factor injections during the following cycles; however, the precise therapeutic safety and efficacy of this strategy require further validation through large-scale cohort studies. As previously mentioned, Schmidt et al (46) reported that hypoacetylation of H3 and H4 of RASL10A, which is a potential tumor suppressor, can contribute to a low RASL10A mRNA level. Notably, patients with glioma and hypoacetylation of H3 and H4 of RASL10A have a higher chance of benefiting from repeated delivery of aqueous panobinostat via convection-enhanced delivery.
ncRNAs
The most extensively studied small ncRNAs are miRNAs that can exert both anticancer and pro-cancer functions, depending on their target genes and the cell context. Most miRNAs mentioned in the present review target different types of small GTPases and function as anticancer genes. In addition, previous studies have shown that miRNAs have strong and complex functions, and the same miRNA can target the mRNA of different genes and different miRNAs can target the mRNA of the same gene (35,38,65). Multiple miRNAs may therefore be available for selection to reverse the abnormal expression of the same gene, and the re-expression of one miRNA may reverse the dysregulation of multiple genes that mediate diverse signaling pathways in glioma. The re-expression of these tumor suppressor miRNAs may be a promising therapeutic approach for glioma. miR-34 is a tumor suppressor that serves an important role in cell proliferation in various types of cancer (233). MRX34 is a lipid-formulated miR-34 that is currently in phase I testing in patients with solid tumors, and is the first miRNA mimic to enter clinical trials (234). However, due to the presence of the blood-brain barrier, the treatment of brain tumors is different from that of other tumors. There is a lack of data supporting whether miRNAs can enter the brain tissue through the blood (235). With technological developments, these limitations are expected to be resolved. For example, biodegradable nanoparticles loaded with miRNA mimics have previously been implanted at the glioma resection site to enable localized therapeutic delivery, which circumvented the delivery bottleneck of miRNA mimics across the blood-brain barrier (236). Exosomes, liposomes and gold nanoparticles with miRNA mimics may also be used for systemic delivery (235). These methods may be applied to the miRNAs discussed in the present study, which could reverse the expression of miRNAs and small GTPases to suppress the development of glioma. To date, to the best of our knowledge, no clinical trials have been conducted to investigate the efficacy of miRNA in glioma treatment. However, some studies have shown that serum miRNAs are valuable prognostic factors for glioma (237,238). Compared with tissue biopsies, serum miRNA detection provides a simpler and less painful alternative for patients; however, the diagnostic value of serum miRNAs that target small GTPases in glioma needs to be explored. Furthermore, lncRNA and circRNA are rarely studied as therapeutic tools; however, they are better prognostic and progression markers than miRNAs due to their stability. Some studies have revealed that serum lncRNAs and serum exosome circRNAs are valuable diagnostic biomarkers for glioma (239-242). Notably, LINC00152 may have the potential to become a prognostic marker of glioma; however, further studies are needed to validate its application as a peripheral biomarker for glioma.
Conclusions
The biological functions of small GTPases are diverse, and have an important role in the initiation and development of glioma. Notably, the incidence of changes in small GTPase gene structure is low in glioma; therefore, researchers have focused more on studying their epigenetic regulation. The present study reviewed relevant studies that explored the epigenetic regulation mechanisms of small GTPases in glioma to provide a reference for future research and to promote the clinical translation of relevant study results. Although translating these research achievements into clinical practice requires further research, the findings of the aforementioned studies provide novel prognostic factors for glioma and lay the theoretical groundwork for epigenetic therapeutic approaches in glioma. It is anticipated that progress in epigenetic drug discovery and delivery methodologies will lead to marked improvements in the prognosis of patients with glioma.
Availability of data and materials
Not applicable.
Authors' contributions
MZ designed the study and wrote the original draft. YH conducted the literature search and study selection. QZ and XZ conducted comprehensive data extraction, data analysis and critical analysis of all literature, subsequently creating standardized tables and figures. JW and LK got funding acquisition and reviewed the manuscript. Data authentication is not applicable. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Abbreviations:
|
ARF |
ADP-ribosylation factor |
|
CDC42 |
cell division cycle 42 |
|
circRNAs |
circular RNAs |
|
CNS |
central nervous system |
|
DNMTs |
DNA methyltransferases |
|
EGFR |
epidermal growth factor receptor |
|
ER |
endoplasmic reticulum |
|
GBM |
glioblastoma |
|
GDP |
guanosine diphosphate |
|
GSCs |
glioblastoma stem cells |
|
GTP |
guanosine triphosphate |
|
lncRNAs |
long non-coding RNAs |
|
MET |
mesenchymal to epithelial transition factor |
|
miRNAs |
microRNAs |
|
NHAs |
normal human astrocytes |
|
PTMs |
post-translational modifications |
|
RAC1 |
RAC family small GTPase 1 |
|
RAP1 |
RAS-associated protein-1 |
|
RASL10A |
RAS like protein family member 10A |
|
TOR |
target of rapamycin |
Acknowledgments
Not applicable.
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province (grant no. LQ21H160040).
References
|
Price M, Neff C, Nagarajan N, Kruchko C, Waite KA, Cioffi G, Cordeiro BB, Willmarth N, Penas-Prado M, Gilbert MR, et al: CBTRUS statistical report: American brain tumor association & NCI system tumors neuro-oncology branch adolescent and young adult primary brain and other central nervous diagnosed in the United States in 2016-2020. Neuro Oncol. 26(Suppl 3): iii1–iii53. 2024. View Article : Google Scholar | |
|
Weller M, Wen PY, Chang SM, Dirven L, Lim M, Monje M and Reifenberger G: Glioma. Nat Rev Dis Primers. 10:332024. View Article : Google Scholar : PubMed/NCBI | |
|
Sanders S, Herpai DM, Rodriguez A, Huang Y, Chou J, Hsu FC, Seals D, Mott R, Miller LD and Debinski W: The presence and potential role of ALDH1A2 in the glioblastoma microenvironment. Cells. 10:24852021. View Article : Google Scholar : PubMed/NCBI | |
|
Schaff LR and Mellinghoff IK: Glioblastoma and other primary brain malignancies in adults: A Review. JAMA. 329:574–587. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Yasinjan F, Xing Y, Geng H, Guo R, Yang L, Liu Z and Wang H: Immunotherapy: A promising approach for glioma treatment. Front Immunol. 14:12556112023. View Article : Google Scholar : PubMed/NCBI | |
|
Xu S, Tang L, Li X, Fan F and Liu Z: Immunotherapy for glioma: Current management and future application. Cancer Lett. 476:1–12. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Weller M, Wick W, Aldape K, Brada M, Berger M, Pfister SM, Nishikawa R, Rosenthal M, Wen PY, Stupp R and Reifenberger G: Glioma. Nat Rev Dis Primers. 1:150172015. View Article : Google Scholar : PubMed/NCBI | |
|
Wang LM, Englander ZK, Miller ML and Bruce JN: Malignant glioma. Adv Exp Med Biol. 1405:1–30. 2023. View Article : Google Scholar | |
|
Chen R, Smith-Cohn M, Cohen AL and Colman H: Glioma subclassifications and their clinical significance. Neurotherapeutics. 14:284–297. 2017. View Article : Google Scholar | |
|
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P and Ellison DW: The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, et al: The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 23:1231–1251. 2021. View Article : Google Scholar : | |
|
Luo Q, Liu Y, Yuan Z, Huang L and Diao B: Expression of Rab3b in human glioma: Influence on cell proliferation and apoptosis. Curr Pharm Des. 27:989–995. 2021. View Article : Google Scholar | |
|
Wang M, Jiang X, Yang Y, Chen H, Zhang C, Xu H, Qi B, Yao C and Xia H: Rhoj is a novel target for progression and invasion of glioblastoma by impairing cytoskeleton dynamics. Neurotherapeutics. 17:2028–2040. 2020. View Article : Google Scholar | |
|
Xu J, Galvanetto N, Nie J, Yang Y and Torre V: Rac1 promotes cell motility by controlling cell mechanics in human glioblastoma. Cancers (Basel). 12:16672020. View Article : Google Scholar | |
|
Reiner DJ and Lundquist EA: Small GTPases. WormBook. 2018:1–65. 2018. View Article : Google Scholar | |
|
Simanshu DK, Nissley DV and McCormick F: RAS proteins and their regulators in human disease. Cell. 170:17–33. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Ridley AJ: Life at the leading edge. Cell. 145:1012–1022. 2011. View Article : Google Scholar | |
|
Cherfils J and Zeghouf M: Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 93:269–309. 2013. View Article : Google Scholar | |
|
Chen K, Zhang Y, Qian L and Wang P: Emerging strategies to target RAS signaling in human cancer therapy. J Hematol Oncol. 14:1162021. View Article : Google Scholar : PubMed/NCBI | |
|
Zuo T, Wong S, Buza N and Hui P: KRAS mutation of extraovarian implants of serous borderline tumor: Prognostic indicator for adverse clinical outcome. Mod Pathol. 31:350–357. 2018. View Article : Google Scholar | |
|
Zhao S, Li Z, Zhang M, Zhang L, Zheng H, Ning J, Wang Y, Wang F, Zhang X, Gan H, et al: A brain somatic RHEB doublet mutation causes focal cortical dysplasia type II. Exp Mol Med. 51:1–11. 2019. | |
|
Gómez Del Pulgar T, Valdés-Mora F, Bandrés E, Pérez-Palacios R, Espina C, Cejas P, García-Cabezas MA, Nistal M, Casado E, González-Barón M, et al: Cdc42 is highly expressed in colorectal adenocarcinoma and downregulates ID4 through an epigenetic mechanism. Int J Oncol. 33:185–193. 2008. | |
|
Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, Harbeck N, Schmitt M and Lengyel E: Rac1 in human breast cancer: Overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene. 19:3013–3020. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Li XR, Ji F, Ouyang J, Wu W, Qian LY and Yang KY: Overexpression of RhoA is associated with poor prognosis in hepatocellular carcinoma. Eur J Surg Oncol. 32:1130–1134. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Mazieres J, Antonia T, Daste G, Muro-Cacho C, Berchery D, Tillement V, Pradines A, Sebti S and Favre G: Loss of RhoB expression in human lung cancer progression. Clin Cancer Res. 10:2742–2750. 2004. View Article : Google Scholar | |
|
Shimada K, Uzawa K, Kato M, Endo Y, Shiiba M, Bukawa H, Yokoe H, Seki N and Tanzawa H: Aberrant expression of RAB1A in human tongue cancer. Br J Cancer. 92:1915–1921. 2005. View Article : Google Scholar | |
|
Li Y, Sun X, Ji D, Kong X, Liu D, Zhao Z, Yan J and Chen S: Expression of Rab5a correlates with tumor progression in pancreatic carcinoma. Virchows Arch. 470:527–536. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Hu X, Yin J, He R, Chao R and Zhu S: Circ_KCNQ5 participates in the progression of childhood acute myeloid leukemia by enhancing the expression of RAB10 via binding to miR-622. Hematology. 27:431–440. 2022. View Article : Google Scholar | |
|
Ge J, Chen Q, Liu B, Wang L, Zhang S and Ji B: Knockdown of Rab21 inhibits proliferation and induces apoptosis in human glioma cells. Cell Mol Biol Lett. 22:302017. View Article : Google Scholar : PubMed/NCBI | |
|
Yin Y, Zhang B, Wang W, Fei B, Quan C, Zhang J, Song M, Bian Z, Wang Q, Ni S, et al: miR-204-5p inhibits proliferation and invasion and enhances chemotherapeutic sensitivity of colorectal cancer cells by downregulating RAB22A. Clin Cancer Res. 20:6187–6199. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang JX, Huang XX, Cai MB, Tong ZT, Chen JW, Qian D, Liao YJ, Deng HX, Liao DZ, Huang MY, et al: Overexpression of the secretory small GTPase Rab27B in human breast cancer correlates closely with lymph node metastasis and predicts poor prognosis. J Transl Med. 10:2422012. View Article : Google Scholar : PubMed/NCBI | |
|
You X, Liu F, Zhang T, Li Y, Ye L and Zhang X: Hepatitis B virus X protein upregulates oncogene Rab18 to result in the dysregulation of lipogenesis and proliferation of hepatoma cells. Carcinogenesis. 34:1644–1652. 2013. View Article : Google Scholar | |
|
de Bruijn I, Kundra R, Mastrogiacomo B, Tran TN, Sikina L, Mazor T, Li X, Ochoa A, Zhao G, Lai B, et al: Analysis and visualization of longitudinal genomic and clinical data from the AACR Project GENIE biopharma collaborative in cBioPortal. Cancer Res. 83:3861–3867. 2023. View Article : Google Scholar : | |
|
Wang XF, Shi ZM, Wang XR, Cao L, Wang YY, Zhang JX, Yin Y, Luo H, Kang CS, Liu N, et al: MiR-181d acts as a tumor suppressor in glioma by targeting K-ras and Bcl-2. J Cancer Res Clin Oncol. 138:573–584. 2012. View Article : Google Scholar | |
|
Wang XR, Luo H, Li HL, Cao L, Wang XF, Yan W, Wang YY, Zhang JX, Jiang T, Kang CS, et al: Overexpressed let-7a inhibits glioma cell malignancy by directly targeting K-ras, independently of PTEN. Neuro Oncol. 15:1491–1501. 2013. View Article : Google Scholar : | |
|
Shi Z, Chen Q, Li C, Wang L, Qian X, Jiang C, Liu X, Wang X, Li H, Kang C, et al: MiR-124 governs glioma growth and angiogenesis and enhances chemosensitivity by targeting R-Ras and N-Ras. Neuro Oncol. 16:1341–1353. 2014. View Article : Google Scholar : | |
|
Zhang Y, Kim J, Mueller AC, Dey B, Yang Y, Lee DH, Hachmann J, Finderle S, Park DM, Christensen J, et al: Multiple receptor tyrosine kinases converge on microRNA-134 to control KRAS, STAT5B, and glioblastoma. Cell Death Differ. 21:720–734. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao Y, Pang D, Wang C, Zhong S and Wang S: MicroRNA-134 modulates glioma cell U251 proliferation and invasion by targeting KRAS and suppressing the ERK pathway. Tumour Biol. 37:11485–11493. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
She X, Yu Z, Cui Y, Lei Q, Wang Z, Xu G, Luo Z, Li G and Wu M: miR-181 subunits enhance the chemosensitivity of temozolomide by Rap1B-mediated cytoskeleton remodeling in glioblastoma cells. Med Oncol. 31:8922014. View Article : Google Scholar : PubMed/NCBI | |
|
She X, Yu Z, Cui Y, Lei Q, Wang Z, Xu G, Xiang J, Wu M and Li G: miR-128 and miR-149 enhance the chemosensitivity of temozolomide by Rap1B-mediated cytoskeletal remodeling in glioblastoma. Oncol Rep. 32:957–964. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Li Z, Xu C, Ding B, Gao M, Wei X and Ji N: Long non-coding RNA MALAT1 promotes proliferation and suppresses apoptosis of glioma cells through derepressing Rap1B by sponging miR-101. J Neurooncol. 134:19–28. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Wan J, Guo AA and Chowdhury I: TRPM7 induces mechanistic target of Rap1b through the downregulation of miR-28-5p in glioma proliferation and invasion. Front Oncol. 9:14132019. View Article : Google Scholar | |
|
Besse A, Sana J, Lakomy R, Kren L, Fadrus P, Smrcka M, Hermanova M, Jancalek R, Reguli S, Lipina R, et al: MiR-338-5p sensitizes glioblastoma cells to radiation through regulation of genes involved in DNA damage response. Tumour Biol. 37:7719–7727. 2016. View Article : Google Scholar | |
|
Kalhori MR, Irani S, Soleimani M, Arefian E and Kouhkan F: The effect of miR-579 on the PI3K/AKT pathway in human glioblastoma PTEN mutant cell lines. J Cell Biochem. 120:16760–16774. 2019. View Article : Google Scholar | |
|
Kalhori MR, Arefian E, Fallah Atanaki F, Kavousi K and Soleimani M: miR-548x and miR-4698 controlled cell proliferation by affecting the PI3K/AKT signaling pathway in Glioblastoma cell lines. Sci Rep. 10:15582020. View Article : Google Scholar : PubMed/NCBI | |
|
Schmidt N, Windmann S, Reifenberger G and Riemenschneider MJ: DNA hypermethylation and histone modifications downregulate the candidate tumor suppressor gene RRP22 on 22q12 in human gliomas. Brain Pathol. 22:17–25. 2012. View Article : Google Scholar | |
|
Shi C, Ren L, Sun C, Yu L, Bian X, Zhou X, Wen Y, Hua D, Zhao S, Luo W, et al: miR-29a/b/c function as invasion suppressors for gliomas by targeting CDC42 and predict the prognosis of patients. Br J Cancer. 117:1036–1047. 2017. View Article : Google Scholar | |
|
Sun G, Cao Y, Shi L, Sun L, Wang Y, Chen C, Wan Z, Fu L and You Y: Overexpressed miRNA-137 inhibits human glioma cells growth by targeting Rac1. Cancer Biother Radiopharm. 28:327–334. 2013.PubMed/NCBI | |
|
Qin W, Rong X, Dong J, Yu C and Yang J: miR-142 inhibits the migration and invasion of glioma by targeting Rac1. Oncol Rep. 38:1543–1550. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Tang H, Wang Z, Liu X, Liu Q, Xu G, Li G and Wu M: LRRC4 inhibits glioma cell growth and invasion through a miR-185-dependent pathway. Curr Cancer Drug Targets. 12:1032–1042. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Q, Guo W, Zhang Y, Wu Y and Xiang J: MiR-19a promotes cell proliferation and invasion by targeting RhoB in human glioma cells. Neurosci Lett. 628:161–166. 2016. View Article : Google Scholar | |
|
Cai S, Shi CJ, Lu JX, Wang YP, Yuan T and Wang XP: miR-124-3p inhibits the viability and motility of glioblastoma multiforme by targeting RhoG. Int J Mol Med. 47:692021. View Article : Google Scholar | |
|
Quan Y, Song Q, Wang J, Zhao L, Lv J and Gong S: MiR-1202 functions as a tumor suppressor in glioma cells by targeting Rab1A. Tumour Biol. 39:10104283176975652017. View Article : Google Scholar : PubMed/NCBI | |
|
Xu D, Yu J, Gao G, Lu G, Zhang Y and Ma P: LncRNA DANCR functions as a competing endogenous RNA to regulate RAB1A expression by sponging miR-634 in glioma. Biosci Rep. 38:BSR201716642018. View Article : Google Scholar : | |
|
Fu Z, Luo W, Wang J, Peng T, Sun G, Shi J, Li Z and Zhang B: Malat1 activates autophagy and promotes cell proliferation by sponging miR-101 and upregulating STMN1, RAB5A and ATG4D expression in glioma. Biochem Biophys Res Commun. 492:480–486. 2017. View Article : Google Scholar | |
|
Gao W, Qiao M and Luo K: Long noncoding RNA TP53TG1 contributes to radioresistance of glioma cells via miR-524-5p/RAB5A axis. Cancer Biother Radiopharm. 36:600–612. 2021. | |
|
Zhang L, Chen H, Tian C and Zheng D: Propofol represses cell growth and metastasis by modulating the circular RNA Non-SMC condensin I complex subunit G/MicroRNA-200a-3p/RAB5A axis in glioma. World Neurosurg. 153:e46–e58. 2021. View Article : Google Scholar | |
|
Wu YJ, Yang QS, Chen H, Wang JT, Wang WB and Zhou L: Long non-coding RNA CASC19 promotes glioma progression by modulating the miR-454-3p/RAB5A axis and is associated with unfavorable MRI features. Oncol Rep. 45:728–737. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Shen G, Mao Y, Su Z, Du J, Yu Y and Xu F: PSMB8-AS1 activated by ELK1 promotes cell proliferation in glioma via regulating miR-574-5p/RAB10. Biomed Pharmacother. 122:1096582020. View Article : Google Scholar | |
|
Zhang X, Wang S, Lin G and Wang D: Down-regulation of circ-PTN suppresses cell proliferation, invasion and glycolysis in glioma by regulating miR-432-5p/RAB10 axis. Neurosci Lett. 735:1351532020. View Article : Google Scholar : PubMed/NCBI | |
|
Peng G, Su J, Xiao S and Liu Q: LINC00152 acts as a potential marker in gliomas and promotes tumor proliferation and invasion through the LINC00152/miR-107/RAB10 axis. J Neurooncol. 154:285–299. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Song D, Ye L, Xu Z, Jin Y and Zhang L: CircRNA hsa_ circ_0030018 regulates the development of glioma via regulating the miR-1297/RAB21 axis. Neoplasma. 68:391–403. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Xia Z, Liu F, Zhang J and Liu L: Decreased expression of MiRNA-204-5p contributes to glioma progression and promotes glioma cell growth, migration and invasion. PLoS One. 10:e01323992015. View Article : Google Scholar | |
|
Wang H, Wang Y, Bao Z, Zhang C, Liu Y, Cai J and Jiang C: Hypomethylated Rab27b is a progression-associated prognostic biomarker of glioma regulating MMP-9 to promote invasion. Oncol Rep. 34:1503–1509. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Q, Tang H, Liu X, Liao Y, Li H, Zhao Z, Yuan X and Jiang W: miR-200b as a prognostic factor targets multiple members of RAB family in glioma. Med Oncol. 31:8592014. View Article : Google Scholar | |
|
López-Ginés C, Navarro L, Muñoz-Hidalgo L, Buso E, Morales JM, Gil-Benso R, Gregori-Romero M, Megías J, Roldán P, Segura-Sabater R, et al: Association between epidermal growth factor receptor amplification and ADP-ribosylation factor 1 methylation in human glioblastoma. Cell Oncol (Dordr). 40:389–399. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Tamura K, Stecher G and Kumar S: MEGA11: Molecular evolutionary genetics analysis version 11. Mol Biol Evol. 38:3022–3027. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Chen QW, Zhu XY, Li YY and Meng ZQ: Epigenetic regulation and cancer (review). Oncol Rep. 31:523–532. 2014. View Article : Google Scholar | |
|
Gu M, Ren B, Fang Y, Ren J, Liu X, Wang X, Zhou F, Xiao R, Luo X, You L and Zhao Y: Epigenetic regulation in cancer. MedComm (2020). 5:e4952024. View Article : Google Scholar : PubMed/NCBI | |
|
Zou Y and Xu H: Involvement of long noncoding RNAs in the pathogenesis of autoimmune diseases. J Transl Autoimmun. 3:1000442020. View Article : Google Scholar | |
|
Zong L, Zhou L, Hou Y, Zhang L, Jiang W, Zhang W, Wang L, Luo X, Wang S, Deng C, et al: Genetic and epigenetic regulation on the transcription of GABRB2: Genotype-dependent hydroxymethylation and methylation alterations in schizophrenia. J Psychiatr Res. 88:9–17. 2017. View Article : Google Scholar | |
|
Bai B, Chen S, Zhang Q, Jiang Q and Li H: Abnormal epigenetic regulation of the gene expression levels of Wnt2b and Wnt7b: Implications for neural tube defects. Mol Med Rep. 13:99–106. 2016. View Article : Google Scholar | |
|
Li P, Han M, Zhao X, Ren G, Mei S and Zhong C: Abnormal epigenetic regulations in the immunocytes of sjögren's syndrome patients and therapeutic potentials. Cells. 11:17672022. View Article : Google Scholar | |
|
Agirre X, Martínez-Climent JÁ, Odero M and Prosper F: Epigenetic regulation of miRNA genes in acute leukemia. Leukemia. 26:395–403. 2012. View Article : Google Scholar | |
|
Zeng C, Tsoi LC and Gudjonsson JE: Dysregulated epigenetic modifications in psoriasis. Exp Dermatol. 30:1156–1166. 2021. View Article : Google Scholar | |
|
Ramazi S, Dadzadi M, Sahafnejad Z and Allahverdi A: Epigenetic regulation in lung cancer. MedComm (2020). 4:e4012023. View Article : Google Scholar | |
|
Kondo Y, Katsushima K, Ohka F, Natsume A and Shinjo K: Epigenetic dysregulation in glioma. Cancer Sci. 105:363–369. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Lu Y, Chan YT, Tan HY, Li S, Wang N and Feng Y: Epigenetic regulation in human cancer: The potential role of epi-drug in cancer therapy. Mol Cancer. 19:792020. View Article : Google Scholar : PubMed/NCBI | |
|
Robertson KD: DNA methylation and chromatin-unraveling the tangled web. Oncogene. 21:5361–5379. 2002. View Article : Google Scholar | |
|
Moore LD, Le T and Fan G: DNA methylation and its basic function. Neuropsychopharmacology. 38:23–38. 2013. View Article : Google Scholar | |
|
Illingworth RS and Bird AP: CpG islands-'a rough guide'. FEBS Lett. 583:1713–1720. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Antequera F: Structure, function and evolution of CpG island promoters. Cell Mol Life Sci. 60:1647–1658. 2003. View Article : Google Scholar | |
|
Lopez-Serra L, Ballestar E, Fraga MF, Alaminos M, Setien F and Esteller M: A profile of methyl-CpG binding domain protein occupancy of hypermethylated promoter CpG islands of tumor suppressor genes in human cancer. Cancer Res. 66:8342–8346. 2006. View Article : Google Scholar | |
|
Hervouet E, Peixoto P, Delage-Mourroux R, Boyer-Guittaut M and Cartron PF: Specific or not specific recruitment of DNMTs for DNA methylation, an epigenetic dilemma. Clin Epigenetics. 10:172018. View Article : Google Scholar : | |
|
Rasmussen KD and Helin K: Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 30:733–750. 2016. View Article : Google Scholar : | |
|
Hatakeyama A, Hartmann B, Travers A, Nogues C and Buckle M: High-resolution biophysical analysis of the dynamics of nucleosome formation. Sci Rep. 6:273372016. View Article : Google Scholar | |
|
Torres IO and Fujimori DG: Functional coupling between writers, erasers and readers of histone and DNA methylation. Curr Opin Struct Biol. 35:68–75. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Wang G, Weng R, Lan Y, Guo X, Liu Q, Liu X, Lu C and Kang J: Synergetic effects of DNA methylation and histone modification during mouse induced pluripotent stem cell generation. Sci Rep. 7:395272017. View Article : Google Scholar : PubMed/NCBI | |
|
Ng MK and Cheung P: A brief histone in time: Understanding the combinatorial functions of histone PTMs in the nucleosome context. Biochem Cell Biol. 94:33–42. 2016. View Article : Google Scholar | |
|
Ke XS, Qu Y, Rostad K, Li WC, Lin B, Halvorsen OJ, Haukaas SA, Jonassen I, Petersen K, Goldfinger N, et al: Genome-wide profiling of histone h3 lysine 4 and lysine 27 trimethylation reveals an epigenetic signature in prostate carcinogenesis. PLoS One. 4:e46872009. View Article : Google Scholar : PubMed/NCBI | |
|
Tamura I, Maekawa R, Jozaki K, Ohkawa Y, Takagi H, Doi-Tanaka Y, Shirafuta Y, Mihara Y, Taketani T, Sato S, et al: Transcription factor C/EBPβ induces genome-wide H3K27ac and upregulates gene expression during decidualization of human endometrial stromal cells. Mol Cell Endocrinol. 520:1110852021. View Article : Google Scholar | |
|
Sungalee S, Liu Y, Lambuta RA, Katanayeva N, Donaldson Collier M, Tavernari D, Roulland S, Ciriello G and Oricchio E: Histone acetylation dynamics modulates chromatin conformation and allele-specific interactions at oncogenic loci. Nat Genet. 53:650–662. 2021. View Article : Google Scholar | |
|
Reda A, Hategan LA, McLean TAB, Creighton SD, Luo JQ, Chen SES, Hua S, Winston S, Reeves I, Padmanabhan A, et al: Role of the histone variant H2A.Z.1 in memory, transcription, and alternative splicing is mediated by lysine modification. Neuropsychopharmacology. 49:1285–1295. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Nemeth K, Bayraktar R, Ferracin M and Calin GA: Non-coding RNAs in disease: From mechanisms to therapeutics. Nat Rev Genet. 25:211–232. 2024. View Article : Google Scholar | |
|
Sana J, Faltejskova P, Svoboda M and Slaby O: Novel classes of non-coding RNAs and cancer. J Transl Med. 10:1032012. View Article : Google Scholar : | |
|
Brosnan CA and Voinnet O: The long and the short of noncoding RNAs. Curr Opin Cell Biol. 21:416–425. 2009. View Article : Google Scholar | |
|
Qin T, Li J and Zhang KQ: Structure, regulation, and function of linear and circular long non-coding RNAs. Front Genet. 11:1502020. View Article : Google Scholar : | |
|
Friedman RC, Farh KK, Burge CB and Bartel DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19:92–105. 2009. View Article : Google Scholar : | |
|
Shang R, Lee S, Senavirathne G and Lai EC: microRNAs in action: Biogenesis, function and regulation. Nat Rev Genet. 24:816–833. 2023. View Article : Google Scholar | |
|
Uszczynska-Ratajczak B, Lagarde J, Frankish A, Guigó R and Johnson R: Towards a complete map of the human long non-coding RNA transcriptome. Nat Rev Genet. 19:535–548. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Khorkova O, Hsiao J and Wahlestedt C: Basic biology and therapeutic implications of lncRNA. Adv Drug Deliv Rev. 87:15–24. 2015. View Article : Google Scholar | |
|
Iwakiri J, Terai G and Hamada M: Computational prediction of lncRNA-mRNA interactionsby integrating tissue specificity in human transcriptome. Biol Direct. 12:152017. View Article : Google Scholar : PubMed/NCBI | |
|
Pisignano G, Michael DC, Visal TH, Pirlog R, Ladomery M and Calin GA: Going circular: History, present, and future of circRNAs in cancer. Oncogene. 42:2783–2800. 2023. View Article : Google Scholar | |
|
Ma S, Kong S, Wang F and Ju S: CircRNAs: Biogenesis, functions, and role in drug-resistant Tumours. Mol Cancer. 19:1192020. View Article : Google Scholar : | |
|
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function as efficient microRNA sponges. Nature. 495:384–388. 2013. View Article : Google Scholar | |
|
Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB and Kjems J: The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 20:675–691. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Yan H and Bu P: Non-coding RNA in cancer. Essays Biochem. 65:625–639. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Elam C, Hesson L, Vos MD, Eckfeld K, Ellis CA, Bell A, Krex D, Birrer MJ, Latif F and Clark GJ: RRP22 is a farnesylated, nucleolar, Ras-related protein with tumor suppressor potential. Cancer Res. 65:3117–3125. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Riemenschneider MJ, Reifenberger J and Reifenberger G: Frequent biallelic inactivation and transcriptional silencing of the DIRAS3 gene at 1p31 in oligodendroglial tumors with 1p loss. Int J Cancer. 122:2503–2510. 2008. View Article : Google Scholar | |
|
Tan Y, Zhang S, Xiao Q, Wang J, Zhao K, Liu W, Huang K, Tian W, Niu H, Lei T and Shu K: Prognostic significance of ARL9 and its methylation in low-grade glioma. Genomics. 112:4808–4816. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Rothhammer-Hampl T, Liesenberg F, Hansen N, Hoja S, Delic S, Reifenberger G and Riemenschneider MJ: Frequent epigenetic inactivation of DIRAS-1 and DIRAS-2 contributes to chemo-resistance in gliomas. Cancers (Basel). 13:51132021. View Article : Google Scholar | |
|
Bernal Astrain G, Nikolova M and Smith MJ: Functional diversity in the RAS subfamily of small GTPases. Biochem Soc Trans. 50:921–933. 2022. View Article : Google Scholar | |
|
Rásó E: Splice variants of RAS-translational significance. Cancer Metastasis Rev. 39:1039–1049. 2020. View Article : Google Scholar | |
|
Prior IA, Hood FE and Hartley JL: The frequency of ras mutations in cancer. Cancer Res. 80:2969–2974. 2020. View Article : Google Scholar | |
|
Knobbe CB, Reifenberger J and Reifenberger G: Mutation analysis of the Ras pathway genes NRAS, HRAS, KRAS and BRAF in glioblastomas. Acta Neuropathol. 108:467–470. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Wee P and Wang Z: Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel). 9:522017. View Article : Google Scholar : PubMed/NCBI | |
|
Sugiura R, Satoh R and Takasaki T: ERK: A double-edged sword in cancer. ERK-dependent apoptosis as a potential therapeutic strategy for cancer. Cells. 10:25092021. View Article : Google Scholar : PubMed/NCBI | |
|
Kim EK and Choi EJ: Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 1802:396–405. 2010. View Article : Google Scholar | |
|
An Z, Aksoy O, Zheng T, Fan QW and Weiss WA: Epidermal growth factor receptor and EGFRvIII in glioblastoma: Signaling pathways and targeted therapies. Oncogene. 37:1561–1575. 2018. View Article : Google Scholar | |
|
Yang J, Yan J and Liu B: Targeting EGFRvIII for glioblastoma multiforme. Cancer Lett. 403:224–230. 2017. View Article : Google Scholar | |
|
Maruyama C, Tomisawa M, Wakana S, Yamazaki H, Kijima H, Suemizu H, Ohnishi Y, Urano K, Hioki K, Usui T, et al: Overexpression of human H-ras transgene is responsible for tumors induced by chemical carcinogens in mice. Oncol Rep. 8:233–237. 2001.PubMed/NCBI | |
|
Tsunematsu S, Saito H, Kagawa T, Morizane T, Hata J, Nakamura T, Ishii H, Tsuchiya M, Nomura T and Katsuki M: Hepatic tumors induced by carbon tetrachloride in transgenic mice carrying a human c-H-ras proto-oncogene without mutations. Int J Cancer. 59:554–559. 1994. View Article : Google Scholar : PubMed/NCBI | |
|
Weber SM and Carroll SL: The role of R-Ras proteins in normal and pathologic migration and morphologic change. Am J Pathol. 191:1499–1510. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Gutierrez-Erlandsson S, Herrero-Vidal P, Fernandez-Alfara M, Hernandez-Garcia S, Gonzalo-Flores S, Mudarra-Rubio A, Fresno M and Cubelos B: R-RAS2 overexpression in tumors of the human central nervous system. Mol Cancer. 12:1272013. View Article : Google Scholar : PubMed/NCBI | |
|
Nakada M, Niska JA, Tran NL, McDonough WS and Berens ME: EphB2/R-Ras signaling regulates glioma cell adhesion, growth, and invasion. Am J Pathol. 167:565–576. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang YL, Wang RC, Cheng K, Ring BZ and Su L: Roles of Rap1 signaling in tumor cell migration and invasion. Cancer Biol Med. 14:90–99. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Altschuler D and Lapetina EG: Mutational analysis of the cAMP-dependent protein kinase-mediated phosphorylation site of Rap1b. J Biol Chem. 268:7527–7531. 1993. View Article : Google Scholar : PubMed/NCBI | |
|
Wittchen ES, Aghajanian A and Burridge K: Isoform-specific differences between Rap1A and Rap1B GTPases in the formation of endothelial cell junctions. Small GTPases. 2:65–76. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Lin KT, Yeh YM, Chuang CM, Yang SY, Chang JW, Sun SP, Wang YS, Chao KC and Wang LH: Glucocorticoids mediate induction of microRNA-708 to suppress ovarian cancer metastasis through targeting Rap1B. Nat Commun. 6:59172015. View Article : Google Scholar : PubMed/NCBI | |
|
Yang Y, Li M, Yan Y, Zhang J, Sun K, Qu JK, Wang JS and Duan XY: Expression of RAP1B is associated with poor prognosis and promotes an aggressive phenotype in gastric cancer. Oncol Rep. 34:2385–2394. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Heard JJ, Fong V, Bathaie SZ and Tamanoi F: Recent progress in the study of the Rheb family GTPases. Cell Signal. 26:1950–1957. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Armijo ME, Campos T, Fuentes-Villalobos F, Palma ME, Pincheira R and Castro AF: Rheb signaling and tumorigenesis: mTORC1 and new horizons. Int J Cancer. 138:1815–1823. 2016. View Article : Google Scholar | |
|
Basso AD, Mirza A, Liu G, Long BJ, Bishop WR and Kirschmeier P: The farnesyl transferase inhibitor (FTI) SCH66336 (lonafarnib) inhibits Rheb farnesylation and mTOR signaling. Role in FTI enhancement of taxane and tamoxifen anti-tumor activity. J Biol Chem. 280:31101–31108. 2005. View Article : Google Scholar | |
|
Mecca C, Giambanco I, Donato R and Arcuri C: Targeting mTOR in Glioblastoma: Rationale and Preclinical/Clinical Evidence. Dis Markers. 2018:92304792018. View Article : Google Scholar | |
|
Colardo M, Segatto M and Di Bartolomeo S: Targeting RTK-PI3K-mTOR axis in gliomas: An update. Int J Mol Sci. 22:48992021. View Article : Google Scholar | |
|
Liu M, Bi F, Zhou X and Zheng Y: Rho GTPase regulation by miRNAs and covalent modifications. Trends Cell Biol. 22:365–373. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Hodge RG and Ridley AJ: Regulating Rho GTPases and their regulators. Nat Rev Mol Cell Biol. 17:496–510. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
O'Connor K and Chen M: Dynamic functions of RhoA in tumor cell migration and invasion. Small GTPases. 4:141–147. 2013. View Article : Google Scholar | |
|
Yin M, Lu Q, Liu X, Wang T, Liu Y and Chen L: Silencing Drp1 inhibits glioma cells proliferation and invasion by RHOA/ROCK1 pathway. Biochem Biophys Res Commun. 478:663–668. 2016. View Article : Google Scholar | |
|
Xiong W, Yin A, Mao X, Zhang W, Huang H and Zhang X: Resveratrol suppresses human glioblastoma cell migration and invasion via activation of RhoA/ROCK signaling pathway. Oncol Lett. 11:484–490. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Talamillo A, Grande L, Ruiz-Ontañon P, Velasquez C, Mollinedo P, Torices S, Sanchez-Gomez P, Aznar A, Esparis-Ogando A, Lopez-Lopez C, et al: ODZ1 allows glioblastoma to sustain invasiveness through a Myc-dependent transcriptional upregulation of RhoA. Oncogene. 36:1733–1744. 2017. View Article : Google Scholar | |
|
Ridley AJ: Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 16:522–529. 2006. View Article : Google Scholar | |
|
Huang M and Prendergast GC: RhoB in cancer suppression. Histol Histopathol. 21:213–218. 2006. | |
|
Zaoui K and Duhamel S: RhoB as a tumor suppressor: It's all about localization. Eur J Cell Biol. 102:1513132023. View Article : Google Scholar : PubMed/NCBI | |
|
Wei LJ, Li JA, Bai DM and Song Y: miR-223-RhoB signaling pathway regulates the proliferation and apoptosis of colon adenocarcinoma. Chem Biol Interact. 289:9–14. 2018. View Article : Google Scholar | |
|
Prendergast GC: Actin' up: RhoB in cancer and apoptosis. Nat Rev Cancer. 1:162–168. 2001. View Article : Google Scholar | |
|
Liu M, Tang Q, Qiu M, Lang N, Li M, Zheng Y and Bi F: miR-21 targets the tumor suppressor RhoB and regulates proliferation, invasion and apoptosis in colorectal cancer cells. FEBS Lett. 585:2998–3005. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Cimbora-Zovko T, Fritz G, Mikac N and Osmak M: Downregulation of RhoB GTPase confers resistance to cisplatin in human laryngeal carcinoma cells. Cancer Lett. 295:182–190. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Kazanietz MG and Caloca MJ: The Rac GTPase in cancer: From old concepts to new paradigms. Cancer Res. 77:5445–5451. 2017. View Article : Google Scholar : | |
|
Steffen A, Ladwein M, Dimchev GA, Hein A, Schwenkmezger L, Arens S, Ladwein KI, Margit Holleboom J, Schur F, Victor Small J, et al: Rac function is crucial for cell migration but is not required for spreading and focal adhesion formation. J Cell Sci. 126:4572–4588. 2013.PubMed/NCBI | |
|
Bosco EE, Mulloy JC and Zheng Y: Rac1 GTPase: A 'Rac' of all trades. Cell Mol Life Sci. 66:370–374. 2009. View Article : Google Scholar | |
|
Leng R, Liao G, Wang H, Kuang J and Tang L: Rac1 expression in epithelial ovarian cancer: effect on cell EMT and clinical outcome. Med Oncol. 32:3292015. View Article : Google Scholar | |
|
Dokmanovic M, Hirsch DS, Shen Y and Wu WJ: Rac1 contributes to trastuzumab resistance of breast cancer cells: Rac1 as a potential therapeutic target for the treatment of trastuzumab-resistant breast cancer. Mol Cancer Ther. 8:1557–1569. 2009. View Article : Google Scholar | |
|
Heid I, Lubeseder-Martellato C, Sipos B, Mazur PK, Lesina M, Schmid RM and Siveke JT: Early requirement of Rac1 in a mouse model of pancreatic cancer. Gastroenterology. 141:719–730. 730.e1–7. 2011. View Article : Google Scholar | |
|
Kwiatkowska A, Didier S, Fortin S, Chuang Y, White T, Berens ME, Rushing E, Eschbacher J, Tran NL, Chan A and Symons M: The small GTPase RhoG mediates glioblastoma cell invasion. Mol Cancer. 11:652012. View Article : Google Scholar : PubMed/NCBI | |
|
Cerione RA: Cdc42: New roads to travel. Trends Cell Biol. 14:127–132. 2004. View Article : Google Scholar | |
|
Etienne-Manneville S: Cdc42-the centre of polarity. J Cell Sci. 117:1291–1300. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Kamai T, Yamanishi T, Shirataki H, Takagi K, Asami H, Ito Y and Yoshida K: Overexpression of RhoA, Rac1, and Cdc42 GTPases is associated with progression in testicular cancer. Clin Cancer Res. 10:4799–4805. 2004. View Article : Google Scholar | |
|
Chen QY, Jiao DM, Yao QH, Yan J, Song J, Chen FY, Lu GH and Zhou JY: Expression analysis of Cdc42 in lung cancer and modulation of its expression by curcumin in lung cancer cell lines. Int J Oncol. 40:1561–1568. 2012. | |
|
Zucman-Rossi J, Legoix P and Thomas G: Identification of new members of the Gas2 and Ras families in the 22q12 chromosome region. Genomics. 38:247–254. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Zhen Y and Stenmark H: Cellular functions of Rab GTPases at a glance. J Cell Sci. 128:3171–3176. 2015. | |
|
Stenmark H: Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 10:513–525. 2009. View Article : Google Scholar | |
|
Homma Y, Hiragi S and Fukuda M: Rab family of small GTPases: An updated view on their regulation and functions. FEBS J. 288:36–55. 2021. View Article : Google Scholar : | |
|
Liu Y, Wang X, Zhang Z, Xiao B, An B and Zhang J: The overexpression of Rab9 promotes tumor progression regulated by XBP1 in breast cancer. Onco Targets Ther. 12:1815–1824. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Li Z, Li Y, Jia Y, Ding B and Yu J: Rab1A knockdown represses proliferation and promotes apoptosis in gastric cancer cells by inhibition of mTOR/p70S6K pathway. Arch Biochem Biophys. 685:1083522020. View Article : Google Scholar | |
|
Zhao Z, Liu XF, Wu HC, Zou SB, Wang JY, Ni PH, Chen XH and Fan QS: Rab5a overexpression promoting ovarian cancer cell proliferation may be associated with APPL1-related epidermal growth factor signaling pathway. Cancer Sci. 101:1454–1462. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang D, Lu C and Ai H: Rab5a is overexpressed in oral cancer and promotes invasion through ERK/MMP signaling. Mol Med Rep. 16:4569–4576. 2017. View Article : Google Scholar | |
|
Ge J and Ge C: Rab14 overexpression regulates gemcitabine sensitivity through regulation of Bcl-2 and mitochondrial function in pancreatic cancer. Virchows Arch. 474:59–69. 2019. View Article : Google Scholar | |
|
Yang XZ, Li XX, Zhang YJ, Rodriguez-Rodriguez L, Xiang MQ, Wang HY and Zheng XF: Rab1 in cell signaling, cancer and other diseases. Oncogene. 35:5699–5704. 2016. View Article : Google Scholar | |
|
Zenner HL, Yoshimura S, Barr FA and Crump CM: Analysis of Rab GTPase-activating proteins indicates that Rab1a/b and Rab43 are important for herpes simplex virus 1 secondary envelopment. J Virol. 85:8012–8021. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Nuoffer C, Davidson HW, Matteson J, Meinkoth J and Balch WE: A GDP-bound of rab1 inhibits protein export from the endoplasmic reticulum and transport between Golgi compartments. J Cell Biol. 125:225–237. 1994. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Wang L, Lv Y, Jiang C, Wu G, Dull RO, Minshall RD, Malik AB and Hu G: The GTPase Rab1 is required for NLRP3 inflammasome activation and inflammatory lung injury. J Immunol. 202:194–206. 2019. View Article : Google Scholar | |
|
Yang XZ, Cui SZ, Zeng LS, Cheng TT, Li XX, Chi J, Wang R, Zheng XF and Wang HY: Overexpression of Rab1B and MMP9 predicts poor survival and good response to chemotherapy in patients with colorectal cancer. Aging (Albany NY). 9:914–931. 2017. View Article : Google Scholar | |
|
Wang X, Liu F, Qin X, Huang T, Huang B, Zhang Y and Jiang B: Expression of Rab1A is upregulated in human lung cancer and associated with tumor size and T stage. Aging (Albany NY). 8:2790–2798. 2016. View Article : Google Scholar | |
|
Xu M, Shao X, Kuai X, Zhang L, Zhou C and Cheng Z: Expression analysis and implication of Rab1A in gastrointestinal relevant tumor. Sci Rep. 9:133842019. View Article : Google Scholar : PubMed/NCBI | |
|
Ren B, Wang L, Nan Y, Liu T, Zhao L, Ma H, Li J, Zhang Y and Ren X: RAB1A regulates glioma cellular proliferation and invasion via the mTOR signaling pathway and epithelial-mesenchymal transition. Future Oncol. 17:3203–3216. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Lanzetti L, Palamidessi A, Areces L, Scita G and Di Fiore PP: Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases. Nature. 429:309–314. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Chiariello M, Bruni CB and Bucci C: The small GTPases Rab5a, Rab5b and Rab5c are differentially phosphorylated in vitro. FEBS Lett. 453:20–24. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Tan JY, Jia LQ, Shi WH, He Q, Zhu L and Yu B: Rab5a-mediated autophagy regulates the phenotype and behavior of vascular smooth muscle cells. Mol Med Rep. 14:4445–4453. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Zhao Y, Hu J, Xiao J, Qu L, Wang Z, Ma D and Chen Y: A novel ER-localized transmembrane protein, EMC6, interacts with RAB5A and regulates cell autophagy. Autophagy. 9:150–163. 2013. View Article : Google Scholar | |
|
Yu MH, Luo Y, Qin SL and Zhong M: Increased expression of Rab5A predicts metastasis and poor prognosis in colorectal cancer patients. Int J Clin Exp Pathol. 8:6974–6980. 2015. | |
|
Engebraaten O, Yau C, Berg K, Borgen E, Garred Ø, Berstad MEB, Fremstedal ASV, DeMichele A, Veer LV', Esserman L and Weyergang A: RAB5A expression is a predictive biomarker for trastuzumab emtansine in breast cancer. Nat Commun. 12:64272021. View Article : Google Scholar | |
|
English AR and Voeltz GK: Rab10 GTPase regulates ER dynamics and morphology. Nat Cell Biol. 15:169–178. 2013. View Article : Google Scholar | |
|
Chen YT, Holcomb C and Moore HP: Expression and localization of two low molecular weight GTP-binding proteins, Rab8 and Rab10, by epitope tag. Proc Natl Acad Sci USA. 90:6508–6512. 1993. View Article : Google Scholar | |
|
Leaf DS and Blum LD: Analysis of rab10 localization in sea urchin embryonic cells by three-dimensional reconstruction. Exp Cell Res. 243:39–49. 1998. View Article : Google Scholar | |
|
Cardoso CM, Jordao L and Vieira OV: Rab10 regulates phagosome maturation and its overexpression rescues Mycobacterium-containing phagosomes maturation. Traffic. 11:221–235. 2010. View Article : Google Scholar | |
|
Babbey CM, Bacallao RL and Dunn KW: Rab10 associates with primary cilia and the exocyst complex in renal epithelial cells. Am J Physiol Renal Physiol. 299:F495–F506. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Lerner DW, McCoy D, Isabella AJ, Mahowald AP, Gerlach GF, Chaudhry TA and Horne-Badovinac S: A Rab10-dependent mechanism for polarized basement membrane secretion during organ morphogenesis. Dev Cell. 24:159–168. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Isabella AJ and Horne-Badovinac S: Rab10-mediated secretion synergizes with tissue movement to build a polarized basement membrane architecture for organ morphogenesis. Dev Cell. 38:47–60. 2016. View Article : Google Scholar | |
|
Etoh K and Fukuda M: Rab10 regulates tubular endosome formation through KIF13A and KIF13B motors. J Cell Sci. 132:jcs2269772019. View Article : Google Scholar | |
|
Zhuo J, Han J, Zhao Y, Hao R, Shen C, Li H, Dai L, Sheng A, Yao H, Yang X and Liu W: RAB10 promotes breast cancer proliferation migration and invasion predicting a poor prognosis for breast cancer. Sci Rep. 13:152522023. View Article : Google Scholar : | |
|
Wang W, Jia WD, Hu B and Pan YY: RAB10 overexpression promotes tumor growth and indicates poor prognosis of hepatocellular carcinoma. Oncotarget. 8:26434–26447. 2017. View Article : Google Scholar : | |
|
Scita G and Di Fiore PP: The endocytic matrix. Nature. 463:464–473. 2010. View Article : Google Scholar | |
|
Simpson JC and Jones AT: Early endocytic Rabs: Functional prediction to functional characterization. Biochem Soc Symp. 72:99–108. 2005. View Article : Google Scholar | |
|
Simpson JC, Griffiths G, Wessling-Resnick M, Fransen JA, Bennett H and Jones AT: A role for the small GTPase Rab21 in the early endocytic pathway. J Cell Sci. 117(Pt 26): 6297–6311. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Evans TM, Ferguson C, Wainwright BJ, Parton RG and Wicking C: Rab23, a negative regulator of hedgehog signaling, localizes to the plasma membrane and the endocytic pathway. Traffic. 4:869–884. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Pellinen T, Arjonen A, Vuoriluoto K, Kallio K, Fransen JA and Ivaska J: Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins. J Cell Biol. 173:767–780. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou Y, Wu B, Li JH, Nan G, Jiang JL and Chen ZN: Rab22a enhances CD147 recycling and is required for lung cancer cell migration and invasion. Exp Cell Res. 357:9–16. 2017. View Article : Google Scholar | |
|
Takeda M, Koseki J, Takahashi H, Miyoshi N, Nishida N, Nishimura J, Hata T, Matsuda C, Mizushima T, Yamamoto H, et al: Disruption of Endolysosomal RAB5/7 efficiently eliminates colorectal cancer stem cells. Cancer Res. 79:1426–1437. 2019. View Article : Google Scholar | |
|
Chen Y, Ng F and Tang BL: Rab23 activities and human cancer-emerging connections and mechanisms. Tumour Biol. 37:12959–12967. 2016. View Article : Google Scholar | |
|
Lledo PM, Johannes L, Vernier P, Zorec R, Darchen F, Vincent JD, Henry JP and Mason WT: Rab3 proteins: Key players in the control of exocytosis. Trends Neurosci. 17:426–432. 1994. View Article : Google Scholar | |
|
Martin S and Parton RG: Characterization of Rab18, a lipid droplet-associated small GTPase. Methods Enzymol. 438:109–129. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Gerondopoulos A, Bastos RN, Yoshimura S, Anderson R, Carpanini S, Aligianis I, Handley MT and Barr FA: Rab18 and a Rab18 GEF complex are required for normal ER structure. J Cell Biol. 205:707–720. 2014. View Article : Google Scholar : | |
|
Dejgaard SY, Murshid A, Erman A, Kizilay O, Verbich D, Lodge R, Dejgaard K, Ly-Hartig TB, Pepperkok R, Simpson JC and Presley JF: Rab18 and Rab43 have key roles in ER-Golgi trafficking. J Cell Sci. 121(Pt 16): 2768–2781. 2008. View Article : Google Scholar | |
|
Fukuda M: Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic. 14:949–963. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, et al: Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 12:19–30. 2010. View Article : Google Scholar | |
|
Li Z, Fang R, Fang J, He S and Liu T: Functional implications of Rab27 GTPases in cancer. Cell Commun Signal. 16:442018. View Article : Google Scholar | |
|
Donaldson JG and Jackson CL: ARF family G proteins and their regulators: Roles in membrane transport, development and disease. Nat Rev Mol Cell Biol. 12:362–375. 2011. View Article : Google Scholar | |
|
D'Souza-Schorey C and Chavrier P: ARF proteins: Roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 7:347–358. 2006. View Article : Google Scholar | |
|
Donaldson JG and Honda A: Localization and function of Arf family GTPases. Biochem Soc Trans. 33(Pt 4): 639–642. 2005. View Article : Google Scholar | |
|
Gillingham AK and Munro S: The small G proteins of the Arf family and their regulators. Annu Rev Cell Dev Biol. 23:579–611. 2007. View Article : Google Scholar | |
|
Schlienger S, Campbell S and Claing A: ARF1 regulates the Rho/MLC pathway to control EGF-dependent breast cancer cell invasion. Mol Biol Cell. 25:17–29. 2014. View Article : Google Scholar | |
|
Boulay PL, Schlienger S, Lewis-Saravalli S, Vitale N, Ferbeyre G and Claing A: ARF1 controls proliferation of breast cancer cells by regulating the retinoblastoma protein. Oncogene. 30:3846–3861. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Ma H, Fang W, Li Q, Wang Y and Hou SX: Arf1 ablation in colorectal cancer cells activates a super signal complex in DC to enhance anti-tumor immunity. Adv Sci (Weinh). 10:e23050892023. View Article : Google Scholar | |
|
Wen PY and Kesari S: Malignant gliomas in adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Rong L, Li N and Zhang Z: Emerging therapies for glioblastoma: Current state and future directions. J Exp Clin Cancer Res. 41:1422022. View Article : Google Scholar : PubMed/NCBI | |
|
Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, et al: EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 18:170–186. 2021. View Article : Google Scholar | |
|
Li T, Li J, Chen Z, Zhang S, Li S, Wageh S, Al-Hartomy OA, Al-Sehemi AG, Xie Z, Kankala RK and Zhang H: Glioma diagnosis and therapy: Current challenges and nanomaterial-based solutions. J Control Release. 352:338–370. 2022. View Article : Google Scholar | |
|
Liu F, Wang Y, Gu H and Wang X: Technologies and applications of single-cell DNA methylation sequencing. Theranostics. 13:2439–2454. 2023. View Article : Google Scholar : | |
|
Alshammari E, Zhang Y, Sobota J and Yang Z: Aberrant DNA methylation of tumor suppressor genes and oncogenes as cancer biomarkers. Genomic and Epigenomic Biomarkers of Toxicology and Disease: Clinical and Therapeutic Actions. Sahu SC: Wiley; Oxford: pp. 251–271. 2022, View Article : Google Scholar | |
|
Da Costa EM, McInnes G, Beaudry A and Raynal NJ: DNA Methylation-targeted drugs. Cancer J. 23:270–276. 2017. View Article : Google Scholar | |
|
Diesch J, Zwick A, Garz AK, Palau A, Buschbeck M and Götze KS: A clinical-molecular update on azanucleoside-based therapy for the treatment of hematologic cancers. Clin Epigenetics. 8:712016. View Article : Google Scholar : | |
|
Federici L, Capelle L, Annereau M, Bielle F, Willekens C, Dehais C, Laigle-Donadey F, Hoang-Xuan K, Delattre JY, Idbaih A, et al: 5-Azacitidine in patients with IDH1/2-mutant recurrent glioma. Neuro Oncol. 22:1226–1228. 2020. View Article : Google Scholar | |
|
He W, Lin S, Guo Y, Wu Y, Zhang LL, Deng Q, Du ZM, Wei M, Zhu W, Chen WJ, et al: Targeted demethylation at ZNF154 promotor upregulates ZNF154 expression and inhibits the proliferation and migration of Esophageal squamous carcinoma cells. Oncogene. 41:4537–4546. 2022. View Article : Google Scholar | |
|
Zaib S, Rana N and Khan I: Histone modifications and their role in epigenetics of cancer. Curr Med Chem. 29:2399–2411. 2022. View Article : Google Scholar | |
|
Chen R, Zhang M, Zhou Y, Guo W, Yi M, Zhang Z, Ding Y and Wang Y: The application of histone deacetylases inhibitors in glioblastoma. J Exp Clin Cancer Res. 39:1382020. View Article : Google Scholar : PubMed/NCBI | |
|
Wang F, Jin Y, Wang M, Luo HY, Fang WJ, Wang YN, Chen YX, Huang RJ, Guan WL, Li JB, et al: Combined anti-PD-1, HDAC inhibitor and anti-VEGF for MSS/pMMR colorectal cancer: A randomized phase 2 trial. Nat Med. 30:1035–1043. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Gao Y, He H, Li X, Zhang L, Xu W, Feng R, Li W, Xiao Y, Liu X, Chen Y, et al: Sintilimab (anti-PD-1 antibody) plus chidamide (histone deacetylase inhibitor) in relapsed or refractory extranodal natural killer T-cell lymphoma (SCENT): A phase Ib/II study. Signal Transduct Target Ther. 9:1212024. View Article : Google Scholar : | |
|
Monje M, Cooney T, Glod J, Huang J, Peer CJ, Faury D, Baxter P, Kramer K, Lenzen A, Robison NJ, et al: Phase I trial of panobinostat in children with diffuse intrinsic pontine glioma: A report from the Pediatric brain tumor consortium (PBTC-047). Neuro Oncol. 25:2262–2272. 2023. View Article : Google Scholar : | |
|
Mueller S, Kline C, Stoller S, Lundy S, Christopher L, Reddy AT, Banerjee A, Cooney TM, Raber S, Hoffman C, et al: PNOC015: Repeated convection-enhanced delivery of MTX110 (aqueous panobinostat) in children with newly diagnosed diffuse intrinsic pontine glioma. Neuro Oncol. 25:2074–2086. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Xu K, Ramesh K, Huang V, Gurbani SS, Cordova JS, Schreibmann E, Weinberg BD, Sengupta S, Voloschin AD, Holdhoff M, et al: Final report on clinical outcomes and tumor recurrence patterns of a pilot study assessing efficacy of belinostat (PXD-101) with chemoradiation for newly diagnosed glioblastoma. Tomography. 8:688–700. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Raedler LA: Farydak (Panobinostat): First HDAC inhibitor approved for patients with relapsed multiple myeloma. Am Health Drug Benefits. 9(Spec Feature): 84–87. 2016.PubMed/NCBI | |
|
Zhang L, Liao Y and Tang L: MicroRNA-34 family: A potential tumor suppressor and therapeutic candidate in cancer. J Exp Clin Cancer Res. 38:532019. View Article : Google Scholar : | |
|
Li Z and Rana TM: Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov. 13:622–638. 2014. View Article : Google Scholar | |
|
Petrescu GED, Sabo AA, Torsin LI, Calin GA and Dragomir MP: MicroRNA based theranostics for brain cancer: Basic principles. J Exp Clin Cancer Res. 38:2312019. View Article : Google Scholar : PubMed/NCBI | |
|
Mathupala SP, Mittal S, Guthikonda M and Sloan AE: MicroRNA and brain tumors: A cause and a cure? DNA Cell Biol. 26:301–310. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Morokoff A, Jones J, Nguyen H, Ma C, Lasocki A, Gaillard F, Bennett I, Luwor R, Stylli S, Paradiso L, et al: Serum microRNA is a biomarker for post-operative monitoring in glioma. J Neurooncol. 149:391–400. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Yue X, Lan F, Hu M, Pan Q, Wang Q and Wang J: Downregulation of serum microRNA-205 as a potential diagnostic and prognostic biomarker for human glioma. J Neurosurg. 124:122–128. 2016. View Article : Google Scholar | |
|
Sun Y, Jing Y and Zhang Y: Serum lncRNA-ANRIL and SOX9 expression levels in glioma patients and their relationship with poor prognosis. World J Surg Oncol. 19:2872021. View Article : Google Scholar : | |
|
Tan SK, Pastori C, Penas C, Komotar RJ, Ivan ME, Wahlestedt C and Ayad NG: Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol Cancer. 17:742018. View Article : Google Scholar : | |
|
Stella M, Falzone L, Caponnetto A, Gattuso G, Barbagallo C, Battaglia R, Mirabella F, Broggi G, Altieri R, Certo F, et al: Serum extracellular vesicle-derived circHIPK3 and circS-MARCA5 are two novel diagnostic biomarkers for glioblastoma multiforme. Pharmaceuticals (Basel). 14:6182021. View Article : Google Scholar | |
|
Li P, Xu Z, Liu T, Liu Q, Zhou H, Meng S, Feng Z, Tang Y, Liu C, Feng J, et al: Circular RNA sequencing reveals serum exosome circular RNA panel for high-grade astrocytoma diagnosis. Clin Chem. 68:332–343. 2022. View Article : Google Scholar |