Novel non‑metal‑based contrast agents for MR imaging: Emerging approaches and clinical perspectives (Review)
- Authors:
- Published online on: July 15, 2025 https://doi.org/10.3892/ijo.2025.5776
- Article Number: 70
-
Copyright: © Du et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Magnetic Resonance Imaging (MRI) is an indispensable non-invasive technique for acquiring detailed physiological and anatomical information. Proton MRI (¹H-MRI) generates high-resolution three-dimensional images with excellent inherent soft tissue contrast, allowing precise visualization of internal structures (1-4). This capability aids in diagnosis, treatment planning, and monitoring of various medical conditions without exposing patients to ionizing radiation.
The foundation of ¹H-MRI lies in the nuclear magnetic resonance (NMR) phenomenon discovered in the early 20th century. In the 1970s, Paul Lauterbur introduced spatial encoding using magnetic field gradients, enabling the creation of two-dimensional images from NMR signals. Peter Mansfield refined the technique by developing echo-planar imaging, which improved image acquisition speed and resolution. Their pioneering work transformed NMR from a spectroscopic method into an imaging modality, laying the groundwork for modern MRI technology. ¹H-MRI exploits the nuclear spin properties of hydrogen atoms abundant in water and organic molecules within the body (1-4). In an external magnetic field, protons align their spins along the field direction, establishing net magnetization. Transverse radiofrequency (RF) pulses perturb this alignment, causing protons to absorb energy and transition to higher energy spin states. Upon cessation of the RF pulse, protons relax back to equilibrium, emitting RF signals characterized by longitudinal relaxation time (T1) and transverse relaxation time (T2) (5). Variations in T1 and T2 among different tissues contribute to the contrast observed in MRI images. Despite its advantages, ¹H-MRI has inherently low sensitivity because only a small population difference exists between low and high energy spin states at thermal equilibrium. To enhance MRI signals, contrast agents (CAs) interact with nearby water protons, altering their relaxation times and increasing image contrast (6-12). Common CAs include gadolinium-based complexes and iron oxide nanoparticles. While effective, reliance on these agents underscores the ongoing need to improve MRI sensitivity.
Paramagnetic contrast agents such as Gadolinium (Gd3+) and Manganese (Mn2+) shorten the T1 relaxation time of water protons, increasing signal intensity on T1-weighted images and making targeted areas appear brighter (13-15). Gadolinium-based agents are widely used to enhance vascular structures and lesions. However, they have limitations: Gadolinium chelates are less readily phagocytosed by cells, which is problematic for cell tracking applications requiring intracellular uptake. High concentrations required for cell labeling increase cytotoxicity risks. Additionally, linear Gd3+ agents accumulate in the brain, raising concerns about neurotoxicity, and gadolinium exposure is associated with nephrogenic systemic fibrosis in patients with impaired renal function (16-18).
Superparamagnetic iron oxide (SPIO) nanoparticles act as T2 contrast agents by affecting the spin-spin relaxation time, causing a reduction in signal intensity on T2-weighted images and resulting in darker areas that enhance contrast (19,20). SPIO particles are highly sensitive and commonly used for cell tracking due to their strong magnetic properties (21). They consist of an iron oxide core coated with hydrophilic materials such as polymers or lipids and can be synthesized in various sizes for different applications. However, challenges such as extracellular accumulation make it difficult to distinguish labeled cells from surrounding tissues. Moreover, quantitative analysis is hindered because MRI signal changes do not proportionally reflect SPIO concentration, complicating the assessment of nanoparticle distribution and dosage (22).
To address the limitations and safety concerns of traditional metal-based agents such as gadolinium, ongoing research focuses on developing novel MRI contrast agents. Advances in nanotechnology offer promising strategies to enhance intracellular uptake, reduce toxicity, and improve specificity and sensitivity. This includes exploring non-metal-based contrast agents that can enhance MRI signals without the associated risks of metal toxicity (23). The present review investigated recent advancements in non-metal-based contrast agents for MRI, including 19F-based agents, polymeric compounds, nanoparticles, small molecules, CEST agents, nitroxide radicals, and hyperpolarized carbon agents. It delved into their mechanisms of action, imaging capabilities, advantages, and limitations. By enhancing MRI sensitivity and specificity without the risks associated with metal-based agents, these novel agents hold potential for improving diagnostic imaging and patient outcomes. The present review advocated continued research and multidisciplinary collaboration to overcome current challenges such as low sensitivity and technical complexities, ultimately advancing the clinical translation of non-metal-based MRI contrast agents and paving the way for more effective and personalized medical diagnostics.
19F MRI
19F MRI exploits the favorable nuclear properties of fluorine-19, whose gyromagnetic ratio (40.08 MHz/T) and nuclear magnetic resonance sensitivity (~83% of 1H) are similar to those of hydrogen (24-27). Importantly, the virtual absence of 19F in biological systems results in negligible endogenous background signals, permitting the specific detection of exogenous fluorinated agents and unambiguous imaging of labeled cells and molecules without interference from surrounding tissues (28-30). An advantage of 19F MRI is its quantitative imaging capability: the MRI signal from 19F nuclei correlates linearly with their concentration, facilitating accurate quantification of cell numbers or agent accumulation in vivo (24,25,31). This property is valuable in cell tracking applications, where fluorinated nanoparticles or nanoemulsions deliver high densities of 19F atoms to target cells, enhancing signal strength and enabling precise localization and tracking of labeled cells. Furthermore, 19F MRI can generate 'hot-spot' images overlaid on conventional 1H-MRI scans, providing both anatomical context and specific functional or molecular information (24,27,31,32) (Fig. 1). The broad chemical shift range of 19F (>350 ppm) allows for simultaneous multiplexed imaging of different fluorinated agents, expanding the potential to investigate multiple biological processes in a single examination. Due to these advantages, 19F MRI has prompted the development of diverse contrast agents, each with distinct fluorine-loading strategies, detection sensitivities, and translational challenges.
Despite these promising features, 19F MRI faces several critical challenges that must be addressed to facilitate broader clinical utility. Chief among these are limited sensitivity relative to 1H-MRI, which can necessitate higher doses of fluorinated agents to achieve sufficient signal-to-noise ratios, and stability issues that may lead to premature agent degradation or unwanted off-target accumulation. Furthermore, quantitative analysis of 19F signals in vivo is complicated by variations in coil sensitivity, partial volume effects, and potential changes in relaxation times associated with different microenvironments (33). Standardized protocols and robust calibration strategies are needed to accurately translate signal intensities into concentrations or cell numbers, an essential step for numerous clinical applications. Efforts to design new fluorinated contrast agents, improve MRI hardware, and develop advanced imaging protocols will be paramount to overcoming these obstacles and advancing the field toward reliable quantitative imaging.
Advances in fluorinated contrast agents have further enhanced the utility of 19F MRI. Agents such as perfluorocarbons (PFCs) offer high densities of 19F nuclei and are biologically inert due to strong carbon-fluorine bonds, ensuring in vivo stability (28,34). Formulated into nanoemulsions, nanocapsules, or nanoparticles, these compounds enhance biocompatibility and facilitate cellular uptake. Clinically approved fluorinated compounds such as perflubron and perflutren underscore the translational potential of 19F MRI technologies (29,35-37). Technological advances continue to address performance issues (38). Improvements in MRI hardware, high-performance 19F probes, and techniques such as hyperpolarization are enhancing signal acquisition and detection. These innovations expand the applications of 19F MRI, making it a powerful tool for quantitative imaging, cell tracking, and advancing molecular imaging in biomedical research.
PFCs are the most widely used non-metal 19F MRI contrast agents (29,37,39,40). These organic compounds, where all hydrogen atoms are replaced by fluorine, possess a high density of 19F nuclei per molecule, markedly enhancing MRI signal sensitivity (25,29,37,39-42). The strong carbon-fluorine (C-F) bond, a result of fluorine's high electronegativity, imparts remarkable thermal, chemical, and oxidative stability to PFCs (41). This chemical inertness renders them resistant to metabolic degradation, making them suitable for in vivo applications. Within the broader PFC family, structural variants such as perfluoro-15-crown-5-ether (PFCE), perfluorooctyl bromide (PFOB), and trans-bis-perfluorobutyl ethylene (F-44E) each exhibit unique imaging characteristics, including differences in biological half-life, signal intensity, and stability that can markedly influence their clinical or preclinical suitability (43,44).
19F-labeled PFC nanoemulsions have emerged as promising MRI contrast agents due to their biocompatibility, lack of background 19F signal, and ability to track cellular and molecular processes in vivo. Their stable 19F signal offers an advantage over fluorescent labels, which can dissociate over time and underestimate PFC deposition when relying solely on fluorescence methods (37,42,45). In infectious and inflammatory disease models, these nanoemulsions have enabled precise visualization and tracking. For instance, in murine models of Staphylococcus aureus infection, 19F-MRI facilitated visualization of abscess formation and immune cell tracking, providing insights into host immune responses and antibacterial therapy efficacy (46). Similarly, in acute cardiac and cerebral ischemia models, 19F-MRI allowed precise localization of infiltrating monocytes/macrophages without background signal interference, enhancing detection of inflammatory processes (47,48). In a collagen-induced arthritis model, 19F MRI signal intensity correlated linearly with disease severity and therapeutic efficacy, highlighting its potential in monitoring inflammatory diseases (48).
In oncology, 19F-labeled PFC nanoemulsions have been instrumental in tumor imaging and analysis (37,49,50). Combining 19F-MRI with 18F-FDG-PET revealed an inverse association between 19F signal intensities and glucose uptake in tumors, suggesting a novel method to study the relationship between tumor-associated macrophages and tumor metabolism (51). Advances in nanoemulsion design, such as uniform-sized PFC droplets, have enabled quantitative measurements of blood volume and capillary permeability in tumors with high spatial resolution (52). Additionally, folate receptor-targeted PFC/rhodamine nanoemulsions have enhanced imaging capabilities for folate receptor-positive tumors through both 19F-MRI and optical imaging, without affecting cell viability (49,53). Labeling human CD34+ hematopoietic stem cells with 19F MRI tracers did not alter their multipotency or therapeutic potential, supporting the safety of this approach for clinical applications (54).
Despite these advantages, PFC-based agents face significant limitations. The restricted fluorine content per particle means that their dispersion in biological systems often results in low local fluorine concentrations. This necessitates administering large quantities of PFCs to achieve sufficient signal intensity, which is impractical and may pose safety concerns (55). Additionally, PFC particles tend to accumulate in the reticuloendothelial system (RES), particularly in the liver and spleen, as they are recognized and phagocytosed by macrophages in these organs (56). This sequestration reduces their availability at target sites, prolongs retention times, diminishes imaging effectiveness, and complicates signal interpretation due to background enhancement from these organs. Potential toxicity concerns further complicate the use of PFC-based contrast agents. While PFCs are generally considered biologically inert, their accumulation raises questions about long-term safety. High doses or repeated exposure can lead to adverse effects such as inflammatory responses and alterations in organ function (57). Moreover, emulsifiers and surfactants used in formulating PFC emulsions may contribute to toxicity by eliciting immune responses or causing cellular stress (58). These challenges highlight the need for alternative non-metal 19F contrast agents with improved properties (59,60). Designing molecules that evade RES uptake would reduce unwanted accumulation in the liver and spleen, improving targeting efficiency and safety profiles. Addressing toxicity concerns using biocompatible materials and thorough preclinical evaluation is essential for advancing the clinical potential of 19F MRI contrast agents.
PFCE
PFCE is a highly sensitive 19F MRI contrast agent due to its 20 magnetically equivalent 19F atoms, enabling effective detection of fluorine-loaded cells in inflammatory processes (49). However, its extremely long biological half-life (>250 days) limits clinical applicability. Compared with other PFCs such as PFOB, PFCE can deliver stronger signal intensities but often poses prolonged organ retention. Investigations into alternative PFCs with shorter half-lives identified PFD, PFOB, and F-44E, with murine liver and spleen half-lives of 9, 12, and 28 days, respectively (61,62). Among these, PFOB emerged as a promising candidate for clinical translation, providing 37% of PFCE's signal intensity in inflammation imaging models (61,63,64).
To overcome the limitations of PFCE and enhance its targeting capabilities, researchers synthesized c-Met-targeting peptide-functionalized PFCE nanoparticles (AH111972-PFCE NPs) with a particle size of 89.3±17.8 nm. These NPs exhibited high specificity and strong c-Met-targeting ability, enabling precise detection of small colorectal liver metastases, particularly ill-defined fused metastases undetectable by ¹H-MRI, with ultralong tumor retention of at least 7 days and minimal side effects (65). Similarly, PFCE encapsulated in PLGA-PEG-mannose nanoparticles targeted tumor-associated macrophages overexpressing the mannose receptor (MRC1/CD206), facilitating in vivo imaging of the tumor microenvironment by 19F MRI. At 48 h post-injection, nanoparticle retention at the tumor site was confirmed, benefiting from robust and specific 19F signals due to the lack of background 19F in the body (66).
Moreover, PFCE demonstrates superior biocompatibility compared with other fluorine reporter probes such as HFB for tissue oxygenation assessment (67,68). Unlike HFB, which induces tissue necrosis and mobility limitations compromising extended pO2 measurements, PFCE exhibits no muscle tissue toxicity and does not affect animal behavior ≤36 h post-injection, allowing accurate and prolonged assessment (67,68). Collectively, these studies highlight the potential of PFCE in 19F MRI applications when strategies are employed to enhance targeting and biocompatibility while mitigating its prolonged biological half-life, thereby advancing its clinical suitability (29,37,63,64).
To further improve the utility of PFCE as a 19F MRI contrast agent, a highly concentrated and stable colloidal nanoemulsion (NE) was developed using the semifluorinated triblock copolymer M2F8H18 to encapsulate PFCE at 35% v/v, enhancing imaging sensitivity for in vivo cancer detection (69,70). The resulting NE nanoparticles mean value 210±38 nm in size with a polydispersity index of 0.03, exhibiting long-term stability of at least 98 days at 4°C and maintaining stability at physiological temperatures and in serum, thus preventing particle growth that could lead to embolism. In vitro cytotoxicity assays using 4T1-Luc murine breast carcinoma cells showed negligible cell death, even at high PFCE concentrations ≤20 mg/ml and incubation periods ≤48 h, indicating good biocompatibility. In a tumor-bearing mouse model, intravenous administration of the NE resulted in high 19F MRI signals with signal-to-noise ratios ≤100 in clinically relevant scan times (~11 min) (71). The NE circulated stably in the vasculature, with visible accumulation in the heart and inferior vena cava at 6 h post-injection, and accumulated in tumors with an estimated concentration of 4-9×1017 19F spins per voxel. The PFCE signal persisted at the tumor site ≤14 days post-injection, with 50% remaining at Day 7 and 33% at Day 14, demonstrating prolonged tumor imaging capability. The high PFCE loading and passive targeting through the enhanced permeability and retention (EPR) effect enabled enhanced 19F MRI contrast and precise, prolonged tumor imaging. However, the nanoparticle size led to uptake by the mononuclear phagocyte system (MPS), resulting in accumulation in the liver and spleen, which may limit specificity. Further optimization, such as reducing particle size, is necessary to minimize MPS uptake. This novel, highly stable NE formulation with unprecedented PFCE loading and prolonged in vivo stability offers significant potential as a 19F MRI contrast agent for cancer diagnostics.
PFOB
Due to its favorable NMR properties and biocompatibility, PFOB has been extensively studied as a 19F MRI contrast agent. Compared with PFCE, PFOB offers a shorter biological half-life and thus lower long-term organ retention, although this advantage comes at the cost of lower signal intensity. Recent efforts to enhance the imaging efficacy and targeting capabilities of PFOB led to the synthesis of PLGA-PEG nanocapsules encapsulating a liquid PFOB core via an emulsion-evaporation process (72,73). Incorporating PEG through PLGA-PEG diblock copolymers enhanced PFOB encapsulation efficiency compared with plain PLGA nanocapsules, as estimated by 19F NMR spectroscopy (74). The PEGylated nanocapsules (mean value 120 nm in diameter) maintained a spherical core-shell morphology confirmed by dynamic light scattering, transmission electron microscopy and scanning electron microscopy analyses. PEGylation was confirmed by zeta potential measurements and X-ray photoelectron spectroscopy, resulting in reduced complement activation in vitro, indicative of stealth properties. In vivo 19F MRI studies in mice demonstrated that PEGylated nanocapsules accumulated in CT26 xenograft tumors 7 h post-intravenous injection, while plain nanocapsules were undetectable. This highlights the efficacy of PEGylation in prolonging circulation time and enhancing tumor targeting via the EPR effect. However, liver accumulation was still observed, indicating a need for further optimization to reduce off-target uptake (72).
Optimizing MRI pulse sequences has also been explored to improve the sensitivity of PFOB-based imaging. Selecting appropriate bandwidths of 180° pulses in spin-echo sequences mitigated detrimental effects of J-coupling, enhancing detection of the CF3 resonance (75). The T2 relaxation time of the CF3 group depended on the interpulse delay in multispin-echo sequences; optimizing this delay yielded an imaging sequence with superior sensitivity over traditional gradient echo and chemical shift imaging sequences. However, the efficacy of this approach relies on precise control of interpulse delays and pulse bandwidths, posing practical challenges in clinical settings (75).
In cell therapies, non-invasive tracking of transplanted cells via 19F MRI is crucial due to its high specificity and negligible background signal (76). A novel nano-contrast agent, termed PSS-NP, was formulated with a PFOB core encapsulated within a PLGA shell and coated with polystyrene sulfonate (PSS) to enhance uptake by MSCs through caveolae-mediated endocytosis. PSS-NPs exhibited a hydrodynamic size of ~140 nm with a zeta potential of −60 mV, indicating good stability, and were efficiently internalized by MSCs without affecting proliferation or osteoblastic differentiation potential, as confirmed by flow cytometry, confocal microscopy, and alkaline phosphatase activity assays (77). In vitro, PSS-NP-labelled cells maintained a detectable 19F MRI signal for ≤14 days. In vivo, these cells could be tracked using 19F MRI for ≤two months post-transplantation in mice, retaining their ability to form mineralized tissue. Importantly, PSS-NP-labelled cells enabled monitoring of immune rejection, evidenced by a 40% loss of 19F MRI signal one week after transplantation in immunocompetent BALB/c mice compared with a 10% loss in immunocompromised NOD/SCID mice. This work demonstrated a safe and efficient method for stem cell labelling that provides insights into cell survival and immune rejection in vivo. A remaining challenge is the reliance on a high-field 16.4 T MRI scanner, which is not directly applicable to clinical settings typically employing 1.5 T to 3 T MRI, necessitating further research for clinical translation (76).
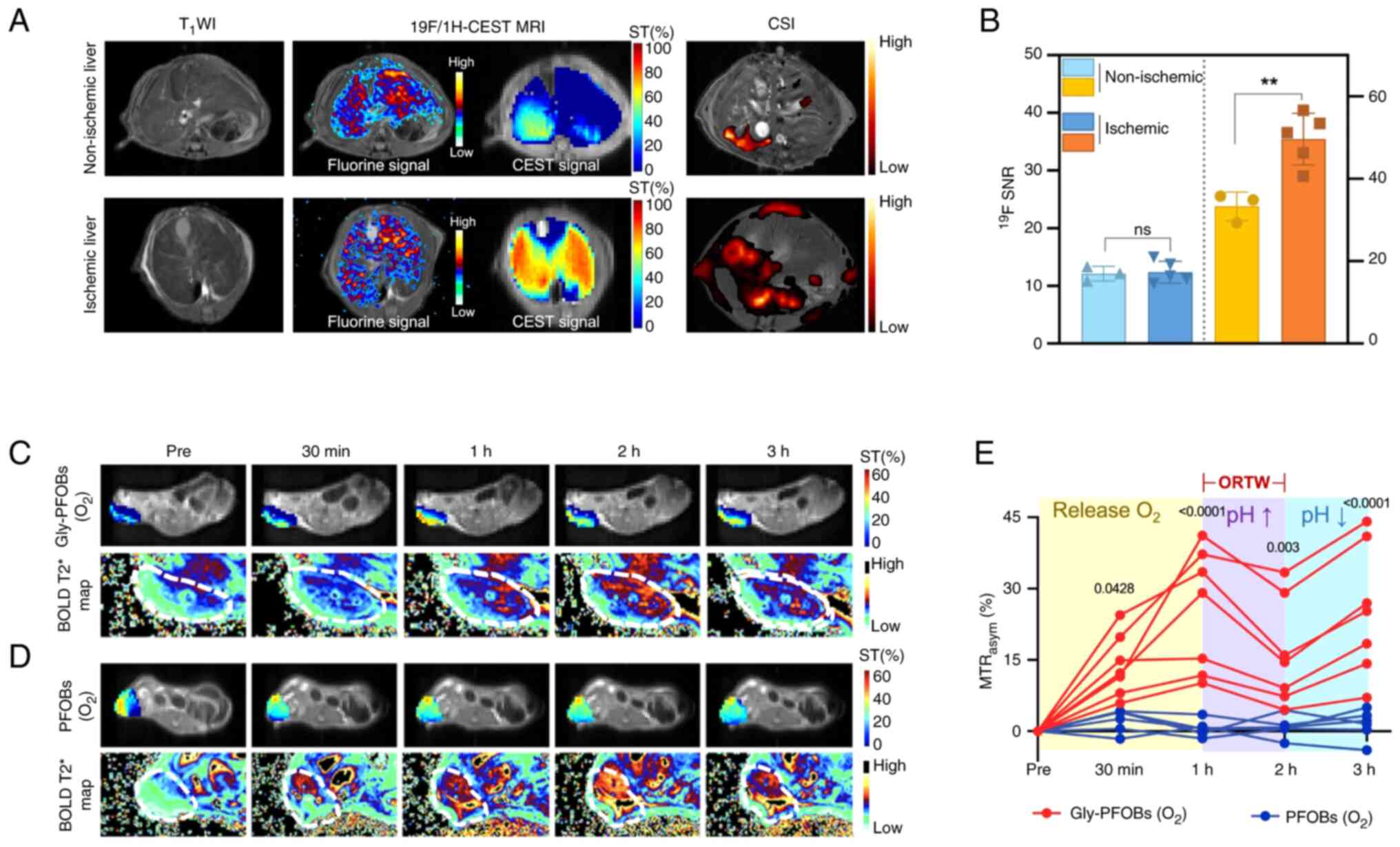
Novel MRI contrast agents play a crucial role in enhancing the precision of cancer therapies, particularly in MRI-guided radiotherapy (RT). Researchers developed pH and O2 dual-sensitive nano-molecular imaging probes (Gly-PFOBs) based on PFOB and glycerol-weighted CEST, exhibiting dual 19F/1H-CEST MRI (Fig. 2) (78). The hypothesis was that Gly-PFOBs could ameliorate tumor hypoxia by delivering oxygen and serve as MRI contrast agents to optimize the radiotherapy time window. The Gly-PFOBs demonstrated excellent pH and oxygen sensitivity in vitro, with CEST signal intensity changes corresponding to pH variations from 5.9-8.0 and oxygen concentration adjustments. In vivo studies using NCI-H460 lung cancer xenograft models showed that oxygenated Gly-PFOBs effectively improved tumor oxygenation, as evidenced by blood oxygen level-dependent MRI and enhanced RT efficacy (78). Specifically, the tumor growth inhibition rate in mice treated with RT and Gly-PFOBs at the optimized time window (1-2 h post-injection) was 81.31%, markedly higher than RT alone (44.72%). The probes exhibited superior therapeutic efficacy and biosafety, with no significant toxicity observed in major organs.
PFPEs
PFPEs have emerged as promising 19F MRI contrast agents for noninvasive in vivo cell tracking due to their biocompatibility. Compared with PFCE or PFOB, PFPE agents can be formulated in a variety of structures that influence mobility and relaxation properties, offering flexibility in optimizing imaging performance. 19F MRI enables selective imaging of PFPE-labeled cells against a background-free environment, since biological tissues lack mobile fluorine atoms, while 1H-MRI provides anatomical context (70). In a pioneering study (79), dendritic cells (DCs) labeled ex vivo with PFPEs retained their function, allowing in vivo tracking of DC migration in mice via 19F MRI (49,79). Antigen-specific T cells labeled with PFPE nanoemulsions were similarly monitored over 21 days, revealing dynamic patterns in lymph nodes and enabling quantification of apparent T-cell numbers, though in vivo cell division complicated accuracy (80,81). To enhance detection, dual-mode PFPE nanoemulsions conjugated with fluorescent dyes (FITC, Alexa647, BODIPy-TR) were developed, facilitating simultaneous 19F MRI and fluorescence detection (67). These nanoemulsions (<200 nm) were rapidly internalized by various cells, and the proportional relationship between intracellular fluorescence and 19F NMR signal enabled calibration of cell loading, improving analysis via fluorescence microscopy and fluorescence-activated cell sorting.
Designing effective polymeric 19F MRI contrast agents is challenging due to the hydrophobicity of fluorine, leading to aggregation and signal attenuation. To address this, thermoresponsive PFPE-based comb-shaped poly(2-oxazoline) s with varying side-chain structures were investigated (82). At increased temperatures, some polymers transitioned to unimers, enhancing imaging intensity, while others formed aggregates, degrading performance, underscoring the importance of polymer self-association in designing smart, thermoresponsive 19F MRI agents (83).
The effect of different hydrophilic segments on imaging performance was further studied using PFPE-containing amphiphilic block copolymers synthesized via RAFT polymerization (84). Block copolymers PMSEA-PFPE, POEGA-PFPE, and POMOXA-PFPE, prepared from hydrophilic monomers MSEA, OEGA, and OMOXA, respectively, formed assemblies with hydrophobic PFPE cores and hydrophilic shells, as confirmed by dynamic light scattering and molecular dynamics simulations. Simulations revealed that POMOXA's rigid, extended chains led to shorter 19F NMR spin-spin relaxation times (T2=33.9 msec), lower 19F spin visibility (59%), and weaker MRI signals [signal-to-noise ratio (SNR)=36.9 at 6.8 mg/ml fluorine concentration]. By contrast, PMSEA-PFPE exhibited higher 19F spin visibility (100%) and stronger MRI signal intensity (SNR=222.0 at 6.8 mg/ml), despite a shorter T2 (41.3 msec) than POEGA-PFPE (62.3 msec). These results emphasize that both high fluorine visibility and long T2 relaxation times are crucial for effective contrast agents, highlighting the significance of hydrophilic segments in influencing self-assembly, chain mobility, and NMR properties.
These studies collectively demonstrate the versatility and potential of PFPE-based 19F MRI agents in noninvasive cell tracking and advancing cellular therapeutics (50,67,85-87). Limitations include quantification challenges due to in vivo cell division and the need to optimize polymer designs to prevent aggregation that diminishes imaging quality. Further in vivo evaluations are necessary to assess imaging efficacy and biocompatibility, as some studies remain at the in vitro or simulation level.
Polymers
Polymer-based 19F MRI contrast agents are promising tools for non-invasive, targeted imaging due to the unique magnetic properties and negligible background signals of fluorine in biological systems. Compared with PFC-based agents, which often exhibit very high 19F loading but potential issues with half-life or RES uptake, polymeric agents can be tailored to balance signal intensity, biocompatibility, and circulation times (33). To enhance imaging performance, various branched and hyperbranched fluorinated polymers have been developed. Fu et al (88) synthesized branched fluorinated glycopolymers via one-pot RAFT polymerization, incorporating glucose units and disulfide cross-linkers. These polymers effectively targeted cancer cells through interactions with overexpressed sugar transporters and showed enhanced 19F MRI signals in reductive environments typical of cancer cells. Similarly, segmented highly branched polymers (SHBPs) composed of fluorinated and PEG-based monomers were reported using RAFT-mediated self-condensing vinyl copolymerization (89). SHBPs with statistical copolymeric acrylate segments exhibited a single 19F signal, long T2 relaxation times, and high fluorine content, making them excellent imaging candidates. A hyperbranched poly(N,N-dimethylacrylamide) conjugated with 5-fluorouracil via an enzyme-degradable peptide linker demonstrated enzyme-responsive 5-FU release, resulting in significant changes in T219F NMR/MRI relaxation times and enabling monitoring of drug release (90).
Modification of natural polysaccharides such as alginic acid, dextran, and polygalacturonic acid with 3-aminobenzotrifluoride produced water-soluble materials containing 1-14% fluorine (91). These materials exhibited low toxicity, lysosomal localization and rapid renal clearance, suggesting suitability for imaging gastrointestinal or genitourinary systems. An injectable 19F-labeled hyaluronic acid hydrogel formed via carbazone reaction was monitored by both 1H and 19F MRI without affecting mechanical properties, indicating potential for minimally invasive, biocompatible tracking (92,93). Superfluorinated polyphosphazene (PPz) polymers modified with sodium mercaptoethanesulfonate achieved exceptional water solubility (>360 mg/ml) and substantial MRI signal enhancement compared with aqueous trifluoroacetic acid controls, showing promise as future MRI contrast agents (94). However, limitations in some perfluorocarbon-based materials necessitate continued optimization for clinical applications (94).
To address these challenges, a novel hyperbranched polymeric 19F MRI contrast agent based on β-cyclodextrin and phosphorylcholine was developed to enhance T2 relaxation time and fluorine content (95,96). Higher branching degrees and optimal fluorine loading prolonged T2 relaxation times, achieving a maximum fluorine content of 11.85% and a T2 relaxation time of ≤612 msec at 9.4 T. In vitro cytotoxicity assays showed excellent biocompatibility against HUVEC and 4T1 cells, with viability exceeding 80% at concentrations ≤1,000 μg/ml (37). In vivo studies indicated no significant tissue damage in mice (39,95,96). This contrast agent provided high-performance 19F MRI capabilities, producing clear phantom images at concentrations as low as 1 mg/ml and enabling successful in vivo tumor imaging in mice following intratumoral injection (95). Incorporating superhydrophilic phosphorylcholine groups adjacent to fluorine atoms in a hyperbranched structure enhanced hydration around fluorine nuclei, resulting in prolonged T2 relaxation times and improved imaging signals (44,95,97). Despite these advantages, the agent showed limited tumor accumulation following intravenous administration due to insufficient targeting capability, indicating the need for further modification to enhance tumor-targeting efficiency.
Hyperbranched polymers with high fluorine content and enhanced molecular mobility have also been explored as effective 19F MRI contrast agents (98). Polymers synthesized via RAFT polymerization of DMAEA (77 mol%) and tFEA (19 mol%), using EGDMA (4 mol%) as a branching agent, resulted in particles ~10 nm in size (98). To improve cytocompatibility, the polymers were chain-extended with PEGMA, producing polymers (P3) with markedly lower cytotoxicity compared with the parent polymer (P1). Functionalization was demonstrated by conjugating mannose to alkyne-terminated polymers (P2) via Huisgen 'click' chemistry (99), yielding a mannose-functionalized polymer (P4). The 19F T2 relaxation time of P1 at 16.4 T was 88 msec at 20 mg/ml, and imaging experiments showed that signal-to-noise ratio increased linearly with concentration, indicating high sensitivity (41). In vivo 19F MRI involved intravenous injection of P1 into mice, with imaging after 2 h revealing prominent 19F signals in the bladder, demonstrating renal excretion and successful imaging within 10 min (37). This work represented the first example of functionalizable hyperbranched fluoropolymers engineered for in vivo 19F MRI with high sensitivity and facile ligand functionalization, offering a promising platform for targeted imaging agents (96). Nevertheless, further investigation into the in vivo efficacy and specificity of the cell-targeted polymers is needed.
19F MRI contrast agents hold significant potential for noninvasive imaging due to the favorable nuclear properties of fluorine and absence of background signals in biological tissues (100). FCy7-NO2, a novel dual-mode near-infrared fluorescence and 19F MRI probe, was designed to image tumor hypoxia by detecting nitroreductase (NTR) activity. FCy7-NO2 incorporates nitro groups as NTR recognition units and fluorine atoms for 19F NMR detection. Upon enzymatic reduction to FCy7-NH2 by NTR, the probe exhibits enhanced fluorescence and significant 19F chemical shift changes. In vitro, FCy7-NO2 showed a sevenfold increase in fluorescence intensity after 1 h incubation with NTR (0.5 μg ml−¹) and a 19F NMR chemical shift change from −118.6 ppm to −123.8 ppm. In hypoxic A549 cells, fluorescence intensity increased proportionally with NTR levels, and 19F MRI quantified intracellular NTR concentrations. In vivo, mice with orthotopic lung tumors injected with FCy7-NO2 exhibited a significant increase in fluorescence intensity in the tumor area 24 h post-injection, with the left lung fluorescence intensity 2.9 times higher than the kidney and 7.4 times higher than the liver. Additionally, 19F MRI detected FCy7-NH2 selectively in the tumor region, confirming NTR activity in vivo. This dual-modal probe enables sensitive and selective detection of NTR over a broad concentration range without depth limitations, offering a promising method for noninvasive imaging of tumor hypoxia and understanding tumor evolution (100-103). However, probe aggregation at high concentrations may reduce 19F MRI signal intensity, necessitating further studies to optimize its pharmacokinetics and biodistribution (41,104).
To overcome the limitations of weak thermal polarization in 19F MRI contrast agents hindering low-concentration target detection (105), a study applied CEST principles to achieve a 900-fold signal amplification of a biocompatible fluorinated agent, enabling micromolar detection in a 'multicolor' imaging format (106,107). In vitro experiments confirmed multiplexed detection capability using 19F-GEST MRI, and cell viability assays verified host biocompatibility. Proof-of-concept in vivo studies delivering G4 via inhalation and hosts via intracranial injection in mouse brains demonstrated localized 19F-GEST effects with potential in vivo translatability. This design exploits dynamic exchange in host-guest supramolecular assemblies to amplify the 19F MRI signal of a single fluorinated agent, markedly enhancing applicability for mapping previously undetectable low-abundance targets with micromolar detectability and multiplexed imaging via distinct chemical shifts (24,28,108-112). Limitations include the need for further in vivo optimization, potential chemical shift overlaps at lower magnetic fields, and the preliminary nature of in vivo studies requiring more extensive research.
Nanoparticles
19F MRI offers significant potential for in vivo molecular and cellular imaging due to its negligible background signal and high specificity. Compared with small-molecule or polymer-based approaches, nanoparticle strategies can load large amounts of fluorine while still permitting functionalization for targeted delivery (25). However, challenges such as MPS clearance and off-target uptake require careful formulation to preserve high signal in desired tissues. Studies have focused on enhancing 19F MRI sensitivity and specificity through innovative nanoparticle designs, addressing issues such as decreased solubility with increased fluorine content, signal attenuation from restricted molecular mobility, and maintaining biocompatibility (108).
Activatable 19F MRI nanoprobes for caspase-1 activity sensing were developed, where tandem repeats of substrate peptide sequences improved turn-on responses, enabling in vivo immune response imaging (112-115). Nanocarriers incorporating fluorinated polyelectrolyte Nafion formed 170 nm particles detectable by 19F MRI, optimized for passive tumor targeting and drug delivery (88,116,117). Dual-mode nanoparticles, such as fluorinated mesoporous silica nanoparticles functionalized with fluorosilane or polyfluorosiloxane and grafted with gadolinium chelates, provided both 1H and 19F MRI capabilities for anatomical and nanoparticle detection, respectively (118,119). Temperature-responsive polymeric nanogels composed of amphiphilic copolymers formed particles (~120 nm) with good 19F MRI sensitivity and non-cytotoxicity (120-122).
To enhance signal, hyperbranched fluoropolymers were synthesized, forming micelles (20-45 nm) where acrylate-based polymers exhibited stronger MRI signals than methacrylate-based ones (123). Perfluorocarbon nanoparticles effectively labeled human islets without compromising viability or glucose responsiveness, detectable by 19F MRI, computed tomography and ultrasound, demonstrating multimodal imaging potential (124). Targeted delivery was improved using αᵥβ3-integrin-targeted PFC nanoparticles, with intratracheal administration in lung cancer models resulting in higher tumor PFC concentrations than intravenous routes (125). Superhydrophilic zwitterionic fluorinated polymers with high 19F content (19.1 wt%) showed resistance to protein adsorption and produced intense whole-body 19F MRI signals following intravenous injection (39,97).
However, challenges remain, such as the need for high concentrations of fluorinated micelles for MRI visualization of labeled cells, adversely affecting cell viability (126). Achieving higher labeling rates without compromising viability and ensuring nanoparticle stability are significant hurdles. These studies collectively highlight advances in nanoparticle-based 19F MRI contrast agents, emphasizing innovations in design and synthesis that enhance imaging sensitivity and specificity while addressing biocompatibility and signal detection challenges.
To address limitations of current 19F MRI probes, researchers developed novel multifunctional core-shell nanoparticles called fluorine accumulated silica nanoparticle for MRI contrast enhancement (FLAME) (87,111,119). FLAME nanoparticles consist of a micelle core filled with liquid PFCE, containing 20 equivalent fluorine atoms yielding a sharp 19F NMR peak, encapsulated within a robust silica shell for surface modification, improved biocompatibility and in vivo stability (127-129). Transmission electron microscopy revealed FLAME particles with an mean value diameter of 76±9 nm, and 19F NMR spectroscopy confirmed a single PFCE-derived peak at −16.4 ppm. FLAME demonstrated high 19F MRI signal intensity proportional to PFCE concentration (87,111).
FLAME nanoparticles modified with ampicillin (FLAME-Amp) enabled the specific detection of BL-tag proteins at concentrations as low as 30 nM, markedly outperforming small-molecule probes such as F-Amp, which required micromolar protein concentrations. Notably, the T2 relaxation times of FLAME-Amp remained stable even after protein binding, ensuring consistent MRI signals. In vivo, PEGylated FLAME (FLAME-PEG) accumulated in tumor tissue via the enhanced permeability and retention effect, with strong 19F MRI signals observed at the tumor site, confirming effective delivery and retention (119). The design of FLAME nanoparticles effectively combines high fluorine content with maintained molecular mobility and surface modifiability, enhancing sensitivity and potential for targeted imaging applications. This work represents a significant advancement in developing highly sensitive and versatile 19F MRI contrast agents capable of detecting gene expression in living cells and tumors with improved stability and functionality. However, further studies are needed to fully evaluate the long-term biocompatibility and targeted delivery capabilities of FLAME nanoparticles in clinical settings (127-129).
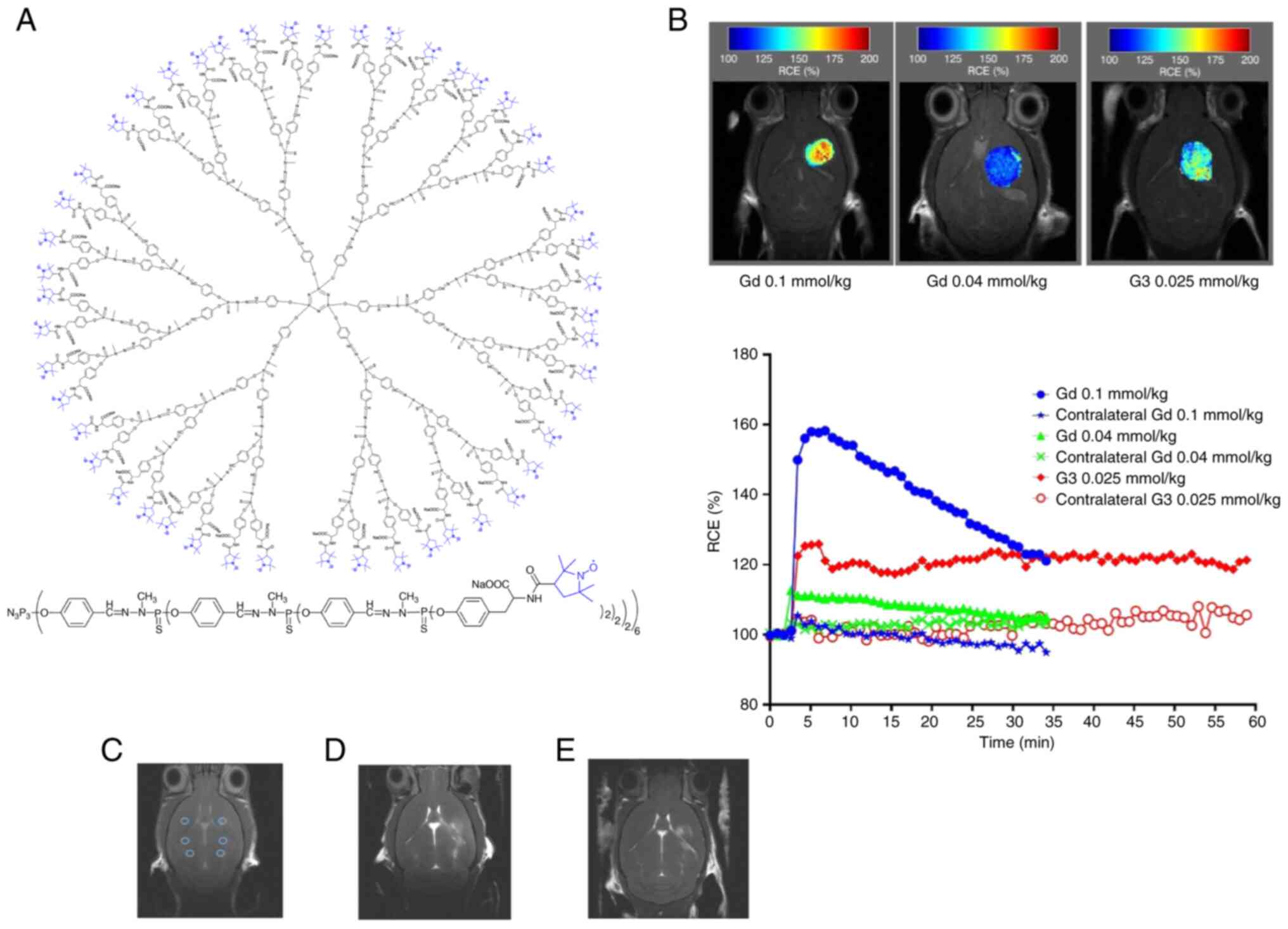
Another promising approach involves self-assembling supramolecular dendrimers for 19F MRI contrast agents (130,131). These amphiphilic dendrimers comprise a hydrophobic alkyl chain and a hydrophilic dendron with multiple negatively charged fluorinated terminals, preventing aggregation via electrostatic repulsion. This design maintains high fluorine nuclei mobility, achieving a high fluorine content (16.7 wt%) with excellent water solubility ≤85 mg/ml. The dendrimer 1c self-assembled into nanomicelles (~25 nm) exhibiting favorable relaxation properties (T1 of 534-593 msec, T2 >190 msec), surpassing common 19F MRI agents. Importantly, dendrimer 1c was non-toxic and capable of encapsulating the near-infrared fluorescence dye DiR and the anticancer drug paclitaxel, enabling multimodal imaging and theranostics for pancreatic cancer. In vivo studies on human pancreatic cancer xenografts in mice demonstrated that PTX-loaded dendrimer markedly inhibited tumor growth Compared with PTX alone, with 19F MRI and NIRF imaging confirming specific tumor localization. This work provides a promising approach for constructing 19F MRI agents and theranostic systems using self-assembling supramolecular dendrimer chemistry. However, the observed toxicity of lower-generation dendrimers highlights the need for careful structural optimization to ensure safety (129,132,133).
A novel dual nanoparticle conjugate (DNC) platform was developed as an aptamer-based 'turn-on' sensor for 19F MRI. The DNC consists of core-shell nanoparticles with a liquid perfluorocarbon core and a mesoporous silica shell (19F-MSNs) of diameter 94±27 nm, providing a robust 19F MR signal, and superparamagnetic iron oxide nanoparticles (SPIONs) of diameter 4.7±0.6 nm serving as magnetic quenchers (134). Due to the strong T2 relaxation enhancement by SPIONs, effective quenching was achieved with only four equivalents of SPIONs relative to 19F-MSNs. The probe functions via target-induced dissociation of DNA aptamers; specifically, the thrombin-binding aptamer was incorporated as a proof-of-concept (DNCThr). Upon incubation with human α-thrombin at concentrations ≤1 μM, the 19F T2 relaxation time increased markedly from a quenched state of 86 msec to 581 msec, indicating successful turn-on of the MR signal. In vivo experiments demonstrated that DNCThr generated a robust 19F MRI 'hot-spot' signal in response to thrombin injected subcutaneously in live mice, with a SNR of 7.3 compared with 3.2 in control injections. The versatility of the platform was further demonstrated by adapting it to sense ATP and kanamycin, showing a similar increase in T2 relaxation times upon target binding.
Overall, advances in nanoparticle-based 19F MRI contrast agents, including FLAME nanoparticles and supramolecular dendrimers, have spurred meaningful progress in imaging sensitivity and specificity. These innovations address key challenges in biocompatibility, stability, and signal detection, bringing 19F MRI closer to practical applications in molecular and cellular imaging (128,135,136). Continued refinement of nanoparticle designs is crucial for translating these technologies into clinical use.
Small molecules
Fluorinated small molecules have emerged as promising 19F MRI contrast agents due to their well-defined chemical structures, enabling precise characterization, reproducible synthesis, and scalable production. While such small-molecule agents are easier to prepare and often clear quickly, they commonly offer fewer 19F atoms per molecule than polymeric or PFC-based systems, limiting their raw signal strength. Nonetheless, the straightforward design and tunable properties of small-molecule probes allow for specific targeting or responsiveness to physiological stimuli, making them valuable tools for disease diagnosis and drug monitoring (39). Their good aqueous solubility and low molecular weight facilitate efficient biological distribution and rapid renal clearance without requiring extensive modification (137-140). Overall, these profiles present a viable alternative to nanoparticle or polymer-based formulations when short circulation times and low accumulation are desirable.
Fluorinated amino acids and sugars
Fluorinated sugars, which mimic natural biomolecules and participate in metabolic processes, are vital metabolic imaging agents for cancer detection and treatment monitoring. The unique properties of 19F, particularly its chemical inertness and small atomic size, allow these compounds to be recognized by biological systems similarly to their non-fluorinated counterparts (25,39,40). Since endogenous 19F is negligible in biological tissues, 19F NMR and MRI can track these molecules in vivo without background interference. A key example is 2-fluoro-2-deoxy-D-glucose (2-FDG), a fluorinated glucose analog (141). Due to its structural similarity to glucose, 2-FDG is taken up by cells via glucose transporters and phosphorylated to form 2-fluoro-2-deoxy-D-glucose-6-phosphate (2-FDG-6-P) but cannot undergo further glycolysis (141). This leads to its accumulation in cells with high glucose uptake, such as cancer cells, providing a basis for imaging tumor metabolism using 19F MRI. Preclinical studies by Kanazawa et al (142) demonstrated the effectiveness of 2-FDG as a metabolic imaging agent. Injecting 2-FDG into tumor-bearing mice, they obtained 19F MR images revealing higher concentrations of 2-FDG and its metabolite 2-fluoro-2-deoxy-D-mannose (2-FDM) within tumor tissues compared with normal tissues, highlighting their potential for non-invasive tumor detection and metabolic assessment. Furthermore, fluorinated sugars such as 2-FDG offer insights into radioactive counterparts used in positron emission tomography (PET), such as 2-[18F]FDG (141). While PET provides high sensitivity, it involves ionizing radiation and limited availability. By contrast, 19F MRI with fluorinated sugars presents a non-radioactive alternative suitable for repeated longitudinal studies, advantageous for ongoing treatment monitoring (37,41,42,143). Beyond detection, fluorinated sugars aid in monitoring therapeutic responses. Changes in their uptake and metabolism can indicate alterations in tumor metabolism post-treatment, providing early signs of efficacy or resistance. Consequently, they serve as valuable tools in personalized medicine, optimizing therapeutic strategies based on the metabolic profiles of individual tumors.
Fluorinated drugs
Fluorinated drugs such as 5-FU and fluoxetine contain fluorine atoms crucial to their therapeutic functions, making them suitable for detection and monitoring via 19F MRI (128,140,144). However, their low fluorine content per molecule limits imaging sensitivity (38,145). To address this, bioorthogonal chemistry strategies have been developed. One approach employs azide-containing small molecules incorporated into cellular components through metabolic processes. These azide groups react with fluorinated cyclooctyne probes via click chemistry, increasing the number of 19F nuclei associated with target cells and amplifying imaging sensitivity (146). This enhancement allows successful deep-tissue visualization of metabolic probes, enabling more accurate and detailed imaging results.
5-FU, a chemotherapeutic agent widely used to treat cancers such as colorectal and breast cancers, undergoes complex metabolic pathways in vivo, converting into active metabolites that disrupt DNA and RNA synthesis in rapidly dividing cells (147). Despite sensitivity challenges, researchers have used 19F MRI to map the biodistribution of 5-FU in tumor-bearing animal models, observing its accumulation in tumors and major organs shortly after administration (128,142). Monitoring the in vivo behavior of 5-FU via 19F MRI aids in optimizing dosing regimens and minimizing systemic toxicity by tailoring treatments to individual patient responses (142). Similarly, fluoxetine, a selective serotonin reuptake inhibitor prescribed for depression and anxiety disorders, contains a fluorine atom detectable by 19F MRI. Visualizing its distribution in the brain and peripheral tissues offers insights into its pharmacokinetics and therapeutic mechanisms. Tracking fluoxetine with 19F MRI could reveal patterns of drug uptake, distribution, and clearance, contributing to personalized medicine approaches in neuropsychiatric treatment.
Responsive 19F MRI probes
The development of responsive 19F MRI probes has markedly advanced non-invasive imaging of pathological conditions, including cancer and inflammation. By altering their NMR properties in response to biological stimuli, such as pH changes, enzyme activity, or metal ions, these probes provide real-time insights into cellular and molecular processes (148). These responsive probes integrate fluorine atoms into molecular structures that undergo chemical transformations upon encountering target stimuli. The high sensitivity of 19F NMR to electronic environment changes, coupled with the absence of endogenous fluorine in biological systems, enhances imaging specificity and makes 19F MRI ideal for detecting these transformations.
In cancer detection, elevated glycolysis in tumor cells leads to an acidic microenvironment. To map intra-tumoral pH variations, responsive 19F MRI probes with pH-sensitive moieties have been developed. These fluorinated compounds undergo detectable chemical shift changes in 19F MRI, enhancing assessment of tumor progression and therapeutic effectiveness through spatial pH mapping. Enzyme-responsive 19F MRI probes provide additional specificity by targeting enzymes overexpressed in tumors, such as matrix metalloproteinases (114,128,148,149). Probes with cleavable linkers alter their 19F NMR signal upon enzymatic cleavage. Remaining quenched until activated by the target enzyme, these probes produce a detectable 19F MRI signal where enzyme activity occurs (134).
In inflammation, 19F MRI probes are instrumental in tracking immune cell infiltration and activity (81,128,129). Metal ion-sensitive probes responsive to Ca2+ or Zn2+ fluctuations during inflammatory responses visualize these processes. Fluorinated chelators binding Zn2+ exhibit changes in 19F NMR relaxation properties, enabling detection of inflammation-associated zinc fluxes. Additionally, reactive oxygen species (ROS)-responsive probes containing boronate esters react with hydrogen peroxide, altering the 19F NMR signal and allowing selective imaging of oxidative stress in diseases such as atherosclerosis or neurodegenerative disorders (150-153). Advanced probe designs incorporate dual functionality by combining 19F MRI with optical fluorescence imaging (154). These dual-modal probes offer complementary information: Fluorescence provides high-resolution localization, while 19F MRI offers deep tissue penetration (154). Using techniques such as aggregation-induced emission fluorophores can circumvent quenching in self-assembled polymeric probes. By mapping physiological changes, pH variations, enzyme activity, and metal ion concentrations, responsive 19F MRI probes provide valuable insights into the onset and progression of diseases such as cancer and inflammation (41,42,128,129).
Other approaches
Advances in 19F MRI contrast agents have led to novel compounds with improved imaging capabilities for diverse biomedical applications. CA-sar-TFMA, a trifluorinated bile acid resistant to CGH-mediated deconjugation, was developed for noninvasive assessment of bile acid transport. It showed favorable in vitro and in vivo stability, acted as a potent inhibitor and substrate of apical sodium dependent bile acid transporter (ASBT) and Na+/taurocholate cotransporting polypeptide (NTCP) and accumulated 16.1-fold more in gallbladders of wild-type mice than Asbt-deficient mice, supporting its potential as an MRI probe for bile acid transport (155).
To improve solubility of fluorine tracers, the hyperfluorinated hydrophilic organofluorine ET1084 (~24 wt% 19F) was developed, achieving water solubility at ≥8 M 19F concentration. Phantom studies at 9.4 T demonstrated a linear increase in SNR with concentration, a detection limit of 5 mM, and preliminary safety ≤20 mM (156). Additionally, water-compatible fluorine-rich polymers were synthesized via nucleophilic addition to enhance 19F MRI signals (37,86). Incorporation of PEG linkers increased T2 without compromising high T1 values, improving NMR signals and peak profiles. Phantom imaging showed bright signals, but clinical translation limitations persist (157).
In pulmonary imaging, octafluorocyclobutane (OFCB) was evaluated as an inhalable 19F MRI contrast agent (29). At 0.5 T, human studies showed anatomically consistent lung images with SNRs of 50 in 2D and 20 in 3D modes using breath-hold durations of 20-40 sec, indicating the clinical potential of OFCB despite resolution limitations due to low field strength (158). Moreover, 19F MRI was used to monitor hydrogel scaffold degradation in vivo, offering precise localization and quantitative degradation rates without endogenous signal interference, suggesting utility for implant evaluation, though further validation is needed (159).
Fluorinated mannoheptulose derivatives (19FMH) have been investigated for imaging GLUT-2-expressing cells. Although 19FMHs preferentially accumulated in GLUT-2-rich tissues and showed potential for cell tracking, rapid clearance and low 19F MRI sensitivity presented challenges requiring optimization (160). Collectively, these findings underscore the wide-ranging efforts to design highly sensitive 19F MRI contrast agents, whether through new small molecules, polymers, nanoparticles, or responsive probes, that highlight promising trends and emerging gaps. Future work must continue improving signal intensity, distribution, safety, and targeted specificity to advance 19F MRI toward widespread clinical adoption.
Chemical exchange saturation transfer (CEST)
CEST MRI is a promising molecular imaging technique for detecting metabolites with exchangeable protons, such as amide, amine, and hydroxyl groups. By exploiting the chemical exchange between these protons and bulk water, it enhances image contrast without traditional metal-based contrast agents, enabling non-invasive assessment of molecular changes within tissues and providing valuable insights into metabolic processes (161,162). Since its introduction by Wolff and Balaban in 1989 (163), CEST MRI has evolved from a conceptual framework to a clinically applicable tool. Initial efforts in brain imaging demonstrated high sensitivity to molecular alterations in brain tumors. Early clinical studies highlighted its potential to differentiate tumor recurrence from radiation necrosis by detecting variations in exchangeable proton signals abundant in malignant tissues but diminished in necrotic areas post-treatment (163-170). Despite these advances, emerging CEST agents still face challenges with agent stability, limited sensitivity, and difficulties in quantitative analysis that require refined acquisition and post-processing methods. Ongoing comparisons among different CEST agents are necessary to clarify their most effective clinical applications and facilitate wider adoption.
Advances in acquisition sequences and post-processing methods have expanded CEST MRI beyond the central nervous system (106). Despite challenges unique to body imaging, such as motion artifacts, B0/B1 inhomogeneities and absence of the blood-brain barrier (BBB), researchers have successfully applied CEST techniques to other tissues. Studies have demonstrated its utility in assessing tumor metabolism, characterizing histological subtypes, and monitoring treatment responses in cancers of the breast, liver, pelvis, and digestive system (171-173). Non-metal CEST contrast agents have been crucial in these developments (174), offering reduced toxicity and improved biocompatibility compared with metal-based agents. By targeting specific metabolites and exploiting endogenous molecules with exchangeable protons, these agents enhance MRI sensitivity and specificity without introducing potentially unsafe exogenous metals. Further comparative evaluations of different non-metal agents are warranted to refine their diagnostic specificity, assess their stability, and address quantification complexities before widespread clinical translation.
Glucose and glucose analogues
D-glucose has emerged as a promising non-metallic CEST contrast agent for brain tumor imaging due to its natural presence and favorable safety profile (175,176). Exploiting exchangeable protons in its hydroxyl groups, D-glucose functions in Dynamic Glucose-Enhanced (DGE) MRI by allowing saturation and detection through MRI, monitoring transient changes in glucose concentration within tissues (176). Intravenous administration of glucose enables real-time tracking of its accumulation and washout in brain tissues, and DGE MRI has been successfully translated to human studies, allowing visualization of brain tumors with enhanced contrast.
A significant advantage of DGE MRI is its ability to detect disruptions in the BBB, a hallmark of malignant brain tumors (177). Tumor-induced BBB breakdown permits increased extravasation of glucose into the tumor interstitium compared with normal brain tissue. This differential uptake results in heightened CEST signals within tumors, providing valuable diagnostic information about tumor location, size, and permeability, and aiding in the assessment of tumor aggressiveness and therapeutic planning (178-182). However, at 3 T, the DGE signal change is modest (~1%) and susceptible to motion artifacts, necessitating effective motion correction and optimized infusion protocols. Prolonged infusion durations of 3-4 min help mitigate transient side effects without compromising the DGE signal change, enhancing the robustness of glucoCEST imaging. The translation of D-glucose glucoCEST MRI to human studies at 7 T demonstrated feasibility in detecting dynamic signal changes in glioma patients, with variations in signal enhancement correlating with perfusion properties and BBB permeability (183,184).
To overcome limitations of D-glucose, other sugar analogues have been explored. Non-metabolizable analogues such as 2-deoxy-D-glucose (2-DG) and 3-O-methyl-D-glucose (3-OMG) are structurally similar to glucose but not fully metabolized, allowing prolonged imaging windows and investigation of glucose transport and uptake mechanisms within tumors. 2-DG enters cells via glucose transporters and becomes trapped after phosphorylation, while 3-OMG is transported without subsequent metabolism. Studies using these agents demonstrated improved tumor visualization and insights into tumor metabolism (185,186). For instance, 3-OMG showed around twice the CEST contrast enhancement compared with D-glucose in brain tumors, with tumor regions exhibiting enhancement of 2.5-5.0% vs. 1.5-3.5% in contralateral brain, and prolonged signal persistence (187). Additionally, 2-DG and 2-fluoro-2-deoxy-D-glucose generated significant CEST effects ≤30% persisting over an hour in mammary tumors, suggesting potential to replace PET imaging in preclinical studies (170). Comparative analyses of these analogues indicate that the lack of phosphorylation of 3-OMG may extend its imaging window, while the phosphorylation of 2-DG increases retention within tumor tissues, each approach offering advantages that can be tailored to specific clinical goals (186,187).
Other agents such as glucosamine (GlcN) have been investigated as exogenous CEST contrast agents (188). The anomeric equilibrium and mutarotation rate constants of GlcN, crucial for CEST effects, markedly depend on concentration, pH, and buffer conditions; for example, at pH 7.0 and GlcN concentration of 0.5 M, the mutarotation rate constant was 5.0×10−4 sec−¹, reaching 95% equilibrium in 1.7 h (189). Sugar alcohols such as maltitol have also been proposed; in vivo studies showed CEST contrast elevation in glioma regions due to permeable BBBs, while not affecting normal brain tissue (190). Xylose demonstrated higher sensitivity than glucose in CEST and CESL MRI techniques, without markedly affecting blood glucose levels or neural activity, making it a promising agent for studying glucose uptake (191).
Researchers developed Dex1, the smallest clinically available dextran (~1 kDa), as a new CEST MRI contrast agent to assess tumor hemodynamics, hypothesizing that its hydroxyl protons provide detectable CEST signals and its established safety profile facilitates clinical translation (192). In vivo CEST MRI studies on mice with orthotopic GL261 brain tumors revealed that intravenous injection of Dex1 (2 g/kg) resulted in markedly higher CEST contrast enhancement in tumors compared with contralateral brain tissue (∆MTR_ asym1.2ppm= 0.010±0.006 vs. 0.002±0.008) at 20 min post-injection. Consistent with dynamic contrast-enhanced MRI and fluorescence microscopy, these findings demonstrate the potential of Dex1 as a highly translatable CEST MRI contrast agent. Overall, these studies underscore that, despite promising safety profiles, glucose and glucose analogues still face challenges in achieving robust sensitivity, stable signal detection, and consistent quantitative analysis protocols for full clinical potential (175).
Endogenous contrast agents
CEST MRI is a novel imaging technique that enables in vivo mapping of metabolites by exploiting proton exchange mechanisms between metabolites and water protons. This method provides contrast based on specific molecular environments, allowing the detection of endogenous molecules such as creatine (Cr), phenol, glycine, and urea (193,194). CEST MRI offers insights into metabolic changes associated with various physiological and pathological conditions, including myocardial infarction (MI), enzymatic activities, neurotransmitter distributions and renal function (168-170). Among these agents, creatine-based contrast is particularly valuable for cardiac remodeling studies, whereas phenol- and glycine-based contrasts facilitate the detection of enzymatic and neurotransmitter abnormalities, respectively, highlighting how each endogenous metabolite addresses different clinical needs.
Cr-weighted CEST MRI can map Cr distribution during MI, offering insights into metabolic changes during myocardial remodeling (195); a study investigated dynamic alterations of myocardial Cr during acute MI using this technique (162). A total of seven adult Bama pigs underwent cardiac cine, Cr-weighted CEST, and late gadolinium-enhanced (LGE) T1-weighted imaging on a 3 T scanner at 3 and 14 days post-MI induction. Cardiac structural and functional indices (MM, EDV, ESV, SV and EF) were assessed, with myocardium categorized as infarct, adjacent, or remote regions based on LGE-determined infarct angle. Cr-weighted CEST MRI signals, reflecting creatine changes, were analyzed using a three-pool Lorentzian model. While MM, EDV, and ESV remained stable (P>0.05), SV and EF rose markedly, and the infarct angle decreased. Cr-weighted CEST signals markedly increased from day 3 to day 14 in infarct, adjacent, and whole myocardium regions. These findings highlight a significant correlation between increased myocardial Cr and structural and functional recovery during acute MI, underscoring the potential of CEST MRI in assessing heart remodeling from a metabolic perspective. Limitations such as small sample size and single-slice imaging may restrict the generalizability of results (196).
Beyond cardiac imaging, CEST MRI has been used with other endogenous compounds to explore various physiological and pathological conditions (106). Phenol has been used as a contrast agent for detecting enzymatic activity. Its exchangeable hydroxyl proton resonates at 4.8 ppm from water and can be detected at sub-millimolar concentrations under acidic conditions (197). Upon acid phosphatase (AcP) activity at pH 5.0, non-CEST-detectable phenyl phosphate is converted to CEST-detectable phenol, enabling direct quantification of AcP activity without a secondary probe (198). This phenolCEST biosensor successfully measured AcP activity in enzyme solutions and prostate cell lysates.
Similarly, GlyCEST MRI has been employed to map glycine levels in the murine brain. Studies revealed higher GlyCEST effects in the thalamus compared with the cerebral cortex (P<0.0001), consistent with biochemical assays (196). In 5xFAD mice, a model of Alzheimer's disease, GlyCEST detected decreased glycine concentrations in the cerebral cortex (P<0.05) and thalamus (P<0.0001), highlighting its potential in investigating neuropsychiatric disorders (196,199).
Additionally, urea has been evaluated as a CEST MRI contrast agent to assess renal concentrating capacity. Phantom experiments demonstrated that urea CEST contrast is concentration and pH-dependent, involving both acid- and base-catalyzed exchange. In vivo studies showed that the inner medulla and papilla exhibited higher pre-injection CEST contrast (2.3±1.9%) compared with the cortex (0.15±0.75%, P = 0.011) and outer medulla (0.12±0.58%, P = 0.008) (200-202). Urea infusion increased CEST contrast in these regions by 2.1±1.9%, whereas saline infusion resulted in a decrease (-0.5±2.0%, P = 0.028 vs. urea), indicating that urea CEST can capture spatial variations in renal function. Practical concerns related to thermal drift, diuretic status and precise pH conditions highlight the need for careful experimental design. Collectively, these studies suggest that endogenous CEST MRI contrast agents offer non-invasive imaging opportunities in diverse contexts, though improving sensitivity and quantitative analysis methods is crucial for broader clinical impact (162).
Exogenous contrast agents
CEST MRI contrast agents use exchangeable protons to enhance MRI signals, enabling functional imaging applications such as pH mapping. Recent developments have focused on designing novel diamagnetic CEST agents with enhanced imaging properties, sensitivity, and specificity (163,203-208). Nonetheless, variations in chemical shifts, exchange rates and in vivo stability highlight the necessity for systematic comparisons of these compounds to identify the most clinically relevant candidates for pH and perfusion imaging.
A total of 14 newly synthesized imidazole-4,5-dicarboxyamides (I45DCs) were developed and evaluated for pH and perfusion imaging applications (209). These aromatic compounds possess large labile proton chemical shifts (≤7.7 ppm from water) due to intramolecular hydrogen bonds and include a second labile proton for ratio-based pH measurements. The I45DCs demonstrated strong CEST contrast across various substitutions, enabling tuning of the measurable pH range by adjusting inflection points in CEST signal ratio vs. pH plots. Notably, the anionic compound I45DC-diGlu exhibited a ring NH proton exchange rate [k(BA)] of 5081 sec−¹ at pH 6.5 and provided a detectable pH range of 5.6-7.0. In vitro studies revealed advantages over currently employed triiodobenzenes for tumor and kidney pH mapping due to larger chemical shifts and tunable pH sensitivity, while cell cytotoxicity assays indicated good tolerability (209-211). In vivo evaluation in a unilateral ureter obstruction mouse model showed that I45DC-diGlu effectively detects functional changes and differences in perfusion and pH between obstructed and unobstructed kidneys, highlighting its potential as a CEST MRI contrast agent for renal imaging. Nevertheless, further investigation into the biocompatibility and quantitative reproducibility of these compounds is necessary prior to clinical adoption.
Additionally, the feasibility of using unlabeled aspirin as an activatable theranostic CEST MRI contrast agent for breast cancer detection has been evaluated (212). By exploiting the conversion of aspirin to salicylic acid (SA), which provides CEST contrast due to exchangeable protons at 9.6 ppm, the study demonstrated that aspirin can serve as a noninvasive theranostic agent. CEST MRI following aspirin treatment showed similar SA CEST contrast (~3%) in both high and low COX-1/-2 expressing breast cancer cell lines, while prostaglandin E2 levels decreased by ~50%. In vivo, mice bearing orthotopic tumor xenografts exhibited tumor contrast enhancement of 5-8% at one h post-injection, with the CEST contrast being dose-dependent. This gadolinium-free imaging approach offers therapeutic effects and imaging capability via a widely used drug. A major limitation is that SA CEST MRI contrast remained independent of COX-1/-2 expression levels, indicating metabolism of aspirin prior to tumor accumulation.
Other studies have advanced the development of diamagnetic CEST agents. Salicylic acid analogues (SAAs) (206), anthranilic acid analogs (213), and phenols with tunable exchangeable protons (214) provide significant contrast at frequencies far from the water resonance (4.8-13.5 ppm), enhancing detection sensitivity. Enzyme-responsive agents synthesized for catalyCEST MRI have demonstrated high specificity in evaluating enzyme activity and inhibition both in vitro and in vivo (215-217), using agents that generate both enzyme-responsive and unresponsive CEST signals for concentration-independent measurements (192). Clinically approved iodinated contrast agents such as iohexol and ioversol have been repurposed as CEST agents, displaying good contrast at 7 T and prolonged tumor enhancement, with significant correlation between CT and CEST-MRI images (R=0.70; P<0.01) (106,218,219). Mannitol, known for osmotic BBB opening, exhibited strong CEST contrast at ~0.8 ppm, enabling non-invasive detection of intracranial accumulation (220). Novel agents such as free-base porphyrins and chlorins provided large upfield shifts (−8 to −13.5 ppm) and suitable exchange rates (500-9,000 sec−¹) for robust detection (221), while citicoline has been explored as a theranostic agent with inherent CEST signals (222,223). Ongoing work aims to reduce the high concentrations occasionally required for detection (197), address small chemical shifts (221) and mitigate potential toxicity issues (224). Collectively, these developments underscore the importance of refining exogenous CEST MRI contrast agents to address stability, sensitivity, and quantitation challenges for future clinical and theranostic applications (106,205,225).
Proteins and peptides
CEST MRI has emerged as a promising metal-free diagnostic imaging technique, enabling the detection of contrast based on endogenous metabolites, peptides and proteins with minimal invasiveness and low toxicity. Recognizing the limitations of existing genetically encoded CEST contrast agents, which often rely on repetitive amino acid sequences and can pose metabolic or stability issues, researchers developed an in silico method to evolve peptide sequences optimized for CEST contrast, hypothesizing that these peptides could be assembled into a de novo biosensor for CEST MRI (226). The authors designed a synthetic gene encoding a recombinant protein, termed superCESTide, by concatenating top-performing peptides identified through in silico optimization. The resultant protein, consisting of 198 amino acids, exhibited a diverse amino acid composition that reduces reliance on any single residue. SuperCESTide was expressed in Escherichia coli and purified using size exclusion chromatography. CEST MRI assessments at 7 T revealed that the magnetization transfer ratio asymmetry (MTR asym) generated by superCESTide reached a maximum of 6% at 3.6 ppm, comparable to protamine sulfate and human protamine. Faster amide proton exchange rates (474 to 902 sec−¹) than poly-L-lysine and numerous endogenous proteins contributed to its enhanced contrast. Challenges remain in purifying superCESTide and characterizing structural stability, suggesting further exploration to ensure reliability and quantify agent performance in complex biological environments.
Researchers modified a lysine-containing peptide (K2) with peptide nucleic acid (PNA) bases at the N-terminus to produce a-K2, c-K2, g-K2, and t-K2 (227), introducing primary amine groups suitable for CEST signal generation. Among these, c-K2 exhibited self-assembly into hydrogels and markedly enhanced the mechanical strength of the hydrogel. The c-K2/g-K2 hydrogel displayed improved mechanical responsivity and good water retention (swelling ratio of 28.6%). These PNA-modified peptide hydrogels generated a detectable CEST signal at ~2.5 ppm due to chemical exchange between exchangeable amine protons and water protons (107). Intratumoral injection into tumor-bearing mice confirmed the capability of hydrogel as an implantable CEST-MRI agent detectable in vivo. Despite their potential, further improvements in gelation properties and evaluation of long-term stability are needed to optimize these systems for clinical feasibility.
Protein and peptide-based CEST MRI contrast agents offer a versatile platform for enhancing imaging specificity via exchangeable protons in amino acid side chains (227-229). Poly(propylene fumarate) scaffolds coated with protamine sulfate demonstrated steady protein release over 24 h, indicating potential for MR-guided drug delivery systems (230). An array of 33 prototype polypeptides showed that the CEST effect can be fine-tuned by altering amino acid sequences (231,232). Methods such as QUEST and QUESP quantified exchange rates in agents such as poly-L-lysine, confirming pH dependence with base-catalyzed exchange predominance (233). Human protamine-1, an arginine-rich peptide, was synthesized as a biocompatible MRI reporter gene, demonstrating markedly higher CEST contrast in engineered cells compared with controls (234,235). Its CEST contrast was highly sensitive to pH, phosphorylation state, and nucleic acids, with binding constants determined by plotting molar concentrations vs. CEST contrast (236). Poly-L-glutamate has been used to map cathepsin expression in vivo, exploiting differences in CEST signals between native and cleaved forms (237). Angiopep-2, an artificial peptide that penetrates the BBB, exhibited a CEST effect peaking at 3.2 ppm with optimal saturation power of 5.5 μT, indicating promise for detecting early Alzheimer's disease (238). A nonmetallic contrast agent, GR-4Am-SA, provided distinct CEST signals at 5.0 and 9.5 ppm to track urokinase plasminogen activator activity with an average reaction coordinate of 80±8% (239). Although these recombinant or modified protein systems can generate high CEST signals, their stability, metabolic effect, and reproducibility must be thoroughly addressed. Continued comparative investigations are essential to determine the optimal strategies for clinical use.
Nanoparticles
A novel furin-mediated self-assembling olsalazine (Olsa) nanoparticle detectable by both CEST MRI and Raman spectroscopy (240) was developed to target furin-overexpressing tumors. Olsa, a DNA-methylation inhibitor, was conjugated to 2-cyano-6-aminobenzothiazole (CBT) and the furin-specific peptide substrate RVRR to create cell-permeable Olsa-RVRR. Intracellular furin cleaves RVRR, exposing a cysteine residue that reacts with the CBT moiety to produce hydrophobic oligomers, which self-assemble into nanoparticles exhibiting a distinct Raman scattering peak at 1,168 cm−¹. In vivo, SCID mice bearing HCT116 xenografts injected with Olsa-RVRR exhibited significant Raman signals in tumors 2 h post-injection, with a 91.7% correct classification rate via support vector machine analysis. This approach offers a potential tool for high-resolution image-guided surgery in furin-overexpressing tumors, though clinical validation is needed to ensure broad specificity.
CEST MRI enables measurement of extracellular pH (pHe) in tumor microenvironments but requires high concentrations of small-molecule contrast agents due to inherent insensitivity (241). To overcome this, nanoscale polymeric CEST agents have been developed to boost CEST sensitivity by increasing the number of exchangeable protons per particle (107,242,243). After optimizing experimental conditions, one study found that a polymer agent enabled acid CEST MRI at concentrations 125-fold lower than a comparable monomer agent, though pH measurements exhibited some concentration dependence (242). In vivo acidoCEST MRI in a xenograft MDA-MB-231 mammary carcinoma model yielded tumor pHe measurements of 6.33±0.12 with iopamidol, 6.70±0.15 with the monomer agent, and 6.85±0.15 with the polymer agent, possibly reflecting differing dosing requirements and complex interactions within the tumor environment. While nanoscale systems can substantially enhance sensitivity, factors such as clearance pathway, potential toxicity, and quantitative accuracy require further optimization.
Nanoparticle-based CEST MRI contrast agents have also been engineered to exploit ionizable tertiary amines (243), carbon dots (244,245) and liposomal formulations (219,222) to enhance imaging contrast in acidic or otherwise specialized physiological conditions. Salicylic acid-conjugated poly(amidoamine) dendrimers produce strong CEST contrast with adjustable proton exchange rates and have shown promise in glioblastoma imaging (246-248). Dual-mode nanoparticles encapsulating perfluoropentane and salicylic acid in hematoporphyrin-poly(lactic acid) polymers have reportedly improved tumor characterization in vitro and in vivo (249). Liposome-based mucus-penetrating particles laden with barbituric acid yielded prolonged vaginal imaging (250). These studies exemplify how nanoparticle strategies can bolster CEST contrast, yet further scrutiny of agent stability and quantitative reproducibility is necessary prior to potential clinical translation.
Other approaches
CEST MRI contrast agents are metal-free alternatives to gadolinium-based agents, providing molecular-level information on key metabolic processes. Using a supramolecular strategy, Pemetrexed was transformed into a molecular hydrogelator with inherent CEST MRI signals; under physiological conditions, it forms filamentous assemblies, creating theranostic hydrogels suitable for injectable delivery and direct monitoring of drug distribution in a mouse glioma model (251). Similarly, paracetamol and acetanilide derivatives have shown significant diamagnetic CEST contrast only when forming intermolecular hydrogen-bonded networks, with paracetamol reaching 12% contrast at 15 mM under physiological conditions (252). A hydrazone-dependent CEST effect (Hydrazo-CEST) derived from N-amino anthranilic acid undergoes a turn-on response upon hydrazone formation with aldehydes, providing an avenue for MRI detection of bioactive aldehydes (253). Meanwhile, N-aryl amides with favorable chemical shifts (4.6-5.8 ppm) allow label-free detection of N-aryl amide drug metabolism (254). A novel 2-HYNIC-based agent for sensing aromatic aldehydes successfully mapped pyridoxal 5′-phosphate in vitro and in vivo in lung cancer models (255). While these supramolecular and label-free strategies expand the range of diamagnetic CEST MRI contrast agents and offer new possibilities for drug delivery monitoring and biomarker detection, further head-to-head comparisons within the broader agent landscape remain essential. Future endeavors should pursue robust validation of safety, improve sensitivity, and address quantitative normalization to enhance the translational potential of these new constructs (106,170,205,225).
Nitroxide radicals
Nitric oxide (NO), a small endogenous signaling molecule integral to various physiological processes, has emerged as a promising MRI contrast agent. By modulating local magnetic environments or participating in CEST processes, NO can effectively enhance MRI signals without the toxicity and accumulation issues associated with metal-based agents. Leveraging a naturally occurring molecule such as NO may also improve biocompatibility and reduce the likelihood of adverse reactions. Compared with nitroxide radicals, which rely on paramagnetic properties and redox sensitivity, NO-based agents target different imaging mechanisms by influencing proton exchange rates rather than focusing primarily on redox-dependent signal changes. Although both agent classes are metal-free and aim to reduce toxicity, additional work is needed to address key challenges such as stability, sensitivity, and the difficulties in quantitative analysis. Future research must clarify whether NO-based approaches can achieve broader clinical applications and longer in vivo half-lives akin to certain nitroxide derivatives (256-258).
Nitroxide radicals
Carbamoyl-proxyl
Carbamoyl-proxyl, a nitroxide radical with paramagnetic properties, has emerged as a promising MRI contrast agent for reflecting tissue redox status. Nitroxide MRI contrast agents undergo in vivo redox reactions, enabling MRI detection of ROS levels through their reduction rates (259). This study hypothesized that the reduction rate of nitroxide radicals could non-invasively differentiate hepatic steatosis from steatohepatitis by detecting excess ROS in the liver. Using diabetic STAM™ mice that sequentially develop hepatic steatosis and steatohepatitis, researchers intravenously injected 3-carbamoyl-PROXYL (CmP) during MRI procedures and calculated the signal intensity reduction rate. The liver's reduction rate was markedly higher in the steatohepatitis group (NAS of 3) than in the hepatic steatosis and control groups, indicating elevated ROS levels in early steatohepatitis; the CmP signal intensity decreased more rapidly, reflecting a faster reduction rate. Immunohistochemical analysis with 4-hydroxynonenal (4-HNE) confirmed excess ROS generation, with a markedly higher positive area ratio (P<0.01) in the steatohepatitis group compared with hepatic steatosis and controls. Monitoring the enhanced reduction rate of CmP in MRI, reflecting rapid conversion of the nitroxide radical to its diamagnetic form via redox reactions with ROS, enabled non-invasive differentiation of early steatohepatitis from hepatic steatosis. This suggested that CmP as an MRI contrast agent reflects organ-specific ROS levels due to redox reaction differences, independent of systemic blood flow variations. The significance of this study lies in its potential to provide a translational, non-invasive method for early detection and differentiation of steatohepatitis from hepatic steatosis in drug development, facilitating timely intervention through drug withdrawal. Further research with other animal models is warranted to verify this method's usefulness and clinical applicability.
Various studies have demonstrated the efficacy of carbamoyl-proxyl in tracking oxidative stress and redox changes across pathological conditions (260-262). In tumor-bearing mice, carbamoyl-proxyl used as a T1-weighted MRI contrast agent at 4.7 T revealed faster nitroxide decay rates in tumor regions Compared with muscle (0.097 min−¹ vs. 0.067 min−¹), indicating higher oxidative stress (263,264). In myocardial infarction models, the reduction rate constant of carbamoyl-proxyl was markedly elevated in infarct regions on Days 1 and 4 post-infarction, associated with increased oxidative stress confirmed by dihydroethidium staining (265). Similarly, in liver fibrosis induced by dimethylnitrosamine, in vivo DNP-MRI with carbamoyl-proxyl allowed reduction rate mapping, showing clear redox status differences between fibrotic and control livers (266). In acute kidney injury models, mitochondrial-targeted carbamoyl-proxyl derivatives improved survival rates and inhibited kidney damage, with T1-weighted MRI enhancement (r1≈0.190 mM−¹ sec−¹) reflecting the ROS scavenging capability of the probe (267). Studies on skeletal muscle inflammation demonstrated that DNP-MRI with carbamoyl-proxyl could non-invasively detect focal redox status changes due to local inflammation, with markedly increased decay rates at 24 h post-injury (268-271). To enhance brain penetration, carbamoyl-proxyl derivatives with varying lipophilicity were designed, with amphiphilic derivatives such as 3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine-N-oxyl showing uniform brain distribution and typical biphasic signal decay profiles (272). In comparison to other nitroxide radicals, carbamoyl-proxyl excels at redox-sensitive detection of early pathological changes in multiple tissues, but BBB-permeable derivatives such as methoxycarbonyl-PROXYL may be more suitable for neurological applications. Continued optimization is also required to improve quantitative imaging reliability in the presence of in vivo reduction.
Methoxycarbonyl-PROXYL
Methoxycarbonyl-PROXYL (MC-PROXYL), a BBB-permeable nitroxide radical, has been extensively investigated as a paramagnetic MRI contrast agent due to its redox-sensitive properties and potential for evaluating oxidative stress in neurological diseases. In rodent studies, MC-PROXYL markedly enhanced MRI signal intensity in brain regions such as the cerebral cortex and thalamus, with post-injection increases ≤50%, compared with only 2.7% with the BBB-impermeable nitroxide 3CxP, indicating effective BBB penetration and suitability for in vivo redox imaging (273). Ex vivo EPR spectroscopy confirmed maximum concentrations of 1.9±0.35 mmol/l in the thalamus and 3.0±0.50 mmol/l in the cerebral cortex.
Advances in multispin nitroxyl contrast agents, where MC-PROXYL molecules are chemically coupled to possess two or three nitroxyl spins, have demonstrated proportional increases in T1-weighted MRI contrast enhancement and T1 relaxivity with the number of nitroxyl spins, enhancing sensitivity while maintaining quantitative behavior ≤3 spins (274). Furthermore, studies using Overhauser-enhanced MRI (OMRI) with MC-PROXYL-loaded liposomes have shown the ability to differentiate intra- and extra-membrane water environments. This is evidenced by splitting of hyperfine lines in DNP spectra and alterations in NMR signal enhancement due to changes in the coupling constant ρ (275). However, at higher concentrations, a decrease in the coupling factor and enhancement was observed, attributed to reduced electron-nuclear spin interactions and increased leakage factor. This highlights the importance of optimizing agent concentration, with significant DNP enhancement noted ≤2 mM in liposomal solutions (276). Taken together, MC-PROXYL provides strong MRI contrast in the brain and can be tailored for improved sensitivity. Compared with carbamoyl-proxyl, the principal advantage of MC-PROXYL lies in its capacity to cross the BBB, rendering it particularly useful for diagnosing or monitoring neurological pathologies. Nonetheless, such as other nitroxides, its stability under physiological reducing conditions remains a challenge for quantitative imaging over extended time frames.
TEMPO
Nitroxide radicals, particularly TEMPO derivatives, are promising MRI contrast agents due to their ability to enhance proton signal intensity via the Overhauser Effect in OMRI (277). In a study (278), various 15N and deuterium-labeled nitroxyl probes were synthesized to improve the enhancement factor in OMRI experiments. Among these, 15N-D-4-oxo-2,2,6,6-tetramethylpiperidine-1-oxyl exhibited the highest enhancement factor. The greater proton signal enhancement observed with 15N-labeled probes compared with their 14N analogues is attributed to the reduced spectral multiplicity of the I=1/2 nucleus. This enhancement is associated with the linewidth and the number of electron spin resonance lines of the nitroxyl radicals. Selective deuteration further improved the signal-to-noise ratio, enhancing spatial and temporal resolutions in OMRI.
Another study demonstrated that hydrophobic, cell-penetrating piperidine-type nitroxide radicals, specifically SLENU and TEMPOL, are effective contrast agents for MRI of cancer based on tissue redox activity (279). Experiments on anesthetized mice with neuroblastoma revealed that cancerous tissues exhibited a long-lived MRI signal (τ1/2 >14 min), indicating high oxidative activity, whereas healthy tissues showed a short-lived signal (1-3 min), indicating high reducing activity. By contrast, hydrophilic, non-penetrating pyrrolidine-type nitroxides did not differentiate between control and cancer-bearing mice. These findings underscore the necessity of cell penetration for nitroxide radicals to function effectively as MRI contrast agents based on redox activity. Compared with carbamoyl-proxyl and MC-PROXYL, TEMPO-based agents display particular strength in Overhauser-based imaging and can achieve high sensitivity under specific conditions. However, the short in vivo half-life of numerous TEMPO derivatives still limits quantitative imaging; modifications to improve stability remain a key research focus.
Polymer nitroxide contrast agents
Nitroxide-based metal-free MRI contrast agents are safer alternatives to gadolinium-based agents but often suffer from low relaxivity and poor in vivo stability (Fig. 3) (280). To address these limitations, a biodegradable nitroxide-based macromolecular contrast agent (mCA), a water-soluble biodegradable nitroxides-based mCA (Linear pDHPMA-mPEG-Ppa-PROXYL) was developed by covalently attaching PROXYL to an enzyme-sensitive linear di-block pDHPMA (281). This mCA achieved a high PROXYL content of 0.111 mmol/g and formed stable nanosized aggregates (~23 nm), resulting in an increased longitudinal relaxivity (r1=0.93 mM−¹ sec−¹), the highest among reported nitroxide-based mCAs. In vitro, it showed low cytotoxicity, with cell viability exceeding 95% in both 4T1 tumor cells and HUVEC cells at concentrations ≤5 μg/ml. In vivo, the mCA exhibited extended blood retention of PROXYL (~8 h), markedly longer than 3-carboxy-PROXYL, which was undetectable within 1 h. MRI studies in mice revealed significant signal enhancements in the liver (197%), kidney (246%) and bladder (304%). In tumor-bearing mice, the mCA accumulated in tumors via passive targeting, providing detectable MRI enhancement using the T1-weighted spin-echo (T1WI SE) sequence, with a maximum signal enhancement of ~134% at 10 min post-injection. Although this enhancement was slightly lower than that of DTPA-Gd (~150%), the mCA demonstrated excellent biosafety, hemocompatibility and biodegradability, with no significant tissue toxicity observed histologically. Therefore, Linear pDHPMA-mPEG-Ppa-PROXYL, featuring high nitroxide content, stable nanostructure formation, and molecular flexibility, represents a promising metal-free MRI contrast agent for potential clinical application. Its longitudinal relaxivity and continuous enhancement time at the tumor site were still inferior to those of DTPA-Gd, highlighting ongoing challenges in matching the performance of established clinical agents while maintaining stability and quantitative accuracy.
Another study reported two water-soluble PROXYL-based macromolecular organic contrast agents (mORCAs), linear and cross-linked poly(carboxylate ester) (PCE)-mPEG-Ppa-PROXYL, by conjugating linear and cross-linked PCE with poly(ethylene glycol; mPEG2000)-modified nitroxides (PROXYL; Fig. 4) (282). The two mORCAs self-assemble in aqueous solutions to form aggregates, with PROXYL molecules protected within hydrophobic cores to enhance resistance to reduction by physiological reducing agents, thereby improving in vivo stability and reducing toxicity. The cross-linked PCE-mPEG-Ppa-PROXYL, due to its branched architecture, forms more stable and compact aggregates with larger particle size compared with the linear analogue. Experimentally, the cross-linked mORCA exhibited a higher longitudinal relaxivity (r1=0.79 mM−¹ sec−¹) than the linear mORCA (r1=0.64 mM−¹ sec−¹), and both exceeded the relaxivity of the best previously reported mORCA (r1=0.42 mM−¹ sec−¹). In vivo studies in mice demonstrated that the cross-linked mORCA had a prolonged blood circulation time of ~48 h, markedly longer than the 10 h observed for the linear counterpart (277,281,283,284). Additionally, the cross-linked PCE-mPEG-Ppa-PROXYL provided superior MRI contrast enhancement in normal organs such as liver and kidney, as well as in tumors, compared with the linear variant.
Polymer-based nitroxide MRI contrast agents have thus emerged as promising metal-free alternatives due to their unique properties for enhanced imaging applications. Compared with small-molecule nitroxides such as carbamoyl-proxyl, MC-PROXYL, or TEMPO, polymeric constructs often display higher longitudinal relaxivities, prolonged circulation times and improved protection against bioreduction, collectively improving sensitivity. Nonetheless, challenges such as short-lived tumor signals, rapid partial reduction and limited quantitative reliability persist. One approach combined PROXYL groups with PEG-modified dendritic poly(l-lysine) to exploit the enhanced permeability and retention EPR effect for tumor accumulation (283). Although significant OMRI signals were observed after intramuscular injection, intravenous administration failed to produce detectable tumor signals due to rapid reduction of nitroxyl radicals in the blood, emphasizing the need for more stable radicals. Another strategy synthesized entirely organic, metal-free MRI contrast agents using polyphosphorhydrazone dendrimers fully functionalized with ≤48 nitroxide radicals (285). Incorporating tyrosine linkers allowed control over water solubility and radical anchoring, achieving high water solubility, low cytotoxicity and remarkable longitudinal relaxivity; four times higher than Gd-DTPA.
To enhance both stability and imaging performance, branched pDHPMA-mPEG-Ppa-PROXYL was developed, forming nanoscale aggregates (~28 nm) with high molecular weight (160 kDa) (286). This macromolecular nitroxide contrast agent displayed a longitudinal relaxivity three times that of 3-carboxy-PROXYL and markedly extended blood retention time to 6 h. It facilitated prolonged MR imaging of tumors, liver, kidneys and, notably, the cardiovascular system. Additionally, human serum albumin conjugated with nitroxide radicals (HSA-NIT) was developed, achieving ≤21 nitroxide residues per protein without altering HSA structure (287). Although HSA-NITs did not exhibit ODNP enhancement initially, under proteolytic conditions simulating cancer tissue, ODNP capabilities were activated, suggesting HSA-NITs can serve as cleavable hyperpolarizing contrast agents in OMRI. These polymer strategies underscore continued efforts to mitigate rapid clearance and bioreduction, central challenges for quantitative imaging.
A third-generation water-soluble poly(phosphorhydrazone) radical dendrimer (G3-Tyr-PROXYL-ONa), functionalized with 48 PROXYL radical units, was reported as a novel organic MRI contrast agent for glioblastoma (GB) imaging (Fig. 5) (288). The G3 dendrimer was designed to act as a T1 contrast agent by leveraging the paramagnetic properties of PROXYL radicals while mitigating concerns about toxic metal accumulation. Ex vivo and in vivo MRI studies were conducted using immunocompetent, orthotopic GL261 murine GB models. Markedly, the G3 dendrimer administered at a dose of 0.025 mmol/kg provided suitable contrast enhancement comparable with commercial Gd-based CAs administered at 0.1 mmol/kg, demonstrating effectiveness at one-quarter the dose. No signs of toxicity were observed in vivo (289,290). The dendrimer showed selective accumulation in brain tumor tissues and prolonged retention, allowing imaging over extended time frames (≥2.5 h). Moreover, the radicals remained stable in biological media for h rather than min, representing a significant step toward addressing the long-standing challenges of short imaging windows and restricted quantitative assessment in nitroxide-based contrast agents (289,291).
Nitroxide-labeled therapeutic agents
Nitroxide-labeled therapeutic agents have emerged as promising MRI contrast agents due to their ability to enhance imaging of specific biochemical processes, such as proteolysis, via OMRI. In OMRI at 0.2 T, nitroxide-labeled proteins demonstrated a significant increase in image intensity from 1-25 upon proteolysis, attributed to the decreased motional correlation time of the substrate, enabling high-sensitivity three-dimensional imaging with good spatial resolution (289). This approach allows specific targeting of any protease using tailor-made cleavable macromolecules, facilitating applications in basic research and therapeutic evaluations in small animal models. Extending this concept, nitroxide-labeled elastin administered orally in mice exhibited high Overhauser enhancements in vitro (19-fold at 18 mM nitroxide) upon cleavage by pancreatic porcine elastase. In vivo, three-dimensional OMRI detected proteolysis in the duodenum with enhancements of 7.2±2.4 (n=7) within 20 sec at 0.125 mm3 resolution, demonstrating the efficacy of the technique in evaluating unregulated proteolytic activities and drug testing (292). Additionally, the development of albumin-nitroxide conjugates, where human serum albumin carriers bear multiple nitroxides conjugated via homocysteine thiolactones, yielded metal-free ORCAs with enhanced relaxivities markedly greater than their individual components and improved resistance to bioreduction. These conjugates retained essential physical and biological properties, offering excellent prospects for optimization in MRI applications (293).
To address signal-to-noise challenges at ultra-low fields (<1 mT), a very-low-field MRI system operating at 206 μT was developed. This system produced Overhauser-enhanced MR images in living rats using stable, non-toxic nitroxides, visualizing them in three dimensions within min post-administration in organs such as the lungs, kidneys and bladder. Concurrent conventional imaging at the same field following pre-polarization at 20 mT was performed, paving the way for molecular imaging of inflammation using protease-specific nitroxide probes (294). Although these studies illustrate significant progress in harnessing nitroxide radicals for sensitive and specific imaging of proteolytic activities, scaling to larger animal or clinical use may require further improvements in sensitivity under low-field conditions, as well as more robust strategies for quantification.
Nitroxide-labeled drugs also offer potential as MRI contrast agents due to their unique redox-sensitive properties and suitability for noninvasive, real-time imaging of physiological parameters such as BBB permeability. A novel nonradioactive methodology using nitroxide radicals as spin-labels was developed to assess BBB permeability for conventional drugs via MRI (295). Two TEMPO-labeled lomustine analogues, SLENU and SLCNUgly, were synthesized by substituting the cyclohexyl group with a nitroxide radical. After intravenous injection into healthy mice, 7.0 T MRI revealed that both compounds exhibited MRI signal dynamics similar to the nonmodified nitroxyl radical TEMPOL, indicating rapid transport and random distribution in brain tissue. This suggests that TEMPO modification does not hinder BBB permeability of the anticancer drug.
These nitroxyl derivatives displayed varying hydrophobicity, cell permeability and blood clearance. Analysis of these structural differences revealed relationships between circulating half-life and MRI signal dynamics in the brain, providing valuable insights for optimizing nitroxide-labeled drugs as MRI contrast agents. Furthermore, in vivo assessment of the paramagnetic and diamagnetic conversions of nitroxide radicals can serve as an index of tissue redox status, which is significant for planning radiation therapy due to their potential as normal tissue-selective radioprotectors (296).
Recent advances include the development of in vivo redox imaging using nitroxide radicals to assess tissue redox status and the design of polymeric nitroxide radical contrast agents and nitroxide-labeled drugs for theranostic applications. While these approaches are promising, each agent class faces unique hurdles involving stability, sensitivity and quantitative analysis. Conventional low-molecular-weight nitroxides may suffer from short in vivo half-lives, whereas polymeric or dendrimer-based constructs can achieve prolonged circulation yet require complex synthesis and frequently show incomplete tumor retention. Future directions involve developing nitroxide contrast agents with high reaction specificity, improved in vivo stability, and robust quantitative performance for translational theranostic applications (261,297).
Nanoparticles
Nitroxide-based organic radical contrast agents offer a metal-free alternative for MRI but face challenges due to low spatial resolution and poor in vivo stability from rapid clearance and bioreduction (261,297-299). To overcome these issues, a study hypothesized that loading nano nitric oxide (NO·) micelles into platelets (PLTs) could enhance glioma targeting and accumulation, improving T1-weighted MRI contrast (299). The authors synthesized NO·@PLT by ultrasonically incorporating nano NO· micelles into pretreated PLTs, preserving PLT morphology and membrane proteins such as CD41. In vitro, NO·@PLT effectively targeted U87 glioma cells via PLT-tumor adhesion, released ~90% of nano NO· micelles within 40 min and exhibited per-nitroxide transverse relaxivities about twice that of free NO· particles. In murine subcutaneous glioma models, systemic administration of NO·@PLT led to selective tumor accumulation from 5 min to 2.5 h, with optimal MRI signal enhancement (1.74-fold increase) at 1.5 h post-injection. The NO·@PLT design leverages the tumor-targeting ability of PLTs and the strong signal of stable nano NO· micelles, achieving significant glioma signal amplification in T1-weighted MRI comparable to metal-based agents. This strategy highlights progress in overcoming the often-limited targeting and stability of nitroxide nanoparticles, although long-term toxicity and persistent signal remain to be optimized.
Organic nitroxide radicals such as 4-carboxy-TEMPO are promising metal-free T1 MRI contrast agents due to their paramagnetic properties and enhanced safety over gadolinium-based agents (300-302). One study developed novel chitosan (CS)-TEMPO-ovalbumin (OVA) nanovaccines by conjugating 4-carboxy-TEMPO with CS and OVA, creating a metal-free nanosystem functioning as both a tumor vaccine and MRI contrast agent. The nanovaccines improved the biocompatibility and circulation time of TEMPO, enhancing T1-weighted MRI contrast in tumors upon intravenous or intramuscular administration (301). In cellular experiments, they demonstrated excellent biocompatibility, effectively stimulating bone marrow-derived dendritic cells to promote maturation and activation of T cells, leading to significant cytokine production. In mouse models, the nanovaccines served as both therapeutic and preventive vaccines, inducing strong immune responses, activating cytotoxic T cells, promoting macrophage M1 polarization, effectively inhibiting melanoma growth, and enhancing survival rates. Combined with αPD-1, they markedly increased infiltration of cytotoxic T lymphocytes within tumors, eliciting robust systemic anti-tumor responses that effectively curbed tumor metastasis. While translating OVA-based findings to other tumor targets remains a future goal, this dual-modality approach exemplifies how nitroxide radicals can facilitate imaging and therapeutic functionalities (303).
Metal-free nitroxide radical-based nanoparticles have continued to improve through incorporation into lyotropic liquid crystals, cubosomes, hexosomes, silica-coated upconversion nanoparticles and magnetic mixed micelles. These designs enhance proton relaxivities and mitigate toxicity Compared with gadolinium complexes. For instance, lyotropic liquid crystal nanoparticles loaded with paramagnetic nitroxide lipids achieved enhanced proton relaxivities and effective liver MRI contrast in vivo (304), while nitroxide lipids in hexosomes provided higher relaxivities and low toxicity for aorta and spleen imaging (305). To address rapid bioreduction, silica-coated nanoparticles doped with TEMPO radicals exhibited prolonged resistance to chemical reduction and a tenfold increase in longitudinal relaxivity Compared with free TEMPO (306). Moreover, all-organic nanotheranostic platforms such as glycol chitosan-linked polypyrrole nanoscaffolds modified with nitroxide radicals and folic acid (GC-PP@TEMPO-FA NPs) achieved high nitroxide loading and extended circulation for effective MRI contrast and near-complete tumor regression under MRI-guided photothermal therapy (307). Although these nanoparticle strategies show multidimensional capabilities, quantitative analysis still presents challenges, particularly in determining precise drug or radical concentrations in vivo when redox processes can alter signals.
Other approaches
Nitroxide MRI contrast agents uniquely enhance magnetic resonance imaging through their paramagnetic properties and redox activity, enabling functional imaging beyond anatomical details. Recent advances have focused on designing nitroxide-based agents that exploit interactions with free radicals and redox reactions in vivo. OMRI, a double resonance technique, amplifies water proton signals via the Overhauser effect, permitting imaging of free radical distributions in small animals. One study demonstrated simultaneous imaging of nitroxyl radicals with different isotopes (14N and 15N) in field-cycled OMRI, enabling dual imaging of oxidation and reduction processes at nanometer scales by labeling membrane-permeable and -impermeable nitroxyl radicals (308). Another study introduced new nitroxyl radicals, Fur-135 and Fur-176, which exhibited low toxicity and improved MRI contrast in transplanted RLS lymphoma in mice; notably, Fur-135 provided high-precision tumor localization distinguishable from Gd3+-based agents (309). An integrated OMRI-Prepolarized MRI system was developed to enable accurate co-registration of radiobiological information with high-quality anatomical images, supporting quantitative longitudinal imaging of tumor hypoxia and redox status (310).
In exploring biological applications, an elastase substrate grafted with stable nitroxide radicals produced high OMRI contrast upon protease digestion, enabling imaging of neutrophil degranulation from as few as 2×104 cells/ml (311). Additionally, nitroxide-enhanced MRI effectively differentiated cancerous from healthy tissue based on redox activity (312). Despite these successes, rapid reduction of common nitroxides within cells remains a primary obstacle to quantitative imaging. Innovations such as tetraethyl-substitution in TEEPONE have achieved extended half-lives over 80 min, but unexpected transformations in hepatic microsomal fractions call for further refinement (313). The exploration of sterically shielded nitroxides and paramagnetic cymantrene derivatives holds promise for increased stability (314,315). Continued research to address sensitivity limitations, quantitative challenges, and tissue-specific delivery will likely expand clinical feasibility, bridging the gap between safety advantages and robust diagnostic performance in metal-free MRI.
Hyperpolarized carbon
Hyperpolarized 13C MRI employs dissolution dynamic nuclear polarization (d-DNP) to enhance the magnetic resonance signals of 13C-enriched metabolites by >10,000 times, enabling detection at physiological concentrations and facilitating real-time study of metabolic reactions in living systems (153,316-318). One of the most extensively studied HP 13C tracers is [1-13C]pyruvate, a central metabolite in glycolysis and the citric acid cycle (318). After administration, hyperpolarized [1-13C]pyruvate enters cells and is converted to lactate by lactate dehydrogenase (LDH), reflecting the cellular redox state. This conversion indicates metabolic alterations associated with diseases such as cancer, where the Warburg effect leads to increased lactate production even in the presence of oxygen (319). Measuring the ratio of hyperpolarized 13C-labeled lactate to pyruvate allows clinicians to assess tumor aggressiveness and monitor therapeutic responses (320-322). Beyond oncology, HP 13C MRI shows potential in evaluating tissue viability and metabolic changes in cardiac ischemia, neurodegenerative disorders, and inflammatory conditions. Tracers such as hyperpolarized [1-13C]fumarate detect cellular necrosis through conversion to malate, while hyperpolarized [1-13C]ascorbate serves as a redox sensor to assess oxidative stress levels (323). Among these agents, [1-13C]pyruvate remains a prominent tracer due to its high polarization levels and direct participation in core metabolic pathways, whereas fumarate or ascorbate target processes such as necrosis detection or oxidative stress, respectively. This growing array of complementary tracers underscores the potential for more comprehensive metabolic profiling of disease states (317).
Utilizing endogenous metabolites as 13C-enriched tracers offers several advantages. These compounds naturally participate in metabolic pathways without altering physiological processes or eliciting adverse immune reactions. The absence of metal ions eliminates concerns regarding metal-related toxicity or accumulation, making the approach safer for repeated clinical use. Advances in HP 13C MRI technology are progressing rapidly, but remain challenged by the transient nature of hyperpolarized signals, specialized hardware requirements and complexities in quantitative analysis. Stability of polarizations can vary across different agents and ensuring consistent sensitivity over the course of imaging is another critical hurdle. Addressing these issues through improved polarization methods, hardware optimization, and robust modeling of signal decay will be crucial for expanding clinical adoption of HP 13C MRI. Collectively, these developments point toward an expanding framework in which multiple 13C-labeled endogenous molecules can be combined or selected for targeted disease assessment, though further investigation into synergy among different tracers is needed.
Cancer imaging
Hyperpolarized 13C MRI contrast agents enable non-invasive metabolic imaging by markedly enhancing the signal intensity of 13C-labeled metabolites, providing valuable insights into physiological and pathological processes, particularly in cancer (321,324-326). Despite their potential, clinical adoption is hindered by the requirement for specialized equipment to excite and detect 13C nuclei with MRI gradients, as well as rapid signal decay and operational complexity. Parahydrogen-induced polarization (PHIP) has emerged as a promising alternative to d-DNP for generating hyperpolarized 13C-labeled compounds, offering simpler methods for preclinical in vivo MRI (316,318,327,328). Studies have demonstrated the application of PHIP in producing hyperpolarized agents and enhancing metabolic imaging (318,327,328).
One study demonstrated that hyperpolarized allyl [1-13C] pyruvate can be detected via stimulated emission of radiation using 13C Radiofrequency Amplification by Stimulated Emission of Radiation (13C RASER), eliminating the need for RF excitation and synchronization (325). Hyperpolarized allyl [1-13C]pyruvate was produced by pairwise addition of parahydrogen to a pyruvate precursor, achieving 13C polarization of 4%. RASER signals were detected with a commercial inductive detector at sample concentrations as low as 0.125 M. The PHIP process yielded a mixture of ketone and hemiketal forms, separated by 10 ppm in 13C NMR spectra, modeling the metabolic conversion of pyruvate to lactate. Selective, background-free detection was possible when the emission threshold was exceeded only for one species. While this approach could enhance accessibility of metabolic imaging on conventional MRI systems, it also introduced non-physiological concentrations and the use of CD3OD solvent.
Another study developed an automated PHIP-based polarizer capable of producing purified, highly concentrated solutions of hyperpolarized [1-13C]fumarate with 13-20% polarization, at physiological pH and in volumes ≤3 ml (326,329). The PHIP process matched d-DNP in polarization levels but reduced preparation time to 10 min compared with 90 min and lowered operational complexity. Cytotoxicity studies confirmed nontoxicity after purification and in vivo experiments showed the use of hyperpolarized fumarate as a perfusion agent and its metabolic conversion to malate in necrotic tumor regions. However, further optimization is needed to reduce residual catalyst contamination for clinical applications and differences in 13C relaxation times between PHIP- and d-DNP-prepared samples require further investigation. Comparisons between HP [1-13C]pyruvate and [1-13C] fumarate highlight how each agent probes a distinct aspect of tumor metabolism, glycolysis vs. necrosis, emphasizing the broader strategy of selecting specific substrates aligned with the biological hallmark of interest.
Additional applications of PHIP include the rapid synthesis of 1-13C-pyruvate-d3, which revealed increased metabolic conversion to lactate and alanine in tumor xenografts (327,328,330). PHIP of 1-13C-phospholactate enabled in vivo delivery of a hyperpolarized agent converting to 1-13C-lactate, showing uptake in multiple organs and tumors (331). Beyond PHIP, hyperpolarized [1-13C]α-ketoglutarate monitored mutant IDH1 activity in glioblastoma, detecting hyperpolarized [1-13C]2-hydroxyglutarate exclusively in mutant tumors (332). Hyperpolarized [5-13C]glutamine revealed elevated glutamine metabolism in prostate cancer cells and significant metabolic reductions upon treatment, suggesting its potential as a biomarker for therapeutic response (333).
Advances in imaging tumor extracellular pH were achieved using hyperpolarized 1,2-glycerol carbonate, producing HP H13CO3− upon hydrolysis and enabling accurate pHₑ measurements in prostate tumors (334). Similarly, 13C-labeled zymonic acid, with a chemical shift change of ≤3.0 ppm per pH unit, allowed reliable extracellular pH mapping in rat kidneys and tumors (335). High-resolution 13C NMR spectroscopy at 48.7 mT using hyperpolarized succinate-1-13C-2,3-d2 achieved narrow linewidths (~3 Hz), enhancing sensitivity for monitoring hyperpolarized agent uptake and metabolism (336). Hyperpolarized perfusion imaging with bis-1,1-(hydroxymethyl)-[1-13C]cyclopropane-d8 exhibited distinct perfusion characteristics in glioblastoma, correlating with gadolinium-based MRI and vascular staining (337,338). Simultaneous imaging of multiple 13C-labeled agents assessed vascular permeability and perfusion, showing elevated parameters in prostate tumors (339).
Ongoing challenges that require further attention include rapid HP 13C signal decay, polarization transfer efficiency, the possibility of residual catalyst contamination with PHIP-based approaches, relaxation time differences between PHIP and d-DNP and complexities in achieving quantitative measurements. These challenges highlight the need for future research into more robust polarization processes, improved agent stability and standardized protocols that improved capture quantitative data from hyperpolarized signals in clinical settings.
Cardiovascular diseases
Hyperpolarized carbon MRI contrast agents have markedly advanced cardiovascular imaging by enhancing the sensitivity of metabolic and structural assessments in myocardial tissue (147,152,316,318). The conversion of hyperpolarized [1,4-13C2]fumarate to [1,4-13C2] malate serves as a specific probe for cardiomyocyte necrosis post-myocardial infarction (MI) (323,340). In vivo studies demonstrated an 82-times increase in malate production one day after infarct and a sustained 31-times increase after one week Compared with controls, indicating sensitivity to cellular energy status when adenosine triphosphate levels deplete by over 50% (323). Compared with other hyperpolarized agents such as [1-13C]acetate, which focuses on oxidative metabolism, [1,4-13C2]fumarate highlights necrosis more directly. Selecting a particular agent thus depends on the specific metabolic process under investigation.
For fast angiography, the SAMBADENA technique enables rapid production of hyperpolarized 13C tracers within the MRI system, visualizing major vessels such as the vena cava and aorta in mice, and offering a simple, low-cost alternative to traditional methods (341). Contrast-enhanced magnetic resonance angiography using hyperpolarized 13C-enriched compounds has achieved remarkable SNRs (~500) in cardiac regions and preserved magnetization across multiple image acquisitions using flip-back techniques (342).
In metabolic imaging, hyperpolarized 13C-labeled glucose analogs have shown myocardial uptake and time-dependent organ distribution in live rats, suggesting applications where radiation-free imaging is preferred over FDG-PET (343). Administration of hyperpolarized [1-13C]acetate has been used for studying myocardial metabolism, achieving a liquid-state polarization of 14.2% and an effective in vivo T1 of 17.6±1.7 sec, although requiring large doses (344). For interventional MRI, hyperpolarized 13C contrast agents in catheter lumens have provided high SNR (~80) for passive catheter tracking, enhancing real-time visualization (345). Although these methods show promise, ongoing work must address polarization longevity and agent selection to achieve routine clinical use.
Metabolism
Hyperpolarized carbon MRI contrast agents markedly enhance sensitivity for carbon-containing metabolites, facilitating detailed investigations of metabolic pathways (152,316,324,346). The sodium iodide with active background rejection (SABRE) technique extends hyperpolarization of 13C nuclei at natural abundance to various α-ketocarboxylates beyond pyruvate, including 2-oxobutyrate (P13C=25%), oxaloacetate (P13C=11%), α-ketoglutarate (P13C=13%), phenylpyruvate (P13C=2%) and phenylglyoxylate (P13C=2%). While these SABRE-based approaches broaden the range of substrates that can be hyperpolarized without chemical modification, the polarization levels often remain lower than those achieved by d-DNP, underscoring a research gap in optimizing SABRE transfer efficiency for clinical use. Temperature-dependent studies showed that hydride and substrate exchange rates affect polarization levels: Maximum polarization occurred at low temperatures for pyruvate and 2-oxobutyrate, while oxaloacetate benefited from higher temperatures. A theoretical kinetic model fitting the hyperpolarization dynamics provides insights into exchange processes and guides optimization (316,324,346).
By transferring spin order from parahydrogen to the 13C nuclei of α-ketocarboxylates via SABRE, NMR signals are enhanced without chemical modification (347). This work broadens SABRE hyperpolarization to more biologically relevant metabolites, but challenges remain for phenyl-substituted substrates due to unfavorable catalyst binding and for compounds, such as oxaloacetate, that undergo decarboxylation under SABRE conditions. Recent advances focus on improving agent design and mechanisms to enhance performance, with proton decoupling techniques such as decoupling pulse sequence (WALTZ-16) eliminating large 13C-1H couplings introduced during enzymatic transformations, boosting detection of hyperpolarized [2-13C]dihydroxyacetone conversion in vivo (348). Innovations in PHIP methods have led to robust, low-cost parahydrogen generators using liquid nitrogen cooling, achieving parahydrogen fractions over 48% and 13C signal enhancements >14,000-fold at 1 T for agents such as sodium [1-13C]pyruvate (349).
Integrating PHIP systems into MRI scanners allows virtually continuous production of hyperpolarized agents, such as [1-13C]succinate-d2 and hydroxyethyl-[1-13C]propionate-d3, with polarizations of ~2 and 19%, respectively, every 15 sec, supporting rapid preclinical studies and dynamic metabolic monitoring (350). Deuterated carbon positions in agents such as [1,1,2,2-D4,2-13C]choline chloride extend 13C T1 relaxation times and achieve favorable polarization levels (24%), broadening the dissolution DNP MRI agent library (351). Such variations highlight a trend toward designing hyperpolarized compounds with optimized T1, polarization yield, and metabolic relevance. Nonetheless, further refinement is needed to achieve stable, biocompatible agents and consistent polarization levels suitable for clinical translation.
Neurodegenerative diseases and brain
Hyperpolarized carbon MRI contrast agents markedly enhance brain imaging sensitivity, advancing neurodegenerative disease diagnosis and understanding. Cerebral perfusion imaging in rats used hyperpolarized 13C-labeled bis-1,1-(hydroxymethyl)-1-13C-cyclopropane-D8, enabling calculation of cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time (MTT) (352). MTT was measured at 2.8±0.8 sec. Although arterial partial-volume effects affected accurate quantification of CBF and CBV, a modified bolus-tracking theory compensated for depolarization effects, improving CBV and MTT accuracy. Hyperpolarized 13C-labeled tert-butanol, with T1 values of 46±4 sec in blood (9.4 T) and 43±24 sec in brain tissue, was evaluated as a freely diffusible contrast agent for brain perfusion imaging (353). Dynamic 13C imaging in rats achieved high temporal (2-4 sec) and spatial (700 μm in-plane, 2 mm slice thickness) resolution, underscoring its promise for robust, quantitative perfusion measurements.
The novel hyperpolarized molecular probe [1,1,2, 2-D4,2-13C] choline chloride, exhibiting 24% polarization and a T1 of 35 sec at 11.8 T, was introduced for monitoring choline metabolism into acetylcholine in the brain (354). Enzymatic assays confirmed the synthesis of hyperpolarized deuterated-acetylcholine (T1=34 sec at 14.1 T). As various hyperpolarized agents are investigated for brain imaging, comparing PHIP and d-DNP demonstrates a trade-off between faster polarization times and higher polarization levels. Continued progress in balancing sensitivity, agent stability, and clinically feasible hardware is essential to harness the full potential of hyperpolarized carbon MRI in neurodegenerative disease research.
Other approaches
Hyperpolarized carbon MRI contrast agents markedly advance molecular imaging by enabling real-time visualization of metabolic processes with enhanced sensitivity (316,318). An open-source, low-cost PHIP hyperpolarizer produced hyperpolarized 13C-succinate with 28% polarization at 30 mM concentration, facilitating ultrafast molecular imaging and multislice 13C MRI in mice following tail vein injection (355). Hyperpolarized [13C, 15N]urea monitored renal function; glucagon infusion increased renal mean transit time by 14%, demonstrating its utility as a biomarker without affecting sodium distribution, glomerular filtration rate, or oxygen consumption (356).
Advances in DNP formulations include using symmetric anhydrides as precursors for 13C-labeled short-chain fatty acids, eliminating the need for glass-forming additives. Employing esterified trityl radicals and lipophilic gadolinium complexes for additive removal further enhances the clinical potential of these MCAs (357). Applications in low-field MRI were demonstrated with hyperpolarized 1-13C-succinate-d2 and 1H-hyperpolarized pyridine, achieving spatial resolutions surpassing micro-PET in 4-8 sec at 47.5 mT, highlighting microscale molecular imaging feasibility at low fields (358). A centrally controlled, automated parahydrogen-based polarizer with in situ detection achieved 20% polarization and a 5,000,000-times signal enhancement at 48 mT, along with an extended T1 relaxation time of 101±7 sec for a 13C hyperpolarized contrast agent in water (316,329,359). Additionally, hyperpolarization of barbituric acid derivatives via parahydrogen-induced polarization achieved a 5000-fold 13C NMR signal enhancement, indicating their potential as active MRI contrast agents (360).
In situ hyperpolarization within an MRI system at 7 T was demonstrated with 1-13C,2,3-2H2-succinate achieving 11% polarization and an 18,000-fold signal enhancement, mitigating polarization loss during transfer and reducing costs (318,361). Nevertheless, continued improvements are necessary to address stability requirements, minimize polarization loss during reactive steps, and refine imaging techniques that capture transient signal enhancements from strategies such as Parkinson's Disease (362). Low-cost hyperpolarizer designs, improved catalyst removal, and extended T1 retention remain critical for widespread adoption. By tackling these challenges, particularly sensitivity, stability, and robust quantitative analysis, hyperpolarized carbon MRI contrast agents can fulfill their potential for clinical diagnostics and real-time metabolic assessment.
Conclusion and perspectives
MRI is a cornerstone of modern medical diagnostics, providing detailed anatomical visualization without ionizing radiation risks. Traditional gadolinium-based contrast agents have enhanced MRI capabilities but raise safety concerns due to potential toxicity and long-term retention in the body (16,17). These issues have spurred the development of novel non-metal-based contrast agents that aim to deliver superior imaging quality while minimizing health risks.
Non-metal-based agents, including non-metallic 19F agents, CEST agents, hyperpolarized 13C compounds, and NO agents, offer promising alternatives. Non-metallic 19F agents exploit the unique properties of 19F, offering high specificity due to the negligible background of 19F in biological tissues. Despite challenges with low imaging sensitivity, advancements in polymer chemistry and smart probe development are paving the way for more effective 19F MRI agents (36,96). Enhancing fluorine atom density without compromising solubility and incorporating stealth properties to improve biodistribution are critical steps forward.
CEST MRI uses exchangeable protons in endogenous or exogenous compounds to enable molecular imaging without metal-based agents (163,167). This technique shows significant promise in brain tumor grading and treatment monitoring by detecting molecular alterations associated with disease states. Technical challenges such as optimizing acquisition parameters and mitigating motion artifacts are being addressed through standardization and advanced post-processing methods. The integration of deep learning algorithms offers potential improvements in data analysis and interpretation, enhancing the clinical applicability of CEST MRI.
Hyperpolarized 13C MRI provides real-time, non-invasive assessment of metabolic processes with unprecedented sensitivity (319,323). Developing redox-sensitive tracers could expand its utility in evaluating cellular redox states, a crucial aspect of numerous pathological conditions. The adoption of PHIP methods presents a cost-effective alternative to traditional hyperpolarization techniques, increasing accessibility and promoting wider research and clinical applications.
NO contrast agents exploit the paramagnetic nature of NO to enhance MRI contrast without metals. These agents offer a unique opportunity to visualize and quantify NO in vivo, providing insights into diseases characterized by dysregulated NO production. Challenges in ensuring stability, biocompatibility, and accurate quantification are being addressed through advancements in nanotechnology and molecular engineering. Developing NO-sensitive probes with controlled release mechanisms and targeted delivery systems enhances their potential clinical utility.
Despite their potential, practical challenges hinder clinical translation of non-metal-based contrast agents. Regulatory hurdles require new agents to demonstrate safety and efficacy through rigorous testing before approval. Lack of standardized manufacturing processes and production limitations affect scalability and consistency. For instance, synthesizing hyperpolarized 13C agents necessitates specialized equipment and expertise, including dynamic nuclear polarizers and rapid dissolution systems, all under sterile conditions and tight time constraints due to rapid polarization decay (363). Large-scale production of agents such as PFC nanoemulsions for 19F MRI requires specialized facilities capable of producing high-purity, sterile preparations under Good Manufacturing Practice (GMP) conditions (33). Stability during storage and transport is a concern, as is the need for cold chain logistics for hyperpolarized compounds with rapidly decaying polarization. Moreover, clinical trial design adds complexity, demanding specialized imaging protocols with multinuclear MRI capabilities, custom radiofrequency coils and fast acquisition sequences for transient signals. Integrating these agents into clinical workflows requires training and adaptation of imaging facilities. Standardizing imaging parameters and quantitative analysis methods is essential for reproducibility and comparability across studies. Overcoming these challenges involves technological advancements and collaborative efforts. High-field MRI systems and improved hardware can enhance sensitivity and resolution, making techniques such as 19F and hyperpolarized 13C MRI more feasible clinically. Advances in coil design, such as cryogenically cooled radiofrequency coils, can increase SNRs without higher field strengths (33). Streamlining manufacturing processes and scaling production under GMP conditions can address production limitations, while early engagement with regulatory agencies may facilitate approval pathways.
Among non-metal-based agents, hyperpolarized 13C MRI holds particular promise for clinical translation. Its ability to provide dynamic metabolic information is valuable in oncology, where metabolic alterations are cancer hallmarks. Implemented at multiple clinical sites, it shows potential in detecting early treatment responses, stratifying tumors and offering insights into tumor aggressiveness (363). However, widespread adoption requires solutions to technical and logistical challenges, including equipment availability, protocol standardization and cost considerations. Similarly, 19F MRI offers unique advantages due to its specificity and quantitative capabilities. The lack of background signal enables precise tracking of labeled cells or therapeutic agents. Achieving sufficient sensitivity remains a challenge, often necessitating high fluorine concentrations or advancements in hardware and imaging sequences (33). CEST MRI provides functional information without exogenous agents by detecting endogenous metabolites, offering a different route to enhancing MRI contrast. Technical challenges such as B0 and B1 inhomogeneities, especially in body imaging outside the brain, currently limit its clinical application (33,363). Developing robust correction techniques and standardized protocols is essential for broader adoption.
So far, only 19F MRI agents are commercially available and used in clinical trials to assess various conditions, particularly those affecting the lungs and gastrointestinal system, as evidenced by studies registered with NCT numbers on ClinicalTrials.gov. Trials such as NCT03532334 (withdrawn) aimed to compare 19F MRI with 133Xe scintigraphy for lung ventilation imaging in lung disease patients, while NCT06066723 (recruiting) investigates its feasibility in children with mild cystic fibrosis vs. healthy controls, using perfluoropropane (PFP) as a contrast agent. Other studies, such as NCT03315065 (completed), incorporated 19F MRI into pre-radiotherapy assessments for lung cancer, and NCT03489590 (completed) evaluated regional ventilation in cystic fibrosis patients. Additionally, NCT01347918 (suspended) explored 19F MRI for gastrointestinal function in irritable bowel syndrome, using fluorine-labeled capsules. These trials collectively highlight the potential of 19F MRI to provide detailed functional and anatomical insights without ionizing radiation, though some studies (for example, NCT02035085 and NCT02921373) were withdrawn before completion, reflecting challenges in implementation or recruitment. Overall, 19F MRI is emerging as a promising tool across diverse clinical applications, with ongoing research refining its utility and safety.
In conclusion, non-metal-based contrast agents have the potential to markedly enhance diagnostic imaging and contribute to personalized medicine by providing molecular and functional imaging capabilities without risks associated with metal-based agents (363,364). Exploring their applications, challenges and future prospects in clinical translation illuminates pathways to integrating these innovative agents into routine clinical practice. Chemists, biologists, pharmacologists, and imaging specialists must work together to innovate and refine these agents. Improvements in chemical design, imaging technology and data analysis are essential to address current limitations. Embracing these innovations will advance radiology and contribute to improved patient outcomes through more precise diagnosis and targeted treatment strategies.
Availability of data and materials
Not applicable.
Authors' contributions
TD and HL conceived the study and revised the manuscript. TD wrote the manuscript. QY, HS and TL reviewed the manuscript. Data authentication is not applicable. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
Not applicable.
Funding
No funding was received.
References
|
Wahsner J, Gale EM, Rodríguez-Rodríguez A and Caravan P: Chemistry of MRI contrast agents: Current challenges and new frontiers. Chem Rev. 119:957–1057. 2019. View Article : Google Scholar : | |
|
Pollack A, Kontorovich AR, Fuster V and Dec GW: Viral myocarditis-diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 12:670–680. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Filippi M, Rocca MA, Ciccarelli O, De Stefano N, Evangelou N, Kappos L, Rovira A, Sastre-Garriga J, Tintorè M, Frederiksen JL, et al: MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 15:292–303. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Wattjes MP, Rovira À, Miller D, Yousry TA, Sormani MP, de Stefano MP, Tintoré M, Auger C, Tur C, Filippi M, et al: Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-establishing disease prognosis and monitoring patients. Nat Rev Neurol. 11:597–606. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Bitar R, Leung G, Perng R, Tadros S, Moody AR, Sarrazin J, McGregor C, Christakis M, Symons S, Nelson A and Roberts TP: MR pulse sequences: What every radiologist wants to know but is afraid to ask. Radiographics. 26:513–537. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Villaraza AJL, Bumb A and Brechbiel MW: Macromolecules, dendrimers, and nanomaterials in magnetic resonance imaging: The interplay between size, function, and pharmacokinetics. Chem Rev. 110:2921–2959. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Angelovski G: Heading toward macromolecular and nanosized bioresponsive MRI probes for successful functional imaging. Acc Chem Res. 50:2215–2224. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Sun C, Lin H, Gong X, Yang Z, Mo Y, Chen X and Gao J: DOTA-branched organic frameworks as giant and potent metal chelators. J Am Chem Soc. 142:198–206. 2020. View Article : Google Scholar | |
|
Lanza GM, Winter PM, Neubauer AM, Caruthers SD, Hockett FD and Wickline SA: 1H/19F magnetic resonance molecular imaging with perfluorocarbon nanoparticles. Curr Top Dev Biol. 70:57–76. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Jahromi AH, Wang C, Adams SR, Zhu W, Narsinh K, Xu H, Gray DL, Tsien RY and Ahrens ET: Fluorous-soluble metal chelate for sensitive fluorine-19 magnetic resonance imaging nanoemulsion probes. ACS Nano. 13:143–151. 2019. View Article : Google Scholar : | |
|
Davies GL, Kramberger I and Davis JJ: Environmentally responsive MRI contrast agents. Chem Commun (Camb). 49:9704–9721. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Major JL and Meade TJ: Bioresponsive, cell-penetrating, and multimeric MR contrast agents. Acc Chem Res. 42:893–903. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou Z and Lu ZR: Gadolinium-based contrast agents for magnetic resonance cancer imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 5:1–18. 2013. View Article : Google Scholar | |
|
Li Y, Yu H, Qian Y, Hu J and Liu S: Amphiphilic star copolymer-based bimodal fluorogenic/magnetic resonance probes for concomitant bacteria detection and inhibition. Adv Mater. 26:6734–6741. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Hu X, Liu G, Li Y, Wang X and Liu S: Cell-penetrating hyperbranched polyprodrug amphiphiles for synergistic reductive milieu-triggered drug release and enhanced magnetic resonance signals. J Am Chem Soc. 137:362–368. 2015. View Article : Google Scholar | |
|
Perazella MA: Current status of gadolinium toxicity in patients with kidney disease. Clin J Am Soc Nephrol. 4:461–469. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, Haruyama T, Kitajima K and Furui S: Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology. 276:228–232. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Bouvain P, Temme S and Flögel U: Hot spot 19 F magnetic resonance imaging of inflammation. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 12:e16392020. View Article : Google Scholar | |
|
Srivastava AK, Kadayakkara DK, Bar-Shir A, Gilad AA, McMahon MT and Bulte JW: Advances in using MRI probes and sensors for in vivo cell tracking as applied to regenerative medicine. Dis Model Mech. 8:323–336. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Shen Z, Wu A and Chen X: Iron oxide nanoparticle based contrast agents for magnetic resonance imaging. Mol Pharm. 14:1352–1364. 2017. View Article : Google Scholar | |
|
Cromer Berman SM, Walczak P and Bulte JW: Tracking stem cells using magnetic nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 3:343–355. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Ribot E and Foster P: In vivo MRI discrimination between live and lysed iron-labelled cells using balanced steady state free precession. Eur Radiol. 22:2027–2034. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Gawel AM, Betkowska A, Gajda E, Godlewska M and Gawel D: Current non-metal nanoparticle-based therapeutic approaches for glioblastoma treatment. Biomedicines. 12:18222024. View Article : Google Scholar : PubMed/NCBI | |
|
Lin H, Tang X, Li A and Gao J: Activatable 19 F MRI nanoprobes for visualization of biological targets in living subjects. Adv Mater. 33:20056572021. View Article : Google Scholar | |
|
Tirotta I, Dichiarante V, Pigliacelli C, Cavallo G, Terraneo G, Bombelli FB, Metrangolo P and Resnati G: (19)F magnetic resonance imaging (MRI): From design of materials to clinical applications. Chem Rev. 115:1106–1129. 2015. View Article : Google Scholar | |
|
Xiang Y, Zheng G, Liang Z, Jin Y, Liu X, Chen S, Zhou K, Zhu J, Lin M, He H, et al: Visualizing the growth process of sodium microstructures in sodium batteries by in-situ 23Na MRI and NMR spectroscopy. Nat Nanotechnol. 15:883–890. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou Z, Deng H, Yang W, Wang Z, Lin L, Munasinghe J, Jacobson O, Liu Y, Tang L, Ni Q, et al: Early stratification of radiotherapy response by activatable inflammation magnetic resonance imaging. Nat Commun. 11:30322020. View Article : Google Scholar : PubMed/NCBI | |
|
Cametti M, Crousse B, Metrangolo P, Milani R and Resnati G: The fluorous effect in biomolecular applications. Chem Soc Rev. 41:31–42. 2012. View Article : Google Scholar | |
|
Li A, Tang X, Gong X, Chen H, Lin H and Gao J: A fluorinated bihydrazide conjugate for activatable sensing and imaging of hypochlorous acid by 19F NMR/MRI. Chem Commun (Camb). 55:12455–12458. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Chirizzi C, De Battista D, Tirotta I, Metrangolo P, Comi G, Bombelli FB and Chaabane L: Multispectral MRI with dual fluorinated probes to track mononuclear cell activity in mice. Radiology. 291:351–357. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Pippard BJ, Neal MA, Maunder AM, Hollingsworth KG, Biancardi A, Lawson RA, Fisher H, Matthews JNS, Simpson AJ, Wild JM and Thelwall PE: Reproducibility of 19 F-MR ventilation imaging in healthy volunteers. Magn Reson Med. 85:3343–3352. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang C, Yan K, Fu C, Peng H, Hawker CJ and Whittaker AK: Biological utility of fluorinated compounds: From materials design to molecular imaging, therapeutics and environmental remediation. Chem Rev. 122:167–208. 2022. View Article : Google Scholar | |
|
Maxouri O, Bodalal Z, Daal M, Rostami S, Rodriguez I, Akkari L, Srinivas M, Bernards R and Beets-Tan R: How to 19F MRI: applications, technique, and getting started. BJR Open. 5:202300192023.PubMed/NCBI | |
|
Yu W, Yang Y, Bo S, Li Y, Chen S, Yang Z, Zheng X, Jiang ZX and Zhou X: Design and synthesis of fluorinated dendrimers for sensitive (19)F MRI. J Org Chem. 80:4443–4449. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Mehta VD, Kulkarni PV, Mason RP, Constantinescu A and Antich PP: Fluorinated proteins as potential 19F magnetic resonance imaging and spectroscopy agents. Bioconjug Chem. 5:257–261. 1994. View Article : Google Scholar : PubMed/NCBI | |
|
Couch MJ, Ball IK, Li T, Fox MS, Littlefield SL, Biman B and Albert MS: Pulmonary ultrashort echo time 19F MR imaging with inhaled fluorinated gas mixtures in healthy volunteers: Feasibility. Radiology. 269:903–909. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Gutberlet M, Kaireit TF, Voskrebenzev A, Lasch F, Freise J, Welte T, Wacker F, Hohlfeld JM and Vogel-Claussen J: Free-breathing dynamic 19F gas MR imaging for mapping of regional lung ventilation in patients with COPD. Radiology. 286:1040–1051. 2018. View Article : Google Scholar | |
|
Xie D, Yu M, Kadakia RT and Que EL: 19F magnetic resonance activity-based sensing using paramagnetic metals. Acc Chem Res. 53:2–10. 2020. View Article : Google Scholar | |
|
Jirak D, Galisova A, Kolouchova K, Babuka D and Hruby M: Fluorine polymer probes for magnetic resonance imaging: Quo vadis? MAGMA. 32:173–185. 2019. View Article : Google Scholar : | |
|
Ruiz-Cabello J, Barnett BP, Bottomley PA and Bulte JWM: Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed. 24:114–129. 2011. View Article : Google Scholar : | |
|
Peterson KL, Srivastava K and Pierre VC: Fluorinated paramagnetic complexes: Sensitive and responsive probes for magnetic resonance spectroscopy and imaging. Front Chem. 6:1602018. View Article : Google Scholar : PubMed/NCBI | |
|
Ahrens ET and Bulte JW: Tracking immune cells in vivo using magnetic resonance imaging. Nat Rev Immunol. 13:755–763. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
O'Hagan D: Understanding organofluorine chemistry. An introduction to the C-F bond. Chem Soc Rev. 37:308–319. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Bona BL, Koshkina O, Chirizzi C, Dichiarante V, Metrangolo P and Baldelli Bombelli F: Multibranched-based fluorinated Materials: Tailor-made design of 19F-MRI probes. Acc Mater Res. 4:71–85. 2022. View Article : Google Scholar | |
|
Bouvain P, Flocke V, Krämer W, Schubert R, Schrader J, Flögel U and Temme S: Dissociation of 19F and fluorescence signal upon cellular uptake of dual-contrast perfluorocarbon nanoemulsions. MAGMA. 32:133–145. 2019. View Article : Google Scholar | |
|
Hertlein T, Sturm V, Kircher S, Basse-Lüsebrink T, Haddad D, Ohlsen K and Jakob P: Visualization of abscess formation in a murine thigh infection model of Staphylococcus aureus by 19F-magnetic resonance imaging (MRI). PLoS One. 6:e182462011. View Article : Google Scholar : PubMed/NCBI | |
|
Flögel U, Ding Z, Hardung H, Jander S, Reichmann G, Jacoby C, Schubert R and Schrader J: In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation. 118:140–148. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Balducci A, Helfer BM, Ahrens ET, O'Hanlon CF III and Wesa AK: Visualizing arthritic inflammation and therapeutic response by fluorine-19 magnetic resonance imaging (19F MRI). J Inflamm (Lond). 9:242012. View Article : Google Scholar : PubMed/NCBI | |
|
De Vries IJM, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, Boerman OC, Oyen WJ, Bonenkamp JJ, Boezeman JB, Adema GJ, et al: Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol. 23:1407–1413. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Tang X, Gong X, Li A, Lin H, Peng C, Zhang X, Chen X and Gao J: Cascaded multiresponsive self-assembled 19F MRI nanoprobes with redox-triggered activation and NIR-induced amplification. Nano Lett. 20:363–371. 2020. View Article : Google Scholar | |
|
Shin SH, Park SH, Kang SH, Kim SW, Kim M and Kim D: Fluorine-19 magnetic resonance imaging and positron emission tomography of tumor-associated macrophages and tumor metabolism. Contrast Media Mol Imaging. 2017:48963102017. View Article : Google Scholar | |
|
Fan X, River JN, Muresan AS, Popescu C, Zamora M, Culp RM and Karczmar GS: MRI of perfluorocarbon emulsion kinetics in rodent mammary tumours. Phys Med Biol. 51:211–220. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Bae PK, Jung J, Lim SJ, Kim D, Kim SK and Chung BH: Bimodal perfluorocarbon nanoemulsions for nasopharyngeal carcinoma targeting. Mol Imaging Biol. 15:401–410. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Helfer BM, Balducci A, Sadeghi Z, O'Hanlon C, Hijaz A, Flask CA and Wesa A: 19F MRI tracer preserves in vitro and in vivo properties of hematopoietic stem cells. Cell Transplant. 22:87–97. 2013. View Article : Google Scholar | |
|
Solanki YS, Agarwal M, Gupta A, Gupta S and Shukla P: Fluoride occurrences, health problems, detection, and remediation methods for drinking water: A comprehensive review. Sci Total Environ. 807:1506012022. View Article : Google Scholar | |
|
Bi J, Mo C, Li S, Huang M, Lin Y, Yuan P, Liu Z, Jia B and Xu S: Immunotoxicity of metal and metal oxide nanoparticles: From toxic mechanisms to metabolism and outcomes. Biomater Sci. 11:4151–4183. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
De A, Jee JP and Park YJ: Why perfluorocarbon nanoparticles encounter bottlenecks in clinical translation despite promising oxygen carriers? Eur J Pharm Biopharm. 199:1142922024. View Article : Google Scholar : PubMed/NCBI | |
|
Mohanto N, Mondal H, Park YJ and Jee JP: Therapeutic delivery of oxygen using artificial oxygen carriers demonstrates the possibility of treating a wide range of diseases. J Nanobiotechnology. 23:252025. View Article : Google Scholar : PubMed/NCBI | |
|
Jennings LE and Long NJ: 'Two is better than one'-probes for dual-modality molecular imaging. Chem Commun (Camb). 3511–3524. 2009. View Article : Google Scholar | |
|
Lee DE, Koo H, Sun IC, Ryu JH, Kim K and Kwon IC: Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem Soc Rev. 41:2656–2672. 2012. View Article : Google Scholar | |
|
Jacoby C, Temme S, Mayenfels F, Benoit N, Krafft MP, Schubert R, Schrader J and Flögel U: Probing different perfluorocarbons for in vivo inflammation imaging by 19F MRI: Image reconstruction, biological half-lives and sensitivity. NMR Biomed. 27:261–271. 2014. View Article : Google Scholar | |
|
Kaneda MM, Caruthers S, Lanza GM and Wickline SA: Perfluorocarbon nanoemulsions for quantitative molecular imaging and targeted therapeutics. Ann Biomed Eng. 37:1922–1933. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Srinivas M, Cruz LJ, Bonetto F, Heerschap A, Figdor CG and De Vries IJM: Customizable, multi-functional fluorocarbon nanoparticles for quantitative in vivo imaging using 19F MRI and optical imaging. Biomaterials. 31:7070–7077. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang Z, Liu X, Jeong E and Yu Y: Symmetry-guided design and fluorous synthesis of a stable and rapidly excreted imaging tracer for 19F MRI. Angew Chem Int Ed. 121:4849–4852. 2009. View Article : Google Scholar | |
|
Li D, Yang J, Xu Z, Li Y, Sun Y, Wang Y, Zou H, Wang K, Yang L, Wu L and Sun X: c-Met-targeting 19F MRI nanoparticles with ultralong tumor retention for precisely detecting small or Ill-defined colorectal liver metastases. Int J Nanomedicine. 18:2181–2196. 2023. View Article : Google Scholar : | |
|
Zambito G, Deng S, Haeck J, Gaspar N, Himmelreich U, Censi R, Löwik C, Di Martino P and Mezzanotte L: Fluorinated PLGA-PEG-mannose nanoparticles for tumor-associated macrophage detection by optical imaging and MRI. Front Med (Lausanne). 8:7123672021. View Article : Google Scholar : PubMed/NCBI | |
|
Janjic JM, Srinivas M, Kadayakkara DKK and Ahrens ET: Self-delivering nanoemulsions for dual fluorine-19 MRI and fluorescence detection. J Am Chem Soc. 130:2832–2841. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Mignion L, Magat J, Schakman O, Marbaix E, Gallez B and Jordan BF: Hexafluorobenzene in comparison with perfluoro-15-crown-5-ether for repeated monitoring of oxygenation using 19F MRI in a mouse model. Magn Reson Med. 69:248–254. 2013. View Article : Google Scholar | |
|
Heaton AR, Lechuga LM, Tangsangasaksri M, Ludwig KD, Fain SB and Mecozzi S: A stable, highly concentrated fluorous nanoemulsion formulation for in vivo cancer imaging via 19F-MRI. NMR Biomed. 37:e51002024. View Article : Google Scholar : | |
|
Helfer BM, Balducci A, Nelson AD, Janjic JM, Gil RR, Kalinski P, de Vries IJ, Ahrens ET and Mailliard RB: Functional assessment of human dendritic cells labeled for in vivo (19) F magnetic resonance imaging cell tracking. Cytotherapy. 12:238–250. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Vu-Quang H, Vinding MS, Nielsen T, Ullisch MG, Nielsen NC and Kjems J: Theranostic tumor targeted nanoparticles combining drug delivery with dual near infrared and 19F magnetic resonance imaging modalities. Nanomedicine. 12:1873–1884. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Diou O, Tsapis N, Giraudeau C, Valette J, Gueutin C, Bourasset F, Zanna S, Vauthier C and Fattal E: Long-circulating perfluorooctyl bromide nanocapsules for tumor imaging by 19FMRI. Biomaterials. 33:5593–5602. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Boissenot T, Fattal E, Bordat A, Houvenagel S, Valette J, Chacun H, Gueutin C and Tsapis N: Paclitaxel-loaded PEGylated nanocapsules of perfluorooctyl bromide as theranostic agents. Eur J Pharm Biopharm. 108:136–144. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Łopuszyńska N and Węglarz WP: Contrasting Properties of polymeric nanocarriers for MRI-guided drug delivery. Nanomaterials (Basel). 13:21632023. View Article : Google Scholar : PubMed/NCBI | |
|
Giraudeau C, Flament J, Marty B, Boumezbeur F, Mériaux S, Robic C, Port M, Tsapis N, Fattal E, Giacomini E, et al: A new paradigm for high-sensitivity 19F magnetic resonance imaging of perfluorooctylbromide. Magn Reson Med. 63:1119–1124. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Quang HV, Chang CC, Song P, Hauge EM and Kjems J: Caveolae-mediated mesenchymal stem cell labelling by PSS-coated PLGA PFOB nano-contrast agent for MRI. Theranostics. 8:2657–2671. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Gao W and Liang L: Effect of polysaccharide sulfate-loaded poly (lactic-co-glycolic acid) nanoparticles on coronary microvascular dysfunction of diabetic cardiomyopathy. J Biomed Nanotechnol. 18:446–452. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
A R, Wang H, Nie C, Han Z, Zhou M, Atinuke OO, Wang K, Wang X, Liu S, Zhao J, et al: Glycerol-weighted chemical exchange saturation transfer nanoprobes allow 19F/1H dual-modality magnetic resonance imaging-guided cancer radiotherapy. Nat Commun. 14:66442023. View Article : Google Scholar | |
|
Ahrens ET, Flores R, Xu H and Morel PA: In vivo imaging platform for tracking immunotherapeutic cells. Nat Biotechnol. 23:983–987. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Srinivas M, Turner MS, Janjic JM, Morel PA, Laidlaw DH and Ahrens ET: In vivo cytometry of antigen-specific t cells using 19F MRI. Magn Reson Med. 62:747–753. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Srinivas M, Morel PA, Ernst LA, Laidlaw DH and Ahrens ET: Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn Reson Med. 58:725–734. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang C, Sanchez RJP, Fu C, Clayden-Zabik R, Peng H, Kempe K and Whittaker AK: Importance of thermally induced aggregation on 19F magnetic resonance imaging of perfluoropolyether-based comb-shaped poly (2-oxazoline)s. Biomacromolecules. 20:365–374. 2019. View Article : Google Scholar | |
|
Kolouchova K, Groborz O, Slouf M, Herynek V, Parmentier L, Babuka D, Cernochova Z, Koucky F, Sedlacek O, Hruby M, et al: Thermoresponsive triblock copolymers as widely applicable 19F magnetic resonance imaging tracers. Chem of Mater. 34:10902–10916. 2022. View Article : Google Scholar | |
|
Wang Y, Tan X, Usman A, Zhang Y, Sawczyk M, Král P, Zhang C and Whittaker AK: Elucidating the impact of hydrophilic segments on 19F MRI sensitivity of fluorinated block copolymers. ACS Macro Lett. 11:1195–1201. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Nakamura T, Matsushita H, Sugihara F, Yoshioka Y, Mizukami S and Kikuchi K: Activatable 19F MRI nanoparticle probes for the detection of reducing environments. Angew Chem Int Ed Engl. 54:1007–1010. 2015. View Article : Google Scholar | |
|
Chen S, Yang Y, Li H, Zhou X and Liu M: pH-Triggered Au-fluorescent mesoporous silica nanoparticles for 19F MR/fluorescent multimodal cancer cellular imaging. Chem Commun (Camb). 50:283–285. 2014. View Article : Google Scholar | |
|
Mizukami S, Takikawa R, Sugihara F, Shirakawa M and Kikuchi K: Dual-function probe to detect protease activity for fluorescence measurement and 19F MRI. Angew Chem Int Ed Engl. 48:3641–3643. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Fu C, Tang J, Pye A, Liu T, Zhang C, Tan X, Han F, Peng H and Whittaker AK: Fluorinated glycopolymers as reduction-responsive 19F MRI agents for targeted imaging of cancer. Biomacromolecules. 20:2043–2050. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Wang K, Peng H, Thurecht KJ, Puttick S and Whittaker AK: Segmented highly branched copolymers: Rationally designed macromolecules for improved and tunable (19)F MRI. Biomacromolecules. 16:2827–2839. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Alhaidari LM and Spain SG: Synthesis of 5-fluorouracil polymer conjugate and 19F NMR analysis of drug release for MRI monitoring. Polymers (Basel). 15:17782023. View Article : Google Scholar | |
|
Krawczyk T, Minoshima M, Sugihara F and Kikuchi K: Modified polysaccharides as potential (19)F magnetic resonance contrast agents. Carbohydr Res. 428:72–78. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Yang X, Sun Y, Kootala S, Hilborn J, Heerschap A and Ossipov D: Injectable hyaluronic acid hydrogel for 19F magnetic resonance imaging. Carbohydr Polym. 110:95–99. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Bermejo-Velasco D, Dou W, Heerschap A, Ossipov D and Hilborn J: Injectable hyaluronic acid hydrogels with the capacity for magnetic resonance imaging. Carbohydr Polym. 197:641–648. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Strasser P, Schinegger V, Friske J, Brüggemann O, Helbich TH, Teasdale I and Pashkunova-Martic I: Superfluorinated, highly water-soluble polyphosphazenes as potential 19F magnetic resonance imaging (MRI) contrast agents. J Funct Biomater. 15:402024. View Article : Google Scholar | |
|
Han J, Duan Z, Liu C, Liu Y, Zhao X, Wang B, Cao S and Wu D: Hyperbranched polymeric 19F MRI contrast agents with long T2 relaxation time based on β-cyclodextrin and phosphorycholine. Biomacromolecules. 25:5860–5872. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Rolfe BE, Blakey I, Squires O, Peng H, Boase NR, Alexander C, Parsons PG, Boyle GM, Whittaker AK and Thurecht KJ: Multimodal polymer nanoparticles with combined 19F magnetic resonance and optical detection for tunable, targeted, multimodal imaging in vivo. J Am Chem Soc. 136:2413–2419. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Feng Z, Li Q, Wang W, Ni Q, Wang Y, Song H, Zhang C, Kong D, Liang XJ and Huang P: Superhydrophilic fluorinated polymer and nanogel for high-performance 19F magnetic resonance imaging. Biomaterials. 256:1201842020. View Article : Google Scholar | |
|
Thurecht KJ, Blakey I, Peng H, Squires O, Hsu S, Alexander C and Whittaker AK: Functional hyperbranched polymers: Toward targeted in vivo 19F magnetic resonance imaging using designed macromolecules. J Am Chem Soc. 132:5336–5337. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Le Droumaguet B and Velonia K: Click chemistry: A powerful tool to create polymer-based macromolecular chimeras. Macromol Rapid Commun. 29:1073–1089. 2008. View Article : Google Scholar | |
|
Chen S, Xiao L, Li Y, Qiu M, Yuan Y, Zhou R, Li C, Zhang L, Jiang ZX, Liu M and Zhou X: In vivo nitroreductase imaging via fluorescence and chemical shift dependent 19F NMR. Angew Chem. 134:e2022134952022. View Article : Google Scholar | |
|
Xu SY, Guo C, Pan K and Wang L: Combined fluorescence and MRI in bioimaging. Imaging Tools for Chemical Biology. 157–179. 2024. View Article : Google Scholar | |
|
Fu Q, Yang X, Wang M, Zhu K, Wang Y and Song J: Activatable probes for ratiometric imaging of endogenous biomarkers in vivo. ACS Nano. 18:3916–3968. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Yang L, Hou H and Li J: Frontiers in fluorescence imaging: tools for the in situ sensing of disease biomarkers. J Mater Chem B. 13:1133–1158. 2025. View Article : Google Scholar | |
|
Akazawa K, Sugihara F, Nakamura T, Mizukami S and Kikuchi K: Highly sensitive detection of caspase-3/7 activity in living mice using enzyme-responsive 19F MRI nanoprobes. Bioconjug Chem. 29:1720–1728. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Shusterman-Krush R, Tirukoti ND, Bandela AK, Avram L, Allouche-Arnon H, Cai X, Gibb BC and Bar-Shir A: Single fluorinated agent for multiplexed 19F-MRI with micromolar detectability based on dynamic exchange. Angew Chem Int Ed Engl. 60:15405–15411. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Jones KM, Pollard AC and Pagel MD: Clinical applications of chemical exchange saturation transfer (CEST) MRI. J Magn Reson Imaging. 47:11–27. 2018. View Article : Google Scholar : | |
|
Banerjee SR, Song X, Yang X, Minn I, Lisok A, Chen Y, Bui A, Chatterjee S, Chen J, van Zijl PCM, et al: Salicylic acid-based polymeric contrast agents for molecular magnetic resonance imaging of prostate cancer. Chemistry. 24:7235–7242. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Janasik D and Krawczyk T: 19F MRI probes for multimodal imaging. Chemistry. 28:e2021025562022. View Article : Google Scholar | |
|
Chen H, Viel S, Ziarelli F and Peng L: 19F NMR: A valuable tool for studying biological events. Chem Soc Rev. 42:7971–7982. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Yang J, Li Y, Sun J, Zou H, Sun Y, Luo J, Xie Q, A R, Wang H, Li X, et al: An osimertinib-perfluorocarbon nanoemulsion with excellent targeted therapeutic efficacy in non-small cell lung cancer: Achieving intratracheal and intravenous administration. ACS Nano. 16:12590–12605. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Takaoka Y, Sakamoto T, Tsukiji S, Narazaki M, Matsuda T, Tochio H, Shirakawa M and Hamachi I: Self-assembling nanoprobes that display off/on 19F nuclear magnetic resonance signals for protein detection and imaging. Nat Chem. 1:557–561. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Akazawa K, Sugihara F, Nakamura T, Matsushita H, Mukai H, Akimoto R, Minoshima M, Mizukami S and Kikuchi K: Perfluorocarbon-based 19F MRI nanoprobes for in vivo multicolor imaging. Angew Chem. 130:16984–16989. 2018. View Article : Google Scholar | |
|
Akazawa K, Sugihara F, Minoshima M, Mizukami S and Kikuchi K: Sensing caspase-1 activity using activatable 19F MRI nanoprobes with improved turn-on kinetics. Chem Commun (Camb). 54:11785–11788. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Yue X, Wang Z, Zhu L, Wang Y, Qian C, Ma Y, Kiesewetter DO, Niu G and Chen X: Novel 19F activatable probe for the detection of matrix metalloprotease-2 activity by MRI/MRS. Mol Pharm. 11:4208–4217. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Guo C, Zhang Y, Li Y, Xu S and Wang L: 19F MRI nanoprobes for the turn-on detection of phospholipase A2 with a low background. Anal Chem. 91:8147–8153. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Szczęch M, Łopuszyńska N, Tomal W, Jasiński K, Węglarz WP, Warszyński P and Szczepanowicz K: Nafion-based nanocarriers for fluorine magnetic resonance imaging. Langmuir. 36:9534–9539. 2020. View Article : Google Scholar | |
|
Hill LK, Frezzo JA, Katyal P, Hoang DM, Ben Youss Gironda Z, Xu C, Xie X, Delgado-Fukushima E, Wadghiri YZ and Montclare JK: Protein-engineered nanoscale micelles for dynamic 19F magnetic resonance and therapeutic drug delivery. ACS Nano. 13:2969–2985. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Bouchoucha M, van Heeswijk RB, Gossuin Y, Kleitz F and Fortin MA: Fluorinated mesoporous silica nanoparticles for binuclear probes in 1H and 19F magnetic resonance imaging. Langmuir. 33:10531–10542. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Chen H, Song M, Tang J, Hu G, Xu S, Guo Z, Li N, Cui J, Zhang X, Chen X and Wang L: Ultrahigh (19)F loaded Cu1.75S nanoprobes for simultaneous (19)F magnetic resonance imaging and photothermal therapy. ACS Nano. 10:1355–1362. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Kolouchova K, Sedlacek O, Jirak D, Babuka D, Blahut J, Kotek J, Vit M, Trousil J, Konefał R, Janouskova O, et al: Self-assembled thermoresponsive polymeric nanogels for 19F MR imaging. Biomacromolecules. 19:3515–3524. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Oishi M, Sumitani S and Nagasaki Y: On-off regulation of 19F magnetic resonance signals based on pH-sensitive PEGylated nanogels for potential tumor-specific smart 19F MRI probes. Bioconjug Chem. 18:1379–1382. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Munkhbat O, Canakci M, Zheng S, Hu W, Osborne B, Bogdanov AA and Thayumanavan S: 19F MRI of polymer nanogels aided by improved segmental mobility of embedded fluorine moieties. Biomacromolecules. 20:790–800. 2019. View Article : Google Scholar : | |
|
Peng H, Blakey I, Dargaville B, Rasoul F, Rose S and Whittaker AK: Synthesis and evaluation of partly fluorinated block copolymers as MRI imaging agents. Biomacromolecules. 10:374–381. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Barnett BP, Ruiz-Cabello J, Hota P, Ouwerkerk R, Shamblott MJ, Lauzon C, Walczak P, Gilson WD, Chacko VP, Kraitchman DL, et al: Use of perfluorocarbon nanoparticles for non-invasive multimodal cell tracking of human pancreatic islets. Contrast Media Mol Imaging. 6:251–259. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Xu X, Zhang R, Liu F, Ping J, Wen X, Wang H, Wang K, Sun X, Zou H, Shen B and Wu L: 19F MRI in orthotopic cancer model via intratracheal administration of ανβ3-targeted perfluorocarbon nanoparticles. Nanomedicine (Lond). 13:2551–2562. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Jirát-Ziółkowska N, Panakkal VM, Jiráková K, Havlíček D, Sedláček O and Jirák D: Cationic fluorinated micelles for cell labeling and 19F-MR imaging. Sci Rep. 14:226132024. View Article : Google Scholar | |
|
Matsushita H, Mizukami S, Sugihara F, Nakanishi Y, Yoshioka Y and Kikuchi K: Multifunctional core-shell silica nanoparticles for highly sensitive 19F magnetic resonance imaging. Angew Chem. 126:1026–1029. 2014. View Article : Google Scholar | |
|
Staal AHJ, Becker K, Tagit O, Koen van Riessen N, Koshkina O, Veltien A, Bouvain P, Cortenbach KRG, Scheenen T, Flögel U, et al: In vivo clearance of 19F MRI imaging nanocarriers is strongly influenced by nanoparticle ultrastructure. Biomaterials. 261:1203072020. View Article : Google Scholar | |
|
Cho MH, Shin SH, Park SH, Kadayakkara DK, Kim D and Choi Y: Targeted, stimuli-responsive, and theranostic 19F magnetic resonance imaging probes. Bioconjug Chem. 30:2502–2518. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Lyu Z, Ralahy B, Perles-Barbacaru TA, Ding L, Jiang Y, Lian B, Roussel T, Liu X, Galanakou C, Laurini E, et al: Self-assembling dendrimer nanosystems for specific fluorine magnetic resonance imaging and effective theranostic treatment of tumors. Proc Natl Acad Sci USA. 121:e23224031212024. View Article : Google Scholar : PubMed/NCBI | |
|
Criscione JM, Le BL, Stern E, Brennan M, Rahner C, Papademetris X and Fahmy TM: Self-assembly of pH-responsive fluorinated dendrimer-based particulates for drug delivery and noninvasive imaging. Biomaterials. 30:3946–3955. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Xu H, Kim D, Zhao YY, Kim C, Song G, Hu Q, Kang H and Yoon J: Remote control of energy transformation-based cancer imaging and therapy. Adv Mater. 36:e24028062024. View Article : Google Scholar : PubMed/NCBI | |
|
Svenson S: The dendrimer paradox-high medical expectations but poor clinical translation. Chem Soc Rev. 44:4131–4144. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Cooke DJ, Maier EY, King TL, Lin H, Hendrichs S, Lee S, Mafy NN, Scott KM, Lu Y and Que EL: Dual nanoparticle conjugates for highly sensitive and versatile sensing using 19F magnetic resonance imaging. Angew Chem Int Ed Engl. 63:e2023123222024. View Article : Google Scholar | |
|
Wang C, Adams SR and Ahrens ET: Emergent fluorous molecules and their uses in molecular imaging. Acc Chem Res. 54:3060–3070. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Bo S, Yuan Y, Chen Y, Yang Z, Chen S, Zhou X and Jiang ZX: In vivo drug tracking with 19F MRI at therapeutic dose. Chem Commun (Camb). 54:3875–3878. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Li L, Li A, Lin Y, Chen D, Kang B, Lin H and Gao J: An activatable 19F MRI molecular probe for sensing and imaging of norepinephrine. ChemistryOpen. 11:e2022001102022. View Article : Google Scholar | |
|
Koshkina O, White PB, Staal AHJ, Schweins R, Swider E, Tirotta I, Tinnemans P, Fokkink R, Veltien A, van Riessen NK, et al: Nanoparticles for 'two color' 19F magnetic resonance imaging: Towards combined imaging of biodistribution and degradation. J Colloid Interface Sci. 565:278–287. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Kadjane P, Platas-Iglesias C, Boehm-Sturm P, Truffault V, Hagberg G, Hoehn M, Logothetis N and Angelovski G: Dual-frequency calcium-responsive MRI agents. Chem Eur J. 20:7351–7362. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Doura T, Hata R, Nonaka H, Sugihara F, Yoshioka Y and Sando S: An adhesive (19)F MRI chemical probe allows signal off-to-on-type molecular sensing in a biological environment. Chem Commun (Camb). 49:11421–11423. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Southworth R, Parry CR, Parkes HG, Medina RA and Garlick PB: Tissue-specific differences in 2-fluoro-2-deoxyglucose metabolism beyond FDG-6-P: A 19F NMR spectroscopy study in the rat. NMR Biomed. 16:494–502. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Kanazawa Y, Umayahara K, Shimmura T and Yamashita T: 19F NMR of 2-deoxy-2-fluoro-D-glucose for tumor diagnosis in mice. An NDP-bound hexose analog as a new NMR target for imaging. NMR Biomed. 10:35–41. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Pujales-Paradela R, Savić T, Esteban-Gómez D, Angelovski G, Carniato F, Botta M and Platas-Iglesias C: Gadolinium(III)-based dual 1H/19F magnetic resonance imaging probes. Chem Eur J. 25:4782–4792. 2019. View Article : Google Scholar | |
|
Yu JX, Kodibagkar VD, Cui W and Mason RP: 19F: A versatile reporter for non-invasive physiology and pharmacology using magnetic resonance. Curr Med Chem. 12:819–848. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Bo S, Song C, Li Y, Yu W, Chen S, Zhou X, Yang Z, Zheng X and Jiang ZX: Design and synthesis of fluorinated amphiphile as (19)F MRI/fluorescence dual-imaging agent by tuning the self-assembly. J Org Chem. 80:6360–6366. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Chen D, Lin Y, Li A, Luo X, Yang C, Gao J and Lin H: Bio-orthogonal metabolic fluorine labeling enables deep-tissue visualization of tumor cells in vivo by 19F magnetic resonance imaging. Anal Chem. 94:16614–16621. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Cabanac S, Malet-Martino MC, Bon M, Martino R, Nedelec JF and Dimicoli JL: Direct 19f NMR spectroscopic observation of 5-fluorouracil metabolism in the isolated perfused mouse liver model. NMR Biomed. 1:113–120. 1988. View Article : Google Scholar : PubMed/NCBI | |
|
Wei H, Frey AM and Jasanoff A: Molecular fMRI of neurochemical signaling. J Neurosci Methods. 364:1093722021. View Article : Google Scholar : PubMed/NCBI | |
|
Matsuo K, Kamada R, Mizusawa K, Imai H, Takayama Y, Narazaki M, Matsuda T, Takaoka Y and Hamachi I: Specific detection and imaging of enzyme activity by signal-amplifiable self-assembling (19)F MRI probes. Chemistry. 19:12875–12883. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Wibowo A, Park JM, Liu SC, Khosla C and Spielman DM: Real-time in vivo detection of H2O2 using hyperpolarized 13C-thiourea. ACS Chem Biol. 12:1737–1742. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Doura T, Hata R, Nonaka H, Ichikawa K and Sando S: Design of a 13C magnetic resonance probe using a deuterated methoxy group as a long-lived hyperpolarization unit. Angew Chem Int Ed Engl. 51:10114–10117. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Nonaka H, Hata R, Doura T, Nishihara T, Kumagai K, Akakabe M, Tsuda M, Ichikawa K and Sando S: A platform for designing hyperpolarized magnetic resonance chemical probes. Nat Commun. 4:24112013. View Article : Google Scholar : PubMed/NCBI | |
|
Lippert AR, Keshari KR, Kurhanewicz J and Chang CJ: A hydrogen peroxide-responsive hyperpolarized 13C MRI contrast agent. J Am Chem Soc. 133:3776–3779. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao J, Chen J, Ma S, Liu Q, Huang L, Chen X, Lou K and Wang W: Recent developments in multimodality fluorescence imaging probes. Acta Pharm Sin B. 8:320–338. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Vivian D, Cheng K, Khurana S, Xu S, Dawson PA, Raufman JP and Polli JE: Design and evaluation of a novel trifluorinated imaging agent for assessment of bile acid transport using fluorine magnetic resonance imaging. J Pharm Sci. 103:3782–3792. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Tanifum EA, Devkota L, Ngwa C, Badachhape AA, Ghaghada KB, Romero J, Pautler RG and Annapragada AV: A hyperfluorinated hydrophilic molecule for aqueous 19F MRI contrast media. Contrast Media Mol Imaging. 2018:16935132018. View Article : Google Scholar | |
|
Du L, Helsper S, Nosratabad NA, Wang W, Fadool DA, Amiens C, Grant S and Mattoussi H: A multifunctional contrast agent for 19F-based magnetic resonance imaging. Bioconjug Chem. 33:881–891. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Pavlova OS, Anisimov NV, Gervits LL, Gulyaev MV, Semenova VN, Pirogov YA and Panchenko VY: 19 F MRI of human lungs at 0.5 Tesla using octafluorocyclobutane. Magn Reson Med. 84:2117–2123. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Li Q, Feng Z, Song H, Zhang J, Dong A, Kong D, Wang W and Huang P: 19F magnetic resonance imaging enabled real-time, non-invasive and precise localization and quantification of the degradation rate of hydrogel scaffolds in vivo. Biomater Sci. 8:3301–3309. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Liang S, Louchami K, Kolster H, Jacobsen A, Zhang Y, Thimm J, Sener A, Thiem J, Malaisse W, Dresselaers T and Himmelreich U: In vivo and ex vivo 19-fluorine magnetic resonance imaging and spectroscopy of beta-cells and pancreatic islets using GLUT-2 specific contrast agents. Contrast Media Mol Imaging. 11:506–513. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Wu B, Warnock G, Zaiss M, Lin C, Chen M, Zhou Z, Mu L, Nanz D, Tuura R and Delso G: An overview of CEST MRI for non-MR physicists. EJNMMI Phys. 3:192016. View Article : Google Scholar : PubMed/NCBI | |
|
Goldenberg JM and Pagel MD: Assessments of tumor metabolism with CEST MRI. NMR Biomed. 32:e39432019. View Article : Google Scholar | |
|
Wolff SD and Balaban RS: Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med. 10:135–144. 1989. View Article : Google Scholar : PubMed/NCBI | |
|
Pavuluri K, Manoli I, Pass A, Li Y, Vernon HJ, Venditti CP and McMahon MT: Noninvasive monitoring of chronic kidney disease using pH and perfusion imaging. Sci Adv. 5:eaaw83572019. View Article : Google Scholar : PubMed/NCBI | |
|
Rivlin M and Navon G: Glucosamine and N-acetyl glucosamine as new CEST MRI agents for molecular imaging of tumors. Sci Rep. 6:326482016. View Article : Google Scholar : PubMed/NCBI | |
|
Nasrallah FA, Pagès G, Kuchel PW, Golay X and Chuang KH: Imaging brain deoxyglucose uptake and metabolism by glucoCEST MRI. J Cereb Blood Flow Metab. 33:1270–1278. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou J, Lal B, Wilson DA, Laterra J and Van Zijl PCM: Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med. 50:1120–1126. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Ngen EJ, Bar-Shir A, Jablonska A, Liu G, Song X, Ansari R, Bulte JW, Janowski M, Pearl M, Walczak P and Gilad AA: Imaging the DNA alkylator melphalan by CEST MRI: An advanced approach to theranostics. Mol Pharm. 13:3043–3053. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Cai K, Xu HN, Singh A, Moon L, Haris M, Reddy R and Li LZ: Breast cancer redox heterogeneity detectable with chemical exchange saturation transfer (CEST) MRI. Mol Imaging Biol. 16:670–679. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Rivlin M, Horev J, Tsarfaty I and Navon G: Molecular imaging of tumors and metastases using chemical exchange saturation transfer (CEST) MRI. Sci Rep. 3:30452013. View Article : Google Scholar : PubMed/NCBI | |
|
Gao T, Zou C, Li Y, Jiang Z, Tang X and Song X: A brief history and future prospects of CEST MRI in clinical non-brain tumor imaging. Int J Mol Sci. 22:115592021. View Article : Google Scholar : PubMed/NCBI | |
|
Tang Y, Xiao G, Shen Z, Zhuang C, Xie Y, Zhang X, Yang Z, Guan J, Shen Y, Chen Y, et al: Noninvasive detection of extracellular pH in human benign and malignant liver tumors using CEST MRI. Front Oncol. 10:5789852020. View Article : Google Scholar : PubMed/NCBI | |
|
Kraiger M, Klein-Rodewald T, Rathkolb B, Calzada-Wack J, Sanz-Moreno A, Fuchs H, Wolf E, Gailus-Durner V and de Angelis MH: Monitoring longitudinal disease progression in a novel murine Kit tumor model using high-field MRI. Sci Rep. 12:146082022. View Article : Google Scholar : PubMed/NCBI | |
|
Barenholz Y: Doxil®-the first FDA-approved nano-drug: Lessons learned. J Control Release. 160:117–134. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Chan KW, McMahon MT, Kato Y, Liu G, Bulte JW, Bhujwalla ZM, Artemov D and van Zijl PC: Natural D-glucose as a biodegradable MRI contrast agent for detecting cancer. Magn Reson Med. 68:1764–1773. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Xu X, Sehgal AA, Yadav NN, Laterra J, Blair L, Blakeley J, Seidemo A, Coughlin JM, Pomper MG, Knutsson L and van Zijl PCM: d-glucose weighted chemical exchange saturation transfer (glucoCEST)-based dynamic glucose enhanced (DGE) MRI at 3T: Early experience in healthy volunteers and brain tumor patients. Magn Reson Med. 84:247–262. 2020. View Article : Google Scholar | |
|
Durmo F, Rydhög A, Testud F, Lätt J, Schmitt B, Rydelius A, Englund E, Bengzon J, van Zijl P, Knutsson L and Sundgren PC: Assessment of Amide proton transfer weighted (APTw) MRI for pre-surgical prediction of final diagnosis in gliomas. PLoS One. 15:e02440032020. View Article : Google Scholar : PubMed/NCBI | |
|
Paech D, Windschuh J, Oberhollenzer J, Dreher C, Sahm F, Meissner JE, Goerke S, Schuenke P, Zaiss M, Regnery S, et al: Assessing the predictability of IDH mutation and MGMT methylation status in glioma patients using relaxation-compensated multipool CEST MRI at 7.0 T. Neuro Oncol. 20:1661–1671. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Schmitt B, Zamecnik P, Zaiss M, Rerich E, Schuster L, Bachert P and Schlemmer HP: A new contrast in MR mammography by means of chemical exchange saturation transfer (CEST) imaging at 3 Tesla: Preliminary results. Rofo. 183:1030–1036. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou Y, van Zijl PCM, Xu X, Li Y, Chen L and Yadav NN: Magnetic resonance imaging of glycogen using its magnetic coupling with water. Proc Natl Acad Sci USA. 117:3144–3149. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Yuwen Zhou I, Wang E, Cheung JS, Lu D, Ji Y, Zhang X, Fulci G and Sun PZ: Direct saturation-corrected chemical exchange saturation transfer MRI of glioma: Simplified decoupling of amide proton transfer and nuclear Overhauser effect contrasts. Magn Reson Med. 78:2307–2314. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou J, Payen JF and Van Zijl PC: The interaction between magnetization transfer and blood-oxygen-level-dependent effects. Magn Reson Med. 53:356–366. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Seidemo A, Lehmann PM, Rydhög A, Wirestam R, Helms G, Zhang Y, Yadav NN, Sundgren PC, van Zijl PCM and Knutsson L: Towards robust glucose chemical exchange saturation transfer imaging in humans at 3 T: Arterial input function measurements and the effects of infusion time. NMR Biomed. 35:e46242022. View Article : Google Scholar | |
|
Xu X, Yadav NN, Knutsson L, Hua J, Kalyani R, Hall E, Laterra J, Blakeley J, Strowd R, Pomper M, et al: Dynamic glucose-enhanced (DGE) MRI: Translation to human scanning and first results in glioma patients. Tomography. 1:105–114. 2015. View Article : Google Scholar | |
|
Knutsson L, Xu X, van Zijl PCM and Chan KWY: Imaging of sugar-based contrast agents using their hydroxyl proton exchange properties. NMR Biomed. 36:e47842023. View Article : Google Scholar | |
|
Kim M, Torrealdea F, Adeleke S, Rega M, Evans V, Beeston T, Soteriou K, Thust S, Kujawa A, Okuchi S, et al: Challenges in glucoCEST MR body imaging at 3 Tesla. Quant Imaging Med Surg. 9:16282019. View Article : Google Scholar : PubMed/NCBI | |
|
Sehgal AA, Li Y, Lal B, Yadav NN, Xu X, Xu J, Laterra J and van Zijl PCM: CEST MRI of 3-O-methyl-D-glucose uptake and accumulation in brain tumors. Magn Reson Med. 81:1993–2000. 2019. View Article : Google Scholar : | |
|
Ling W, Regatte RR, Navon G and Jerschow A: Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST). Proc Natl Acad Sci USA. 105:2266–2270. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Rivlin M and Navon G: Phosphate buffer-catalyzed kinetics of mutarotation of glucosamine investigated by NMR spectroscopy. Carbohydr Res. 517:1085812022. View Article : Google Scholar : PubMed/NCBI | |
|
Bagga P, Wilson N, Rich L, Marincola FM, Schnall MD, Hariharan H, Haris M and Reddy R: Sugar alcohol provides imaging contrast in cancer detection. Sci Rep. 9:110922019. View Article : Google Scholar : PubMed/NCBI | |
|
Wang J, Fukuda M, Chung JJ, Wang P and Jin T: Chemical exchange sensitive MRI of glucose uptake using xylose as a contrast agent. Magn Reson Med. 85:1953–1961. 2021. View Article : Google Scholar : | |
|
Han Z, Chen C, Xu X, Bai R, Staedtke V, Huang J, Chan KWY, Xu J, Kamson DO, Wen Z, et al: Dynamic contrast-enhanced CEST MRI using a low molecular weight dextran. NMR Biomed. 35:e46492022. View Article : Google Scholar : | |
|
Huang J, Chen Z, Park SW, Lai JHC and Chan KWY: Molecular imaging of brain tumors and drug delivery using CEST MRI: Promises and challenges. Pharmaceutics. 14:4512022. View Article : Google Scholar : PubMed/NCBI | |
|
Pavuluri K, Rosenberg JT, Helsper S, Bo S and McMahon MT: Amplified detection of phosphocreatine and creatine after supplementation using CEST MRI at high and ultrahigh magnetic fields. J Magn Reson. 313:1067032020. View Article : Google Scholar : PubMed/NCBI | |
|
Liu J, Xie CM, Liu Q, Xu J, Zheng LY, Liu X, Zheng H and Wu Y: Dynamic alteration in myocardium creatine during acute infarction using MR CEST imaging. NMR Biomed. 35:e47042022. View Article : Google Scholar : PubMed/NCBI | |
|
Ohno K, Ohkubo M, Zheng B, Watanabe M, Matsuda T, Kwee IL and Igarashi H: GlyCEST: Magnetic resonance imaging of glycine-distribution in the normal murine brain and alterations in 5xFAD mice. Contrast Media Mol Imaging. 2021:89887622021. View Article : Google Scholar | |
|
Jin T, Wang P, Zong X and Kim SG: Magnetic resonance imaging of the amine-proton exchange (APEX) dependent contrast. Neuroimage. 59:1218–1227. 2012. View Article : Google Scholar | |
|
Zhang J, Yuan Y, Han Z, Li Y, van Zijl PCM, Yang X, Bulte JWM and Liu G: Detecting acid phosphatase enzymatic activity with phenol as a chemical exchange saturation transfer magnetic resonance imaging contrast agent (PhenolCEST MRI). Biosens Bioelectron. 141:1114422019. View Article : Google Scholar : PubMed/NCBI | |
|
Huang J, Lai JHC, Tse KH, Cheng GWY, Liu Y, Chen Z, Han X, Chen L, Xu J and Chan KWY: Deep neural network based CEST and AREX processing: Application in imaging a model of Alzheimer's disease at 3 T. Magn Reson Med. 87:1529–1545. 2022. View Article : Google Scholar | |
|
Shin SH, Wendland MF, Zhang B, Tran A, Tang A and Vandsburger MH: Noninvasive imaging of renal urea handling by CEST-MRI. Magn Reson Med. 83:1034–1044. 2020. View Article : Google Scholar | |
|
Shin SH, Wendland MF and Vandsburger MH: Delayed urea differential enhancement CEST (dudeCEST)-MRI with T1 correction for monitoring renal urea handling. Magn Reson Med. 85:2791–2804. 2021. View Article : Google Scholar | |
|
Stabinska J, Keupp J and McMahon MT: CEST MRI for monitoring kidney diseases. Advanced Clinical MRI of the Kidney: Methods and Protocols. Springer International Publishing; pp. 345–360. 2023, View Article : Google Scholar | |
|
Yang X, Song X, Ray Banerjee S, Li Y, Byun Y, Liu G, Bhujwalla ZM, Pomper MG and McMahon MT: Developing imidazoles as CEST MRI pH sensors. Contrast Media Mol Imaging. 11:304–312. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Longo DL, Sun PZ, Consolino L, Michelotti FC, Uggeri F and Aime S: A general MRI-CEST ratiometric approach for pH imaging: Demonstration of in vivo pH mapping with iobitridol. J Am Chem Soc. 136:14333–14336. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Sherry AD and Woods M: Chemical exchange saturation transfer contrast agents for magnetic resonance imaging. Annu Rev Biomed Eng. 10:391–411. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Song X, Walczak P, He X, Yang X, Pearl M, Bulte JWM, Pomper MG, McMahon MT and Janowski M: Salicylic acid analogues as chemical exchange saturation transfer MRI contrast agents for the assessment of brain perfusion territory and blood-brain barrier opening after intra-arterial infusion. J Cereb Blood Flow Metab. 36:1186–1194. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Yang X, Song X, Li Y, Liu G, Banerjee SR, Pomper MG and McMahon MT: Salicylic acid and analogues as diaCEST MRI contrast agents with highly shifted exchangeable proton frequencies. Angew Chem Int Ed Engl. 52:8116–8119. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Bar-Shir A, Liu G, Liang Y, Yadav NN, McMahon MT, Walczak P, Nimmagadda S, Pomper MG, Tallman KA, Greenberg MM, et al: Transforming thymidine into a magnetic resonance imaging probe for monitoring gene expression. J Am Chem Soc. 135:1617–1624. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Bo S, Stabinska J, Wu Y, Pavuluri KD, Singh A, Mohanta Z, Choudhry R, Kates M, Sedaghat F, Bhujwalla Z, et al: Exploring the potential of the novel imidazole-4,5-dicarboxyamide chemical exchange saturation transfer scaffold for pH and perfusion imaging. NMR Biomed. 36:e48942023. View Article : Google Scholar : | |
|
Longo DL, Carella A, Corrado A, Pirotta E, Mohanta Z, Singh A, Stabinska J, Liu G and McMahon MT: A snapshot of the vast array of diamagnetic CEST MRI contrast agents. NMR Biomed. 36:e47152023. View Article : Google Scholar | |
|
Bo S, Sedaghat F, Pavuluri K, Rowe SP, Cohen A, Kates M and McMahon MT: Dynamic contrast enhanced-MR CEST urography: An emerging tool in the diagnosis and management of upper urinary tract obstruction. Tomography. 7:80–94. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Pavuluri K, Yang E, Ayyappan V, Sonkar K, Tan Z, Tressler CM, Bo S, Bibic A, Glunde K and McMahon MT: Unlabeled aspirin as an activatable theranostic MRI agent for breast cancer. Theranostics. 12:1937–1951. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Song X, Yang X, Ray Banerjee S, Pomper MG and McMahon MT: Anthranilic acid analogs as diamagnetic CEST MRI contrast agents that feature an intramolecular-bond shifted hydrogen. Contrast Media Mol Imaging. 10:74–80. 2015. View Article : Google Scholar | |
|
Yang X, Yadav NN, Song X, Ray Banerjee S, Edelman H, Minn I, van Zijl PC, Pomper MG and McMahon MT: Tuning phenols with intra-molecular bond shifted HYdrogens (IM-SHY) as diaCEST MRI contrast agents. Chemistry. 20:15824–15832. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Sinharay S, Randtke EA, Howison CM, Ignatenko NA and Pagel MD: Detection of enzyme activity and inhibition during studies in solution, in vitro and in vivo with catalyCEST MRI. Mol Imaging Biol. 20:240–248. 2018. View Article : Google Scholar : | |
|
Kombala CJ, Lokugama SD, Kotrotsou A, Li T, Pollard AC and Pagel MD: Simultaneous evaluations of pH and enzyme activity with a CEST MRI contrast agent. ACS Sens. 6:4535–4544. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Sinharay S, Randtke EA, Jones KM, Howison CM, Chambers SK, Kobayashi H and Pagel MD: Noninvasive detection of enzyme activity in tumor models of human ovarian cancer using catalyCEST MRI. Magn Reson Med. 77:2005–2014. 2017. View Article : Google Scholar | |
|
Hingorani DV, Montano LA, Randtke EA, Lee YS, Cárdenas-Rodríguez J and Pagel MD: A single diamagnetic catalyCEST MRI contrast agent that detects cathepsin B enzyme activity by using a ratio of two CEST signals. Contrast Media Mol Imaging. 11:130–138. 2016. View Article : Google Scholar | |
|
Longo DL, Michelotti F, Consolino L, Bardini P, Digilio G, Xiao G, Sun PZ and Aime S: In vitro and in vivo assessment of nonionic iodinated radiographic molecules as chemical exchange saturation transfer magnetic resonance imaging tumor perfusion agents. Invest Radiol. 51:155–162. 2016. View Article : Google Scholar | |
|
Liu J, Chu C, Zhang J, Bie C, Chen L, Aafreen S, Xu J, Kamson DO, van Zijl PCM, Walczak P, et al: Label-free assessment of mannitol accumulation following osmotic blood-brain barrier opening using chemical exchange saturation transfer magnetic resonance imaging. Pharmaceutics. 14:25292022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang X, Yuan Y, Li S, Zeng Q, Guo Q, Liu N, Yang M, Yang Y, Liu M, McMahon MT and Zhou X: Free-base porphyrins as CEST MRI contrast agents with highly upfield shifted labile protons. Magn Reson Med. 82:577–585. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Liu H, Jablonska A, Li Y, Cao S, Liu D, Chen H, Van Zijl PC, Bulte JW, Janowski M, Walczak P and Liu G: Label-free CEST MRI detection of citicoline-liposome drug delivery in ischemic stroke. Theranostics. 6:1588–1600. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Aime S, Calabi L, Biondi L, De Miranda M, Ghelli S, Paleari L, Rebaudengo C and Terreno E: Iopamidol: Exploring the potential use of a well-established x-ray contrast agent for MRI. Magn Reson Med. 53:830–834. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Feng X, Zhu W, Oskolkov N, Zhou T, Kim BK, Baig N, McMahon MT and Oldfield E: Chemical exchange saturation transfer (CEST) agents: Quantum chemistry and MRI. Chemistry. 22:264–271. 2016. View Article : Google Scholar : | |
|
Zhang H, Zhou J and Peng Y: Amide proton transfer-weighted MR imaging of pediatric central nervous system diseases. Magn Reson Imaging Clin N Am. 29:631–641. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Fillion AJ, Bricco AR, Lee HD, Korenchan DE, Farrar CT and Gilad AA: Development of a synthetic biosensor for chemical exchange MRI utilizing in silico optimized peptides. NMR Biomed. 36:e50072023. View Article : Google Scholar : PubMed/NCBI | |
|
Rosa E, Di Gregorio E, Ferrauto G, Diaferia C, Gallo E, Terreno E and Accardo A: Hybrid PNA-peptide hydrogels as injectable CEST-MRI agents. J Mater Chem B. 12:6371–6383. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Sartoretti E, Sartoretti T, Wyss M, Reischauer C, van Smoorenburg L, Binkert CA, Sartoretti-Schefer S and Mannil M: Amide proton transfer weighted (APTw) imaging based radiomics allows for the differentiation of gliomas from metastases. Sci Rep. 11:55062021. View Article : Google Scholar : PubMed/NCBI | |
|
Liang Y, Bar-Shir A, Song X, Gilad AA, Walczak P and Bulte JW: Label-free imaging of gelatin-containing hydrogel scaffolds. Biomaterials. 42:144–150. 2015. View Article : Google Scholar | |
|
Wu Y, Evbuomwan M, Melendez M, Opina A and Sherry AD: Advantages of macromolecular to nanosized chemical-exchange saturation transfer agents for MRI applications. Future Med Chem. 2:351–366. 2010. View Article : Google Scholar | |
|
Choi J, Kim K, Kim T, Liu G, Bar-Shir A, Hyeon T, McMahon MT, Bulte JW, Fisher JP and Gilad AA: Multimodal imaging of sustained drug release from 3-D poly(propylene fumarate) (PPF) scaffolds. J Control Release. 156:239–245. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
McMahon MT, Gilad AA, DeLiso MA, Cromer Berman SM, Bulte JWM and Van Zijl PCM: New 'multicolor' polypeptide diamagnetic chemical exchange saturation transfer (DIACEST) contrast agents for MRI. Magn Reson Med. 60:803–812. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou J, Payen JF, Wilson DA, Traystman RJ and Van Zijl PCM: Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 9:1085–1090. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
McMahon MT, Gilad AA, Zhou J, Sun PZ, Bulte JWM and Van Zijl PCM: Quantifying exchange rates in chemical exchange saturation transfer agents using the saturation time and saturation power dependencies of the magnetization transfer effect on the magnetic resonance imaging signal (QUEST and QUESP): pH calibration for poly-L-lysine and a starburst dendrimer. Magn Reson Med. 55:836–847. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Bar-Shir A, Liu G, Chan KWY, Oskolkov N, Song X, Yadav NN, Walczak P, McMahon MT, van Zijl PCM, Bulte JWM and Gilad AA: Human protamine-1 as an MRI reporter gene based on chemical exchange. ACS Chem Biol. 9:134–138. 2014. View Article : Google Scholar : | |
|
Bar-Shir A, Liang Y, Chan KWY, Gilad AA and Bulte JWM: Supercharged green fluorescent proteins as bimodal reporter genes for CEST MRI and optical imaging. Chem Commun (Camb). 51:4869–4871. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Oskolkov N, Bar-Shir A, Chan KWY, Song X, van Zijl PCM, Bulte JWM, Gilad AA and McMahon MT: Biophysical characterization of human protamine-1 as a responsive CEST MR contrast agent. ACS Macro Lett. 4:34–38. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Haris M, Singh A, Mohammed I, Ittyerah R, Nath K, Nanga RP, Debrosse C, Kogan F, Cai K, Poptani H, et al: In vivo magnetic resonance imaging of tumor protease activity. Sci Rep. 4:60812014. View Article : Google Scholar : PubMed/NCBI | |
|
Wang C, Lin G, Shen Z and Wang R: Angiopep-2 as an exogenous chemical exchange saturation transfer contrast agent in diagnosis of Alzheimer's disease. J Healthc Eng. 2022:74805192022.PubMed/NCBI | |
|
Sinharay S, Howison CM, Baker AF and Pagel MD: Detecting in vivo urokinase plasminogen activator activity with a catalyCEST MRI contrast agent. NMR Biomed. Mar 29–2017.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI | |
|
Yuan Y, Raj P, Zhang J, Siddhanta S, Barman I and Bulte JWM: Furin-mediated self-assembly of olsalazine nanoparticles for targeted Raman imaging of tumors. Angew Chem. 133:3969–3973. 2021. View Article : Google Scholar | |
|
Kombala CJ, Kotrotsou A, Schuler FW, de la Cerda J, Ma JC, Zhang S and Pagel MD: Development of a nanoscale chemical exchange saturation transfer magnetic resonance imaging contrast agent that measures pH. ACS Nano. 15:20678–20688. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Goffeney N, Bulte JW, Duyn J, Bryant LH Jr and Van Zijl PC: Sensitive NMR detection of cationic-polymer-based gene delivery systems using saturation transfer via proton exchange. J Am Chem Soc. 123:8628–8629. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Langereis S, Keupp J, van Velthoven JLJ, de Roos IHC, Burdinski D, Pikkemaat JA and Grüll H: A temperature-sensitive liposomal 1H CEST and 19F contrast agent for MR image-guided drug delivery. J Am Chem Soc. 131:1380–1381. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang J, Yuan Y, Gao M, Han Z, Chu C, Li Y, van Zijl PCM, Ying M, Bulte JWM and Liu G: Carbon dots as a new class of diamagnetic chemical exchange saturation transfer (diaCEST) MRI contrast agents. Angew Chem Int Ed Engl. 58:9871–9875. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Chan KWY, Liu G, Song X, Kim H, Yu T, Arifin DR, Gilad AA, Hanes J, Walczak P, van Zijl PCM, et al: MRI-detectable pH nanosensors incorporated into hydrogels for in vivo sensing of transplanted-cell viability. Nat Mater. 12:268–275. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Tyler B, Fowers KD, Li KW, Recinos VR, Caplan JM, Hdeib A, Grossman R, Basaldella L, Bekelis K, Pradilla G, et al: A thermal gel depot for local delivery of paclitaxel to treat experimental brain tumors in rats. J Neurosurg. 113:210–217. 2010. View Article : Google Scholar | |
|
Lesniak WG, Oskolkov N, Song X, Lal B, Yang X, Pomper M, Laterra J, Nimmagadda S and McMahon MT: Salicylic acid conjugated dendrimers are a tunable, high performance CEST MRI nanoplatform. Nano Lett. 16:2248–2253. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Pikkemaat J, Wegh R, Lamerichs R, van de Molengraaf RA, Langereis S, Burdinski D, Raymond AY, Janssen HM, de Waal BF, Willard NP, et al: Dendritic PARACEST contrast agents for magnetic resonance imaging. CContrast Media Mol Imaging. 2:229–239. 2007. View Article : Google Scholar | |
|
Ding L, Xu F, Luo B, Cheng L, Huang L, Jia Y and Ding J: Preparation of hematoporphyrin-poly(lactic acid) nanoparticles encapsulated perfluoropentane/salicylic acid for enhanced US/CEST MR bimodal imaging. Int J Nanomedicine. 19:4589–4605. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Yu T, Chan KWY, Anonuevo A, Song X, Schuster BS, Chattopadhyay S, Xu Q, Oskolkov N, Patel H, Ensign LM, et al: Liposome-based mucus-penetrating particles (MPP) for mucosal theranostics: Demonstration of diamagnetic chemical exchange saturation transfer (diaCEST) magnetic resonance imaging (MRI). Nanomedicine. 11:401–405. 2015. View Article : Google Scholar | |
|
Lock LL, Li Y, Mao X, Chen H, Staedtke V, Bai R, Ma W, Lin R, Li Y, Liu G and Cui H: One-component supramolecular filament hydrogels as theranostic label-free magnetic resonance imaging agents. ACS Nano. 11:797–805. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Chakraborty S, Peruncheralathan S and Ghosh A: Paracetamol and other acetanilide analogs as inter-molecular hydrogen bonding assisted diamagnetic CEST MRI contrast agents. RSC Adv. 11:6526–6534. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Dang T, Suchy M, Truong YJ, Oakden W, Lam WW, Lazurko C, Facey G, Stanisz GJ and Shuhendler AJ: Hydrazo-CEST: hydrazone-dependent chemical exchange saturation transfer magnetic resonance imaging contrast agents. Chemistry. 24:9148–9156. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Cai X, Zhang J, Lu J, Yi L, Han Z, Zhang S, Yang X and Liu G: N-Aryl amides as chemical exchange saturation transfer magnetic resonance imaging contrast agents. Chemistry. 26:11705–11709. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Barandov A, Ghosh S and Jasanoff A: Probing nitric oxide signaling using molecular MRI. Free Radic Biol Med. 191:241–248. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Barandov A, Ghosh S, Li N, Bartelle BB, Daher JI, Pegis ML, Collins H and Jasanoff A: Molecular magnetic resonance imaging of nitric oxide in biological systems. ACS Sens. 5:1674–1682. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Xue X, Bo R, Qu H, Jia B, Xiao W, Yuan Y, Vapniarsky N, Lindstrom A, Wu H, Zhang D, et al: A nephrotoxicity-free, iron-based contrast agent for magnetic resonance imaging of tumors. Biomaterials. 257:1202342020. View Article : Google Scholar : PubMed/NCBI | |
|
Brun EMSPT, Calvert ND, Suchý M, Kirby A, Melkus G, Garipov R, Addison CL and Shuhendler AJ: Mapping vitamin B6 metabolism by hydrazoCEST magnetic resonance imaging. Chem Commun (Camb). 57:10867–10870. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Hosain MZ, Hyodo F, Mori T, Takahashi K, Nagao Y, Eto H, Murata M, Akahoshi T, Matsuo M and Katayama Y: Development of a novel molecular probe for the detection of liver mitochondrial redox metabolism. Sci Rep. 10:164892020. View Article : Google Scholar : PubMed/NCBI | |
|
Matsumoto KI, Nakanishi I, Zhelev Z, Bakalova R and Aoki I: Nitroxyl radical as a theranostic contrast agent in magnetic resonance redox imaging. Antioxid Redox Signal. 36:95–121. 2022. View Article : Google Scholar : | |
|
Adachi K, Hyodo F, Elhelaly A, Mori T, Taniguchi T, Nakaya S and Matsuo M: Spatiotemporal evaluation of the early inflammatory response of the gut to radiation using non-invasive in vivo redox imaging. Int J Radiat Oncol Biol Phys. 120:e346–e347. 2024. View Article : Google Scholar | |
|
Koyasu N, Hyodo F, Iwasaki R, Eto H, Elhelaly AE, Tomita H, Shoda S, Takasu M, Mori T, Murata M, et al: Spatiotemporal imaging of redox status using in vivo dynamic nuclear polarization magnetic resonance imaging system for early monitoring of response to radiation treatment of tumor. Free Radic Biol Med. 179:170–180. 2022. View Article : Google Scholar | |
|
Yoshino Y, Fujii Y, Chihara K, Nakae A, Enmi JI, Yoshioka Y and Miyawaki I: Non-invasive differentiation of hepatic steatosis and steatohepatitis in a mouse model using nitroxyl radical as an MRI-contrast agent. Toxicol Rep. 12:1–9. 2023. | |
|
Matsumoto KI, Hyodo F, Matsumoto A, Koretsky AP, Sowers AL, Mitchell JB and Krishna MC: High-resolution mapping of tumor redox status by magnetic resonance imaging using nitroxides as redox-sensitive contrast agents. Clin Cancer Res. 12:2455–2462. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Shah SA, Cui SX, Waters CD, Sano S, Wang Y, Doviak H, Leor J, Walsh K, French BA and Epstein FH: Nitroxide-enhanced MRI of cardiovascular oxidative stress. NMR Biomed. 33:e43592020. View Article : Google Scholar : PubMed/NCBI | |
|
Kawano T, Murata M, Hyodo F, Eto H, Kosem N, Nakata R, Hamano N, Piao JS, Narahara S, Akahoshi T and Hashizume M: Noninvasive mapping of the redox status of dimethylnitrosamine-induced hepatic fibrosis using in vivo dynamic nuclear polarization-magnetic resonance imaging. Sci Rep. 6:326042016. View Article : Google Scholar : PubMed/NCBI | |
|
Kuroda Y, Togashi H, Uchida T, Haga K, Yamashita A and Sadahiro M: Oxidative stress evaluation of skeletal muscle in ischemia-reperfusion injury using enhanced magnetic resonance imaging. Sci Rep. 10:108632020. View Article : Google Scholar : PubMed/NCBI | |
|
Hyodo F, Eto H, Naganuma T, Koyasu N, Elhelaly AE, Noda Y, Kato H, Murata M, Akahoshi T, Hashizume M, et al: In vivo dynamic nuclear polarization magnetic resonance imaging for the evaluation of redox-related diseases and theranostics. Antioxid Redox Signal. 36:172–184. 2022. View Article : Google Scholar | |
|
Eto H, Murata M, Kawano T, Tachibana Y, Elhelaly AE, Noda Y, Kato H, Matsuo M and Hyodo F: Evaluation of the redox alteration in Duchenne muscular dystrophy model mice using in vivo DNP-MRI. Npj Imaging. 2:522024. View Article : Google Scholar | |
|
Tao Q, Zhang D, Zhang Q, Liu C, Ye S, Feng Y and Liu R: Mitochondrial targeted ROS scavenger based on nitroxide for treatment and MRI imaging of acute kidney injury. Free Radic Res. 56:303–315. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Eto H, Hyodo F, Kosem N, Kobayashi R, Yasukawa K, Nakao M, Kiniwa M and Utsumi H: Redox imaging of skeletal muscle using in vivo DNP-MRI and its application to an animal model of local inflammation. Free Radic Biol Med. 89:1097–1104. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Matsumoto KI, Yamasaki T, Nakamura M, Ishikawa J, Ueno M, Nakanishi I, Sekita A, Ozawa Y, Kamada T, Aoki I and Yamada K: Brain contrasting ability of blood-brain-barrier-permeable nitroxyl contrast agents for magnetic resonance redox imaging. Magn Reson Med. 76:935–945. 2016. View Article : Google Scholar | |
|
Hyodo F, Chuang KH, Goloshevsky AG, Sulima A, Griffiths GL, Mitchell JB, Koretsky AP and Krishna MC: Brain redox imaging using blood-brain barrier-permeable nitroxide MRI contrast agent. J Cereb Blood Flow Metab. 28:1165–1174. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Matsumoto KI, Yakumaru H, Narazaki M, Nakagawa H, Anzai K, Ikehira H and Ikota N: Modification of nitroxyl contrast agents with multiple spins and their proton T(1) relaxivity. Magn Reson Imaging. 26:117–121. 2008. View Article : Google Scholar | |
|
Benial AMF, Utsumi H, Ichikawa K, Murugesan R, Yamada K, Kinoshita Y, Naganuma T and Kato M: Dynamic nuclear polarization studies of redox-sensitive nitroxyl spin probes in liposomal solution. J Magn Reson. 204:131–138. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Yuan X, Yu H, Wang L, Uddin MA and Ouyang C: Nitroxide radical contrast agents for safe magnetic resonance imaging: Progress, challenges, and perspectives. Mater Horiz. 12:1726–1756. 2025. View Article : Google Scholar : PubMed/NCBI | |
|
Meenakumari V, Utsumi H, Jawahar A and Franklin Benial AM: Concentration dependence of nitroxyl spin probes in liposomal solution: Electron spin resonance and Overhauser-enhanced magnetic resonance studies. J Liposome Res. 28:87–96. 2018. View Article : Google Scholar | |
|
Yamada KI, Kinoshita Y, Yamasaki T, Sadasue H, Mito F, Nagai M, Matsumoto S, Aso M, Suemune H, Sakai K and Utsumi H: Synthesis of nitroxyl radicals for Overhauser-enhanced magnetic resonance imaging. Arch Pharm (Weinheim). 341:548–553. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Zhelev Z, Gadjeva V, Aoki I, Bakalova R and Saga T: Cell-penetrating nitroxides as molecular sensors for imaging of cancer in vivo, based on tissue redox activity. Mol Biosyst. 8:2733–2740. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Guo S, Wang X, Li Z, Pan D, Dai Y, Ye Y, Tian X, Gu Z, Gong Q, Zhang H and Luo K: A nitroxides-based macromolecular MRI contrast agent with an extraordinary longitudinal relaxivity for tumor imaging via clinical T1WI SE sequence. J Nanobiotechnology. 19:2442021. View Article : Google Scholar : PubMed/NCBI | |
|
Nguyen HVT, Chen Q, Paletta JT, Harvey P, Jiang Y, Zhang H, Boska MD, Ottaviani MF, Jasanoff A, Rajca A and Johnson JA: Nitroxide-based macromolecular contrast agents with unprecedented transverse relaxivity and stability for magnetic resonance imaging of tumors. ACS Cent Sci. 3:800–811. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Guo S, Wang X, Dai Y, Dai X, Li Z, Luo Q, Zheng X, Gu Z, Zhang H, Gong Q and Luo K: Enhancing the efficacy of metal-free MRI contrast agents via conjugating nitroxides onto PEGylated cross-linked poly(carboxylate ester). Adv Sci (Weinh). 7:20004672020. View Article : Google Scholar : PubMed/NCBI | |
|
Wang X, Guo S, Li Z, Luo Q, Dai Y, Zhang H, Ye Y, Gong Q and Luo K: Amphiphilic branched polymer-nitroxides conjugate as a nanoscale agent for potential magnetic resonance imaging of multiple objects in vivo. J Nanobiotechnology. 19:2052021. View Article : Google Scholar : PubMed/NCBI | |
|
Niidome T, Gokuden R, Watanabe K, Mori T, Naganuma T, Utsumi H, Ichikawa K and Katayama Y: Nitroxyl radicals-modified dendritic poly (l-lysine) as a contrast agent for Overhauser-enhanced MRI. J Biomater Sci Polym Ed. 25:1425–1439. 2014. View Article : Google Scholar | |
|
Pinto LF, Lloveras V, Zhang S, Liko F, Veciana J, Muñoz-Gómez JL and Vidal-Gancedo J: Fully water-soluble polyphosphorhydrazone-based radical dendrimers functionalized with Tyr-PROXYL radicals as metal-free MRI T1 contrast agents. ACS Appl Bio Mater. 3:369–376. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Wang X, Guo S, Li Z, Luo Q, Dai Y, Zhang H, Ye Y, Gong Q and Luo K: Amphiphilic branched polymer-nitroxides conjugate as a nanoscale agent for potential magnetic resonance imaging of multiple objects in vivo. J Nanobiotechnology. 19:2052021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang S, Lloveras V, Lope-Piedrafita S, Calero-Pérez P, Wu S, Candiota AP and Vidal-Gancedo J: Metal-free radical dendrimers as MRI contrast agents for glioblastoma diagnosis: Ex vivo and in vivo approaches. Biomacromolecules. 23:2767–2777. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Mitin D, Bullinger F, Dobrynin S, Engelmann J, Scheffler K, Kolokolov M, Krumkacheva O, Buckenmaier K, Kirilyuk I and Chubarov A: Contrast agents based on human serum albumin and nitroxides for 1H-MRI and Overhauser-enhanced MRI. Int J Mol Sci. 25:40412024. View Article : Google Scholar | |
|
Tian C, Zhang S, Lloveras V and Gancedo JV: Application of radical dendrimers as organic radical contrast agents for magnetic resonance imaging. Adv Ind Eng Polym Res. 7:255–261. 2024. | |
|
Pagar RR, Musale SR, Pawar G, Kulkarni D and Giram PS: Comprehensive review on the degradation chemistry and toxicity studies of functional materials. ACS Biomater Sci Eng. 8:2161–2195. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Mellet P, Massot P, Madelin G, Marque SR, Harte E, Franconi JM and Thiaudière E: New concepts in molecular imaging: Non-invasive MRI spotting of proteolysis using an Overhauser effect switch. PLoS One. 4:e52442009. View Article : Google Scholar : PubMed/NCBI | |
|
Koonjoo N, Parzy E, Massot P, Lepetit-Coiffé M, Marque SR, Franconi JM, Thiaudiere E and Mellet P: In vivo Overhauser-enhanced MRI of proteolytic activity. Contrast Media Mol Imaging. 9:363–371. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Dobrynin S, Kutseikin S, Morozov D, Krumkacheva O, Spitsyna A, Gatilov Y, Silnikov V, Angelovski G, Bowman MK, Kirilyuk I and Chubarov A: Human serum albumin labelled with sterically-hindered nitroxides as potential MRI contrast agents. Molecules. 25:17092020. View Article : Google Scholar : PubMed/NCBI | |
|
Boudries D, Massot P, Parzy E, Seren S, Mellet P, Franconi JM, Miraux S, Bezançon E, Marque SRA, Audran G, et al: A system for in vivo on-demand ultra-low field Overhauser-enhanced 3D-magnetic resonance imaging. J Magn Reson. 348:1073832023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhelev Z, Bakalova R, Aoki I, Matsumoto K, Gadjeva V, Anzai K and Kanno I: Nitroxyl radicals for labeling of conventional therapeutics and noninvasive magnetic resonance imaging of their permeability for blood-brain barrier: Relationship between structure, blood clearance, and MRI signal dynamic in the brain. Mol Pharm. 6:504–512. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Haugland MM, Lovett JE and Anderson EA: Advances in the synthesis of nitroxide radicals for use in biomolecule spin labelling. Chem Soc Rev. 47:668–680. 2018. View Article : Google Scholar | |
|
Akakuru OU, Iqbal MZ, Saeed M, Liu C, Paunesku T, Woloschak G, Hosmane NS and Wu A: The transition from metal-based to metal-free contrast agents for T1 magnetic resonance imaging enhancement. Bioconjug Chem. 30:2264–2286. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Ding Y, Ge M, Zhang C, Yu J, Xia D, He J and Jia Z: Platelets as delivery vehicles for targeted enrichment of NO• to cerebral glioma for magnetic resonance imaging. J Nanobiotechnology. 21:4992023. View Article : Google Scholar | |
|
Brasch R: Work in progress: methods of contrast enhancement for NMR imaging and potential applications. A subject review. Radiology. 147:781–788. 1983. View Article : Google Scholar : PubMed/NCBI | |
|
Hou Y, Kong F, Tang Z, Zhang R, Li D, Ge J, Yu Z, Wahab A, Zhang Y, Iqbal MZ and Kong X: Nitroxide radical conjugated ovalbumin theranostic nanosystem for enhanced dendritic cell-based immunotherapy and T1-magnetic resonance imaging. J Control Release. 373:547–563. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Rohrer M, Bauer H, Mintorovitch J, Requardt M and Weinmann HJ: Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 40:715–724. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Xu W, Su Y, Ma Y, Wei Q, Yang J, Zhuang X, Ding J and Chen X: Immunologically effective poly (D-lactic acid) nanoparticle enhances anticancer immune response. Sci China Chem. 66:1150–1160. 2023. View Article : Google Scholar | |
|
Keana JF, Pou S and Rosen GM: Nitroxides as potential contrast enhancing agents for MRI application: Influence of structure on the rate of reduction by rat hepatocytes, whole liver homogenate, subcellular fractions, and ascorbate. Magn Reson Med. 5:525–536. 1987. View Article : Google Scholar : PubMed/NCBI | |
|
Muir BW, Acharya DP, Kennedy DF, Mulet X, Evans RA, Pereira SM, Wark KL, Boyd BJ, Nguyen TH, Hinton TM, et al: Metal-free and MRI visible theranostic lyotropic liquid crystal nitroxide-based nanoparticles. Biomaterials. 33:2723–2733. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Bye N, Hutt OE, Hinton TM, Acharya DP, Waddington LJ, Moffat BA, Wright DK, Wang HX, Mulet X and Muir BW: Nitroxide-loaded hexosomes provide MRI contrast in vivo. Langmuir. 30:8898–8906. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Chen C, Kang N, Xu T, Wang D, Ren L and Guo X: Core-shell hybrid upconversion nanoparticles carrying stable nitroxide radicals as potential multifunctional nanoprobes for upconversion luminescence and magnetic resonance dual-modality imaging. Nanoscale. 7:5249–5261. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Akakuru OU, Xu C, Liu C, Li Z, Xing J, Pan C, Li Y, Nosike EI, Zhang Z, Iqbal ZM, et al: Metal-free organo-theranostic nanosystem with high nitroxide stability and loading for image-guided targeted tumor therapy. ACS Nano. 15:3079–3097. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Utsumi H, Yamada KI, Ichikawa K, Sakai K, Kinoshita Y, Matsumoto S and Nagai M: Simultaneous molecular imaging of redox reactions monitored by Overhauser-enhanced MRI with 14N- and 15N-labeled nitroxyl radicals. Proc Natl Acad Sci USA. 103:1463–1468. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Letyagin AY, Sorokina KN, Tolstikova TG, Zhukova NA, Popova NA, Fursova EY, Savelov AA and Ovcharenko VI: Evaluation of magnetic resonance imaging characteristics of new nitroxyl radicals on the model of RLS lymphoma. Bull Exp Biol Med. 143:240–243. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Ahn KH, Scott G, Stang P, Conolly S and Hristov D: Multiparametric imaging of tumor oxygenation, redox status, and anatomical structure using Overhauser-enhanced MRI-prepolarized MRI system. Magn Reson Med. 65:1416–1422. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Parzy E, Bouchaud V, Massot P, Voisin P, Koonjoo N, Moncelet D, Franconi JM, Thiaudière E and Mellet P: Overhauser-enhanced MRI of elastase activity from in vitro human neutrophil degranulation. PLoS One. 8:e579462013. View Article : Google Scholar : PubMed/NCBI | |
|
Zhelev Z, Aoki I, Gadjeva V, Nikolova B, Bakalova R and Saga T: Tissue redox activity as a sensing platform for imaging of cancer based on nitroxide redox cycle. Eur J Cancer. 49:1467–1478. 2013. View Article : Google Scholar | |
|
Babić N, Orio M and Peyrot F: Unexpected rapid aerobic transformation of 2,2,6,6-tetraethyl-4-oxo(piperidin-1-yloxyl) radical by cytochrome P450 in the presence of NADPH: Evidence against a simple reduction of the nitroxide moiety to the hydroxylamine. Free Radic Biol Med. 156:144–156. 2020. View Article : Google Scholar | |
|
Maryunina K, Letyagin G, Romanenko G, Bogomyakov A, Morozov V, Tumanov S, Veber S, Fedin M, Saverina E, Syroeshkin M, et al: 2-imidazoline nitroxide derivatives of cymantrene. Molecules. 27:75452022. View Article : Google Scholar : PubMed/NCBI | |
|
Dobrynin SA, Gulman MM, Morozov DA, Zhurko IF, Taratayko AI, Sotnikova YS, Glazachev YI, Gatilov YV and Kirilyuk IA: Synthesis of sterically shielded nitroxides using the reaction of nitrones with alkynylmagnesium bromides. Molecules. 27:76262022. View Article : Google Scholar : PubMed/NCBI | |
|
Keshari KR and Wilson DM: Chemistry and biochemistry of 13C hyperpolarized magnetic resonance using dynamic nuclear polarization. Chem Soc Rev. 43:1627–1659. 2014. View Article : Google Scholar : | |
|
Woitek R and Gallagher FA: The use of hyperpolarised 13C-MRI in clinical body imaging to probe cancer metabolism. Br J Cancer. 124:1187–1198. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Ardenkjær-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M and Golman K: Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc Natl Acad Sci USA. 100:10158–10163. 2003. View Article : Google Scholar | |
|
Vander Heiden MG, Cantley LC and Thompson CB: Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Woitek R, McLean MA, Gill AB, Grist JT, Provenzano E, Patterson AJ, Ursprung S, Torheim T, Zaccagna F, Locke M, et al: Hyperpolarized 13C MRI of tumor metabolism demonstrates early metabolic response to neoadjuvant chemotherapy in breast cancer. Radiol Imaging Cancer. 2:e2000172020. View Article : Google Scholar | |
|
Gallagher FA, Woitek R, McLean MA, Gill AB, Manzano Garcia R, Provenzano E, Riemer F, Kaggie J, Chhabra A, Ursprung S, et al: Imaging breast cancer using hyperpolarized carbon-13 MRI. Proc Natl Acad Sci USA. 117:2092–2098. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Golman K, Zandt RI, Lerche M, Pehrson R and Ardenkjaer-Larsen JH: Metabolic imaging by hyperpolarized 13C magnetic resonance imaging for in vivo tumor diagnosis. Cancer Res. 66:10855–10860. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Miller JJ, Lau AZ, Nielsen PM, McMullen-Klein G, Lewis AJ, Jespersen NR, Ball V, Gallagher FA, Carr CA, Laustsen C, et al: Hyperpolarized [1,4-13C2]fumarate enables magnetic resonance-based imaging of myocardial necrosis. JACC Cardiovasc Imaging. 11:1594–1606. 2018. View Article : Google Scholar : | |
|
Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, et al: Metabolic imaging of patients with prostate cancer using hyperpolarized [1-¹3C]pyruvate. Sci Transl Med. 5:198ra1082013. View Article : Google Scholar | |
|
Nantogma S, de Maissin H, Adelabu I, Abdurraheem A, Nelson C, Chukanov NV, Salnikov OG, Koptyug IV, Lehmkuhl S, Schmidt AB, et al: Carbon-13 radiofrequency amplification by stimulated emission of radiation of the hyperpolarized ketone and hemiketal forms of Allyl [1-13C]pyruvate. ACS Sens. 9:770–780. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Gierse M, Nagel L, Keim M, Lucas S, Speidel T, Lobmeyer T, Winter G, Josten F, Karaali S, Fellermann M, et al: Parahydrogen-polarized fumarate for preclinical in vivo metabolic magnetic resonance imaging. J Am Chem Soc. 145:5960–5969. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Cavallari E, Carrera C, Sorge M, Bonne G, Muchir A, Aime S and Reineri F: The 13C hyperpolarized pyruvate generated by ParaHydrogen detects the response of the heart to altered metabolism in real time. Sci Rep. 8:83662018. View Article : Google Scholar | |
|
Bhattacharya P, Chekmenev EY, Reynolds WF, Wagner S, Zacharias N, Chan HR, Bünger R and Ross BD: Parahydrogen-induced polarization (PHIP) hyperpolarized MR receptor imaging in vivo: A pilot study of 13C imaging of atheroma in mice. NMR Biomed. 24:1023–1028. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Hövener JB, Pravdivtsev AN, Kidd B, Bowers CR, Glöggler S, Kovtunov KV, Plaumann M, Katz-Brull R, Buckenmaier K, Jerschow A, et al: Parahydrogen-based hyperpolarization for biomedicine. Angew Chem Int Ed Engl. 57:11140–11162. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Fries LM, Hune TLK, Sternkopf S, Mamone S, Schneider KL, Schulz-Heddergott R, Becker D and Glöggler S: Real-time metabolic magnetic resonance spectroscopy of pancreatic and colon cancer tumor-xenografts with parahydrogen hyperpolarized 1-13C Pyruvate-d3. Chemistry. 30:e2024001872024. View Article : Google Scholar | |
|
Shchepin RV, Pham W and Chekmenev EY: Dephosphorylation and biodistribution of 1-¹3C-phospholactate in vivo. J Labelled Comp Radiopharm. 57:517–524. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Chaumeil MM, Larson PE, Yoshihara HA, Danforth OM, Vigneron DB, Nelson SJ, Pieper RO, Phillips JJ and Ronen SM: Non-invasive in vivo assessment of IDH1 mutational status in glioma. Nat Commun. 4:24292013. View Article : Google Scholar : PubMed/NCBI | |
|
Canapè C, Catanzaro G, Terreno E, Karlsson M, Lerche MH and Jensen PR: Probing treatment response of glutaminolytic prostate cancer cells to natural drugs with hyperpolarized [5-(13) C] glutamine. Magn Reson Med. 73:2296–2305. 2015. View Article : Google Scholar | |
|
Mu C, Liu X, Kim Y, Riselli A, Korenchan DE, Bok RA, Delos Santos R, Sriram R, Qin H, Nguyen H, et al: Clinically translatable hyperpolarized 13C bicarbonate pH imaging method for use in prostate cancer. ACS Sens. 8:4042–4054. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Düwel S, Hundshammer C, Gersch M, Feuerecker B, Steiger K, Buck A, Walch A, Haase A, Glaser SJ, Schwaiger M and Schilling F: Imaging of pH in vivo using hyperpolarized 13C-labelled zymonic acid. Nat Commun. 8:151262017. View Article : Google Scholar | |
|
Coffey AM, Feldman MA, Shchepin RV, Barskiy DA, Truong ML, Pham W and Chekmenev EY: High-resolution hyperpolarized in vivo metabolic 13C spectroscopy at low magnetic field (48.7mT) following murine tail-vein injection. J Magn Reson. 281:246–252. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Park I, von Morze C, Lupo JM, Ardenkjaer-Larsen JH, Kadambi A, Vigneron DB and Nelson SJ: Investigating tumor perfusion by hyperpolarized 13C MRI with comparison to conventional gadolinium contrast-enhanced MRI and pathology in orthotopic human GBM xenografts. Magn Reson Med. 77:841–847. 2017. View Article : Google Scholar | |
|
Von Morze C, Larson PEZ, Hu S, Yoshihara HA, Bok RA, Goga A, Ardenkjaer-Larsen JH and Vigneron DB: Investigating tumor perfusion and metabolism using multiple hyperpolarized (13)C compounds: HP001, pyruvate and urea. Magn Reson Imaging. 30:305–311. 2012. View Article : Google Scholar | |
|
Von Morze C, Bok RA, Reed GD, Ardenkjaer-Larsen JH, Kurhanewicz J and Vigneron DB: Simultaneous multiagent hyperpolarized (13)C perfusion imaging. Magn Reson Med. 72:1599–1609. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Laustsen C, Nielsen PM, Qi H, Løbner MH, Palmfeldt J and Bertelsen LB: Hyperpolarized [1,4-13C]fumarate imaging detects microvascular complications and hypoxia mediated cell death in diabetic nephropathy. Sci Rep. 10:96502020. View Article : Google Scholar | |
|
Schmidt AB, Berner S, Braig M, Zimmermann M, Hennig J, von Elverfeldt D and Hövener JB: In vivo 13C-MRI using SAMBADENA. PLoS One. 13:e02001412018. View Article : Google Scholar : PubMed/NCBI | |
|
Svensson J, Månsson S, Johansson E, Petersson JS and Olsson LE: Hyperpolarized 13C MR angiography using trueFISP. Magn Reson Med. 50:256–262. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Allouche-Arnon H, Wade T, Waldner LF, Miller VN, Gomori JM, Katz-Brull R and McKenzie CA: In vivo magnetic resonance imaging of glucose-initial experience. Contrast Media Mol Imaging. 8:72–82. 2013. View Article : Google Scholar | |
|
Flori A, Liserani M, Frijia F, Giovannetti G, Lionetti V, Casieri V, Positano V, Aquaro GD, Recchia FA, Santarelli MF, et al: Real-time cardiac metabolism assessed with hyperpolarized [1-(13) C]acetate in a large-animal model. Contrast Media Mol Imaging. 10:194–202. 2015. View Article : Google Scholar | |
|
Magnusson P, Johansson E, Månsson S, Petersson JS, Chai CM, Hansson G, Axelsson O and Golman K: Passive catheter tracking during interventional MRI using hyperpolarized 13C. Magn Reson Med. 57:1140–1147. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
McBride SJ, MacCulloch K, TomHon P, Browning A, Meisel S, Abdulmojeed M, Goodson BM, Chekmenev EY and Theis T: Carbon-13 hyperpolarization of α-ketocarboxylates with parahydrogen in reversible exchange. CChemMedChem. 20:e2024003782025. View Article : Google Scholar | |
|
Roig ES, Magill AW, Donati G, Meyerspeer M, Xin L, Ipek O and Gruetter R: A double-quadrature radiofrequency coil design for proton-decoupled carbon-13 magnetic resonance spectroscopy in humans at 7T. Magn Reson Med. 73:894–900. 2015. View Article : Google Scholar | |
|
Von Morze C, Tropp J, Chen AP, Marco-Rius I, Van Criekinge M, Skloss TW, Mammoli D, Kurhanewicz J, Vigneron DB, Ohliger MA and Merritt ME: Sensitivity enhancement for detection of hyperpolarized 13C MRI probes with 1H spin coupling introduced by enzymatic transformation in vivo. Magn Reson Med. 80:36–41. 2018. View Article : Google Scholar | |
|
Chapman B, Joalland B, Meersman C, Ettedgui J, Swenson RE, Krishna MC, Nikolaou P, Kovtunov KV, Salnikov OG, Koptyug IV, et al: Low-cost high-pressure clinical-scale 50% parahydrogen generator using liquid nitrogen at 77 K. Anal Chem. 93:8476–8483. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Schmidt AB, Zimmermann M, Berner S, de Maissin H, Müller CA, Ivantaev V, Hennig J, Elverfeldt DV and Hövener JB: Quasi-continuous production of highly hyperpolarized carbon-13 contrast agents every 15 sec within an MRI system. Commun Chem. 5:212022. View Article : Google Scholar | |
|
Shchepi n RV, Coffey AM, Waddell K and Wand Chekmenev EY: Parahydrogen induced polarization of 1-(13)C-phospholactate-d(2) for biomedical imaging with >30,000,000-fold NMR signal enhancement in water. Anal Chem. 86:5601–5605. 2014. View Article : Google Scholar | |
|
Johansson E, Månsson S, Wirestam R, Svensson J, Petersson JS, Golman K and Ståhlberg F: Cerebral perfusion assessment by bolus tracking using hyperpolarized 13C. Magn Reson Med. 51:464–472. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Grant AK, Vinogradov E, Wang X, Lenkinski RE and Alsop DC: Perfusion imaging with a freely diffusible hyperpolarized contrast agent. Magn Reson Med. 66:746–755. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Allouche-Arnon H, Gamliel A, Barzilay CM, Nalbandian R, Gomori JM, Karlsson M, Lerche MH and Katz-Brull R: A hyperpolarized choline molecular probe for monitoring acetylcholine synthesis. Contrast Media Mol Imaging. 6:139–147. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Coffey AM, Shchepin RV, Truong ML, Wilkens K, Pham W and Chekmenev EY: Open-source automated parahydrogen hyperpolarizer for molecular imaging using (13)C metabolic contrast agents. Anal Chem. 88:8279–8288. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Qi H, Mariager CØ, Nielsen PM, Schroeder M, Lindhardt J, Nørregaard R, Klein JD, Sands JM and Laustsen C: Glucagon infusion alters the hyperpolarized 13C-urea renal hemodynamic signature. NMR Biomed. 32:e40282019. View Article : Google Scholar | |
|
Colombo Serra S, Karlsson M, Giovenzana GB, Cavallotti C, Tedoldi F and Aime S: Hyperpolarized (13) C-labelled anhydrides as DNP precursors of metabolic MRI agents. Contrast Media Mol Imaging. 7:469–477. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Coffey AM, Kovtunov KV, Barskiy DA, Koptyug IV, Shchepin RV, Waddell KW, He P, Groome KA, Best QA, Shi F, et al: High-resolution low-field molecular magnetic resonance imaging of hyperpolarized liquids. Anal Chem. 86:9042–9049. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Waddell KW, Coffey AM and Chekmenev EY: In situ detection of PHIP at 48 mT: Demonstration using a centrally controlled polarizer. J Am Chem Soc. 133:97–101. 2011. View Article : Google Scholar : | |
|
Roth M, Koch A, Kindervater P, Bargon J, Spiess HW and Münnemann K: (13)C hyperpolarization of a barbituric acid derivative via parahydrogen induced polarization. J Magn Reson. 204:50–55. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Berner S, Schmidt AB, Zimmermann M, Pravdivtsev AN, Glöggler S, Hennig J, von Elverfeldt D and Hövener JB: SAMBADENA hyperpolarization of 13C-succinate in an MRI: Singlet-triplet mixing causes polarization loss. ChemistryOpen. 8:728–736. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Deen SS, Rooney C, Shinozaki A, McGing J, Grist JT, Tyler DJ, Serrão E and Gallagher FA: Hyperpolarized carbon 13 MRI: Clinical applications and future directions in oncology. Radiol Imaging Cancer. 5:e2300052023. View Article : Google Scholar : PubMed/NCBI | |
|
Vinogradov E, Keupp J, Dimitrov IE, Seiler S and Pedrosa I: CEST-MRI for body oncologic imaging: Are we there yet? NMR Biomed. 36:e49062023. View Article : Google Scholar : PubMed/NCBI |