Inextricable association of connective tissue disease with B‑cell lymphoma (Review)
- Authors:
- Published online on: April 1, 2025 https://doi.org/10.3892/mco.2025.2843
- Article Number: 48
-
Copyright: © Ruan et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
1. Introduction
Non-Hodgkin lymphoma (NHL) represents a group of malignancies that are histologically and genetically diverse, originating from B- and T-lymphocytes (1). Connective tissue disease (CTD) encompasses a diverse range of autoimmune disorders driven by B cells or T cells, such as systemic sclerosis, rheumatoid arthritis (RA), Sjögren's syndrome (SS), systemic lupus erythematosus (SLE), polymyositis, dermatomyositis and mixed CTD (2). RA, SS and SLE, characterized by B-cell responses, are known to be linked with an elevated risk of diffuse large B-cell lymphoma and marginal zone lymphoma (MZL). Conversely, CTDs driven by T-cell responses, such as dermatomyositis/polymyositis, inflammatory bowel disease, multiple sclerosis, psoriasis and sarcoidosis, are also thought to be associated with peripheral T-cell lymphoma (3). This association of CTD with lymphoproliferative diseases is in two ways: Autoimmune diseases are often accompanied by a higher incidence of lymphoid neoplasms and individuals with lymphocytic malignancies exhibit higher rates of autoimmune disorders (4,5). According to a descriptive epidemiological study of autoimmune diseases associated with malignant lymphoma by Váróczy et al (6), autoimmunity precedes a lymphoma diagnosis in NHL, whereas autoimmunity primarily emerges following the treatment of the malignancy in Hodgkin's lymphoma.
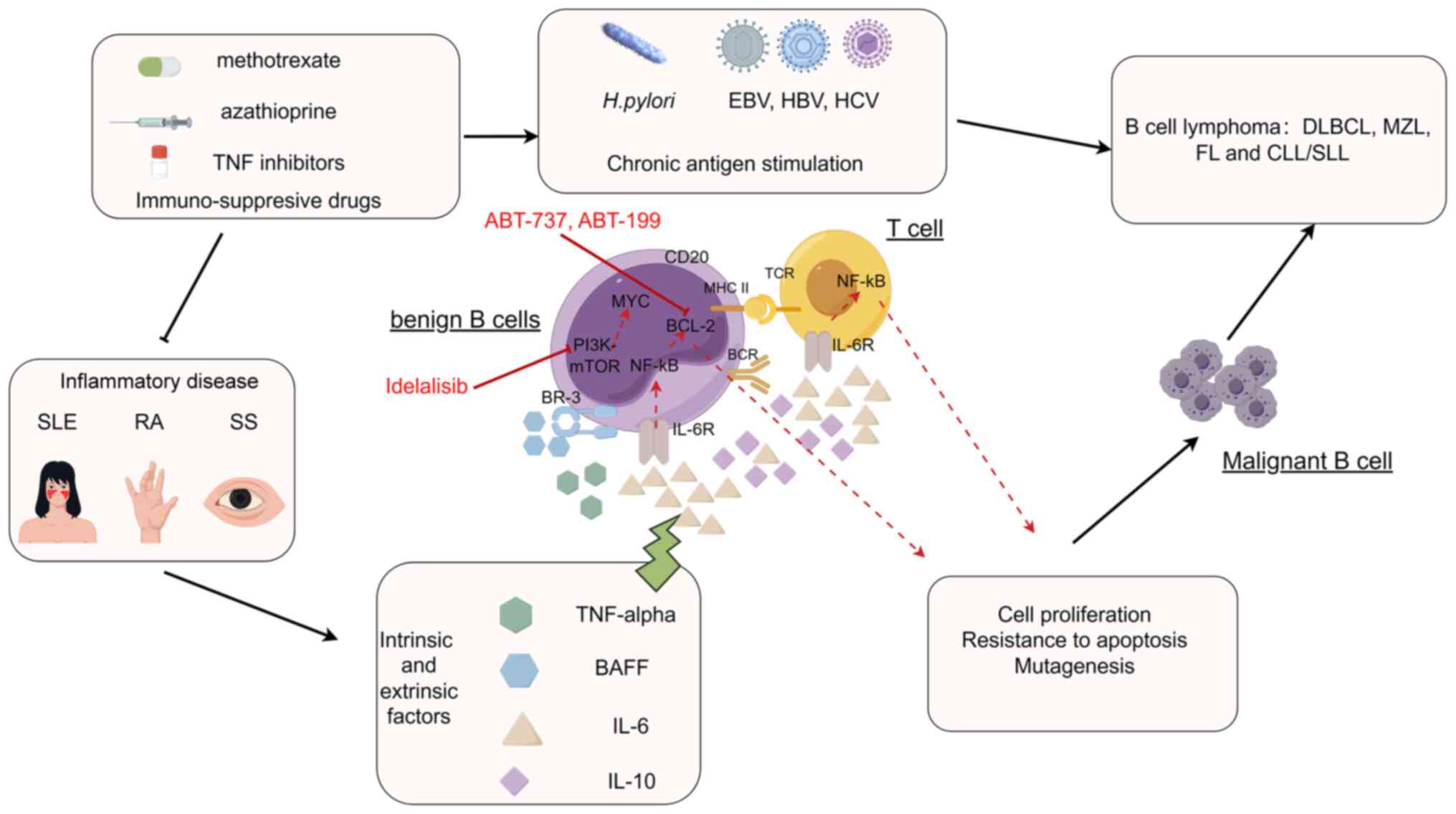
The relationships between the risk of NHL and particular autoimmune conditions may not be universal, but instead influenced by distinct NHL subtypes. Particularly, specific NHL subtypes show notable positive correlations, with a primary emphasis on diffuse large B-cell lymphoma (linked to RA, SS, SLE and celiac disease), as well as MZL (associated with SS), lymphoplasmacytic lymphoma (linked to RA) and T-cell lymphomas (connected to celiac disease) (7). In terms of the anatomical site of extranodal NHL, a significantly elevated risk of gastrointestinal lymphoma was noted in connection with celiac disease, while there was a higher occurrence of parotid NHL in cases of primary SS (pSS) (8,9). The present review consolidates disease-related, environmental and genetic risk factors, along with the suggested role of immunosuppressive medications or specific viral infections, notably Epstein-Barr virus (EBV), as potential factors that may contribute to the progression towards lymphoma development in individuals with autoimmune disorders. Given the critical role of B-cell and T-cell activation in autoimmune and lymphoma pathogeneses, chronic inflammation and antigenic stimulation are potential factors contributing to the development of lymphoma in this scenario.
New insights into the pathogenesis of autoimmune and lymphoma along with the concept of a strong association between the two have been reinforced by the emergence of innovative treatments. This review focuses on the link between CTD and B-cell lymphoma. The association between lymphomas and common CTDs is discussed in the subsequent chapters, following closely behind several mechanisms for this association and recent advances in therapies for patients with these CTDs and haematological malignancies.
2. Association between autoimmune diseases and B-cell lymphoma
Numerous studies have been carried out to unveil the connection between CTDs and lymphoma. In the literature, RA, SLE and SS have been extensively investigated, revealing a significant positive association between these CTDs and lymphoma (Table I).
Table IStandardized incidence ratio of lymphoma in SLE, RA and pSS reported in selected cohort studies. |
SLE
The autoimmune disease SLE has the potential to impact various organs, such as the skin, joints, central nervous system and kidneys (10). Factors such as genetic predisposition and environmental stimuli, as well as activation of the innate immune system and the adaptive immune system, contribute to determining the risk of SLE in the general population. Over the past 30 years, data have shown a higher risk of NHL in patients with SLE (11). The likelihood of haematologic malignancy in patients with SLE is two to three times higher than that in the general population. An increased occurrence of malignancies has been validated in individuals with SLE through a cross-sectional population-based study (odds ratio, 95% CI: 3.02-3.72) (12). The results of other cohort studies corroborate this association [Pettersson et al (13), 95% CI: 11.9-111.0; Cibere et al (14), 95% CI: 1.9-18.0; Sweeney et al (15), 95% CI: 0.25-55.7; Abu-Shakra et al (16), 95% CI: 1.1-15.7; and Mellemkler et al, (17) 95% CI: 2.2-10.3]. In mainly Caucasian cohorts, the clinical case study by King and Costenbader (18) showed that the predominant form of lymphoma in SLE is diffuse large B lymphoma, with an incidence of ~64%.
RA
RA is characterized as a chronic, progressive inflammatory condition that presents as symmetric polyarthritis affecting both small and large joints, potentially resulting in joint and periarticular structural harm and systemic inflammation (19). Swedish (20), Finnish (21) and Danish (22) population-based cohort studies provide evidence for an increased risk for NHL, Hodgkin's lymphoma and lung cancer, while the risk of colorectal cancer and female breast cancer was decreased. Thus, clinicians treating patients with rheumatoid disease cannot exclude the heightened risk of haematologic malignancies linked with it.
According to a Finnish cohort study of 9,469 patients with RA, patients with RA exhibited a higher incidence of NHL compared to the general population [standardized incidence ratio (SIR)=2.2] (23). The results of other cohort studies corroborate this association [Prior (24), 95% CI: 11.5-50.6; Kauppi et al (23), 95% CI: 1.5-3.1; Hakulinen et al (25), 95% CI: 2.0-3.9; Gridley et al (20), 95% CI: 1.3-2.6; Mellemkjaer et al (22), 95% CI: 1.9-2.9; Silman et al (26), 95% CI: 3.8-26.6; Mariette et al (27), 95% CI: 0.7-1.9; and Kinlen (28), 95% CI: 4.8-34.4].
pSS
SS is a chronic inflammatory and autoimmune disorder with distressing symptoms of dry mouth and dry eyes (29). The disease exists in two forms: PSS and secondary SS (sSS). According to the American College of Rheumatology/European League Against Rheumatism Classification Criteria for pSS from 2016, diagnosis relies on ruling out other systemic CTDs and conditions that can mimic pSS, such as active hepatitis, IgG4-related disease or sarcoidosis (30). In essence, the diagnosis of sSS involves the presence of SS symptoms alongside other systemic CTDs. Nevertheless, identification of sSS commonly relies on dryness symptoms in conjunction with other CTDs, leading to a highly subjective sSS diagnosis. Sebastian et al (31) proposed the necessity of performing labial salivary gland biopsy in all suspected sSS cases for confirmation of diagnosis. In general, the physical findings linked with a heightened risk of lymphoma-parotid enlargement, splenomegaly and lymphadenopathy-are associated with an increased risk of lymphoma in patients with SS (32).
Among autoimmune connective tissue diseases, lymphomas are most commonly observed in patients with SS (33), with a relative risk estimated to be 4 to 40 times greater than that in the general population (32,34). In a large cohort of patients, Kauppi et al (23) reported that the risk ratio for developing NHL was 8.7 in patients with pSS, compared to 4.5 in cases of sSS. Among the 676 patients with pSS studied, half were diagnosed with NHL. The results of other cohort studies corroborate this association [Kauppi et al (23), 95% CI 4.3-15.5; Kassan et al (32), 95% CI 16.7-118.4; Pertovaara et al (35), 95% CI 2.7-3.8].
3. Risk factors for lymphoma development in autoimmune conditions
Potential mechanisms for the progression of autoimmune diseases to lymphoma encompass the dysregulation of B cells linked to persistent antigenic stimulation, shared genetic or environmental factors, the level of inflammatory response in the disease, concurrent viral infections such as EBV and external factors including the use of cytotoxic or immunosuppressive agents (5,36) (Fig. 1). Nevertheless, research by Shaffer et al (37) suggests that a history of autoimmune disease in the family does not serve as a reliable indicator of the risk of developing lymphoma. Similarly, a study by Ekström et al (38) on relatives of individuals with RA did not find a higher likelihood of developing lymphoma. Conclusive findings have not been reached in studies investigating the risk of lymphoma post-autoimmune therapies (cytotoxicity and immunosuppressive application resulting in iatrogenic deficiency). In patients with severe immunodeficiency, congenital, iatrogenic or acquired immune deficiency, NHL is frequently attributed to widespread EBV infection. Overall, patients with congenital or acquired immunodeficiency have an incidence of NHL that is 50 times higher or more than that in the general population (39), which may be because immune deficiency results in chronic antigen triggering of lymphatic hyperplasia and development of lymphoma. Within the patients with pSS, the emergence of lymphoma seems to be closely connected to the abnormal stimulation of B-cells or the compromised repair mechanisms, and stronger disease activity or more persistent disease may be a sign of increased likelihood of lymphoma development in individuals diagnosed with RA and SLE.
Adequate clinical data exist to establish an association between CTD and NHL, yet the molecular mechanisms underlying this association have not been thoroughly explored. Peng et al (40) employed bioinformatics analysis to investigate the shared molecular genetic mechanisms between diffuse large B-cell lymphoma (DLBCL) and SLE. The analysis revealed that the NF-κB pathway is highly activated, representing a common pathophysiological characteristic of both SLE and lymphoma. The four core shared genes identified, including proteasome 20S subunit β10 (PSMB10), PSMB4, TATA-box binding protein associated factor 10 and NF-κB inhibitor α (IκBα), exhibited significant upregulation of DNA damage and inflammatory factors (such as IL-6, IL-10, TNF, etc.), thereby activating the NF-κB pathway through the proteasomal degradation of IκBα in both diseases. The activation of the NF-κB pathway induces BAFF production, facilitating immune evasion and contributing significantly to the pathogenesis and progression of both SLE and DLBCL (40).
Likewise, in their study, Li et al (41) employed a bioinformatics analysis to investigate the shared molecular genetic mechanisms between DLBCL and RA. The core gene of utmost significance, galectin 2 (LGALS2), was identified through screening. Functional enrichment analysis revealed that LGALS2 was found to be involved in regulating various immune cells, particularly CD4 T cells. LGALS2 is additionally implicated in the inflammatory response, the complement pathway and the interferon signaling pathway. One potential mechanism underlying the susceptibility of patients with RA to DLBCL involves LGALS2's involvement in DLBCL development by generating numerous inflammatory factors and autoantibodies. This process can result in sustained chronic inflammation and B-cell hyperactivation. Enrichment analyses further demonstrated that mitochondrial dysfunction is implicated in the modulation of transcriptional and apoptotic levels, along with p53-like mediators of intrinsic apoptotic signaling pathways. These processes are closely linked to the progression of diseases such as RA and DLBCL (41).
Role of chronic antigen stimulation of B cells
The dysregulation of B-cell differentiation may arise from extended or uncontrolled antigenic stimulation, leading to the development of lymphomas and certain autoimmune disorders. The stages of B-cell differentiation in lymphoid malignancies frequently bear similarities to the normal ones. In normal B-cell development and differentiation, the facilitation of antigen recognition is a critical function performed by the rearrangement of immunoglobulin genes in the early stages of B-cell precursor development. However, the recombination of immunoglobulin variable-(diversity)-joining genes, along with somatic hypermutation, can result in chromosomal translocations commonly observed in mature B-cell lymphomas. These translocations can cause dysregulation of the expression of critical proto-oncogenes such as myc, bcl-2 or bcl-6, impacting cell proliferation, differentiation and survival (42). Direct evidence for a pathogenic role of antigens was provided by research on gastric mucosa-assisted lymphoid tissue (MALT) lymphoma associated with H. pylori (43) and of lymphoma associated with the hepatitis C virus (44). EBV leads to extensive methylation of both the host and viral genome, which promotes viral persistence and propagation. The host mounts an ongoing immune response to microbes that generates an actively proliferating B-cell population, which may account for these phenomena. In the host body, early lymphomatous lesions may regress up to a certain point upon eradication of the antigen stimulus (45).
B-cell proliferation and autoantibody production are hallmarks of RA, pSS and SLE, as they lead to overstimulation and impaired apoptosis of B cells (46-48). DLBCL, MZL and lymphoplasmacytic lymphoma all stem from B cells that possess somatic hypermutations in the immunoglobulin variable region genes, which occur in the germinal center phase following exposure to T-cell-dependent antigens. Thus, the pattern of NHL subtypes observed supports the theory that both persistent B-cell stimulation and antigenic stimulation contribute to the development of autoimmunity-related lymphoma (49).
‘Cell-intrinsic’ and ‘cell-extrinsic’ factors. NF-κB signalling pathway
The NF-κB signaling pathway plays a crucial role in the pathogenesis of autoimmune diseases (such as SLE and RA) and B-cell lymphoma, regulating the proliferation and apoptosis of B cells and the development and activity of immune cells to maintain immune balance and acceptance, thus influencing immune homeostasis and tolerance. The NF-κB signaling pathway initiates transcriptional activation of genes that inhibit apoptosis, such as Bcl-2, A1 and Bcl-XL, leading to the survival and positive selection of cells that favour lymphomagenesis. Chronic antigens, EBV or unknown oncogenic alterations can activate the NF-κB pathway (37). High levels of NF-κB target genes are observed in activated B-cell-like diffuse large B-cell lymphoma (ABC DLBCLs), primary mediastinal B-cell lymphoma (PMBL) and MALT lymphoma, rendering them attractive therapeutic targets (50).
TNF-α. TNF-α is a crucial growth factor for lymphoma (particularly for DLBCL), RA, SLE and SS. In a meta-analysis, Wang et al (1) found that individuals with the AG/AA genotype of TNF-α exhibited a higher risk of B-cell-mediated autoimmune conditions contributing to the development of various subtypes of NHL [such as DLBCL, MZL, follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL)] compared to those with the GG genotype. This correlation was consistent across different B-cell NHL subtypes, such as MZL and FL (1). Elevated TNF-α levels result in the upregulation of antiapoptotic regulators and proinflammatory effectors through the NF-κB pathway, whereby imbalance of proapoptotic and antiapoptotic processes increases cell survival and promotes lymphomagenesis.
IL-6 and IL-10. Among the proinflammatory mediators, IL-6 and IL-10 enhance the growth and differentiation of lymphocytes and inhibit apoptosis by binding to their respective receptors. The signaling of proinflammatory cytokines is transduced by NF-κB, which functions as the central intracellular transducer. IL-6, a pleiotropic cytokine, regulates antibody production and inflammation. It has the ability to induce the expression of BCL-2, which in turn promotes the survival of immune cells activated by antigens (51,52). In RA, the IL-6 cytokine family stimulate synovial hyperplasia, leading to joint inflammation and causing damage to the underlying cartilage and bone. IL-6 can also potentiate MYC and BCL-XL, which may play a role in the initiation and rapid progression of B-cell lymphoma.
According to one study, the association between IL-6 polymorphisms and NHL risk may be linked to abnormalities in the type 2 T-helper cell (Th2) immune response, potentially enhancing the development of lymphoma (53). Changes in the Th cell response have also been linked to autoimmune disorders. SLE is associated with a Th2 response, while SS is characterized by high serum levels of both Th1 and Th2 cytokines (45). In conclusion, IL-6 is involved in the pathogenesis of CTDs and lymphoma, potentially by perturbing the equilibrium between Th1 and Th2 cell populations.
IL-10 is a multifunctional cytokine generated by Th2 cells, monocytes and macrophages, as well as normal and neoplastic B lymphocytes, and decreases the expression of Th1 cytokines to exert an immunosuppressive effect (54,55). B-cell lymphomas display a marked elevation in IL-10 levels, which act as an autocrine stimulator of tumor growth (56). According to Rothman et al (57), the presence of the IL10-3575T variant is linked to a higher susceptibility to B-cell lymphoma, particularly in cases of diffuse large B-cell lymphoma (P for trend=0.006). IL10 plays a crucial role in regulating the inflammatory response and maintaining the Th1/Th2 balance, and single nucleotide polymorphisms in the encoding gene may be susceptibility loci for NHL.
BAFF (BLyS). As a member of the TNF family of proteins, BAFF can bind to its receptor BR-3 and thereby regulate the survival and proliferation of B lymphocytes. This mechanism may be responsible for the pathogenic activation of B cells observed in a range of systemic autoimmune diseases and lymphomas. In addition, BAFF secretion can trigger a positive loop of activation that further stimulates B cells. In patients with RA, SLE and pSS, elevated levels of BAFF have been detected in serum or synovial fluid. These levels may be positively correlated with autoantibody levels, particularly in patients with pSS (58). In patients with NHL, lymphomatous B cells are known to secrete BAFF, which can cause autocrine activation in B cells regardless of whether autoimmunity is present (59). Novak et al (59) have also presented evidence indicating that BLyS is also expressed in tumors of patients diagnosed with NHL and that increased BLyS levels correlate with reduced median overall survival (OS) in patients with newly diagnosed large B-cell lymphoma. Thus, the mechanism associated with BAFF levels or function may be a common link between autoimmunity and lymphoma.
Immunosuppressive drugs: Controversial contributors to lymphoma development
The risk of lymphoproliferative disorders caused by immunosuppressant therapy is highly controversial. Studies have shown that nonbiological disease-modifying anti-rheumatic drugs (DMARDs), namely methotrexate (MTX) or azathioprine, increase the likelihood of NHL in patients with RA (60-62). This may be due to cytotoxicity itself, which relieves control of immune surveillance and inhibits immune defence against malignant cells (23). However, a study of cancer and RA epidemiology reported that this heightened risk does not seem to be linked to the usage of immunosuppressive medications (24), which is consistent with a cohort study by Symmons et al (63). Ekström Smedby et al (64) showed that the occurrence of NHL in patients with SLE and pSS remained unaffected by the administration of MTX or azathioprine. However, a potential increase in lymphoma risk associated with cyclophosphamide exposure was indicated in a multi-site SLE cohort (65). Such research can examine the independent effects of medication.
Since biological DMARDS (TNF inhibitors) were first introduced in the late 1990s, there has been apprehension regarding the elevated occurrence of lymphoma in patients receiving these inhibitors. Wolfe and Michaud (66) reported a slight rise in lymphoma cases among 18,572 patients with RA who underwent anti-TNF therapy, resulting in an SIR of 2.9 for the use of biologics (infliximab and etanercept). However, the higher rates of lymphoma observed in these patients with RA undergoing anti-TNF therapy may be attributed to channeling bias, where individuals with a greater predisposition to lymphoma tend to preferentially receive anti-TNF treatment. In a comprehensive meta-analysis of 18 randomized trials consisting of 8,808 patients with RA, no elevated risk of lymphoma was detected when the recommended doses of TNF antagonists were administered (67). A recent extensive pooled study conducted across 12 countries that involved nearly 30,000 subjects demonstrated that neither the use of DMARDs nor treatment with TNF inhibitors had any impact on the association between SS or SLE and the risk of developing various NHL subtypes (64). There are some explanations for these disputable findings, such as the limited duration of observation for TNF-blocking therapies, inadequate data on the anticipated lymphoma incidence among patients receiving TNF inhibitors, as well as considerations regarding disease severity and biases related to time dependency (68,69).
Hence, while it is challenging to dismiss the possibility that immunosuppressive drugs may contribute to lymphomagenesis, there is currently insufficient compelling evidence to support the notion that immunosuppressive therapy is the primary determiner of lymphoma development in individuals with autoimmune disorders.
Intensity of inflammatory disease
Autoimmune disorders encompass two fundamental elements within the broader term inflammatory activity: Continuous inflammation and continual stimulation of autoimmune B cells. These two underlying mechanisms can result in diverse forms of lymphoma. In RA, it is common to observe indications of both autoimmune B-cell activation (such as the presence of rheumatoid factor or anti-citrullinated protein autoantibodies) and systemic inflammation (elevated erythrocyte sedimentation rate or C-reactive protein levels). A case-control study conducted in Sweden offered compelling evidence for a link between inflammation activity in RA and the risk of developing lymphoma. The study revealed that patients with RA with the highest cumulative disease activity faced a 70-fold greater risk of lymphoma compared to those with minimal disease activity (60). Prolonged systemic inflammatory activity could contribute to the emergence of non-localized lymphoma, as a notable correlation was identified between elevated RA disease activity and DLBCL (60). Conversely, continuous activation of autoreactive B cells may play a more significant role in the development of lymphoma in organ-specific autoimmune conditions. As an illustration, the inflammatory response in SS primarily revolves around the sustained stimulation of autoimmune B cells, encompassing antinuclear antibody, anti-Sjogren syndrome A antibody (anti-SSA), anti-SSB or cryoglobulinaemia. In SS, marginal zone autoreactive B cells become active in salivary glands and other mucosal areas targeted by the autoimmune response, resulting in a higher incidence of extranodal marginal zone lymphomas such as MALT lymphomas and other marginal zone B-cell lymphomas (MZBCL) (70). In addition, certain studies have indicated a heightened likelihood of DLBCL development, a phenomenon that could be equally linked to the progression of indolent lymphoma, e.g. MZBCL (64,71,72).
Hence, differentiating between these two pathways will necessitate the creation of prospective patient cohorts to assess the predictive potential of systemic inflammatory markers and autoreactive B-cell markers for lymphoma onset (73).
4. Treatment of lymphoma
The treatment strategy for NHL involves a combination of chemotherapeutic radiotherapy. Cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) is the standard treatment for invasive lymphoma, with a 5-year progression-free survival (PFS) rate of 41-80%. For indolent lymphomas such as MZL, lymphoplasmacytic lymphoma and FL, chemoradiotherapy is effective but not well tolerated, and FC (fludarabine, cyclophosphamide) can be used. For cases of relapsed or treatment-resistant aggressive lymphoma, haematopoietic stem cell transplantation (HSCT) could be a viable therapeutic option. BCL6 has emerged as a potential novel target for patients diagnosed with DLBCL (74), the NF-κB pathway located in the nucleus and perturbed in the case of ABC DLBCL or PMBL, as well as respective components of the BCR activator of RhoGEF and GTPase (BCR) signalling pathway, namely Bruton's tyrosine kinase (BTK) or spleen tyrosine kinase (SYK) in cases of ABC DLBCL, and finally the PI3K-mTOR pathway, have demonstrated potential as promising therapeutic targets (75), and BCL2. Due to the high expression of BCL2 in both FL and MCL, targeting BCL2 could be a promising approach for these types of lymphoma. Utilizing novel compounds tailored to the specific biology of malignant lymphoma holds the potential to bring personalized treatments into clinical practice (76).
Classic treatment
The standard treatment for high-grade lymphoma is the CHOP regimen, which includes cyclophosphamide, doxorubicin, vincristine and prednisone (77). Over the past three decades, monoclonal antibody therapy using rituximab, which targets CD20, has emerged as a significant breakthrough in the management of lymphoproliferative disorders. The addition of rituximab, an anti-CD20 monoclonal antibody, to the standard CHOP regimen (R-CHOP) has significantly revolutionized the frontline treatment of patients diagnosed with DLBCL (78), leading to significant improvement in clinical outcomes for patients with invasive lymphoma, particularly in those with DLBCL and FL subtypes (79-82).
B-cell receptor signalling pathway inhibitors
The initiation and perpetuation of B-cell malignancies are closely related to the BCR complex and protein tyrosine kinases. An investigation was conducted on the BTK inhibitor ibrutinib among 70 patients with relapsed or refractory DLBCL, resulting in an objective response rate (ORR) of 40% (10/25), with a complete response rate of 8% (2/25) and partial response rate of 32% (8/25). Furthermore, it was found that patients with ABC DLBCL had a PFS of 5.5 months (83). In a separate phase II trial, 68 patients who received treatment with the SYK inhibitor fostamatinib demonstrated an ORR of 22% (5/23) for DLBCL, 10% (2/21) for FL, 55% (6/11) for SLL/CLL and 11% (1/9) for MCL (84). The disruption of BCR-mediated chemotaxis and adhesion of malignant cells to the tumor microenvironment results in an elevation of peripheral blood lymphocyte counts. Treatment with Ibrutinib heightens the likelihood of low-grade hemorrhage, presenting as contusions, petechiae and mild mucocutaneous bleeding. Subjects enrolled in clinical trials and treated with ibrutinib exhibited a 3- to 4-fold heightened risk of developing atrial fibrillation. In a single-arm phase 2 trial involving 111 patients with relapsed or refractory MCL, the primary adverse events associated with ibrutinib consisted of low-grade nonhematologic toxicities, such as diarrhea, malaise, fatigue, mild nausea, vomiting and decreased appetite, peripheral edema, dyspnea, constipation and upper respiratory tract infections. Neutropenia emerged as the most prevalent hematologic adverse event in the trial, with 16% of patients experiencing grade 3 or 4 neutropenia (85,86). Instances of invasive aspergillosis and Pneumocystis carinii pneumonia observed in patients undergoing ibrutinib treatment in clinical settings indicate an elevated susceptibility to opportunistic infections. This underscores the necessity of vigilance regarding the immunosuppressive effects of ibrutinib in clinical practice (86). The most frequently reported adverse events associated with fostamatinib included diarrhea, fatigue, cytopenia, nausea and hypertension. Hypertension induced by fostamatinib typically responds to a single antihypertensive medication within one month of starting the drug and resolves promptly upon discontinuation (85).
The PI3K-mTOR pathway
PI3Kδ is responsible for transmitting B-cell receptor signaling and supportive signals from the microenvironment that enhance the proliferation and viability of malignant B lymphocytes. A phase 2 clinical trial, conducted in a single-group, open-label setting, demonstrated the antitumor efficacy of idelalisib, an orally administered selective inhibitor of PI3Kδ, in patients with relapsed indolent NHL (75). mTOR is thought to have a critical role in supporting tumors driven by MYC in B lymphocytes (87,88). A phase 2 clinical trial conducted by the University of Chicago consortium revealed that the mTOR inhibitor temsirolimus administered as a single agent exhibited notable efficacy in patients with diffuse large B-cell lymphoma and FL (87). Adverse events associated with PI3K inhibitors are predominantly immune-mediated, with notable manifestations such as hepatotoxicity, pneumonia and colitis accompanied by severe diarrhea. In a clinical trial involving 72 patients with FL, the prevailing adverse effects associated with idelalisib comprised diarrhea, cough, fever, fatigue, malaise and nausea; the most prevalent severe (grade ≥3) adverse effects were diarrhea, pneumonia and fever (89).
BCL2 inhibitors
Proteins belonging to the antiapoptotic BCL-2 family, including Bcl-2, Bcl-XL, Bcl-w, Mcl-1, Bfl1/A-1 and Bcl-B, prevent cell death by binding to BH3-domain proapoptotic proteins (90). ABT-737 and its oral counterpart ABT-263 are designed to specifically target various antiapoptotic proteins within the BCL-2 family. In addition, ABT-199 exhibits potent and selective inhibition of BCL-2, leading to the sequestration of proapoptotic proteins and ultimately promoting the death of cancerous cells (91,92). Clinical trials were hindered by inadequate oral absorption of ABT-737, while ABT-263 was ceased due to thrombocytopenia limitations resulting from the targeted inhibition of BCL-XL (93). ABT-199 exhibited dose-limiting toxicity characterized by thrombocytopenia, and also had hematologic adverse effects, such as anemia or neutropenia. Around 28% of patients encountered a swift decrease in platelet counts (grades 3-5) (94,95). A phase 1 clinical trial comprising 106 patients with relapsed or refractory B-NHL was conducted, demonstrating the favorable tolerability of ABT-199, with gastrointestinal toxicity and neutropenia being the predominant adverse events. In the subsequent phase 2 segment of the trial (ABT-199+R-CHOP), increased rates of hematologic toxicity and infections were observed (95).
HSCT
High-dose chemotherapy followed by autologous HSC transplantation (auto-HCT) is now considered the preferred therapeutic approach for individuals experiencing a relapse of aggressive NHL (96). Auto-HCT has been shown to enhance survival rates among patients diagnosed with primary refractory or relapsed aggressive B-cell lymphoma and mantle cell lymphoma, as well as those suffering from relapsed FL or peripheral T-cell lymphoma (97). A prospective multicenter study investigating the cellular composition of autografts in NHL revealed that higher levels of CD34+ cells (>2.5x106/kg), CD34+CD133+CD38-counts (>0.08x106/kg) and infused CD3+CD4+ cell counts (>37x106/kg) were associated with improved PFS and OS. These findings suggest that the cellular composition of autografts may influence the outcomes of patients with NHL (98). In general, HSCT is a treatment possibility that could provide a survival advantage for these individuals.
5. Conclusion
This review aimed to explore the shared characteristics between CTDs and malignant lymphoma. Furthermore, the observed connections between particular autoimmune conditions and the likelihood of developing NHL indicate that these associations may be specific to certain NHL subtypes rather than being universally applicable. Currently, it is unnecessary to rely on extensive cohorts to establish the typical relative risk of lymphoma compared to the general populace. Instead, studies that combine genotypic and phenotypic data about the autoimmune disease and its treatment are preferable. Gene expression analysis can be employed to classify lymphoma subtypes and to evaluate the potential hazards posed by standard therapy. Based on recent research, it has been found that the deregulated immunological environment, characterized by B-cell activation, proliferation, chronic antigen stimulation and inflammation, plays a crucial role in the development of malignant cell clones. In ongoing clinical trials, several new compounds that target oncogenic signaling pathways are being investigated for their efficacy in patients with lymphoma. The utilization of these innovative compounds, in conjunction with an understanding of the lymphoma's underlying biology, holds the potential to enable personalized treatments tailored to individual patients, making it a plausible clinical reality.
Novel targeted therapies exhibit significant potential in treating both indolent and aggressive lymphomas, although overcoming the eventual emergence of drug resistance poses a persistent challenge. Future endeavors should encompass endeavors to comprehend and address mechanisms of resistance to BCR signaling pathway inhibitors, investigations into combined therapeutic approaches integrating BCR-targeted medications to refine therapeutic sequencing strategies, and the exploration of novel pharmaceutical agents. As the focus of treatment transitions towards prolonged administration of oral BCR pathway inhibitors, pharmacoeconomic evaluations of individual agents and potential combination protocols gain significance to guarantee continuous access to therapy. As additional medications enter the market, there will be a necessity to develop fresh payment and reimbursement strategies to ensure that the heightened expenses of these therapies stay within reach for both payers and patients with B-cell malignancies. This is essential to enable patients to maintain access to these effective and well-tolerated treatments. Additional future avenues involve addressing resistance to BTK inhibitors, employing combination therapies with BCR-targeted agents and investigating novel pharmaceuticals.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by grants from the Taishan Youth Scholar Foundation of Shandong Province (grant no. tsqn201812140), the Academic Promotion Program of Shandong First Medical University (grant no. 2019RC018) and the Natural Science Foundation of Shandong Province (grant nos. ZR2021MH319 and ZR2020MH112).
Availability of data and materials
Not applicable.
Authors' contributions
JSR contributed to writing the first manuscript draft and preparation of the figure. SX contributed to revising the manuscript. NNS conceived the review and revised the manuscript. All authors read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Wang SS, Vajdic CM, Linet MS, Slager SL, Voutsinas J, Nieters A, de Sanjose S, Cozen W, Alarcón GS, Martinez-Maza O, et al: Associations of non-Hodgkin lymphoma (NHL) risk with autoimmune conditions according to putative NHL loci. Am J Epidemiol. 181:406–421. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Cereser L, Passarotti E, De Pellegrin A, Patruno V, Poi ED, Marchesini F, Zuiani C and Girometti R: Chest high-resolution computed tomography in patients with connective tissue disease: Pulmonary conditions beyond ‘the usual suspects’. Curr Probl Diagn Radiol. 51:759–767. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Khanmohammadi S, Shabani M, Tabary M, Rayzan E and Rezaei N: Lymphoma in the setting of autoimmune diseases: A review of association and mechanisms. Crit Rev Oncol Hematol. 150(102945)2020.PubMed/NCBI View Article : Google Scholar | |
|
Stern M, Buser AS, Lohri A, Tichelli A and Nissen-Druey C: Autoimmunity and malignancy in hematology-more than an association. Crit Rev Oncol Hematol. 63:100–110. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Ehrenfeld M, Abu-Shakra M, Buskila D and Shoenfeld Y: The dual association between lymphoma and autoimmunity. Blood Cells Mol Dis. 27:750–756. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Váróczy L, Gergely L, Zeher M, Szegedi G and Illés A: Malignant lymphoma-associated autoimmune diseases-a descriptive epidemiological study. Rheumatol Int. 22:233–237. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Smedby KE, Hjalgrim H, Askling J, Chang ET, Gregersen H, Porwit-MacDonald A, Sundström C, Akerman M, Melbye M, Glimelius B and Adami HO: Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst. 98:51–60. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Mariette X: Lymphomas in patients with Sjögren's syndrome: Review of the literature and physiopathologic hypothesis. Leuk Lymphoma. 33:93–99. 1999.PubMed/NCBI View Article : Google Scholar | |
|
Cellier C, Delabesse E, Helmer C, Patey N, Matuchansky C, Jabri B, Macintyre E, Cerf-Bensussan N and Brousse N: Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French coeliac disease study group. Lancet. 356:203–208. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Kaul A, Gordon C, Crow MK, Touma Z, Urowitz MB, van Vollenhoven R, Ruiz-Irastorza G and Hughes G: Systemic lupus erythematosus. Nat Rev Dis Primers. 2(16039)2016.PubMed/NCBI View Article : Google Scholar | |
|
Bernatsky S, Ramsey-Goldman R, Rajan R, Boivin JF, Joseph L, Lachance S, Cournoyer D, Zoma A, Manzi S, Ginzler E, et al: Non-Hodgkin's lymphoma in systemic lupus erythematosus. Ann Rheum Dis. 64:1507–1509. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Azrielant S, Tiosano S, Watad A, Mahroum N, Whitby A, Comaneshter D, Cohen AD and Amital H: Correlation between systemic lupus erythematosus and malignancies: A cross-sectional population-based study. Immunol Res. 65:464–469. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Pettersson T, Pukkala E, Teppo L and Friman C: Increased risk of cancer in patients with systemic lupus erythematosus. Ann Rheum Dis. 51:437–439. 1992.PubMed/NCBI View Article : Google Scholar | |
|
Cibere J, Sibley J and Haga M: Systemic lupus erythematosus and the risk of malignancy. Lupus. 10:394–400. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Sweeney DM, Manzi S, Janosky J, Selvaggi KJ, Ferri W, Medsger TA Jr and Ramsey-Goldman R: Risk of malignancy in women with systemic lupus erythematosus. J Rheumatol. 22:1478–1482. 1995.PubMed/NCBI | |
|
Abu-Shakra M, Gladman DD and Urowitz MB: Malignancy in systemic lupus erythematosus. Arthritis Rheum. 39:1050–1054. 1996.PubMed/NCBI View Article : Google Scholar | |
|
Mellemkler L, Andersen V, Linet MS, Gridley G, Hoover R and Olsen JH: Non-Hodgkin's lymphoma and other cancers among a cohort of patients with systemic lupus erythematosus. Arthritis Rheum. 40:761–768. 1997.PubMed/NCBI View Article : Google Scholar | |
|
King JK and Costenbader KH: Characteristics of patients with systemic lupus erythematosus (SLE) and non-Hodgkin's lymphoma (NHL). Clin Rheumato. 26:1491–1494. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Cush JJ: Rheumatoid arthritis: Early diagnosis and treatment. Med Clin North Am. 105:355–365. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Gridley G, McLaughlin JK, Ekbom A, Klareskog L, Adami HO, Hacker DG, Hoover R and Fraumeni JF Jr: Incidence of cancer among patients with rheumatoid arthritis. J Natl Cancer Inst. 85:307–311. 1993.PubMed/NCBI View Article : Google Scholar | |
|
Isomäki HA, Hakulinen T and Joutsenlahti U: Excess risk of lymphomas, leukemia and myeloma in patients with rheumatoid arthritis. J Chron Dis. 31:691–696. 1978.PubMed/NCBI View Article : Google Scholar | |
|
Mellemkjaer L, Linet MS, Gridley G, Frisch M, Møller H and Olsen JH: Rheumatoid arthritis and cancer. Eur J Cancer. 32A:1753–1757. 1996.PubMed/NCBI View Article : Google Scholar | |
|
Kauppi M, Pukkala E and Isomäki H: Elevated incidence of hematologic malignancies in patients with Sjögren's syndrome compared with patients with rheumatoid arthritis (Finland). Cancer Causes Control. 8:201–204. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Prior P: Cancer and rheumatoid arthritis: epidemiologic considerations. Am J Med. 78:15–21. 1985.PubMed/NCBI View Article : Google Scholar | |
|
Hakulinen T, Isomaki H and Knekt P: Rheumatoid arthritis and cancer studies based on linking nationwide registries in Finland. Am J Med. 78:29–32. 1985.PubMed/NCBI View Article : Google Scholar | |
|
Silman AJ, Petrie J, Hazleman B and Evans SJ: Lymphoproliferative cancer and other malignancy in patients with rheumatoid arthritis treated with azathioprine: A 20 year follow up study. Ann Rheum Dis. 47:988–992. 1988.PubMed/NCBI View Article : Google Scholar | |
|
Mariette X, Cazals-Hatem D, Warszawki J, Liote F, Balandraud N and Sibilia J: Investigators of the Club Rhumatismes et Inflammation. Lymphomas in rheumatoid arthritis patients treated with methotrexate: A 3-year prospective study in France. Blood. 99:3909–3915. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Kinlen LJ: Incidence of cancer in rheumatoid arthritis and other disorders after immunosuppressive treatment. Am J Med. 78:44–49. 1985.PubMed/NCBI View Article : Google Scholar | |
|
Thorne I and Sutcliffe N: Sjögren's syndrome. Br J Hosp Med (Lond). 78:438–442. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, Rasmussen A, Scofield H, Vitali C, Bowman SJ, et al: 2016 American college of rheumatology/European league against rheumatism classification criteria for primary Sjögren's syndrome: A consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 69:35–45. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Sebastian A, Szachowicz A and Wiland P: Classification criteria for secondary Sjögren's syndrome. Current state of knowledge. Reumatologia. 57:277–280. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Kassan SS, Thomas TL, Moutsopoulos HM, Hoover R, Kimberly RP, Budman DR, Costa J, Decker JL and Chused TM: Increased risk of lymphoma in sicca syndrome. Ann Intern Med. 89:888–892. 1978.PubMed/NCBI View Article : Google Scholar | |
|
Váróczy L, Páyer E, Kádár Z, Gergely L, Miltényi Z, Magyari F, Szodoray P and Illés A: Malignant lymphomas and autoimmunity-a single center experience from Hungary. Clin Rheumatol. 31:219–224. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Zintzaras E, Voulgarelis M and Moutsopoulos H: The risk of lymphoma development in autoimmune diseases: A meta-analysis. Arch Intern Med. 165:2337–2344. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Pertovaara M, Pukkala E, Laippala P, Miettinen A and Pasternack A: A longitudinal cohort study of Finnish patients with primary Sjögren's syndrome: Clinical, immunological, and epidemiological aspects. Ann Rheum Dis. 60:467–472. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Bernatsky S, Ramsey-Goldman R and Clarke A: Malignancy and autoimmunity. Curr Opin Rheumatol. 18:129–134. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Shaffer AL, Rosenwald A and Staudt LM: Lymphoid malignancies: The dark side of B-cell differentiation. Nat Rev Immunol. 2:920–932. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Ekström K, Hjalgrim H, Brandt L, Baecklund E, Klareskog L, Ekbom A and Askling J: Risk of malignant lymphomas in patients with rheumatoid arthritis and in their first-degree relatives. Arthritis Rheum. 48:963–970. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Grulich AE, Vajdic CM and Cozen W: Altered immunity as a risk factor for non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 16:405–408. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Peng Z, Liang X, Lin X, Lin W, Lin Z and Wei S: Exploration of the molecular mechanisms, shared gene signatures, and MicroRNAs between systemic lupus erythematosus and diffuse large B cell lymphoma by bioinformatics analysis. Lupus. 31:1317–1327. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Li H, Yu L, Zhang X, Shang J and Duan X: Exploring the molecular mechanisms and shared gene signatures between rheumatoid arthritis and diffuse large B cell lymphoma. Front Immunol. 13(1036239)2022.PubMed/NCBI View Article : Google Scholar | |
|
Mackay LR and Rose NR: Autoimmunity and lymphoma: Tribulations of B cells. Nature Immunol. 2:793–795. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M and Isaacson PG: Regression of primary lowgrade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 324:575–577. 1993.PubMed/NCBI View Article : Google Scholar | |
|
Mariette X: Lymphomas complicating Sjögren's syndrome and hepatitis C virus infection may share a common pathogenesis: Chronic stimulation of rheumatoid factor B cells. Ann Rheum Dis. 60:1007–1010. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Smedby KE, Baecklund E and Askling J: Malignant lymphomas in autoimmunity and inflammation: A review of risks, risk factors, and lymphoma characteristics. Cancer Epidemiol Biomarkers Prev. 15:2069–2077. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Firestein GS: Evolving concepts of rheumatoid arthritis. Nature. 423:356–361. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Bernatsky S, Clarke A and Ramsey-Goldman R: Malignancy and systemic lupuserythematosus. Curr Rheumatol Rep. 4:351–358. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Yamamoto K: Pathogenesis of Sjogren's syndrome. Autoimmun Rev. 2:13–18. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Baecklund E, Askling J, Rosenquist R, Ekbom A and Klareskog L: Rheumatoid arthritis and malignant lymphomas. Curr Opin Rheumatol. 16:254–261. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Lenz G and Staudt LM: Aggressive lymphomas. N Engl J Med. 362:1417–1429. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Jones SA and Jenkins BJ: Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 18:773–789. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Qin B, Zhou Z, He J, Yan C and Ding S: IL-6 inhibits starvation-induced autophagy via the STAT3/Bcl-2 signaling pathway. Sci Rep. 5(15701)2015.PubMed/NCBI View Article : Google Scholar | |
|
Lan Q, Zheng T, Rothman N, Zhang Y, Wang SS, Shen M, Berndt SI, Zahm SH, Holford TR, Leaderer B, et al: Cytokine polymorphisms in the Th1/Th2 pathway and susceptibility to non-Hodgkin lymphoma. Blood. 107:4101–4108. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Cortes J and Kurzrock R: Interleukin-10 in non-Hodgkin's lymphoma. Leuk Lymphoma. 26:251–259. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Khatri VP and Caligiuri MA: A review of the association between interleukin-10 and human B-cell malignancies. Cancer Immunol Immunother. 46:239–244. 1998.PubMed/NCBI View Article : Google Scholar | |
|
Emilie D, Zou W, Fior R, Llorente L, Durandy A, Crevon MC, Maillot MC, Durand-Gasselin I, Raphael M, Peuchmaur M and Galamaud P: Production and roles of IL-6, IL-10, and IL-13 in B-lymphocyte malignancies and in B-lymphocyte hyperactivity of HIV infection and autoimmunity. Methods. 11:133–142. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Rothman N, Skibola CF, Wang SS, Morgan G, Lan Q, Smith MT, Spinelli JJ, Willett E, De Sanjose S, Cocco P, et al: Genetic variation in TNF and IL10 and risk of non-Hodgkin lymphoma: A report from the InterLymph consortium. Lancet Oncol. 7:27–38. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Szodoray P and Jonsson R: The BAFF/APRIL system in systemic autoimmune diseases with a special emphasis on Sjögren's syndrome. Scand J Immunol. 62:421–428. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Novak AJ, Grote DM, Stenson M, Ziesmer SC, Witzig TE, Habermann TM, Harder B, Ristow KM, Bram RJ, Jelinek DF, et al: Expression of BLyS and its receptors in B-cell non-Hodgkin lymphoma: Correlation with disease activity and patient outcome. Blood. 104:2247–2253. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Baecklund E, Iliadou A, Askling J, Ekbom A, Backlin C, Granath F, Catrina AI, Rosenquist R, Feltelius N, Sundström C and Klareskog L: Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 54:692–701. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Bachman TR, Sawitzke AD, Perkins SL, Ward JH and Cannon GW: Methotrexate-associated lymphoma in patients with rheumatoid arthritis: Report of two cases. Arthritis Rheum. 39:325–329. 1996.PubMed/NCBI View Article : Google Scholar | |
|
Baltus JA, Boersma JW, Hartman AP and Vandenbroucke JP: The occurrence of malignancies in patients with rheumatoid arthritis treated with cyclophosphamide: A controlled retrospective follow-up. Ann Rheum Dis. 42:368–373. 1983.PubMed/NCBI View Article : Google Scholar | |
|
Symmons DP, Ahern M, Bacon PA, Hawkins CF, Amlot PL, Jones EL, Prior P and Scott DL: Lymphoproliferative malignancy in rheumatoid arthritis: A study of 20 cases. Ann Rheum Dis. 43:132–135. 1984.PubMed/NCBI View Article : Google Scholar | |
|
Ekström Smedby K, Vajdic CM, Falster M, Engels EA, Martínez-Maza O, Turner J, Hjalgrim H, Vineis P, Seniori Costantini A, Bracci PM, et al: Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: A pooled analysis within the InterLymph consortium. Blood. 111:4029–4038. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Bernatsky S, Ramsey-Goldman R, Joseph L, Boivin JF, Costenbader KH, Urowitz MB, Gladman DD, Fortin PR, Nived O, Petri MA, et al: Lymphoma risk in systemic lupus: Effects of disease activity versus treatment. Ann Rheum Dis. 73:138–142. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Wolfe F and Michaud K: Lymphoma in rheumatoid arthritis: The effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum. 50:1740–1751. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Leombruno JP, Einarson TR and Keystone EC: The safety of anti-tumour necrosis factor treatments in rheumatoid arthritis: Meta and exposure-adjusted pooled analyses of serious adverse events. Ann Rheum Dis. 68:1136–1145. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Askling J, Fored CM, Baecklund E, Brandt L, Backlin C, Ekbom A, Sundström C, Bertilsson L, Cöster L, Geborek P, et al: Haematopoietic malignancies in rheumatoid arthritis: Lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann Rheum Dis. 64:1414–1420. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Wolfe F and Michaud K: The effect of methotrexate and anti-tumor necrosis factor therapy on the risk of lymphoma in rheumatoid arthritis in 19,562 patients during 89,710 person-years of observation. Arthritis Rheum. 56:1433–1439. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Royer B, Cazals-Hatem D, Sibilia J, Agbalika F, Cayuela JM, Soussi T, Maloisel F, Clauvel JP, Brouet JC and Mariette X: Lymphomas in patients with Sjogren's syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood. 90:766–775. 1997.PubMed/NCBI | |
|
Ghesquières H, Berger F, Felman P, Callet-Bauchu E, Bryon PA, Traverse-Glehen A, Thieblemont C, Baseggio L, Michallet AS, Coiffier B and Salles G: Clinicopathologic characteristics and outcome of diffuse large B-cell lymphomas presenting with an associated low-grade component at diagnosis. J Clin Oncol. 24:5234–5241. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Zucca E, Conconi A, Pedrinis E, Cortelazzo S, Motta T, Gospodarowicz MK, Patterson BJ, Ferreri AJ, Ponzoni M, Devizzi L, et al: Nongastric marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Blood. 101:2489–2495. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Smedby KE, Askling J, Mariette X and Baecklund E: Autoimmune and inflammatory disorders and risk of malignant lymphomas-an update. J Intern Med. 264:514–527. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Cerchietti LC, Yang SN, Shaknovich R, Hatzi K, Polo JM, Chadburn A, Dowdy SF and Melnick A: A peptomimetic inhibitor of BCL6 with potent antilymphoma effects in vitro and in vivo. Blood. 113:3397–3405. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, Flinn IW, Flowers CR, Martin P, Viardot A, et al: PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 370:1008–1018. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Nogai H, Dörken B and Lenz G: Pathogenesis of non-Hodgkin's lymphoma. J Clin Oncol. 29:1803–1811. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Hiddemann W: Non-Hodgkin's lymphomas-current status of therapy and future perspectives. Eur J Cancer. 31A:2141–2145. 1995.PubMed/NCBI View Article : Google Scholar | |
|
Nowakowski GS and Czuczman MS: ABC, GCB, and double-hit diffuse large B-cell lymphoma: Does subtype make a difference in therapy selection? Am Soc Clin Oncol Educ Book. 35:e449–e457. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R, MacPherson N, O'Reilly S, Spinelli JJ, Sutherland J, et al: Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 23:5027–5033. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Forstpointner R, Dreyling M, Repp R, Hermann S, Hänel A, Metzner B, Pott C, Hartmann F, Rothmann F, Rohrberg R, et al: The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German low-grade lymphoma study group. Blood. 104:3064–3071. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Marcus R, Imrie K, Belch A, Cunningham D, Flores E, Catalano J, Solal-Celigny P, Offner F, Walewski J, Raposo J, et al: CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 105:1417–1423. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, et al: CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 346:235–242. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Wilson WH, Gerecitano JF, Goy A, de Vos S, Kenkre VP, Barr PM, Blum KA, Shustov AR, Advani RH, Lih J, et al: The Bruton's tyrosine kinase (BTK) inhibitor, ibrutinib (PCI-32765), has preferential activity in the ABC subtype of relapsed/refractory de novo diffuse large B-cell lymphoma (DLBCL): Interim results of a multicenter, open-label, phase 2 study. Blood. 120(686)2012. | |
|
Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A, Schaefer-Cutillo J, De Vos S, Sinha R, Leonard JP, et al: Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 115:2578–2585. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Valla K, Flowers CR and Koff JL: Targeting the B cell receptor pathway in non-Hodgkin lymphoma. Expert Opin Investig Drugs. 27:513–522. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Aw A and Brown JR: Current status of Bruton's tyrosine kinase inhibitor development and use in B-cell malignancies. Drugs Aging. 34:509–527. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Smith SM, van Besien K, Karrison T, Dancey J, McLaughlin P, Younes A, Smith S, Stiff P, Lester E, Modi S, et al: Temsirolimus has activity in non-mantle cell non-Hodgkin's lymphoma subtypes: The University of Chicago phase II consortium. J Clin Oncol. 28:4740–4746. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Pourdehnad M, Truitt ML, Siddiqi IN, Ducker GS, Shokat KM and Ruggero D: Myc and mTOR converge on a common node in protein synthesis control that confers synthetic lethality in Myc-driven cancers. Proc Natl Acad Sci USA. 110:11988–11993. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Smolewski P and Rydygier D: Efficacy and safety of idelalisib for the treatment of indolent B-cell malignancies. Expert Opin Pharmacother. 21:1915–1926. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Kang MH and Reynolds CP: Bcl-2 inhibitors: Targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 15:1126–1132. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, et al: ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 19:202–208. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Vandenberg CJ and Cory S: ABT-199, a new Bcl-2-specific BH3 mimetic, has in vivo efficacy against aggressive Myc-driven mouse lymphomas without provoking thrombocytopenia. Blood. 121:2285–2288. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Lin VS, Xu ZF, Huang DCS and Thijssen R: BH3 mimetics for the treatment of B-cell malignancies-insights and lessons from the clinic. Cancers (Basel). 12(3353)2020.PubMed/NCBI View Article : Google Scholar | |
|
Davids MS, Roberts AW, Seymour JF, Pagel JM, Kahl BS, Wierda WG, Puvvada S, Kipps TJ, Anderson MA, Salem AH, et al: Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 35:826–833. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Klanova M and Klener P: BCL-2 proteins in pathogenesis and therapy of B-cell non-Hodgkin lymphomas. Cancers (Basel). 12(938)2020.PubMed/NCBI View Article : Google Scholar | |
|
Nademanee A: Transplantation for non-Hodgkin lymphoma. Expert Rev Hematol. 2:425–442. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Bhatt VR and Vose JM: Hematopoietic stem cell transplantation for non-Hodgkin lymphoma. Hematol Oncol Clin North Am. 28:1073–1095. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Turunen A, Valtola J, Partanen A, Ropponen A, Kuittinen O, Kuitunen H, Vasala K, Ågren L, Penttilä K, Keskinen L, et al: Autograft cellular composition and outcome in NHL patients: Results of the prospective multicenter GOA study. Leuk Lymphoma. 61:2082–2092. 2020.PubMed/NCBI View Article : Google Scholar |