Bevacizumab‑associated intracerebral hemorrhage in patients with malignant glioma
- Authors:
- Published online on: April 17, 2025 https://doi.org/10.3892/mco.2025.2852
- Article Number: 57
-
Copyright: © Hama et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Malignant glioma including glioblastoma has poor prognosis, despite recent advances in molecular diagnostics and in modern individual treatment (1). The current standard of care comprises surgical resection followed by adjuvant radiotherapy and chemotherapy (2). Glioblastoma is characterized by overexpression of vascular endothelial growth factor (VEGF), a crucial factor of tumor-associated angiogenesis, and these tumors are composed of highly disorganized vessels (3). A promising alternative therapeutic approach for glioblastoma is the inhibition of angiogenesis through VEGF, which may slow down tumor growth and enhance the effects of radiotherapy and chemotherapy (4). Bevacizumab is a recombinant humanized monoclonal antibody targeting VEGF, which suppresses tumor angiogenesis and growth (5). In the AVAglio study, bevacizumab combined with standard treatment for patients with newly-diagnosed glioblastoma was associated with a 4.4-month increase in median progression-free survival (6). In the RTOG 0825 trial, first-line use of bevacizumab did not improve overall survival in patients with newly diagnosed glioblastoma, however, progression-free survival was longer in the bevacizumab group than in the placebo group (10.7 months vs. 7.3 months) (7).
Bevacizumab has some specific adverse effects, however, including proteinuria, hypertension, severe bleeding, protracted wound healing, and gastrointestinal perforation (5). In the RTOG 0825 trial, some adverse events were more prevalent in the bevacizumab group than in the placebo group, including hypertension (4.2% vs. 0.9%), thromboembolic disease (7.7% vs. 4.7%), and wound dehiscence (1.5% vs. 0.9%) (7). Administration of bevacizumab in patients with glioblastoma has been associated with an increased risk of intracerebral hemorrhage (ICH) (8). However, risk factors for the occurrence of ICH as a result of bevacizumab treatment are unclear. In this study, we therefore analyzed patients treated with bevacizumab for malignant glioma and investigated the characteristics of the patients in the cohort that developed ICH.
Materials and methods
Patients
We retrospectively identified 118 patients with malignant glioma (newly diagnosed or recurrent) that were treated at Wakayama Medical University Hospital between January 2015 and December 2022. Among them, 64 had been treated with bevacizumab, of which 61 were men (51.7%) and 57 were women (48.3%), with a median age of 66 years (range: 18-91 years). We extracted their clinical and molecular biological information, treatment details, and the presence of ICH after bevacizumab administration from the hospital database. We retrospectively analyzed potential risk factors involved in ICH. This study was carried out in accordance with the principles of the Declaration of Helsinki. Approval was obtained from the Wakayama Medical University Institutional Review Board (approval no. 98). As this is a retrospective chart review involving de-identified patient data, patient consent was not required for this study.
Statistical analyses
Statistical analysis was performed using the SAS package and JMP Pro version 16 (SAS Institute, Cary, NC, USA). The overall survival was measured from date of diagnosis until death and the overall survival curves were obtained by the Kaplan-Meier method and compared with log-rank test. Categorized data were compared between subgroups using the Fisher's exact test. Continuous variables were compared using unpaired Student's t-tests. Multivariate analyses were performed using logistic regression analysis. P<0.05 was considered to indicate a statistically significant difference. All incidences of ICH were graded according to the common terminology criteria for adverse events version 5.0(9).
Results
Patient characteristics
Within the study cohort were 64 patients that had been treated with bevacizumab; their demographic and clinical characteristics are summarized in Table I. There were 38 men (59.4%) and 26 women (40.6%) with a mean (standard deviation) age of 69 (14.6) years. Based on integrated diagnosis by 2021 WHO Classification, 54 patients (84.4%) had glioblastoma, IDH-wildtype, four patients (6.3%) had astrocytoma, IDH-mutant, and two patients (3.1%) had oligodendroglioma, IDH-mutant, and 1p/19q-codeleted. Of the 64 patients, seven (10.9%) developed ICH while on bevacizumab.
Univariate and multivariate analyses for the determination the factor of ICH
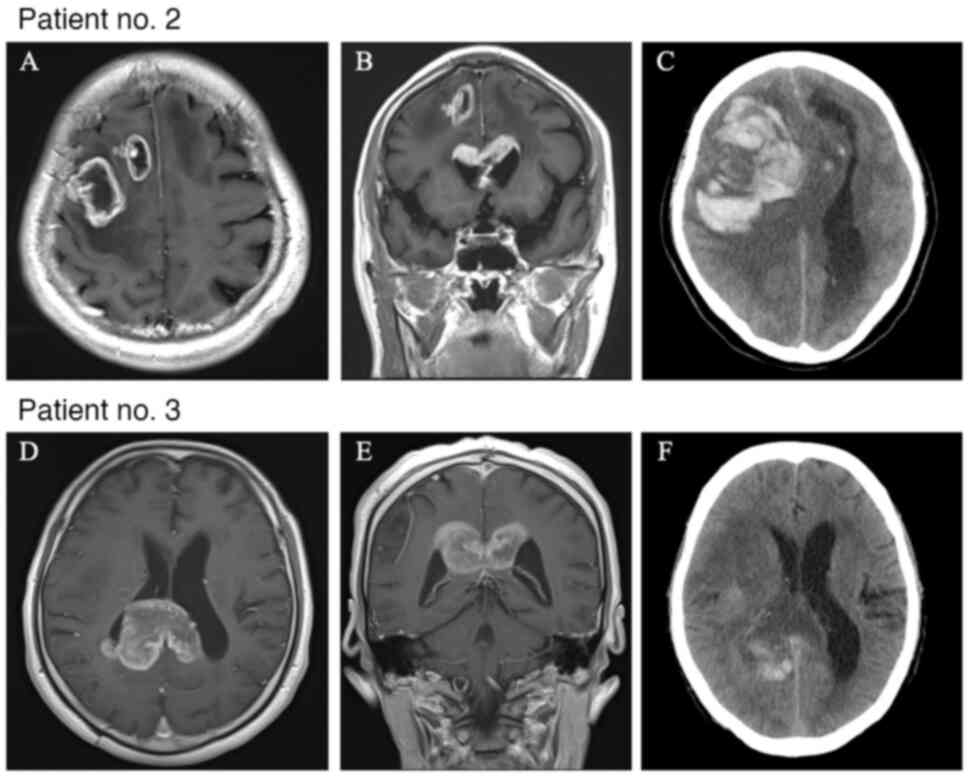
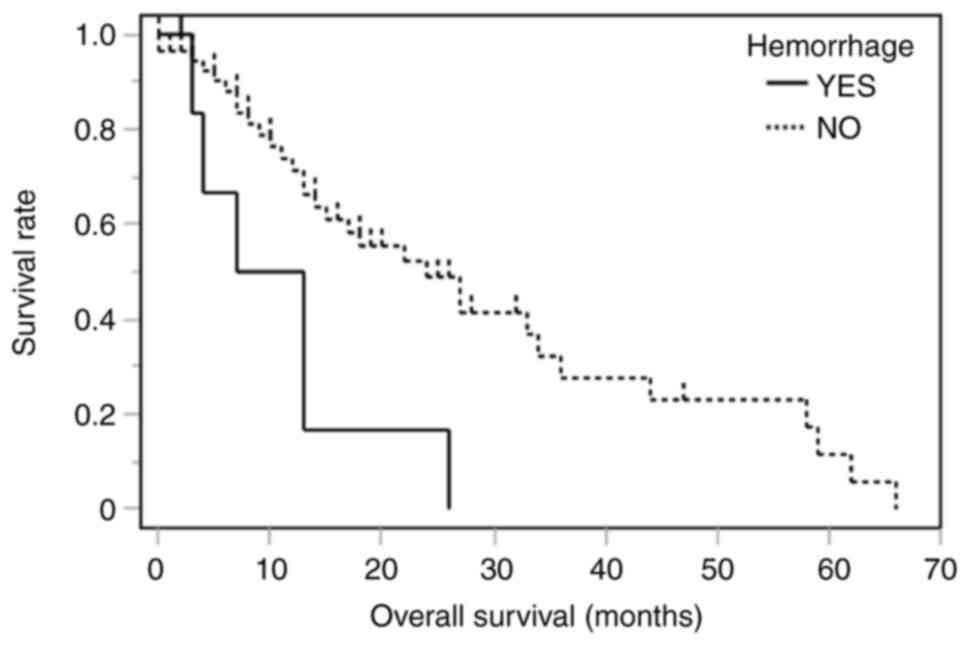
The clinical and molecular characteristics of patients who developed ICH after bevacizumab administration are summarized in Table II. ICH occurred in four men and three women, with a mean age (standard deviation) of 64.4 (11.6) years. Regarding the extent of resection, six patients had a needle biopsy and one patient had a subtotal resection. Based on the 2021 WHO Classification, six patients had glioblastoma, IDH-wildtype, and the other patient had oligodendroglioma, IDH-mutant, and 1p/19q-codeleted. Five patients received radiation treatment and seven patients received temozolomide. Two patients (28.6%) had grade ≥3 hemorrhage (Table II). At the time of ICH, computed tomography showed peritumoral hemorrhage rather than intra-tumor hemorrhage (Fig. 1). The median number of administrations of bevacizumab before the onset of ICH was seven times (range: 1-32), and the median duration from first administration to ICH was 4 months (range: 1-22). One patient (14.2%) was taking anti-thrombotic medication. ICH itself was associated with shorter overall survival (Fig. 2, log-rank, P=0.008). Tumor invasion into the corpus callosum on contrast-enhanced magnetic resonance imaging before administration of bevacizumab was associated with ICH in both univariate analysis (Table III, odds ratio, 9.38; 95% confidence interval, 1.61-54.4; P=0.01) and multivariate analysis (Table IV, odds ratio, 9.76; 95% confidence interval, 1.44-65.8; P=0.02).
Table IISummary of patients in our cohort with intracerebral hemorrhage after bevacizumab administration (n=7). |
Discussion
Bevacizumab has a specific toxicity profile related to its anti-angiogenic effect, and some bevacizumab-associated adverse events can be life-threatening (8). We investigated the overall incidence and risk of ICH among patients with high-grade glioma that underwent bevacizumab therapy. We found ICH to be associated with shorter overall survival. Also, tumor invasion into the corpus callosum before administration of bevacizumab was associated with an increased risk of developing ICH.
Among brain tumors, gliomas and metastasis tend to bleed most frequently (10). ICH occurs in 3.7-7.2% of gliomas, mainly in glioblastoma and oligodendroglioma (10). The most important etiopathogenesis of ICH in glioblastoma is considered to be the abnormalities of tumor vascularization (10). Glioblastomas are highly angiogenic and infiltrative tumors. Tumor cells invade along blood vessels to increase tumor growth, and glioblastomas displace the endfeet of astrocytes and affect pericyte stability, leading to perivascular niches and cell evasion (11). Microvascular proliferation with hyperplasia of endothelial cells following obliteration and the presence of numerous thin-walled, poorly formed, or dilated vessels may induce hemorrhages (10,11).
In the AVAglio study, the incidence of ICH was 2.5% in the placebo group and 4.3% in the bevacizumab group, suggesting that bevacizumab may contribute to development of ICH (6). A post-marketing surveillance study of bevacizumab for patients with newly diagnosed or recurrent malignant glioma in Japan reported an ICH incidence of 5.4% (12). In the current study, the hemorrhage rates were higher than in these reports. The AVAglio study reported that just 13% of patients in the bevacizumab-treated group underwent a biopsy (6), as compared with 58% in this study. We suggest the high hemorrhagic rate may be related to the patient population in this study, which includes a large portion of patients who underwent biopsy rather than tumor removal due to old age, poor preoperative performance status, and dissemination.
The pathophysiology underlying ICH in high-grade glioma with bevacizumab still requires elucidation. VEGF is thought to be important for endothelial cells to maintain the architecture and integrity of the microvasculature (5). In metastatic brain tumors, overexpression of VEGF and metalloproteinase, which degrade the extracellular matrix, may play a role in causing hemorrhages through rapid growth and breakdown of vessels around the tumor (13). Bevacizumab may cause hemorrhage by reducing the regenerative capacity of endothelial cells (14). However, dysfunction of endothelial cells alone cannot explain the ICH in patients that have received bevacizumab (13,14).
In our cohort, tumor invasion into the corpus callosum before administration of bevacizumab was associated with an increased risk of ICH. Gliomas invading the corpus callosum reportedly account for approximately 14% of gliomas (15,16). Tumor invasion into the corpus callosum has been said to be a more aggressive subtype of an already aggressive and incurable disease (16). In a recent retrospective cohort study, it was significantly associated with glioma WHO grade and PDGFRA mutation (17). Endocan, an endothelial-secreted proteoglycan reportedly activates PDGFRA and promotes a hypervascular phenotype of glioblastoma (18). The aggressive biological behavior and hypervascularization of the tumor might affect ICH after bevacizumab administration.
In relation to tumor-related ICH, a number of reports have discussed feeding arteries (11,19), but there has been little mention of the draining veins. Brain arteriovenous malformations with deep venous drainage have shown higher rates of hemorrhage, suggesting arteriovenous malformation pressurization due to draining veins (20). Inhibition of VEGF signaling suppresses the endothelial cell activation, predisposing to thromboembolic events (14). Even in malignant gliomas, the lesions invading the corpus callosum are associated with deep venous drainage, and we suggest that thrombosis of deep cerebral veins due to the use of bevacizumab may contribute to ICH.
This study has several limitations. Unlike a randomized study, there is a possibility of selection bias in the decision-making on treatment strategy. Second, the limited number of patients could be responsible for the absence of statistical power to detect differences between groups. The sample size of this study is insufficient to establish an association between invasion of corpus callosum and ICH after bevacizumab administration. Perhaps due to the small sample size, no risk factors other than corpus callosum invasion could be observed in this study. Further investigation in a larger population is required to examine other factors, such as antithrombotic medications, angiographical features, and microbleeds in the magnetic resonance imaging, which would contribute to a better understanding of bevacizumab-associated ICH in patients with malignant glioma.
In conclusion, in our cohort of patients with malignant glioma treated with bevacizumab, ICH was associated with shorter overall survival. It was suggested that invasion of the corpus callosum shown on magnetic resonance imaging might be a predictor of development of ICH in some patients that receive bevacizumab. Further studies in larger cohorts are required to confirm these results. Meticulous management, such as frequent follow-up and treatment of hypertension, may be necessary in patients at high risk for ICH after administration of bevacizumab.
Acknowledgements
The authors would like to thank Mr. Benjamin Phillis (Clinical Study Support Center at Wakayama Medical University) for proofreading and editing the manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
All authors (YH, TS, JF and NN) contributed to the study conception and design. YH and TS wrote the final manuscript and acquired all data. YH and TS confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Approval was obtained from the Wakayama Medical University Institutional Review Board (approval no. 98). This was a retrospective chart review involving de-identified patient data, so patient consent was not required for this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, Batchelor TT, Bindra RS, Chang SM, Chiocca EA, et al: Glioblastoma in adults: A society for neuro-oncology (SNO) and European society of neuro-oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 22:1073–1113. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Das S and Marsden PA: Angiogenesis in glioblastoma. N Engl J Med. 369:1561–1563. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Yang SB, Gao KD, Jiang T, Cheng SJ and Li WB: . Bevacizumab combined with chemotherapy for glioblastoma: A meta-analysis of randomized controlled trials. Oncotarget. 8:57337–57344. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Liu ZL, Chen HH, Zheng LL, Sun LP and Shi L: Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. 8(198)2023.PubMed/NCBI View Article : Google Scholar | |
|
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, et al: Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 370:709–722. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, et al: A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 370:699–708. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Brandes AA, Bartolotti M, Tosoni A, Poggi R and Franceschi E: Practical management of bevacizumab-related toxicities in glioblastoma. Oncologist. 20:166–175. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available at https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Accessed on May 20, 2024. | |
|
Ostrowski RP, He Z, Pucko EB and Matyja E: Hemorrhage in brain tumor-an unresolved issue. Brain Hemorrhages. 3:98–102. 2022. | |
|
Guyon J, Chapouly C, Andrique L, Bikfalvi A and Daubon T: The normal and brain tumor vasculature: Morphological and functional characteristics and therapeutic targeting. Front Physiol. 12(622615)2021.PubMed/NCBI View Article : Google Scholar | |
|
Motoo N, Hayashi Y, Shimizu A, Ura M and Nishikawa R: Safety and effectiveness of bevacizumab in Japanese patients with malignant glioma: A post-marketing surveillance study. Jpn J Clin Oncol. 49:1016–1023. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Kubo H, Fujiwara T, Jussila L, Hashi H, Ogawa M, Shimizu K, Awane M, Sakai Y, Takabayashi A, Alitalo K, et al: Involvement of vascular endothelial growth factor receptor-3 in maintenance of integrity of endothelial cell lining during tumor angiogenesis. Blood. 96:546–553. 2000.PubMed/NCBI | |
|
Kamba T and McDonald DM: Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 96:1788–1795. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Chen KT, Wu TW, Chuang CC, Hsu YH, Hsu PW, Huang YC, Lin TK, Chang CN, Lee ST, Wu CT, et al: Corpus callosum involvement and postoperative outcomes of patients with gliomas. J Neurooncol. 124:207–214. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Dayani F, Young JS, Bonte A, Chang EF, Theodosopoulos P, McDermott MW, Berger MS and Aghi MK: Safety and outcomes of resection of butterfly glioblastoma. Neurosurg Focus. 44(E4)2018.PubMed/NCBI View Article : Google Scholar | |
|
Shen S, Feng S, Liu H, Jiang J and Yu X: Associations of histological and molecular alterations with invasion of the corpus callosum in gliomas. Acta Neurochir (Wien). 162:1691–1699. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Bastola S, Pavlyukov MS, Sharma N, Ghochani Y, Nakano MA, Muthukrishnan SD, Yu SY, Kim MS, Sohrabi A, Biscola NP, et al: Endothelial-secreted Endocan activates PDGFRA and regulates vascularity and spatial phenotype in glioblastoma. Nat Commun. 16(471)2025.PubMed/NCBI View Article : Google Scholar | |
|
Liebelt B, Boghani Z, Takei H, Fung S and Britz G: Epithelioid glioblastoma presenting as massive intracerebral hemorrhage: Case report and review of the literature. Surg Neurol Int. 6 (Suppl 2):S97–S100. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Alexander MD, Cooke DL, Nelson J, Guo DE, Dowd CF, Higashida RT, Halbach VV, Lawton MT, Kim H and Hetts SW: Association between venous angioarchitectural features of sporadic brain arteriovenous malformations and intracranial hemorrhage. Am J Neuroradiol. 36:949–952. 2015.PubMed/NCBI View Article : Google Scholar |