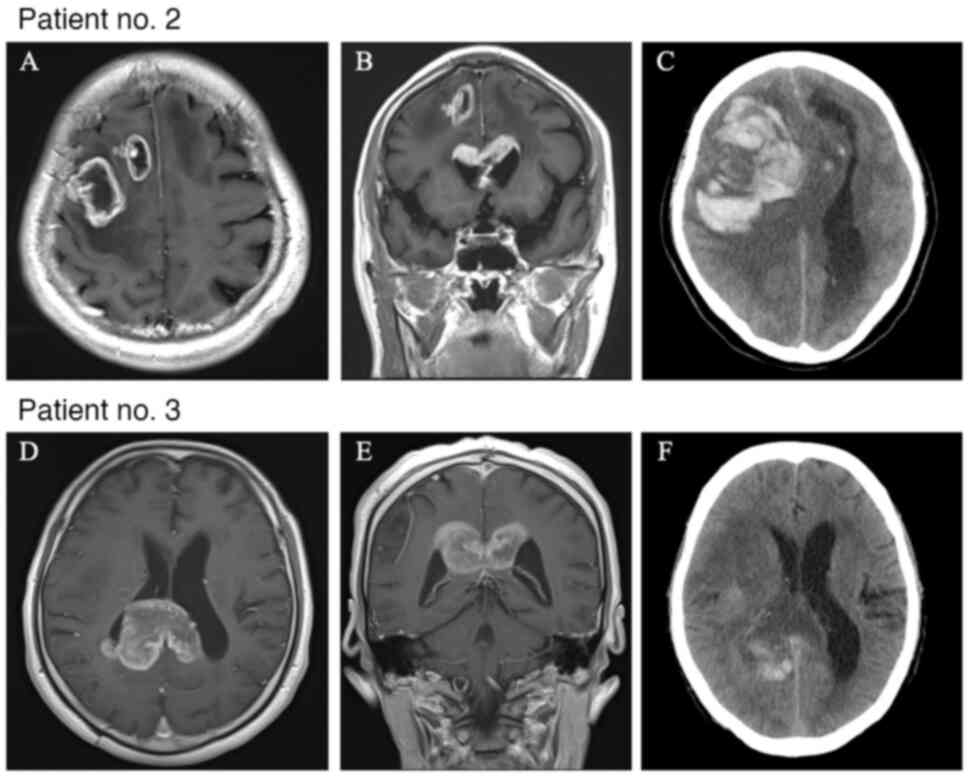
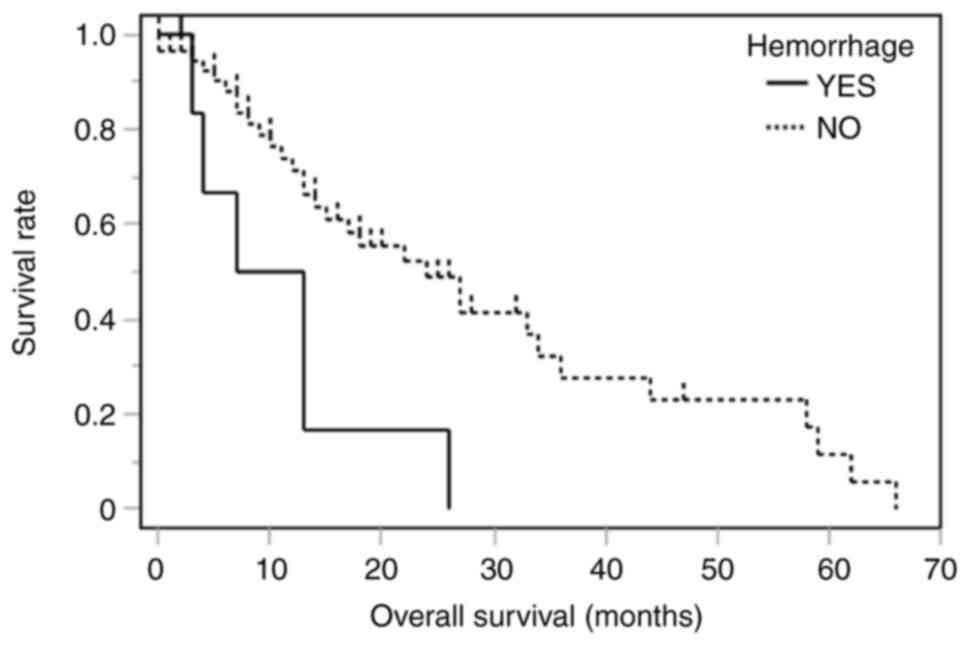
|
1
|
Wen PY, Weller M, Lee EQ, Alexander BM,
Barnholtz-Sloan JS, Barthel FP, Batchelor TT, Bindra RS, Chang SM,
Chiocca EA, et al: Glioblastoma in adults: A society for
neuro-oncology (SNO) and European society of neuro-oncology (EANO)
consensus review on current management and future directions. Neuro
Oncol. 22:1073–1113. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Das S and Marsden PA: Angiogenesis in
glioblastoma. N Engl J Med. 369:1561–1563. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yang SB, Gao KD, Jiang T, Cheng SJ and Li
WB: . Bevacizumab combined with chemotherapy for glioblastoma: A
meta-analysis of randomized controlled trials. Oncotarget.
8:57337–57344. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu ZL, Chen HH, Zheng LL, Sun LP and Shi
L: Angiogenic signaling pathways and anti-angiogenic therapy for
cancer. Signal Transduct Target Ther. 8(198)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chinot OL, Wick W, Mason W, Henriksson R,
Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea
D, et al: Bevacizumab plus radiotherapy-temozolomide for newly
diagnosed glioblastoma. N Engl J Med. 370:709–722. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gilbert MR, Dignam JJ, Armstrong TS, Wefel
JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S,
Won M, et al: A randomized trial of bevacizumab for newly diagnosed
glioblastoma. N Engl J Med. 370:699–708. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Brandes AA, Bartolotti M, Tosoni A, Poggi
R and Franceschi E: Practical management of bevacizumab-related
toxicities in glioblastoma. Oncologist. 20:166–175. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Common Terminology Criteria for Adverse
Events (CTCAE) v5.0. Available at https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50.
Accessed on May 20, 2024.
|
|
10
|
Ostrowski RP, He Z, Pucko EB and Matyja E:
Hemorrhage in brain tumor-an unresolved issue. Brain Hemorrhages.
3:98–102. 2022.
|
|
11
|
Guyon J, Chapouly C, Andrique L, Bikfalvi
A and Daubon T: The normal and brain tumor vasculature:
Morphological and functional characteristics and therapeutic
targeting. Front Physiol. 12(622615)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Motoo N, Hayashi Y, Shimizu A, Ura M and
Nishikawa R: Safety and effectiveness of bevacizumab in Japanese
patients with malignant glioma: A post-marketing surveillance
study. Jpn J Clin Oncol. 49:1016–1023. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kubo H, Fujiwara T, Jussila L, Hashi H,
Ogawa M, Shimizu K, Awane M, Sakai Y, Takabayashi A, Alitalo K, et
al: Involvement of vascular endothelial growth factor receptor-3 in
maintenance of integrity of endothelial cell lining during tumor
angiogenesis. Blood. 96:546–553. 2000.PubMed/NCBI
|
|
14
|
Kamba T and McDonald DM: Mechanisms of
adverse effects of anti-VEGF therapy for cancer. Br J Cancer.
96:1788–1795. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen KT, Wu TW, Chuang CC, Hsu YH, Hsu PW,
Huang YC, Lin TK, Chang CN, Lee ST, Wu CT, et al: Corpus callosum
involvement and postoperative outcomes of patients with gliomas. J
Neurooncol. 124:207–214. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dayani F, Young JS, Bonte A, Chang EF,
Theodosopoulos P, McDermott MW, Berger MS and Aghi MK: Safety and
outcomes of resection of butterfly glioblastoma. Neurosurg Focus.
44(E4)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shen S, Feng S, Liu H, Jiang J and Yu X:
Associations of histological and molecular alterations with
invasion of the corpus callosum in gliomas. Acta Neurochir (Wien).
162:1691–1699. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bastola S, Pavlyukov MS, Sharma N,
Ghochani Y, Nakano MA, Muthukrishnan SD, Yu SY, Kim MS, Sohrabi A,
Biscola NP, et al: Endothelial-secreted Endocan activates PDGFRA
and regulates vascularity and spatial phenotype in glioblastoma.
Nat Commun. 16(471)2025.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liebelt B, Boghani Z, Takei H, Fung S and
Britz G: Epithelioid glioblastoma presenting as massive
intracerebral hemorrhage: Case report and review of the literature.
Surg Neurol Int. 6 (Suppl 2):S97–S100. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Alexander MD, Cooke DL, Nelson J, Guo DE,
Dowd CF, Higashida RT, Halbach VV, Lawton MT, Kim H and Hetts SW:
Association between venous angioarchitectural features of sporadic
brain arteriovenous malformations and intracranial hemorrhage. Am J
Neuroradiol. 36:949–952. 2015.PubMed/NCBI View Article : Google Scholar
|