Clinical significance of post‑progression survival after chemoradiotherapy on overall survival in limited‑disease small cell lung cancer
- Authors:
- Published online on: April 23, 2025 https://doi.org/10.3892/mco.2025.2853
- Article Number: 58
Abstract
Introduction
Lung cancer is the primary contributor to cancer-related fatalities globally (1). Small-cell lung cancer (SCLC) represents 10-15% of lung cancer cases worldwide, manifesting as an aggressive tumor characterized by rapid proliferation and early-onset widespread metastasis (2,3). Limited-stage SCLC (LD-SCLC), which accounts for approximately one-third of all diagnosed SCLC cases (4), is confined to the hemithorax and adjacent regional lymph nodes, allowing for safe treatment with definitive radiation doses. The standard treatment protocol for LD-SCLC in patients with a favorable performance status (PS) typically includes concurrent platinum/etoposide chemotherapy and thoracic radiotherapy (TRT), followed by prophylactic cranial irradiation (PCI) for those who respond to chemoradiotherapy [CRT] (5,6). In LD-SCLC, concurrent cisplatin-etoposide chemotherapy with TRT has demonstrated superior efficacy compared to sequential TRT following cisplatin-etoposide combination therapy (7).
Progression-free survival (PFS) and overall survival (OS) are common endpoints in clinical oncology trials. OS, easily calculated by recording the date of death, is a reliable and precise measure that offers distinct advantages. Various treatment strategies influence the impact of front-line therapy on OS (8). In contrast, PFS is evaluated at earlier stages than OS, as its components chronologically precede those of OS (9). If a statistically significant correlation exists between PFS and OS, PFS could serve as a viable surrogate marker of OS. In non-SCLC (NSCLC), a prolonged PFS does not consistently correlate with extended OS (10); however, post-progression survival (PPS) has been strongly associated with OS beyond initial treatment (11,12). Multiple studies employing individual-level analyses have consistently shown a strong correlation between PPS following first-line treatment and OS in metastatic NSCLC (13-15). OS comprises the combined duration of PFS and PPS (8). Patients with LD-SCLC have exhibited a robust relationship between PPS and OS after concurrent CRT at an individual level (16). Since immune checkpoint inhibitors (ICIs) have only recently been administered to patients with relapsed SCLC, the correlation between PPS and OS in the context of ICI treatment is yet to be elucidated. Additionally, the effect of PPS on patients with LD-SCLC treated with CRT remains unclear. Examining the correlation between PFS-OS and PPS-OS post-CRT in patients with LD-SCLC using individual-level data is imperative.
We aimed to ascertain the effects of PFS and PPS on OS following CRT in patients with LD-SCLC. Additionally, we examined patient characteristics to identify the clinical factors associated with PPS.
Materials and methods
Patients
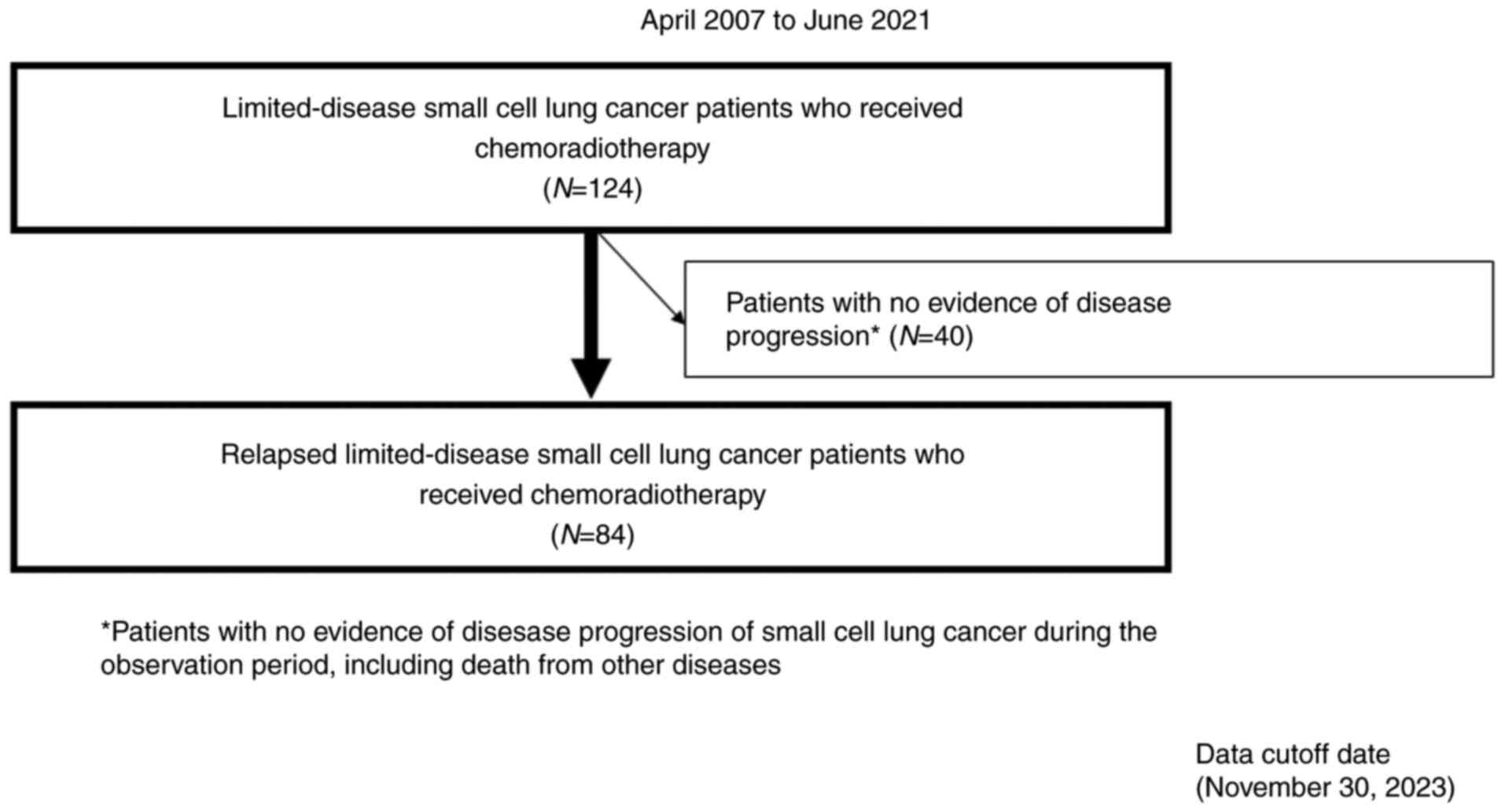
This retrospective study included 84 patients with recurrent LD-SCLC receiving CRT between April 2007 and June 2021. Patients from the International Medical Center of Saitama Medical University and Gunma Prefectural Cancer Center were enrolled in this study. The eligibility criteria were as follows: i) cytologically or histologically diagnosed SCLC; ii) involvement of unilateral hemithorax and regional lymph node metastasis treatable with a single radiotherapy field; iii) patients who received first-line CRT (concurrent/sequential); and iv) disease relapse after CRT.
Fig. 1 illustrates the patient selection process. Prior to treatment initiation, all patients underwent thorough evaluation and standardized staging procedures. A comprehensive assessment, including physical examination, chest radiography, thoracic and abdominal computed tomography (CT), brain magnetic resonance imaging or CT, and bone scintigraphy or 18F-fluorodeoxyglucose positron emission tomography (PET), was performed to determine the tumor-node-metastasis (TNM) stages. SCLC was histologically categorized and clinically staged according to the 2015 World Health Organization classification system and 7th Edition of the Union for International Cancer Control Tumor-Node-Metastasis (TNM) Classification, respectively (17). Medical records of the eligible patients were used to retrieve their data, with information collected from January to March 2024. For this study, we accessed the medical records of patients from the International Medical Center of Saitama Medical University and the Gunma Prefectural Cancer Center. The Ethics Committee of Saitama Medical University waived the need for written informed consent due to the retrospective nature of this study. All study protocols adhered to the principles outlined in the Declaration of Helsinki. The Institutional Ethics Committee of the International Medical Center of Saitama Medical University (approval number 2023-033) approved the study design.
Treatments. Chemotherapy
For all patients, the therapeutic regimen included the administration of cisplatin/carboplatin and etoposide. The attending physician determined the dosage according to product labeling. Etoposide (60-100 mg/m2) was intravenously administered on days 1-3, cisplatin (60-80 mg/m2) on day 1, or carboplatin [area under the curve (AUC) 3-6] on day 1 of every 3-4 weeks. Both institutions adhered to consistent criteria to initiate subsequent chemotherapy cycles and followed the protocols for concurrent or sequential CRT. In cases where the patients did not meet these criteria, their chemotherapy cycles were delayed until the requirements were met. Chemotherapy was discontinued if the dosing criteria were not fulfilled within 7 weeks of cycle initiation. Dosage adjustments or alternative chemotherapeutic regimens were considered for patients experiencing a Grade 4 decrease in platelet count, persistent Grade 4 decrease in white blood cell/neutrophil count, or Grade 3 or higher non-hematological toxicities during the preceding cycle. The specific dosage was adjusted by the attending physician in charge, as needed. For cisplatin, an initial dose of 80 mg/m2 was reduced by one or two levels to 60 and 40 mg/m2, respectively, but was not increased beyond 80 mg/m2. For carboplatin, an initial dose of AUC 5 was reduced to AUC 4 in a one-step reduction but was discontinued if further reduction was required. For etoposide, an initial dose of 100 mg/m2 was reduced to 80 mg/m2 in a one-step reduction and to 60 mg/m2 in a two-step reduction but was discontinued at that level. The granulocyte colony-stimulating factor for neutropenia was prophylactically administered at the discretion of the attending physician, whereas treatment was terminated in cases of disease progression, intolerable toxicity, or withdrawal of patient consent.
Radiotherapy
Most patients received three-dimensional conformal radiotherapy, although a few with more advanced disease stages received intensity-modulated radiotherapy to ensure adequate tumor coverage. The prescribed doses were 45 Gy in 30 fractions (twice daily) and 60 Gy in 30 fractions (once daily) for the accelerated hyperfractionated and sequential radiotherapy regimens, respectively. The gross tumor volume consisted of the primary tumor and metastatic lymph node. Metastatic lymph nodes were defined as those with a diameter of >10 mm or abnormal 18F-fluorodeoxyglucose uptake. Notably, the prophylactic lymph node area was not irradiated in this study. All patients who received PCI were administered a total dose of 25 Gy in 10 fractions.
Assessment of treatment efficacy
Tumor responses were evaluated based on the overall response and degree of maximum tumor shrinkage. Radiographic tumor responses were categorized according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1(18). Tumor responses to treatment were classified into the following categories: complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), or not evaluable (NE). Patients experiencing PD who desired further treatment were offered subsequent therapeutic options despite initial treatment failure. Treatment efficacy was assessed after two or four cycles of chemotherapy, and in the sequential chemoradiotherapy group, after the completion of radiotherapy. Following chemoradiotherapy, CT imaging was performed at ~3-month intervals, with additional imaging conducted as needed upon detection of signs of recurrence.
Definition of PFS, PPS, and OS
PFS was defined as the time interval between treatment initiation and disease progression. The diagnostic methods for detecting local recurrence and distant metastasis included CT for the trunk and MRI for the brain. Following chemoradiotherapy, imaging evaluation was primarily conducted using CT at ~3 month intervals, at the discretion of the attending physician. However, when signs of recurrence were observed, imaging evaluation, including PET-CT, was performed as appropriate. PPS was calculated from the onset of PD to death in patients treated with CRT. Patients who were alive within the observation period were censored on the date of their last visit or follow-up. OS was measured from treatment initiation to death or censoring on the date of the last follow-up.
Statistical analyses
Survival curves were generated using the Kaplan-Meier method, and PPS values were compared using the log-rank test. Spearman's rank correlation and linear regression analyses were employed to assess the correlations. The potential clinical factors for PPS were prognostically evaluated using univariate and multivariate analyses, with the Cox proportional hazards model employing a stepwise regression procedure. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated. Statistically significant differences were determined using a two-tailed P-value of <0.05. All statistical analyses were performed using JMP version 11.0 for Windows (SAS Institute).
Results
Patient characteristics and therapeutic efficacy
Table I outlines the characteristics of the 84 patients included in our study. A total of 69 males and 15 females [median age, 70 (range, 48-92) years] were included in the current analysis. The PS of 78 (92.8%) patients was 0-1. Eighty-three (98.8%) patients were current or former smokers. Combined small cell carcinoma were present in three (3.6%) patients. At diagnosis, 66 (78.6%) patients were classified as stage III. The starting dose of platinum-based combination therapy with etoposide were carboplatin (AUC 5) + etoposide (100 mg/m2) for 23 (27.3%) patients and cisplatin (80 mg/m2) + etoposide (100 mg/m2) for 24 (28.6%). Reasons for discontinuing platinum and etoposide included adverse events in nine (10.7%) patients, PS deterioration in one (1.2%), and other reasons in four (4.8%). Among the 59 patients in the concurrent chemoradiotherapy group, 26 received conventional irradiation at a dose of 60 Gy in 30 fractions, while 33 received accelerated hyperfractionated irradiation at 45 Gy in 30 fractions. In principle, radiotherapy commenced from the first cycle of chemotherapy; however, 21 and five patients started radiotherapy from the second and third cycles, respectively.
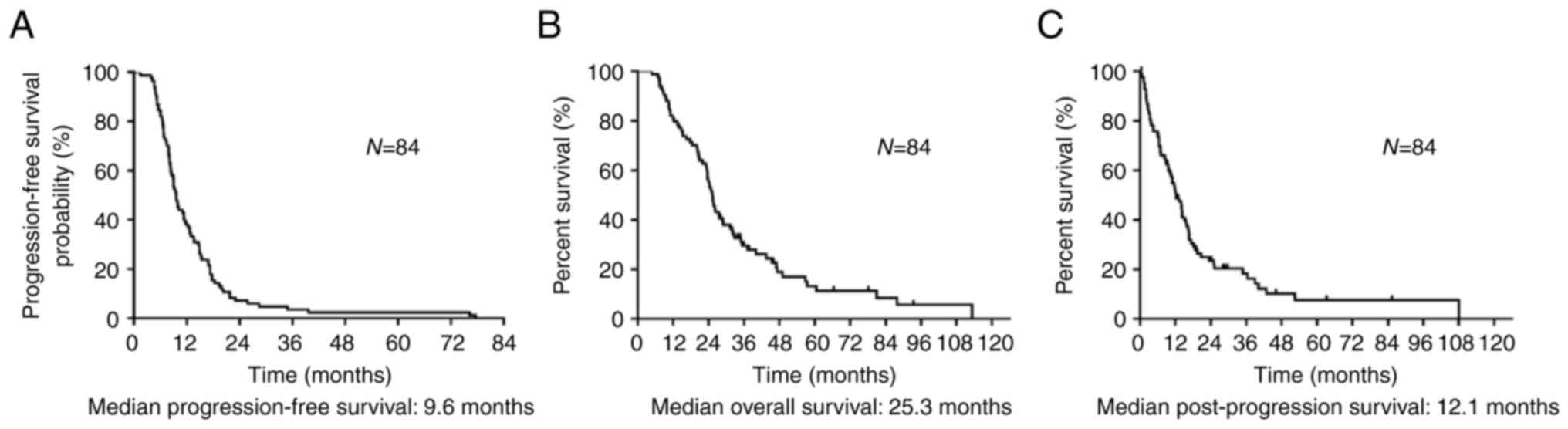
Of the included patients, 70 patients died during the median follow-up period of 25.2 (range: 4.8-113.2) months. Treatment responses after recurrence were as follows: CR, PR, and SD in 18, 62, and four patients, respectively, with no cases of PD observed (Table SI). The overall response rate was 95.2% (95% CI: 88.0-98.5), and the disease control rate was 100% (95% CI: 94.7-100). Median PFS, OS, and PPS were 9.6, 25.3, and 12.1 months, respectively (Fig. 2A-C). Of the 84 patients who relapsed after CRT, 21 did not receive further chemotherapy. Table II lists the chemotherapy regimens administered after disease progression following CRT, with a median of one (range: 0-6 regimens) for the entire cohort. Carboplatin and etoposide were the most used first-line treatments, with amrubicin monotherapy being the most frequent second-line treatment. Table SII shows the comparison between the group of patients who received subsequent chemotherapy after recurrence (n=63) and those who were only observed but without subsequent chemotherapy (n=21). The reasons for not receiving subsequent chemotherapy after relapse in the observation group are outlined in Table SII. Of the 21 patients, 10 had complications, four were older adults, three had worsened PS, and two developed brain metastases after whole brain irradiation.
Correlations between PFS-OS and PPS-OS
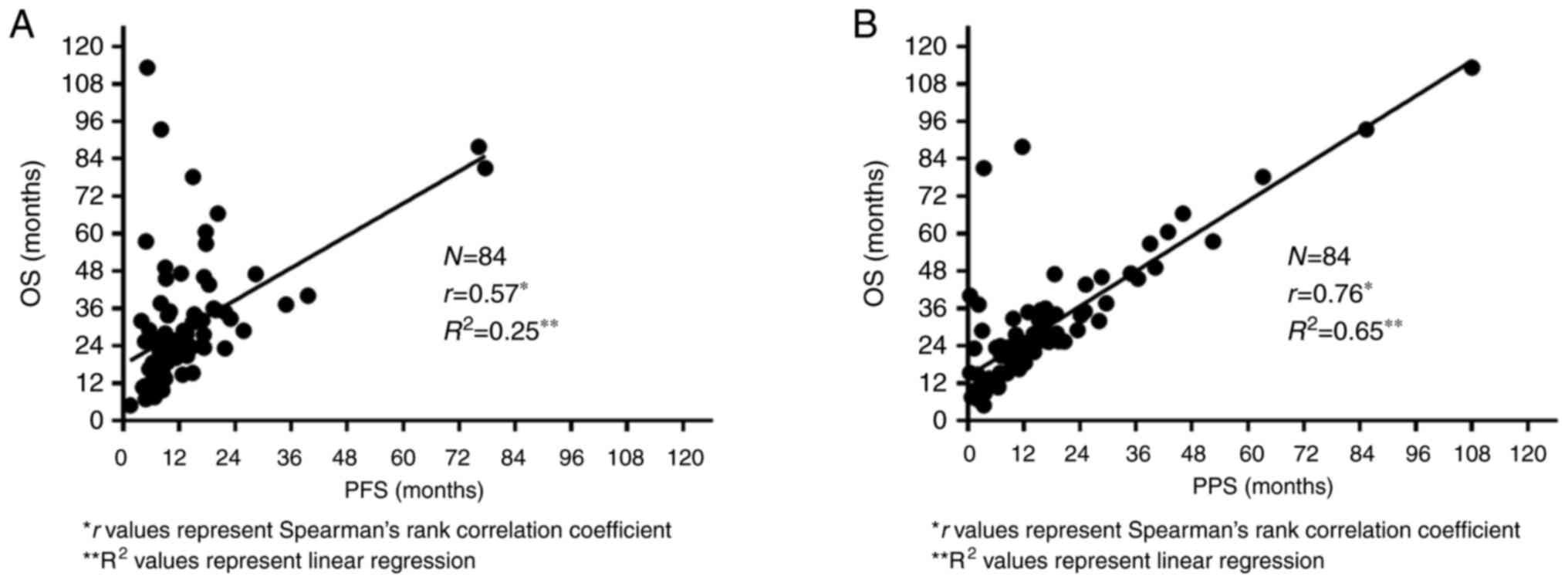
The correlations between PFS and OS and between PPS and OS are shown in Fig 3A and B, respectively. Spearman's rank correlation coefficient and linear regression analysis revealed a significant correlation between PPS and OS (r=0.76, P<0.05, R2=0.65). PFS moderately correlated with OS (r=0.57, P<0.05, R2=0.25).
Clinical factors affecting PPS
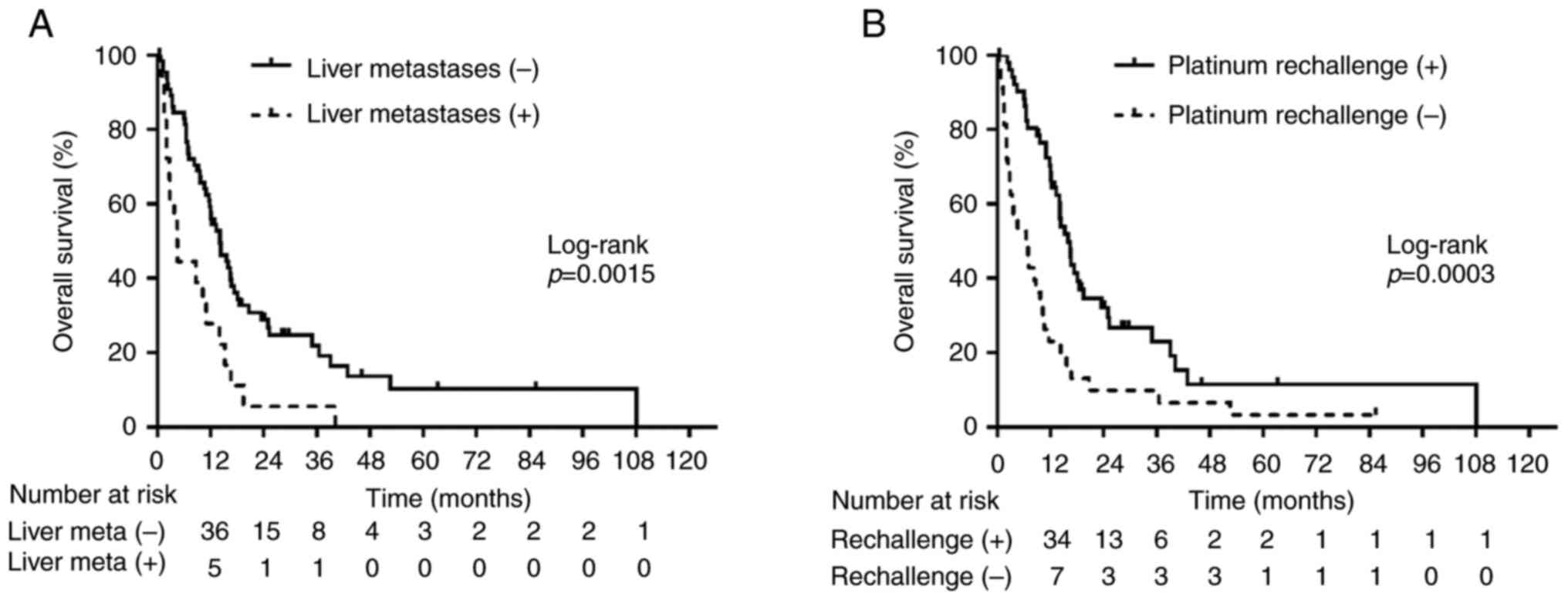
Our analysis demonstrated a significantly strong correlation between PPS and OS. We explored various clinical factors influencing PPS. Univariate analysis (Table III) revealed significant correlations between PPS and age at CRT initiation, type of platinum agent, presence of liver metastases at relapse, administration of platinum re-challenge chemotherapy (PRC), and administration of ICIs (P<0.05). Multivariate analysis (Table III) identified the presence of liver metastases at relapse and the administration of PRC as statistically associated with PPS (P<0.05). The significance of these factors was further confirmed using the log-rank test (P<0.05; Fig. 4A and B). Patients without liver metastases at relapse exhibited a median PPS of 14.1 months, which was significantly longer than those with liver metastases (PPS: 4.5 months; log-rank test, P=0.0015; Fig. 4A). Patients who received PRC had a longer PPS of 15.9 months compared with those who did not (PPS: 6.4 months; log-rank test, P=0.0003; Fig. 4B). These findings were consistent with the results obtained from the adjusted Cox proportional hazards models (Table III). Table SIII shows the characteristics of patients with or without liver metastases at relapse and those who received or did not receive PRC. When comparing 18 patients with, and 66 patients without liver metastases, radiotherapy timing in CRT (concurrent/sequential) was the only variable significantly related to the presence of liver metastases (P<0.05). The type of platinum agent (cisplatin- or carboplatin-based), presence of prophylactic cranial irradiation, and ICI administration were also closely related to the receipt of PRC (all P<0.05) in 51 patients receiving PRC compared to 33 patients without. Table SIV provides information regarding post-treatment of liver metastases identified as exhibiting a short PPS. Among the 18 patients with liver metastases, 13 received chemotherapy at relapse, of which eight received chemotherapy at relapse with platinum combination chemotherapy.
Table IIIUnivariate and multivariate Cox regression analyses of patient characteristics for post-progression survival. |
Discussion
We investigated the relationship between PFS-OS and PPS-OS in individual patients with LD-SCLC who relapsed after curative-intent CRT. Spearman's rank correlation coefficient and linear regression analyses revealed a strong correlation between PPS and OS, whereas PFS was moderately correlated with OS. Furthermore, PPS was independently influenced by the presence of liver metastases at relapse and the administration of PRC. These findings provide insights into individual-level factors affecting PPS in patients with LD-SCLC following CRT.
Biostatisticians have proposed various methodologies to evaluate the reliability of alternative endpoints (19,20). In patients with extensive disease-small cell lung cancer (ED-SCLC), PFS has been shown to correlate with OS and could potentially serve as a surrogate endpoint for assessing survival efficacy (21,22); however, its suitability in LD-SCLC remains controversial (21). One study explored the concept of PPS, defined as OS minus PFS, assuming that a given treatment influences PFS but not PPS (8). This study indicated that a longer PPS in OS may dilute the significance of PFS. Some studies have identified a robust correlation between PPS after first-line chemotherapy in advanced NSCLC and OS at the clinical trial level (11,12). Similarly, others have explored the influence of PPS in ED-SCLC and advanced NSCLC at the individual patient level and suggested that PPS plays a significant role in OS (13-15,23-25).
Our study revealed that PFS does not consistently reflect OS in patients with relapsed LD-SCLC treated with CRT. Instead, PPS exerted a strong influence on OS. The data demonstrated that PFS tended to be shorter than PPS, whereas PPS significantly affected OS in a linear manner. This close correlation underscores that, although PFS is a crucial component of OS, its impact on OS might be less pronounced in this context. Prolonged PPS diminishes the relative significance of PFS after first-line therapy on OS. Hence, prioritizing OS as the primary efficacy endpoint across all lines of treatment seems crucial for aggressive malignancies such as SCLC. Although PPS surpasses PFS in terms of duration, it relies on death events, limiting its utility for prognostication in clinical practice. Nonetheless, PPS remains clinically significant because subsequent treatment following disease progression after front-line therapy can substantially affect OS. This suggests that optimizing subsequent therapy may improve OS outcomes. Our finding that OS exhibits a stronger correlation with PPS than with PFS implies that post-relapse chemotherapy exerts a greater impact on OS than initial CRT. Therefore, clinical trials for LD-SCLC should consider factors that could influence PPS to prevent potential confounding effects on OS.
In our study, patients with liver metastases during recurrence exhibited a significantly shorter PPS than those without. The liver is the most frequent site of metastasis in SCLC, with ~17% of the patients manifesting liver metastases at diagnosis, which is markedly higher than that observed in patients with NSCLC [4%] (26). SCLC has the highest incidence of liver metastasis (27). In ED-SCLC, the proportion of liver metastases can exceed 30%, reaching 60% in some cases (28,29). Patients with SCLC manifesting liver metastases have worsened prognoses (29-31). Some reports have demonstrated liver metastasis as an independent adverse prognostic factor of SCLC (29-31). In our patients, the timing of radiotherapy in CRT significantly differed between patients with or without liver metastases (Table SIII). Concurrent CRT, being more powerful and invasive than sequential CRT, is often administered to younger patients with good PS and organ function, which may have influenced the presence of liver metastasis at relapse. For patients with liver metastases, the lower incidence of liver metastases at relapse in the concurrent combination group, compared to the sequential combination therapy, is likely related to the increased intensity of treatment in the concurrent combination therapy group, which may contribute to better overall control. Ongoing clinical trials are evaluating the combination of CRT with ICIs, such as atezolizumab and durvalumab, for the treatment of LD-SCLC (32,33). In the future, as immune maintenance therapies are increasingly integrated into standard treatment, changes may occur in the management of liver metastases following CRT. Table SIV shows the breakdown of subsequent treatment regimens for 18 patients with liver metastases at relapse; 13/18 (72.2%) received chemotherapy, of which 9/13 (69.2%) received PRC. However, PPS was poor compared to the population without liver metastases. Of the patients included in this analysis, 50 (59.5%) received conventional fractionated radiotherapy of 60 Gy in 30 fractions, while 34 (40.5%) received accelerated hyperfractionated radiotherapy of 45 Gy in 30 fractions. The use of conventional fractionation in the sequential chemoradiotherapy group was primarily driven by its widespread adoption in clinical practice. This approach aligns with existing reports on sequential chemoradiotherapy, where conventional fractionation is the standard irradiation method (34). In the concurrent chemoradiotherapy group, many patients began radiotherapy with chemotherapy from the second cycle, as it could not be initiated in the first cycle of chemotherapy due to the risk of markedly enlarging the radiotherapy field. Additionally, in cases where the tumor growth was rapid, chemotherapy was prioritized to initiate treatment as soon as possible.
Prognostic factors for PPS, such as PRC, suggested that stabilizing the disease post-progression after CRT could prolong PPS and OS. A phase III study (GFPC01-13) demonstrated significantly prolonged PFS in patients receiving carboplatin and etoposide (platinum re-challenge) compared to oral topotecan monotherapy (35). Although this prospective study did not include ICIs in the platinum-based combination chemotherapy regimen, our findings indicate that PRC remains a viable option. Although we did not specifically consider the number of post-progression chemotherapeutic regimens administered, our examination of subsequent therapy regimens revealed PRC as an independent prognostic factor for PPS. Notably, although the number of regimens administered after disease progression may correlate with a longer PPS, selecting patients with good organ function for PRC may have contributed to a higher number of administered regimens. Despite the limited treatment options available for SCLC compared to NSCLC, the considerable variety of treatment regimens utilized for LD-SCLC after CRT in our study can be attributed to the expanding array of chemotherapeutic options like amrubicin, irinotecan, and topotecan, for post-relapse chemotherapy. Amrubicin or ICI administration following CRT did not emerge as significant prognostic factors for PPS, which indicated that these agents may not influence PPS or OS. This could be attributed to insufficient statistical power in detecting significant differences in the presence of ICI administration, considering the recent integration of ICIs into clinical practice for SCLC and the relatively small number of patients who received chemotherapy, including ICIs, in our study population. Table SIII depicts the clinical background differences between patients who received platinum re-challenge and those who did not. ICI administration is considered confounding because it is typically administered only in combination with PRC in clinical practice. However, cisplatin is less commonly used in combination with CRT for patients who are not receiving PRC, potentially due to their poorer health status or organ function. The lower number of patients who underwent PCI among those who did not receive PRC further suggests that a favorable patient background that allows for such procedures may be a factor influencing the use of PRC.
In conventional clinical trials, PFS encompasses the period from treatment initiation to either disease progression or death from any cause. However, in this analysis, PFS was defined as the time from the start of treatment to disease progression. This is because some patients with LD-SCLC who are cured would have a long-term prognosis and likely not experience disease progression or death from underlying disease due to SCLC relapse. If death from any cause were included in PFS, a patient with no disease progression but death from other causes would have a PPS of zero, hindering the original PPS assessment.
Ongoing clinical trials are evaluating the use of combining ICIs such as atezolizumab and durvalumab with CRT for LD-SCLC treatment (33,34). Whether the significance of PPS observed in our study will yield similar outcomes once these agents become more widely incorporated into clinical practice remains to be determined.
Our study had some limitations. First, its retrospective design relied on subjective assessments by physicians, which may introduce variability in treatment response and survival data. Second, treatment decisions were made at the discretion of the treating physicians, possibly resulting in treatment modifications, omissions, or delays. To mitigate this bias, we included consecutive patients treated at the participating institutions and meticulously reviewed their medical records. Third, the inclusion of cases with censored survival data may have some effect on the analysis. In cases where patients did not die during the follow-up period, the duration of PFS remained unchanged, leading to a prolonged PPS and OS and reinforcing the strong correlation between PPS and OS. Fourth, the results of the present study indicate that PPS may be more strongly associated with OS than PFS. This applies specifically to patients with LD-SCLC who received chemoradiotherapy and subsequently relapsed. However, this finding may not be applicable to the entire population, as 40 patients survived without recurrence and did not require further treatment. This limitation raises concerns about the generalizability of the impact of post-relapse treatment on survival, as the study's design does not fully support such a broad conclusion. Therefore, the results should be interpreted with caution until further analysis validates these results. Lastly, the retrospective nature and small size of the study, coupled with heterogeneous treatment procedures, may introduce variability and increase susceptibility to the effects of interactions between variables, making it challenging to isolate the independent effects of each factor. Nonetheless, the study provides valuable insights into the disease course after recurrence in consecutive patients with recurrent LD-SCLC who received chemoradiotherapy.
In summary, the results of this study on patients with relapsed LD-SCLC treated with CRT suggest that PPS has a greater impact on OS than on PFS in patients wherein CRT was not curative. Factors such as the presence of liver metastases or PRC may affect PPS. Further research should involve larger multicenter studies to validate our findings in diverse patient populations across various clinical settings.
Supplementary Material
Tumor response.
Patient characteristics (subsequent treatment vs. observation group).
Patient characteristics based on the presence of liver metastases at relapse and administration of platinum re-challenge chemotherapy.
Chemotherapy regimens administered following disease progression after chemoradiotherapy among patients with liver metastases.
Acknowledgements
The authors thank Ms. Kyoko Nakagawa, Dr. Kosuke Hashimoto, Dr. Yu Miura, Dr. Kenji Masaki, Dr. Ou Yamaguchi and Dr. Fuyumi Nisahihara for their assistance in preparing the manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
AS, SE and HI conceptualized the study and designed methodology. HI and KKa collected and analyzed the data. AS, SE and HI were project administrators, visualized the diagram and wrote the original draft. KKa, KMi and HK supervised the study. SO, TA, AM, KMa, KKo, KMi, SK and HK performed the experiments and provided resources. AS and HI confirm the authenticity of all the raw data. All authors wrote, reviewed and edited the paper. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All procedures complied with the ethical standards of the institutional and/or national research committee, and the 1964 Declaration of Helsinki and its subsequent amendments or comparable ethical standards. This study was approved by the Institutional Review Board of the International Medical Center at Saitama Medical University (approval no. 2023-033). Ethics approval was also provided by Gunma Prefectural Cancer Center (approval no. 405-05038). Informed consent was waived due to the retrospective nature of this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Siegel RL, Miller KD, Fuchs HE and Jemal A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL and Piccirillo J: Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 24:4539–4544. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Dómine M, Moran T, Isla D, Martí JL, Sullivan I, Provencio M, Olmedo ME, Ponce S, Blasco A and Cobo M: SEOM clinical guidelines for the treatment of small-cell lung cancer (SCLC) (2019). Clin Transl Oncol. 22:245–255. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Dingemans AC, Früh M, Ardizzoni A, Besse B, Faivre-Finn C, Hendriks LE, Lantuejoul S, Peters S, Reguart N, Rudin CM, et al: Small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 32:839–853. 2021.PubMed/NCBI View Article : Google Scholar | |
|
NCCN Guidelines®-Small cell lung cancer. version 2.2024. Available from: https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. Accepted at April 12, 2024. | |
|
Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, Nishiwaki Y, Watanabe K, Noda K, Tamura T, et al: Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: Results of the Japan clinical oncology group study 9104. J Clin Oncol. 20:3054–3060. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Broglio KR and Berry DA: Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 101:1642–1649. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Soria JC, Massard C and Le Chevalier T: Should progression-free survival be the primary measure of efficacy for advanced NSCLC therapy? Ann Oncol. 21:2324–2332. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N and Manegold C: Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab ss first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 27:1227–1234. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Hotta K, Kiura K, Fujiwara Y, Takigawa N, Hisamoto A, Ichihara E, Tabata M and Tanimoto M: Role of survival post-progression in phase III trials of systemic chemotherapy in advanced non-small-cell lung cancer: A systematic review. PLoS One. 6(e26646)2011.PubMed/NCBI View Article : Google Scholar | |
|
Hayashi H, Okamoto I, Morita S, Taguri M and Nakagawa K: Postprogression survival for first-line chemotherapy of patients with advanced non-small-cell lung cancer. Ann Oncol. 23:1537–1541. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Imai H, Takahashi T, Mori K, Ono A, Akamatsu H, Shukuya T, Taira T, Kenmotsu H, Naito T, Murakami H, et al: Individual-level data on the relationships of progression-free survival, post-progression survival, and tumor response with overall survival in patients with advanced non-squamous non-small cell lung cancer. Neoplasma. 61:233–240. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Imai H, Yamada Y, Sugiyama T, Minemura H, Kaira K, Kanazawa K, Kasai T, Kaburagi T and Minato K: Gunma-Ibaraki-Fukushima-Tochigi (GIFT) group. Clinical impact of post-progression survival on overall survival in elderly patients with non-small-cell lung cancer harboring sensitive EGFR mutations treated with first-line EGFR tyrosine kinase inhibitors. Chemotherapy. 63:181–189. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Imai H, Kishikawa T, Minemura H, Yamada Y, Ibe T, Mori K, Yamaguchi O, Mouri A, Hamamoto Y, Kanazawa K, et al: Post-progression survival influences overall survival among patients with advanced non-small cell lung cancer undergoing first-line Pembrolizumab monotherapy. Oncology. 99:562–570. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Kasahara N, Imai H, Kaira K, Mori K, Wakuda K, Ono A, Taira T, Kenmotsu H, Harada H, Naito T, et al: Clinical impact of post-progression survival on overall survival in patients with limited-stage disease small cell lung cancer after first-line chemoradiotherapy. Radiol Oncol. 49:409–415. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L: International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions. The IASLC lung cancer staging project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2:706–714. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al: New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer (version 1.1). 45:228–247. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Weir CJ and Walley RJ: Statistical evaluation of biomarkers as surrogate endpoints: A literature review. Stat Med. 25:183–203. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Fleischer F, Gaschler-Markefski B and Bluhmki E: A statistical model for the dependence between progression-free survival and overall survival. Stat Med. 28:2669–2686. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Foster NR, Qi Y, Shi Q, Krook JE, Kugler JW, Jett JR, Molina JR, Schild SE, Adjei AA and Mandrekar SJ: Tumor response and progression-free survival as potential surrogate endpoints for overall survival in extensive stage small-cell lung cancer: Findings on the basis of North central cancer treatment group trials. Cancer. 117:1262–1271. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Foster NR, Renfro LA, Schild SE, Redman MW, Wang XF, Dahlberg SE, Ding K, Bradbury PA, Ramalingam SS, Gandara DR, et al: Multitrial evaluation of progression-free survival as a surrogate end point for overall survival in first-line extensive-stage small-cell lung cancer. J Thorac Oncol. 10:1099–1106. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Imai H, Mori K, Wakuda K, Ono A, Akamatsu H, Shukuya T, Taira T, Kenmotsu H, Naito T, Kaira K, et al: Progression-free survival, post-progression survival, and tumor response as surrogate markers for overall survival in patients with extensive small cell lung cancer. Ann Thorac Med. 10:61–66. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Imai H, Mori K, Watase N, Fujimoto S, Kaira K, Yamada M and Minato K: Clinical significance of the relationship between progression-free survival or postprogression survival and overall survival in patients with extensive disease-small-cell lung cancer treated with carboplatin plus etoposide. Can Respir J. 2016(5405810)2016.PubMed/NCBI View Article : Google Scholar | |
|
Imai H, Kaira K and Minato K: Clinical significance of post-progression survival in lung cancer. Thorac Cancer. 8:379–386. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Kagohashi K, Satoh H, Ishikawa H, Ohtsuka M and Sekizawa K: Liver metastasis at the time of initial diagnosis of lung cancer. Med Oncol. 20:25–28. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Wang X, Wang Z, Pan J, Lu ZY, Xu D, Zhang HJ, Wang SH, Huang DY and Chen XF: Patterns of extrathoracic metastases in different histological types of lung cancer. Front Oncol. 10(715)2020.PubMed/NCBI View Article : Google Scholar | |
|
Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J and Hemminki K: Metastatic sites and survival in lung cancer. Lung Cancer. 86:78–84. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Arriola E, Trigo JM, Sánchez-Gastaldo A, Navarro A, Perez C, Crama L and Ponce-Aix S: Prognostic value of clinical staging according to TNM in patients with SCLC: A real-world surveillance epidemiology and end-results database analysis. JTO Clin Res Rep. 3(100266)2022.PubMed/NCBI View Article : Google Scholar | |
|
Nakazawa K, Kurishima K, Tamura T, Kagohashi K, Ishikawa H, Satoh H and Hizawa N: Specific organ metastases and survival in small cell lung cancer. Oncol Lett. 4:617–620. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Zhang S, Wang Y, Li S, Liu Y and Cheng Y: A retrospective analysis of prognostic factors and treatment choices in small cell lung cancer with liver metastasis. J Thorac Dis. 15:6776–6787. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Ross HJ, Hu C, Higgins KA, Jabbour SK, Kozono DE, Owonikoko TK, Movsas B, Solberg T, Xiao C, Williams TM, et al: NRG Oncology/Alliance LU005: A phase II/III randomized clinical trial of chemoradiation versus chemoradiation plus atezolizumab in limited stage small cell lung cancer. J Clin Oncol. 38(TPS9082)2020. | |
|
Senan S, Okamoto I, Lee GW, Chen Y, Niho S, Mak G, Yao W, Shire N, Jiang H and Cho BC: Design and rationale for a phase III, randomized, placebo-controlled trial of Durvalumab with or without tremelimumab after concurrent chemoradiotherapy for patients with limited-stage small-cell lung cancer: The Adriatic study. Clin Lung Cancer. 21:e84–e88. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Fried DB, Morris DE, Poole C, Rosenman JG, Halle JS, Detterbeck FC, Hensing TA and Socinski MA: Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol. 22:4837–4845. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Baize N, Monnet I, Greillier L, Geier M, Lena H, Janicot H, Vergnenegre A, Crequit J, Lamy R, Auliac JB, et al: Carboplatin plus etoposide versus topotecan as second-line treatment for patients with sensitive relapsed small-cell lung cancer: An open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 21:1224–1233. 2020.PubMed/NCBI View Article : Google Scholar |