Treatment patterns and clinical outcomes in Chinese patients with NSCLC with MET alterations resistant to EGFR‑TKI therapy
- Authors:
- Published online on: July 4, 2025 https://doi.org/10.3892/mco.2025.2876
- Article Number: 81
-
Copyright: © Shen et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Non-small cell lung cancer (NSCLC) accounts for ~85% of all lung cancer cases (1), with its high prevalence driving substantial advances in molecular genetics and targeted therapies (2). A major breakthrough in the understanding and treatment of NSCLC is the discovery of mutations in the epidermal growth factor receptor (EGFR), which are found in ~10% of Caucasian patients and up to 50% of Asian patients (3). In patients with locally advanced or metastatic NSCLC harboring EGFR mutations (EGFRm), EGFR tyrosine kinase inhibitors (EGFR-TKI) have shown superior efficacy over conventional chemotherapy and are now established as the standard first-line treatment (4).
It has been previously reported that ~20-30% of patients with NSCLC with activated EGFRm exhibit primary resistance to EGFR-TKI (5). Furthermore, nearly all patients eventually develop acquired resistance (6). Understanding the mechanisms underlying EGFR-TKI resistance is critical to developing strategies to overcome it in NSCLC (7). Studies have identified three primary mechanisms of secondary resistance to EGFR-TKI: on-target resistance, off-target resistance and histological transformation (8). Among these, dysregulation of the mesenchymal to epithelial transition (MET) signaling pathway, including MET amplification or MET overexpression, is one of the most common mechanisms of resistance to EGFR-TKI in patients with EGFRm advanced NSCLC (9-11). Research data indicates that ~2-26% of patients with EGFRm NSCLC exhibit de novo MET amplification, while ~20% of patients develop MET amplification following EGFR-TKI therapy, emphasizing its pivotal role in resistance mechanisms (9,12,13).
Although several clinical trials have evaluated the efficacy of combining MET-TKI and EGFR-TKI in NSCLC patients with MET amplification and/or MET overexpression in the context of acquired resistance to EGFR-TKI, the results have shown promising clinical efficacy and a manageable safety profile (14,15). However, real-world data on different treatment patterns and clinical outcomes for these patients remain scarce, particularly in the Chinese population, underscoring the need for further region-specific studies. To address this gap, the treatment regimens and clinical outcomes of 32 patients with locally advanced or metastatic EGFRm NSCLC and MET amplification or protein overexpression who experienced progression on EGFR-TKI were analyzed. These patients were treated at the Affiliated Hospital of Jiangnan University in China.
Materials and methods
Patients' eligibility
This retrospective study analyzed data which were collected between February 2022 and April 2024 from patients with NSCLC characterized by MET amplification or MET overexpression who experienced disease progression following EGFR-TKI therapy. The inclusion criteria included the following: i) Age >18 years; ii) an Eastern Cooperative Oncology Group Performance Status of 0 to 2; iii) a diagnosis of locally advanced or metastatic lung cancer; iv) confirmed EGFRm status; v) and tumor tissue testing showing MET amplification or overexpression either before EGFR-TKI treatment or after the onset of EGFR-TKI resistance. Patients who received MET-TKI treatment prior to developing resistance to EGFR-TKI were excluded. Patients who did not receive systemic anticancer treatment following EGFR-TKI resistance were excluded. Additionally, patients with incomplete clinical information or those with concurrent malignancies were also excluded. Treatment regimens were determined at the discretion of the attending physician. The present study was approved (approval no. LS2024553) by the Ethical Committee of the Affiliated Hospital of Jiangnan University (Wuxi, China) and conducted in accordance with the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all participants.
MET IHC and MET FISH
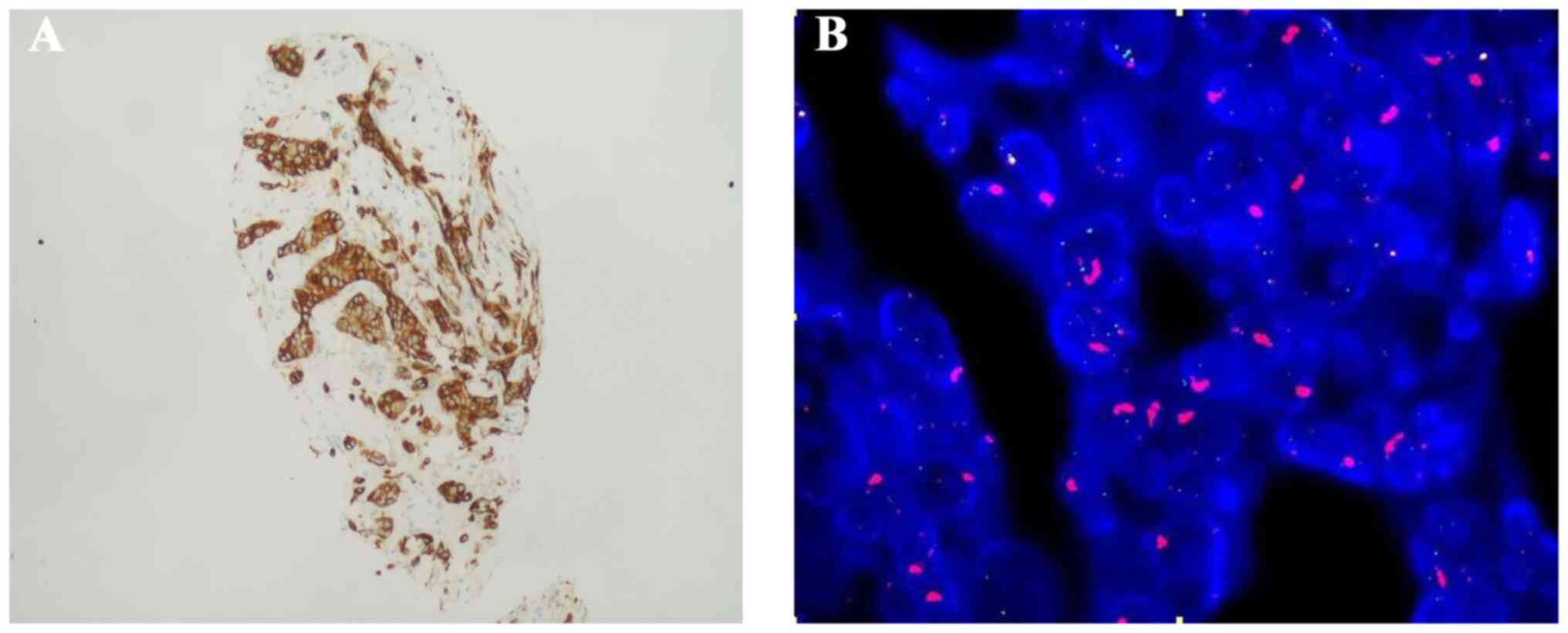
Immunohistochemistry (IHC) staining for MET was performed on 4-µm-thick sections obtained from formalin-fixed paraffin-embedded tumor tissue using the SP44 clone. MET protein overexpression was defined as positive if at least 50% of tumor cells exhibited strong staining intensity (3+ score) (Fig. 1A). IHC scoring was carried out by a trained histopathologist to ensure accuracy. In addition, MET gene amplification was evaluated using fluorescence in situ hybridization (FISH) on tissue biopsy samples. MET amplification was defined as positive if the MET gene copy number was ≥5 or the MET-to-centromere of chromosome 7 (MET/CEP7) ratio was ≥2 (Fig. 1B).
Treatment regimens
Based on different treatment regimens and patients' compliance, the study population was categorized into three subgroups. For ease of classification, patients treated with MET-TKI (savolitinib: 600 mg daily for those weighing ≥50 kg, 400 mg daily for those weighing <50 kg) in combination with EGFR-TKI (osimertinib 80 mg daily, furmonertinib 80 mg daily, or aumolertinib 110 mg daily) were designated as the MET-TKI plus EGFR-TKI group. Patients receiving cytotoxic chemotherapy agents, either alone or in combination with additional antitumor therapies, were classified into the chemotherapy-based regimen group. Patients who received other anticancer therapies-including anti-angiogenic agents such as bevacizumab or anlotinib, immune checkpoint inhibitors (ICIs) such as sintilimab or tislelizumab, or combination therapy with EGFR-TKIs-were categorized into the other regimens group.
Primary outcomes measures
The clinical outcomes evaluated in the present study included time to treatment failure (TTF), objective response rate (ORR), post-progression survival (PPS) and adverse events (AEs). TTF was defined as the time from treatment initiation to the discontinuation of next-line therapy following EGFR-TKI progression due to any of the following reasons: Disease progression, treatment-related toxicity, patient preference, physician decision, initiation of a subsequent treatment line, or death/loss to follow-up. Loss to follow-up was addressed as censoring values. ORR was defined as the proportion of patients achieving a complete response (CR) or partial response (PR). The PPS was defined as the duration from the initiation of subsequent therapy following EGFR-TKI progression to death from any cause. Tumor response was evaluated based on the Response Evaluation Criteria in Solid Tumors version 1.1, while AEs were graded according to the Common Terminology Criteria for Adverse Events (version 5.0) (15).
Statistical analysis
Patients' demographic and clinical characteristics were summarized using descriptive statistics. Survival analyses were conducted using the Kaplan-Meier method, which provided medians and 95% confidence intervals (CI). Statistical significance was evaluated using log-rank tests. P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were conducted using SPSS version 27.0 (IBM Corp.).
Results
Patients' demographics and clinical characteristics
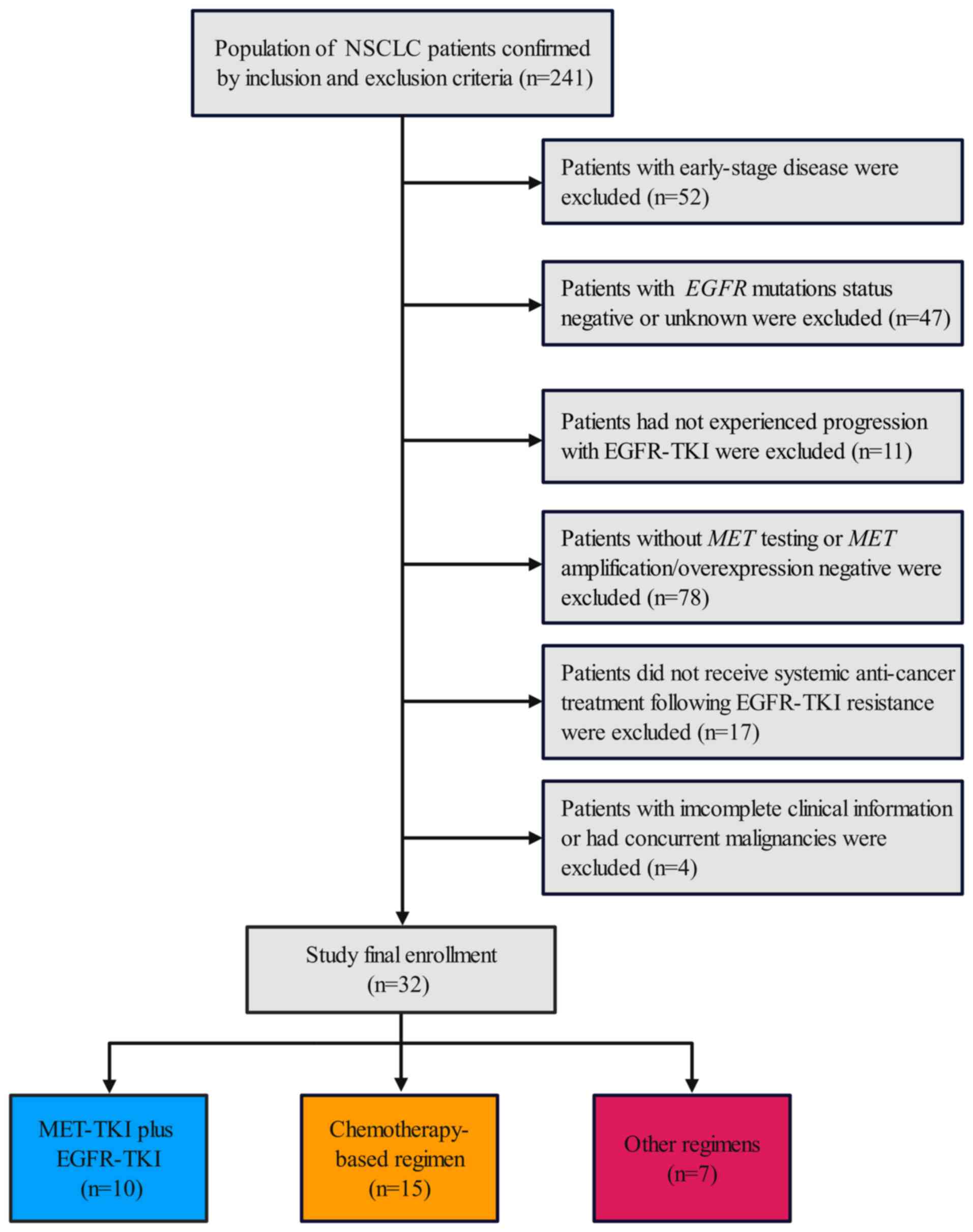
Between February 2022 and April 2024, a total of 241 individuals were assessed for eligibility. Of these, 209 participants were excluded for reasons outlined in the patient flowchart (Fig. 2) and 32 patients formed the analyzable cohort.
The median age of the cohort was 61.5 years (range, 42-80 years). Male patients accounted for 43.8% of the group, and 14 patients (43.8%) had an ECOG PS score of 2. Most patients (93.7%) presented with stage IV disease, including 40.6% with brain metastases. A total of eight patients (25.0%) had primary MET alterations, whereas 24 (75.0%) developed secondary alterations. Molecular profiling identified MET amplification in 31.3% and overexpression in 68.7% of cases (Table I). In the cohort, 81.3% of patients exhibited resistance to third-generation EGFR-TKI, while 18.7% experienced disease progression on first- or second-generation EGFR-TKI. A total of 10 patients (31.3%) received combination therapy with MET-TKI and EGFR-TKI, 15 (46.9%) underwent chemotherapy-based regimen, and 7 (21.9%) received other therapeutic regimens.
Therapeutic effects various regimens
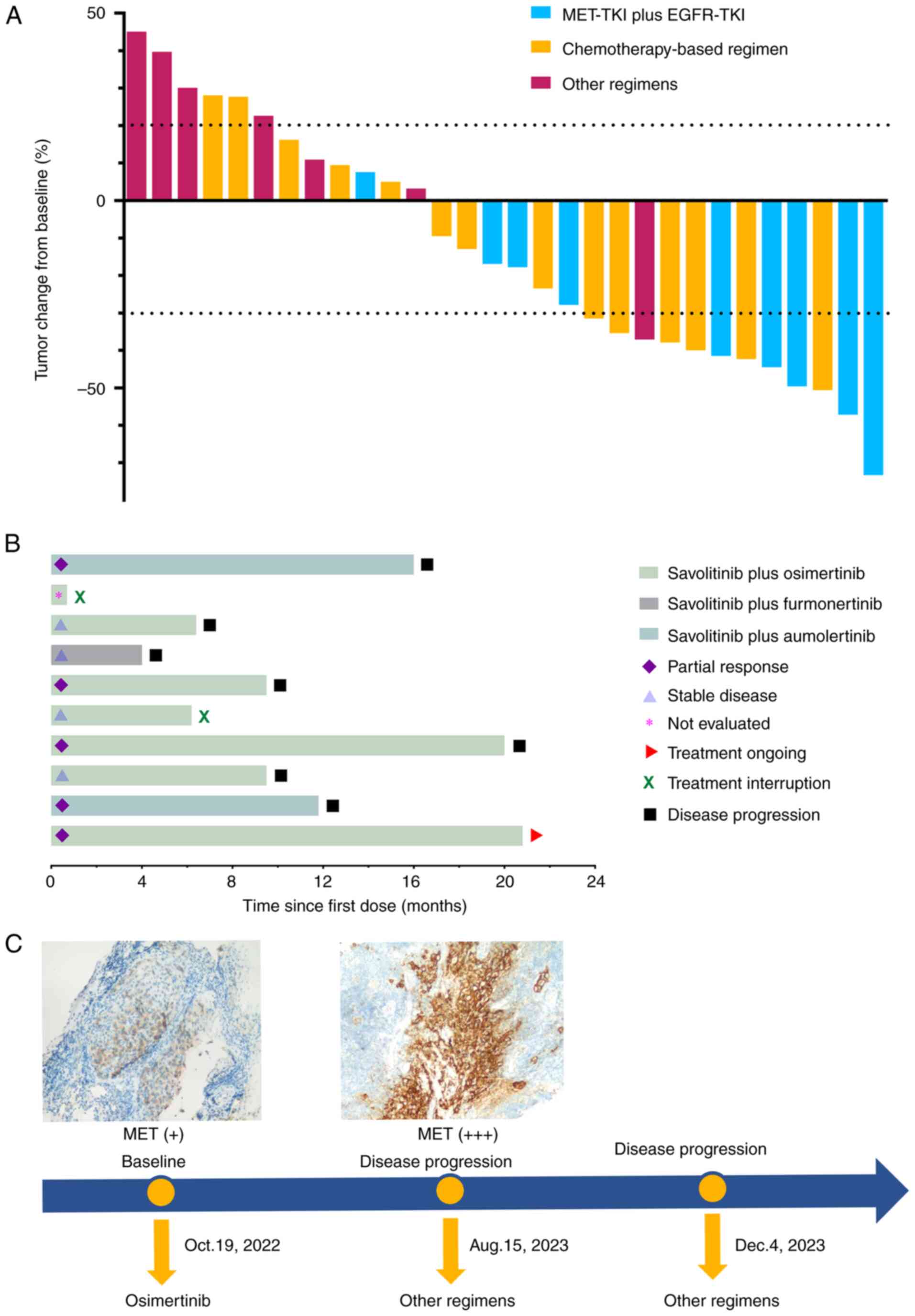
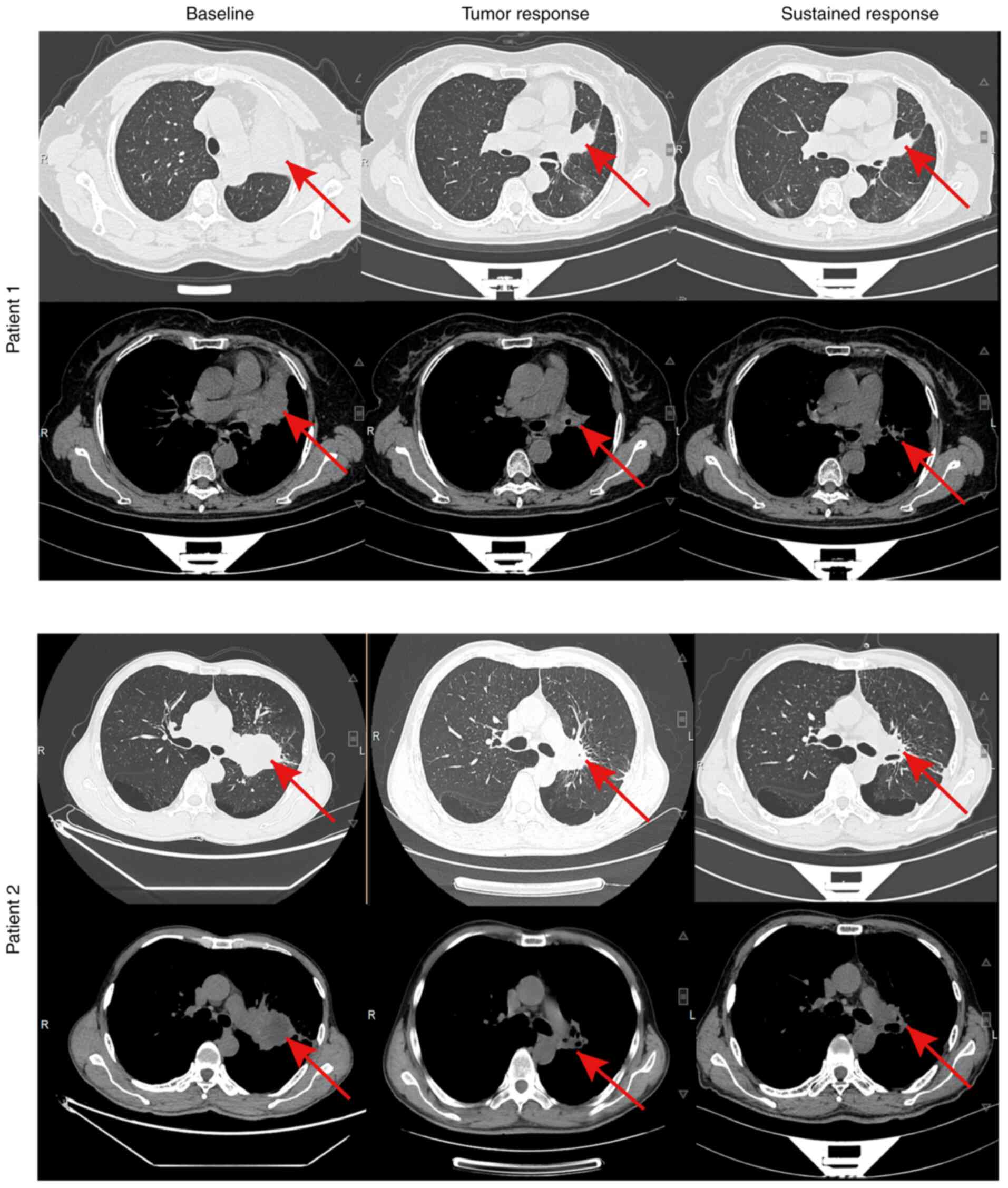
In total, 30 patients (93.7%) underwent at least one imaging follow-up after treatment. A waterfall plot illustrating tumor response is shown in Fig. 3A. Among the patients, 12 achieved a PR, while no CR were observed, resulting in an ORR of 40.0%. Patients treated with a combination of MET-TKI and EGFR-TKI achieved the highest ORR at 55.6%, compared with an ORR of 42.9% for those receiving chemotherapy-based therapy. By contrast, patients treated with other regimens demonstrated a markedly lower ORR of only 14.3%. A swimmer plot depicting the treatment duration for patients receiving MET-TKI plus EGFR-TKI is also shown in Fig. 3B. Treatment was interrupted in two patients: one discontinued treatment due to worsening clinical symptoms, while the other did so for personal reasons. At the last follow-up, one patient remained on dual oral TKI therapy with stable disease (Table SI). The treatment timeline of a patient who developed secondary resistance to MET-driven EGFR-TKI and was subsequently treated with other regimens is demonstrated in Fig. 3C.
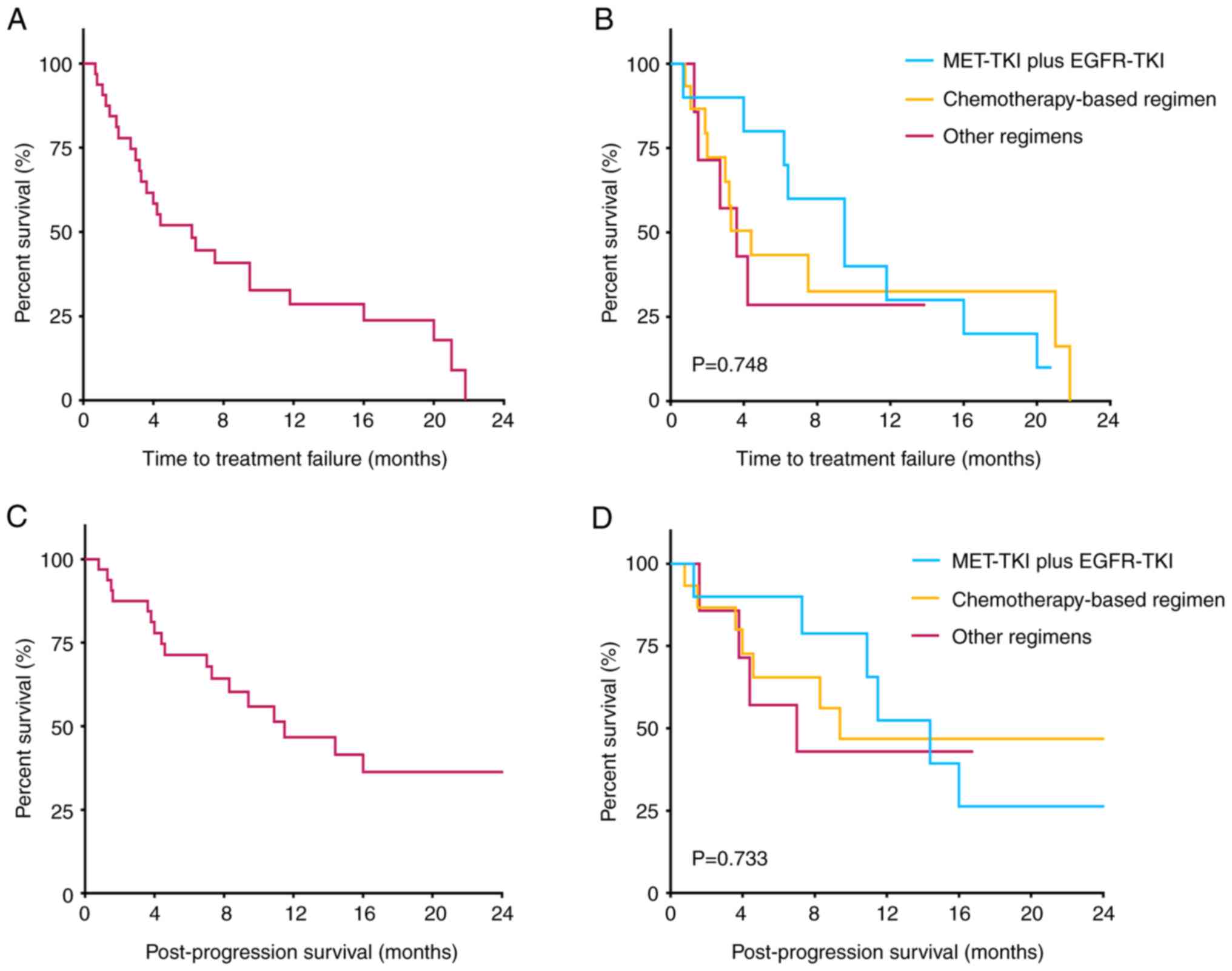
The follow-up period concluded on October 24, 2024, with one patient lost to follow-up. The median follow-up durations were 16.8 months (95% CI, 12.3-21.3 months) for the entire cohort, 20.8 months (95% CI, 3.4-38.2 months) for the MET-TKI plus EGFR-TKI group, 16.8 months (95% CI, 9.5-24.1 months) for the chemotherapy-based group, and 9.5 months (95% CI, 7.1-11.9 months) for the group receiving other regimens. The median TTF for all patients was 6.2 months (95% CI, 2.5-9.9 months). Among the subgroups, patients receiving MET-TKI combined with EGFR-TKI achieved the longest median TTF at 9.5 months (95% CI, 4.8-14.2 months), compared with 4.4 months (95% CI, 2.2-6.6 months) in the chemotherapy-based group and 3.6 months (95% CI, 1.3-5.9 months) in the other regimens group; these differences, however, were not statistically significant (P=0.748). By the end of the follow-up period, 19 patients (59.4%) had died, with a median PPS of 11.5 months (95% CI, 4.8-18.2 months) across all patients. Subgroup analysis showed that the MET-TKI plus EGFR-TKI group had the longest median PPS at 14.4 months (95% CI, 9.8-18.9 months), compared with 9.4 months (95% CI, 0-23.2 months) in the chemotherapy-based group and 7.0 months (95% CI, 0.3-13.7 months) in the group receiving other regimens. However, the differences in survival outcomes between these groups did not reach statistical significance (P=0.733) (Table II and Fig. 4).
Treatment-related AEs
Nausea, peripheral edema, decreased appetite, and rash were the most frequently observed treatment-related AEs in patients receiving dual oral TKI combination therapy, with incidence rates of 40, 30, 30 and 10%, respectively. Notably, one patient experienced a grade 3 or higher adverse event characterized by severe loss of appetite.
In patients receiving a chemotherapy-based regimen, leukopenia was the most common adverse reaction, observed in 66.7% of cases, including three instances of severe leukopenia. Thrombocytopenia was reported in 7 patients, while anemia was documented in 2 patients. Additionally, 4 cases experienced chemotherapy-induced nausea or vomiting. Among patients treated with other regimens, hypertension and fatigue were the most frequently reported AEs, each occurring in 42.8% of cases. Furthermore, liver function injury was observed in two out of seven patients (28.5%) (Table III).
Discussion
The present study provides real-world evidence to guide subsequent treatments for patients with EGFRm NSCLC with MET amplification or protein overexpression who experience progression after EGFR-TKI therapy. Resistance mechanisms driven by MET alterations, including MET amplification, MET exon 14 skipping mutations, and MET overexpression, present significant clinical challenges by reactivating downstream pathways such as PI3K/AKT and MAPK, thereby bypassing EGFR inhibition (16). Although various strategies have been explored to overcome resistance, no universally effective therapies have yet been established for patients with concurrent MET alterations. Our findings confirm the clinical benefit of a dual-targeted TKI strategy-combining MET-TKI with EGFR-TKI-in a Chinese patient cohort, demonstrating its potential to improve outcomes beyond those achieved with conventional chemotherapy. Comparison with other salvage regimens provided additional insights into the relative efficacy and tolerability of available options. These real-world data enhance the clinical applicability of our findings and support the need for personalized, context-specific treatment strategies.
A significant observation in the present study is the improvement in both median TTF and PPS by over five months for patients treated with MET-TKI plus EGFR-TKI compared with chemotherapy-based or other regimens. This dual-targeted TKI strategy achieved an ORR of 55.6%. To illustrate this, a representative imaging group of two patients treated with a combination of MET-TKI and EGFR-TKI was selected, demonstrating sustained efficacy (Fig. 5). This finding aligns with the INSIGHT 2 study by Wu et al (17), which explored tepotinib combined with osimertinib as a strategy for treating patients with EGFRm NSCLC with MET amplification resistant to osimertinib, reporting an ORR of 50.0%. These results underscore the promise of this dual-targeting approach, suggesting that simultaneously inhibiting both the EGFR and MET pathways may address key resistance mechanisms arising during EGFR-TKI treatment. Nevertheless, this ORR remains lower than the initial response rates to EGFR-TKI, which has been reported to range between 60-80% (18-21), highlighting the ongoing challenges in overcoming resistance in this context. Additional factors, such as mutations in human epidermal growth factor receptor 2 (HER2), Kirsten rat sarcoma viral oncogene homolog (KRAS), v-raf murine sarcoma viral oncogene homolog B1 (BRAF), anaplastic lymphoma kinase (ALK), or ROS proto-oncogene 1 (ROS1), may further complicate treatment outcomes. These genetic alterations likely contribute to resistance and are not sufficiently addressed by the combination of MET-TKI and EGFR-TKI alone. Furthermore, eight patients in the present study exhibited primary MET amplification or overexpression. Whether the combination of EGFR-TKI and MET-TKI can delay resistance in these patients when used during initial therapy remains uncertain. Ongoing prospective trials, such as the FLOWERS study (NCT05163249), are expected to provide critical insights into the efficacy of early dual-targeted therapy in this subgroup.
Chemotherapy continues to be the standard salvage therapy for patients with EGFRm NSCLC who progress on EGFR-TKI treatment, despite its limited efficacy (22). Several studies have reported that chemotherapy after EGFR-TKI resistance yields an ORR of ~20-30%, with median overall survival ranging from 6 to 12 month (23-25). In the present study, patients receiving chemotherapy-based treatment had a shorter median PPS compared with those treated with MET-TKI plus EGFR-TKI (9.4 vs. 14.4 months), which aligns with the lower ORR observed in this group (42.9 vs. 55.6%). Our results surpass those reported in previous studies, possibly due to the use of platinum-based doublet chemotherapy combined with anti-angiogenic agents or ICIs in some patients. Emerging evidence suggests that combining chemotherapy with targeted agents or ICIs, could improve efficacy compared with chemotherapy alone (25-27). Although chemotherapy-based regimens may be less effective than MET-TKI plus EGFR-TKI therapy in overcoming MET-driven resistance to EGFR-TKI, they remain essential for certain patients, especially when dual TKI therapies are unavailable or poorly tolerated. A notable observation is that patients receiving other regimens, including ICIs and anti-angiogenic agents, experienced the poorest clinical outcomes. This finding aligns with prior evidence suggesting that patients with EGFRm NSCLC derive limited benefit from ICIs due to the ‘cold’ immune microenvironment of these tumors (28). It was hypothesized that, in the absence of cytotoxic chemotherapy, which primes the immune system by inducing immunogenic cell death, the efficacy of ICIs is diminished.
The effectiveness of third-generation EGFR-TKI has been shown to exceed that of first-generation TKI in the first-line treatment of EGFRm advanced NSCLC (20,29,30). Re-biopsy plays a crucial role in determining the next course of treatment following progression on initial EGFR-TKI therapy (31). In the present study, 81.3% of patients underwent a second biopsy after developing resistance to EGFR-TKI. A smaller subset of patients, who experienced progression following first- or second-generation EGFR-TKI therapy and were identified as harboring both the T790M mutation and MET amplification, were subsequently switched to a combination therapy of third-generation EGFR-TKI and MET-TKI. Although genetic testing was not performed on all patients, analysis of the available data from several individuals revealed no concurrent actionable alterations, including KRAS, BRAF, ALK, ROS1, or HER2. We acknowledge that broader genetic testing across the entire cohort may provide a more comprehensive understanding of the genomic landscape associated with resistance mechanisms.
The incidence of severe drug-related AEs was low in the present study. The safety profiles of the three therapeutic approaches evaluated exhibited distinct patterns of AEs, reflecting their differing mechanisms of action and systemic impacts. Patients receiving dual oral TKI combination therapy experienced AEs that are commonly associated with targeted therapies. Nausea, peripheral edema and decreased appetite were the most frequent AEs. These findings are consistent with the known safety profiles of MET-TKI and EGFR-TKI, as gastrointestinal disturbances, dermatologic reactions and edema are well-documented AEs of these agents (17). Such AEs may be attributed to off-target effects, as both MET-TKI and EGFR-TKI interact with multiple signaling pathways involved in homeostatic processes. By contrast, leukopenia, thrombocytopenia and anemia were the most prevalent AEs in patients receiving chemotherapy-based regimens. These findings are consistent with the cytotoxic effects of chemotherapeutic agents, which target rapidly dividing cells and consequently impair bone marrow function (32). Patients treated with other regimens, including ICIs and anti-angiogenic agents, experienced AEs such as hypertension, fatigue and liver function injury. Hypertension reflects the vascular effects of anti-angiogenic agents, while ICIs are known to induce immune-mediated fatigue and hepatotoxicity.
Several limitations of the present study warrant careful consideration. First, the relatively small sample size of 32 patients limits the generalizability of the current findings, potentially reducing their applicability to larger and more diverse populations. Additionally, the retrospective nature of the study introduces potential biases, such as selection and information bias, which could undermine the validity of the results. Second, although treatment responses were evaluated using the RECIST criteria, the lack of next-generation sequencing data for certain patients limits our ability to fully understand the spectrum of genetic alterations contributing to EGFR-TKI resistance. The molecular heterogeneity of the tumors, including potential co-occurring mutations, could have influenced clinical outcomes, but these factors were not thoroughly investigated in our study. Third, while the ratio of loss to follow-up in the present study was low, loss to follow-up always carries the potential to introduce bias. Moreover, the follow-up period in our study was relatively short, with a median duration of 16.8 months. Longer follow-up is essential to assess the durability of treatment responses and the long-term survival benefits associated with the combination therapy of MET-TKI and EGFR-TKI. Finally, to further investigate the role of this dual-target therapy, future prospective studies with larger sample sizes, extended follow-up periods, and more comprehensive molecular profiling are required to provide clearer insights into its effectiveness in overcoming resistance mechanisms in EGFRm NSCLC.
In conclusion, the results of a real-world study were reported, which evaluated the therapeutic strategies and clinical outcomes of patients with NSCLC with MET alterations and progression on EGFR-TKI therapy. The combination of MET-TKI and EGFR-TKI demonstrates promising benefits in terms of TTF, PPS and ORR, underscoring its potential as a viable treatment option. However, the modest improvements observed, coupled with the inherent limitations of the present study, emphasize the necessity for further research to optimize treatment strategies and gain a deeper understanding of the long-term efficacy of combination therapy in addressing MET-driven resistance to EGFR-TKI.
Supplementary Material
Detailed information of individual patients enrolled in the present study.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Wuxi City Social Development Science and Technology Demonstration Project (grant no. N20201005).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
CLS, SYZ, XSG and YM carried out the studies, participated in collecting data, analysis, and drafted the manuscript. XLW and DS supported data analysis and provided valuable feedback on this manuscript. CLS and YM confirm the authenticity of all the raw data. All authors contributed to the article, read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved (approval no. LS2024553) by the Institutional Review Boards of Affiliated Hospital of Jiangnan University (Wuxi, China). Written informed consent was obtained from every patient for participation in this study prior to undergoing treatment.
Patient consent for publication
Written informed consent for publication of patient data and associated images obtained from the participants.
Competing interests
The authors declare that they have no competing interests.
References
|
Beaudet M, Lacasse Y and Labbé C: Palliative systemic therapy given near the end of life for metastatic non-small cell lung cancer. Curr Oncol. 29:1316–1325. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Ichikawa M, Muramatsu N, Matsunaga W, Ishikawa T, Okuda T, Okamoto H and Gotoh A: Effects of inhalable gene transfection as a novel gene therapy for non-small cell lung cancer and malignant pleural mesothelioma. Sci Rep. 12(8634)2022.PubMed/NCBI View Article : Google Scholar | |
|
Yang W, Gao Y, Li X, Zhang J, Liu T, Feng X, Pan H, Yang X, Xie S, Feng X, et al: Postoperative survival of EGFR-TKI-targeted therapy in non-small cell lung cancer patients with EGFR 19 or 21 mutations: a retrospective study. World J Surg Oncol. 15(197)2017.PubMed/NCBI View Article : Google Scholar | |
|
Dong Y, Li Q, Miao Q and Li D: Erlotinib as a salvage treatment after gefitinib failure for advanced non-small-cell lung cancer patients with brain metastasis: A successful case report and review. Medicine (Baltimore). 100(e26450)2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhu Y, Guo YB, Xu D, Zhang J, Liu ZG, Wu X, Yang XY, Chang DD, Xu M, Yan J, et al: A computed tomography (CT)-derived radiomics approach for predicting primary co-mutations involving TP53 and epidermal growth factor receptor (EGFR) in patients with advanced lung adenocarcinomas (LUAD). Ann Transl Med. 9(545)2021.PubMed/NCBI View Article : Google Scholar | |
|
Liu HE, Vuppalapaty M, Wilkerson C, Renier C, Chiu M, Lemaire C, Che J, Matsumoto M, Carroll J, Crouse S, et al: Detection of EGFR mutations in cfDNA and CTCs, and comparison to tumor tissue in non-small-cell-lung-cancer (NSCLC) patients. Front Oncol. 10(572895)2020.PubMed/NCBI View Article : Google Scholar | |
|
Chen H, Yang D, Wang Y, Tao H, Luo Y, Wu A, Li S, Yang Z and Chen M: Activation of the Hedgehog pathway mediates resistance to epidermal growth factor receptor inhibitors in non-small cell lung cancer. J Cancer. 13:987–997. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Xu M, Xie Y, Ni S and Liu H: The latest therapeutic strategies after resistance to first generation epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) in patients with non-small cell lung cancer (NSCLC). Ann Transl Med. 3(96)2015.PubMed/NCBI View Article : Google Scholar | |
|
Peng KC, Su JW, Xie Z, Wang HM, Fang MM, Li WF, Chen YQ, Guan XH, Su J, Yan HH, et al: Clinical outcomes of EGFR+/METamp+ vs. EGFR+/METamp-untreated patients with advanced non-small cell lung cancer. Thorac Cancer. 13:1619–1630. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Calabrese F, Pezzuto F, Lunardi F, Fortarezza F, Tzorakoleftheraki SE, Resi MV, Tiné M, Pasello G and Hofman P: Morphologic-molecular transformation of oncogene addicted non-small cell lung cancer. Int J Mol Sci. 23(4164)2022.PubMed/NCBI View Article : Google Scholar | |
|
Qin K, Hong L, Zhang J and Le X: MET amplification as a resistance driver to TKI therapies in lung cancer: Clinical challenges and opportunities. Cancers (Basel). 15(612)2023.PubMed/NCBI View Article : Google Scholar | |
|
Feng W, Xie Q, Liu S, Ji Y, Li C, Wang C and Jin L: Krüppel-like factor 4 promotes c-Met amplification-mediated gefitinib resistance in non-small-cell lung cancer. Cancer Sci. 109:1775–1786. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Hu X, Zheng X, Yang S, Wang L, Hao X, Cui X, Ding L, Mao L, Hu P and Shi Y: First-in-human phase I study of BPI-9016M, a dual MET/Axl inhibitor, in patients with non-small cell lung cancer. J Hematol Oncol. 13(6)2020.PubMed/NCBI View Article : Google Scholar | |
|
Wu YL, Zhang L, Kim DW, Liu X, Lee DH, Yang JCH, Ahn MJ, Vansteenkiste JF, Su WC, Felip E, et al: Phase Ib/II study of capmatinib (INC280) plus gefitinib after failure of epidermal growth factor receptor (EGFR) inhibitor therapy in patients with EGFR-mutated, MET factor-dysregulated non-small-cell lung cancer. J Clin Oncol. 36:3101–3109. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Wu YL, Cheng Y, Zhou J, Lu S, Zhang Y, Zhao J, Kim DW, Soo RA, Kim SW, Pan H, et al: Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): An open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir Med. 8:1132–1143. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Overbeck TR, Cron DA, Schmitz K, Rittmeyer A, Körber W, Hugo S, Schnalke J, Lukat L, Hugo T, Hinterthaner M, et al: Top-level MET gene copy number gain defines a subtype of poorly differentiated pulmonary adenocarcinomas with poor prognosis. Transl Lung Cancer Res. 9:603–616. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Wu YL, Guarneri V, Voon PJ, Lim BK, Yang JJ, Wislez M, Huang C, Liam CK, Mazieres J, Tho LM, et al: Tepotinib plus osimertinib in patients with EGFR-mutated non-small-cell lung cancer with MET amplification following progression on first-line osimertinib (INSIGHT 2): A multicentre, open-label, phase 2 trial. Lancet Oncol. 25:989–1002. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, et al: Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 362:2380–2388. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, et al: Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 13:239–246. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 378:113–125. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, et al: Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 41:2869–2876. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Zhou S, Ren F and Meng X: Efficacy of immune checkpoint inhibitor therapy in EGFR mutation-positive patients with NSCLC and brain metastases who have failed EGFR-TKI therapy. Front Immunol. 13(955944)2022.PubMed/NCBI View Article : Google Scholar | |
|
Jiang K, Wu L, Zheng X, Xu Y, Miao Q, Zheng X, Zhang L, Huang C and Lin G: Chemotherapy versus personalized therapy for EGFR mutant lung adenocarcinoma resistance to EGFR-tyrosine kinase inhibitors: A retrospective dual-center study. BMC Pulm Med. 24(96)2024.PubMed/NCBI View Article : Google Scholar | |
|
Gridelli C, Ciardiello F, Gallo C, Feld R, Butts C, Gebbia V, Maione P, Morgillo F, Genestreti G, Favaretto A, et al: First-line erlotinib followed by second-line cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung cancer: The TORCH randomized trial. J Clin Oncol. 30:3002–3011. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Auliac JB, Chouaid C, Greillier L, Monnet I, Le Caer H, Falchero L, Corre R, Descourt R, Bota S, Berard H, et al: Randomized open-label non-comparative multicenter phase II trial of sequential erlotinib and docetaxel versus docetaxel alone in patients with non-small-cell lung cancer after failure of first-line chemotherapy: GFPC 10.02 study. Lung Cancer. 85:415–419. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, Ma Z, Li X, Zhuang W, Liu Y, et al: Tislelizumab plus chemotherapy as first-line treatment for locally advanced or metastatic nonsquamous NSCLC (RATIONALE 304): A randomized phase 3 trial. J Thorac Oncol. 16:1512–1522. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S, Feng J, He J, Han B, Wang J, et al: BEYOND: A randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol. 33:2197–2204. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Ma L, Diao B, Huang Z, Wang B, Yu J and Meng X: The efficacy and possible mechanisms of immune checkpoint inhibitors in treating non-small cell lung cancer patients with epidermal growth factor receptor mutation. Cancer Commun (Lond). 41:1314–1330. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Lu S, Dong X, Jian H, Chen J, Chen G, Sun Y, Ji Y, Wang Z, Shi J, Lu J, et al: AENEAS: A randomized phase III trial of aumolertinib versus gefitinib as first-line therapy for locally advanced or metastaticnon-small-cell lung cancer with EGFR exon 19 deletion or L858R mutations. J Clin Oncol. 40:3162–3171. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Shi Y, Chen G, Wang X, Liu Y, Wu L, Hao Y, Liu C, Zhu S, Zhang X, Li Y, et al: Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): A multicentre, double-blind, randomised phase 3 study. Lancet Respir Med. 10:1019–1028. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Asami K, Ando M, Nishimura T, Yokoi T, Tamura A, Minato K, Mori M, Ogushi F, Yamamoto A, Yoshioka H, et al: A randomized phase II study of docetaxel or pemetrexed with or without the continuation of gefitinib after disease progression in elderly patients with non-small cell lung cancer harboring EGFR mutations (JMTO LC12-01). Thorac Cancer. 13:1827–1836. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Chen Y, Liu Z, An N, Zhang J, Meng W, Wang W, Wu X, Hu X, Chen Y and Yin W: Platelet-derived mitochondria attenuate 5-FU-induced injury to bone-associated mesenchymal stem cells. Stem Cells Int. 2023(7482546)2023.PubMed/NCBI View Article : Google Scholar |