Durvalumab consolidation after chemoradiotherapy for locoregional recurrent non‑small cell lung cancer
- Authors:
- Published online on: July 10, 2025 https://doi.org/10.3892/mco.2025.2879
- Article Number: 84
-
Copyright: © Suminaga et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY_NC 4.0].
Abstract
Introduction
Surgery is the standard treatment for early-stage non-small cell lung cancer (NSCLC) (1); however, postoperative recurrence occurs in 20-50% of cases (2-4). The types of postoperative recurrence are locoregional, systemic, or a combination of both, with locoregional recurrence accounting for 8-24% of cases (5). Similarly, patients treated with definitive chemoradiotherapy (CRT) for locally advanced NSCLC and stereotactic body radiotherapy (SBRT) for early-stage NSCLC may develop locoregional recurrence; subsequent treatment strategies are important. Local therapy with curative intent has often been selected for patients with locoregional recurrence and has demonstrated a favorable prognosis and safety (6,7). Several retrospective studies have reported that CRT has a prolonged prognosis compared with radiotherapy alone in patients with locoregional recurrence of NSCLC (8,9). Therefore, although a standard of care for locoregional NSCLC is yet to be established in prospective trials, CRT is widely used in clinical practice.
In comparison with placebo, a phase III PACIFIC study (10) involving patients with unresectable locally advanced stage III NSCLC demonstrated significantly longer progression-free survival (PFS) and overall survival with CRT followed by the programmed death-ligand 1 (PD-L1) inhibitor durvalumab, thereby establishing this treatment as the standard of care (10,11). It has been reported in vitro that radiation therapy increases PD-L1 expression, and that anti-PD-L1 therapy enhances the antitumor effects of radiation (12). The PACIFIC trial was conducted based on these results, and durvalumab has been used in clinical practice with promising results. Other agents, such as pembrolizumab, have demonstrated promising results in phase II trials (13), but have not been further investigated for clinical use. Therefore, in Japan, durvalumab is currently the only immune checkpoint inhibitor used after CRT.
The efficacy of durvalumab consolidation following CRT for unresectable stage III NSCLC, as established by the PACIFIC trial, has been validated in real-world settings (14,15). An international retrospective study (PACIFIC-R) (14) analyzed data from a broad stage III NSCLC population, confirming the PFS benefit of durvalumab observed in the PACIFIC trial. This study included a large, international, real-world cohort and showed that durvalumab consolidation is effective in diverse patient populations. Similarly, a Japanese multicenter retrospective study (15) investigated recurrence patterns and PFS after CRT with or without durvalumab. This study, conducted within Japan, demonstrated the effectiveness of durvalumab in a Japanese population and provided insights into how the treatment affects recurrence patterns; however, while it included some patients with locoregional recurrence, they were not analyzed as a separate subgroup. Thus, while the PACIFIC trial and these real-world studies have significantly advanced the treatment of unresectable stage III NSCLC by establishing the role of durvalumab consolidation after CRT, they primarily focused on the initial treatment setting. There remains limited evidence specifically addressing durvalumab use after CRT for locoregional recurrence. To address this knowledge gap, the present study examined the efficacy of durvalumab treatment after CRT, specifically in patients with locally recurrent NSCLC.
Patients and methods
Study design
Patients with NSCLC who developed locoregional recurrence after definitive therapy (including surgery, CRT, and SBRT) and were subsequently treated with CRT at Kyoto University Hospital (Kyoto, Japan) between January 2010 and December 2022, were retrospectively analyzed. Patients who received concurrent platinum-based chemotherapy with definitive radiotherapy and showed no disease progression after radiotherapy were included. Patients who showed disease progression immediately after completion of CRT, those who failed to complete CRT, and those who were lost to follow-up were excluded. A total of 43 patients were eventually included. There were 12 patients in the durvalumab group, with a median age of 71.5 years, 8 men and 4 women. There were 31 patients in the non-durvalumab group, with a median age of 68 years, 22 men and 9 women. The PFS, objective response rate, and adverse events between patients treated with and without durvalumab were next compared. Locoregional recurrence was defined as disease recurrence in pulmonary, hilar, mediastinal, or supraclavicular regions. In addition, as a reference group, patients with locally advanced NSCLC who underwent definitive CRT and received durvalumab for consolidation with those who did not were compared. Durvalumab was administered intravenously at a dose of 10 mg/kg once every 2 weeks for up to 12 months. The collected data included age, sex, smoking history, Eastern Cooperative Oncology Group (ECOG) performance status (PS), histology, driver mutation, type of initial definitive therapy, time to recurrence after initial definitive therapy, PD-L1 expression by tumor proportion score (TPS), pathological stage according to the TNM system (eighth edition) (16), chemotherapy regimen used in CRT at recurrence, and radiation dose. Adverse events were graded using the Common Terminology Criteria for Adverse Events (17). Esophagitis, neutropenia, pneumonitis, and other grade 3 or higher adverse events in patients treated with and without durvalumab were examined.
The present study was approved (approval no. R4482) by the Kyoto University Ethics Committee (Kyoto, Japan). Due to the retrospective study design, the requirement for informed consent for the use of clinical records was waived. However, all the patients were informed about the study and withdraw at will, with an opt-out option provided on the website of the institution.
Statistical analysis
Individual clinical factors were compared using the Fisher's exact test and Student's t-tests. PFS was defined as the time from the end of radiotherapy to the date of the first documented event of tumor progression or death. The cut-off date for data collection was September 30, 2023. The survival curve was estimated using the Kaplan-Meier method and compared using the log-rank test. The best objective response was evaluated using computed tomography according to the Response Evaluation Criteria for Solid Tumors (18). Imaging findings after the completion of radiotherapy were used as the baseline to evaluate the objective response. P<0.05 was considered to indicate a statistically significant difference. All statistical analyses and data processing were performed using JMP 17 (SAS Institute, Inc.) and GraphPad Prism 10 (Dotmatics).
Results
Patient characteristics
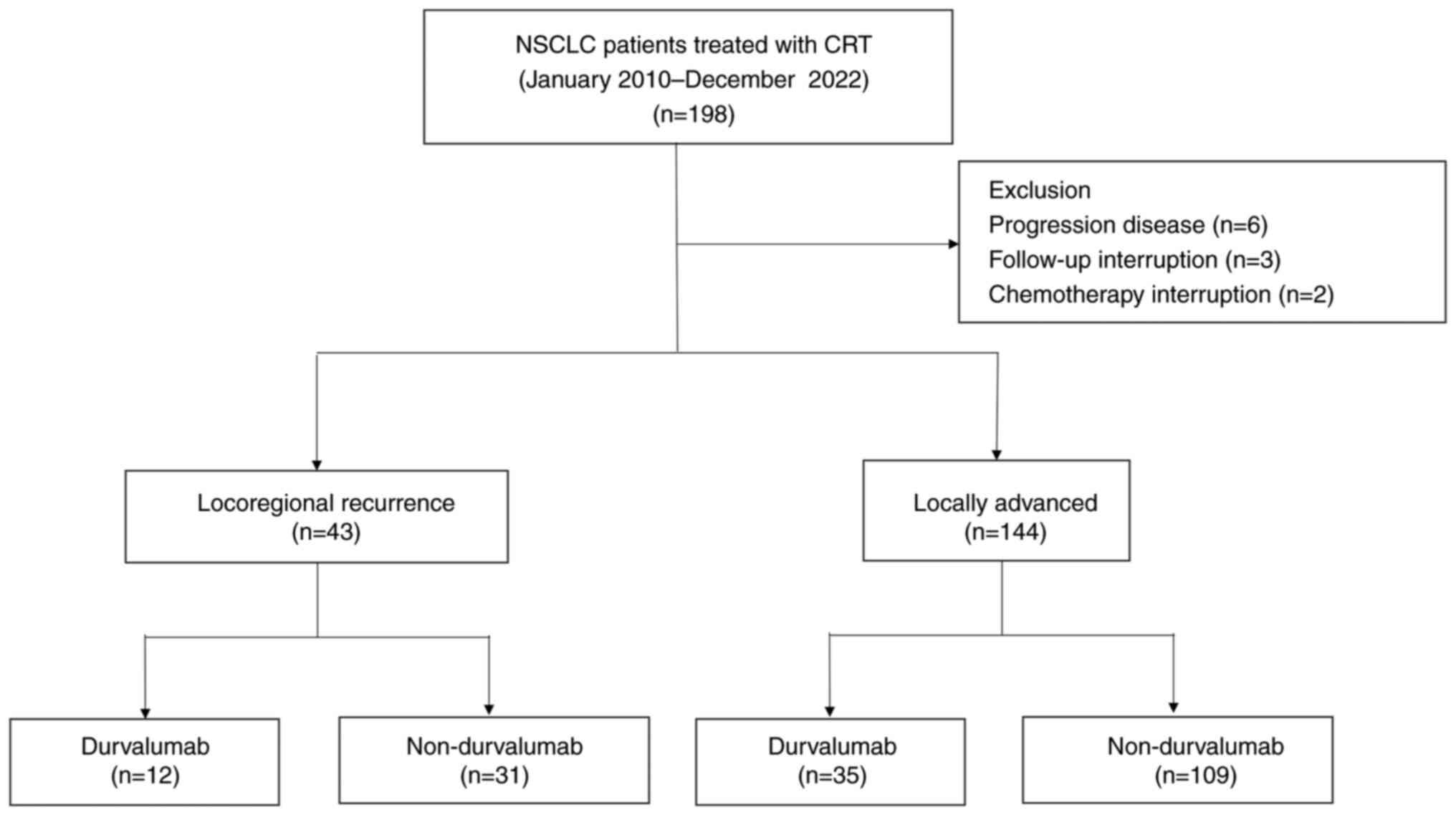
Between January 2010 and December 2022, 198 patients with NSCLC were treated with CRT (Fig. 1). A total of 11 patients were excluded from this study, including 6 patients who were excluded due to disease progression immediately after CRT, 3 patients who were lost to follow-up and 2 patients who failed to complete CRT. In total, 187 patients were included in the analysis. In total 43 patients had locoregional recurrence and 144 had locally advanced disease. Among the patients with locoregional recurrent NSCLC, 12 were treated with durvalumab consolidation therapy. Durvalumab was administered to 35 patients with locally advanced NSCLC. Most patients in both groups who did not receive durvalumab were treated before the advent of durvalumab.
The characteristics of patients with locoregional recurrence are listed in Table I. No statistically significant differences in age, sex, smoking status, ECOG PS, or histological type were observed between patients treated with and without durvalumab. The proportion of driver mutation-positive patients was lower among those treated with durvalumab than among those who were not. In both the treatment groups, surgery was the most common initial definitive therapy. The median number of months until recurrence was longer in the patients who did not receive durvalumab. Most patients who did not receive durvalumab were treated before it became available in Japan (July 2018); therefore, they did not undergo PD-L1 immunostaining. The results of the PD-L1 staining of the samples at the time of recurrence were used. No statistically significant differences in the clinical T and N factors at recurrence were observed between the treatment groups. Regarding the chemotherapy regimen used for CRT at recurrence, all patients in the durvalumab group received concurrent CRT with carboplatin and paclitaxel. Radiotherapy was delivered at 54-66 Gy (median, 60 Gy) using three-dimensional radiotherapy or intensity-modulated radiotherapy in all patients.
The characteristics of patients with locally advanced NSCLC are listed in Table SI. The proportion of patients with an unknown PD-L1 staining status was high among those who did not receive durvalumab. The frequency of chemotherapy regimens combining cisplatin and vinorelbine was lower in the durvalumab group. Among patients who did not receive durvalumab, 86% were treated before durvalumab became available. Other clinical factors did not differ between the treatment groups.
Efficacy
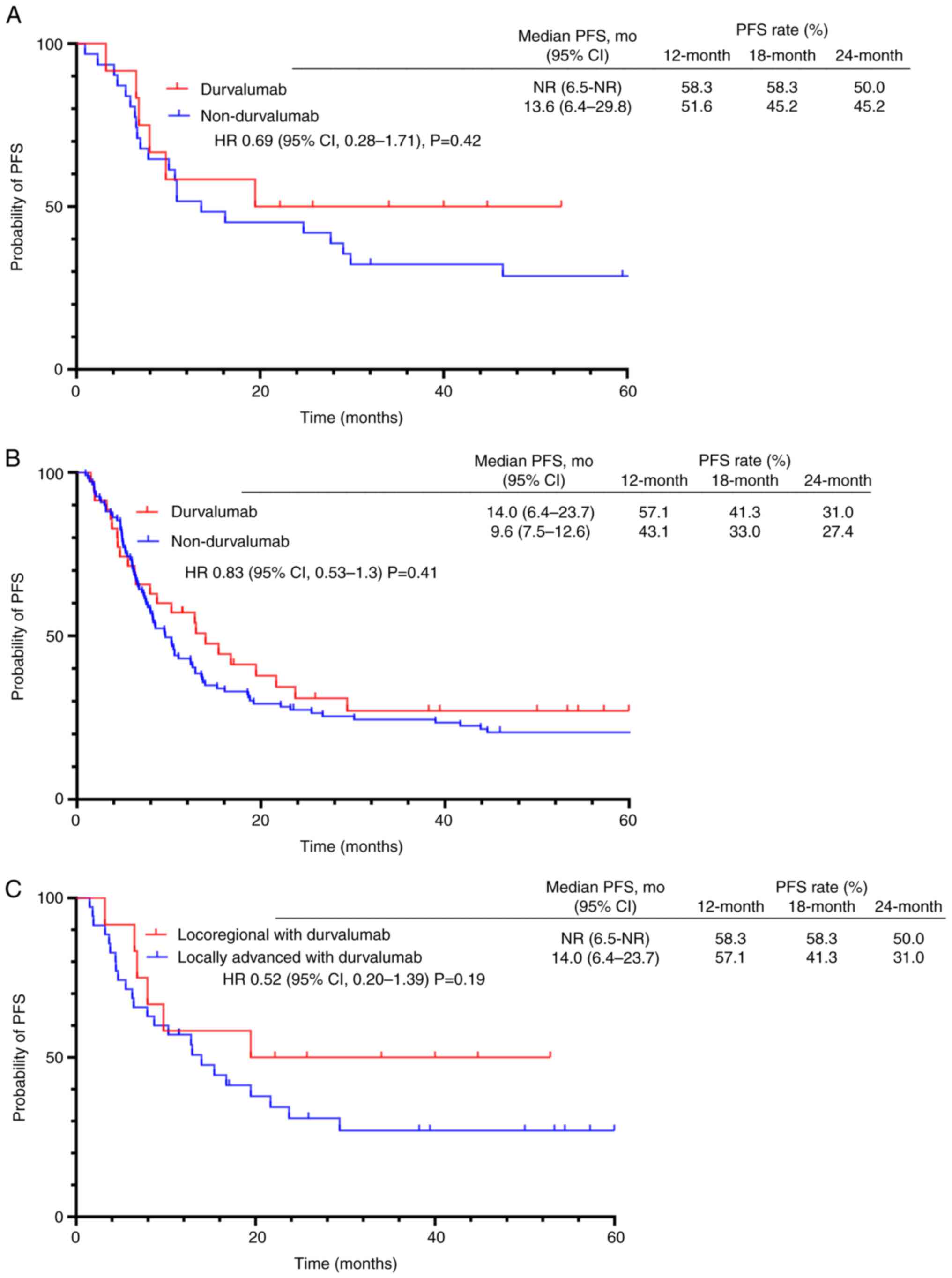
In locoregional recurrent NSCLC, the median follow-up period was 24.2 months (range, 5.9-52.8 months) for patients treated with durvalumab, and 59.3 months (range, 13.4-131.0 months) for patients who did not receive durvalumab. At the time of analysis, 6 patients in the durvalumab group had disease progression, 5 of whom subsequently succumbed to disease. In the group not treated with durvalumab, 24 patients showed disease progression, 15 of whom succumbed to disease. The median PFS was not reached [95% confidence interval (CI), 6.5 months-not reached] in the durvalumab group, and it was 13.6 months (95% CI, 6.9-29.8) in the group treated without durvalumab (HR, 0.69; 95% CI, 0.28-1.71; P=0.42). The 12-, 18-, and 24-month PFS rates in the durvalumab group were 58.3, 58.3, and 50.0%, respectively. The median PFS in the durvalumab group was not reached. This is attributed to the shorter observation period and fewer events compared with the non-durvalumab group. Shorter observation period limited the time available for progression events to occur; however, this lower event rate, even within the shorter follow-up, suggested a potential delay in disease progression with durvalumab. By contrast, the rates in the non-durvalumab group were 51.6, 45.2, and 45.2%, respectively (Fig. 2A). Although the difference was not statistically significant, a trend toward prolonged PFS was observed in the durvalumab group. The objective response rate (ORR) and disease control rate (DCR) were similar in both treatment groups. The ORR values were 41.7 and 41.9% in the durvalumab and non-durvalumab groups, respectively (P>0.999). The DCR in the durvalumab and non-durvalumab groups were 100 and 93.5%, respectively (P>0.999) (Table II).
In locally advanced NSCLC, the median follow-up period was 28.0 months (range, 2.4-60.0 months) for patients treated with durvalumab, and 26.1 months (range, 2.5-151.7) for patients who did not receive durvalumab. The median PFS was 14.0 months (95% CI, 6.4-23.7) and 9.6 months (95% CI, 7.5-12.6) in the durvalumab and non-durvalumab group, respectively (HR, 0.83; 95% CI, 0.53-1.30; P=0.41). The 12-, 18-, and 24-month PFS rates in the durvalumab group were 57.1, 41.3, and 31.0%, respectively. By contrast, the rates in the non-durvalumab group were 43.1, 33.0, and 27.4%, respectively (Fig. 2B). The median PFS of patients treated with durvalumab was longer; however, the difference was not statistically significant.
The PFS of patients with locoregional recurrence to that of patients with locally advanced NSCLC who were treated with durvalumab (Fig. 2C) was also compared. The median PFS was longer in patients with locoregional recurrent NSCLC, but the difference was not statistically significant (HR, 0.52; 95% CI, 0.20-1.39; P=0.19).
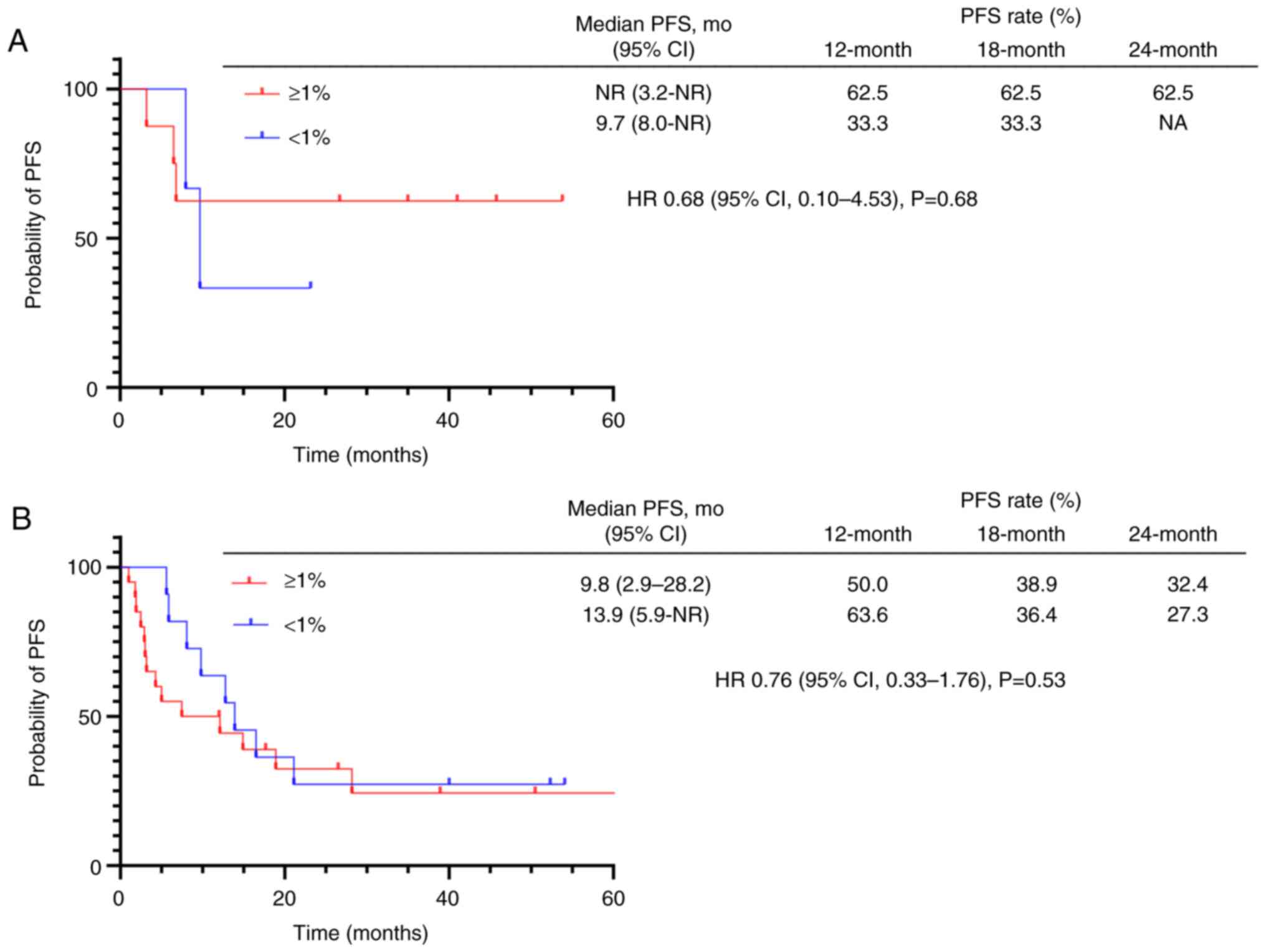
Among the patients who received durvalumab consolidation therapy, PFS was comparable regardless of PD-L1 expression for both locoregional recurrent NSCLC (not reached in PD-L1 positive vs. 9.7 months in PD-L1 negative; HR 0.68; P=0.68) (Fig. 3A) and locally advanced NSCLC (9.8 in PD-L1 positive vs. 13.9 months in PD-L1 negative; HR 0.76; P=0.53) (Fig. 3B); however, these results should be interpreted with caution due to the limited number of cases studied.
Toxicity
Major adverse events are described in Table III. Esophagitis, neutropenia, pneumonitis, and other grade 3 adverse events were focused on in the present study. No other grade 4 adverse events were observed. No difference in the frequency of esophagitis, neutropenia, or other grade 3 adverse events was observed between the durvalumab and non-durvalumab groups. Pneumonitis was the most common adverse event in the durvalumab group and was significantly more frequent than that in the non-durvalumab group. However, none of the patients in either group developed grade 3 or higher pneumonitis. Other grade 3 or higher adverse events included hyponatremia, eosinophilia, and pneumothorax in the durvalumab group, and thrombocytopenia, hypertension, pneumothorax, peripheral neuropathy, and febrile neutropenia in the non-durvalumab group. No treatment-related deaths occurred during the study period.
Discussion
In the present study, the efficacy of durvalumab after CRT in patients with locoregional recurrent NSCLC was examined. Among the patients with locoregional recurrence, those who received durvalumab after CRT showed a trend toward longer PFS compared with those who were not treated with durvalumab, although the difference was not statistically significant. The adverse events in patients treated with durvalumab were manageable.
Previous studies examining the efficacy of CRT for locoregional recurrence have reported a PFS of 13-20 months (8,19-21). The median PFS of patients who did not receive durvalumab in the present study was 13.6 months, which appears comparable, considering that the population was slightly older than that previously reported. The NCCN guidelines (22) recommend surgery or radiation for operable local recurrence, and CRT for mediastinal lymph node recurrence. While the NCCN guidelines do not explicitly recommend durvalumab after CRT for locoregional recurrence, its use is becoming increasingly common in clinical practice. This is largely driven by the significant survival benefits observed with durvalumab consolidation following CRT in the PACIFIC trial for locally advanced NSCLC (10). Although direct evidence supporting durvalumab in locoregional recurrence is limited, clinicians often extrapolate from the PACIFIC trial results, anticipating similar benefits in this patient population. This highlights the urgent need for robust clinical trials to evaluate the efficacy of durvalumab in this specific setting.
To the best of our knowledge, only two studies have reported the results of adding durvalumab after CRT for local recurrence (23,24). In one study (23), 24 patients were retrospectively examined and the median PFS was 15 months. The 12-, 18-, and 24-month PFS rates were 68.7, 45.8, and 34.3%, respectively, with acceptable adverse events. However, the significance of adding durvalumab could not be determined owing to the lack of a control group that did not receive durvalumab. Similar to the present study, another retrospective study (24) compared the efficacy and safety of durvalumab in patients with locoregional recurrent lung cancer treated with CRT, with or without durvalumab, at multiple facilities. The median PFS was 25.4 and 11.5 months, for the durvalumab group and non-durvalumab group, respectively. Consistent with this, the present study expands upon the findings of a previous study (24) in several important ways. First, unlike the previous study that focused exclusively on patients with recurrence following complete resection, the present study includes a broader population with locoregional recurrence after various definitive local therapies (surgery, CRT, and SBRT), reflecting diverse clinical scenarios. Second, the present study uniquely compares outcomes of consolidation durvalumab in locoregional recurrence to those in locally advanced NSCLC, providing novel insights into potential prognostic differences and treatment benefits. These distinctions offer additional context to the existing literature on durvalumab consolidation therapy for NSCLC. Additionally, a recent study evaluated the feasibility and safety of salvage surgery for patients who developed locoregional failure (LRF) after CRT and consolidation durvalumab (25). While both that study and the present research address locoregional recurrence, they have distinct focuses. The aforementioned study (25) specifically investigates the feasibility of salvage surgery as a treatment option for LRF, whereas the present study examines the efficacy of durvalumab consolidation after CRT in patients with locoregional recurrence. However, it is important to note that the present study did not include cases of recurrence following CRT and durvalumab, which represents a potential area for future research. A comparison of the present study with previous studies is presented in Table IV.
Regarding locally advanced NSCLC, a similar trend toward longer PFS was observed in the durvalumab group; however, the difference was not as statistically significant as that in the PACIFIC study (10). Compared with the PACIFIC trial, the durvalumab group in the present study had comparable PFS results, whereas the non-durvalumab group tended to have a longer PFS of 9.6 months. The PACIFIC study only administered a placebo to the control group. However, in this study, many control patients received consolidation chemotherapy, which could explain the difference. In addition to the results of the present study, real-world data have shown a median PFS of ~10 months for CRT without durvalumab (26). Therefore, the results obtained in the present study are not unexpected. This may be attributed to the fact that numerous patients in the non-durvalumab group received consolidation therapy with cytotoxic chemotherapy, which was widely used in clinical practice prior to the advent of durvalumab.
The present study found a difference in the positivity rates of driver mutations between the two groups. This is probably because real-world clinical data showed the limited efficacy of durvalumab in EGFR-positive, locally advanced NSCLC (14,27,28), which influenced the decisions of physicians and patients. However, as most previous reports on locoregional recurrent NSCLC did not mention driver mutations, more cases are required to determine the significance of durvalumab in driver mutation-positive locoregional recurrence. In the present study, differences in efficacy due to driver mutations such as EGFR were not examined due to the small sample size. This may be related to the fact that EGFR-positive lung cancer occurs more often in the form of distant metastasis than as locoregional recurrence (29).
PD-L1 expression based on TPS also differed between the two groups, likely due to the high number of patients with unknown TPS in the non-durvalumab group. The group not treated with durvalumab included numerous patients from a time period in which PD-L1 staining was not routinely performed, resulting in a high percentage of patients with unknown TPS. In locally advanced NSCLC, an association between a high TPS and favorable outcomes of durvalumab treatment has been reported (30,31); however, these associations have not been validated in locoregional recurrent NSCLC. In the present study, PD-L1-positive patients treated with durvalumab consolidation tended to have an improved PFS. However, this finding was based on a limited number of patients, and further research is required to better understand the role of TPS in locoregional recurrent NSCLC.
The chemotherapy regimens at relapse also differed between the two groups, reflecting differences in the time period in which the patients were treated. A network meta-analysis revealed no significant differences in the efficacy of various CRT regimens for the treatment of locally advanced NSCLC (32). Therefore, it is considered that the differences in the treatment regimens in the present study had a less significant impact on the efficacy results.
The present study has several limitations. This study was conducted at a single center with a limited number of patients. Although a trend toward improved PFS with durvalumab following CRT was noted, the difference was not statistically significant, likely due to the small sample size. Larger multicenter studies are needed to validate and generalize these findings. In particular, the differences in the efficacy of PD-L1 should be interpreted with caution. Locally advanced NSCLC is estimated to account for ~1/3 of cases at initial diagnosis, while locally recurrent NSCLC accounts for as few as 9-37% of cases that recur after complete resection (33-35), and most studies to date have been retrospective. Large-scale multicenter prospective studies are warranted in the future. Due to the retrospective nature of the study, patient characteristics were partially unbalanced between the durvalumab and non-durvalumab groups. For example, there may have been a selection bias in the administration of durvalumab to patients with driver mutations. The shorter median follow-up in the durvalumab group also represents a study limitation. This difference in observation period may have impacted the number of observed progression events and the maturity of PFS estimates. However, the data in the present study are promising and suggest new treatment options for patients with locoregionally recurrent NSCLC. Future validation in a large number of patients stratified by driver mutations and PD-L1 TPS is required.
In conclusion, patients who received durvalumab following CRT for locoregional recurrent NSCLC tended to have a longer PFS than those who did not. This treatment strategy may benefit patients with locoregional recurrent NSCLC, similar to those with unresectable locally advanced stage III NSCLC.
Supplementary Material
Patient characteristics of locally advanced NSCLC.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by internal funding (grant no. 021515) from the Kyoto University Hospital (Kyoto, Japan).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
KS contributed to data curation, investigation, validation, formal analysis, methodology, visualization, and writing of the original draft. HironoriY contributed to the conceptualization, data curation, formal analysis, investigation, methodology, project administration, funding acquisition, supervision, validation, and writing (review and editing). KS and HironoriY confirm the authenticity of all the raw data. TH and HO contributed to the funding acquisition, supervision, validation of methods, as well as writing, reviewing, and editing of the manuscript. YS, HA, and TN contributed to the data curation, resource acquisition, investigation, writing, review, and editing. KHo, TO, HiroshiY, KHa, MY, NK, and TF contributed to formal analysis, data curation, investigation, and writing (review and editing). All authors contributed to the resources, writing (review), and editing and have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved (approval no. R4482) by the Kyoto University Ethics Committee (Kyoto, Japan). Due to the retrospective study design, the requirement for informed consent for the use of clinical records was waived. However, all the patients were informed about the study and withdraw at will, with an opt-out option provided on the website of the institution.
Consent for publication
Not applicable.
Competing interests
HO reports personal fees from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., MSD K.K., Pfizer Japan Inc., Novartis Pharmaceuticals, Takeda Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Sanofi K.K., Eli Lilly Japan K.K., and Ono Pharmaceutical Co., Ltd. outside the submitted work. HironoriY reports personal fees from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., MSD K.K., Takeda Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., and Ono Pharmaceutical Co., Ltd. outside of the submitted work. All the other authors declare that they have no competing interests.
References
|
Cao C, Wang D, Chung C, Tian D, Rimner A, Huang J and Jones DR: A systematic review and meta-analysis of stereotactic body radiation therapy versus surgery for patients with non-small cell lung cancer. J Thorac Cardiovasc Surg. 157:362–373.e8. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Okami J, Shintani Y, Okumura M, Ito H, Ohtsuka T, Toyooka S, Mori T, Watanabe SI, Date H, Yokoi K, et al: Demographics, safety and quality, and prognostic information in both the seventh and eighth editions of the TNM classification in 18,973 surgical cases of the Japanese joint committee of lung cancer registry database in 2010. J Thorac Oncol. 14:212–222. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Potter AL, Costantino CL, Suliman RA, Haridas CS, Senthil P, Kumar A, Mayne NR, Panda N, Martin LW and Yang CJ: Recurrence after complete resection for non-small cell lung cancer in the national lung screening trial. Ann Thorac Surg. 116:684–692. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Lou F, Sima CS, Rusch VW, Jones DR and Huang J: Differences in patterns of recurrence in early-stage versus locally advanced non small cell lung cancer. Ann Thorac Surg. 98:1755–1760; discussion 1760-1. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Fedor D, Johnson WR and Singhal S: Local recurrence following lung cancer surgery: Incidence, risk factors, and outcomes. Surg Oncol. 22:156–161. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Matsuguma H, Nakahara R, Wakamatsu I, Kishikawa T, Sugiyama T, Nakamura Y, Kasai T, Kamiyama Y, Hoshi N, Inoue K, et al: Definitive local therapy for oligo-recurrence in patients with completely resected non-small cell lung cancer. Am J Clin Oncol. 43:210–217. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Matsuo Y: A systematic literature review on salvage radiotherapy for local or regional recurrence after previous stereotactic body radiotherapy for lung cancer. Technol Cancer Res Treat. 17(1533033818798633)2018.PubMed/NCBI View Article : Google Scholar | |
|
Nakamichi S, Horinouchi H, Asao T, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Ito Y, Watanabe SI and Ohe Y: Comparison of radiotherapy and chemoradiotherapy for locoregional recurrence of non-small-cell lung cancer developing after surgery. Clin Lung Cancer. 18:e441–e448. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Ma L, Qiu B, Zhang J, Li QW, Wang B, Zhang XH, Qiang MY, Chen ZL, Guo SP and Liu H: Survival and prognostic factors of non-small cell lung cancer patients with postoperative locoregional recurrence treated with radical radiotherapy. Chin J Cancer. 36(93)2017.PubMed/NCBI View Article : Google Scholar | |
|
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et al: PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non-small cell cancer. N Engl J Med. 377:1919–1929. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz Ares L, Vansteenkiste JF, Garassino MC, Hui R, Quantin X, et al: Five-year survival outcomes from the PACIFIC trial: Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 40:1301–1311. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR and Fu YX: Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 124:687–695. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Jabbour SK, Lee KH, Frost N, Breder V, Kowalski DM, Pollock T, Levchenko E, Reguart N, Martinez-Marti A, Houghton B, et al: Pembrolizumab plus concurrent chemoradiation therapy in patients with unresectable, locally advanced, stage III non-small cell lung cancer: The phase 2 KEYNOTE-799 nonrandomized trial. JAMA Oncol. 7:1–9. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Girard N, Bar J, Garrido P, Garassino MC, McDonald F, Mornex F, Filippi AR, Smit HJM, Peters S, Field JK, et al: Treatment characteristics and real-world progression-free survival in patients with unresectable stage III NSCLC who received durvalumab after chemoradiotherapy: Findings from the PACIFIC-R study. J Thorac Oncol. 18:181–193. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Kishi N, Matsuo Y, Shintani T, Ogura M, Mitsuyoshi T, Araki N, Fujii K, Okumura S, Nakamatsu K, Kishi T, et al: Recurrence patterns and progression-free survival after chemoradiotherapy with or without consolidation durvalumab for stage III non-small cell lung cancer. J Radiat Res. 64:142–153. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Brierley JD, Gospodarowicz MK and Wittekind C (eds): TNM Classification of Malignant Tumours, 8th Edition. Wiley-Blackwell, Oxford, 2017. | |
|
U.S. Department of Health and Human Services, National Institutes of Health and National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE) v5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm. | |
|
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al: New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Takenaka T, Takenoyama M, Toyozawa R, Inamasu E, Yoshida T, Toyokawa G, Shiraishi Y, Hirai F, Yamaguchi M, Seto T and Ichinose Y: Concurrent chemoradiotherapy for patients with postoperative recurrence of surgically resected non-small-cell lung cancer. Clin Lung Cancer. 16:51–56. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Bar J, Ng D, Moretto P, Goss GD, Sun A, Macrae R, Laurie SA, Leighl N and Nicholas G: Chemoradiotherapy for locoregional recurrence of non-small-cell lung cancer after surgical resection: A retrospective analysis. Clin Lung Cancer. 14:200–204. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Hisakane K, Yoh K, Nakamura N, Udagawa H, Kirita K, Umemura S, Matsumoto S, Niho S, Akimoto T, Tsuboi M and Goto K: Salvage chemoradiotherapy with cisplatin and vinorelbine for postoperative locoregional recurrence of non-small cell lung cancer. Medicine (Baltimore). 96(e8635)2017.PubMed/NCBI View Article : Google Scholar | |
|
National Comprehensive Cancer Network: Non-Small Cell Lung Cancer (version 3.2025). https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. | |
|
Borghetti P, Imbrescia J, Volpi G, Scotti V, Aquilano M, Bruni A, Franceschini D, Ursino S, Ciammella P, Piperno G, et al: Chemo-radiotherapy plus durvalumab for loco-regional relapse of NSCLC. Radiat Oncol. 17(124)2022.PubMed/NCBI View Article : Google Scholar | |
|
Furuta M, Horinouchi H, Yokota I, Yamaguchi T, Itoh S, Fukui T, Iwashima A, Sugisaka J, Miura Y, Tanaka H, et al: Durvalumab after chemoradiotherapy for locoregional recurrence of completely resected non-small-cell lung cancer (NEJ056). Cancer Sci. 115:3705–3717. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Dickhoff C, Unal S, Heineman DJ, Winkelman JA, Braun J, Bahce I, van Dorp M, Senan S and Dahele M: Feasibility of salvage resection following locoregional failure after chemoradiotherapy and consolidation durvalumab for unresectable stage III non-small cell lung cancer. Lung Cancer. 182(107294)2023.PubMed/NCBI View Article : Google Scholar | |
|
Saad A, Goldstein J, Appel S, Daher S, Urban D, Onn A, Gantz-Sorotsky H, Lobachov A, Gottfried T, Spieler B and Bar J: Chemoradiation followed by adjuvant durvalumab in stage III non small cell lung cancer: Real-world comparison of treatment outcomes to historical controls treated with chemoradiation alone. Thorac Cancer. 13:1763–1771. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Aredo JV, Mambetsariev I, Hellyer JA, Amini A, Neal JW, Padda SK, McCoach CE, Riess JW, Cabebe EC, Naidoo J, et al: Durvalumab for stage III EGFR-mutated NSCLC after definitive chemoradiotherapy. J Thorac Oncol. 16:1030–1041. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Hellyer JA, Aredo JV, Das M, Ramchandran K, Padda SK, Neal JW and Wakelee HA: Role of consolidation durvalumab in patients with EGFR- and HER2-mutant unresectable stage III NSCLC. J Thorac Oncol. 16:868–872. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Qin Q, Peng B and Li B: The impact of epidermal growth factor receptor mutations on the efficacy of definitive chemoradiotherapy in patients with locally advanced unresectable stage III non-small cell lung cancer: A systematic review and meta-analysis. Expert Rev Anticancer Ther. 19:533–539. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Park CK, Oh HJ, Kim YC, Kim YH, Ahn SJ, Jeong WG, Lee JY, Lee JC, Choi CM, Ji W, et al: Korean real-world data on patients with unresectable stage III NSCLC treated with durvalumab after chemoradiotherapy: PACIFIC-KR. J Thorac Oncol. 18:1042–1054. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Jazieh K, Gad M, Saad A, Wei W and Pennell NA: Tumor PD-L1 expression is associated with outcomes in stage III non-small cell lung cancer (NSCLC) patients treated with consolidation durvalumab. Transl Lung Cancer Res. 10:3071–3078. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Zheng Q, Min S and Zhou Y: A network meta-analysis for efficacies and toxicities of different concurrent chemoradiotherapy regimens in the treatment of locally advanced non-small cell lung cancer. BMC Cancer. 22(674)2022.PubMed/NCBI View Article : Google Scholar | |
|
Yano T, Hara N, Ichinose Y, Asoh H, Yokoyama H, Ohta M and Hata K: Local recurrence after complete resection for non-small-cell carcinoma of the lung. Significance of local control by radiation treatment. J Thorac Cardiovasc Surg. 107:8–12. 1994.PubMed/NCBI | |
|
Saynak M, Veeramachaneni NK, Hubbs JL, Nam J, Qaqish BF, Bailey JE, Chung W and Marks LB: Local failure after complete resection of N0-1 non-small cell lung cancer. Lung Cancer. 71:156–165. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Casal-Mouriño A, Ruano-Ravina A, Lorenzo-González M, Rodríguez-Martínez Á, Giraldo-Osorio A, Varela-Lema L, Pereiro-Brea T, Barros-Dios JM, Valdés-Cuadrado L and Pérez-Ríos M: Epidemiology of stage III lung cancer: frequency, diagnostic characteristics, and survival. Transl Lung Cancer Res. 10:506–518. 2021.PubMed/NCBI View Article : Google Scholar |