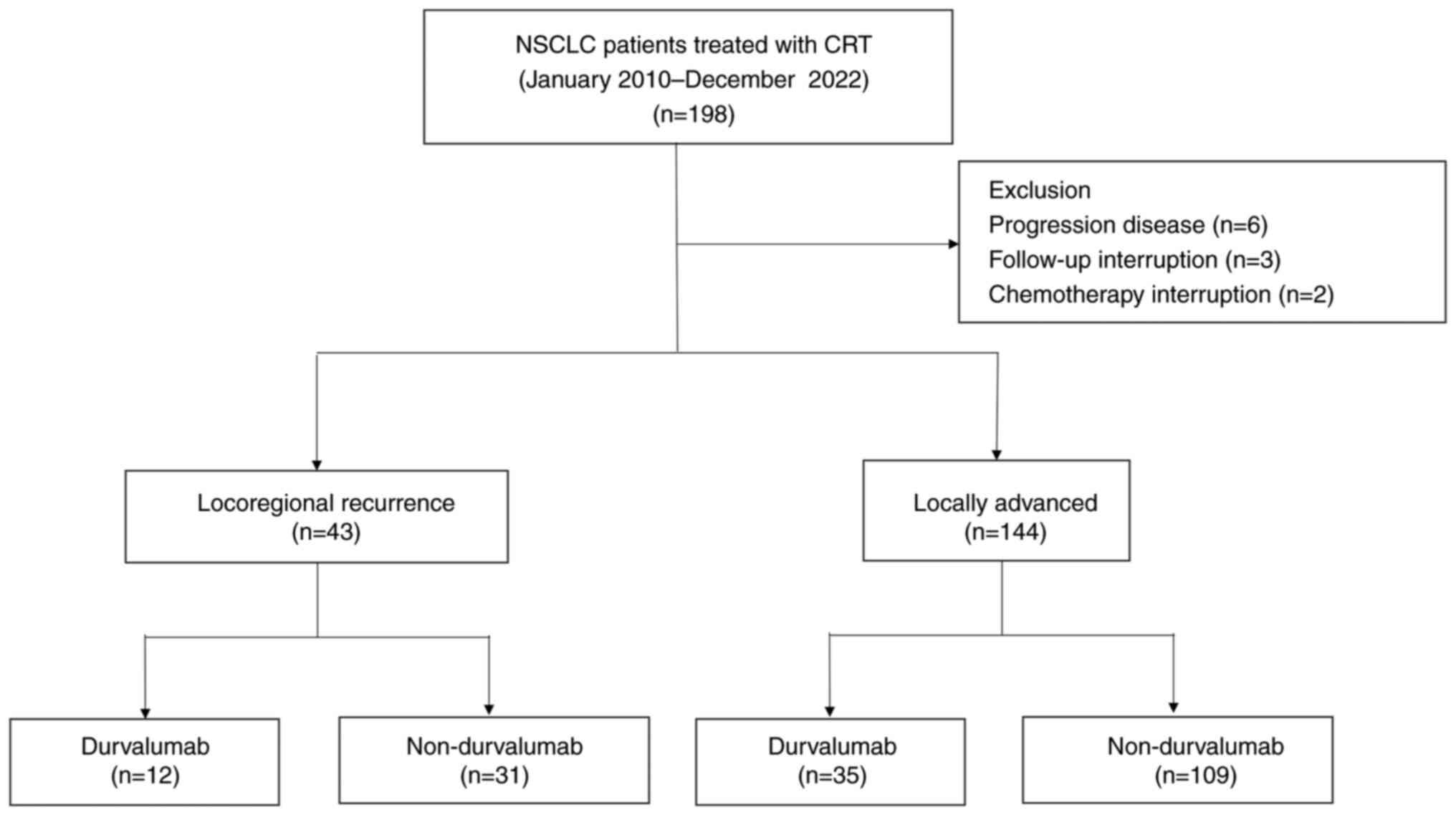
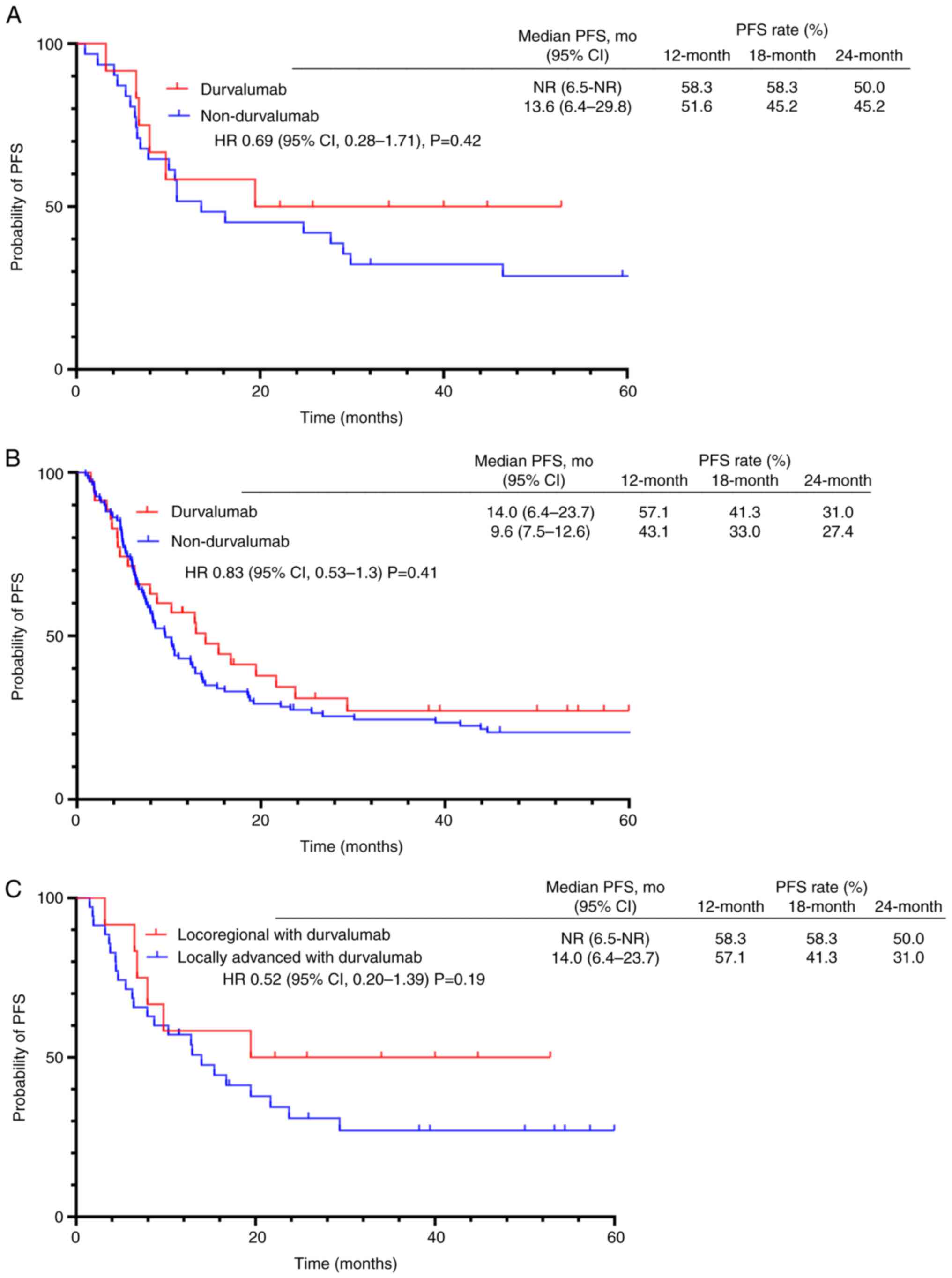
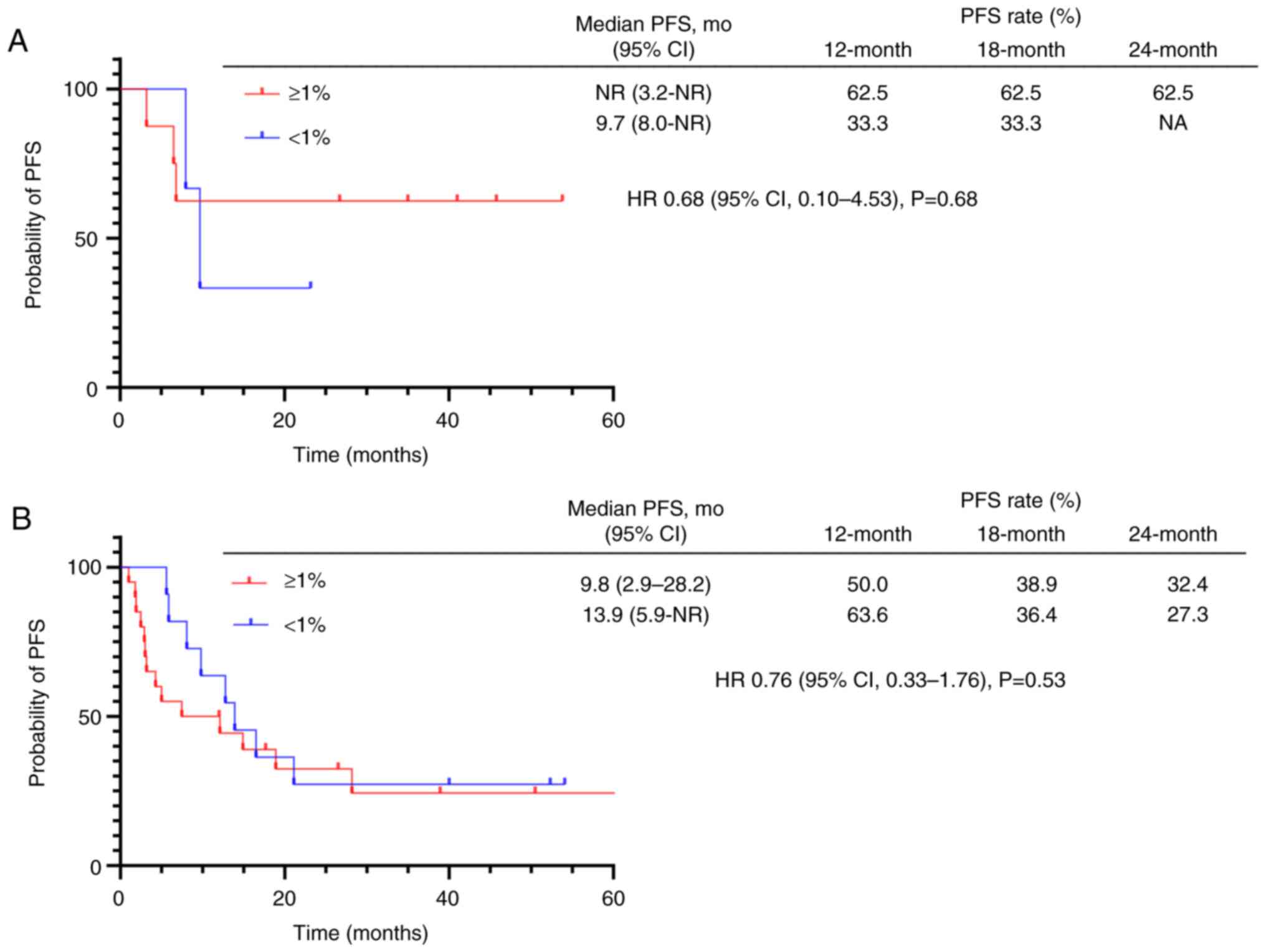
|
1
|
Cao C, Wang D, Chung C, Tian D, Rimner A,
Huang J and Jones DR: A systematic review and meta-analysis of
stereotactic body radiation therapy versus surgery for patients
with non-small cell lung cancer. J Thorac Cardiovasc Surg.
157:362–373.e8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Okami J, Shintani Y, Okumura M, Ito H,
Ohtsuka T, Toyooka S, Mori T, Watanabe SI, Date H, Yokoi K, et al:
Demographics, safety and quality, and prognostic information in
both the seventh and eighth editions of the TNM classification in
18,973 surgical cases of the Japanese joint committee of lung
cancer registry database in 2010. J Thorac Oncol. 14:212–222.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Potter AL, Costantino CL, Suliman RA,
Haridas CS, Senthil P, Kumar A, Mayne NR, Panda N, Martin LW and
Yang CJ: Recurrence after complete resection for non-small cell
lung cancer in the national lung screening trial. Ann Thorac Surg.
116:684–692. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lou F, Sima CS, Rusch VW, Jones DR and
Huang J: Differences in patterns of recurrence in early-stage
versus locally advanced non small cell lung cancer. Ann Thorac
Surg. 98:1755–1760; discussion 1760-1. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fedor D, Johnson WR and Singhal S: Local
recurrence following lung cancer surgery: Incidence, risk factors,
and outcomes. Surg Oncol. 22:156–161. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Matsuguma H, Nakahara R, Wakamatsu I,
Kishikawa T, Sugiyama T, Nakamura Y, Kasai T, Kamiyama Y, Hoshi N,
Inoue K, et al: Definitive local therapy for oligo-recurrence in
patients with completely resected non-small cell lung cancer. Am J
Clin Oncol. 43:210–217. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Matsuo Y: A systematic literature review
on salvage radiotherapy for local or regional recurrence after
previous stereotactic body radiotherapy for lung cancer. Technol
Cancer Res Treat. 17(1533033818798633)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nakamichi S, Horinouchi H, Asao T, Goto Y,
Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Ito Y, Watanabe SI and
Ohe Y: Comparison of radiotherapy and chemoradiotherapy for
locoregional recurrence of non-small-cell lung cancer developing
after surgery. Clin Lung Cancer. 18:e441–e448. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ma L, Qiu B, Zhang J, Li QW, Wang B, Zhang
XH, Qiang MY, Chen ZL, Guo SP and Liu H: Survival and prognostic
factors of non-small cell lung cancer patients with postoperative
locoregional recurrence treated with radical radiotherapy. Chin J
Cancer. 36(93)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et
al: PACIFIC Investigators. Durvalumab after chemoradiotherapy in
stage III non-small cell cancer. N Engl J Med. 377:1919–1929.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Spigel DR, Faivre-Finn C, Gray JE, Vicente
D, Planchard D, Paz Ares L, Vansteenkiste JF, Garassino MC, Hui R,
Quantin X, et al: Five-year survival outcomes from the PACIFIC
trial: Durvalumab after chemoradiotherapy in stage III
non-small-cell lung cancer. J Clin Oncol. 40:1301–1311.
2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Deng L, Liang H, Burnette B, Beckett M,
Darga T, Weichselbaum RR and Fu YX: Irradiation and anti-PD-L1
treatment synergistically promote antitumor immunity in mice. J
Clin Invest. 124:687–695. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Jabbour SK, Lee KH, Frost N, Breder V,
Kowalski DM, Pollock T, Levchenko E, Reguart N, Martinez-Marti A,
Houghton B, et al: Pembrolizumab plus concurrent chemoradiation
therapy in patients with unresectable, locally advanced, stage III
non-small cell lung cancer: The phase 2 KEYNOTE-799 nonrandomized
trial. JAMA Oncol. 7:1–9. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Girard N, Bar J, Garrido P, Garassino MC,
McDonald F, Mornex F, Filippi AR, Smit HJM, Peters S, Field JK, et
al: Treatment characteristics and real-world progression-free
survival in patients with unresectable stage III NSCLC who received
durvalumab after chemoradiotherapy: Findings from the PACIFIC-R
study. J Thorac Oncol. 18:181–193. 2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kishi N, Matsuo Y, Shintani T, Ogura M,
Mitsuyoshi T, Araki N, Fujii K, Okumura S, Nakamatsu K, Kishi T, et
al: Recurrence patterns and progression-free survival after
chemoradiotherapy with or without consolidation durvalumab for
stage III non-small cell lung cancer. J Radiat Res. 64:142–153.
2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Brierley JD, Gospodarowicz MK and
Wittekind C (eds): TNM Classification of Malignant Tumours, 8th
Edition. Wiley-Blackwell, Oxford, 2017.
|
|
17
|
U.S. Department of Health and Human
Services, National Institutes of Health and National Cancer
Institute: Common Terminology Criteria for Adverse Events (CTCAE)
v5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm.
|
|
18
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Takenaka T, Takenoyama M, Toyozawa R,
Inamasu E, Yoshida T, Toyokawa G, Shiraishi Y, Hirai F, Yamaguchi
M, Seto T and Ichinose Y: Concurrent chemoradiotherapy for patients
with postoperative recurrence of surgically resected non-small-cell
lung cancer. Clin Lung Cancer. 16:51–56. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bar J, Ng D, Moretto P, Goss GD, Sun A,
Macrae R, Laurie SA, Leighl N and Nicholas G: Chemoradiotherapy for
locoregional recurrence of non-small-cell lung cancer after
surgical resection: A retrospective analysis. Clin Lung Cancer.
14:200–204. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hisakane K, Yoh K, Nakamura N, Udagawa H,
Kirita K, Umemura S, Matsumoto S, Niho S, Akimoto T, Tsuboi M and
Goto K: Salvage chemoradiotherapy with cisplatin and vinorelbine
for postoperative locoregional recurrence of non-small cell lung
cancer. Medicine (Baltimore). 96(e8635)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
National Comprehensive Cancer Network:
Non-Small Cell Lung Cancer (version 3.2025). https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
|
|
23
|
Borghetti P, Imbrescia J, Volpi G, Scotti
V, Aquilano M, Bruni A, Franceschini D, Ursino S, Ciammella P,
Piperno G, et al: Chemo-radiotherapy plus durvalumab for
loco-regional relapse of NSCLC. Radiat Oncol.
17(124)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Furuta M, Horinouchi H, Yokota I,
Yamaguchi T, Itoh S, Fukui T, Iwashima A, Sugisaka J, Miura Y,
Tanaka H, et al: Durvalumab after chemoradiotherapy for
locoregional recurrence of completely resected non-small-cell lung
cancer (NEJ056). Cancer Sci. 115:3705–3717. 2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dickhoff C, Unal S, Heineman DJ, Winkelman
JA, Braun J, Bahce I, van Dorp M, Senan S and Dahele M: Feasibility
of salvage resection following locoregional failure after
chemoradiotherapy and consolidation durvalumab for unresectable
stage III non-small cell lung cancer. Lung Cancer.
182(107294)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Saad A, Goldstein J, Appel S, Daher S,
Urban D, Onn A, Gantz-Sorotsky H, Lobachov A, Gottfried T, Spieler
B and Bar J: Chemoradiation followed by adjuvant durvalumab in
stage III non small cell lung cancer: Real-world comparison of
treatment outcomes to historical controls treated with
chemoradiation alone. Thorac Cancer. 13:1763–1771. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Aredo JV, Mambetsariev I, Hellyer JA,
Amini A, Neal JW, Padda SK, McCoach CE, Riess JW, Cabebe EC, Naidoo
J, et al: Durvalumab for stage III EGFR-mutated NSCLC after
definitive chemoradiotherapy. J Thorac Oncol. 16:1030–1041.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hellyer JA, Aredo JV, Das M, Ramchandran
K, Padda SK, Neal JW and Wakelee HA: Role of consolidation
durvalumab in patients with EGFR- and HER2-mutant unresectable
stage III NSCLC. J Thorac Oncol. 16:868–872. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Qin Q, Peng B and Li B: The impact of
epidermal growth factor receptor mutations on the efficacy of
definitive chemoradiotherapy in patients with locally advanced
unresectable stage III non-small cell lung cancer: A systematic
review and meta-analysis. Expert Rev Anticancer Ther. 19:533–539.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Park CK, Oh HJ, Kim YC, Kim YH, Ahn SJ,
Jeong WG, Lee JY, Lee JC, Choi CM, Ji W, et al: Korean real-world
data on patients with unresectable stage III NSCLC treated with
durvalumab after chemoradiotherapy: PACIFIC-KR. J Thorac Oncol.
18:1042–1054. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jazieh K, Gad M, Saad A, Wei W and Pennell
NA: Tumor PD-L1 expression is associated with outcomes in stage III
non-small cell lung cancer (NSCLC) patients treated with
consolidation durvalumab. Transl Lung Cancer Res. 10:3071–3078.
2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zheng Q, Min S and Zhou Y: A network
meta-analysis for efficacies and toxicities of different concurrent
chemoradiotherapy regimens in the treatment of locally advanced
non-small cell lung cancer. BMC Cancer. 22(674)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yano T, Hara N, Ichinose Y, Asoh H,
Yokoyama H, Ohta M and Hata K: Local recurrence after complete
resection for non-small-cell carcinoma of the lung. Significance of
local control by radiation treatment. J Thorac Cardiovasc Surg.
107:8–12. 1994.PubMed/NCBI
|
|
34
|
Saynak M, Veeramachaneni NK, Hubbs JL, Nam
J, Qaqish BF, Bailey JE, Chung W and Marks LB: Local failure after
complete resection of N0-1 non-small cell lung cancer. Lung Cancer.
71:156–165. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Casal-Mouriño A, Ruano-Ravina A,
Lorenzo-González M, Rodríguez-Martínez Á, Giraldo-Osorio A,
Varela-Lema L, Pereiro-Brea T, Barros-Dios JM, Valdés-Cuadrado L
and Pérez-Ríos M: Epidemiology of stage III lung cancer: frequency,
diagnostic characteristics, and survival. Transl Lung Cancer Res.
10:506–518. 2021.PubMed/NCBI View Article : Google Scholar
|