Link between multiple human papillomavirus 16 and 18 infection and prostate cancer, and relevance of tumor characteristics
- Authors:
- Published online on: July 11, 2025 https://doi.org/10.3892/mco.2025.2880
- Article Number: 85
-
Copyright: © Rodríguez‑Romero et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Worldwide, prostate cancer (PCa) is the second most common cancer type in men, with the highest incidence in North and South America, Europe, Australia and the Caribbean (1). Early detection of PCa is becoming increasingly popular and acceptable among the male population. Screening with biomarkers and magnetic resonance imaging aims to enable timely diagnosis and reduce mortality. However, multiple factors can contribute to its development; among the risk factors that have been identified are family history, germline mutations, ethnicity, age, metabolic syndrome, obesity, smoking, lifestyle and infectious diseases (2).
Human papillomavirus (HPV) is a 55 nm enveloped virus with a circular double-stranded deoxyribonucleic acid (DNA) chain that encodes eight genes: late structural proteins L1 and L2 and early proteins E1, E2, E4, E5, E6, and E7. The proteins E6 and E7 are considered the most important in cell transformation due to their interactions with p53 and pRB, respectively. VPH is one of the most common sexually transmitted infections worldwide (3). In total, ~200 HPV types have been identified and classified as low-risk (LR) and high-risk (HR) based on their oncogenic potential (4,5). Certain types of cancer, such as cervical, oropharyngeal, breast, anal, penile and PCa, have been linked to HPV infection (3).
There is sufficient evidence for the association between HPV and PCa in several meta-analyses and case-control studies (6-13). However, differences in HPV prevalence have been described by geographical location (14). Due to the mode of transmission of a pathogen and the fact that HPV infection has been linked in male genital and urinary tumors, potential for intra-organ transmission (15), vaccination is a critical factor in developing effective preventive strategies, and investigation of HPV prevalence and its distribution of genotypes are of critical importance for the implementation of vaccination programs. In addition, HPV infection in PCa and their causal role remains unclear, and existing reports often lack information about the clinical characteristics of tumors. The study of clinical tumor characteristics is critical to our understanding of the molecular mechanisms underlying the link between HPV and PCa. The objective of the present study was to analyze the association between HPV and PCa, the genotype distribution and the potential association with tumor development.
Materials and methods
Study population
The present study is cross-sectional in the Central Military Hospital of the National Defense Ministry (Mexico City, Mexico). A total of 177 paraffin-embedded tissue prostate samples from men were analyzed. Tissue collection was performed between June and November 2024, from the biopsy sample repertoire of the pathology department of the Central Military Hospital, from patients who had previously undergone prostatectomy.
The inclusion and exclusion criteria were as follows: Inclusion criteria: i) Unrelated men, recruited between May 2022 and September 2023, who had undergone a prostate biopsy based on clinical findings (urinary symptoms and digital rectal examination) and had a histopathological diagnosis of benign prostatic hyperplasia (BPH) or PCa; ii) controlled comorbidities (diabetes and/or hypertension); iii) no history of HPV infection; and iv) no evidence of infectious diseases or inflammatory disorders. Exclusion criteria: i) Patients who were presented with cancer elsewhere in the body; ii) Cases with incomplete or inconsistent data in clinical history; iii) patients without follow-up after the biopsy (missing or not attending further consultations); and iv) missing biopsy samples or those with insufficient tissue for analysis. The samples were divided into two groups, a group including 117 tissue samples from men diagnosed by histological analysis with adenocarcinoma, and a group including 60 tissue samples from men with BPH. Tissue samples for histological examination were preserved in formaldehyde, embedded in paraffin, sectioned into 3 mm-thick slices, stained with hematoxylin and eosin, and examined microscopically. Histopathological analysis from tissue prostate samples of the patients were evaluated by Pathology Department, and the clinical characteristics of the patients were explored by a urologist. The histopathological and clinical data of all patients were collected from digital expedients. The present study was approved (approval no. CInv-045) by the Ethics and Research Committee of the Teaching and Research Department of the Central Military Hospital (Mexico City, Mexico).
DNA extraction and molecular assays
DNA was extracted from paraffin-embedded prostate tissue samples. The sample deparaffination was carried out using xylene (J.T. Baker; Avantor) and DNA extraction was carried out via an organic method using UltraPureTM: mixture 1:24:25, v/v of isoamyl alcohol: chloroform: phenol (cat. no. 1593049; Invitrogen; Thermo Fisher Scientific, Inc.). HPV detection and genotyping were analyzed using a Thermal Cycler for Real-Time PCR System Techne Prime Pro 48 (Antylia Scientific) and the Genotyping Real-time PCR Diagnostic kit (PCR-Fluorescenc Probing for 23 genotypes of HPV DNA (cat no. S3108E Sansure Biotech Inc.). The technology is based on the use of specific primers and Taqman fluorescence probes with DNA fragments specific to 23 HPV types for genotyping (6, 11, 16, 18, 26, 31, 33, 35, 39, 42, 43, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 81, 82) in groups of 4 different genotypes in 6 reaction tubes.
The PCR test used a contamination-proof system using the uracil-N-glycosylase + deoxy-uridine triphosphate enzyme to eliminate false positives by degrading potentially unwanted amplified products. To evaluate the efficiency of nucleic acid extraction, it uses β-globin in human epidermal cells as an internal control to ensure amplification and avoid false negatives by detecting potential PCR inhibitors. The limit of detection for each genotype was determined to be 400 copies/ml. The quality control was evaluated simultaneously with sample analysis using an HPV genotype-negative control, with no Ct values at specific fluorescence channels of HPV genotype assays and an HPV genotype-positive control which shows typical S-type amplification curves and the tested Ct values range from 24 to 30 at specific fluorescence channels of HPV genotype assays.
Statistical analysis
The clinical results are presented as percentages, and the mean ± SD. The analysis of variables was performed using the χ2 test for categorical variables or Fisher's exact test when the expected frequencies were lower than 5, and the unpaired Student's t-test for comparing variables between groups. The odds ratio (OR) with 95% confidence intervals was determined as a measure of the association between HPV infection and PCa risk. P<0.05 was considered to indicate a statistically significant difference. Data were analyzed using SPSS version 24 (IBM Corp.).
Results
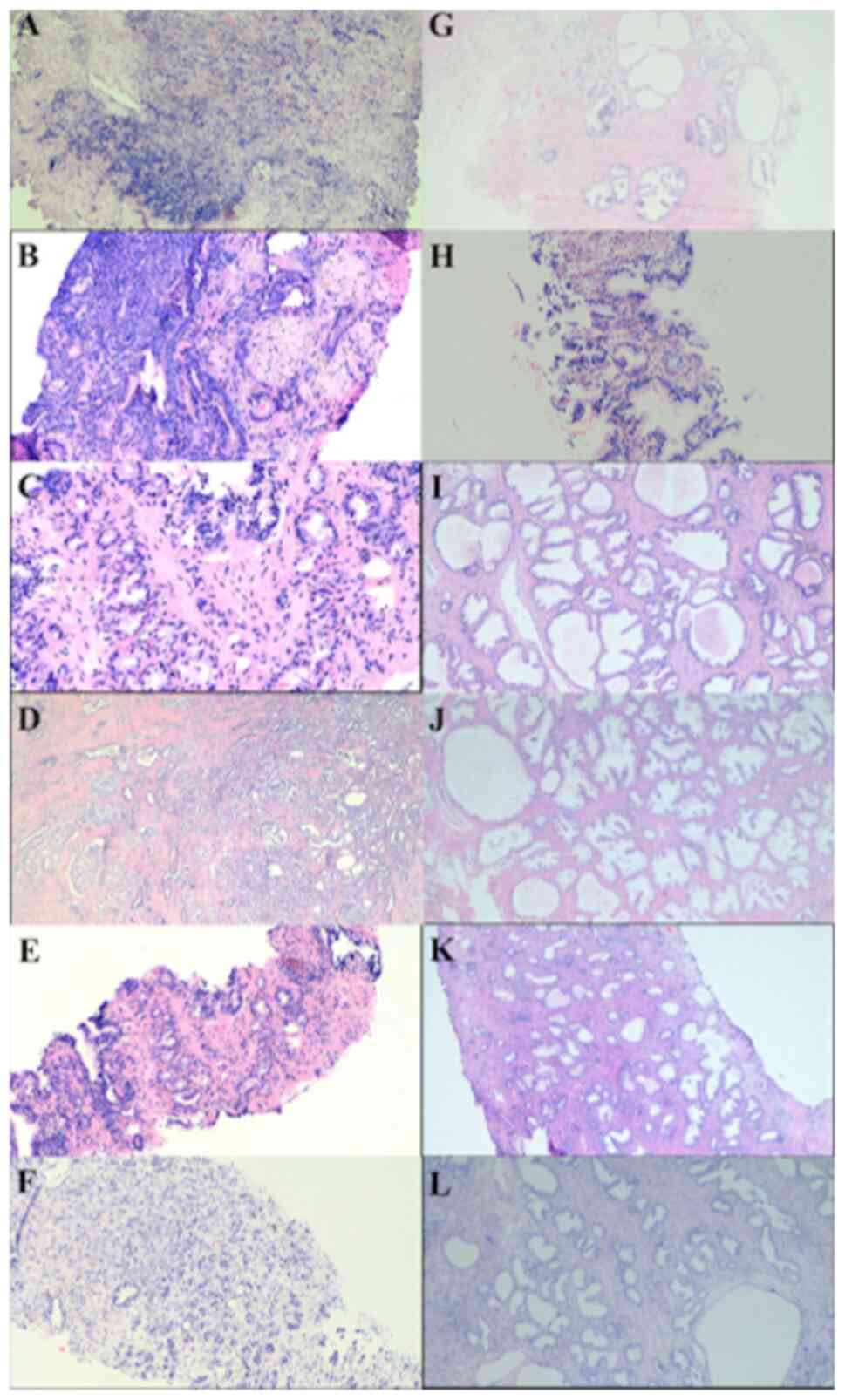
In the present study, paraffin-embedded prostate tissue samples from a group of men with PCa and a group of men with BPH were used (Fig. 1). The prevalence and HPV genotypes were studied in the patients, who were aged between 51-96 years, with a mean age of 69.6±8.7 years.
Clinical characteristics
Similar clinical characteristics were detected between groups: Most had no family history of cancer, tobacco use, or alcohol, and presented similar proportions of controlled comorbidities. There was no case with a history of HPV infection or other STIs. No statistically significant differences were found in the clinical characteristics of the groups (Table I).
Prevalence and HPV genotypes
HPV was detected in 84.1% (149/177) of all analyzed samples, exhibiting a higher prevalence in tissues with PCa (109/117 or 93%) than in tissues with BPH (40/60 or 67%), evidencing that HPV infection is associated with PCa (OR, 6.8; 95% CI, 2.77-16.69; P<0.0001).
The viral genotypes detected overall in the study, and the distribution between groups are summarized in Table II. The main viral genotypes observed in the samples, in order of decreasing prevalence, were 16, 11, 18 and 6. Genotypes 16, 11, 18 and 53 were detected with higher frequency in the PCa group and genotype 58 was detected with higher frequency in the HPB group (Table II).
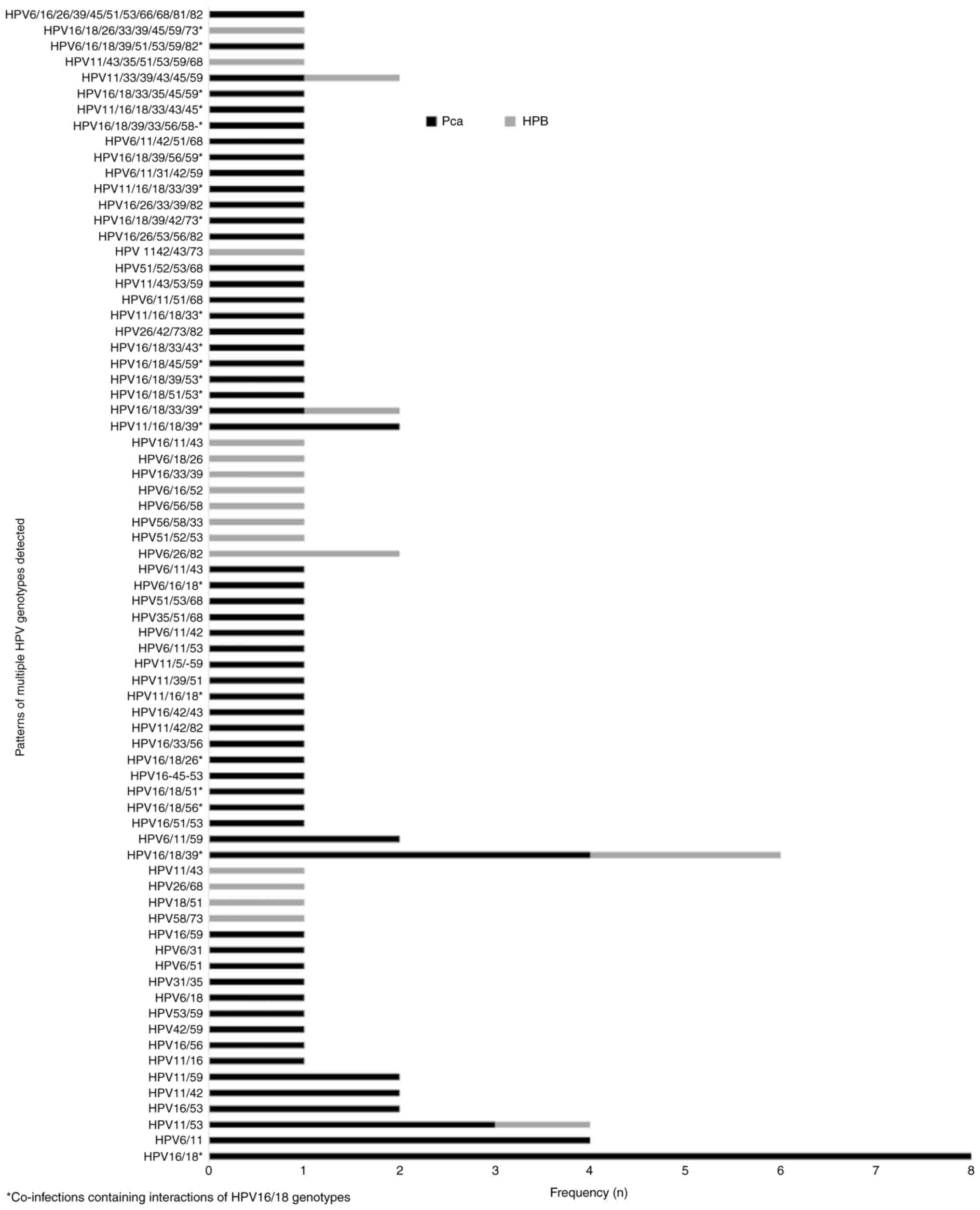
The detection of multiple HPV infection in the same sample (2-11 different genotypes) in the population studied was 55.9% (99/177), which was higher than the detection of a single HPV infection (28.2%; 50/177) (Table III). A higher single HPV infection rate was observed in HPB (31.6%; 19/60) compared with PCa (26.5%; 31/117); however, no statistical significance was detected between groups. The detection rate of multiple HPV infection was significantly higher in PCa (66.7%; 78/117) compared with HPB (35%; 21/60), which suggested that multiple HPV infection represents a risk factor for PCa (OR 3.7; 95% CI, 1.929-7.151; P=0.0001). A total of 73 different patterns of multiple HPV infections were identified (Fig. 2). The co-infections containing two different HPV genotypes were the most frequent. Of note, co-infections containing interactions of HPV16-18 types were associated with PCa: HPB (4 cases) and PCa (33 cases) (OR, 3.9079; CI, 1.2867-11.8685; P=0.0162) indicating new epidemiological data regarding the specific combination of HPV genotypes in PCa.
HR-HPV genotypes were found in 48/149 (32.2%) cases, LR-HPV genotypes in 50/149 (33.6%), and both genotypes in 51/149 (34.2%). Similarly, the distribution of genotypes was detected in study groups. In the PCa group the HR-HPV genotype was detected in 36/109 (33%) of cases, LR-HPV in 32/109 (29.4%) and both in 41/109 (37.6%). In the BHP group, the HR-HPV genotype was detected in 12/40 (30%) of cases, LR-HPV in 18/40 (45%) and both in 10/40 (25%) (Table IV). The proportion of HR-HPV, LR-HPV and both in multiple- and single-infection HPV was analyzed, detecting similar frequencies between the infected population and the study groups (Table IV).
Patients with PCa were staged according to the differentiation and aggressiveness of the prostate tumor using the Gleason score (a scale of 6 to 10) according to the histopathological findings; the higher the score, the greater the aggressiveness of the lesion and staging according to the ISUP grade (classification of 1 to 5 grades). As the grade increases, the risk of the cancer being aggressive and spreading rapidly also increases (Table V).
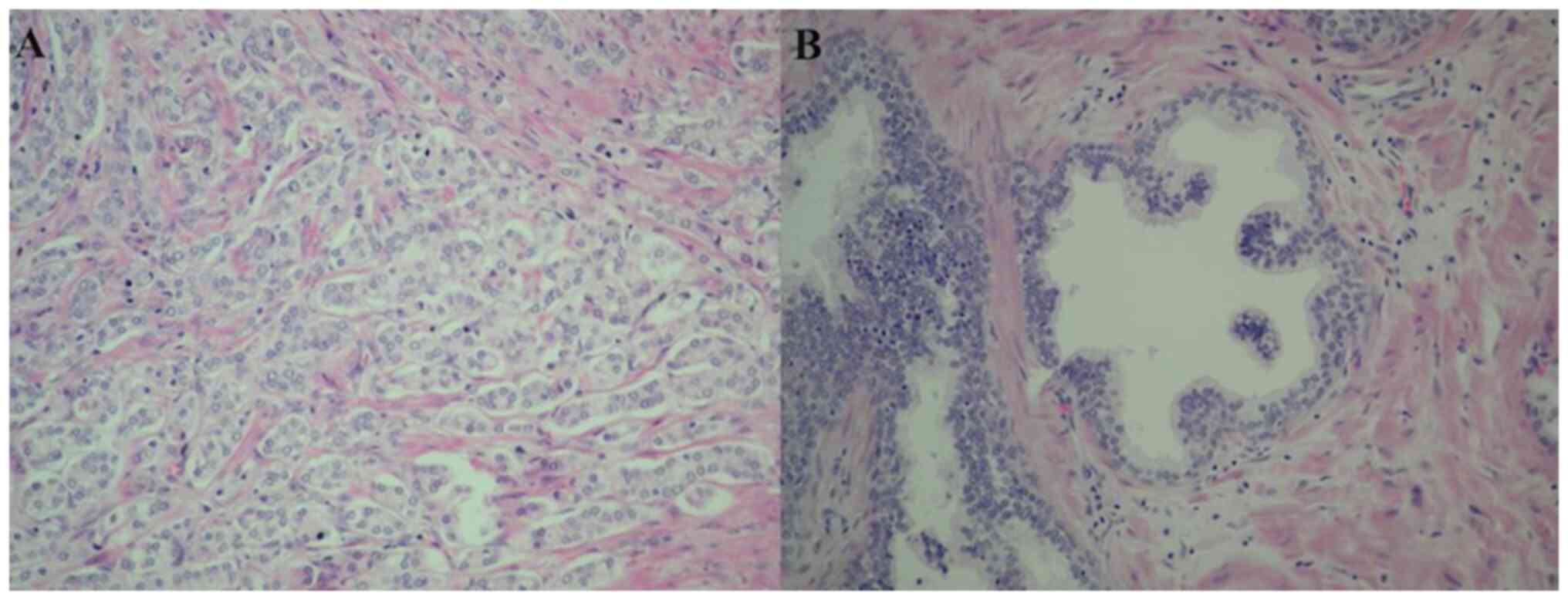
Prostatic specific antigen was evaluated, and normal values (<4 ng/µl) were more frequent in the study population (64.1%), which was concordant with a higher frequency of a Gleason Score of 6-7 (78.6%). In terms of histopathological type, all samples in the PCa group were carcinoma acinar, and the comparison of histopathological characteristics in prostatic tissues between HPV-infected and not infected patients was performed. Most cases of PCa are found in the first stages of cancer (Gleason 6-7 and ISUP 1-2), but they did not present a significant association with the presence of HPV; the results showed that cribriform cells, prostatic atrophy and prostatitis were more frequent in patients not infected with HPV (Table V). The clinical and histopathological characteristics were also compared between single and multiple infections with HR-HPV, LR-HPV and cribriform cells were also more frequent in patients with a single LR-HPV infection (Table V and Fig. 3).
Discussion
In the present study, the prevalence of HPV in prostate tissue samples from patients with PCa and BPH, and their HPV genotype distribution, were analyzed. Epidemiological studies on HPV prevalence in patients with PCa have been previously conducted; in the present study, the detected prevalence of HPV was 84.1%, which was consistent with the findings of a previous study (16), but inconsistent with our previous study, where 20% HPV prevalence was found (17). One explanation for the variation in the detection rate of HPV in our studies could be the use of different diagnostic methods. HPV detection and genotyping using genotyping Real-time PCR used in the present study has as advantage the use of a high number of HPV type-specific PCR primers, resulting in high sensitivity and specificity of the HPV detection. Despite being the most rapid diagnostic method, a disadvantage is that it is limited to numbers of HPV type-specific PCR primers according to kit design. Nested PCR used in the authors' previous study (17) also has as advantage the high sensitivity. However, an important disadvantage is the use of L1 primers for HPV detection (universal degenerated primers MY09/MY11, GP5+/6+ and L1C1), which could decrease the detection rate, due to the loss of the L1 gene during HPV integration into the host genome.
On the other hand, a pathogenetic link has been described between HPV and various malignancies other than PCa, which involve the genitourinary system in men; A previous study has suggested that HPV infection of the prostate gland could occur due to its anatomic proximity to the genitourinary system, which is considered as a virus reservoir (18). This suggestion is supported by the following: i) Certain studies have found positive associations between PCa, and sexual activities and sexually transmitted diseases (19,20); ii) HPV transmission during sexual activities through non-penetrative sexual contact (21); iii) evidence of HPV transmission via circulating extracellular vesicles throughout the body (22); and iv) a recent study revealed that a patient with prostate and bladder cancer had a history of recurrent HPV infection, highlighting the potential intra-organ spread of the virus (15). Based on the aforementioned, the increased prevalence in the present study could be due to the high prevalence of HPV in the genitourinary system in Mexican men (23).
Regarding HPV genotypes, the simultaneous presence of different HPV types suggested that they originated from the samples analyzed and are not the result of contamination. With regards to the prevalence of HPV genotypes detected in the present study, HPV16 was the most detected genotype, which was in accordance with previous meta-analysis exploring the link between HPV infection and PCa (7,9,10). The high frequency of HPV16 is important, due to certain studies reporting that the clearance of genital HPV infection in men is higher for the HR-HPV genotype compared with the LR-HPV genotype; namely, the clearance of genital HPV16 infection and some HVP 16 lineages are nearly twice as long (24) as in other oncogenic HPV types, which based on another studies could lead to persistent infection, a chronically inflammatory microenvironment and immune dysregulation, and promote carcinogenesis (25,26). The presence of HPV11 and HPV6 was also detected in a high proportion of patients in both groups, suggesting the presence of a precancerous inflammatory state in PCa, although a previous study also reported the presence of LR-HPV in cancer (27). Furthermore, some recent systematic reviews (9,28) reported that HPV16 and HPV6 were the most common genotypes in genital HPV infections in men. Consistently, another study reported a high prevalence of HPV16 (63.41%) in urethral scraping samples in men from Mexico City (29).
Multiple HPV infection was more common in PCa group (66.7%) than HBP group (35%), demonstrating that multiple HPV infection was associated with PCa as a risk factor, which represents a relevant finding in the present study. Moreover, multiple infection with HPV16/18, was higher in the PCa group, than the HPB group (PCa, 41% vs. HPB, 19%) evidenced new epidemiological data, which suggest synergistic interactions between HPV 16/18 associated with an increased risk of PCa. Similarly, the findings of our previous study showed that the most frequent combination of genotypes detected was 16/18 for the PCa group although not significant difference was detected with HPB (17).
Specific antagonist and synergic interactions between HPV genotypes have not yet been fully described in PCa. However, some studies have reported that coinfection with HR-HPVs can significantly increase the risk of high grade squamous intraepithelial lesions and high-grade anal intraepithelial neoplasia in men (30) such as persistent infection (31); and also anal infection with HPV16/18 and other oncogenic genotypes have been reported as predominant in HIV-positive men who have sex with men (32).
In addition, recent findings demonstrated that precancerous lesions, cervical cancer and changes in vaginal microecology were strongly linked to HPV16/18, although the molecular mechanisms underlying this association remain unclear (33,34).
Moreover, evaluation of HPV whole genome sequences has allowed genetic variation within each HPV type (lineages and sublineages), and cancer risk (35). Some studies have shown that HPV types differ in their frequencies of integration into the host genome (36); moreover, certain sublineages have even shown to be significatively associated with increased risks of cervical cancer (37), and sublineages risks of cancer also vary by histologic subtype (35) and are influenced by host ethnicity (38).
Therefore, in the present study, the specific combination of HPV genotypes detected may contribute to allow viral persistence within the host, modulating the developing PCa and in patients infected with HPB and HPV could be a risk factor to developing PCa.
Furthermore, a hit-and-run mechanism in oncogenesis was evidenced, since there were differences in the cellular transformation capacity between HPV16 and HPV18. In HPV16, all cell lines obtained by DNA transfection contained >1 copy/cell viral DNA, while HPV18 viral DNA was not detected in all cell lines obtained by transfection, which suggested that the presence of the virus is not always required to maintain a transformed cellular state (39). Therefore, according to this hypothesis that HPV16 is more frequent in PCa tissues than in HPV18 tissues, which was the finding of the present study. In addition, a previous study showed that in the presence of HR-HPVs, including HPV16 and HPV18 from 28 different patients with BPH prior to the development of HPV-positive PCa in the same patients, HPV causality in PCa was indicated (40). On the other hand, HPV18 has been associated with PCa using the epidemiological method of Mendelian Randomization, which uses single nucleotide polymorphisms as instrumental variables to clarify the causal relationship between exposure and outcome (41). HPV18 has also been linked with PCa when it is found as a co-infection with Epstein Barr virus; this infection status has been found to be correlated with microbial dysbiosis (16,42).
Regarding the distribution of the HR-HPV and LR-HPV, and HR-HPV + LR-HPV genotypes, no significant differences were detected between the multiple- and single infection groups. These findings were consistent with the ones of previous studies, who have shown that HPVs, predominantly HPV16 and HPV18, were detected as transcriptionally active and showed evidence of an early influence of the infection on oncogenesis, through the detection of HPVE6 and HPVE7 overexpression in prostate tissues with BPH in a period of 1-10 years prior to debuting with PCA (39,40).
In the present study, the prostatic specific antigen was evaluated, and normal values (<4 ng/µl) were more frequent in the study population (64.1%), which was concordant with a higher frequency (78.6%) of a Gleason Score of 6-7 (grades with low and intermediate risk not associated with a fatal outcome) (43,44). Comparisons between patients in the PCa group infected with HPV and those not infected revealed a higher level >4 ng/µl in infected patients; however, no statistical difference was detected.
No associations were detected regarding tumor progression because, in terms of histopathological type, all samples in the CaP group were acinar carcinoma, of which 76% presented Gleason Score of 6-7 (grades with low and intermediate risk not associated with fatal outcome), then differences were only detected in cribriform cells, prostatic atrophy and prostatitis.
No histological differences were found between HPV-HPB and HPV + HPB, while that comparison of the histopathological characteristics of tumors between HPV-PCa and HPV + PCa showed that cribriform cells, prostatic atrophy and prostatitis were more frequent in patients not infected with HPV.
Cribriform morphologies in prostate tissue are associated with a worse prognosis in PCa; certain studies have even found an association between cribriform morphologies and germline mutations in DNA repair genes (45), linked to oncological pathologies following radical prostatectomy (46), as well as higher mortality and biochemical recurrence (47).
On the other hand, prostatitis is a frequent disease that can manifest as acute or chronic inflammation (48). The connection between inflammation and PCa carcinogenesis is known (26). In tissues with BPH and PCa, chronic inflammation is commonly present; this finding is attributed as carcinogenic potential to trigger the progression of PCa (49-52). The inflammatory state causes DNA damage due to the release of cytokines and free radicals, causing cellular damage and atrophy (53).
The clinical and histopathological characteristics of tumors between single vs. multiple HPV infection were compared; and HR-HPV, LR-HPV were evaluated detected that cribriform cells also were more frequent in single infection and, LR-HPV infection. The differences in histopathological characteristics revealed a worse prognosis for patients with a single infection, LR-HPV infection, or no infection. One explanation for this finding could be due to differences in the tumor microenvironment between HPV-positive and -negative in PCa such as have been demonstrated in certain studies, as evidenced by the direct association between the oncogenic proteins E6 and E7 of high-risk HPVs and their ability to act on tumor suppressor signaling pathways through various mechanisms, such as the overexpression of antiapoptotic mediators (Bcl-2, survivin) with the progression of PCa and the induction of p53 and pRB degradation through E6 and E7, respectively (54,55).
Integration of the high-risk HPV genome into the host leads to a significant decrease in p53 expression levels in cancer cells; in detail, E6 requires binding to the E3 ligase protein E6-associated protein (E6AP) in order to bind to p53, which leads to p53 degradation by E6AP-mediated ubiquitination (56), this causes the inactivation of p21 (p21WAF1/Cip1), a cyclin-dependent kinase inhibitor, which under normal conditions promotes cell cycle arrest in the G1 phase, preventing cells from entering the S phase of the cell cycle (57,58); therefore, p53 inactivation, mediated by high-risk HPV, results in the suppression of cell cycle checkpoints, causing cell immortalization, and consequently leading to uncontrolled cell proliferation with the potential for progression to malignancy (59).
This mechanism has been described experimentally in mice, where conditional inactivation of the p53 gene (Trp53) in normal prostate epithelial cells resulted in the formation of prostate adenocarcinoma and NEPC (neuroendocrine PCa). PCa with neuroendocrine features may be resistant to castration as part of the treatment, since it presents attenuated androgen receptor (AR) signaling, suggesting that the absence of TP53 drives lineage plasticity, manifesting as a phenotypic switch from AR-dependent cells to AR-independent neuroendocrine cells, which in turn may lead to resistance to androgen deprivation therapy (60).
Moreover, the accumulation of p53 mutations may lead to an increase in MRP1 expression (61,62) and thus a decrease in the accumulation and sensitivity to drugs (63), a multidrug resistance phenotype observed in this disease (63,64).
Furthermore, a previous study detected viral modulation in the behavior of PCa cells by stimulating chronic inflammation, as observed by the increase of cytokines (IL-1, IL-6, IL-7, IL-8, IL-11, IL-17, TNF-α and TGF-β), whose expression pattern varied according to the different types of HPV studied (65). In addition, in PCa tissue with HPV infection, the overexpression of the vascular endothelial growth factor has also been detected and has been attributed to tissue inflammation leading to hypoxia, and thus angiogenesis and tumorigenesis (54); Therefore, the variants that contribute to the modulation of the immune response are attributed to the complex viral interaction between different variants presents (65). Thus, probable discrepancy results in the present study and other respect to aggressivity tumor in patients with a single infection, LR-HPV infection, or no infection, could be due to the complex interaction among different HPV types that exist within individuals as mutation, integration and host ethnicity. Similar findings have been reported into HPV-positive women, where the co-infection of HPV 16 and other genotypes of HPV except 18 was associated with decrease of cervical intraepithelial neoplasia grade 3, (34); likewise, interactions between high and low risk HPV types reduced the risk of squamous cervical cancer (66). Finally, a previous study reported that E6 oncoprotein varied depending on the HPV genotypes composition in multiple infection; however, expression was greatest in women with single HPV-type infections compared with those with multiple HPV types regardless of histopathology (67). Then studies evaluating differences in the tumor microenvironment are needed to clarify the association between co-infections and histopathological tumor characteristics. The present study has an important limitation: The sample type analyzed (paraffin-embedded prostate tissue), which resulted insufficient to analyze ‘tumor environment’ and the ‘immune response’; however, studies in progress analyzing fresh tissue could help us to clarify the association between viral infections and histopathological tumor characteristics for the control and management of the disease.
Although in the present study HPV showed a higher prevalence in patients with PCa with Gleason stages 6-7 and ISUP 1-2, and uninfected PCa patients have a worse prognosis, it cannot be suggested that the male population should be infected with HPV16/18 to improve their survival against PCa; rather, it is intended to show that HPV has a role yet to be demonstrated in the development of PCa in the early stages, therefore preventive measures such as vaccination focused on the most frequent genotypes according to the population should be reinforced to prevent high-risk HPV infection, as well as promoting health promotion for screening for the early detection of PCa. According to the data obtained, HPV may play a role in the initiation of PCa development, thus the benefit of vaccination would be to prevent infection before PCa develops. The benefits of HPV vaccination in patients diagnosed with PCa would need to be studied.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Polytechnic Institute (grant nos. 20240195 and 20250552) and the SECITHTI scholarship.
Availability of data and materials
The data generated in the present study are included in the figures and/or tables of this article.
Authors' contributions
BIRR was responsible for the data acquisition, analysis and writing of the manuscript. NMPV, MGL and MOMF were responsible for data acquisition and analysis. ACC was responsible for sample collection. VSM conceptualized the study, analyzed data and contributed to writing of the manuscript. All authors read and approved the final version of the manuscript. BIRR and VSM confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The study protocol was approved (approval no. CInv-045) by The Institutional Human Ethical Committee of Hospital Central Military of the National Defense Ministry (Mexico City, Mexico).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Bergengren O, Pekala KR, Matsoukas K, Fainberg J, Mungovan SF, Bratt O, Bray F, Brawley O, Luckenbaugh AN, Mucci L, et al: Update on prostate cancer epidemiology and risk Factors-A systematic review. Eur Urol. 84:191–206. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Gandaglia G, Leni R, Bray F, Fleshner N, Freedland SJ, Kibel A, Stattin P, Van Poppel H and La Vecchia C: Epidemiology and prevention of prostate cancer. Eur Urol Oncol. 4:877–892. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Gamboa-Hoil SI: Human papillomavirus in men. Rev Int Androl. 21(100325)2023.PubMed/NCBI View Article : Google Scholar | |
|
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans: Human Immunodeficiency Viruses and Human T-Cell Lymphotropic Viruses. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. IARC, Lyon, France, pp250-270, 1996. https://www.ncbi.nlm.nih.gov/books/NBK321760/. | |
|
Jensen JE, Becker GL, Jackson JB and Rysavy MB: Human papillomavirus and associated cancers: A Review. Viruses. 16(680)2024.PubMed/NCBI View Article : Google Scholar | |
|
Yin SH, Chung SD, Hung SH, Liu TC and Lin HC: Association of prostate cancer with human papillomavirus infections: A Case-control study. Prostate Cancer Prostatic Dis. 27:743–748. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Arriaga D, Morales F and Canizalez A: Human papillomavirus and prostate cancer in Mexican men: A systematic review and meta-analysis. Cancer Causes Control: March 15, 2025 (Epub ahead of print). | |
|
Sosse SA, Laraqui A, Mrabti M, Alami M, Mzibri ME and Ennaji M: Molecular evaluation of human papillomavirus as an oncogenic biomarker in prostate cancer. Mol Biol Rep. 50:5719–5724. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Russo GI, Calogero AE, Condorelli RA, Scalia G, Morgia G and La Vignera S: Human papillomavirus and risk of prostate cancer: A systematic review and meta-analysis. Aging Male. 23:132–138. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Lawson JS and Glenn WK: Evidence for a causal role by human papillomaviruses in prostate cancer-a systematic review. Infect Agent Cancer. 15(41)2020.PubMed/NCBI View Article : Google Scholar | |
|
Moghoofei M, Keshavarz M, Ghorbani S, Babaei F, Nahand JS, Tavakoli A, Mortazavi HS, Marjani A, Mostafaei S and Monavari SH: Association between human papillomavirus infection and prostate cancer: A global systematic review and meta-analysis. Asia Pac J Clin Oncol. 15:e59–e67. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Tsydenova IA, Ibragimova MK, Tsyganov MM and Litviakov NV: Human papillomavirus and prostate cancer: Systematic review and meta-analysis. Sci Rep. 3(16597)2023.PubMed/NCBI View Article : Google Scholar | |
|
Opeyemi Bello R, Willis-Powell L, James O, Sharma A, Marsh E, Ellis L, Gaston K and Siddiqui Y: Does human papillomavirus play a causative role in prostate cancer? A systematic review using bradford Hill's Criteria. Cancers (Basel). 15(3897)2023.PubMed/NCBI View Article : Google Scholar | |
|
Mahmoudi S, Jafari-Sales A, Nasiri R and Baghi H: Prostate cancer and human papillomavirus infection: A recent literature review. Rev Res Med Microbiol. 33:100–108. 2022. | |
|
Ahmed MY, Cakir MO, Salman NA, Sandhu S and Ashrafi GH: Concurrent high risk HPV35, HPV45, and HPV59 infections in prostate and bladder cancer tissues of a single patient: A case report. Heliyon. 10(e35074)2024.PubMed/NCBI View Article : Google Scholar | |
|
Whitaker NJ, Glenn WK, Sahrudin A, Orde MM, Delprado W and Lawson JS: Human papillomavirus and Epstein Barr virus in prostate cancer: Koilocytes indicate potential oncogenic influences of human papillomavirus in prostate cancer. Prostate. 73:236–241. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Medel-Flores O, Valenzuela-Rodríguez VA, Ocadiz-Delgado R, Castro-Muñoz LJ, Hernández-Leyva S, Lara-Hernández G, Silva-Escobedo JG, Vidal PG and Sánchez-Monroy V: Association between HPV infection and prostate cancer in a Mexican population. Genet Mol Biol. 41:781–789. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Giuliano AR, Nyitray AG, Kreimer AR, Pierce Campbell CM, Goodman MT, Sudenga SL, Monsonego J and Franceschi S: EUROGIN 2014 roadmap: Differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int J Cancer. 136:2752–2760. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Taylor ML, Mainous AG and Wells BJ: Prostate cancer and sexually transmitted diseases: A meta-analysis. Fam Med. 37:506–512. 2005.PubMed/NCBI | |
|
Huang WY, Hayes R, Pfeiffer R, Viscidi RP, Lee FK, Wang YF, Reding D, Whitby D, Papp JR and Rabkin CS: Sexually transmissible infections and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 17:2374–2381. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Liu Z, Nyitray AG, Hwang LY, Swartz MD, Abrahamsen M, Lazcano-Ponce E, Salmerón J, Quiterio M, Villa LL, Baggio ML, et al: Human papillomavirus prevalence among 88 male virgins residing in brazil, mexico, and the united states. J Infect Dis. 214:1188–1191. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Gameiro SF and Flondra KM: Human Papillomavirus-associated tumor extracellular vesicles in HPV+ tumor microenvironments. J Clin Med. 12(5668)2023.PubMed/NCBI View Article : Google Scholar | |
|
Luna-Aguirre CM, Reyes-Cortés LM, Torres-Grimaldo AA, Karr-de-León SF, Cerda-Flores RM, Melo-Nava B, Aizpuru-Akel VE and Barrera-Saldaña HA: Prevalence of human papillomavirus types in north and central regions of Mexico. Epidemiol Infect. 146:1724–1730. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Ferreira MT, Mendoza López RV, Gonçalves MG, Ferreira S, Sirak B, Baggio ML, Lazcano-Ponce E, Nyitray AG, Giuliano AR, Villa LL, et al: Human papillomavirus 16 lineage a variants associated with persistent genital infections in men: The HPV infection in men (HIM) study. J Infect Dis. 228:1748–1757. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Abidi SH, Bilwani F, Ghias K and Abbas F: Viral etiology of prostate cancer: Genetic alterations and immune response. A literature review. Int J Surg. 52:136–140. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Sfanos KS, Yegnasubramanian S, Nelson WG and De Marzo AM: The inflammatory microenvironment and microbiome in prostate cancer development. Nat Rev Urol. 15:11–24. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Silva LLD, Teles AM, Santos JMO, Souza de Andrade M, Medeiros R, Faustino-Rocha AI, Oliveira PA, Dos Santos APA, Ferreira Lopes F, Braz G, et al: Malignancy associated with Low-Risk HPV6 and HPV11: A systematic review and implications for cancer prevention. Cancers (Basel). 15(4068)2023.PubMed/NCBI View Article : Google Scholar | |
|
Kusters JMA, Brouwer JGM, van Benthem BHB, Heijne JCM and Schim van der Loeff MF: Global Type-specific genital human papillomavirus prevalence in men, by sexual orientation: A systematic review and Meta-analysis. J Infect Dis. 228:1023–1032. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Gallegos-Bolaños J, Rivera-Domínguez JA, Presno-Bernal JM and Cervantes-Villagrana RD: High prevalence of co-infection between human papillomavirus (HPV) 51 and 52 in Mexican population. BMC Cancer. 17(531)2017.PubMed/NCBI View Article : Google Scholar | |
|
Zhang X, Lu D, Szporn AH, Zakowski MF and Si Q: A comparative study of the genotype profiles of high-risk human papillomavirus infection in male and female HIV-positive patients and their correlation with anal cytology and biopsy. J Am Soc Cytopathol. 11:21–30. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Gaisa MM, Liu Y, Deshmukh AA, Stone KL and Sigel KM: Electrocautery ablation of anal high-grade squamous intraepithelial lesions: Effectiveness and key factors associated with outcomes. Cancer. 126:1470–1479. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Torres-Ibarra L, Conde-Glez CJ, Salmerón J, Palefsky J, Hernández-Nevares P, Sánchez-Alemán MA, Magis-Rodríguez C and Lazcano-Ponce E: Risk factors for anal HPV-16/18 infection in Mexican HIV-infected men who have sex with men. Prev Med. 69:157–64. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Zhang Z, Yang Y, Zhang L, Wu Y, Jia P, Ma Q and Wang D: Relationship between cervicovaginal microecological changes and HPV16/18 infection and cervical cancer in women of childbearing age. Ann Clin Lab Sci. 53:825–834. 2023.PubMed/NCBI | |
|
Ao M, Yao X, Zheng D, Gu X and Xi M: Risk of cervical intraepithelial neoplasia grade 3 or more diagnoses for human papillomavirus16/18-positive women by cytology and co-infection status. Infect Agent Cancer. 18(57)2023.PubMed/NCBI View Article : Google Scholar | |
|
Nelson C and Mirabello L: Human papillomavirus genomics: Understanding carcinogenicity. Tumour Virus Res. 15(20025)2023.PubMed/NCBI View Article : Google Scholar | |
|
The Cancer Genome Atlas Research Network. Integrated genomic and molecular characterization of cervical cancer. Nature. 543:378–384. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Mirabello L, Yeager M, Cullen M, Cullen M, Boland JF, Chen Z, Wentzensen N, Zhang X, Yu K, Yang Q, et al: HPV16 sublineage associations with Histology-specific cancer risk using HPV Whole-genome sequences in 3200 women. J Natl Cancer Inst. 108(djw100)2016.PubMed/NCBI View Article : Google Scholar | |
|
Xi LF, Kiviat NB, Hildesheim A, Galloway DA, Wheeler CM, Ho J and Koutsky LA: Human papillomavirus type 16 and 18 variants: Race-related distribution and persistence. J Natl Cancer Inst. 98:1045–1052. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Iwasaka T, Hayashi Y, Yokoyama M, Hara K, Matsuo N and Sugimori H: ‘Hit and run’ oncogenesis by human papillomavirus type 18 DNA. Acta Obstet Gynecol Scand. 7:219–223. 1992.PubMed/NCBI View Article : Google Scholar | |
|
Glenn WK, Ngan CC, Amos TG, Edwards RJ, Swift J, Lutze-Mann L, Shang F, Whitaker NJ and Lawson JS: High risk human papilloma viruses (HPVs) are present in benign prostate tissues before development of HPV associated prostate cancer. Infect Agent Cancer. 12(46)2017.PubMed/NCBI View Article : Google Scholar | |
|
Sun J, Xiang J, An Y, Xu J, Xiong Y, Wang S and Xia Q: Unveiling the Association between HPV and Pan-Cancers: A Bidirectional Two-sample mendelian randomization study. Cancers (Basel). 15(5147)2023.PubMed/NCBI View Article : Google Scholar | |
|
Sarkar P, Malik S, Banerjee A, Datta C, Pal DK, Ghosh A and Saha A: Differential microbial signature associated with benign prostatic hyperplasia and prostate cancer. Front Cell Infect Microbiol. 12(894777)2022.PubMed/NCBI View Article : Google Scholar | |
|
Sehn JK: Prostate cancer pathology: Recent updates and controversies. Mo Med. 115:151–155. 2018.PubMed/NCBI | |
|
Humphrey PA: Histopathology of prostate cancer. Cold Spring Harb Perspect Med. 7(a030411)2017.PubMed/NCBI View Article : Google Scholar | |
|
Hesterberg AB, Gordetsky JB and Hurley PJ: Cribriform prostate cancer: Clinical pathologic and molecular considerations. Urology. 155:47–54. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Sayan M, Tuac Y, Akgul M, Pratt GK, Rowan MD, Akbulut D, Kucukcolak S, Tjio E, Moningi S, Leeman JE, et al: Prognostic significance of the cribriform pattern in prostate cancer: Clinical outcomes and genomic alterations. Cancers (Basel). 16(1248)2024.PubMed/NCBI View Article : Google Scholar | |
|
Russo GI, Soeterik T, Puche-Sanz I, Broggi G, Lo Giudice A, De Nunzio C, Lombardo R, Marra G and Gandaglia G: European Association of Urology Young Academic Urologists. Oncological outcomes of cribriform histology pattern in prostate cancer patients: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 26:646–654. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Woenckhaus J and Fenic I: Proliferative inflammatory atrophy: A background lesion of prostate cancer? Andrologia. 40:134–137. 2008.PubMed/NCBI View Article : Google Scholar | |
|
De Marzo AM, Marchi VL, Epstein JI and Nelson WG: Proliferative inflammatory atrophy of the prostate: Implications for prostatic carcinogenesis. Am J Pathol. 155:1985–1992. 1999.PubMed/NCBI View Article : Google Scholar | |
|
Sfanos KS and De Marzo AM: Prostate cancer and inflammation: The evidence. Histopathology. 60:199–215. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Mani RS, Amin MA, Li X, Kalyana-Sundaram S, Veeneman BA, Wang L, Ghosh A, Aslam A, Ramanand SG, Rabquer BJ, et al: Inflammation-induced oxidative stress mediates gene fusion formation in prostate cancer. Cell Rep. 17:2620–2631. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Kwon OJ, Zhang L, Ittmann MM and Xin L: Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. Proc Natl Acad Sci USA. 111:E592–E600. 2014.PubMed/NCBI View Article : Google Scholar | |
|
de Bono JS, Guo C, Gurel B, De Marzo AM, Sfanos KS, Mani RS, Gil J, Drake CG and Alimonti A: Prostate carcinogenesis: Inflammatory storms. Nat Rev Cancer. 20:455–469. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Sadri Nahand J, Esghaei M, Hamidreza Monavari S, Moghoofei M, Jalal Kiani S, Mostafaei S, Mirzaei H and Bokharaei-Salim F: The assessment of a possible link between HPV-mediated inflammation, apoptosis, and angiogenesis in Prostate cancer. Int Immunopharmacol. 88(106913)2020.PubMed/NCBI View Article : Google Scholar | |
|
Fatemipour M, Nahand JS, Fard Azar ME, Baghi HB, Taghizadieh M, Sorayyayi S, Hussen BM, Mirzaei H, Moghoofei M and Bokharaei-Salim F: Human papillomavirus and prostate cancer: The role of viral expressed proteins in the inhibition of anoikis and induction of metastasis. Microb Pathog. 152(104576)2021.PubMed/NCBI View Article : Google Scholar | |
|
Li S, Hong X, Wei Z, Xie M, Li W, Liu G, Guo H, Yang J, Wei W and Zhang S: Ubiquitination of the HPV Oncoprotein E6 Is Critical for E6/E6AP-Mediated p53 degradation. Front Microbiol. 10(2483)2019.PubMed/NCBI View Article : Google Scholar | |
|
El-Deiry WS: p21(WAF1) mediates Cell-cycle inhibition, relevant to cancer suppression and therapy. Cancer Res. 76:5189–5191. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Barr AR, Cooper S, Heldt FS, Butera F, Stoy H, Mansfeld J, Novák B and Bakal C: DNA damage during S-phase mediates the proliferation-quiescence decision in the subsequent G1 via p21 expression. Nat Commun. 8(14728)2017.PubMed/NCBI View Article : Google Scholar | |
|
Szymonowicz KA and Chen J: Biological and clinical aspects of HPV-related cancers. Cancer Biol Med. 17:864–878. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Wang G, Zhao D, Spring DJ and DePinho RA: Genetics and biology of prostate cancer. Genes Dev. 32:1105–1140. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Sullivan GF, Yang JM, Vassil A, Yang J, Bash-Babula J and Hait WN: Regulation of expression of the multidrug resistance protein MRP1 by p53 in human prostate cancer cells. J Clin Invest. 105:1261–1267. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Rigalli JP, Reichel M and Tocchetti GN: Human papilloma virus (HPV) 18 proteins E6 and E7 up-regulate ABC transporters in oropharyngeal carcinoma. Involvement of the nonsense-mediated decay (NMD) pathway. Cancer Lett. 428:69–76. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Khooshemehri P, Jamaldini S, Ziaee S, Afshari M, Sattari M, Narouie B, Sotoudeh M, Montazeri V, Sarhangi N and Hasanzad M: Genetic polymorphism of mismatch repair genes and susceptibility to prostate cancer. Urol J. 17:271–275. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Poku VO and Iram SH: A critical review on modulators of Multidrug Resistance Protein 1 in cancer cells. PeerJ. 10(e12594)2022.PubMed/NCBI View Article : Google Scholar | |
|
Boccardo E, Lepique AP and Villa LL: The role of inflammation in HPV carcinogenesis. Carcinogenesis. 31:1905–1912. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Sundström K, Ploner A, Arnheim-Dahlström L, Eloranta S, Palmgren J, Adami HO, Ylitalo Helm N, Sparén P and Dillner J: Interactions between high- and low-risk HPV types reduce the risk of squamous cervical cancer. J Natl Cancer Inst. 107(djv185)2015.PubMed/NCBI View Article : Google Scholar | |
|
Wu Z, Li TY, Jiang M, Yu L, Zhao J, Wang H, Zhang X, Chen W and Qiao Y: Human papillomavirus (HPV) 16/18 E6 oncoprotein expression in infections with single and multiple genotypes. Cancer Prev Res (Phila). 12:95–102. 2019.PubMed/NCBI View Article : Google Scholar |