Epidemiology of autonomic dysfunction in Parkinson's disease (Review)
- Authors:
- Published online on: September 1, 2025 https://doi.org/10.3892/mi.2025.267
- Article Number: 68
-
Copyright : © Rissardo et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
1. Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disorder that is recognized mainly for presenting clinically with motor symptoms of tremors, rigidity and bradykinesia (Fig. 1) (1). However, non-motor symptoms are often underrecognized in PD, particularly those related to autonomic functions (2). Autonomic dysfunction can affect a wide array of systems, including cardiovascular (3), gastrointestinal (4), genitourinary (5), sweat glands (6), sexual function (7) and even temperature regulation (8).
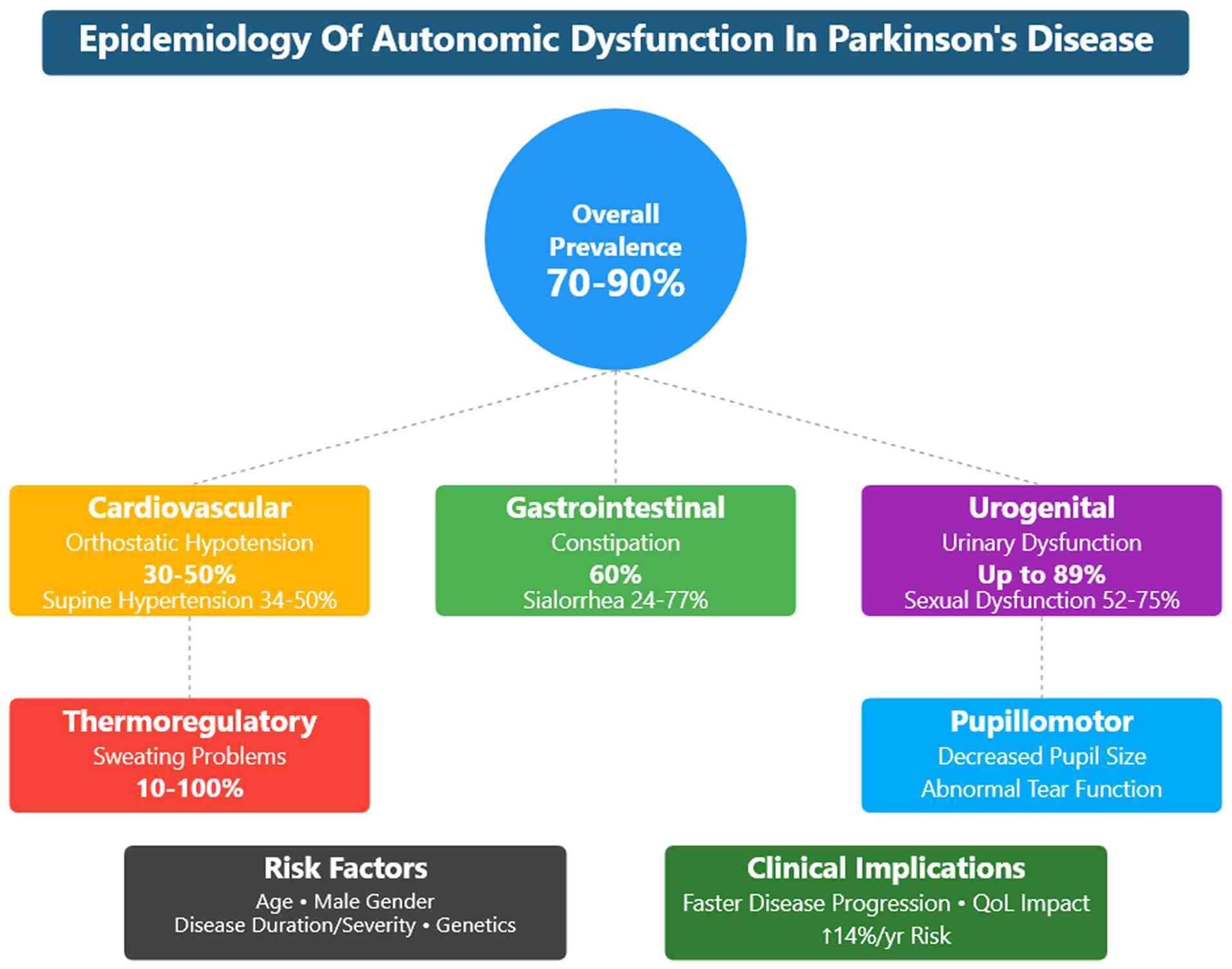
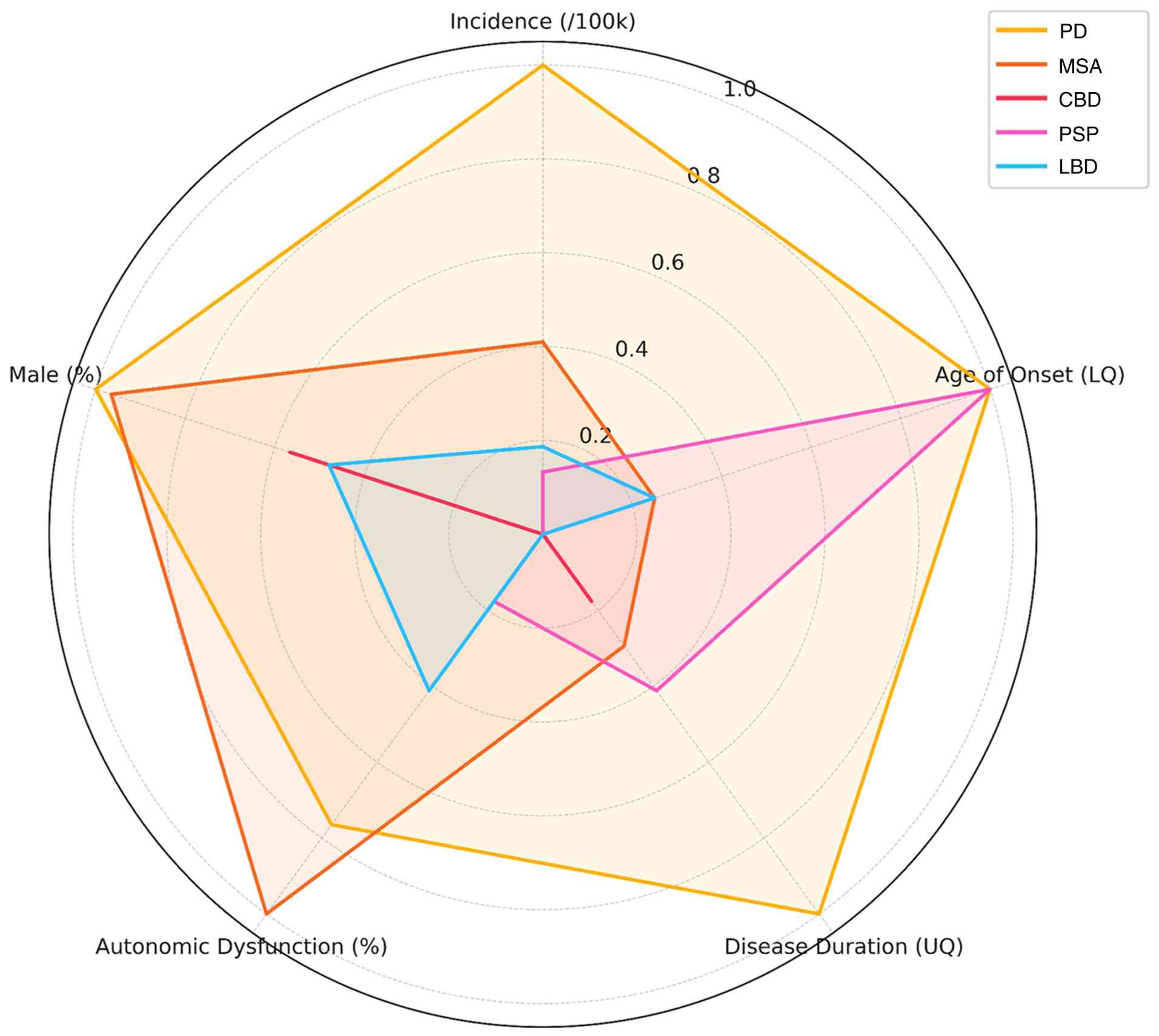
While less visible than the motor symptoms, autonomic dysfunction significantly affects the quality of life (QoL) of patients with PD (9). It can manifest early in the course of the disease, sometimes even preceding the onset of motor symptoms (10), and its prevalence increases with disease progression (11,12). In this context, orthostatic hypotension (OH) is related to significant increases in the health care burden from $9831 to $25,205/person/year (13). Notably, PD and atypical parkinsonian syndromes are affected differently by autonomic symptoms (Fig. 2, and Tables SI and SII) (14-16).
Patients with autonomic dysfunction may have a distinct phenotype. Research has demonstrated that dysautonomia mainly manifests in older individuals with bilateral onset and lower frequency of dystonic symptoms (17). On the other hand, there are some contradictory results in the literature reporting patients with dysautonomia and worsening motor and cognitive outcomes (18). Of note, as demonstrated in a previous study, three main clusters of patients with autonomic dysfunction have been found: Cluster F (hypertension and sexual dysfunction), cluster G (depression and weight loss), and cluster H (sweating and anxiety) (19). The authors of that study did not find a specific constellation of manifestations for urinary and gastrointestinal symptoms (19). Another study found two groups of individuals defined by cluster A (thermoregulatory, gastrointestinal, and pupillomotor) and cluster B (cardiovascular and gastrointestinal). Only cluster B was associated with cognitive impairment (20). Some authors have hypothesized that non-motor symptoms, including autonomic symptoms, also fluctuate and are dependent on the levodopa equivalent daily dose (LEDD) (21).
Stanković et al (22) reported that autonomic dysfunction is common at the time of diagnosis of PD and progresses independently from motor symptoms during the first 3 years. De Pablo-Fernandez et al (23), reported a shorter survival rate with early autonomic dysfunction symptoms, a similar pattern of survival rate and autonomic dysfunction was found in patients with PD dementia (24). Moreover, patients with drug-induced parkinsonism that develop autonomic failure features more likely develop PD (25).
Understanding the autonomic dysfunction spectrum in PD is essential for clinicians and researchers. The appreciation of its early presence may provide a critical window for intervention with disease-modifying therapy, which could affect the eventual course of the disease (26). In addition, studies on autonomic dysfunction, particularly studies on individuals with idiopathic REM sleep behavior disorder (RBD), have contributed to the understanding of the pathophysiology of neurodegenerative synucleinopathies, as certain autonomic symptoms are associated with an increased risk of phenoconversion to PD or Lewy body dementia (LBD) (27). Notably, the female sex and gastrointestinal dysfunctions have been shown to be associated with phenoconversion (28,29). Furthermore, the presence of autonomic dysfunction in patients with idiopathic RBD is associated with a higher phenoconversion rate, although it is not commonly studied in clinical trials (30,31).
Recent results from the Parkinson's Progression Markers Initiative (PPMI) revealed no association between biomarkers and autonomic dysfunction. Thus, the diagnosis is still based on clinical evaluation (32). There are several challenges in diagnosing and instituting therapy for autonomic dysfunction. The symptoms are frequently non-specific and diverse, mimicking other conditions, such as adverse effects from medications (33). Moreover, several patients with PD may be hesitant to report such autonomic symptoms, fearing the loss of independence or underestimating their impact on the QoL. It is worth mentioning that autonomic dysfunction and the presence of at least one of the 44 most common single-nucleotide polymorphisms which are linked to PD can predict the diagnosis of PD in 82% of cases (34).
The present literature review on the epidemiology of autonomic dysfunction in PD is crucial due to the significant impact of these often-overlooked symptoms on the lives of individuals living with PD (9). Therefore, proactive screening and thorough assessment are paramount for the detection and management of autonomic dysfunction in PD. In recognition of these diverse presentations of this commonly hidden burden, clinicians can provide more holistic care for patients with PD, enhancing the QoL and probably altering the course of the disease.
2. General prevalence and incidence of autonomic dysfunction in Parkinson's disease
Despite its significant impact, the true prevalence of dysautonomia in PD remains unclear. Existing studies report a wide range of estimates, depending on the specific autonomic function being investigated and the methods used to assess it (35). This variability underscores the need to synthesize existing knowledge and identify potential gaps in the understanding of how frequently different types of autonomic dysfunction occur in people with PD.
Autonomic dysfunction is common among patients with PD, affecting up to 70-90% of patients (36,37). The dysfunctions in the cardiovascular system manifests as orthostatic hypotension (OH) (38). OH is a sustained fall in blood pressure (BP) ≥20 mmHg systolic or ≥10 mmHg diastolic when moving from the supine to the standing position. OH is a clinical sign, and it can be symptomatic or asymptomatic. When symptomatic, it manifests as lightheadedness, syncope (39), blurry vision and feeling faint, which can be explained by tissue hypoperfusion. Other less specific symptoms include tiredness, cognitive impairment, dyspnea, neck and shoulder discomfort, or angina (38). The prevalence of OH in PD is 30-50%, although it is symptomatic in approximately one-third of patients (13-16%). The prevalence increases with age and disease progression (23). Supine hypertension (SH) is also common among patients with PD. The American Heart Association defined three degrees of arterial hypertension in the case of BP as ≥140 mmHg systolic or ≥90 mmHg diastolic (40). The prevalence of SH was found in 34-50% of patients with PD (41,42). OH can cause acute morbidities, such as syncope and falls, whereas SH causes end-target organ damage over time (38). SH and OH have been proposed as negative prognostic factors for survival, cardiological and cerebrovascular outcomes, as well as cognitive decline in PD (43).
The gastrointestinal system is also affected by autonomic dysfunction, with a number of clinical manifestations. Upper and lower gastrointestinal symptoms, such as constipation, are common in patients with PD and contribute to a decreased QoL (44). At least 35% of patients with PD complain of dysphagia (45). As shown by videofluoroscopy, the majority of patients with PD have abnormal swallowing with reduced efficiency and frequency. This manifests as sialorrhea or the drooling of saliva in 50-60% of patients with PD, particularly those with advanced disease (46). Constipation is the most common autonomic and gastrointestinal symptom and occurs in up to 90% of patients with PD (47). Autonomic dysfunction of the gastrointestinal system may present as acute emergencies, such as colonic volvulus, intestinal pseudo-obstruction, megacolon, fecal impaction, or overflow diarrhea (48).
Urinary dysfunction often affects patients with PD due to the high prevalence of detrusor overactivity. Up to 85% of patients with PD suffer from lower urinary tract symptoms (49). Nocturia is the most commonly reported symptom (57-86%), followed by frequency (32-71%), urgency (32-68%) and urge incontinence (21-40%). Hesitancy and incomplete emptying affect 1-38% and 8-28% of patients with PD, respectively (50-52). Sexual dysfunction is also common among patients with PD and can appear years prior to the diagnosis of PD (53). Of note, 79% of males with PD complain of erectile dysfunction, ejaculation issues and difficulties achieving orgasm. In addition, 75% of females with PD and multiple system atrophy (MSA) report sexual-related issues, such as as vaginal dryness, decreased libido and difficulties reaching orgasm (38).
3. Demographics and risk factors
Age. Age is associated with autonomic dysfunction and has direct and indirect associations with this dysfunction. Age has been found to be related to autonomic dysfunction, with older patients having more autonomic dysfunction symptoms (23). Szewczyk-Krolikowski et al (54) found only a trend without statistical significance between age and autonomic dysfunction, whereas Zhou et al (55) revealed that age was an independent predictor of dysautonomia. Furthermore, older patients with PD are most likely to have more severe disease with a longer disease duration. Autonomic dysfunction has been found to be prevalent in patients with more severe motor disease (47,56).
Female/male sex. The male sex is more commonly associated with autonomic dysfunction (23), and males have more drastic impairment of the autonomic functions (57). In this context, males are more likely to be treated for autonomic dysfunction (58). One of the possible explanations for this finding is that physicians screen for symptoms based on the sex of patients (59). It has been found that urinary and sexual symptoms are more common in males with PD, while cardiovascular dysfunction is more common among females (59,60). However, some researchers have found that the prevalence of dysautonomia is more common among females, which may be explained by regional and cultural factors of where the study was conducted (61).
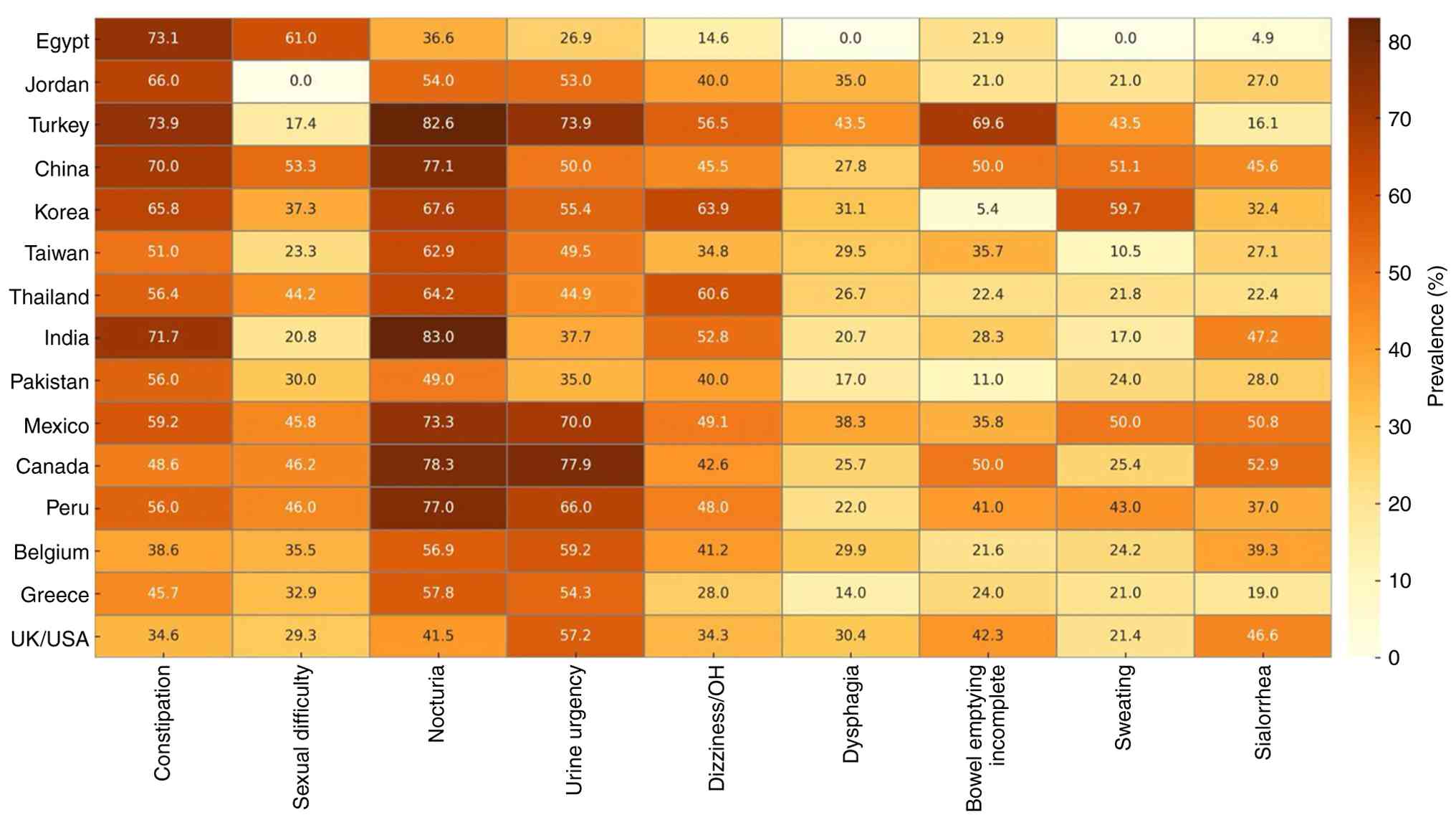
Geographic and ethnic variations. There is limited knowledge about geographic and ethnic variations in autonomic dysfunction. However, some studies have tried to bridge the gap (Fig. 3), and (Tables SIII and SIV) (62-76). For example, Sauerbier et al (77) discussed the prevalence of non-motor symptoms in Asian patients. They found that autonomic dysfunction symptoms were common among Asian patients. Constipation, memory impairment and nocturia were the most frequently self-reported symptoms (77). Chen et al (78) also found differences in non-motor symptoms between Chinese patients with PD and their fellow Western patients. Okubadejo et al (79) found that autonomic dysfunction symptoms were more common among patients of African origin when compared to German patients. Sidahmed et al (80) suggested that this difference may be due to genetic or environmental factors. These variations can also be due to cultural norms; for example, Zhou et al (37) reported lower sexual symptoms and suggested that it may be due to the hesitation of Chinese patients to answer such questions.
Seasonal variation may influence the reporting and severity of autonomic dysfunction in patients with PD. While some studies have suggested that autonomic symptoms are less prevalent in summer compared to winter (81), this seasonal difference appears to be most pronounced in cardiovascular dysautonomia (82). Additionally, winter months are associated with increased mortality rates in PD, primarily due to respiratory infections, such as pneumonia (83); however, some researchers have only found statistical significance for cardiovascular dysautonomia (84). Notably, the impact of seasonal changes on autonomic symptoms may be partially mediated by their influence on sleep-related disturbances, which are common in PD.
Disease duration, severity and phenotype. Studies have found that autonomic dysfunction is more common among patients with a longer disease duration (56,85,86), and with more severe motor symptoms (Hoehn and Yahr Scale scores 4 and 5) (47). Previous studies have also demonstrated that the severity of autonomic dysfunction symptoms is associated with the severity of motor symptoms (47,56), and mainly occurs in individuals with non-tremor dominant PD forms (87). Furthermore, right-sided motor presentation has been shown to be related to a higher frequency of autonomic functions (88); however, other research has not revealed significance with laterality at presentation (89). Moreover, patients with normal neuroimaging characterized by non-dopaminergic loss usually have worsening autonomic features (90,91).
Gu et al (92) found a predilection for autonomic dysfunction among specific PD subtypes. Patients with PD with mainly gait impairment more frequently had pupillomotor, thermoregulatory and sexual dysfunction. On the other hand, other phenotypes were more commonly associated cardiovascular issues. Urinary and gastrointestinal dysfunctions were not associated with any specific subtype (92). Wang et al (93) found similar results with primary gait impairment being correlated with OH and thermoregulatory abnormalities.
Autonomic dysfunction is not only associated with the severity of the disease, but the early development of autonomic dysfunction has been found to increase the risk of reaching the first disease milestone by 14% per year. It has also been shown to be associated with disability, a shorter survival rate and earlier reaching PD endpoints (23,94). Moreover, patients with PD primarily affected by non-motor symptoms with prodromal symptoms have longer disease duration and worse autonomic dysfunction over time (95), particularly Caucasians (32). In addition, some researchers have found that autonomic dysfunction is part of a cluster of patients with cognitive impairment, psychosis and depression (96).
Longardner et al (97) reported that only cardiovascular dysautonomia was associated with cognitive impairment. Apparently, patients in the early stages of PD that have abnormal increased sympathetic response more commonly develop cognitive impairment (98). In addition, some researchers have found an association between the overall autonomic dysfunction and cognitive decline (99).
A high frequency of autonomic symptoms is associated with specific gait features in PD involving decreasing swing, cadence and velocity. Nevertheless, subdomain analysis has demonstrated that only urinary symptoms are directly associated with gait impairment (100). In other studies, freezing of gait (101) and postural instability (102,103) were shown to be directly associated with the overall autonomic dysfunction. However, the association with freezing of gait and autonomic symptoms were not observed in other research (104).
Yoon et al (105) found another cluster of patients with hyposmia and autonomic dysfunction with overall lower uptake in dopamine transporter imaging and a higher risk of dementia. However, the risk of motor complications was the same (105). Pan et al (106) found that patients with dysautonomia may have more rapid brain atrophy, particularly in the temporal region. A similar subgroup termed ‘biotype 1’ characterized by subcortical volume loss was found by Wang et al (107). In addition, an inverse proportionality was found between dopamine transporter single-photon emission computed tomography and overall autonomic symptoms (94).
Non-motor fluctuations in autonomic symptoms. Autonomic symptoms in PD are not only persistent, but may also exhibit fluctuations that parallel motor ‘on-off’ states. These non-motor fluctuations include episodic changes in BP, sweating, gastrointestinal motility and urinary urgency. For instance, hyperhidrosis is frequently reported during ‘off’ periods and may improve during ‘on’ states, suggesting a dopaminergic modulation of sudomotor pathways (108,109). Similarly, orthostatic hypotension can worsen during ‘off’ periods due to a reduced sympathetic tone, while some patients experience transient postprandial hypotension that varies with medication timing (110). Gastrointestinal symptoms such as bloating, nausea and constipation may also fluctuate, potentially linked to delayed gastric emptying during ‘off’ states (111). Urinary urgency and frequency have been observed to intensify during periods of reduced dopaminergic stimulation, although evidence remains limited. These fluctuations are often underrecognized, yet they significantly affect QoL and may complicate symptom management (112). Recognizing the dynamic nature of autonomic dysfunction in PD is essential for tailoring treatment strategies and improving patient outcomes.
Effects of medication. The study by De Pablo-Fernandez et al (23) found that the development of autonomic dysfunction was related to a poorer response to levodopa treatment. It was also associated with lower maximum LEDD (23), although there are contradictory results in the literature (113). Dopamine agonists are theoretically known to worsen the severity of autonomic dysfunction symptoms (47,114); however, some studies have revealed an improvement in heart rate (HR) due to vagal tone response with levodopa administration (115,116). On the other hand, in another study, there was no cardiovascular dysregulation with rasagiline (117); furthermore another study demonstrated that entacapone intake reduced the risk of OH in patients with PD (118). Of note, Malek et al did not find the same association with other medications apart from dopamine agonists (56). A previous systematic review found no association between OH and dopamine agonist or monoamine oxidase-B inhibitor medications (119).
Aris et al (120) demonstrated that unmedicated patients with PD rarely manifested autonomic symptoms, and those taking medications usually developed symptoms later in the disease course, which they hypothesized of being a side-effect. Similar results were also observed by other authors (121). The autonomic side-effects of medications for PD are presented in Table SV.
Comorbid conditions. The occurrence of comorbidities, which are a hallmark of this mostly elderly population affected by PD, strongly complicates the condition of autonomic dysfunction (1). The evaluation and treatment of autonomic dysfunction in PD should take these comorbidities into account, as they may independently cause or exacerbate autonomic symptoms and impact their presentation and severity (36).
Guseva and Zhukova (122) reported that hypertension was associated with the progression of PD and the worsening of motor functions. Similar results were reported by Korchounov et al (123), with arterial hypertension being an independent risk factor of dysautonomia. Therefore, autonomic dysfunction may be mainly associated with demographic, but not to PD-related factors.
Several comorbidities common to the PD population are established causes of secondary autonomic failure, which are known to contribute to the symptom complex of PD, including conditions, such as diabetes mellitus (124), various cardiovascular diseases (hypertension) and renal insufficiency (58,125). For example, diabetes mellitus may cause autonomic neuropathy, resulting in OH, constipation and gastroparesis that can be difficult to differentiate from those derived from PD (36,58). Cardiovascular diseases pose particular challenges, and previous hypertension may either coexist with diabetes or contribute to SH, a frequent issue for PD-related autonomic dysfunction (38,41). Moreover, drugs for these cardiovascular comorbidities are likely to exacerbate OH episodes in patients with PD (e.g., diuretics and other antihypertensive agents) (26,38).
Autonomic dysregulation can also be worsened by cerebrovascular disease through alteration in the central control pathways, further aggravating the burden of dysautonomia and the intrinsic neurodegeneration (126). Urinary tract pathology, such as benign prostatic hypertrophy in men, may also produce symptoms of frequency and urgency, indistinguishable from the bladder dysfunction commonly encountered in PD (5,49). Furthermore, psychiatric comorbidities, such as depression and anxiety, which are common in PD, can influence the recognition and reporting of autonomic symptoms of the patient, also affecting QoL (127). Hence, comorbid conditions need to be carefully considered when diagnosing autonomic dysfunction in PD and formulating personal management strategies for these non-motor symptoms (35,36).
Environmental factors. A number of environmental factors have been studied with PD. For example, cigarette smoking is a known modifiable risk factor associated with a lower incidence of PD (128). Caffeine is also associated with a lower risk of developing PD (129). Dietary vitamin E has been shown to be a protective factor against PD (130). In a recent study by Dorsey and Bloem (131), environmental toxins, including certain pesticides, industrial chemicals and air pollution, were investigated as triggers in the pathophysiology of PD.
Genetic factors. Autonomic dysfunction is more common in familial PD cases when compared to idiopathic PD (58,132). For example, synuclein alpha (SNCA) multiplication is an autosomal dominant trait in familial PD, and it is associated with cardiac sympathetic denervation (133); cardiac sympathetic denervation is also common among symptomatic and asymptomatic carriers of the E46K mutation of the SNCA gene (114). In this context, PARK 20 (SYNJ1 gene) is frequently observed with gastrointestinal and urinary symptoms (134).
It has been found that autonomic dysfunction in general and particularly cardiac sympathetic denervation, are less pronounced in patients with familial PD with parkin RING-between-RING E3 ubiquitin protein ligase (PARK2) (135) and ATPase cation transporting 13A2 (PARK9) (134). Song et al (136) reported no difference in autonomic symptoms frequency between PARK2 individuals and idiopathic PD.
Patients with PD who are carriers of leucine rich repeat kinase 2 (LRRK2) have been found to have slightly less gastrointestinal dysfunction and RBD than patients with idiopathic PD (137). Similar results have been observed for cardiac dysautonomia (138). However, other research found no difference in the incidence of autonomic dysfunction between individuals who are carriers of LRRK2 compared to non-LRRK2 individuals (139).
Patients with glucosylceramidase beta-1 (GBA-1) mutation, when compared with idiopathic PD, more frequently complain of cardiovascular (140) and gastrointestinal (141) dysautonomia. More specifically, constipation has been shown to be more statistically significant among the gastrointestinal symptoms reported in GBA-PD (142). In addition, GBA-PD has been shown to be associated with a more pronounced involvement of systolic BP than diastolic BP (143).
4. Cardiovascular dysfunctions
Cardiovascular sympathetic denervation is common among patients with PD (144), and mild impairment can be noted early in the course of PD (145). The symptoms associated with this dysfunction are OH, postprandial hypotension, SH and non-dipping BP. Nevertheless, half of the patients report non-specific complaints, such as dizziness, lightheadedness, nausea and transient visual impairment (146). In this context, cardiovascular symptoms were not found to be associated with motor fluctuations (147), although they were associated with olfactory dysfunction (148). More specifically, anosmia was associated with a lower baroreflex, a pronounced decrease in systolic BP and a lower increase in norepinephrine during orthostasis (149). A summary of the prevalence of cardiovascular dysfunctions among patients with PD is presented in Table SVI (41,42,150-153).
OH. OH is prevalent in ~20% to 40% of patients with PD (150). In drug-naïve patients with PD, OH was found in 11.1% of cases (154). Hiorth et al (155) revealed an increase in the prevalence of OH, affecting 65.4% of individuals in 7 years, with only 29.2% exhibiting clinical symptoms. However, the management was uncommon, occurring in only 0.5% of cases (155). The wide variation in prevalence may be attributed to a number of factors, including different populations, inconsistent diagnostic criteria applied, and the type of medications used by the patients (156). In this context, the height or weight of the individual do not influence the incidence of OH in patients with PD (157). However, Umehara et al (158) found an association with underweight and cardiovascular dysfunction. On the other hand, Mochizuki et al (159) revealed a positive direct assocsation between weight and the risk of cardiovascular dysautonomia.
OH is associated with the duration and severity of the LBD (150), and it is an independent predictor of overall disability (160). Merola et al (161) demonstrated that the incidence of OH increased from 31 to ~46% in 1 year Nevertheless, Josta and Augustis (162) found a similar percentage of OH throughout the course of PD, not demonstrating predilection with disease severity.
OH can be asymptomatic or can present with symptoms varying from lightheadedness to a loss of consciousness (156). Among patients with PD, only 43% experience the typical symptoms, whereas 33% are completely asymptomatic (152). OH is associated with a decreased QoL and an increased risk of falls (OR, 10.70) (163), leading to fractures and prolonged peirods of hospitalization (164). Notably, from all the autonomic symptoms, only OH and urinary dysfunction have been associated with increased risk of falls (165), although this has not been reproduced in other research (166). The gait findings that are worse in patients with PD and OH are speed, stride length, postural transition and postural sway (167).
OH is associated with an increased risk of cognitive impairment in executive functions (168). In addition, mild cognitive impairment is related to OH (169), particularly among females (170). Another fact is the white matter changes in patients with PD and OH, which were already linked with cognitive impairment (171). The white matter microvascular changes are mainly observed in the fronto-subcortical and posterior cortical regions (172). Furthermore, the cognitive impairment and the white matter changes are not only linked to cardiovascular dysfunction, but with the overall dysautonomic symptoms (173,174).
Nakamura et al (175) noticed that up to 85% of wheelchair-bound individuals with PD were asymptomatic with OH. It would be valuable to have a direct comparison involving wheelchair bound and non-wheelchair-bound individuals with PD regarding their cardiovascular dysfunction.
Postprandial hypotension. Postprandial hypotension is a frequent manifestation, affecting up to 60% of patients with PD (176), and it has been found to be independent of meal type (177). Risk factors for postprandial hypotension include OH, constipation and the female sex (178). Consistently, Yalcin et al (179) demonstrated that postprandial hypotension affected 94% of patients with OH (153). Diagnosing postprandial hypotension is essential because it is associated with an increased risk of falling. In addition, although postprandial hypotension is more prevalent in individuals with severe PD, levodopa does not influence the hypotensive features (180).
Non-dipping BP. Non-dipping BP is having BP that does not decline at night (181), and it is independent of hypertension or anti-hypertensive therapy (182). Non-dipping is prevalent among patients with PD (183). Sommer et al (182) demonstrated that non-dipping occurred in 88% of patients with PD and occurred more frequently among patients with OH than those without. Furthermore, it occurred in 100% of patients with SH (182). Similarly, Kapoor et al (151) reported that 83% of patients with PD had non-dipping, while 64% had SH. This is clinically relevant, as patients with PD and nocturnal hypertension have a higher rate of left ventricular hypertrophy (184). Furthermore, the non-dipping degree is associated with the severity of PD psychosis (185).
SH. SH occurs in ~34% of patients with PD, often in conjunction with OH (41). Goldstein demonstrated that in patients with PD or MSA, OH was associated with significantly higher mean BP at the supine position (109±3 mmHg) compared to patients without OH (96±3 mmHg) (33). In addition, patients with asymptomatic OH had a higher prevalence of SH than symptomatic patients (42). Identifying SH and OH is essential, as they are associated with a higher risk of end-organ damage, cardiovascular events and the overall risk of mortality (186). A previous study reported the case of a patient with posterior reversible encephalopathy syndrome due to the pronounced BP fluctuation and SH (187).
Others. Other common cardiac autonomic dysfunction observed with the tilt test in patients with PD apart from OH are chronotropic incompetence (39%) and postural orthostatic tachycardia syndrome (1%) (188). Sebastian et al (189) revealed that 47% of individuals with idiopathic PD had a reverse dipping pattern and 80% variability in BP. Milazzo et al (190) found reverse dipping in 69% of patients with symptomatic cardiovascular dysfunction. Furthermore, 41% of patients with PD have decreased power spectral analysis of HR variability, indicating early autonomic dysfunction without symptoms (191). Some specialists recommend the early evaluation of cardiovascular function since PD is associated with sudden cardiac death and sudden unexpected death (192). Notably, there is abnormal cerebral autoregulation in PD independent of cardiac dysautonomia (193), and levodopa have been found to increase the local cerebral blood flow (194).
HR variability is affected by PD, and its prevalence is unknown due to the requirement of cardiac testing for its diagnosis (Table SVII) (121,195-202). Abnormal variability was only noted in off-periods with dyskinesia, suggesting that on-periods were associated with a normal HR variability (203). The HR variation is associated with worsening gait impairment and more episodes of freezing of gait (204). However, other researchers have not found significant results on the association between autonomic dysfunction and freezing of gait (205). Carricarte Naranjo et al (206) found that the deceleration capacity of HR in patients with idiopathic PD was impaired, and may be an early marker of autonomic failure. In addition, the abnormal cardiac rhythms observed in patients with PD assessed by deep machine learning were diagnostic in up to 90% of cases (207). In another study, the HR variability was shown to be associated with the parasympathetic tone impairment only in a short-term evaluation (208); in another study, there was no significance for long-term measures or worsening of the autonomic function (209). Of note, cardiac rhythm abnormalities may be related to dysautonomia without sinus node dysfunction (210).
Vallelonga et al (211) assessed ambulatory BP monitoring with a prevalence of autonomic dysfunction of 36% and accuracy of 91%. During the COVID-19 pandemic, the remote monitoring of cardiac function was effective in the diagnosis and management of OH (212). Notably, the continuous monitoring of BP and HR, compared to episodic, may be more effective in diagnosing the beginning of autonomic failure in PD (213). Apparently, the most effective evaluation of sympathetic and parasympathetic cardiac function in PD is performed by combining the analysis of frequency and time domains of HR (214). Netten et al (215) used a device for evaluation of five responses for diagnosis PD and identified 23% of individuals with cardiovascular autonomic failure.
A marker of the cardiac sympathetic dysfunction in PD is OH; however, in the absence of OH, the most effective indirect predictor of sympathetic failure is the low-frequency diastolic BP (216). Park et al (217) also reported increased neurofilament light chain levels in patients with PD and OH compared to non-OH.
During traditional aerobic exercise or aerobic exercise performed with self-selected intensity, individuals with PD have been shown to have the same cardiologic response compared to the controls (218). Of note, in another study, a similar response to the controls was observed in patients with decreased hemoglobin levels (219). The stability of the BP may be related to a positive inotropic response against vasodilation even in individuals with impaired cardiac sympathetic function (220). Miyasato et al (221) found contradictory results with a lower increase in systolic BP and HR during exercise in patients with PD; this is associated with overt cardiovascular autonomic failure (222) and the magnitude of hypotension is related to the exercise intensity (223). Furthermore, a reduced sympathetic response has been noted during hypoxia without any influence by dopaminergic therapy (224).
5. Gastrointestinal dysfunctions
Patients with PD experience gastrointestinal symptoms in the early and prodromal stages of the disease (225). These manifestations include weight loss, sialorrhea, dysphagia, gastroparesis, small intestinal bacterial overgrowth syndrome, constipation and defecatory dysfunction (4). Urinary and gastrointestinal symptoms are the most frequently reported autonomic symptoms, affecting up to 100% of individuals with PD (226).
Dizziness is associated with cardiovascular, but also with gastrointestinal dysfunction (227), although other researchers have not found statistically significant results for these associations (228). Furthermore, apart from the influence of gastrointestinal dysfunction in the cognitive function involving the visuospatial, letter fluency and memory (229,230); olfactory dysfunction is associated with gastrointestinal and urinary dysfunction (231). The prevalence of gastrointestinal dysfunction in PD is summarized in Table SVIII (44,229,232-239).
Weight loss. Weight loss is a frequent symptom, affecting almost half of patients with PD (232). The underlying mechanism is not yet understood. However, it may be related to the disease progression rather than reduced appetite (232). Weight loss is associated with malnutrition, falls, fractures and a poor QoL (240). Furthermore, it is associated with cognitive impairment, dependency and mortality (126,241). In addition, patients with early-stage PD that have weight loss usually develop more severe cardinal motor symptoms, cognitive function and executive function; on the other hand, patients with weight gain may have slower impairment in processing speed, attention and motor symptoms (242).
Sialorrhea and xerostomia. The frequency of sialorrhea ranges from 32 to 77% in patients with PD (243), with a mean frequency of almost 50% (244). Patients with PD could experience sialorrhea in the early or late stages of the disease (56,233). Müller et al (245) found that the most frequently reported autonomic symptom observed in patients with untreated PD at the time of diagnosis was drooling. van Wamelen et al (233) demonstrated that following 3 years of follow-up, the frequency of sialorrhea increased from 37 to 40%; however, its severity was not affected. Nevertheless, other authors have found that sialorrhea is associated with an older age and the frequency of other non-motor symptoms (246). Furthermore, sialorrhea is often associated with dysphagia and could be an indicator of subclinical dysphagia (233,247).
Xerostomia is reported in ~50% of individuals with PD (234), with a higher mean prevalence than drooling. But, it is rarely mentioned as an autonomic symptom (248), and only 12% of the patients complain to their physicians of dry mouth (249). Xerostomia is associated with dysphagia, and unfortunately does not improve with drooling. It can severely affect the QoL (250), and it is a commonly side-effect of medications in patients with PD (251). It is worth mentioning that patients complaining of xerostomia have increased concentrations of acetylcholinesterase activity (252).
Dysphagia. Dysphagia is a frequent gastrointestinal symptom in patients with PD, and its incidence surprisingly increases after 15 years of the PD onset (253). Gong et al (254) found that dysphagia occurred in 36% of patients, varying from 9 to 77%, and Oceania has the highest prevalence followed by Africa, Asia, Europe and America. Moreover, the prevalence was higher when dysphagia was confirmed using instrumental examination, 44 to 69.1% (254). Dysphagia is associated with an older age, disease duration, body mass index, severity of motor symptoms and depression (255). Dysphagia affects the QoL of patients with PD (256). Moreover, it can result in malnutrition, dehydration and aspiration pneumonia (257). Nevertheless, some researchers have not find a strong association between dysphagia and nutritional status (258).
Gastroparesis. Gastroparesis is delayed gastric emptying without obstruction on the upper GI system, along with symptoms, such as nausea, fullness, bloating and distension for a period ≥12 weeks (259). One of the clinical findings of decreased gastrointestinal motility is the reduced bowel sounds with digital auscultation that can be observed in patients with PD (260).
Delayed gastric emptying is highly prevalent among patients with PD, affecting ~70 to 100% of this population. However, the exact prevalence of symptomatic gastroparesis remains unknown due to limited data (261). Soykan et al (236) reported that among patients with gastroparesis, 7.5% of them had PD. Trahair et al (262) observed an inverse association between the gastric delayed time and the blood glucose in PD. Gastroparesis is less prevalent than other gastrointestinal symptoms. However, it is associated with a significantly reduced QoL (263).
Small intestinal bacterial overgrowth syndrome (SIBO). SIBO involves an increase in the count of small intestinal bacteria >100,000 cells per ml (264). The prevalence of SIBO in patients with PD is ~36 to 56% (265). SIBO manifests as bloating, abdominal pain and diarrhea (264). Moreover, SIBO is associated with poor motor functions among patients with PD (266). One of the factors associated with a high percentage of SIBO in PD is the prolonged small intestine passage observed in almost 100% of patients (267).
Constipation. Constipation is a common autonomic symptom, affecting ~60% of patients with PD (44). Moreover, it affects ~20 to 25% of individuals in the prodromal stage; thus, constipation is considered one of the Movement Disorders Society (MDS) criteria for prodromal PD (268). Furthermore, it is associated with cognitive impairment and apathy among older patients (269,270). Notably, it is an independent factor of the onset of cognitive impairment (271), and more severe motor and non-motor symptoms (44). Some researchers have revealed the occurrence of diarrhea in 28% of patients with PD, although this symptom is not associated with QoL (272).
Defecatory dysfunction. Defecatory dysfunction affects ~60% of patients with PD (238). Patients with PD and defecatory dysfunction have impaired squeezing pressure and an impaired recto-anal gradient during defecation (273). Defecatory dysfunction may be the leading cause of constipation in PD. Ramu et al (273 reported that among patients with PD and constipation, 40% of them had defecatory dysfunction. Zhou et al (274) reported that 94% of patients had at least dyssynergia, and 19% of patients reported anal hypotension, 47% hypocontractility, and 8% of patients had both. Krogh et al (275) revealed that levodopa therapy was associated with an improvement in the defecatory response.
6. Urinary dysfunctions
Urinary and sexual dysfunctions are prevalent among patients with PD. Lower urinary tract symptoms affect up to 89% of patients with PD (276). At the same time, sexual dysfunction affects up to 88% of patients with PD (277). As opposed to other autonomic dysfunctions, evidently, urinary symptoms are not associated with cardiovascular manifestations (278). The prevalence of urinary and sexual dysfunctions in PD is summarized in Table SIX (50,276,279-282).
Urinary dysfunction. Urinary dysfunction symptoms can be classified as voiding or storage symptoms. Voiding symptoms involve incomplete emptying and intermittent voiding (279). Storage symptoms involve frequency, urgency and urge incontinence (283). Both voiding and storage are affected in patients with PD, and this can severely affect QoL, particularly at the early stages of the disease (284). These symptoms are associated with an increased risk and recurrence of urinary tract infections (285). Notably, confounding factors of urinary symptoms should be excluded prior to the diagnosis of associating with PD. Valentino et al (286) found that 42.8% of patients with PD complaining of lower urinary tract symptoms had benign prostatic hyperplasia.
Micturition disturbances in PD can be formally classified into distinct etiological categories: secondary urinary symptoms, such as those caused by benign prostatic hyperplasia and adverse effects medications; lower urinary tract dysfunction resulting directly from the neurodegenerative process of PD; the infrequent occurrence of detrusor hypoactivity; and the rare presence of detrusor-sphincter dyssynergia. Of note, Galloway et al (287) found abnormal sphincter contractions in patients with PD, which is known as ‘urethrismus’ (288). Detrusor hyperactivity can be also be observed in individuals with PD, although no association with motor impairment has been noted (289).
Sexual dysfunction. Sexual dysfunction is a prevalent non-motor symptom in PD. However, it is often underreported (290). Sexual dysfunctions involve reduced sexual urge, hypersexuality and ejaculatory or erectile dysfunction (290). It occurs in the late and early stages of the disease (279). Moreover, Durcan et al (225) reported the incidence of 15% of sexual dysfunction in the prodromal stage of PD. Yu et al (291) found that more than half of individuals had normal sexual fantasy and this was associated with the duration of PD; thus, complaints of sexual dysfunction in patients with PD with advanced disease are clinically relevant.
Sexual dysfunction is associated with age, anxiety and depression (277). Evidently, depression is the main predictor for loss of libido in patients with PD, and this is not influenced by antidepressant therapy (292). Furthermore, it is related to the age of onset of the disease. Özcan et al (279) demonstrated that sexual dysfunction was more frequent among patients with late-onset PD (80%) compared to those with early-onset PD (59%). However, available evidence on the association between sexual dysfunction and sex is inconsistent. Shalash et al (280) reported that sexual dysfunction was more prevalent among males. Haktanır and Yılmaz (277) reported that it was more common among females. There was a positive association between sexual dysfunction and other autonomic dysfunction, including gastrointestinal (293); however, this finding was not consistent in other research (294).
Erectile dysfunction. Shalash et al (280) demonstrated that ~70% of male patients with PD experienced erectile dysfunction, and the severity of sexual dysfunction was associated with age. Erectile dysfunction affects the QoL of patients and is associated with cognitive dysfunction (280). Notably, sexual dysfunction in patients with PD is managed with evidence-based drugs (295).
Female sexual dysfunction. Females with sexual dysfunction can experience reduced sexual desire, hypersexuality, vaginismus, loss of lubrication, involuntary urination, or orgasm dysfunction (290,296). In this context, all autonomic spheres evidently worsen with the progression of PD, apart from female sexual dysfunction (297). The most prevalent among these symptoms are orgasm dysfunction (50%) and reduced sexual desire (48%) (281); this can negatively affect QoL (298). Furthermore, only a small number of females seek medical advice for sexual dysfunction. Deraz et al (298) revealed that only 2% of females sought medical advice compared to 30% of males.
Restless genital syndrome. Restless genital syndrome is defined as excessive and persistent arousal of the genitalia not associated with sexual desire. It is often described as tingling, burning, throbbing, or pain. These unwanted feelings may persist for the entire day. Moreover, they are not relieved by ordinary orgasm (299). Although these genital symptoms are considered rare to occur in PD, they could be severe and disabling (300). Aquino et al (301) reported a case of PD and disabling genital discomfort in a female aged 65 years.
7. Thermoregulatory dysfunction
The first description of thermoregulatory dysfunction as a ‘special sense of coldness in the affected limbs’ in PD can be found in the textbook of Gowers (302) entitled ‘A Manual of Diseases of the Nervous System’ in published 1983. Gowers emphasized the progressive nature of these symptoms and also noted hyperhidrosis on the side of maximum motor impairment. This preferential affection of the side with maximum motor deficits has been reported in later studies as well (108,197).
Thermoregulatory dysfunction in PD arises from both central and peripheral autonomic impairment. Alpha-synuclein pathology and Lewy body formation in the hypothalamus, brainstem nuclei, and spinal preganglionic neurons disrupt central autonomic regulation (303,304). Peripherally, small fiber neuropathy affecting sweat glands, erector pili muscles, and vascular innervation has been reported, and may be related to the length of levodopa exposure (305,306). Neuropathy in PD has also been shown to be associated with vitamin B12 deficiency and the neurotoxic effects of elevated homocysteine and methylmalonic acid levels (307). Several functional studies have reported abnormalities contributing to thermoregulatory impairment in PD. These include asymmetric sympathetic skin response (SSR) dysfunction favoring the more affected motor side (308,309), reduced frequency of skin sympathetic nerve activity observed across disease stages (310,311), diminished cutaneous vasoconstriction (312) and levodopa-associated reductions in venous tone (313).
In general, patients with PD report a spectrum of sweating disturbances, ranging from excess sweating to reduced sweating (Table SX) (6,197,308,314-319). When queried to compare their sweating patterns to prior to their diagnosis of PD, sweating issues were reported in ~2 in every 3 individuals with PD; however, studies report a broad range of these complaints between 10 to 100% of patients (6,314,320). Hyperhidrosis is more commonly reported than hypohidrosis (6).
Siepmann et al (321) revealed defects in the pilomotor function, but not in the sudomotor axon-reflex with PD progression, and this effect was associated with motor symptoms. Asahina et al (322) found that the sudomotor response impairment was observed even at the diagnosis of PD prior to commencing any medication. In addition, Xu et al (323) found association with LEDD and the development of electrochemical skin conductance tests. Nevertheless, no association has been found between seborrheic dermatitis and autonomic dysfunction in patients with PD (324).
Anbalagan et al (325) assessed infrared thermography in PD, and they found that the recovery rate was impaired in PD and was dependent on dopaminergic agents and motor impairment. Purup et al (326) reported similar findings, although there was no association of the thermography difference with other autonomic dysfunctions. Noteworthy, Rocchi et al (327) assessed patients complaining of sudomotor dysfunction, although the electrochemical skin conductance was normal. Similarly, Roy et al (328) did not observe abnormality in the standard cold pressor tests.
Hyperhidrosis. Hyperhidrosis is mostly episodic in PD and may be generalized or asymmetric, the latter being more common in the side of greatest motor involvement (308,329). In cases when hyperhidrosis is not related to motor impairment, it tends to involve mainly the trunk and the head (6). Hyperhidrosis is more often reported during ‘off’ periods (330), and it can improve during ‘on’ periods, which is known as non-motor fluctuations (109). The study by Pursiainen et al (108) demonstrated a significant increase in sweating at the hand site using an evaporimeter in patients with motor fluctuations but not in those without notable ‘off’ symptoms. Increased sweating has also been found problematic in patients with severe dyskinesias, which is considered to result from excessive physical activity (6). The underlying pathophysiology of this possible connection between sweating and motor fluctuations is likely poorly understood and may involve disordered sympathetic activation, inadequate central dopaminergic stimulation, or alterations in dopamine levels (110,197). Hyperhidrosis is also in part deemed to be compensatory to lower body hypohidrosis (315). In this manner, the hypohidrosis of the hands and feet can lead to thoracic (331) and head (332) increased sweating due to reduced sympathetic function in extremities. Selective norepinephrine reuptake inhibitors may also cause hyperhidrosis (333).
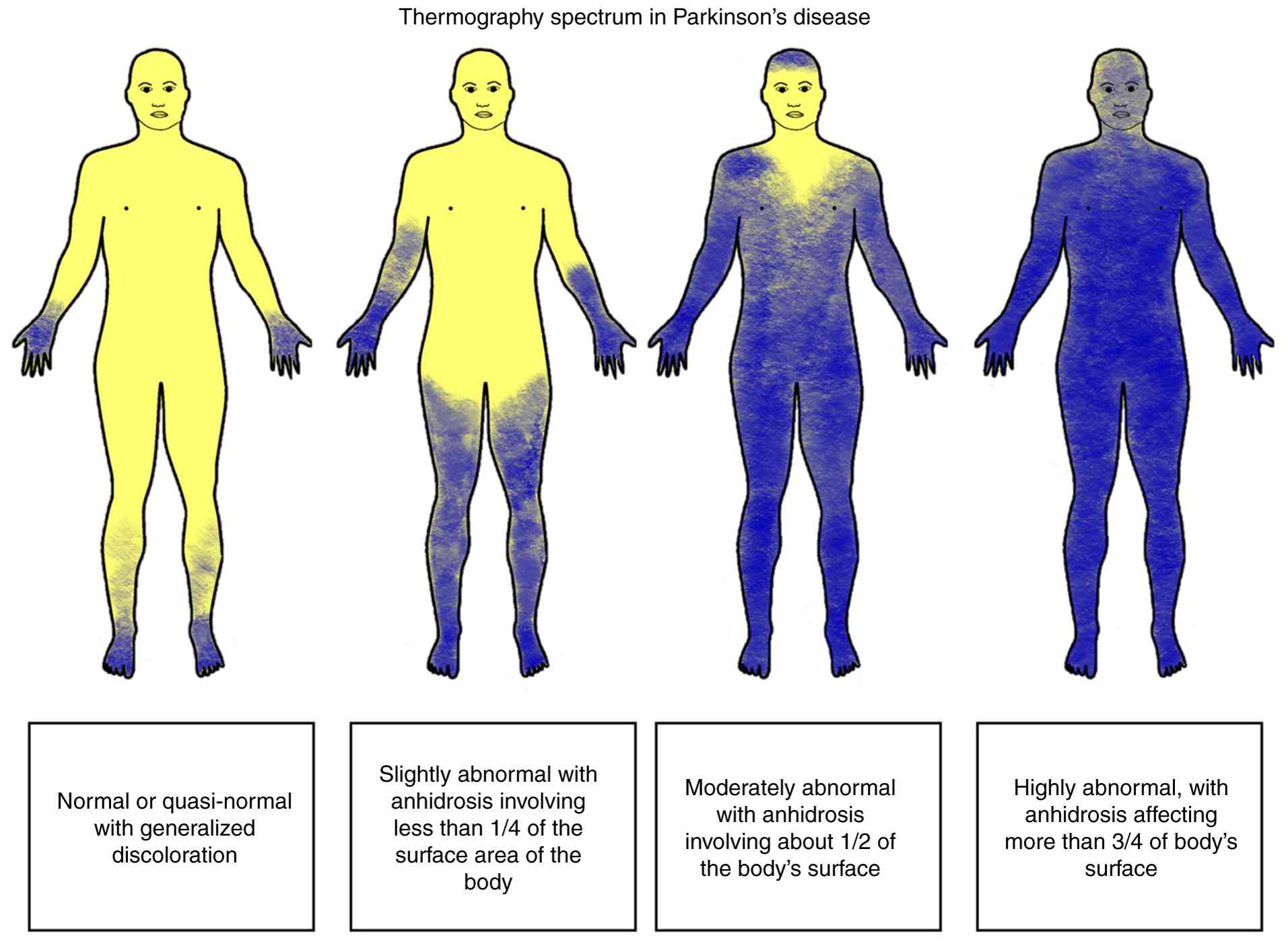
Hypohidrosis. Hypohidrosis is less commonly reported by patients with PD than hyperhidrosis (6). It is likely due to the fact that the latter is more problematic for patients; however, it is important to note that hyperhidrosis may in part be compensatory to lower-body hypohidrosis (315,334). Decreased sweating is often observed in a length-dependent pattern involving the distal part of the lower extremities (335). The only clinical symptom of hypohidrosis or anhidrosis in PD may be heat intolerance and is reported in approximately two thirds of patients (6). Saito (319) reported that only 30% of the individuals with PD had normal thermoregulatory function, and 9% had severe anhidrosis, affecting >75% of the body. The main part of the body being affected is the lower limbs, and less commonly, the upper extremities and the face (Fig. 4) (319,336). Notably, individuals with tremor-dominant PD are less commonly affected by sudomotor impairment (319). Patients with PD in later stages of the disease, based on Hoehn and Yahr staging, demonstrate a more pronounced reduction in sweat volumes at leg sites compared to patients with early-stage disease, consistent with the established observation that autonomic dysfunction tends to worsen with disease progression (12,337). Hypohidrosis and heat intolerance may also result from the use of anticholinergic medications (336).
Hypothermia. Hypothermia is generally described as a body temperature of <35˚C (95˚F). Temperatures as low as 30.9˚C have been reported in the literature in patients with PD, and it mainly occurs during the nighttime (338). Clinically, these patients may present with worsening bradykinesia and rigidity, myoclonus and somnolence or coma (317,339-341). Electroencephalography may show widespread slowing and triphasic discharges (341). Mortality in these hypothermic patients is primarily linked to their comorbid conditions rather than their core temperature (342). Other reported thermoregulatory symptoms in PD are heat or cold intolerance and vasomotor issues such as blue mottling of the skin (336).
Parkinsonism-hyperpyrexia syndrome. Parkinsonism-hyperpyrexia syndrome usually results from abrupt discontinuation of anti-Parkinson's medications and can be a fatal, albeit rare, complication. Clinically, it resembles neuroleptic malignant syndrome and is a result of depletion of central dopaminergic transmission (343). Dehydration, hot weather, febrile infection and the concurrent use of neuroleptic medication may all contribute to this syndrome in addition to the withdrawal of dopaminergic medications (344,345).
Sebum secretion, sympathetic skin response and others. Another tool for controlling body temperature is sebum production. Kitagawa et al (346) found a direct correlation between the sebum production and cardiac sympathetic function.
Sympathetic skin response can be measured by different methods. Ozawa et al (347) assessed the electric shock stimulation of the frontal head region, and they found an association between the amplitudes of response and the cardiac sympathetic function. However, other studies have reported that the SSR is not related to cardiac dysautonomia (348) and the duration of disease (349) or motor, behavioral, and cognitive changes in PD (350). It has been shown that sex, medication in use and PD phenotype do not influence SSR (351), even in the presence of other autonomic dysfunctions (352). When specifically analyzing motor symptoms, bradykinesia and rigidity, but not tremor, have been found to be associated with an abnormal skin response (353). Of note, manganism in PD can cause abnormal SSR and RR interval variation (354).
Patients with PD commonly report ‘severe cold in lower limbs’, which mainly occurs during the winter, although this has also been in the summer, and it does not improve with dopaminergic medications (355). The fact that patients with PD have abnormal cold stress responses may support this finding (356). Another phenomenon observed to be impaired in 50% of patients with PD is the skin wrinkling test (357), and is more prevalent in the side most affected by motor symptoms (358).
8. Pupillo-motor and tear abnormalities
There are numerous pupillo-motor and tear abnormalities studies on patients with PD. Pupil size is decreased in patients with PD, but also in individuals with prodromal phases such as idiopathic RBD (359). Patients with PD may have a slower peak constriction, and dilation amplitude and velocity, which is worse in the presence of cardiac dysfunction (360). Manohar and Husain demonstrated the resolution of the pupillary reward with dopamine agonists (361). Dietz et al (362) found normal pupillary sympathetic responses, although the movement of the eyes was slow. Hori et al (363) revealed abnormal parasympathetic response and pupillary supersensitivity to light.
There is an association between spontaneous changes in pupil diameter and motor symptoms in PD (364). In addition, patients with PD are more sensitive to the instillation of parasympathomimetic and sympathomimetic agents (363); however, this finding is not associated with disease duration (365). Furthermore, abnormal tear function has been noted in patients with PD, and it is more commonly observed in patients with severe PD (366,367). In this context, as demonstrated in a previous study, patients with PD have half of the quantity of tears than the controls, which may be related to autonomic and emotional disturbances (368). However, other researchers have not found an association with the pupillary abnormalities and the severity of PD (369). Autonomic dysfunction is related to corneal nerve loss in PD by unclear mechanisms, mainly in individuals reporting gastrointestinal and urinary symptoms (370).
9. Sleep autonomic dysregulation
Sleep disorders affect 40 to 90% of patients with PD (371). Abnormalities in the autonomic parts of the sleep is observed in 23% of the individuals (372), and they are mainly characterized by abnormal breathing patterns (373). The most common abnormal respiratory sleep pattern is tachypnea with awakening, and central and obstructive apnea in patients with other autonomic dysfunctions (374). In addition, the fact that there is impairment of motor function and arousal mechanisms can lead to further abnormalities in sleep (375).
In general, the severity of autonomic dysfunction is correlated with prevalence of sleep disorders (85,376-378). Wüllner et al (379) found that sleep abnormalities were more common in females, and there was an association of their severity and the disease duration. Another study also revealed an inverse association with HR variability during sleep and motor symptoms severity (380), during REM and non-REM sleep (381). Notably, patients with idiopathic RBD that later develop PD have more commonly autonomic dysfunction, particularly nocturia and sleep fragmentation (382) or gastrointestinal motility impairment (383).
OH and constipation are the main dysfunctions associated with sleep behavioral disorders, and the atonic time during sleep is directly associated with OH (384). Fujita et al (385) reported that urinary and cardiovascular domains were associated with sleep disorders in PD. Matsubara et al (386) reported an association between sleep issues and only nocturia in a stepwise regression, which indicates that there is overlap among autonomic symptoms as a factor associated with sleep.
Excessive daytime sleepiness (EDS) was associated with autonomic dysfunction, in a bidirectional model, suggesting that the treatment of EDS may lead to an improvement of autonomic functions (387). However, there is no association with all the autonomic domains; for example, OH is not associated with EDS (388).
Cho et al (389) studied some autonomic features of the sleep architecture, and they found that patients with PD and idiopathic RBD had abnormal activation, and this finding was not associated with sleep spindles. Thermoregulatory response has also been normally observed during sleep in patients with PD (390).
10. Impact on quality of life
The definition of QoL, according to the World Health Organization (WHO), is as follows ‘an individual's perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns’ (391). QoL encompasses physical, psychological, autonomy, cognitive, social relations and environmental factors (391).
Numerous aspects of QoL (activity of daily living, emotion, cognitive functions, communication and social support) are affected by autonomic dysfunctions (392). In addition, autonomic symptoms are among the nonmotor symptoms with highest influence in QoL (393). In this context, there is an association between the emotional disturbances and the autonomic dysfunction, which can significantly impact the QoL (394). Of note, dysautonomia is associated with the worsening of depression (395) and fatigue (396) in individuals with PD. Depression is directly associated with autonomic symptoms independently of the motor symptoms in patients with PD (397), mainly constipation and sensation of residual urine (398). In addition, the bidirectional association is also observed with OH and depression (399). Some studies have evaluated this association and found that activities of daily living is the confounding factor between these variables (400). On the other hand, fatigue is associated with gastrointestinal, urinary and cardiovascular dysfunction (401); notably, the strongest factor related to fatigue is the presence of OH (202). Nevertheless, some reports describe no influence in fatigue by autonomic symptoms (402).
Autonomic dysfunction symptoms significantly impair the QoL scores of patients with PD, even more than motor symptoms (403,404). Gastrointestinal symptoms are among the autonomic domains most likely associated with this finding (405). Magerkurth et al (406) reported that only bladder dysfunction significant impaired QoL. Notably, in another study, logistic regression analysis did not reveal an association between autonomic symptoms and QoL (407). The PRIAMO study revealed that urinary and gastrointestinal symptoms are the most common autonomic and among the five most frequently reported nonmotor symptoms that negatively affect QoL (408).
Functional impairment. The majority of patients with PD are cared for by friends or family, who also suffer from significant psychological burden, and issues that affect their socioeconomic, mental, and physical well-being. The QoL of caregivers tends to be reportedly decrease due to these factors, which affects patient care (409). Furthermore, the increased caregiving burden and increased illness severity over time are key factors for the institutionalization of patients with PD (410), particularly in individuals that develop cardiovascular dysautonomia (411). Grün et al (412) found that autonomic dysfunction was the strongest factor contributing to the burden of caregivers. Of note, institutionalized individuals with PD suffer from severe impairments in motor and cognitive functions, and activities of daily living (413).
Psychosocial impact. Patients with PD are highly susceptible to developing psychiatric comorbidities. A previous study found that 36% of patients had depression, 33% had anxiety, 40% had fatigue and 47% had sleep disturbances (414). Social isolation is more common among those who suffer from depression symptoms (415). Notably, 21% of patients with PD are also affected by impulsive control behaviors. In this context, autonomic dysfunction was directly associated with this neuropsychiatric symptom (416).
Autonomic dysfunction also interferes significantly with the social life of patients with PD. For example, a previous study demonstrated that patients with urinary incontinence reported significantly higher levels of depression and stress and lower levels of self-esteem compared to healthy controls (417). Urinary incontinence was significantly associated with impairment in activities of daily living, disability and less social network integration (418). Apart from urinary symptoms, other dysautonomic manifestations can affect the social life of patients with PD. For example, the drooling of saliva becomes more prominent with hyperkyphosis, and the tendency for the mouth to remain open often causes social anxiety in patients with PD (419).
11. Diagnostic challenges and future directions
Despite the high prevalence and significant impact of autonomic symptoms in PD, epidemiological studies investigating these symptoms remain markedly limited. This gap may be attributed to multiple interrelated factors. Clinician awareness of autonomic dysfunction is often limited, and even when recognized, assessment is hindered by a lack of standardized, validated diagnostic tools and clear definitions. Autonomic symptoms often overlap with those observed in other parkinsonian and non-parkinsonian disorders, such as MSA, LBD and diabetic autonomic neuropathy, which often delays proper diagnosis. As researchers over the past decades have tended to focus on the motor manifestations of PD, non-motor symptoms have been historically neglected, including those related to autonomic dysfunction. In addition, a number of patients underreport these symptoms, either as they perceive them as unrelated to PD, are not significantly bothered by them (e.g., hypohidrosis), or may feel anxious or hesitant discussing issues such as sexual dysfunction.
The scarcity of autonomic specialists and limited exposure to dysautonomia during neurology training further exacerbate the underrecognition of these clinical symptoms. Even when recommended tools are available, such as those endorsed by the International Movement Disorder Society, they are often underutilized. Numerous of these scales are time-consuming, require multiple instruments to assess different symptoms, and are thus difficult to integrate into busy clinical workflows, contributing to under-diagnosis and misdiagnosis. Numerous providers may also not feel confident to assess these symptoms as they may not be familiar with these presentations or may not know how to recognize them.
A 2009 task force from the Movement Disorder Society systematically reviewed rating scales for sialorrhea, dysphagia and constipation in PD (420). Their findings reinforce a number of the challenges outlined above. Of the disease-specific scales, none met the full criteria for ‘Recommended’ status, with the majority falling into the ‘Suggested’ category due to limited validation. For example, the Drooling Severity and Frequency Scale (DSFS), the Swallowing Disturbance Questionnaire (SDQ) and the Sialorrhea Clinical Scale for Parkinson's Disease (SCS-PD) are among the most commonly used, but each has either limited psychometric testing or insufficient use beyond the original validation study. No scale for constipation met even the ‘Suggested’ designation due to a lack of validation in PD populations, despite the widespread clinical use of tools such as the Rome III Constipation Module. Among global tools, the Scales for Outcomes in Parkinson's Disease-Autonomic (SCOPA-AUT) and the Non-Motor Symptoms Questionnaire for Parkinson's Disease (NMSQuest) were the only instruments to receive a ‘Recommended’ designation, due to their broader use and more robust validation. The Non-Motor Symptoms Scale (NMSS) was rated as ‘Suggested’, as it exhibits promising clinimetric properties, but has not yet been widely adopted outside of the original studies. These broader tools, however, provide limited granularity in symptom tracking and treatment response, and were not designed specifically for gastrointestinal autonomic dysfunction. The task force concluded that while new scales may not be immediately necessary, existing instruments should be more thoroughly validated, particularly for their use in PD populations, and applied more consistently in both clinical and research settings (420). A summary of the scales developed for the evaluation of autonomic symptoms in patients with PD is provided in Table SXI (72,86,197,421-427).
The underrepresentation of autonomic symptoms in PD research and care warrants a multidisciplinary approach and increased awareness through improved clinician education, the broader use of validated scales and patient-centered evaluation frameworks. For example, cardiovascular abnormalities have a significant impact in clinical practice, and when associated with side-effects related to medication in patients already susceptible to developing abnormalities, this may lead to poor adherence to therapy and worse outcomes (428). In addition, patients usually do not complain of autonomic symptoms in the early stages of the disease, and the diagnosis can only be performed with cardiovascular reflex tests (429). It is worth mentioning that a number of these individuals will not be referred to the neurologist; instead, they will likely have their first visit with the family medicine doctor, cardiologists and urologists (430,431). Therefore, the development of links between the neurologists and these specialties needs to be reinforced, particularly in cases concerning neurodegenerative conditions. Moreover, some autonomic specialists recommend that all patients with PD should undergo a bedside evaluation of BP and HR (432,433).
Biomarkers have been studied in autonomic dysfunction, and positive results were found with thermoregulatory, gastrointestinal and urinary dysfunctions (434). However, it is recommended to perform first cardiovascular autonomic neuropathy (CAN) risk score before more sophisticated tests (435), particularly in patients with PD aged >65 years (79).
Most autonomic dysfunctions in patients with PD are treated with the same algorithm as other conditions. It is possible that these individuals may benefit from specific approaches, as indicated by Panicker et al (436). Some evidence for this hypothesis is the fact that patients with PD undergoing deep brain stimulation (DBS) were occasionally found to exhibit an improvement of thermoregulatory (437), urinary (438), gastrointestinal (439) and cardiovascular functions (440-443). Most common location of stimulation was the subthalamic nucleus (444,445). However, the benefits in the autonomic function were not consistent throughout the literature (446,447). Some studies have reported a temporary effect of DBS in autonomic symptoms (448), while others have revealed that the benefit may be related with the amount of daily activity leading to improvement of cardiac autonomic symptoms (449). Cani et al (450) found that OH in patients with DBS taking and not taking levodopa had different responses.
It is a common conception that autonomic dysfunction is mainly observed in patients with atypical parkinsonism. Grażyńska et al (451) reported that there was no difference between autonomic and neuropsychiatric manifestations in patients with atypical parkinsonism when compared to those with idiopathic PD.
Another critical aspect that warrants investigation is the association between autonomic symptoms and cognitive function. It is well-known that patients with PD can develop impairment of cognition over time, although it is interesting to note that cardiovascular dysautonomia was already associated with cognitive impairment. There is recent evidence to indicate that not only cardiovascular dysautonomia, but overall dysautonomia is associated with cognitive decline in individuals with PD, particularly in de novo cases (452). Apparently, only PD dementia is related to dysautonomia, when compared to Alzheimer's disease, vascular dementia and dementia with Lewy bodies (453). Of note, there is a cluster of autonomic symptoms (gastrointestinal, sexual dysfunction, thermoregulatory and urinary) that worsen together with the progression of PD (297). Nevertheless, some authors have reported no association between cognitive function and autonomic function, and they claimed that these two spheres may progress independently from each other (454).
A critical limitation for the development of new therapies for autonomic dysfunction in PD is unreliable animal models. The majority of the current models are based on indirect findings and do not reflect the pathophysiology observed in patients with PD. In this way, animal studies may lead to results with unknown clinical significance and should be cautiously analyzed (455). Further research with the development of synuclein disease and specific observations of time required to clinical manifestations is warranted.
12. Conclusions
Autonomic dysfunction is a common, yet frequently underrecognized component of PD, with symptoms that often precede motor onset and contribute significantly to disease burden. The present review underscores the complex and heterogeneous nature of dysautonomia in PD, shaped by factors, such as age, disease duration and phenotype. Early autonomic involvement is linked to faster clinical decline and increased risk of cognitive impairment, yet these symptoms are often underreported or misattributed.
These findings highlight the need for proactive screening, tailored diagnostic tools and PD-specific treatment strategies. Addressing autonomic dysfunction holistically, alongside motor symptoms, may improve quality of life and potentially alter disease progression. Future research is required to prioritize the development of targeted therapies, explore the interplay between autonomic and cognitive domains, and refine animal models to better reflect the clinical spectrum of PD.
Supplementary Material
Summary of autonomic dysfunction features across neurodegenerative disorders [adapted from the study by Niimi et al (16)]a.
Data of the radar chart.
Prevalence of NMSQuest in different studies from different countries.
Prevalence NMSQuest.
Autonomic side-effects of medications for Parkinson’s diseasea.
Prevalence of cardiovascular dysfunction In Parkinson’s disease.
Detailed overview of autonomic function test findings in Parkinson’s disease across selected studies.
Prevalence of gastrointestinal dysfunction in Parkinson’s disease.
Prevalence of urinary and sexual dysfunctions in Parkinson’s disease.
Prevalence of thermoregulatory dysfunction in Parkinson’s disease.
Clinical scales specifically designed for Parkinson’s disease.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
All authors (JPR, AFG, IK, AA, KSB and ALFC) designed the study and drafted the manuscript. All authors have read and approved the final version. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Kalia LV and Lang AE: Parkinson's disease. Lancet. 386:896–912. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Goldstein DS and Low PA: Clinical evaluation of the autonomic nervous system. Continuum: Lifelong Learning Neurol. 13:33–49. 2007. | |
|
Jain S and Goldstein DS: Cardiovascular dysautonomia in Parkinson disease: From pathophysiology to pathogenesis. Neurobiol Dis. 46:572–580. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Fasano A, Visanji NP, Liu LWC, Lang AE and Pfeiffer RF: Gastrointestinal dysfunction in Parkinson's disease. Lancet Neurol. 14:625–639. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Winge K and Fowler CJ: Bladder dysfunction in Parkinsonism: Mechanisms, prevalence, symptoms, and management. Mov Disord. 21:737–745. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Swinn L, Schrag A, Viswanathan R, Bloem BR, Lees A and Quinn N: Sweating dysfunction in Parkinson's disease. Mov Disord. 18:1459–1463. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Meco G, Rubino A, Caravona N and Valente M: Sexual dysfunction in Parkinson's disease. Parkinsonism Relat Disord. 14:451–456. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Pierangeli G, Provini F, Maltoni P, Barletta G, Contin M, Lugaresi E, Montagna P and Cortelli P: Nocturnal body core temperature falls in Parkinson's disease but not in Multiple-System Atrophy. Mov Disord. 16:226–232. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Martinez-Martin P: The importance of non-motor disturbances to quality of life in Parkinson's disease. J Neurol Sci. 310:12–16. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Rissardo JP and Caprara AL: A narrative review on biochemical markers and emerging treatments in prodromal synucleinopathies. Clin Pract. 15(65)2025.PubMed/NCBI View Article : Google Scholar | |
|
Fereshtehnejad SM, Romenets SR, Anang JBM, Latreille V, Gagnon JF and Postuma RB: New clinical subtypes of Parkinson disease and their longitudinal progression: A prospective cohort comparison with other phenotypes. JAMA Neurol. 72:863–873. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Kim JB, Kim BJ, Koh SB and Park KW: Autonomic dysfunction according to disease progression in Parkinson's disease. Parkinsonism Relat Disord. 20:303–307. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Merola A, Sawyer RP, Artusi CA, Suri R, Berndt Z, Lopez-Castellanos JR, Vaughan J, Vizcarra JA, Romagnolo A and Espay AJ: Orthostatic hypotension in Parkinson disease: Impact on health care utilization. Parkinsonism Relat Disord. 47:45–49. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Lo RY: Epidemiology of atypical parkinsonian syndromes. Tzu Chi Med J. 34:169–181. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Alster P and Madetko-Alster N: Significance of dysautonomia in Parkinson's disease and atypical parkinsonisms. Neurol Neurochir Pol. 58:147–149. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Niimi Y, Ieda T, Hirayama M, Koike Y, Sobue G, Hasegawa Y and Takahashi A: Clinical and physiological characteristics of autonomic failure with Parkinson's disease. Clin Auton Res. 9:139–144. 1999.PubMed/NCBI View Article : Google Scholar | |
|
Angelopoulou E, Bozi M, Simitsi AM, Koros C, Antonelou R, Papagiannakis N, Maniati M, Poula D, Stamelou M, Vassilatis DK, et al: Clinical differences between early-onset and mid-and-late-onset Parkinson's disease: Data analysis of the Hellenic Biobank of Parkinson's disease. J Neurol Sci. 442(120405)2022.PubMed/NCBI View Article : Google Scholar | |
|
Yoo HS, Lee S, Jeong SH, Ye BS, Sohn YH, Yun M and Lee PH: Clinical and dopamine depletion patterns in Hyposmia- and Dysautonomia-dominant Parkinson's disease. J Parkinsons Dis. 11:1703–1713. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Rodriguez-Sanchez F, Rodriguez-Blazquez C, Bielza C, Larrañaga P, Weintraub D, Martinez-Martin P, Rizos A, Schrag A and Chaudhuri KR: Identifying Parkinson's disease subtypes with motor and non-motor symptoms via model-based multi-partition clustering. Sci Rep. 11(23645)2021.PubMed/NCBI View Article : Google Scholar | |
|
Leclair-Visonneau L, Magy L, Volteau C, Clairembault T, Le Dily S, Préterre C, Peyre A, Damier P, Neunlist M, Péréon Y and Derkinderen P: Heterogeneous pattern of autonomic dysfunction in Parkinson's disease. J Neurol. 265:933–941. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Martínez-Fernández R, Schmitt E, Martinez-Martin P and Krack P: The hidden sister of motor fluctuations in Parkinson's disease: A review on nonmotor fluctuations. Mov Disord. 31:1080–1094. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Stanković I, Petrović I, Pekmezović T, Marković V, Stojković T, Dragašević-Mišković N, Svetel M and Kostić V: Longitudinal assessment of autonomic dysfunction in early Parkinson's disease. Parkinsonism Relat Disord. 66:74–79. 2019.PubMed/NCBI View Article : Google Scholar | |
|
De Pablo-Fernandez E, Tur C, Revesz T, Lees AJ, Holton JL and Warner TT: Association of autonomic dysfunction with disease progression and survival in parkinson disease. JAMA Neurol. 74:970–976. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Stubendorff K, Aarsland D, Minthon L and Londos E: The impact of autonomic dysfunction on survival in patients with dementia with Lewy bodies and Parkinson's disease with dementia. PLoS One. 7(e45451)2012.PubMed/NCBI View Article : Google Scholar | |
|
Morley JF, Pawlowski SM, Kesari A, Maina I, Pantelyat A and Duda JE: Motor and non-motor features of Parkinson's disease that predict persistent drug-induced Parkinsonism. Parkinsonism Relat Disord. 20:738–742. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Goldstein DS: Dysautonomia in Parkinson disease. Compr Physiol. 4:805–826. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Joza S, Hu MT, Jung KY, Kunz D, Stefani A, Dušek P, Terzaghi M, Arnaldi D, Videnovic A, Schiess MC, et al: Progression of clinical markers in prodromal Parkinson's disease and dementia with Lewy bodies: A multicentre study. Brain. 146:3258–3272. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Miller-Patterson C, Hsu JY, Chahine LM, Morley JF and Willis AW: Selected autonomic signs and symptoms as risk markers for phenoconversion and functional dependence in prodromal Parkinson's disease. Clin Auton Res. 32:463–476. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Liepelt-Scarfone I, Pilotto A, Müller K, Bormann C, Gauss K, Wurster I, Streffer J and Berg D: Autonomic dysfunction in subjects at high risk for Parkinson's disease. J Neurol. 262:2643–2652. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Bhatti KS, Kaleru T, Vankeshwaram V, Maheshwary A and Khan S: The significance of rapid eye movement sleep behavior disorder in neurodegenerative diseases: An updated review. Neurol India. 70:19–24. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Riboldi GM, Russo MJ, Pan L, Watkins K and Kang UJ: Dysautonomia and REM sleep behavior disorder contributions to progression of Parkinson's disease phenotypes. NPJ Parkinsons Dis. 8(110)2022.PubMed/NCBI View Article : Google Scholar | |
|
Yang L, Gao H and Ye M: Baseline prevalence and longitudinal assessment of autonomic dysfunction in early Parkinson's disease. J Neural Transm (Vienna). 131:127–139. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Goldstein DS: Dysautonomia in Parkinson's disease: Neurocardiological abnormalities. Lancet Neurol. 2:669–676. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Cao LX, Jiang Y, Piao YS and Huang Y: Rapid motor progression of Parkinson's disease associates with clinical and genetic variants. Front Biosci (Landmark Ed). 26:1503–1512. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Asahina M, Vichayanrat E, Low DA, Iodice V and Mathias CJ: Autonomic dysfunction in parkinsonian disorders: Assessment and pathophysiology. J Neurol Neurosurg Psychiatry. 84:674–680. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Pfeiffer RF: Autonomic dysfunction in Parkinson's disease. Neurotherapeutics. 17:1464–1479. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Zhou Z, Zhou X, Zhou X, Xiang Y, Zhu L, Qin L, Wang Y, Pan H, Zhao Y, Sun Q, et al: Characteristics of autonomic dysfunction in Parkinson's disease: A large Chinese multicenter cohort study. Front Aging Neurosci. 13(761044)2021.PubMed/NCBI View Article : Google Scholar | |
|
Palma JA and Kaufmann H: Treatment of autonomic dysfunction in Parkinson disease and other synucleinopathies. Mov Disord. 33:372–390. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Leńska-Mieciek M, Derecka-Charzyńska I, Fiszer U, Królicki L and Kułakowski P: Syncope and autonomic cardiovascular dysfunction in Parkinson disease. Neurol Neurochir Pol. 45:335–341. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT, et al: Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 42:1206–1252. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Fanciulli A, Göbel G, Ndayisaba JP, Granata R, Duerr S, Strano S, Colosimo C, Poewe W, Pontieri FE and Wenning GK: Supine hypertension in Parkinson's disease and multiple system atrophy. Clin Auton Res. 26:97–105. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Palma JA, Gomez-Esteban JC, Norcliffe-Kaufmann L, Martinez J, Tijero B, Berganzo K and Kaufmann H: Orthostatic hypotension in Parkinson disease: How much you fall or how low you go? Mov Disord. 30:639–645. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Fanciulli A, Strano S, Colosimo C, Caltagirone C, Spalletta G and Pontieri FE: The potential prognostic role of cardiovascular autonomic failure in α-synucleinopathies. Eur J Neurol. 20:231–235. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Yu QJ, Yu SY, Zuo LJ, Lian TH, Hu Y, Wang RD, Piao YS, Guo P, Liu L, Jin Z, et al: Parkinson disease with constipation: Clinical features and relevant factors. Sci Rep. 8(567)2018.PubMed/NCBI View Article : Google Scholar | |
|
Kalf JG, Borm GF, de Swart BJ, Bloem BR, Zwarts MJ and Munneke M: Reproducibility and validity of patient-rated assessment of speech, swallowing, and saliva control in Parkinson's disease. Arch Phys Med Rehabil. 92:1152–1158. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Pfeiffer RF: Autonomic dysfunction in Parkinson's disease. Expert Rev Neurother. 12:697–706. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM and van Hilten JJ: Patient-reported autonomic symptoms in Parkinson disease. Neurology. 69:333–341. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Pfeiffer RF: Management of autonomic dysfunction in Parkinson's disease. Semin Neurol. 37:176–185. 2017.PubMed/NCBI View Article : Google Scholar | |
|
McDonald C, Winge K and Burn DJ: Lower urinary tract symptoms in Parkinson's disease: Prevalence, aetiology and management. Parkinsonism Relat Disord. 35:8–16. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Zhang LM and Zhang XP: Investigation of urination disorder in Parkinson's disease. Chin Med J (Engl). 128:2906–2912. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Kapoor S, Bourdoumis A, Mambu L and Barua J: Effective management of lower urinary tract dysfunction in idiopathic Parkinson's disease. Int J Urol. 20:79–84. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Ragab MM and Mohammed ES: Idiopathic Parkinson's disease patients at the urologic clinic. Neurourol Urodyn. 30:1258–1261. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Gao X, Chen H, Schwarzschild MA, Glasser DB, Logroscino G, Rimm EB and Ascherio A: Erectile function and risk of Parkinson's disease. Am J Epidemiol. 166:1446–1450. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Szewczyk-Krolikowski K, Tomlinson P, Nithi K, Wade-Martins R, Talbot K, Ben-Shlomo Y and Hu MTM: The influence of age and gender on motor and non-motor features of early Parkinson's disease: Initial findings from the oxford Parkinson disease center (OPDC) discovery cohort. Parkinsonism Relat Disord. 20:99–105. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Zhou MZ, Gan J, Wei YR, Ren XY, Chen W and Liu ZG: The association between non-motor symptoms in Parkinson's disease and age at onset. Clin Neurol Neurosurg. 115:2103–2107. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Malek N, Lawton MA, Grosset KA, Bajaj N, Barker RA, Burn DJ, Foltynie T, Hardy J, Morris HR, Williams NM, et al: Autonomic dysfunction in early Parkinson's disease: Results from the United Kingdom Tracking Parkinson's study. Mov Disord Clin Pract. 4:509–516. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Guidez D, Behnke S, Halmer R, Dillmann U, Faßbender K, Kirsch CM, Hellwig D and Spiegel J: Is reduced myocardial sympathetic innervation associated with clinical symptoms of autonomic impairment in idiopathic Parkinson's disease? J Neurol. 261:45–51. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Chen Z, Li G and Liu J: Autonomic dysfunction in Parkinson's disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol Dis. 134(104700)2020.PubMed/NCBI View Article : Google Scholar | |
|
Miller-Patterson C, Edwards KA and Chahine LM: Sex Disparities in autonomic symptom treatment in Parkinson's disease. Mov Disord Clin Pract. 7:718–719. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Bansal NR, Paul BS, Paul G and Singh G: Gender differences and impact of autonomic disturbance on fatigue and quality of life in Parkinson's disease. Neurol India. 70:203–208. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Donzuso G, Cicero CE, Vinciguerra E, Sergi R, Luca A, Mostile G, Terravecchia C, Zappia M and Nicoletti A: Gender differences in Non-motor fluctuations in Parkinson's disease. J Neural Transm (Vienna). 130:1249–1257. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Ragab OA, Elheneedy YA and Bahnasy WS: Non-motor symptoms in newly diagnosed Parkinson's disease patients. Egyptian J Neurol Psychiat Neurosurgery. 55:1–7. 2019. | |
|
Dahbour SS, Al Murr MJ, Oweis LH, Al Antary NT, Mohsen M and Al Fegi S: Non-motor manifestation of Parkinson's disease: A cross-sectional study in a teaching hospital in Jordan. Egyptian J Neurol Psychiatry Neurosurgery. 58(148)2022. | |
|
Sengul Y, Sengul HS, Sural MK, Bakim B and Forta H: A comparison between rate of nonmotor symptom development in essential tremor and Parkinson's disease. Acta Neurol Belg. 115:289–294. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Yu B, Xiao Z, Li J, Yuan J and Liu Y: Study of an integrated Non-motor symptoms questionnaire for Parkinson's disease. Chin Med J (Engl). 123:1436–1440. 2010.PubMed/NCBI | |
|
Cheon SM, Ha MS, Park MJ and Kim JW: Nonmotor symptoms of Parkinson's disease: Prevalence and awareness of patients and families. Parkinsonism Relat Disord. 14:286–290. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Liu WM, Lin RJ, Yu RL, Tai CH, Lin CH and Wu RM: The impact of nonmotor symptoms on quality of life in patients with Parkinson's disease in Taiwan. Neuropsychiatr Dis Treat. 11:2865–2873. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Vongvaivanich K, Nidhinandana S, Udommongkol C, Chairungsaris P, Chinvarun Y, Wongmek W, Suphakasem S, Suwantamee J and Sithinamsuwan P: Non-motor symptoms in Thai patients with Parkinson's disease studied at Phramongkutklao Hospital. J Med Assoc Thai. 97 (Suppl 2):S159–S167. 2014.PubMed/NCBI | |
|
Rukmini Mridula K, Borgohain R, Jabeen SA, Padmaja G, Bandaru VS, Ankathi P, Kanikannan MA and Ali Khan MS: Comparison of frequencies of non motor symptoms in Indian Parkinson's disease patients on medical management versus deep brain stimulation: A case-control study. Iran J Neurol. 14:86–93. 2015.PubMed/NCBI | |
|
Mukhtar S, Imran R, Zaheer M and Tariq H: Frequency of non-motor symptoms in Parkinson's disease presenting to tertiary care centre in Pakistan: An observational, cross-sectional study. BMJ Open. 8(e019172)2018.PubMed/NCBI View Article : Google Scholar | |
|
Sánchez-Martínez CM, Choreño-Parra JA, Placencia-Álvarez N, Nuñez-Orozco L and Guadarrama-Ortiz P: Frequency and dynamics of Non-motor symptoms presentation in hispanic patients with parkinson disease. Front Neurol. 10(1197)2019.PubMed/NCBI View Article : Google Scholar | |
|
Romenets SR, Wolfson C, Galatas C, Pelletier A, Altman R, Wadup L and Postuma RB: Validation of the non-motor symptoms questionnaire (NMS-Quest). Parkinsonism Relat Disord. 18:54–58. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Cosentino C, Nuñez Y and Torres L: Frequency of non-motor symptoms in Peruvian patients with Parkinson's disease. Arq Neuropsiquiatr. 71:216–219. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Crosiers D, Pickut B, Theuns J, Deyn PP, Van Broeckhoven C, Martinez-Martin P, Chaudhuri KR and Cras P: Non-motor symptoms in a Flanders-Belgian population of 215 Parkinson's disease patients as assessed by the Non-motor symptoms questionnaire. Am J Neurodegener Dis. 1:160–167. 2012.PubMed/NCBI | |
|
Bostantjopoulou S, Katsarou Z, Karakasis C, Peitsidou E, Milioni D and Rossopoulos N: Evaluation of non-motor symptoms in Parkinson's disease: An underestimated necessity. Hippokratia. 17:214–219. 2013.PubMed/NCBI | |
|
Rodriguez-Blazquez C, Schrag A, Rizos A, Chaudhuri KR, Martinez-Martin P and Weintraub D: Prevalence of Non-motor symptoms and Non-motor fluctuations in Parkinson's disease using the MDS-NMS. Mov Disord Clin Pract. 8:231–239. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Sauerbier A, Rosa-Grilo M, Qamar MA and Chaudhuri KR: Nonmotor subtyping in Parkinson's disease. Int Rev Neurobiol. 133:447–478. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Chen W, Xu ZM, Wang G and Chen SD: Non-motor symptoms of Parkinson's disease in China: A review of the literature. Parkinsonism Relat Disord. 18:446–452. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Okubadejo NU and Danesi MA: Frequency and predictors of autonomic dysfunction in Parkinson's disease: A study of African patients in Lagos, Nigeria. Niger Postgrad Med J. 11:45–49. 2004.PubMed/NCBI | |
|
Sidahmed AM and Ali HA: Frequency and associated factors of autonomic dysfunction in patients with Parkinson's disease in Khartoum State. Adv Parkinson's Dis. 8(63)2019. | |
|
Fonseca MCM, Sansone D, Farah D, Fiorini AC, Scorza CA and Scorza FA: Seasonality as a risk factor for deaths in Parkinson's disease. Clinics (Sao Paulo). 79(100506)2024.PubMed/NCBI View Article : Google Scholar | |
|
Elmandouh O, Waseem H, Abideen Z, Rissardo JP, Aimen S, Fornari Caprara AL, Byroju V, Yadav Tirupathi S and Fawad Tahir M: Temporal Trends in Parkinson's disease and aspiration pneumonia-related mortality in older adults in the United States (1999-2020): A 21-year retrospective analysis (P4-5.016). Neurology. 104(5938)2025. | |
|
Wang J, Xiong K, Chao J, Zhuang S, Li J and Liu C: Seasonal variations of nonmotor symptoms in patients with Parkinson's disease in Southeast China. Chin Med J (Engl). 136:415–422. 2023.PubMed/NCBI View Article : Google Scholar | |
|
van Wamelen DJ, Podlewska AM, Leta V, Śmiłowska K, Rizos A, Martinez-Martin P, Bloem BR and Chaudhuri KR: Slave to the rhythm: Seasonal differences in non-motor symptoms in Parkinson's disease. Parkinsonism Relat Disord. 63:73–76. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Arnao V, Cinturino A, Valentino F, Perini V, Mastrilli S, Bellavia G, Savettieri G, Realmuto S and D'Amelio M: In Patient's with Parkinson disease, autonomic symptoms are frequent and associated with other non-motor symptoms. Clin Auton Res. 25:301–307. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Visser M, Marinus J, Stiggelbout AM and Van Hilten JJ: Assessment of autonomic dysfunction in Parkinson's disease: The SCOPA-AUT. Mov Disord. 19:1306–1312. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Kwon KY, Joo BE, You J and Kim RO: Impact of motor features on non-motor symptoms in patients with de novo Parkinson's disease: Cognition, depression, anxiety, fatigue, and dysautonomia. Geriatr Gerontol Int. 25:392–397. 2025.PubMed/NCBI View Article : Google Scholar | |
|
Kurlawala Z, Shadowen PH, McMillan JD, Beverly LJ and Friedland RP: Progression of nonmotor symptoms in Parkinson's disease by sex and motor laterality. Parkinsons Dis. 2021(8898887)2021.PubMed/NCBI View Article : Google Scholar | |
|
Cubo E, Martín PM, Martin-Gonzalez JA, Rodríguez-Blázquez C and Kulisevsky J: Motor laterality asymmetry and nonmotor symptoms in Parkinson's disease. Mov Disord. 25:70–75. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Taylor S, Gafton J, Shah B, Pagano G, Chaudhuri KR, Brooks DJ and Pavese N: Progression of nonmotor symptoms in subgroups of patients with non-dopamine-deficient Parkinsonism. Mov Disord. 31:344–351. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Sprenger FS, Seppi K, Djamshidian A, Reiter E, Nocker M, Mair K, Göbel G and Poewe W: Nonmotor symptoms in subjects without evidence of dopaminergic deficits. Mov Disord. 30:976–981. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Gu SC, Shi R, Gao C, Yuan XL, Wu Y, Liu ZG, Wang CD, Zhao SR, Chen X, Yuan CX and Ye Q: Autonomic function and motor subtypes in Parkinson's disease: A multicentre cross-sectional study. Sci Rep. 13(14548)2023.PubMed/NCBI View Article : Google Scholar | |
|
Wang JY, Wang MY, Liu RP, Li Y, Zhang WY, Ovlyakulov B, Zhang X and Zhu JH: Association analyses of autonomic dysfunction and sympathetic skin response in motor subtypes of Parkinson's disease. Front Neurol. 11(577128)2020.PubMed/NCBI View Article : Google Scholar | |
|
Fujita H, Ogaki K, Shiina T, Sakuramoto H, Nozawa N and Suzuki K: Impact of autonomic symptoms on the clinical course of Parkinson's disease. Neurol Sci. 45:3799–3807. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Qin K, Li Y, Liu Y, Xue L, Wang Z, Xian W, Tu R, Yang B, Ning F and Xie A: Divergent amygdala function in proposed brain-first and body-first Parkinson's disease: A resting-state functional magnetic resonance imaging study. J Affect Disord. 382:123–130. 2025.PubMed/NCBI View Article : Google Scholar | |
|
van Rooden SM, Visser M, Verbaan D, Marinus J and van Hilten JJ: Patterns of motor and non-motor features in Parkinson's disease. J Neurol Neurosurg Psychiatry. 80:846–850. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Longardner K, Merola A, Litvan I, De Stefano AM, Maule S, Vallelonga F, Lopiano L and Romagnolo A: Differential impact of individual autonomic domains on clinical outcomes in Parkinson's disease. J Neurol. 269:5510–5520. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Umehara T, Oka H, Nakahara A, Matsuno H and Toyoda C: High norepinephrinergic orthostatic hypotension in early Parkinson's disease. Parkinsonism Relat Disord. 55:97–102. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Mahajan A, Morrow CB, Seemiller J, Mills KA and Pontone GM: The effect of dysautonomia on motor, behavioral, and cognitive fluctuations in Parkinson's disease. Mov Disord. 40:157–162. 2025.PubMed/NCBI View Article : Google Scholar | |
|
Lee SM, Lee M, Lee EJ, Kim RO, Kim Y and Kwon KY: Association between gait and dysautonomia in patients with de novo Parkinson's disease: Forward gait versus backward gait. J Mov Disord. 16:59–67. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Pavelka L, Rawal R, Sapienza S, Klucken J, Pauly C, Satagopam V and Krüger R: Genetically stratified Parkinson's disease with freezing of gait is related to specific pattern of cognitive impairment and non-motor dominant endophenotype. Front Aging Neurosci. 16(1479572)2024.PubMed/NCBI View Article : Google Scholar | |
|
You S, Kim HA and Lee H: Association of postural instability with autonomic dysfunction in early Parkinson's disease. J Clin Med. 9(3786)2020.PubMed/NCBI View Article : Google Scholar | |
|
Khoo TK, Yarnall AJ, Duncan GW, Coleman S, O'Brien JT, Brooks DJ, Barker RA and Burn DJ: The spectrum of nonmotor symptoms in early Parkinson disease. Neurology. 80:276–281. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Hall JM, Shine JM, Walton CC, Gilat M, Kamsma YPT, Naismith SL and Lewis SJG: Early phenotypic differences between Parkinson's disease patients with and without freezing of gait. Parkinsonism Relat Disord. 20:604–607. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Yoon SH, You DH, Na HK, Kang S, Baik K, Park M, Lyoo CH, Sohn YH and Lee PH: Parkinson's disease with hyposmia and dysautonomia: Does it represent a distinct subtype? J Neurol. 271:5064–5073. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Pan G, Jiang Y, Zhang W, Zhang X, Wang L and Cheng W: Identification of Parkinson's disease subtypes with distinct brain atrophy progression and its association with clinical progression. Psychoradiology. 4(kkae002)2024.PubMed/NCBI View Article : Google Scholar | |
|
Wang L, Cheng W, Rolls ET, Dai F, Gong W, Du J, Zhang W, Wang S, Liu F, Wang J, et al: Association of specific biotypes in patients with Parkinson disease and disease progression. Neurology. 95:e1445–e1460. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Pursiainen V, Haapaniemi TH, Korpelainen JT, Sotaniemi KA and Myllylä VV: Sweating in Parkinsonian patients with wearing-off. Mov Disord. 22:828–832. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Witjas T, Kaphan E, Azulay JP, Blin O, Ceccaldi M, Pouget J, Poncet M and Chérif AA: Nonmotor fluctuations in Parkinson's disease: Frequent and disabling. Neurology. 59:408–413. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Baratti M and Calzetti S: Fluctuation of arterial blood pressure during end-of-dose akinesia in Parkinson's disease. J Neurol Neurosurg Psychiatry. 47:1241–1243. 1984.PubMed/NCBI View Article : Google Scholar | |
|
Hardoff R, Sula M, Tamir A, Soil A, Front A, Badarna S, Honigman S and Giladi N: Gastric emptying time and gastric motility in patients with Parkinson's disease. Mov Disord. 16:1041–1047. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Lei HB, Fang TC, Lin YH, Chiu SC, Chang MH and Guo YJ: What does it mean when the pleasant smells come and go? Correlation between UPSIT odor identification status and fluctuation of non-motor symptoms in Parkinson's disease. Acta Neurol Belg. 125:469–479. 2025.PubMed/NCBI View Article : Google Scholar | |
|
Jost WH, Altmann C, Fiesel T, Becht B, Ringwald S and Hoppe T: Influence of levodopa on orthostatic hypotension in Parkinson's disease. Neurol Neurochir Pol. 54:200–203. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Dumbravă EL, Cojocaru DC and Postolache P: Orthostatic intolerance-an expression of autonomic disfunction in Parkinson's disease. Rev Med Chir Soc Med Nat Iasi. 118:75–80. 2014.PubMed/NCBI | |
|
Meng L, Dunckley ED and Xu X: Effects of a single dose levodopa on heart rate variability in Parkinson's disease. Zhonghua Yi Xue Za Zhi. 95:493–495. 2015.PubMed/NCBI(In Chinese). | |
|
Quadri Z, Johnson N, Zamudio F, Miller A, Peters M, Smeltzer S, Hunt JB Jr, Housley SB, Brown B, Kraner S, et al: Overexpression of human wtTDP-43 causes impairment in hippocampal plasticity and behavioral deficits in CAMKII-tTa transgenic mouse model. Mol Cell Neurosci. 102(103418)2020.PubMed/NCBI View Article : Google Scholar | |
|
Oka H, Sengoku R, Nakahara A and Yamazaki M: Rasagiline does not exacerbate autonomic blood pressure dysregulation in early or mild Parkinson's disease. Clin Park Relat Disord. 6(100124)2022.PubMed/NCBI View Article : Google Scholar | |
|
Perez-Lloret S, Rey MV, Fabre N, Ory F, Spampinato U, Senard JM, Pavy-Le Traon A, Montastruc JL and Rascol O: Factors related to orthostatic hypotension in Parkinson's disease. Parkinsonism Relat Disord. 18:501–505. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Nimmons D, Bhanu C, Orlu M, Schrag A and Walters K: Orthostatic hypotension and antiparkinsonian drugs: A systematic review and Meta-analysis. J Geriatr Psychiatry Neurol. 35:639–654. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Aris E, Dotchin CL, Gray WK and Walker RW: Autonomic function in a prevalent Tanzanian population with Parkinson's disease and its relationship to disease duration and 5-year mortality. BMC Res Notes. 6(535)2013.PubMed/NCBI View Article : Google Scholar | |
|
van Dijk JG, Haan J, Zwinderman K, Kremer B, van Hilten BJ and Roos RA: Autonomic nervous system dysfunction in Parkinson's disease: Relationships with age, medication, duration, and severity. J Neurol Neurosurg Psychiatry. 56:1090–1095. 1993.PubMed/NCBI View Article : Google Scholar | |
|
Guseva OV and Zhukova NG: The high blood pressure in patients with Parkinson's disease. Zh Nevrol Psikhiatr Im S S Korsakova. 121:13–17. 2021.PubMed/NCBI View Article : Google Scholar : (In Russian). | |
|
Korchounov A, Kessler KR, Yakhno NN, Damulin IV and Schipper HI: Determinants of autonomic dysfunction in idiopathic Parkinson's disease. J Neurol. 252:1530–1536. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Li L, Guo P, Ding D, Lian T, Zuo L, Du F and Zhang W: Parkinson's disease with orthostatic hypotension: Analyses of clinical characteristics and influencing factors. Neurol Res. 41:734–741. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Zalewski P, Slomko J and Zawadka-Kunikowska M: Autonomic dysfunction and chronic disease. Br Med Bull. 128:61–74. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Kim JS, Oh YS, Lee KS, Kim YI, Yang DW and Goldstein DS: Association of cognitive dysfunction with neurocirculatory abnormalities in early Parkinson disease. Neurology. 79:1323–1331. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Kwon KY, Park S, Kim RO, Lee EJ and Lee M: Associations of cognitive dysfunction with motor and non-motor symptoms in patients with de novo Parkinson's disease. Sci Rep. 12(11461)2022.PubMed/NCBI View Article : Google Scholar | |
|
Thacker EL, O'Reilly EJ, Weisskopf MG, Chen H, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ and Ascherio A: Temporal relationship between cigarette smoking and risk of Parkinson disease. Neurology. 68:764–768. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Hong CT, Chan L and Bai CH: The effect of caffeine on the risk and progression of Parkinson's disease: A Meta-Analysis. Nutrients. 12(1860)2020.PubMed/NCBI View Article : Google Scholar | |
|
Schirinzi T, Martella G, Imbriani P, Di Lazzaro G, Franco D, Colona VL, Alwardat M, Sinibaldi Salimei P, Mercuri NB, Pierantozzi M and Pisani A: Dietary vitamin E as a protective factor for Parkinson's disease: Clinical and experimental evidence. Front Neurol. 10(148)2019.PubMed/NCBI View Article : Google Scholar | |
|
Dorsey ER and Bloem BR: Parkinson's disease is predominantly an environmental disease. J Parkinsons Dis. 14:451–465. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Chelban V, Vichayanrat E, Schottlaende L, Iodice V and Houlden H: Autonomic dysfunction in genetic forms of synucleinopathies. Mov Disord. 33:359–371. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Orimo S, Uchihara T, Nakamura A, Mori F, Ikeuchi T, Onodera O, Nishizawa M, Ishikawa A, Kakita A, Wakabayashi K and Takahashi H: Cardiac sympathetic denervation in Parkinson's disease linked to SNCA duplication. Acta Neuropathol. 116:575–577. 2008.PubMed/NCBI View Article : Google Scholar | |
|
De Rosa A, Pellegrino T, Pappatà S, Lieto M, Bonifati V, Palma V, Topa A, Santoro L, Bilo L, Cuocolo A and De Michele G: Non-motor symptoms and cardiac innervation in SYNJ1-related parkinsonism. Parkinsonism Relat Disord. 23:102–105. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Tijero B, Gabilondo I, Lezcano E, Teran-Villagrá N, Llorens V, Ruiz-Martinez J, Marti-Masso JF, Carmona M, Luquin MR, Berganzo K, et al: Autonomic involvement in Parkinsonian carriers of PARK2 gene mutations. Parkinsonism Relat Disord. 21:717–722. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Song J, Shen B, Yang YJ, Liu FT, Zhao J, Tang YL, Chen C, Ding ZT, An Y, Wu JJ, et al: Non-motor symptoms in Parkinson's disease patients with parkin mutations: More depression and less executive dysfunction. J Mol Neurosci. 70:246–253. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Hentati F, Trinh J, Thompson C, Nosova E, Farrer MJ and Aasly JO: LRRK2 parkinsonism in Tunisia and Norway: A comparative analysis of disease penetrance. Neurology. 83:568–569. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Tijero B, Gómez Esteban JC, Somme J, Llorens V, Lezcano E, Martinez A, Rodríguez T, Berganzo K and Zarranz JJ: Autonomic dysfunction in parkinsonian LRRK2 mutation carriers. Parkinsonism Relat Disord. 19:906–909. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Nasri A, Kacem I, Farhat N, Gharbi A, Sakka S, Souissi A, Zidi S, Damak M, Bendjebara M, Gargouri A, et al: Heart rate variability and sympathetic skin response for the assessment of autonomic dysfunction in leucine-rich repeat kinase 2 associated Parkinson's disease. Neurophysiol Clin. 52:81–93. 2022.PubMed/NCBI View Article : Google Scholar | |
|
De Santis T, Cocco A, Castiglioni P, Ferrarin M, Mineri R, Morenghi E, Avenali M and Albanese A: Parasympathetic dysfunction prevails in GBA1-associated Parkinson's disease. Mov Disord Clin Pract. 12:364–370. 2025.PubMed/NCBI View Article : Google Scholar | |
|
Zhou Y, Wang Y, Wan J, Zhao Y, Pan H, Zeng Q, Zhou X, He R, Zhou X, Xiang Y, et al: Mutational spectrum and clinical features of GBA1 variants in a Chinese cohort with Parkinson's disease. NPJ Parkinsons Dis. 9(129)2023.PubMed/NCBI View Article : Google Scholar | |
|
Carandina A, Lazzeri G, Rodrigues GD, Franco G, Monfrini E, Arienti F, Frattini E, Trezzi I, da Silva Soares PP, Bellocchi C, et al: Dysautonomia in Parkinson's disease: Impact of glucocerebrosidase gene mutations on cardiovascular autonomic control. Front Neurosci. 16(842498)2022.PubMed/NCBI View Article : Google Scholar | |
|
Usnich T, Hanssen H, Lohmann K, Lohse C, Klein C, Kasten M and Brüggemann N: Pronounced orthostatic hypotension in GBA-related Parkinson's disease. J Parkinsons Dis. 12:1539–1544. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Pitton Rissardo J and Fornari Caprara AL: Cardiac 123I-metaiodobenzylguanidine (MIBG) Scintigraphy in Parkinson's disease: A comprehensive review. Brain Sci. 13(1471)2023.PubMed/NCBI View Article : Google Scholar | |
|
Bouhaddi M, Vuillier F, Fortrat JO, Cappelle S, Henriet MT, Rumbach L and Regnard J: Impaired cardiovascular autonomic control in newly and long-term-treated patients with Parkinson's disease: Involvement of L-dopa therapy. Auton Neurosci. 116:30–38. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Jost WH: Autonomic dysfunction in Parkinson's disease: Cardiovascular symptoms, thermoregulation, and urogenital symptoms. Int Rev Neurobiol. 134:771–785. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Storch A, Schneider CB, Wolz M, Stürwald Y, Nebe A, Odin P, Mahler A, Fuchs G, Jost WH, Chaudhuri KR, et al: Nonmotor fluctuations in Parkinson disease: Severity and correlation with motor complications. Neurology. 80:800–809. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Mizutani Y, Nakamura T, Okada A, Suzuki J, Watanabe H, Hirayama M and Sobue G: Hyposmia and cardiovascular dysautonomia correlatively appear in early-stage Parkinson's disease. Parkinsonism Relat Disord. 20:520–524. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Goldstein DS, Sewell L and Holmes C: Association of anosmia with autonomic failure in Parkinson disease. Neurology. 74:245–251. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Velseboer DC, de Haan RJ, Wieling W, Goldstein DS and de Bie RMA: Prevalence of orthostatic hypotension in Parkinson's disease: A systematic review and meta-analysis. Parkinsonism Relat Disord. 17:724–729. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Kapoor S, Saluja A, Margekar SL, Agarwal M, Mondal S and Dhamija RK: Neurogenic supine hypertension and cardiovascular autonomic dysfunction in patients with Parkinson's Disease. Ann Indian Acad Neurol. 26:33–38. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Arbogast SD, Alshekhlee A, Hussain Z, McNeeley K and Chelimsky TC: Hypotension unawareness in profound orthostatic hypotension. Am J Med. 122:574–580. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Yalcin A, Atmis V, Cengiz OK, Cinar E, Aras S, Varli M and Atli T: Evaluation of cardiac autonomic functions in older Parkinson's disease patients: A Cross-sectional study. Aging Dis. 7:28–35. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Quarracino C, Otero-Losada M, Capani F and Pérez-Lloret S: Prevalence and factors related to orthostatic syndromes in recently diagnosed, drug-naïve patients with Parkinson disease. Clin Auton Res. 30:265–271. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Hiorth YH, Pedersen KF, Dalen I, Tysnes OB and Alves G: Orthostatic hypotension in Parkinson disease: A 7-year prospective population-based study. Neurology. 93:e1526–e1534. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Goldstein DS, Holmes C and Imrich R: Clinical laboratory evaluation of autoimmune autonomic ganglionopathy: Preliminary observations. Auton Neurosci. 146:18–21. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Campese N, Goebel G, Leys F, Ndayisaba JP, Eschlboeck S, Eckhardt C, Raccagni C, Granata R, Ceravolo R, Kiechl S, et al: Orthostatic Hypotension in Parkinson's Disease: Do Height and Weight Matter? Mov Disord. 36:2703–2705. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Umehara T, Nakahara A, Matsuno H, Toyoda C and Oka H: Body weight and dysautonomia in early Parkinson's disease. Acta Neurol Scand. 135:560–567. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Mochizuki H, Taniguchi A, Nakazato Y, Ishii N, Ebihara Y, Sugiyama T, Shiomi K and Nakazato M: Increased body mass index associated with autonomic dysfunction in Parkinson's disease. Parkinsonism Relat Disord. 24:129–131. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Tulbă D, Tănăsoiu AC, Constantinescu AM, Blidaru N, Buzea A, Băicuș C, Dumitrescu L, Davidescu EI and Popescu BO: Cardiovascular dysautonomia in Patients with Parkinson's disease and hypertension: A Cross-sectional pilot study. J Clin Med. 14(2225)2025.PubMed/NCBI View Article : Google Scholar | |
|
Merola A, Romagnolo A, Rosso M, Suri R, Berndt Z, Maule S, Lopiano L and Espay AJ: Autonomic dysfunction in Parkinson's disease: A prospective cohort study. Mov Disord. 33:391–397. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Jost WH and Augustis S: Severity of orthostatic hypotension in the course of Parkinson's disease: No correlation with the duration of the disease. Parkinsonism Relat Disord. 21:314–316. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Romagnolo A, Zibetti M, Merola A, Canova D, Sarchioto M, Montanaro E, Artusi CA, Vallelonga F, Maule S and Lopiano L: Cardiovascular autonomic neuropathy and falls in Parkinson disease: A prospective cohort study. J Neurol. 266:85–91. 2019.PubMed/NCBI View Article : Google Scholar | |
|
LeWitt PA and Chaudhuri KR: Unmet needs in Parkinson disease: Motor and non-motor. Parkinsonism Relat Disord. 80 (Suppl 1):S7–S12. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Li Z, Jiang X, Yang M and Pan Y: Association between falls and nonmotor symptoms in patients with Parkinson's disease. J Clin Neurosci. 118:143–146. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Altmann CF, Koschel J and Jost WH: Predictors of falls in Parkinson's disease, progressive supranuclear palsy, and multiple system atrophy: A retrospective study. Neurol Neurochir Pol. 57:297–304. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Imbalzano G, Ledda C, Tangari MM, Artusi CA, Montanaro E, Rizzone MG, Zibetti M, Lopiano L and Romagnolo A: Unraveling the stride: Exploring the influence of neurogenic orthostatic hypotension on gait and balance in Parkinson's disease. Clin Auton Res. 34:593–601. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Sforza M, Assogna F, Rinaldi D, Sette G, Tagliente S and Pontieri FE: Orthostatic hypotension acutely impairs executive functions in Parkinson's disease. Neurol Sci. 39:1459–1462. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Cicero CE, Raciti L, Monastero R, Mostile G, Donzuso G, Sciacca G, Luca A, Terravecchia C, Giuliano L, Baschi R, et al: Cardiovascular autonomic function and MCI in Parkinson's disease. Parkinsonism Relat Disord. 69:55–58. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Loureiro D, Bilbao R, Bordet S, Grasso L, Otero-Losada M, Capani F, Ponzo OJ and Perez-Lloret S: A systematic review and meta-analysis on the association between orthostatic hypotension and mild cognitive impairment and dementia in Parkinson's disease. Neurol Sci. 44:1211–1222. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Khalil I, Sayad R, Kedwany AM, Sayed HH, Caprara ALF and Rissardo JP: Cardiovascular dysautonomia and cognitive impairment in Parkinson's disease (Review). Med Int (Lond). 4(70)2024.PubMed/NCBI View Article : Google Scholar | |
|
Chung SJ, Bae YJ, Jun S, Yoo HS, Kim SW, Lee YH, Sohn YH, Lee SK, Seong JK and Lee PH: Dysautonomia is associated with structural and functional alterations in Parkinson disease. Neurology. 92:e1456–e1467. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Jurcau A and Nunkoo VS: Clinical markers may identify patients at risk for early Parkinson's disease dementia: A prospective study. Am J Alzheimers Dis Other Demen. 36(15333175211021369)2021.PubMed/NCBI View Article : Google Scholar | |
|
Ashraf-Ganjouei A, Majd A, Javinani A and Aarabi MH: Autonomic dysfunction and white matter microstructural changes in drug-naïve patients with Parkinson's disease. PeerJ. 6(e5539)2018.PubMed/NCBI View Article : Google Scholar | |
|
Nakamura T, Suzuki M, Ueda M, Harada Y, Hirayama M and Katsuno M: Impact of orthostatic hypotension on wheelchair use in patients with Parkinson's disease. J Neural Transm (Vienna). 127:379–383. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Nozaki S, Kang J, Miyai I and Matsumura T: Postprandial hypotension in Parkinson's disease-the incidence and risk factor. Rinsho Shinkeigaku. 33:1135–1139. 1993.PubMed/NCBI(In Japanese). | |
|
Loew F, Gauthey L, Koerffy A, Herrmann FR, Estade M, Michel JP and Vallotton MB: Postprandial hypotension and orthostatic blood pressure responses in elderly Parkinson's disease patients. J Hypertens. 13:1291–1297. 1995.PubMed/NCBI View Article : Google Scholar | |
|
Umehara T, Matsuno H, Toyoda C and Oka H: Clinical characteristics of supine hypertension in de novo Parkinson disease. Clin Auton Res. 26:15–21. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Ali Abdelhamid Y, Weinel LM, Hatzinikolas S, Summers M, Nguyen TAN, Kar P, Phillips LK, Horowitz M, Deane AM and Jones KL: Autonomic function, postprandial hypotension and falls in older adults at one year after critical illness. Crit Care Resusc. 22:53–62. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Mehagnoul-Schipper DJ, Boerman RH, Hoefnagels WH and Jansen RW: Effect of levodopa on orthostatic and postprandial hypotension in elderly Parkinsonian patients. J Gerontol A Biol Sci Med Sci. 56:M749–M755. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Fanciulli A, Jordan J, Biaggioni I, Calandra-Buonaura G, Cheshire WP, Cortelli P, Eschlboeck S, Grassi G, Hilz MJ, Kaufmann H, et al: Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the european federation of autonomic societies (EFAS): Endorsed by the european academy of neurology (EAN) and the european society of hypertension (ESH). Clin Auton Res. 28:355–362. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Sommer S, Aral-Becher B and Jost W: Nondipping in Parkinson's disease. Parkinsons Dis. 2011(897586)2011.PubMed/NCBI View Article : Google Scholar | |
|
Arici Duz O and Helvaci Yilmaz N: Nocturnal blood pressure changes in Parkinson's disease: Correlation with autonomic dysfunction and vitamin D levels. Acta Neurol Belg. 120:915–920. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Di Stefano C, Sobrero G, Milazzo V, Vallelonga F, Romagnolo A, Zibetti M, Milan A, Veglio F and Maule S: Cardiac organ damage in patients with Parkinson's disease and reverse dipping. J Hypertens. 38:289–294. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Stuebner E, Vichayanrat E, Low DA, Mathias CJ, Isenmann S and Haensch CA: Non-dipping nocturnal blood pressure and psychosis parameters in Parkinson disease. Clin Auton Res. 25:109–116. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Palma JA and Kaufmann H: Orthostatic hypotension in Parkinson disease. Clin Geriatr Med. 36:53–67. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Morozumi S, Kato S, Yasui K and Hasegawa Y: Posterior reversible encephalopathy syndrome in Parkinson disease probably caused by prominent supine hypertension and blood pressure fluctuation. Rinsho Shinkeigaku. 56:754–758. 2016.PubMed/NCBI View Article : Google Scholar : (In Japanese). | |
|
Huckemann S, Mueller K, Averdunk P, Kühn E, Hilker L, Kools S, Scholz L, Bulut Y, Brünger J, Fiegert S, et al: Vagal cross-sectional area correlates with parasympathetic dysfunction in Parkinson's disease. Brain Commun. 5(fcad006)2023.PubMed/NCBI View Article : Google Scholar | |
|
Sebastian I, Kate MP, Khatter H, Singh B and Pandian JD: Spectrum of cardiovascular autonomic dysfunction and 24-hour blood pressure variability in idiopathic Parkinson's disease. Ann Indian Acad Neurol. 25:902–908. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Milazzo V, Di Stefano C, Vallelonga F, Sobrero G, Zibetti M, Romagnolo A, Merola A, Milan A, Espay AJ, Lopiano L, et al: Reverse blood pressure dipping as marker of dysautonomia in Parkinson disease. Parkinsonism Relat Disord. 56:82–87. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Miyagi T, Yamazato M, Nakamura T, Tokashiki T, Namihira Y, Kokuba K, Ishihara S, Sakima H and Ohya Y: Power spectral analysis of heart rate variability is useful as a screening tool for detecting sympathetic and parasympathetic nervous dysfunctions in Parkinson's disease. BMC Neurol. 22(339)2022.PubMed/NCBI View Article : Google Scholar | |
|
Scorza FA, Fiorini AC, Scorza CA and Finsterer J: Cardiac abnormalities in Parkinson's disease and Parkinsonism. J Clin Neurosci. 53:1–5. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Vokatch N, Grötzsch H, Mermillod B, Burkhard PR and Sztajzel R: Is cerebral autoregulation impaired in Parkinson's disease? A transcranial doppler study. J Neurol Sci. 254:49–53. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Kobari M, Fukuuchi Y, Shinohara T, Obara K and Nogawa S: Levodopa-induced local cerebral blood flow changes in Parkinson's disease and related disorders. J Neurol Sci. 128:212–218. 1995.PubMed/NCBI View Article : Google Scholar | |
|
Sachs C, Berglund B and Kaijser L: Autonomic cardiovascular responses in Parkinsonism: Effect of levodopa with dopa-decarboxylase inhibition. Acta Neurol Scand. 71:37–42. 1985.PubMed/NCBI View Article : Google Scholar | |
|
Camerlingo M, Aillon C, Bottacchi E, Gambaro P, D'Alessandro G, Franceschi M, Devoti G and Mamoli A: Parasympathetic assessment in Parkinson's disease. Adv Neurol. 45:267–269. 1987.PubMed/NCBI | |
|
Goetz CG, Lutge W and Tanner CM: Autonomic dysfunction in Parkinson's disease. Neurology. 36:73–75. 1986.PubMed/NCBI View Article : Google Scholar | |
|
Ludin SM, Steiger UH and Ludin HP: Autonomic disturbances and cardiovascular reflexes in idiopathic Parkinson's disease. J Neurol. 235:10–15. 1987.PubMed/NCBI View Article : Google Scholar | |
|
Turkka JT, Tolonen U and Myllylä VV: Cardiovascular reflexes in Parkinson's disease. Eur Neurol. 26:104–112. 1987.PubMed/NCBI View Article : Google Scholar | |
|
Piha SJ, Rinne JO, Rinne UK and Seppänen A: Autonomic dysfunction in recent onset and advanced Parkinson's disease. Clin Neurol Neurosurg. 90:221–226. 1988.PubMed/NCBI View Article : Google Scholar | |
|
Meco G, Pratesi L and Bonifati V: Cardiovascular reflexes and autonomic dysfunction in Parkinson's disease. J Neurol. 238:195–199. 1991.PubMed/NCBI View Article : Google Scholar | |
|
Ahn JH, Kim M, Mun JK, Cho Y, Kim JS, Youn J, Kim JS and Cho JW: The dysfunctional autonomic function and ‘Dysfunctional’ fatigue in drug naïve Parkinson's disease. J Parkinsons Dis. 10:605–612. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Devos D, Kroumova M, Bordet R, Vodougnon H, Guieu JD, Libersa C and Destee A: Heart rate variability and Parkinson's disease severity. J Neural Transm (Vienna). 110:997–1011. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Heimler B, Koren O, Inzelberg R, Rosenblum U, Hassin-Baer S, Zeilig G, Bartsch RP and Plotnik M: Heart-rate variability as a new marker for freezing predisposition in Parkinson's disease. Parkinsonism Relat Disord. 113(105476)2023.PubMed/NCBI View Article : Google Scholar | |
|
Hall JM, Shine JM, O'Callaghan C, Walton CC, Gilat M, Naismith SL and Lewis SJG: Freezing of gait and its associations in the early and advanced clinical motor stages of Parkinson's disease: A Cross-sectional study. J Parkinsons Dis. 5:881–891. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Carricarte Naranjo C, Marras C, Visanji NP, Cornforth DJ, Sanchez-Rodriguez L, Schüle B, Goldman SM, Estévez M, Stein PK, Lang AE, et al: Short-term deceleration capacity of heart rate: A sensitive marker of cardiac autonomic dysfunction in idiopathic Parkinson's disease. Clin Auton Res. 31:729–736. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Yoo H, Chung SH, Lee CN and Joo HJ: Deep Learning algorithm of 12-Lead electrocardiogram for Parkinson disease screening. J Parkinsons Dis. 13:71–82. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Bidikar MP, Jagtap GJ and Chakor RT: Influence of deep breathing on heart rate variability in Parkinson's disease: Co-relation with severity of disease and non-motor symptom scale score. J Clin Diagn Res. 8:BC01–BC03. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Heimrich KG, Lehmann T, Schlattmann P and Prell T: Heart rate variability analyses in Parkinson's disease: A systematic review and meta-analysis. Brain Sci. 11(959)2021.PubMed/NCBI View Article : Google Scholar | |
|
Yamamoto T, Tamura N, Kinoshita S, Itokawa K, Sumita N, Fukui M, Shimazu K and Kato R: A case of sick sinus syndrome and autonomic failure with Parkinson's disease. Auton Neurosci. 146:115–117. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Vallelonga F, Sobrero G, Merola A, Valente M, Giudici M, Di Stefano C, Milazzo V, Burrello J, Burrello A, Veglio F, et al: Machine learning applied to ambulatory blood pressure monitoring: A new tool to diagnose autonomic failure? J Neurol. 269:3833–3840. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Polverino P, Ajčević M, Catalan M, Bertolotti C, Furlanis G, Marsich A, Buoite Stella A, Accardo A and Manganotti P: Comprehensive telemedicine solution for remote monitoring of Parkinson's disease patients with orthostatic hypotension during COVID-19 pandemic. Neurol Sci. 43:3479–3487. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Hellman AM, Shah SP, Pawlowski SM, Duda JE and Morley JF: Continuous non-invasive monitoring to detect covert autonomic dysfunction in Parkinson's disease. Parkinsonism Relat Disord. 21:723–728. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Rodríguez M, Sabaté M and Troncoso E: Time and frequency domain analysis for the assessment of heart autonomic control in Parkinson's disease. J Neural Transm (Vienna). 103:447–454. 1996.PubMed/NCBI View Article : Google Scholar | |
|
Netten PM, de Vos K, Horstink MW and Hoefnagels WH: Autonomic dysfunction in Parkinson's disease, tested with a computerized method using a Finapres device. Clin Auton Res. 5:85–89. 1995.PubMed/NCBI View Article : Google Scholar | |
|
Pérez T, Tijero B, Gabilondo I, Luna A, Llorens V, Berganzo K, Acera M, Gonzalez A, Sanchez-Ferro A, Lezcano E, et al: Cardiocirculatory manifestations in Parkinson's disease patients without orthostatic hypotension. J Hum Hypertens. 29:604–609. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Park DG, Kim JW, An YS, Chang J and Yoon JH: Plasma neurofilament light chain level and orthostatic hypotension in early Parkinson's disease. J Neural Transm (Vienna). 128:1853–1861. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Kanegusuku H, Cucato GG, Longano P, Okamoto E, Piemonte MEP, Correia MA and Ritti-Dias RM: Acute Cardiovascular Responses to Self-selected Intensity Exercise in Parkinson's Disease. Int J Sports Med. 43:177–182. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Umehara T, Oka H, Nakahara A, Shiraishi T, Sato T, Matsuno H, Komatsu T, Omoto S, Murakami H and Iguchi Y: Sympathetic nervous activity and hemoglobin levels in de novo Parkinson's disease. Clin Auton Res. 30:273–278. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Nakamura T, Hirayama M, Hara T, Mizutani Y, Suzuki J, Watanabe H and Sobue G: Role of cardiac sympathetic nerves in preventing orthostatic hypotension in Parkinson's disease. Parkinsonism Relat Disord. 20:409–414. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Miyasato RS, Silva-Batista C, Peçanha T, Low DA, de Mello MT, Piemonte MEP, Ugrinowitsch C, Forjaz CLM and Kanegusuku H: Cardiovascular responses during resistance exercise in patients with parkinson disease. PM R. 10:1145–1152. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Strano S, Fanciulli A, Rizzo M, Marinelli P, Palange P, Tiple D, De Vincentis G, Calcagnini G, Censi F, Meco G and Colosimo C: Cardiovascular dysfunction in untreated Parkinson's disease: A multi-modality assessment. J Neurol Sci. 370:251–255. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Low DA, Vichayanrat E, Iodice V and Mathias CJ: Exercise hemodynamics in Parkinson's disease and autonomic dysfunction. Parkinsonism Relat Disord. 20:549–553. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Seccombe LM, Rogers PG, Hayes MW, Farah CS, Veitch EM and Peters MJ: Reduced hypoxic sympathetic response in mild Parkinson's disease: Further evidence of early autonomic dysfunction. Parkinsonism Relat Disord. 19:1066–1068. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Durcan R, Wiblin L, Lawson RA, Khoo TK, Yarnall AJ, Duncan GW, Brooks DJ, Pavese N and Burn DJ: ICICLE-PD Study Group. Prevalence and duration of non-motor symptoms in prodromal Parkinson's disease. Eur J Neurol. 26:979–985. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Martinez-Ramirez D, Velazquez-Avila ES, Almaraz-Espinoza A, Gonzalez-Cantú A, Vazquez-Elizondo G, Overa-Posada D, Cervantes-Arriaga A, Rodriguez-Violante M and Gonzalez-Gonzalez M: Lower urinary tract and gastrointestinal dysfunction are common in Early Parkinson's disease. Parkinsons Dis. 2020(1694547)2020.PubMed/NCBI View Article : Google Scholar | |
|
Kwon KY, Park S, Lee EJ, Lee M and Ju H: Impact of subjective dizziness on motor and non-motor symptoms in patients with early stages of Parkinson's disease. J Integr Neurosci. 21(3)2022.PubMed/NCBI View Article : Google Scholar | |
|
Park JH and Kang SY: Dizziness in Parkinson's disease patients is associated with vestibular function. Sci Rep. 11(18976)2021.PubMed/NCBI View Article : Google Scholar | |
|
Jones JD, Rahmani E, Garcia E and Jacobs JP: Gastrointestinal symptoms are predictive of trajectories of cognitive functioning in de novo Parkinson's disease. Parkinsonism Relat Disord. 72:7–12. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Kim S, Woo KA, Choi H, Shin JH and Kim HJ: Monoaminergic degeneration, cognition, and autonomic symptom trajectory in early Parkinson's disease. Parkinsonism Relat Disord. 127(107086)2024.PubMed/NCBI View Article : Google Scholar | |
|
Wang XY, Han YY, Li G and Zhang B: Association between autonomic dysfunction and olfactory dysfunction in Parkinson's disease in southern Chinese. BMC Neurol. 19(17)2019.PubMed/NCBI View Article : Google Scholar | |
|
Cersosimo MG, Raina GB, Pellene LA, Micheli FE, Calandra CR and Maiola R: Weight Loss in Parkinson's disease: The relationship with motor symptoms and disease progression. Biomed Res Int. 2018(9642524)2018.PubMed/NCBI View Article : Google Scholar | |
|
van Wamelen DJ, Leta V, Johnson J, Ocampo CL, Podlewska AM, Rukavina K, Rizos A, Martinez-Martin P and Chaudhuri KR: Drooling in Parkinson's disease: Prevalence and progression from the non-motor international longitudinal study. Dysphagia. 35:955–961. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Barbe AG, Heinzler A, Derman S, Hellmich M, Timmermann L and Noack MJ: Hyposalivation and xerostomia among Parkinson's disease patients and its impact on quality of life. Oral Dis. 23:464–470. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Mohamed AAB, Mohamed GF, Elnady HM, Sayed MA, Imam AM, Hassan MM and Ahmed SR: Evaluation of dysphagia in different phenotypes of early and idiopathic Parkinsonism. Egypt J Neurol Psychiatr Neurosurg. 54(28)2018.PubMed/NCBI View Article : Google Scholar | |
|
Soykan I, Sivri B, Sarosiek I, Kiernan B and McCallum RW: Demography, clinical characteristics, psychological and abuse profiles, treatment, and Long-term follow-up of patients with gastroparesis. Dig Dis Sci. 43:2398–2404. 1998.PubMed/NCBI View Article : Google Scholar | |
|
Sung HY, Park JW and Kim JS: The frequency and severity of gastrointestinal symptoms in patients with early Parkinson's disease. J Mov Disord. 7:7–12. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Cersosimo MG, Raina GB, Pecci C, Pellene A, Calandra CR, Gutiérrez C, Micheli FE and Benarroch EE: Gastrointestinal manifestations in Parkinson's disease: Prevalence and occurrence before motor symptoms. J Neurol. 260:1332–1338. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Qin X, Li X, Xin Z and Li Z: Gastrointestinal dysfunction in Chinese patients with Parkinson's disease. Parkinsons Dis. 2019(3897315)2019.PubMed/NCBI View Article : Google Scholar | |
|
Ma K, Xiong N, Shen Y, Han C, Liu L, Zhang G, Wang L, Guo S, Guo X, Xia Y, et al: Weight loss and malnutrition in patients with Parkinson's disease: Current Knowledge and future prospects. Front Aging Neurosci. 10(1)2018.PubMed/NCBI View Article : Google Scholar | |
|
Cumming K, Macleod AD, Myint PK and Counsell CE: Early weight loss in Parkinsonism predicts poor outcomes: Evidence from an incident cohort study. Neurology. 89:2254–2261. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Kim R, Choi S, Byun K, Kang N, Suh YJ, Jun JS and Jeon B: Association of early weight change with cognitive decline in patients with Parkinson disease. Neurology. 100:e232–e241. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Kalf JG, de Swart BJM, Borm GF, Bloem BR and Munneke M: Prevalence and definition of drooling in Parkinson's disease: A systematic review. J Neurol. 256:1391–1396. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Rascol O, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destée A, Fabbri M, Meissner WG, Rachdi A, Tison F, et al: Excessive buccal saliva in patients with Parkinson's disease of the French COPARK cohort. J Neural Transm (Vienna). 127:1607–1617. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Müller B, Larsen JP, Wentzel-Larsen T, Skeie GO and Tysnes OB: Autonomic and sensory symptoms and signs in incident, untreated Parkinson's disease: Frequent but mild. Mov Disord. 26:65–72. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Santos-García D, de Deus Fonticoba T, Cores Bartolomé C, Feal Painceiras MJ, Íñiguez-Alvarado MC, Jesús S, Buongiorno MT, Planellas L, Cosgaya M, García Caldentey J, et al: Prevalence and factors associated with drooling in Parkinson's disease: Results from a longitudinal prospective cohort and comparison with a control group. Parkinsons Dis. 2023(3104425)2023.PubMed/NCBI View Article : Google Scholar | |
|
Nicaretta DH, Rosso AL, Mattos JP de, Maliska C and Costa MMB: Dysphagia and sialorrhea: The relationship to Parkinson's disease. Arq Gastroenterol. 50:42–49. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Špiljak B, Lisak M, Pašić H, Trkanjec Z, Lovrenčić Huzjan A and Bašić Kes V: Sialorrhea and xerostomia in parkinson's disease patients. Acta Clin Croat. 61:320–326. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Cersosimo MG, Raina GB, Calandra CR, Pellene A, Gutiérrez C, Micheli FE and Benarroch EE: Dry mouth: An overlooked autonomic symptom of Parkinson's disease. J Parkinsons Dis. 1:169–173. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Müller B, Assmus J, Herlofson K, Larsen JP and Tysnes OB: Importance of motor vs. non-motor symptoms for Health-related quality of life in early Parkinson's disease. Parkinsonism Relat Disord. 19:1027–1032. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Ayala A, Triviño-Juárez JM, Forjaz MJ, Rodríguez-Blázquez C, Rojo-Abuin JM and Martínez-Martín P: Parkinson's disease severity at 3 years can be predicted from non-motor symptoms at baseline. Front Neurol. 8(551)2017.PubMed/NCBI View Article : Google Scholar | |
|
Fedorova T, Knudsen CS, Mouridsen K, Nexo E and Borghammer P: Salivary acetylcholinesterase activity is increased in Parkinson's disease: A potential marker of parasympathetic dysfunction. Parkinsons Dis. 2015(156479)2015.PubMed/NCBI View Article : Google Scholar | |
|
Umemoto G and Furuya H: Management of dysphagia in patients with Parkinson's disease and related disorders. Intern Med. 59:7–14. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Gong S, Gao Y, Liu J, Li J, Tang X, Ran Q, Tang R and Liao C: The prevalence and associated factors of dysphagia in Parkinson's disease: A systematic review and meta-analysis. Front Neurol. 13(1000527)2022.PubMed/NCBI View Article : Google Scholar | |
|
Manor Y, Balas M, Giladi N, Mootanah R and Cohen JT: Anxiety, depression and swallowing disorders in patients with Parkinson's disease. Parkinsonism Relat Disord. 15:453–456. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Plowman-Prine EK, Sapienza CM, Okun MS, Pollock SL, Jacobson C, Wu SS and Rosenbek JC: The relationship between quality of life and swallowing in Parkinson's disease. Mov Disord. 24:1352–1358. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Suttrup I and Warnecke T: Dysphagia in Parkinson's disease. Dysphagia. 31:24–32. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Leite Silva ABR, Gonçalves de Oliveira RW, Diógenes GP, de Castro Aguiar MF, Sallem CC, Lima MPP, de Albuquerque Filho LB, Peixoto de Medeiros SD, Penido de Mendonça LL, de Santiago Filho PC, et al: Premotor, nonmotor and motor symptoms of Parkinson's disease: A new clinical state of the art. Ageing Res Rev. 84(101834)2023.PubMed/NCBI View Article : Google Scholar | |
|
Soliman H, Coffin B and Gourcerol G: Gastroparesis in Parkinson disease: Pathophysiology, and clinical management. Brain Sci. 11(831)2021.PubMed/NCBI View Article : Google Scholar | |
|
Ozawa T, Saji E, Yajima R, Onodera O and Nishizawa M: Reduced bowel sounds in Parkinson's disease and multiple system atrophy patients. Clin Auton Res. 21:181–184. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Heetun ZS and Quigley EMM: Gastroparesis and Parkinson's disease: A systematic review. Parkinsonism Relat Disord. 18:433–440. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Trahair LG, Kimber TE, Flabouris K, Horowitz M and Jones KL: Gastric emptying, postprandial blood pressure, glycaemia and splanchnic flow in Parkinson's disease. World J Gastroenterol. 22:4860–4867. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Lubomski M, Davis RL and Sue CM: Gastrointestinal dysfunction in Parkinson's disease. J Neurol. 267:1377–1388. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, Vorisek V and Kopacova M: Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 16:2978–2990. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Li X, Feng X and Jiang Z and Jiang Z: Association of small intestinal bacterial overgrowth with Parkinson's disease: A systematic review and meta-analysis. Gut Pathog. 13(25)2021.PubMed/NCBI View Article : Google Scholar | |
|
Niu XL, Liu L, Song ZX, Li Q, Wang ZH, Zhang JL and Li HH: Prevalence of small intestinal bacterial overgrowth in Chinese patients with Parkinson's disease. J Neural Transm (Vienna). 123:1381–1386. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Dutkiewicz J, Szlufik S, Nieciecki M, Charzyńska I, Królicki L, Smektała P and Friedman A: Small intestine dysfunction in Parkinson's disease. J Neural Transm (Vienna). 122:1659–1661. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, Gasser T, Goetz CG, Halliday G, Joseph L, et al: MDS research criteria for prodromal Parkinson's disease. Mov Disord. 30:1600–1611. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Picillo M, Palladino R, Erro R, Alfano R, Colosimo C, Marconi R, Antonini A and Barone P: PRIAMO study group. The PRIAMO study: Age- and sex-related relationship between prodromal constipation and disease phenotype in early Parkinson's disease. J Neurol. 268:448–454. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Yao L, Liang W, Chen J, Wang Q and Huang X: Constipation in Parkinson's disease: A systematic review and Meta-analysis. Eur Neurol. 86:34–44. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Leta V, Urso D, Batzu L, Weintraub D, Titova N, Aarsland D, Martinez-Martin P, Borghammer P, van Wamelen DJ, Yousaf T, et al: Constipation is associated with development of cognitive impairment in de novo Parkinson's disease: A longitudinal analysis of two international cohorts. J Parkinsons Dis. 11:1209–1219. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Pilipovich AA, Vorob'eva OV, Makarov SA and Kuchuk AV: Lower gastrointestinal dysfunction in patients with Parkinson's disease. Zh Nevrol Psikhiatr Im S S Korsakova. 123:42–49. 2023.PubMed/NCBI View Article : Google Scholar : (In Russian). | |
|
Ramu SK, Oblizajek NR, Savica R, Chunawala ZS, Deb B and Bharucha AE: Defecatory disorders are a common cause of chronic constipation in Parkinson disease. Neurogastroenterol Motil. 36(e14767)2024.PubMed/NCBI View Article : Google Scholar | |
|
Zhou W, Triadafilopoulos G, Gurland B, Halawi H, Becker L, Garcia P, Nguyen L, Miglis M, Muppidi S, Sinn DI, et al: Differential findings on anorectal manometry in patients with Parkinson's disease and defecatory dysfunction. Mov Disord Clin Pract. 10:1074–1081. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Krogh K, Ostergaard K, Sabroe S and Laurberg S: Clinical aspects of bowel symptoms in Parkinson's disease. Acta Neurol Scand. 117:60–64. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Xu D, Han S, Wang J and Feng J: Relationship between lower urinary tract dysfunction and clinical features in Chinese Parkinson's disease patients. Parkinsons Dis. 2019(6820937)2019.PubMed/NCBI View Article : Google Scholar | |
|
Haktanır D and Yılmaz S: Sexual Dysfunction and related factors in patients with Parkinson's disease. J Psychosoc Nurs Ment Health Serv. 61:45–55. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Brusa L, Rocchi C, Ponzo V, Stanzione P, Finazzi E and Attanasio A: Is there a correlation between urological and cardiovascular dysfunction in Parkinson's disease? Funct Neurol. 33:225–228. 2018.PubMed/NCBI | |
|
Özcan T, Benli E, Özer F, Demir EY, Kaya Y and Ayyıldız A: The association between symptoms of sexual dysfunction and age at onset in Parkinson's disease. Clin Auton Res. 26:205–209. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Shalash A, Hamid E, Elrassas H, Abushouk AI and Salem HH: Sexual dysfunction in male patients with Parkinson's disease: Related factors and impact on quality of life. Neurol Sci. 41:2201–2206. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Kinateder T, Marinho D, Gruber D, Hatzler L, Ebersbach G and Gandor F: Sexual Dysfunctions in Parkinson's disease and their influence on Partnership-data of the PRISM Study. Brain Sci. 12(159)2022.PubMed/NCBI View Article : Google Scholar | |
|
Lee YH, Lee JE, Ryu DW, Oh YS, Lee KS, Hong SH and Kim JS: Urinary dysfunctions and Post-void residual urine in typical and atypical Parkinson diseases. J Parkinsons Dis. 8:145–152. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Vichayanrat E, Hentzen C, Batla A, Simeoni S, Iodice V and Panicker JN: Lower urinary tract dysfunction in Parkinsonian syndromes. Neurol Sci. 42:4045–4054. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Pilipovich AA, Vorob'eva OV and Makarov SA: Bladder dysfunction in patients with stages I-III of Parkinson's disease. Zh Nevrol Psikhiatr Im S S Korsakova. 124:91–99. 2024.PubMed/NCBI View Article : Google Scholar : (In Russian). | |
|
Hogg E, Frank S, Oft J, Benway B, Rashid MH and Lahiri S: Urinary tract infection in Parkinson's Disease. J Parkinsons Dis. 12:743–757. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Valentino F, Bartolotta TV, Cosentino G, Mastrilli S, Arnao V, Aridon P, Scurria S, Pavone A, Pavone C and D'Amelio M: Urological dysfunctions in patients with Parkinson's disease: Clues from clinical and non-invasive urological assessment. BMC Neurol. 18(148)2018.PubMed/NCBI View Article : Google Scholar | |
|
Galloway NT: Urethral sphincter abnormalities in Parkinsonism. Br J Urol. 55:691–693. 1983.PubMed/NCBI View Article : Google Scholar | |
|
Jost WH, Merkle W and Müller-Lobeck H: Urethrismus accounting for voiding disorder. Urology. 52(352)1998.PubMed/NCBI View Article : Google Scholar | |
|
Campos-Sousa RN, Quagliato EMAB, Almeida KJ, Castro IA and Campelo V: Urinary dysfunction with detrusor hyperactivity in women with Parkinson's disease cannot be blamed as a factor of worsening motor performance. Arq Neuropsiquiatr. 71:591–595. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Bhattacharyya KB and Rosa-Grilo M: Sexual dysfunctions in Parkinson's disease: An underrated problem in a much discussed disorder. Int Rev Neurobiol. 134:859–876. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Yu M, Roane DM, Miner CR, Fleming M and Rogers JD: Dimensions of sexual dysfunction in Parkinson disease. Am J Geriatr Psychiatry. 12:221–226. 2004.PubMed/NCBI | |
|
Kummer A, Cardoso F and Teixeira AL: Loss of libido in Parkinson's disease. J Sex Med. 6:1024–1031. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Varlıbaş H, Erdoğan HA, Acir I and Yayla V: Relationship between autonomic dysfunction and sexual dysfunctions in Parkinson's patients. Front Aging Neurosci. 17(1562003)2025.PubMed/NCBI View Article : Google Scholar | |
|
Manuli A, Maggio MG, De Pasquale P, Raciti L, Filoni S, Portaro S, Pucciarelli G and Calabrò RS: Determinants of sexual dysfunction in Parkinson's disease patients: A secondary analysis of a multicenter cross-sectional study. J Clin Med. 14(152)2024.PubMed/NCBI View Article : Google Scholar | |
|
Ng YF, Chen CYT, Chia GTH, Tan BBJW, Chan LL and Tan EK: The association between Parkinson's disease and Sexual dysfunction: Clinical correlation and therapeutic implications. Ageing Res Rev. 79(101665)2022.PubMed/NCBI View Article : Google Scholar | |
|
Bronner G and Vodušek DB: Management of sexual dysfunction in Parkinson's disease. Ther Adv Neurol Disord. 4:375–383. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Stewart CB, Ledingham D, Foster VK, Anderson KN, Sathyanarayana S, Galley D, Pavese N and Pasquini J: The longitudinal progression of autonomic dysfunction in Parkinson's disease: A 7-year study. Front Neurol. 14(1155669)2023.PubMed/NCBI View Article : Google Scholar | |
|
Deraz HADA, Amer HAH, Suleiman MR and Dahshan A: Sexual dysfunction in a sample of Egyptian patients with Parkinson's disease. Neurol Sci. 45:1071–1077. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Facelle TM, Sadeghi-Nejad H and Goldmeier D: Persistent genital arousal disorder: Characterization, etiology, and management. J Sex Med. 10:439–450. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Ford B, Louis ED, Greene P and Fahn S: Oral and genital pain syndromes in Parkinson's disease. Mov Disord. 11:421–426. 1996.PubMed/NCBI View Article : Google Scholar | |
|
Aquino CC, Mestre T and Lang AE: Restless genital syndrome in Parkinson disease. JAMA Neurol. 71:1559–1561. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Gowers WR: A Manual of diseases of the nervous system. J. & A. Churchill. 1893. | |
|
Langston JW and Forno LS: The hypothalamus in Parkinson disease. Ann Neurol. 3:129–133. 1978.PubMed/NCBI View Article : Google Scholar | |
|
Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH and Braak E: Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 24:197–211. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Dabby R, Djaldetti R, Shahmurov M, Treves TA, Gabai B, Melamed E, Sadeh M and Avinoach I: Skin biopsy for assessment of autonomic denervation in Parkinson's disease. J Neural Transm (Vienna). 113:1169–1176. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Nolano M, Provitera V, Estraneo A, Selim MM, Caporaso G, Stancanelli A, Saltalamacchia AM, Lanzillo B and Santoro L: Sensory deficit in Parkinson's disease: Evidence of a cutaneous denervation. Brain. 131:1903–1911. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Toth C, Breithaupt K, Ge S, Duan Y, Terris JM, Thiessen A, Wiebe S, Zochodne DW and Suchowersky O: Levodopa, methylmalonic acid, and neuropathy in idiopathic Parkinson disease. Ann Neurol. 68:28–36. 2010.PubMed/NCBI View Article : Google Scholar | |
|
De Marinis M, Argenta G, Mele D, Carbone A, Baffigo G and Agnoli A: Evaluation of vesico-urethral and sweating function in disorders presenting with Parkinsonism. Clin Auton Res. 3:125–130. 1993.PubMed/NCBI View Article : Google Scholar | |
|
Haapaniemi TH, Korpelainen JT, Tolonen U, Suominen K, Sotaniemi KA and Myllylä VV: Suppressed sympathetic skin response in Parkinson disease. Clin Auton Res. 10:337–342. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Ishida G, Nakashima K and Takahashi K: Skin nerve sympathetic activity reflex latency in Parkinson's disease. Acta Neurol Scand. 81:121–124. 1990.PubMed/NCBI View Article : Google Scholar | |
|
Shindo K, Iida H, Watanabe H, Ohta E, Nagasaka T and Shiozawa Z: Sympathetic sudomotor and vasoconstrictive neural function in patients with Parkinson's disease. Parkinsonism Relat Disord. 14:548–552. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Siepmann T, Frenz E, Penzlin AI, Goelz S, Zago W, Friehs I, Kubasch ML, Wienecke M, Löhle M, Schrempf W, et al: Pilomotor function is impaired in patients with Parkinson's disease: A study of the adrenergic axon-reflex response and autonomic functions. Parkinsonism Relat Disord. 31:129–134. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Wolf JP, Bouhaddi M, Louisy F, Mikehiev A, Mourot L, Cappelle S, Vuillier F, Andre P, Rumbach L and Regnard J: Side-effects of L-dopa on venous tone in Parkinson's disease: A Leg-weighing assessment. Clin Sci (Lond). 110:369–377. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Mano Y, Nakamuro T, Takayanagi T and Mayer RF: Sweat function in Parkinson's disease. J Neurol. 241:573–576. 1994.PubMed/NCBI View Article : Google Scholar | |
|
Appenzeller O and Goss JE: Autonomic deficits in Parkinson's syndrome. Arch Neurol. 24:50–57. 1971.PubMed/NCBI View Article : Google Scholar | |
|
Sage JI and Mark MH: Drenching sweats as an off phenomenon in Parkinson's disease: Treatment and relation to plasma levodopa profile. Ann Neurol. 37:120–122. 1995.PubMed/NCBI View Article : Google Scholar | |
|
Gubbay SS and Barwick DD: Two cases of accidental hypothermia in Parkinson's disease with unusual E.E.G. findings. J Neurol Neurosurg Psychiatry. 29:459–466. 1966.PubMed/NCBI View Article : Google Scholar | |
|
Hashimoto T, Tokuda T, Hanyu N, Tabata K and Yanagisawa N: Withdrawal of levodopa and other risk factors for malignant syndrome in Parkinson's disease. Parkinsonism Relat Disord. 9 (Suppl 1):S25–S30. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Saito H: Sudomotor deficits in Parkinson's disease with special reference to motor subtypes. Parkinsonism Relat Disord. 114(105489)2023.PubMed/NCBI View Article : Google Scholar | |
|
Hirayama M: Sweating dysfunctions in Parkinson's disease. J Neurol. 253 (Suppl 7):VII42–VII47. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Siepmann T, Arndt M, Sedghi A, Szatmár S Jr, Horváth T, Takáts A, Bereczki D, Moskopp ML, Buchmann S, Skowronek C, et al: Two-Year observational study of autonomic skin function in patients with Parkinson's disease compared to healthy individuals. Eur J Neurol. 30:1281–1292. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Asahina M, Mathias CJ, Katagiri A, Low DA, Vichayanrat E, Fujinuma Y, Yamanaka Y and Kuwabara S: Sudomotor and cardiovascular dysfunction in patients with early untreated Parkinson's disease. J Parkinsons Dis. 4:385–393. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Xu X, Liao J, Dong Q, Qin F, Li J, Sun X, Lu T, Fang L, Peng F, Lu Z and Qiu W: Clinical utility of SUDOSCAN in predicting autonomic neuropathy in patients with Parkinson's disease. Parkinsonism Relat Disord. 64:60–65. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Tomic S, Kuric I, Kuric TG, Popovic Z, Kragujevic J, Zubonja TM, Rajkovaca I and Matosa S: Seborrheic dermatitis is related to motor symptoms in Parkinson's disease. J Clin Neurol. 18:628–634. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Anbalagan B, Karnam Anantha S, Arjunan SP, Balasubramanian V, Murugesan M and R K: A Non-invasive IR sensor technique to differentiate Parkinson's disease from other neurological disorders using autonomic dysfunction as diagnostic criterion. Sensors (Basel). 22(266)2021.PubMed/NCBI View Article : Google Scholar | |
|
Purup MM, Knudsen K, Karlsson P, Terkelsen AJ and Borghammer P: Skin temperature in Parkinson's disease measured by infrared thermography. Parkinsons Dis. 2020(2349469)2020.PubMed/NCBI View Article : Google Scholar | |
|
Rocchi C, Cerroni R, Conti M, Lauretti B, Mercuri NB, Stefani A and Pierantozzi M: Sudomotor and cardiovascular autonomic function in de novo Parkinson's disease assessed by sudoscan and cardiovascular reflexes. J Neurol Sci. 427(117502)2021.PubMed/NCBI View Article : Google Scholar | |
|
Roy S, Srivastava AK, Jaryal AK and Deepak KK: Cardiovascular responses during cold pressor test are different in Parkinson disease and multiple system atrophy with Parkinsonism. Clin Auton Res. 25:219–224. 2015.PubMed/NCBI View Article : Google Scholar | |
|
De Marinis M, Stocchi F, Testa SR, De Pandis F and Agnoli A: Alterations of thermoregulation in Parkinson's disease. Funct Neurol. 6:279–283. 1991.PubMed/NCBI | |
|
Dutkiewicz J, Szlufik S and Friedman A: ‘New methods of assessing autonomic disorders in Parkinson disease patients: Skin-galvanic reaction’. J Neural Transm (Vienna). 126:1421–1424. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Schestatsky P, Valls-Solé J, Ehlers JA, Rieder CRM and Gomes I: Hyperhidrosis in Parkinson's disease. Mov Disord. 21:1744–1748. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Yoshioka M, Oka H, Morita M and Inoue K: Sudomotor dysfunction in Parkinson's disease. Rinsho Shinkeigaku. 43:379–384. 2003.PubMed/NCBI(In Japanese). | |
|
Lemke MR: Effect of reboxetine on depression in Parkinson's disease patients. J Clin Psychiatry. 63:300–304. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Lipp A, Sandroni P, Ahlskog JE, Fealey RD, Kimpinski K, Iodice V, Gehrking TL, Weigand SD, Sletten DM, Gehrking JA, et al: Prospective differentiation of multiple system atrophy from Parkinson disease, with and without autonomic failure. Arch Neurol. 66:742–750. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Kimpinski K, Iodice V, Sandroni P, Fealey RD, Vernino S and Low PA: Sudomotor dysfunction in autoimmune autonomic ganglionopathy: A follow-up study. Clin Auton Res. 22:131–136. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Coon EA and Low PA: Thermoregulation in Parkinson disease. Handb Clin Neurol. 157:715–725. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Magalhães M, Wenning GK, Daniel SE and Quinn NP: Autonomic dysfunction in pathologically confirmed multiple system atrophy and idiopathic Parkinson's disease-a retrospective comparison. Acta Neurol Scand. 91:98–102. 1995.PubMed/NCBI View Article : Google Scholar | |
|
Meigal A and Lupandin Y: ‘Thermoregulation-dependent component’ in pathophysiology of motor disorders in Parkinson's disease? Pathophysiology. 11:187–196. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Hama K, Miwa H and Kondo T: Life-threatening hypothermia in Parkinson's disease. Mov Disord. 24:945–946. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Sehara Y: Hypothermia with Osborn waves in Parkinson's disease. Intern Med. 48:615–618. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Li BY and Lou M: Severe hypothermia in Parkinson's disease. CNS Neurosci Ther. 18:864–866. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Muszkat M, Durst RM and Ben-Yehuda A: Factors associated with mortality among elderly patients with hypothermia. Am J Med. 113:234–237. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Newman EJ, Grosset DG and Kennedy PGE: The parkinsonism-hyperpyrexia syndrome. Neurocrit Care. 10:136–140. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Gaig C, Martí MJ, Tolosa E, Gómez-Choco MJ and Amaro S: Parkinsonism-hyperpyrexia syndrome not related to antiparkinsonian treatment withdrawal during the 2003 summer heat wave. J Neurol. 252:1116–1119. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Douglas A and Morris J: It was not just a heatwave! Neuroleptic malignant-like syndrome in a patient with Parkinson's disease. Age Ageing. 35:640–641. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Kitagawa T, Sengoku R, Ozawa M, Matsuno H, Umehara T, Nakahara A and Oka H: Association between sebum secretion and cardiac sympathetic dysfunction in Parkinson's disease. Parkinsonism Relat Disord. 129(107178)2024.PubMed/NCBI View Article : Google Scholar | |
|
Ozawa M, Morishima R, Shimizu T and Takahashi K: Correlation with sympathetic skin response, 123I-MIBG scintigraphy, and 123I-FP-CIT SPECT in Parkinson's disease. Neurophysiol Clin. 54(102956)2024.PubMed/NCBI View Article : Google Scholar | |
|
De Marinis M, Stocchi F, Gregori B and Accornero N: Sympathetic skin response and cardiovascular autonomic function tests in Parkinson's disease and multiple system atrophy with autonomic failure. Mov Disord. 15:1215–1220. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Jost WH, Kirchhöfer U, Houy S and Schimrigk K: Sympathetic skin response in Parkinson syndrome. Nervenarzt. 66:777–780. 1995.PubMed/NCBI(In German). | |
|
Maetzler W, Karam M, Berger MF, Heger T, Maetzler C, Ruediger H, Bronzova J, Lobo PP, Ferreira JJ, Ziemssen T and Berg D: Time- and frequency-domain parameters of heart rate variability and sympathetic skin response in Parkinson's disease. J Neural Transm (Vienna). 122:419–425. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Braune HJ, Korchounov AM and Schipper HI: Autonomic dysfunction in Parkinson's disease assessed by sympathetic skin response: A prospective clinical and neurophysiological trial on 50 patients. Acta Neurol Scand. 95:293–297. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Andersen EB and Boesen F: Sympathetic vasoconstrictor reflexes in Parkinson's disease with autonomic dysfunction. Clin Auton Res. 7:5–11. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Giza E, Katsarou Z, Georgiadis G and Bostantjopoulou S: Sympathetic skin response in Parkinson's disease before and after mental stress. Neurophysiol Clin. 42:125–131. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Chu NS, Huang CC and Calne DB: Sympathetic skin response and RR interval variation in manganism and a comparison with Parkinson's disease. Parkinsonism Relat Disord. 2:23–28. 1996.PubMed/NCBI View Article : Google Scholar | |
|
Kataoka H and Ueno S: Severe cold lower limbs in patients with Parkinson's disease During the Summer. Neurol Int. 8(6676)2016.PubMed/NCBI View Article : Google Scholar | |
|
Antonio-Rubio I, Madrid-Navarro CJ, Salazar-López E, Pérez-Navarro MJ, Sáez-Zea C, Gómez-Milán E, Mínguez-Castellanos A and Escamilla-Sevilla F: Abnormal thermography in Parkinson's disease. Parkinsonism Relat Disord. 21:852–857. 2015.PubMed/NCBI View Article : Google Scholar | |
|
de Araújo DF, de Melo Neto AP, Oliveira ÍSC, Brito BS, de Araújo IT, Barros IS, Lima JW, Horta WG and Gondim Fde A: Small (autonomic) and large fiber neuropathy in Parkinson disease and parkinsonism. BMC Neurol. 16(139)2016.PubMed/NCBI View Article : Google Scholar | |
|
Djaldetti R, Melamed E and Gadoth N: Abnormal skin wrinkling in the less affected side in hemiparkinsonism-a possible test for sympathetic dysfunction in Parkinson's disease. Biomed Pharmacother. 55:475–478. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Habibi M, Coe BC, Brien DC, Huang J, Riek HC, Bremmer F, Timmermann L, Janzen A, Oertel WH and Munoz DP: Saccade, pupil, and blink abnormalities in prodromal and manifest alpha-synucleinopathies. J Parkinsons Dis. 15:300–310. 2025.PubMed/NCBI View Article : Google Scholar | |
|
Tsitsi P, Nilsson M, Waldthaler J, Öqvist Seimyr G, Larsson O, Svenningsson P and Markaki I: Pupil light reflex dynamics in Parkinson's disease. Front Integr Neurosci. 17(1249554)2023.PubMed/NCBI View Article : Google Scholar | |
|
Manohar SG and Husain M: Reduced pupillary reward sensitivity in Parkinson's disease. NPJ Parkinsons Dis. 1(15026)2015.PubMed/NCBI View Article : Google Scholar | |
|
Dietz J, Bradley MM, Okun MS and Bowers D: Emotion and ocular responses in Parkinson's disease. Neuropsychologia. 49:3247–3253. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Hori N, Takamori M, Hirayama M, Watanabe H, Nakamura T, Yamashita F, Ito H, Mabuchi N and Sobue G: Pupillary supersensitivity and visual disturbance in Parkinson's disease. Clin Auton Res. 18:20–27. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Jain S, Siegle GJ, Gu C, Moore CG, Ivanco LS, Studenski S, Greenamyre JT and Steinhauer SR: Pupillary unrest correlates with arousal symptoms and motor signs in Parkinson disease. Mov Disord. 26:1344–1347. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Yamashita F, Hirayama M, Nakamura T, Takamori M, Hori N, Uchida K, Hama T and Sobue G: Pupillary autonomic dysfunction in multiple system atrophy and Parkinson's disease: An assessment by eye-drop tests. Clin Auton Res. 20:191–197. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Tamer C, Melek IM, Duman T and Oksüz H: Tear film tests in Parkinson's disease patients. Ophthalmology. 112(1795)2005.PubMed/NCBI View Article : Google Scholar | |
|
Bagheri H, Berlan M, Senard JM, Rascol O and Montastruc JL: Lacrimation in Parkinson's disease. Clin Neuropharmacol. 17:89–91. 1994.PubMed/NCBI | |
|
Kwon OY, Kim SH, Kim JH, Kim MH and Ko MK: Schrimer test in Parkinson's disease. J Korean Med Sci. 9:239–242. 1994.PubMed/NCBI View Article : Google Scholar | |
|
Giza E, Fotiou D, Bostantjopoulou S, Katsarou Z and Karlovasitou A: Pupil light reflex in Parkinson's disease: Evaluation with pupillometry. Int J Neurosci. 121:37–43. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Che NN, Chen S, Jiang QH, Chen SY, Zhao ZX, Li X, Malik RA, Ma JJ and Yang HQ: Corneal confocal microscopy differentiates patients with Parkinson's disease with and without autonomic involvement. NPJ Parkinsons Dis. 8(114)2022.PubMed/NCBI View Article : Google Scholar | |
|
Suzuki K, Miyamoto M, Miyamoto T and Hirata K: Parkinson's disease and sleep/wake disturbances. Curr Neurol Neurosci Rep. 15(8)2015.PubMed/NCBI View Article : Google Scholar | |
|
Jain S, Park SY and Comer D: Patterns of motor and Non-motor features in Medication-Naïve Parkinsonism. Neuroepidemiology. 45:59–69. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Suzuki K, Miyamoto M, Miyamoto T and Hirata K: Sleep and autonomic function: Sleep related breathing disorders in Parkinson's disease and related disorders. Rinsho Shinkeigaku. 54:1041–1043. 2014.PubMed/NCBI View Article : Google Scholar : (In Japanese). | |
|
Apps MC, Sheaff PC, Ingram DA, Kennard C and Empey DW: Respiration and sleep in Parkinson's disease. J Neurol Neurosurg Psychiatry. 48:1240–1245. 1985.PubMed/NCBI View Article : Google Scholar | |
|
Laihinen A, Alihanka J, Raitasuo S and Rinne UK: Sleep movements and associated autonomic nervous activities in patients with Parkinson's disease. Acta Neurol Scand. 76:64–68. 1987.PubMed/NCBI View Article : Google Scholar | |
|
Liu Y, Xue L, Zhao J, Dou K, Wang G and Xie A: Clinical characteristics in early Parkinson's disease with excessive daytime sleepiness: A cross-sectional and longitudinal study. Clin Transl Sci. 16:2033–2045. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Ashraf-Ganjouei A, Moradi K, Aarabi M, Abdolalizadeh A, Kazemi SZ, Kasaeian A and Vahabi Z: The Association between REM Sleep behavior disorder and autonomic dysfunction in Parkinson's disease. J Parkinsons Dis. 11:747–755. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhu K, van Hilten JJ and Marinus J: Course and risk factors for excessive daytime sleepiness in Parkinson's disease. Parkinsonism Relat Disord. 24:34–40. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Wüllner U, Schmitz-Hübsch T, Antony G, Fimmers R, Spottke A, Oertel WH, Deuschl G, Klockgether T and Eggert K: KNP e.V. Autonomic dysfunction in 3414 Parkinson's disease patients enrolled in the German Network on Parkinson's disease (KNP e.V.): The effect of ageing. Eur J Neurol. 14:1405–1408. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Palma JA, Urrestarazu E, Alegre M, Pastor MA, Valencia M, Artieda J and Iriarte J: Cardiac autonomic impairment during sleep is linked with disease severity in Parkinson's disease. Clin Neurophysiol. 124:1163–1168. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Schaller S, Anderer P, Dorffner G, Klösch G, Machatschke IH, Schlögl A and Zeitlhofer J: Autonomic dysfunction in PD during sleep. Mov Disord. 27(454)2012.PubMed/NCBI View Article : Google Scholar | |
|
Bjørnarå KA, Dietrichs E and Toft M: Clinical features associated with sleep disturbances in Parkinson's disease. Clin Neurol Neurosurg. 124:37–43. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Ferini-Strambi L, Oertel W, Dauvilliers Y, Postuma RB, Marelli S, Iranzo A, Arnulf I, Högl B, Manni R, Miyamoto T, et al: Autonomic symptoms in idiopathic REM behavior disorder: A multicentre case-control study. J Neurol. 261:1112–1118. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Eckhardt C, Fanciulli A, Högl B, Heidbreder A, Eschlböck S, Raccagni C, Krismer F, Leys F, Kiechl S, Ransmayr G, et al: Analysis of sleep, daytime sleepiness, and autonomic function in multiple system atrophy and Parkinson disease: A prospective study. J Clin Sleep Med. 19:63–71. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Fujita H, Shiina T, Sakuramoto H, Nozawa N, Ogaki K and Suzuki K: Sleep and autonomic manifestations in Parkinson's disease complicated with probable rapid eye movement sleep behavior disorder. Front Neurosci. 16(874349)2022.PubMed/NCBI View Article : Google Scholar | |
|
Matsubara T, Suzuki K, Fujita H, Watanabe Y, Sakuramoto H, Matsubara M and Hirata K: Autonomic symptoms correlate with Non-autonomic non-motor symptoms and sleep problems in patients with Parkinson's disease. Eur Neurol. 80:193–199. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Huang Y, Du S, Chen D, Qin Y, Cui J, Han H, Ge X, Bai W, Zhang X and Yu H: The path linking excessive daytime sleepiness and activity of daily living in Parkinson's disease: The longitudinal mediation effect of autonomic dysfunction. Neurol Sci. 43:4777–4784. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Mahajan A, Duque KR, Dwivedi AK, Abanto J, Marsili L, Hill EJ, Saraf A, McDonald KJ, Arowosegbe A, Deraz HA, et al: Exploring the intersection between orthostatic hypotension and daytime sleepiness in Parkinson's disease. J Neurol Sci. 468(123366)2025.PubMed/NCBI View Article : Google Scholar | |
|
Cho Y, Levendowski DJ, Walsh CM, Tsuang D, Lee-Iannotti JK, Berka C, Mazeika G, Salat D, Hamilton JM, Boeve BF, et al: Autonomic dysregulation during sleep in Parkinsonian spectrum disorders-A proof of concept. Parkinsonism Relat Disord. 117(105905)2023.PubMed/NCBI View Article : Google Scholar | |
|
Al-Qassabi A, Pelletier A, Fereshtehnejad S-M and Postuma RB: Autonomic sweat responses in REM Sleep Behavior disorder and Parkinsonism. J Parkinsons Dis. 8:463–468. 2018.PubMed/NCBI View Article : Google Scholar | |
|
The World Health Organization quality of life assessment (WHOQOL): Position paper from the World Health Organization. Soc Sci Med. 41:1403–1409. 1995.PubMed/NCBI View Article : Google Scholar | |
|
Tomic S, Rajkovaca I, Pekic V, Salha T and Misevic S: Impact of autonomic dysfunctions on the quality of life in Parkinson's disease patients. Acta Neurol Belg. 117:207–211. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Gallagher DA, Lees AJ and Schrag A: What are the most important nonmotor symptoms in patients with Parkinson's disease and are we missing them? Mov Disord. 25:2493–2500. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Qin Y, Meng DT, Jin ZH, Du WJ and Fang BY: Association between autonomic dysfunction with motor and non-motor symptoms in patients with Parkinson's disease. J Neural Transm (Vienna). 131:323–334. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Ogaki K, Fujita H, Nozawa N, Shiina T, Sakuramoto H and Suzuki K: Factors contributing to sleep disturbances and excessive daytime sleepiness in patients with Parkinson's disease. Front Neurol. 14(1097251)2023.PubMed/NCBI View Article : Google Scholar | |
|
Pitton Rissardo J, Jayasinghe M, Rashidi M, Rashidi M, Rashidi F, Moharam H, Khalil I, Dway A, Elhassan WA, Elbadawi MH, et al: Exploring fatigue in Parkinson's disease: A comprehensive literature review. Cureus. 17(e81129)2025.PubMed/NCBI View Article : Google Scholar | |
|
Sklerov M, Browner N, Dayan E, Rubinow D and Frohlich F: Autonomic and depression symptoms in Parkinson's disease: Clinical evidence for overlapping physiology. J Parkinsons Dis. 12:1059–1067. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Sakakibara R, Ogata T, Aiba Y, Tateno F, Uchiyama T and Yamamoto T: Does depression contribute to the bladder and bowel complaint in Parkinson's disease Patients? Mov Disord Clin Pract. 8:240–244. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Park HE, Kim JS, Oh YS, Park IS, Park JW, Song IU and Lee KS: Autonomic nervous system dysfunction in patients with Parkinson disease having depression. J Geriatr Psychiatry Neurol. 29:11–17. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Cui J, Qin Y, Tian Y, Ge X, Han H, Yang Z and Yu H: Activities of daily living as a longitudinal moderator of the effect of autonomic dysfunction on anxiety and depression of Parkinson's patients. Brain Behav. 11(e2297)2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhao Z, Liu H, Xue J, Shi Z and You N: Association between subjective autonomic dysfunction and fatigue in Parkinson's disease in southern Chinese. Neurol Sci. 42:2951–2954. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Olivola E, Brusa L, Rocchi C, Schillaci O, Liguori C, Cerroni R, Pierantozzi M, Chiaravalloti A, Stefani A and Stocchi F: Does fatigue in Parkinson's disease correlate with autonomic nervous system dysfunction? Neurol Sci. 39:2169–2174. 2018.PubMed/NCBI View Article : Google Scholar | |
|
van Uem JMT, Marinus J, Canning C, van Lummel R, Dodel R, Liepelt-Scarfone I, Berg D, Morris ME and Maetzler W: Health-related quality of life in patients with Parkinson's disease-A systematic review based on the ICF model. Neurosci Biobehav Rev. 61:26–34. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Prakash KM, Nadkarni NV, Lye WK, Yong MH and Tan EK: The impact of non-motor symptoms on the quality of life of Parkinson's disease patients: A longitudinal study. Eur J Neurol. 23:854–860. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Pilipovich AA, Vorob'eva OV, Makarov SA, Shindryaeva NN and Vorob'eva YD: Gastrointestinal dysfunction impact on life quality in a cohort of Russian patients with Parkinson's disease I-III H&Y Stage. Parkinsons Dis. 2022(1571801)2022.PubMed/NCBI View Article : Google Scholar | |
|
Magerkurth C, Schnitzer R and Braune S: Symptoms of autonomic failure in Parkinson's disease: Prevalence and impact on daily life. Clin Auton Res. 15:76–82. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Kuhlman GD, Flanigan JL, Sperling SA and Barrett MJ: Predictors of Health-related quality of life in Parkinson's disease. Parkinsonism Relat Disord. 65:86–90. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, Bottacchi E, Cannas A, Ceravolo G, Ceravolo R, et al: The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov Disord. 24:1641–1649. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Martínez-Martín P, Benito-León J, Alonso F, Catalán MJ, Pondal M, Zamarbide I, Tobías A and de Pedro J: Quality of life of caregivers in Parkinson's disease. Qual Life Res. 14:463–472. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Abendroth M: Development and initial validation of a Parkinson's disease caregiver strain risk screen. J Nurs Meas. 23:4–21. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Ledda C, Montanaro E, Imbalzano G, Merola A, Bruno I, Artusi CA, Zibetti M, Rizzone MG, Bozzali M, Sobrero G, et al: Burden of caregiving for cardiovascular dysautonomia in Parkinson's disease. Clin Auton Res. 32:455–461. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Grün D, Pieri V, Vaillant M and Diederich NJ: Contributory factors to caregiver burden in Parkinson disease. J Am Med Dir Assoc. 17:626–632. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Keränen P, Martikainen K, Keränen T and Marttila R: Parkinson's disease patients in institutionalized care. European Geriatric Medicine. 4:376–379. 2013. | |
|
Shulman LM, Taback RL, Bean J and Weiner WJ: Comorbidity of the nonmotor symptoms of Parkinson's disease. Mov Disord. 16:507–510. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Elmer T, Geschwind N, Peeters F, Wichers M and Bringmann L: Getting stuck in social isolation: Solitude inertia and depressive symptoms. J Abnorm Psychol. 129:713–723. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Zhu X, Gan J, Wu N, Wan Y, Song L, Liu Z and Zhang Y: Assessing impulse control behaviors in early Parkinson's disease: A longitudinal study. Front Neurol. 14(1275170)2023.PubMed/NCBI View Article : Google Scholar | |
|
Li FF, Cui YS, Yan R, Cao SS and Feng T: Prevalence of lower urinary tract symptoms, urinary incontinence and retention in Parkinson's disease: A systematic review and meta-analysis. Front Aging Neurosci. 14(977572)2022.PubMed/NCBI View Article : Google Scholar | |
|
Stickley A, Santini ZI and Koyanagi A: Urinary incontinence, mental health and loneliness among community-dwelling older adults in Ireland. BMC Urol. 17(29)2017.PubMed/NCBI View Article : Google Scholar | |
|
Srivanitchapoom P, Pandey S and Hallett M: Drooling in Parkinson's disease: A review. Parkinsonism Relat Disord. 20:1109–1118. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Evatt ML, Chaudhuri KR, Chou KL, Cubo E, Hinson V, Kompoliti K, Yang C, Poewe W, Rascol O, Sampaio C, et al: Dysautonomia rating scales in Parkinson's disease: Sialorrhea, dysphagia, and constipation-critique and recommendations by movement disorders task force on rating scales for Parkinson's disease. Mov Disord. 24:635–646. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Perez Lloret S, Pirán Arce G, Rossi M, Caivano Nemet ML, Salsamendi P and Merello M: Validation of a new scale for the evaluation of sialorrhea in patients with Parkinson's disease. Mov Disord. 22:107–111. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Camacho M, Greenland JC and Williams-Gray CH: The gastrointestinal dysfunction scale for Parkinson's disease. Mov Disord. 36:2358–2366. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Sakakibara R, Shinotoh H, Uchiyama T, Sakuma M, Kashiwado M, Yoshiyama M and Hattori T: Questionnaire-based assessment of pelvic organ dysfunction in Parkinson's disease. Auton Neurosci. 92:76–85. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Pavy-Le Traon A, Cotterill N, Amarenco G, Duerr S, Kaufmann H, Lahrmann H, Tison F, Wenning GK, Goetz CG, Poewe W, et al: Clinical rating scales for urinary symptoms in Parkinson Disease: Critique and recommendations. Mov Disord Clin Pract. 5:479–491. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Chaudhuri KR, Martinez-Martin P, Brown RG, Sethi K, Stocchi F, Odin P, Ondo W, Abe K, Macphee G, Macmahon D, et al: The metric properties of a novel non-motor symptoms scale for Parkinson's disease: Results from an international pilot study. Mov Disord. 22:1901–1911. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, et al: Movement disorder Society-sponsored revision of the Unified Parkinson's Disease rating scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord. 23:2129–2170. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Jenkinson C, Fitzpatrick R, Peto V, Greenhall R and Hyman N: The Parkinson's disease Questionnaire (PDQ-39): Development and validation of a Parkinson's disease summary index score. Age Ageing. 26:353–357. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Cuenca-Bermejo L, Almela P, Navarro-Zaragoza J, Fernández Villalba E, González-Cuello AM, Laorden ML and Herrero MT: Cardiac changes in Parkinson's disease: Lessons from clinical and experimental evidence. Int J Mol Sci. 22(13488)2021.PubMed/NCBI View Article : Google Scholar | |
|
Baschieri F, Sambati L, Guaraldi P, Barletta G, Cortelli P and Calandra-Buonaura G: Neurogenic orthostatic hypotension in early stage Parkinson's disease: New insights from the first 105 patients of the BoProPark study. Parkinsonism Relat Disord. 93:12–18. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Fanciulli A and Wenning GK: Autonomic failure: A neglected presentation of Parkinson's disease. Lancet Neurol. 20:781–782. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Csoti I, Jost WH and Reichmann H: Parkinson's disease between internal medicine and neurology. J Neural Transm (Vienna). 123:3–17. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Vallelonga F, Romagnolo A, Merola A, Sobrero G, Di Stefano C, Milazzo V, Burrello J, Burrello A, Zibetti M, Milan A, et al: Detection of orthostatic hypotension with ambulatory blood pressure monitoring in parkinson's disease. Hypertens Res. 42:1552–1560. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Vallelonga F, Sobrero G, Giudici M, Valente M, Milazzo V, Di Stefano C and Maule S: Screening indexes for cardiovascular autonomic failure in Parkinson's disease. J Neurol Sci. 428(117571)2021.PubMed/NCBI View Article : Google Scholar | |
|
Yu Z and Li Y: Association of autonomic symptoms with cerebrospinal fluid biomarkers in Parkinson disease and scans without evidence of dopaminergic deficit. Medicine (Baltimore). 100(e24837)2021.PubMed/NCBI View Article : Google Scholar | |
|
Huang CC, Lai YR, Lien CY, Cheng BC, Tsai NW and Lu CH: The role of electrochemical skin conductance as a screening test of cardiovascular autonomic neuropathy in patients with Parkinson's disease. Int J Environ Res Public Health. 17(7751)2020.PubMed/NCBI View Article : Google Scholar | |
|
Panicker JN, Fanciulli A, Skoric MK, Kaplan T, Aleksovska K, Adamec I, Averbeck MA, Campese N, Guaraldi P, Leys F, et al: European academy of neurology (EAN)/European federation of autonomic societies (EFAS)/International Neuro-urology society (INUS) guidelines for practising neurologists on the assessment and treatment of neurogenic urinary and sexual symptoms (NEUROGED guidelines). Eur J Neurol. 32(e70119)2025.PubMed/NCBI View Article : Google Scholar | |
|
Zhang F, Wang F, Li CH, Wang JW, Han CL, Fan SY, Gao DM, Xing YJ, Yang C, Zhang JG and Meng FG: Subthalamic nucleus-deep brain stimulation improves autonomic dysfunctions in Parkinson's disease. BMC Neurol. 22(124)2022.PubMed/NCBI View Article : Google Scholar | |
|
Winge K, Nielsen KK, Stimpel H, Lokkegaard A, Jensen SR and Werdelin L: Lower urinary tract symptoms and bladder control in advanced Parkinson's disease: Effects of deep brain stimulation in the subthalamic nucleus. Mov Disord. 22:220–225. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Pietraszko W, Furgala A, Gorecka-Mazur A, Thor P, Moskala M, Polak J, Surowka AD and Krygowska-Wajs A: Efficacy of deep brain stimulation of the subthalamic nucleus on autonomic dysfunction in patients with Parkinson's disease. Folia Med Cracov. 53:15–22. 2013.PubMed/NCBI | |
|
Cucinotta F, Swinnen B, Makovac E, Hirschbichler S, Pereira E, Little S, Morgante F and Ricciardi L: Short term cardiovascular symptoms improvement after deep brain stimulation in patients with Parkinson's disease: A systematic review. J Neurol. 271:3764–3776. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Yavasoglu NG and Comoglu SS: The effect of subthalamic deep brain stimulation on autonomic dysfunction in Parkinson's disease: Clinical and electrophysiological evaluation. Neurol Res. 43:894–899. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Hyam JA, Roy HA, Huang Y, Martin S, Wang S, Rippey J, Coyne TJ, Stewart I, Kerr G, Silburn P, et al: Cardiovascular autonomic responses in patients with Parkinson disease to pedunculopontine deep brain stimulation. Clin Auton Res. 29:615–624. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Combs HL, Garcia-Willingham NE, Berry DTR, van Horne CG and Segerstrom SC: Psychological functioning in Parkinson's disease post-deep brain stimulation: Self-regulation and executive functioning. J Psychosom Res. 111:42–49. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Rissardo JP, Vora NM, Tariq I, Mujtaba A and Caprara ALF: Deep brain stimulation for the management of refractory neurological disorders: A comprehensive review. Medicina (Kaunas). 59(1991)2023.PubMed/NCBI View Article : Google Scholar | |
|
Basiago A and Binder DK: Effects of deep brain stimulation on autonomic function. Brain Sci. 6(33)2016.PubMed/NCBI View Article : Google Scholar | |
|
Kalhoro A and Hashim ASM: Effectiveness of deep brain stimulation in Parkinson's disease treatment with Single-center experience in Pakistan. Pak J Med Sci. 39:1018–1023. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Bunjo Z, Bacchi S, Chandran AS and Zacest A: Orthostatic hypotension following deep brain stimulation in Prkinson's disease: A systematic review. Br J Neurosurg. 34:587–590. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Romagnolo A, Fabbri M, Merola A, Montanaro E, Palermo S, Martone T, Seresini A, Goldwurm S, Rizzone MG and Lopiano L: Beyond 35 years of Parkinson's disease: A comprehensive clinical and instrumental assessment. J Neurol. 265:1989–1997. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Sumi K, Katayama Y, Otaka T, Obuchi T, Kano T, Kobayashi K, Oshima H, Fukaya C, Yamamoto T, Ogawa Y and Iwasaki K: Effect of subthalamic nucleus deep brain stimulation on the autonomic nervous system in Parkinson's disease patients assessed by spectral analyses of R-R interval variability and blood pressure variability. Stereotact Funct Neurosurg. 90:248–254. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Cani I, Giannini G, Guaraldi P, Barletta G, Sambati L, Baldelli L, Cortelli P and Calandra-Buonaura G: Exploring cardiovascular autonomic function before and after chronic deep brain stimulation in Parkinson's disease. Mov Disord Clin Pract. 11:698–703. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Grażyńska A, Urbaś W, Antoniuk S, Adamczewska K, Bień M, Chmiela T and Siuda J: Comparative analysis of non-motor symptoms in patients with Parkinson's disease and atypical parkinsonisms. Clin Neurol Neurosurg. 197(106088)2020.PubMed/NCBI View Article : Google Scholar | |
|
Joo BE, You J, Kim RO and Kwon KY: Association between cognitive and autonomic dysfunctions in patients with de novo Parkinson's disease. Sci Rep. 15(13535)2025.PubMed/NCBI View Article : Google Scholar | |
|
Allan LM, Ballard CG, Allen J, Murray A, Davidson AW, McKeith IG and Kenny RA: Autonomic dysfunction in dementia. J Neurol Neurosurg Psychiatry. 78:671–677. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Idiaquez J, Benarroch EE, Rosales H, Milla P and Ríos L: Autonomic and cognitive dysfunction in Parkinson's disease. Clin Auton Res. 17:93–98. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Wichmann T, Nelson A, Torres ERS, Svenningsson P and Marongiu R: Leveraging animal models to understand non-motor symptoms of Parkinson's disease. Neurobiol Dis. 208(106848)2025.PubMed/NCBI View Article : Google Scholar |