Differential expression of haematopoietic prostaglandin D synthase by POU2F3‑positive tuft cells in conventional bilayered oncocytic and metaplastic epithelia of Warthin tumours
- Authors:
- Published online on: July 17, 2025 https://doi.org/10.3892/mmr.2025.13624
- Article Number: 259
-
Copyright: © Hosomi et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Warthin tumours (WT), the second most common benign salivary gland neoplasm, are predominantly seen in males and are restricted to the parotid glands (1). This tumour is histopathologically characterised by papillary cystic proliferation of bilayered oncocytic epithelial cells without nuclear atypia, surrounded by various amounts of lymphoid stroma occasionally accompanying the germinal centre (1). WT can induce various epithelial changes (2). The transition from typical bilayered oncocytic epithelial cells to monomorphic bland metaplastic epidermoid epithelial cells composed of several layers, as well as goblet cells, is a common histopathological finding (1,2).
Tuft cells, also referred to as brush cells, are minor chemosensory epithelial cells located on the normal surfaces of the gastrointestinal and respiratory tracts (3); they express taste receptors and are luminal sensors (3,4). Tuft cells play important roles in antibacterial reactions, initiation of immune responses, and tissue repair, depending on their location, by secreting various physiologically active substances, including prostaglandins (PGs) (5,6). POU class 2 homeobox 3 (POU2F3) is a useful marker for identifying tuft cells, as this gene is a master regulator of tuft cell differentiation and gene deficiency, leading to a lack of tuft cells (7–10). The presence of tuft cells in the salivary glands has been reported (11,12). One study reported tuft cells in the normal striated ducts of mouse, pig, and human submandibular glands but not in the acinus, intercalated ducts, or excretory ducts (11). Recently, Hoki et al (12) demonstrated that POU2F3-positive tuft cells are primarily present in the striated ducts of the normal human salivary glands, including parotid, submandibular, sublingual, and minor glands, but not in the acini, although these cells are rare epithelial components of the striated ducts, accounting for <1% of the epithelial cells. In addition, the authors showed that WT, as well as pleomorphic adenoma, the most common salivary gland tumour, had POU2F3-positive tuft cells within the tumour and the most abundant tuft cells in WT among various types of benign and malignant salivary gland tumours (12).
PGs are lipid mediators in inflammation, smooth muscle contraction or dilatation, and vasodilation in various tissues or organs (13) and are synthesised from arachidonic acid in the cell membrane by specific PG synthases (14). PGD2 is synthesised by two distinct types of PGD synthase (PGDS): haematopoietic PGDS (H-PGDS) and lipocalin-type PGDS (L-PGDS) (15). H-PGDS is expressed in immune cells, such as macrophages, mast cells, and a subset of T lymphocytes (Th2), whereas the expression of L-PGDS is restricted to the brain and cardiac muscle and is thought to be related to homeostasis of these organs (15–17). PGD2 released from tuft cells in pancreatic lesions plays a role in tissue repair and suppresses the development and acceleration of pancreatic carcinogenesis in a transgenic mouse model (18). Although the presence of tuft cells in normal salivary glands and WT has been shown, whether these cells produce PGD2 has not been elucidated. The development of WT is suspected to be related to epithelial injury with mitochondrial dysfunction (19). PGD2 secreted by tuft cells plays a role in response to injury and tissue repair, although one of the major secretion substances of tuft cells is IL-25, which plays a role in anti-helminth response (5). The roles of tissue injury and repair in L-PGDS have not been reported (15). Therefore, this study aimed to clarify the production of PGD2 in POU2F3-positive cells in normal salivary glands and WT by detecting the expression of H-PGDS.
Materials and methods
Patient selection
Consecutive patients diagnosed with WT by postoperative pathological examination at Osaka Medical and Pharmaceutical University Hospital (Osaka, Japan) (from 1 January 2021 to 31 December 2023) were included in this study. Clinical data, including smoking history, were obtained from medical records.
This retrospective, single-institution study was conducted in accordance with the tenets of the Declaration of Helsinki, and the study protocol was approved by the Institutional Review Board of Osaka Medical and Pharmaceutical University Hospital (Approval #2023-198). All data were anonymised. Informed consent was obtained from patients using the opt-out methodology because of the retrospective study design, as medical records and archived samples were used with no risk to the participants. Moreover, the present study did not include minors. Information regarding this study, such as the inclusion criteria and opportunity to opt out, was provided through the institutional website (https://www.ompu.ac.jp/u-deps/path/img/file23.pdf).
Histopathological analysis
Surgically resected salivary gland tumours were fixed in 10% neutral buffered formalin, dehydrated, embedded in paraffin, sectioned (4 µm), and stained with haematoxylin and eosin. Three authors (KH, AS, and MI) independently evaluated the histopathological features of the specimens.
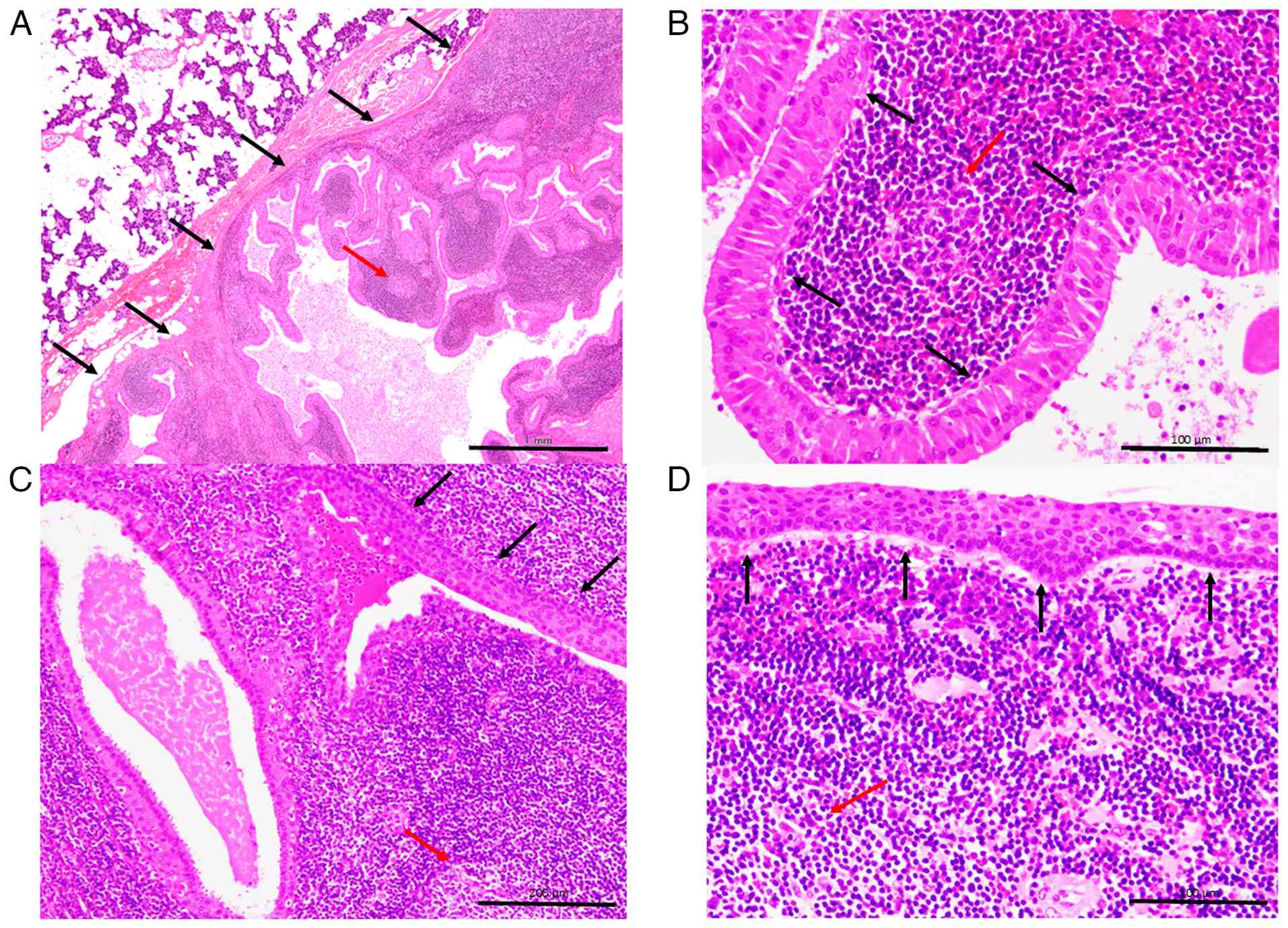
The diagnostic criterion for the WT was papillary cystic proliferation of bilayered oncocytic epithelial cells without nuclear atypia surrounded by lymphoid stroma. Bilayered epithelial cells are composed of inner columnar and outer cuboidal cells, which have a rich granular eosinophilic cytoplasm. Multilayered squamous, mucous, and ciliated cells are occasionally observed (1,2).
Histopathological features, such as the type of epithelium (bilayered oncocytic epithelial cells only or the presence of squamous cells), were evaluated. Metaplastic squamous epithelium was defined as multi-layered polygonal epithelial cells with occasional intercellular bridges and a lack of oncocytic cytoplasm (1).
Immunohistochemical analysis
Immunohistochemical staining was performed using an autostainer (Leica Bond-MAX; Leica Biosystems GmbH, Nußloch, Germany) according to the manufacturer's instructions. The BOND Polymer Refine Detection Kit (DS9800; Leica) and BOND Polymer Refine Red Detection Kit (DS9390; Leica) were used for dual immunohistochemical staining. A rabbit monoclonal antibody against POU2F3 (E5N2D; Cell Signalling Technology, Danvers, MA, USA, diluted 1:200) and a rabbit polyclonal antibody against H-PGDS [(20), diluted 1:4,000] were used. Squamous cells of the skin were used as positive controls for POU2F3 (21), and placental trophoblasts were used for H-PGDS (22). Negative controls were prepared without primary antibodies. Nuclear and cytoplasmic staining was recognised as positive immunoreactivity for POU2F3 (12,21) and H-PGDS (20,22), respectively. Three authors (KH, AS, and MI) independently evaluated the immunohistochemical features. POU2F3+/H-PGDS+ (nuclei stained black and cytoplasm stained red), POU2F3+/H-PGDS− (only nuclei stained brown), and POU2F3−/H-PGDS+ cells (both nuclei and cytoplasm stained red) were separately counted in five high-power fields (×400) within the tumour epithelial cell nests in each tumour, as well as within the non-neoplastic salivary gland tissue around the tumour. Moreover, these analyses were performed in the conventional bilayered oncocytic and metaplastic squamous epithelia (if present).
Statistical analysis
Data are presented as means ± standard deviation. The correlation between the two groups was analysed using the Wilcoxon signed-rank test (Statcel 3; OMS Ltd., Tokyo, Japan). Statistical significance was set at P<0.05.
Results
Patient characteristics
Table SI lists the clinicopathological features of the study cohort, which included 28 patients with WT of the salivary gland. The median patient age was 68 years (range, 54–84 years). The study population comprised only male patients. The tumours were located in the parotid gland in all patients (right and left sides in 13 and 15 patients, respectively). Patient 18 also had a pleomorphic adenoma of the parotid gland on the same side as the WT; however, no continuity was observed between the two lesions.
Smoking history was observed in 22 patients (10/12 patients with only conventional bilayered oncocytic epithelium and 12/16 patients with both bilayered oncocytic and metaplastic squamous epithelia). Ten of 12 patients with only conventional bilayered oncocytic epithelium and 11 of 16 patients with both bilayered oncocytic and metaplastic squamous epithelia were current smokers. No significant differentiation of smoking status was observed between the two groups (Table SI).
Histopathological features
The typical histopathological features of WT are presented in Fig. 1. The well-circumscribed tumour was surrounded by a nonneoplastic parotid gland (Fig. 1A). The tumour comprised a proliferation of bilayered epithelial cells in rich lymphoid stroma, occasionally accompanied by lymphoid follicles with a germinal centre (Fig. 1A and B). The bilayered epithelial cells were composed of inner columnar and outer cuboidal cells, which had rich granular eosinophilic cytoplasm and lacked nuclear atypia (Fig. 1B). The presence of multilayered squamoid cells, that is, squamous metaplasia, was observed in 16 patients (Fig. 1C and D) a transition from conventional bilayered oncocytic epithelial cells to squamous metaplasia was also observed (Fig. 1C). The remaining 12 tumours contained only conventional bilayered oncocytic epithelial cells. This cohort was divided into two subgroups: WT with metaplastic squamous epithelium (Patients No. 1-16) and WT with only conventional oncocytic epithelium (Patients No. 17-28).
Immunohistochemical features
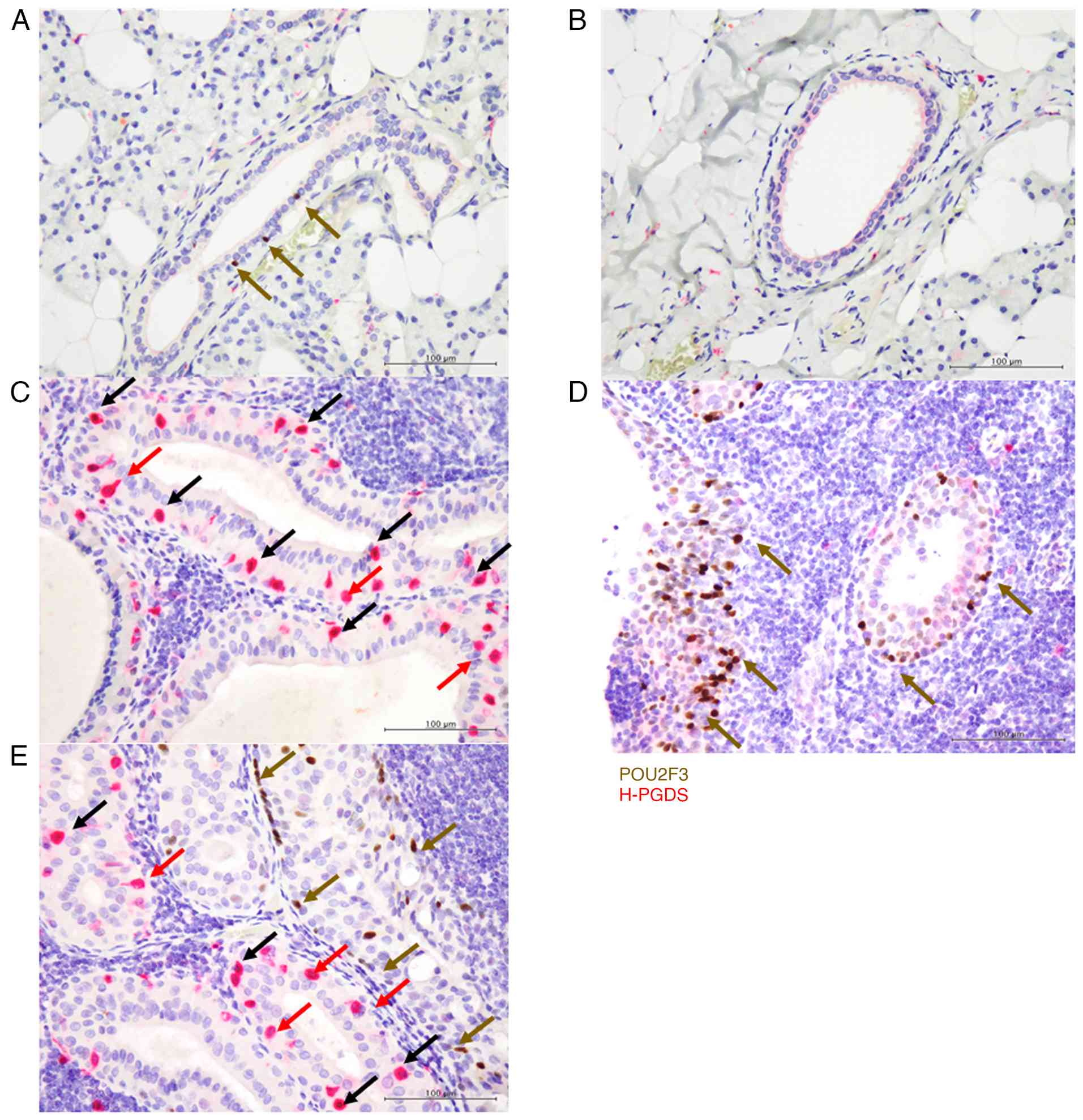
In the normal parotid glands, a few POU2F3-positive tuft cells were observed in the striated duct (three cells (median) in five high-power fields of all specimens) (Fig. 2A) but not in the acinus, intercalated ducts, or excretory ducts in five high-power fields of all specimens (Fig. 2A and B) (Table I). No H-PGDS expression was detected in POU2F3-positive cells in five high-power fields of all specimens (Fig. 2A).
In the conventional bilayered epithelium in WT of both subgroups (Patients No. 1-28), POU2F3+/H-PGDS+ cells were abundant in most tumours, and almost all POU2F3-positive cells expressed H-PGDS (the median ratio of tuft cells expressing H-PGDS [POU2F3+/H-PGDS+ cells/(POU2F3+/H-PGDS+ + POU2F3+/H-PGDS− cells)] was 91.9%). The median ratio of POU2F3-positive cells expressing H-PGDS in the region of the conventional oncocytic epithelium in the subgroup of WT with only conventional oncocytic epithelium (Patients No. 17-28) was 95.1%, while the ratio in that with metaplastic squamous epithelium (Patients No. 1-16) was 89.9%; there was no significant difference in the ratio between the two subgroups. These POU2F3-positive cells were particularly present on the abluminal side of the bilayered epithelium (Fig. 2C) (Table SI).
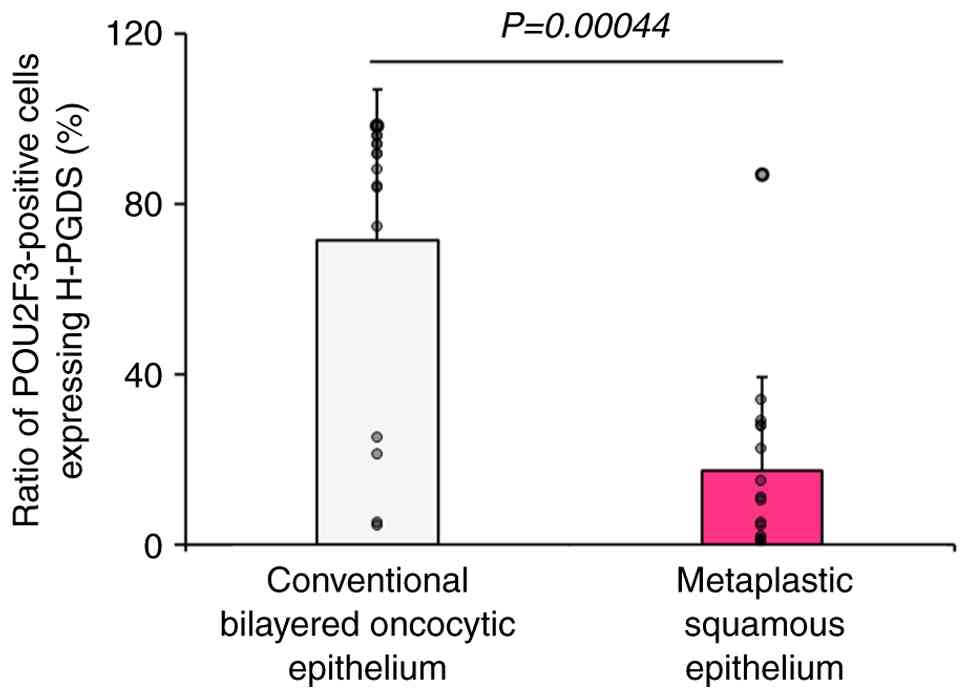
In the subgroup of WT with metaplastic squamous epithelium (Patients No. 1-16), POU2F3+/H-PGDS− cells were abundant in the metaplastic squamous epithelium (Fig. 2D and E) (Table SI). The proportion of POU2F3-positive cells expressing H-PGDS was significantly higher in the conventional bilayered oncocytic epithelium (median 89.9%) than in the metaplastic squamous epithelium (median 10.6%) (P=0.00044) (Fig. 3, Table SI).
Discussion
In the present study, we observed many POU2F3-positive cells in both the conventional bilayered oncocytic and metaplastic squamous epithelia in WT and demonstrated that the ratio of POU2F3-positive cells expressing H-PGDS (POU2F3+/H-PGDS+ cells) was significantly higher in the conventional bilayered oncocytic epithelium than in the metaplastic f epithelium in WT.
Tuft cells are chemosensory epithelial cells that perform multiple functions by secreting various physiologically activating substances (3–6). Tuft cells in the nasal mucosa produce acetylcholine during bacterial infection, inducing the production of antimicrobial peptides and inflammation (23). Tuft cells in the small intestinal mucosa play important roles in parasitic infections via the production of IL-25, the activation of type 2 inflammation, and tissue remodelling (4–6). Moreover, tuft cells are present in the human pancreas; their presence is restricted to the pancreatic ducts but not the acinus (24). Tuft cells appear in acinar-to-ductal metaplasia (ADM) lesions in response to tissue injury in the mouse pancreas (18). ADM is considered de-differentiation or trans-differentiation of pancreatic acinar cells due to pancreatic acinar cell injury from various causes, including acute pancreatitis, using a mouse model (25). These tuft cells present in the pancreatic ADM produce PGD2, leading to tissue remodelling and repair. Moreover, PGD2 in ADM plays a role in inhibiting the progression to pancreatic carcinogenesis using the K-rasG12D transgenic mouse model (18). Tuft cells play various important roles in inflammation, tissue repair, and remodelling, depending on the site and type of the stimuli (3–6,18,23).
Only a few studies have reported the presence of tuft cells in normal human salivary glands and salivary gland neoplasms (11,12). The present and previous studies demonstrated that POU2F3-positive tuft cells were present in the striated duct but not in the acinus and the intercalated and excretory ducts of the human salivary gland (11,12). The location of tuft cells in the human salivary glands is similar to that in the human pancreas, in which tuft cells are present in the normal pancreatic duct but not in acinar cells (24), although tuft cells are not present in the normal conditions of the mice pancreas (26). Recently, Hoki et al (12) analysed the frequency and distribution of tuft cells in various types of salivary gland neoplasms. Tuft cells are present in WT, pleomorphic adenoma, basal cell adenoma, oncocytoma, mucoepidermoid carcinoma, adenoid cystic carcinoma, and salivary duct carcinoma; WT is the tumour with the highest number of tuft cells (12). The authors also demonstrated that POU2F3-positive cells were present on the abluminal side of the bilayered oncocytic epithelium in WT (12). These findings support our results regarding the location of POU2F3-positive cells. The functions of tuft cells in WT remain to be determined, although Hoki et al (12) suspected that POU2F3-positive cells might be aberrantly induced, possibly by tissue injury, such as smoking. Some studies have addressed the association between tuft cells and tissue injury. Tuft cells in the lungs are caused by various types of severe lung injury, arise from basal-like cells, and migrate to damaged alveoli (27). The occurrence of majority of WT is linked to smoking, and epithelial injury with mitochondrial dysfunction is speculated to be related to WT development (19). Moreover, the possible origin of WT is the striated ducts present in the salivary duct included in the intraparotid lymph node (28). Accordingly, tissue injury caused by smoking in the striated duct of the intraparotid lymph node may be linked to the presence of tuft cells in WT, as a few tuft cells are present in the striated ducts of the normal salivary gland. Further studies are warranted to clarify the mechanisms underlying the presence of tuft cells in WT.
PGD2, a lipid mediator, plays an important role in inflammation and homeostasis via DP1 receptors and a chemoattractant receptor-homologous molecule expressed on Th2 cell (CRTH2) receptors (13). H-PGDS is involved in the production of PGD2 in immune cells, such as macrophages, mast cells, and a subset of Th2 cells, which leads to the regulation of inflammation (16,17). Immunohistochemical staining for H-PGDS is useful for demonstrating PGD2 production (17,18,20). The present study demonstrated that POU2F3-positive tuft cells present in the normal striated duct of the parotid gland did not express H-PGDS, and most POU2F3-positive cells present in the bilayered oncocytic epithelium of WT expressed H-PGDS. The ratio of POU2F3-positive cells expressing H-PGDS to the total tuft cells in the bilayered oncocytic epithelium (median 89.9%) was significantly higher than that in the metaplastic squamous epithelium (median 10.6%). This finding indicates that most POU2F3-positive cells present in the conventional oncocytic epithelium express H-PGDS, unlike most of those in the metaplastic squamous epithelium. The function of POU2F3-positive cells in WT might differ between the types of epithelium in WT. PGD2 plays various roles in tissue injury (17), and the degree of tissue injury may be related to the ratio of POU2F3-positive cells expressing H-PGDS in WT. A higher rate of POU2F3-positive cells expressing H-PGDS within the bilayered oncocytic epithelium in WT might be related to stronger ongoing tissue injury, whereas the metaplastic squamous epithelium in WT may be the result of previously existing tissue damage, with ongoing tissue injury being weaker in the metaplastic epithelium. Smoking status was not significantly different between patients with WT with and without metaplastic squamous epithelium; thus, the degree of tissue damage might vary in each tumour. Moreover, tuft cells in the normal striated duct, a possible origin of WT, showed no H-PGDS expression. Increased functional changes in tuft cells might occur during WT development, possibly related to tissue injury or the microenvironment in the striated duct. Further analysis is needed to clarify the functions and production of substances by POU2F3-positive cells in WT, which will provide deeper insights into the pathogenesis of WT. The presence of POU2F3-positive cells has been reported in some types of salivary gland tumours (12); however, data on whether H-PGDS expression is observed in POU2F3-positive cells in other types of salivary gland neoplasms are lacking. Further analysis is needed.
This study has some limitations. First, the functions of POU2F3-positive cells and PGD2 in WT remain unknown. It has been speculated that tuft cells in WT are related to tissue injury, such as smoking; however, the mechanism of emergence of POU2F3-positive cells in WT remains unclear. Second, although this study clearly demonstrated H-PGDS expression in POU2F3-positive cells in WT, no biochemical assay of PGD2 was performed. Although immunohistochemical analysis for H-PGDS is recognized as useful for demonstrating PGD2 production (17,18,20), direct measurement of PGD2 production was not performed in the present study. Further analyses are needed to clarify the functions of tuft cells in normal salivary glands and WT. This will expand our understanding of the pathogenesis of WT. Third, this study included a relatively small number of patients with WT, and the cohort comprised only male patients. Although WT shows a male predominance (1), the present cohort may potentially lead to a bias in the statistical power.
In conclusion, the proportion of POU2F3-positive cells expressing H-PGDS was significantly higher in the conventional bilayered oncocytic epithelium than in the metaplastic squamous epithelium of WT. The results indicate that PGD2 produced by POU2F3-positive cells in WT may be related to tissue injury during WT development. Further studies are warranted to clarify the function of POU2F3-positive cells in WT and normal salivary glands, which will provide novel insights into the pathogenesis of WT.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Shizuka Ono, Mr. Yusuke Ohnishi and Mr. Naoto Kohno (Department of Pathology, Osaka Medical and Pharmaceutical University, Takatsuki, Japan) for their technical assistance in the present study.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included in the figures and/or tables of this article.
Authors' contributions
KH and MI conceptualised and designed the study. KH, AS and MI performed histopathological and immunohistochemical examinations. KH, AS, MI, KN, TT, SIH, YH and KF acquired and analysed the data. KH, AS and MI drafted the manuscript, tables and figures. KH, AS and MI confirmed the authenticity of the raw data. All authors have read and approved the final version of the manuscript.
Ethical approval and consent to participate
The present study was conducted in accordance with the tenets of The Declaration of Helsinki, and the study protocol was approved by the Institutional Review Board of Osaka Medical and Pharmaceutical University (protocol no. 2023-198; Takatsuki, Japan). Informed consent was obtained from all the patients using an opt-out methodology. All data were anonymised.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
ADM |
acinar-to-ductal metaplasia |
|
H-PGDS |
haematopoietic prostaglandin D synthase |
|
L-PGDS |
lipocalin-type prostaglandin D synthase |
|
PG |
prostaglandin |
|
PGDS |
prostaglandin D synthase |
|
POU2F3 |
POU class 2 homeobox 3 |
|
WT |
Warthin tumours |
References
|
Simpson RHW, Di Palma S, Faquin WC and Pasricha S: Warthin tumour. In: WHO Classification of Tumours Head and Neck Tumours. 5th edition. IARC Press; Lyon: pp. 173–175. 2024 | |
|
Seifert G, Bull HG and Donath K: Histologic subclassification of the cystadenolymphoma of the parotid gland. Analysis of 275 cases. Virchows Arch A Pathol Anat Histol. 388:13–38. 1980. View Article : Google Scholar : PubMed/NCBI | |
|
Billipp TE, Nadjsombati MS and von Moltke J: Tuning tuft cells: New ligands and effector functions reveal tissue-specific function. Curr Opin Immunol. 68:98–106. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Gerbe F and Jay P: Intestinal tuft cells: Epithelial sentinels linking luminal cues to the immune system. Mucosal Immunol. 9:1353–1359. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Kotas ME, O'Leary CE and Locksley RM: Tuft cells: Context- and tissue-specific programming for a conserved cell lineage. Annu Rev Pathol. 18:311–335. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Schneider C, O'Leary CE and Locksley RM: Regulation of immune responses by tuft cells. Nat Rev Immunol. 19:584–593. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Matsumoto I, Ohmoto M, Narukawa M, Yoshihara Y and Abe K: Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat Neurosci. 14:685–687. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Ohmoto M, Yamaguchi T, Yamashita J, Bachmanov AA, Hirota J and Matsumoto I: Pou2f3/Skn-1a is necessary for the generation or differentiation of solitary chemosensory cells in the anterior nasal cavity. Biosci Biotechnol Biochem. 77:2154–2156. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, et al: Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 529:226–230. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Yamashita J, Ohmoto M, Yamaguchi T, Matsumoto I and Hirota J: Skn-1a/Pou2f3 functions as a master regulator to generate Trpm5-expressing chemosensory cells in mice. PLoS One. 12:e01893402017. View Article : Google Scholar : PubMed/NCBI | |
|
Tavares Dos Santos H, Nam K, Maslow FM, Small T, Galloway TLI, Dooley LM, Tassone PT, Zitsch RP III, Weisman GA and Baker OJ: Tuft cells are present in submandibular glands across species. J Histochem Cytochem. 70:659–667. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Hoki M, Yamada Y, Hiratomo E, Hirata M, Takeuchi Y, Yoshimatsu M, Kikuchi M, Kishimoto Y, Marx A and Haga H: Expression of FOXI1 and POU2F3 varies among different salivary gland neoplasms and is higher in Warthin tumor. Discov Oncol. 15:362024. View Article : Google Scholar : PubMed/NCBI | |
|
Ricciotti E and FitzGerald GA: Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 31:986–1000. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Seo MJ and Oh DK: Prostaglandin synthases: Molecular characterization and involvement in prostaglandin biosynthesis. Prog Lipid Res. 66:50–68. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Urade Y and Eguchi N: Lipocalin-type and hematopoietic prostaglandin D synthases as a novel example of functional convergence. Prostaglandins Other Lipid Mediat. 68–69. 375–382. 2002.PubMed/NCBI | |
|
Kanaoka Y and Urade Y: Hematopoietic prostaglandin D synthase. Prostaglandins Leukot Essent Fatty Acids. 69:163–167. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Redensek A, Rathore KI, Berard JL, López-Vales R, Swayne LA, Bennett SA, Mohri I, Taniike M, Urade Y and David S: Expression and detrimental role of hematopoietic prostaglandin D synthase in spinal cord contusion injury. Glia. 59:603–614. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
DelGiorno KE, Chung CY, Vavinskaya V, Maurer HC, Novak SW, Lytle NK, Ma Z, Giraddi RR, Wang D, Fang L, et al: Tuft cells inhibit pancreatic tumorigenesis in mice by producing prostaglandin D2. Gastroenterology. 159:1866–1881.e8. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Aoki R and Tanaka T: Pathogenesis of Warthin's tumor: Neoplastic or non-neoplastic? Cancers (Basel). 16:9122024. View Article : Google Scholar : PubMed/NCBI | |
|
Mohri I, Eguchi N, Suzuki K, Urade Y and Taniike M: Hematopoietic prostaglandin D synthase is expressed in microglia in the developing postnatal mouse brain. Glia. 42:263–274. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Yamada Y, Simon R, Iwane K, Nakanishi Y, Takeuchi Y, Yoshizawa A, Takada M, Toi M, Haga H, Marx A and Sauter G: An exploratory study for tuft cells in the breast and their relevance in triple-negative breast cancer: The possible relationship of SOX9. BMC Cancer. 23:4382023. View Article : Google Scholar : PubMed/NCBI | |
|
Helliwell RJ, Keelan JA, Marvin KW, Adams L, Chang MC, Anand A, Sato TA, O'Carroll S, Chaiworapongsa T, Romero RJ and Mitchell MD: Gestational age-dependent up-regulation of prostaglandin D synthase (PGDS) and production of PGDS-derived antiinflammatory prostaglandins in human placenta. J Clin Endocrinol Metab. 91:597–606. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Nevo S, Kadouri N and Abramson J: Tuft cells: From the mucosa to the thymus. Immunol Lett. 210:1–9. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Schütz B, Ruppert AL, Strobel O, Lazarus M, Urade Y, Büchler MW and Weihe E: Distribution pattern and molecular signature of cholinergic tuft cells in human gastro-intestinal and pancreatic-biliary tract. Sci Rep. 9:174662019. View Article : Google Scholar : PubMed/NCBI | |
|
Storz P: Acinar cell plasticity and development of pancreatic ductal adenocarcinoma. Nat Rev Gastroenterol Hepatol. 14:296–304. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
DelGiorno KE, Naeem RF, Fang L, Chung CY, Ramos C, Luhtala N, O'Connor C, Hunter T, Manor U and Wahl GM: Tuft cell formation reflects epithelial plasticity in pancreatic injury: Implications for modeling human pancreatitis. Front Physiol. 11:882020. View Article : Google Scholar : PubMed/NCBI | |
|
Barr J, Gentile ME, Lee S, Kotas ME, de Mello Costa MF, Holcomb NP, Jaquish A, Palashikar G, Soewignjo M, McDaniel M, et al: Injury-induced pulmonary tuft cells are heterogenous, arise independent of key type 2 cytokines, and are dispensable for dysplastic repair. Elife. 11:e780742022. View Article : Google Scholar : PubMed/NCBI | |
|
McLean-Holden AC and Bishop JA: Low molecular weight cytokeratin immunohistochemistry reveals that most salivary gland Warthin tumors and lymphadenomas arise in intraparotid lymph nodes. Head Neck Pathol. 15:438–442. 2021. View Article : Google Scholar : PubMed/NCBI |