Pan‑cancer analysis of the carcinogenic role of WSB2 in human tumors
- Authors:
- Published online on: July 21, 2025 https://doi.org/10.3892/mmr.2025.13625
- Article Number: 260
-
Copyright: © Deng et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
According to the World Health Organization, cancer has become one of the main factors endangering human health across the world (1). Currently, cancer treatment mainly includes surgery, chemotherapy, radiotherapy and immune targeted therapy. Although cancer treatment strategies have made great progress, the 5-year overall survival (OS) remains unsatisfactory due to drug resistance, the tumor immune microenvironment (TIME) and potential adverse reactions to drugs (2,3). The development of cancer involves the interaction of numerous genes and signaling pathways. Further study of the potential molecular mechanism of cancer occurrence and development is important to develop new treatments and improve patient prognosis.
The gene WD repeat and SOCS box containing 2 (WSB2, also known as SBA2) is located on human chromosome 12q24.23. Members of the WD repeat protein subfamily are divided into WSB1 and WSB2 according to the number of WD motifs. The family members contain two highly conserved motifs, a WD repeat sequence and a SOCS box. Members of the WSB family participate in several important biological processes, such as cell signal transduction, protein transport, apoptosis, cell cycle control, chromatin modification and transcriptional regulation, performing important regulatory roles (4–6).
WSB1 is the core element of the E3 ubiquitin ligase complex, which has an important function in mediating protein degradation through the ubiquitin proteasome pathway. WSB1 can enhance the ubiquitination and proteasome degradation of the Von Hippel-Lindau tumor suppressor, and promote tumor invasion and metastasis (7). In addition, WSB1 can also break oncogene-induced senescence by promoting the ubiquitination degradation of ataxic Ataxia telangiectasia mutated protein, leading to abnormal cell proliferation and transformation, eventually generating a tumor (8). Compared with the established functions of WSB1, the biological roles of WSB2 are less well defined. Elevated WSB2 expression has been documented in breast cancer, hepatocellular carcinoma (HCC) and melanoma tissues (4,9,10), and it is associated with the OS of patients with estrogen receptor-positive breast cancer (11). Knockdown of WSB2 in melanoma cells (A375 and G361) significantly downregulate the expression of phosphorylated retinoblastoma, CDK4 and cyclin D3, ultimately impeding cell cycle progression (4). WSB2 interacts with the carboxyl-terminal domain of the granulocyte colony-stimulating factor receptor (G-CSF-R), modulating receptor stability/functionality and contributing to myeloid leukemogenesis (12). WSB1 and 2 also mediate polyubiquitination of methylated RelA to target for proteasomal degradation, thereby facilitating the termination of NF-κB-dependent transcription (13). Furthermore, WSB2 mediates Krüppel-like factor transcription factor (KLF) 15 ubiquitination-dependent degradation, which inhibits PDZ and LIM domain 2 expression and consequently enhancing the activation of the NF-κB pathway, thereby promoting hepatic lipogenesis and HCC progression (14). However, there are no reports on the role of WSB2 in pan-cancer. Consequently, the present study aimed to carry out the first pan-cancer analysis of WSB2 using The Cancer Genome Atlas (TCGA) database. The present study discussed the expression of WSB2, and the association between the WSB2 expression levels and clinical prognosis and pathological stages in several types of pan-cancer. In addition, the mechanisms by which WSB2 influences the proliferation and migration of breast cancer were explored.
Materials and methods
Analysis of WSB2 expression and patient prognosis
TIMER2.0 (http://timer.cistrome.org) and UALCAN (http://ualcan.path.uab.edu/analysis-prot.html) databases were used to analyze the expression of WSB2 in normal and pan-cancer tissues. TIMER2.0 collected 10,897 samples of 32 types of cancer from TCGA (https://portal.gdc.cancer.gov), and the statistical significance of differential expression was evaluated using the Wilcoxon test (15). The data acquisition and analysis methods of UALCAN were as follows, TCGA-Assembler was used to download TCGA 3-level RNA-seq data associated with 33 types of cancer, Student's t-test was used to evaluate whether differences in expression levels between normal tumors and primary tumors were significant (16). P-values were downloaded from the two databases, types of cancer that expressed WSB2 in both two databases were identified and the expression P-values of 23 types of cancer were obtained. Finally, an expression heat map was drawn using Microsoft Excel (Microsoft). P<0.05 was considered to indicate a statistically significant difference.
The Human Protein Atlas (HPA; http://www.proteinatlas.org/) provides a comprehensive map of the expression and distribution of nearly all human proteins (~26,000) across human tissues and organs (17). Entering ‘WSB2’ into the database and clicking on the ‘CANCER’ module provides access to immunohistochemical images of cancerous tissues. Alternatively, clicking on the ‘Tissue’ module allows for the retrieval of immunohistochemical images of normal tissues. In this context, the antibody selected was HPA077139.
The Kaplan-Meier Plotter (18) was used to analyze the relationship between WSB2 expression levels and the OS rate and relapse-free survival (RFS) in patients with various types of cancer. After entering the website, mRNA was selected as the data type, ‘Start KM Plotter for pan-cancer module’ was clicked, the target gene ‘WSB2’ was input to generate the survival analysis visualization. This platform automatically stratifies patient samples into two cohorts based on predefined biomarker expression quantiles. Subsequently, it carries out a comparative survival analysis between these cohorts by generating a Kaplan-Meier survival curve. Statistical outputs include the log-rank test P-value, hazard ratio with 95% confidence intervals and corresponding significance estimates.
Cell culture and transfection
SKOV3 (ovarian adenocarcinoma) and U2OS (osteosarcoma) cells were cultured in McCoy's 5A medium (Procell Life Science & Technology Co., Ltd.). A2780 cells (ovarian endometroid adenocarcinoma) were cultured in 1640 medium (Gibco, Thermo Fisher Scientific, Inc.). SVCT cells (human breast epithelial cells), MDA-MB-231 cells (triple-negative breast cancer), MCF-7 cells (estrogen receptor positive breast cancer), T47D cells (infiltrating ductal carcinoma of the breast), MCF-10A cells (non-tumorigenic epithelial cells), HeLa cells (cervical cancer), U87 MG cells (glioblastoma of unknown origin) and 293T cells (embryonic kidney cells) were cultured in Dulbecco's modified Eagle's medium (Gibco, Thermo Fisher Scientific, Inc.).
Plasmids were transfected into cells according to the instructions of Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). MDA-MB-231 and MCF-7 cells were transfected with pCMV-HA-WSB2 or pCMV-HA (cat. no. HG-VPC0051, HonorGene) plasmids and assessed using reverse transcription-quantitative PCR (RT-qPCR), Cell Counting Kit 8 (CCK-8), 5-ethynyl-2′-deoxyuridine (EdU), cell proliferation, wound healing, Transwell and colony formation assays. MDA-MB-231, A2780, U87 MG, HeLa and MCF-7 cells were transfected with pCMV-SP1(human)-3×FLAG-Neo (cat. no. P39378; Wuhan Miaoling Biotechnology Co., Ltd.) or control pCMV-3×FLAG-Neo (cat. no. P1303; Wuhan Miaoling Biotechnology Co., Ltd.) plasmids for RT-qPCR.
RT-qPCR
Total RNA from SVCT, 293T, U87 MG, MCF-10A, MCF-7, A2780, T47D, MDA-MB-231, U2OS, SKOV3, and HeLa cells was extracted using the Trizol (cat. no. 15596018; Invitrogen; Thermo Fisher Scientific, Inc.) reagent, and the RNA was reverse-transcribed into cDNA using a FastKing cDNA first strand synthesis kit (cat. no. KR116; Tiangen Biotech Co., Ltd.). The cDNA was used as the template for the qPCR step of the RT-qPCR protocol, which used a SYBR Green kit (cat. no. MF013; Mei5 Biotechnology Co., Ltd.). The RT-qPCR thermocycling conditions were as follows: Initial denaturation at 95°C for 3 mins, 40 cycles of 95°C for 15 sec, 60°C for 15 sec, and 72°C for 30 sec WSB2, specificity protein 1 (SP1), NUPR1 (encoding nuclear protein 1, transcriptional regulator), LDLRAD4 (encoding low density lipoprotein receptor class A domain containing 4) and MDM2 (encoding double min 2 Protein) mRNA were quantified using the 2−ΔΔCq method (19), with GAPDH mRNA (encoding glyceraldehyde-3-phosphate dehydrogenase) as the internal reference. The sequences of the primers used are shown in Table I.
Construction of plasmid vectors
Primers (Table II) were used to amplify the coding sequence region of WSB2 and inserted into the EcoRI and KpnI sites of the pCMV-HA vector. The −1624/-787 region of the WSB2 promoter was analyzed according to the TRANSFAC (http://www.gene-regµlation.com) and TESS (http://www.cbil.upenn.edu./cgi-bin/tess/tess) databases. The promoter region of WSB2 was also amplified using the primers shown in Table II, inserted into the KpnI and HindIII sites of the pGL3-basic vector and named as pWSB2-1.6k, pWSB2-0.8k, pWSB2-0.3k respectively.
Dual luciferase reporter assay
MDA-MB-231 cells (7×10⁴/well) were cultured in 24-well plates until reaching >85% confluency. A total of 1 µg pWSB2-1.6k, pWSB2-0.8k, pWSB2-0.3k or pGL3-basic plasmids was transfected into the cells using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), followed by incubation at 37°C for 6 h. Then, the cells were treated with 150 or 200 nM plicamycin or DMSO (Sigma-Aldrich; Merck KgaA). At 24 h after treatment, a luciferase assay was conducted as previously described (20).
Chromatin immunoprecipitation (ChIP) assay
MDA-MB-231 cells were transfected with 24 µg of pCMV-SP1(human)-3×FLAG-Neo or control pCMV-3×FLAG-Neo per 10-cm dish, followed by incubation at 37°C for 48 h. Then, 1×106 cells were lysed with 200 µl lysis buffer (P0013C, Beyotime), and 50 µl lysate was used per CHIP reaction. ChIP assays were carried out according to the manufacturer's protocol using a Millipore ChIP assay kit (cat. no. 17-295, Millipore Sigma). The following 10 µg primary antibodies were used: Anti-SP1 (cat. no. ab231778; Abcam) and anti-immunoglobulin G (IgG; cat. no. sc-2345; Santa Cruz Biotechnology, Inc.). The amount of each specific DNA fragment in the immunoprecipitants was determined using PCR and RT-qPCR as aforementioned, using the primers CHIP-SP1-F and CHIP-SP1-R, as shown in Table I.
CCK-8 assay
At 24 h after transfection, the cell density was adjusted to 2×104 cells /ml and 100 µl of MDA-MB-231 or MCF-7 cells were inoculated into 96-well plates. The cells were incubated for 24, 48 and 72 h, respectively, before being incubated with 10 µl CCK-8 (cat. no. CA1210; Beijing Solarbio Science & Technology Co., Ltd.) reagent to each well. Following incubation at 37°C for 2 h, the absorbance value of 450 nm was measured using a plate reader.
EdU cell proliferation assay
At 48 h after transfection, the MDA-MB-231 or MCF-7 cells were incubated with 50 µM EdU (cat. no. C10310-1; Guangzhou RiboBio Co., Ltd.) at 37°C for 1 h. The cells were fixed, stained, images were captured and the number of EdU-positive cells was determined as described previously (21).
Clonal formation assays
After transfection, the cells were cultured for 2–3 weeks in DMEM, fixed with 4% paraformaldehyde (cat. no. BL539A; Biosharp Life Sciences) for 30 min at room temperature, stained with 850 µl of 0.5% crystal violet (cat. no. G1063; Beijing Solarbio Science & Technology Co., Ltd.) for 30 mins at room temperature, washed twice with PBS, dried and the cell clone count was determined manually.
Wound healing assays
Transfected MDA-MB-231 or MCF-7 cells were grown in serum-starved DMEM at 37°C for 6 h until a monolayer formed. The cell monolayer was scratched vertically using a 200 µl pipette tip, cultured in DMEM at 37°C and imaged using an inverted optical microscope at 0, 24 and 48 h, respectively. ImageJ (version 1.54; National Institutes of Health) was used to quantify the average degree of wound healing.
Transwell assays
A Transwell chamber was purchased from Corning Inc. (cat. no. 3422) and was placed in a 24-well plate. MDA-MB-231 or MCF-7 cells were resuspended in serum-free medium. After counting, 1×104 cells were taken and inoculated into the upper chamber of the Transwell plate. Subsequently, 750 µl of medium containing 10% serum was added to the lower chamber. The Transwell chambers were incubated in the culture dish for 12–48 h at 37°C and fixed with 4% paraformaldehyde for 30 min at room temperature. The cells were stained using 850 µl 0.5% crystal violet for 15 min at room temperature, washed with PBS and imaged using an inverted microscope. Each sample was imaged in five random fields and the number of cells that passed through the filter membrane were counted.
Western blotting
Standard technique was used to carry out western blotting (22) and the following specific primary antibodies were used: Anti-WSB2 (cat. no. ab127176; Abcam, 1:2,000), anti-GADPH (cat. no. CSB-MA000071Mom; Cusabio Technology, LLC, 1:10,000), anti-β-Actin (cat. no. AC026; ABclonal Biotech Co., Ltd. 1:10,000 dilution), anti-HA-tag (cat. no. B1021; Suzhou Botelon Immunotechnology Co., Ltd. 1:5,000), anti-E-cadherin (cat. no. BD-PT1454; Suzhou Botelon Immunotechnology Co., Ltd.; 1:500 dilution), anti-proliferating cell nuclear antigen (PCNA; cat. no. D220014-0025; Sangon Biotech Co., Ltd. 1:1,000 dilution), anti-Vimentin (cat. no. BD-PB4686; Suzhou Botelon Immunotechnology Co., Ltd. 1:500), anti-p53 antibody (p53) (cat. no. CSB-PA07889A0Rb; Cusabio Technology, LLC. 1:1,000 dilution), anti- regulated kinase 1/2 (ERK1/2) (cat. no. PTM-5850; Hangzhou Jingjie Biotechnology Co., Ltd.; 1:1,000), anti-Snail (cat. no. CY3066; Shanghai Aibei Biotechnology Co., Ltd.; 1:2,000). The labeled secondary antibodies were obtained from Sangon Biotech Co., Ltd. (cat. no. D110058-0025, 1:5,000 dilution) and Cusabio Technology, LLC (cat. no. CSB-PA573747, 1:5,000 dilution). Primary antibodies were incubated overnight at 4°C and secondary antibodies were incubated for 2 h at room temperature.
Transcriptome sequencing analysis
MDA-MB-231 cells were transfected with pCMV-HA-WSB2 and pCMV-HA. At 48 h after transfection, total RNA was extracted and purified as previously described (23). cDNA library construction and RNA-seq were performed by GeneChem (Shanghai, China) as previously described (24). A total of three samples were used for the transcriptome sequencing analysis. Differentially expressed mRNAs were identified, and Gene Ontology (geneontology.org/) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis were conducted as previously described (25,26). The data from the transcriptome sequencing analysis were deposited at the Sequence Read Archive database (ncbi.nlm.nih.gov/sra) and are accessible via the accession no. PRJNA1189658.
Statistical analysis
Statistical analysis was carried out using GraphPad Prism 8.2.1. (Dotmatics). The results are presented as the mean ± standard deviation from three independent experiments. Unpaired Student's t-test was used to compare the significant differences between the two groups of samples, and one-way ANOVA was employed for comparisons among multiple groups followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of WSB2 in pan-cancer
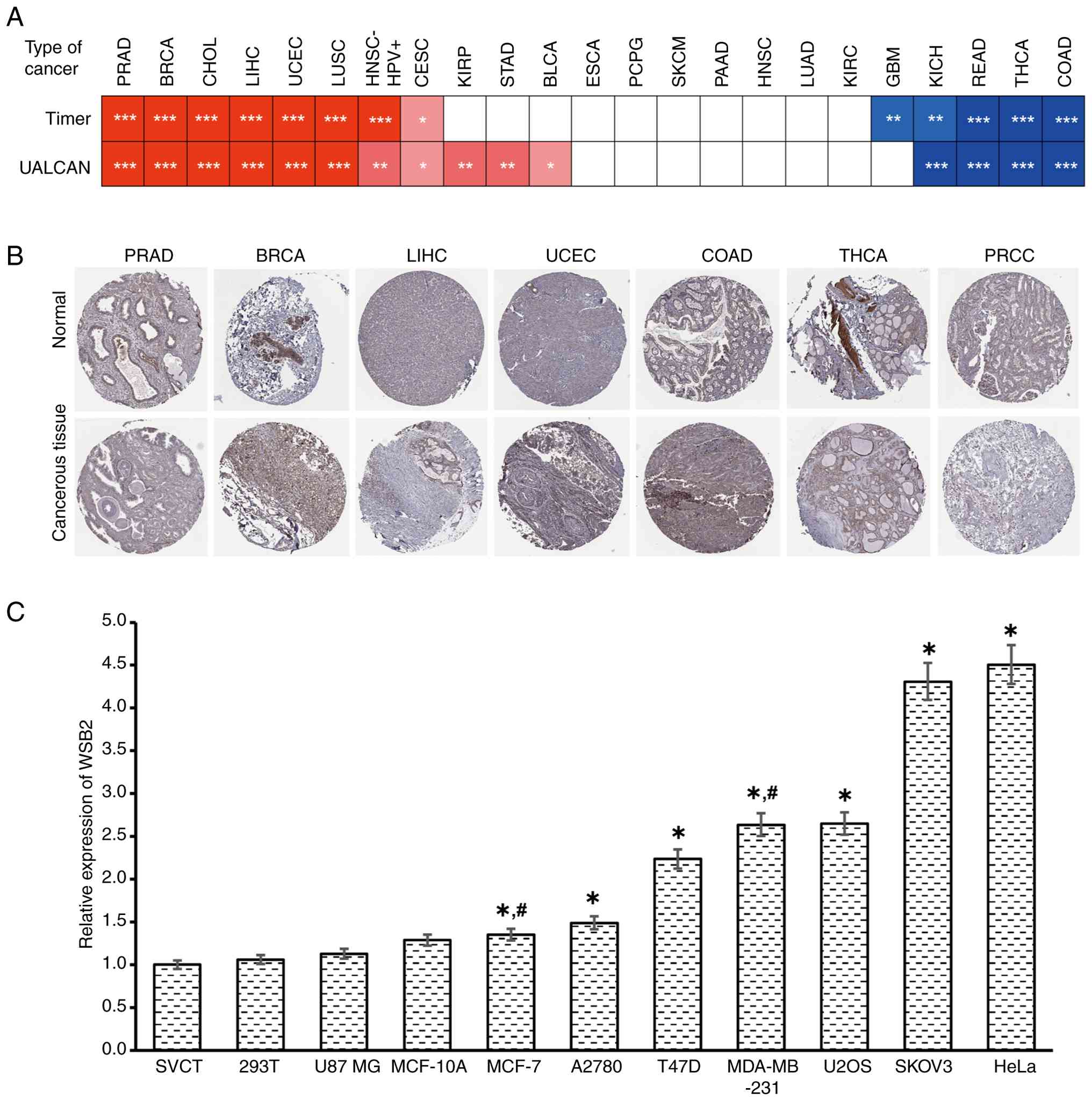
Expression of WSB2 in pan-cancer was primarily analyzed. First, the mRNA expression levels of WSB2 in pan-cancer were evaluated using the UALCAN and TIMER2.0 databases. The results were consistent between UALCAN and TIMER2.0 (Fig. 1A). Online pan-cancer analysis revealed that the expression of WSB2 was increased in breast invasive carcinoma (BRCA), cervical and endocervical cancer types (CESC), cholangiocarcinoma (CHOL), liver HCC (LIHC), lung squamous cell carcinoma (LUSC), prostate adenocarcinoma (PRAD) and uterine corpus endometrial carcinoma (UCEC), but reduced in colon adenocarcinoma (COAD), kidney chromophobe (KICH), rectum adenocarcinoma (READ) and thyroid carcinoma (THCA), compared with that in their corresponding normal tissues according to TIMER2.0 and UALCAN (Fig. 1A). Subsequently, the HPA database was used to evaluate the protein expression of WSB2 in pan-cancer which revealed that WSB2 protein expression was different in tumor and normal tissues (Fig. 1B). WSB2 expression levels in 11 cell lines were examined using RT-qPCR. Analysis revealed that WSB2 was expressed at different levels in these 11 cell lines (Fig. 1C).
SP1 promotes WSB2 expression by binding to its promoter
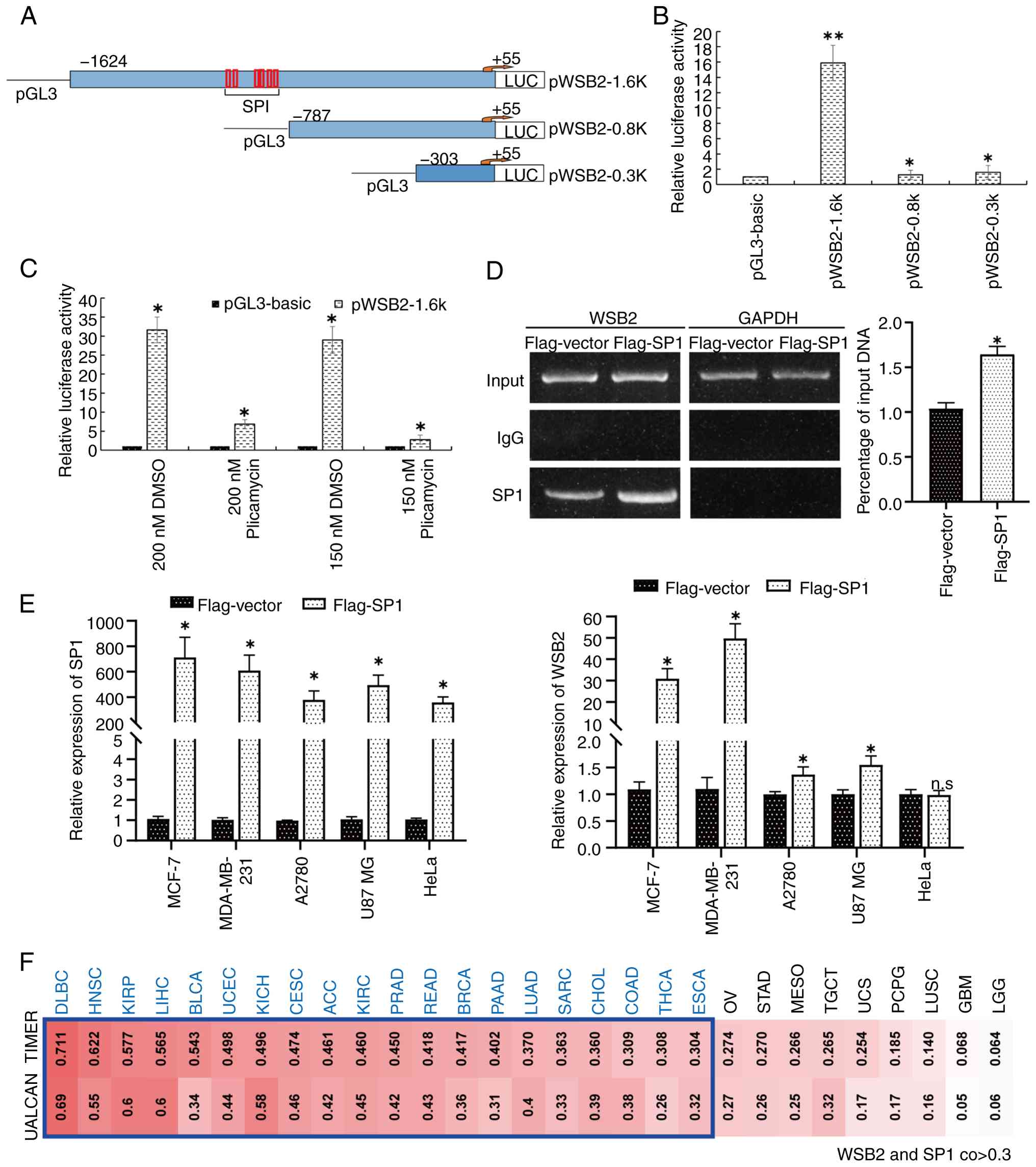
Transcription factors are proteins that can bind to the promoter of a gene, thereby regulating its expression (27). Transcription factors also regulate the expression of WSB2. In the present study, three different truncated WSB2 promoter-luciferase expression vectors, pWSB2-1.6k, pWSB2-0.8k and pWSB2-0.3k, were constructed (Fig. 2A). Analysis of the luciferase reporter assays revealed that pWSB2-1.6k had the highest activity (Fig. 2B), indicating that the −1624/-787 region of the WSB2 promoter may be important for WSB2 transcription. The −1624/-787 region was then analyzed according to the TRANSFAC and TESS databases, and several binding sites for the transcription factor SP1 were found in the −990/-798 region (Fig. 2A). Subsequently, analysis revealed that plicamycin, an SP1 specific inhibitor, dose-dependently reduced pWSB2-1.6k luciferase activity (Fig. 2C). In addition, the results of ChIP-qPCR analysis indicated significantly enhanced recruitment of SP1 to the promoter of endogenous WSB2 (Fig. 2D). Overexpression of SP1 upregulated the mRNA levels of WSB2 in MDA-MB-231, MCF-7, A2780 and U87 MG cells, but not in HeLa cells when compared with Flag-vector (Fig. 2E). Combined TIMER and UALCAN analysis revealed a strong association between WSB2 and SP1 expression in multiple types of cancer (R>0.3; Fig. 2F). These results indicate that SP1 carries out a key role in regulating WSB2 expression levels by binding to its promoter region.
The association between WSB2 expression and prognostic value and pathological stage
UALCAN was used to evaluate the relationship between the WSB2 expression levels and the tumor pathological stage. The expression of WSB2 was positively associated with the pathological stage of BRCA, CESC, CHOL, LIHC, LUSC and UCEC; but was negatively associated with the pathological stages of COAD, KICH, READ and THCA (Fig. S1).
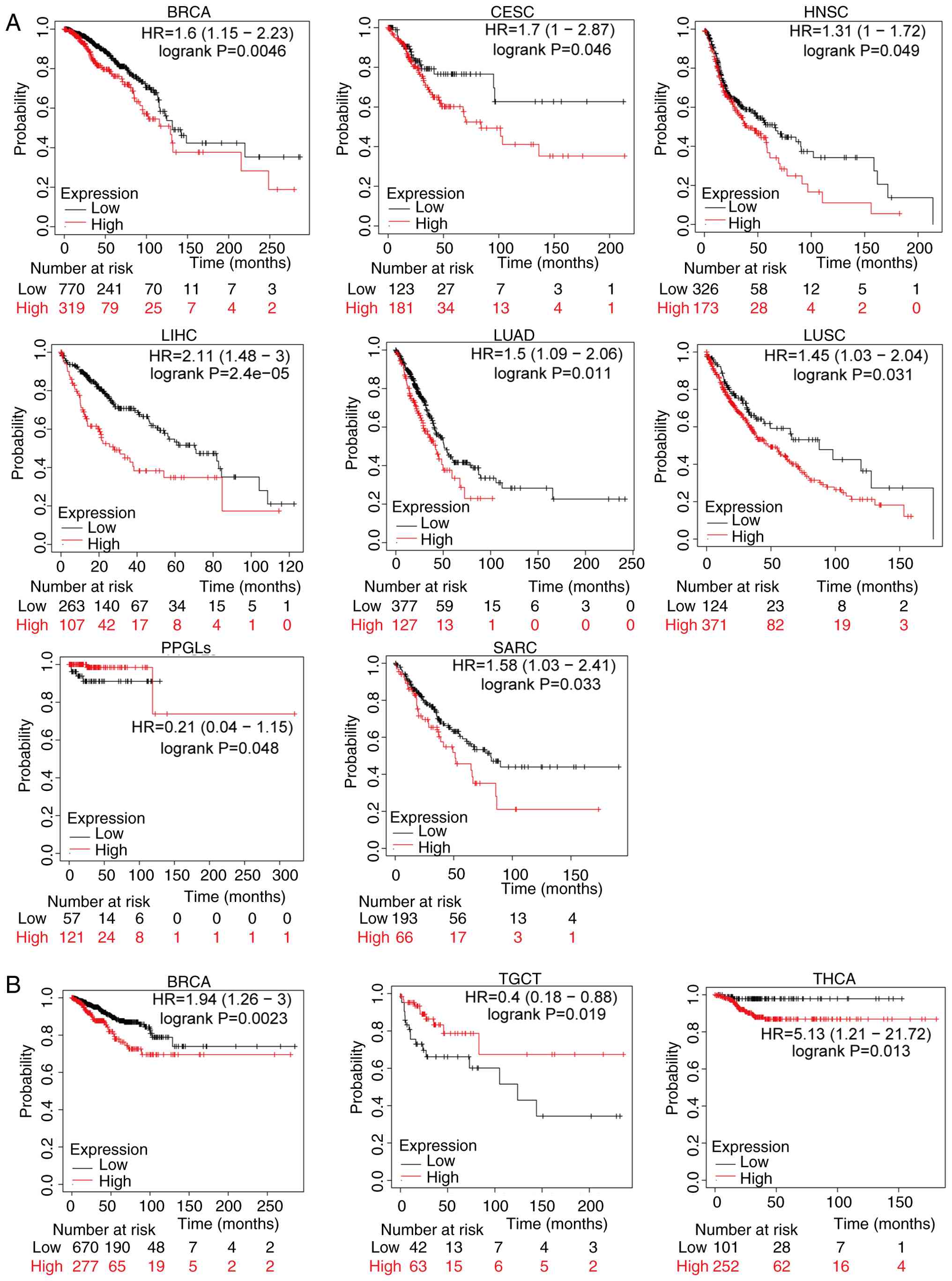
Kaplan-Meier analysis was used to assess the relationship between WSB2 expression levels and survival prognosis in patients with cancer. Analysis revealed that in BRCA, CESC, HNSC, LIHC, LUAD, LUSC, paraganglioma-pheochromocytoma syndromes and sarcoma), increased expression of WSB2 was associated with shorter OS (Fig. 3A). In addition, increased expression of WSB2 was associated with shorter RFS in BRCA and THCA, and decreased WSB2 expression was associated with poor prognosis of patients with TGCT (Fig. 3B)
WSB2 promotes the proliferation and migration of breast cancer cells
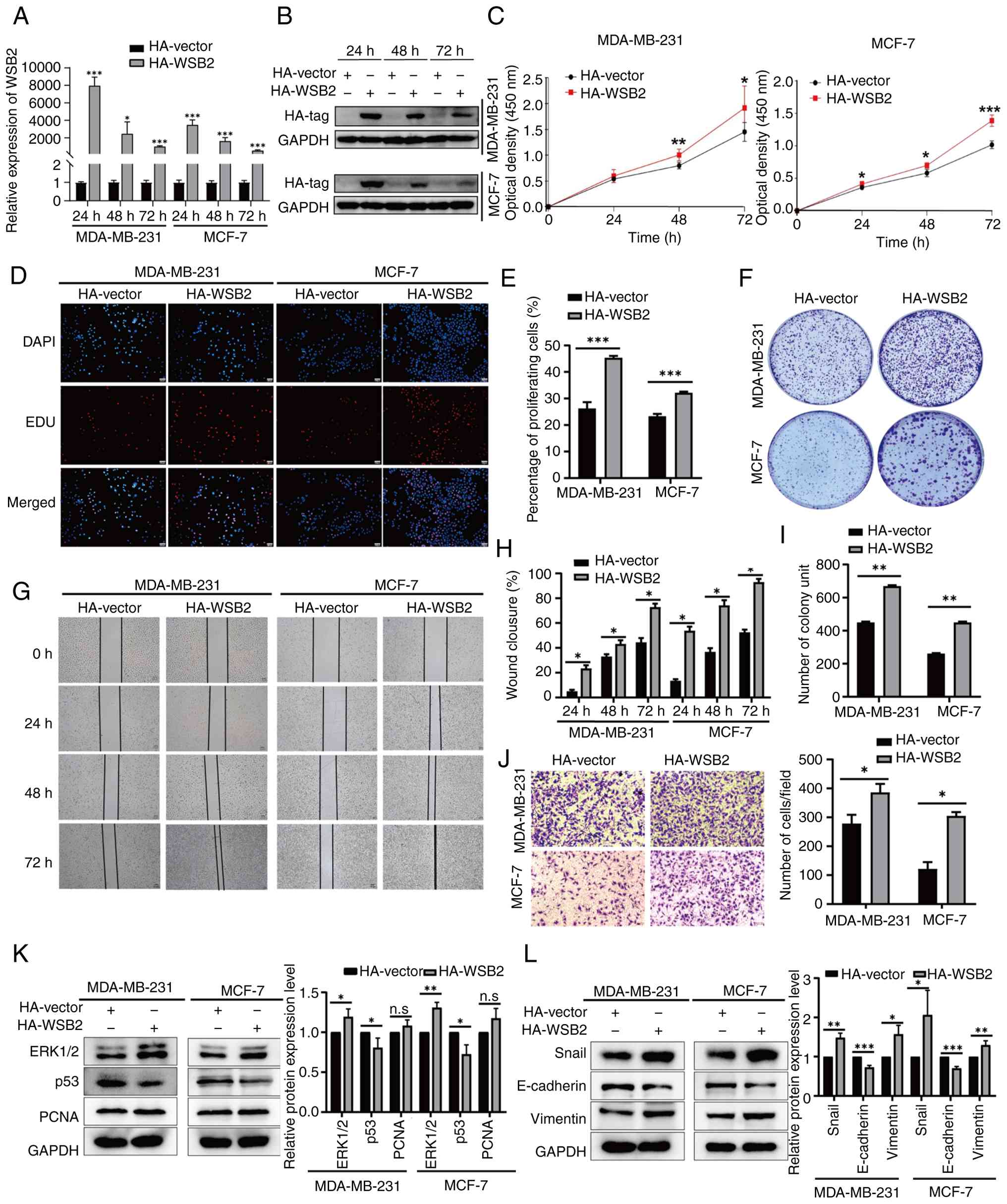
Bioinformatic analysis revealed that WSB2 is highly expressed in BRCA (Fig. 1A) and is associated with its pathological stage (Fig. S1) and OS (Fig. 3A). Our previous research demonstrated that microRNA miR-28-5p inhibits the migration of breast cancer cells by regulating WSB2 expression levels (9). In addition, compared with that in the normal breast cell lines SVCT and MCF-10A, WSB2 is highly expressed in MCF-7 and MDA-MB-231 breast cancer cells (Fig. 1C). To investigate the biological function of WSB2 in breast cancer, MDA-MB-231 and MCF-7 cells were transiently transfected with pCMV-HA-WSB2. RT-qPCR and western blotting were used to determine the transfection efficiency (Fig. 4A and B). Overexpression of WSB2 in MDA-MB-231 and MCF-7 increased cell viability compared with that of the control group (Fig. 4C). Furthermore, analysis of the EdU proliferation assay revealed that overexpression of WSB2 increased the number of cells in the S phase (Fig. 4D and E). Next, the results of colony formation experiments revealed that overexpression of WSB2 increased the number of colonies (Fig. 4F and I). Wound healing and Transwell assays revealed that overexpression of WSB2 promoted the migration of MCF-7 and MDA-MB-231 cells (Fig. 4G-H and Fig. 4J). Western blotting was used to validate the possible proteins involved in WSB2-regulated cell proliferation and metastasis. The results revealed that WSB2 increased the expression levels of vimentin, Snail and ERK1/2, and inhibited the expression of p53 and E-cadherin in MDA-MB-231 and MCF-7 cells (Fig. 4K and L). In summary, these results suggest that WSB2 may promote the proliferation, migration and colony formation of breast cancer cells.
Genes regulated by WSB2
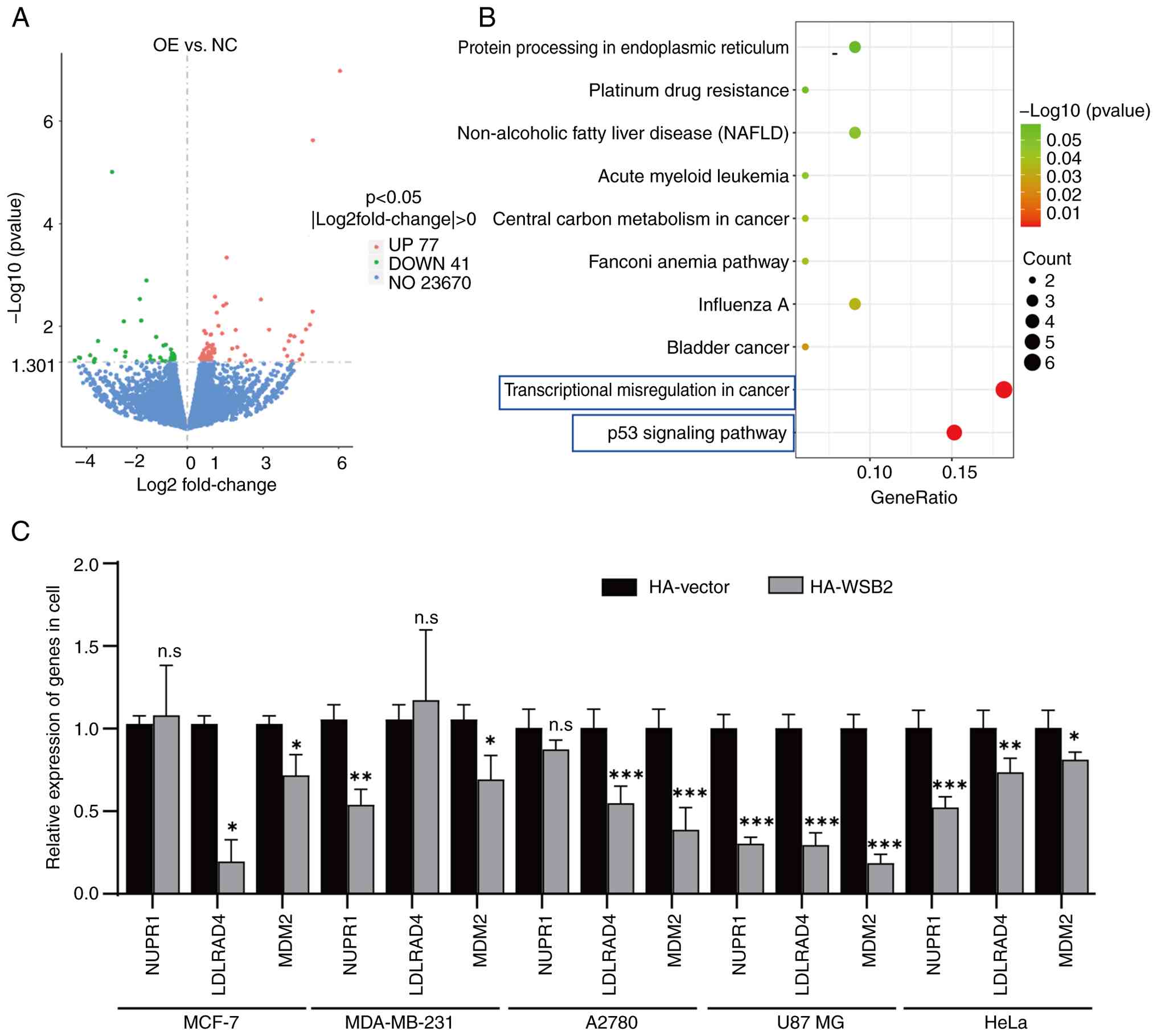
Transcriptome sequencing analysis was used to identify the differentially expressed genes (DEGs) between MDA-MB-231 cells overexpressing WSB2 and MDA-MB-231 cells transfected with the negative control. Analysis revealed that compared with the control group, 118 DEGs were identified, including 77 up- and 41 downregulated genes (Fig. 5A). The results of KEGG enrichment analysis revealed that the DEGs were mainly enriched in the ‘p53 signaling pathway’ and ‘transcriptional misregulation in cancer’ (Fig. 5B). Furthermore, NUPR1 and MDM2, which were enriched in the aforementioned two signaling pathways, and LDLRAD4, which was randomly selected, were used to validate the results of the transcriptome sequencing analysis using RT-qPCR. Overexpression of WSB2 significantly decreased the expression of LDLRAD4 and MDM2 in MCF-7 and A2780 cells, the expression of NUPR1 and MDM2 in MDA-MB-231 cells, and the expression of NUPR1, MDM2 and LDLRAD4 in U87 MG and HeLa cells Fig. 5C. Further screening and validation are needed to identify the target genes regulated by WSB2.
Discussion
Pan-cancer research crosses the boundaries of tumor types and provides information to develop new intervention strategies for clinical trials. In the present study, integrative bioinformatic (TCGA pan-cancer cohort) and experimental validation (RT-qPCR across 11 cell lines) analysis revealed heterogeneity of WSB2 expression patterns in cell lines and tissues. Importantly, immunohistochemistry also confirmed the increased expression of WSB2 in BRCA (9) and HCC (10). In general, WSB2 may have different roles in different types of cancer.
The regulation of gene transcription is influenced by factors such as gene spatial structure, folding state (28), the interaction between regulatory sequences and regulatory factors on DNA (29) and DNA methylation (30). SP1 is a zinc finger transcription factor that can bind to the GC rich motifs of several promoters (31,32). The present study revealed that SP1 may carry out a key role in regulating WSB2 expression by binding to its promoter region.
WSB2 carries out a key role in the development of melanoma (4), BRCA (9) and HCC (10). The present study revealed that WSB2 can promote the proliferation, migration and colony formation of breast cancer cells, corroborating the aforementioned studies. WSB2 is considered to be an E3 ubiquitin ligase, which can directly bind to p53 in HCC cells, disrupt p53 stability through ubiquitination and promote the carcinogenesis and metastasis of HCC through the AKT-mTOR axis (10). WSB2 regulates the ubiquitination and proteasome-mediated degradation of cyclin D1 in a phosphorylation-dependent manner, thereby controlling cell cycle progression and cellular proliferation (33). Western blotting results of the present study revealed that overexpression of WSB2 can reduce the expression of p53. Therefore, it was hypothesized that WSB2 may also promote cell proliferation by ubiquitinating and degrading p53 in breast cancer. In the present study, the transcriptome sequencing results revealed that the WSB2 overexpression-related DEGs were mainly enriched in the ‘p53 signaling pathway’ and ‘transcriptional misregulation in cancer’. These findings suggest that WSB2 may carry put a key role in the proliferation of breast cancer by regulating the p53 signaling pathway.
While the present study focused on the functional role of WSB2 in breast cancer, emerging evidence suggests that WSB2 may interact with diverse signaling pathways across malignancies. For instance, WSB2 regulates the stability of the membrane-bound proteins IL-21 receptor (34) and G-CSF-R (12) and proliferation related proteins, cyclin D1 (33), p53 (35) and retinoblastoma binding protein 5 (10). Additionally, WSB2 has been implicated in modulating the stability of NF-κB pathway related proteins KLF15 (14) and chromatin-bound lysine-methylated RelA (13). Deeper investigation into the molecular mechanisms of WSB2 across different cancer types, particularly its role in various signaling pathways, would provide a more comprehensive understanding of its function in cancer biology. Future studies should map the molecular mechanisms and signaling pathways of WSB2 in other types of cancer.
In summary, comprehensive experimental and bioinformatic analyses were used to conduct a pan-cancer analysis of WSB2. The present study explored the WSB2 expression profile in pan-cancer, revealing that SP1 upregulates the expression of WSB2 by binding to its promoter region. Elevated WSB2 promotes the proliferation and migration of MDA-MB-231 and MCF-7 cells. The present study deepens the understanding of the role of WSB2 in tumorigenesis. However, the transcriptome sequencing analysis in the manuscript is based solely on MDA-MB-231 cells, limiting the generalizability of the results. Experimental validation in other cancer cell lines or tissue samples is still required to enhance the universality and generalizability of the results.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the Natural Science Foundation of Hebei Province (grant nos. H2025209057 and C2023204100) and the S&T Program of Tangshan (grant nos. 23130221E).
Availability of data materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
YD and YFL conceived the study, carried out the experiments and wrote original draft. RL and XG were responsible for data processing and visualization. YL, SW, ML, and JZ performed experiments. LZ, YL, and HC analyzed and interpreted data. YZ and FH contributed to conceptualization, data processing and edited the manuscript. YD and YFL confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, Kramer J and Siegel RL: Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 72:409–436. 2022.PubMed/NCBI | |
|
Hu C, Li Q, Xiang L, Luo Y, Li S, An J, Yu X, Zhang G, Chen Y, Wang Y and Wang D: Comprehensive pan-cancer analysis unveils the significant prognostic value and potential role in immune microenvironment modulation of TRIB3. Comput Struct Biotechnol J. 23:234–250. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Kirchhammer N, Trefny MP, Auf der Maur P, Läubli H and Zippelius A: Combination cancer immunotherapies: Emerging treatment strategies adapted to the tumor microenvironment. Sci Transl Med. 14:eabo36052022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Li Z, Zhao W, Hu H, Zhao L, Zhu Y, Yang X, Gao B, Yang H, Huang Y and Song X: WD repeat and SOCS box containing protein 2 in the proliferation, cycle progression, and migration of melanoma cells. Biomed Pharmacother. 116:1089742019. View Article : Google Scholar : PubMed/NCBI | |
|
Yu L, Gaitatzes C, Neer E and Smith TF: Thirty-plus functional families from a single motif. Protein Sci. 9:2470–2476. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Jain BP and Pandey S: WD40 repeat proteins: Signalling scaffold with diverse functions. Protein J. 37:391–406. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Kim JJ, Lee SB, Jang J, Yi SY, Kim SH, Han SA, Lee JM, Tong SY, Vincelette ND, Gao B, et al: WSB1 promotes tumor metastasis by inducing pVHL degradation. Genes Dev. 29:2244–2257. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Kim JJ, Lee SB, Yi SY, Han SA, Kim SH, Lee JM, Tong SY, Yin P, Gao B, Zhang J and Lou Z: WSB1 overcomes oncogene-induced senescence by targeting ATM for degradation. Cell Res. 27:274–293. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Ma L, Zhang Y and Hu F: miR-28-5p inhibits the migration of breast cancer by regulating WSB2. Int J Mol Med. 46:1562–1570. 2020.PubMed/NCBI | |
|
Li X, Zhang CC, Lin XT, Zhang J, Zhang YJ, Yu HQ, Liu ZY, Gong Y, Zhang LD and Xie CM: Elevated expression of WSB2 degrades p53 and activates the IGFBP3-AKT-mTOR-dependent pathway to drive hepatocellular carcinoma. Exp Mol Med. 56:177–191. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou H, Lv Q and Guo Z: Transcriptomic signature predicts the distant relapse in patients with ER+ breast cancer treated with tamoxifen for five years. Mol Med Rep. 17:3152–3157. 2018.PubMed/NCBI | |
|
Erkeland SJ, Aarts LH, Irandoust M, Roovers O, Klomp A, Valkhof M, Gits J, Eyckerman S, Tavernier J and Touw IP: Novel role of WD40 and SOCS box protein-2 in steady-state distribution of granulocyte colony-stimulating factor receptor and G-CSF-controlled proliferation and differentiation signaling. Oncogene. 26:1985–1994. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang J, Yu Y, Zou X, Du Y, Liang Q, Gong M, He Y, Luo J, Wu D, Jiang X, et al: WSB1/2 target chromatin-bound lysine-methylated RelA for proteasomal degradation and NF-κB termination. Nucleic Acids Res. 52:4969–4984. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Chen J, Chen X, Cai H, Yang Y, Zhu Q, Sun D and Gao C: The ubiquitination degradation of KLF15 mediated by WSB2 promotes lipogenesis and progression of hepatocellular carcinoma via inhibiting PDLIM2 expression. J Gastroenterol Hepatol. 40:192–207. 2025. View Article : Google Scholar : PubMed/NCBI | |
|
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, Li B and Liu XS: TIMER: A web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 77:e108–e110. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Chandrashekar DS, Karthikeyan SK, Korla PK, Patel H, Shovon AR, Athar M, Netto GJ, Qin ZS, Kumar S, Manne U, et al: UALCAN: An update to the integrated cancer data analysis platform. Neoplasia. 25:18–27. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Pontén F, Schwenk JM, Asplund A and Edqvist PH: The human protein atlas as a proteomic resource for biomarker discovery. J Intern Med. 270:428–446. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Goel MK, Khanna P and Kishore J: Understanding survival analysis: Kaplan-Meier estimate. Int J Ayurveda Res. 1:274–278. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Hu F, Meng Y, Gou L and Zhang X: Analysis of promoters and CREB/AP-1 binding sites of the human TMEM174 gene. Exp Ther Med. 6:1290–1294. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Hu F, Meng X, Tong Q, Liang L, Xiang R, Zhu T and Yang S: BMP-6 inhibits cell proliferation by targeting microRNA-192 in breast cancer. Biochim Biophys Acta. 1832:2379–2390. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Wang J, Zou Y, Guan R, Tan S, Su L, Zhao Z, Cao Z, Jiang K, Wang T and Zheng G: Copper supplementation alleviates hypoxia-induced ferroptosis and oxidative stress in neuronal cells. Int J Mol Med. 54:1172024. View Article : Google Scholar : PubMed/NCBI | |
|
Hu F, Zhang Y, Li M, Bai Y and Zhang X: Expression and role of HEPIS in breast cancer. Oncol Lett. 18:6648–6656. 2019.PubMed/NCBI | |
|
Li J, Jiang H, Lv Z, Sun Z, Cheng C, Tan G, Wang M, Liu A, Sun H, Guo H, et al: Articular fibrocartilage-targeted therapy by microtubule stabilization. Sci Adv. 8:eabn84202022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang X, Hao L, Meng L, Liu M, Zhao L, Hu F, Ding C, Wang Y, He B, Pan Y, et al: Digital gene expression tag profiling analysis of the gene expression patterns regulating the early stage of mouse spermatogenesis. PLoS One. 8:e586802013. View Article : Google Scholar : PubMed/NCBI | |
|
Kanehisa M and Goto S: KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Lambert SA, Jolma A, Campitelli LF, Das PK, Yin Y, Albu M, Chen X, Taipale J, Hughes TR and Weirauch MT: The human transcription factors. Cell. 172:650–665. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Gothe HJ, Bouwman BAM, Gusmao EG, Piccinno R, Petrosino G, Sayols S, Drechsel O, Minneker V, Josipovic N, Mizi A, et al: Spatial chromosome folding and active transcription drive DNA fragility and formation of oncogenic MLL translocations. Mol Cell. 75:267–283.e12. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Lu F and Lionnet T: Transcription factor dynamics. Cold Spring Harb Perspect Biol. 13:a0409492021. View Article : Google Scholar : PubMed/NCBI | |
|
Moore LD, Le T and Fan G: DNA methylation and its basic function. Neuropsychopharmacology. 38:23–38. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Vellingiri B, Iyer M, Devi Subramaniam M, Jayaramayya K, Siama Z, Giridharan B, Narayanasamy A, Abdal Dayem A and Cho SG: Understanding the role of the transcription factor Sp1 in ovarian cancer: From theory to practice. Int J Mol Sci. 21:11532020. View Article : Google Scholar : PubMed/NCBI | |
|
Vizcaíno C, Mansilla S and Portugal J: Sp1 transcription factor: A long-standing target in cancer chemotherapy. Pharmacol Ther. 152:111–124. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang M, Lu K, Wei G, Xiao G, Tong L and Chen D: Multiple cullin-associated E3 ligases regulate cyclin D1 protein stability. Elife. 12:e803272023. View Article : Google Scholar : PubMed/NCBI | |
|
Nara H, Onoda T, Rahman M, Araki A, Juliana FM, Tanaka N and Asao H: Regulation of interleukin-21 receptor expression and its signal transduction by WSB-2. Biochem Biophys Res Commun. 392:171–177. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Yang Z, Jia Y, Wang S, Zhang Y, Fan W, Wang X, He L, Shen X, Yang X, Zhang Y and Yang H: Retinoblastoma-binding protein 5 regulates H3K4 methylation modification to inhibit the proliferation of melanoma cells by inactivating the Wnt/β-catenin and epithelial-mesenchymal transition pathways. J Oncol. 2023:50939412023. View Article : Google Scholar : PubMed/NCBI |