Evidence of a functional Smad2/3 signaling axis and TGFβ‑mediated autocrine transcriptional regulation of in vitro vasculogenic mimicry in mesenchymal stem/stromal cells
- Authors:
- Published online on: June 23, 2025 https://doi.org/10.3892/ol.2025.15151
- Article Number: 405
-
Copyright: © Gnao et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Transforming growth factor β (TGFβ), a well-known angiogenic and immunosuppressive cytokine, significantly affects mesenchymal stromal cells (MSCs) through different processes. Among those, inhibition of their terminal differentiation into osteoblasts (1), and regulation of chondrogenic differentiation (2), both of which requiring TGFβ signaling. In pathological conditions, TGFβ signaling in MSCs contributes to the development of myelofibrosis, a condition characterized by increased collagen deposition in the bone marrow (3,4). On the other hand, overexpressing TGFβ in MSCs has been shown to enhance their therapeutic effects, particularly in reducing organ injury and inflammation during septic conditions (5). Collectively, these effects highlight the complex multifaceted role of TGFβ in regulating MSCs functions.
Efforts to enhance MSCs' therapeutic potential have increasingly focused on their response to TGFβ. For instance, overexpressing TGFβ1 in MSCs was found to attenuate organ dysfunction in septic mice, by reducing macrophage-driven inflammation, and also by promoting the mobilization of MSCs for tissue repair (5,6). Additionally, strategies exploiting specific inhibitors of TGFβ signaling, along with genetic engineering techniques have also been employed to overexpress or knock down TGFβ receptors (TGFβR) or downstream signaling molecules in MSCs (7). Hypoxic preconditioning, a condition that mimics the low oxygen tension present in ischemic tissues or that found within solid tumors (8,9), has been shown to upregulate TGFβ and other immunosuppressive factors, thereby improving MSCs' ability to modulate immune responses (4). These strategies help harness the beneficial effects of TGFβ while minimizing potential adverse effects, thereby improving the efficacy of MSC-based therapies.
MSCs play a complex role in tumor angiogenesis, acting as both promoters and inhibitors of tumor growth [10]. This dual role makes them a double-edged sword in cancer therapy, as their ability to home to tumor sites and modulate the tumor microenvironment (TME) is being explored for therapeutic purposes, including targeted drug delivery and modulation of the immune response (10,11). Recently, MSCs have been suggested to be involved in vasculogenic mimicry (VM), a process where cells form vascular-like structures without the involvement of endothelial cells, contributing in pathological settings to tumor blood supply and metastasis (12). Interestingly, MSCs' ability to migrate and invade tissues is closely linked to epithelial-to-mesenchymal transition (EMT), a process closely linked to VM, as it enables cells to acquire properties necessary for forming these vessel-like structures (13). MSCs share characteristics with cancer stem cells (CSCs), which are known to play a crucial role in VM. CSCs can differentiate into various cell types, including those that contribute to VM (14). Moreover, MSCs interact with the TME, promoting conditions that support VM through their paracrine activity, including TGFβ secretion, and that enhance local angiogenesis and VM that support tumor growth and metastasis (15,16). Understanding the role of MSCs in VM could facilitate the development of targeted therapies aimed at inhibiting this process, thereby potentially reduce tumor progression and metastasis.
As cancer cells often adapt and develop resistance to TGFβ's tumor-suppressive effects and start secreting TGFβ themselves, the autocrine effects of such secretion role in MSCs' ability to promote tumor growth, invasion, metastasis, and immune evasion remains unknown (17). For instance, in colorectal cancer, TGFβ secretion by tumor cells contributes to the TME, facilitating immune suppression and neovascularization (18). Similarly, in breast cancer, TGFβ secreted by tumor cells and stromal cells within the TME supports tumor maintenance and progression (19).
Tumors have therefore a remarkable ability to regulate their environment and influence body homeostasis through several mechanisms including metabolic reprogramming to support their rapid growth and survival. This involves altering their energy production pathways, such as increasing glycolysis not only to support the energy demands of tumor cells but to also create an acidic and hypoxic tumor microenvironment TME (20), which can suppress the immune response (21). In addition, interaction with stromal cells within the TME can further promote tumor growth and new blood vessel formation (21). Finally, tumors can hijack normal homeostatic processes in the body as they can alter cytokine and chemokine secretion, leading to systemic inflammation and immune dysregulation (22,23). These mechanisms highlight the complex interplay between tumors and their environment, demonstrating how MSC recruitment can support their growth and evade the body's defense mechanisms. In this study, we aim to investigate the potential impact of TGFβ and particularly, the interrelation between TGFβ and Smad2/3-dependant signaling on the in vitro formation of 3D capillary-like structures.
Materials and methods
Reagents
Micro bicinchoninic acid (BCA) protein assay reagents were from Pierce (Micro BCA™ Protein Assay Kit; Thermo Fisher Scientific, Inc.). The polyclonal antibodies against Snail (3879S), FOXC2 (12974S), and Fibronectin (30903S), as well as the monoclonal antibody against GAPDH (D4C6R) were all from Cell Signaling Technology. HRP-conjugated donkey anti-rabbit and anti-mouse immunoglobulin (Ig) G secondary antibodies were from Jackson ImmunoResearch Laboratories. All other reagents were from Sigma-Aldrich; Merck KGaA.
Cell culture and capillary-like structure formation assay
Human bone marrow-derived mesenchymal stromal/stem cells (MSCs, PCS-500-012) were purchased from the American Type Culture Collection. Cell culture media was from Life Technologies Corp. Cells were plated in high glucose aMEM supplemented with 10% FBS and 50 units/ml penicillin/streptomycin and cultured in a humidified incubator at 37°C with 5% CO2. MSCs were kept subconfluent and expanded for not more than 10 passages by a 1:2 split on a weekly basis. VM was assessed in vitro using Cultrex (3432-010-01, R&D Systems) to monitor 3D capillary-like structures formation (24). In brief, each well of a 96-well plate was pre-coated with 50 µl of Cultrex. MSCs suspension in culture media (104 cells/100 µl) was then seeded on top of polymerized Cultrex and incubated at 37°C in a CO2 incubator for different time points of vascular network formations. Phase contrast pictures were taken over time using a digital camera coupled to an inverted microscope. For each loop and tube measurement, the pixels that belong to its edge are considered its border or perimeter. The number of loops and tubes, as well as tube branching formed by the cells were quantified using the Wimasis analysis software (https://www.wimasis.com; Cordoba, Spain) or the ImageJ software (https://imagej.net) (25).
Total RNA isolation, cDNA synthesis, and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from cell monolayers using 1 ml of TRIzol reagent for a maximum of 3×106 cells as recommended by the manufacturer (Life Technologies). For cDNA synthesis, 2 µg of total RNA was reverse-transcribed using a high-capacity cDNA reverse transcription kit (Applied Biosystems). The cDNA was stored at −20°C prior to PCR. Gene expression was quantified qPCR using iQ SYBR-Green Supermix (Bio-Rad Laboratories). DNA amplification was carried out using an Icycler iQ5 (Bio-Rad Laboratories) and product detection was performed by measuring the binding of the fluorescent dye SYBR-Green I to double-stranded DNA. The following primer sets were from Qiagen: FOXC2 (Hs_FOXC2_1_SG, QT00220871), SNAI1 (Hs_SNAI1_SG, QT00010010), Fibronectin (Hs_FN1_1_SG, QT00038024), TGFβ (Hs_TGFB1_1_SG, QT00000728), GAPDH (Hs_GAPDH_1_SG, QT00079247) and Peptidylprolyl Isomerase A (PPIA) (Hs_PPIA_4_SG, QT01866137). The relative quantities of target gene mRNA were normalized against internal housekeeping genes PPIA and GAPDH. The RNA was measured by following a ∆CT method employing an amplification plot (fluorescence signal vs. cycle number). The difference (∆CT) between the mean values in the triplicate samples of the target gene and the housekeeping genes was calculated with the CFX manager Software version 2.1 (Bio-Rad Laboratories) and the relative quantified value (RQV) was expressed as 2−ΔΔCq (26). Single amplicons and appropriate melting curves were indicative of specific qPCR conditions and efficacy (not shown).
Transfection method and RNA interference
For gene silencing experiments, MSCs were transiently transfected with siRNA sequences using Lipofectamine-2000 transfection reagent (Thermo Fisher Scientific, Inc.). Gene silencing was performed over 24 h using 20 nM siRNA against TGFβ (Hs_TGFB1_2 FlexiTube siRNA Geneglobe ID: SI00013601), Smad2 (Hs_SMAD2_1 FlexiTube siRNA GeneGlobe ID: SI00082460), Smad3 (Hs_SMAD3_1 FlexiTube siRNA GeneGlobe ID: SI00082481), or scrambled sequences (AllStar Negative Control siRNA, 1027281). The above small interfering RNA and mismatch siRNA were all synthesized by Qiagen and annealed to form duplexes. Gene silencing efficacy was assessed by RT-qPCR as described above.
Nuclear extraction
Nuclear extraction was performed as described by us previously (27). Briefly, cell monolayers were first lysed with a cytoplasmic buffer and then with a nuclear buffer according to the manufacturer's instructions (Invent Biotechnologies, SC-003). In the case of the cells cultured on Cultrex, they were first detached from the matrix using a non-enzymatic Cultrex organoid harvesting and dissociation solution (3700-100-01) from R&D Systems. Nucleus enrichment was assessed upon Fibrillarin protein expression, whereas protein GAPDH protein expression was used to assess cytosolic purity/contamination of the nuclear fraction.
Western blot
Electrophoresis reagents origin, total cell lysis procedure, SDS-polyacrylamide gel electrophoresis, electro transfer to low-fluorescence polyvinylidene difluoride membranes, and immunodetection were conducted as described in detail previously (28). Immunoreactive material was visualized by enhanced chemiluminescence.
Chemotactic cell migration assay
Cell migration assays were carried out using the Real-Time Cell Analyzer (RTCA) Dual-Plate (DP) Instrument of the xCELLigence system (Roche Diagnostics). Adherent MSC monolayers were trypsinized and seeded (30,000 cells/well) onto CIM-Plates 16 (Roche Diagnostics). These migration plates are similar to conventional transwells (8 µm pore size) but have gold electrode arrays on their bottom side of the membrane to provide real-time data acquisition of cell migration. Prior to cell seeding, the underside of the wells from the upper chamber were coated with 25 µl of 0.15% gelatin in PBS and incubated for 1 h at 37°C. Cell migration was continuously monitored for up to 6 h using serum-free media, in the presence or absence of 30 ng/ml TGFβ. In all cases, the impedance values were measured by the RTCA DP Instrument software and were expressed as Normalized Cell Migration Index. Each experiment was performed two times in triplicates.
Statistical data analysis
All statistical analyses were conducted using the GraphPad Prism 7 software (Dotmatics). Data and error bars are presented as the mean ± standard error of the mean from three or more independent experiments, unless otherwise specified. Hypothesis testing was performed using the Kruskal-Wallis test followed by a Dunn Tukey's post-test (>2 groups). P<0.05 was considered to indicate a statistically significant difference.
Results
MSCs in vitro VM triggers the expression of EMT biomarkers and Smad2/3 phosphorylation
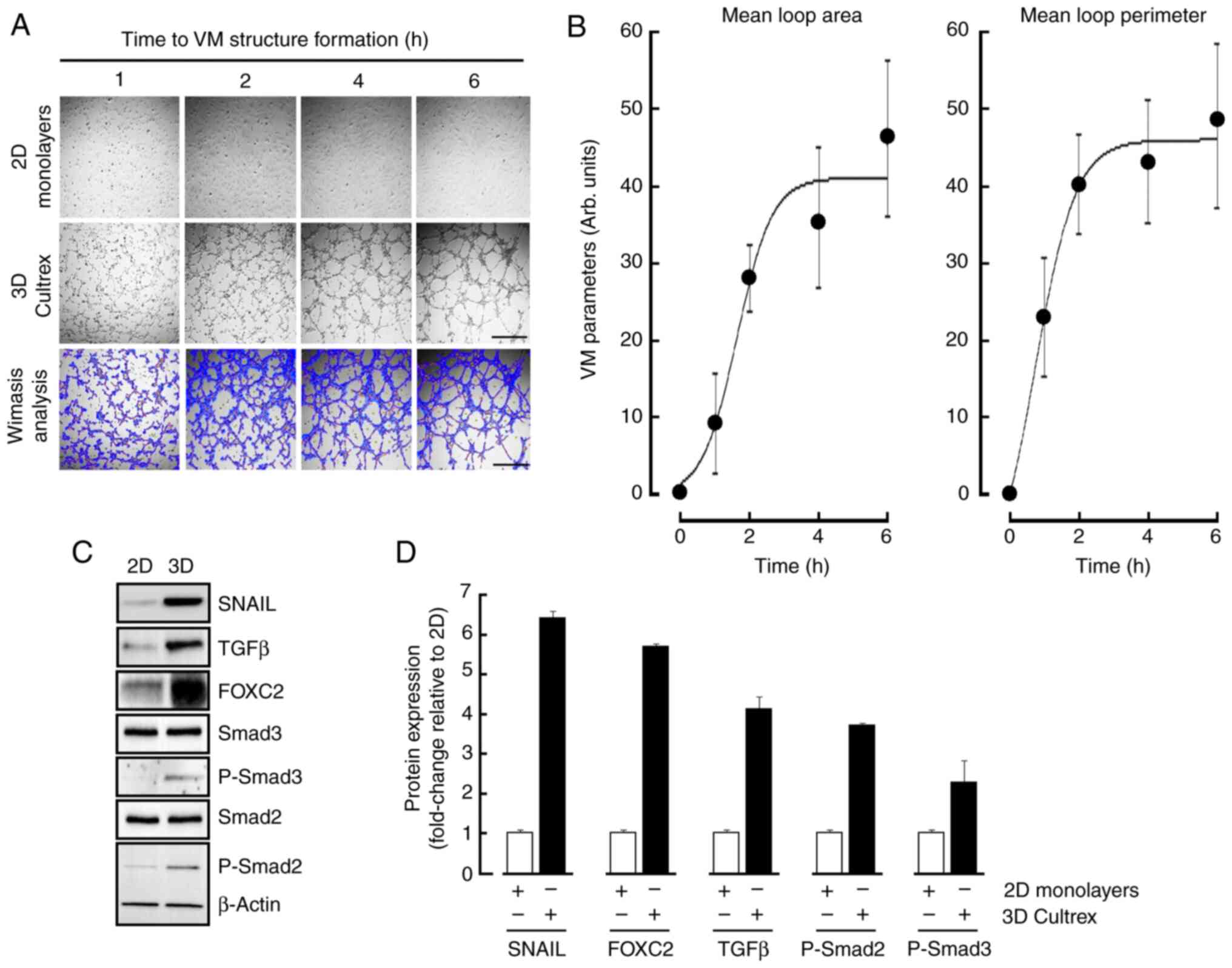
The ability of MSCs to form in vitro 3D capillary-like structures mimicking VM was first assessed as described in the Methods section. Mature structures were formed upon 6 h (Fig. 1A, middle panels) when compared to 2D monolayers (Fig. 1A, upper panels). VM parameters analysis, namely mean loop area and perimeter, were performed as described in the Methods section (Fig. 1A, lower panels) and reflected such in vitro maturation in time (Fig. 1B). Cell lysates were isolated from 2D and 3D cultures and immunoblotting performed in an attempt to characterize the acquisition of an epithelial-to-mesenchymal transition (EMT) molecular phenotype as well as TGFβ signaling (Fig. 1C). VM structures were found to significantly trigger the expression of EMT biomarker Snail and FOXC2 as reported elsewhere (24), whereas inductions in TGFβ and of the phosphorylated states of Smad2/3, but not that of total Smad2/3 or β-Actin, were also observed (Fig. 1D, black bars). Harmonized densitometric normalization was performed to β-Actin for the expression of all those tested biomarkers that were found to be changed only. Altogether, these data suggest that a potential TGFβ signaling axis appears to correlate with EMT and be involved during VM in MSCs.
Transient silencing of TGFβ and pharmacological inhibition of TGFβR1 kinase activity alters in vitro VM
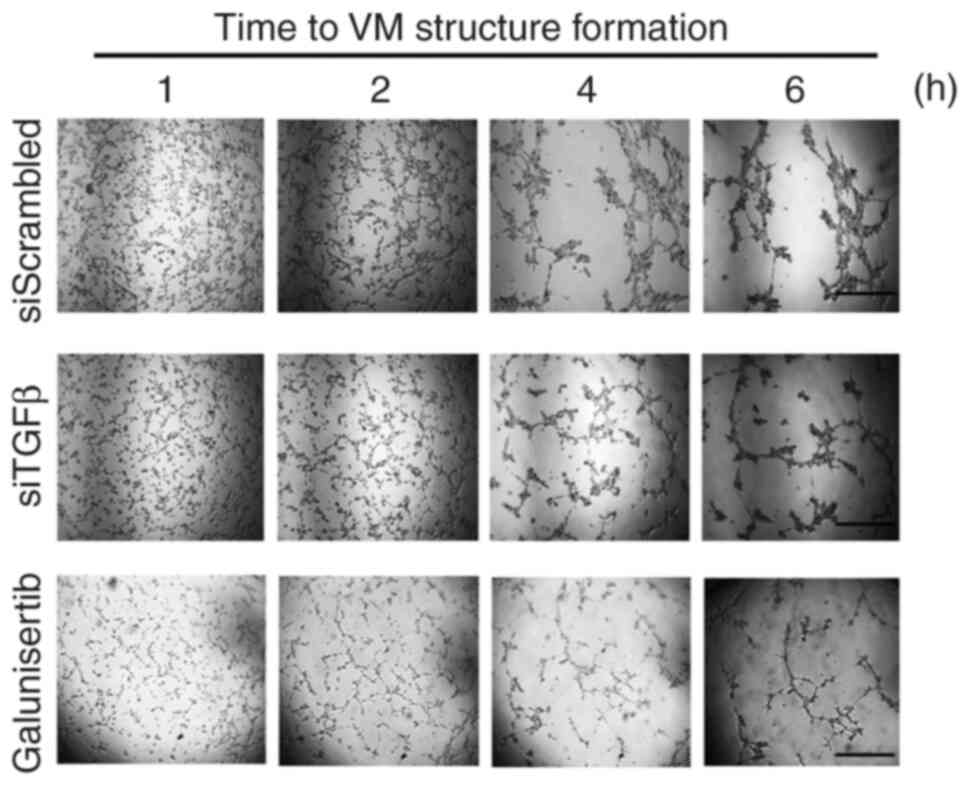
The contribution of the TGFβ signaling axis involving the TGFβR was next assessed using Galunisertib, a pharmacological inhibitor well known to alter the kinase activity of TGFβR (29–31). Moreover, the requirement of TGFβ, as induced upon VM (Fig. 1), was also addressed. Pre-transient silencing of TGFβ was performed for 24 h, then cells seeded on top of Cultex for 6 h. VM structures were reduced when TGFβ was silenced (Fig. 2, middle panels). This suggests a potential requirement for an autocrine regulation process to take place in order to trigger VM. Similarly, when cells were treated with Galunisertib, VM formation was also inhibited (Fig. 2, lower panels). Altogether, these data confirm that a TGFβ signaling axis requiring the kinase activity of the TGFβR is a prerequisite to VM and further support the increase in downstream phosphorylation status of Smad2/3 observed (Fig. 1). Whether TGFβ could further solely and specifically regulate any downstream transcriptional activity was next assessed.
TGFβ triggers activation of Smad2/3 and nuclear translocation of Snail
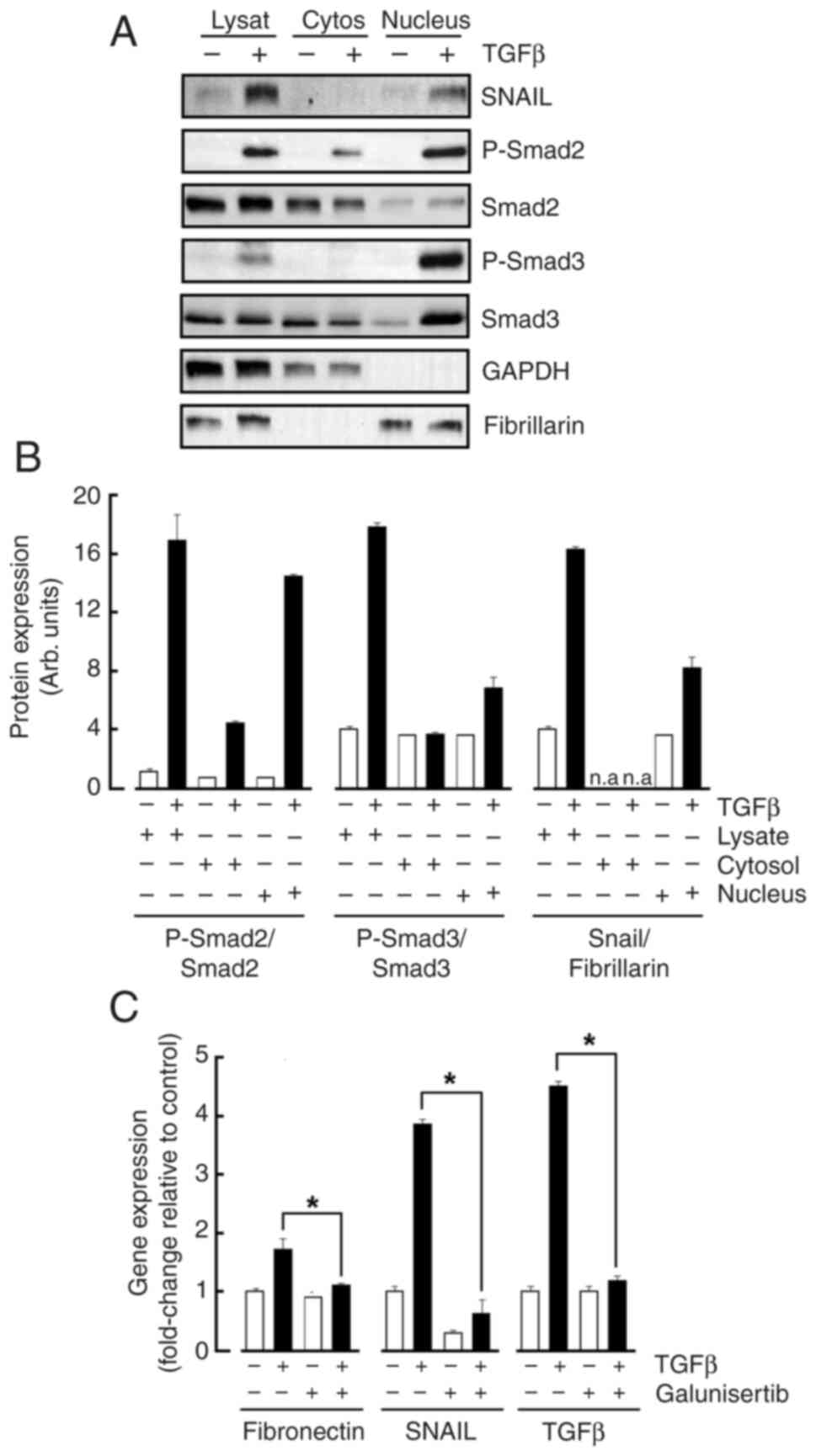
MSCs were treated or not with TGFβ and nuclear fractionation performed as described in the Methods section. Cell lysates were harvested along cytoplasmic and nuclear fractions and immunoblotting performed. The purity control of the cytosolic fraction and of the nuclear material was respectively attested when GAPDH and Fibrillarin were immunoblotted (Fig. 3A). While TGFβ effects in total cell lysates were confirmed (Fig. 3B, Lysate), Snail as well as the phosphorylated Smad2/3 proteins were found to significantly translocate to the Fibrillarin-enriched nucleus fraction (Fig. 3B, Nucleus). Collectively, efficient nuclear translocation in response to TGFβ treatment prompts for the exploration of gene regulation processes. Total RNA was therefore extracted from treated cells and genes of interest assessed by RT-qPCR. TGFβ indeed significantly increased Snail, Fibronectin, and TGFβ gene expression levels confirming increased transcriptional activity in treated MSC (Fig. 3C). Galunisertib pharmacological inhibition of the TGFβR activity prevented those TGFβ-mediated inductions and confirms the necessity of an active signal transducing process (Fig. 3C).
VM triggers differential EMT and TGFβ biomarker gene expression and requires Smad2/3 signaling
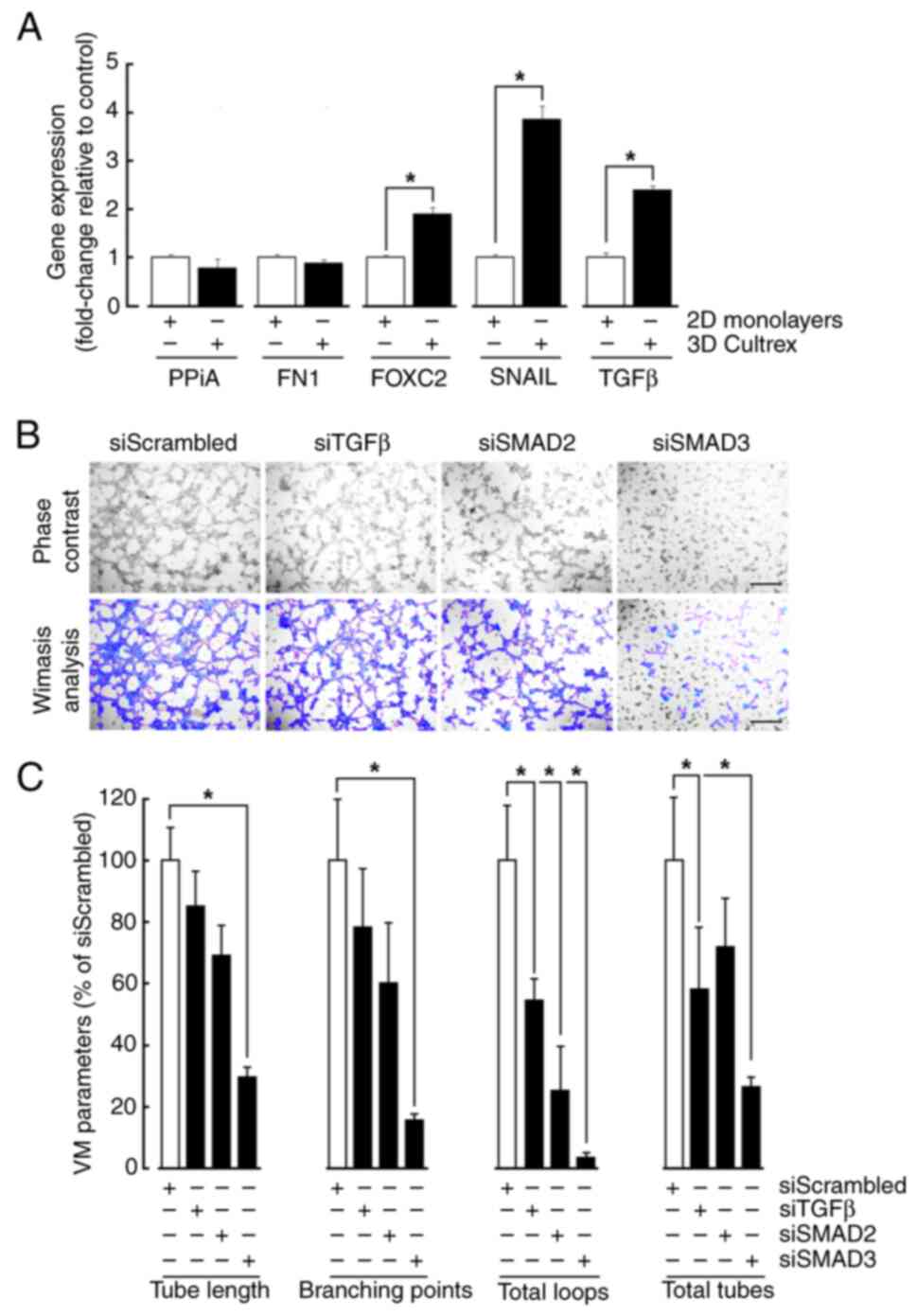
Given the active transcriptional process induced in TGFβ-treated cells, total RNA was extracted from cell monolayers and from cells forming VM on Cultrex. While increased gene expression of Snail, FOXC2, and TGFβ upon VM recapitulated that increase observed in response to TGFβ, gene expression of Fibronectin remained however unchanged (Fig. 4A). As FOXC2 and SNAIL have been previously reported to alter VM formation in MSC (24), transient gene silencing was performed to repress TGFβ, as well as Smad2/3 and cells subsequently seeded on top of Cultrex (Fig. 4B). Accordingly with the downstream effect of Galunisertib on Smad2/3 phosphorylation, silencing of Smad3 reduced all the VM parameters associated with vascular structure formation, including tube length, branching points, total loops, and total tubes (Fig. 4C). This was strongly associated with significant reduction in total loops, and a tendency to reduction of all other parameters in Smad2-silenced cells. Intriguingly, silencing of TGFβ also only altered total loops and tubes formation without affecting other VM parameters (Fig. 4C). Collectively, this evidence suggests that common signaling cues are triggered upon either TGFβ treatment or VM formation. Moreover, given the selective regulation of EMT biomarkers expression, namely that of Fibronectin, complex interplay between these cues will require further investigation although evidence suggests that possible autocrine regulation by TGFβ may regulate VM.
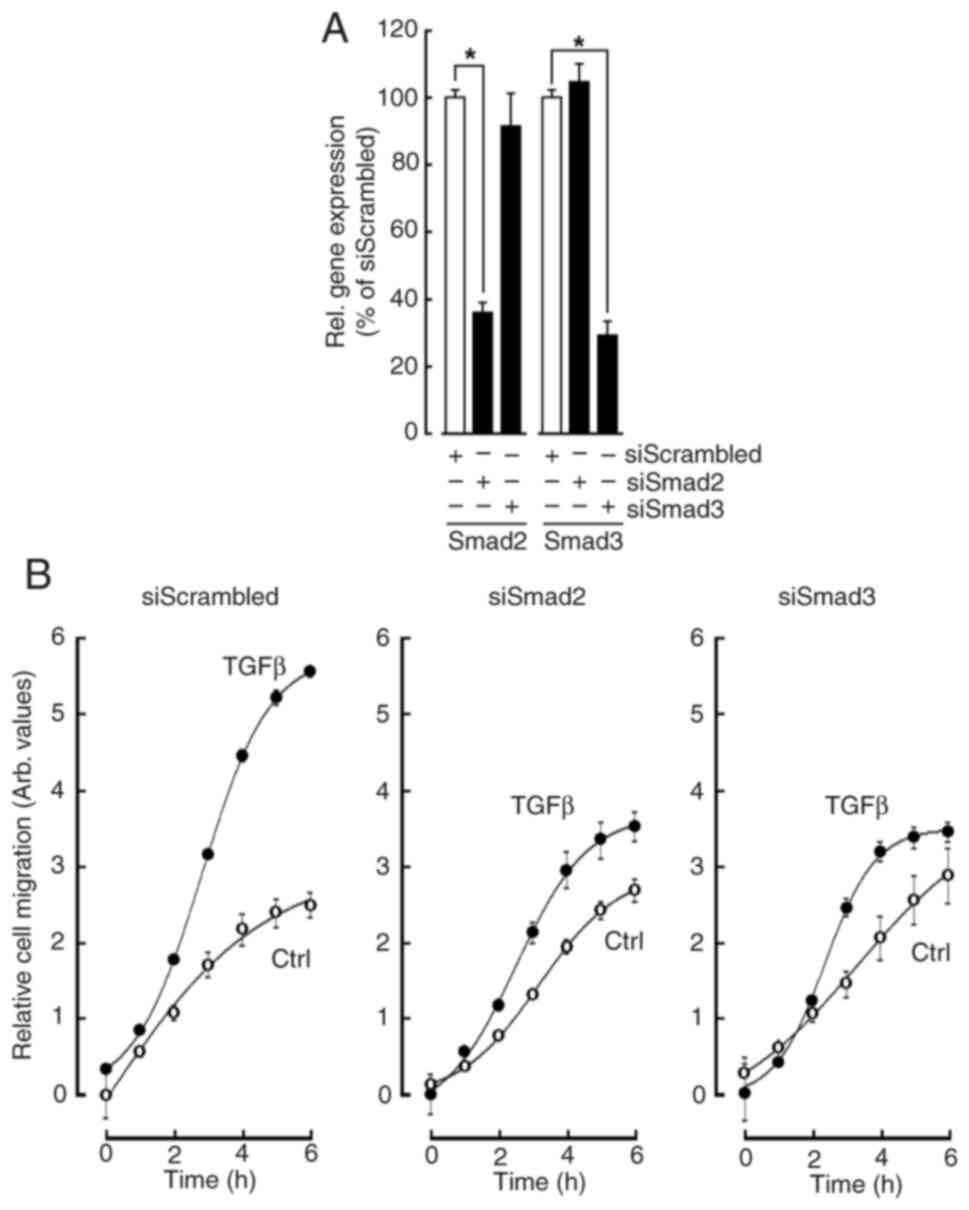
Silencing of Smad2/3 in MSC inhibits their chemotactic response to TGFβ
Given some of the common acquisition of an EMT phenotype between TGFβ treatment and VM formation, the involvement of the Smad2/3 signaling required for MSCs to migrate and form 3D capillary-like structures was next assessed. Coupled to the increased TGFβ expression and a possible autocrine regulation, MSC chemotaxis in response to TGFβ was performed in siRNA transiently silenced cells for Smad2 and Smad3 as described in the Methods section and validated (Fig. 5A). Real-time cell migration was monitored for up to 6 h and found to significantly increase in response to TGFβ (Fig. 5B, left panel, closed circles). When gene silencing was performed to suppress either Smad2 or Smad3, TGFβ chemotaxis was significantly reduced in both conditions (Fig. 5B, middle and right panels respectively). This evidence supports the hypothesis that an autocrine TGFβ-mediated process could regulate in vitro VM formation. More importantly, and along their role in VM formation described above, this represents strong evidence for the involvement of Smad2/3 transducing events in response to such autocrine regulation.
Discussion
Several signaling pathways such as Wnt/β-Catenin, Notch signaling, PI3K/Akt, MAPK/ERK, and Hedgehog pathways regulate MSCs plasticity (32), impacting their ability to differentiate into various cell types and compromising their adaptive capacity within different environments including the TME. In the current study we focused on the TGFβ/SMAD pathway which is recognized to regulate MSCs differentiation into 3D capillary-like structures, a process also believed to maintain stemness (33). As TGFβ signaling through Smad2/3 is particularly important for inducing EMT (34), one can thereafter safely assume that these pathways collectively interact with each other, and with the extracellular matrix (ECM) proteins to regulate MSC plasticity and adaptability, facilitating their role in VM (35). Understanding these interconnected pathways can help to develop novel therapeutic strategies in tissue regeneration repair, or in anticancer therapies.
Cytokines' regulation that promotes MSC mobilization include Stromal Cell-Derived Factor-1 (SDF-1), Granulocyte Colony-Stimulating Factor (G-CSF), Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF), Substance P (SP), and incidentally, TGFβ (36). These cytokines interact through complex networks to regulate MSC motility and migration (37). While MSCs can exhibit anti-tumor effects, such as through modulation of the immune response within the TME (11), tumors create an inflammatory microenvironment that releases several of these cytokines and chemokines which enable MSCs recruitment to the tumor site (38). Notably, vascular progenitors derived from murine bone marrow stromal cells were found to be avidly recruited by vascularizing tumors (39). Once recruited, MSC can interact with tumor cells through paracrine signaling, promoting angiogenesis, tumor growth, and metastasis (40).
Additionally, MSCs are involved in VM a process where vessel-like structures form without endothelial cells, providing a blood supply to the tumor. MSC can contribute to these pseudo-vascular networks by differentiating into endothelial-like cells (24). This involvement of MSCs in VM highlights their role in tumor progression and metastasis (41). While TGFβ signaling is known to promote tumor vasculature by strengthening the association between pericytes and endothelial cells (42), which is crucial for the formation of stable blood vessels, the role of TGFβ-primed MSC was yet to be explored. Here, we demonstrate that TGFβ and TGFβ-mediated signaling play a role in regulating in vitro VM.
TGF-β also plays a crucial role in immunosuppression within the TME through several molecular mechanisms involving, in part, the Smad2 and Smad3 proteins (43). These mechanisms collectively help TGFβ maintain immune homeostasis and prevent overactive immune responses that could lead to autoimmunity or chronic inflammation (44). Strategies, such as using antibodies to block TGFβ receptors have shown promise in reversing immunosuppression. These antibodies have been shown to modulate macrophage polarization and enhance immune cell infiltration, leading to significant anti-tumor effects (45). Since VM is associated with immunosuppression in cancer, our study provides the first evidence of Smad2 and Smad3 involvement in MSC-driven. These signaling intermediates are part of the TGFβ signaling pathway and, through this nuclear translocation, appeared to be crucial in the in vitro formation of VM. Pharmacological evidence further highlights the importance of TGFβR kinase activity in VM. The nuclear translocation of phosphorylated Smad2/3 is necessary for transcriptional regulation, reinforcing their role in MSC-driven VM (46). While we show that TGFβ potentially plays a significant role in regulating MSCs within the context of cancer, in part through the acquisition of mesenchymal properties which enhance their migratory capabilities and VM, more work will be required to better assess how increased TGFβ secreted by MSCs forming VM can shape the TME through the secretion of ECM components and cytokines that would support tumor growth and immune evasion. MSCs secretion of TGFβ and contribution to an immunosuppressive environment may also affect immune cells within the TME and help tumors evade immune detection. Whether MSCs can maintain the cancer stem cells crucial for tumor initiation, progression, and resistance to therapy will also require to be addressed. Altogether, these multifaceted roles of TGFβ definitely make it a critical target for therapeutic strategies aimed at disrupting its signaling pathways to inhibit cancer progression.
On the other hand, the contribution of MSCs to carcinogenic processes can be relatively well exploited in clinical settings by employing them as drug delivery vehicles (47). Thanks to their natural tumor-homing abilities, MSCs can be engineered to deliver anticancer drugs like doxorubicin, paclitaxel, and cisplatin directly to tumor sites (11). This approach has the potential to enhance treatment efficacy while minimizing side effects. Moreover, MSCs can also be engineered to alter the TME so that to inhibit cancer progression by, modifying them to secrete anti-tumor cytokines or to disrupt the supportive stroma around tumors (48). Accordingly, several clinical trials are underway to evaluate the safety and efficacy of MSC-based therapies in cancer treatment (49). Although these strategies are still under investigation, they hold promise for advancing cancer treatment by targeting the complex and diverse roles MSCs play in tumor biology.
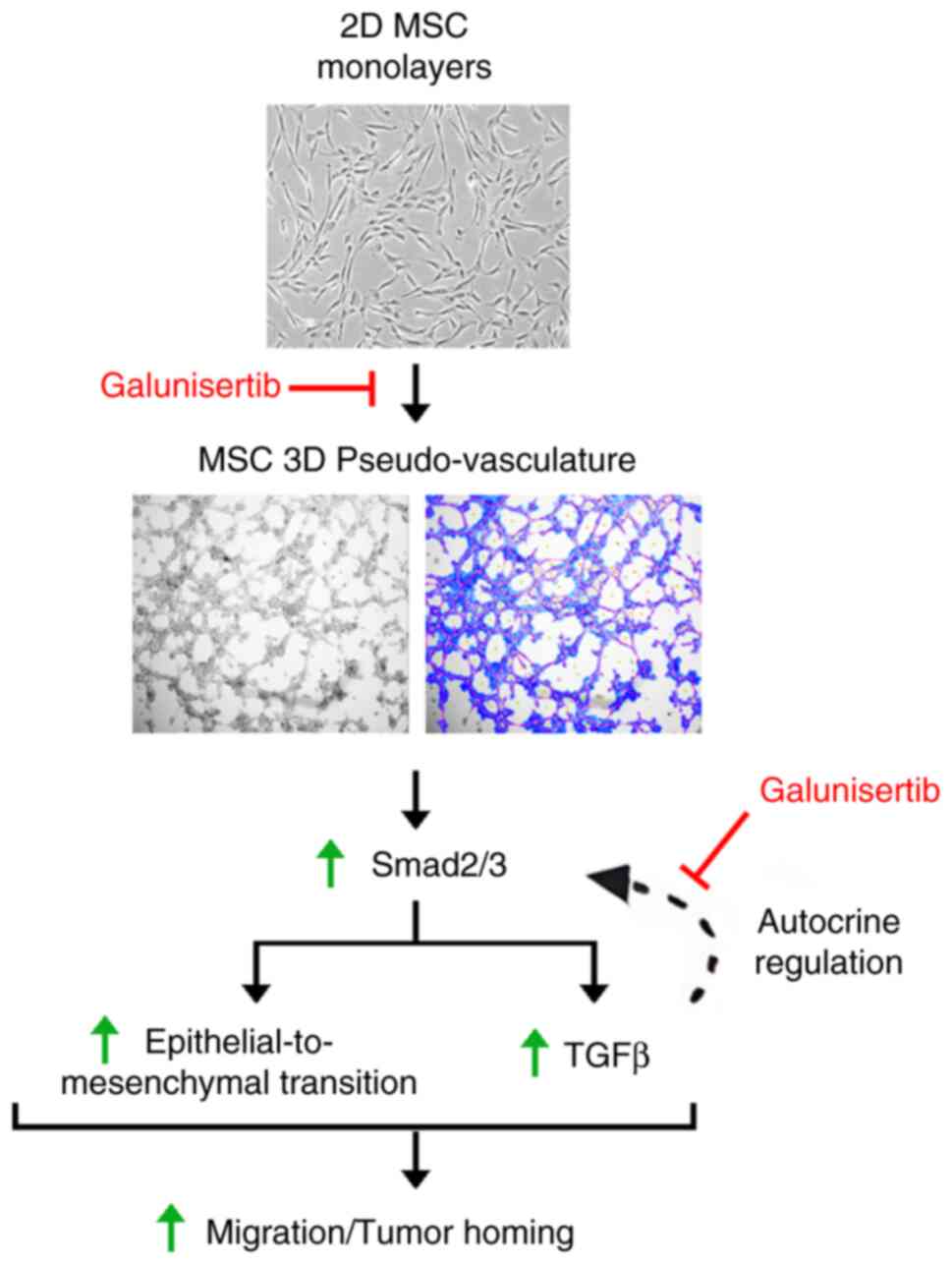
In conclusion, MSCs' key contributions and guiding significance for future research and clinical practice include their tumor homing ability, and capacity to modulate the immune response, which is crucial in the TME (50). Our study highlights in vitro an alternative mechanism involving VM and allowing MSCs to adopt a vascular-like phenotype. The role of Smad2/3 signaling and TGFβ-mediated autocrine regulation in MSCs mobilization and in vitro VM is summarized (Fig. 6). The involvement of TGFβ-induced autocrine signaling in VM could be a target for future anticancer strategies targeting Smad2/3 signaling in MSCs. Unraveling the roles of Smad2/3 in VM highlights their potential as therapeutic targets in cancer treatment. Modulating this pathway could provide means to disrupt the VM process and inhibit tumor progression. Drugs that inhibit TGFβR activity can indirectly modulate Smad2/3 signaling. Several of these inhibitors are currently being investigated for their ability to disrupt TGFβ-mediated cancer progression (51,52). While these experimental approaches are still in early stages of research, further studies are essential to evaluate their safety and effectiveness in cancer treatment. Despite their potential, MSCs face several challenges in clinical applications. Among those, combining MSC-based therapies with other treatments like chemotherapy, radiotherapy, and immunotherapy could eventually enhance overall treatment efficacy but may show limitations in clinical trials (53). Importantly, in the current study, additional research is needed to explore their potential in specifically targeting MSCs mobilization processes.
Acknowledgements
Not applicable.
Funding
This work was funded by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC, grant no. RGPIN-2024-04541).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
KPDG, MER, AZ, MD and BA contributed to the study conception and design. Material preparation, data collection and analysis were performed by KPDG, MER, AZ and MD. KPDG, AZ and BA confirm the authenticity of all the raw data. The first draft of the manuscript was written by KPDG and BA. All authors commented on previous versions of the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The need for ethics approval for the use of human bone marrow-derived mesenchymal stromal/stem cells was waived by the Université du Québec à Montréal ethics committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Authors' information
BA holds an institutional Research Chair in Cancer Prevention and Treatment.
Glossary
Abbreviations
Abbreviations:
|
CSCs |
cancer stem cells |
|
ECM |
extracellular matrix |
|
EMT |
epithelial-to-mesenchymal transition |
|
PPIA |
peptidylprolyl isomerase A |
|
TGFβ |
transforming growth factor β |
|
TGFβR |
transforming growth factor β receptor |
|
TME |
tumor microenvironment |
|
VM |
vasculogenic mimicry |
References
|
Grafe I, Alexander S, Peterson JR, Snider TN, Levi B, Lee B and Mishina Y: TGF-β family signaling in mesenchymal differentiation. Cold Spring Harb Perspect Biol. 10:a0222022018. View Article : Google Scholar : PubMed/NCBI | |
|
Yang X, Tian S, Fan L, Niu R, Yan M, Chen S, Zheng M and Zhang S: Integrated regulation of chondrogenic differentiation in mesenchymal stem cells and differentiation of cancer cells. Cancer Cell Int. 22:1692022. View Article : Google Scholar : PubMed/NCBI | |
|
Yao JC, Oetjen KA, Wang T, Xu H, Abou-Ezzi G, Krambs JR, Uttarwar S, Duncavage EJ and Link DC: TGF-β signaling in myeloproliferative neoplasms contributes to myelofibrosis without disrupting the hematopoietic niche. J Clin Invest. 132:e1540922022. View Article : Google Scholar : PubMed/NCBI | |
|
Wei E, Hu M, Wu L, Pan X, Zhu Q, Liu H and Liu Y: TGF-β signaling regulates differentiation of MSCs in bone metabolism: Disputes among viewpoints. Stem Cell Res Ther. 15:1562024. View Article : Google Scholar : PubMed/NCBI | |
|
Liu F, Xie J, Zhang X, Wu Z, Zhang S, Xue M, Chen J, Yang Y and Qiu H: Overexpressing TGF-β1 in mesenchymal stem cells attenuates organ dysfunction during CLP-induced septic mice by reducing macrophage-driven inflammation. Stem Cell Res Ther. 11:3782020. View Article : Google Scholar : PubMed/NCBI | |
|
Wan M, Li C, Zhen G, Jiao K, He W, Jia X, Wang W, Shi C, Xing Q, Chen YF, et al: Injury-activated transforming growth factor β controls mobilization of mesenchymal stem cells for tissue remodeling. Stem Cells. 30:2498–2511. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Fan XL, Zhang Y, Li X and Fu QL: Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 77:2771–2794. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Denko NC and Giaccia AJ: Tumor hypoxia, the physiological link between Trousseau's syndrome (carcinoma-induced coagulopathy) and metastasis. Cancer Res. 61:795–798. 2001.PubMed/NCBI | |
|
Emami Nejad A, Najafgholian S, Rostami A, Sistani A, Shojaeifar S, Esparvarinha M, Nedaeinia R, Haghjooy Javanmard S, Taherian M, Ahmadlou M, et al: The role of hypoxia in the tumor microenvironment and development of cancer stem cell: A novel approach to developing treatment. Cancer Cell Int. 21:622021. View Article : Google Scholar : PubMed/NCBI | |
|
Liang W and Chen X, Zhang S, Fang J, Chen M, Xu Y and Chen X: Mesenchymal stem cells as a double-edged sword in tumor growth: Focusing on MSC-derived cytokines. Cell Mol Biol Lett. 26:32021. View Article : Google Scholar : PubMed/NCBI | |
|
Aravindhan S, Ejam SS, Lafta MH, Markov A, Yumashev AV and Ahmadi M: Mesenchymal stem cells and cancer therapy: Insights into targeting the tumour vasculature. Cancer Cell Int. 21:1582021. View Article : Google Scholar : PubMed/NCBI | |
|
Andonegui-Elguera MA, Alfaro-Mora Y, Cáceres-Gutiérrez R, Caro-Sánchez CHS, Herrera LA and Díaz-Chávez J: An overview of vasculogenic mimicry in breast cancer. Front Oncol. 10:2202020. View Article : Google Scholar : PubMed/NCBI | |
|
Yao J, Sun L, Gao F and Zhu W: Mesenchymal stem/stromal cells: Dedicator to maintain tumor homeostasis. Hum Cell. 38:212024. View Article : Google Scholar : PubMed/NCBI | |
|
den Hollander P, Maddela JJ and Mani SA: Spatial and temporal relationship between epithelial-mesenchymal transition (EMT) and stem cells in cancer. Clin Chem. 70:190–205. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Hass R: Role of MSC in the tumor microenvironment. Cancers (Basel). 12:21072020. View Article : Google Scholar : PubMed/NCBI | |
|
Hill BS, Pelagalli A, Passaro N and Zannetti A: Tumor-educated mesenchymal stem cells promote pro-metastatic phenotype. Oncotarget. 8:73296–73311. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Fasano M, Pirozzi M, Miceli CC, Cocule M, Caraglia M, Boccellino M, Vitale P, De Falco V, Farese S, Zotta A, et al: TGF-β modulated pathways in colorectal cancer: New potential therapeutic opportunities. Int J Mol Sci. 25:74002024. View Article : Google Scholar : PubMed/NCBI | |
|
Sritharan S and Sivalingam N: Secretion of IL-6 and TGF-β2 by colon cancer cells may promote resistance to chemotherapy. Ind J Clin Biochem. 2024. View Article : Google Scholar | |
|
Dumont N and Arteaga CL: Transforming growth factor-beta and breast cancer: Tumor promoting effects of transforming growth factor-beta. Breast Cancer Res. 2:125–132. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Huang K, Han Y, Chen Y, Shen H, Zeng S and Cai C: Tumor metabolic regulators: Key drivers of metabolic reprogramming and the promising targets in cancer therapy. Mol Cancer. 24:72025. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang H, Li S, Wang D, Liu S, Xiao T, Gu W, Yang H, Wang H, Yang M and Chen P: Metabolic reprogramming and immune evasion: The interplay in the tumor microenvironment. Biomark Res. 12:962024. View Article : Google Scholar : PubMed/NCBI | |
|
Paul D and Nedelcu AM: The underexplored links between cancer and the internal body climate: Implications for cancer prevention and treatment. Front Oncol. 12:10400342022. View Article : Google Scholar : PubMed/NCBI | |
|
Slominski RM, Raman C, Chen JY and Slominski AT: How cancer hijacks the body's homeostasis through the neuroendocrine system. Trends Neurosci. 46:263–275. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Roy ME, Veilleux C and Annabi B: In vitro biomaterial priming of human mesenchymal stromal/stem cells: Implication of the Src/JAK/STAT3 pathway in vasculogenic mimicry. Sci Rep. 14:214442024. View Article : Google Scholar : PubMed/NCBI | |
|
Schneider CA, Rasband WS and Eliceiri KW: NIH image to ImageJ: 25 Years of image analysis. Nat Methods. 9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Roy ME, Veilleux C, Paquin A, Gagnon A and Annabi B: Transcriptional regulation of CYR61 and CTGF by LM98: A synthetic YAP-TEAD inhibitor that targets in-vitro vasculogenic mimicry in glioblastoma cells. Anticancer Drugs. 35:709–719. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Pratt J, Haidara K and Annabi B: MT1-MMP expression levels and catalytic functions dictate LDL receptor-related protein-1 ligand internalization capacity in U87 glioblastoma cells. Int J Mol Sci. 23:142142022. View Article : Google Scholar : PubMed/NCBI | |
|
Holmgaard RB, Schaer DA, Li Y, Castaneda SP, Murphy MY, Xu X, Inigo I, Dobkin J, Manro JR, Iversen PW, et al: Targeting the TGFβ pathway with galunisertib, a TGFβRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J Immunother Cancer. 6:472018. View Article : Google Scholar : PubMed/NCBI | |
|
Sicard AA, Suarez NG, Cappadocia L and Annabi B: Functional targeting of the TGF-βR1 kinase domain and downstream signaling: A role for the galloyl moiety of green tea-derived catechins in ES-2 ovarian clear cell carcinoma. J Nutr Biochem. 87:1085182021. View Article : Google Scholar : PubMed/NCBI | |
|
Djediai S, Gonzalez Suarez N, El Cheikh-Hussein L, Rodriguez Torres S, Gresseau L, Dhayne S, Joly-Lopez Z and Annabi B: MT1-MMP cooperates with TGF-β receptor-mediated signaling to trigger SNAIL and Induce epithelial-to-mesenchymal-like transition in U87 glioblastoma cells. Int J Mol Sci. 22:130062021. View Article : Google Scholar : PubMed/NCBI | |
|
Antoon R, Overdevest N, Saleh AH and Keating A: Mesenchymal stromal cells as cancer promoters. Oncogene. 43:3545–3555. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Liu S, Ren J, Hu Y, Zhou F and Zhang L: TGFβ family signaling in human stem cell self-renewal and differentiation. Cell Regen. 13:262024. View Article : Google Scholar : PubMed/NCBI | |
|
Wang J, Peng J, Chen Y, Nasser MI and Qin H: The role of stromal cells in epithelial-mesenchymal plasticity and its therapeutic potential. Discov Oncol. 15:132024. View Article : Google Scholar : PubMed/NCBI | |
|
Novoseletskaya ES, Evdokimov PV and Efimenko AY: Extracellular matrix-induced signaling pathways in mesenchymal stem/stromal cells. Cell Commun Signal. 21:2442023. View Article : Google Scholar : PubMed/NCBI | |
|
Nam D, Park A, Dubon MJ, Yu J, Kim W, Son Y and Park KS: Coordinated regulation of mesenchymal stem cell migration by various chemotactic stimuli. Int J Mol Sci. 21:85612020. View Article : Google Scholar : PubMed/NCBI | |
|
Cottler-Fox MH, Lapidot T, Petit I, Kollet O, DiPersio JF, Link D and Devine S: Stem cell mobilization. Hematology Am Soc Hematol Educ Program. 419–437. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Ridge SM, Sullivan FJ and Glynn SA: Mesenchymal stem cells: Key players in cancer progression. Mol Cancer. 16:312017. View Article : Google Scholar : PubMed/NCBI | |
|
Annabi B, Naud E, Lee YT, Eliopoulos N and Galipeau J: Vascular progenitors derived from murine bone marrow stromal cells are regulated by fibroblast growth factor and are avidly recruited by vascularizing tumors. J Cell Biochem. 91:1146–1158. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Frisbie L, Buckanovich RJ and Coffman L: Carcinoma-associated mesenchymal stem/stromal cells: Architects of the pro-tumorigenic tumor microenvironment. Stem Cells. 40:705–715. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang J, Qiao L, Liang N, Xie J, Luo H, Deng G and Zhang J: Vasculogenic mimicry and tumor metastasis. J BUON. 21:533–541. 2016.PubMed/NCBI | |
|
Zonneville J, Safina A, Truskinovsky AM, Arteaga CL and Bakin AV: TGF-β signaling promotes tumor vasculature by enhancing the pericyte-endothelium association. BMC Cancer. 18:6702018. View Article : Google Scholar : PubMed/NCBI | |
|
Yoshimura A and Muto G: TGF-β function in immune suppression. Curr Top Microbiol Immunol. 350:127–147. 2011.PubMed/NCBI | |
|
Konkel JE, Zhang D, Zanvit P, Chia C, Zangarle-Murray T, Jin W, Wang S and Chen W: Transforming growth factor-β signaling in regulatory T cells controls T helper-17 cells and tissue-specific immune responses. Immunity. 46:660–674. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Guo C, Sun H, Du Y, Dai X, Pang Y, Han Z, Xiong X, Li S, Zhang J, Zheng Q and Gui X: Specifically blocking αvβ8-mediated TGF-β signaling to reverse immunosuppression by modulating macrophage polarization. J Exp Clin Cancer Res. 44:12025. View Article : Google Scholar : PubMed/NCBI | |
|
Itoh F, Itoh S, Adachi T, Ichikawa K, Matsumura Y, Takagi T, Festing M, Watanabe T, Weinstein M, Karlsson S and Kato M: Smad2/Smad3 in endothelium is indispensable for vascular stability via S1PR1 and N-cadherin expressions. Blood. 119:5320–5628. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Tashima T: Mesenchymal stem cell (MSC)-based drug delivery into the brain across the blood-brain barrier. Pharmaceutics. 16:2892024. View Article : Google Scholar : PubMed/NCBI | |
|
Minev T, Balbuena S, Gill JM, Marincola FM, Kesari S and Lin F: Mesenchymal stem cells-the secret agents of cancer immunotherapy: Promises, challenges, and surprising twists. Oncotarget. 15:793–805. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Wu X, Jiang J, Gu Z, Zhang J, Chen Y and Liu X: Mesenchymal stromal cell therapies: Immunomodulatory properties and clinical progress. Stem Cell Res Ther. 11:3452020. View Article : Google Scholar : PubMed/NCBI | |
|
Lan T, Luo M and Wei X: Mesenchymal stem/stromal cells in cancer therapy. J Hematol Oncol. 14:1952021. View Article : Google Scholar : PubMed/NCBI | |
|
Kim BG, Malek E, Choi SH, Ignatz-Hoover JJ and Driscoll JJ: Novel therapies emerging in oncology to target the TGF-β pathway. J Hematol Oncol. 14:552021. View Article : Google Scholar : PubMed/NCBI | |
|
Guo Y, Wang Z, Zhou H, Pan H, Han W, Deng Y, Li Q, Xue J, Ge X, Wang S, et al: First-in-human study of GFH018, a small molecule inhibitor of transforming growth factor-β receptor I inhibitor, in patients with advanced solid tumors. BMC Cancer. 24:4442024. View Article : Google Scholar : PubMed/NCBI | |
|
Margiana R, Markov A, Zekiy AO, Hamza MU, Al-Dabbagh KA, Al-Zubaidi SH, Hameed NM, Ahmad I, Sivaraman R, Kzar HH, et al: Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review. Stem Cell Res Ther. 13:3662022. View Article : Google Scholar : PubMed/NCBI |