Post‑irradiation mastoid mucosa disease in patients with nasopharyngeal carcinoma after radiotherapy: A large‑cohort and long‑term study
- Authors:
- Published online on: June 23, 2025 https://doi.org/10.3892/ol.2025.15152
- Article Number: 406
-
Copyright: © Xie et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Nasopharyngeal carcinoma (NPC) is a specific type of head and neck cancer and is endemic in East Asia and Southeast Asia (1). Clinically, radiotherapy (RT) is the primary treatment for NPC. As epithelial cells are radiation-sensitive, RT causes these cells to become degenerative and prone to desquamation, increasing the susceptibility to pathogenic organisms in the irradiated target regions (2). Diseases of the middle ear include eustachian tube dysfunction (ETD), otitis media with effusion (OME) and mastoiditis (2). After curative RT treatment for newly diagnosed head and neck cancer, the incidence of mastoiditis varies from 4.9 to 33% (2–4). The peak incidence of mastoiditis occurs within 6 months after RT before decreasing and stabilizing (3,5). Patients with mastoid mucosa disease (MMD) often have complaints including tinnitus, clogged ears and hearing loss. Hence, there is no doubt that these persistent conditions impair patients' quality of life. Mild mastoiditis is self-limited, but severe mastoiditis requires a visit to an otolaryngologist due to the risk of life-threatening complications such as sinus thrombosis (6,7). Mastoiditis reportedly induces intracranial abscesses in patients with NPC (8). Persistent severe MMD, which is frequently associated with chronic OME or mastoiditis, can have significant long-term consequences, including anatomical and structural damage, auditory deficits and neurological or cranial complications if left untreated or inadequately managed.
Few studies have focused on the risks of post-irradiation MMD, and to the best of our knowledge, no studies have investigated persistent MMD. Age (3), tumor invasion (3,9), radiation dose (2,5,10–12), time after radiation (3), chemotherapy (3) and chronic otitis media with effusion (2) are reported risk factors for post-irradiation mastoiditis. However, the risks of inflammation indicators, drug administration and targeted therapy in patients with MMD after RT have remained elusive. Furthermore, the long-term incidence of post-RT MMD has remained to be determined. Hence, in the present study, the incidence and risk of persistent MMD were explored in a large-scale and long-term follow-up cohort after exposure to radiation.
Patients and methods
Study design
One of the goals of the GARTP study (ChiCTRROC-17012658), a prospective observational clinical trial, is to identify risk factors for radiation-induced normal tissue complications in patients with NPC (13). The present study included 1,238 eligible patients from the cohort of the GARTP study, for which 1,410 patients with non-metastatic NPC were recruited from Sun Yat-sen University Cancer Center (SYSUCC, Guangzhou, China) from January 2010 to December 2015. The inclusion criteria were as follows: i) Newly diagnosed NPC proven by histology; ii) initial diagnostic magnetic resonance imaging (MRI) available at SYSUCC; iii) medical records available for review; iv) no prior history of otologic pathology; v) no pathologically confirmed residual tumor after RT; vi) RT with or without chemotherapy; vii) no malignancy other than NPC; and viii) at least 3 consecutive post-RT MRIs performed at SYSUCC.
Patients and characteristics
Two clinicians, blinded to the patients' MMD status, independently restaged each patient's pretreatment clinical records using the 8th edition of the International Union against Cancer/American Joint Committee on Cancer staging manual (14). All of the final decisions concerning the different findings were unanimous. Nasopharyngeal recurrence was defined as pathologically confirmed recurrence of a nasopharyngeal tumor. Inflammation markers were measured via blood tests within one week before RT. Ambroxol, budesonide, clarityne, myrtol and nasal irrigation were prescribed during RT.
All patients received intensity-modulated RT (IMRT) or non-IMRT with or without chemotherapy. RT was delivered at 66–70 Gy at 1.8–2 Gy per fraction once a day and five fractions per week. Platinum-based chemotherapy was prescribed, with or without a combination of one or two of the following regimens, including paclitaxel, gemcitabine and fluorouracil.
Image assessment of MMD
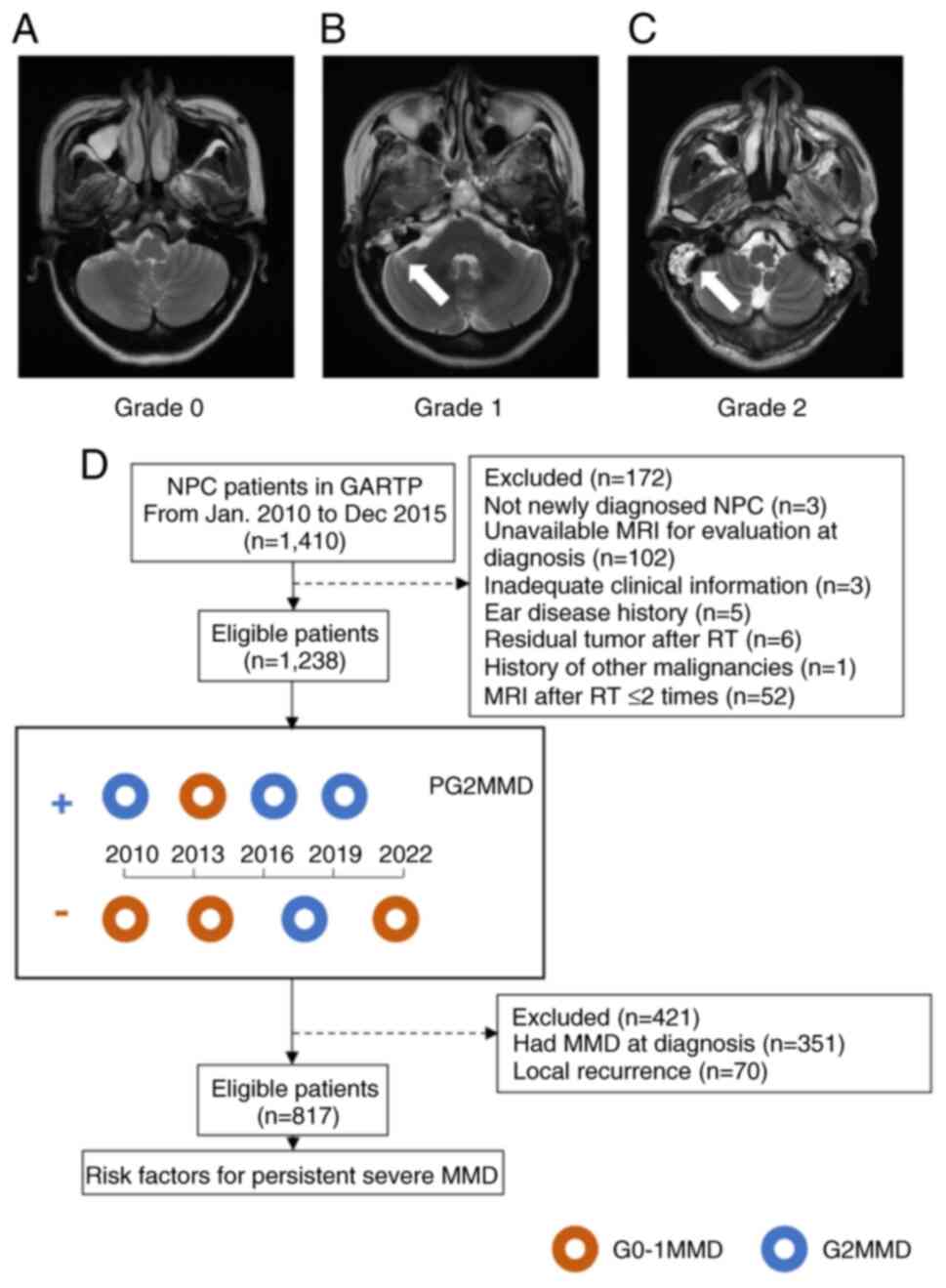
MRI evaluation was performed at 1.5- or 3.0-Tesla MRI units using a standard head coil. The published criteria were used as a measure to evaluate the severity of MMD (15). The severity of MMD was graded according to the high signal of mastoid opacification on T2-weighted MRI as follows: Grade 0 (not detected), Grade 1 (observed <50% air cells involved), and Grade 2 (observed in all mastoid air cells) (Fig. 1A-C) (5,10). Representative MRI scans are shown in Fig. 1. Owing to a discrepancy between the left and right mastoids in patients with NPC, both sides were examined independently for this study. If either the left or right mastoid was classified as Grade 2 MMD (G2MMD), the patient was recorded as Grade 2, which was referred to as severe MMD. The incidence of G2MMD was calculated as follows: Patients with MRI-based G2MMD/total patients at the indicated follow-up period × 100. The missing data were not included in the calculation. The primary endpoint was persistent G2MMD (PG2MMD) after RT, which was defined as at least half of ipsilateral MRI-based MMD scored as Grade 2 after RT (Fig. 1D). When a patient suffered from G2MMD for more than half of the follow-up period, the case was recorded as persistent severe MMD (PG2MMD) (Fig. 1D). The time to event for actuarial analysis of PG2MMD was defined as the time from RT completion to the first occurrence of G2MMD (for the PG2MMD cohort) or to death or the last follow-up (for the non-PG2MMD cohort), whichever occurred first. The MRIs taken at diagnosis, and 3, 6, 12, 24, 36, 48, 60, 72, 84, 96, 108 and 120 months after the end of radiation, were examined. The last follow-up was in December 2022.
Statistical analysis
The statistical tests were performed using R software (version 4.2.1). The clinical and pathological characteristics and parameters were analyzed with the chi-squared test and t-test. The mean radiation dose to mastoid (mastoid Dmean) and the volume to mastoid (mastoid volume) were transformed into binary variables using the optimal cut-off point based on the area under the receiver operating characteristic (ROC) curve (AUC). The Kaplan-Meier method and the log-rank test were applied to compare patient survival curves among the groups of MMD. The Schoenfeld individual test was used to assess the proportional hazards assumption for the Cox regression model fit. After the validity to assume the proportional hazards, the univariate Cox proportional hazards model was applied to evaluate the association with the time to PG2MMD of patients. Baseline characteristics, inflammation indicators and pretreatment mastoid status before RT, treatment-related factors and drug administration were entered into the model. Missing MRI-based MMD evaluations were retained as missing data and not subjected to statistical imputation. Factors with P<0.200 in the univariate analysis were included into the multivariate Cox regression. The final models were determined by backward stepwise selection. All of the Kaplan-Meier curves of the adjusted Cox model were generated with the R package ‘adjustedCurves’ and the method was ‘conditional’. A value of P<0.05 was considered to indicate statistical significance.
Results
Clinical characteristics
The sample of patients with NPC consisted of 1,238 eligible patients (915 males and 323 females), with a mean age of 42.82 years (range, 18–76 years) at the time of diagnosis. A total of 275 (22.21%) patients had T1-2 disease and 963 (77.79%) had T3-4 disease. A total of 138 patients (11.15%) were N0 and 1,100 (88.85%) were N1-3 (Table SI). Prior to RT, 271 (21.89%) patients with NPC had G2MMD at diagnosis, whereas 887 (71.65%) did not have any signal of mastoid opacification on MRI. The median post-RT time was 90.56 (range, 6.00–151.86) months. Due to patient compliance and the judgment of the radio-oncologists, not every patient was able to complete all of the post-RT MRI follow-ups. Overall, 8,827 MRI group scans were evaluated, each representing the nasopharyngeal and neck MRIs at the follow-up visits.
Incidence of post-irradiation MMD
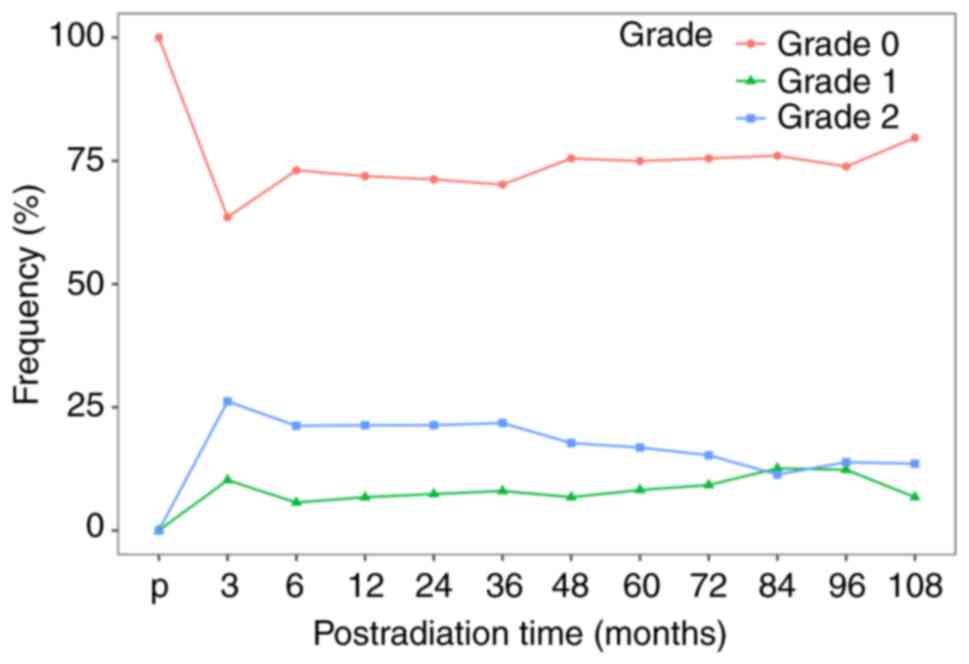
A total of 746 (60.3%) patients were diagnosed with G2MMD at least once after RT. The median post-RT time to develop G2MMD at the first follow-up was 3 (range, 3 to 120) months (data not shown). The peak incidence of G2MMD was at 3 months after RT and continued to decrease thereafter (Fig. S1). The incidences of G2MMD at 3, 6, 12, 24, 60 and 120 months after RT were 35.8% (388/1,083), 28.9% (300/1,039), 26.9% (301/1121), 27.3% (297/1,089), 22.7% (160/706) and 18.8% (6/32), respectively. The incidence of the PG2MMD was 25.5% (316/1,238) in this cohort.
To identify the risk factors for PG2MMD, patients with NPC without MMD pretreatment or local recurrence in the nasopharynx (n=817) were selected. The baseline characteristics are shown in Table I. The mean age was 42.33 years (range, 18–76 years), with 592 male patients. In this cohort, the incidences of G2MMD at 3, 6, 12, 24, 60 and 120 months after RT were 26.2% (187/714), 21.3% (146/687), 21.4% (158/740), 21.4% (153/716), 16.8% (82/487) and 9.5% (2/21), respectively (Fig. 2). The incidence of PG2MMD was 17.9% (146/817).
Table I.Baseline characteristics of patients with nasopharyngeal carcinoma without pretreatment mastoid mucosa disease or nasopharyngeal recurrence (n=817). |
Risk factors associated with persistent G2MMD after RT
Radiation dose was reported to be a significant predictor of MMD among the dosimetry factors of RT (2,5,10–12). Patients who received a higher mean dose of RT to the mastoids were more likely to suffer from P2GMMD. However, these indicators related to radiation dose were not measured in a large-scale cohort and none of them were assessed for persistent disease. In addition, it was investigated whether the volume of mastoid (mastoid volume) associated with radiation-induced MMD. Hence, the mastoid Dmean and mastoid volume were transformed into binary variables according to the ROC curves (Table SII). Patients with a mastoid Dmean >37.30 Gy were assigned to the high mastoid Dmean group, while patients with a mastoid volume ≤18.45 mm2 were classified as the low mastoid volume group.
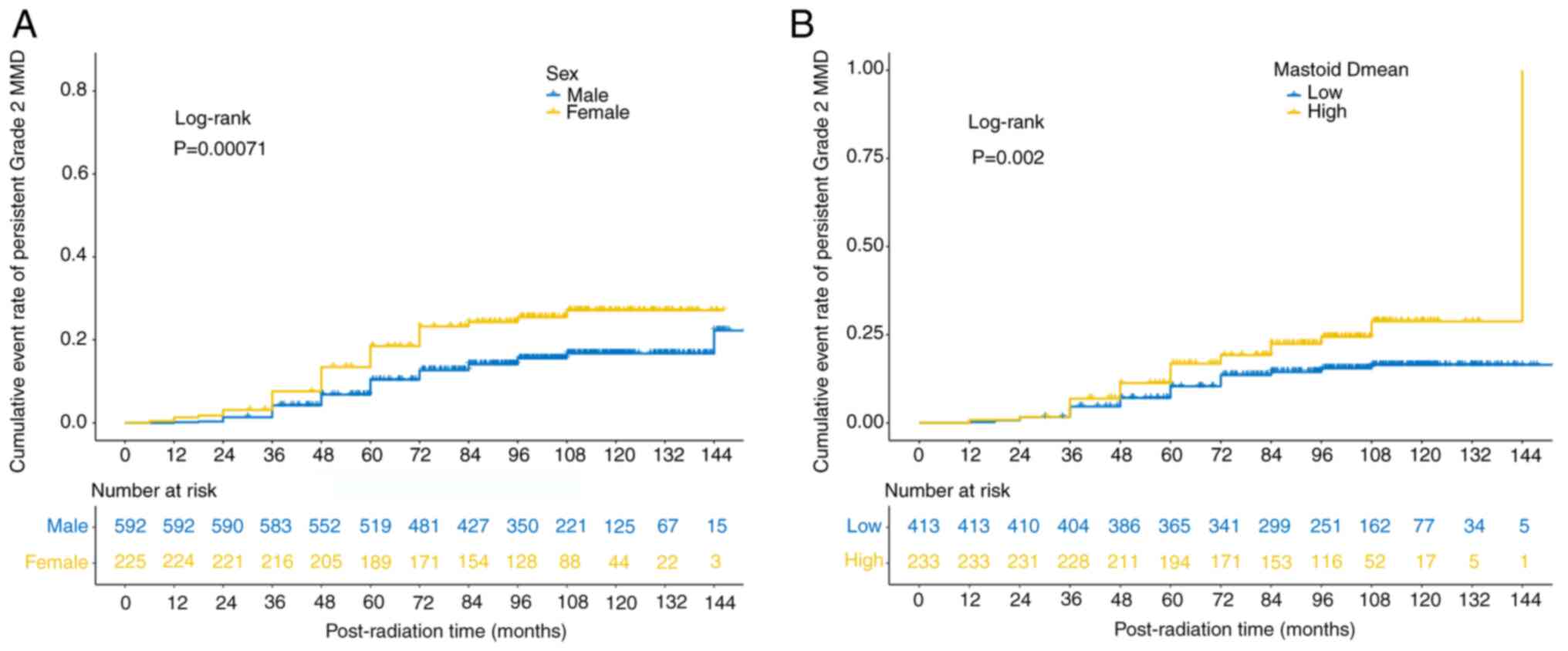
After analyzing the factors affecting MMD aggravation after RT, it was found that smoking, tumor invasion of the ipsilateral wall, the pharyngeal recess (PR), the pharyngeal opening of the eustachian tube (POET), the levator veli palatine muscle (LVPM), the tensor veli palatine muscle (TVPM) and mastoid volume were significant risk factors in the univariate analyses (P<0.05, Table II), but not in the multivariate analyses (P>0.05; Table II). Age, sex and mastoid Dmean were risk factors in both the univariate and multivariate analyses (P<0.05; Fig. 3A and B, Table II).
Table II.Prognostic factors of persistent grade 2 mastoid mucosa disease by univariate and multivariate analyses for patients with nasopharyngeal without pretreatment mastoiditis or local recurrence in the nasopharynx. |
The ROC curves of age, sex and mastoid Dmean were used to evaluate the prediction efficacy of the final Cox model. The AUC values for the 1-, 2- and 10-year incidences of PG2MMD after RT were 0.894, 0.746 and 0.780, respectively (Fig. S2).
Discussion
In the present study, the incidences and risk factors for persistent severe MMD in patients with NPC after RT over a long-term period were investigated. Age, sex and mastoid Dmean were favorable factors for developing PG2MMD after radiation in patients with NPC. The location of tumor invasion, inflammatory indicators within one week before RT, targeted therapy and drugs administered during radiation were not significant risk factors for PG2MMD. To the best of our knowledge, this was the largest cohort to report persistent MMD in patients with NPC after RT with long-term follow-up.
The present study revealed similar results for the incidence of severe MMD as reported within the early post-RT period (2–5). The radiation-induced acute response requires a standard 6- to 8-week period to turn over (16). During this period, the tissue around the irradiated nasopharynx swells, which results in a narrow orifice of the eustachian tube and ETD. Deterioration of eustachian tube function persisted and an inflammatory reaction was noted in the upper respiratory tract, including the maxillary sinus and nasopharynx. Unlike OME in patients with NPC at the pre-RT stage, post-RT OME is due to both organic obstruction and functional impairment of the tube. Functional impairment is caused by inflammatory processes within the radiation field due to radiation-induced tissue reactions (i.e., mucositis, desquamation or edema) and tissue damage (i.e., atrophy, fibrosis, degeneration or necrosis) in the radiation-exposed area. The destruction of the effusion evacuation system subsequently causes MMD and contributes to the peak incidence of G2MMD after 3 months of RT. Reactive edema may be gradually absorbed and removed, which leads to a lower incidence of MMD as time progresses. As the acute effect of RT receded, the incidence of G2MMD decreased 3 months after RT.
Patients who received more radiation to the mastoids (a higher mastoid Dmean) from RT were more likely to suffer from PG2MMD. In the long-term study, it was discovered that the mastoid Dmean value was the only significant irradiation factor associated with PG2MMD, and there was a limited difference in the prescribed RT, such as the total dose and fractions (P>0.05), between patients with NPC with and without PG2MMD. Radiation dose was reported to be a significant predictor of MMD among the dose volume factors of RT, but, to the best of our knowledge, it has not been used in a large-scale cohort (2,5,10–12). A mastoid Dmean of 37.30 Gy was suggested as the dose limit for PG2MMD. Both univariate and multivariate analyses revealed that a higher mastoid Dmean was a significant factor associated with PG2MMD (P<0.05). As aforementioned, radiation injury to the eustachian tubal region causes post-irradiation OME, particularly at high radiation doses, and functional impairment is caused by radiation-induced inflammation (2). The destruction of the effusion evacuation system leads to a higher incidence of PG2MMD.
Female patients with NPC were more likely to have PG2MMD (P=0.001), with an HR of 1.594 (95% CI, 1.035 to 2.455) in the multivariate analysis. Anatomic variability in mastoid morphology and sex-specific hormonal profiles may contribute to the differential incidence of PG2MMD. Consistent with a previous study (17), in the present study, significantly smaller mastoid volumes were observed in females than in males (P<0.001), a structural disparity that could increase mechanical vulnerability and reduce mucosal drainage efficiency, potentially elevating PG2MMD susceptibility. In general, estrogens at physiological concentrations are thought to play an immune-stimulating role by upregulating both cellular and humoral immunity, whereas androgens have an anti-inflammatory effect (18). This interplay between anatomic constraints and hormonally mediated immune regulation may contribute to sex-specific disparities in the incidence of PG2MMD.
Patients with NPC aged >50 years at diagnosis were more likely to have PG2MMD (P=0.016), with an HR of 1.699 (95% CI, 1.105 to 2.613). An age-related decline in immune function (19) increases susceptibility to infections. Comorbidities, such as diabetes, hypertension and cardiovascular disease, which impair microvascular circulation and tissue repair ability, are common in older patients. These factors collectively exacerbate radiation-induced damage in the mastoid region, disrupting mucosal integrity and increasing the risk of MMD. The interplay of immunosenescence, chronic inflammation from comorbidities and the cytotoxic effect of radiation creates a pathophysiological cascade that compromises the regenerative capacity of the mastoid, facilitating MMD progression.
Notably, the indicators of tumor invasion, including invasion of the ipsilateral wall, the PR, POET, LVPM, TVPM and the T stage (P>0.05), were not able to predict PG2MMD in the multivariate analyses. These results indicated that the tumor invasion before RT did not influence the incidence of PG2MMD directly due to the involvement of various complex factors and changes in nasopharyngeal tissues after RT.
Remarkably, no drug applied during RT was a significant risk factor for PG2MMD. In addition to the lack of efficacy of anti-EGFR therapy (P>0.05), the application of ambroxol, budesonide, clarityne, myrtol or nasal irrigation during RT played a significant role in PG2MMD (P>0.05). Histopathological images of otomastoiditis samples revealed numerous vessels involved in angiogenesis (20), suggesting that antiangiogenic therapy, as a targeted therapy, may play a role in PG2MMD. Ambroxol therapy is a safe proven cough linctus and has improved the therapeutic effect of secretory otitis media, one of whose complications is MMD (21,22). Hence, it was hypothesized that ambroxol prevented irradiated patients with NPC from developing PG2MMD. In contrast to expectations, neither targeted therapy nor ambroxol influenced the incidence of P2GMMD after RT in patients with NPC. The reasons may be as follows: First, the effects of these drugs were not as lasting as those of radiation. Furthermore, the drug combination could affect the treatment of secretory otitis media. For instance, the addition of ambroxol reduces tympanic pressure after glucocorticoid treatment (22), which is not commonly applied in the treatment of NPC due to lacking anti-tumor properties. In addition, the role of the above medications in nasopharyngeal ciliary motility remains elusive, even though certain drugs serve as mucolytic agents in respiratory diseases (21). To conclude, the use of medicine to relieve PG2MMD requires further investigation.
Inflammatory indicators within one week before RT, such as the WBC count, neutrophil count, SAA, CRP and LDH, did not show any utility in predicting PG2MMD (P>0.05). The inflammation indicators reflect the status of the whole body; therefore, they may not be correlated with regional inflammation, such as nasal cavity stenosis and atresia (23), and MMD.
There were several limitations to this study. First, no audiometric findings were analyzed because our center restarted audiometry tests beginning in 2023. The omission of audiometric data weakens the study's ability to fully characterize MMD-related disease, assess treatment efficacy and inform holistic patient care. This highlights a gap in understanding the functional consequences of the disease to a certain extent. Second, even though the results were obtained from patients from prospective cohorts, they were based on retrospective groups, leading to biases, data quality issues and challenges in establishing causality. Third, treatments for severe MMD were not fully included in this study. The treatments for post-irradiation OME include simple tympanic aspiration, incisional myringotomy, ventilation tube insertion and laser myringotomy. As the cancer center, we were unable to perform surgery, such as mastoidectomy to severe MMD due to a lack of specialists for the mastoid. Hence, cooperation between our center and the general hospital with the otolaryngology head and neck department is necessary. Finally, no efficient medicines have been shown to improve PGMMD. Future studies should integrate audiometric assessments to address these gaps and better align findings with patient-centered outcomes.
In conclusion, for patients with NPC after irradiation, the peak incidence of G2MMD occurred at the 3 months after RT and decreased thereafter. Female sex, advanced age at diagnosis and high mastoid Dmean were significant risk factors for PG2MMD, which has potential risks leading to sensorineural/mixed hearing loss. Thus, clinicians should consider appropriate strategies to reverse the inflammatory reaction.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (grant nos. 82230034, 82071024 and 82261160399), the Key-Area Research and Development of Guangdong Province (grant no. 2020B1111190001), the Special Support Program for High-level Talents in Sun Yat-sen University Cancer Center, the Guangzhou Science and Technology Plan Project (grant no. 202103010001), the National Ten Thousand Talents Program Science and Technology Innovation Leading Talents (grant no. 84000-41180005), the Jiangxi Technology Innovation Guidance Program (grant no. S2021KJHZH0161), the Nanchang Regional Innovation System Construction Project (grant no. 20212BDH80023), the Guangdong Clinical Teaching Research Project (grant no. 2021JD014), the Guangdong Basic and Applied Basic Research Foundation (grant no. 2022A1515111113), and the China Postdoctoral Science Foundation (grant no. 2020M683116). The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
RLX was involved in the conceptualization, methodology, formal analysis, investigation, data curation, writing and revising the original draft, and funding acquisition. SYC contributed by performing formal analysis and investigation. ZKF, WBW, XTD, JHW and RY contributed to data collection and interpretation. SYF and PH taught other authors how to recognize and evaluate images of mastoid mucosa disease and performed data interpretation. CYD and CL selected the methods of statistical analysis used in the study and performed data interpretation. BL interpreted the data and wrote the original draft of the manuscript. RLX and SYC confirm the authenticity of all the raw data. WHJ was involved in the study conceptualization, resources and supervision. MYC contributed to the study conceptualization, supervision, project administration and funding acquisition. YPL was involved in the conceptualization and supervision of the study. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
This retrospective cohort study was approved and the written informed consents were waived by the ethics committee of SYSUCC (Guangzhou, China; Institutional Review Board no. B2023-050-01).
Patient consent for publication
The patients provided written informed consent for the publication of accompanying images.
Competing interests
The authors declare that they have no competing interests.
References
|
Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y and Ma J: Nasopharyngeal carcinoma. Lancet. 394:64–80. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Bhandare N, Antonelli PJ, Morris CG, Malayapa RS and Mendenhall WM: Ototoxicity after radiotherapy for head and neck tumors. Int J Radiat Oncol Biol Phys. 67:469–479. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Yao JJ, Zhou GQ, Yu XL, Tang LL, Chen L, Mao YP, Lin L, Zhang LL, Shao JY, Guo Y, et al: Incidence of and risk factors for mastoiditis after intensity modulated radiotherapy in nasopharyngeal carcinoma. PLoS One. 10:e01312842015. View Article : Google Scholar : PubMed/NCBI | |
|
Kew J, Tong MC, King AD, Leung SF, Metreweli C and van Hasselt CA: Magnetic resonance imaging and audiologic assessment of middle ear effusions in patients with nasopharyngeal carcinoma before radiation therapy. Am J Otol. 20:74–76. 1999.PubMed/NCBI | |
|
Nishimura R, Baba Y, Murakami R, Baba T, Furusawa M, Ishikawa T, Ushio Y and Takahashi M: MR evaluation of radiation otomastoiditis. Int J Radiat Oncol Biol Phys. 39:155–160. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
McDonald MH, Hoffman MR and Gentry LR: When is fluid in the mastoid cells a worrisome finding? J Am Board Fam Med. 26:218–220. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Jose J, Coatesworth AP, Anthony R and Reilly PG: Life threatening complications after partially treated mastoiditis. BMJ. 327:41–42. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Chen JF and Lee ST: Nasopharyngeal carcinoma presenting as an intracranial abscess. Surg Neurol. 49:553–557. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
King AD, Kew J, Tong M, Leung SF, Lam WW, Metreweli C and van Hasselt CA: Magnetic resonance imaging of the eustachian tube in nasopharyngeal carcinoma: Correlation of patterns of spread with middle ear effusion. Am J Otol. 20:69–73. 1999.PubMed/NCBI | |
|
Yao JJ, Zhou GQ, Lin L, Zhang WJ, Peng YL, Chen L, Tang LL, Mao YP, Ma J and Sun Y: Dose-volume factors associated with ear disorders following intensity modulated radiotherapy in nasopharyngeal carcinoma. Sci Rep. 5:135252015. View Article : Google Scholar : PubMed/NCBI | |
|
Walker GV, Ahmed S, Allen P, Gidley PW, Woo SY, DeMonte F, Chang EL and Mahajan A: Radiation-induced middle ear and mastoid opacification in skull base tumors treated with radiotherapy. Int J Radiat Oncol Biol Phys. 81:e819–e823. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Yao JJ, Zhou GQ, Jin YN, Zhang WJ, Lin L, Yu XL, Shao JY, Ma J and Sun Y: Predictors of mastoiditis after intensity-modulated radiotherapy in nasopharyngeal carcinoma: A dose-volume analysis. J Cancer. 7:276–282. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Wang TM, Shen GP, Chen MY, Zhang JB, Sun Y, He J, Xue WQ, Li XZ, Huang SY, Zheng XH, et al: Genome-wide association study of susceptibility loci for radiation-induced brain injury. J Natl Cancer Inst. 111:620–628. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, et al: AJCC Cancer Staging Manual. Springer; Heidelberg: 2017 | |
|
Saat R, Mahmood G, Laulajainen-Hongisto A, Lempinen L, Aarnisalo AA, Jero J and Markkola A: Comparison of MR imaging findings in paediatric and adult patients with acute mastoiditis and incidental intramastoid bright signal on T2-weighted images. Eur Radiol. 26:2632–2639. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Halperin E, Wazer D, Perez C and Brady L: Perez & Brady's Principles and Practice of Radiation Oncology. Lippincott Williams & Wilkins (LWW); Philadelphia, PA: 2018 | |
|
Boussaid M, Brahim O, Bouanen I, Kenani M, Limem H, Mahjoub Y, Mesrati MA and Aissaoui A: Sex determination by Ct -scan analysis of the mastoid bone: A cross-sectional study. Heliyon. 10:e337122024. View Article : Google Scholar : PubMed/NCBI | |
|
Yalcinkaya A, Yalcinkaya R, Sardh F and Landegren N: Immune dynamics throughout life in relation to sex hormones and perspectives gained from gender-affirming hormone therapy. Front Immunol. 15:15013642025. View Article : Google Scholar : PubMed/NCBI | |
|
Kaiser M, Semeraro MD, Herrmann M, Absenger G, Gerger A and Renner W: Immune aging and immunotherapy in cancer. Int J Mol Sci. 22:70162021. View Article : Google Scholar : PubMed/NCBI | |
|
Popescu C, Ioniţă E, Mogoantă CA, Simionescu C and Pătru E: Clinical and histopathological aspects in otomastoiditis. Rom J Morphol Embryol. 50:453–460. 2009.PubMed/NCBI | |
|
Mullin S, Smith L, Lee K, D'Souza G, Woodgate P, Elflein J, Hällqvist J, Toffoli M, Streeter A, Hosking J, et al: Ambroxol for the treatment of patients with parkinson disease with and without glucocerebrosidase gene mutations: A nonrandomized, noncontrolled trial. JAMA Neurol. 77:427–434. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou X, Jin X, Yang L and Wei X: Efficacy and safety of ambroxol hydrochloride in the treatment of secretory otitis media: A systematic review and meta-analysis. Ann Transl Med. 10:1422022. View Article : Google Scholar : PubMed/NCBI | |
|
Yan JJ, Guo SS, Lin DF, Liu LT, Liu SL, Xiao BB, Yang JH, Wen DX, Yang ZC, Liang YJ, et al: Development and validation of a normal tissue complication probability model for acquired nasal cavity stenosis and atresia after radical radiotherapy for nasopharyngeal carcinoma. Radiother Oncol. 160:9–17. 2021. View Article : Google Scholar : PubMed/NCBI |