Risk factors for bone metastasis in lung cancer and the efficacy of palliative radiotherapy and opioid analgesics in alleviating bone metastasis pain
- Authors:
- Published online on: July 7, 2025 https://doi.org/10.3892/ol.2025.15175
- Article Number: 429
-
Copyright: © Zhang et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Lung cancer is the most common malignancy worldwide. According to the 2022 International Agency for Research on Cancer report, there were an estimated 2.5 million novel cases globally. The incidence rate is as high as 74.31% in men and 39.08% in women (1). Advanced-stage lung cancer accounts for a notable proportion of cases, highlighting the importance of effective treatment strategies, particularly the need for individualized approaches based on different metastatic sites.
Among the various complications associated with lung cancer, bone metastasis occurs in 10–15% of cases (2,3). Particularly in patients with lung adenocarcinoma, the incidence of bone metastasis can reach 30–40% (4). Bone-related events (such as pathological fractures, spinal cord compression and hypercalcemia) are common and further worsen the prognosis and quality of life for patients with lung cancer. Bone metastasis can lead to intense bone pain, fractures and other complications, which impose substantial physical and psychological burdens on patients (5–7). Following bone metastasis, survival rates drop sharply, with a 1-year survival rate of 5.3%, a 2-year survival rate of 2.1% and a median survival time of 6–10 months (8,9). The occurrence of bone-related events not only markedly increases mortality risk but also exacerbates physical pain, functional limitations and emotional distress (10). Therefore, the early identification of bone metastasis in lung cancer is key for the improvement of patient outcomes.
Currently, bone metastasis in patients with cancer is mainly detected through local computed tomography (CT), radionuclide bone scans and positron emission tomography (PET)-CT (11,12). While these methods are widely used, they are costly, involve high radiation exposure and have limited specificity. Previous studies have increasingly focused on identifying non-invasive methods for the detection of bone metastases in patients with lung cancer. However, non-invasive approaches remain in the exploratory stage and face challenges such as inconsistent sensitivity, lack of standardization and limited clinical validation. Although various treatment options, such as bisphosphonates, denosumab, radiotherapy and surgery, are available to manage bone metastasis, the effectiveness varies among individuals. However, these treatments primarily focus on symptom relief rather than prolonging survival and their efficacy may be influenced by patient-specific factors (13). These limitations highlight the need for further investigation to optimize treatment strategies and improve outcomes for patients with bone metastasis.
Given the high incidence and notable impact of bone metastasis on the prognosis of patients with lung cancer, there is an urgent need to identify early diagnostic markers and optimize treatment strategies. The present study aims to identify key risk factors for bone metastasis based on clinical data and to develop an effective predictive model for early screening. Additionally, it systematically evaluates the pain management outcomes of radiotherapy, opioid therapy and their combination in patients with bone metastasis. Through this research, clinical risk assessment could be enhanced, individualized treatment approaches could be improved, and valuable insights for early detection and comprehensive management of lung cancer bone metastasis could be provided.
Materials and methods
Study participants
The present retrospective study included 100 patients with lung cancer with bone metastasis and 100 without metastasis, who were treated at The First Affiliated Hospital, Qiqihar Medical University Hospital (Qiqihar, China) between January 2019 and December 2023. The selection of 100 patients in each group was based on a consecutive sampling method, where eligible patients who met the inclusion criteria were recruited until the predetermined sample size was reached. The exact number of patients in each group was determined by available clinical data during the study period, which ensured balanced representation for comparison. The inclusion criteria were as follows: i) Newly diagnosed primary lung cancer, confirmed pathologically; ii) single-photon emission CT bone scintigraphy, bone magnetic resonance imaging or PET-CT performed to assess the presence of bone metastasis; iii) complete clinical data; and iv) expected survival time ≥3 months. Exclusion criteria were as follows: i) Presence of any other primary tumors; ii) concurrent diseases that affect bone metabolism, traumatic fractures and severe liver or kidney dysfunction; iii) incomplete clinical data; and iv) expected survival time <3 months. Due to this heterogeneity in chemotherapy types, doses and durations, chemotherapy was not included as a uniform variable in the comparative pain analysis. The expected survival time was evaluated by the attending physicians, who considered factors such as the general health status of the patient, the type and pathological staging of the primary tumor, and any comorbidities. The present study adhered to ethical principles and informed consent was obtained from all participants. The present study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Qiqihar Medical University (approval no. 2023-008-01).
Data collection
Clinical data on age, sex, occupation, family history, smoking status, initial symptoms, primary tumor location, primary tumor diameter, pathology type, EGFR status and tumor markers [such as carcinoembryonic antigen (CEA), cancer antigen 125 (CA125), CA199, cancer antigen 153 (CA153), neuron-specific enolase (NSE) and alkaline phosphatase (ALP)] were retrospectively collected for the present study. For patients with lung cancer with bone metastasis, data on pain treatment (radiotherapy, opioid therapy or combination therapy) were collected, along with the visual analog scale (VAS) and Pittsburgh Sleep Quality Index (PSQI) scores before and after treatment (14,15). The VAS is a tool used to assess pain intensity, ranging from 0 (no pain) to 10 (worst imaginable pain). The PSQI is a questionnaire designed to evaluate sleep quality, with total scores ranging from 0 to 21; higher scores indicate poorer sleep quality.
Pain management protocols
For patients in the radiotherapy group, external beam radiotherapy was delivered to symptomatic bone lesions using a linear accelerator. The total dose was 30 Gy in 10 fractions (3 Gy per fraction; five times per week over 2 weeks). Opioid therapy was administered via oral formulations, including oxycodone sustained-release tablets and morphine hydrochloride sustained-release tablets. The specific dosages were individually adjusted according to the pain severity of the patient, in accordance with the World Health Organization three-step analgesic ladder (16). The combined treatment group received both modalities simultaneously and followed the aforementioned protocols.
Statistical analysis
All patients with lung cancer in the present study were classified into two groups: 100 patients without bone metastasis and 100 patients with bone metastasis. Each group was then randomly divided into a training cohort (n=70) and a validation cohort (n=30) in a 7:3 ratio. This approach ensured that the distribution of bone metastasis and non-metastasis cases remained consistent between the training and validation cohorts. The training cohort was used to identify high-risk factors for bone metastasis and to develop a predictive model, while the validation cohort assessed the predictive performance and stability of the model. Continuous variables with a normal distribution are expressed as the mean (standard deviation). Differences between two groups were analyzed by independent sample Student's t-tests and differences between three groups were analyzed by one-way ANOVA. For cases with balanced sample sizes and homogeneity of variances, Tukey's Honestly Significant Difference test was used for pairwise comparisons. For cases with unbalanced sample sizes, Bonferroni correction was applied for post hoc comparisons to ensure robustness and control the type I error rate caused by multiple comparisons. Non-normally distributed variables are presented as the median and interquartile range, and differences between two groups were assessed using the Mann-Whitney U test, while comparisons among multiple groups were performed using the Kruskal-Wallis test. When the Kruskal-Wallis test indicated statistical significance, Dunn's post hoc test with Bonferroni correction was used for pairwise comparisons to control for type I error. Categorical variables are represented as frequency and percentage. Group differences were assessed using the χ2 test or Fisher's exact test, as appropriate, with the latter applied when expected cell counts were ≤5 in >20% of cells. For paired data (such as VAS and PSQI scores before and after treatment in the same patients) Wilcoxon signed-rank tests were used. The normality of variables was assessed using the Shapiro-Wilk test. Univariate and multivariate logistic regression models were used to analyze risk factors, including age, sex and smoking status. P<0.05 was considered to indicate a statistically significant difference. Significant factors from the univariate analysis were subsequently included in the multivariate analysis. Receiver operating characteristic (ROC) curves, calibration curves, nomograms and decision curve analysis (DCA) were generated using R software (v4.3.2; R Core Team).
Results
Baseline characteristics of patients
A comparative analysis was conducted between 100 patients with lung cancer with bone metastasis and 100 patients with lung cancer without metastasis to investigate their clinical characteristics and demographic factors, as shown in Table I. There were no significant differences between patients with and without bone metastasis in terms of age, sex, occupation, family history, primary tumor location and smoking status (P>0.05). Symptoms were absent in 62.0% of patients in the non-metastasis group but only in 2.0% of patients in the metastasis group, revealing a significant difference (P<0.001). A post hoc pairwise comparison of initial symptoms using Fisher's exact test with Bonferroni correction (significance threshold set at P<0.0167) revealed significant differences in the proportions of asymptomatic (P<0.001) and pain symptoms (P<0.001) between the metastasis and non-metastasis groups. The difference in respiratory symptoms did not reach statistical significance after correction (P=0.019). The median diameter of primary tumors was significantly lower in the non-metastasis group (1.80 cm) compared with that in the metastasis group (2.80 cm) (P<0.001). There was no significant difference in pathology type between the two groups (P=0.928). However, there was a significant difference in EGFR mutation positivity between the non-metastasis group (35.0%; n=35) and the metastasis group (85.0%; n=85) (P<0.001). Tumor markers CEA and CA199 were significantly higher in the metastasis group compared with those in the non-metastasis group (P<0.001 and P=0.011). NSE and ALP levels were also significantly higher in the metastasis group compared with those in the non-metastasis group (both P<0.001). These results suggested that tumor size, the presence of symptoms, EGFR mutations and elevated tumor markers were strongly associated with the occurrence of bone metastasis in patients with lung cancer.
Table I.Comparison of clinical and demographic characteristics between non-metastasis and metastasis groups. |
Identification of potential variables for predictive model construction
To construct a predictive model, all 200 patients were randomly divided into a training cohort (n=140) and a validation cohort (n=60) (Table II). In the training cohort, univariate logistic regression analysis identified several significant factors (Table III). Initial symptoms (OR, 71.067; 95% CI, 15.669–1,257.246; P<0.001), primary tumor diameter (OR, 18.021; 95% CI, 7.680–51.901; P<0.001), CEA (OR, 2.952; 95% CI, 2.172–4.308; P<0.001), CA199 (OR, 1.042; 95% CI, 1.002–1.086; P=0.043), NSE (OR, 1.176; 95% CI, 1.115–1.253; P<0.001), ALP (OR, 1.043; 95% CI, 1.027–1.060; P<0.001) and EGFR status (OR, 13.141; 95% CI, 5.888–31.792; P<0.001) were significantly associated with bone metastasis. In multivariate logistic regression, primary tumor diameter (OR, 151.425; 95% CI, 3.084–7,435.895; P=0.012), CEA (OR, 3.048; 95% CI, 1.229–7.557, P=0.016), initial symptoms (OR, 183.618, 95% CI, 2.299–14,667.163, P=0.020) and EGFR status (OR, 49.892; 95% CI, 1.264–1,969.338; P=0.037) remained statistically significant. CA199, NSE and ALP were not significantly associated with bone metastasis (P>0.05) (Table IV). These findings indicated that initial symptoms, primary tumor diameter, CEA levels and EGFR mutations were the most reliable predictors of bone metastasis and formed a solid foundation for model construction.
Model construction
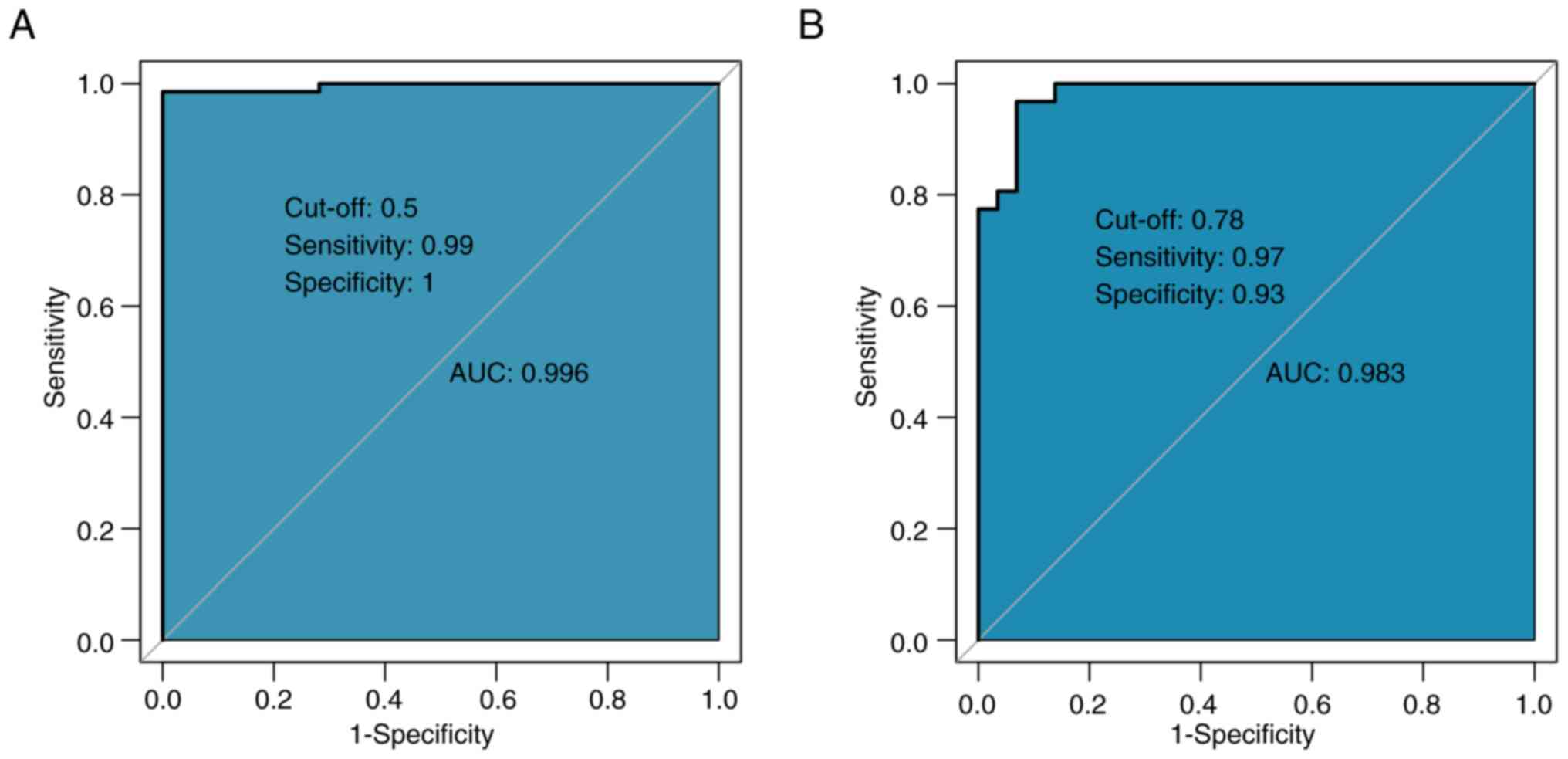
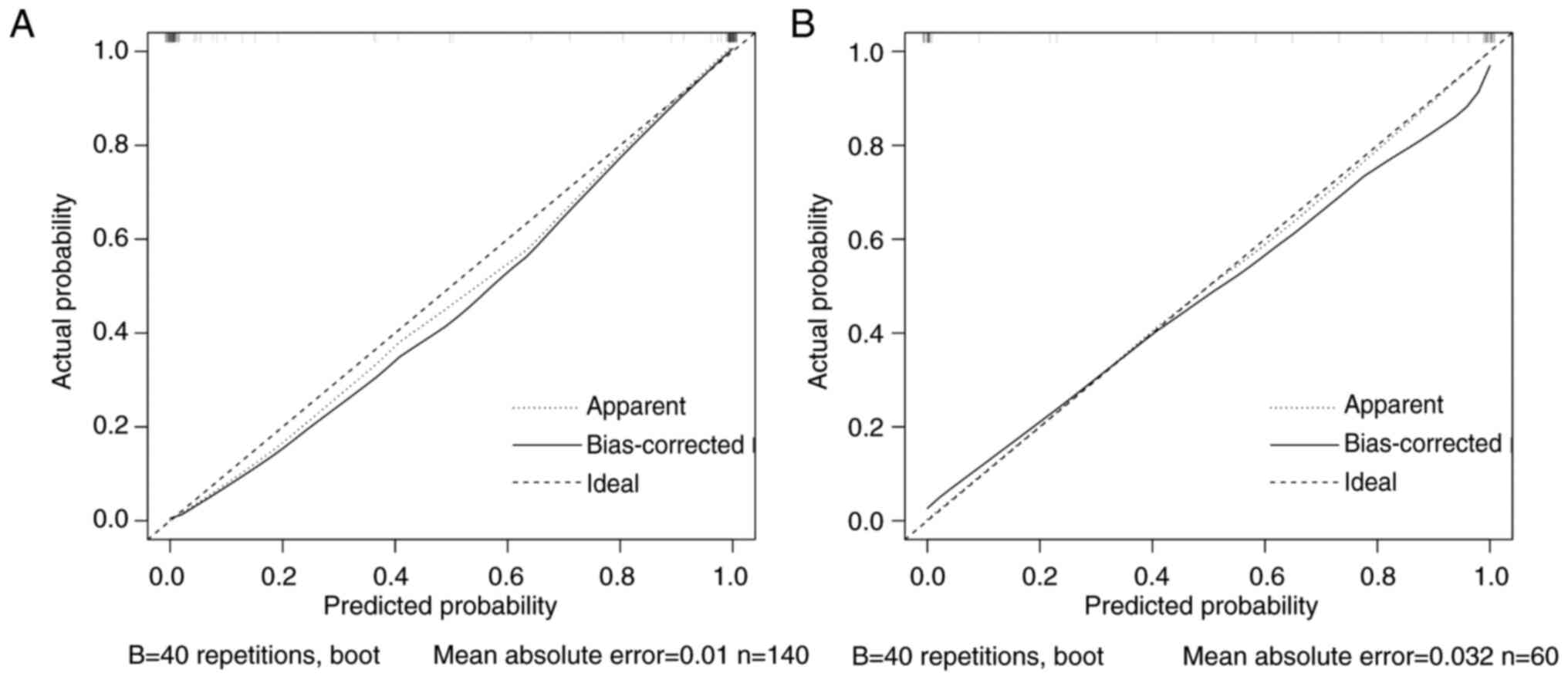
Based on the aforementioned analysis, four high-risk factors, namely primary diameter, CEA, initial symptoms and EGFR mutation, were selected to construct a predictive model for bone metastasis. The ROC curve demonstrated high predictive performance, with an AUC of 0.996, which indicated an outstanding diagnostic performance (Fig. 1A). The optimal cut-off value was 0.5, with a sensitivity of 0.99 and specificity of 1.00, which indicated enhanced diagnostic performance. In the validation cohort, the discriminatory performance of the model was further validated, wherein results demonstrated an AUC of 0.983 (Fig. 1B). The calibration curve demonstrated a high level of agreement between predicted probabilities and actual results, with a mean absolute error of 0.009, which indicated strong calibration and low predictive error (Fig. 2). Overall, the model demonstrated notable predictive power and reliability in both internal and external validations.
Nomogram construction
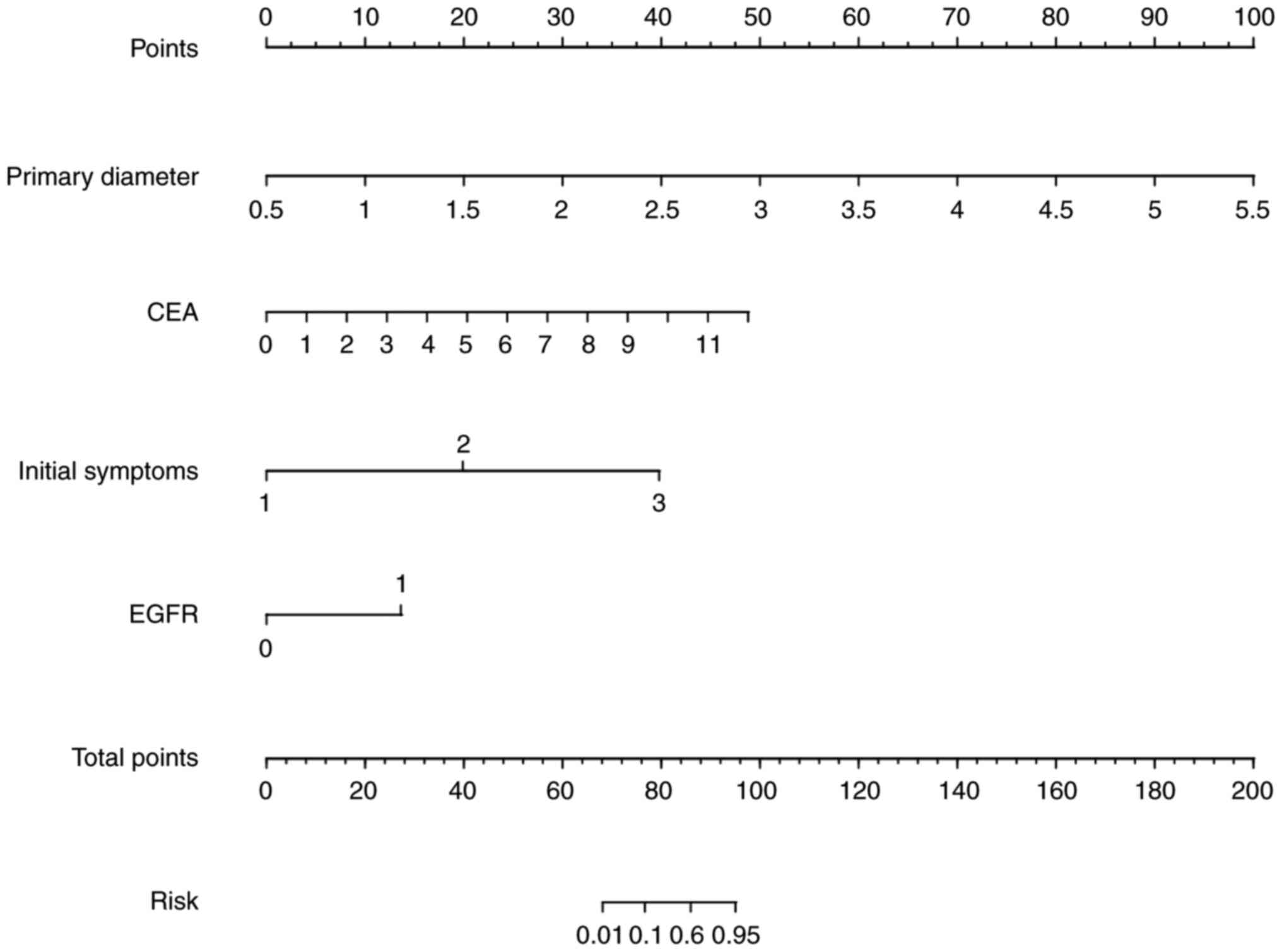
A nomogram was developed based on the four aforementioned risk indicators to provide a visual high-risk scoring system for bone metastasis in patients with lung cancer (Fig. 3). Each variable corresponded to a specific score and the total score indicated the predicted probability of metastasis. This approach provided clinicians with a rapid and practical risk assessment tool. The nomogram allowed for intuitive interpretation of model predictions and facilitated personalized clinical decision-making.
Clinical utility assessment
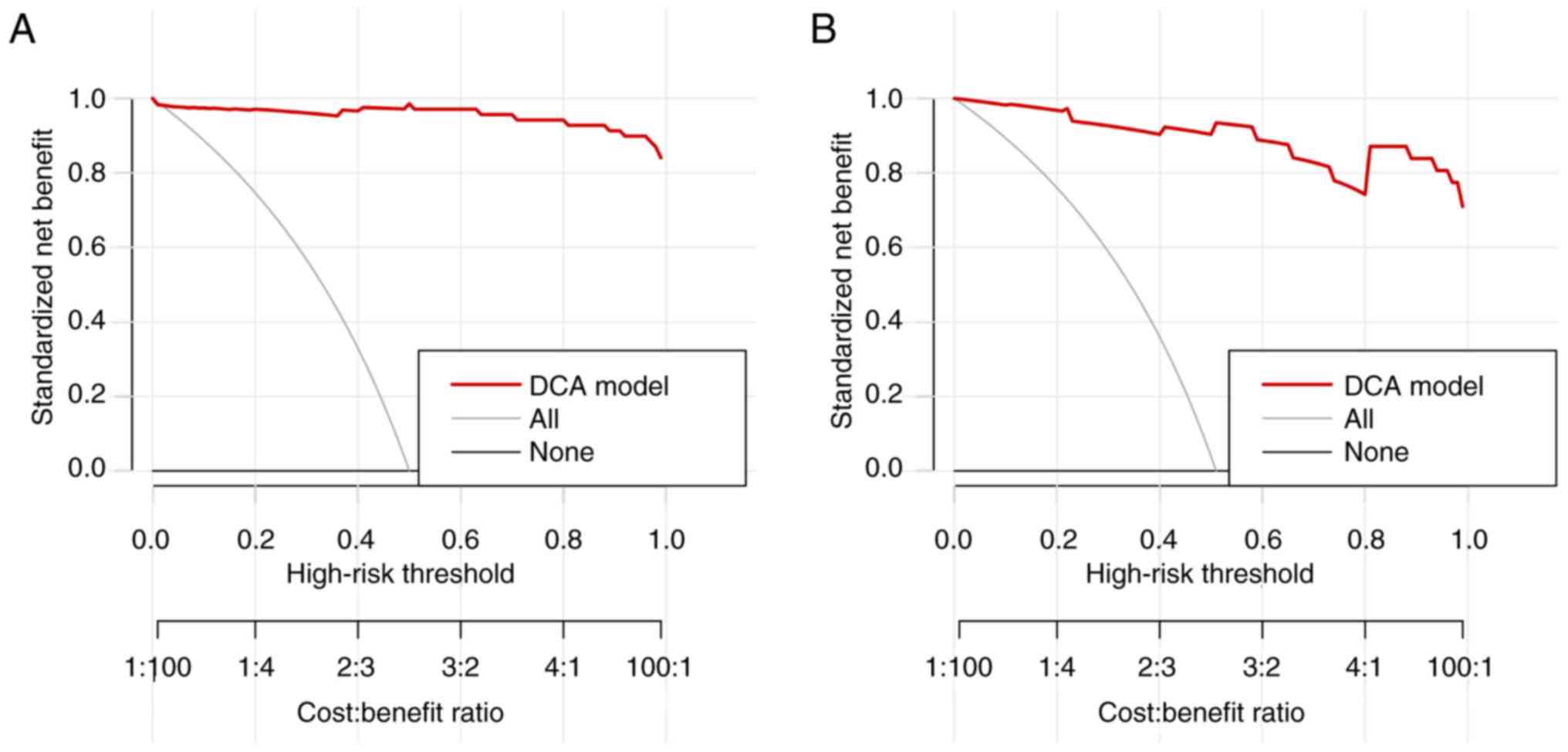
DCA was conducted to evaluate the clinical utility of the predictive model. The DCA curve (Fig. 4) demonstrated a higher net benefit across various risk thresholds in both the training and validation cohorts, which suggested greater net benefit in clinical decision-making across different high-risk thresholds. These results highlighted the practical value of the model for the identification of high-risk patients and supporting early intervention strategies.
Baseline and efficacy analysis of different treatment methods
The present study conducted a comparative analysis of baseline characteristics across three pain management approaches (radiotherapy, opioid therapy and combined therapy) for patients with tumors and bone metastasis, as shown in Table V. No significant differences were observed among the three groups regarding age, sex, occupation, or family history. However, smoking status varied significantly between patients in the radiotherapy group (81.8%; n=27), the opioid therapy group (97.0%; n=32) and the combined therapy group (97.1%; n=33) (overall P=0.031). There were no significant differences among the groups in terms of initial symptoms, primary tumor location, primary tumor diameter, pathological type, metastatic site or EGFR mutation status. Similarly, comparisons of tumor markers (CEA, CA125, CA199, CA153, NSE and ALP) indicated no significant differences between the radiotherapy, opioid therapy and combined therapy groups.
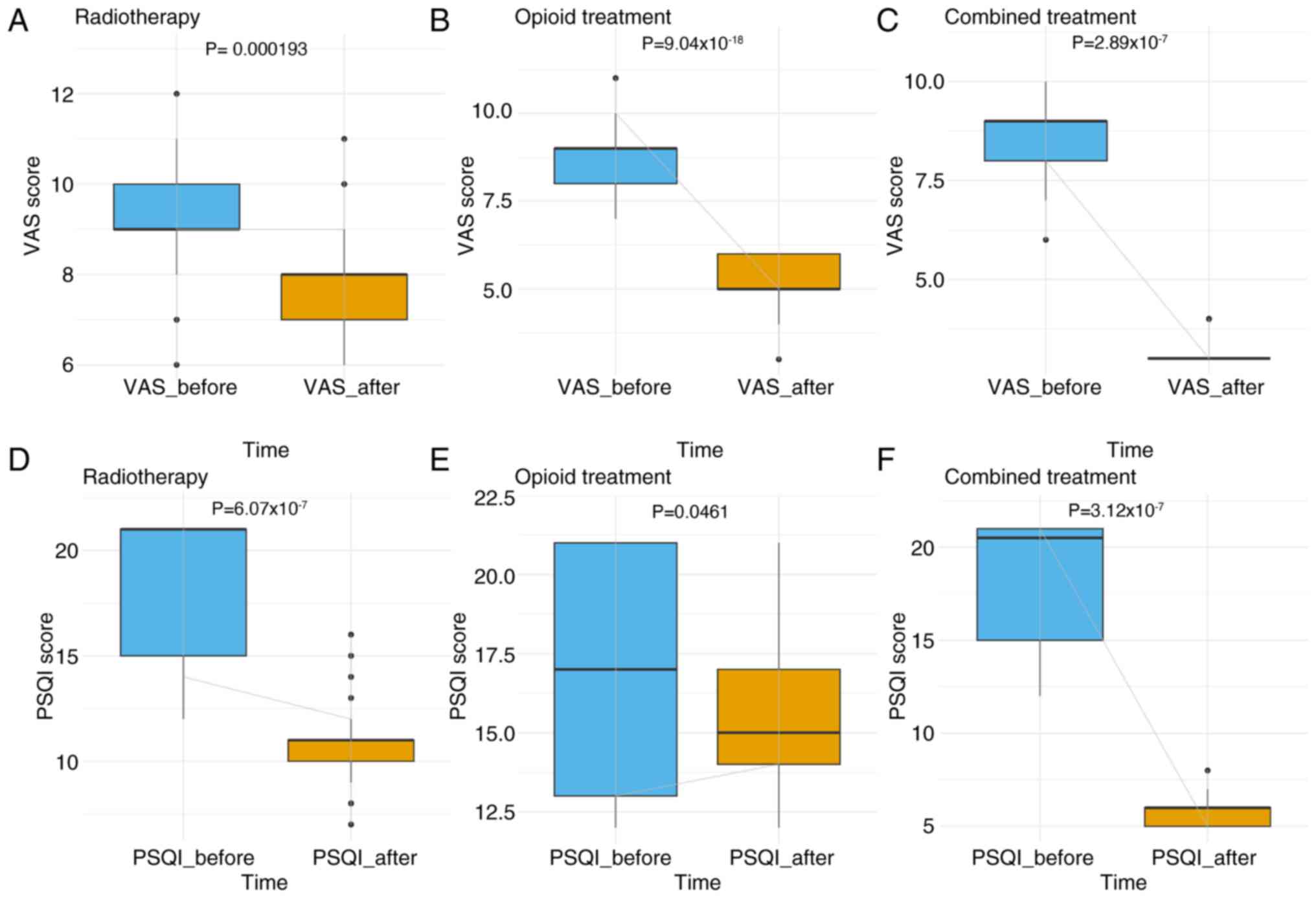
Before treatment, there were no significant differences in VAS scores among the three groups: Radiotherapy, opioid treatment, and combined treatment (P>0.05; Table VI). Post-treatment, the VAS scores ranked from highest to lowest were as follows: i) Radiotherapy group; ii) opioid therapy group; and iii) combined treatment group (wherein lower VAS scores indicated reduced levels of pain). Similarly, there was no significant difference in PSQI scores in the three groups when comparing before treatment and after treatment scores (P>0.05; Fig. 5). After treatment, the PSQI scores ranked from lowest to highest were as follows: i) Combined treatment group; ii) radiotherapy group; and iii) opioid therapy group (wherein higher PSQI scores indicated improved sleep quality) (17). These findings demonstrated that combined therapy provided superior efficacy in the management of bone metastasis-related pain symptoms compared with monotherapy.
Table VI.Comparison of VAS and PSQI before and after treatment for different therapeutic modalities. |
Discussion
The present study focused on the identification of high-risk factors associated with bone metastasis in lung cancer and evaluating the efficacy of palliative radiotherapy and opioid treatments for bone metastasis-related pain. Results from the present study indicated that initial symptoms, primary tumor diameter, CEA levels and EGFR mutations were significant independent risk factors for bone metastasis in lung cancer. Furthermore, the combination of palliative radiotherapy and opioid treatment proved more effective in managing pain compared with either radiotherapy or opioid treatment alone.
Although the relationship between lung cancer and bone metastasis has been widely studied, the present study aimed to fill a gap in the literature by constructing a predictive model specifically based on clinical characteristics, tumor markers and EGFR mutation status. These factors were most significant in the prediction of bone metastasis risk, which made the model particularly valuable for early screening and prognostic assessment in patients with lung cancer. The novelty of the current study arose in the use of clinical data to create a practical tool for clinical decision-making.
Firstly, initial symptoms, especially respiratory symptoms and pain, are closely associated to the occurrence of bone metastasis (18). The present study demonstrated that the proportion of patients with pain was significantly higher in the bone metastasis group, which suggested that early symptoms might reflect a more aggressive tumor. Pain in bone metastasis may arise from bone destruction, changes in the bone marrow microenvironment, and the release of exosomes and inflammatory factors by tumor cells (19,20). Previous studies have also reported that pain is common in patients with bone metastasis and is closely associated with disease progression (6,21). Thus, pain should be considered as a clinical indicator for bone metastasis while screening high-risk patients.
Secondly, primary tumor diameter and bone metastasis are significantly associated. The present study reported that patients in the bone metastasis group had significantly larger median tumor diameters compared with patients without metastasis. Larger median tumor diameters may be correlated with increased tumor burden, higher cell proliferation and potential enhancement in invasiveness. Larger tumor sizes can facilitate the ability of the tumor to breach the tissue barriers of the primary site and spread to distant tissues via blood or lymphatic systems (22). This finding aligned with the present study results, which demonstrated that larger primary tumor diameters were associated with a higher risk of bone metastasis, which underscored the importance of tumor size as a predictive factor for bone metastasis, particularly in the guidance of non-invasive imaging evaluations and follow-up strategies.
Elevated CEA levels are a significant high-risk factor for lung cancer bone metastasis. In the multivariate logistic regression analysis of the present study, CEA demonstrated strong predictive efficacy for bone metastasis. As a traditional tumor marker in lung cancer, CEA levels are generally positively associated with tumor burden, differentiation and disease progression, and previous studies have reported that elevated CEA is associated with an increased risk of lymph node and brain metastases (23,24). Potential mechanisms include the increased adhesion of high CEA-expressing tumor cells to vascular walls or bone tissues, which promote the formation of metastatic foci via interactions with platelets or endothelial cells (25,26). Therefore, close monitoring and timely intervention for bone metastasis should be considered for patients with elevated CEA levels.
Previous studies have supported the association between EGFR mutations and bone metastasis in NSCLC (27–29). EGFR mutations are commonly observed in patients with bone metastases, and EGFR mutation is associated with increased tumor invasiveness and metastasis (30–32). EGFR activation enhances tumor cell proliferation and migration, while PI3K/AKT and MAPK/ERK pathways further amplify signaling, which accelerate cell proliferation and promote metastasis (33,34). In the formation of bone metastasis, enhanced EGFR signaling may assist tumor cells in bone growth and in modifying the bone remodeling environment to create metastatic foci (35). This finding further supports the need to monitor bone metastasis in EGFR mutation-positive patients and suggests that EGFR inhibitors may serve a more active role in the treatment of patients with EGFR mutations. In the present study, the construction of the predictive model for bone metastasis included retrospectively collected data from patients who were newly diagnosed with primary lung adenocarcinoma and had not received any prior treatments, including surgery, chemotherapy or radiotherapy.
In terms of pain relief treatment strategies, the present study indicated that the combined use of palliative radiotherapy and opioid medications was significantly more effective compared with single treatment. Radiotherapy may act directly on bone metastatic sites, which reduces bone destruction and the proliferation of tumor cells within the bone tissue and thereby reduces pain (36). Opioids, on the other hand, directly alleviate pain through neurotransmitter regulation (37). A combined approach not only improves pain management but also enhances the quality of life for patients. In light of the present study findings, clinicians are advised to consider using combined radiotherapy and opioid therapy as a more effective pain management strategy for patients with bone metastasis. Regarding pain management, 87 patients had received chemotherapy either prior to or during the course of treatment as part of a comprehensive therapeutic strategy. However, since the primary aim of this part of the study was to evaluate the efficacy of palliative radiotherapy, opioid therapy and their combination in managing bone metastasis-related pain, steps were taken to minimize confounding factors. Specifically, only patients who did not receive any new chemotherapy regimens during the pain treatment evaluation period were included in the pain efficacy analysis. Due to this heterogeneity in chemotherapy types, doses and durations, chemotherapy was not included as a uniform variable in the comparative pain analysis. Future prospective studies may further explore the interactions between chemotherapy and pain management efficacy.
The present study has several limitations. First, the risk factors identified in the study were influenced by multiple inclusion variables, including clinical characteristics (such as age, sex and smoking status), tumor markers (ALP, CEA and NSE) and EGFR mutation status. Altering the inclusion or exclusion of any of these variables in the logistic regression models might have led to changes in the final set of risk factors identified. Second, the results were based solely on data from The First Affiliated Hospital, Qiqihar Medical University Hospital, and the findings might differ if applied to patient populations from other centers, which highlights the need for multi-center validation to improve generalizability.
In summary, the present study identified high-risk factors for lung cancer bone metastasis through clinical characteristics, tumor markers and gene mutations, which established an effective prediction model that provides an important reference for early clinical screening, precise diagnosis and treatment. In pain management, the present study demonstrated that combined palliative radiotherapy and opioid therapy can markedly relieve pain and improve the quality of life in patients, which highlighted its value in patients with late-stage lung cancer. Future studies could further optimize the predictive efficacy of the model and verify its applicability with multi-center data. Additionally, with advancements in targeted therapy and immunotherapy, interventions targeting EGFR mutations and other key signaling pathways may offer novel approaches for the prevention and treatment of lung cancer bone metastasis.
The present study elucidated key risk factors for bone metastasis in primary lung cancer, including initial symptoms, tumor diameter, CEA levels and EGFR mutation status. A predictive model was developed to facilitate early screening and targeted interventions. Additionally, the combination of palliative radiotherapy and opioid therapy demonstrated notable efficacy in pain management and thereby improved the quality of life in patients. The present study findings underscored the importance of personalized therapeutic strategies for the management of bone metastasis and highlighted the necessity of integrated approaches for pain alleviation.
Acknowledgements
Not applicable.
Funding
The present study received funding from the Qiqihar Science and Technology Bureau (grant no. LSFGG-2023059).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
QZ and BS designed the manuscript. CC and YG helped with the data analysis. BQ, CL and JF collected the data and participated in data analysis. QZ and BS analyzed the data. QZ and YG confirm the authenticity of all the raw data. All authors contributed to drafting and revising the manuscript and approved the final version for publication.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of the First Affiliated Hospital of Qiqihar Medical University (Qiqihar, China; approval no. 2023-008-01). All participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 74:229–63. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Huang X, Shi X, Huang D, Li B, Lin N, Pan W, Yan X, Li H, Hao Q and Ye Z: Mutational characteristics of bone metastasis of lung cancer. Ann Palliat Med. 10:8818–8826. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Sugiura H, Yamada K, Sugiura T, Hida T and Mitsudomi T: Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop Relat Res. 466:729–736. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Hernandez RK, Wade SW, Reich A, Pirolli M, Liede A and Lyman GH: Incidence of bone metastases in patients with solid tumors: Analysis of oncology electronic medical records in the United States. BMC Cancer. 18:442018. View Article : Google Scholar : PubMed/NCBI | |
|
Jimenez-Andrade JM, Mantyh WG, Bloom AP, Ferng AS, Geffre CP and Mantyh PW: Bone cancer pain. Ann N Y Acad Sci. 1198:173–181. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Wu S, Pan Y, Mao Y, Chen Y and He Y: Current progress and mechanisms of bone metastasis in lung cancer: A narrative review. Transl Lung Cancer Res. 10:439–451. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Coleman RE: Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 12:6243s–6249s. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Hoda MA, Rozsas A, Lang E, Klikovits T, Lohinai Z, Torok S, Berta J, Bendek M, Berger W, Hegedus B, et al: High circulating activin A level is associated with tumor progression and predicts poor prognosis in lung adenocarcinoma. Oncotarget. 7:13388–13399. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Fornetti J, Welm AL and Stewart SA: Understanding the bone in cancer metastasis. J Bone Miner Res. 33:2099–2113. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Kommalapati A, Tella SH, Esquivel MA and Correa R: Evaluation and management of skeletal disease in cancer care. Crit Rev Oncol Hematol. 120:217–226. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
O'Sullivan GJ, Carty FL and Cronin CG: Imaging of bone metastasis: An update. World J Radiol. 7:202–211. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Çayir D, Bozkurt M, Gültekin SS and Karaçalioğlu AÖ: Earlier phases of bone scintigraphy can better delineate the extent of soft tissue involvement of bone metastasis. Clin Nucl Med. 43:854–856. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Bozzo A, Deng J, Abbas U, Bhasin R, Deodat M, Wariach S, Sanger S, Axelrod D, Masrouha K, Turcotte R, et al: Which bone-modifying agent is associated with better outcomes in patients with skeletal metastases from lung cancer? A systematic review and network meta-analysis. Clin Orthop Relat Res. 479:2047–2057. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Huskisson EC: Measurement of pain. Lancet. 2:1127–1131. 1974. View Article : Google Scholar : PubMed/NCBI | |
|
Nam H, Lim JS, Kim JS, Lee KJ, Koo DL and Lee C: Sleep perception in obstructive sleep apnea: A study using polysomnography and the multiple sleep latency test. J Clin Neurol. 12:230–235. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Jadad AR and Browman GP: The WHO analgesic ladder for cancer pain management. Stepping up the quality of its evaluation. JAMA. 274:1870–1873. 1995. View Article : Google Scholar : PubMed/NCBI | |
|
Chai X, Yinwang E, Wang Z, Wang Z, Xue Y, Li B, Zhou H, Zhang W, Wang S, Zhang Y, et al: Predictive and prognostic biomarkers for lung cancer bone metastasis and their therapeutic value. Front Oncol. 11:6927882021. View Article : Google Scholar : PubMed/NCBI | |
|
Ursavas A, Karadag M, Uzaslan E, Rodoplu E, Demirdögen E, Burgazlioglu B and Gozu RO: Can clinical factors be determinants of bone metastases in non-small cell lung cancer? Ann Thorac Med. 2:9–13. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Yang Q, Wang W, Cheng D, Wang Y, Han Y, Huang J and Peng X: Non-coding RNA in exosomes: Regulating bone metastasis of lung cancer and its clinical application prospect. Transl Oncol. 46:1020022024. View Article : Google Scholar : PubMed/NCBI | |
|
Arakil N, Akhund SA, Elaasser B and Mohammad KS: Intersecting paths: Unraveling the complex journey of cancer to bone metastasis. Biomedicines. 12:10752024. View Article : Google Scholar : PubMed/NCBI | |
|
Maroni P, Gomarasca M and Lombardi G: Long non-coding RNAs in bone metastasis: Progresses and perspectives as potential diagnostic and prognostic biomarkers. Front Endocrinol (Lausanne). 14:11564942023. View Article : Google Scholar : PubMed/NCBI | |
|
Lv T, Li Z, Wang D, Guo X, Zhang X, Cao J and Wang Z: Role of exosomes in prostate cancer bone metastasis. Arch Biochem Biophys. 748:1097842023. View Article : Google Scholar : PubMed/NCBI | |
|
Xu S, Hu T, Zhou C, Wang M, Jiang J, Wang Y and Wang C: The ratio of lymph node eluate/serum cytokeratin 21-1 is a potential predictor of lymph node metastasis in lung adenocarcinoma. J Thorac Dis. 16:5709–5717. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Y, Dai S, Liu Z, He L, Zhu L, Qin Z, Fan H, Fang F, Xie Y and Peng X: Serum tumor markers and outcomes in lung cancer patients with brain metastases: A retrospective longitudinal cohort study. Transl Lung Cancer Res. 13:2282–2295. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Yu M and Wang P: A comparative study of ProGRP and CEA as serological markers in small cell lung cancer treatment. Discov Oncol. 15:4852024. View Article : Google Scholar : PubMed/NCBI | |
|
Passaro A, Al Bakir M, Hamilton EG, Diehn M, André F, Roy-Chowdhuri S, Mountzios G, Wistuba II, Swanton C and Peters S: Cancer biomarkers: Emerging trends and clinical implications for personalized treatment. Cell. 187:1617–1635. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Laganà M, Gurizzan C, Roca E, Cortinovis D, Signorelli D, Pagani F, Bettini A, Bonomi L, Rinaldi S, Berardi R, et al: High prevalence and early occurrence of skeletal complications in EGFR mutated NSCLC patients with bone metastases. Front Oncol. 10:5888622020. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Zheng Z, Wang L, Han L, Du Y, Zhang X, Liu X and Xie J: Association of mutation profiles with metastasis in patients with non-small cell lung cancer. Front Oncol. 14:14515762024. View Article : Google Scholar : PubMed/NCBI | |
|
Knapp BJ, Devarakonda S and Govindan R: Bone metastases in non-small cell lung cancer: A narrative review. J Thorac Dis. 14:1696–1712. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang C, Wu Q, Yang H, Zhang H, Liu C, Yang B and Hu Q: Ferroptosis-related gene signature for predicting prognosis and identifying potential therapeutic drug in EGFR wild-type lung adenocarcinoma. Commun Biol. 7:14162024. View Article : Google Scholar : PubMed/NCBI | |
|
Li N, Bian Z, Cong M and Liu Y: Survival outcomes of patients with epidermal growth factor receptor mutations in non-small cell lung cancer with leptomeningeal metastasis. Front Oncol. 11:7235622022. View Article : Google Scholar : PubMed/NCBI | |
|
Passaro A, Jänne PA, Mok T and Peters S: Overcoming therapy resistance in EGFR-mutant lung cancer. Nat Cancer. 2:377–391. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Tabei Y and Nakajima Y: IL-1β-activated PI3K/AKT and MEK/ERK pathways coordinately promote induction of partial epithelial-mesenchymal transition. Cell Commun Signal. 22:3922024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Yang W, Han X, Qiao Y, Wang H, Chen T, Li T and Ou WB: Knockdown of HE4 suppresses tumor growth and invasiveness in lung adenocarcinoma through regulation of EGFR signaling. Oncol Res. 32:1119–1128. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Gao A and Wang H: Targeting tumour-osteoclast interactions: A trigger-explosion system to combat bone metastasis. Biomater Transl. 5:328–330. 2024.PubMed/NCBI | |
|
De Felice F, Piccioli A, Musio D and Tombolini V: The role of radiation therapy in bone metastases management. Oncotarget. 8:25691–25699. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Hong S, Youk T, Lee SJ, Kim KM and Vajdic CM: Bone metastasis and skeletal-related events in patients with solid cancer: A Korean nationwide health insurance database study. PLoS One. 15:e02349272020. View Article : Google Scholar : PubMed/NCBI |