Research progress of anti‑angiogenic therapy combined with immunotherapy and radiotherapy for the treatment of brain metastases in non‑small cell lung cancer (Review)
- Authors:
- Published online on: July 8, 2025 https://doi.org/10.3892/ol.2025.15180
- Article Number: 434
-
Copyright: © Li et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Lung cancer is one of the most common cancers, with a 5-year survival rate of only 27%, and non-small cell lung cancer (NSCLC) is the most common subtype (1). Of note, ~57% of patients with NSCLC are initially diagnosed at an advanced stage and 40–50% of patients develop brain metastases (BMs) during the disease progression due to the limited screening methods (2,3). Currently, the primary treatment of patients with NSCLC with BMs is systemic therapy coupled with local therapy, such as surgery and radiotherapy (RT) (4). For instance, the 2022 American Society of Clinical Oncology-Society for Neuro-Oncology-American Society for Radiation Oncology guidelines (5) recommend that patients with asymptomatic BMs from NSCLC receive stereoscopic radiosurgery (SRS) or stereoscopic body RT alone for treating one to four lesions. RT should be provided after surgery in patients with one or two BMs, because it is indispensable in the treatment of brain metastases. Furthermore, targeted therapy is preferred for patients with BMs that are vital for driver genes, such as Epidermal Growth Factor Receptor gene mutations can be treated with 1–3 generation targeted drugs. But there is insufficient evidence to recommend any systemic therapy, specifically for intracranial tumour control in patients who are negative for driver genes (4–6). In summary, the current treatments for BMs are limited, which markedly affects the overall prognosis of patients with NSCLC with BMs (7).
Angiogenesis and immune escape are key elements in tumour formation and the growth of metastatic brain tumours in NSCLC is critically dependent on angiogenesis (8). Anti-angiogenic drugs (AADs) normalise blood vessels, improving access to the tumours, which is useful when combined with immunotherapy (IT) methods. This combined treatment may have a synergistic effect on the tumour immune microenvironment (TIM) to inhibit the occurrence of tumours (9–11). In addition, anti-angiogenic therapy (AAT) can modulate the tumor immunosuppressive microenvironment and help to reverse IT resistance (ITR) (12). Previous studies have reported that IT combined with AAT has tolerable toxicity and efficient antitumour activity in patients with advanced NSCLC receiving later-line treatment (13). Furthermore, AADs cause dehydration, which creates conditions for RT to further control tumour progression and effectively improve radiation-induced brain injury (14).
The emergence of IT has become a milestone in antitumour therapy. However, the frequency of ITR is also increasing with the use of immunosuppressants (15). Although the TIM is an abundant source of therapeutic targets (16), the understanding of the TIM in the brain lacks comprehensive and integrated analyses. Nevertheless, the importance of the TIM cannot be ignored in the whole treatment process of patients with NSCLC with BMs (17). Research into the mechanisms will provide a more comprehensive understanding of the treatment of diseases. Accordingly, the present article aimed to elaborate on the TIM and clinical application of AAT, IT and RT in patients with NSCLC with BMs, in order to broaden the ideas for subsequent clinical research and treatment strategy design.
Intracerebral immune microenvironment of NSCLC
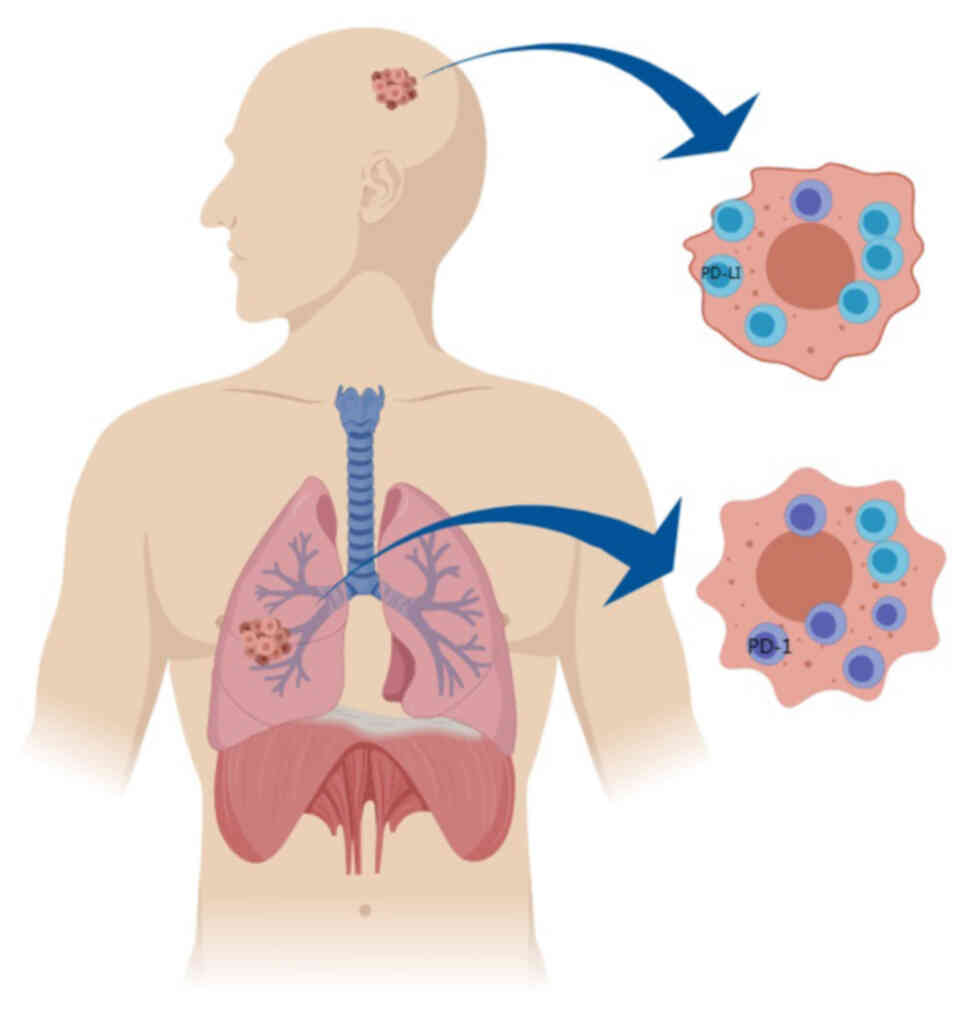
The occurrence of BMs from NSCLC is a complex process and the TIM serves an indispensable role (18). Studies have demonstrated that monocyte-macrophages, neutrophils, CD4+ T cells and CD8+ T cells serve a decisive role in the TIM of BMs from lung cancer (9,19). When disseminated tumour cells meet these immune cells, complex interactions occur, forming tumour-promoting and tumour-suppressing environments (20). Due to differences in the unique composition of the extracellular matrix in terms of collagen, hyaluronic acid and enzymes, the TIM of the brain has unique characteristics, which are radically different from those of the TIM of primary lung cancer (21). In NSCLC BM, tumour cells change the function of cell junctions after penetrating the blood-brain barrier, which leads to an altered intracranial TIM (22). For instance, patients with lung adenocarcinoma show a more distinctive pattern of low infiltration of CD3+ and CD8+ lymphocytes, suggesting that most patients lack a meaningful local immune response associated with BMs (23,24). An analysis of immunohistochemical results by Kim et al (25) showed that the number of programmed cell death protein 1 (PD-1) tumour-infiltrating lymphocytes was markedly lower in BMs compared with primary lung cancer. As shown in Fig. 1, another study revealed that PD-1-positive tumour-infiltrating lymphocytes had low intracranial expression and high primary lung sites in patients with NSCLC with BMs due to their lower permeability. Programmed cell death protein ligand 1 (PD-L1) is generally highly expressed in metastatic sites and is highly expressed in the brain, which is one of the reasons for the application of IT (26). These findings reveal that the efficacy of immune checkpoint inhibitor (ICI) agents is different. In other words, tumors with high PD-L1 expression are more likely to respond to ICI. Besides, chemotherapy, targeted therapy, RT and other antitumour therapies also affect the TIM (27–29).
In 2022, Li et al (30) performed ribonucleic acid sequencing on 86 samples from 43 patients with primary lung tumours and corresponding BMs to analyse the TIM. The results revealed that BMs were more immunosuppressed compared with primary lung tumours. In addition, the article by Wang et al (31) explored the mechanism of BMs in NSCLC, further highlighting the role of the TIM in the treatment of metastatic tumours. A previous study has concluded that combination therapy can increase the expression of PD-L1 in NSCLC BMs, thereby enhancing the treatment efficacy in these patients (32). In conclusion, in the era of IT, numerous treatment methods have different effects on the intracranial immune microenvironment of patients with BMs. The combined treatment has a synergistic effect and the impact on the microenvironment has become more evident (33,34).
RT
RT is an effective local treatment for NSCLC and it can affect the intracranial immune microenvironment. It eliminates tumour cells by inducing double-stranded deoxyribonucleic acid (DNA) damage and regulating antitumour immune responses at both irradiated and non-irradiated sites (35). As a commonly used treatment for BMs, RT can promote tumour-associated anti-immunity and enhance reoxygenation in the TIM, thereby promoting the recruitment of several immune effector cells and helping them infiltrate tumours (36,37). In addition, RT can cause immunogenic cell death and promote the release of tumour associated antigens, thus serving a synergistic role with IT (38). RT combined with IT has shown notable efficacy and can control tumour progression and prolong the survival of patients with NSCLC. Notably, studies have demonstrated that PD-L1 expression is mediated by the PI3K/AKT/mTOR pathway, which controls numerous cellular processes, including PD-L1 translation and transcription (39). Stimulation to enhance the AKT/mTOR pathway increases PD-L1 expression and vice versa. Downstream elements of AKT stimulate gene transcription by binding to the PD-L1 promoter. Furthermore, NF-κB, a downstream signal transducer of AKT, also acts on the promoter to induce PD-L1 mRNA expression (40). RT activates PI3K/AKT signalling through various pathways, such as the DNA damage response, reactive oxygen species (ROS) activation, inflammatory factor expression and growth receptor expression, leading to PD-1 expression and radioresistance (41).
Immunosuppressive agents can eliminate radioresistance caused by the upregulation of PD-L1 induced by RT and enhance its efficacy (42). Ikarashi et al (43) reported that CD4+ T cells, CD8+ T cells and CD4 Foxp3+ T cells were significantly higher in the tumor and stromal regions in primary lung cancer specimens, whereas only CD204+ cells were elevated in the tumor regions of brain metastases. CD4+ and CD4 Foxp3+ T cells were significantly increased in brain metastases with radiation therapy in the cancer and stromal regions compared with patients without radiation therapy. These macrophages may be immunosuppressive and make the immune environment less reactive (44,45).
Combination therapy can increase the local control rate of RT
Whole-brain RT (WBRT) and stereoscopic body RT (SBRT) are effective treatments for NSCLC with BMs, resulting in marked improvements in the local control rate (46). A study suggests RT combined with IT has an improved survival benefit compared with the sequential mode in patients with NSCLC BMs (47). Some data also show that RT combined with IT has a relatively high local intracranial control rate and results in an improved disease prognosis (48). ICIs coupled with RT have a strong intracranial efficacy and show a clinical benefit for patients with EGFR-mutant NSCLC with BMs whose previous EGFR-tyrosine kinase inhibitor (TKI) treatment failed (49,50). A meta-analysis of 32 preclinical and 9 clinical studies revealed that the radiosensitising effect of immunosuppressive agents exhibited a range with sensitizer enhancement ratios from 0.4 to 80, and it was improved when immunosuppressive agents were administered simultaneously (51). In addition, a study reported that RT combined with anti-vascular targeted therapy can also cause immune effects in patients with BMs. For instance, bevacizumab combined with radiotherapy can increase the infiltration of immune cells (52).
Cerebral radiation necrosis is one of the side effects of RT. The primary mechanism is the increased permeability caused by neovascularization, resulting brain edema with symptoms such as dizziness and headache. AAT is one of the most important treatment methods for these patients with cerebral edema (53). Therefore, if radiation-induced brain injury occurs after RT, AAT can be protective (42,54). The mechanism of the synergistic action between AAT and ionising radiation is complex. Drugs that target the tumour vasculature and angiogenesis in new tumours can regulate the TIM and improve blood flow and oxygenation, thereby increasing radiosensitivity (55). Previous studies have demonstrated that bevacizumab is a feasible and favourable supportive treatment for cerebral necrosis after WBRT or SBRT, which can notably relieve patient symptoms and improve RT tolerance (56,57). The results of the meta-analysis by Khan et al (58) showed a marked improvement in both radiographic and clinical responses with bevacizumab, without any serious adverse events. In addition, anlotinib can promote vascular normalisation and increase tumour tissue oxygenation, thereby improving the efficacy of radio-surgical treatment (59). Furthermore, it can also increase the infiltration and activation of CD8+ T cells encouraged by RT, promote the cytotoxicity and proliferation of CD8+ T cells, promote the activation of immune memory and enhance radiosensitivity (60). In summary, combination therapy can increase the local control rate of RT.
Anti-angiogenesis
AADs influence the TIM of patients with NSCLC with BMs
Animal studies have demonstrated that AAT can reduce the infiltration of intracranial tumour T cells and reduce the number of immunosuppressive tumour-associated macrophages (TAMs) (61). At the same time, it can also decrease the number of blood vessels in tumours, promote the stability of the endothelium, enhance endothelial barrier function, promote the migration of white blood cells and heighten antitumour activity (62). At present, AATs approved for clinical use are mainly classified into three main categories: Small-molecule multitarget angiogenesis inhibitors, large-molecule single-target angiogenesis inhibitors and endogenous pan-target angiogenesis inhibitors (63,64). The role of these drugs has been explored in clinical trials of various diseases, including breast cancer (65), gastric cancer (66) and liver cancer (67). For instance, previous trials have demonstrated that bevacizumab can improve the objective response rate. However, the effects on the progression-free survival (PFS) and the overall survival (OS) were not clinically meaningful in patients with NSCLC with BMs (68,69). A previous study demonstrated that apatinib was effective in patients with advanced NSCLC, but the improvement of PFS was limited (70). However, several larger prospective studies reported that apatinib was effective and well tolerated in patients with advanced NSCLC, and PFS and OS were markedly improved (71,72). Regorafenib can inhibit tumour growth and angiogenesis in vivo, enhance the antitumour efficacy of antigen-specific T cells and regulate macrophage polarisation to enhance antitumour immunity (73). In a retrospective study, anti-PD-1 therapy combined with anlotinib had tolerable toxicity and superior antitumour activity in treating patients with advanced NSCLC treated beyond second-line therapy (13). In addition, a number of AADs, such as lenvatinib and Endostar, have shown beneficial effects on the treatment of lung cancer, but the effects of single drugs on the TIM are relatively unknown (62,74). Therefore, further exploration of the mechanisms of action of different drugs is needed.
AAT enhances the efficacy of combined therapy by affecting the TIM
A preclinical study demonstrated that AAT can induce vascular normalization (75). Therefore, consolidation therapy with AAT after RT is an effective approach (76). AAT restores microvasculature in the tumour, thereby alleviating hypoxia, and its combination with SBRT may synergistically inhibit tumour growth (77). Park et al (78) performed further animal experiments and demonstrated that the combination of AAT inhibiting VEGF and placental growth factor and RT could effectively inhibit tumour growth.
Through anti-angiogenesis, tumour vascular normalization and TAM polarisation from the protumour M2 phenotype to the antitumour M1 phenotype occur. Bevacizumab inhibits angiogenesis by increasing perivascular cell coverage and decreasing tumour hyperpermeability, vessel calibre, hypoxia and interstitial fluid stress. This reduces peritumoral oedema and alleviates subsequent radiation necrosis (79). Furthermore, it can inhibit DNA double-strand break repairs, enhance tumour microvasculature recovery and increase NSCLC radiosensitivity (80). For instance, Li et al (81) explored the combination of bevacizumab with SRS or WBRT for BMs and found that the combination therapy improved the overall efficacy and reduced peritumoral oedema of BMs caused by NSCLC, without any notable side effects. Chen et al (82) also found that the combination of bevacizumab and SRS was effective in the treatment of BMs with improved radiographic response and less treatment-related toxicity. In addition, anlotinib can improve the vascular structure and permeability of tumours and reduce brain oedema, making it a useful alternative to glucocorticoids in treating refractory brain oedema (83). Previous studies on combination therapy have demonstrated that effective low-dose anlotinib combined with PD-1 blockade can induce durable antitumour effects with few side effects (84). Research on the use of anlotinib combined with RT in the treatment of brain malignant tumours has found that RT reduces the effect on the blood-brain barrier, thereby enhancing the antitumour activity of anlotinib (85). Apatinib can improve radiation-induced brain necrosis in patients with head and neck cancer and is well tolerated when combined with RT (86). Sunitinib can inhibit tumour growth and angiogenesis, showing efficient synergistic tumour inhibition ability (87). In conclusion, AAT has clinical importance for treating patients with advanced NSCLC with BMs.
Combination of AADs may contribute to reversing resistance to IT
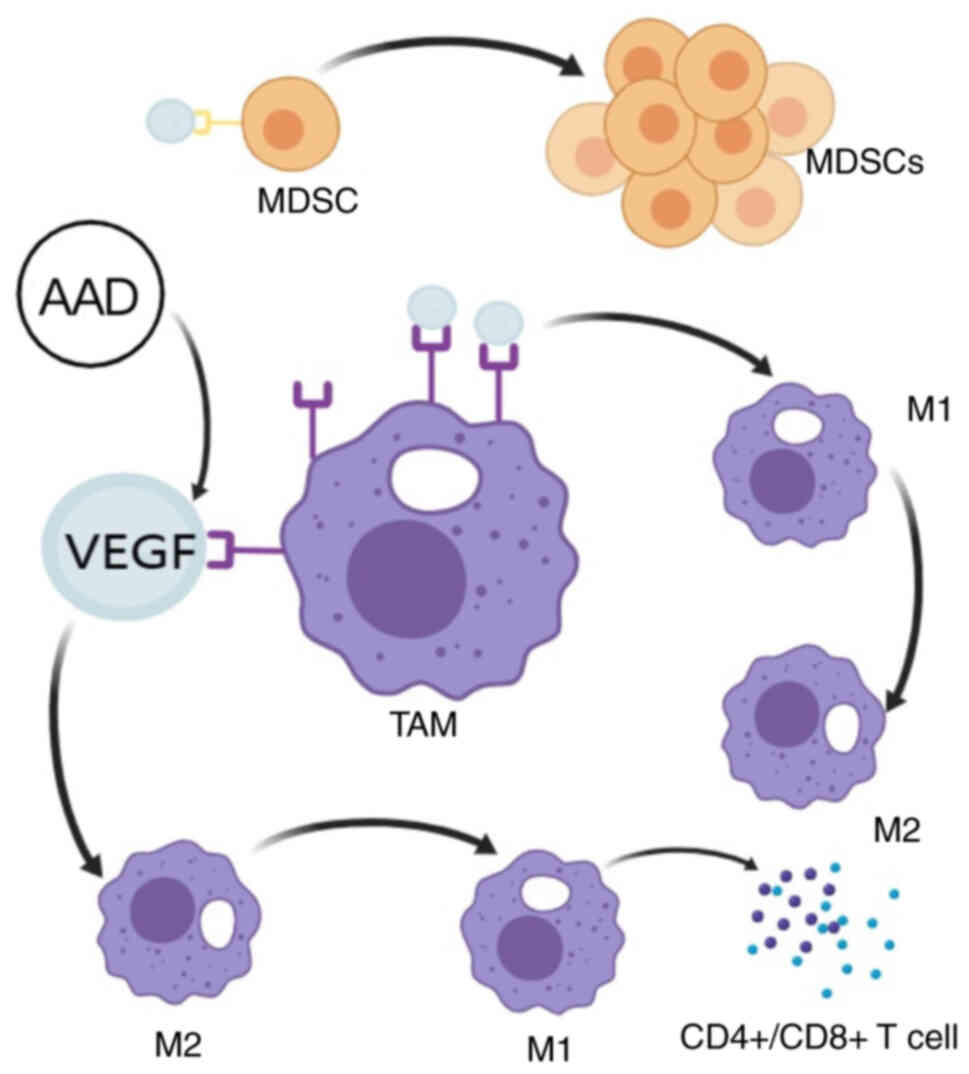
The tumour vasculature is an essential component of the TIM. Improving access to tumours by vascular normalization with AAT is an effective combination strategy with IT, and this combination therapy may have a synergistic effect on the TIM to inhibit tumour occurrence (9,88,89). As shown in Fig. 2, VEGF can bind to VEGFR on the surface of bone marrow-derived suppressor cells (MDSCs) and promote their continuous proliferation as a chemoattractant. MDSCs are immunosuppressive cells, and anti-VEGF therapy can inhibit their proliferation, enhance the immune response and exert synergistic antitumour effects when combined with IT drugs (90). Furthermore, the expression of VEGFR is regulated by hypoxia in a hypoxia-inducible factor (HIF)-dependent manner, while the stable expression of HIF-1α was demonstrated to be dependent on ROS production, indicating ROS regulates the expression of VEGFR (91). Under hypoxic conditions, VEGFR expression in TAMs is increased, and TAMs bind more VEGF, activate the PI3K/AKT signalling pathway, inhibit proinflammatory M1 polarisation and promote the expression of anti-inflammatory and repair-associated M-type markers (92).
On the other hand, VEGFR-activated tumour cells secrete factors including IL-10 and TGF-β, which directly induce the transformation of macrophages into the M2 type. Several mechanisms work together to promote the transformation of TAMs from M1 to M2, enhance immunity and angiogenesis and promote tumour development (93). Therefore, anti-VEGF therapy normalises blood vessels, improves hypoxia, reduces VEGFR expression and converts TAMs from immunosuppressive M2 cells to immune-stimulating M1 cells through multiple mechanisms (92). M1 cells can produce large amounts of ROS and nitric oxide, which can directly induce DNA damage and apoptosis in tumour cells. Furthermore, IL-12 and IL-18 are secreted to inhibit VEGF expression, block tumour angiogenesis and serve a tumour suppressor role (94). In addition, M1-type macrophages can present tumour antigens to CD4+ T cells, recruit CD8+ T cells to the tumour site and mediate the ability of IL-12 to increase their killing activity and promote CD4+ and CD8+ T-lymphocyte infiltration, thereby improving the tumour immunosuppressive microenvironment (95).
Enhancing IT and reversing immune resistance are important for AAT combined with IT to achieve notable antitumour effects. The addition of bevacizumab to atezolizumab produced sustained biochemical and radiographic responses that appeared to overcome resistance to nivolumab monotherapy (96). Anlotinib can regulate the tumour immunosuppressive microenvironment and may help reverse ITR (12,13). Apatinib can alleviate high angiogenesis and hypoxia in the microenvironment, improve immunosuppressive agent efficiency in tumours with VEGFA overexpression and overcome innate resistance to immunosuppressive agents in tumour models with high angiogenesis (97). An increasing number of clinical studies support that combined AAT and IT can improve ITR and improve the prognosis of malignant tumours (98–100).
Immunosuppressants
IT and immune resistance
In tumour tissues, cancer cells, immune cells and other stromal cells interact to generate an immunosuppressive microenvironment through a variety of immunosuppressive factors, such as interleukin-10 and hyaluronic acid (101). Therefore, PD-1/PD-L1 has become the main target of IT (102). With immunosuppressant development, IT has been recommended as the first-line treatment for patients with NSCLC. However, certain patients are considered resistant to IT during treatment, and the mechanism of ITR is still being explored (103). Contemporary ideas point to a variety of factors that may contribute to immune resistance. Regardless of genomic, immune system, cancer microenvironment-associated or host cell-generated factors, ITR can emerge during treatment and lead to treatment failure (104–106). Various combined therapies, such as immunotherapy combined with radiotherapy, have prevented and reversed immunosuppressant resistance to a certain extent (107–109). The study by Abou Khouzam et al (110) proposed that hypoxia induces angiogenesis and subsequent immunosuppression, which is the main reason for IT failure. The study by Larroquette et al (111) proposed that combining chemotherapy and IT can disrupt the ITR of the NSCLC TME. However, chemotherapy is not well tolerated in patients with NSCLC with BMs, and it is thus important to develop other methods.
Immunosuppressive agents affect the microenvironment of BMs
Due to differences in the TIM, the response of primary tumour lesions and BMs to the IT of NSCLC may be inconsistent (112). The antitumour CD8+ T cells are relatively deficient in intracranial tumours and there are more dysfunctional CD8+ T cells compared with primary tumours. Furthermore, intracranial macrophages and dendritic cells have increased protumour and anti-inflammatory effects (113). Additionally, PD-1 tumour-infiltrating lymphocytes are less permeable in BMs, which may markedly reduce the ICI effect of anti-PD-1 therapy. However, PD-L1 expression is usually higher in metastatic sites compared with primary tumours, which is also associated with the efficacy of ICIs (114). Studies have demonstrated that PD-1/PD-L1 expression can be employed as an independent predictor of survival in patients with NSCLC with BMs who receive IT (19,114). When BMs develop from NSCLC, PD-1 is expressed on activated T cells, monocytes and dendritic cells, and PD-L1 is also expressed on tumour and immune cells in the brain. In this immune microenvironment, PD-1/PD-L1 inhibitors may have a notable antitumour effect on BM (115,116). Therefore, for patients with NSCLC with BMs, the use of immunosuppressive agents can achieve improved efficacy.
Combined IT improves treatment efficacy in patients with NSCLC with BMs
IT is an effective treatment for a variety of advanced cancers and the IT combined with targeted therapy, chemotherapy, or radiotherapy is the standard first-line treatment for patients with advanced NSCLC with negative driver genes (117,118). The results of a study by Yu et al (119) showed that patients with lung cancer with liver metastases derive less benefit from IT compared with patients with BMs. Therefore, patients with BMs have an improved basis for organ-specific IT. In a meta-analysis published in 2023, the authors investigated the potential benefit of different treatment options for intracranial lesions in patients with NSCLC that is negative for driver genes (120). The results showed that immunosuppressant-based combination therapy provided a long-term survival benefit for patients receiving non-targeted therapy compared with patients who did not receive immunotherapy. The results of another meta-analysis also showed that IT was effective in patients with non-targeted NSCLC (121). Therefore, IT serves an important role in cancer treatment, and combination therapy can delay disease progression and prolong patient survival to a certain extent (122,123).
IT combined with RT provides the best local control for patients with BM without increasing the risk of toxicity (124). A multicentre retrospective study by the Italian Society of Radiotherapy and Clinical Oncology reached similar conclusions (125). Additional studies have also reported that using ICIs combined with RT improves OS and reduces the risk of brain failure and neurological mortality in patients with NSCLC with BMs compared with a single treatment (126–128). ICIs have effective radiosensitizing effects and can synergistically enhance antitumour effects in combination with brain RT (129,130). Furthermore, since AAD promotes antitumour immune responses (131,132), the combination of IT and AAT may notably impact the BMs (133). Other studies have demonstrated increased anticancer efficacy after adding AAT to immunosuppressive agents (134,135). IT combined with AAT can markedly improve the OS of patients with NSCLC driver gene mutation-negative BMs (136). In conclusion, ICIs combined with AAT and RT may have notable survival benefits for patients with NSCLC.
Triple-injection therapy can increase treatment efficacy and reverse immune resistance
Previous studies have demonstrated that IT combined with RT, IT combined with AAT and RT combined with AAT achieve notable clinical benefits in the treatment of patients with NSCLC with BMs, and no serious grade 4–5 adverse reactions were reported in the process of combined treatment (137–141). However, no clinical trials have investigated the combination of these three treatments in patients with NSCLC with BMs. On the one hand, there is no clear indication for the combination therapy with these three treatments and most clinicians use combination therapy with two treatments (142,143). On the other hand, although there are safety reports on combination therapy, these reports are rare, and most are basic experiments and have not been translated into clinical practice. For example, combination therapy with aspirin, afatinib and vinorelbine was significantly more effective than monotherapy in NSCLC cell models (144,145). As a result, research into the simultaneous application of multiple treatments has been hindered.
AAT, IT and RT can affect the intracranial immune microenvironment of patients with BMs and exert antitumour effects. However, it currently remains elusive whether the simultaneous use of these three methods will synergistically change the TME and achieve stronger antitumour effects. To date, numerous studies have investigated the mechanism of AAT combined with IT and demonstrated that the combined therapies can have a stronger antitumour effect (146,147). However, relatively few studies have investigated the combination with RT. In animal experiments, RT combined with anti-PD-L1 and anlotinib therapy increased the number of tumour-infiltrating lymphocytes and reversed the immunosuppressive effect of RT on the TIM. These findings suggest that AAT may be a potential radioimmunotherapy synergistic therapy to achieve improved antitumour efficacy in patients with NSCLC by enhancing the TIM (148,149).
Previous mechanistic studies have shown that AAT promotes vascular normalisation and immune cell infiltration, RT provides antigen release and in situ vaccine effects and IT maintains T-cell activity, thereby forming a beneficial cycle (42,150). The molecular mechanisms by which these three factors regulate the TIM through multiple dimensions include the following: First, AAT serves a central role by targeting VEGF/VEGFR and other pathways to reduce tumour vessel density, inhibit abnormal angiogenesis, alleviate hypoxia, improve vascular permeability and promote T-cell penetration and drug delivery (89,151). Second, IT enhances antitumour efficacy by restoring T-cell function and promoting the antitumour immune response while synergistically activating T cells in combination with RT (89,152). Finally, RT can upregulate PD-L1 expression and enhance immune recognition while providing an antigen ‘immune’ effect, maintaining a long-term response to IT and increasing the anti-angiogenic therapeutic window (153). The combination of these three treatments not only reverses ‘immune-excluded tumours’ to ‘immune-inflamed tumours’ in the presence or absence of a strong immune response within the TME to enhance the antitumour effect but also alleviates single-drug resistance, providing long-term benefits for the treatment of advanced tumours (150).
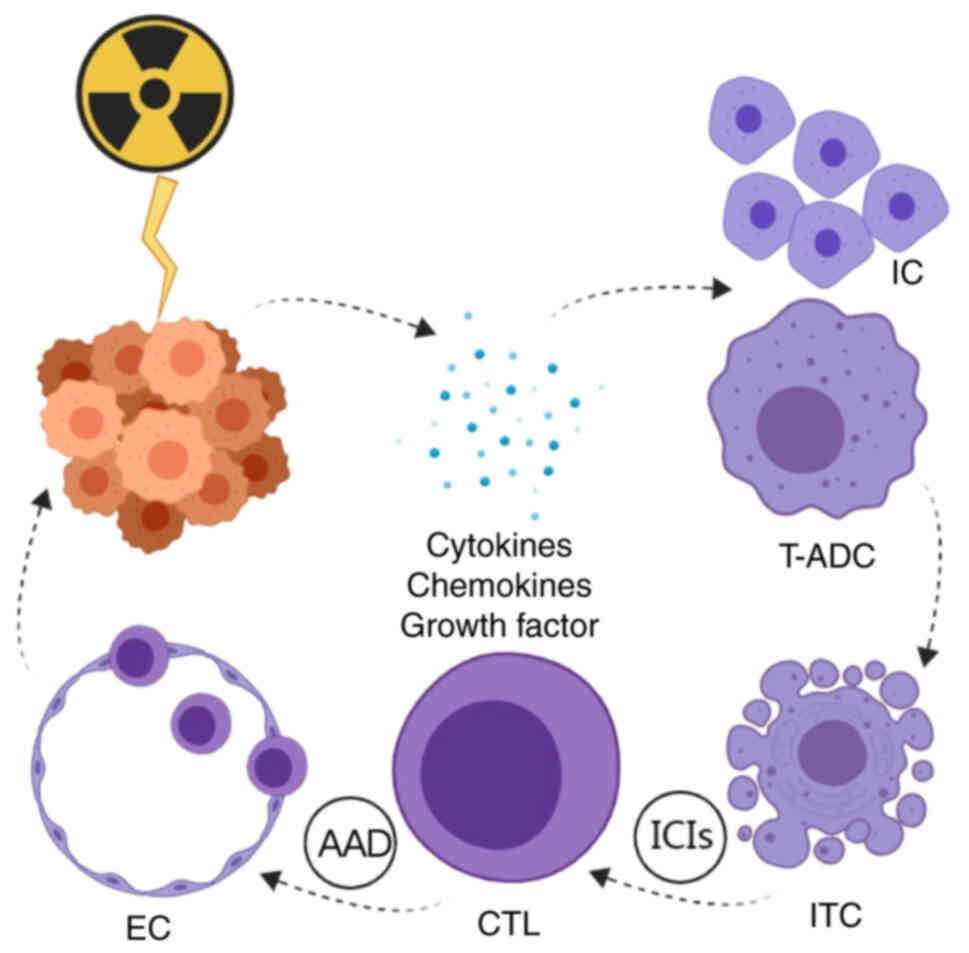
The molecular mechanisms of RT in combination with IT and AAT for malignant tumour treatment are described in Fig. 3. RT irradiation leads to tumour cell death and the recruitment of numerous cytokines, chemokines and growth factors in the TME. In addition, RT causes the recruitment of immune cells while activating tumour antigen-presenting dendritic cells through the apoptosis of immunogenic tumour cells, the activation of innate immunity and the activation of cytotoxic T lymphocytes (CTLs). This antigenic ‘vaccine’ effect provided by RT could allow anti-PD-1 agents to further enhance CTL activation, induce immune memory and amplify systemic antitumour responses through an ‘abnormal effect’. AAT can inhibit abnormal angiogenesis, improve vascular structure and function in the short term and restore the blood vessels to normal. PD-1 IT releases interferon, which allows CTLs to have an antitumour effect through endothelial cells in structurally normalised blood vessels and cooperate with RT and IT (42,52).
The meta-analysis performed by Xian et al (154) demonstrated that in the treatment of solid tumours, ICIs combined with RT and AAT showed improved survival benefits compared with single or dual drug combination therapy, and triple therapy was tolerable and safe. However, the analysis did not identify specific cancer conditions to evaluate tolerability, and the safety of triple therapy in patients with NSCLC BMs has not been evaluated in the published literature. Therefore, all patients in Table I screened for NSCLC BMs were treated with double therapy, and the safety evaluation was acceptable. The main adverse reactions included leukopenia and other haematological toxicities, such as hypertension. The incidence of radiation-induced brain necrosis was not high, possibly because AADs increase vascular permeability, which not only improves brain oedema during RT but also reduces the occurrence of radiation-induced brain necrosis. However, AADs can markedly increase blood pressure. In clinical practice, certain patients cannot use these drugs due to difficulty in blood pressure control (155). For these patients, the dose should be reduced or used cautiously. In addition, no obvious immune-related adverse events occurred in patients receiving the two-combination therapy. The 2023 case report by Long et al (156) described a 55-year-old patient with BMs from advanced small-cell lung cancer who received combined treatment consisting of anlotinib and WBRT, with anti-PD-L1 IT in combination with anlotinib as long-term maintenance therapy. The OS was 41 months after the onset of BM and no serious adverse reactions occurred. The application of IR and TT has improved BM treatment and several studies have suggested that IT or TT is effective for patients with NSCLC with BMs (102,157). Therefore, combining the three therapies may have clinical benefits in the treatment of patients with NSCLC with BMs with no serious adverse reactions.
Table I.Clinical trials evaluating the efficacy and safety of combination therapy for patients with non-small cell lung cancer with brain metastases. |
The results of previously published meta-analyses indicate that most studies on AAT combined with IT and RT consist primarily of animal trials, retrospective analyses, a limited number of case reports and single-arm trials with small sample sizes (154,158). These studies demonstrate that triple therapy can enhance the efficacy of antitumour treatment without inducing severe adverse reactions. To date, IT plus chemotherapy combined with AAT has achieved good results in a number of randomized controlled trials (159,160), and the results of certain clinical trials have demonstrated that RT plus chemotherapy combined with IT or AAT has effective antitumour treatment effects (161,162). However, there is still a lack of large clinical randomized controlled trials of RT combined with IT and AAT. Results of a phase II trial indicated that triple therapy plus chemotherapy showed promising antitumor activity and was well tolerated in advanced solid tumours (163). Therefore, further investigation is warranted into the integration of AAT with RT and IT (164,165). The present article provides an in-depth feasibility analysis of triple therapy for patients with NSCLC with BMs, concluding that such therapy improves patient survival with controllable safety. Based on these findings, a clinical observation project has been initiated for triple therapy in patients with NSCLC with BMs. The project has received ethical approval and is currently recruiting participants, aiming to encourage more institutions to perform large-scale clinical trials, which represents one of the key directions for future research.
WBRT is currently the first choice for patients with NSCLC with extensive BMs. SRS alone can be performed for patients with 1–4 metastatic lesions, SR can be provided after craniocerebral surgery for patients with 1–2 metastatic lesions and WBRT + SRS can be performed for patients with numerous metastatic lesions but no extensive metastases (5). A previous study demonstrated that patients receiving WBRT + SRS or SRS alone have similar intracranial control rates and survival benefits; however, patients receiving WBRT + SRS have more severe cognitive impairment (166). Therefore, regardless of the number of BMs, different RT methods can result in similar therapeutic responses, but when combined with systemic therapy, RT needs to be selected. For triple therapy, patients who can undergo SRS are more inclined to be selected to reduce adverse reactions. The application of local therapy is suitable for most patients with NSCLC with BMs, and their treatment response mainly differs with respect to the choice of systemic therapy (167).
According to the current clinical guidelines, targeted therapy is the first choice of systemic therapy for patients with NSCLC with BMs and positive driver genes (5). However, due to the blood-brain barrier, there is no strong evidence-based foundation for the systemic treatment of patients with negative driver genes (5). The results from the phase III IMpower150 study (168) revealed statistically and clinically notable PRS with combination therapy regardless of PD-L1 expression or EGFR or anaplastic lymphoma kinase mutation status. However, in patients with positive driver genes, targeted therapy seems to achieve improved survival benefits compared with patients without driver genes (168). Sintilimab plus anlotinib showed durable efficacy in metastatic NSCLC with rare EGFR mutations in a phase II study (169). Similarly, in another study, benmelstobart combined with anlotinib showed promising antitumor efficacy and a tolerable safety profile in patients with EGFR-positive advanced NSCLC after the failure of EGFR TKI (170).
As previously discussed, AAT can reverse immune resistance and exert more powerful antitumour effects in combination with IT. Therefore, it is important to evaluate the factors affecting IT and AAT efficacy. Tumours with high expression of PD-L1 (such as NSCLC) may be more sensitive to PD-1/PD-L1 inhibitors, and certain PD-L1-negative patients may still benefit. Therefore, the population suitable for IT may be selected by detecting PD-L1 expression in clinical practice. By selecting patients with a tumor proportion score (TPS) ≥1%, partially negative patients can also be evaluated for IT. In addition, different drugs can be selected based on the TPS value for more precise IT (171). The VEGF expression level can affect AAT efficacy; tumours with high VEGF expression may be more sensitive to AAT, but certain tumours with low VEGF expression may also be responsive. Serum/plasma VEGF levels can be used to predict treatment efficacy, and high VEGF levels may have greater effects on patients (172). However, it is necessary to carefully select anti-VEGF drugs for patients with hypertension and closely observe blood pressure changes during their use as hypertension is the most common adverse reaction to anti-VEGF drugs (172). Therefore, triple therapy is mainly used in patients with negative driver genes but can also be used in patients with positive driver genes where targeted therapy failed.
In addition, due to the increased risk of life-threatening radiation necrosis and oedema in the brainstem and cerebellum after RT, the treatment strategy should be carefully considered in triple therapy, including the RT dose, AADs, IT drug dose and duration (173,174). For BMs, both functional and non-functional areas should be considered in RT. The performance of RT in functional areas is similar to that in the brainstem, and the combination of AADs can effectively relieve oedema and reduce the side effects of RT to a certain extent, while there is no limitation in non-functional areas (175). However, the treatment effect and survival of patients with leptomeningeal metastasis are poor, and these patients are not sensitive to palliative RT. Combined therapy may increase the burden on patients, and accordingly, triple therapy is not recommended (176).
Since the approval of IT for NSCLC treatment, patients treated with ICIs have obtained notable survival benefits compared with previous treatments (177,178). To date, with the continuous application of IT, numerous patients have developed ITR, which severely affects disease treatments (179,180). Exploring the mechanisms of resistance to immunosuppressants is crucial for further improving their clinical benefit (181). A variety of IT combination therapies have prevented and reversed ITR (182,183). Currently, an increasing number of clinical trials are exploring the safety and efficacy of combination therapy in patients with NSCLC with ICI resistance (183,184). AAT, RT, IT and other treatments will affect the TIM, which may reverse ITR and improve clinical efficacy (153,185,186). However, previous studies choose two of the three treatment methods: RT, IT and TT for combined treatment and their efficacy achieved satisfactory results with relatively few side effects (187–189). However, as the disease progressed, the two treatments were not sufficient, so triple therapy was explored. The results from animal experiments demonstrate that triple therapy can notably delay tumour growth and enhance the survival rate compared with two or single treatments (148,190), which is expected to be further extended to clinical application and bring new survival benefits.
Triple therapy is currently being explored in animal models; however, only one study has been published to date (148). Although the effect of triple therapy in animal models is notable, these models still have certain limitations. First, the survival observation time and the establishment form of the animal model were insufficient, and the effect of triple therapy on survival and tumour recurrence has not been observed in the experimental process. Second, there are no animal models to investigate whether different RT dose fractionations affect the efficacy of triple therapy. Finally, the effects of RT and different dosing intervals on efficacy have not been explored. There remains a need to study triple-therapy animal models in the future. However, owing to the notable differences in gene number, expression profile and ligand binding and function between human and murine TIM proteins, the same therapeutic strategy that works well in animal models may not work well in humans (191–194). Therefore, phase II or III clinical trials should be performed under ethical conditions to assess the efficacy and safety of triple therapy. This will not only provide new treatment strategies for patients with advanced NSCLC with BMs but also provide new ideas for the treatment of other cancers.
There are still numerous directions for future research. First, based on the evidence discussed in the present review, triple therapy can achieve good efficacy and controllable safety in patients with NSCLC with BMs, but there is a lack of large-scale prospective clinical trials to verify this; therefore, further studies are needed to evaluate its clinical benefits. Second, triple therapy can be used in the treatment of other cancers, such as liver cancer or pancreatic cancer, to provide new ideas for tumour inhibition, but this also needs to be evaluated by clinical trials. Third, the mechanism by which triple therapy jointly regulates the TIM is not fully understood and changes in the TIM when the three treatments are used simultaneously need to be explored further. Finally, at present, most studies on TIM regulation by triple therapy are carried out in animal models; therefore, future studies using human tissues or blood are needed. This article also has certain limitations. There are relatively few relevant studies including triple therapy and there is a lack of prospective clinical trials to prove its effectiveness. Secondly, it only provides a new treatment idea for patients with NSCLC with BMs and does not extend to include more methods. This is expected to be addressed in future studies.
Conclusions
RT combined with AAT and IT has a synergistic effect in the treatment of patients with BMs from NSCLC and may reverse the ITR during treatment. Combination therapy may provide additional survival benefits to patients with NSCLC with BMs, but its specific efficacy and safety need to be explored further.
Acknowledgements
Not applicable.
Funding
The present work was financially supported by the 2023 Yunnan Xingdian Talents Support Program ‘Young Talents’ project (grant no. XDYC-QNRC-2023-0189).
Availability of data and materials
Not applicable.
Authors' contributions
ML was involved in the conceptualization, validation, formal analysis, investigation, data curation and writing the original draft. JG was involved in formal analysis, investigation, and manuscript review and editing. FL was involved in formal analysis, visualization and methodology. CG was involved in visualisation and methodology. JZ was involved in conceptualization and formal analysis. LW was involved in project administration, conceptualisation, manuscript review and editing, and resources/funding acquisition. YX was involved in resources, project administration, conceptualisation, project administration, manuscript review and editing, and funding acquisition. Data authentication is not applicable. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
BMs |
brain metastases |
|
NSCLC |
non-small cell lung cancer |
|
RT |
radiotherapy |
|
SRS |
stereoscopic radiosurgery |
|
AAD |
anti-angiogenic drugs |
|
IT |
immunotherapy |
|
TIM |
tumour immune microenvironment |
|
AAT |
anti-angiogenic therapy |
|
ICI |
immune checkpoint inhibitors |
|
ITR |
immunotherapy resistance |
|
PD-1 |
programmed cell death protein 1 |
|
PD-L1 |
programmed cell death protein ligand 1 |
|
DNA |
deoxyribonucleic acid |
|
WBRT |
whole-brain radiotherapy |
|
SBRT |
stereoscopic body radiotherapy |
|
PFS |
progression-free survival |
|
OS |
overall survival |
|
MDSC |
marrow-derived suppressor cell |
|
TAM |
tumour-associated macrophage |
|
CTL |
cytotoxic T lymphocyte |
References
|
Siegel RL, Kratzer TB, Giaquinto AN, Sung H and Jemal A: Cancer statistics, 2025. CA Cancer J Clin. 75:10–45. 2025. View Article : Google Scholar : PubMed/NCBI | |
|
Gansler T, Ganz PA, Grant M, Greene FL, Johnstone P, Mahoney M, Newman LA, Oh WK, Thomas CR Jr, Thun MJ, et al: Sixty years of CA. Cancer J Clin. 60:345–350. 2010. View Article : Google Scholar | |
|
Soffietti R, Ahluwalia M, Lin N and Rudà R: Management of brain metastases according to molecular subtypes. Nat RevNeurol. 16:557–574. 2020. | |
|
El Rassy E, Botticella A, Kattan J, Le Péchoux C, Besse B and Hendriks L: Non-small cell lung cancer brain metastases and the immune system: From brain metastases development to treatment. Cancer Treat Rev. 68:69–79. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D, Dunn IF, Gaspar LE, Gatson NTN, Gondi V, et al: Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol. 40:492–516. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Sung KS: Clinical practice guidelines for brain metastasis from solid tumors. Brain Tumor Res Treat. 12:14–22. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Berger A, Mullen R, Bernstein K, Alzate JD, Silverman JS, Sulman EP, Donahue BR, Chachoua A, Shum E, Velcheti V, et al: Extended survival in patients with non-small-cell lung cancer-associated brain metastases in the modern era. Neurosurgery. 93:50–59. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Berghoff AS, Ilhan-Mutlu A, Dinhof C, Magerle M, Hackl M, Widhalm G, Hainfellner JA, Dieckmann K, Pichler J, Hutterer M, et al: Differential role of angiogenesis and tumour cell proliferation in brain metastases according to primary tumour type: Analysis of 639 cases. Neuropathol Appl Neurobiol. 41:e41–e55. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Fang L, Zhao W, Ye B and Chen D: Combination of immune checkpoint inhibitors and anti-angiogenic agents in brain metastases from non-small cell lung cancer. Front Oncol. 11:6703132021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang X, Song M, Wang A, Zhao Y, Wei Z and Lu Y: Microbiome crosstalk in immunotherapy and antiangiogenesis therapy. Front Immunol. 12:7479142021. View Article : Google Scholar : PubMed/NCBI | |
|
Tu J, Liang H, Li C, Huang Y, Wang Z, Chen X and Yuan X: The application and research progress of anti-angiogenesis therapy in tumor immunotherapy. Front Immunol. 14:11989722023. View Article : Google Scholar : PubMed/NCBI | |
|
Sun Y, Niu W, Du F, Du C, Li S, Wang J, Li L, Wang F, Hao Y, Li C and Chi Y: Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. 9:1052016. View Article : Google Scholar : PubMed/NCBI | |
|
Wang P, Fang X, Yin T, Tian H, Yu J and Teng F: Efficacy and safety of Anti-PD-1 plus anlotinib in patients with advanced non-small-cell lung cancer after previous systemic treatment failure-a retrospective study. Front Oncol. 11:6281242021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang YX, Cheng C and Zhuang HQ: The safety and efficacy of anlotinib in combination with stereotactic radiotherapy for the treatment of brain metastases from non-small cell lung cancer. Zhonghua Yi Xue Za Zhi. 102:930–934. 2022.PubMed/NCBI | |
|
Dutta S, Ganguly A, Chatterjee K, Spada S and Mukherjee S: Targets of immune escape mechanisms in cancer: Basis for development and evolution of cancer immune checkpoint inhibitors. Biology (Basel). 12:2182023.PubMed/NCBI | |
|
Vilariño N, Bruna J, Bosch-Barrera J, Valiente M and Nadal E: Immunotherapy in NSCLC patients with brain metastases. Understanding brain tumor microenvironment and dissecting outcomes from immune checkpoint blockade in the clinic. Cancer Treat Rev. 89:1020672020. View Article : Google Scholar : PubMed/NCBI | |
|
Xiao G, Liu Z, Gao X, Wang H, Peng H, Li J, Yang L, Duan H and Zhou R: Immune checkpoint inhibitors for brain metastases in non-small-cell lung cancer: From rationale to clinical application. Immunotherapy. 13:1031–1051. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Spano D and Zollo M: Tumor microenvironment: A main actor in the metastasis process. Clin Exp Metastasis. 29:381–395. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Lv B, Wang Y, Ma D, Cheng W, Liu J, Yong T, Chen H and Wnag C: Immunotherapy: Reshape the tumor immune microenvironment. Front Immunol. 13:8441422022. View Article : Google Scholar : PubMed/NCBI | |
|
Rios-Hoyo A and Arriola E: Immunotherapy and brain metastasis in lung cancer: Connecting bench side science to the clinic. Front Immunol. 14:12210972023. View Article : Google Scholar : PubMed/NCBI | |
|
Mansfield AS, Aubry MC, Moser JC, Harrington SM, Dronca RS, Park SS and Dong H: Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol. 27:1953–1958. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Guan Z, Lan H, Cai X, Zhang Y, Liang A and Li J: Blood-Brain barrier, cell junctions, and tumor microenvironment in brain metastases, the biological prospects and dilemma in therapies. Front Cell Dev Biol. 9:7229172021. View Article : Google Scholar : PubMed/NCBI | |
|
Harter PN, Bernatz S, Scholz A, Zeiner PS, Zinke J, Kiyose M, Blasel S, Beschorner R, Senft C, Bender B, et al: Distribution and prognostic relevance of tumor-infiltrating lymphocytes (TILs) and PD-1/PD-L1 immune checkpoints in human brain metastases. Oncotarget. 6:40836–40849. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Téglási V, Reiniger L, Fábián K, Pipek O, Csala I, Bagó AG, Várallyai P, Vízkeleti L, Rojkó L, Tímár J, et al: Evaluating the significance of density, localization, and PD-1/PD-L1 immunopositivity of mononuclear cells in the clinical course of lung adenocarcinoma patients with brain metastasis. Neuro Oncol. 19:1058–1067. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Kim R, Keam B, Kim S, Kim M, Kim SH, Kim JW, Kim YJ, Kim TM, Jeon YK, Kim DW, et al: Differences in tumor microenvironments between primary lung tumors and brain metastases in lung cancer patients: Therapeutic implications for immune checkpoint inhibitors. BMC Cancer. 19:192019. View Article : Google Scholar : PubMed/NCBI | |
|
Mehdizadeh S, Bayatipoor H, Pashangzadeh S, Jafarpour R, Shojaei Z and Motallebnezhad M: Immune checkpoints and cancer development: Therapeutic implications and future directions. Pathol Res Pract. 223:1534852021. View Article : Google Scholar : PubMed/NCBI | |
|
Yu WD, Sun G, Li J, Xu J and Wang X: Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett. 452:66–70. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Bader JE, Voss K and Rathmell JC: Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol Cell. 78:1019–1033. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Darvin P, Toor SM, Nair VS and Elkord E: Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp Mol Med. 50:1–11. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Li M, Hou X, Sai K, Wu L, Chen J, Zhang B, Wang N, Wu L, Zheng H, Zhang J, et al: Immune suppressive microenvironment in brain metastatic non-small cell lung cancer: Comprehensive immune microenvironment profiling of brain metastases versus paired primary lung tumors (GASTO 1060). Oncoimmunology. 11:20598742022. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y, Chen R, Wa Y, Ding S, Yang Y, Liao J, Tong L and Xiao G: Tumor immune microenvironment and immunotherapy in brain metastasis from non-small cell lung cancer. Front Immunol. 13:8294512022. View Article : Google Scholar : PubMed/NCBI | |
|
Takamori S, Toyokawa G, Takada K, Shoji F, Okamoto T and Maehara Y: Combination therapy of radiotherapy and Anti-PD-1/PD-L1 treatment in non-small-cell lung cancer: A mini-review. Clin Lung Cancer. 19:12–16. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Popat S, Grohé C, Corral J, Reck M, Novello S, Gottfried M, Radonjic D and Kaiser R: Anti-angiogenic agents in the age of resistance to immune checkpoint inhibitors: Do they have a role in non-oncogene-addicted non-small cell lung cancer? Lung Cancer. 144:76–84. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Xie M and Su C: Microenvironment and the progress of immunotherapy in clinical practice of NSCLC brain metastasis. Front Oncol. 12:10062842022. View Article : Google Scholar : PubMed/NCBI | |
|
Meng L, Xu J, Ye Y, Wang Y, Luo S and Gong X: The combination of radiotherapy with immunotherapy and potential predictive biomarkers for treatment of non-small cell lung cancer patients. Front Immunol. 12:7236092021. View Article : Google Scholar : PubMed/NCBI | |
|
Suwinski R: Combination of immunotherapy and radiotherapy in the treatment of brain metastases from non-small cell lung cancer. J Thorac Dis. 13:3315–3322. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Chen YA, Zhuang H and Wang J: The rationale and toxicity of combined cranial radiotherapy and immune checkpoint inhibitors in non-small cell lung cancer. Asia Pac J Clin Oncol. 18:165–170. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Xu L, Chen Y and Wang M: Efficacy and safety of radiotherapy combined with immunotherapy for brain metastases from lung cancer: A meta-analysis. Zhongguo Fei Ai Za Zhi. 25:715–722. 2022.(In Chinese). PubMed/NCBI | |
|
Lastwika KJ, Wilson W III, Li QK, Norris J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, et al: Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. 76:227–238. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Quan Z, Yang Y, Zheng H, Zhan Y, Luo J, Ning Y and Fan S: Clinical implications of the interaction between PD-1/PD-L1 and PI3K/AKT/mTOR pathway in progression and treatment of non-small cell lung cancer. J Cancer. 13:3434–3443. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Perri F, Pacelli R, Scarpati GD, Cella L, Giuliano M, Caponigro F and Pepe S: Radioresistance in head and neck squamous cell carcinoma: Biological bases and therapeutic implications. Head Neck. 37:763–770. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Goedegebuure RSA, de Klerk LK, Bass AJ, Derks S and Thijssen V: Combining radiotherapy with anti-angiogenic therapy and immunotherapy; A therapeutic triad for cancer? Front Immunol. 14:31072019. View Article : Google Scholar | |
|
Ikarashi D, Okimoto T, Shukuya T, Onagi H, Hayashi T, Sinicropi-Yao SL, Amann JM, Nakatsura T, Kitano S and Carbone DP: Comparison of tumor microenvironments between primary tumors and brain metastases in patients with NSCLC. JTO Clin Res Rep. 2:1002302021.PubMed/NCBI | |
|
Nanda VGY, Peng W, Hwu P, Davies MA, Ciliberto G, Fattore L, Malpicci D, Aurisicchio L, Ascierto PA, Croce CM, et al: Melanoma and immunotherapy bridge 2015 : Naples, Italy. 1–5 December 2015. J Transl Med. 14:652016. View Article : Google Scholar : PubMed/NCBI | |
|
Patel RR, He K, Barsoumian HB, Chang JY, Tang C, Verma V, Comeaux N, Chun SG, Gandhi S, Truong MT, et al: High-dose irradiation in combination with non-ablative low-dose radiation to treat metastatic disease after progression on immunotherapy: Results of a phase II trial. Radiother Oncol. 162:60–67. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Levis M, Gastino A, De Giorgi G, Mantovani C, Bironzo P, Mangherini L, Ricci AA, Ricardi U, Cassoni P and Bertero L: Modern stereotactic radiotherapy for brain metastases from lung cancer: Current trends and future perspectives based on integrated translational approaches. Cancers (Basel). 15:46222023. View Article : Google Scholar : PubMed/NCBI | |
|
Khan M, Zhao Z, Li X and Liao G: Anti-PD1 therapy plus whole-brain radiation therapy May Prolong PFS in selected non-small cell lung cancer patients with brain metastases: A retrospective study. Int J Gen Med. 14:8903–8918. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Chen ZY, Duan XT, Qiao SM and Zhu XX: Radiotherapy combined with PD-1/PD-L1 inhibitors in NSCLC brain metastases treatment: The mechanisms, advances, opportunities, and challenges. Cancer Med. 12:995–1006. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Guo T, Zhou Y, Liang F, Wang Z, Bourbonne V, Käsmann L, Sundahl N, Wu AJ, Ni J and Zhu Z: Potential synergistic effects of cranial radiotherapy and atezolizumab in non-small cell lung cancer: An analysis of individual patient data from seven prospective trials. Transl Lung Cancer Res. 13:126–138. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Luo S, Li P, Zhang A, Meng L, Huang L, Wu X, Cheng H, Tu H and Gong X: G-CSF improving combined whole brain radiotherapy and immunotherapy prognosis of non-small cell lung cancer brain metastases. Int Immunopharmacol. 130:1117052024. View Article : Google Scholar : PubMed/NCBI | |
|
Vanneste BGL, Van Limbergen EJ, Dubois L, Samarska IV, Wieten L, Aarts MJB, Marcelissen T and De Ruysscher D: Immunotherapy as sensitizer for local radiotherapy. Oncoimmunology. 9:18327602020. View Article : Google Scholar : PubMed/NCBI | |
|
Bendavid J and Modesto A: Radiation therapy and antiangiogenic therapy: Opportunities and challenges. Cancer Radiother. 26:962–967. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Yang X, Ren H and Fu J: Treatment of radiation-induced brain necrosis. Oxid Med Cell Longev. 2021:47935172021. View Article : Google Scholar : PubMed/NCBI | |
|
Król K, Mazur A, Stachyra-Strawa P and Grzybowska-Szatkowska L: Non-Small cell lung cancer treatment with molecularly targeted therapy and concurrent radiotherapy-A review. Int J Mol Sci. 24:58582023. View Article : Google Scholar : PubMed/NCBI | |
|
Hsu HW, Wall NR, Hsueh CT, Kim S, Ferris RL, Chen CS and Mirshahidi S: Combination antiangiogenic therapy and radiation in head and neck cancers. Oral Oncol. 50:19–26. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Lévy C, Allouache D, Lacroix J, Dugué AE, Supiot S, Campone M, Mahe M, Kichou S, Leheurteur M, Hanzen C, et al: REBECA: A phase I study of bevacizumab and whole-brain radiation therapy for the treatment of brain metastasis from solid tumours. Ann Oncol. 25:2351–2356. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, He J, Cai L, Lai M, Hu Q, Ren C, Wen L, Wang J, Zhou J, Zhou Z, et al: Bevacizumab as a treatment for radiation necrosis following stereotactic radiosurgery for brain metastases: Clinical and radiation dosimetric impacts. Ann Palliat Med. 10:2018–2026. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Khan M, Zhao Z, Arooj S and Liao G: Bevacizumab for radiation necrosis following radiotherapy of brain metastatic disease: A systematic review & meta-analysis. BMC Cancer. 21:1672021. View Article : Google Scholar : PubMed/NCBI | |
|
Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, Cheng Y, He J, Shi Y, Zhao Y, et al: Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: The ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 4:1569–1575. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Han D, Zhang J, Bao Y, Liu L, Wang P and Qian D: Anlotinib enhances the antitumor immunity of radiotherapy by activating cGAS/STING in non-small cell lung cancer. Cell Death Discov. 8:4682022. View Article : Google Scholar : PubMed/NCBI | |
|
Dirkx AE, oude Egbrink MG, Castermans K, van der Schaft DW, Thijssen VL, Dings RP, Kwee L, Mayo KH, Wagstaff J, Bouma-ter Steege JC and Griffioen AW: Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB J. 20:621–630. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Tran TT, Caulfield J, Zhang L, Schoenfeld D, Djureinovic D, Chiang VL, Oria V, Weiss SA, Olino K, Jilaveanu LB and Kluger HM: Lenvatinib or anti-VEGF in combination with anti-PD-1 differentially augments antitumor activity in melanoma. JCI Insight. 8:e1573472023. View Article : Google Scholar : PubMed/NCBI | |
|
Ramadan WS, Zaher DM, Altaie AM, Talaat IM and Elmoselhi A: Potential therapeutic strategies for lung and breast cancers through understanding the anti-angiogenesis resistance mechanisms. Int J Mol Sci. 21:5652020. View Article : Google Scholar : PubMed/NCBI | |
|
Qi S, Deng S, Lian Z and Yu K: Novel drugs with high efficacy against tumor angiogenesis. Int J Mol Sci. 23:69342022. View Article : Google Scholar : PubMed/NCBI | |
|
Fakhrejahani E and Toi M: Antiangiogenesis therapy for breast cancer: An update and perspectives from clinical trials. Jpn J Clin Oncol. 44:197–207. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Wu LC and Zhang WD: Clinical trials of antiangiogenesis therapy on gastric cancer. Gastroenterol Res. 1:14–19. 2008. | |
|
Ribatti D, Vacca A, Nico B, Sansonno D and Dammacco F: Angiogenesis and anti-angiogenesis in hepatocellular carcinoma. Cancer Treat Rev. 32:437–444. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Giantonio BJ, Levy DE, O'Dwyer PJ, Meropol NJ, Catalano PJ and Benson AB III; Eastern Cooperative Oncology Group, : A phase II study of high-dose bevacizumab in combination with irinotecan, 5-fluorouracil, leucovorin, as initial therapy for advanced colorectal cancer: Results from the eastern cooperative oncology group study E2200. Ann Oncol. 17:1399–1403. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Xu J, Liu X, Yang S, Zhang X and Shi Y: Clinical response to apatinib monotherapy in advanced non-small cell lung cancer. Asia Pac J Clin Oncol. 14:264–269. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Ren S, He J, Fang Y, Chen G, Ma Z, Chen J, Guo R, Lin X, Yao Y, Wu G, et al: Camrelizumab plus apatinib in treatment-naive patients with advanced nonsquamous NSCLC: A multicenter, open-label, single-arm, phase 2 trial. JTO Clin Res Rep. 3:1003122022.PubMed/NCBI | |
|
Zhou C, Wang Y, Zhao J, Chen G, Liu Z, Gu K, Huang M, He J, Chen J, Ma Z, et al: Efficacy and biomarker analysis of camrelizumab in combination with apatinib in patients with advanced nonsquamous NSCLC previously treated with chemotherapy. Clin Cancer Res. 27:1296–1304. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Ou DL, Chen CW, Hsu CL, Chung CH, Feng ZR, Lee BS, Cheng AL, Yang MH and Hsu C: Regorafenib enhances antitumor immunity via inhibition of p38 kinase/Creb1/Klf4 axis in tumor-associated macrophages. J Immunothera Cancer. 9:e0016572021. View Article : Google Scholar | |
|
Huang G and Chen L: Discrepancies between antiangiogenic and antitumor effects of recombinant human endostatin. Cancer Biother Radiopharm. 24:589–596. 2009.PubMed/NCBI | |
|
Rani V and Prabhu A: Combining angiogenesis inhibitors with radiation: Advances and challenges in cancer treatment. Curr Pharm Des. 27:919–931. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Riesterer O: Angiogenesis inhibitors and radiotherapy. Praxis (Bern 1994). 101:1031–1037. 2012.(In German). View Article : Google Scholar : PubMed/NCBI | |
|
Sun X, Deng L and Lu Y: Challenges and opportunities of using stereotactic body radiotherapy with anti-angiogenesis agents in tumor therapy. Chin J Cancer Res. 30:147–156. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Park I, Yang H, Park JS, Koh GY and Choi EK: VEGF-Grab enhances the efficacy of radiation therapy by blocking VEGF-A and treatment-induced PlGF. Int J Radiat Oncol Biol Phys. 102:609–618. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Dickson PV, Hamner JB, Sims TL, Fraga CH, Ng CY, Rajasekeran S, Hagedorn NL, McCarville MB, Stewart CF and Davidoff AM: Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. 13:3942–3950. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Gao H, Xue J, Zhou L, Lan J, He J, Na F, Yang L, Deng L and Lu Y: Bevacizumab radiosensitizes non-small cell lung cancer xenografts by inhibiting DNA double-strand break repair in endothelial cells. Cancer Lett. 365:79–88. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Li L, Feng M, Xu P, Wu YL, Yin J, Huang Y, Tan MY and Jinyi L: Stereotactic radiosurgery with whole brain radiotherapy combined with bevacizumab in the treatment of brain metastases from NSCLC. Int J Neurosci. 133:334–341. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Chen YL, Huang AP, Wang CC, Chen HY, Chen YF, Xiao F, Lu SL, Cheng JC and Hsu FM: Peri-radiosurgical administration of bevacizumab improves radiographic response to single and fractionated stereotactic radiosurgery for large brain metastasis. J Neurooncol. 153:455–465. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Yang S, Sun J, Xu M, Wang Y, Liu G and Jiang A: The value of anlotinib in the treatment of intractable brain edema: Two case reports. Front Oncol. 11:6178032021. View Article : Google Scholar : PubMed/NCBI | |
|
Fan P, Qiang H, Liu Z, Zhao Q, Wang Y, Liu T, Wang X, Chu T, Huang Y, Xu W and Qin S: Effective low-dose Anlotinib induces long-term tumor vascular normalization and improves anti-PD-1 therapy. Front Immunol. 13:9379242022. View Article : Google Scholar : PubMed/NCBI | |
|
Li PJ, Lai SZ, Jin T, Ying HJ, Chen YM, Zhang P, Hang QQ, Deng H, Wang L, Feng JG, et al: Radiotherapy opens the blood-brain barrier and synergizes with anlotinib in treating glioblastoma. Radiother Oncol. 183:1096332023. View Article : Google Scholar : PubMed/NCBI | |
|
He L, Pi Y, Li Y, Wu Y, Jiang J, Rong X, Cai J, Yue Z, Cheng J, Li H, et al: Efficacy and safety of apatinib for radiation-induced brain injury among patients with head and neck cancer: An open-label, single-arm, phase 2 study. Int J Radiat Oncol Biol Phys. 113:796–804. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Xu W, Yang M, Du X, Peng H, Yang Y, Wang J and Zhang Y: Multifunctional nanoplatform based on sunitinib for synergistic phototherapy and molecular targeted therapy of hepatocellular carcinoma. Micromachines (Basel). 14:6132023. View Article : Google Scholar : PubMed/NCBI | |
|
Rahma OE and Hodi FS: The intersection between tumor angiogenesis and immune suppression. Clin Cancer Res. 25:5449–5457. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Hu H, Chen Y, Tan S, Wu S, Huang Y, Fu S, Luo F and He J: The research progress of antiangiogenic therapy, immune therapy and tumor microenvironment. Front Immunol. 13:8028462022. View Article : Google Scholar : PubMed/NCBI | |
|
Kusmartsev S, Eruslanov E, Kübler H, Tseng T, Sakai Y, Su Z, Kaliberov S, Heiser A, Rosser C, Dahm P, et al: Oxidative stress regulates expression of VEGFR1 in myeloid cells: Link to tumor-induced immune suppression in renal cell carcinoma. J Immunol. 181:346–353. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Zafar MI, Zheng J, Kong W, Ye X, Gou L, Regmi A and Chen LL: The role of vascular endothelial growth factor-B in metabolic homoeostasis: Current evidence. Biosci Rep. 37:BSR201710892017. View Article : Google Scholar : PubMed/NCBI | |
|
Bourhis M, Palle J, Galy-Fauroux I and Terme M: Direct and indirect modulation of T cells by VEGF-A counteracted by anti-angiogenic treatment. Front Immunol. 12:6168372021. View Article : Google Scholar : PubMed/NCBI | |
|
Szebeni GJ, Vizler C, Kitajka K and Puskas LG: Inflammation and cancer: Extra- and intracellular determinants of tumor-associated macrophages as tumor promoters. Mediators Inflamm. 2017:92940182017. View Article : Google Scholar : PubMed/NCBI | |
|
Liu QP, Chen YY, An P, Rahman K, Luan X and Zhang H: Natural products targeting macrophages in tumor microenvironment are a source of potential antitumor agents. Phytomedicine. 109:1546122023. View Article : Google Scholar : PubMed/NCBI | |
|
Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR, Vianello F, et al: Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proce Natil Acad Sci USA. 109:17561–17566. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Swed B, Ryan K, Gandarilla O, Shah MA and Brar G: Favorable response to second-line atezolizumab and bevacizumab following progression on nivolumab in advanced hepatocellular carcinoma: A case report demonstrating that anti-VEGF therapy overcomes resistance to checkpoint inhibition. Medicine (Baltimore). 100:e264712021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Q, Gao J, Di W and Wu X: Anti-angiogenesis therapy overcomes the innate resistance to PD-1/PD-L1 blockade in VEGFA-overexpressed mouse tumor models. Cancer Immunol Immunother. 69:1781–1799. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Manegold C, Dingemans AC, Gray JE, Nakagawa K, Nicolson M, Peters S, Reck M, Wu YL, Brustugun OT, Crinò L, et al: The potential of combined immunotherapy and antiangiogenesis for the synergistic treatment of advanced NSCLC. J Thorac Oncol. 12:194–207. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Fukumura D, Kloepper J, Amoozgar Z, Duda DG and Jain RK: Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat Rev Clin Oncol. 15:325–340. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Huang Y, Kim BYS, Chan CK, Hahn SM, Weissman IL and Jiang W: Improving immune-vascular crosstalk for cancer immunotherapy. Nat Rev Immunol. 18:195–203. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Yaguchi T, Sumimoto H, Kudo-Saito C, Tsukamoto N, Ueda R, Iwata-Kajihara T, Nishio H, Kawamura N and Kawakami Y: The mechanisms of cancer immunoescape and development of overcoming strategies. Int J Hematol. 93:294–300. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Xia L, Liu Y and Wang Y: PD-1/PD-L1 blockade therapy in advanced non-small-cell lung cancer: Current status and future directions. Oncologist. 24 (Suppl 1):S31–S41. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Horvath L, Thienpont B, Zhao L, Wolf D and Pircher A: Overcoming immunotherapy resistance in non-small cell lung cancer (NSCLC)-novel approaches and future outlook. Mol Cancer. 19:1412020. View Article : Google Scholar : PubMed/NCBI | |
|
Xu J, Gan C, Yu S, Yao S, Li W and Cheng H: Analysis of immune resistance mechanisms in TNBC: Dual effects inside and outside the tumor. Clin Breast Cancer. 24:e91–e102. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Khalaf K, Hana D, Chou JT, Singh C, Mackiewicz A and Kaczmarek M: Aspects of the tumor microenvironment involved in immune resistance and drug resistance. Front Immunol. 12:6563642021. View Article : Google Scholar : PubMed/NCBI | |
|
Dobosz P, Stępień M, Golke A and Dzieciątkowski T: Challenges of the immunotherapy: Perspectives and limitations of the immune checkpoint inhibitor treatment. Int J Mol Sci. 23:28472022. View Article : Google Scholar : PubMed/NCBI | |
|
Kudo-Saito C, Ishida A, Shouya Y, Teramoto K, Igarashi T, Kon R, Saito K, Awada C, Ogiwara Y and Toyoura M: Blocking the FSTL1-DIP2A axis improves anti-tumor immunity. Cell Rep. 24:1790–1801. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Codony-Servat J and Rosell R: Cancer stem cells and immunoresistance: Clinical implications and solutions. Transl Lung Cancer Res. 4:689–703. 2015.PubMed/NCBI | |
|
Wu L, Cheng D, Yang X, Zhao W, Fang C, Chen R and Ji M: M2-TAMs promote immunoresistance in lung adenocarcinoma by enhancing METTL3-mediated m6A methylation. Ann Transl Med. 10:13802022. View Article : Google Scholar : PubMed/NCBI | |
|
Khouzam RA, Janji B, Thiery J, Zaarour RF, Chamseddine AN, Mayr H, Savagner P, Kieda C, Gad S, Buart S, et al: Hypoxia as a potential inducer of immune tolerance, tumor plasticity and a driver of tumor mutational burden: Impact on cancer immunotherapy. Semin Cancer Biol. 97:104–123. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Larroquette M, Domblides C, Lefort F, Lasserre M, Quivy A, Sionneau B, Bertolaso P, Gross-Goupil M, Ravaud A and Daste A: Combining immune checkpoint inhibitors with chemotherapy in advanced solid tumours: A review. Eur J Cancer. 158:47–62. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Luo L, Liu P, Zhao K, Zhao W and Zhang X: The immune microenvironment in brain metastases of non-small cell lung cancer. Front Oncol. 11:6988442021. View Article : Google Scholar : PubMed/NCBI | |
|
Wu Y, Kang K, Han C, Wang L, Wang Z and Zhao A: Single-Cell profiling comparisons of tumor microenvironment between primary advanced lung adenocarcinomas and brain metastases and machine learning algorithms in predicting immunotherapeutic responses. Biomolecules. 13:1852023. View Article : Google Scholar : PubMed/NCBI | |
|
Hulsbergen AFC, Mammi M, Nagtegaal SHJ, Lak AM, Kavouridis V, Smith TR, Iorgulescu JB, Mekary RA, Verhoeff JJC, Broekman MLD and Phillips JG: Programmed death receptor ligand one expression may independently predict survival in patients with non-small cell lung carcinoma brain metastases receiving immunotherapy. Int J Radiat Oncol Biol Phys. 108:258–267. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Hargadon KM, Johnson CE and Williams CJ: Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 62:29–39. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Cha JH, Chan LC, Li CW, Hsu JL and Hung MC: Mechanisms controlling PD-L1 expression in cancer. Mol Cell. 76:359–370. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 31:2078–2092. 2018. View Article : Google Scholar | |
|
Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Şenler FC, Csőszi T, Fülöp A, et al: Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 379:2040–2051. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Yu S, Zhang S, Xu H, Yang G, Xu F, Yang L, Chen D, An G and Wang Y: Organ-specific immune checkpoint inhibitor treatment in lung cancer: A systematic review and meta-analysis. BMJ Open. 13:e0594572023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou S, Ren F and Meng X: Efficacy of immune checkpoint inhibitor therapy in EGFR mutation-positive patients with NSCLC and brain metastases who have failed EGFR-TKI therapy. Front Immunol. 13:9559442022. View Article : Google Scholar : PubMed/NCBI | |
|
Chu X, Niu L, Xiao G, Peng H, Deng F, Liu Z, Wu H, Yang L, Tan Z, Li Z and Zhou R: The long-term and short-term efficacy of immunotherapy in non-small cell lung cancer patients with brain metastases: A systematic review and meta-analysis. Front Immunol. 13:8754882022. View Article : Google Scholar : PubMed/NCBI | |
|
Shepard MJ, Xu Z, Donahue J, Muttikkal TJ, Cordeiro D, Hansen L, Mohammed N, Gentzler RD, Larner J, Fadul CE and Sheehan JP: Stereotactic radiosurgery with and without checkpoint inhibition for patients with metastatic non-small cell lung cancer to the brain: A matched cohort study. J Neurosurg. 133:685–692. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Khalifa J, Amini A, Popat S, Gaspar LE and Faivre-Finn C; International Association for the Study of Lung Cancer Advanced Radiation Technology Committee, : Brain metastases from NSCLC: Radiation therapy in the era of targeted therapies. J Thorac Oncol. 11:1627–1643. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Porte J, Saint-Martin C, Frederic-Moreau T, Massiani MA, Bozec L, Cao K, Verrelle P, Otz J, Jadaud E, Minsat M, et al: Efficacy and safety of combined brain stereotactic radiotherapy and immune checkpoint inhibitors in non-small-cell lung cancer with brain metastases. Biomedicines. 10:22492022. View Article : Google Scholar : PubMed/NCBI | |
|
Scoccianti S, Olmetto E, Pinzi V, Osti MF, Di Franco R, Caini S, Anselmo P, Matteucci P, Franceschini D, Mantovani C, et al: Immunotherapy in association with stereotactic radiotherapy for non-small cell lung cancer brain metastases: Results from a multicentric retrospective study on behalf of AIRO. Neuro Oncol. 23:1750–1764. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Gagliardi F, De Domenico P, Snider S, Roncelli F, Pompeo E, Barzaghi LR, Bulotta A, Gregorc V, Lazzari C, Cascinu S, et al: Role of stereotactic radiosurgery for the treatment of brain metastasis in the era of immunotherapy: A systematic review on current evidences and predicting factors. Crit Rev Oncol Hematol. 165:1034312021. View Article : Google Scholar : PubMed/NCBI | |
|
Enright TL, Witt JS, Burr AR, Yadav P, Leal T and Baschnagel AM: Combined immunotherapy and stereotactic radiotherapy improves neurologic outcomes in patients with non-small-cell lung cancer brain metastases. Clin Lung Cancer. 22:110–119. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Dohm AE, Tang JD, Mills MN, Liveringhouse CL, Sandoval ML, Perez BA, Robinson TJ, Creelan BC, Gray JE, Etame AB, et al: Clinical outcomes of non-small cell lung cancer brain metastases treated with stereotactic radiosurgery and immune checkpoint inhibitors, EGFR tyrosine kinase inhibitors, chemotherapy and immune checkpoint inhibitors, or chemotherapy alone. J Neurosurg. 138:1600–1607. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Guo T, Chu L, Chu X, Yang X, Li Y, Zhou Y, Xu D, Zhang J, Wang S, Hu J, et al: Brain metastases, patterns of intracranial progression, and the clinical value of upfront cranial radiotherapy in patients with metastatic non-small cell lung cancer treated with PD-1/PD-L1 inhibitors. Transl Lung Cancer Res. 11:173–187. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Kang S, Jeong H, Park JE, Kim HS, Kim YH, Lee DH, Kim SW, Lee JC, Choi CM and Yoon S: Central nervous systemic efficacy of immune checkpoint inhibitors and concordance between intra/extracranial response in non-small cell lung cancer patients with brain metastasis. J Cancer Res Clin Oncol. 149:4523–4532. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Guo F and Cui J: Anti-angiogenesis: Opening a new window for immunotherapy. Life Sci. 258:1181632020. View Article : Google Scholar : PubMed/NCBI | |
|
Yang J, Yan J and Liu B: Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol. 9:9782018. View Article : Google Scholar : PubMed/NCBI | |
|
Yang Y, Li L, Jiang Z, Wang B and Pan Z: Anlotinib optimizes anti-tumor innate immunity to potentiate the therapeutic effect of PD-1 blockade in lung cancer. Cancer Immunol Immunother. 69:2523–2532. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Song Y, Fu Y, Xie Q, Zhu B, Wang J and Zhang B: Anti-angiogenic agents in combination with immune checkpoint inhibitors: A promising strategy for cancer treatment. Front Immunol. 11:19562020. View Article : Google Scholar : PubMed/NCBI | |
|
Rivera LB, Meyronet D, Hervieu V, Frederick MJ, Bergsland E and Bergers G: Intratumoral myeloid cells regulate responsiveness and resistance to antiangiogenic therapy. Cell Rep. 11:577–591. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Song JQ, Wang X, Zeng ZM, Liang PA, Zhong CY and Liu AW: Efficacy of PD-1 inhibitors combined with anti-angiogenic therapy in driver gene mutation negative non-small-cell lung cancer with brain metastases. Discov Med. 35:321–331. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Altan M, Wang Y, Song J, Welsh J, Tang C, Guha-Thakurta N, Blumenschein GR, Carter BW, Wefel JS, Ghia AJ, et al: Nivolumab and ipilimumab with concurrent stereotactic radiosurgery for intracranial metastases from non-small cell lung cancer: Analysis of the safety cohort for non-randomized, open-label, phase I/II trial. J Immunother Cancer. 11:e0068712023. View Article : Google Scholar : PubMed/NCBI | |
|
Cho A, Untersteiner H, Hirschmann D, Shaltout A, Göbl P, Dorfer C, Rössler K, Marik W, Kirchbacher K, Kapfhammer I, et al: Gamma knife radiosurgery for brain metastases in non-small cell lung cancer patients treated with immunotherapy or targeted therapy. Cancers (Basel). 12:36682020. View Article : Google Scholar : PubMed/NCBI | |
|
Geng Y, Zhang Q, Feng S, Li C, Wang L, Zhao X, Yang Z, Li Z, Luo H, Liu R, et al: Safety and efficacy of PD-1/PD-L1 inhibitors combined with radiotherapy in patients with non-small-cell lung cancer: A systematic review and meta-analysis. Cancer Med. 10:1222–1239. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Ma K, Guo Q and Li X: Efficacy and safety of combined immunotherapy and antiangiogenic therapy for advanced non-small cell lung cancer: A real-world observation study. BMC Pulm Med. 23:1752023. View Article : Google Scholar : PubMed/NCBI | |
|
Chen B, Wang J, Pu X, Li J, Wang Q, Liu L, Xu Y, Xu L, Kong Y, Li K, et al: The efficacy and safety of immune checkpoint inhibitors combined with chemotherapy or anti-angiogenic therapy as a second-line or later treatment option for advanced non-small cell lung cancer: A retrospective comparative cohort study. Transl Lung Cancer Res. 11:2111–2124. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang S, Li S, Liu J, Yang C, Zhang L, Bao H and Cheng Y: Comparative efficacy and safety of TKIs alone or in combination with antiangiogenic agents in advanced EGFR-mutated NSCLC as the first-line treatment: A systematic review and meta-analysis. Clin Lung Cancer. 23:159–169. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Wu J, Ni T, Deng R, Li Y, Zhong Q, Tang F, Zhang Q, Fang C, Xue Y, Zha Y and Zhang Y: Safety and efficacy of radiotherapy/chemoradiotherapy combined with immune checkpoint inhibitors for non-small cell lung cancer: A systematic review and meta-analysis. Front Immunol. 14:10655102023. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Chen Y, Hu F, Qiang H, Chang Q, Qian J, Shen Y, Cai Y and Chu T: Comparison of the efficacy and safety in the treatment strategies between chemotherapy combined with antiangiogenic and with immune checkpoint inhibitors in advanced non-small cell lung cancer patients with negative PD-L1 expression: A network meta-analysis. Front Oncol. 12:10015032022. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Z, Cui L, Wang J, Zhao W and Teng Y: Aspirin boosts the synergistic effect of EGFR/p53 inhibitors on lung cancer cells by regulating AKT/mTOR and p53 pathways. Cell Biochem Funct. 42:e39022024. View Article : Google Scholar : PubMed/NCBI | |
|
Qian C, Liu C, Liu W, Zhou R and Zhao L: Targeting vascular normalization: A promising strategy to improve immune-vascular crosstalk in cancer immunotherapy. Front Immunol. 14:12915302023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou S and Zhang H: Synergies of targeting angiogenesis and immune checkpoints in cancer: From mechanism to clinical applications. Anticancer Agents Med Chem. 20:768–776. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Yuan M, Zhai Y, Men Y, Zhao M, Sun X, Ma Z, Bao Y, Yang X, Sun S, Liu Y, et al: Anlotinib enhances the antitumor activity of high-dose irradiation combined with anti-PD-L1 by potentiating the tumor immune microenvironment in murine lung cancer. Oxid Med Cell Longev. 2022:54794912022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao X, Zhao R, Wen J, Zhang X, Wu S, Fang J, Ma J, Zheng W, Zhang X, Lu Z, et al: Anlotinib reduces the suppressive capacity of monocytic myeloid-derived suppressor cells and potentiates the immune microenvironment normalization window in a mouse lung cancer model. Anticancer Drugs. 34:1018–1024. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Swamy K: Vascular normalization and immunotherapy: Spawning a virtuous cycle. Front Oncol. 12:10029572022. View Article : Google Scholar : PubMed/NCBI | |
|
Lee WS, Yang H, Chon HJ and Kim C: Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med. 52:1475–1485. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Oliveira G and Wu CJ: Dynamics and specificities of T cells in cancer immunotherapy. Nat Rev Cancer. 23:295–316. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Charpentier M, Spada S, Van Nest SJ and Demaria S: Radiation therapy-induced remodeling of the tumor immune microenvironment. Semin Cancer Biol. 86:737–747. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Xian F, Wu J, Zhong L and Xu G: Efficacy and safety of PD1/PDL1 inhibitors combined with radiotherapy and anti-angiogenic therapy for solid tumors: A systematic review and meta-analysis. Medicine (Baltimore). 102:e332042023. View Article : Google Scholar : PubMed/NCBI | |
|
Sun L, Zhao Q, Wang Y, Wang Y, Zheng M, Ding X and Miao L: Efficacy and safety of anlotinib-containing regimens in advanced non-small cell lung cancer: A real-world study. Int J Gen Med. 16:4165–4179. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Long YY, Chen J, Xie Y, Wang Y, Wu YZ, Xv Y, Weng KG and Zhou W: Long-term survival with a combination of immunotherapy, anti-angiogenesis, and traditional radiotherapy in brain metastatic small cell lung cancer: A case report. Front Oncol. 13:12097582023. View Article : Google Scholar : PubMed/NCBI | |
|
Schettino C, Bareschino MA, Rossi A, Maione P, Sacco PC, Colantuoni G, Rossi E and Gridelli C: Targeting angiogenesis for treatment of NSCLC brain metastases. Curr Cancer Drug Targets. 12:289–299. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Gao RL, Song J, Sun L, Wu ZX, Yi XF, Zhang SL, Huang LT, Ma JT and Han CB: Efficacy and safety of combined immunotherapy and antiangiogenesis with or without chemotherapy for advanced non-small-cell lung cancer: A systematic review and pooled analysis from 23 prospective studies. Front Pharmacol. 13:9201652022. View Article : Google Scholar : PubMed/NCBI | |
|
Li D, Xu L, Ji J, Bao D, Hu J, Qian Y, Zhou Y, Chen Z, Li D, Li X, et al: Sintilimab combined with apatinib plus capecitabine in the treatment of unresectable hepatocellular carcinoma: A prospective, open-label, single-arm, phase II clinical study. Front Immunol. 13:9440622022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang B, Qi L, Wang X, Xu J, Liu Y, Mu L, Wang X, Bai L and Huang J: Phase II clinical trial using camrelizumab combined with apatinib and chemotherapy as the first-line treatment of advanced esophageal squamous cell carcinoma. Cancer Commun (Lond). 40:711–720. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Lai S, Li P, Liu X, Liu G, Xie T, Zhang X, Wang X, Huang J, Tang Y, Liu Z, et al: Efficacy and safety of anlotinib combined with the STUPP regimen in patients with newly diagnosed glioblastoma: A multicenter, single-arm, phase II trial. Cancer Biol Med. 21:433–444. 2024.PubMed/NCBI | |
|
Theelen W, Chen D, Verma V, Hobbs BP, Peulen HMU, Aerts J, Bahce I, Niemeijer ALN, Chang JY, de Groot PM, et al: Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Respir Med. 9:467–475. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Gu D, Yu H, Ding N, Xu J, Qian P, Zhu J, Jiang M, Tao H and Zhu X: A phase II study of anlotinib plus whole brain radiation therapy for patients with NSCLC with multiple brain metastases. Ann Med. 56:24016182024. View Article : Google Scholar : PubMed/NCBI | |
|
Li S, Yu W, Xie F, Luo H, Liu Z, Lv W, Shi D, Yu D, Gao P, Chen C, et al: Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nat Commun. 14:82023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhai Y, Ma H, Hui Z, Zhao L, Li D, Liang J, Wang X, Xu L, Chen B, Tang Y, et al: HELPER study: A phase II trial of continuous infusion of endostar combined with concurrent etoposide plus cisplatin and radiotherapy for treatment of unresectable stage III non-small-cell lung cancer. Radiother Oncol. 131:27–34. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, Carrero XW, Barker FG II, Deming R, Burri SH, et al: Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA. 316:401–409. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Abdallah M, Voland R, Decamp M, Flickinger J, Pacioles T, Jamil M, Silbermins D, Shenouda M, Valsecchi M, Bir A, et al: Evaluation of anti-angiogenic therapy combined with immunotherapy and chemotherapy as a strategy to treat locally advanced and metastatic non-small-cell lung cancer. Cancers (Basel). 16:42072024. View Article : Google Scholar : PubMed/NCBI | |
|
Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, et al: Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 7:387–401. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Chen K, Xu Y, Huang Z, Yu X, Hong W, Li H, Xu X, Lu H, Xie F, Chen J, et al: Sintilimab plus anlotinib as second- or third-line therapy in metastatic non-small cell lung cancer with uncommon epidermal growth factor receptor mutations: A prospective, single-arm, phase II trial. Cancer Med. 12:19460–19470. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Shi M, Chen P, Cui B, Yao Y, Wang J, Zhou T and Wang L: Benmelstobart plus anlotinib in patients with EGFR-positive advanced NSCLC after failure of EGFR TKIs therapy: A phase I/II study. Signal Transduct Targeted Ther. 9:2832024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang F, Chen G, Yin Y, Chen X, Nie R and Chen Y: First-line immune checkpoint inhibitors in low programmed death-ligand 1-expressing population. Front Pharmacol. 15:13776902024. View Article : Google Scholar : PubMed/NCBI | |
|
Lorenc P, Sikorska A, Molenda S, Guzniczak N, Dams-Kozlowska H and Florczak A: Physiological and tumor-associated angiogenesis: Key factors and therapy targeting VEGF/VEGFR pathway. Biomed Pharmacother. 180:1175852024. View Article : Google Scholar : PubMed/NCBI | |
|
Patel A, Dong T, Ansari S, Cohen-Gadol A, Watson GA, Moraes FY, Nakamura M, Murovic J, Chang SD, Hatiboglu MA, et al: Toxicity of radiosurgery for brainstem metastases. World Neurosurg. 119:e757–e764. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Chen WC, Baal UH, Baal JD, Pai JS, Boreta L, Braunstein SE and Raleigh DR: Efficacy and safety of stereotactic radiosurgery for brainstem metastases: A systematic review and meta-analysis. JAMA Oncol. 7:1033–1040. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Shaw MG and Ball DL: Treatment of brain metastases in lung cancer: Strategies to avoid/reduce late complications of whole brain radiation therapy. Curr Treat Options Oncol. 14:553–567. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Yang TJ, Wijetunga NA, Yamada J, Wolden S, Mehallow M, Goldman DA, Zhang Z, Young RJ, Kris MG, Yu HA, et al: Clinical trial of proton craniospinal irradiation for leptomeningeal metastases. Neuro Oncol. 23:134–143. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Mehmi I and Hamid O: Immunotherapy of cancer in the era of checkpoint inhibitor. Clin Exp Metastasis. 39:231–237. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Rossi G, Russo A, Tagliamento M, Tuzi A, Nigro O, Vallome G, Sini C, Grassi M, Dal Bello MG, Coco S, et al: Precision medicine for NSCLC in the era of immunotherapy: New biomarkers to select the most suitable treatment or the most suitable patient. Cancers (Basel). 12:11252020. View Article : Google Scholar : PubMed/NCBI | |
|
Chang K, Jiao Y, Zhang B, Hou L, He X, Wang D, Li D, Li R, Wang Z, Fan P and Zhang J: MGP(+) and IDO1(+) tumor-associated macrophages facilitate immunoresistance in breast cancer revealed by single-cell RNA sequencing. Int Immunopharmacol. 131:1118182024. View Article : Google Scholar : PubMed/NCBI | |
|
Tímár J and Ladányi A: Immunogenomic aspects of tumor progression. Magy Onkol. 63:173–182. 2019.(In Hungarian). PubMed/NCBI | |
|
Albesiano E, Han JE and Lim M: Mechanisms of local immunoresistance in glioma. Neurosurg Clin N Am. 21:17–29. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Cabezón-Gutiérrez L, Custodio-Cabello S, Palka-Kotlowska M, Alonso-Viteri S and Khosravi-Shahi P: Biomarkers of immune checkpoint inhibitors in non-small cell lung cancer: Beyond PD-L1. Clin Lung Cancer. 22:381–389. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou S and Yang H: Immunotherapy resistance in non-small-cell lung cancer: From mechanism to clinical strategies. Front Immunol. 14:11294652023. View Article : Google Scholar : PubMed/NCBI | |
|
Jing Y, Zeng H, Cheng R, Tian P and Li Y: Advances of immunotherapy resistance and coping strategies in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 26:66–77. 2023.PubMed/NCBI | |
|
Zhang Y and Brekken RA: Direct and indirect regulation of the tumor immune microenvironment by VEGF. J Leukoc Biol. 111:1269–1286. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Li Z, Lai X, Fu S, Ren L, Cai H, Zhang H, Gu Z, Ma X and Luo K: Immunogenic cell death activates the tumor immune microenvironment to boost the immunotherapy efficiency. Adv Sci (Weinh). 9:e22017342022. View Article : Google Scholar : PubMed/NCBI | |
|
Guo S, Yao Y, Tang Y, Xin Z, Wu D, Ni C, Huang J, Wei Q and Zhang T: Radiation-induced tumor immune microenvironments and potential targets for combination therapy. Signal Transduct Target Ther. 8:2052023. View Article : Google Scholar : PubMed/NCBI | |
|
Sun D, Liu J, Zhou H, Shi M, Sun J, Zhao S, Chen G, Zhang Y, Zhou T, Ma Y, et al: Classification of tumor immune microenvironment according to programmed death-ligand 1 expression and immune infiltration predicts response to immunotherapy plus chemotherapy in advanced patients with NSCLC. J Thorac Oncol. 18:869–881. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y, Liu S, Yang Z, Algazi AP, Lomeli SH, Wang Y, Othus M, Hong A, Wang X, Randolph CE, et al: Anti-PD-1/L1 lead-in before MAPK inhibitor combination maximizes antitumor immunity and efficacy. Cancer Cell. 39:1375–1387.e6. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Caetano MS, Younes AI, Barsoumian HB, Quigley M, Menon H, Gao C, Spires T, Reilly TP, Cadena AP, Cushman TR, et al: Triple therapy with MerTK and PD1 inhibition plus radiotherapy promotes abscopal antitumor immune responses. Clin Cancer Res. 25:7576–7584. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Yeung MY, McGrath M and Najafian N: The emerging role of the TIM molecules in transplantation. Am J Transplant. 11:2012–2019. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Chen L, Tong F, Peng L, Huang Y, Yin P, Feng Y, Cheng S, Wang J and Dong X: Efficacy and safety of recombinant human endostatin combined with whole-brain radiation therapy in patients with brain metastases from non-small cell lung cancer. Radiother Oncol. 174:44–51. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Yang RF, Yu B, Zhang RQ, Wang XH, Li C, Wang P, Zhang Y, Han B, Gao XX, Zhang L, et al: Bevacizumab and gefitinib enhanced whole-brain radiation therapy for brain metastases due to non-small-cell lung cancer. Braz J Med Biol Res. 51:e60732017. View Article : Google Scholar : PubMed/NCBI | |
|
Xu Y, Chen K, Xu Y, Li H, Huang Z, Lu H, Huang D, Yu S, Han N, Gong L, et al: Brain radiotherapy combined with camrelizumab and platinum-doublet chemotherapy for previously untreated advanced non-small-cell lung cancer with brain metastases (C-Brain): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 26:74–84. 2025. View Article : Google Scholar : PubMed/NCBI |