Usefulness of the preoperative Prognostic Immune and Nutritional Index as a prognostic predictor for patients with gastric cancer
- Authors:
- Published online on: July 8, 2025 https://doi.org/10.3892/ol.2025.15181
- Article Number: 435
Abstract
Introduction
Gastric cancer (GC) is the fifth most common malignant tumor and the fifth leading cause of cancer-related deaths worldwide; in 2022, it accounted for ~968,350 new cases and 659,853 deaths globally (1). Despite advancements in targeted therapies, neoadjuvant chemotherapy and minimally invasive surgical techniques, the 5-year overall survival (OS) rate for patients with GC remains suboptimal (2). Although the eighth edition of the American Joint Committee on Cancer (AJCC) Tumor-Node-Metastasis (TNM) staging system (3) serves as the gold standard for prognostic assessment, its predictive accuracy is limited by its inability to account for key biological features such as immune microenvironment heterogeneity, metabolic dysregulation and systemic inflammatory responses (2). This limitation underscores the need for novel prognostic biomarkers that integrate multidimensional biological parameters.
In recent years, composite inflammatory-nutritional indices derived from peripheral blood components have emerged as promising prognostic tools in GC research. The Systemic Immune-Inflammation Index (calculated as platelets × neutrophils/lymphocytes) (109/l) and the Prognostic Nutritional Index (calculated as albumin (g/l) + 5 × [lymphocytes (109/l)] have been independently associated with postoperative recurrence risk and OS in patients with GC (4,5). However, whether single-dimensional biomarkers adequately capture the complexity of tumor-host interactions remains debatable. Addressing this question, Jung et al (6) innovatively proposed the Prognostic Immune and Nutritional Index (PINI), calculated as follows: [albumin (g/dl) × 0.9]-[monocytes (mm3) × 0.0007]. This is a multidimensional model integrating nutritional status (albumin) and innate immune response (monocytes) (6). Mechanistically, hypoalbuminemia reflects not only nutritional depletion but also systemic inflammation driven by IL-6 (7), while tumor-associated monocytes secrete VEGF-A and MMP-9 to promote angiogenesis and extracellular matrix remodeling, facilitating micrometastasis (8). Notably, Xie et al (9) recently validated the prognostic utility of PINI in colorectal cancer, suggesting its potential applicability across tumor types.
To date, the prognostic role of PINI in GC following curative resection remains unexplored. The present single-center retrospective cohort study aimed to systematically evaluate the association between PINI and recurrence-free survival (RFS), as well as OS, in patients with GC, assessing its prognostic value in those undergoing radical gastrectomy. PINI may serve as a critical risk stratification tool to identify patients with high-risk GC, enabling personalized treatment strategies and optimized clinical decision-making to improve long-term outcomes.
Patients and methods
Patients
The present retrospective study analyzed clinicopathological data and laboratory hematological parameters (measured within 1 week preoperatively) from patients with GC who underwent radical gastrectomy at the Department of Gastrointestinal Surgery, The First People's Hospital of Jingdezhen (Jingdezhen, China) between January 2016 and December 2019. Clinical and preoperative (1 week prior) laboratory data were collected from a maintained patient database. The inclusion criteria were as follows: i) Primary GC with histological confirmation; ii) no previous radiation or neoadjuvant chemotherapy prior to surgery; and iii) radical GC resection. The exclusion criteria were as follows: i) Patients with additional malignancies; ii) patients with granulocytopenia or other hematological disorders; iii) a history of severe infection or an immunocompromised status within the last month; and iv) a lack of crucial baseline data and missing follow-up information.
Treatment and follow-up
The disease staging established by the Japanese Gastric Cancer Association was determined with reference to the TNM classification system (5th edition) (10). Postoperative complications occurring within 30 days were graded according to the Clavien-Dindo classification system (11), with grade III or higher complications being recorded. Following Japanese GC treatment guidelines (12), patients with stage II or III GC received adjuvant chemotherapy when deemed clinically appropriate based on their overall health status. Postoperative surveillance included contrast-enhanced computed tomography scans performed at minimum every 6 months and blood tests conducted every 3 months. Patients were followed regularly through outpatient visits or telephone interviews every 3 months starting from postoperative day 1 until reaching the study endpoint, defined as either patient death or December 31, 2024, whichever occurred first. For outcome assessment, RFS was calculated as the time from surgery to GC recurrence, last follow-up or death, while OS was defined as the time from surgery to death from any cause or last follow-up for surviving patients.
Determination of PINI
The PINI was calculated using the following formula: [albumin (g/dl) × 0.9]-[monocytes (mm3) × 0.0007], with measurements taken preoperatively (5).
Statistical analysis
Statistical analyses were performed using SPSS software (v25.0; IBM Corp.). The predictive performance of PINI for patient outcomes was evaluated through receiver operating characteristic (ROC) curve analysis, with the area under the curve (AUC) calculated to determine the optimal cutoff value using the Youden index. Based on this cutoff, patients were stratified into high-PINI and low-PINI groups for subsequent comparative analyses of clinicopathological characteristics. Continuous variables with normal distribution are expressed as the mean ± standard deviation and compared using independent samples t-tests, while non-normally distributed continuous variables are presented as median (Q1-Q3) and were analyzed using the Mann-Whitney U test. Categorical variables are reported as n (%) and compared using either the χ2 test or Fisher's exact test. P<0.05 was considered to indicate a statistically significant difference. Univariate and multivariate Cox proportional hazards regression models were employed to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for RFS and OS. Variables demonstrating statistical significance (P<0.05) in univariate analysis were included in the multivariate model. Survival probabilities for RFS and OS were estimated using the Kaplan-Meier method, with between-group differences assessed by log-rank tests.
Ethical approval
The present study received ethical approval from the Institutional Review Board of Jingdezhen First People's Hospital (approval no. jdzyykt202425) and was conducted in full compliance with the ethical principles outlined in the Declaration of Helsinki. The requirement for informed consent was waived by the ethics committee as this retrospective study utilized anonymized clinical data without any identifiable patient information.
Results
ROC analysis and survival outcomes stratified by PINI
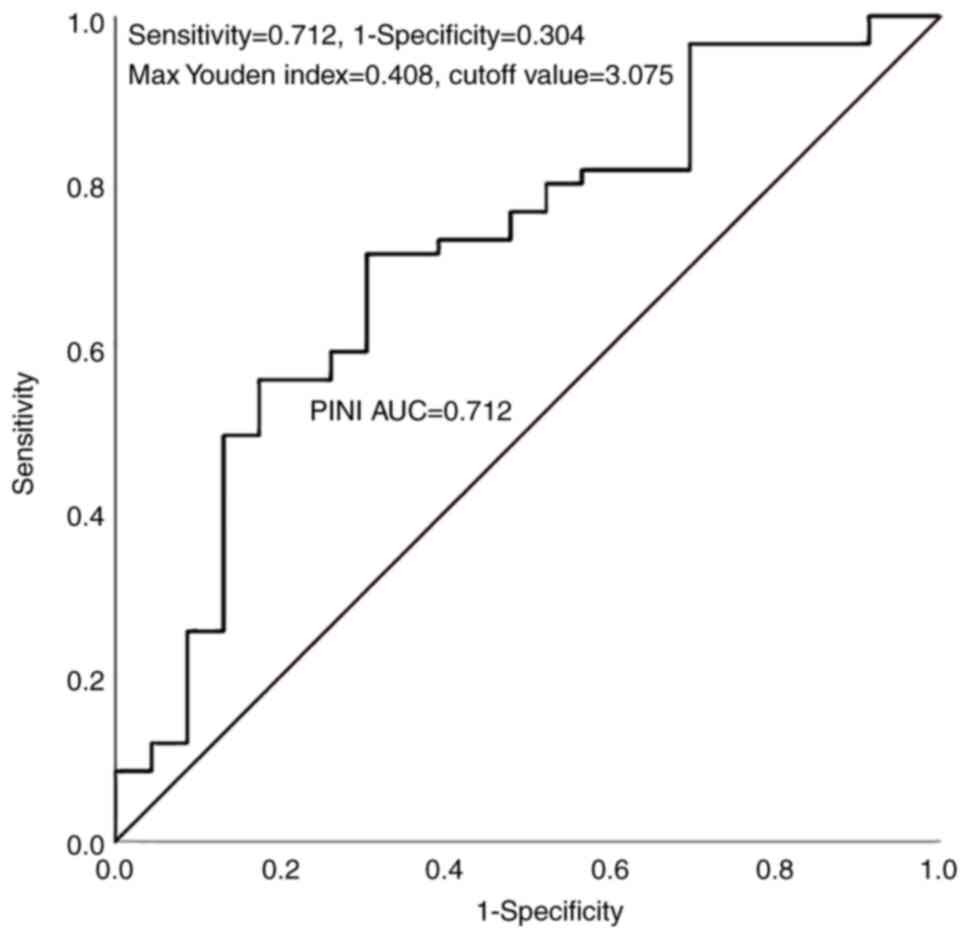
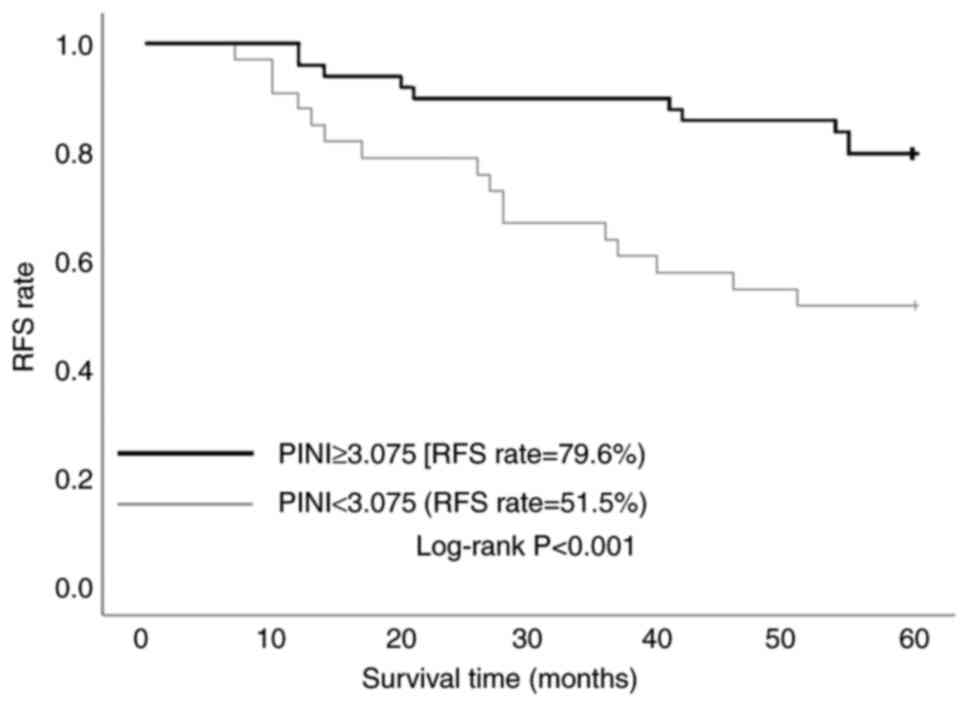
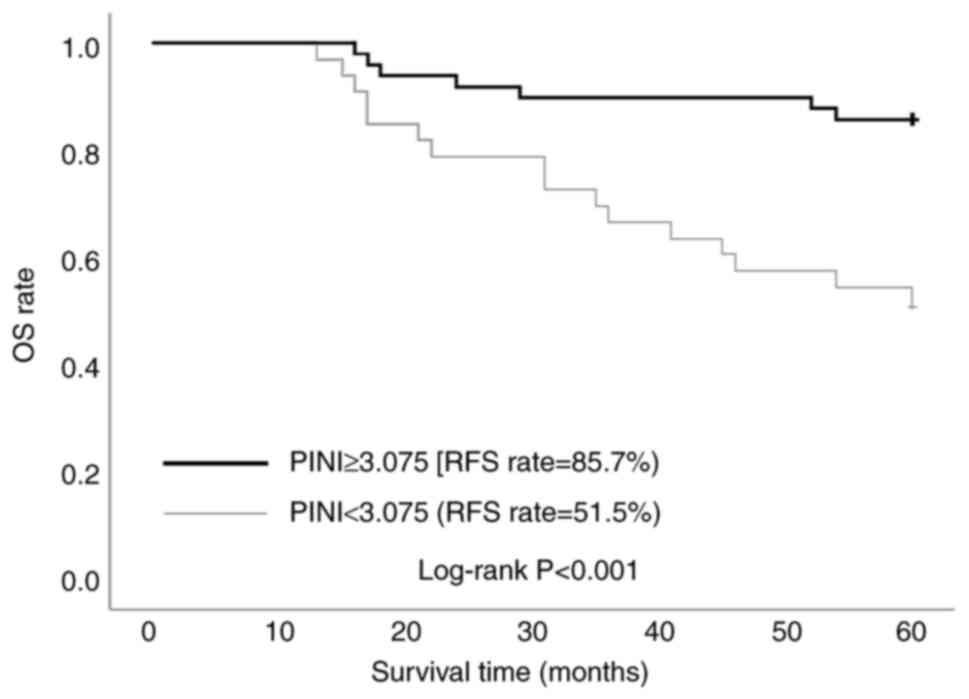
ROC curve analysis identified an optimal PINI cutoff value of 3.075 (AUC, 0.712), demonstrating moderate predictive accuracy with 71.2% sensitivity and 69.6% specificity (Fig. 1). A total of 82 patients (aged 39–85 years) were divided into the low-PINI group (n=33; 40.2%) and the high-PINI group (n=49; 59.8%). Figs. 2 and 3 present the Kaplan-Meier analysis of RFS and OS stratified by PINI values. In Fig. 2, the 5-year RFS rate was significantly higher in the high-PINI group (PINI ≥3.075) at 79.6% compared with 51.5% in the low-PINI group (PINI<3.075) (log-rank P<0.001). Similarly, in Fig. 3, a higher 5-year OS rate was demonstrated in the high-PINI group (85.7%) compared with that in the low-PINI group (51.5%) (log-rank P<0.001). These findings consistently indicate that elevated PINI levels are strongly associated with improved long-term survival outcomes, underscoring its potential as a significant prognostic biomarker in GC.
COX regression analysis of 5-year RFS in patients with GC after radical surgery
Table I presents the univariate and multivariate Cox regression analyses of factors associated with RFS in patients with GC. Univariate Cox regression analysis identified several significant prognostic factors for patients with GC, including surgical method (open vs. laparoscopic: HR, 0.33; 95% CI, 0.11–0.96; P=0.042), anemia (yes vs. no: HR, 3.16; 95% CI, 1.46–6.85; P=0.004), transfusion requirement (yes vs. no: HR, 3.04; 95% CI, 1.37–6.74; P=0.006), intraoperative blood loss (≥150 ml, yes vs. no: HR, 2.28; 95% CI, 1.05–4.94; P=0.036), operative time ≥210 min (yes vs. no: HR, 9.47; 95% CI, 2.84–31.63; P<0.001), tumor size ≥3.5 cm (yes vs. no: HR, 3.46; 95% CI, 1.50–7.98; P=0.004), T stage (T0-1 vs. T2-4: HR, 0.18; 95% CI, 0.06–0.51; P=0.001), nodal involvement (N0 vs. N1-3: HR, 0.14; 95% CI, 0.03–0.59; P=0.008), pathological stage (I vs. II–III: HR, 0.17; 95% CI, 0.05–0.57; P=0.004) and PINI ≥3.075 (yes vs. no: HR, 0.30; 95% CI, 0.13–0.68; P=0.004). In multivariate analysis, operative time ≥210 min (yes vs. no: HR, 5.38; 95% CI, 1.32–21.93; P=0.019) and pathological stage (I vs. II–III: HR, 0.63; 95% CI, 0.25–0.88; P=0.037) remained independent predictors, while PINI ≥3.075 retained significance as a protective factor (yes vs. no: HR, 0.59; 95% CI, 0.38–0.85; P=0.022).
Table I.Univariate and multivariate analysis for recurrence-free survival in patients with gastric cancer. |
COX regression analysis of 5-year OS in GC patients after radical surgery
Table II demonstrates the prognostic factors influencing OS in patients with GC through comprehensive Cox regression analyses. Univariate Cox regression analysis revealed several significant predictors of OS in patients with GC, including anemia (yes vs. no: HR, 2.89; 95% CI, 1.25–6.71; P=0.013), hypertension (yes vs. no: HR, 3.62; 95% CI, 1.07–12.28; P=0.039), hypoproteinemia (yes vs. no: HR, 3.62; 95% CI, 1.07–12.28; P=0.039), transfusion requirement (yes vs. no: HR, 3.13; 95% CI, 1.34–7.34; P=0.009), operative time ≥210 min (yes vs. no: HR, 11.56; 95% CI, 2.70–49.54; P<0.001), tumor size ≥3.5 cm (yes vs. no: HR, 3.95; 95% CI, 1.54–10.10; P=0.004), T stage (T0-1 vs. T2-4: HR, 0.23; 95% CI, 0.08–0.67; P=0.007), N stage (N0 vs. N1-3: HR, 0.08; 95% CI, 0.01–0.62; P=0.015), pathological stage (I vs. II–III: HR, 0.21; 95% CI, 0.06–0.72; P=0.013) and PINI ≥3.075 (yes vs. no: HR, 0.29; 95% CI, 0.12–0.69; P=0.005). Multivariate analysis confirmed that operative time ≥210 min (yes vs. no: HR, 7.12; 95% CI, 1.41–35.96; P=0.017) and pathological stage (I vs. II–III: HR, 0.32; 95% CI, 0.08–0.89; P=0.045) maintained their status as independent prognostic indicators. Importantly, a PINI score ≥3.075 continued to demonstrate significant protective effects (yes vs. no: HR, 0.45; 95% CI, 0.25–0.72; P=0.012) in the final model.
Patient characteristics
This comparative analysis of 82 patients with GC stratified by PINI levels (low-PINI: <3.075, n=33; high-PINI: ≥3.075, n=49) revealed significant differences in clinical and pathological characteristics (Table III). The low-PINI group demonstrated substantially higher rates of adverse features, including anemia (39.4 vs. 16.3%; P=0.020), hypoproteinemia (15.2 vs. 0.0%; P=0.005), pyloric stenosis (12.1 vs. 0%; P=0.013), transfusion requirements (33.3 vs. 12.2%; P=0.022) and postoperative complications (36.4 vs. 16.3%; P=0.039). Pathologically, patients in the low-PINI group showed more advanced disease with higher T2-4 stage (75.8 vs. 36.5%; P=0.002), nodal involvement (63.6 vs. 44.9%; P=0.002) and stage II–III tumors (87.9 vs. 44.9%; P<0.001). Notably, the median PINI values differed significantly between groups (2.77 vs. 3.39; P<0.001). These findings collectively demonstrate that low PINI status is strongly associated with worse nutritional parameters, more advanced tumor characteristics and poorer surgical outcomes, suggesting its potential as a comprehensive marker integrating both inflammatory and nutritional status for risk stratification in patients with GC.
Discussion
Hematological biomarkers offer distinct advantages, including accessibility, cost-effectiveness and reliable accuracy, with high patient acceptance and compliance. The PINI, as a representative hematological biomarker, has garnered increasing attention for its role in tumorigenesis, metastasis and antitumor immunity (13). The present retrospective analysis of patients with GC demonstrated that elevated PINI levels were significantly associated with improved RFS and OS rates. To the best of our knowledge, this represents the first study in China to systematically integrate PINI with clinicopathological parameters in patients with GC, not only providing novel insights for personalized treatment but also validating the prognostic value of PINI in this population. These findings establish PINI as both an independent prognostic factor and a potential biomarker for guiding nutritional support and optimizing therapeutic strategies. The optimal PINI cutoff value identified in the present study (3.075) warrants discussion in the context of existing literature. Jung et al (6) initially proposed a cutoff value of 3.0 for risk stratification in patients with colorectal cancer undergoing radical resection, while Xie et al (9) subsequently recommended 2.85 based on Chinese colorectal cancer cohorts. The observed variations in optimal thresholds may stem from several factors, including methodological differences in statistical approaches, inherent tumor heterogeneity across cancer types and demographic variations among study populations. These factors collectively contribute to the observed fluctuations in establishing prognostic PINI thresholds.
The clinical relevance of PINI stems from its integration of albumin and monocyte counts, reflecting both inflammatory and nutritional status. Inflammatory cytokines disrupt protein metabolism by altering the balance between synthesis and degradation. Albumin, a classical nutritional marker, is regulated by proinflammatory cytokines, including IL-1, IL-6 and TNF-α (14,15). Patients with GC frequently develop malnutrition (prevalence up to 80%) due to increased protein catabolism and reduced intake, leading to elevated mortality, prolonged hospitalization, postoperative complications and treatment toxicity (16,17). Systemic inflammation plays a pivotal role in tumor progression and metastasis (18–21), while monocytes, as key inflammatory markers, facilitate tumor progression by creating an immunosuppressive microenvironment (22). Elevated monocyte counts correlate with a poor prognosis and lymph node metastasis in multiple malignancies, including Kaposi sarcoma (23), chronic lymphocytic leukemia (24), myelodysplastic neoplasms (25), pancreatic cancer (26), lung cancer (27) and solid tumors (28). The present study findings revealed that patients with PINI≥3.075 had significantly higher 5-year RFS (P<0.001) and OS (P<0.001) rates, with PINI emerging as an independent protective factor (P<0.05) following radical gastrectomy.
Multivariate analysis confirmed pathological stage as an independent prognostic factor for both RFS and OS (P<0.05). Advanced tumors create a vicious cycle where increased tumor burden exacerbates systemic catabolism, while tumor-induced inflammation further suppresses immune function, accelerating disease progression (29,30). In the present study, the low-PINI group exhibited significantly higher rates of anemia, hypoproteinemia, pyloric stenosis, transfusion requirements and postoperative complications. Notably, the median PINI values differed significantly between groups. Pathological evaluation revealed more advanced disease in the low-PINI group, including deeper tumor invasion, greater nodal metastasis and advanced-stage tumors, suggesting that low PINI reflects exacerbated inflammation and malnutrition, which predict adverse outcomes. These findings support the potential clinical value of nutritional and immunomodulatory interventions for patients with low PINI. Prolonged operative time (≥210 min) was identified as an independent prognostic factor for both RFS and OS in patients with GC following surgery. This association may be attributed to several potential factors, including a larger tumor size with advanced staging, tumor invasion into the surrounding tissues leading to unclear surgical margins, technical difficulties in tissue dissection, intraoperative bleeding requiring hemostasis or conversion to open surgery in some cases.
The present study has several limitations that warrant consideration. First, the single-center retrospective design and relatively limited sample size may introduce selection bias, highlighting the need for future multicenter studies with larger cohorts to validate the findings. Second, the proposed PINI cutoff value was derived from a single institutional dataset, necessitating external validation through collaborative multicenter research to confirm its generalizability across diverse populations. Finally, the exclusive reliance on single-timepoint PINI measurements precluded assessment of dynamic changes in this parameter over time, suggesting that future prospective studies incorporating serial measurements would provide more comprehensive insights into its clinical utility.
In conclusion, the present study establishes PINI as a simple yet powerful prognostic tool for patients with GC undergoing radical resection. The study findings demonstrate the following: i) PINI ≥3.075 independently predicts superior long-term survival outcomes; ii) the index effectively stratifies patients by surgical risk and tumor aggressiveness; and iii) it provides complementary value to conventional TNM staging. The biological plausibility of PINI, reflecting both systemic inflammation and nutritional status, strengthens its clinical relevance. While requiring external validation, these results suggest PINI could be readily incorporated into routine preoperative assessment to identify high-risk patients who may benefit from nutritional optimization or intensified surveillance. Future research should focus on prospective multicenter validation and investigate whether PINI-guided interventions can improve clinical outcomes. The present study advances the field by providing clinicians with a practical, cost-effective tool to enhance personalized decision-making in gastric cancer management.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Science and Technology Project of Jingdezhen City, Jiangxi Province, China (number 20241SFZC012).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
JL and SZ were responsible for the study conceptualization and methodology. JL and LY were responsible for validation, investigation and resources. Data curation, which consisted of data collection, data cleaning, data organization, data analysis preparation, and quality assurance, was performed by JL, LY, XH, TH and MC. JL and LY confirm the authenticity of all the raw data. JL wrote the original draft, and JL and SZ reviewed and edited the manuscript. Study visualization was performed by JL and LY. SZ supervised the study, while project administration was performed by JL, LY, XH, TH and MC. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee at The First People's Hospital of Jingdezhen (Jingdezhen, China; approval no. jdzyykt202425). The requirement for informed consent was waived due to the retrospective nature of the study and data anonymization.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Li JJ, Rogers JE, Waters RE, Gan Q, Blum Murphy M and Ajani JA: Evolution of Therapeutics for Locally Advanced Upper Gastrointestinal Adenocarcinoma. Cancers (Basel). 17:13072025. View Article : Google Scholar : PubMed/NCBI | |
|
Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, et al: AJCC Cancer Staging Manual. 8th edition. Springer; New York, NY: 2017 | |
|
Aoyama T, Hashimoto I, Maezawa Y, Hara K, Tamagawa A, Cho H, Morita J, Tanabe M, Numata M, Kawahara S, et al: The clinical impact of the prognostic immune and nutritional index in gastric cancer patients who received curative treatment. Anticancer Res. 44:2231–2238. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Wang LJ, Li QY, Yuan Z, Zhang DC, Xu H, Yang L, Gu XH and Xu ZK: Prognostic value of preoperative immune-nutritional scoring systems in remnant gastric cancer patients undergoing surgery. World J Gastrointest Surg. 15:211–221. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Jung SH, Hao J, Shivakumar M, Nam Y, Kim J, Kim MJ, Ryoo SB, Choe EK, Jeong SY, Park KJ, et al: Development and validation of a novel strong prognostic index for colon cancer through a robust combination of laboratory features for systemic inflammation: A prognostic immune nutritional index. Br J Cancer. 126:1539–1547. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Evans DC, Corkins MR, Malone A, Miller S, Mogensen KM, Guenter P and Jensen GL; ASPEN Malnutrition Committee, : The use of visceral proteins as nutrition markers: An ASPEN position paper. Nutr Clin Pract. 36:22–8. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Chittezhath M, Dhillon MK, Lim JY, Laoui D, Shalova IN, Teo YL, Chen J, Kamaraj R, Raman L, Lum J, et al: Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Immunity. 41:815–829. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Xie H, Wei L, Liu M, Liang Y, Yuan G, Gao S, Wang Q, Lin X, Tang S and Gan J: Prognostic significance of preoperative prognostic immune and nutritional index in patients with stage I–III colorectal cancer. BMC Cancer. 22:13162022. View Article : Google Scholar : PubMed/NCBI | |
|
Japanese Gastric Cancer Association, . Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 24:1–21. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, et al: The Clavien-Dindo classification of surgical complications: Five-year experience. Ann Surg. 250:187–196. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Japanese Gastric Cancer Association, . Japanese classification of gastric carcinoma-2nd english edition -. Gastric Cancer. 1:10–24. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Kayikcioglu E and Iscan G: A novel prognostic index for metastatic colon cancer: The prognostic immune nutritional index. Cureus. 15:e338082023.PubMed/NCBI | |
|
Gupta D and Lis CG: Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr J. 9:692010. View Article : Google Scholar : PubMed/NCBI | |
|
Mantovani A, Allavena P, Sica A and Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Kanda M, Tanaka H, Shimizu D, Miwa T, Umeda S, Tanaka C, Kobayashi D, Hattori N, Suenaga M, Hayashi M, et al: SYT7 acts as a driver of hepatic metastasis formation of gastric cancer cells. Oncogene. 37:5355–5366. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Deftereos I, Kiss N, Isenring E, Carter VM and Yeung JM: A systematic review of the effect of preoperative nutrition support on nutritional status and treatment outcomes in upper gastrointestinal cancer resection. Eur J Surg Oncol. 46:1423–1434. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Aoyama T, Maezawa Y, Hashimoto I, Rino Y and Oshima T: Clinical impact of nutrition and inflammation assessment tools in pancreatic cancer treatment. Anticancer Res. 43:3849–3860. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Aoyama T, Kazama K, Maezawa Y and Hara K: Usefulness of nutrition and inflammation assessment tools in esophageal cancer treatment. In Vivo. 37:22–35. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Aoyama T, Hara K, Kazama K and Maezawa Y: Clinical impact of nutrition and inflammation assessment tools in gastric cancer treatment. Anticancer Res. 42:5167–5180. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Ju M, Aoyama T, Fukuda M, Ishiguro T, Kano K, Kazama K, Sawazaki S, Tamagawa H, Yukawa N and Rino Y: Prognostic value of the perioperative systemic inflammation score for patients with curatively resected gastric cancer. Cancer Diagn Progn. 2:627–633. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Mildner A, Kim KW and Yona S: Unravelling monocyte functions: From the guardians of health to the regulators of disease. Discov Immunol. 3:kyae0142024. View Article : Google Scholar : PubMed/NCBI | |
|
Neumeyer S and Tagawa T: The Kaposi sarcoma herpesvirus control of monocytes, macrophages, and the tumour microenvironment. Virology. 601:1102862025. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang Q, Peng Y, Herling CD and Herling M: The immunomodulatory mechanisms of BTK inhibition in CLL and beyond. Cancers (Basel). 16:35742024. View Article : Google Scholar : PubMed/NCBI | |
|
Janssen LLG, van Leeuwen-Kerkhoff N, Westers TM, de Gruijl TD and van de Loosdrecht AA: The immunoregulatory role of monocytes and thrombomodulin in myelodysplastic neoplasms. Front Oncol. 14:14141022024. View Article : Google Scholar : PubMed/NCBI | |
|
Li H, Peng S, An R, Du N, Wu H, Zhen X, Gao Y, Li Z and Min J: The prognostic role of lymphocyte-to-monocyte ratio in patients with resectable pancreatic cancer: A systematic review and meta-analysis. PeerJ. 12:e175852024. View Article : Google Scholar : PubMed/NCBI | |
|
Li MY, Wang YH, Zhang YL, Zhu WC, Li FF and Bian L: Research advances in tissue-resident macrophages and monocyte-derived macrophages in lung cancer. Zhonghua Bing Li Xue Za Zhi. 53:515–520. 2024.(In Chinese). PubMed/NCBI | |
|
Ammarah U, Pereira-Nunes A, Delfini M and Mazzone M: From monocyte-derived macrophages to resident macrophages-how metabolism leads their way in cancer. Mol Oncol. 18:1739–1758. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Liu QW, Liu L, Hu JX, Hou JQ, He WB, Shu YS and Wang XL: Nomogram based on a novel nutritional immune-inflammatory status score to predict postoperative outcomes in esophageal squamous cell carcinoma. World J Gastroenterol. 31:1017492025. View Article : Google Scholar : PubMed/NCBI | |
|
Wu C, Qi Z, Chen J, Yan X, Li S, Wang L, Yu L and Jiang Y: Prognostic value of inflammation and nutritional indicators for sinonasal squamous cell carcinoma: A single-center retrospective study. Laryngoscope Investig Otolaryngol. 10:e700462025. View Article : Google Scholar : PubMed/NCBI |