Analysis and exploration of the association between serum tumor marker status and clinical pathological features, as well as efficacy, in colorectal cancer
- Authors:
- Published online on: July 9, 2025 https://doi.org/10.3892/ol.2025.15184
- Article Number: 438
-
Copyright: © Xing et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
For patients with unresectable locally advanced or metastatic colorectal cancer (mCRC), pharmacotherapy remains the primary treatment modality, including chemotherapy, targeted therapy and treatment with immune checkpoint inhibitors (ICIs) (1,2). To optimize outcomes, the timely initiation of treatment, rapid assessment of its efficacy and more accurate identification of effective treatment populations continue to be hot topics in oncology research.
Tumor markers (TMs) are a class of bioactive substances produced and secreted into the bloodstream by cells in malignant tumor tissues, allowing for the quantification of serum-related biomarkers (3). These substances can indirectly reflect the occurrence, development, metastasis and degree of invasion of tumors, and may indicate the differentiation type of a tumor to some extent (4). Therefore, these markers play a positive role in the diagnosis, treatment and prognosis of tumors (5).
Currently, research on TMs of CRC predominantly focuses on three key aspects, including early screening and diagnosis, monitoring for postoperative recurrence, and evaluating pharmacotherapeutic efficacy in patients with advanced disease. However, in clinical practice, some patients exhibit no abnormal elevation of TMs in serum, whereas others show an abnormal elevation of one or more TMs, and it remains unclear whether these differences are related to the patients' clinical stage, treatment outcomes or prognosis. Furthermore, as tumor-associated antigens, the presence of TMs may be indicative of higher immunogenicity. In recent years, numerous attempts have been made to treat patients with advanced CRC using chemotherapy or targeted therapy combined with programmed cell death protein 1 (PD-1) monoclonal antibody therapy; however, the results have been unsatisfactory, and identifying an effective population remains challenging. Therefore, it is worth investigating whether patients with elevated levels of multiple TMs derive greater benefits from immunotherapy.
Microsatellite instability (MSI) refers to variations in the coding and non-coding sequences of microsatellites on chromosomes, specifically within repetitive DNA sequences (6). Immunotherapy has become the standard first-line treatment for patients with high MSI (MSI-H)/deficient mismatch repair (dMMR) CRC (7). However, non-MSI-H/dMMR patients comprise ~95% of the population (8), and only a small number of them benefit from immunotherapy. Therefore, the ability to accurately distinguish between these populations has significant implications for identifying those most likely to exhibit a treatment response.
The aim of the present study was to analyze the associations between TM expression and the clinical stage, tumor differentiation and tumor location in patients with CRC, as well as the number of metastatic sites, metastatic lesion diameter and MSI status in patients with advanced-stage disease, and their impact on treatment efficacy and prognosis.
Patients and methods
Patients
The present retrospective study analyzed data from 293 patients with CRC at stages I–III (n=103) or IV (n=190) who received treatment at the Oncology Center of Henan Provincial People's Hospital (Zhengzhou, China) between January 1, 2020, and June 30, 2023. In terms of sex, 178 patients were male, and 115 were female, with a median age of 59 years (range, 19–86 years). There were 223 and 70 cases involving adenocarcinoma in the left colon (including the rectum) and the right colon (including the transverse colon), respectively. In terms of pathological grading, 186 cases were classified as moderately to well-differentiated and 107 as poorly differentiated. The inclusion criteria for patients were as follows: i) A pathological diagnosis of colorectal adenocarcinoma; ii) early resectable CRC; iii) unresectable locally advanced or metastatic CRC; iv) an Eastern Cooperative Oncology Group (9) performance status of 0–2; v) an accurate patient history and data availability; and vi) availability of follow-up data. The exclusion criteria were as follows: i) A diagnosis of non-colorectal adenocarcinoma; ii) severe metabolic or cellular immune abnormalities with organ failure; and iii) the presence of other concurrent tumors. The follow-up period concluded in October 2024. Informed consent was obtained from all patients before initiating treatment.
Detection of serum carcinoembryonic antigen (CEA) and cancer antigen (CA)19-9, CA72-4 and CA125
Serum concentrations of CEA, CA19-9, CA72-4 and CA125 were determined from routine clinical tests immediately before initiating treatment. The quantitative detection of serum tumor markers (CEA, CA19-9, CA72-4 and CA125) was performed using the Electrochemiluminescence Immunoassay method, utilizing the Cobas 6000 602 fully automated electrochemiluminescence immunoassay analyzer produced by Roche Diagnostics GmbH. All testing reagents were used with the corresponding Elecsys® reagent kits: Elecsys CEA (cat. no. 04491777190), Elecsys CA19-9 (cat. no. 11776193122), Elecsys CA72-4 (cat. no. 09005692190) and Elecsys CA 125 II (cat. no. 11776223190), also manufactured by Roche Diagnostics GmbH. The normal levels of CEA, CA19-9, CA72-4 and CA125 were defined as ≤5.0 ng/ml, ≤35.0 U/ml, ≤6.9 U/ml and ≤35.0 U/ml, respectively. When the serum levels exceeded the critical values for a marker, the results were considered positive, and patients were categorized into negative, single TM elevation and multiple TM elevation (≥2 TMs) groups according to the number of positive markers.
Clinical response evaluation
Treatment efficacy was evaluated using the Response Evaluation Criteria in Solid Tumors (version 1.1) (10). Responses were categorized as complete remission (CR), partial remission (PR), stable disease (SD) and progressive disease (PD). The objective response rate (ORR) included CR and PR cases, whereas the disease control rate (DCR) included CR, PR and SD cases.
Statistical analysis
Data analysis was performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp.). Count data are represented as n (%), and χ2 tests were used for comparisons between two or more groups. For cases that did not meet the conditions of the χ2 tests, Fisher's tests were used. Progression-free survival (PFS) time was calculated from the first day of chemotherapy until the occurrence of disease progression. PFS was assessed using one-way ANOVA, with Bonferroni's post hoc test. Univariate survival analysis was conducted using the Kaplan-Meier method and the log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical and pathological characteristics
The clinical and pathological characteristics of the 293 patients are summarized in Table I. Among the 103 patients with disease stages of I–III, the highest proportion of individuals with negative TM elevation was observed in those with a T stage of 1–2. The results demonstrate an association between tumor marker status and T-stage (P=0.01). Among the 293 patients, the negative group had the highest proportion of stage I–II patients, whereas the multiple TM elevation group had the highest proportion of patients with stage IV disease, and there was a significant association between the number of elevated TMs and clinical staging (P<0.001). The multiple TM elevation group had the largest proportion of patients with poorly differentiated tumors, whereas the negative TM elevation group had the largest proportion of patients with moderately to well-differentiated tumors (P<0.001). The multiple-elevation group showed the highest proportions of both peritoneal metastasis and liver metastasis (P<0.001). The number of elevated TMs was significantly associated with tumor differentiation status and metastatic sites. The TRUSTY study prospectively established 6 cm as the biologically and clinically validated cutoff for the sum of metastatic lesion diameters (11), which was similarly adopted in the present study. When the sum of the diameters of the metastatic lesions was ≥6 cm, there were significant differences between the three groups (P<0.001), whereas no significant differences were observed when the diameter was <6 cm. The number of elevated TMs was significantly associated with the tumor burden. TM expression levels were not associated with the N stage, tumor location or MSI status (Table II).
TM positivity rates
In advanced-stage patients, the number of cases and positivity rates for CEA, CA19-9, CA72-4 and CA125 were 121 (63.7%), 80 (42.1%), 38 (20.0%) and 40 (21.1%), respectively. In this subset, the number of cases and proportions of patients with negative elevation, single TM elevation and multiple TM elevations were 43 (22.6%), 64 (33.7%) and 83 (43.7%), respectively. When any two markers were combined, the number of cases and positivity rate ranged from 61 (32.1%) to 138 (72.6%).
Analysis of first-line treatment efficacy in patients with stage IV disease
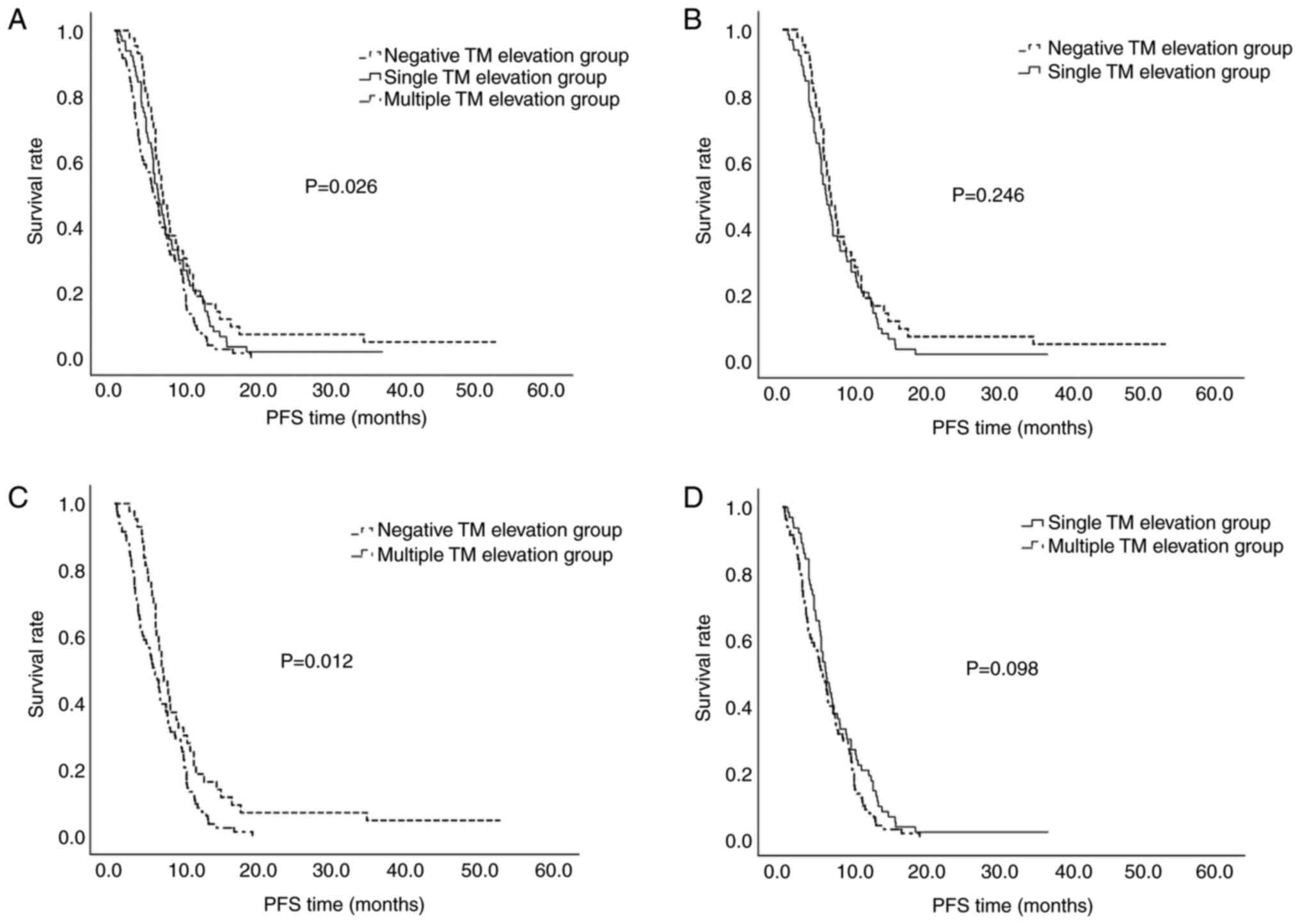
Treatment responses were compared between the TM elevation groups in the 190 patients with stage IV CRC. There were no significant differences in the ORR and DCR between the three groups; however, there was a decreasing trend in PFS among them (P=0.007, Table III; P=0.026, Fig. 1). The difference between the negative TM elevation group and the multiple TM elevation group was particularly pronounced (P=0.012; Fig. 1).
Table III.Comparison of treatment responses between TM elevation groups among patients with stage IV colorectal cancer. |
Analysis of third-line treatment efficacy in non-MSI-H/dMMR patients
There were 73 non-MSI-H/dMMR patients who received combination therapy with ICIs and tyrosine kinase inhibitors (TKIs) as a third-line treatment regimen. The number of cases and proportions of patients receiving certain ICIs were as follows: Sintilimab, 39 (53.4%); camrelizumab, 9 (12.3%); tislelizumab, 15 (20.5%); triplimab, 3 (4.1%); pembrolizumab, 3 (4.1%); and other ICIs, 4 (5.5%). For TKIs, regorafenib was administered in 25 (34.2%) of cases, while the other 48 (65.8%) received fruquintinib.
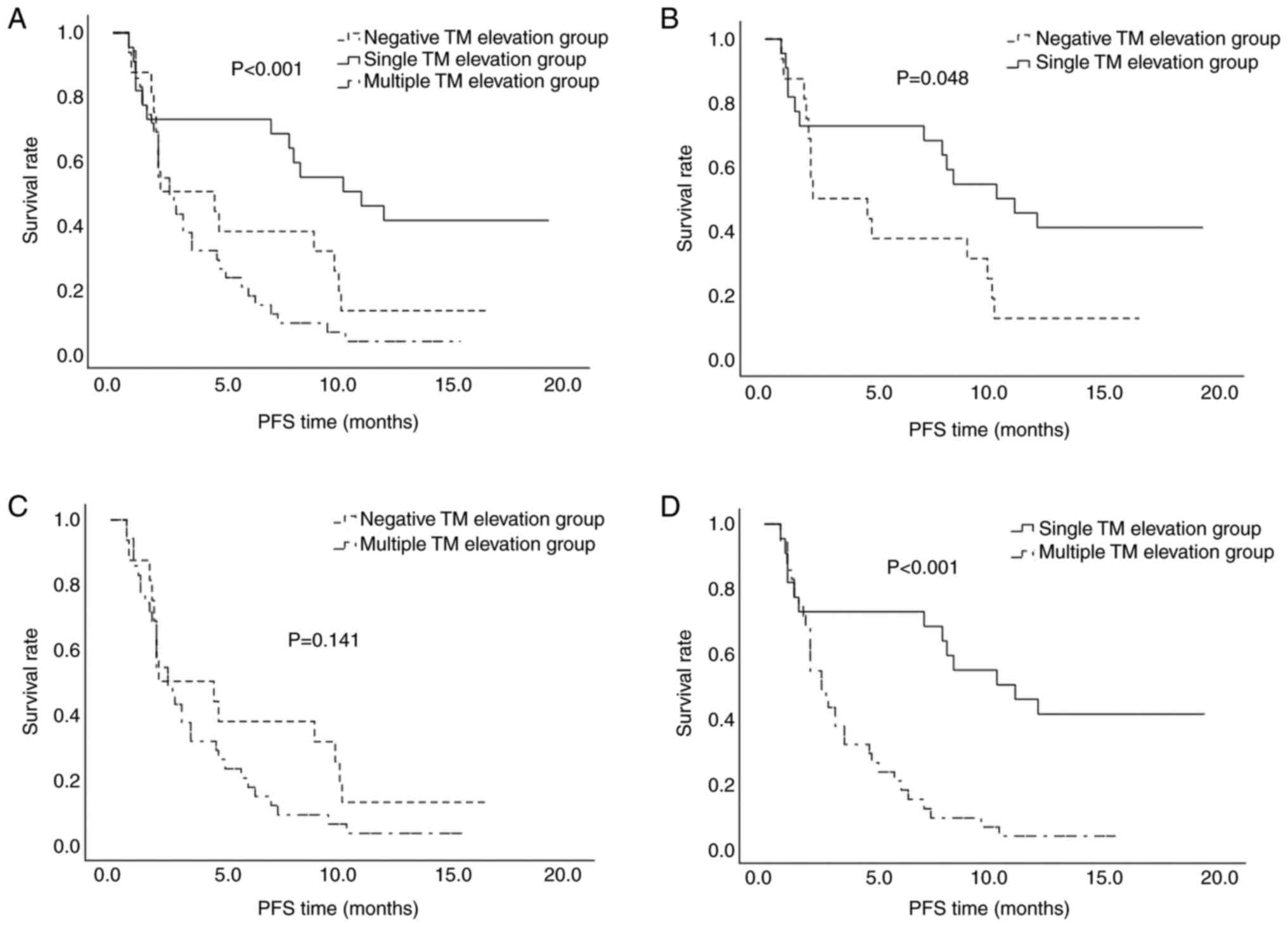
There were no significant differences in the ORR between the three groups, whereas the DCR for the single TM elevation group was significantly higher than that of the other groups at 72.7%. The results demonstrate significant differences in DCR among the three groups (P=0.017) (Table III). PFS also differed significantly based on the number of elevated TMs (P<0.001) (Table III; Fig. 2). Pairwise comparisons revealed a significant difference between the negative TM and single TM elevation groups (P=0.031), as well as between the single and multiple TM elevation groups (P<0.001) (Fig. 2).
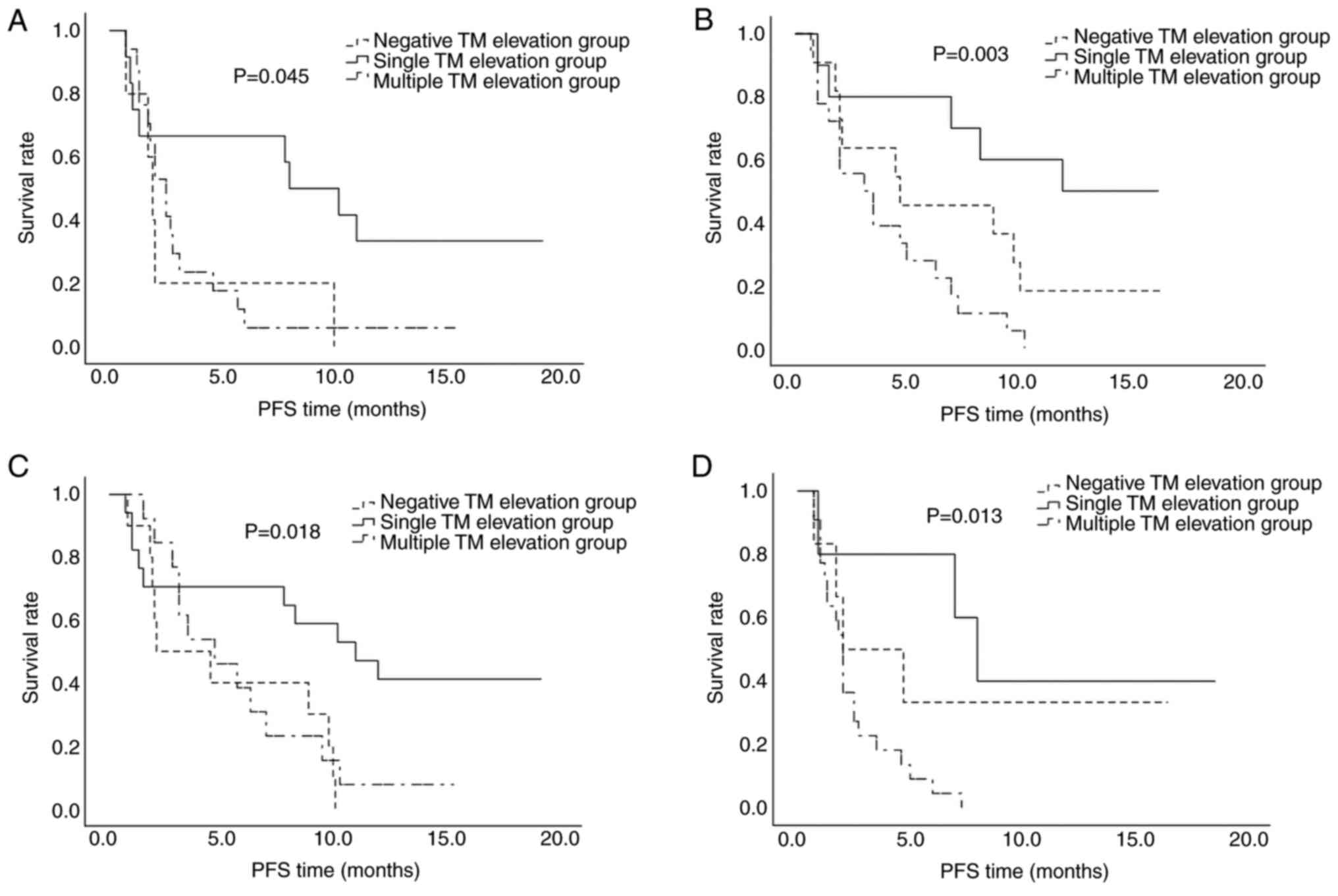
When the sum of the diameters of the metastatic lesions was ≥6 cm, a significant intergroup difference was observed in the DCR, with the highest value (66.7%) in the single TM elevation group (P=0.046). The median PFS time for the negative, single TM elevation and multiple TM elevation groups was 1.9, 8.0 and 2.5 months, respectively (P=0.015, Table IV; P=0.037, Fig. 3), indicating superior disease control in the single TM elevation group. When the diameter of the metastatic lesions was <6 cm, the median PFS time for the negative, single TM elevation and multiple TM elevation groups was 4.7, 12.2 and 3.1 months, respectively (P=0.004, Table IV; P=0.002, Fig. 3), indicating that when the tumor diameter was <6 cm, the PFS time was longer.
Table IV.Comparison of differences in immune checkpoint inhibitor + tyrosine kinase inhibitor treatment for non-high microsatellite instability/deficient mismatch repair patients with metastatic lesion diameter at different TM levels. |
Differential analysis of the neutrophil-to-lymphocyte ratio (NLR) in non-MSI-H/dMMR patients
The patients were divided into two groups according to their NLR. Based on previous studies, the cut-off value of NLR was defined as 3 (12,13). In those with an NLR <3, the median PFS time for the negative, single and multiple TM elevation groups was 2.1, 11 and 4.7 months, respectively (P=0.021, Table V; P=0.013, Fig. 3). In those with an NLR >3, the DCR was the highest in the single elevation group (P=0.017), and the median PFS for the same three groups was 2.0, 8.0 and 2.0 months, respectively (P=0.002, Table V; P=0.013, Fig. 3). Patients with an NLR <3 tended to benefit more from treatment with ICIs + TKIs.
Table V.Comparison of differences in patients with different NLR ratios at various TM levels in non-high microsatellite instability/deficient mismatch repair patients. |
Discussion
The pathogenesis and progression of CRC are progressive evolutionary processes involving multiple genes (14–16). Owing to the complex etiology and numerous risk factors associated with the disease (17,18), the molecular mechanisms underlying the pathophysiology of CRC remain unclear (19). Numerous studies have shown that certain TMs, such as CEA, CA125, CA19-9 and CA72-4, are highly correlated with both the occurrence and progression of CRC (20). Given the lack of specific serum biomarkers for CRC, the use of these markers could have significant clinical implications, especially when three or more indicators are combined to facilitate the early screening and diagnosis of CRC (20,21). In the present study, the serum levels of these TMs were analyzed in 190 patients with unresectable advanced or metastatic CRC. The positivity rates for CEA, CA19-9, CA72-4 and CA125 in this population were 63.7, 42.1, 20.0 and 21.1%, respectively, which is consistent with previous research findings (22,23).
Large and numerous metastatic lesions are usually associated with a poor prognosis (24). In the present study, in patients with advanced-stage disease, a total metastatic lesion diameter of ≥6 cm was significantly associated with the elevation of multiple TMs. Among these patients, 61.7% exhibited elevations in multiple TMs, and combined TM elevations can be indicative of the individual tumor burden. Concurrently, metastatic sites demonstrated a significant association with concurrent elevation of multiple TMs. Peritoneal metastasis often contributes to poor prognostic outcomes in patients with cancer, and these findings indicate that patients with peritoneal metastasis are likely to have a greater number of elevated TMs, which may be associated with worse outcomes.
TMs can also be used to monitor the prognosis of patients undergoing chemotherapy (25,26). In a previous study, in patients with CRC with elevated levels of either CEA or CA19-9, the 5-year survival rates were reported to be 53 and 51%, respectively, whereas patients with elevated levels of both markers had a significantly lower 5-year survival rate of only 23% (23). The present study found that the median PFS time for the three groups of patients with advanced-stage disease was 6.6, 5.9 and 5.4 months, respectively. As the number of elevated TMs increased, PFS decreased, and the number of elevated TMs was negatively associated with PFS after treatment.
Studies have demonstrated that MSI-H individuals are likely to benefit from ICI therapy. For example, in the KEYNOTE-016 study, patients with dMMR or MSI-H mCRC who failed to respond to standard treatment exhibited an ORR as high as 40% after receiving immunotherapy targeting PD-1 (pembrolizumab) (27,28). The subsequent CheckMate-142 study confirmed that dMMR or MSI-H populations do experience significant benefits from PD-1 monoclonal antibody immunotherapy (29). Collectively, these findings indicate that MSI can serve as a potential predictive biomarker of a patient's response to immunotherapy. Such populations typically exhibit higher immunogenicity; however, the MSI and MSI-H status was evaluated in both the TM-negative and TM-positive groups in the present study, and the results did not support this notion. Nonetheless, there was an observed trend towards an increase in the proportion of elevated TMs in the MSI-H group. Notably, the number of patients with MSI-H included in this study was small, necessitating further validation with a larger sample size.
The non-MSI-H/dMMR population accounts for ~95% of patients with advanced CRC (8); however, these patients often struggle to achieve a beneficial effect from ICI monotherapy. Therefore, combination therapies with ICIs and TKIs are being increasingly administered; however, a number of patients still do not respond to such treatment. The present study sought to identify which patients would be the most likely to benefit from this combination therapy. A puzzling phenomenon was observed in the 73 patients receiving third-line ICI + TKI treatment; more specifically, those with a single elevated TM derived more benefits from the combination therapy compared to that achieved by the negative TM and multiple TM elevation groups, and this effect was unrelated to the tumor burden.
Previous studies reported a 5-year survival rate of 77.2% among patients with localized CRC who have a low preoperative NLR, whereas the rate was much lower at only 50.8% in those with a high preoperative NLR (30,31). Therefore, a combined analysis of the effect of elevated TMs and the NLR on treatment efficacy was conducted in the present study, which revealed that the patients with a single elevated TM exhibited higher response rates and improved PFS times when treated with ICIs in combination with TKIs, particularly in the subgroup with an NLR <3; those patients experienced a PFS time of up to 11 months. These data suggest that patients with a single elevated TM and an NLR <3 may represent a favorable treatment population for combination therapies involving ICIs and TKIs.
In summary, quantifying multiple TMs simultaneously can improve diagnostic sensitivity. The elevation of multiple TMs is associated with factors such as staging, differentiation, metastatic site, tumor burden and advanced stages of cancer. In the present study, an increase in the number of elevated TMs was linked to poorer PFS. Among the non-MSI-H/dMMR patients, those with a single elevated TM were more likely to benefit from combination therapies involving ICIs and TKIs, particularly in those who also had an NLR <3. Collectively, these findings suggest that this group may represent an optimal population for combination therapy with ICI + TKI, and the study provides valuable insights that could facilitate the design of treatment regimens and improve the prognosis of patients with CRC, albeit needing further validation at a larger scale.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
ZX, YX, SL, GJ, PC, YC, JZ, LS and CL were involved in the conception and design of the study. Preparation of materials, data collection and analysis were conducted by ZX and YX. ZX, YX and CL confirm the authenticity of all the raw data. The first draft of the manuscript was written by ZX, and all authors commented on the previous manuscript version. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Medicine Ethics Committee of Henan Provincial People's Hospital (Zhengzhou, China; approval no. 2023-221-02). Informed consent was obtained in writing from all individual participants included in the study.
Patient consent for publication
Written informed consent was obtained from the patients for publication of this original article and accompanying images.
Competing interests
The authors declare that they have no competing interests.
References
|
Rawla P and Barsouk A, Hadjinicolaou AV and Barsouk A: Immunotherapies and targeted therapies in the treatment of metastatic colorectal cancer. Med Sci (Basel). 7:832019.PubMed/NCBI | |
|
Morris VK, Kennedy EB, Baxter NN, Benson AB III, Cercek A, Cho M, Ciombor KK, Cremolini C, Davis A, Deming DA, et al: Treatment of metastatic colorectal cancer: ASCO Guideline. J Clin Oncol. 41:678–700. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou Y, Tao L, Qiu J, Xu J, Yang X, Zhang Y, Tian X, Guan X, Cen X and Zhao Y: Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct Target Ther. 9:1322024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang C, Qin C, Dewanjee S, Bhattacharya H, Chakraborty P, Jha NK, Gangopadhyay M, Jha SK and Liu Q: Tumor-derived small extracellular vesicles in cancer invasion and metastasis: Molecular mechanisms, and clinical significance. Mol Cancer. 23:182024. View Article : Google Scholar : PubMed/NCBI | |
|
Cao Y and Wang X: Effects of molecular markers on the treatment decision and prognosis of colorectal cancer: A narrative review. J Gastrointest Oncol. 12:1191–1196. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Lorenzi M, Amonkar M, Zhang J, Mehta S and Liaw KL: Epidemiology of microsatellite instability high (MSI-H) and deficient mismatch repair (dMMR) in solid tumors: A structured literature review. J Oncol. 2020:18079292020. View Article : Google Scholar | |
|
Schrock AB, Ouyang C, Sandhu J, Sokol E, Jin D, Ross JS, Miller VA, Lim D, Amanam I, Chao J, et al: Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol. 30:1096–1103. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Maio M, Ascierto PA, Manzyuk L, Motola-Kuba D, Penel N, Cassier PA, Bariani GM, De Jesus Acosta A, Doi T, Longo F, et al: Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: Updated analysis from the phase II KEYNOTE-158 study. Ann Oncol. 33:929–938. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Azam F, Latif MF, Farooq A, Tirmazy SH, AlShahrani S, Bashir S and Bukhari N: Performance status assessment by using ECOG (Eastern Cooperative Oncology Group) score for cancer patients by oncology healthcare professionals. Case Rep Oncol. 12:728–736. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al: New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Masuishi T, Kuboki Y, Terazawa T, Nakamura M, Watanabe J, Ojima H, Makiyama A, Kotaka M, Hara H, Ohta T, et al: Exploratory analysis of baseline tumor burden in the TRUSTY study: A randomized phase 2/3 study of trifluridine/tipiracil plus bevacizumab versus irinotecan and fluoropyrimidine plus bevacizumab as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 40 (4_suppl):S872022. View Article : Google Scholar | |
|
Hoshino S, Takeuchi M, Kawakubo H, Matsuda S, Mayanagi S, Irino T, Fukuda K, Nakamura R, Wada N and Kitagawa Y: Usefulness of Neutrophil to Lymphocyte ratio at recurrence for predicting long-term outcomes in patients with recurrent esophageal squamous cell carcinoma. Ann Surg Oncol. 28:3001–3008. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang GY, Yang Y, Li H, Zhang J, Jiang N, Li MR, Zhu HB, Zhang Q and Chen GH: A scoring model based on neutrophil to lymphocyte ratio predicts recurrence of HBV-associated hepatocellular carcinoma after liver transplantation. PLoS One. 6:e252952011. View Article : Google Scholar : PubMed/NCBI | |
|
Ogino S and Goel A: Molecular classification and correlates in colorectal cancer. J Mol Diagn. 10:13–27. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Grady WM and Carethers JM: Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 135:1079–1099. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Abolghasemi Fard A and Mahmoodzadeh A: Unraveling the progression of colon cancer pathogenesis through epigenetic alterations and genetic pathways. Cureus. 16:e595032024.PubMed/NCBI | |
|
Keum N and Giovannucci E: Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 16:713–732. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Roshandel G, Ghasemi-Kebria F and Malekzadeh R: Colorectal cancer: Epidemiology, risk factors, and prevention. Cancers (Basel). 16:15302024. View Article : Google Scholar : PubMed/NCBI | |
|
Malki A, ElRuz RA, Gupta I, Allouch A, Vranic S and Al Moustafa AE: Molecular mechanisms of colon cancer progression and metastasis: Recent insights and advancements. Int J Mol Sci. 22:1302021. View Article : Google Scholar | |
|
Gao Y, Wang J, Zhou Y, Sheng S, Qian SY and Huo X: Evaluation of Serum CEA, CA19-9, CA72-4, CA125 and ferritin as diagnostic markers and factors of clinical parameters for colorectal cancer. Sci Rep. 8:27322018. View Article : Google Scholar : PubMed/NCBI | |
|
Lech G, Słotwiński R, Słodkowski M and Krasnodębski IW: Colorectal cancer tumour markers and biomarkers: Recent therapeutic advances. World J Gastroenterol. 22:1745–1755. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Yang AP, Liu J, Lei HY, Zhang QW, Zhao L and Yang GH: CA72-4 combined with CEA, CA125 and CAl9-9 improves the sensitivity for the early diagnosis of gastric cancer. Clin Chim Acta. 437:183–186. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Lakemeyer L, Sander S, Wittau M, Henne-Bruns D, Kornmann M and Lemke J: Diagnostic and prognostic value of CEA and CA19-9 in colorectal cancer. Diseases. 9:212021. View Article : Google Scholar : PubMed/NCBI | |
|
Shan Q, Fan Y, Guo J, Han X, Wang H and Wang Z: Relationship between tumor size and metastatic site in patients with stage IV non-small cell lung cancer: A large SEER-based study. PeerJ. 7:e78222019. View Article : Google Scholar : PubMed/NCBI | |
|
Zeng WG, Liang JW, Wang Z, Zhang XM, Hu JJ, Hou HR, Zhou HT and Zhou ZX: Clinical parameters predicting pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer. Chin J Cancer. 34:468–474. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Li C, Zhao K, Zhang D, Pang X, Pu H, Lei M, Fan B, Lv J, You D, Li Z and Zhang T: Prediction models of colorectal cancer prognosis incorporating perioperative longitudinal serum tumor markers: A retrospective longitudinal cohort study. BMC Med. 21:632023. View Article : Google Scholar : PubMed/NCBI | |
|
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Le DT, Uram JN, Wang H, Bartlett B, Kemberling H, Eyring A, Saba Azad N, Donehower RC, Crocenzi TS, Goldberg RM, et al: Programmed death-1 blockade in mismatch repair deficient colorectal cancer. J Clin Oncol. 34 (15_suppl):S1032016. View Article : Google Scholar | |
|
Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, et al: Durable clinical benefit with Nivolumab Plus Ipilimumab in DNA mismatch Repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 36:773–779. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM and Kerin MJ: The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review. J Surg Oncol. 115:470–479. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Kim JH, Lee JH, Lee HS, Shin SJ, Park EJ, Cho ES, Baik SH, Lee KY and Kang J: Elevated Neutrophil-to-lymphocyte ratio in perioperative periods is suggestive of poor prognosis in patients with colorectal cancer. J Inflamm Res. 14:4457–4466. 2021. View Article : Google Scholar : PubMed/NCBI |