Proteomics analysis reveals elevated RAB21 in serum‑derived extracellular vesicles from patients with follicular thyroid carcinoma
- Authors:
- Published online on: July 16, 2025 https://doi.org/10.3892/ol.2025.15190
- Article Number: 444
-
Copyright : © Kawakami et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
Introduction
Papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC) are major components of differentiated primary thyroid carcinoma. PTC is the most common type, followed by FTC, with the latter accounting for approximately 10–15% of all thyroid malignancies (1). Although both originate from thyroid cells, they have rather different clinical behaviors. PTC frequently invades adjacent organs, such as the recurrent laryngeal nerve, esophagus, and trachea, but this is rare in FTC. Regarding metastases and recurrences, PTC frequently metastasizes to regional lymph nodes, whereas distant metastasis/recurrence is predominant for FTC (2). When determining diagnoses, it is important to differentiate both malignancies from benign types; namely, between PTC and adenomatous nodule (AN) and between FTC and follicular thyroid adenoma (FTA). Cytological examination, with relatively low invasiveness, can distinguish PTC from AN. However, preoperative differentiation between FTC and FTA has remained difficult, because FTC and FTA have overlapping cytomorphological features and the diagnosis of FTC requires histopathological proof of tumor capsule invasion and/or vascular invasion. Although FTC, like PTC, generally has a favorable prognosis if appropriately managed, its prognosis can be limited in patients with certain clinicopathological features. Therefore, diagnostic surgery is recommended for patients presenting with suspicious follicular nodules, with the aim of evaluating the presence of capsular or vascular invasion; however, the malignancy rate is quite low, leading to a large proportion of suspicious follicular nodules being pathologically confirmed as benign tumors after surgery (3,4). Based on these limitations, there is an urgent need to develop a preoperative noninvasive approach to discriminate between FTC and FTA because this would enable the accurate diagnosis of thyroid follicular tumors in clinical practice.
Extracellular vesicles (EVs) are membrane particles released by cells. Although various classification systems exist for EVs, they are commonly categorized based on their size, such as small EVs (50–200 nm) and large EVs (200–1000 nm), or by their biogenesis pathways, such as exosomes and microvesicles (5). Exosomes, a type of small EV, are formed through the endosomal pathway, a cellular membrane trafficking route. They originate from early endosomes, progress to multivesicular bodies, and are released into the extracellular space. A notable characteristic of exosomes is that they contain proteins, nucleic acids, and lipids from the cells from which they derive (6).
EVs are present not only in the immediate vicinity of the cells that release them, but also in bodily fluids, including the blood. This suggests that analyzing EVs released by diseased tissues and circulating in the bloodstream could provide insights into pathological conditions. Consequently, EVs are considered a promising tool for liquid biopsy and are expected to facilitate disease diagnosis (7,8).
In light of their potential, our study implemented an unbiased exploratory approach to compare the protein profiles of serum EVs between FTC and FTA patients. Through the comprehensive proteomic screening of EVs using nano liquid chromatography-tandem mass spectrometry (nanoLC-MS/MS), we aimed to identify differentially expressed proteins that could distinguish between these conditions. Rather than focusing on predetermined targets, we sought to discover novel biomarkers and potential therapeutic targets for FTC, addressing the critical need for the improved preoperative diagnosis of thyroid follicular tumors.
Materials and methods
Patients
The protocol of this study was approved by the Medical Review Boards of Teikyo University (approval no. 14-019-6), Tokyo Metropolitan Institute for Geriatrics and Gerontology (approval no. 6179), and Kanaji Thyroid Hospital (approval no. 9). All methods were carried out in accordance with the relevant guidelines and regulations (Declaration of Helsinki). Serum from patients with FTC (10 patients), FTA (14 patients), and follicular tumor of uncertain malignant potential (FT-UMP) (1 patient) was collected between May 2015 and January 2018, and the serum samples were stored at −80°C until use. Patient demographics are summarized in Table SI. Pathological diagnoses were made according to the WHO classification (5th edition) (9).
Purification of EVs
EVs were purified from human serum by the phosphatidylserine affinity method (10,11). Briefly, the extracellular domain of mouse Tim4 and the Fc region of human IgG1 (FUJIFILM Wako Pure Chemical, Osaka, Japan) were biotinylated and conjugated to streptavidin magnetic beads (Thermo Fisher Scientific, Waltham, MA, USA). Serum (500 µl) was centrifuged at 10,000 × g for 30 min and then filtered through a 0.22-µm filter. The filtered sample was diluted twofold with Tris-buffered saline (TBS) and incubated for 1 h at room temperature (RT) with Tim4-conjugated beads in the presence of 2 mM CaCl2. After washing with 0.01% Tween-TBS containing 2 mM CaCl2, the captured EVs were eluted with 2 mM EDTA in phosphate-buffered saline (PBS). Purified EVs were stored at −80°C until later use.
Measurement of protein concentration
The protein concentrations of purified EVs were quantified using the CBQCA protein quantitation kit (Thermo Fisher Scientific) according to the manufacturer's protocol. The fluorescence intensity was measured using an EnVision plate reader (PerkinElmer, Waltham, MA, USA).
Measurement of EV size
The size of the purified EVs was measured using the qNano particle analyzer (Izon Science, Christchurch, New Zealand). Purified EVs were diluted in 0.01% Tween-PBS buffer and measured using an NP100 nanopore. Calibration was performed using CPC100 as a standard control. Data were analyzed using Izon control software (version 3.4.2.51).
Western blot analysis
Purified EVs were mixed with sample buffer (62.5 mM Tris-HCl pH 6.8, 2% SDS, 10% glycerol, 0.01% bromophenol blue, and 5% 2-mercaptoethanol) and 130 ng of protein was loaded for SDS-polyacrylamide gel electrophoresis. Equal protein loading was used as there is no established loading control for EVs, particularly for human serum-derived EVs where the amount of purified EVs varies significantly between individuals even from the same volume. The separated proteins were then transferred to PVDF membranes (Millipore, Burlington, MA, USA). After blocking with 1% skim milk, the membranes were probed with a primary antibody, followed by a horseradish peroxidase (HRP)-linked secondary antibody. After washing, bound proteins were visualized using the ImmunoStar LD (FUJIFILM Wako Pure Chemical) as an HRP substrate and the FUSION Chemiluminescence Imaging System (Vilber, Marne-la-Vallée, France) as a detection instrument. The antibodies used in this study were as follows: anti-Alix (ab186429; Abcam, Cambridge, UK), anti-flotillin-2 (610383; BD Transduction Laboratories, Franklin Lakes, NJ, USA), anti-CD9 (13174; Cell Signaling Technology, Danvers, MA, USA), anti-RAB21 (NBP2-93771; Novus Biologicals, Centennial, CO, USA), and anti-GAPDH (2118; Cell Signaling Technology), along with secondary anti-mouse IgG (715-035-151) and anti-rabbit IgG (711-035-152) antibodies (Jackson ImmunoResearch, West Grove, PA, USA). The signal intensities of the obtained data were measured using ImageJ software (version 1.54d) (https://imagej.net/software/imagej/). Uncropped western blot images are provided in Fig. S1.
Proteomic analysis
Protein digestion with trypsin was performed prior to proteomic analysis. EVs were dissolved in 0.1% Rapigest SF (Waters, Milford, MA, USA), reduced with 10 mM dithiothreitol in 50 mM triethylammonium bicarbonate (TEAB) at RT for 30 min, and then alkylated with 55 mM iodoacetamide in 50 mM TEAB at RT for 30 min in the dark. The samples were digested with trypsin (Promega, Madison, WI, USA) in 50 mM TEAB at 37°C for 16 h. The cleaved peptides were desalted using a GL-Tip SDB column (GL Sciences, Tokyo, Japan) and evaporated in a SpeedVac vacuum concentrator (Thermo Fisher Scientific). The peptides were reconstituted in 0.1% formic acid (FA) and subjected to nanoLC-MS/MS analysis.
Measurements were performed by nanoLC-MS/MS using an Ultimate 3000 RSLCnano system (Thermo Fisher Scientific) coupled to a Q Exactive hybrid quadrupole-Orbitrap mass spectrometer with a nanoESI source (Thermo Fisher Scientific), as previously described (12). The nanoLC system was equipped with a trap column (C18 PepMap 100, 0.3×5 mm, 5 µm particle size; Thermo Fisher Scientific) and an analytical column (NTCC-360/75-3-125, 75 µm × 12 cm, 3 µm particle size, C18; Nikkyo Technos, Tokyo, Japan). Peptides were separated over a period of 90 min using a gradient of water containing 0.1% FA (mobile phase A) and acetonitrile containing 0.1% FA (mobile phase B) at a flow rate of 300 nl/min. The elution gradient was set as follows: 0–3 min, 2% B; 3–93 min, 2–40% B; 93–95 min, 40–95% B; 95–105 min, 95% B; 105–107 min, 95–2% B; and 107–120 min, 2% B. The mass spectrometer was operated in data-dependent acquisition mode. All data were analyzed for protein identification and label-free quantification using Proteome Discoverer 2.4 software (Thermo Fisher Scientific). The analytical parameters used for the database search were as follows: search engine, Sequest HT; protein database, Swiss-prot (Homo Sapiens); enzyme name, trypsin (full); dynamic modification, oxidation (methionine); static modification, carbamidomethyl (cysteine); precursor mass tolerance: 10 ppm, fragment mass tolerance: 0.02 Da, missed cleavage: 2, and false discovery rate (FDR) <0.05. The protein abundances were normalized to the median of all proteins detected in each sample and compared between FTC and FTA using NormalyzerDE (https://normalyzerde.immunoprot.lth.se/) (13).
Gene expression analysis of FTC using a public database
To estimate the expressions of genes in thyroid cancer tissues, we used the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/gds/). We analyzed the GSE15045 dataset, which comprises gene expression data from 4 FTA patients and 8 FTC patients, using the GEO2R web tool (https://www.ncbi.nlm.nih.gov/geo/geo2r/). This analysis allowed us to compare the gene expression profiles of FTA and FTC tissues.
Cell culture
The human FTC cell line, FTC-133, was obtained from the European Collection of Authenticated Cell Cultures (UK Health Security Agency, Porton Down, UK). FTC-133 cells were cultured in DMEM/Ham's F12 medium (FUJIFILM Wako Pure Chemical) containing 10% fetal bovine serum (FBS) (Thermo Fisher Scientific), 100 units/ml penicillin, and 100 µg/ml streptomycin (FUJIFILM Wako Pure Chemical).
Small interfering RNA transfection
Dicer-substrate short interfering RNAs (DsiRNAs) for the human RAB21 gene (109142326) and negative control DsiRNA (51-01-14-03) were purchased from Integrated DNA Technologies (Coralville, IA, USA). The sequences of DsiRNAs are shown in Table SII. DsiRNAs were transfected into FTC-133 cells at a concentration of 2 nM using the Lipofectamine RNAiMax transfection reagent (13778150; Thermo Fisher Scientific), in accordance with the manufacturer's instructions. After 6 h of incubation, the medium was changed to DMEM/Ham's F-12 medium supplemented with 10% FBS. After a 2-day culture, the transfected cells were used for western blot analysis, and cell invasion and migration assays.
Cell invasion assay
DsiRNA-transfected cells were seeded at 2×104 cells onto a Matrigel invasion chamber (354480; Corning, Corning, NY, USA) and incubated at 37°C under a 5% CO2 atmosphere. After 24 h, the cells were fixed with 100% methanol and stained with crystal violet, and then, the number of invaded cells was determined. The invasion rate was represented as the ratio of the number of invaded cells for RAB21-knockdown cells compared with that for control cells.
Cell migration assay
Transfected cells were cultured in a six-well plate until they reached confluence. Cell layers were wounded using a 1 ml pipette tip and cultured for 30 h. The wound area was analyzed based on photographs taken at 0 and 30 h using ImageJ software, and the cell migration rate was calculated from the ratio of each void area at 30 h to that at 0 h, as follows:
Migration rate (%)=[1-(void area at 30 h/void area at 0 h)]x100.
Statistical analysis
The statistical significance of differences was determined using limma (14) for proteomic analysis. Unpaired Student's t-test was used for analysis of RAB21 in serum-derived EVs from FTC and FTA patients. For analysis of RAB21 mRNA expression from the GEO database, Welch's t-test was performed. For cell invasion and migration assays, data were analyzed using Mann-Whitney U test. P<0.05 was considered to indicate a statistically significant difference.
Results
Confirmation of purified EVs
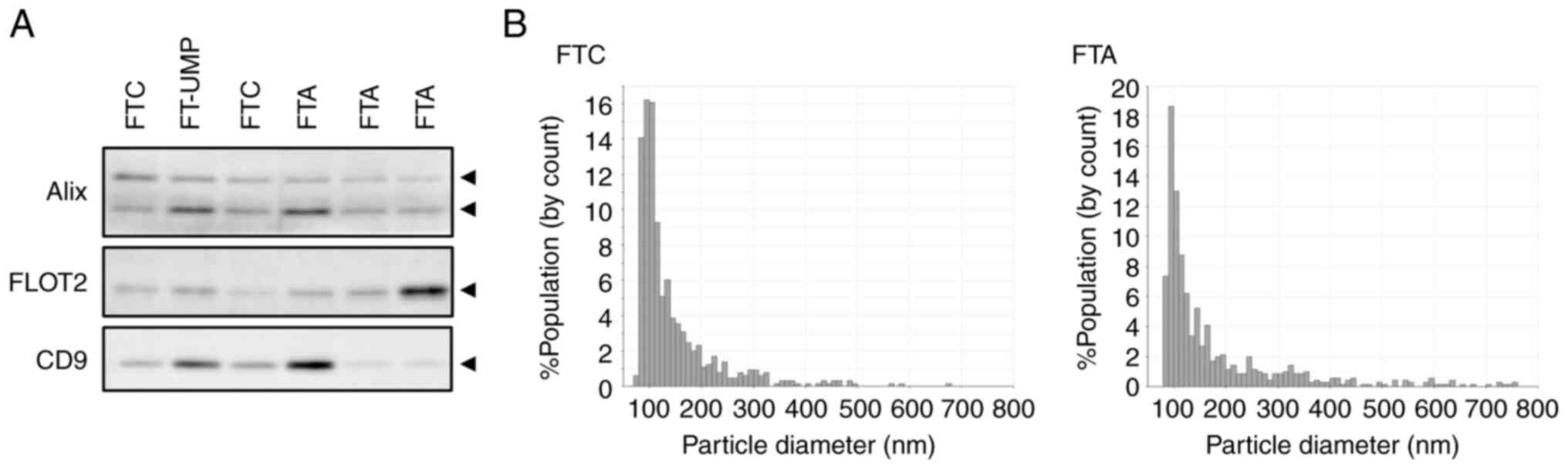
Serum EVs from patients were purified using the Tim4 molecule, which has affinity for phosphatidylserine. The purified serum EVs were confirmed by detecting EV marker proteins and measuring their particle size characteristics. The analysis of serum EVs purified from patients revealed the presence of the EV markers Alix, Flotillin-2, and CD9, which are commonly found in small EVs (Figs. 1A and S1A). Furthermore, the particle sizes were mainly distributed around 100 nm, which corresponds to the size of small EVs, and there were no substantial differences in this variable among the patients (Fig. 1B).
Proteomics of serum EVs purified from FTA and FTC patients
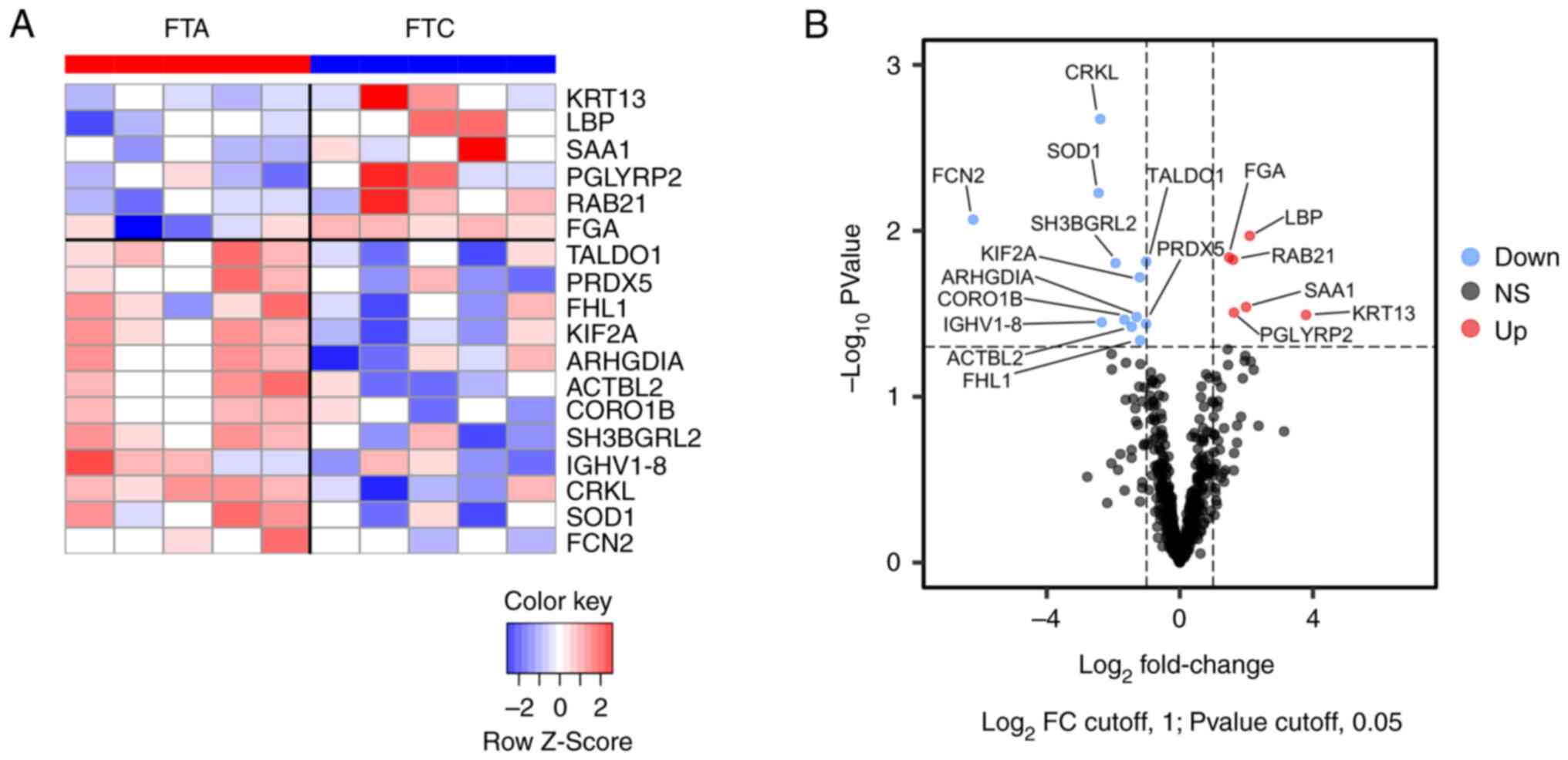
Proteomic analysis was performed on the EVs purified from the serum of FTC and FTA patients using an LC-MS/MS instrument to identify differentially expressed proteins. The results identified 639 proteins (Table SIII). Upon comparing the normalized protein abundances between FTC and FTA EVs, we found that 6 proteins had more than a 2-fold increase and 12 proteins had less than 0.5-fold expression in EVs from FTC patients (Fig. 2 and Table I).
Table I.Up- and downregulated proteins in serum EVs of FTC patients isolated using PS affinity method. |
Several proteins related to cancer development and progression were identified in the list of abundant proteins in FTC, including Ras-related protein Rab-21 (RAB21) (15), Keratin 13 (KRT13) (16), and Serum amyloid A1 (SAA1) (17). In contrast, the deficient proteins in FTC included antioxidative proteins such as Peroxiredoxin 5 (PRDX5), Superoxide dismutase 1 (SOD1), and Transaldolase (TALDO1). Furthermore, Four and a half LIM domains protein 1 (FHL1), which is a tumor suppressor gene in thyroid cancer (18,19), was downregulated in FTC.
Validation of upregulated RAB21 in EVs purified from the serum of FTC patients
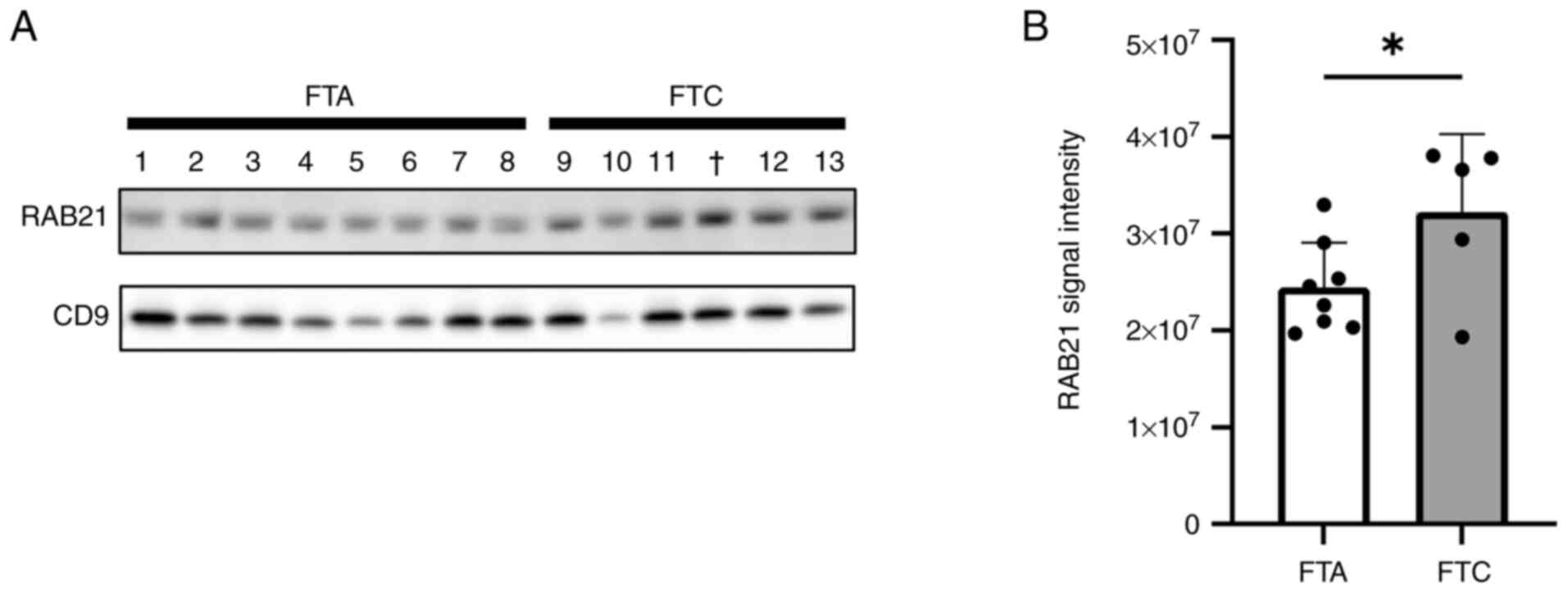
RAB21, a member of the Rab family of small GTPases, has been reported to modulate cell adhesion and migration processes (15). Therefore, we focused on RAB21, and attempted to validate its upregulation in FTC serum EVs using western blot analysis, as well as in FTC tissues through in silico analyses. First, we examined the RAB21 levels in serum EVs by western blot analysis using CD9 as an EV marker and confirmed that RAB21 was more abundant in the serum EVs of FTC patients than in those of FTA patients (Figs. 3 and S1B). Second, we determined whether RAB21 was actually present in EVs, rather than being a contaminant from the blood, because proteins that are abundant in the blood inevitably contaminate EVs purified from serum. We analyzed the levels of RAB21 in blood using the Protein Atlas database (https://www.proteinatlas.org/humanproteome/blood/proteins+detected+in+ms). Compared with the other upregulated proteins, the RAB21 level was remarkably low (Fig. S2), so we considered that RAB21 was derived from EVs and was not a contaminant from the blood, and that FTC tissues might contain abundant RAB21. Third, we estimated RAB21 mRNA expression in FTC and FTA tissues using the GSE15045 dataset from the GEO public database. This suggests that RAB21 mRNA was significantly upregulated in FTC compared with the level in FTA (Fig. S3). These findings indicate that RAB21 is upregulated in FTC tissues and that its levels are increased in serum EVs from FTC patients.
Effects of RAB21 knockdown on the malignant phenotype of FTC-133 cells
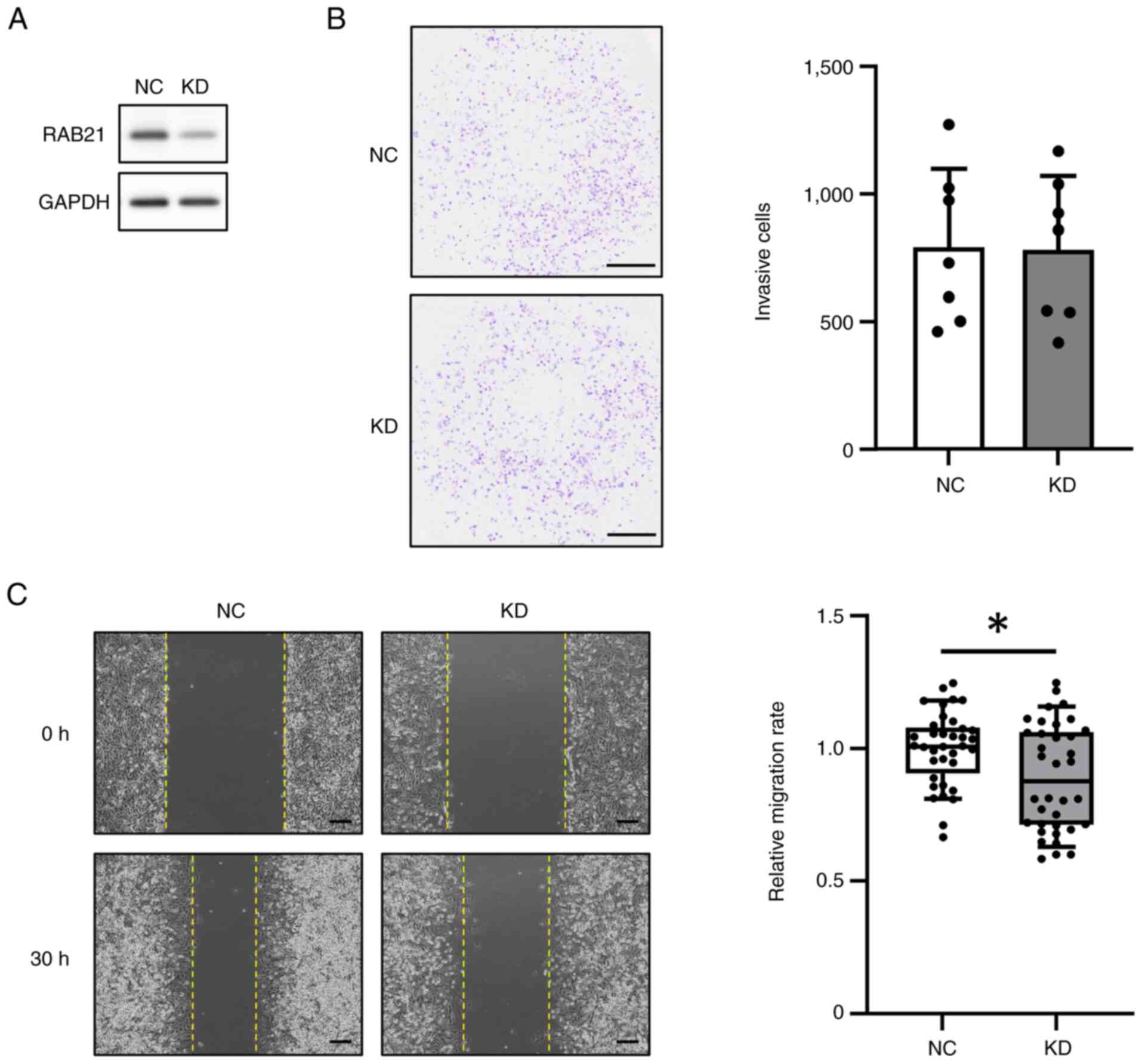
Because RAB21 gene expression was increased in the tissues of FTC patients, we considered that RAB21 might be involved in the malignant phenotype of tumors. Therefore, we examined whether the knockdown (KD) of RAB21 expression in FTC-133 cells, a follicular thyroid carcinoma cell line, might affect malignant phenotypes such as invasion and migration. RAB21-KD in FTC-133 cells was performed using DsiRNA and Lipofectamine RNAiMAX, and confirmed by western blot analysis (Figs. 4A and S1C). Cell invasion was performed using a Matrigel invasion chamber for 24 h. The results showed that the invasion of RAB21-KD cells did not differ from that of the negative control (NC) (Fig. 4B). However, the cell migration rate calculated from the change in wound area during 30 h was significantly decreased in RAB21-KD cells (Fig. 4C). Taken together, these findings suggest that RAB21-KD did not affect cell invasion but reduced the migration of FTC-133 cells under these experimental conditions.
Discussion
A major problem in the clinical diagnosis of FTC is that discriminating between this malignant carcinoma and benign FTA is difficult. As a result, a definitive diagnosis is typically made only after the surgical removal of the tumor.
Recent efforts to differentiate FTC from FTA have primarily focused on identifying molecular markers using formalin-fixed, paraffin-embedded tissues. Regarding gene markers, genetic mutation analysis revealed FLT3, TP53, and RET were candidate markers for detecting malignancies in follicular lesions (20). Moreover, another study using a gene expression microarray and qRT-PCR identified CPQ, PLVAP, TFF3, and ACVRL1 as potential biomarkers (21). Regarding protein markers, CD56, HBME-1, Galectin-3, and CK19 were reported to be useful for differentiating FTC from FTA based on an immunohistochemical approach (22). Moreover, proteomic analysis demonstrated that ferroptosis pathways were altered in malignant follicular carcinoma (23). Regarding DNA methylation markers, Zhang et al (24) identified 70 DNA methylation haplotype block markers, many of which were located in promoter regions (24). Furthermore, combining the detection of thyrotropin receptor mRNA in circulating tumor cells with fine-needle aspiration biopsy results has been reported to improve diagnostic accuracy (25). Additionally, Zabegina et al (26) demonstrated that miRNAs of the let-7 family were upregulated in plasma EVs from FTC patients (26).
In this study, we focused on proteins in serum EVs because they might be directly involved in the malignant phenotype of FTC and thus, provide therapeutic targets for FTC. The comparison of protein profiles of serum EVs between FTC and FTA demonstrated that RAB21 protein in serum EVs was elevated in FTC patients and was a potential marker for discriminating FTC preoperatively. Moreover, blood can be collected repeatedly with minimal invasiveness, so this approach might enable the monitoring of disease recurrence (27).
RAB21 is involved in intracellular vesicular trafficking, specifically in the early endosome pathway of endocytosis, the mechanism by which cells internalize external substances (28). In terms of the relationship between RAB21 expression in tissues and cancer, Lin et al (29) and Anand et al (30) demonstrated that high RAB21 expression was significantly correlated with poor overall survival in breast invasive carcinoma (BRCA) and pancreatic ductal adenocarcinoma (PDAC), using Gene Expression Profiling Interactive Analysis and The Cancer Genome Atlas databases, respectively (29,30). Regarding the intracellular functions of RAB21, Pellinen et al (15) reported that it regulated cancer invasion and migration via integrin-β1 in breast cancer cell lines (15). Integrins expressed on the cell surface provide adhesion between cells and the extracellular matrix (31). Mai et al (32) revealed that RAB21 indirectly influenced cancer cell invasion and migration capabilities by mediating integrin endocytosis in breast cancer cells, forming part of a dynamic trafficking system where its competitive binding to p120RasGAP determined integrin localization between endosomes and the cell surface (32). While RAB21 knockdown affected cell migration in our study, no significant difference was observed in invasion. This observation suggests that RAB21 primarily regulates integrin-mediated migration, whereas invasion requires additional processes such as extracellular matrix degradation through matrix metalloproteinases (33). In addition, the silencing of RAB21 in glioma cell lines has been shown to induce apoptosis and inhibit cell proliferation (34). Pei et al (35) also demonstrated that RAB21 plays a crucial role in regulating the recycling of glucose transporter SLC2A1/GLUT1 to the cell membrane, thus maintaining glucose uptake and energy homeostasis in cancer cells. Their findings suggested that RAB21 is essential for cancer cell survival and proliferation, particularly in the glucose-deprived tumor microenvironment (35). Moreover, a recent study reported that EVs derived from head and neck squamous cell carcinoma carrying RAB21 homed to lung macrophages and were incorporated into them through an interaction with integrin-β1 on the macrophage surface, eventually inducing the immunosuppression of these cells (36). These findings suggest that increased RAB21 expression may contribute to the malignant progression of tumors. Therefore, we analyzed the function of RAB21 in malignant FTC-133 cells and found that RAB21 knockdown reduced their migratory ability. The results are consistent with those of previous studies, and indicate that RAB21 may be involved in the malignant phenotype of FTC.
Our study had several limitations. The Tim4-based phosphatidylserine affinity method may introduce false-positive results because of potential contamination and false-negative findings by missing certain EV subpopulations. Sample collection limited to specific Japanese hospitals may create geographic and demographic biases, potentially restricting the generalizability of the results. Additionally, our relatively small sample size affects statistical power and reliability, and certain experimental conditions may require further optimization. Future research should employ multiple EV isolation techniques in larger, more diverse cohorts through multi-center collaborations to validate and extend our findings.
In conclusion, this study searched for biomarkers to distinguish between FTC and FTA, focusing on the proteins contained in serum EVs. Based on the proteomic analysis of serum EVs in patients and functional cell culture studies, we found that RAB21 in serum EVs might be a discriminant marker for FTC and that it could play an important role in the malignant phenotype of FTC. Further larger-scale clinical studies are necessary to validate our findings; however, to the best of our knowledge, this is the first study to describe a protein marker found in serum EVs with the potential to discriminate between FTC and FTA. This is particularly valuable because serum EVs are readily available for preoperative evaluation via a minimally invasive procedure. Moreover, our findings on the functional role of RAB21 in cancer cell migration provide insights into the molecular mechanisms underlying the malignant progression of FTC, potentially opening new avenues for therapeutic interventions.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgments
Not applicable.
Funding
This work was supported by JSPS KAKENHI [grant no. 18K08490 (to KM)].
Availability of data and materials
The data generated in the present study may be requested from the corresponding author. The mass spectrometry proteomics data generated in the present study may be found in the ProteomeXchange Consortium via the jPOST partner repository under accession numbers PXD064847 for ProteomeXchange and JPST003852 for jPOST or at the following URL: https://repository.jpostdb.org/entry/JPST003852.
Authors' contributions
KK conceived and designed the study, performed data curation and formal analysis, conducted the investigation, managed project administration, performed data validation, created figures and wrote the original manuscript. NE, KM, TI, HO and TF contributed to study design, histopathological evaluation and sample selection, provided clinical interpretation, and contributed to reviewing and editing the manuscript. HT and KU participated in proteome data analysis and interpretation, and contributed to reviewing and editing the manuscript. MI contributed to study design, and to reviewing and editing the manuscript. YM contributed to data analysis and provided expertise in the interpretation of all data, supervised the study, wrote the original draft, and contributed to reviewing and editing the manuscript. KK and YM confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The protocol of this study was approved by the Medical Review Boards of Teikyo University (approval no. 14-019-6), Tokyo Metropolitan Institute for Geriatrics and Gerontology (approval no. 6179), and Kanaji Thyroid Hospital (approval no. 9). All methods were carried out in accordance with relevant guidelines and regulations (Declaration of Helsinki). Written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
AN |
adenomatous nodule |
|
DsiRNAs |
Dicer-substrate short interfering RNAs |
|
EVs |
extracellular vesicles |
|
FA |
formic acid |
|
FBS |
fetal bovine serum |
|
FTA |
follicular thyroid adenoma |
|
FTC |
follicular thyroid carcinoma |
|
FT-UMP |
follicular tumor of uncertain malignant potential |
|
GEO |
Gene Expression Omnibus |
|
HRP |
horseradish peroxidase |
|
KD |
knockdown |
|
nanoLC-MS/MS |
nano liquid chromatography-tandem mass spectrometry |
|
PBS |
phosphate-buffered saline |
|
PTC |
papillary thyroid carcinoma |
|
RT |
room temperature |
|
TBS |
Tris-buffered saline |
|
TEAB |
triethylammonium bicarbonate |
References
|
Veschi V, Verona F, Lo Iacono M, D'Accardo C, Porcelli G, Turdo A, Gaggianesi M, Forte S, Giuffrida D, Memeo L and Todaro M: Cancer stem cells in thyroid tumors: From the origin to metastasis. Front Endocrinol (Lausanne). 11:5662020. View Article : Google Scholar : PubMed/NCBI | |
|
Ito Y and Miyauchi A: Prognostic factors of papillary and follicular carcinomas based on pre-, intra-, and post-operative findings. Eur Thyroid J. 13:e2401962024. View Article : Google Scholar : PubMed/NCBI | |
|
Conzo G, Avenia N, Ansaldo GL, Calò P, De Palma M, Dobrinja C, Docimo G, Gambardella C, Grasso M, Lombardi CP, et al: Surgical treatment of thyroid follicular neoplasms: Results of a retrospective analysis of a large clinical series. Endocrine. 55:530–538. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Staubitz JI, Musholt PB and Musholt TJ: The surgical dilemma of primary surgery for follicular thyroid neoplasms. Best Pract Res Clin Endocrinol Metab. 33:1012922019. View Article : Google Scholar : PubMed/NCBI | |
|
Welsh JA, Goberdhan DCI, O'Driscoll L, Buzas EI, Blenkiron C, Bussolati B, Cai H, Di Vizio D, Driedonks TAP, Erdbrugger U, et al: Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. 13:e124042024. View Article : Google Scholar : PubMed/NCBI | |
|
Raposo G and Stoorvogel W: Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. 200:373–383. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Andre M, Caobi A, Miles JS, Vashist A, Ruiz MA and Raymond AD: Diagnostic potential of exosomal extracellular vesicles in oncology. BMC Cancer. 24:3222024. View Article : Google Scholar : PubMed/NCBI | |
|
Feng X, Iliuk A, Zhang X, Jia S, Shen A, Zhang W, Hu L and Tao WA: Supramolecular exosome array for efficient capture and in situ detection of protein biomarkers. Anal Chem. 95:2812–2821. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
WHO classification of tumours online, Endocrine and neuroendocrine Tumours (5th ed.), . https://tumourclassification.iarc.who.int/chapters/532024 18–October. 2024 | |
|
Kawakami K, Fujita Y, Kato T, Horie K, Koie T, Umezawa K, Tsumoto H, Miura Y, Katagiri Y, Miyazaki T, et al: Diagnostic potential of serum extracellular vesicles expressing prostate-specific membrane antigen in urologic malignancies. Sci Rep. 11:150002021. View Article : Google Scholar : PubMed/NCBI | |
|
Nakai W, Yoshida T, Diez D, Miyatake Y, Nishibu T, Imawaka N, Naruse K, Sadamura Y and Hanayama R: A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci Rep. 6:339352016. View Article : Google Scholar : PubMed/NCBI | |
|
Hishida S, Kawakami K, Fujita Y, Kato T, Takai M, Iinuma K, Nakane K, Tsuchiya T, Koie T, Miura Y, et al: Proteomic analysis of extracellular vesicles identified PI3K pathway as a potential therapeutic target for cabazitaxel-resistant prostate cancer. Prostate. 81:592–602. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Willforss J, Chawade A and Levander F: NormalyzerDE: Online tool for improved normalization of omics expression data and high-sensitivity differential expression analysis. J Proteome Res. 18:732–740. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Smyth GK: Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 3:Article32004. View Article : Google Scholar : PubMed/NCBI | |
|
Pellinen T, Arjonen A, Vuoriluoto K, Kallio K, Fransen JA and Ivaska J: Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins. J Cell Biol. 173:767–780. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Li Q, Yin L, Jones LW, Chu GC, Wu JB, Huang JM, Li Q, You S, Kim J, Lu YT, et al: Keratin 13 expression reprograms bone and brain metastases of human prostate cancer cells. Oncotarget. 7:84645–84657. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang G, Sun X, Lv H, Yang X and Kang X: Serum amyloid A: A new potential serum marker correlated with the stage of breast cancer. Oncol Lett. 3:940–944. 2012.PubMed/NCBI | |
|
Fryknas M, Wickenberg-Bolin U, Goransson H, Gustafsson MG, Foukakis T, Lee JJ, Landegren U, Hoog A, Larsson C, Grimelius L, et al: Molecular markers for discrimination of benign and malignant follicular thyroid tumors. Tumour Biol. 27:211–220. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Chen J, Zeng C, Jin J, Zhang P, Zhang Y, Zhang H, Li Y and Guan H: Overexpression of FHL1 suppresses papillary thyroid cancer proliferation and progression via inhibiting Wnt/β-catenin pathway. Endocrine. 85:238–249. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Borowczyk M, Szczepanek-Parulska E, Debicki S, Budny B, Verburg FA, Filipowicz D, Wieckowska B, Janicka-Jedyńska M, Gil L, Ziemnicka K and Ruchała M: Differences in mutational profile between follicular thyroid carcinoma and follicular thyroid adenoma identified using next generation sequencing. Int J Mol Sci. 20:31262019. View Article : Google Scholar : PubMed/NCBI | |
|
Wojtas B, Pfeifer A, Oczko-Wojciechowska M, Krajewska J, Czarniecka A, Kukulska A, Eszlinger M, Musholt T, Stokowy T, Swierniak M, et al: Gene expression (mRNA) markers for differentiating between malignant and benign follicular thyroid tumours. Int J Mol Sci. 18:11842017. View Article : Google Scholar : PubMed/NCBI | |
|
Alshenawy HA: Utility of immunohistochemical markers in differential diagnosis of follicular cell-derived thyroid lesions. J Microsc Ultrastruct. 2:127–136. 2014. View Article : Google Scholar | |
|
Sun Y, Li L, Zhou Y, Ge W, Wang H, Wu R, Liu W, Chen H, Xiao Q, Cai X, et al: Stratification of follicular thyroid tumours using data-independent acquisition proteomics and a comprehensive thyroid tissue spectral library. Mol Oncol. 16:1611–1624. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang H, Zhang Z, Liu X, Duan H, Xiang T, He Q, Su Z, Wu H and Liang Z: DNA methylation haplotype block markers efficiently discriminate follicular thyroid carcinoma from follicular adenoma. J Clin Endocrinol Metab. 106:1011–1021. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Milas M, Mazzaglia P, Chia SY, Skugor M, Berber E, Reddy S, Gupta M and Siperstein A: The utility of peripheral thyrotropin mRNA in the diagnosis of follicular neoplasms and surveillance of thyroid cancers. Surgery. 141:137–146; discussion 146. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Zabegina L, Nazarova I, Knyazeva M, Nikiforova N, Slyusarenko M, Titov S, Vasilyev D, Sleptsov I and Malek A: MiRNA let-7 from TPO(+) extracellular vesicles is a potential marker for a differential diagnosis of follicular thyroid nodules. Cells. 9:19172020. View Article : Google Scholar : PubMed/NCBI | |
|
Vasconcelos MH, Caires HR, Ābols A, Xavier CPR and Linē A: Extracellular vesicles as a novel source of biomarkers in liquid biopsies for monitoring cancer progression and drug resistance. Drug Resist Updat. 47:1006472019. View Article : Google Scholar : PubMed/NCBI | |
|
Simpson JC, Griffiths G, Wessling-Resnick M, Fransen JA, Bennett H and Jones AT: A role for the small GTPase Rab21 in the early endocytic pathway. J Cell Sci. 117:6297–6311. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Lin S, Cao C, Meng Y, Wu P, Gao P, Zhi W, Peng T, Wu P and Gui L: Comprehensive analysis of the value of RAB family genes in prognosis of breast invasive carcinoma. Biosci Rep. 40:BSR202011032020. View Article : Google Scholar : PubMed/NCBI | |
|
Anand S, Khan MA, Khushman M, Dasgupta S, Singh S and Singh AP: Comprehensive analysis of expression, clinicopathological association and potential prognostic significance of RABs in pancreatic cancer. Int J Mol Sci. 21:55802020. View Article : Google Scholar : PubMed/NCBI | |
|
Paul NR, Jacquemet G and Caswell PT: Endocytic trafficking of integrins in cell migration. Curr Biol. 25:R1092–R1105. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Mai A, Veltel S, Pellinen T, Padzik A, Coffey E, Marjomäki V and Ivaska J: Competitive binding of Rab21 and p120RasGAP to integrins regulates receptor traffic and migration. J Cell Biol. 194:291–306. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Friedl P and Wolf K: Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat Rev Cancer. 3:362–374. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Ge J, Chen Q, Liu B, Wang L, Zhang S and Ji B: Knockdown of Rab21 inhibits proliferation and induces apoptosis in human glioma cells. Cell Mol Biol Lett. 22:302017. View Article : Google Scholar : PubMed/NCBI | |
|
Pei Y, Lv S, Shi Y, Jia J, Ma M, Han H, Zhang R, Tan J and Zhang X: RAB21 controls autophagy and cellular energy homeostasis by regulating retromer-mediated recycling of SLC2A1/GLUT1. Autophagy. 19:1070–1086. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Wu K, Li Y, Ji Y, Liu C, Wang X, Guo H, Zhang J and He Y: Tumor-derived RAB21+ABHD12+ sEVs drive the premetastatic microenvironment in the lung. Cancer Immunol Res. 12:161–179. 2024. View Article : Google Scholar : PubMed/NCBI |